associated with increased risk of oral mucositis, including chemotherapy and/or radiotherapy, and/or hematopoietic stem cell transplantation.
|
|
|
- Helen Palmer
- 5 years ago
- Views:
Transcription
1 Medical Coverage Policy Low-Level Laser Therapy EFFECTIVE DATE: POLICY LAST UPDATED: OVERVIEW Low-level laser therapy (LLLT), also called photobiomodulation, is being evaluated to treat various conditions including oral mucositis, myofascial pain, joint pain, lymphedema, and chronic wounds. MEDICAL CRITERIA PRIOR AUTHORIZATION POLICY STATEMENT BlueCHiP for Medicare Low-level laser therapy is covered for prevention of oral mucositis in patients undergoing cancer treatment associated with increased risk of oral mucositis, including chemotherapy and/or radiotherapy, and/or hematopoietic stem cell transplantation. Low-level laser therapy is not covered for all other indications, as the evidence is insufficient to determine the effects of the technology on health outcomes, including but not limited to: Carpal tunnel syndrome Neck pain Subacromial impingement Adhesive capsulitis Temporomandibular joint pain Low back pain Osteoarthritic knee pain Heel pain (ie, Achilles tendinopathy, plantar fasciitis) Rheumatoid arthritis Bell palsy Fibromyalgia Wound healing Lymphedema Commercial Products Low-level laser therapy is covered for prevention of oral mucositis in patients undergoing cancer treatment associated with increased risk of oral mucositis, including chemotherapy and/or radiotherapy, and/or hematopoietic stem cell transplantation. Low-level laser therapy is not medically necessary for all other indications, as the evidence is insufficient to determine the effects of the technology on health outcomes, including but not limited to: Carpal tunnel syndrome Neck pain 500 EXCHANGE STREET, PROVIDENCE, RI MEDICAL COVERAGE POLICY 1
2 Subacromial impingement Adhesive capsulitis Temporomandibular joint pain Low back pain Osteoarthritic knee pain Heel pain (ie, Achilles tendinopathy, plantar fasciitis) Rheumatoid arthritis Bell palsy Fibromyalgia Wound healing Lymphedema COVERAGE Benefits may vary between groups and contracts. Please refer to the appropriate Benefit Booklet, Evidence of Coverage, or Subscriber Agreement for applicable not medically necessary/not covered benefits/coverage. BACKGROUND Low-level laser therapy (LLLT) refers to the use of red-beam or near-infrared lasers with a wavelength between 600 and 1000 nm and power between 5 and 500 MW. In contrast, lasers used in surgery typically use 300 Watts. When applied to the skin, LLLT produces no sensation and does not burn the skin. Because of the low absorption by human skin, it is hypothesized that the laser light can penetrate deeply into the tissues where it has a photobiostimulative effect. The exact mechanism of its effect on tissue healing is unknown; hypotheses have included improved cellular repair and stimulation of the immune, lymphatic, and vascular systems. LLLT is being evaluated to treat a wide variety of conditions, including soft tissue injuries, myofascial pain, tendinopathies, nerve injuries, and joint pain. LLLT has also been evaluated for lymphedema. ORAL MUCOSITIS Oral mucositis describes inflammation of the oral mucosa and typically manifests as erythema or ulcerations that appear 7 to 10 days after initiation of high-dose cancer therapy. Oral mucositis can cause significant pain and increased risk of systemic infection, dependency on total parenteral nutrition, and use of narcotic analgesics. Treatment Treatment planning may also need to be modified due to dose-limiting toxicity. There are a number of interventions for oral mucositis that may partially control symptoms, but none is considered a criterion standard treatment. When uncomplicated by infection, oral mucositis is self-limited and usually heals within 2 to 4 weeks after cessation of cytotoxic chemotherapy. Low-level laser therapy (LLLT) has been used in cancer therapy induced oral mucositis in patients treated with radiotherapy and/or chemotherapy and hematopoietic cell transplantation. MUSCULOSKELETAL AND NEUROLOGIC DISORDERS Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy and the most commonly performed surgery of the hand. The syndrome is related to the bony anatomy of the wrist. The carpal tunnel is bound dorsally and laterally by the carpal bones and ventrally by the transverse carpal ligament. Through this contained space run the 9 flexor tendons and the median nerve. Therefore, any space-occupying lesion can compress the median nerve and produce the typical symptoms of CTS - pain, numbness, and tingling in the distribution of the median nerve. Symptoms of more severe cases include hypesthesia, clumsiness, loss of dexterity, and weakness of pinch. In the most severe cases, patients experience marked sensory loss and significant functional impairment with thenar atrophy. Treatment 500 EXCHANGE STREET, PROVIDENCE, RI MEDICAL COVERAGE POLICY 2
3 Mild-to-moderate cases of CTS are usually first treated conservatively with splinting and cessation of aggravating activities. Other conservative therapies include oral steroids, diuretics, nonsteroidal antiinflammatory drugs, and steroid injections into the carpal tunnel itself. Patients who do not respond to conservative therapy or who present with severe CTS with thenar atrophy may be considered candidates for surgical release of the carpal ligament, using either an open or endoscopic approach. LLLT is also used to treat CTS. For individuals who have increased risk of oral mucositis due to some cancer treatments (eg, chemotherapy, radiotherapy) and/or hematopoietic cell transplantation who receive LLLT, the evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. The evidence for LLLT is insufficient to determine the effects of the technology on health outcomes in individuals who have the following conditions: orthopedic pain (ie, neck pain, osteoarthritic knee pain, low back pain, carpal tunnel syndrome) shoulder conditions (eg, subacromial impingement syndrome, adhesive capsulitis), heel pain, or temporomandibular joint pain bone, ligament, and joint conditions (eg, rheumatoid arthritis, fibromyalgia) Bell palsy lymphedema chronic non-healing wounds CODING BlueCHiP for Medicare and Commercial Products Providers should file the following HCPCS code, as there isn't a specific CPT code for the service. The following code is covered when filed with the ICD-10 diagnosis codes below. S8948 Application of a modality (requiring constant provider attendance) to one or more areas; low-level laser; each 15 minutes ICD-10 CM: C00 - D49 RELATED POLICIES PUBLISHED Provider Update, September 2018 Provider Update, January 2018 Provider Update, January 2017 Provider Update, February 2016 Provider Update, May 2014 Provider Update, April 2013 Provider Update, March 2012 REFERENCES 1. Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. May ;120(10): PMID Schubert MM, Eduardo FP, Guthrie KA, et al. A phase III randomized double-blind placebocontrolled clinical trial to determine the efficacy of low level laser therapy for the prevention of oral mucositis in patients undergoing hematopoietic cell transplantation. Support Care Cancer. Oct 2007;15(10): PMID Figueiredo AL, Lins L, Cattony AC, et al. Laser therapy in the control of oral mucositis: a metaanalysis. Rev Assoc Med Bras (1992). Sep-Oct 2013;59(5): PMID EXCHANGE STREET, PROVIDENCE, RI MEDICAL COVERAGE POLICY 3
4 4. Oberoi S, Zamperlini-Netto G, Beyene J, et al. Effect of prophylactic low level laser therapy on oral mucositis: a systematic review and meta-analysis. PLoS One. Sep 2014;9(9):e PMID Doeuk C, Hersant B, Bosc R, et al. Current indications for low level laser treatment in maxillofacial surgery: a review. Br J Oral Maxillofac Surg. Apr 2015;53(4): PMID Gautam AP, Fernandes DJ, Vidyasagar MS, et al. Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients - A triple blinded randomized controlled trial. Radiother Oncol. Sep 2012;104(3): PMID Gautam AP, Fernandes DJ, Vidyasagar MS, et al. Low Level Helium Neon Laser therapy for chemoradiotherapy induced oral mucositis in oral cancer patients - A randomized controlled trial. Oral Oncol. Sep 2012;48(9): PMID Gautam AP, Fernandes DJ, Vidyasagar MS, et al. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy--a randomized controlled trial. Support Care Cancer. May 2013;21(5): PMID Gautam AP, Fernandes DJ, Vidyasagar MS, et al. Low level laser therapy against radiation induced oral mucositis in elderly head and neck cancer patients-a randomized placebo controlled trial. J Photochem Photobiol B. Mar 2015;144: PMID Oton-Leite AF, Silva GB, Morais MO, et al. Effect of low-level laser therapy on chemoradiotherapyinduced oral mucositis and salivary inflammatory mediators in head and neck cancer patients. Lasers Surg Med. Apr 2015;47(4): PMID Ferreira B, da Motta Silveira FM, de Orange FA. Low-level laser therapy prevents severe oral mucositis in patients submitted to hematopoietic stem cell transplantation: a randomized clinical trial. Support Care Cancer. Mar 2016;24(3): PMID Blue Cross and Blue Shield Technology Evaluation Center (TEC). Low-level laser therapy for carpal tunnel syndrome and chronic neck pain. TEC Assessment. Nov 2010;Volume 25:Tab 4. PMID Li ZJ, Wang Y, Zhang HF, et al. Effectiveness of low-level laser on carpal tunnel syndrome: A metaanalysis of previously reported randomized trials. Medicine (Baltimore). Aug 2016;95(31):e4424. PMID Fusakul Y, Aranyavalai T, Saensri P, et al. Low-level laser therapy with a wrist splint to treat carpal tunnel syndrome: a double-blinded randomized controlled trial. Lasers Med Sci. May 2014;29(3): PMID Chow RT, Heller GZ, Barnsley L. The effect of 300 mw, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. Sep 2006;124(1-2): PMID Gross AR, Dziengo S, Boers O, et al. Low level laser therapy (LLLT) for neck pain: a systematic review and meta-regression. Open Orthop J. Oct 2013;7: PMID Yeldan I, Cetin E, Ozdincler AR. The effectiveness of low-level laser therapy on shoulder function in subacromial impingement syndrome. Disabil Rehabil. Nov 2009;31(11): PMID Dogan SK, Ay S, Evcik D. The effectiveness of low laser therapy in subacromial impingement syndrome: a randomized placebo controlled double-blind prospective study. Clinics (Sao Paulo). Dec 2010;65(10): PMID Abrisham SM, Kermani-Alghoraishi M, Ghahramani R, et al. Additive effects of low-level laser therapy with exercise on subacromial syndrome: a randomised, double-blind, controlled trial. Clin Rheumatol. May ;30(10): PMID Bal A, Eksioglu E, Gurcay E, et al. Low-level laser therapy in subacromial impingement syndrome. Photomed Laser Surg. Feb 2009;27(1): PMID Calis HT, Berberoglu N, Calis M. Are ultrasound, laser and exercise superior to each other in the treatment of subacromial impingement syndrome? A randomized clinical trial. Eur J Phys Rehabil Med. Mar ;47(3): PMID Page MJ, Green S, Kramer S, et al. Electrotherapy modalities for adhesive capsulitis (frozen shoulder). Cochrane Database Syst Rev. Oct ;10:CD PMID EXCHANGE STREET, PROVIDENCE, RI MEDICAL COVERAGE POLICY 4
5 23. Stergioulas A. Low-power laser treatment in patients with frozen shoulder: preliminary results. Photomed Laser Surg. Apr 2008;26(2): PMID Chen J, Huang Z, Ge M, et al. Efficacy of low-level laser therapy in the treatment of TMDs: a metaanalysis of 14 randomised controlled trials. J Oral Rehabil. Apr 2015;42(4): PMID Chang WD, Lee CL, Lin HY, et al. A meta-analysis of clinical effects of low-level laser therapy on temporomandibular joint pain. J Phys Ther Sci. Aug 2014;26(8): PMID Shobha R, Narayanan VS, Jagadish Pai BS, et al. Low-level laser therapy: A novel therapeutic approach to temporomandibular disorder - A randomized, double-blinded, placebo-controlled trial. Indian J Dent Res. Jul-Aug 2017;28(4): PMID i CLICK THE ENVELOPE ICON BELOW TO SUBMIT COMMENTS This medical policy is made available to you for informational purposes only. It is not a guarantee of payment or a substitute for your medical judgment in the treatment of your patients. Benefits and eligibility are determined by the member's subscriber agreement or member certificate and/or the employer agreement, and those documents will supersede the provisions of this medical policy. For information on member-specific benefits, call the provider call center. If you provide services to a member which are determined to not be medically necessary (or in some cases medically necessary services which are non-covered benefits), you may not charge the member for the services unless you have informed the member and they have agreed in writing in advance to continue with the treatment at their own expense. Please refer to your participation agreement(s) for the applicable provisions. This policy is current at the time of publication; however, medical practices, technology, and knowledge are constantly changing. BCBSRI reserves the right to review and revise this policy for any reason and at any time, with or without notice. Blue Cross & Blue Shield of Rhode Island is an independent licensee of the Blue Cross and Blue Shield Association. 500 EXCHANGE STREET, PROVIDENCE, RI MEDICAL COVERAGE POLICY 5
