Randomized Comparison of Linezolid (PNU ) versus Oxacillin-Dicloxacillin for Treatment of Complicated Skin and Soft Tissue Infections
|
|
|
- Catherine Long
- 5 years ago
- Views:
Transcription
1 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 2000, p Vol. 44, No /00/$ Copyright 2000, American Society for Microbiology. All Rights Reserved. Randomized Comparison of (PNU ) versus Oxacillin-Dicloxacillin for Treatment of Complicated Skin and Soft Tissue Infections DENNIS L. STEVENS, 1,2 * LEON G. SMITH, 3 JON B. BRUSS, 4 MAUREEN A. MCCONNELL-MARTIN, 4 SUE E. DUVALL, 4 WESLEY MARK TODD, 4 AND BARRY HAFKIN 4 FOR THE LINEZOLID SKIN AND SOFT TISSUE INFECTIONS STUDY GROUP Infectious Diseases Section, Veterans Administration Medical Center, Boise, Idaho 1 ; University of Washington School of Medicine, Seattle, Washington 2 ; Saint Michael s Medical Center, Newark, New Jersey 3 ; and Pharmacia & Upjohn, Kalamazoo, Michigan 4 Received 14 February 2000/Returned for modification 12 July 2000/Accepted 6 September 2000 This randomized, double-blind, multicenter trial compared the efficacy and safety of linezolid, an oxazolidinone, with those of oxacillin-dicloxacillin in patients with complicated skin and soft tissue infections. A total of 826 hospitalized adult patients were randomized to receive linezolid (600 mg intravenously [i.v.]) every 12 h or oxacillin (2 g i.v.) every 6 h; following sufficient clinical improvement, patients were switched to the respective oral agents (linezolid [600 mg orally] every 12 h or dicloxacillin [500 mg orally] every 6 hours). Primary efficacy variables were clinical cure rates in both the intent-to-treat (ITT) population and clinically evaluable (CE) patients and microbiological success rate in microbiologically evaluable (ME) patients. Safety and tolerability were evaluated in the ITT population. Demographics and baseline characteristics were similar across treatment groups in the 819 ITT patients. In the ITT population, the clinical cure rates were 69.8 and 64.9% in the linezolid and oxacillin-dicloxacillin groups, respectively (P 0.141; 95% confidence interval 1.58 to 11.25). In 298 CE linezolid-treated patients, the clinical cure rate was 88.6%, compared with a cure rate of 85.8% in 302 CE patients who received oxacillin-dicloxacillin. In 143 ME linezolid-treated patients, the microbiological success rate was 88.1%, compared with a success rate of 86.1% in 151 ME patients who received oxacillin-dicloxacillin. Both agents were well tolerated; most adverse events were of mild-to-moderate intensity. No serious drug-related adverse events were reported in the linezolid group. These data support the use of linezolid for the treatment of adults with complicated skin and soft tissue infections. * Corresponding author. Mailing address: Infectious Diseases Section, Bldg. 45, VA Medical Center, 500 W. Fort St., Boise, ID Phone: (208) Fax: (208) dlsteven@primenet.com. Skin and soft tissue infections are frequently encountered in clinical practice, and gram-positive bacteria are a leading cause (18). These infections are classified as complicated when surgical intervention is required and/or the infectious process is suspected or confirmed to involve deeper tissue (e.g., subcutaneous tissues, fascia, and/or skeletal muscle) (18). Complications of improperly treated skin and soft tissue infections may include endocarditis, osteomyelitis, brain abscess or meningitis, lung abscess, or pneumonia. Skin and soft tissue infections include superficial infections such as erysipelas, cellulitis, simple abscesses, furuncles, wound infections, and deeper infections such as necrotizing fasciitis, myositis, and gas gangrene. Streptococcus pyogenes, Staphylococcus aureus, Streptococcus agalactiae, and group C and G streptococci are the most commonly involved pathogens (27, 28). Intravenous antibiotics are often used in patients with complicated infections, and most patients are hospitalized for management of their infection. In addition, some patients acquire such infections while hospitalized for surgical procedures or trauma (18). The usual treatment for most gram-positive skin and soft tissue infections is a penicillinase-resistant penicillin or a cephalosporin (15). However, the worldwide emergence of pathogens with decreased susceptibility to available therapies has created a need for new antimicrobial agents (3, 4, 11, 12, 20, 21, 29, 30). (PNU ) is the first of the oxazolidinones, a new class of antimicrobial agents that inhibit bacterial protein synthesis by blocking formation of the initiation complex (26, 31). has demonstrated in vitro and in vivo antibacterial activity against staphylococci, streptococci, and enterococci, including resistant strains such as methicillin-resistant S. aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci (6, 8, 9, 10, 14, 17, 19, 23, 31). In early, phase II clinical trials, linezolid was safe and effective in the treatment of gram-positive skin and soft tissue infections and pneumonia (S.-K. Cammarata, B. Hafkin, W. M. Todd, and D. H. Batts, Am. J. Respir. Crit. Care Med. 159(Suppl.): 844, part 2, abstr., 1999; S.-K. Cammarata, B. Hafkin, D. M. Demke, S. M. Eckert, and D. H. Batts, Clin. Microbiol. Infect. 5(Suppl. 3):133, abstr., 1999). This randomized trial compared the efficacy and safety of linezolid with oxacillin-dicloxacillin in the treatment of adults with complicated skin and soft tissue infections. MATERIALS AND METHODS Study design. This prospective, randomized, double-blind, double-dummy, multicenter, multinational study was conducted from November 1998 to June 1999 at 133 sites. Study objectives included assessment of the comparative clinical and microbiological efficacy, safety, and tolerability of linezolid versus oxacillin-dicloxacillin in the treatment of adults with complicated skin and soft tissue infections. The study consisted of (i) a baseline or screening visit, (ii) an end-of-treatment visit, and (iii) a follow-up visit 15 to 21 days after the final dose of study medication. A test-of-cure (TOC) evaluation was conducted at the follow-up visit. Patients underwent daily clinical assessments while hospitalized (one intravenous [i.v.] dose minimum) and every 6 days after discharge. The protocol, informed consent, and all other forms of patient information related to the study were reviewed and approved by each investigator s institutional review 3408
2 VOL. 44, 2000 LINEZOLID FOR SKIN AND SOFT TISSUE INFECTION 3409 board and/or independent ethics committee. All patients provided written informed consent prior to enrollment. Patient selection. Hospitalized patients who were at least 18 years of age with a suspected gram-positive complicated skin and soft tissue infection were eligible for participation. Infections included those that involved deep soft tissue (e.g., major abscess, infected ulcer, major burn, or deep and extensive cellulitis) with at least two of the following symptoms: drainage and/or discharge, erythema, fluctuance, heat and/or localized warmth, pain and/or tenderness to palpation, or swelling and/or induration. At least one of the following conditions must have been present: fever (defined as body temperature of 38 C [orally]), elevated total peripheral white blood cell (WBC) count ( 10,000/mm 3 ), or 15% immature neutrophils (bands) irrespective of total peripheral WBC count. In addition, patients must have been able to take i.v. and oral (p.o.) medications, have an accessible infection site for Gram staining and culture, and have been willing to return for end-of-treatment and follow-up visits. Patients meeting any of the following criteria were excluded from the study: previous antibiotic therapy for 24 h within 7 days of study entry unless the pathogen showed drug resistance or the treatment failed (no clinical improvement after 3 days); uncomplicated skin infection; abscesses requiring only surgical drainage; self-limited infection; diabetic foot ulcer, decubitus ulcer, ischemic ulcers, necrotizing fasciitis, gas gangrene, or burns on more than 20% of total body surface; superinfected eczema; infections requiring concomitant systemic corticosteroids or antimicrobial agents (other than aztreonam); infections complicated by prosthetic materials; inability to comply with treatment period and evaluation; treatment with another investigational medication within the past 30 days; or prior enrollment in this or another linezolid protocol. Exclusionary medical conditions included pheochromocytoma, carcinoid syndrome, liver disease, osteomyelitis, uncontrolled hypertension, neutropenia, untreated hyperthyroidism, and hypersensitivity to study medications. In addition, female patients were excluded if they were pregnant, nursing, or unable to take adequate contraceptive precautions. Treatment. Patients were randomized in a 1:1 ratio to receive either linezolid (supplied by Pharmacia & Upjohn, Kalamazoo, Mich.) (600 mg i.v.) every 12 h or oxacillin (Marsam Pharmaceuticals, Inc., Cherry Hill, N.J.) (2 g i.v.) every 6 h for 10 to 21 days. and placebo were alternated every 6 h to maintain the study blind. Patients in both treatment groups who required empiric gramnegative coverage were allowed to receive i.v. aztreonam (1 to 2 g) three or four times daily as appropriate. When a patient demonstrated clinical improvement, the patient could be switched to oral study medication at the investigator s discretion. Patients initially randomized to i.v. linezolid were switched to linezolid tablets (600 mg p.o.) every 12 h (study medication and placebo were alternated every 6 h tomaintain the study blind), while those initially randomized to oxacillin were switched to dicloxacillin (Apothecon, Princeton, N.J.) (500 mg capsules p.o.) every 6 h. In each treatment group, placebo dummies were identical to the active antibiotic for the respective group. Clinical assessments. Clinical evaluations including a medical history and physical examination, with vital signs, clinical observations (i.e., chills, drainage and/or discharge, swelling and/or induration, tenderness and/or pain to palpation, heat and/or localized warmth, and fluctuance), clinical laboratory assays (i.e., hematology, clinical chemistries, urinalysis), and electrocardiogram, were performed at baseline, during