Subject: Infrared Energy Therapy and Low Level Laser Therapy
 09-E0000-44 Original Effective Date: 08/15/03 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Infrared Energy Therapy and Low Level Laser Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-44 Original Effective Date: 08/15/03 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Infrared Energy Therapy and Low Level Laser Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Low-Level Laser Therapy
 Protocol Low-Level Laser Therapy (20156) Medical Benefit Effective Date: 04/01/17 Next Review Date: 01/19 Preauthorization No Review Dates: 04/07, 05/08, 05/09, 01/10, 01/11, 07/11, 07/12, 07/13, 07/14,
Protocol Low-Level Laser Therapy (20156) Medical Benefit Effective Date: 04/01/17 Next Review Date: 01/19 Preauthorization No Review Dates: 04/07, 05/08, 05/09, 01/10, 01/11, 07/11, 07/12, 07/13, 07/14,
Low-Level Laser Therapy
 Protocol Low-Level Laser Therapy (20156) Medical Benefit Effective Date: 04/01/17 Next Review Date: 01/20 Preauthorization No Review Dates: 04/07, 05/08, 05/09, 01/10, 01/11, 07/11, 07/12, 07/13, 07/14,
Protocol Low-Level Laser Therapy (20156) Medical Benefit Effective Date: 04/01/17 Next Review Date: 01/20 Preauthorization No Review Dates: 04/07, 05/08, 05/09, 01/10, 01/11, 07/11, 07/12, 07/13, 07/14,
Low-Level Laser Therapy
 Low-Level Laser Therapy Policy Number: 2.01.56 Last Review: 6/2018 Origination: 6/2005 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Low- Level
Low-Level Laser Therapy Policy Number: 2.01.56 Last Review: 6/2018 Origination: 6/2005 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Low- Level
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Low-Level Laser Therapy Page 1 of 28 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Low-Level Laser Therapy Professional Institutional Original Effective Date:
Low-Level Laser Therapy Page 1 of 28 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Low-Level Laser Therapy Professional Institutional Original Effective Date:
Low-Level Laser Therapy. Description
 Subject: Low-Level Laser Therapy Page: 1 of 20 Last Review Status/Date: December 2016 Low-Level Laser Therapy Description Low-level laser therapy (LLLT), also called photobiomodulation, is being evaluated
Subject: Low-Level Laser Therapy Page: 1 of 20 Last Review Status/Date: December 2016 Low-Level Laser Therapy Description Low-level laser therapy (LLLT), also called photobiomodulation, is being evaluated
MEDICAL POLICY POLICY TITLE TRANSCUTANEOUS LASER THERAPY POLICY NUMBER MP Original Issue Date (Created): July 26, 2004
 Original Issue Date (Created): July 26, 2004 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY Low-level laser therapy is considered investigational for all
Original Issue Date (Created): July 26, 2004 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY Low-level laser therapy is considered investigational for all
LASER THERAPY FOR PHYSIOTHERAPISTS
 BioFlex Laser Therapy presents LASER THERAPY FOR PHYSIOTHERAPISTS Expand your knowledge. Build your practice. Did you know? Laser Therapy is one of the strongest evidence-based therapies according to Clinical
BioFlex Laser Therapy presents LASER THERAPY FOR PHYSIOTHERAPISTS Expand your knowledge. Build your practice. Did you know? Laser Therapy is one of the strongest evidence-based therapies according to Clinical
d EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Epidural Injections for Pain Management d EFFECTIVE DATE: 04 01 2018 POLICY LAST UPDATED: 03 20 2018 OVERVIEW Epidural injections are generally performed to treat pain arising from
Medical Coverage Policy Epidural Injections for Pain Management d EFFECTIVE DATE: 04 01 2018 POLICY LAST UPDATED: 03 20 2018 OVERVIEW Epidural injections are generally performed to treat pain arising from
BlueCHiP for Medicare Not applicable
 Medical Coverage Policy Hearing Aid Mandate EFFECTIVE DATE: 01 14 2014 POLICY LAST UPDATED: 09 04 2018 OVERVIEW As defined by the Mandate, "hearing aid is any nonexperimental, wearable instrument or device
Medical Coverage Policy Hearing Aid Mandate EFFECTIVE DATE: 01 14 2014 POLICY LAST UPDATED: 09 04 2018 OVERVIEW As defined by the Mandate, "hearing aid is any nonexperimental, wearable instrument or device
ONE of the following:
 Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Medical Coverage Policy Percutaneous Vertebroplasty and Scaroplasty
 Medical Coverage Policy Percutaneous Vertebroplasty and Scaroplasty EFFECTIVE DATE: 02 01 2011 POLICY LAST UPDATED: 07 02 2013 OVERVIEW Percutaneous vertebroplasty is an interventional technique involving
Medical Coverage Policy Percutaneous Vertebroplasty and Scaroplasty EFFECTIVE DATE: 02 01 2011 POLICY LAST UPDATED: 07 02 2013 OVERVIEW Percutaneous vertebroplasty is an interventional technique involving
Osteochondral allografting for all other joints is not covered as the evidence is insufficient to determine the
 Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
PROLIA: Medical Coverage Policy Denosumab (Prolia and. Xgeva) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Monitored Anesthesia care (MAC) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Monitored Anesthesia care (MAC) EFFECTIVE DATE: 09 01 2004 POLICY LAST UPDATED: 01 08 2013 OVERVIEW Monitored anesthesia care is a specific anesthesia service for a diagnostic or
Medical Coverage Policy Monitored Anesthesia care (MAC) EFFECTIVE DATE: 09 01 2004 POLICY LAST UPDATED: 01 08 2013 OVERVIEW Monitored anesthesia care is a specific anesthesia service for a diagnostic or
Clinical Scenario. Focused Clinical Question. Summary of Search, Best Evidence Appraised, and Key Findings
 Journal of Sport Rehabilitation, 2013, 22, 72-78 2013 Human Kinetics, Inc. Effectiveness of Low-Level Laser Therapy Combined With an Exercise Program to Reduce Pain and Increase Function in Adults With
Journal of Sport Rehabilitation, 2013, 22, 72-78 2013 Human Kinetics, Inc. Effectiveness of Low-Level Laser Therapy Combined With an Exercise Program to Reduce Pain and Increase Function in Adults With
Abstracts - Mucositis
 s - Mucositis Support Care Cancer. 2013 May;21(5):1421-8. doi: 10.1007/s00520-012-1684-4. Epub 2012 Dec 8. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of
s - Mucositis Support Care Cancer. 2013 May;21(5):1421-8. doi: 10.1007/s00520-012-1684-4. Epub 2012 Dec 8. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of
Name of Policy: Low Level Laser and High Power Laser Therapies
 Name of Policy: Low Level Laser and High Power Laser Therapies Policy #: 270 Latest Review Date: January 2014 Category: Therapy Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Name of Policy: Low Level Laser and High Power Laser Therapies Policy #: 270 Latest Review Date: January 2014 Category: Therapy Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Payment Policy Drug Testing EFFECTIVE DATE: POLICY LAST UPDATED:
 Payment Policy Drug Testing EFFECTIVE DATE: 05 23 2013 POLICY LAST UPDATED: 06 05 2018 OVERVIEW This policy documents the criteria and documentation requirements for immunoassay (IA) testing (also called
Payment Policy Drug Testing EFFECTIVE DATE: 05 23 2013 POLICY LAST UPDATED: 06 05 2018 OVERVIEW This policy documents the criteria and documentation requirements for immunoassay (IA) testing (also called
For Commercial products, please refer to the following policy: Preauthorization via Web-Based Tool for Procedures
 Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Medical Coverage Policy Circulating Tumor DNA and. Circulating Tumor Cells for Cancer Management (Liquid Biopsy)
 Medical Coverage Policy Circulating Tumor DNA and Circulating Tumor Cells for Cancer Management (Liquid Biopsy) EFFECTIVE DATE: 12 01 2016 POLICY LAST UPDATED: 07 17 2018 OVERVIEW Circulating tumor DNA
Medical Coverage Policy Circulating Tumor DNA and Circulating Tumor Cells for Cancer Management (Liquid Biopsy) EFFECTIVE DATE: 12 01 2016 POLICY LAST UPDATED: 07 17 2018 OVERVIEW Circulating tumor DNA
Plantar Fasciitis. Medical Coverage Policy Extracorporeal Shock Wave Treatment for Plantar Fasciitis and Other Musculoskeletal Conditions
 Medical Coverage Policy Extracorporeal Shock Wave Treatment for Plantar Fasciitis and Other Musculoskeletal Conditions EFFECTIVE DATE: 10 01 2015 POLICY LAST UPDATED: 10 16 2018 OVERVIEW Extracorporeal
Medical Coverage Policy Extracorporeal Shock Wave Treatment for Plantar Fasciitis and Other Musculoskeletal Conditions EFFECTIVE DATE: 10 01 2015 POLICY LAST UPDATED: 10 16 2018 OVERVIEW Extracorporeal
This policy is applicable to Commercial Products only. For BlueCHiP for Medicare, see Related Policy section.
 Medical Coverage Policy Multimarker Serum Testing Related to Ovarian Cancer EFFECTIVE DATE: 10 01 2015 POLICY LAST UPDATED: 04 17 2018 OVERVIEW This policy documents the coverage determination for Multimarker
Medical Coverage Policy Multimarker Serum Testing Related to Ovarian Cancer EFFECTIVE DATE: 10 01 2015 POLICY LAST UPDATED: 04 17 2018 OVERVIEW This policy documents the coverage determination for Multimarker
sad EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Outpatient Pulmonary Rehabilitation sad EFFECTIVE DATE: 07 07 2009 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Pulmonary rehabilitation (PR) is a multidisciplinary approach to reducing
Medical Coverage Policy Outpatient Pulmonary Rehabilitation sad EFFECTIVE DATE: 07 07 2009 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Pulmonary rehabilitation (PR) is a multidisciplinary approach to reducing
Nutrition Therapy. Medical Coverage Policy Enteral/Parenteral EFFECTIVE DATE: POLICY LAST UPDATED: 11/20/2018 OVERVIEW
 Medical Coverage Policy Enteral/Parenteral Nutrition Therapy EFFECTIVE DATE: 01 20 2007 POLICY LAST UPDATED: 11/20/2018 OVERVIEW This policy describes the reimbursement for enteral and parenteral nutrition
Medical Coverage Policy Enteral/Parenteral Nutrition Therapy EFFECTIVE DATE: 01 20 2007 POLICY LAST UPDATED: 11/20/2018 OVERVIEW This policy describes the reimbursement for enteral and parenteral nutrition
The number of Chiropractic visits allowed per year may vary according to the member s specific benefit.
 Payment Policy Chiropractic Services EFFECTIVE DATE:07 21 2009 POLICY LAST UPDATED: 04 17 2018 OVERVIEW Chiropractic is a healthcare profession that focus on disorders of the musculoskeletal and nervous
Payment Policy Chiropractic Services EFFECTIVE DATE:07 21 2009 POLICY LAST UPDATED: 04 17 2018 OVERVIEW Chiropractic is a healthcare profession that focus on disorders of the musculoskeletal and nervous
Cold Laser Therapy (Low Level Laser Therapy) -
 The Truth about Cold Laser Therapy Dr. Torri Gambacorta DC Myrtle Beach Spine Center 100 Legends Dr. Myrtle Beach SC 29579 843-236-9090 Cold Laser Therapy, also known as Low Level Laser Therapy (LLLT),
The Truth about Cold Laser Therapy Dr. Torri Gambacorta DC Myrtle Beach Spine Center 100 Legends Dr. Myrtle Beach SC 29579 843-236-9090 Cold Laser Therapy, also known as Low Level Laser Therapy (LLLT),
Considered Judgement Form
 Considered Judgement Form This form is a checklist of issues that may be considered by the Purchasing Guidance Advisory Group when making purchasing recommendations. Meeting date: 14/10/2014 Topic: Low-level
Considered Judgement Form This form is a checklist of issues that may be considered by the Purchasing Guidance Advisory Group when making purchasing recommendations. Meeting date: 14/10/2014 Topic: Low-level
This policy is applicable to Commercial Products only. For BlueCHiP for Medicare, see related policy section.