inpatient and/or outpatient treatment, at the end of treatment, and at follow-up. These assessments also were performed as patients were switched from i.v. to p.o. therapy. The follow-up evaluation scheduled for 15 to 21 days following treatment was considered the TOC evaluation. TOC assessments were based upon improvement and/or resolution of clinical signs and symptoms of the skin and soft tissue infection. Microbiological assessments. Prior to the first infusion of either study drug, deep culture specimens of the area contiguous to the primary infected area were obtained for Gram s stain, culture, and susceptibility testing. All isolated pathogens were submitted to a central laboratory for identification. Complete identification of each bacterial isolate was performed, and each organism was classified as a pathogen or a nonpathogen. Susceptibility testing was performed in accordance with National Committee for Clinical Laboratory Standards guidelines. Sponsor-defined breakpoints were used for linezolid susceptibility testing ( 4 g/ml, sensitive; 4 g/ml, resistant). Patients whose cultures grew gram-positive or gram-negative pathogens that were not susceptible to study medications were discontinued from the study unless they demonstrated clinical improvement and did not require concomitant antimicrobial therapy (other than aztreonam). Efficacy variables. Primary efficacy variables were clinical outcome and microbiological outcome based on resolution or improvement of clinical signs and symptoms of infection at the end of treatment compared with those at baseline. Objective and subjective clinical observations recorded throughout the course of the study included anatomical site of infection, extent of infection, degree of involvement, infected-site description, and body temperature. Criteria for assessing clinical outcome were as follows: cure was defined as resolution of baseline clinical signs and symptoms of infection or improvement such that no further antimicrobial treatment was necessary after at least 5 days and 10 doses of study medication; failure was defined as a need for nonstudy antibiotic due to lack of efficacy after at least 2 days and 8 doses of study medication or absence of clinical assessments at the end of treatment and follow-up; indeterminate response was defined as clinically improved or cured at the end of treatment and no assessment at TOC; and missing was defined as those patients receiving less than 2 days of therapy or fewer than 8 doses. Criteria for assessing microbiological outcome were as follows: success was defined as documented eradication (absence of original pathogen[s] from culture at TOC) or presumed eradication (clinical cure at TOC with no microbiological data); failure was defined as documented persistence (presence of original pathogen [one or more] from culture at TOC) or presumed persistence, superinfection, or reinfection (clinical failures at TOC with no microbiological data or those who received nonstudy antibiotic therapy); indeterminate was defined as those patients classified as indeterminate in clinical assessment; and missing was defined as the absence of clinical determination and no microbiological data at TOC. Other variables evaluated included body temperature, WBC counts, clinical signs and symptoms of infection (chills, erythema, drainage and/or discharge, swelling and/or induration, tenderness and/or pain to palpation, heat and/or localized warmth, and fluctuance), and selected organism or pathogen eradication rates. Safety variables. The safety of linezolid and oxacillin-dicloxacillin therapy was monitored throughout the study by physical examination, vital signs, laboratory evaluations, and assessment of adverse events. All patients who received at least one dose of study medication were included in the safety analysis. Physical examination was conducted at baseline and follow-up visits, and vital signs were assessed at all visits. The following laboratory evaluations were conducted: hematology, chemistry, urinalysis, pregnancy test (for females of childbearing potential), site culture and Gram staining, blood culture, and bacterial isolate susceptibility testing. Adverse events were reported from the time of first dose of study drug to the final study visit and were monitored until they resolved or until the patient s participation in the study ended. Population for analysis. Three patient populations were evaluated in this study: the intent-to-treat (ITT) population, the clinically evaluable patients, and the microbiologically evaluable patients. The ITT population included all patients who received at least one dose of double-blind study drug. Clinically evaluable patients included patients from the ITT population who (i) had not received concomitant antibiotic therapy (other than aztreonam) during the study, (ii) had received at least 7 days and 24 doses of study medication (unless the patient discontinued the study for any reason other than lack of efficacy), (iii) had taken at least 80% of prescribed study medications throughout the study and did not miss two or more consecutive doses through day 7 of treatment, and (iv) had a postbaseline assessment during the follow-up period (15 to 21 days posttreatment). Microbiologically evaluable patients included clinically evaluable patients who had a confirmed pathogen from the infection site or blood culture at baseline that was not resistant to study medications. Statistical methods. Analyses were performed to compare the efficacy and safety of linezolid with oxacillin-dicloxacillin. All data listings, summaries, and statistical analyses were generated using the Statistical Analysis System (version 6; SAS Institute Inc., Cary, N.C.). All statistical tests were two-sided, and P values of 0.05 were considered statistically significant. Analyses of efficacy variables were performed for the ITT, clinically evaluable, and microbiologically evaluable patient populations. Assuming each treatment group would yield a 90% success rate, 142 evaluable patients per treatment group were required to determine, with 80% power, equivalence between the groups to within 10%. Assuming an evaluability rate of 45%, this translated to a requirement of 316 enrolled patients per treatment group. All 95% confidence intervals (95% CI) were based on the normal approximation to the binomial distribution and were considered consistent with equivalence if the following conditions were met: there were at least 142 patients per treatment group, the CI included 0, and the lower limit of the CI exceeded 10%. Due to the expected small numbers of evaluable patients in each center, terms for investigator effect and treatment group-by-investigator interaction were not included in the models for statistical analysis. Comparability of baseline demographics between treatment groups was assessed using one-way analysis of variance fixed-effects models (age, weight, vital signs, and selected quantitative laboratory analyses) or using the chi-square test for two-way contingency tables (gender, race, physical examination, clinical signs and symptoms). Patient clinical outcome was assessed by clinical cure rate (the number of cures divided by the number of cures and failures) in the clinically evaluable patient cohort. Microbiological outcome was assessed by the microbiological success rate, defined as the number of successes divided by the sum of the number of successes and failures in the microbiologically evaluable patient cohort. Comparability of clinical cure rates and microbiological success rates were assessed using 95% CI for the difference in these rates and a chi-square test for homogeneity of proportions for the distribution of clinical cures, microbiological successes, and failures between treatment groups. RESULTS Patient demographics. Of the 826 patients enrolled in 133 centers, 403 and 423 were randomized to the linezolid and oxacillin-dicloxacillin treatment groups, respectively. The ITT population consisted of 819 patients who received at least one dose of study drug (400 received linezolid and 419 received oxacillin-dicloxacillin). The study evaluation groups and rea-
3 3410 STEVENS ET AL. ANTIMICROB. AGENTS CHEMOTHER. TABLE 1. Disposition of patients Population a or detail No. (%) treated with: ITT patients 400 (100) 419 (100) Clinically evaluable patients 298 (74.5) 302 (72.1) Reasons for nonevaluability: Prior antibiotic usage 3 (0.8) 4 (1.0) Insufficient therapy 29 (7.3) 43 (10.3) Concomitant antibiotics 11 (2.8) 15 (3.6) Noncompliance with regimen 39 (9.8) 47 (11.2) No clinical outcome postbaseline 64 (16.0) 64 (15.3) Microbiologically evaluable patients 143 (35.8) 151 (36.0) Reasons for nonevaluability: Clinically nonevaluable 102 (25.5) 117 (27.9) No baseline pathogens 189 (47.3) 201 (48.0) Baseline pathogen resistant to study medication 11 (2.8) 11 (2.6) a Patients may have had multiple reasons for nonevaluability. All reasons are summarized; therefore, percentages may total more than 100%. sons for nonevaluability are shown in Table 1. Six hundred patients (298 receiving linezolid and 302 receiving oxacillindicloxacillin) made up the clinically evaluable subgroup. The microbiologically evaluable patients included 143 linezolid and 151 oxacillin-dicloxacillin patients. The most common reason for clinical nonevaluability was lack of a baseline assessment. Lack of a baseline pathogen was the most frequent reason for microbiological nonevaluability. Demographics and baseline characteristics of the ITT population (Table 2) and clinically evaluable patients were similar in both treatment groups. The most common complicated skin and soft tissue diagnoses at baseline were cellulitis, skin abscess, and erysipelas. The majority of patients in the study had relatively serious infections, with 319 of 397 (80.4%) of the linezolid-treated patients and 322 of 417 (77.2%) of the oxacillin-dicloxacillin-treated patients having deep involvement of the skin at the primary site of infection. Clinically relevant pathogens isolated at baseline included: S. aureus in 140 linezolid patients and 143 oxacillindicloxacillin patients; S. pyogenes in 41 linezolid patients and 46 oxacillin-dicloxacillin patients; and S. agalactiae in 10 linezolid patients and 12 