 Medical Coverage Policy Ophthalmologic Techniques that Evaluate the Posterior Segment for Glaucoma EFFECTIVE DATE: 01 01 2017 POLICY LAST UPDATED: 09 18 2018 OVERVIEW Several techniques have been developed
Medical Coverage Policy Ophthalmologic Techniques that Evaluate the Posterior Segment for Glaucoma EFFECTIVE DATE: 01 01 2017 POLICY LAST UPDATED: 09 18 2018 OVERVIEW Several techniques have been developed
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Anodyne -Skin Contact Monochromatic Infrared Energy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Anodyne - Skin Contact Monochromatic Infrared
Anodyne -Skin Contact Monochromatic Infrared Energy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Anodyne - Skin Contact Monochromatic Infrared
CARPAL TUNNEL SYNDROME
 CARPAL TUNNEL SYNDROME Carpal tunnel syndrome results from the pinching or entrapping of the median nerve in the underside of the wrist. The actual pathology in most cases is due to either a decrease in
CARPAL TUNNEL SYNDROME Carpal tunnel syndrome results from the pinching or entrapping of the median nerve in the underside of the wrist. The actual pathology in most cases is due to either a decrease in
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Anodyne -Skin Contact Monochromatic Infrared Energy as a Page 1 of 7 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Anodyne - Skin Contact Monochromatic Infrared
Anodyne -Skin Contact Monochromatic Infrared Energy as a Page 1 of 7 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Anodyne - Skin Contact Monochromatic Infrared
Clinical Policy: Trigger Point Injections for Pain Management
 Clinical Policy: for Pain Management Reference Number: CP.MP.169 Last Review Date: 08/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Clinical Policy: for Pain Management Reference Number: CP.MP.169 Last Review Date: 08/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Referral Criteria: Carpal Tunnel Syndrome Feb
 Referral Criteria: Carpal Tunnel Syndrome Feb 2019 1 5.2. Carpal Tunnel Syndrome Background Carpal tunnel syndrome present with non-traumatic tingling of the fingers due to compression of the median nerve
Referral Criteria: Carpal Tunnel Syndrome Feb 2019 1 5.2. Carpal Tunnel Syndrome Background Carpal tunnel syndrome present with non-traumatic tingling of the fingers due to compression of the median nerve
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions
 Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Medical Coverage Policy Functional Neuromuscular Electrical Stimulation EFFECTIVE DATE: 10/16/212 POLICY LAST UPDATED: 09/11/2014
 Medical Coverage Policy Functional Neuromuscular Electrical Stimulation EFFECTIVE DATE: 10/16/212 POLICY LAST UPDATED: 09/11/2014 OVERVIEW Neuromuscular Electrical Stimulation, (NMES), involves the use
Medical Coverage Policy Functional Neuromuscular Electrical Stimulation EFFECTIVE DATE: 10/16/212 POLICY LAST UPDATED: 09/11/2014 OVERVIEW Neuromuscular Electrical Stimulation, (NMES), involves the use
sad EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Bone Mineral Density Studies sad EFFECTIVE DATE: 06 07 2011 POLICY LAST UPDATED: 11 06 2018 OVERVIEW Bone density studies can be used to identify individuals with osteoporosis and
Medical Coverage Policy Bone Mineral Density Studies sad EFFECTIVE DATE: 06 07 2011 POLICY LAST UPDATED: 11 06 2018 OVERVIEW Bone density studies can be used to identify individuals with osteoporosis and
PRIOR AUTHORIZATION Prior authorization is recommended and obtained via the online tool for participating providers.
 Medical Coverage Policy Intensity-Modulated Radiotherapy: Central Nervous System Tumors EFFECTIVE DATE: 02 15 2016 POLICY LAST UPDATED: 09 05 2017 OVERVIEW Radiotherapy (RT) is an integral component in
Medical Coverage Policy Intensity-Modulated Radiotherapy: Central Nervous System Tumors EFFECTIVE DATE: 02 15 2016 POLICY LAST UPDATED: 09 05 2017 OVERVIEW Radiotherapy (RT) is an integral component in
Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow.
 Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow. Prepared for The Canadian Orthopaedic Association Contents Executive
Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow. Prepared for The Canadian Orthopaedic Association Contents Executive
Low-level laser therapy: a standard of supportive care for cancer therapy-induced oral mucositis in head and neck cancer patients?
 Low-level laser therapy: a standard of supportive care for cancer therapy-induced oral mucositis in head and neck cancer patients? E Jadaud 1, RJ Bensadoun 2 1: Institut de Cancérologie de l Ouest, Paul
Low-level laser therapy: a standard of supportive care for cancer therapy-induced oral mucositis in head and neck cancer patients? E Jadaud 1, RJ Bensadoun 2 1: Institut de Cancérologie de l Ouest, Paul
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
High Dose Laser Therapy Revolutionizing Pain Management
 High Dose Laser Therapy Revolutionizing Pain Management The Best New Profit Center In Physical Medicine & Rehabilitation TECHNOLOGICAL MEDICAL ADVANCEMENTS, LLC High Dosage Laser Therapy (HDLT) Common
High Dose Laser Therapy Revolutionizing Pain Management The Best New Profit Center In Physical Medicine & Rehabilitation TECHNOLOGICAL MEDICAL ADVANCEMENTS, LLC High Dosage Laser Therapy (HDLT) Common
Carpal Tunnel Release
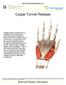 Carpal Tunnel Release Carpal tunnel syndrome is a condition in the hand and wrist caused by excessive pressure on the median nerve in the wrist. Compression of the nerve typically causes numbness and tingling
Carpal Tunnel Release Carpal tunnel syndrome is a condition in the hand and wrist caused by excessive pressure on the median nerve in the wrist. Compression of the nerve typically causes numbness and tingling
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions
 Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Medical Coverage Policy Computer-Assisted. Musculoskeletal Surgical Navigational Orthopedic Procedure
 Medical Coverage Policy Computer-Assisted Musculoskeletal Surgical Navigational Orthopedic Procedure EFFECTIVE DATE: 06 06 2017 POLICY LAST UPDATED: 04 03 2018 OVERVIEW Computer-assisted navigation (CAN)
Medical Coverage Policy Computer-Assisted Musculoskeletal Surgical Navigational Orthopedic Procedure EFFECTIVE DATE: 06 06 2017 POLICY LAST UPDATED: 04 03 2018 OVERVIEW Computer-assisted navigation (CAN)
Corporate Medical Policy
 Corporate Medical Policy Epidural Steroid Injections for Back Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: epidural_steroid_injections_for_back_pain 2/2016 4/2017 4/2018
Corporate Medical Policy Epidural Steroid Injections for Back Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: epidural_steroid_injections_for_back_pain 2/2016 4/2017 4/2018
Some of the electrothermal intradiscal procedures are briefly described.
 Medical Coverage Policy Minimally Invasive Intradiscal and Annular Procedures for Back Pain EFFECTIVE DATE: 10 01 2004 POLICY LAST UPDATED: 07 18 2017 OVERVIEW This policy addresses a variety of minimally
Medical Coverage Policy Minimally Invasive Intradiscal and Annular Procedures for Back Pain EFFECTIVE DATE: 10 01 2004 POLICY LAST UPDATED: 07 18 2017 OVERVIEW This policy addresses a variety of minimally
Commercial Products. Medical Coverage Policy Measurement of Serum. Antibodies to Infliximab and Adalimumab
 Medical Coverage Policy Measurement of Serum Antibodies to Infliximab and Adalimumab EFFECTIVE DATE: 10 02 2012 POLICY LAST UPDATED: 09 18 2018 OVERVIEW This policy documents secondary loss of response
Medical Coverage Policy Measurement of Serum Antibodies to Infliximab and Adalimumab EFFECTIVE DATE: 10 02 2012 POLICY LAST UPDATED: 09 18 2018 OVERVIEW This policy documents secondary loss of response
sad EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Prior Authorization via Web-Based Tool for Procedures sad EFFECTIVE DATE: 09 01 2015 POLICY LAST UPDATED: 12 18 2018 OVERVIEW This policy documents the prior authorization request
Medical Coverage Policy Prior Authorization via Web-Based Tool for Procedures sad EFFECTIVE DATE: 09 01 2015 POLICY LAST UPDATED: 12 18 2018 OVERVIEW This policy documents the prior authorization request
Medical Coverage Policy Homocysteine Testing in the Screening and Diagnosis and Management of Cardiovascular Disease
 Medical Coverage Policy Homocysteine Testing in the Screening and Diagnosis and Management of Cardiovascular Disease EFFECTIVE DATE:05 02 2017 POLICY LAST UPDATED:10 02 2018 OVERVIEW Homocysteine is an
Medical Coverage Policy Homocysteine Testing in the Screening and Diagnosis and Management of Cardiovascular Disease EFFECTIVE DATE:05 02 2017 POLICY LAST UPDATED:10 02 2018 OVERVIEW Homocysteine is an
Eastern Bodywork & Neuromuscular Sports Injury and Medicine Massage Therapy Explained
 Eastern Bodywork & Neuromuscular Sports Injury and Medicine Massage Therapy Explained LA#8419 You Can Experience Ease of Movement Once Again! Call Today for your appointment! Higher Purpose Healing 1 (504)
Eastern Bodywork & Neuromuscular Sports Injury and Medicine Massage Therapy Explained LA#8419 You Can Experience Ease of Movement Once Again! Call Today for your appointment! Higher Purpose Healing 1 (504)
sad EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Chelation Therapy for Off-Label Uses sad EFFECTIVE DATE: 01 01 2017 POLICY LAST UPDATED: 08 21 2018 OVERVIEW Chelation therapy, an established treatment for heavy metal toxicities
Medical Coverage Policy Chelation Therapy for Off-Label Uses sad EFFECTIVE DATE: 01 01 2017 POLICY LAST UPDATED: 08 21 2018 OVERVIEW Chelation therapy, an established treatment for heavy metal toxicities
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Commissioning Policy Individual Funding Request
 Commissioning Policy Individual Funding Request Carpal Tunnel Syndrome Surgery Criteria Based Access Policy Date Adopted: 6 th February 2017 Version: 1617.1.02 Individual Funding Request Team Bristol,
Commissioning Policy Individual Funding Request Carpal Tunnel Syndrome Surgery Criteria Based Access Policy Date Adopted: 6 th February 2017 Version: 1617.1.02 Individual Funding Request Team Bristol,
Number: Policy *Please see amendment for Pennsylvania Medicaid at the end. Last Review 06/09/2016 Effective: 08/14/2001 Next Review: 06/08/2017
 1 of 6 Number: 0552 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers laser peripheral nerve block (laser neurolysis) experimental and investigational for any
1 of 6 Number: 0552 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers laser peripheral nerve block (laser neurolysis) experimental and investigational for any
Peripheral Subcutaneous Field Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Medical Coverage Policy Progenitor Cell Therapy for the Treatment of Damaged Myocardium due to Ischemia
 Medical Coverage Policy Progenitor Cell Therapy for the Treatment of Damaged Myocardium due to Ischemia EFFECTIVE DATE: 02 01 2017 POLICY LAST UPDATED: 02 20 2018 OVERVIEW Progenitor cell therapy describes
Medical Coverage Policy Progenitor Cell Therapy for the Treatment of Damaged Myocardium due to Ischemia EFFECTIVE DATE: 02 01 2017 POLICY LAST UPDATED: 02 20 2018 OVERVIEW Progenitor cell therapy describes
medically necessary as the evidence is insufficient to determine the effects of the technology on health outcomes.
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Limb Pain (Tendonitis, Osteoarthritis, Plantar Fasciitis, Piriformis Syndrome)
 Limb Pain (Tendonitis, Osteoarthritis, Plantar Fasciitis, Piriformis Syndrome) Martin Taylor, DO, PhD Neurology / OrthoNeuro Clinical Associate Professor, Neurology Ohio University College of Osteopathic
Limb Pain (Tendonitis, Osteoarthritis, Plantar Fasciitis, Piriformis Syndrome) Martin Taylor, DO, PhD Neurology / OrthoNeuro Clinical Associate Professor, Neurology Ohio University College of Osteopathic
Nonpharmacologic Treatment of Rosacea Corporate Medical Policy
 Nonpharmacologic Treatment of Rosacea Corporate Medical Policy File Name: Nonpharmacologic Treatment of Rosacea. File Code: UM.SURG.11 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Nonpharmacologic Treatment of Rosacea Corporate Medical Policy File Name: Nonpharmacologic Treatment of Rosacea. File Code: UM.SURG.11 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Subject: Neuromuscular Electrical Stimulation (NMES)
 09-E0000-25 Original Effective Date: 09/15/02 Reviewed: 08/23/18 Revised: 09/15/18 Subject: Neuromuscular Electrical Stimulation (NMES) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-E0000-25 Original Effective Date: 09/15/02 Reviewed: 08/23/18 Revised: 09/15/18 Subject: Neuromuscular Electrical Stimulation (NMES) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
The intent of this policy is to address only those allergy tests that are considered not medically necessary.