oxacillin-dicloxacillin patients. Treatment. The total durations of treatment (i.v. and p.o.) were similar in both treatment groups; the mean duration of treatment was days in the linezolid group and days in the oxacillin-dicloxacillin group. (Unless otherwise noted, values are means standard deviations.) Among the clinically evaluable patients, the total durations of treatment (i.v. and p.o.) also were similar, with a mean duration of days in the linezolid group and days in the oxacillin-dicloxacillin group. Most ITT patients in both treatment groups received 5 days of i.v. therapy, with a mean duration of days for linezolid-treated patients and days for oxacillin-dicloxacillin-treated patients. Similar results were observed in clinically evaluable patients. Among the ITT patients, 49 patients (12.3%) in the linezolid group and 77 patients (18.4%) in the oxacillin-dicloxacillin group received concomitant noninvestigational antimicrobial therapy after the first day of study medication. The most common classes of antimicrobials used were cephalosporins, penicillins, fluoroquinolones, and parenteral aminoglycosides. Of these patients, 26 linezolid-treated patients (6.5%) and 40 oxacillin-dicloxacillin-treated patients (9.5%) used cephalosporins, and eight linezolid-treated patients (2.0%) and 10 oxacillin-dicloxacillin-treated patients (2.4%) used topical antibiotics. Discontinuations. Overall, the percentages of patients who completed treatment and follow-up were similar between treatment groups (336 of 403 [84%] in the linezolid group and 327 of 423 [78%] in the oxacillin-dicloxacillin group). In the ITT population, more patients in the oxacillin-dicloxacillin group (70 of 419 [16.7%]) than in the linezolid group (43 of 400 [10.8%]) discontinued participation during treatment. The most frequent reason for discontinuation, regardless of treatment group, was lack of efficacy (9 of 400 [2.3%] patients in the linezolid group and 15 of 419 [3.6%] patients in the oxacillindicloxacillin group). Similarly, the percentage of patient discontinuations during follow-up was slightly higher in the oxacillin-dicloxacillin group (73 of 419 [17.4%] patients) than in the linezolid group (54 of 400 [13.5%] patients), with loss to follow-up representing the most common reason for discontinuation (27 of 400 [6.8%] patients in the linezolid group and 32 of 419 [7.6%] patients in the oxacillin-dicloxacillin group). Efficacy. In the ITT population, the clinical cure rates at the TOC visit were comparable in the two treatment groups, with 279 of 400 (69.8%) linezolid-treated patients and 272 of 419 (64.9%) oxacillin-dicloxocillin-treated patients achieving a clinical cure (P 0.141; 95% CI, 1.58 to [point estimate, 4.9]). In clinically evaluable patients, clinical cure rates at the TOC visit also were comparable in linezolid and oxacillin-dicloxacillin groups (264 of 298 [88.6%] patients versus 259 of 302 [85.8%] patients, respectively) (P 0.300; 95% CI, 2.5 to 8.2 [point estimate, 2.8]) (Table 3). Subgroup analysis of clinical outcome by gender, age, and race demonstrated similar results between treatment groups, except for males in the ITT population, for whom cure rates were 85.3% (174 of 204 patients) and 76.7% (171 of 223 patients) in the linezolid and TABLE 2. Demographic and baseline characteristics for ITT population Characteristic No. (%) of ITT patients treated with: (n 400) Oxacillindicloxacillin Oxacillindicloxacillin (n 419) Gender [no. (%) a ] Male 252 (63.0) 255 (60.9) Female 148 (37.0) 164 (39.1) Mean age SD (yr) Mean weight SD (kg) Race [no. (%)] White 227 (56.8) 230 (54.9) Black 49 (12.3) 69 (16.5) Asian or Pacific Islander 38 (9.5) 42 (10.0) Other or unknown 86 (21.5) 78 (18.6) Diagnosis [no. (%) a ] Cellulitis 178 (44.8) 186 (44.6) Skin abscesses 58 (14.6) 64 (15.3) Skin ulcer 15 (13.8) 14 (3.4) Erysipelas 41 (10.3) 40 (9.6) Infected surgical incision 25 (6.3) 26 (6.2) Infected wound 24 (6.0) 40 (9.6) Infected bite 7 (1.8) 3 (0.7) Other 49 (12.3) 43 (10.3) Missing 0 1 (0.2) Mean duration of infection (days) a Percentages are based on the number of patients reporting.
4 VOL. 44, 2000 LINEZOLID FOR SKIN AND SOFT TISSUE INFECTION 3411 TABLE 3. Assessment of efficacy in ITT, clinically evaluable, and microbiologically evaluable patients Patient group Treatment Total no. (%) of patients assessed a No. (%) of patients with assessment Cure Failure c P (95% CI b [point estimate]) b ITT 400 (100) 279 (69.8) 121 (30.8) ( 1.58, [4.9]) Oxacillin-dicloxacillin 419 (100) 272 (64.9) 147 (35.1) Clinically evaluable 298 (100) 264 (88.6) 34 (11.4) ( 2.5, 8.2 [2.8]) Oxacillin-dicloxacillin 302 (100) 259 (85.8) 43 (14.2) Microbiologically evaluable 143 (100) 126 (88.1) 17 (11.9) ( 5.6, 9.7 [2.0]) Oxacillin-dicloxacillin 151 (100) 130 (86.1) 21 (13.9) a Percentages based on number of assessed patients. b Confidence interval based on normal approximation, expressed as a percentage. c Includes patients with missing or indeterminate outcomes. oxacillin-dicloxacillin groups, respectively (P 0.024; 95% CI, 1.2 to 16.5 [point estimate, 8.6]). In addition, no statistically significant differences in clinical cure rate were observed between treatment groups when analyzed by diagnosis. In the microbiologically evaluable patients, the microbiological success rate at the TOC visit was similar between treatment groups, with 126 of 143 (88.1%) patients in the linezolid group and 130 of 151 (86.1%) patients in the oxacillin-dicloxacillin group achieving microbiological success (P 0.606; 95% CI, 5.6 to 9.7 [point estimate, 2.0]). Subgroup analysis of microbiological outcome by gender, age, and race demonstrated comparable results between treatment groups. Eradication rates of selected baseline pathogens (S. aureus, S. pyogenes, and S. agalactiae) at the TOC visit are summarized for the microbiologically evaluable patients in Table 4. Eradication rates generally were similar between treatment groups for these pathogens. For S. aureus, the eradication rate in the linezolid group was 91.4% (85 of 93 patients) compared with 84.5% (87 of 103 patients) in the oxacillin-dicloxacillin group (P 0.139; 95% CI, 2.1 to 16.0 [point estimate, 6.9]). Consistent with the resolution of infection, a comparable improvement in clinical signs and symptoms of infection was observed from baseline to follow-up. At baseline, the incidences of moderate to severe swelling and/or induration were similar in both treatment groups (273 of 298 patients [91.6%] in the linezolid group and 279 of 301 patients [92.7%] in the oxacillin-dicloxacillin group). At the follow-up visit, the most common symptom of infection still present in clinically evaluable patients was swelling and/or induration, in 39 of 298 (13.1%) linezolid-treated patients and 53 of 302 (17.5%) oxacillin-dicloxacillin-treated patients, with moderate to severe swelling and/or induration reported in 3 patients in each treatment group. Mean changes in WBC count, absolute neutrophil count, and temperature during the study in both treatment groups also were consistent with the resolution of infection. In the ITT population, less than 1% of all patients experienced reinfection, superinfections, or colonizations. Of the 400 linezolid-treated patients, none had a reinfection; superinfection was observed in 1 patient and colonization was observed in 2 patients. Of the 419 patients who received oxacillin-dicloxacillin, 1 had a reinfection, 2 had a superinfection, and 1 had colonization. Safety. Safety assessments were performed on the ITT population. The frequencies of adverse events reported, regardless of causality, were comparable between treatment groups. A total of 47.3% (189 of 400) of the patients in the linezolid group and 41.3% (173 of 419) of the patients in the oxacillindicloxacillin treatment group experienced at least one adverse TABLE 4. Eradication rates of selected baseline pathogens a Pathogen Eradication rate (%) b with: event. Frequencies of reported adverse events reported in 2% of patients in either treatment group are presented in Table 5. The most frequently reported adverse events in the linezolid group were nausea (23 of 400 patients [5.8%]), headache (22 of 400 patients [5.5%]), and vomiting (13 of 400 patients [3.3%]), while those most frequently reported in the oxacillin-dicloxacillin group were nausea (24 of 419 patients [5.7%]), headache (16 of 419 patients [3.8%]), and constipation (13 of 419 patients [3.1%]). The percentages of patients with at least one adverse event considered to be drug related were similar between the linezolid and oxacillin-dicloxacillin groups (67 of 400 patients [16.8%] versus 72 of 419 patients [17.2 %], respectively). The most common drug-related adverse events in the linezolid group were nausea (14 of 400 patients [3.5%]) and headache (10 of 400 patients [2.5%]). Nausea (12 of 419 patients [2.9%]) was the most common drug-related adverse event in the oxacillin-dicloxacillin group. No statistically significant differences in the frequency of drug-related adverse events were observed between treatment groups. In addition, most drugrelated adverse events reported in both treatment groups were characterized as mild or moderate in intensity. Serious adverse events were reported in 5.5% (22 of 400) of linezolid-treated patients and in 4.5% (19 of 419) of oxacillindicloxacillin-treated patients. In the linezolid group, none was considered to be drug related, while four were considered possibly or probably drug-related in the oxacillin-dicloxacillin group. Death was reported in three linezolid-treated patients and in one oxacillin-dicloxacillin-treated patient; none of these was considered by the investigator to be drug related. Hypertension was reported in 12 of 400 (3.0%) of linezolidtreated patients and 1 of 419 (0.2%) of oxacillin-dicloxacillin- Oxacillindicloxacillin P (95% CI c [point estimate]) S. aureus 85/93 (91.4) 87/103 (84.5) ( 2.1, 16.0 [6.9]) S. pyogenes 23/29 (79.3) 27/32 (84.4) ( 24.4, 14.3 [5.1]) S. agalactiae 7/7 (100) 4/6 (66.7) ( 4.4, 71.1 [33.3]) a In microbiologically evaluable patients. b Eradication rate number of eradicated pathogens divided by the total of eradicated and noneradicated pathogens. c Confidence interval based on normal approximation, expressed as a percentage.
5 3412 STEVENS ET AL. ANTIMICROB. AGENTS CHEMOTHER. TABLE 5. Adverse events reported in 2% of patients in the ITT population Adverse event (n 400) No. (%) of patients in treatment group Oxacillin-dicloxacillin (n 419) Nausea 23 (5.8) 24 (5.7) Headache 22 (5.5) 16 (3.8) Vomiting 13 (3.3) 8 (1.9) Hypertension 12 (3.0) 1 (0.2) Diarrhea 11 (2.8) 12 (2.9) Localized pain 11 (2.8) 3 (0.7) Dyspepsia 10 (2.5) 7 (1.7) Insomnia 10 (2.5) 9 (2.1) Dizziness 9 (2.3) 3 (0.7) Abdominal pain (localized) 8 (2.0) 5 (1.2) Constipation 7 (1.8) 13 (3.1) Pruritus (nonapplication site) 6 (1.5) 9 (2.1) Fever 5 (1.3) 11 (2.6) treated patients. Seven of the linezolid-treated patients had baseline hypertension, which was not adversely affected by the subsequent administration of linezolid. Four patients had normal baseline blood pressure measurements and intermittently elevated blood pressure measurements during treatment; three of these four had normal measurements at follow-up. One patient with no prior history of hypertension had a single isolated elevated (170/110 mm Hg) measurement on day 9 of treatment, but all other measurements were normal. Since linezolid is classified as a mild inhibitor of monoamine oxidase (MAO), an analysis was performed to examine a potential MAO interaction in these patients with hypertension. Eleven of these 12 patients were not on any concomitant medications that had MAO-interacting or -inhibitory properties, therefore decreasing the likelihood that this was attributable to MAO interaction. In addition, for all enrolled patients in the study who were not receiving a concomitant potent MAO inhibitor but receiving a MAO-interacting drug, there was no difference between linezolid-treated patients and oxacillin-dicloxacillintreated patients in the change from baseline for mean systolic blood pressure, mean diastolic blood pressure, and mean arterial pressure. Similar percentages of patients withdrew from the study due to adverse events in the linezolid group (12 of 400 patients [3.0%]) and the oxacillin-dicloxacillin group (23 of 419 patients [5.5%]). However, significantly more patients in the oxacillindicloxacillin group withdrew due to adverse events judged to be drug-related than did patients in the linezolid group (15 of 419 patients [3.6%] versus 4 of 400 [1.0%], respectively; P 0.014). No clinically relevant changes in physical examination observations, vital sign results, or hematological or clinical chemistry panels were observed from baseline to follow-up. No potential drug interactions between linezolid and MAO inhibitors or any other concomitant medications were observed. DISCUSSION This well-designed randomized trial compared the efficacy and safety of linezolid, a new oxazolidinone, with those of oxacillin-dicloxacillin, a therapy of choice in many parts of the world, for patients with complicated skin and soft tissue infections. Both treatment groups were similar with respect to their demographics, baseline infections, and evaluability of the patients. Results of this study indicated that linezolid is as effective as oxacillin-dicloxacillin in the treatment of these infections. Clinical cure rates and microbiological success rates for linezolid-treated patients were high (88.6 and 88.1%, respectively) and compared favorably with those observed in oxacillin-dicloxacillin-treated patients (85.8 and 86.1%, respectively). also was as effective as oxacillin-dicloxacillin in eradicating S. aureus, S. pyogenes, and S. agalactiae. Most patients in both treatment groups were able to switch from i.v. to p.o. therapy (based on clinical improvement) within 5 days of therapy initiation, and 80% of patients completed both the treatment and follow-up phases of the study. Gram-positive bacteria are important pathogens among patients with skin and soft tissue infections. Historically, oxacillin has been a drug of choice for many gram-positive skin and soft tissue infections (27). However, the emergence of multidrugresistant gram-positive species, particularly MRSA, is an increasing concern; in recent surveys in the United States and Europe, methicillin resistance has been observed in 22 to 25% of S. aureus isolates from patients with skin and soft tissue infections (5, 13). Although patients with MRSA were excluded from this study, the increasing prevalence of resistant gram-positive pathogens suggests that many patients will require treatment with an antimicrobial that has activity against these resistant strains. may substantially impact the approach to treatment of skin and soft tissue infections caused by many grampositive species because it has a unique mechanism of action, possesses significant activity against gram-positive pathogens (including MRSA), and has excellent clinical efficacy as demonstrated in this and other studies (6, 22). Thus, it is a promising empiric treatment for either community-acquired or nosocomial skin and soft tissue infections. s efficacy in treating skin and soft tissue infections may be due, in part, to the high concentrations achieved in the skin (K. M. Donaldson, P. Blood., T. J. Parker, P. T. Daly-Yates, and J. D. Harry, unpublished data) and its ability to inhibit bacterial virulence factor and toxin production in S. aureus and S. pyogenes at concentrations well below the MICs (C. G. Gemmell and C. W. Ford, Abstr. 39th Intersci Conf. Antimicrob. Agents Chemother., abstr. 1537, 1994). It has been suggested that antimicrobials that have both antibacterial properties as well as the ability to inhibit the synthesis of bacterial toxins may provide greater efficacy and improved outcomes in these gram-positive bacterial toxin-mediated diseases (1, 24, 25). Unlike other antibiotics, the oral formulation of linezolid is 100% bioequivalent to the intravenous formulation, ensuring that patients receive adequate serum and tissue concentrations of drug upon switch to oral therapy. This will allow physicians to switch to the oral formulation earlier in hospitalized patients and may result in earlier discharge (16). Further studies evaluating the use of oral linezolid alone in the treatment of complicated skin and soft tissue infections are needed. In the present study, linezolid proved to be safe and well tolerated regardless of the site of infection. The majority of adverse events reported were mild or moderate in intensity. No serious drug-related adverse events were reported in the linezolid group. In addition, there was no evidence of a drug interaction between linezolid and MAO inhibitors or any other concomitant medications. Hypertension was reported in 3.0% of patients receiving linezolid; however, a direct causal relationship could not be determined. In addition, although linezolid may cause mild MAO inhibition (2, 7), the hypertension observed in this trial did not appear to be related to this property of the drug. Complicated skin and soft tissue infections are a significant cause of morbidity and mortality in hospitalized patients. The
6 VOL. 44, 2000 LINEZOLID FOR SKIN AND SOFT TISSUE INFECTION 3413 emergence of resistant pathogens has created the need for newer, more effective antimicrobial therapies, which can be given parenterally or p.o. In conclusion, linezolid is well tolerated and as effective as oxacillin-dicloxacillin for complicated skin and soft tissue infections, with the added advantages of convenient twice-daily dosing administered either i.v. or p.o. ACKNOWLEDGMENTS This study was supported by Pharmacia Corporation. We thank the participating investigators in the Skin and Soft Tissue Infections Study Group and the investigator site staff for their efforts in providing care to the patients. REFERENCES 1. Bisno, A. L., and D. L. Stevens Current concepts: streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334: Brickner, S. J Oxazolidinone antibacterial agents. Curr. Pharm. Des. 2: Centers for Disease Control and Prevention Staphylococcus aureus with reduced susceptibility to vancomycin United States, Morb. Mortal. Wkly. Rep. 46: Centers for Disease Control and Prevention Update: Staphylococcus aureus with reduced susceptibility to vancomycin United States, Morb. Mortal. Wkly. Rep. 46: Colsky, A. S., R. S. Kirsner, and F. A. Kerdel Analysis of antibiotic susceptibilities of skin wound flora in hospitalized dermatology patients. The crisis of antibiotic resistance has come to the surface. Arch. Dermatol. 134: Daly, J. S., G. M. Eliopoulos, S. Willey, and R. C. Moellering, Jr Mechanism of action and in vitro and in vivo activities of S-6123, a new oxazolidinone compound. Antimicrob. Agents Chemother. 32: Dostert, P. L., M. Strolin Benedetti, and K. F. Tipton Interactions of monoamine oxidase with substrates and inhibitors. Med. Res. Rev. 9: Dresser, L. D., and M. J. Rybak The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18: Eliopoulos, G. M., C. B. Wennersten, H. S. Gold, and R. C. Moellering, Jr In vitro activities of new oxazolidinone antimicrobial agents against enterococci. Antimicrob. Agents Chemother. 40: Ford, C. W., J. C. Hamel, D. M. Wilson, J. K. Moerman, D. Stapert, R. J. Yancey, Jr., D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner In vivo activities of U and U , novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob. Agents Chemother. 40: Hiramatsu, K The emergence of Staphylococcus aureus with reduced susceptibility to vancomycin in Japan. Am. J. Med. 104:7S 10S. 12. Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogenously resistant to vancomycin. Lancet 350: Jones, M. E., F. J. Schmitz, A. C. Fluit, J. Acar, R. Gupta, J. Verhoef, and the SENTRY Participants Group Frequency of occurrence and antimicrobial susceptibility of bacterial pathogens associated with skin and soft tissue infections during 1997 from an international surveillance programme. Eur. J. Clin. Microbiol. Infect. Dis. 18: Jorgensen, J. H., M. L. McElmeel, and C. W. Trippy In vitro activities of the oxazolidinone antibiotics U and U , two novel fluorinated oxazolidinones. Antimicrob. Agents Chemother. 47: Lewis, R. T Soft tissue infections. World J. Surg. 22: Li, Z., L. Pinto, H. A. Glick, R. J. Willke, B. E. Rittenhouse, and B. Hafkin Comparing hospital length of stay (LOS) between linezolid and vancomycin in the treatment of methicillin-resistant Staphylococci species (MRSS) infections: a randomized, multi-center clinical trial. Proceedings of the Annual Meeting of the American College of Clinical Pharmacy, Monterey, Calif. 17. Mercier, R. C., S. R. Penzak, and M. J. Rybak In vitro activities of investigational quinolone, glycylcycline, glycopeptide, streptogramin, and oxazolidinone tested alone and in combinations against vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 41: Nichols, R. L Optimal treatment of complicated skin and skin structure infections. J. Antimicrob. Chemother. 44: Noskin, G. A., F. Siddiqui, V. Stosor, D. Hacek, and L. R. Peterson In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43: Panlilio, A. L., D. H. Culver, R. P. Gaynes, S. Banerjee, T. S. Henderson, J. S. Tolson, and W. J. Martone Methicillin-resistant Staphylococcus aureus in U.S. hospitals, Infect. Control Hosp. Epidemiol. 13: Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351: Rubinstein, E., and R. G. Wunderink for the Nosocomial Pneumonia Study Group. (PNU-10076) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis., in press. 23. Rybak, M. J., D. M. Cappelletty, T. Moldovan, J. R. Aeschlimann, and G. W. Kaatz Comparative in vitro activity and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU ) and linezolid (PNU ) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob. Agents Chemother. 42: Stevens, D. L, A. E. Bryant, and S. P. Hackett Antibiotic effects on bacterial viability, toxin production, and host response. Clin. Infect. Dis. 20:S154 S Stevens, D. L., K. A. Maier, and J. E. Mitten Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob. Agents Chemother. 31: Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42: Swartz, M. N Cellulitis and superficial infections, p In G. Mandell, R. Douglas, and J. Bennett (ed.), Principles and practice of infectious diseases Churchill Livingstone Inc., New York, N.Y. 28. Swartz, M. N Subcutaneous tissue infections and abscesses, p In G. Mandell, R. Douglas, and J. Bennett (ed.), Principles and practice of infectious diseases Churchill Livingstone Inc., New York, N.Y. 29. Voss, A., D. Milatovic, C. Wallrauch-Schwarz, V. T. Rosdahl, and I. Braveny Methicillin-resistant Staphylococcus aureus in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 13: Wong, S. S. Y., P. L. Ho, P. C. Y. Woo, and K. Y. Yuen Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin. Infect. Dis. 29: Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner In vitro activities of U and U , novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH)
 Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Infections Amenable to OPAT. (Nabin Shrestha + Ajay Mathur)
 3 Infections Amenable to OPAT (Nabin Shrestha + Ajay Mathur) Decisions regarding outpatient treatment of infections vary with the institution, the prescribing physician, the individual patient s condition
3 Infections Amenable to OPAT (Nabin Shrestha + Ajay Mathur) Decisions regarding outpatient treatment of infections vary with the institution, the prescribing physician, the individual patient s condition
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers
 Journal of Antimicrobial Chemotherapy (2003) 51, 1239 1246 DOI: 10.1093/jac/dkg180 Advance Access publication 28 March 2003 Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous
Journal of Antimicrobial Chemotherapy (2003) 51, 1239 1246 DOI: 10.1093/jac/dkg180 Advance Access publication 28 March 2003 Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous
CLINICAL USE OF GLYCOPEPTIDES. Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel
 CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more)
 Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry
 doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
PFIZER INC. Study Initiation Date and Completion Dates: Information not available (Date of Statistical Report: 16 May 2004)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Methicillin-Resistant Staphylococcus aureus (MRSA) S urveillance Report 2008 Background Methods
 Methicillin-Resistant Staphylococcus aureus (MRSA) Surveillance Report 2008 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services
Methicillin-Resistant Staphylococcus aureus (MRSA) Surveillance Report 2008 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Henry A. McKinnell, Jr., Ph.D. Chairman of the Board and Chief Executive
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Henry A. McKinnell, Jr., Ph.D. Chairman of the Board and Chief Executive
Steven D. Brown* and Maria M. Traczewski. The Clinical Microbiology Institute, 9725 SW Commerce Circle, Wilsonville, Oregon 97070
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2010, p. 2063 2069 Vol. 54, No. 5 0066-4804/10/$12.00 doi:10.1128/aac.01569-09 Copyright 2010, American Society for Microbiology. All Rights Reserved. Comparative
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2010, p. 2063 2069 Vol. 54, No. 5 0066-4804/10/$12.00 doi:10.1128/aac.01569-09 Copyright 2010, American Society for Microbiology. All Rights Reserved. Comparative
General surgery department of SGMU Lecturer ass. Khilgiyaev R.H. Anaerobic infection. Gas gangrene
 Anaerobic infection Gas gangrene Anaerobic bacteria Anaerobic bacteria are the most numerous inhabitants of the normal gastrointestinal tract, including the mouth Bacteroides fragilis and Clostridium The
Anaerobic infection Gas gangrene Anaerobic bacteria Anaerobic bacteria are the most numerous inhabitants of the normal gastrointestinal tract, including the mouth Bacteroides fragilis and Clostridium The
L. Montero. Carrera 16# , Consultorio 303, Santafe de Bogotd, D.C., Colombia
 Journal of Antimicrobial Chemotherapy (1996) 37, Suppl. C, 125-131 A comparative study of the efficacy, safety and tolerability of azithromycin and cefaclor in the treatment of children with acute skin
Journal of Antimicrobial Chemotherapy (1996) 37, Suppl. C, 125-131 A comparative study of the efficacy, safety and tolerability of azithromycin and cefaclor in the treatment of children with acute skin
December 3, 2015 Severe Sepsis and Septic Shock Antibiotic Guide
 Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
ORIGINAL ARTICLE DOI: (e) ISSN Online: (p) ISSN Print: Anand Kumar Singh 1, Poonam Verma 2. Sciences, Dehradun
 ORIGINAL ARTICLE CLINICAL ASSESSMENT OF NEPHROTOXICITY ASSOCIATED WITH VANCOMYCIN TROUGH CONCENTRATIONS DURING TREATMENT OF DEEP-SEATED INFECTIONS: A RETROSPECTIVE ANALYSIS Anand Kumar Singh 1, Poonam
ORIGINAL ARTICLE CLINICAL ASSESSMENT OF NEPHROTOXICITY ASSOCIATED WITH VANCOMYCIN TROUGH CONCENTRATIONS DURING TREATMENT OF DEEP-SEATED INFECTIONS: A RETROSPECTIVE ANALYSIS Anand Kumar Singh 1, Poonam
Severe β-lactam allergy. Alternative (use for mild-moderate β-lactam allergy) therapy
 Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger
 Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
An evaluation of 2.0 McFarland Etest method for detection of heterogeneous vancomycin-intermediate Staphylococcus aureus
 Asian Biomedicine Vol. 4 No. 1 February 2010; 141-145 Brief communication (Original) An evaluation of 2.0 McFarland Etest method for detection of heterogeneous vancomycin-intermediate Staphylococcus aureus
Asian Biomedicine Vol. 4 No. 1 February 2010; 141-145 Brief communication (Original) An evaluation of 2.0 McFarland Etest method for detection of heterogeneous vancomycin-intermediate Staphylococcus aureus
Drug Class Review on Macrolides
 Drug Class Review on Macrolides Preliminary Scan Report 5 July 2014 Last Report: Original August 2006 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review on Macrolides Preliminary Scan Report 5 July 2014 Last Report: Original August 2006 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis
 Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Morbidity & Mortality Conference Downstate Medical Center. Daniel Kaufman, MD
 Morbidity & Mortality Conference Downstate Medical Center University Case Presentation Hospital of Brooklyn Daniel Kaufman, MD Necrotizing Fasciitis and Soft- Tissue Infections Necrotizing Fasciitis Deep
Morbidity & Mortality Conference Downstate Medical Center University Case Presentation Hospital of Brooklyn Daniel Kaufman, MD Necrotizing Fasciitis and Soft- Tissue Infections Necrotizing Fasciitis Deep
! Macrolide antibacterial. Fidaxomicin (Dificid ) package labeling. Optimer Pharmaceuticals, Inc. May 2011.
 Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
MRSA: A TEAM APPROACH
 Eric Bosley, MD Laura Stadler, MD John MD J h Draus, D MRSA: A TEAM APPROACH PART I: OUTPATIENT ISSUES AND MANAGEMENT NOT REQUIRING I&D OR HOSPITALIZATION Eric L. Bosley, MD, FAAP Pediatric Associates,
Eric Bosley, MD Laura Stadler, MD John MD J h Draus, D MRSA: A TEAM APPROACH PART I: OUTPATIENT ISSUES AND MANAGEMENT NOT REQUIRING I&D OR HOSPITALIZATION Eric L. Bosley, MD, FAAP Pediatric Associates,
Of 142 cases where sex was known, 56 percent were male; of 127cases where race was known, 90 percent were white, 4 percent were
 Group B Streptococcus Surveillance Report 2014 Oregon Active Bacterial Core Surveillance (ABCs) Center for Public Health Practice Updated: November 2015 Background The Active Bacterial Core surveillance
Group B Streptococcus Surveillance Report 2014 Oregon Active Bacterial Core Surveillance (ABCs) Center for Public Health Practice Updated: November 2015 Background The Active Bacterial Core surveillance
MRSA Micro Scan Pos Combo 6J DADE BEHRING VCM
 PKPD MRSA 1 1 2 1 1 2 17 1 26 17 3 16 vancomycinvcm methicillin-resistant Staphylococcus aureusmrsa 31 pharmacokineticpkparameter retrospective VCM 21 10 PK parameter Mann- Whitney U-test Cmax 37.1 µ gml29.942
PKPD MRSA 1 1 2 1 1 2 17 1 26 17 3 16 vancomycinvcm methicillin-resistant Staphylococcus aureusmrsa 31 pharmacokineticpkparameter retrospective VCM 21 10 PK parameter Mann- Whitney U-test Cmax 37.1 µ gml29.942
Foot infections in persons with diabetes are
 DIAGNOSIS AND MANAGEMENT OF DIABETIC FOOT INFECTION * James S. Tan, MD, MACP, FCCP ABSTRACT According to the American Diabetes Association, approximately 82 000 nontraumatic lower-limb amputations were
DIAGNOSIS AND MANAGEMENT OF DIABETIC FOOT INFECTION * James S. Tan, MD, MACP, FCCP ABSTRACT According to the American Diabetes Association, approximately 82 000 nontraumatic lower-limb amputations were
Webposting Clinical Trial Results Synopsis
 Study Summary This summary information is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This summary information is not intended to replace
Study Summary This summary information is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This summary information is not intended to replace
URINARY TRACT INFECTIONS
 URINARY TRACT INFECTIONS Learning Objectives Identify signs and symptoms that may indicate presence of UTI (both complicated and uncomplicated) List common causative organisms and risk factors for UTIs
URINARY TRACT INFECTIONS Learning Objectives Identify signs and symptoms that may indicate presence of UTI (both complicated and uncomplicated) List common causative organisms and risk factors for UTIs
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
The Challenge of Managing Staphylococcus aureus Bacteremia
 The Challenge of Managing Staphylococcus aureus Bacteremia M A R G A R E T G R A Y B S P F C S H P C L I N I C A L P R A C T I C E M A N A G E R N O R T H / I D P H A R M A C I S T A L B E R T A H E A
The Challenge of Managing Staphylococcus aureus Bacteremia M A R G A R E T G R A Y B S P F C S H P C L I N I C A L P R A C T I C E M A N A G E R N O R T H / I D P H A R M A C I S T A L B E R T A H E A
Beta-lactamase production should have no effect on Azithromycin activity.
 AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
Resistance to new anti-grampositive. Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France
 Resistance to new anti-grampositive agents Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France Recently available antimicrobials against MDR Gram-positive infections Cyclic lipopeptide: daptomycin
Resistance to new anti-grampositive agents Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France Recently available antimicrobials against MDR Gram-positive infections Cyclic lipopeptide: daptomycin
Cubicin A Guide to Dosing
 Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens to tedizolid, a novel oxazolidinone
 Zurenko et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:46 RESEARCH Open Access Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens
Zurenko et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:46 RESEARCH Open Access Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens
GROUP A STREPTOCOCCUS (GAS) INVASIVE
 GROUP A STREPTOCOCCUS (GAS) INVASIVE Case definition CONFIRMED CASE Laboratory confirmation of infection with or without clinical evidence of invasive disease: isolation of group A streptococcus (Streptococcus
GROUP A STREPTOCOCCUS (GAS) INVASIVE Case definition CONFIRMED CASE Laboratory confirmation of infection with or without clinical evidence of invasive disease: isolation of group A streptococcus (Streptococcus
Aciphin Ceftriaxone Sodium
 Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
TOUGH ON IMPETIGO GENTLE ON THE1 PATIENT. Demonstrated efficacy and safety1. New treatment option now available!
 New treatment option now available! TOUGH ON IMPETIGO GENTLE ON THE1 PATIENT Demonstrated efficacy and safety1 Home Efficacy Safety Convenience (ozenoxacin) is indicated for the topical treatment of impetigo
New treatment option now available! TOUGH ON IMPETIGO GENTLE ON THE1 PATIENT Demonstrated efficacy and safety1 Home Efficacy Safety Convenience (ozenoxacin) is indicated for the topical treatment of impetigo
ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: 07/November/2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING
 EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
D DAVID PUBLISHING. 1. Introduction. Kathryn Koliha 1, Julie Falk 1, Rachana Patel 1 and Karen Kier 2
 Journal of Pharmacy and Pharmacology 5 (2017) 607-615 doi: 10.17265/2328-2150/2017.09.001 D DAVID PUBLISHING Comparative Evaluation of Pharmacist-Managed Vancomycin Dosing in a Community Hospital Following
Journal of Pharmacy and Pharmacology 5 (2017) 607-615 doi: 10.17265/2328-2150/2017.09.001 D DAVID PUBLISHING Comparative Evaluation of Pharmacist-Managed Vancomycin Dosing in a Community Hospital Following
Objectives. Define classes of uncomplicated skin and soft tissue infection (SSTI) that drive empiric antimicrobial selection
 Objectives Define classes of uncomplicated skin and soft tissue infection (SSTI) that drive empiric antimicrobial selection Purulent SSTI Non-purulent SSTI Recognize conditions that suggest complications
Objectives Define classes of uncomplicated skin and soft tissue infection (SSTI) that drive empiric antimicrobial selection Purulent SSTI Non-purulent SSTI Recognize conditions that suggest complications
Open Forum Infectious Diseases MAJOR ARTICLE
 Open Forum Infectious Diseases MAJOR ARTICLE Efficacy and Safety of Oritavancin Relative to Vancomycin for Patients with Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in the Outpatient Setting:
Open Forum Infectious Diseases MAJOR ARTICLE Efficacy and Safety of Oritavancin Relative to Vancomycin for Patients with Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in the Outpatient Setting:
Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia
 Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia Brandon Dionne, PharmD, BCPS, AAHIVP Assistant Clinical Professor Northeastern University Seth Housman, PharmD, MPA Clinical Assistant
Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia Brandon Dionne, PharmD, BCPS, AAHIVP Assistant Clinical Professor Northeastern University Seth Housman, PharmD, MPA Clinical Assistant
EDUCATIONAL COMMENTARY CLOSTRIDIUM DIFFICILE UPDATE
 EDUCATIONAL COMMENTARY CLOSTRIDIUM DIFFICILE UPDATE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
EDUCATIONAL COMMENTARY CLOSTRIDIUM DIFFICILE UPDATE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
Fusidic acid and erythromycin in the treatment of skin and soft tissue infection: a double blind study Wall A R, Menday A P
 Fusidic acid and erythromycin in the treatment of skin and soft tissue infection: a double blind study Wall A R, Menday A P Record Status This is a critical abstract of an economic evaluation that meets
Fusidic acid and erythromycin in the treatment of skin and soft tissue infection: a double blind study Wall A R, Menday A P Record Status This is a critical abstract of an economic evaluation that meets
ADJUVANT TIGECYCLINE FOR SEVERE CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA
 ADJUVANT TIGECYCLINE FOR SEVERE CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA Candace Marr, DO Catholic Health System University at Buffalo, NY Kevin Shiley, MD Catholic Health System Buffalo, NY FINANCIAL
ADJUVANT TIGECYCLINE FOR SEVERE CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA Candace Marr, DO Catholic Health System University at Buffalo, NY Kevin Shiley, MD Catholic Health System Buffalo, NY FINANCIAL
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Treatment of febrile neutropenia in patients with neoplasia
 Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Aerobic bacteria isolated from diabetic septic wounds
 Aerobic bacteria isolated from diabetic septic wounds Eithar Mohammed Mahgoub*, Mohammed Elfatih A. Omer Faculty of Pharmacy, Omdurman Islamic University Department of Pharmaceutical Microbiology, Omdurman
Aerobic bacteria isolated from diabetic septic wounds Eithar Mohammed Mahgoub*, Mohammed Elfatih A. Omer Faculty of Pharmacy, Omdurman Islamic University Department of Pharmaceutical Microbiology, Omdurman
Xerava (eravacycline) NEW PRODUCT SLIDESHOW
 Xerava (eravacycline) NEW PRODUCT SLIDESHOW Introduction Brand name: Xerava Generic name: Eravacycline Pharmacological class: Tetracycline antibiotic Strength and Formulation: 50mg; per vial; lyophilized
Xerava (eravacycline) NEW PRODUCT SLIDESHOW Introduction Brand name: Xerava Generic name: Eravacycline Pharmacological class: Tetracycline antibiotic Strength and Formulation: 50mg; per vial; lyophilized
Pneumonia Community-Acquired Healthcare-Associated
 Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Endocardite infectieuse
 Endocardite infectieuse 1. Raccourcir le traitement: jusqu où? 2. Proposer un traitement ambulatoire: à partir de quand? Endocardite infectieuse A B 90 P = 0.014 20 P = 0.0005 % infective endocarditis
Endocardite infectieuse 1. Raccourcir le traitement: jusqu où? 2. Proposer un traitement ambulatoire: à partir de quand? Endocardite infectieuse A B 90 P = 0.014 20 P = 0.0005 % infective endocarditis
VANLID Capsules (Vancomycin hydrochloride)
 Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Osteomyelitis Samir S. Shah, MD, MSCE
 Osteomyelitis Samir S. Shah, MD, MSCE Professor, Department of Pediatrics University of Cincinnati College of Medicine Director, Division of Hospital Medicine Attending Physician in Infectious Diseases
Osteomyelitis Samir S. Shah, MD, MSCE Professor, Department of Pediatrics University of Cincinnati College of Medicine Director, Division of Hospital Medicine Attending Physician in Infectious Diseases
Trust Guideline for the Use of Parenteral Vancomycin and Teicoplanin in Adults
 A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
ZINEX. Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg
 ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
Vancomycin: Class: Antibiotic.
 Vancomycin: Class: Antibiotic. Indications: Treatment of patients with infections caused by staphylococcal species and streptococcal Species. Available dosage form in the hospital: 1G VIAL, 500MG VIAL.
Vancomycin: Class: Antibiotic. Indications: Treatment of patients with infections caused by staphylococcal species and streptococcal Species. Available dosage form in the hospital: 1G VIAL, 500MG VIAL.
Incidence per 100,
 Group B Streptococcus Surveillance Report 2005 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services Updated: January 2007 Background
Group B Streptococcus Surveillance Report 2005 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services Updated: January 2007 Background
Zinplava. (bezlotoxumab) New Product Slideshow
 Zinplava (bezlotoxumab) New Product Slideshow Introduction Brand name: Zinplava Generic name: Bezlotoxumab Pharmacological class: Human IgG1 monoclonal antibody Strength and Formulation: 25mg/mL; solution
Zinplava (bezlotoxumab) New Product Slideshow Introduction Brand name: Zinplava Generic name: Bezlotoxumab Pharmacological class: Human IgG1 monoclonal antibody Strength and Formulation: 25mg/mL; solution
Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil
 224 BJID 2003; 7 (June) Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil Larissa Lutz, Adão Machado, Nadia Kuplich and Afonso Luís
224 BJID 2003; 7 (June) Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil Larissa Lutz, Adão Machado, Nadia Kuplich and Afonso Luís
Received 24 August 2010/Returned for modification 7 November 2010/Accepted 7 February 2011
 JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2011, p. 1583 1587 Vol. 49, No. 4 0095-1137/11/$12.00 doi:10.1128/jcm.01719-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Clinical and
JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2011, p. 1583 1587 Vol. 49, No. 4 0095-1137/11/$12.00 doi:10.1128/jcm.01719-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Clinical and
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Rapenin (phenoxymethylpenicillin potassium) is indicated for the treatment of infections caused by penicillin-sensitive bacteria.
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Rapenin (phenoxymethylpenicillin potassium) is indicated for the treatment of infections caused by penicillin-sensitive bacteria.
ABSTRACT PURPOSE METHODS
 ABSTRACT PURPOSE The purpose of this study was to characterize the CDI population at this institution according to known risk factors and to examine the effect of appropriate evidence-based treatment selection
ABSTRACT PURPOSE The purpose of this study was to characterize the CDI population at this institution according to known risk factors and to examine the effect of appropriate evidence-based treatment selection
SKIN AND SOFT TISSUE INFECTIONS
 SKIN AND SOFT TISSUE INFECTIONS ZAIN CHAGLA SEA COURSES - PATAGONIA COPYRIGHT 2017 BY SEA COURSES INC. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in
SKIN AND SOFT TISSUE INFECTIONS ZAIN CHAGLA SEA COURSES - PATAGONIA COPYRIGHT 2017 BY SEA COURSES INC. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in
Evaluation of Vancomycin Continuous Infusion in Trauma Patients
 OBJECTIVES Evaluation of Vancomycin Continuous Infusion in Trauma Patients Brittany D. Bissell, Pharm.D. PGY-2 Critical Care Pharmacy Resident Jackson Memorial Hospital Miami, Florida Evaluate the potential
OBJECTIVES Evaluation of Vancomycin Continuous Infusion in Trauma Patients Brittany D. Bissell, Pharm.D. PGY-2 Critical Care Pharmacy Resident Jackson Memorial Hospital Miami, Florida Evaluate the potential
2018 CNISP HAI Surveillance Case definitions
 2018 CNISP HAI Surveillance Case definitions The following case definitions for the surveillance of healthcare-associated infections (HAIs) are used by all acute-care hospitals that participate in the
2018 CNISP HAI Surveillance Case definitions The following case definitions for the surveillance of healthcare-associated infections (HAIs) are used by all acute-care hospitals that participate in the
Updates to pharmacological management in the prevention of recurrent Clostridium difficile
 Updates to pharmacological management in the prevention of recurrent Clostridium difficile Julia Shlensky, PharmD PGY2 Internal Medicine Resident September 12, 2017 2017 MFMER slide-1 Clinical Impact Increasing
Updates to pharmacological management in the prevention of recurrent Clostridium difficile Julia Shlensky, PharmD PGY2 Internal Medicine Resident September 12, 2017 2017 MFMER slide-1 Clinical Impact Increasing
Cefotaxime Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Antimicrobial Guidelines for the Empirical Management of Diabetic Foot Infections
 Antimicrobial Guidelines for the Empirical Management of Diabetic Foot Infections Version 7.2 PAGL Inclusion Approved at January 2017 PGC APPROVED BY: TRUST REFERENCE: B3/2017 AWP REF: UHL Policies and
Antimicrobial Guidelines for the Empirical Management of Diabetic Foot Infections Version 7.2 PAGL Inclusion Approved at January 2017 PGC APPROVED BY: TRUST REFERENCE: B3/2017 AWP REF: UHL Policies and
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Mupirocin Rationale for the EUCAST clinical breakpoints, version th July 2010
 Mupirocin Rationale for the EUCAST clinical breakpoints, version 1.0 6 th July 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Mupirocin Rationale for the EUCAST clinical breakpoints, version 1.0 6 th July 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Disclosure. Patient Case. Objectives. Patient Case. Patient Case 7/25/2015. An update on the treatment of skin and soft tissue infections
 Disclosure 49th Annual Meeting An update on the treatment of skin and soft tissue infections I do not have a vested interest in or affiliation with any corporate organization offering financial support
Disclosure 49th Annual Meeting An update on the treatment of skin and soft tissue infections I do not have a vested interest in or affiliation with any corporate organization offering financial support
Chapter 19. Pathogenic Gram-Positive Bacteria. Staphylococcus & Streptococcus
 Chapter 19 Pathogenic Gram-Positive Bacteria Staphylococcus & Streptococcus Staphylococcus Normal members of every human's microbiota Can be opportunistic pathogens Facultative anaerobes Cells occur in
Chapter 19 Pathogenic Gram-Positive Bacteria Staphylococcus & Streptococcus Staphylococcus Normal members of every human's microbiota Can be opportunistic pathogens Facultative anaerobes Cells occur in
OSTEOMYELITIS. If it occurs in adults, then the axial skeleton is the usual site.
 OSTEOMYELITIS Introduction Osteomyelitis is an acute or chronic inflammatory process of the bone and its structures secondary to infection with pyogenic organisms. Pathophysiology Osteomyelitis may be
OSTEOMYELITIS Introduction Osteomyelitis is an acute or chronic inflammatory process of the bone and its structures secondary to infection with pyogenic organisms. Pathophysiology Osteomyelitis may be
Spectrum of vancomycin and susceptibility testing
 Spectrum of vancomycin and susceptibility testing Olivier Denis Service de Microbiologie Laboratoire de bactériologie Service de Microbiologie Hôpital Erasme Glycopeptides Vancomycin 1958 < Amycolatopsis
Spectrum of vancomycin and susceptibility testing Olivier Denis Service de Microbiologie Laboratoire de bactériologie Service de Microbiologie Hôpital Erasme Glycopeptides Vancomycin 1958 < Amycolatopsis
Mesporin TM. Ceftriaxone sodium. Rapid onset, sustained action, for a broad spectrum of infections
 Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Linezolid-related adverse effects in clinical practice in children
 Original article Arch Argent Pediatr 2017;115(5):470-475 / 470 Linezolid-related adverse effects in clinical practice in children Nuri Bayram, M.D., Associate professor a, Mine Düzgöl, M.D. a, Ahu Kara,
Original article Arch Argent Pediatr 2017;115(5):470-475 / 470 Linezolid-related adverse effects in clinical practice in children Nuri Bayram, M.D., Associate professor a, Mine Düzgöl, M.D. a, Ahu Kara,
Staphylococci. What s to be Covered. Clinical Scenario #1
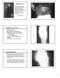 Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
What s to be Covered. Microbiology of staphylococci Epidemiology of S. aureus infections Pathogenesis of S. aureus infections
 Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
Cellulitis: a practical guide
 Cellulitis: a practical guide Dr John Day Consultant in Infectious Diseases & General Medicine Southend University Hospital NHS Foundation Trust 77 yr old retired civil servant A&E presentation c/o rigors
Cellulitis: a practical guide Dr John Day Consultant in Infectious Diseases & General Medicine Southend University Hospital NHS Foundation Trust 77 yr old retired civil servant A&E presentation c/o rigors
Necrotizing Fasciitis. By Lisa Banks
 Necrotizing Fasciitis By Lisa Banks Foot infections are the most common softtissue infections in pts with diabetes Necrotizing fasciitis is the most important soft tissue infection in DM pts involving
Necrotizing Fasciitis By Lisa Banks Foot infections are the most common softtissue infections in pts with diabetes Necrotizing fasciitis is the most important soft tissue infection in DM pts involving
Comparison of the Microbiology and Antibiotic Treatment Among Diabetic and Nondiabetic Patients Hospitalized for Cellulitis or Cutaneous Abscess
 ORIGINAL RESEARCH Comparison of the Microbiology and Antibiotic Treatment Among Diabetic and Nondiabetic atients Hospitalized for Cellulitis or Cutaneous Abscess Timothy C. Jenkins, MD 1,2,3,4 *, Bryan
ORIGINAL RESEARCH Comparison of the Microbiology and Antibiotic Treatment Among Diabetic and Nondiabetic atients Hospitalized for Cellulitis or Cutaneous Abscess Timothy C. Jenkins, MD 1,2,3,4 *, Bryan
Aerobic gram-positive microorganisms Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pneumonia, Streptococcus pyogenes
 AZIMEX Composition Each 5 ml contains Azithromycin (as dihydrate) 200 mg Powder Action Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and, thus, interfering with
AZIMEX Composition Each 5 ml contains Azithromycin (as dihydrate) 200 mg Powder Action Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and, thus, interfering with
Beta-lactamase production should have no effect on Azithromycin activity.
 AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules & Powder Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Azimex
AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules & Powder Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Azimex
ALTARGO TM Retapamulin. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w)
 ALTARGO TM Retapamulin QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) PHARMACEUTICAL FORM Ointment. CLINICAL PARTICULARS Indications ALTARGO (retapamulin) 1% ointment is indicated
ALTARGO TM Retapamulin QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) PHARMACEUTICAL FORM Ointment. CLINICAL PARTICULARS Indications ALTARGO (retapamulin) 1% ointment is indicated
IH Pharmacy Live Rounds
 IH Pharmacy Live Rounds Effect of Vancomycin plus Rifampicin in the Treatment of Nosocomial Methicillin-resistant Staphylococcus aureus Pneumonia Sandra Katalinic, Pharmacy Resident Kelowna General Hospital
IH Pharmacy Live Rounds Effect of Vancomycin plus Rifampicin in the Treatment of Nosocomial Methicillin-resistant Staphylococcus aureus Pneumonia Sandra Katalinic, Pharmacy Resident Kelowna General Hospital
Safety and tolerability of linezolid
 Journal of Antimicrobial Chemotherapy (2003) 51, Suppl. S2, ii45 ii53 DOI: 10.1093/jac/dkg253 Safety and tolerability of linezolid Gary French* Department of Infection, Guy s and St Thomas Hospital and
Journal of Antimicrobial Chemotherapy (2003) 51, Suppl. S2, ii45 ii53 DOI: 10.1093/jac/dkg253 Safety and tolerability of linezolid Gary French* Department of Infection, Guy s and St Thomas Hospital and
Antibiotic treatment of streptococcal and enterococcal endocarditis: an overview
 European Heart Journal (1995) 16 {Supplement B), 75-79 Antibiotic treatment of streptococcal and enterococcal endocarditis: an overview P. FRANCIOLI Division of Hospital Preventative Medicine and Department
European Heart Journal (1995) 16 {Supplement B), 75-79 Antibiotic treatment of streptococcal and enterococcal endocarditis: an overview P. FRANCIOLI Division of Hospital Preventative Medicine and Department
Department of Pharmacy Services, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA; 2
 Pathogens 2015, 4, 599-605; doi:10.3390/pathogens4030599 Article OPEN ACCESS pathogens ISSN 2076-0817 www.mdpi.com/journal/pathogens Assessing the Surrogate Susceptibility of Oxacillin and Cefoxitin for
Pathogens 2015, 4, 599-605; doi:10.3390/pathogens4030599 Article OPEN ACCESS pathogens ISSN 2076-0817 www.mdpi.com/journal/pathogens Assessing the Surrogate Susceptibility of Oxacillin and Cefoxitin for
Bone and Joint Infections in Diabetics: Diagnosis and Management of Diabetic Foot and Other Common Lower Extremity Infections
 Bone and Joint Infections in Diabetics: Diagnosis and Management of Diabetic Foot and Other Common Lower Extremity Infections Objectives How do you to diagnose, classify and manage DFI? How do you diagnose
Bone and Joint Infections in Diabetics: Diagnosis and Management of Diabetic Foot and Other Common Lower Extremity Infections Objectives How do you to diagnose, classify and manage DFI? How do you diagnose
Synopsis Style Clinical Study Report SAR ACT sarilumab Version number : 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
Continuous vs Intermittent Dosing of Antibiotics in Critically-Ill Patients
 Continuous vs Intermittent Dosing of Antibiotics in Critically-Ill Patients Jan O Friedrich, MD DPhil Associate Professor of Medicine, University of Toronto Medical Director, MSICU St. Michael s Hospital,
Continuous vs Intermittent Dosing of Antibiotics in Critically-Ill Patients Jan O Friedrich, MD DPhil Associate Professor of Medicine, University of Toronto Medical Director, MSICU St. Michael s Hospital,
Probiotics for Primary Prevention of Clostridium difficile Infection
 Probiotics for Primary Prevention of Clostridium difficile Infection Objectives Review risk factors for Clostridium difficile infection (CDI) Describe guideline recommendations for CDI prevention Discuss
Probiotics for Primary Prevention of Clostridium difficile Infection Objectives Review risk factors for Clostridium difficile infection (CDI) Describe guideline recommendations for CDI prevention Discuss