 Medical Coverage Policy Allergy Testing EFFECTIVE DATE: 12 01 2016 POLICY LAST UPDATED: 09 05 2017 OVERVIEW Allergic or hypersensitivity disorders can manifest themselves as generalized systemic reactions
Medical Coverage Policy Allergy Testing EFFECTIVE DATE: 12 01 2016 POLICY LAST UPDATED: 09 05 2017 OVERVIEW Allergic or hypersensitivity disorders can manifest themselves as generalized systemic reactions
Carpal Tunnel Syndrome. Relieving Pressure in Your Wrist
 Carpal Tunnel Syndrome Relieving Pressure in Your Wrist Understanding Carpal Tunnel Syndrome Carpal tunnel syndrome (CTS) is a problem that affects the wrist and hand. If you have CTS, tingling and numbness
Carpal Tunnel Syndrome Relieving Pressure in Your Wrist Understanding Carpal Tunnel Syndrome Carpal tunnel syndrome (CTS) is a problem that affects the wrist and hand. If you have CTS, tingling and numbness
Shockwave Therapies for Musculoskeletal Problems Useful Literature
 Shockwave Therapies for Musculoskeletal Problems Useful Literature I have some 1600 references related to shockwave therapy. Those listed below are the main musculoskeletal related papers from 2005-07
Shockwave Therapies for Musculoskeletal Problems Useful Literature I have some 1600 references related to shockwave therapy. Those listed below are the main musculoskeletal related papers from 2005-07
Premier Health Plan considers Iontophoresis for Musculoskeletal Conditions medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL MP.036.PH - Iontophoresis for Musculoskeletal Conditions This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Premier Health Plan POLICY AND PROCEDURE MANUAL MP.036.PH - Iontophoresis for Musculoskeletal Conditions This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
sad EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Prior Authorization via Web-Based Tool for Procedures sad EFFECTIVE DATE: 09 01 2015 POLICY LAST UPDATED: 12 19 2017 OVERVIEW This policy documents the prior authorization request
Medical Coverage Policy Prior Authorization via Web-Based Tool for Procedures sad EFFECTIVE DATE: 09 01 2015 POLICY LAST UPDATED: 12 19 2017 OVERVIEW This policy documents the prior authorization request
A Patient s Guide to Carpal Tunnel Syndrome
 A Patient s Guide to Carpal Tunnel Syndrome Concord Orthopaedics 264 Pleasant Street Concord, NH 03301 Phone: 6032243368 Fax: 6032287268 marketing.copa@concordortho.com DISCLAIMER: The information in this
A Patient s Guide to Carpal Tunnel Syndrome Concord Orthopaedics 264 Pleasant Street Concord, NH 03301 Phone: 6032243368 Fax: 6032287268 marketing.copa@concordortho.com DISCLAIMER: The information in this
Clinical Policy: Mechanical Stretching Devices for Joint Stiffness and Contracture
 Clinical Policy: Mechanical Stretching Devices for Joint Stiffness and Contracture Reference Number: PA.CP.MP.144 Last Review Date: 09/18 Effective Date: 09/18 Coding Implications Revision Log Description
Clinical Policy: Mechanical Stretching Devices for Joint Stiffness and Contracture Reference Number: PA.CP.MP.144 Last Review Date: 09/18 Effective Date: 09/18 Coding Implications Revision Log Description
Medical Coverage Policy Allergy Testing EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Allergy Testing EFFECTIVE DATE: 03 01 2019 POLICY LAST UPDATED: 11 06 2018 OVERVIEW Allergy is a hypersensitive reaction that is usually manifested in the clinical form of allergic
Medical Coverage Policy Allergy Testing EFFECTIVE DATE: 03 01 2019 POLICY LAST UPDATED: 11 06 2018 OVERVIEW Allergy is a hypersensitive reaction that is usually manifested in the clinical form of allergic
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION. POLICY NUMBER: CATEGORY: Therapy/Rehabilitation
 MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
Corporate Medical Policy
 Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
10/8/14. Revision Date(s): Policy Number: MCP-207. Review Date: 12/16/15, 9/15/16
 Subject: Platelet-rich Plasma (PRP) Policy Number: MCP-207 Review Date: 12/16/15, 9/15/16 Revision Date(s): Original Effective Date: 10/8/14 DISCLAIMER This Molina Clinical Policy (MCP) is intended to
Subject: Platelet-rich Plasma (PRP) Policy Number: MCP-207 Review Date: 12/16/15, 9/15/16 Revision Date(s): Original Effective Date: 10/8/14 DISCLAIMER This Molina Clinical Policy (MCP) is intended to
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: facet_joint_denervation 6/2009 4/2017 4/2018 4/2017 Description of Procedure or Service Facet joint denervation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: facet_joint_denervation 6/2009 4/2017 4/2018 4/2017 Description of Procedure or Service Facet joint denervation
Medical Coverage Policy Measurement of Exhaled Nitric Oxide and Exhaled Breath Condensate in the Diagnosis and Management of Respiratory Disorders
 Medical Coverage Policy Measurement of Exhaled Nitric Oxide and Exhaled Breath Condensate in the Diagnosis and Management of Respiratory Disorders EFFECTIVE DATE: 03 03 2015 POLICY LAST UPDATED: 10 16
Medical Coverage Policy Measurement of Exhaled Nitric Oxide and Exhaled Breath Condensate in the Diagnosis and Management of Respiratory Disorders EFFECTIVE DATE: 03 03 2015 POLICY LAST UPDATED: 10 16
Intelect High Power Laser HPL7 & HPL15
 Intelect High Power Laser HPL7 & HPL15 Intelect High Power Laser HPL7 & HPL15 The New High Power Laser for True Pain Relief The Chattanooga High Power Laser raises the game of laser treatment; its higher
Intelect High Power Laser HPL7 & HPL15 Intelect High Power Laser HPL7 & HPL15 The New High Power Laser for True Pain Relief The Chattanooga High Power Laser raises the game of laser treatment; its higher
Subject: Chemoresistance and Chemosensitivity Assays
 05-86000-11 Original Effective Date: 12/15/02 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Chemoresistance and Chemosensitivity Assays THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
05-86000-11 Original Effective Date: 12/15/02 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Chemoresistance and Chemosensitivity Assays THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Corporate Medical Policy
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy
 Corporate Medical Policy Orthotics File Name: Origination: Last CAP Review: Next CAP Review: Last Review: orthotics 6/1990 2/2017 2/2018 2/2017 Description of Procedure or Service An orthotic (orthosis)
Corporate Medical Policy Orthotics File Name: Origination: Last CAP Review: Next CAP Review: Last Review: orthotics 6/1990 2/2017 2/2018 2/2017 Description of Procedure or Service An orthotic (orthosis)
MEDICAL POLICY. 1 Proprietary Information of YourCare Health Plan
 MEDICAL POLICY INTERFERENTIAL STIMULATORS Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized
MEDICAL POLICY INTERFERENTIAL STIMULATORS Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized
A Patient s Guide to Carpal Tunnel Syndrome
 A Patient s Guide to Carpal Tunnel Syndrome 15195 Heathcote Blvd Suite 334 Haymarket, VA 20169 Phone: 703-369-9070 Fax: 703-369-9240 DISCLAIMER: The information in this booklet is compiled from a variety
A Patient s Guide to Carpal Tunnel Syndrome 15195 Heathcote Blvd Suite 334 Haymarket, VA 20169 Phone: 703-369-9070 Fax: 703-369-9240 DISCLAIMER: The information in this booklet is compiled from a variety
Dry Needling of Myofascial Trigger Points
 Dry Needling of Myofascial Trigger Points Policy Number: 2.01.100 Last Review: 3/2018 Origination: 3/1/2016 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Dry Needling of Myofascial Trigger Points Policy Number: 2.01.100 Last Review: 3/2018 Origination: 3/1/2016 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: surgical_deactivation_of_migraine_headache_trigger_sites 10/2012 5/2017 5/2018 5/2017 Description of Procedure
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: surgical_deactivation_of_migraine_headache_trigger_sites 10/2012 5/2017 5/2018 5/2017 Description of Procedure
TECHNOLOGY AND HOW WE USE IT TO DAMAGE OURSELVES WILLIAM A. DELP, DO ASSISTANT PROFESSOR OF OMM GA PCOM
 TECHNOLOGY AND HOW WE USE IT TO DAMAGE OURSELVES WILLIAM A. DELP, DO ASSISTANT PROFESSOR OF OMM GA PCOM OBJECTIVES Understand how we interact with technology new and old Understand how injury occurs Texting
TECHNOLOGY AND HOW WE USE IT TO DAMAGE OURSELVES WILLIAM A. DELP, DO ASSISTANT PROFESSOR OF OMM GA PCOM OBJECTIVES Understand how we interact with technology new and old Understand how injury occurs Texting
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
FROM BASIC SCIENCE. to ADVANCED TECHNOLOGY
 FROM BASIC SCIENCE to ADVANCED TECHNOLOGY CONTENTS INTRODUCTION TO LASER THERAPY 1 PHYSIOLOGICAL EFFECTS OF LASER THERAPY 2-3 INFLAMMATION PATHWAY 4-5 CLINICAL EFFECTS OF LASER THERAPY 6-7 PAIN PATHWAY
FROM BASIC SCIENCE to ADVANCED TECHNOLOGY CONTENTS INTRODUCTION TO LASER THERAPY 1 PHYSIOLOGICAL EFFECTS OF LASER THERAPY 2-3 INFLAMMATION PATHWAY 4-5 CLINICAL EFFECTS OF LASER THERAPY 6-7 PAIN PATHWAY
Low-level Infrared Laser Therapy for Chemoor Radiotherapy-induced Oral Mucositis: A Randomized, Placebo-controlled Study
 Publication Low-level Infrared Laser Therapy Chemoor -induced Oral Mucositis: A Randomized, Placebo-controlled Study Alessandra Kuhn a, Giovana Vacaro b, Denise Almeida b, Álvaro Machado b, Pedro B. Braghini
Publication Low-level Infrared Laser Therapy Chemoor -induced Oral Mucositis: A Randomized, Placebo-controlled Study Alessandra Kuhn a, Giovana Vacaro b, Denise Almeida b, Álvaro Machado b, Pedro B. Braghini
A Patient s Guide to Carpal Tunnel Syndrome. William T. Grant, MD
 A Patient s Guide to Carpal Tunnel Syndrome Dr. Grant is a talented orthopedic surgeon with more than 30 years of experience helping people return to their quality of life. He and GM Pugh, PA-C pride themselves
A Patient s Guide to Carpal Tunnel Syndrome Dr. Grant is a talented orthopedic surgeon with more than 30 years of experience helping people return to their quality of life. He and GM Pugh, PA-C pride themselves
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Orthopaedic Section of the APTA Grant Program Annual Progress Report Form
 Orthopaedic Section of the APTA Grant Program Annual Progress Report Form Date: 9/21/2015 Name of Investigators: Name of Grant: Shane McClinton, Timothy Flynn, Bryan Heiderscheit Comparison of Usual Podiatric
Orthopaedic Section of the APTA Grant Program Annual Progress Report Form Date: 9/21/2015 Name of Investigators: Name of Grant: Shane McClinton, Timothy Flynn, Bryan Heiderscheit Comparison of Usual Podiatric
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: charged_particle_radiotherapy 3/12/96 5/2017 5/2018 5/2017 Description of Procedure or Service Charged-particle
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: charged_particle_radiotherapy 3/12/96 5/2017 5/2018 5/2017 Description of Procedure or Service Charged-particle
Dynamic Splinting Devices
 Dynamic Splinting Devices Policy Number: 1.01.500 Last Review: 8/2017 Origination: 8/2003 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for dynamic
Dynamic Splinting Devices Policy Number: 1.01.500 Last Review: 8/2017 Origination: 8/2003 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for dynamic
HTE introduces new product - 830nm-POINTER. Laser Medical Device
 HTE introduces new product - 830nm-POINTER Laser Medical Device For over 20 years, the low-level laser has been widely applied in medical field to treat health issues such as soft tissue injuries, neck
HTE introduces new product - 830nm-POINTER Laser Medical Device For over 20 years, the low-level laser has been widely applied in medical field to treat health issues such as soft tissue injuries, neck
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14, 09/15/15,09/21/17. THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS
 Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title COMPUTERIZED MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS Policy Number 2.01.13 Category Technology Assessment Effective Date
Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title COMPUTERIZED MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS Policy Number 2.01.13 Category Technology Assessment Effective Date
Dry Needling of Myofascial Trigger Points Corporate Medical Policy
 Dry Needling of Myofascial Trigger Points Corporate Medical Policy File Name: Dry Needling of Myofascial Trigger Points File Code: UM.REHAB.09 Origination: 04/2015 Last Review: 09/2018 Next Review: 09/2019
Dry Needling of Myofascial Trigger Points Corporate Medical Policy File Name: Dry Needling of Myofascial Trigger Points File Code: UM.REHAB.09 Origination: 04/2015 Last Review: 09/2018 Next Review: 09/2019
CARPAL TUNNEL RELEASE
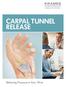 CARPAL TUNNEL RELEASE Relieving Pressure in Your Wrist What Is Carpal Tunnel Syndrome? Carpal tunnel syndrome (CTS) is a condition that affects nerves in the wrist and hand. CTS causes tingling and numbness
CARPAL TUNNEL RELEASE Relieving Pressure in Your Wrist What Is Carpal Tunnel Syndrome? Carpal tunnel syndrome (CTS) is a condition that affects nerves in the wrist and hand. CTS causes tingling and numbness
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION DEVICES FOR VENOUS THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION REVISED DATE: 06/26/14, 10/15/15, 06/16/16, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION REVISED DATE: 06/26/14, 10/15/15, 06/16/16, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria
