The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten
|
|
|
- Ashley Fields
- 5 years ago
- Views:
Transcription
1 University of Groningen The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 1998 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Blok, B. F. M. (1998). The organization of the central control of micturition in cats and humans: anatomical and physiological investigations s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date:
2 The Organization of the Central Control of Micturition in Cats and Humans Anatomical and Physiological Investigations
3 1998, B.F.M. Blok All rights reserved. No part of this book may be reproduced or transmitted in any form or by any means, without permission from the author. ISBN: Cover design: P.O. Gerrits Printed by: Joh. Enschedé en Zonen, Amsterdam This thesis was supported by: Johan Vermeij Stichting Medical Measurements Systems Medtronic Interstim Remmert Adriaan Laan fonds Van Leersumfonds KNAW
4 RIJKSUNIVERSITEIT GRONINGEN The Organization of the Central Control of Micturition in Cats and Humans Anatomical and Physiological Investigations Proefschrift ter verkrijging van het doctoraat in de Medische Wetenschappen aan de Rijksuniversiteit Groningen op gezag van de Rector Magnificus, dr. F. van der Woude in het openbaar te verdedigen op woensdag 20 mei 1998 des namiddags te 2.45 uur door Bertil Feddo Maarten Blok geboren op 15 december 1962 te Noordwijk
5 Promotor: Prof. dr. G. Holstege
6 Promotion Committee: Prof. dr. W.C. de Groat Prof. dr. D. Griffiths Prof. dr. R.A. Janknegt Prof. dr. E.A. Tanagho University of Pittsburgh, Pittsburgh University of Alberta, Edmonton University of Maastricht, Maastricht University of California, San Francisco Paranymphs: Drs. L.J. Mouton Dr. P.O. Gerrits Voor Wessel en Willemijn
7 Contents General Introduction 9 Chapter 1 Ultrastructural Evidence for a Paucity of 19 Projections from the Lumbosacral Cord to the Pontine Micturition Center or M-Region in the Cat: A New Concept for the Organization of the Micturition Reflex with the Periaqueductal Gray as Central Relay Bertil F.M. Blok, Henk de Weerd, and Gert Holstege J. Comp. Neurol. 359: (1995) Chapter 2 Ultrastructural Evidence for a Direct Pathway 33 from the Pontine Micturition Center to the Parasympathetic Preganglionic Motoneurons of the Bladder of the Cat Bertil F.M. Blok, and Gert Holstege Neurosci. Lett. 222: (1997) Chapter 3 The Pontine Micturition Center Projects to 39 Sacral Cord GABA Immunoreactive Neurons in the Cat Bertil F.M. Blok, Henk de Weerd, and Gert Holstege Neurosci. Lett. 233: (1997) Chapter 4 Electrical Stimulation of the Sacral Dorsal Gray 45 Commissure evokes Relaxation of the External Urethral Sphincter in the Cat Bertil F.M. Blok, Jos T.P.W. van Maarseveen, and Gert Holstege Neurosci. Lett., in press Chapter 5 Location of External Anal Sphincter Motoneurons in 49 the Sacral Cord of the Female Domestic Pig Bertil F.M. Blok, Gert Roukema, Bas Geerdes, and Gert Holstege Neurosci. Lett. 216: (1996) Chapter 6 The Two Pontine Micturition Centers in the Cat are 53 not Interconnected; Implications for the Central Organization of Micturition Bertil F.M. Blok, and Gert Holstege J. Comp. Neurol., submitted
8 Chapter 7 Direct Projections from the Periaqueductal 63 Gray to the Pontine Micturition Center (M-region). An Anterograde and Retrograde Tracing Study in the Cat Bertil F.M. Blok, and Gert Holstege Neurosci. Lett. 166:93-96 (1994) Chapter 8 A PET Study on Brain Control of Micturition 69 in Humans Bertil F.M. Blok, Antoon T.M. Willemsen, and Gert Holstege Brain 120: (1997) Chapter 9 A PET Study on Cortical and Subcortical Control 83 of Pelvic Floor Musculature in Women Bertil F.M. Blok, Leontien M. Sturms, and Gert Holstege J. Comp. Neurol. 389: (1997) Chapter 10 Brain Activation during Micturition in Women 95 Bertil F.M. Blok, Leontien M. Sturms, and Gert Holstege Brain, in press General Discussion 105 References 111 Abbreviations 118 Summary 119 Samenvatting 121 Dankwoord 123 List of Publications 125 Curriculum Vitae 127
9 General Introduction General Introduction The kidneys continuously produce urine, which, for practical reasons, is first collected in the bladder where it is stored until disposal or micturition is possible. Since the individual animal is relatively vulnerable during the release of urine, micturition only takes place when the environment is relatively safe. Furthermore, in many animals urine is used as a marker for territorial demarcation or sexual attraction (a female lets the males know that she is in estrus by leaving a scent trace). Thus, micturition does not take place at random, but is part of a rather complicated behavior, directly related to the survival of the individual or species. In order to investigate this rather complicated behavior it is necessary to identify and define the nature of the muscular and neural structures involved in micturition. This chapter gives a general description of the central organization of motor control. The structures involved in micturition will be introduced, and the aim of the work, described in this thesis, will be explained. Motor system Motoneurons All motor behavior, including micturition, is the result of the activation of specific sets of striated and smooth muscles. Striated or skeletal muscles are innervated by somatic motoneurons, whereas smooth musculature is innervated by sympathetic or parasympathetic postganglionic motoneurons, which, in turn, are innervated by preganglionic motoneurons. The sympathetic and parasympathetic motoneurons and their fibers form the autonomic nervous system. Each muscle is innervated by its own group of motoneurons. Somatic motoneurons innervating muscles of the head are located in various cell groups in the brainstem, those of the remaining parts of the body in the ventral horn of the spinal cord. The axial muscles of the neck and back are innervated by motoneurons in the medial part of the ventral horn throughout the length of the spinal cord, whereas those innervating the muscles of the extremities are located in the lateral part of the ventral horn of the cervical and lumbosacral enlargements. Sympathetic preganglionic motoneurons are present in the lateral horn of the thoracic and upper lumbar cord, whereas parasympathetic preganglionics are located in certain brain stem nuclei, and in the sacral cord (see Holstege, 1996 for review). All central somatic and autonomic motoneuronal cell groups are controlled by other structures in the central nervous system (CNS). The motoneurons together with their control structures form the motor system as defined by Holstege (1991; Fig. 1). The basic premotor interneuronal system Motoneurons receive afferents from several sources. Most are excitatory in nature, but normal motor behavior is not possible without inhibitory afferent input to antagonist muscles. In the spinal cord most of the premotor interneurons are located in the intermediate zone of the spinal cord (laminae V to VIII; Rexed, 1952). Premotor interneurons for the somatic motoneurons of the brainstem are located in the pontine and medullary reticular formation, which can be seen as the rostral extent of the spinal intermediate zone (Holstege et al., 1977). Most of these interneurons project to motoneurons at the same level or at levels rostral or caudal to where interneurons are located. The majority of the premotor interneurons terminate on motoneurons nearby, but in some cases they project to motoneurons much further away, for example C2 interneurons projecting to C8 (see Holstege, 1988), the respiratory interneurons in the medulla projecting to the phrenic, intercos- 9
10 General Introduction Voluntary motor system Lateral Medial independent movements of the extremities Motor system eye, neck, axial and proximal body movements Basic system (premotor interneurons) Emotional motor system Lateral specific emotional behaviors Medial gain setting systems including triggering mechanisms of rhythmical and other spinal reflexes Motoneurons Fig. 1. Schematic overview of the three subdivisions of the motor system (from Holstege, 1996). tal or abdominal motoneurons, and the nucleus retroambiguus (NRA) interneurons in the caudal medulla projecting to motoneurons in the lumbosacral cord involved in mating behavior (see VanderHorst and Holstege, 1996). The basic motor system consists of all the premotor interneuronal cell groups in the spinal cord and caudal brainstem (Fig. 1). 10 The voluntary or somatic motor system The influence of the voluntary motor system is evident in hemiplegic patients who are unable to lift their arm or leg voluntarily on one side of their body. The somatic motor system is first described by Kuijpers (for review, see Kuijpers, 1981; Holstege 1991; 1996) and involves structures controlling voluntary non-emotionally directed movements. It consists of a medial and a lateral component. The medial component controls the voluntary control of proximal musculature of the trunk and back, and is important for postural control against gravity and the coordination of the head-body movements. This component consists of the ventral cortico-, interstitio-, tecto-, vestibulo-, and reticulospinal tracts, which are located at the level of the spinal cord in the ventral funiculus. These tracts terminate bilaterally on premotor interneurons located medially in the ventral horn, which in turn project to the motoneurons of back and trunk muscles. The lateral component concerns the voluntary control of distal musculature of arms and legs, and is important for behavior like grasping objects and typing on a computer. In most mammals the lateral component consists of the lateral corticobulbospinal tract and the rubrospinal tract. The lateral corticospinal tract originates in the motor and premotor cortex, passes through the internal capsule, cerebral peduncle and pyramidal tract to cross via its decussation at the transition between brainstem and spinal cord. The fibers terminate on premotor interneurons and motoneurons in the lateral
11 General Introduction part of the ventral horn. Interruption of the lateral corticospinal tract by a cerebral stroke in the internal capsule results in a paralysis of one or more contralateral limbs. The rubrospinal tract, originating in the nucleus ruber in the mesencephalon, also plays an important role in the lateral component, but not in humans, where it seems to be overgrown by the corticospinal tract. The emotional motor system Many studies demonstrate that certain parts of the limbic system give rise to a descending system, which is completely separate from the somatic motor system. Holstege (1992; 1996) used the term emotional motor system for the other motor system, originating in parts of the limbic system or in structures strongly related to it. All emotionally related activities together are crucial for the survival of the individual or its species. Also the emotional motor system can be subdivided into a medial and a lateral component (Fig. 1). The medial component, via its projections to the neurons of origin of the diffuse (sub-)coeruleo-, ventral medullary reticulospinal and raphespinal pathways, has a global effect on the level of activity of all the somatosensory and motoneurons by changing their membrane excitability. The medial component has its origin in the medial hypothalamus and mesencephalic periaqueductal gray (PAG). The lateral component represents several distinct pathways underlying specific emotional behaviors, such as vocalization (Jürgens, 1979; Holstege, 1989), blood pressure control (Lovick, 1993; 1996), sexual behavior (Pfaff et al., 1994; VanderHorst and Holstege, 1995), and micturition (Blok and Holstege, 1996). The lateral component has its origin in the central nucleus of the amygdala, the bed nucleus of the stria terminalis, the lateral hypothalamus, but also the mesencephalic PAG. The PAG controls several relatively uncomplicated primary reactions important for the survival of the individual, like aggression and defensive behavior, and important for the survival of the species, like maternal and reproductive behavior. Each of these reactions consists of a specific array of motor activities and level setting mechanisms. In order to control this specific array specific parts of the PAG project to specific premotor interneurons (Fig. 2). Micturition related motoneurons and premotor interneurons Micturition is a coordinated action between the bladder muscle and the striated external urethral sphincter (EUS), which closes the bladder outlet. The EUS is part of the pelvic floor musculature. During the storage of urine, the detrusor muscle is relaxed and the EUS is tonically contracted. When micturition takes place, this activation pattern is reversed: the EUS is relaxed and the bladder contracts, resulting in expulsion of urine. Although the preganglionic motoneurons of the bladder and the somatic motoneurons of the EUS are located in the sacral cord (Fig. 3), their premotor interneurons are located in the brainstem. Motoneurons innervating the urinary bladder and external urethral sphincter The parasympathetic preganglionic motoneurons of the smooth muscle of the bladder (musculus detrusor) are located in the sacral intermediolateral cell group (IML). In the cat these neurons are located at the spinal segments S2-S3 and their axons reach the bladder via the pelvic nerve (Morgan et al., 1979). In the rat the parasympathetic preganglionic motoneurons are located in the spinal segments L6-S1 (Hancock and Peveto, 1979; Nadelhaft and Booth, 1984) and in humans in S2-S4 (Pick, 1970). The preganglionic fibers terminate on ganglion cells in the bladder wall. These postganglionic neurons innervate the smooth bladder muscle. The majority of the sympathetic preganglionic motoneurons innervating the bladder 11
12 12 Pedunculopontine and cuneiform nuclei Limbic system Periaqueductal gray (PAG) General Introduction Ventral 1/3 of caudal pontine and medullary medial tegmentum Barrington's nucleus subretrofacial nucleus nucleus retroambiguus sympathetic preganglionics in the intermediolateral cell column sensory neurons in the dorsal horn motoneurons and premotor interneurons in the ventral horn and intermediate zone parasympathetic preganglionics in the sacral cord sympathetic preganglionics in the intermediolateral cell column motoneurons of the larynx, pharynx, soft palate, and expiratory muscles motoneurons of the iliopsoas, adductor longus, hamstring, pelvic floor, and axial muscles spinal cord laminae VIII and medial VII T1-T2 intermediolateral cell column C4-T8 lamina X general level of sympathetic activity nociception control locomotion micturition (this thesis) cardiovascular changes vocalization receptive behavior defensive posture? pupil dilatation?? Fig. 2. Schematic overview of the descending projections from the PAG to different regions in the caudal brainstem and spinal cord, and their possible functions.
13 (Onufrowicz, 1899). General Introduction Parasympathetic motoneurons Onuf's nucleus (+) (+) External urethral sphincter Bladder S2 Fig. 3. Schematic overview of the sacral motoneurons involved in micturition. are located in the intermediolateral cell group of the caudal thoracic and rostral lumbar cord and send their axons via the splanchnic nerves to sympathetic ganglion cells in the lumbosacral sympathetic chain, inferior mesenteric ganglia and the major pelvic ganglia (Applebaum et al., 1980; Andersson and Sjögren, 1982; Vera and Nadelhaft, 1992). Postganglionic sympathetic fibers run via the pelvic and hypogastric nerves before innervating the bladder. The sympathetic outflow innervates the smooth muscle fibers of the bladder neck, and is believed to play a role during the urine storage phase (de Groat and Saum, 1972; Vaughan and Satchell, 1992) and during sexual behavior (Kimura et al., 1975). The EUS is innervated by the pudendal nerve. Motoneurons of the EUS in the cat are located in the ventrolateral part of the so-called nucleus of Onuf of the ventral horn at the level of S1 and S2 (Sato et al., 1978; Kuzuhara et al., 1983). The motoneurons of the external anal sphincter are located in the dorsomedial part of the nucleus of Onuf (Kuzuhara et al., 1983). In humans Onuf s nucleus is located in the segments S1-S3 (+) Premotor interneurons involved in micturition Since the work of Barrington (1925) it is known that the coordinating component of the micturition reflex is not located in the sacral cord, but in the dorsolateral portion of the pontine tegmental field. Bilateral lesions in this area in the cat result in urinary retention. The same is true when the fiber pathways originating in Barrington s area are interrupted e.g. at the level of the spinal cord. Also in humans interruption of the descending fibers from the pons to the sacral cord, for example in patients with a transection of the spinal cord, results in retention of urine and finally in dyssynergic micturition. In such patients the contraction of the bladder is accompanied by simultaneous contraction of the sphincter. Several studies in various animals have attempted to identify the area originally described by Barrington. Pontine micturition center Barrington s area or nucleus is also called the pontine micturition center (PMC; Loewy et al., 1979), or M (medial)-region (Holstege et al., 1986). The latter term was chosen because another group of neurons in more lateral parts of the same dorsolateral pontine tegmental field was found to influence micturition and urinary continence. These lateral cells are referred to as L (lateral)-region (Holstege et al., 1986). Anterograde tracing studies in the rat (Loewy et al., 1979), opossum (Martin et al, 1979), cat (Holstege et al., 1979, 1986), and monkey (Westlund and Coulter, 1980) have shown that neurons in the PMC project, via fibers in the spinal lateral and dorsolateral funiculi, directly to the sacral intermediolateral cell group, which contain the parasympathetic preganglionic motoneurons of the urinary bladder. Neurons in the PMC in the cat project also to the sacral dorsal gray com- 13
14 General Introduction Fig. 4. Brightfield photomicrographs of autoradiographs showing [ 3 H] leucine injection areas and darkfield photomicrographs showing the spinal distribution of labeled fibers after injections in the M-region (on the left) and in the L-region (on the right) in the cat. Note the dense distribution of labeled fibers to the sacral intermediolateral (parasympathetic motoneurons) and intermediomedial cell groups from the M-region (S2 segment on the left). Note also the pronounced projection to the nucleus of Onuf (S1 segment on the right) from the L-region. Note further the contralateral pathway in the dorsolateral funiculus, terminating in lamina I, the outer part of II, and laminae V and VI throughout the length of the spinal cord (from Holstege et al., 1986). 14
15 General Introduction missure (DGC) or intermedio-medial cell group (IMM), but do not project to the nucleus of Onuf (Holstege et al.,1979, 1986; Fig. 4, on the left). Electrical stimulation in the PMC in the cat produces an immediate and sharp decrease in the urethral pressure and pelvic floor electromyogram (EMG), followed in about 2 seconds by a steep rise in the intravesical pressure (Holstege et al. 1986), mimicking normal micturition. L-region The L-region projects, via fibers in the lateral funiculus, bilaterally to the nucleus of Onuf (Holstege et al., 1979; 1986; Fig. 4, on the right). Stimulation in the L-region results in strong excitation of the pelvic floor musculature and an increase in the urethral pressure (Holstege et al., 1986). Bilateral lesions in the L-region give rise to an inability to store urine; bladder capacity is reduced and urine is expelled prematurely by excessive detrusor activity accompanied by urethral relaxation (Griffiths et al., 1990). Apart from the afferents from the PMC- and L-regions, the parasympathetic preganglionic bladder motoneurons and Onuf s nucleus motoneurons receive also afferents from other sources, like the NRA (VanderHorst and Holstege, 1995) and the paraventricular nucleus of the hypothalamus (Holstege, 1987). However, their descending systems are thought to play a role in other functions as mating behavior, but not in micturition. Ascending pathways involved in micturition Peripheral afferent nerves Most afferent fibers from the bladder enter the sacral cord via the pelvic nerve. The peripheral fibers of the dorsal root ganglia neurons of the pelvic nerve contact the bladder wall mechanoreceptors. The proximal fibers enter Lissauer s tract and terminate mainly in Rexed s (1954) laminae I, V, VII, and X of the lumbosacral spinal cord at segments L4-S2 (Morgan et al., 1979 in the cat). The majority of these afferents are thin myelinated and unmyelinated axons, and their conduction velocities are in the A and C- fiber range, respectively (Hulsebosch and Coggeshall, 1982). Most A fibers originate from slowly adapting mechanoreceptors in the bladder wall, and excitation of these fibers results in activation of the micturition reflex. In all likelihood, the A fibers are the peripheral afferent fibers for this reflex (De Groat et al., 1982; Mallory et al., 1989), because the unmyelinated C-fibers in the pelvic nerve do not respond to distention and contraction of the urinary bladder (Jänig and Morrison, 1982). Spinal cord-brainstem pathways involved in the micturition reflex In order to function properly the PMC must be informed about the amount of bladder filling, since micturition can take place only when the bladder contains a certain amount of urine. The current view is that the micturition reflex is a spinobulbospinal reflex. De Groat (1975), on the basis of physiological recording studies in the dorsolateral pons of the rat and cat, suggested that the lumbosacral neurons receiving bladder afferents relay bladder filling information directly to the PMC, which project to the preganglionic bladder motoneurons. This would imply a micturition circuit in which lumbosacral interneurons convey information concerning bladder filling directly to the pontine micturition centers. In a later paper the same author found evidence that the PAG receives bladder information before the PMC (Noto et al., 1989). Aim of the thesis The investigations presented in this thesis are focussed on two main issues. First, which neurons are involved in the micturition reflex, and, second, which structures, although they are not part of the micturition reflex pathway itself, still influence the re- 15
16 General Introduction flex. Clarification of these issues is very important for the understanding of the control mechanisms in normal individuals, and, moreover, in incontinent patients. It is highly probable that dysfunction of certain brain areas cause urinary incontinence in many elderly (Andrew and Nathan, 1964; Blaivas, 1982). Urge incontinence occurs when patients sense the urge to void, but are unable to delay it long enough to reach the toilet. In healthy individuals this urge is not immediately followed by micturition and usually disappears when micturition is not appropriate at that particular time and place. Urge incontinence is also frequently found in patients with stroke (Khan et al., 1981) or with neurodegenerative diseases, as multiple sclerosis (Blaivas et al., 1979). Urge incontinence should not be confused with genuine stress incontinence, which is not the result of lesions in the central nervous system and will not be discussed in this thesis. Anterograde and retrograde tracers were used to identify pathways and neurons involved in micturition. Most of the tracer experiments were done in cats. The tracers were visualized using histo- and immunocytochemical techniques, and the results were analyzed with light- and electron microscopy. Electrical stimulation was used in order to study the effects on the function of the bladder and EUS. In order to localize micturition related neurons in humans positron emission tomography (PET) was used. PET is a non-invasive technique to study changes in regional cerebral blood flow (rcbf) in humans performing specific tasks (Fox and Mintun, 1989). The first part of the study is focussed on the neuronal components of the micturition reflex itself. The micturition reflex consists of sensory (ascending) and motor (descending) components. Afferents from the bladder to sensory neurons in lamina I and V of the lumbosacral cord were thoroughly described in the cat (Morgan et al., 1981). Since it was anatomically unclear whether these lumbosacral cells projected directly to the PMC, ultrastructural experiments were done which resulted in the data presented in the first chapter. It was demonstrated that the PMC of the cat received only a very small projection from the lumbosacral cord, and that none of these afferents contacted retrogradely labeled cells in the PMC. This means that the bladder information reaches the PMC indirectly. The main target of lumbosacral projections to the caudal brainstem of the cat appeared to be the ventrolateral PAG. An important feature of micturition is the synergic action between the bladder muscle and the EUS. Stimulation of the PMC results in the same effect (Holstege et al., 1986). The study presented in chapter two demonstrates that from the PMC terminals in the IML more than 75% are directly in contact with parasympathetic preganglionic bladder motoneurons, and all are excitatory in nature. Chapter three investigated ultrastructurally the nature of the PMC fiber terminations in the dorsal gray commissure (DGC). From the PMC terminals in the DGC 55% made contact with inhibitory GABA-ergic interneurons. This investigation provided evidence that the GABA-ergic interneurons in the DGC play a role in the relaxation of the EUS during micturition, which is substantiated by the observation that electrical stimulation of the sacral DGC results in a relaxation of the EUS. The sacral DGC of the domestic pig contains the motoneurons of the external anal sphincter. Chapter 6 demonstrates that the PMC and L-regions are not interconnected, which makes it probable that the PMC controls micturition only via its sacral projections. Stimulation of the PAG elicits micturition, which indicates a possible role in the motor control of micturition. Since the majority of the afferents from the lumbosacral cord terminate in the PAG, and not in the PMC, the existence of PAG projections to the PMC 16
17 General Introduction was investigated in chapter 7. The presence of this projection completes the concept of the normal micturition reflex. The chapters 8, 9 and 10 present the findings of the PET scanning experiments in humans. Since nothing was known about the central control of micturition in humans the study was done in male and female volunteers. The results demonstrate that the brainstem structures, which control micturition and continence, seem to be the same in cats and humans. Additionally, emotional related cortical areas play a role in the onset of micturition, but are not part of the micturition reflex itself. Chapter 9 reports the results of a study on the voluntary motor control of the pelvic floor and abdominal musculature. The general discussion puts the results of all chapters into perspective, presenting a scheme for the sensory and motor pathways in the spinal cord and brainstem involved in micturition and continence. 17
18
19 Sacral projections to the PMC and PAG Chapter 1 Ultrastructural Evidence for a Paucity of Projections from the Lumbosacral Cord to the Pontine Micturition Center or M-Region in the Cat: A New Concept for the Organization of the Micturition Reflex with the Periaqueductal Gray as Central Relay Bertil F.M. Blok, Henk de Weerd, and Gert Holstege J. Comp. Neurol. 359: (1995) ABSTRACT Information concerning the rate of bladder filling is determined by receptors in the bladder wall, and conveyed via afferent fibers in the pelvic nerve to sensory neurons in the lumbosacral cord. It was assumed that this information is relayed from the lumbosacral cord to a medial cell group in the dorsolateral pontine tegmentum, called M-region, pontine micturition center, or Barrington s nucleus. The M-region, in turn, projects via long descending pathways to the sacral parasympathetic motoneurons. In the present electron microscopic study it was investigated in cats whether monosynaptic projections from lumbosacral neurons to the M-region indeed exist. Wheat germ-agglutinin horseradish peroxidase injections were made in the lumbosacral cord. Many retrogradely labeled dendrites and somata were found in the M-region, but no labeled terminals were found on retrogradely labeled dendrites or somata. Only a small number of anterogradely labeled terminals, filled with mainly round vesicles, contacted unlabeled dendrites in the M-region. In contrast, many more anterogradely labeled terminals, filled with mainly round and to a limited extent dense core vesicles, and with asymmetrical synapses were found on dendrites in the lateral part of the periaqueductal gray (PAG). Previously (Neurosci. Lett., vol. 166, pp , 1994), it was demonstrated that the lateral part of the PAG contains neurons projecting to the M-region. A concept for the central organization of the micturition reflex is presented in which ascending projections from the lumbosacral cord convey information on bladder filling to the PAG. When the bladder contains so much urine that voiding is necessary, the PAG, in turn, triggers the M-region. The M-region, however, also receives afferents from the preoptic area, which might be involved in the final decision to start micturition. INTRODUCTION The bladder and its sphincter have two main functions: storage of urine and voiding or micturition. In the storage phase the external urethral sphincter is tonically contracted, while the detrusor, the muscle of the bladder, is relaxed. In the micturition phase the opposite takes place. The detrusor contracts, and the external urethral sphincter relaxes (Tanagho and Miller, 1970). The synergy between detrusor and external urethral sphincter is under brainstem control (in human, Blaivas, 1982; in cat, Holstege et al., 1986). The brainstem neurons are located in a medial and a lateral cell group in the dorsolateral pons (Holstege et al., 1979). The medial cell group specifically projects via long descending pathways to the sacral intermediomedial cell group and to the intermediolateral cell column containing autonomic motoneurons of the detrusor muscle of the bladder. The medial cell group in the dorsolateral pons called M-region (Holstege et al., 1986), Barrington s (1925) nucleus, or pontine micturition center (Loewy et al., 19
20 Chapter ). The lateral cell group is called L-region (Holstege et al., 1986), and sends fibers throughout the length of the spinal cord to the nucleus of Onuf in the S1 and S2 spinal segments. Onuf s nucleus contains motoneurons innervating the pelvic floor, including the external urethral sphincter (Sato et al., 1978). Electrical stimulation of the M-region in the cat mimics normal micturition, which encompasses relaxation of the external urethral sphincter, followed by contraction of the bladder (Holstege et al., 1986). Bilateral lesions of the M-region result in long-term retention of urine (Griffiths et al., 1990). Electrical stimulation in the L-region elicits a strong contraction of the external urethral sphincter (Holstege et al., 1986), while bilateral lesions of the L-region produce urine incontinence (Griffiths et al., 1990). The M-region may be regarded as the micturition control center and the L-region as the control center for the storage of urine. In order to function properly the brainstem micturition areas have to be informed about the rate of bladder filling, since micturition can take place only when the bladder contains a certain amount of urine. The question arises how the information concerning bladder filling reaches the micturition centers in the brainstem. Afferents in the pelvic nerve, including those from the bladder, have been shown to terminate in Rexed s (1954) laminae I, V and VII of the lumbosacral cord (Morgan et al., 1981), and Bahns et al. (1986) have demonstrated that thin myelinated afferent fibers in the pelvic nerve convey information concerning bladder pressure. Subsequently, lumbosacral neurons receiving bladder afferents relay this information to the brainstem. Hida and Shimizu (1982), on the basis of retrograde tracer injections in the M-region of the rat, and De Groat (1975), on the basis of physiological recording studies in the dorsolateral pons of the rat and cat, suggest that these lumbosacral neurons project directly to the M-region. This would imply a micturition circuit in which lumbosacral interneurons convey information concerning bladder filling directly to the pontine micturition centers. However, apart from the study of Hida and Shimizu, spinal projections to the M-region have not been described despite many anterograde and retrograde tracing studies in rat, cat and monkey on spinal projections to the brainstem (for review see Willis and Coggeshall, 1991). Main targets are the dorsal column nuclei, lateral pontine reticular formation and lateral parabrachial nuclei as well as the periaqueductal gray (PAG), but the M-region is not among these structures. The present study, using retrograde and anterograde tracing methods, is an attempt to solve the question whether, or not, neurons in the lumbosacral cord project to the M- region. Lumbosacral tracer injections not only result in anterogradely labeled fibers but also in retrogradely labeled neurons in the brainstem, including the M-region. These retrogradely labeled neurons obscure termination patterns of the anterogradely labeled fibers in this area at the light microscopic level. Only electron microscopic studies can overcome this problem. The electron microscopic results demonstrate a paucity of lumbosacral spinal fibers and terminals in the M-region and an abundance of such fibers and terminals in the PAG. The importance of latter projection in the micturition reflex is emphasized since the PAG is the only brainstem structure to be known to project to the M-region. MATERIALS AND METHODS Five adult cats were used for this study. The surgery procedures, pre- and postoperative care, handling and housing of the animals are in accordance with protocols approved by the Committee on Animal Experiments of the Faculty of Medicine of the University of Groningen. The animals were se- 20
21 Sacral projections to the PMC and PAG dated with ketamine (0.1 ml/kg) and sedamun (0.1 ml/kg) intramuscular and anaesthetized with a mixture of halothane, nitrous oxide and oxygen. During surgery heart rate and body temperature were monitored. The tracer injections were placed as described below. After a survival time of 3 days, the animals were deeply anaesthetized with 6% sodium pentobarbital, perfused intracardially with 1.5 l phosphate buffered saline (PBS) (ph 7.4) at room temperature, followed by 1.5 l fixative containing 1% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer (ph 7.4, room temperature). The brains and spinal cords were removed and placed in the same fixative for 2-3 hours at 4 o C. Subsequently, the material was processed according to light or electron microscopic procedures. Light microscopic study In order to localize the neurons in the lumbosacral cord projecting to the M-region in case 2178, 50 nl 5% wheat germ-agglutinin horseradish peroxidase (WGA-HRP) was injected in the dorsolateral pons. In a control case (2179) a 200 nl WGA-HRP-injection was made in the caudal PAG. After fixation and postfixation, the brainstems and lumbosacral segments were dehydrated overnight in 25% sucrose in 0.1 M phosphate buffer (ph 7.4). The next day the tissue was frozen to -55 C in a isopentane bath and cut in 40 µm sections on a cryostat. One out of four sections of the dorsolateral pons were incubated according to the diaminobenzidine (DAB) method to determine the extent of the injection site. Every fifth section of the third lumbar (L3) to first coccygeal (Co1) segments were incubated with tetramethylbenzidine (TMB) and nitroprusside for detection of tracer (Mesulam, 1982). The reaction product was visualized and photographed under combined darkfield and polarized light microscopy using a Zeiss Axioplan microscope. The sections, including the retrogradely labeled neurons, were drawn using a camera lucida. To identify the lumbosacral projections to the dorsolateral pons and the PAG, in three cases multiple injections of 2.5-5% WGA- HRP were made in the lumbosacral cord unilaterally cases 2261 and 2265, and bilaterally in case The injected segments, the amount and concentration of injected tracer are listed in table 1. Every second 50 µm vibratome section of the rostral pons and caudal midbrain was prepared for light microscopy and the alternate sections for electron microscopy. Serial sections of the lumbosacral cord were processed with the DAB method to disclose the injection sites. Electron microscopic study The vibratome sections were incubated with TMB and ammoniumheptamolybdate overnight (Olucha et al., 1985). The next day these sections were processed using a slow osmication method for postfixation (Henry et al., 1985). Two areas were selected for ultrastructural study. The first area was part of the M-region containing many retrogradely labeled neurons and the second area was part of the central division of the lateral PAG which contained intense anterograde labeling were selected. The selected tissue was stained en bloc in 1% uranylacetate in bidest, and the slabs were dehydrated in graded series of alcohol and embedded in Epon between dimethyldi- Table 1 Cases with WGA-HRP injections into the lumbosacral or sacral cord case injected segments µl WGA-HRP percentage 2236 L7-rostral S caudal L7-S caudal S1-S
22 Chapter 1 chlorosilane-coated glass-slides (Vinores et al., 1984). Semithin sections of 1 µm were made to determine areas of interest. Ultrathin sections from these areas were cut and studied with an electron microscope. To determine the density of labeled terminals per area in the M-region and the PAG, the labeled and unlabeled terminals were counted at a magnification 7000 times in 4 selected mazes of both areas (Table 2). Each maze measured µm 2. To determine whether this part of the reflex loop is excitatory or inhibitory in nature, the symmetry or asymmetry of the synaptic membrane specialization was established. RESULTS Light microscopy Retrograde tracing In case 2178 the injection site was located in the M-region and ventromedially adjacent pontine tegmentum (Fig. 1). Anterogradely labeled fibers were observed to descend through the dorsolateral funiculus of the spinal cord and to terminate in the sacral intermediomedial and intermediolateral cell group, and some to more ventral parts of the ventral horn. Retrogradely labeled neurons were found also. In the L3- Co1 segments they were located mainly contralaterally in laminae V, VII and VIII, but not in lamina I and X. In control case 2179 the injection occupied the lateral part of the caudal half of the PAG, and was larger than in 2178 (Fig. 1). In case 2179 no anterogradely labeled fibers were observed in the sacral intermediomedial and intermediolateral cell group, but many retrogradely labeled neurons were present. They were located in laminae I, V, VII, VIII and X with a strong contralateral predominance. The main conclusion from retrograde labeling in the two cases is that both the M-region and/ or adjacent areas as well as the caudal ventrolateral PAG receive lumbosacral afferent projections originating in laminae V, VII and VIII. Only the PAG receives additional projections from neurons in lamina I and X. Anterograde tracing The spinal cord injections involved both the ventral and dorsal portions of the gray matter of L7 and rostral S1 bilaterally (case 2236), caudal L7 and S1 unilaterally (case 2261) and caudal S1 and S2 unilaterally (case 2265). In all 3 cases a specific group of retrogradely labeled neurons was found bilaterally in the dorsolateral pons just medial and ventromedial to the mesencephalic trigeminal tract (Fig. 2, left). This group formed a rostrocaudally oriented column, extending from the level of the inferior colliculus, rostrally, to the level just rostral to the motor trigeminal nucleus, caudally. It corresponds with the M-region as described previously (Holstege et al., 1986). Many labeled structures were observed around the retrogradely labeled neurons in the dorsolateral pons. At the light microscopic level using the Mesulam TMB-impregnation technique it was not possible to distinguish between anterogradely labeled axons and terminals and retrogradely labeled axons and dendrites in the M-region. Using the Olucha TMBimpregnation technique on sections to be processed for EM at the light microscopic level only retrogradely labeled cells were observed in the M-region (Fig. 2, right). However, in the caudal PAG in the Mesulam TMB- as well as in the Olucha TMB-impregnation technique a strong anterograde projection was present. In cases 2236 (the L7-rostral S1 injection) the labeling was found in the dorsal and lateral parts of the PAG bilaterally, in case 2261 (caudal L7- S1 injection) in the dorsal and lateral parts of the PAG bilaterally with a contralateral predominance (Fig. 3). In case 2265 (with the caudal S1-S2 injection) only the dorsal and more central portion of the lateral part of the caudal PAG contained anterogradely labeled fibers bilaterally with a contralateral predominance. In this case the most lat- 22
23 SC PAG BIC 2179 III IC PAG 2179 ML IV IC IN PC MLF PON ML BC PC CSN BP PC Fig. 1. Schematic drawing of the WGA-HRP injection sites of the retrograde tracing study. BP BC 2178 RST NLL NTB P MesV MLF Sacral projections to the PMC and PAG
24 Chapter 1 eral parts of the caudal PAG did not receive anterograde labeling. Although very few retrogradely labeled neurons can be found in the lateral PAG after WGA-HRP injections in the sacral cord (Mouton and Holstege, 1994), they were not present in the sections processed for EM (Fig. 4). Electron microscopy M-region Thirty-five ultrathin sections from different rostrocaudal levels of the M-region were examined for the presence of labeled structures. In each ultrathin section 2-3 retrogradely labeled perikarya were found. A total of 47 retrogradely labeled perikarya in the M-region were studied. The perikarya had a spherical to fusiform shape with a round or oval nucleus and abundant cytoplasm. The reaction product was homogeneously distributed throughout the cytoplasm and the proximal dendrites (Fig. 5). When a retrogradely labeled perikaryon and its dendrite (observed maximum length of such a dendrite was 72 µm) were visible in a single section, the reaction product was Fig. 2. Left: A combined darkfield and polarized light photomicrograph of the dorsolateral pons after a WGA-HRP injection in the S1 and S2 segments (case 2265). Note the retrogradely labeled cells medial from the brachium conjunctivum. This cell group corresponds with the M-region. The area indicated by thick white lines corresponds with the figure on the right. Scale is 500 µm. Right: Brightfield photomicrograph of a portion of the M-region as indicated on the left. The vibratome section was osmicated for electron microscopy. Arrows point to some of the retrogradely labeled neurons. Scale is 40 µm. Fig. 3. Combined darkfield and polarized light photomicrographs of the caudal (on the left) and intermediate (on the right) levels of the PAG after a WGA-HRP injection in the L7 and S1 segments (case 2261). The area indicated by thick white lines corresponds with figure 4. Scale is 500 µm. 24
25 Sacral projections to the PMC and PAG heterogeneously distributed. In these cases the reaction product in the perikaryon and the proximal dendrite was denser than in the distal parts of the dendrite, although retrograde label was also found in the distal dendrites. Many axonal terminal profiles with round, pleomorphic and, to a limited extent, flat vesicles were found contacting the retrogradely labeled somata and dendrites, but none of them contained WGA-HRP. In 43.6% (954/2188) of all labeled and unlabeled terminals in the selected area of the M-region a synapse was found (Table 2), most of which (866 = 90.1%) were asymmetric. Throughout the M-region only a few anterogradely labeled terminals were observed, which terminated exclusively on unlabeled dendrites. These labeled terminals (80%) contained a mixture of round and some dense core vesicles. The synapses found in the labeled terminals were all asymmetric (Table 2). Some labeled axons and terminal profiles were present in the area directly dorsal to the M-region, which area did not contain retrogradely labeled dendrites or somata. Caudal lateral PAG Thirty-five ultrathin sections of different rostrocaudal levels of the caudal lateral PAG were examined for the presence of labeled profiles. In all 3 cases these sections con- Table 2. Density of labeled and unlabeled terminals in selected areas of the M-region and the PAG M-region labeled + or - a synapse unlabeled + or - a synapse total - symm. asymm.? - symm. asymm. not clear terminals % total lateral PAG labeled + or - a synapse unlabeled + or - a synapse total - symm asymm.? - symm.asymm. not clear terminals % total
26 Chapter 1 Fig. 4. Brightfield photomicrograph of osmificated section of the PAG in case 2261 as indicated in figure 3, right. Arrows point to labeled punctata. Note the absence of retrogradely labeled neurons. Scale is 40 µm. tained numerous labeled profiles, the majority of which were terminals (61-67%), or myelinated axons (4-11%). The remaining labeled profiles (27%) were not recognizable, for example because the reaction product completely covered the profile. Approximately 43% (1431/3313) of all labeled and unlabeled terminals in the selected area of the PAG contained a synaps (Fig. 6; Table 2). The majority of these synapses (1322 = 92.4%) was asymmetric. In 23% (32/141) of the labeled terminals in the selected region a synapse was visible, all of which were asymmetric. Forty-five percent of the labeled terminals exclusively contained round vesicles. Fifty-five percent contained a mixture of round vesicles and dense-core vesicles, although in these terminals the round vesicles usually outnumbered the dense core vesicles (Fig. 6). In a few terminals the dense-core vesicles were more numerous. The bulk of the labeled terminals had axo-dendritic synapses, while less than 26 5% were axosomatic. Axo-axonic contacts were not found. Density of labeling In order to quantify the difference between the density of lumbosacral cord labeling in the M-region and the caudal part of the lateral PAG, in all 3 cases from both regions an area was selected for ultrastructural inspection. Four mazes, each measuring µm 2, were taken from these two areas as indicated in figure 2, right, and figure 4, and were examined. The data (Table 2) indicate that the absolute number of labeled terminals is 6 to 12 times higher in the selected PAG region than in the selected portion of the M-region. Since the total number of terminals (labeled and unlabeled) per maze was higher in the PAG than in the M- region, the relative density of labeled terminals in both areas was determined. In the PAG this relative density appeared to be 4 to 5 times as high as in the M-region.
27 Sacral projections to the PMC and PAG The PAG not only contains a much higher density of sacral afferents, but the area which receives these sacral afferents is much larger than in the M-region. Thus, the results clearly indicate that the sacral cord projection to the PAG is much stronger than the very limited projection to the M-region. DISCUSSION The present study provides evidence for a paucity of direct lumbosacral projections to M-region neurons, which is in contrast to the current views on the organization of the supraspinal micturition reflex (Blaivas, 1982; McMahon, 1986; De Groat and Steers, 1990; Noto et al., 1991; however see De Groat et al., 1993). Moreover, a strong direct sacral projection to neurons in the caudal lateral PAG was observed, which led us to propose a new concept for the central organization of the micturition reflex. Central components of the micturition reflex, current view Several anatomical and physiological studies provided evidence that the bulbospinal or descending component of the micturition reflex originates in a distinct area in the dorsolateral pons, called the M-region (Holstege, 1986), pontine micturition center (Loewy et al., 1979), or Barrington s (1925) nucleus. Anterograde tracing experiments in rat (Loewy et al., 1979), and in cat (Holstege et al., 1979; Holstege et al., 1986) Fig. 5. Electron microscopic photograph of a retrogradely labeled perikaryon in the M-region in case Scale bar = 5 µm. 27
28 Chapter 1 Fig. 6. Electron microscopic photograph of an anterogradely labeled terminal making contact with a dendrite (asterisk) in the caudal lateral PAG in case An asymmetric synaptic cleft is indicated by arrow heads. Note the presence of many round and a few dense core vesicles in the terminal. Scale bar = 0.5 µm. demonstrated that the M-region projects, via long descending pathways, to the sacral intermediolateral cell column, which contains the parasympathetic preganglionic motoneurons of the bladder. Electrical and chemical stimulation in the M-region evoked bladder contractions (Holstege et al., 1986; Noto et al., 1989; Mallory et al., 1991), while bilateral lesioning of this area results in chronic retention of urine (Barrington, 1925; Holstege et al., 1986; Griffiths et al., 1990). On the other hand, the organization of the spinobulbar or ascending component of the micturition reflex is not well understood. Many anatomical studies exist on the spinal projections to the brainstem in rat, cat and monkey, which point to the the lateral reticular nucleus (Mehler, 1969; Künzle, 1973), inferior olive (Mizuno, 1966; Mehler, 1969), dorsal column nuclei (Mehler, 1969), lateral pontine reticular formation (Mehler, 1969), lateral parabrachial nuclei (Mehler, 1969; Cechetto et al, 1985), and PAG (Mehler, 1969; Björkeland and Boivie, 1984; Wiberg and Blomquist, 1984; Yezierski, 1988) as the main targets receiving spinal afferents. The M-region was not among these targets. 28
29 Sacral projections to the PMC and PAG Only a few studies were focussed on the ascending component of the micturition reflex. In a light microscopic study Hida and Shimuzu (1982) found retrogradely labeled neurons in the intermediate zone of the sacral cord after an injection of HRP in the M- region of the rat, which led them to conclude that there exists a strong projection from the sacral cord to the M-region. Stimulation of afferents in the pelvic nerve evoked negative field potentials in the rostral pontine areas at latencies of msec (De Groat, 1975). On the basis of these results it was suggested that information concerning bladder filling is conveyed via the sensory neurons in the dorsolateral part of the lumbosacral cord directly to the M-region (De Groat and Steers, 1990). In the present study an injection in the M- region and adjacent pontine tegmentum (case 2178) resulted in retrogradely labeled neurons in lamina V, VII and VIII of the L3- Co1 segments of the spinal cord. This is in agreement with the findings of Hida and Shimuzu (1982), who described retrogradely labeled neurons in the intermediate zone of the lumbosacral cord in the rat after an injection of HRP in the M-region. In the present study using WGA-HRP injections in the lumbosacral cord at the light microscopic level it was difficult to discriminate in the M-region between anterogradely labeled axons and terminals and retrogradely labeled cell bodies, axons and dendrites. However, at the electron microscopic level in the M-region no anterogradely labeled terminals on retrogradely labeled dendrites and cell bodies were observed. The few labeled terminals found in this area made contact with unlabeled somata and dendrites only. In some ultrathin sections it was clear that the distribution of WGA-HRP in the perikaryon and its proximal dendrites was denser than in its distal dendrites. Therefore, it can not be excluded that the few labeled terminals in the M-region contacted unlabeled distal dendrites of spinally-projecting neurons in which only the perikaryon and proximal dendrites contained label. However, the scarcity of anterogradely labeled terminals, and the presence of retrogradely labeled distal dendrites which did not receive contact from anterogradely labeled terminals, strongly suggest that eventual sacral projections to labeled distal dendrites play a minor role in the transmission of bladder filling information. This suggestion is further corroborated by evidence from the literature on latency potentials to be presented in the next paragraph. Another possibility is that some of the anterogradely labeled terminals originate from lumbosacral dorsal horn neurons and terminate on neurons of the locus coeruleus and the subcoerulear area (Craig, 1992). In the framework of micturition it is not very likely that locus coeruleus or subcoeruleus plays a major role in the micturition reflex, because destruction of the descending noradrenergic pathways in the rat neither alters micturition nor diminishes the retrograde labeling of cells in the M-region after injection of tracer in the lumbosacral cord (Satoh et al., 1978; Loewy et al. 1979). Obviously, the M-region must be informed about the grade of bladder filling, but the results of this study strongly suggest that it does not receive this information directly from the lumbosacral cord. This implies an indirect pathway to the M-region. Since the micturition reflex is not abolished by precollicular or intercollicular decerebration (Barrington, 1921), this pathway does not involve telencephalic or diencephalic structures. Central components of the micturition reflex, revised view After WGA-HRP injections in the lateral PAG retrogradely labeled neurons were located in the Rexed s (1954) laminae I, V, VII, VIII and X of the lumbosacral cord with a strong contralateral predominance. Injections in the lumbosacral cord resulted in 29
30 Chapter 1 strong anterograde labeling in the caudal lateral PAG and less dense labeling in the dorsal PAG. Ultrastructural analysis of this projection showed that the anterogradely labeled terminals in the PAG were filled with round vesicles and contained an asymmetric synapse, suggesting an excitatory function. The projections from the spinal cord to the PAG are mostly viewed in the light of nociceptive control (Mehler, 1969; Willis and Coggeshall, 1991), but in our concept these projections, especially those from the caudal lumbar and the sacral cord, also contain the ascending loop of the micturition reflex. There are several arguments for this hypothesis. First, the lateral and the dorsal PAG portions receiving afferents from the sacral cord, project specifically to the M-region (Blok and Holstege, 1994). Second, electrical stimulation of the caudal PAG can evoke complete micturition (Skultety, 1959), facilitates bladder reflexes, and reduces bladder capacity (Kruse et al., 1990). Third, stimulation of afferent pathways in bladder nerves in the rat evokes short latency potentials (13 ± 3 ms) in the most caudal dorsal PAG, and much longer latency potentials (42 ± 7 ms) in the M-region (Noto et al., 1991), suggesting that the PAG receives the spinal afferents directly and the M-region indirectly. Functional implications In our concept (see also De Groat et al., 1993) the micturition reflex is organized as follows: information concerning the grade of bladder filling enters the lumbosacral cord via afferents in the pelvic nerves. The afferents terminate on sensory neurons in the dorsolateral part of the caudal lumbar and sacral spinal cord. These sensory neurons, in turn, project to the lateral and dorsal parts of the PAG. If the bladder is sufficiently filled, the PAG turns on the switch to start micturition via its projection to the M-region. Finally, the M-region starts micturition by inhibiting the urethral sphincter and activating the bladder via descending pathways to the sacral parasympathetic motoneurons (Fig. 7). It should be emphasized that the PAG is involved in specific functions such as vocalization (Holstege, 1989; Davis and Zhang, 1991), cardiovascular control (Lovick, 1985; Bandler et al., 1991) and lordosis (Sakuma and Pfaff, 1979; VanderHorst and Holstege, 1995). Since Cd PU Cl CL Preoptic area M-region BP (+) Parasympathetic motoneurons Onuf's nucleus LL IC BP PON (+) (+) Striated urethral sphincter BC ML P F CA OC CSN PON IV Bladder BNST Fig. 7. Schematic representation of the spinal and supraspinal structures involved in the control of micturition (after Blok and Holstege, 1994). SC PC SON ML Periaqueductal gray BC IC S2 (+) (+) L-region 30
31 Sacral projections to the PMC and PAG micturition can also be considered as a specific emotional behavior (Holstege, 1992), it seems obvious to have an integration of micturition control together with the control of other vital functions. This first line integration probably takes place in the PAG. Precollicular decerebration does not disturb the micturition reflex. In such a preparation the bladder can be filled until a certain threshold, after which the micturition reflex automatically starts (Barrington, 1921). In unanaesthetized intact animals, including man, with a filled bladder, micturition does not start automatically. It is not known which structures determine the moment of beginning of micturition. Emotional motor system and the control of micturition Micturition control can be viewed in the framework of the emotional motor system, as defined by Holstege (1992). The emotional motor system contains descending pathways from limbic structures to the caudal brainstem and spinal cord and can be subdivided into medial and lateral components. The medial component has a global effect on the level of activity of all somatosensory neurons and motoneurons, including those of the lower urinary tract. The lateral component of the emotional motor system regulates specific functions such as vocalization, cardiovascular regulation and lordosis. Although stimulation in the cat in structures belonging to the lateral component of the emotional motor system (Gjone, 1966) can elicit micturition, most of these structures are not known to project directly to the M-region. However, such a direct projection has been described for the dorsolateral part of the preoptic area only (Holstege, 1987). It should be emphasized that in territorial and sexual behavior in many animals micturition is of great importance, and that the preoptic area is known to play a role in these behaviors (Grossman and Wang, 1955; Malsbury, 1971; Powers and Valenstein, 1972; Scouten et al., 1980). Structures belonging to the lateral component of the emotional motor system, such as the central nucleus of the amygdala (Hopkins and Holstege, 1978), bed nucleus of the stria terminalis (Holstege et al., 1985), lateral hypothalamus, as well as the preoptic area (Holstege, 1987), project heavily to the lateral part of the caudal PAG. Possibly, these structures influence, via their projections to the PAG, the M-region, and thus control micturition. In Figure 7 a final scheme is presented showing the spinal and supraspinal structures involved in the control of micturition. 31
32
33 PMC terminates directly on bladder motoneurons Chapter 2 Ultrastructural Evidence for a Direct Pathway from the Pontine Micturition Center to the Parasympathetic Preganglionic Motoneurons of the Bladder of the Cat Bertil F.M. Blok, and Gert Holstege Neurosci. Lett. 222: (1997) ABSTRACT Light microscopy tracing studies have provided evidence that the pontine micturition center (PMC) projects to the area of the intermediolateral cell column of the sacral spinal cord. Although this region contains parasympathetic preganglionic motoneurons of the bladder and colon, it also contains many local interneurons and neurons projecting to supraspinal levels. The present study demonstrates that neurons in the PMC indeed project to preganglionic bladder motoneurons. Wheat germ agglutinin horseradish peroxidase was injected in the PMC and cholera toxin B subunit was injected in the bladder wall. Many anterogradely labeled fibers from the PMC were found to terminate on somata and dendrites of the retrogradely labeled preganglionic bladder motoneurons. The terminals were filled with many round vesicles and possessed an asymmetric synaptic cleft, suggesting an excitatory function. INTRODUCTION Tracing studies in the cat (Holstege et al., 1979) and the rat (Loewy et al., 1979) revealed that a distinct cell group in the dorsal pontine tegmentum, the pontine micturition center (PMC), projects directly to the sacral cord intermediolateral cell column (IML). Since this area contains parasympathetic preganglionic motoneurons of the bladder (Petras and Cummings, 1978) it was suggested that this pathway might represent a direct PMC projection to preganglionic bladder motoneurons. This assumption was further corroborated by the finding that stimulation in the PMC results in bladder contractions (Holstege et al., 1986; Mallory et al., 1990). However, the situation was more complicated when it became apparent that the sacral IML not only contains preganglionic bladder motoneurons, but also other neurons. Examples are the parasympathetic motoneurons innervating the distal colon (De Groat et al., 1981) and possibly other pelvic organs (Marson et al, 1993; Vizard et al., 1995), but also dendrites of the nucleus of Onuf (Beattie et al, 1990), neurons supraspinally projecting to the periaqueductal gray (Blok et al., 1995; VanderHorst et al., 1996), and locally projecting interneurons (McMahon and Morrison, 1982). This means that the projections of the PMC to the sacral IML do not represent direct connections to the preganglionic bladder motoneurons, but to other parasympathetic motoneurons, local interneurons or to neurons giving rise to ascending projections. In order to solve this problem, in 4 adult male cats a double labeling electron microscopic study was performed in which the bladder was injected with the retrograde tracer cholera toxin B subunit (CTB) and the PMC with the an- 33
34 Chapter 2 terograde and retrograde tracer wheat germ agglutinin horseradish peroxidase (WGA- HRP).WGA-HRP was usedto anterogradely label PMC terminals on bladder motoneurons, which were retrogradely labeled with CTB. MATERIALS AND METHODS The surgery procedures, pre- and postoperative care, handling and housing of the cats are in accordance with protocols approved by the Committee on Animal Experiments of the Faculty of Medicine of the University of Groningen. During surgery, heart rate and body temperature were monitored. The animals (weight kg) were sedated with ketamine (30 mg/kg) intramuscular, followed by additional doses of 15 mg/kg as necessary. In order to localize the bladder motoneurons 30 µl of 1% CTB was injected bilaterally in the bladder wall using a Hamilton syringe. Five days later WGA-HRP was injected into the left PMC in order to label its fibers to the preganglionic bladder motoneurons. In order to properly place the needle, prior to the WGA-HRP injection, the PMC was identified by means of electrical stimulation. For this reason the intravesical and urethral pressures were measured with two transducers mounted on a single 6 French catheter (Raumedic, Germany). The distance between the two transducers was 3 cm. The transducer at the tip of the catheter was placed intravesically. Electrical stimulation was performed with a 5 sec train of negative going pulses, 5 ms duration, repetition frequency of 80 Hz, and 100 µa amplitude. The stimulation started about 5 mm dorsal to the dorsolateral pontine tegmentum and the electrode advanced in successive steps of 0.5 mm. A positive response consisted of a sharp decrease in the transurethral pressure followed by an IC Mes V MLF 2378 BC PMC LL 2366 MLF SC BP RST ML ML NLL P NTB P Fig. 1. Schematic drawings of wheat germ agglutinin horseradish peroxidase (WGA-HRP) injections in the dorsolateral pontine tegmentum in the four cats. The injection sites of 2366, 2370 and 2380 included the pontine micturition center (PMC; indicated by dark cells; see Blok and Holstege, 1994) since numerous anterogradely labeled fibers were found in the sacral IML. The injection site of 2378 did not include the PMC and no anterograde labeling was observed in the sacral cord. 34
35 PMC terminates directly on bladder motoneurons Fig. 2. Electron microscopic photograph of an anterogradely labeled terminal (WGA- HRP reaction product marked with arrows) making contact with the soma of a retrogradely labeled bladder motoneuron (cholera toxin B subunit (CTB) reaction product marked with arrowheads) in the second sacral segment of case Note the asymmetric synaptic cleft in the center of the photograph, and the presence of many round vesicles in the terminal. Bar represents 400 nm. increase in the intravesical pressure (Holstege et al., 1986). At the optimal stimulation site 40 nl 2.5% WGA-HRP was injected. Three days after the WGA-HRP injection, the animals were deeply anaesthetized with 60 mg/kg sodium pentobarbital, perfused intracardially with 1.5 l heparinized saline (ph 7.4) at room temperature, followed by 1.5 l fixative containing 1% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (ph 7.4, room temperature). The spinal cords were removed and placed in the same fixative for 2 hours at 4 o C. The brainstems were dehydrated overnight in 25% sucrose in 0.1 M phosphate buffer (ph 7.4), and the next day the tissue was frozen to -55 C in a isopentane bath and cut in 40 µm sections on a cryostat. One out of four sections were incubated according to the diaminobenzidine (DAB) method to determine the extent of the injection site. Vibratome sections of 60 µm thick were made of the second and third sacral segment, and every fourth section was prepared for electron microscopy. The vibratome sec- 35
36 Chapter 2 tions were incubated with tetramethyl benzidin (TMB) and ammoniumheptamolybdate overnight (Olucha et al., 1985), and the reaction product was stabilized with the DAB-cobalt method (Rye et al.., 1984). The sections were incubated in goat anti-ctb antibody (List Biological Laboratories) diluted 1: 10,000 in Tris Buffered Saline containing 0.05% Triton X-100 (TBS + ) overnight at 4 C, transferred to rabbit anti-goat IgG (DAKO) diluted 1:250 in TBS + and 1% normal rabbit serum during 1 hour at room temperature, and placed in TBS + solution containing goat-pap (1:250; DAKO) and 1% normal rabbit serum (Ericson and Blomquist, 1988). Subsequently, the sections were incubated with DAB, and were osmicated during 45 minutes at 4 C. The sections were stained en bloc in 1% uranylacetate in bidest, dehydrated in graded series of alcohol, and embedded in Epon between dimethyldichlorosilanecoated glass-slides (Vinores et al., 1984). Ultrathin sections were cut from selected areas in the dorsolateral part of the ventral horn containing the sacral IML and studied with an electron microscope. Ten mazes (total surface of 100,000 µm 2 ) from ultrathin sections of case 2366 were screened for a quantitative and qualitative investigation on the presence of anterogradely labeled WGA-HRP terminals on retrogradely labeled neurons with CTB reaction product. Cryostat sections of 40 µm were cut of the area of the dorsolateral pons and were incubated using DAB during 25 minutes. The sections were mounted on slides, dehydrated and coverslipped with DePeX mounting medium. The injection sites were studied with a Zeiss Axioplan microscope. RESULTS Three injection sites of the cases 2366, 2370 and 2380 were placed in the dorsolateral pontine tegmentum, including the pontine micturition center (Fig. 1 left). In one control case (2378) the injection was placed dorsolateral to the PMC (Fig. 1 right). In the first 3 cases, at the light microscopic level, a large number of anterogradely labeled fibers and presumptive terminal labeling was observed bilaterally in the sacral IML, which contained retrogradely labeled neurons with CTB to be recognized by their brown reaction product. The labeled dendrites of the CTB-labeled bladder motoneurons extended mediolaterally into laminae V and VII, and dorsally into the lateral part of lamina V and the ventral part of lamina I. Some retrogradely labeled WGA-HRP neurons with a black reaction product were present just dorsal to the bladder motoneurons in lamina V. Presumably, these neurons project to the most caudal part of the PAG, just dorsal to the PMC, because the PMC itself does receive only scarcely sacral cord afferents (Blok et al., 1995). In the control case 2378 no anterogradely labeled fibers were observed in the sacral IML neither light nor electron microscopically. However, many retrogradely WGA- HRP labeled neurons were visible in lamina I and lamina V, just dorsal to the retrogradely labeled CTB bladder motoneurons. At the ultrastructural level in cases 2366, 2370 and 2380 retrogradely labeled bladder motoneurons had a fusiform shape and a round nucleus. The CTB reaction product was mostly found in the Golgi apparatus. In all three cases the anterogradely labeled terminals with WGA-HRP crystals were filled with many round and occasionally dense core vesicles and were often in close apposition with dendrites or somata. When a synaptic cleft was present it was always asymmetric, and no labeled profiles with a symmetric cleft were found. In the area of 100,000 µm 2 used for ultrastructural quantification a total 1488 terminals were observed, of which 57 (3.8%) were anterogradely labeled with WGA-HRP. Twenty of the 57 analyzed labeled termi- 36
37 PMC terminates directly on bladder motoneurons nals had an asymmetric synaptic cleft, and 15 of these 20 labeled terminals were in contact with a retrogradely labeled soma (9/ 15) or dendrite (6/15). In the other 37 terminals no synaptic cleft was found. Twentyone of these 37 terminals were adjacent to a dendrite or soma filled with anti-ctb reaction product. The percentage of labeled synapses with an asymmetric cleft terminating on retrogradely labeled bladder motoneurons is probably underestimated, since the CTB, due to the presence of 1% glutaraldehyde in the fixative, was mainly visible in the somata and proximal dendrites, but not in more distal dendrites. DISCUSSION These results provide final proof for a direct connection between the PMC and the bladder parasympathetic preganglionic motoneurons. Naturally, these findings do not exclude the possibility that there also exist direct PMC projections to other neurons in the sacral cord, such as those projecting to Onuf s nucleus (Nadelhaft and Vera, 1996), or neurons relaying dorsal root information to the PAG (Blok et al., 1995). The finding that the anterogradely labeled terminals on retrogradely labeled dendrites or somata always contained round vesicles and an asymmetric synapse strongly suggest that the PMC has an excitatory effect on bladder motoneurons This corresponds with the finding that electrical (Holstege et al., 1986) and chemical stimulation with excitatory amino acids (Mallory et al., 1990) in the PMC produce contractions of the bladder. Apart from the PMC afferents the preganglionic bladder motoneurons also receive afferents from other sources, which might explain the many unlabeled terminals on retrogradely labeled preganglionic bladder motoneurons. These unlabeled terminals not only contained round, but also dense core or pleiomorphic vesicles. According to Mawe et al. (1986) some of these unlabeled terminals originate from the pelvic nerve. They contain mainly round vesicles, indicating an excitatory input from the pelvic nerve to sacral parasympathetic motoneurons. The remaining unlabeled terminals may originate from spinal or supraspinal structures, which have been described to project to the sacral IML. Examples are the sacral interneurons dorsolateral to the central canal (Nadelhaft and Vera, 1996), raphe nuclei and adjacent ventromedial medullary tegmental field (Holstege and Kuijpers, 1982), and the paraventricular nucleus of the hypothalamus (Holstege, 1987). The latter two supraspinal sources are probably not specifically involved in micturition. The present results represent a further step in our understanding of the micturition control system in the central nervous system. 37
38
39 PMC projects to GABA neurons in sacral DGC Chapter 3 The Pontine Micturition Center Projects to Sacral Cord GABA Immunoreactive Neurons in the Cat Bertil F.M. Blok, Henk de Weerd, and Gert Holstege Neurosci. Lett. 233: (1997) ABSTRACT Stimulation of the pontine micturition center (PMC) results in micturition, i.e. an immediate relaxation of the external urethral sphincter (EUS) and a contraction of the detrusor muscle of the bladder. Earlier studies have shown that the bladder contraction is brought about by a direct excitatory pathway from the PMC to the parasympathetic preganglionic bladder motoneurons in the sacral cord. How the PMC produces the inhibition of the EUS is not known. The present study in two adult male cats demonstrates at the ultrastructural level a direct pathway from the PMC to the dorsal gray commissure of the sacral cord. More than half (55%) of these terminals made contact with γ amino butyric acid (GABA) immunoreactive dendrites or somata, the others with non-gaba immunoreactive profiles. The PMC terminals contained many round vesicles, some dense cored vesicles and exclusively asymmetric synaptic clefts, which corresponds with an excitatory pathway. A concept is put forward in which this pathway produces the relaxation of the EUS during micturition. INTRODUCTION Micturition depends on a coordinated action of the smooth detrusor muscle of the bladder and the striated external urethral sphincter (EUS), which closes the bladder. During urine storage, the bladder is relaxed and the EUS is tonically contracted. When micturition takes place, this activation pattern is reversed, i.e. the bladder contracts and the EUS relaxes, resulting in elimination of urine. The bladder and the bladder sphincter are innervated by respectively the sacral cord parasympathetic motoneurons (De Groat et al., 1981) and the motoneurons in the ventrolateral part of the nucleus of Onuf (Kuzuhara et al., 1980). The coordination between these two motoneuronal cell groups during micturition does not take place in the spinal cord, but in the caudal brainstem (Blaivas, 1982; Holstege et al., 1986; Blok et al., 1997). Tracing studies in rat (Loewy et al., 1979) and cat (Holstege et al., 1979; Holstege et al., 1986; Blok and Holstege, 1997) have shown that a distinct cell group in the dorsal pontine tegmentum, the pontine micturition center (PMC), projects to the parasympathetic bladder motoneurons in the sacral intermediolateral cell group (IML), as well as to the dorsal gray commissure (DGC), but not to the nucleus of Onuf. Stimulation in the PMC results in an immediate and sharp decrease in the urethral pressure and pelvic floor electromyogram (EMG), followed in about 2 sec by a steep rise in the intravesical pressure, mimicking complete micturition (Holstege et al., 1986). The increased bladder pressure during this stimulation is probably caused by a direct projection from the PMC to bladder motoneurons, because the PMC terminals on bladder motoneuronal somata and dendrites contain round vesicles and have an asymmetric cleft, which corresponds with an excitatory func- 39
40 Chapter 3 IC MLF Mes V BC 2350 PMC ML 2366 P MLF ML SC RST BP NLL NTB P Fig. 1. Schematic drawings of WGA-HRP injections in the dorsolateral pontine tegmentum in the two cats. The PMC is indicated by the dark cells (on the basis of Blok and Holstege, 1994). tion (Blok and Holstege, 1997). However, this does not explain the decrease in the urethral pressure during micturition, because a direct PMC projection to the nucleus of Onuf does not exist (Holstege et al., 1979; Holstege et al., 1986). The PMC, in order to inhibit the bladder sphincter, might use inhibitory interneurons as relay to the sphincter motoneurons in Onuf s nucleus. In this respect the following observations are important: 1. Onuf s nucleus receives a relatively large number of γ amino butyric acid (GABA) immunoreactive terminals (Ramirez-Léon et al., 1994); 2. Almost all Onuf s nucleus afferents from the lumbosacral cord originate from neurons in the DGC Konishi et al., 1985; Nadelhaft and Vera, 1996), a few from the IML (Nadelhaft and Vera, 1996) but none from other areas in the lumbosacral cord (Holstege and Tan, 1987; Nadelhaft and Vera, 1996); 3. Many GABA immunoreactive neurons are located in the DGC and a few in the IML (Alvarez et al., 1996); and 4. The PMC projects directly to the DGC and IML bilaterally (Holstege et al., 1979; Loewy et al., 1979; Holstege et al., 1986). In our concept the PMC uses GABA-ergic relay cells in the DGC, and probably also the few GABA-ergic cells in the IML, to inhibit the bladder sphincter motoneurons in Onuf s nucleus. If that is true there must exist a direct PMC excitatory projection to GABA immunoreactive neurons in the DGC the demonstration of which is the object of this study. MATERIALS AND METHODS Two adult male cats were used. The PMC was injected with the anterograde tracer wheat germ agglutinin horseradish peroxidase (WGA-HRP) to anterogradely label PMC terminals in the IMM. The surgery procedures, pre- and postoperative care, and handling and housing of the cats were in accordance with protocols approved by the Committee on Animal Experiments of the Faculty of Medicine of the University of 40
41 PMC projects to GABA neurons in sacral DGC Groningen. During surgery, heart rate and body temperature were monitored. The animals (weight 4.0 and 4.4 kg) were sedated with ketamin (30 mg/kg) intramuscular, followed by additional doses of 15 mg/kg as necessary. In order to properly place the needle in the PMC, it was identified by means of electrical stimulation prior to the WGA-HRP injection (for details see reference 4). At the optimal stimulation site 40 nl 2.5% WGA- HRP was injected. Three days after the WGA-HRP injection, the animals were deeply anaesthetized with 60 mg/kg sodium pentobarbital, perfused intracardially with 1.5 l heparinized saline (ph 7.4) at room temperature, followed by 1.5 l fixative containing 1% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (ph 7.4, room temperature). The spinal cords were removed and placed in the same fixative for 2 hours at 4 o C. The brainstems were dehydrated overnight in 25% sucrose in 0.1 M phosphate buffer (ph 7.4), and the next day the tissue was frozen to -55 C in a isopentane bath and cut in 40 µm sections on a cryostat. One out of four sections were incubated according to the diaminobenzidine (DAB; Sigma, USA) method to determine the extent of the injection site. Vibratome sections of 60 µm thick were made of the second and third sacral segment, and every fourth section was prepared for electron microscopy using the protocol described earlier (Blok and Holstege, 1997). Ultrathin sections were collected on formvar-coated nickel grids and processed for the ultrastructural localization of GABA (De Zeeuw et al., 1988). The ultrathin sections were etched in 1% periodic acid and 1% sodium periodate and incubated for 2 hours at room temperature with 1:1000 rabbit GABA antiserum (Sigma, USA). After rinsing, the sections were incubated for 1 hour with 1:30 goat anti-rabbit IgG coupled to colloidal gold particles (15 nm; Aurion, The Netherlands). Method specificity was controlled by omitting the primary antibody. After contrasting with uranyl acetate for 2 minutes and lead citrate for 1 minute, the sections were studied with an electron microscope. RESULTS The injection sites of the cases 2350 and 2366 were placed in the left dorsolateral pontine tegmentum, including the PMC (Fig. 1). At the light microscopic level, a large number of anterogradely labeled fibers and presumptive terminal labeling was observed bilaterally in the sacral IML and DGC (Fig. 2). At the ultrastructural level numerous anterogradely labeled terminals with WGA-HRP crystals were found bilaterally in the sacral DGC. These terminals were filled with many round and occasionally dense core vesicles and were often in close apposition with dendrites or somata. When a synaptic cleft was present it was always asymmetric, and no labeled profiles with a symmetric cleft were found. In both cases WGA-HRP labeled terminals were found contacting dendrites or somata immunoreactive for GABA. The GABA immunoreactive somata contained many immunoreactive particles (Fig. 3 left), but their dendrites contained only a few (5-10; Fig. 3 right), which is normal for dendrites of IML DGC Fig. 2. Polarized light photomicrograph of a section of the second sacral segment of case Anterogradely labeled fibers are visible in the IML and the DGC. Bar represents 300 µm. 41
42 Chapter 3 Table 1 Number of WGA-HRP labeled terminals on GABA immunoreactive profiles WGA-HRP labeled terminals Case Case Total Percentage GABA immunoreactive dendrites GABA immunoreactive somata GABA negative profiles Total GABA immunoreactive neurons (De Biasi et al., 1997). Since the background labeling in our preparations was extremely low, it was not necessary to make a statistical analysis of the distribution of gold particles to judge a profile as labeled or not (see also De Biasi et al., 1997). A selected area of the DGC was screened on the presence of WGA-HRP positive terminals with a synaptic cleft, and their postsynaptic contacts were characterized (Table 1). In each maze (=10,000 µm 2 ), 2 to 3 WGA-HRP positive terminals were found to contact GABA immunoreactive profiles. A total of 109 labeled terminals were found, of which more than half (60 of 109 =55%) contacted GABA im- Fig. 3. Left: Electron microscopic photograph of a WGA-HRP labeled terminal (asterisk) making contact with a GABA immunoreactive dendrite (PSD = postsynaptic dendrite) in the second sacral segment of case Arrowheads indicate anti-gaba immunogold particles. Right: Electron microscopic photograph of an anterogradely labeled terminal (asterisk) making contact with a GABA immunoreactive soma in the second sacral segment of case S = GABA immunoreactive soma. Bar represents 400 nm. 42
43 PMC projects to GABA neurons in sacral DGC munoreactive profiles. Also many GABA immunoreactive terminals with flat and pleiomorphic vesicles were observed, but these never contained WGA-HRP crystals or contacted GABA immunoreactive profiles. DISCUSSION The results are in agreement with the concept that the PMC produces micturition via a direct excitatory connection with the bladder detrusor muscle motoneurons and with the GABA immunoreactive inhibitory interneurons in the DGC (Fig. 4). Although not yet demonstrated, it seems likely that the PMC fibers to the DGC GABA immunoreactive neurons are collaterals of PMC fibers to the IML (Nadelhaft and Vera, 1996). Theoretically, it is possible that the GABA immunoreactive DGC cells, which receive afferents from the PMC do not project to Onuf s nucleus. However, this seems extremely unlikely, because almost all premotor interneurons projecting to Onuf s nucleus are located in the DGC, and a few in the IML. Other sources of GABA immunoreactive innervation of the Onuf s nucleus do not exist, because it is not affected by spinal cord transection rostral to the sacral cord or dorsal rhizotomy (Ramirez-Léon et al., 1994). Thus, although GABA immunoreactive neurons are also located in the dorsal horn, the GABA immunoreactive premotor interneu- IC Pontine micturition center (+) (+) GABA-ergic neurons Bladder motoneurons ( ) S2 Bladder sphincter motoneurons Bladder (+) (+) Bladder Sphincter Fig. 4. Schematic overview of the pathways involved in the control of the bladder and bladder sphincter motoneurons during micturition. Pathways are indicated on one side only. 43
44 Chapter 3 rons projecting to Onuf s nucleus, must be located in the DGC and to a very limited extend in the IML. Both regions receive PMC projections. This leads to the concept that the PMC excites the bladder motoneurons via a direct pathway to their parasympathetic motoneurons, and inhibits the bladder sphincter motoneurons via an excitatory projection to the GABA-ergic premotor interneurons of Onuf s nucleus. 44
45 DGC stimulation results in urethral relaxation Chapter 4 Electrical Stimulation of the Sacral Dorsal Gray Commissure evokes Relaxation of the External Urethral Sphincter in the Cat Bertil F.M. Blok, Jos T.P.W. van Maarseveen, and Gert Holstege Neurosci. Lett., in press ABSTRACT Stimulation of the pontine micturition center (PMC) results in micturition, i.e. an immediate relaxation of the urethral sphincter and a contraction of the detrusor muscle of the bladder. The PMC generates the bladder contraction by way of a direct excitatory pathway to the parasympathetic bladder motoneurons in the sacral cord. The idea is that the PMC produces the relaxation of the urethral sphincter via direct projections to GABA-ergic neurons in the dorsal gray commissure (DGC), which, in turn, inhibit the urethral sphincter motoneurons. According to this hypothesis electrical stimulation in the dorsal gray commissure in three cats should result in a relaxation of the urethral sphincter. The results were in total agreement with this concept. During DGC stimulation a sharp decrease of the urethral pressure was found, the strength of which depended completely on the amplitude of the electrical stimulation. INTRODUCTION Micturition depends on a coordinated action of the smooth detrusor muscle of the bladder and its striated external urethral sphincter (EUS). During urine storage, the bladder is relaxed and the EUS is contracted. During micturition a reversed pattern is observed; the bladder contracts and the EUS is relaxed. The bladder and the EUS are innervated by respectively sacral preganglionic parasympathetic motoneurons (Morgan et al., 1979) and motoneurons in the ventrolateral part of the nucleus of Onuf (Sato. During micturition the activity of these two motoneuronal cell groups is coordinated by the pontine micturition center (PMC) in the brainstem (Holstege et al., 1986; Blok et al., 1997; Blok et al., 1998). Tracing studies in the rat (Loewy et al., 1979) and cat (Holstege et al., 1979; Holstege et al., 1986; Blok and Holstege, 1997; Blok et al., 1997) have shown that the PMC projects to the parasympathetic bladder motoneurons in the sacral intermediolateral cell group (IML), as well as to the dorsal gray commissure (DGC) or intermediomedial cell column (IMM). However, the PMC does not project to the nucleus of Onuf (Holstege et al., 1979; Holstege et al., 1986). Ultrastructural studies have shown that the PMC-fibers to the IML terminate directly on the preganglionic bladder motoneurons. This projection is excitatory, not only because PMC stimulation results in bladder contraction (Holstege et al., 1986), but also because the PMC terminals on bladder motoneurons contain asymmetric clefts and round vesicles (Blok and Holstege, 1997). Similar excitatory projections were found to the GABA-ergic interneurons in the dorsal gray commissure (DGC) (Blok et al., 1997). Many of the local neurons projecting to Onuf s nucleus are located in the DGC (Konishi et al., 1985; Nadelhaft and Vera, 1996) and Onuf s nucleus receives a substantial afferent input from GABA-neurons 45
46 Chapter 4 Stimulation tract A. p urethra (kpa) time (s) 0 10 time (s) 0 10 time (s) S1 B. p urethra (kpa) p bladder (kpa) time (s) Fig. 1. The intraurethral pressure effects after electrical stimulation (80 µa) in the medial part of the funiculus gracilis (A) and in the dorsal gray commissure (B) of the segment S1. in the lumbosacral cord (Ramirez-Léon et al., 1994), most of which are located in the DGC (Alvarez et al., 1996). Combining these anatomical findings with the observation that stimulation of the PMC not only produces a steep rise in the intravesical pressure, but also a decrease in the intraurethral pressure (Holstege et al., 1986), leads to the following concept of micturition control. The bladder contraction during micturition is the result of direct PMC projections to the parasympathetic bladder motoneurons, and the decrease of intraurethral pressure during micturition is the result of the PMC projections to the GABA-neurons in the DGC. If this concept is true, electrical stimulation of the DGC would result in a decrease of the urethral sphincter pressure as during micturition or stimulation of the PMC. Therefore, the DGC was electrically stimulated and the bladder and urethral sphincter pressures were measured. MATERIALS AND METHODS The surgery procedures, pre- and postoperative care, and handling and housing of the cats were in accordance with protocols approved by the Committee on Animal Experiments of the Faculty of Medicine of the University of Groningen. During surgery, heart rate and body temperature were monitored. The animals (weight 4.0 and 4.4 kg) were initially sedated with ketamin (30 mg/ kg) intramuscular and anaesthetized with a mixture of halothane, nitric oxide and oxygen. A laminectomy of the lumbar vertebrae was made and the lumbosacral enlargement with its caudal extent was exposed. The intravesical and intraurethral pressures were measured with two microtip transducers 46
47 DGC stimulation results in urethral relaxation RESULTS Electrical stimulation in the medial part of the fasciculus gracilis almost invariably resulted in a strong increase of the intrauretral pressure without a significant effect on the bladder pressure (Fig. 1). This effect was not only obtained at sacral levels but also at lower lumbar levels (L5, L6 and L7). When the electrode was advanced further ventrally and reached the DGC, a completely reverse effect was observed. A strong and immediate decrease of the intraurethral pressure was observed without a significant effect on the bladder pressure (Fig. 1). This effect was observed in the caudal L7, and in S1, S2 and S3 segments, but not at levels rostral to caudal L7. In S1 the decrease in urethral pressure was observed over a dorsoventral distance of 1.2 mm. In the S2 and S3 segments this effect was observed at a wider range corresponding with a dorsoventral distance of 1.7 mm. The intraurethral pressure immediately decreased, at the beginning of the stimulus, remained decreased during stimulation, independent of the length of stimulation, but increased sharply to resting levels when the stimulus was stopped. Occasionally, stimulation of the ventral funiculus ventral to the DGC resulted in an increased intraurethral pressure. The halothane anesthesia was of great influence on the stimulation responses. When the animal was deeply anesthetized the intrauremounted on a single 6 French catheter with a lumen (Raumedic, Germany). The distance between the two transducers was 3.5 cm. The transducer at the tip of the catheter was placed intravesically, the second transducer was placed at the point were the intraurethral pressure was at its highest. The bladder was emptied and subsequently filled with 30 ml of saline at body temperature. The spinal cord was electrically stimulated with a monopolar stainless steel electrode with an exposed tip of 0.25 mm. The stimulus consisted of a 2 sec long pulse train of negative going 80 µa pulses with pulse width 25 ms and pulse rate 20 pps. The dura was opened and the caudal lumbar and sacral segments of the spinal cord were exposed. The stimulation was started dorsally just through the pia in the gracile column and the electrode advanced in successive steps of 0.3 mm. At each location the tissue was stimulated with one or more pulse trains. Computer recordings of the intravesical and intraurethral pressures were made during a time period beginning 5 sec before the first stimulation until 15 sec after the last stimulation. The output of the pressure transducers was amplified with Neurolog pressure amplifiers (NL108, Digitimer Ltd., England), low-pass filtered (third order Bessel filter with cut-off frequency at 7.9 Hz) and digitized at a rate of 40 samples/ channel/s with a CED 1401plus (Cambridge Electronic Design Ltd, England). Data acquisition and off-line analysis were performed with Spike 2 software (Cambridge Electronic Design Ltd, England) on a Macintosh computer. At the location at which a strong decrease of the intraurethral pressure could be evoked 50 nl of 2% pontamine sky blue was injected with a picopomp. Immediately after the experiments, the animals were deeply anaesthetized with 6% sodium pentobarbital, perfused intracardially with 1.5 l heparinized phosphate buffered saline (PBS) (ph 7.4) at room temperature, followed by 1.5 l fixa- tive containing 4% paraformaldehyde in 0.1 M phosphate buffer (ph 7.4, room temperature). The lumbosacral spinal cords were removed and placed in the same fixative for 2-3 hours at 4 o C. The tissue was dehydrated overnight in 25% sucrose in 0.1 M phosphate buffer (ph 7.4), and the next day the tissue was frozen to -55 C in a isopentane bath and cut in 40 µm sections on a cryostat. Sections of 40 µm thick were made of the spinal segments L7, S1, S2 and S3, and the sections were studied light microscopically with a Zeiss axioplan. 47
48 Chapter 4 IC Pontine micturition center (+) (+) GABA-ergic neurons Bladder motoneurons ( ) S2 Bladder sphincter motoneurons Bladder (+) (+) Bladder Sphincter Fig. 2. Schematic overview of the pathways involved in the control of the bladder and bladder sphincter motoneurons during micturition. Pathways are indicated on one side only. thral pressure was low (1-2 kpa) and did not show pressure variations. Under such circumstances a decrease of the intraurethral pressure could not be observed during stimulation in the DGC. DISCUSSION The results are in agreement with the concept that the PMC produces micturition via a direct excitatory connection both with the bladder detrusor muscle motoneurons and with the GABA-ergic inhibitory interneurons in the DGC. Previous investigations on sacral microstimulation did not report a locally evoked decrease in intraurethral pressure (Jonas et al., 1975), possibly because the stimulation was aimed at the IML and not at the DGC. Theoretically, it is possible that the GABAergic DGC cells, which receive afferents from the PMC do not project to Onuf s nucleus. However, this seems extremely unlikely, because almost all local premotor interneurons, independent of whether they are GABA-ergic, projecting to Onuf s nucleus are located in the DGC, and a few in the IML. Other sources of GABA-ergic innervation of the Onuf s nucleus do not exist, because it is not affected by spinal cord transection rostral to the sacral cord or dorsal rhizotomy (Ramirez-Léon et al., 1994). In conclusion, the present results give further support to the concept that the PMC excites the bladder via a direct pathway to their parasympathetic preganglionic motoneurons, and inhibits the bladder sphincter via an excitatory projection to the GABAergic premotor interneurons of the urethral sphincter motoneurons in Onuf s nucleus (Fig. 2). 48
49 Chapter 5 Anal sphincter motoneurons in pig Location of External Anal Sphincter Motoneurons in the Sacral Cord of the Female Domestic Pig Bertil F.M. Blok, Gert Roukema, Bas Geerdes, and Gert Holstege Neurosci. Lett. 216: (1996) ABSTRACT The location of the striated external anal sphincter motoneurons in the spinal cord was investigated in 12, between 3 and 4 months old, female domestic pigs using the retrograde tracer horseradish peroxidase (HRP). Their motoneuronal cell bodies were found in the spinal segments S1-S3, and were not located in the ventral horn, but dorsolateral to the central canal. This location within the spinal gray matter strongly differs from the location of the external anal sphincter motoneurons in rat, cat, dog, monkey and humans, but is similar to that in the Mongolian gerbil. The possible relevance of this aberrant location is discussed. INTRODUCTION Anal atresia or imperforate anus is a congenital disease in humans caused by agenesis of the dorsal part of the cloacal plate. In order to study how to reestablish normal continence for faeces in humans, the pig is often used as an animal model (VanderPutte, 1986; Lambrecht and Lierse, 1987), because anal atresia also exists in this species, and pigs with this disease can be obtained relatively easy. Moreover, the anal anatomy in the pig is similar to that in humans (VanderPutte, 1986). Usually, in anal atresia the striated external anal sphincter (EAS) is rudimentary and dysfunctional (Stephens and Smith, 1971). The surgical methods used at present to reestablish normal continence for faeces in anal atresia are not effective in the long term (Langemeijer and Molenaar, 1991), possibly because of neuronal dysfunction. However, neither in sick, nor in healthy humans and pigs it is known how the neuronal control of the EAS is organized. In order to elucidate this organization, in the present study the motoneurons of the EAS were localized in the spinal cord of the healthy pig. The EAS forms the main closure muscle of the anus. Together with the levator ani, the coccygeus muscle and the external urethral sphincter (EUS), the EAS takes part in the pelvic floor, and all pelvic floor muscles are innervated by the pudendal nerve. In cat, dog, rhesus monkey, and probably also in humans, the motoneurons innervating the pelvic floor are located in a cell group (Onufrowicz, 1899; Sato et al., 1978; Kuzuhara et al., 1980; Roppolo et al., 1985), which was first described in 1899 by Onufrowicz, who called himself Onuf. This nucleus of Onuf extended from the caudal S1 to the rostral S3 segments of the human spinal cord. Later retrograde tracing studies in cat, dog and rhesus monkey using horseradish peroxidase (HRP) demonstrated that the EAS and EUS motoneurons are located, respectively, in the ventrolateral and the dorsomedial part of Onuf s nucleus (Sato et al., 1978; Kuzuhara et al., 1980; Roppolo et al., 1985). However, some other animals show a different somatotopic organization. For example, in the rat the EAS motoneurons are located in a cell group at the medial border of the ventral horn, and the EUS motoneurons in another cell group 49
50 Chapter 5 at the lateral border (Schrøder, 1980). Finally, in the Mongolian gerbil the EAS motoneurons are not located in the ventral horn, but dorsolateral to the central canal (Ulibarri et al., 1995). On the other hand, the EUS in this animal is located laterally in the ventral horn in a position similar to Onuf s nucleus in cat, dog, monkey and man. MATERIALS AND METHODS The surgery procedures, pre- and postoperative care, handling and housing of the animals followed protocols approved by the Faculty of Medicine of the University of Maastricht. The animals were preanaesthetized with intravenous pentobarbital sodium 20 mg/kg diluted with 1:1 sodium hydrochloride and with Stressnil (Janssen Pharmaceutics, Belgium). The animals were ventilated with 1-2% Halothane in a mixture of 66% nitric oxide and 34% oxygen. Twelve adolescent female pigs were injected (weighing kg; between 3 and 4 months old). In 7 animals (B1, B2, B6, B9, B11, B13, B14) a total of 20 µl of the retrograde tracer HRP (type VI Sigma; 20% solution dissolved in saline) was injected in the left EAS using a Hamilton syringe. In 5 other pigs (B20, B21, B27, B29 and B30) the EAS was injected bilaterally with a total of 40 µl HRP in order to label a maximum number of motoneurons. After a survival time of 48 hours the pigs were deeply anaesthetized and perfused with 8 liters of saline, followed by 4 liters of a fixative containing 2% glutaraldehyde (GA) and 1% paraformaldehyde (PF) in 0.1 M phosphate buffer, ph 7.4. The lumbosacral cord was removed and postfixed for 2 hours using the same fixation solution, and dehydrated overnight in a 25% sucrose solution. The segments of the lumbosacral cord were identified on the basis of their dorsal roots. In all cases the L5-S4 segments were cut transversally, with the exception of cases B29 and B30 which were cut longitudinally, in 60 µm thick sections on a cryostat and processed using the tetramethylbenzidine (TMB) impregnation method. For each case, except the cases with longitudinal sections, the total number of labeled neurons was counted in every other section. The labeled neurons were plotted. The diameter of 41 labeled neurons was determined in the S2 segment of cases B9, B14 and B27. The largest diameter of a cell body was measured, and only labeled cells with two or more labeled dendrites were considered labeled motoneurons. RESULTS In 7 animals (B1, B2, B6, B9, B11, B13, B14), which were injected unilaterally in the left EAS, many retrogradely labeled motoneurons were found ipsilaterally to the injected muscle. Sporadically, a contralat- Fig. 1. Polarized light photomicrograph of a section of the second sacral segment of case B27. Retrogradely labeled motoneurons of the external anal sphincter are located dorsolateral from the central canal. Note that the ventral horn does not contain motoneurons. The border of the gray and the white matter is indicated by a white line. CC = central canal. Bar, 300 µm. 50
51 Anal sphincter motoneurons in pig B11 B 13 B14 S1 Middle S1 Caudal S2 Rostral S2 Middle 1 S2 Middle 2 S2 Caudal S2 Rostral S2 Middle Fig. 2. Schematic drawings with small dots indicating the location of the retrogradely labeled motoneurons in cases B11, B13 and B14. Each drawing represents 12 alternate sections. eral located cell was observed, probably due to spread of the tracer to the contralateral side. The labeled motoneurons were not located ventrally in the ventral horn as in most other mammalian species, but dorsolateral to the central canal. In the cases with bilateral injections in the EAS (B20, B21, B27, B29 and B30) labeled motoneurons were found bilaterally in the same location as in the ipsilateral cases (Fig. 1). In none of the cases labeled cells were found in the ventral horn. Occasionally, retrogradely labeled axons could be followed from their exit from the ventral white matter through the ventral horn and intermediate zone to their soma of origin. Some retrogradely labeled dendrites of EAS motoneurons were found to cross the midline towards the contralaterally located motoneurons. No clear rostrocaudal periodicity was found in the labeled motoneuron pool. The precise rostrocaudal distribution of the labeled motoneurons varied among the cases. Consistently, motoneurons were found in the caudal S1 to rostral S3 seg- 51
52 Chapter 5 ments, but in three cases (B1, B11 and B20) labeled motoneurons were already found in the middle of S1. Caudally, in two other cases (B13 and B14) the motoneuronal cell group extended until the level of middle S3 instead of upper S3 (Fig. 2). Most of the cells were round to polygonal, and some cells had a fusiform shape. The mean diameter was 47 µm, varying between 31 and 65 µm. The number of EAS motoneurons counted in the ipsilaterally injected cases (B1, B2, B6, B9, B11, B13, B14) varied between 54 and 90. In the cases B20, B21 and B22, which were injected bilaterally in the EAS, the number of EAS motoneurons on the left varied between 42 and 78 and on the right between 43 and 80. In all cases the bulk of the labeled motoneurons was present in the second sacral segment. DISCUSSION The results demonstrate that the EAS motoneurons of the female domestic pig are located dorsolateral to the central canal in the sacral cord. In the cat, according to Rexed (Rexed, 1954), this area corresponds with the transition area of the medial part of lamina VII and the lateral part of lamina X. Lamina X contains many neurons of yet unknown nature, but motoneurons have never been described in this area. In the cat the medial part of the intermediate zone (lamina V to VII) is known to contain many interneurons, as well as a few preganglionic parasympathetic motoneurons forming the most medial extent of the dorsal band of the sacral parasympathetic nucleus of Nadelhaft et al. (1980). Since somatic motoneurons in species as rat, cat, dog, monkey and man are exclusively located in the ventral horn, one might wonder why in the pig and the Mongolian gerbil (Ulibarri et al., 1995) the EAS motoneurons are located in such an aberrant location. First, EAS motoneurons cannot be considered as normal somatic motoneurons. Although they partly function in the framework of abdominal pressure control, they also play a role in the more autonomic function of defaecation. Although EAS motoneurons innervate a striated muscle, which is clearly under voluntary control, they also behave as autonomic motoneurons in respect to defaecation and receive, unlike somatic motoneuronal cell groups, direct hypothalamic afferents (Holstege and Tan, 1987). Moreover, they also behave as autonomic motoneurons in respect to the disease of amyotrophic lateral sclerosis (ALS), in which all somatic but no autonomic preganglionic motoneurons are affected (Mannen et al., 1977). It has, therefore, been proposed to consider these motoneurons to belong to a separate class of motoneurons between somatic and autonomic (Holstege and Tan, 1987). The fact that autonomic motoneurons are located much more dorsal in the spinal gray than somatic ones, might explain the dorsal location of the EAS motoneurons in the pig. Second, in a recent study, in which pseudorabies virus was injected in the EUS of the rat [8], it was found that the strongest input to the EUS motoneurons originates from premotor interneurons dorsal and dorsolateral to the central canal, the same location as that of the EAS motoneurons in the pig. In the cat dendrites of Onuf s nucleus motoneurons (EAS and EUS) also extend into this area (Sasaki, 1994). These findings indicate that the area dorsolateral to the central canal, in which in the pig the EAS motoneurons are located, contains premotor interneurons for pelvic floor motoneurons in other species. Since in rat and cat this same area receives strong input from Barrington s nucleus in the pons (Holstege et al., 1979; Loewy et al., 1979), one might speculate that in the pig Barrington s nucleus might have direct access to the EAS motoneurons. 52
53 Pontine micturition centers are not interconnected Chapter 6 The Two Pontine Micturition Centers in the Cat are not Interconnected; Implications for the Central Organization of Micturition Bertil F.M. Blok, and Gert Holstege J. Comp. Neurol., submitted ABSTRACT In the cat the detrusor muscle of the urinary bladder is innervated by sacral cord parasympathetic preganglionic motoneurons in the intermediolateral cell column and the external urethral sphincter by motoneurons in the ventrolateral part of the nucleus of Onuf. Neurons coordinating the activity of the urinary bladder and the external urethral sphincter during micturition and urinary continence are not located in the sacral spinal cord, but in two pontine regions, the M (=medial)-region or pontine micturition center and the L (=lateral)-region or pontine storage center. The M-region excites the detrusor muscle of the bladder via direct projections to its parasympathetic preganglionic motoneurons, and inhibits the bladder sphincter via direct excitatory projections to the GABA interneurons, that in turn inhibit the bladder sphincter motoneurons. The L-region, via direct projections, excites the bladder sphincter motoneurons. The present study investigated whether there are interconnections between the M- and L-regions. If so, micturition is the result of a coordinated activity of the two pontine regions, if not, they control micturition and continence independently. Ten adult cats were injected with antero- and retrograde tracers. First, WGA-HRP was injected in the segments caudal S1 and S2 in order to delimit the M- and L-regions in the pons. In all cases with M-region injections anterogradely labeled fibers were found to the sacral intermediolateral cell column containing bladder motoneurons, but not to the nucleus of Onuf. In these cases no specific projections were found to either to the ipsilateral L- region or to the contralateral M- and L-regions. In the cases with L-region injections a distinct projection of labeled fibers was found to the sacral nucleus of Onuf, but not to the sacral intermediolateral cell column. In the cases no specific projections was observed to either the ipsilateral M-region, or to the contralateral M- and L-regions. The results indicate that the M-region produces micturition via direct projections to the sacral cord, but not via an inhibitory projection to the L-region. The L-region has a continuous excitatory effect on the bladder sphincter, but does not influence the M-region. It is concluded that the M- and L-regions are two separate functional systems which act independently. INTRODUCTION Micturition depends on a coordinated action or synergy between the urinary bladder and the external urethral sphincter (EUS). When the bladder contracts, the EUS relaxes and micturition takes place. An opposite action is necessary during continence: the EUS is tonically contracted and the bladder is relaxed while it is slowly filled with urine. The motoneurons innervating the detrusor muscle of the bladder in the cat are the preganglionic parasympathetic neurons in the sacral cord intermediolateral cell column (IML; Morgan et al., 1979). The motoneurons of the EUS are located in the socalled nucleus of Onuf, in the cat located at the level S1-S2 (Sato et al., 1978). M-region In normal adult animals the coordination between bladder and sphincter does not take place in the spinal cord, but in the dorsolateral pontine tegmentum. Tracing studies in 53
54 Chapter 6 the rat (Loewy et al., 1979) and cat (Holstege et al., 1979, 1986; Blok and Holstege, 1997; Blok et al., 1997b) have shown that a distinct group of neurons in the medial part of the dorsolateral pontine tegmentum, the M (=medial)-region, pontine micturition center (PMC) or Barrington s area, projects to preganglionic parasympathetic bladder motoneurons in the sacral IML, as well as to the dorsal gray commissure (DGC), but not to the nucleus of Onuf. Ultrastructural studies of Blok and Holstege (1997) have demonstrated that the M-region terminals on bladder motoneuronal somata and dendrites in the IML contain round vesicles and have an asymmetric cleft, which corresponds with an excitatory function. Also the M-region terminals in the sacral DGC contain many round vesicles, some dense cored vesicles and exclusively asymmetric synaptic clefts. The majority of them make contact with g amino butyric acid (GABA) immunoreactive dendrites or somata (Blok et al., 1997a). Stimulation in the M-region results in an immediate and sharp decrease in the intraurethral pressure and pelvic floor electromyogram (EMG), followed in about 2 sec by a steep rise in the intravesical pressure, mimicking complete synergic micturition (Holstege et al., 1986). Further evidence for the crucial importance of the M-region in micturition is the finding that bilateral lesioning of the M-region in the cat causes chronic urinary retention (Barrington, 1925; Griffiths et al. 1990; Mallory et al., 1991). L-region Another area, important for maintaining continence, is also located in the pontine tegmentum, but more ventral and lateral than the M-region. This area is called L (=lateral)-region, and sends direct projections to the nucleus of Onuf in the sacral cord (Holstege et al., 1979; 1986). Stimulation of the L-region in the cat results in a contraction of the pelvic floor, including the external urethral sphincter (Holstege et al., 1986), and bilateral lesions in the L-region cause an extreme form of incontinence (Griffiths et al., 1990). Apparently, the L- region serves as a pontine urinary storage center. Griffiths et al. (1990) brought up the idea that the synergic relaxation of the EUS during micturition could be caused by an inhibitory pathway from the M- to the L-region, and that during periods of continence the L-region inhibits, via a direct pathway, the M-region. This concept, however, was not based on a study of the anatomical connections between the M- and L-regions. In the meantime, Blok et al. (1997a) have indicated that the M-region inhibits the urethral sphincter via excitatory projections to GABA neurons in the dorsal gray commissure (DGC). Does that mean that there are no reciprocal connections between the M- and L-regions, and that these two regions act independently? The only way to find out is to determine whether there exist anatomical pathways between the two areas. MATERIALS AND METHODS The surgical procedures, pre- and postoperative care, and the handling and housing of the animals occurred according to protocols approved by the Faculty of Medicine of the University of Groningen. Seven male (cases 696, 863, 1015, 1036, 2350, 2366, and 2419) and three female adult cats (1017, 1224, and 2265) were used. Retrograde tracing cases Four cats (cases 2265, 2350, 2366, and 2419) were sedated with ketamin (30 mg/ kg) intramuscular, followed by additional doses of 15 mg/kg as necessary. During surgery EKG and temperature were monitored. In order to identify the pontine neurons projecting to the sacral cord in one case (2265) wheat germ-agglutinin horseradish peroxidase (WGA-HRP) was unilaterally injected in the caudal S1 and S2 segments (Fig. 1). 54
55 Pontine micturition centers are not interconnected In the other three cats WGA-HRP (cases 2350 and 2366) or cholera toxin subunit B (CTB; case 2419) was injected into the left M-region (Fig 2 and 3). In order to properly place the needle, prior to the injection, the M-region was identified by means of electrical stimulation. For this reason the intravesical and urethral pressures were measured with two transducers mounted on a single 6 French catheter (Raumedic, Germany). The distance between the two transducers was 3.5 cm. The transducer at the tip of the catheter was placed intravesically. Electrical stimulation was performed with a 5 sec train of negative going pulses, 5 ms duration, repetition frequency of 80 Hz, and 100 µa amplitude. The stimulation started about 5 mm dorsal to the dorsolateral pontine tegmentum and the electrode advanced in successive steps of 0.5 mm. A positive response consisted of a sharp decrease in the transurethral pressure followed by an increase in the intravesical pressure (Holstege et al., 1986). At the optimal stimulation site 40 nl 2.5% WGA-HRP (2350 and 2366) or 40 nl 2% CTB (2419) was injected. Three days after the WGA-HRP injection and 18 days after the CTB injection, the animals were initially anesthetized with ketamin and sedamun followed by an intraperitoneal injection 60 mg/kg sodium pentobarbital, perfused intracardially with 1.5 l heparinized saline (ph 7.4) at room temperature, followed by 1.5 l fixative containing in the WGA-HRP cases 2350 and % paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (PB; room temperature; ph 7.4), and in case % paraformaldehyde in 0.1 M PB (room temperature; ph 7.4). The spinal cords and brainstems were removed and placed in the same fixative for 2 hours at 4 o C, and the tissue of the WGA-HRP cases was dehydrated overnight in 25% sucrose in 0.1 M phosphate buffer (ph 7.4). The next day the tissue was frozen to -55 C in a isopentane bath and cut in 40 µm transverse sections on a Leica cryostat. One out of four sections was processed using tetramethylbenzidine (TMB). To determine the extent of the injection sites one out of four sections at the level of the pontomedullary junction were incubated according to the diaminobenzidine (DAB). The brain and spinal cord of the CTB case was postfixed for 2 hours at 4 o C, and 60 µm vibratome sections of the brainstem and sacral segments were cut on a Leica vibratome machine (see Blok and Holstege, 1997). The sections were incubated in goat anti-ctb antibody (List Biological Laboratories) diluted 1: 10,000 in Tris Buffered Saline containing 0.05% Triton X-100 (TBS + ) overnight at 4 C, transferred to rabbit anti-goat IgG (DAKO) diluted 1:250 in TBS + and 1% normal rabbit serum during 1 hour at room temperature, and placed in TBS + solution containing goat-pap (1:250; DAKO) and 1% normal rabbit serum. Subsequently, the sections were incubated with DAB. The sections of both of the WGA- HRP and CTB cases were mounted on slides, dehydrated and coverslipped with DePeX mounting medium. The distribution of retrograde and anterograde labeling was microscopically examined with a Zeiss Axioskop under combination of polarized light and darkfield condensor. Photomicrographs and plottings of representative sections were taken. Tritiated leucine cases In six cats single injections with L-(4,5-3 H)- leucine (specific activity > 100 Ci/mmol) were placed in the dorsolateral pontine tegmentum (Fig. 1). In four cases (696, 863, 1017 and 1036) the injections were made in the ventrolateral, and in the other two cases (1015 and 1224) in the dorsomedial part of the dorsolateral pontine tegmentum. After dorsal approach and exposure of the cerebellar cortex, the injection in each cat was placed stereotactically with a Hamilton microsyringe fitted with a 22-gauge needle. 55
56 Chapter 6 Retrograde labeling after WGA-HRP injection in S1/S2 IC M-region IC MLF MesV M-region BP L-region BC MLF BP M-region MesV A P BP B P L-region LL BP ML C P MLF S1 S2 Fig. 1. Retrogradely labeled neurons in the pons after injection of WGA-HRP in the caudal S1 and S2 segments unilaterally in case The presumed retrograde labeled neurons of the M- and L regions are indicated. In all cases 0.5 µl distilled water containing approximately 50 µci tritiated leucine was injected over a period of 5 minutes. The needle was left in place for an additional 30 minutes to minimize spread along the needle track. After a survival period of 6 weeks (Holstege et al., 1979), the animals were deeply anesthetized and perfused with saline followed by 10% formalin. After postfixation in 10% formalin for at least one week, brain and spinal cords were cut into transverse 25 µm frozen sections. One series of every tenth section was mounted, coated with Ilford G5 emulsion by dipping, and stored in the dark at 5 C for 3 months. Subsequently, the material was developed with Kodak D19 at 16 C, fixed, and counterstained with cresyl violet. The sections were studied with a Wild darkfield M7S microscope, and photomicrographs were taken of representative sections. In each experiment, the injection area was defined as that area in which the silver grains over the cell bodies were either as numerous as, or more numerous than those over the surrounding neuropil. RESULTS Injections in the dorsomedial part of the dorsolateral pontine tegmentum (cases 1015, 1224, 2350, 2366 and 2419) were assumed to be placed in the M-region when a strong distinct projection was found to the sacral IML and DGC. Injections in the ventrolateral part of the dorsolateral pontine tegmentum were considered to be placed in the L-region when a similar projection was found to the nucleus of Onuf (Holstege et al., 1979; 1986). WGA-HRP injection in the sacral cord The spinal cord injection involved both the ventral and dorsal portions of the gray matter of caudal S1 and S2 unilaterally (case 2265), and a specific group of retrogradely labeled neurons was found bilaterally in the dorsolateral pons just medial and ventromedial to the mesencephalic trigeminal tract (Fig. 2). This group formed a rostrocaudally oriented column, extending from the level of the inferior colliculus, rostrally, to the level just rostral to the motor trigeminal 56
57 Pontine micturition centers are not interconnected Injections in the M-region IC 1224 PAG Mes V IC 2350 BP A BC ML P CSN PON L-region BP 1224 LL B 1015 ML PON P 2366 L-region BP C 2350 MesV M-region BC MLF P Injections in the L-region IC 696 BC BP ML P A CSN PON PAG Mes V 696 BP IC LL B M-region MLF 863 ML PON P C M-region MLF 863 P BC PBL L-region BP Fig. 2. Upper row: Schematic drawing of injections sites in the dorsomedial part of dorsolateral pontine tegmental field, including the M-region. On the left are the tritiated leucine injections and on the right the WGA-HRP injections. Lower row: Schematic drawing of tritiated leucine injections sites in the ventrolateral part of dorsolateral pontine tegmental field, including the L-region. nucleus, caudally. It corresponded with the M-region as described previously anatomically and physiologically by Holstege et al. (1986). A second group of retrogradely labeled neurons was found in the lateral part of the dorsolateral pons just ventrally from the brachium conjunctivum and dorsally from the lateral lemniscus. This area corresponds with the L-region (Holstege et al., 1986). Retrogradely labeled cells were also found bilaterally in the nucleus subcoeruleus and in the pontine tegmentum (Fig. 1). Injections in the M-region Both the tritiated leucine cases 1015 and 1224, the WGA-HRP cases 2350 and 2366, and the CTB case 2419 were placed in the dorsomedial part of the dorsolateral pontine tegmentum on the left side (Fig. 2 and Fig. 3), which area corresponds with the M-region. In cases 1015, 2366 and 2419 the injection area involved the M-region and the medial part of the nucleus subcoeruleus. The injection sites extended laterally into the medial parabrachial area, medially into the pontine medial tegmental field, and dorsally into the locus coeruleus. In case 1015 the injection extended rostrally into the caudal part of the ventrolateral periaqueductal gray (PAG). The injection area of case 2350 was located somewhat more dorsally and involved the medial parts of the parabrachial nuclei, the locus coeruleus, and the dorsal parts of the nucleus subcoeruleus. In case 1224 the injection area was located more rostrally than in the other 4 cases and in- 57
58 Chapter 6 Retrograde labeling after CTB injection in the M-region IC IC BP MLF 2419 Mes V BC P L-region BP L-region 2419 MLF BC P BP P Fig. 3. Retrogradely labeled neurons in the pons after injection of CTB in the M-region. Note the scarcity of retrograde labeled neurons in the M- and L regions. volved the M-region, locus coeruleus, the adjoining PAG, the dorsomedial part of the parabrachial area, the dorsal part of the nucleus subcoeruleus and the adjoining parts of the pontine medial tegmental field. In all five cases at the level of the sacral spinal cord, a large number of anterogradely labeled fibers and presumptive terminals were observed in the sacral IML and DGC bilaterally. In the cases 1224, 2350 and 2366 only very few labeled fibers were found in the area of the nucleus of Onuf. In case 1015, which included the medial part of the nucleus subcoeruleus, diffuse projections were found to all parts of the spinal gray matter throughout the lenght of the spinal cord (Holstege et al., 1979; 1986), and such diffuse projections were also seen in the nucleus of Onuf. In none of the five cases with M-region injections anterogradely labeled were found in the ipsi- or contralateral L-regions, and only a few in the contralateral M-region (Fig. 4). In the WGA-HRP cases 2350 and 2366, and the CTB case 2419 many retrogradely labeled neurons were found bilaterally in the nucleus subcoeruleus, the parabrachial area and the pontine tegmentum, but a specific group of retrogradely labeled neurons in the area of the L-region could not be found, neither ipsi- nor contralaterally. Labeled neurons were also absent in the contralateral the M-region (indicated in Fig. 4). Injections in the L-region In four tritiated leucine cases (696, 863, 1017 and 1036) injections were placed ventrally and laterally in dorsolateral pontine tegmentum (Fig. 2), and included the previously described L-region. The injection sites involved the lateral parts of the parabrachial area, including the nucleus of Kölliker-Fuse, and the lateral parts of the nucleus subcoeruleus. The injections in cases 696 and 863 were placed more ventrally than in cases 1017 and In all four cases a strong projection to the nucleus of Onuf was observed bilaterally, but with an ipsilateral predominance. Especially in cases 696 and 863, in which the injection sites extended to only a limited extend into the nucleus subcoeruleus (Wiklund et al., 1981; Jones and Freedman, 1983), very few labeled fibers were seen in the area of the sacral IML and DGC. On the 58
59 Pontine micturition centers are not interconnected Fig. 4. Left: Ipsilateral anterograde labeling in the dorsolateral pons after tritiated leucine injection in the M-region in case Note the absence of labeling in the ipsilateral L-region. Right: contralateral anterograde labeling in the dorsolateral pons in same case. Note the absence of specific labeling in the contralateral M- and L-region. Fig. 5. Left: Ipsilateral anterograde labeling in the dorsolateral pons after tritiated leucine injection in the L-region in case 863. Note the absence of labeling in the ipsilateral M-region. Right: contralateral anterograde labeling in the dorsolateral pons in same case. Note the absence of specific labeling in the contralateral M- and L-region. other hand, in cases 1015, 1017, and 1036, in which the injection sites extensively involved the subcoerulear area, numerous labeled fibers were present throughout the spinal gray matter (see also Holstege and Kuypers, 1982), thus including the nucleus of Onuf and the sacral IML. In the cases 696 and 863 no anterogradely labeled fibers were found in the area of the ipsi- or contralateral M-region (Fig. 5). In the other three cases (1015, 1017 and 1036) only few labeled fibers were found in the ipsi- and contralateral dorsolateral pontine tegmentum, but there was no specific projection to the M-region. In none of the four cases labeled fibers were found in the con- tralateral L-region. DISCUSSION The aim of the present study was to investigate the existence of reciprocal connections between the two micturition related areas in the dorsolateral pontine tegmentum of the cat: the M-region, also known as pontine micturition center (PMC) or Barrington s area, and the L-region or pontine storage center. The results demonstrate that the M- an L-regions do not maintain direct fiber connections, which suggests that the coordination of micturition and continence is controlled by two separate descending systems which exert their influence at the level 59
60 Chapter 6 of the sacral cord. The role of the M-region in micturition Stimulation of the M-region results in an immediate decrease of the intraurethral pressure and pelvic floor EMG, followed by an increase in the intravesical pressure (Holstege et al., 1986). From the present results together with other recent experiments (Blok and Holstege, 1997; Blok et al., 1997a), it is clear that the effect of the M-region stimulation is the result of direct excitatory projections from the M-region to the parasympathetic preganglionic bladder motoneurons and to the GABA-ergic interneurons in the sacral DGC, which in turn inhibit Onuf s nucleus motoneurons. The present results deny the possibility that the M-region produces bladder sphincter inhibition by way of a direct projection to the L-region. Another interesting finding is that the M- regions on both sides in the pontine tegmentum do not have reciprocal connections. One might speculate that such connections are not needed, because the M-region projections to the sacral cord are bilateral. It also explains why a lesion in the M-region on one side of the brainstem does not result in micturition problems. On the other hand, bilateral lesions in the M-regions lead to an immediate and continuous urinary retention (Griffiths et al., 1990). Apparently, cats, and probably also humans (Blok et al., 1997b; 1998), have two independent pontine micturition centers. Such an organization makes the micturition control system less vulnerable for lesions, not only at the level of the pons, but, more importantly, also at the level of the spinal cord, where the long axonal descending pathways travel in the lateral and dorsolateral funiculi (Holstege et al., 1979; 1982; 1986). Whether in the cat, as observed in humans (Blok et al., 1997b; 1998), the right sided micturition center is more active than the left one, remains to be clarified. The role of the L-region in micturition Stimulation of the L-region in the cat results in a strong contraction of the EUS and an increase in the pelvic floor EMG (Holstege et al., 1986). In this respect the PET-scan findings in men and women (Blok et al., 1997; 1998) are of great interest. More than half of the PET-scan volunteers in this study vigorously tried to micturate, but for involuntary, emotional reasons, kept their pelvic floor contracted, and thus kept their anal and bladder sphincters tightly closed. In these volunteers a region in the ventrolateral pontine tegmentum was activated, which might indicate the existence of an L- region in humans. The present results indicate that, because of the lack of afferents from the M-region, the L-region does not seem to play a role during the act of micturition. On the other hand, it is known that bilateral lesions of the L- region in the cat result in an extreme form of incontinence (Griffiths et al., 1990). Apparently, the L-region is continuously active during the time periods between micturition. Only during micturition its excitatory effect on the Onuf motoneurons is counteracted by the M-region, but not at the pontine level, i. e. via direct M-region projections to the L-region, but at the motoneuronal level via its direct projections to Onuf s nucleus. Suprapontine effects on M- and L-region Previous studies have indicated that the M- region receives afferents from the PAG (Blok and Holstege, 1994) and from a distinct region in the preoptic area (Holstege, 1987). Both areas have been demonstrated to affect micturition (Grossman and Wang, 1956; Skultety, 1959). Because of its location in or close to the Kölliker-Fuse area and ventral parabrachial nuclei, the afferents of the L-region are much more difficult to determine, and nothing precise is known about them. It is obvious that conti- 60
61 Pontine micturition centers are not interconnected nence is very important for cats and humans, and that the emotional motor system plays an important role. The study of the precise organization of the suprapontine afferents to the L-region neurons is also important, because these cells might also a role in sexual activities. Since the L-region neurons are located within the region of the ventrolateral parabrachial and Kolliker-Füse nuclei, such studies need to be done on the ultrastructural level, with retrograde labeling from the sacral cord and anterograde labeling from the various parts of the emotional motor system (Holstege, 1997). The present results, however, indicate that the pontine micturition center and the pontine continence center act independently. 61
62
63 PAG projects to PMC Chapter 7 Direct Projections from the Periaqueductal Gray to the Pontine Micturition Center (M-region). An Anterograde and Retrograde Tracing Study in the Cat Bertil F.M. Blok, and Gert Holstege Neurosci. Lett. 166:93-96 (1994) ABSTRACT Micturition is a spino-bulbo-spinal reflex. The bulbospinal part of this reflex is formed by the projections from the M-region, also called the pontine micturition center or Barrington s nucleus, to the preganglionic parasympathetic motoneurons in the sacral cord innervating the bladder. In respect to the spino-bulbar part of the micturition reflex, our group recently showed that the sacral cord projections to the brainstem terminate mainly in the periaqueductal gray (PAG). In this study it was investigated whether the PAG might serve as a link between the sacral cord and the M-region, by examining the possible connections using the tracers wheat germ-agglutin horseradish peroxidase and tritiated leucine. The results demonstrate that a specific circumscribed rostrocaudally oriented cell group within the ventrolateral PAG and parts of the dorsomedial PAG project specifically to the M- region. A concept is put forward in which specific parts of the PAG are involved in the control of micturition and that information concerning bladder filling is conveyed via the PAG to the M-region. INTRODUCTION Normal micturition is a coordinated action between the detrusor muscle of the bladder and the external striated urethral sphincter. In the cat the parasympathetic motoneurons innervating the detrusor muscle are located in the intermediolateral cell group (IML) in the S2-S3 segments of the spinal cord (Nadelhaft et al., 1980). The motoneurons innervating the striated external urethral sphincter are located in the nucleus of Onuf in the S1 and S2 ventral horn (Sato et al., 1978). In adult mammals the area responsible for the synergistic action of both muscles (detrusor-sphincter synergy) is not located in the spinal cord but in the brainstem. The region involved is located in the dorsolateral pons, and is known as Barrington s area (1925), M-region (Holstege et al., 1986) or pontine micturition center (Loewy et al., 1979). In this paper the term M(medial)-region will be used, because there exists a L(lateral)-region also. Neurons in the M-region project to the IML in the sacral cord (Loewy et al., 1979; Holstege et al., 1986), while cells in the L- region maintain connections with the nucleus of Onuf (Holstege et al., 1986). In the cat electrical stimulation in the M-region results in relaxation of the pelvic floor and urethral sphincter, followed in about two seconds by contraction of the bladder, thus mimicking normal micturition (Holstege et al., 1986). Bilateral lesions of the M-region cause an inability to empty the bladder resulting in a retention of urine (Barrington, 1925; Holstege et al., 1986). The question arises which areas in turn control the M-region. Several physiological studies have demonstrated that most of these areas belong to the limbic system, e.g. cingulate gyrus, preoptic area of the hypothalamus, amygdala, and bed nucleus of the stria terminalis (Torrens and Morrison, 1987). Electrical stimulation in these areas and in 63
64 64 Retrogradely labeled cells after a WGA-HRP injection in the dorsolateral pontine tegmentum, including the M-region NOT CP PP CGM D INC BIC ML RN III SN IN PC SC SC SC CGM IC IC BIC BIC III III ML RN SN IN PC PON IV MLF BC IN ML CSN BP PON PC nv PC PON ML A B PC C D E WGA-HRP injection sites in the PAG and adjacent areas IC MesV MesV BC BC 2178 BP MLF RST PC NLL F G NTB P Chapter 7 BIC ML III PAG 2250 BIC PAG 2250 IC PAG 2250 IC PAG 2250 IC A IN PC PON B ML ML PC PC PON Tritiated leucine injection sites in the PAG and adjacent areas B C BP PON D ML PC SC SC SC PP CGM PAG CGM PAG PAG IC BIC ML III BIC III BIC 1434 IV III BC SN RN ML RN SN ML 1481 IN PC A PC IN CSN BP ML PC C PON D PC PON BP BC CSN BP ML E PC PAG IC 1435 BC E ML PC PON Fig. 1. Schematic drawing of retrogradely labeled neurons in the PAG after a WGA-HRP injection in the M-region in case 2178 (on top). The arrowheads indicate an accumulation of retrogradely labeled cells in the ventrolateral PAG (see text). The WGA-HRP and the 3 H-leucine injection sites are shown in the middle and at the bottom, respectively. The injection sites of the cases with projections to the M-region are indicated on the left, those without such projections the right.
65 PAG projects to PMC the periaqueductal gray (PAG), can evoke micturition or micturition-like contractions of the bladder. In the cat specific fiber projections to the M-region have been shown to originate from the preoptic area (Holstege, 1987), but the connections from the PAG to the M-region have not been studied. Therefore, in a retrograde and anterograde tracing study it was attempted to find out whether PAG - M-region projections exist in the cat. MATERIALS AND METHODS A total of 20 adult male cats was used and the surgery procedures, pre- and postoperative care, handling and housing of the animals followed protocols approved by the Faculty of Medicine of the University of Groningen. The animals were anesthetized with intravenous pentobarbital sodium 20 mg/kg diluted with 1:1 sodium hydrochloride. In order to identify the M-region neurons, the caudal lumbar vertebrae were laminectomized and a total of 5 µl 20% HRP was injected bilaterally in the S2 and S3 segments of the sacral cord in two cases (2129 and 2226). To determine whether this pathway was ipsi- or bilateral, a hemisection was made on the right side at upper lumbar levels prior to the injection. All the injections in the brainstem were placed stereotaxically. In order to localize the PAG neurons projecting to the M-region, in 3 cases (2153, 2178 and 2185; Figure 1) injections of nl 5% wheat germagglutinin HRP (WGA-HRP) were made in the M-region, and in two control cases (2189 and 2224) in adjacent areas, but not in the M-region itself. In anterograde transport studies, nl 5% WGA-HRP was injected in the PAG in two cases (2239 and 2241). The injections were centered on those parts of the PAG that had been shown to contain retrogradely labeled neurons after WGA-HRP injections in the M-region. In one further case (2250) the injection was made in adjacent areas of the PAG and mesencephalic tegmentum (Figure 1). After 3 days survival time, the (WGA)-HRP cats were re-anesthetized and perfused through the left ventricle with 1.5 L saline, followed by 1.5 L fixative solution containing 2 % glutaraldehyde and 1 % paraformaldehyde. Brainstems and sacral cords were cut in four series of 40 µm sections. One series was processed using the tetramethylbenzidine (TMB) method, and one series using the ammonium paratungstate (PT) method (Weinberg and VanEyck, 1991). In order to determine the extent of the injections, of the third series the sections with injection sites were processed with the Fig. 2. On the left side a brightfield photomicrograph of the retrogradely labeled cells in the dorsolateral pons forming the M-region (case 2226). On the right a photomicrograph with polarized light of anterograde labeling in the M-region after an injection of WGA-HRP in the lateral PAG (case 2241) using the paratungstate method. Bars represent 300 µm. 65
66 Chapter 7 diaminobenzidine (DAB) method. In additional anterograde studies of projections from the PAG, in 10 cases (1337, 1409; 1410, 1434, 1435, 1481, 1486, 1487, 1495, 1497; Figure 1) 0.5 µl L-[4,5-3 H]- leucine was injected in the PAG and the surrounding tissue. These animals were allowed to survive for 6 weeks after which they were perfused with 1.5 L saline and 1.5 L 10% paraformaldehyde. The brains were processed for autoradiography, as described by Holstege (1987). RESULTS After the HRP injections in the S2 and S3 segments (cases 2129 and 2226) a specific group of labeled neurons was found ipsilaterally in the dorsolateral pons just medial and ventromedial to the mesencephalic trigeminal tract (Figure 2, on the left). The labeled cell group forms a rostro-caudally oriented column, extending from the level of the inferior colliculus, rostrally, to the level just rostral to the motor trigeminal nucleus, caudally. It corresponds with the M-region as described previously (Holstege et al., 1986). In 3 cases with WGA-HRP injections in the M-region and adjacent areas, a large number of anterogradely labeled fibers was observed in the sacral intermediomedial and intermediolateral cell group, containing the preganglionic parasympathetic motoneurons in the sacral cord. In the same 3 cases retrogradely labeled cells were found in the ventral, lateral and dorsomedial parts of the ipsilateral PAG, but they were especially numerous in a specific region of the ventrolateral PAG between the levels the trochlear nucleus and the center of the oculomotor nucleus (Figure 1, indicated by arrow heads in the upper row). In control case 2189, the WGA-HRP injection site involved the superior and lateral vestibular nuclei, deep nuclei of the cerebellum, and the caudal part of locus coeruleus, but not the M- region. In this case only very few retrogradely labeled cells were present in the PAG. In the other control case (2224), with an injection in the pontine tegmentum just ventral to the M-region, retrogradely labeled cells were found scattered throughout the ventrolateral PAG, mainly at caudal levels. A specific accumulation of labeled neurons, however, was not found. In cases 1434, 1435, 2239 and 2241, with injections in the lateral PAG, and in cases 1486 and 1495, with bilateral injections in the dorsal PAG (Figure 1), thin labeled fi- Cd PU Cl CL Preoptic area M-region BP (+) Parasympathetic motoneurons Onuf's nucleus LL IC BP PON (+) (+) Striated urethral sphincter BC ML P F CA OC CSN PON Bladder BNST Fig. 3. Schematic representation of the spinal and supraspinal structures involved in micturition control. Excitatory pathways are indicated by (+). IV SC PC SON ML Periaqueductal gray BC IC S2 (+) (+) L-region 66
67 PAG projects to PMC bers descended from the injection site into the area of the cuneiform nucleus dorsal to brachium coniunctivum. From this fiber bundle labeled fibers passed medially and dorsally to the brachium coniunctivum to terminate in the M-region (Figure 2, on the right). Only a limited number of labeled fibers terminated in the areas surrounding the M-region. In the cases with injections in the dorsomedial PAG the projection to the M- region was less pronounced than in the cases with injections in the ventrolateral PAG. However, in the former cases the projections were bilateral, due to bilaterallity of the injection sites. In the other six 3 H-leucine cases and one WGA-HRP case the injections were placed more ventrally, laterally or dorsolaterally in the PAG, but did not involve the area of the PAG revealing an accumulation of labeled neurons after WGA-HRP injections in the M-region. In these control cases weak diffuse anterograde labeling was observed throughout the dorsolateral pontine tegmentum, but specific anterograde labeling in the M-region was not found. DISCUSSION The present results provide evidence for a major projection from the ventrolateral and a minor projection from the dorsomedial PAG to the M-region in the cat. In a study on the PAG projections to the rostromedial pericoerulear region in the rat (Ennis et al., 1991), PAG fibers were found in Barrington s area as defined by Paxinos and Watson (1986). The present results not only show that a similar projection exists in the cat, but also that it originates mainly from a rostrocaudally oriented cell group in the ventrolateral PAG and from a more loosely arranged rostrocaudally oriented cell group in the dorsal PAG. The results suggest that the PAG plays a substantial role in micturition and they might explain why stimulation in the PAG results in voiding (Torrens and Morrison, 1987; Blok, unpublished results). However, the exact role of the PAG in the framework of micturition is not so clear. Micturition is considered to be a spino-bulbo-spinal reflex (Torrens and Morrison, 1987). The spino-bulbar part of this reflex is formed by the projection from the sacral cord to the brainstem, conveying information on bladder filling or bladder extension. Recent findings in the cat (VanderHorst et al., 1996) have demonstrated that particularly the sacral segments of the spinal cord project strongly to the PAG. In view of the finding that the sacral cord does not, or only to a limited extent, projects directly to the M-region (Blok et al., 1995), it is possible that the PAG serves as a major receiving station for ascending sacral projections to the brainstem. The present results show that the PAG, in turn, might activate the M-region in order to produce voiding. Thus, the PAG may take part in the spino-bulbo-spinal reflex of micturition. In summary, a concept is presented in which the ascending projections from the sacral cord, conveying information on bladder filling, terminate in the PAG. In case the bladder is enough extended that voiding is necessary, the PAG, in turn, stimulates the M-region, which results in micturition. Rostrally located limbic structures, as the preoptic area, might control this spino-bulbo-spinal reflex and possibly determine, in respect to the safety of the individual, the beginning of the act of micturition (Figure 3). The last projection, thus, might serve as a safe signal, i.e. allows micturition only when the individual finds itself in a safe situation. 67
68
69 PET study on micturition control in men Chapter 8 A PET Study on Brain Control of Micturition in Humans Bertil F.M. Blok, Antoon T.M. Willemsen, and Gert Holstege Brain 120: (1997) ABSTRACT Although the brain plays a crucial role in the control of micturition, little is known about the structures involved. Identification of these areas is important, because their dysfunction is thought to cause urge incontinence, a major problem in the elderly. In the cat three areas in the brainstem and diencephalon are specifically implicated in the control of micturition: the dorsomedial pontine tegmentum, the periaqueductal gray, and the preoptic area of the hypothalamus. Positron emission tomography (PET) was used to test whether these areas are also involved in human micturition. Seventeen right handed male volunteers were scanned during the following four conditions: (1) 15 minutes prior to micturition during urine withholding; (2) during micturition; (3) 15 minutes after micturition; (4) 30 minutes after micturition. Ten of the 17 volunteers were able to micturate during scanning. Micturition was associated with increased blood flow in the right dorsomedial pontine tegmentum, the periaqueductal gray, the hypothalamus, and the right inferior frontal gyrus. Decreased blood flow was found in the right anterior cingulate gyrus during urine withholding. The other seven volunteers were not able to micturate during scanning, although they had a full bladder and tried vigorously to urinate. In this group during not successful micturition increased blood flow was found in the right ventral pontine tegmentum, which corresponds with the hypothesis that this area, according to cat results, controls the motoneurons of the pelvic floor. Increased blood flow was also found in the right inferior frontal gyrus during not successful micturition, and decreased blood flow in the right anterior cingulate gyrus was found during withholding of urine. The results suggest that, similar to the cat, the human brainstem contains specific nuclei responsible for the control of micturition, and that the cortical and pontine micturition sites are exclusively on the right side. INTRODUCTION It is generally accepted that the brain plays a crucial role in normal micturition (=urination), but in humans little is known about which specific brain areas are involved. This lack of knowledge is the more surprising, since it is highly probable that dysfunction of certain brain areas cause incontinence in many elderly (Andrew and Nathan, 1964; Blaivas, 1982). Incontinence in elderly is a major social problem. At least 10 million adults in the United States (Consensus conference, 1989), up to 30% of elderly citizens (age 60 years), residing in the community (Teasdale et al., 1988) and over 50% of those living in an institution (Resnick et al., 1989) suffer from urinary incontinence, in particular urge incontinence (Jewett et al., 1981; Ouslander et al., 1982). In 1987 the direct annual costs for care of patients with incontinence were estimated to exceed $10.3 billion in the United States only (Consensus Conference, 1989). Urge incontinence occurs when patients sense the urge to void, but are unable to delay it long enough to reach the toilet (Consensus Conference, 1989). In healthy individuals this urge is not immediately followed by micturition and it usually disappears when micturition is not appropriate at that particular time and place. Urge incontinence is also frequently found in pa- 69
70 Chapter 8 tients with stroke (Khan et al., 1981) or with neurodegenerative diseases, as multiple sclerosis (Blaivas et al., 1979). Urge incontinence should not be confused with genuine stress incontinence, which is not the result of lesions in the central nervous system and will not be discussed in this study. In humans, complete interruption of the brainstem-sacral cord pathways always results in uncoordinated contractions of bladder and sphincter (= bladder-sphincter dyssynergia; Blaivas, 1982). Patients with brain lesions rostral to the pons never show bladder-sphincter dyssynergia, but may suffer from urge incontinence (Blaivas, 1982). Apparently, micturition as such is not coordinated by regions in the spinal cord, but in the caudal brainstem. The beginning of micturition, however, is determined by regions in the forebrain. Evidence from animal experiments supports this conclusion. Cat experiments of Blok and Holstege (1994) and Blok et al. (1995) have led to a concept for the micturition reflex (Fig. 1). The pelvic nerve conveys information about the degree of bladder filling to neurons in the lumbosacral cord (Morgan et al., 1981), which in turn project to the periaqueductal gray (PAG) (Noto et al., 1991; Blok et al., 1995; VanderHorst et al., 1996), a midbrain area known for its role in nociception and emotional responses (Depaulis and Bandler, 1991; Holstege, 1995). When the bladder is filled to such a degree that voiding is appropriate, the PAG, according to this concept, activates an area in the dorsomedial pontine tegmentum (the pontine micturition center (PMC) or M-region; Blok and Holstege, 1994), which produces complete (synergic) micturition via long descending pathways to the parasympathetic bladder motoneurons in the sacral cord (Holstege et al., 1979; 1986). In the cat stimulation of forebrain structures, including the anterior cingulate gyrus, preoptic area of the hypothalamus, Pontine micturition center L-region Secondary bladder afferents Nucleus of Onuf Medial preoptic area Periaqueductal gray PON S2 Fig. 1. A schematic overview of the spinal and supraspinal structures involved in the control of micturition based on experiments in the cat. The locations of the micturition control areas (see text) in the brainstem and diencephalon were used in the null hypotheses for the present study in humans. Pathways are indicated on one side only. amygdala, bed nucleus of the stria terminalis and septal nuclei has been shown to elicit bladder contractions (Gjone and Setekleiv, 1963; Gjone, 1966). All these structures give rise to descending pathways to the PAG and other regions belonging to the so-called emotional motor system (Holstege, 1995), but only one, the medial preoptic area (MPO), being the rostral part of the hypothalamus, projects directly to the PMC or M-region (Holstege, 1987). Possibly, the MPO plays a role in the start of micturition. To maintain continence another pontine area has been implicated. This area, also called L-region, is located more laterally and ventrally than the PMC or M-region, and maintains direct projections to the nucleus of OC SC Bladder motoneurons CA BC IC External Urethral Sphincter Bladder 70
71 PET study on micturition control in men Onuf in the sacral cord (Holstege et al., 1979; 1986). Onuf s nucleus contains motoneurons innervating the pelvic floor, including the anal and urethral sphincters (Sato et al., 1978). In the cat bilateral lesions in the L-regions cause an extreme form of urge incontinence (Holstege et al., 1986; Griffiths et al., 1990). Positron emission tomography (PET) is a non-invasive technique to study changes in regional cerebral blood flow (rcbf) in humans performing specific tasks (Fox and Mintun, 1989). The rcbf is used as an index for the presynaptic activity in the area under consideration (Jueptner and Weiller, 1995). In the present PET-study the hypothesis was tested that in humans, during micturition, the same brain areas are active as in the cat. Therefore, areas in the brainstem and diencephalon, such as the PMC, PAG and the MPO of the hypothalamus, received special attention. In addition, it was investigated whether in humans structures other than those known from the cat have an increased or decreased rcbf during micturition. Because of the limitation of the detection field of the camera areas located at the top of the brain were not scanned. MATERIALS AND METHODS Subjects In all experiments the volunteers completed a general health questionnaire. Nobody reported a history of neurological, psychiatric or urologic illnesses. All subjects were exclusively right-handed, and gave their written informed consent according to the declaration of Helsinki. The protocol of the study was approved by the research ethics committee of the University Hospital of Groningen. During each scan the lights were dimmed, the subjects had their eyes closed and did not move. Experimental design Brain activation was measured in 17 righthanded male volunteers (age range 21-50, mean age 32 years; Table 1). Each scanning session consisted of the following four measurements and lasted 1.5 hours in total. The following scans were planned: Scan 1. Fifteen minutes prior to micturition with a filled bladder (withholding scan), Scan 2. Just after the command to start micturition (micturition scan), Scan 3. Fifteen minutes after micturition (empty bladder scan 15'), Scan 4. Thirty minutes after micturition (empty bladder scan 30'). Before the first scan all volunteers affirmed that their bladder was full. Eight seconds before the second scan and 15 seconds after the beginning of injection of the H 2 15 O bolus the right index finger of the volunteers was touched to indicate that they could start micturition. Prior to the other three scans no specific assignment was given. A few days before the scanning session the volunteers were asked to practice urinating horizontally. All volunteers reported that they had no problems to empty their blad- Table 1 Subjects Subject Age Micturition (years) initiation, time after start of Scan 2 (s) A B C 23 no micturition D 46-2 E 31 no micturition F 43 1 G H 23 0 I J 29 no micturition K 45 no micturition L 26 no micturition M N 49 0 O 41 no micturition P 42-2 Q 21 no micturition Mean
72 Chapter 8 der during such a practice session. During the training and the scanning session a selfadhesive external condom catheter was used which was attached to the volunteer s penis and connected to a plastic bag to collect the urine. Data acquisition The subjects were placed in a horizontal position in the PET camera (Siemens-CTI 951/31, Knoxville, TN, USA) parallel to and 5 cm below the glabella-inion line as determined by external examination. Because of the technical characteristics of the PET camera, the most caudal limit of the scanned area was the pons, and the most rostral limit was the cingulate gyrus. This resulted in images extending between -28 mm below and 44 mm above the intercommissural plane. This implied that for example the motor cortex was not part of the investigated brain regions. An individually constructed head mold was used to minimize head movement between sessions. First, a transmission scan of 20 minutes was made for attenuation correction of the emission scans. Subsequently, the four scans were carried out. Before each scan, the subjects were given 1.85 GBq of H 2 15 O in saline. The H 2 15 O bolus was injected in the right brachial vein, followed by 40 ml saline from an automatic pump. Data acquisition was initiated 23 seconds after the beginning of the injection, at which time the peak in radioactivity was assumed to reach the cerebral blood flow, and continued during 90 seconds. To allow the radiation to reach background levels, there were 15 minutes intervals between the injections. Data analysis The data of each scan were summated and the resulting images were centered to prevent loss of information during sampling. Prior to the statistical procedure the data were sampled to a voxel size of 2.2 x 2.2 x 2.4 mm. The data were further analyzed using the Statistical Parametric Mapping procedure (SPM95 from the Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks Inc. Sherborn MA, USA) on a SPARC workstation (Sun Microsystems Inc., Surrey, UK). The SPM95 software was used for the anatomical realignment, normalization, smoothing and statistical analysis. Anatomical realignment The rcbf was reconstructed for each scan, using an attenuation correction based on the transmission scan. Movement-related components between scans were corrected during the realignment, using the first recorded scan of each subject as a reference. Normalization Following realignment all images were transformed into a stereotactic standard space (Talairach and Tournoux, 1988). This normalizing spatial transformation matches each scan to a reference or template image that conforms to the standard space (Friston et al., 1991a). Stereotactic normalization of PET images allows comparison of scan data in identical pixels across different subjects and scans. Smoothing Prior to the statistical analysis the scans were smoothed in order to increase signal to noise ratio and to suppress effects due to residual differences in functional and gyral anatomy between subjects during intersubject averaging. During smoothing a Gaussian filter of 8x8x8 mm was used (full width, half maximum in the x, y and z axes, respectively). This relatively small filter was used because the main part of the study was aimed at the relatively small brainstem and diencephalon. Statistical analysis Differences in global activity within and 72
73 PET study on micturition control in men Table 2 Study design Groups scan 1 scan 2 scan 3 scan 4 Group 1 (10) Withholding of Micturition Empty bladder Empty bladder urine successful for 15 min for 30 min Group 2 (7) Withholding of Micturition Empty bladder Empty bladder urine unsuccessful for 15 min for 30 min All subjects in Group 2 (unable to micturate when requested: micturition unsuccessful) were able to micturate within 5 min of completion of the second scan. Table 3 Results from Group I subjects (successful micturition during Scan 2) Peak position Min/max of %rcbf Z-score (x, y, z in mm) z (mm)*** increase Micturition, successful withholding urine (Scan 2 - Scan 1) Right hemisphere Inferior frontal gyrus (BA 44, 45, 47) +52, +16, +4-8 / * Anterior cingulate gyrus (BA 24, 32) +12, +32, +16 0/ * Medial temporal gyrus (BA 21) +50, -2, / Left hemisphere Striatum -22, +22, +4 0/ Diencephalon and brainstem Hypothalamus -4, -4, -4-8/ Periaqueductal gray +4, -34, / Right dorsomedial pons +10, -42, / Micturition, successful empty bladder (Scan 2 - Scan 3) Right hemisphere Inferior frontal gyrus (BA 45, 47) +48, +26, -4-8/ * Medial temporal gyrus (BA 21) +50, -2, / Diencephalon and brainstem Hypothalamus -4, -4, -4-8/ Periaqueductal gray +4, -34, / Right dorsomedial pons +10, -42, ** Empty bladder withholding urine (Scan 3 - Scan 1) Right hemisphere Anterior cingulate gyrus (BA 24, 32) +8, +36, -4-8/ * Medial temporal gyrus (BA 21) +50, -18, 0 0/ The location of the peak activation is indicated by the coordinates x, y and z according to the atlas of Talaraich and Tournoux (1988). The differences between scans 2 (Micturition, successful) and scan 4 (Empty bladder 30 minutes) were in the same range as those between scan 2 and 3 (Empty bladder 15 minutes), and are not shown. BA = Brodmann s area; * = Significant after a correction for multiple comparisons with threshold of P<0.05; ** = Extent on the basis of an uncorrected threshold of P<0.004; ***extent in z-axis is based on of an uncorrected theshold of P<0.001, unless otherwise indicated. 73
74 Chapter 8 between subjects were removed by analysis of covariance on a pixel-by-pixel basis with global counts as covariate. This was done because inter- and intra-subject differences in global activity may obscure regional alterations in activity following cognitive processes (Friston et al., 1990). For each pixel in the stereotactic space the analysis of covariance (ANCOVA) generated a condition-specific adjusted mean rcbf value (normalized to 50 ml/dl/min) and an associated adjusted error variance. A repeated-measures ANCOVA was used for the comparison of the second micturition scan with the other scans, each subject being studied under all conditions. The ANCOVA allows comparison of the means across conditions using the t statistic. The resulting set of t values constitutes a statistical parametric map (SPM{t}) (Friston et al., 1991b). During analysis special attention was put on the expected micturition control areas in the diencephalon and brainstem, i.e. the PMC, PAG and hypothalamus, including the MPO. An uncorrected P-value of was used for these areas since the exact location could be predicted on the basis of cat experiments. This (omnibus) approach can be used when the location of the area, in which activation is Fig. 2. Differences in rcbf for the comparison successful micturition (scan 2) minus withholding of urine (scan 1). Pixels that are significant at the threshold of an uncorrected P<0.002 are displayed on single sagittal, coronal and transverse projections of the brain. The numbers refer to the distance in millimeters relative to the intercommissural plane. The activations of the expected micturition control areas have been indicated by arrows. Abbreviations: pmc = pontine micturition center; pag = periaqueductal gray; ht = hypothalamus; R = right side of the brain; VAC = vertical line through the anterior commissure; VPC = vertical line through the posterior commissure. 74
75 75 Fig. 3. Regions with significant higher rcbf during successful micturition (scan 2) compared to withholding of urine (scan 1). Display at uncorrected threshold of P< Numbers refer to the distance in mm relative to the intercommissural plane. Significant activation was observed in the same three areas as have been demonstrated in the cat to be related micturition: pontine micturition center (indicated by pmc), periaqueductal gray (pag), and hypothalamus (ht). The right anterior cingulate gyrus, inferior frontal gyrus, medial temporal gyrus and the left rostral striatum are also activated. Of these regions only the anterior cingulate gyrus and the inferior frontal gyrus are statistically significant activated after a correction for multiple comparisons. L = left side; R = right side. PET study on micturition control in men
76 Chapter 8 expected, is known (Friston et al., 1991b). Trends in activation in the expected micturition control areas are reported when they reach a significance level of P< This level of significance protects sufficiently against false positives (Warburton et al., 1996). Other brain areas observed to be activated were considered statistically significant only after a correction for multiple comparisons (corrected threshold P<0.05), which is necessary when there is no preconceived hypothesis (Friston et al., 1991b). RESULTS Ten of the 17 volunteers were able to urinate within 30 seconds after the beginning of the second scan, i.e. within 53 seconds after the injection of H 2 15 O (Table 1). The mean collected urine volume was 567 ml (± 267 ml). The results obtained in this group will be referred to as Micturition, successful. The other seven volunteers tried but did not succeed to urinate during scanning (Table 1 and Table 2). All seven were able to micturate within 5 minutes after finishing the second scan (mean urine volume amounted 712 ml ± 234 ml). The scanning data of this group were analyzed separately and the results are reported under the heading Micturition, not successful. Micturition, successful The activated brain areas of the 10 subjects of this group are presented in Table 3. The overall activation of the comparison between successful micturition (scan 2) and the condition during withholding of urine (scan 1) is shown in Fig. 2. During micturition the rcbf was significantly increased (uncorrected P<0.001) in the periaqueductal gray (PAG) and hypothalamus and on the right in the dorsomedial pontine tegmentum (Figs. 2 and 3). The activation in the hypothalamus appeared to be located predominantly in its rostral part (Fig. 4). Of the cortex cerebri, only the right inferior frontal gyrus (Brodmann s areas (BA) 45 and 47, and the most dorsal tip of BA 44) and right anterior cingulate gyrus (BA 24 and 32) were significantly more activated than in the urine withholding phase (corrected P<0.05). Additionally, the right medial temporal gyrus (BA 21) and the rostral striatum were also more active, although not significant for multiple comparisons. Comparing successful micturition (scan 2) with the conditions 15 and 30 minutes after micturition (scans 3 and 4) similar results were obtained. During micturition the rcbf was increased (uncorrected P<0.001) in the same areas as when micturition (scan 2) was compared with urine withholding (scan 1). Exceptions were the anterior cingulate gyrus and the rostral striatum which did not show any statistically significant difference in activity (Table 3). A significant increase in activity was found in the anterior cingulate gyrus (corrected P<0.05) during empty bladder (scans 3 and 4) compared with the withholding of urine condition (scan 1). Decreases in rcbf were also found. During micturition (scan 2) compared with scan 1 or with scans 3 or 4, decreased rcbf was found in the left inferior and medial frontal gyri (BA 9 and 44; peak activation -42, +10, +32; Z score 3.1) and the left medial temporal gyrus (BA 21; peak activation - 56, -48, -8; Z score 3.2). However, these differences were not significant after correction for multiple comparisons (corrected P<0.05). Micturition, not successful In the not successful micturition group increased rcbf was found in distinct brain areas during the second scan when the volunteers tried hard, but failed to micturate, compared to the condition during empty bladder (scans 3 and 4). Prior to the statistical analysis it was hypothesized that the ventrolateral pons would be activated dur- 76
77 PET study on micturition control in men Fig. 4. The activation of the hypothalamus (indicated by ht) as seen on the coronal section y=-4 mm relative to the anterior commissure for the comparison successful micturition (scan 2) minus withholding urine (scan 1). The peak activation seems to be located just ventral to the anterior commissure in the preoptic area of the hypothalamus. The activation in the right temporal lobe was not significant after a correction for multiple comparisons. An uncorrected threshold of P<0.002 was used for display. For the Z score scale see Fig. 3. Fig. 5. Left: Significant differences in rcbf in the right dorsomedial tegmentum (indicated by pmc) after the comparison Micturition, successful (scan 2) minus Withholding of urine (scan 1). Right: Significant differences in rcbf in the right ventral tegmentum (indicated by L-region) after the comparison Micturition, not successful (scan 2) minus Empty bladder I (scan 3). Threshold uncorrected P<
78 Chapter 8 ing the not successful micturition (scan 2). Previously, this area has been implicated in the process of maintaining continence on the basis of cat experiments. Indeed, increased activation (uncorrected P<0.0001) was found in the right ventral pons (peak activation x = +8 mm, y = -28 mm, z = -28 mm; Z score 3.7; extent in z-axis relative to the line between the anterior and posterior commissure (AC-PC line): -24 to -28 mm (on the basis of an uncorrected threshold of P<0.001); Fig. 5 on the right). In the right inferior frontal gyrus (BA 45 and 47; peak activation x = +50, y = +26, z = 0; Z score 4.3; extent in z-axis 0 to +4 mm) an activation was found also, but this was not significant after a correction for multiple comparisons (corrected P<0.05). Comparing the second scan with the condition during withholding of urine (scan 1), a slight increase (uncorrected P<0.002) in the rcbf was found during the second scan in the right ventral pons (peak activation x = +8, y = -28, z = -28; Z score 2.9). Furthermore, a significant difference (corrected P<0.05) was observed in the right anterior cingulate gyrus (BA 24 and 32; peak activation x = +10, y = +20, z = +24; Z score 4.7; extent in z-axis +24 to +28 mm). Remarkably, the location of the activated area was more posterior and superior than when successful micturition was compared with withholding of urine (Fig. 6 at the bottom). In the inferior frontal gyrus (BA 45 and 47; peak activation x = +50, y = +26, z = 0; Z score 3.4; extent in z- axis 0 to +4 mm) an increase in rcbf was found, but after correction for multiple comparisons (corrected P<0.05) it appeared not to be significant. Comparing scan 3 (Empty bladder 20 minutes) with scan 1 (withholding of urine), a significant difference (corrected P<0.05) was found in the right anterior cingulate gyrus (BA 24 and 32; peak activation x = +8, y = +24, z = +24; Z score 4.6; extent in z-axis +20 to +28 mm). DISCUSSION The present study for the first time demonstrates that specific human brain structures have an increased rcbf during micturition. In all likelihood, these areas are involved in micturition control. There are not many studies on the central nervous system control of micturition in humans. Blaivas (1982), based on patient studies with lesions in brain and spinal cord, concluded that the coordination between bladder (detrusor) and the urethral sphincter takes place in the pons, similar to the organization of micturition in the cat (Holstege et al., 1986; Griffiths et al., 1990). This led us to pay special attention to the brainstem in the present PET study. In general, the results indicate that in the human brainstem micturition seems to be organized in very much the same way as in the cat (Fig. 1). In the following each of the various brain structures, which have been implicated in this study to play a role in human micturition, will be discussed separately. Micturition related areas Dorsomedial pontine tegmentum In the dorsomedial pontine tegmentum a distinct area was activated during micturition in comparison to the condition during withholding of urine (scan 1) or empty bladder (scan 3 and 4). Retrograde and anterograde tracing studies have shown that in the rat and cat a cell group exists in the dorsomedial pons, which is called pontine micturition center (PMC; Loewy et al, 1979) or M-region (Holstege et al., 1986) or Barrington s nucleus (Barrington, 1925). Cells in this region project directly to the sacral intermediolateral cell column (Holstege et al., 1979; Loewy et al., 1979; Holstege et al., 1986), which contains the parasympathetic motoneurons innervating the bladder. In the cat electrical stimulation of the PMC produces an immediate and sharp decrease in urethral pressure and pelvic floor electromyogram, 78
79 PET study on micturition control in men Fig. 6. Top: Significant differences in rcbf in the anterior cingulate gyrus after the comparison Micturition, successful (scan 2) minus Withholding of urine (scan 1). Bottom: Significant difference in rcbf in the anterior cingulate gyrus after the comparison Micturition, not successful (scan 2) minus Withholding urine (scan 1). Threshold uncorrected P< followed in about 2 seconds by a steep rise in the intravesical pressure, mimicking complete micturition. Bilateral lesions in the PMC result in urinary retention, during which detrusor activity is depressed and bladder capacity increases (Holstege et al., 1986; Griffiths et al., 1990). The present PET results strongly suggest that in the dorsomedial pons of humans a similar group of neurons exists. The periaqueductal gray (PAG) In humans the midbrain PAG appears to be more active during micturition than in the conditions withholding of urine or empty bladder. In the cat electrical stimulation of the PAG has been shown to evoke complete micturition (Skultety, 1959), facilitate bladder reflexes, and reduce bladder capacity (Kruse et al, 1990). The PAG receives fibers from the sacral cord (Blok et al., 1995; VanderHorst et al., 1996), possibly conveying information concerning the degree of bladder filling. In turn, the PAG sends fibers to the PMC (Blok and Holstege, 1994). A previous PET study showed in human the PAG is also involved in the perception of visceral pain which appeared in angina pectoris (Rosen et al., 1994). The present PET results suggest that in humans the PAG is also important in the control of micturition. The hypothalamus Hypothalamic involvement in micturition control in humans, as our results implicate, has been in the cat demonstrated by Tang and Ruch (1956). In this animal stimulation of forebrain structures, including the anterior cingulate gyrus, preoptic area of the hypothalamus, amygdala, bed nucleus of the stria terminalis and septal nuclei elicits bladder contractions (Gjone and Setekleiv, 79
80 Chapter ; Gjone, 1966). All these structures give rise to descending pathways to the PAG and other regions belonging to the so-called emotional motor system (Holstege, 1995; Blok and Holstege, 1996). Of only the preoptic area, being the rostral part of the hypothalamus, it has been shown that it projects directly onto the PMC (Holstege, 1987). Although the present PET results suggest that the preoptic area plays also in humans an important role in the control of micturition, its exact role is unknown. One might speculate that the direct influence of the preoptic area on the PMC determines the beginning of micturition. Whether or not micturition takes place is always related to the environment in which the individual is situated. In other words, when a full bladder via its fibers via the sacral cord and via the PAG signals that micturition should occur, it only takes place when forebrain structures have decided that the situation is safe enough to let micturition take place. Right inferior frontal gyrus The present results demonstrate that the right inferior frontal gyrus is significantly activated during micturition. The same region is involved in attention mechanisms (Pardo et al., 1991) and response selection (Jenkins et al., 1994). In respect to micturition, it might play a role in making the decision whether or not micturition should take place at that particular time and place. Right anterior cingulate gyrus The rcbf in the right anterior cingulate gyrus was significantly decreased during withholding of urine (scan 1) in comparison to the conditions during successful micturition (scans 2) or empty bladder (scan 3 and 4). Possibly, the decrease in rcbf in the anterior cingulate gyrus during withholding of urine (scan 1) reflects a general suppression of sensory input and motor output in order to suppress the sensation of a filled bladder and subsequent urge to void. Additionally, the rcbf during withholding of urine (scan 1) is also lower than in the conditions empty bladder (scans 3 and 4). Apparently, the relatively high rcbf during scans 2, 3 and 4 is not specific for response selection or attention, because that was only the case during scan 2 and not during scans 3 and 4. According to Paus et al. (1993) the anterior cingulate gyrus facilitates possible responses on incoming sensory input (see also Pardo et al., 1990, and Jenkins et al., 1994). In other words it might be considered as a level setting system facilitating certain blue prints of behavior. However, in the condition of withholding of urine the reverse of facilitating of a response is needed, i. e. the urge of voiding has to be inhibited. This might explain the decreased rcbf in the anterior cingulate gyrus during withholding of urine even when compared to the empty bladder condition. On the other hand, lesions in the forebrain including the anterior cingulate gyrus have been reported to cause urge incontinence (Andrew and Nathan, 1964; Maurice-Williams, 1974). This would favour the idea of the anterior cingulate gyrus also plays a specific role in micturition control. Observations during not successful micturition Seven volunteers were not able to micturate. In this group the ventral pontine tegmentum showed increased rcbf during scan 2 in comparison to the other three scans (1, 3, and 4). During the second scan thevolunteers of the not succesful micturition group, probably because of emotional reasons, contracted their urethral sphincter and withheld their urine, although they had a full bladder and tried to urinate. At first glance the condition withholding of urine (scan 1) is the same as scan 2, when the volunteers also withhold their urine. The difference is that 80
81 PET study on micturition control in men the volunteers, albeit involuntarily, powerfully contract their urethral sphincter, much stronger than during the withholding of urine condition of scan 1. From cat experiments it is known that a distinct cell group in the ventrolateral pontine tegmentum has been shown to be able to powerfully contract the bladder sphincter (Holstege et al., 1986). This cell group located ventrolateral to the PMC, is called L- region and projects to the nucleus of Onuf in the sacral cord (Holstege et al., 1979; 1986). The nucleus of Onuf contains motoneurons of the pelvic floor muscles including the external urethral sphincter. Electrical stimulation of the L-region produces closure of the sphincter, and bilateral lesions result in a condition that can best be explained as an extreme kind of urge -incontinence (Holstege et al., 1986; Griffiths et al., 1990). Possibly, the L-region plays an important role in the storage of urine. This led to the prediction by the authors that in the ventral pontine tegmentum in the not successful micturition group a human homologue of the cat s L- region might be present, and, as described in the present paper, the PET results corroborate this idea. Similar to the findings in the group of successful micturition, the anterior cingulate gyrus showed decreased rcbf during the first scan in comparison to the other scans, and the prefrontal cortex showed increased rcbf. The possible involvement of these areas in more general mechanisms than in the regulation of micturition itself has been discussed in the previous paragraph. In summary, this study suggests that in humans, similar to the cat, the dorsomedial and ventral pontine areas are involved in micturition and maintaining continence, respectively. The distance between the peak activation of these areas in the pons was more than 15 mm, well within the resolution of the scanner, which is approximately 6 mm in the x, y and z direction, and within in the smoothing limits of the apllied Gaussian filter of 8 mm in the x, y and z direction. Lateralized activation A striking observation was that the micturition control areas were found predominantly on the right side of the brain (frontal and cingulate cortices) and brainstem (pontine areas). This finding corresponds with studies reporting that urge incontinence is specifically correlated with lesions in the right hemisphere (Maurice-Williams, 1974; Kuroiwa et al., 1987). The importance of the right side of the brain in autonomic behavior in general is also indicated by a PET study on human respiration (Corfield et al., 1995), who found an increase in rcbf in the anterior cingulate gyrus, predominantly at the right side. A SPECT (= Single Photon Emission Computer Tomography) study observed an increase in the right prefrontal cortex during male orgasm (Tiihonen et al., 1994). Future studies using dynamic imaging techniques are underway to find out whether the micturition control structures in the brain, as demonstrated in the present study, are dysfunctional in patients with urge incontinence. 81
82
83 PET study on brain control of pelvic floor muscles Chapter 9 A PET Study on Cortical and Subcortical Control of Pelvic Floor Musculature in Women Bertil F.M. Blok, Leontien M. Sturms, and Gert Holstege J. Comp. Neurol. 389: (1997) ABSTRACT The pelvic floor musculature plays an important role in many behaviors, such as defecation, micturition, mating behavior, and vomiting. A recent positron emission tomography (PET) study revealed that structures belonging to the emotional motor system are involved in the control of the pelvic floor during micturition. However, there also exist brain structures involved in the voluntary motor control of the pelvic floor and the present PET study was designed to identify these structures. Six adult female volunteers were scanned with the bolus injection of H 15 2 O during the following four conditions: (1) rest; (2) repetitive pelvic floor straining; (3) sustained pelvic floor straining; (4) sustained abdominal straining. The results revealed that the superomedial precentral gyrus, the most medial portion of the motor cortex, is activated during pelvic floor contraction and the superolateral precentral gyrus during contraction of the abdominal musculature. In these conditions significant activations were also found in the cerebellum and thalamus. No activations were found in subcortical structures belonging to the emotional motor system. INTRODUCTION In humans the motor cortex is crucial in voluntary motor control, but other areas in the brain are involved in motor activities related to emotional behavior. These areas form the so-called emotional motor system (Holstege, 1997). An example of a motor activity which is strongly influenced by the emotional motor system is micturition (Blok and Holstege, 1996). During micturition the coordination of the smooth detrusor muscle of the bladder and its external striated muscle sphincter does not take place in the sacral spinal cord or in the cerebral cortex but in the so-called pontine micturition center (PMC) in the pons (in the cat Barrington, 1925; De Groat, 1975; Holstege et al., 1986; in humans Blok et al., 1997). The PMC, in turn, is not under control of the motor cortex but of structures belonging to the emotional motor system, such as the periaqueductal gray (in the cat Blok and Holstege, 1994; Blok et al., 1995; in humans Blok et al., 1997) and the dorsolateral part of the preoptic area of the hypothalamus (in the cat Holstege, 1987; in humans Blok et al., 1997). The striated muscle of the bladder sphincter is one of a group of muscles forming the pelvic floor. The pelvic floor not only takes part in micturition and defecation, but also in mating behavior, another component of the emotional motor system (Van der Horst and Holstege, 1995). However, the pelvic floor can also be activated voluntarily, for example during voluntary abdominal or pelvic straining on command. In our search to the supraspinal organization of the pelvic floor in general, in a study using Positron Emission Tomography (PET) scanning, in human subjects the brain regions were identified that were activated 83
84 Chapter 9 during voluntary contraction of the pelvic floor. PET scanning is a non-invasive technique, which is used to identify sites of locally increased blood flow (Fox and Minton, 1989). The regional cerebral blood flow (rcbf) is used as an index for the presynaptic activity in the area under consideration during various physiological conditions (Jueptner and Weiller, 1995). Since the pelvic floor is often used in combination with abdominal muscles, for example during abdominal straining, the volunteers were also asked to use their abdominal muscles in a separate condition. The results not demonstrate which brain areas are active during voluntary control of the pelvic floor, but also show the differences between these pelvic areas and regions involved in the voluntary control of the abdominal muscles. MATERIALS AND METHODS Subjects The 6 volunteers were females between 21 and 24 years of age. They all completed a general health questionnaire. Volunteers, who reported a history of any neurologic, psychiatric or gastroenterologic illness, were excluded from the study. All subjects were right-handed, and gave their written informed consent, according to the declaration of Helsinki. The protocol of the study was approved by the research ethics committee of the University Hospital of Groningen. During each scan the lights were dimmed, the subjects had their eyes closed and they did not move. Each scanning session consisted of four measurements and lasted 1.5 hours in total. Experimental protocol and training Acquisition of scanning data was planned during the following 4 conditions: Condition 1. Rest, no task was given, Condition 2. Repetitive pelvic floor straining, Condition 3. Sustained pelvic floor straining, Condition 4. Sustained abdominal straining. It is important to realize that for each volunteer an unbiased sequence of conditions was determined using a randomized number generator. Prior to a scanning session the volunteers were instructed about this sequence. Twenty three seconds before the beginning of scans 2, 3 and 4 the volunteers received a verbal command to start the task. Prior to the rest condition no assignment was given. Before the scanning session, all the volunteers had practiced the tasks. For each volunteer this was done in two sessions: one session at the subjects home under the guidance of one of the authors (L.M.S.), and another session prior to the scanning procedure at the PET center in Groningen. For the pelvic floor straining conditions, the volunteers were asked to contract their pelvic floor or their anal sphincter. For the abdominal straining condition the volunteers were asked to contract their abdominal muscles as if they would begin to defecate. They were explicitely asked to breath normally and not to move their legs, thighs, glutei muscles, and, during the pelvic floor conditions, their abdominal muscles. During the session at the subjects home the whole procedure was extensively explained and after the scanning session the volunteers reported that they had no problems to contract their anal sphincter. The muscle activity during rest, pelvic and abdominal straining was recorded continuously with bipolar surface electromyographic (EMG) electrodes. The left and right abdominal electrodes were placed 3 cm from the midline and 3 cm below the costal margin. The pelvic floor electrodes were placed 2 cm lateral from the anal orifice. The EMG signals were processed using an ambulatory differential voltage amplifier UPS 2020 (Medical Measure- 84
85 PET study on brain control of pelvic floor muscles ment Systems, The Netherlands), bandwidth Hz. The signal was, after amplification, full-wave rectified and averaged. PET scans The subjects were placed in a horizontal position in the PET camera (Siemens-CTI 951/31, Knoxville, TN, USA) parallel to and 7 cm below the orbitomeatal line as determined by external examination. Because of the technical characteristics of the PET camera, the most caudal limit of the scanned area was the midbrain, and the most rostral limit included the total cerebral cortex. This resulted in images extending between -20 mm below and 76 mm above the intercommissural plane. This implied that the caudal cerebellum and brainstem regions as pons and medulla were not part of the investigated brain areas. An individually constructed head mold was used to minimize head movement between sessions. First, a transmission scan of 20 minutes was made for attenuation correction of the emission scans. Subsequently, the four scans were carried out. Before each scan, the subjects were given 1.85 GBq of H2 15 O diluted in saline. The H2 15 O bolus was injected in the right brachial vein, followed by 40 ml saline from an automatic pump. Data acquisition continued during 90 seconds, and was initiated 23 seconds after the beginning of the injection, at which time the peak in radioactivity was assumed to reach the cerebral blood flow. To allow the radiation to reach background levels, there was a 15 minutes interval between the injections. Data analysis The data of each scan were summated and the resulting images were centered to prevent loss of information during sampling. Prior to the statistical procedure the data were sampled to a voxel size of 2.2 x 2.2 x 2.4 mm. The data were further analyzed using the Statistical Parametric Mapping procedure (SPM96 from the Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks Inc. Sherborn MA, USA) on a SPARC workstation (Sun Microsystems Inc., Surrey, UK). The SPM96 software was used for the anatomical realignment, normalization, smoothing and statistical analysis. Anatomical realignment The rcbf was reconstructed for each scan, using an attenuation correction based on the transmission scan. Movement-related components between scans were corrected during the realignment, using the first recorded scan of each subject as a reference. Normalization Following realignment all images were transformed into a stereotactic standard space (Talairach and Tournoux, 1988). This normalizing spatial transformation matches each scan to a reference or template image that conforms to the standard space (Friston et al., 1991a). Stereotactic normalization of PET images allows comparison of scan data in identical pixels across different subjects and scans. Smoothing Prior to the statistical analysis the scans were smoothed in order to increase signal to noise ratio and to suppress effects due to residual differences in functional and gyral anatomy between subjects during inter-subject averaging. During smoothing a Gaussian filter of 12 x 12 x 12 mm was used (full width, half maximum in the x, y and z axes, respectively). Statistical analysis Differences in global activity within and between subjects were removed by analysis of covariance on a pixel-by-pixel basis with global counts as covariate. This was 85
86 Chapter 9 Table 1 Areas with Significant Increased rcbf x, y, z peak Z-score Repetitive pelvic floor straining - rest Motor related areas Superolateral precentral gyrus (4) right Superomedial precentral gyrus (4) midline Superomedial precentral gyrus (4) midline Thalamus left Cerebellum midline Other areas Superior frontal gyrus (8) right Medial frontal gyrus (mfg; 9) right Temporal lobe (39) right Ant. cingulate (32) & mfg (10) right Sustained pelvic floor straining - rest Motor related areas Superolateral precentral gyrus (4) right Superomedial precentral gyrus (4) midline Thalamus left Cerebellum midline Other areas Superior frontal gyrus (8) right Medial frontal gyrus (9) right Anterior cingulate (32) & mfg (10) right Sustained abdominal straining - rest Motor related areas Superolateral precentral gyrus (4) right Superolateral precentral gyrus (4) left Superomedial precentral gyrus (4) midline Supplementary motor area (6) right Cerebellum midline Thalamus left Other areas Superior frontal gyrus (8) right Sust. abdom. straining - repetit. pelvic straining Superolateral precentral gyrus (4) right Superolateral precentral gyrus (4) left Medial & Superior frontal gyrus (6) midline The location of the peak activations is indicated in millimeters by the coordinates x, y and z according to the atlas of Talaraich and Tournoux (1988). Coordinates in standard stereotactic space: x is distance to right (+) or left (-) of the midsagittal line; y is distance anterior (+) or posterior (-) to vertical plane through the anterior commissure; z is the distance superior (+) or inferior (-) to the intercommissural (AC-PC) line. The Brodmann area is given (where appropriate) in parentheses. Significant activations include Z-scores of 3.0 (P<0.001) or larger, trends in activation include Z- scores between 2.6 and
87 PET study on brain control of pelvic floor muscles done because inter- and intra-subject differences in global activity may obscure regional alterations in activity following cognitive processes (Friston et al., 1990). For each pixel in the stereotactic space the analysis of covariance (ANCOVA) generated a condition-specific adjusted mean rcbf value (normalized to 50 ml/dl/min) and an associated adjusted error variance. A repeated-measures ANCOVA was used for the comparison of the straining conditions with the rest condition, each subject being studied under all conditions. The ANCOVA allows comparison of the means across conditions using the t statistic. The resulting set of t values constitutes a statistical parametric map (SPM{t}) (Friston et al., 1991b). An uncorrected P-value of (Z= 3.0) was used. This (omnibus) approach can be used when the location of the area, in which activation is expected, is known (Friston et al., 1991b). Trends in activation are reported when they reach a significance level of P<0.005 (Z = 2.6). This level of significance protects sufficiently against false positives (Warburton et al., 1996). RESULTS Subjects comments Before the scanning the volunteers confirmed that they had an empty bladder and rectum. During scanning they were awake with their eyes closed and were in no discomfort. After the scanning session all volunteers were confident that the tasks were well performed. EMG pattern of muscle contraction The EMG records of the rectus abdominis and external anal sphincter muscles of one volunteer during the various tasks and scanning are shown in Fig. 1. EMG recorded from the abdominal musculature during rest showed respiratory activity. The strongest amplitude was observed during abdominal straining, but the signal was also increased during sustained pelvic floor contraction. The abdominal EMG signal during repetitive pelvic straining differed only slightly from the abdominal EMG signal during rest. The EMG signal from the external anal sphincter was always increased during all three conditions (repetitive and sustained pelvic floor, and abdominal straining) in comparison to the rest condition. Regional cerebral blood flow Repetitive pelvic floor contraction versus rest condition The strongest significant activation (uncorrected P<0.001) was found in the vermis of the cerebellum (Table 1; Figure 2). Significant focal activity was also found in the superomedial precentral gyrus, possibly representing activation in the primary motor cortex (M1), the superior frontal gyrus and left anterior thalamus. Trends in increased rcbf (0.001<P<0.005) were found in the right anterior cingulate gyrus and superolateral precentral gyrus. Sustained pelvic floor contraction versus rest condition The strongest significant activation (uncorrected P<0.001) was found in superolateral precentral gyrus and the vermis of the cerebellum (Table 1). Other significant activated areas were the superomedial precentral gyrus, superior frontal gyrus, and the right anterior cingulate gyrus. The rcbf increase in the left anterior thalamus was only slight (0.005<P<0.01; Table 1). Sustained abdominal straining versus rest condition The strongest significant increase in rcbf was found the right superolateral precentral gyrus (Figure 3). Furthermore, significantly increased rcbf was found in the superior frontal gyrus and frontal areas just rostral from the primary motor cortex. The latter areas possibly represent premotor 87
88 Chapter 9 Fig. 1. Significant differences in regional cerebral blood flow (rcbf) for the comparison repetitive pelvic floor straining minus rest projected on coronal slices. Pixels are displayed at a threshold of an uncorrected P< The numbers on the slices refer to the distance in millimeters relative to the vertical plane through the anterior commissure. The numbers on the colour scale refer to the corresponding Z-scores. Note the activations in the primary motor cortex in -20 to -32, the left thalamus in 0 to -8, and the cerebellum in -52 and
89 PET study on brain control of pelvic floor muscles Fig. 2. Significant differences in regional cerebral blood flow (rcbf) for the comparisons between the 3 straining conditions and rest, projected on the superior surface of the brain (top), the medial surface of the left hemisphere (middle), and the medial surface of the right hemisphere (bottom). The shown differences include all activations located 5 mm in front of the shown surface of the brain, and 20 mm behind this surface. and supplementary motor areas. Trends in increased rcbf (0.001<P<0.005) were observed in the left anterior thalamus and in the vermis of the cerebellum. No increase in rcbf was found in the anterior cingulate gyrus. Abdominal straining versus pelvic floor straining In order to find the exact location of the part of the primary motor cortex involved in abdominal muscle control a comparison was made between the conditions during abdominal straining and pelvic straining. The abdominal straining condition minus the pelvic floor straining revealed that a significant increase was found bilaterally in the right and left primary motor cortex (M1) 3 cm lateral to the midline (Figure 4). studies have reported that the superolateral precentral gyrus is involved in the control of respiration (Colebatch et al., 1991; Ramsay et al., 1993). 89
90 Chapter 9 Fig. 3. Differences in rcbf for the comparison sustained abdominal straining minus rest projected on coronal slices. Note that the activations in the primary motor cortex in planes -12 to -32 extend much more laterally than in Figure 1. 90
91 PET study on brain control of pelvic floor muscles Fig. 4. Significant activations in the precentral gyrus for the comparisons sustained abdominal wall straining minus rest (left), repetitive pelvic floor straining minus rest (middle), and sustained abdominal wall straining minus repetitive pelvic floor (right) projected on horizontal slices. The arrows in the planes of the right column indicate the bilateral difference in the superolateral precentral gyrus, which is related to abdominal straining. 91
92 Chapter 9 DISCUSSION The pelvic floor consists of striatal muscles that play a role in voluntary as well as in autonomic motor activities. Examples of the latter are micturition and sexual behavior. In a previous PET-scan study (Blok et al., 1997) it was found that during micturition in human males the same brainstem regions were active as in cats. In that study, the voluntary contol of the pelvic floor was not studied. It is possible, albeit difficult, to voluntarily interrupt micturition by contracting the pelvic floor. Also in other circumstances voluntary contraction of the pelvic floor is possible, for example during activities related to abdominal straining. The present PET-scan study investigated which brain areas are activated during voluntary contraction of the pelvic floor. Technical aspects Due to the technical limitations of the camera used in this study, it was necessary to pool data from different subjects and use stereotactic normalization of brain shape in order to detect significant increases resulting from the experiments. Inevitably, such normalization results in some loss of resolution of the specific sites of increased rcbf, but it was very well possible to identify significant local differences in rcbf. Primary motor cortex activations Abdominal motor area in the superolateral precentral gyrus The present PET results found increased rcbf during abdominal straining bilaterally in the superolateral precentral gyrus about 3 cm lateral to the midline. Woolsey et al. (1979) localized their abdominal motor area in the same region, 2 cm from the midline. According to Krause (1911) and Foerster (1936) is the abdominal muscle area located between the arm and leg areas of the primary motor cortex, and electrical stimulation in this area in lightly or locally anesthetized humans evoked abdominal straining. Focal magnetic brain stimulation on the scalp over the same region elicited a strong respons of the rectus abdominis (Carr et al., 1994). Other PET studies have reported that the superolateral precentral gyrus is involved in the control of respiration (Colebatch et al., 1991; Ramsay et al., 1993). Pelvic floor motor area in the superomedial precentral gyrus The present results point to this part of the primary motor, but the classical electrical stimulation studies on the localization of the human motor cortex failed to report a motor cortex area specifically related to the pelvic floor motor (Krause, 1911; Foerster, 1936; Penfield and Boldrey, 1937; Woolsey et al., 1979). The reason for this failure might be quite simple. The superomedial precentral gyrus inhumans is located on the medial surface of the cortex, against which the sagittal sinus with its veins is located in the longitudinal fissure. This makes electrical stimulation in this medial cortical area rather difficult (Penfield and Boldrey, 1937). Another difficulty in determining the pelvic floor region of the human motor cortex is that the much larger leg motor area in this region seems to vary considerably among individuals (Penfield and Boldrey, 1937; Woolsey et al., 1979). On the other hand, animal experiments leave no doubt that a pelvic floor motor cortex exists. Focal electrical stimulation of the dog s medial sigmoid gyrus evoked contraction of the anal and bladder sphincters (Franck, 1887; Von Bechterew and Meyer, 1893). Stimulation of the upper part of the precentral gyrus of the monkey (Sherrington, 1892; Vogt and Vogt, 1907) and chimpanzee (Grünbaum and Sherrington, 1904) resulted in protrusion of the anal canal and closure of the anal sphincter. Clinical studies on World War I injured 92
93 PET study on brain control of pelvic floor muscles soldiers suggested that in humans a cortical pelvic floor motor area was located close to the hip motor area between the arm and leg motor areas in the superior precentral gyrus. It would control the external sphincter muscle during conscious withholding of urine and interruption of micturition (Pfeifer, 1918). Bilateral destruction of this area was accompanied by urine retention due to a hyperrefexia of the pelvic floor musculature, causing inability of the bladder to relax. Usually this condition was combined with a bilateral spastic paralysis of the lower limbs, which combination is known as the paracentral lobule syndrome (Nathan, 1976). Non-invasive transcranial electrical stimulation of the primary motor cortex detected the optimal response of the external anal sphincter after stimulation in the midline of the skull (Merton, 1985; Ertekin et al., 1990). No rcbf differences during the muscle contraction tasks in comparison with the rest condition were observed in the area deeper in the medial motor cortex, i.e. just superior to the cingulate gyrus. This is important because this region has been implicated in the control of the bladder and rectum (Foerster, 1936). Activations in other motor related areas Significant activation during sustained and repetitive pelvic floor straining, and non significantly, during abdominal straining, was found in the thalamus and cerebellum. Given the resolution of the scanner, it was not possible unequivocally to determine which thalamic nuclei are activated, although the impression was gained that the peak activation was in the ventrolateral nucleus. In other PET studies these same regions in cerebellum and thalamus were found to be activated during motor related activities, as respiration (Colebatch et al., 1991; Ramsay et al., 1993), movements of the arm (Fox et al., 1985; Colebatch et al., 1991; Grafton), and leg movements (Fink et al., 1995). Especially during abdominal straining an area was activated rostral to the activated area in the superomedial precentral gyrus. It probably represents the part of Brodmann s area 6, that is called the supplementary motor area (SMA), or, more precisely, SMA proper. This region is thought to be involved in the execution of simple tasks (Picard and Strick, 1996), of which the straining tasks of the present study might be an example. Activation in the right anterior cingulate gyrus This area was significantly activated during repetitive and sustained pelvic floor straining, but not during abdominal straining. Usually, the pelvic floor is tonically contracted, and the individual is unaware of this. This might be the reason that voluntary contraction of the pelvic floor on the top of this tonical contraction is more difficult than voluntary contraction of the abdominal wall. The anterior cingulate gyrus has been implicated in many motor behaviors, but especially when it concerns a novelt and difficult task (Jenkins et al., 1994). The anterior cingulate gyrus has also been implicated in attention to body parts (Pardo et al., 1991). An example is the bladder. Andrew and Nathan (1964) reported that lesions in the anterior cingulate gyrus and the adjacent superior prefrontal cortex can cause urge incontinence. According to our PET study (Blok et al., 1997) the same part of the anterior cingulate gyrus as indicated by Andrew and Nathan plays a role in micturition. The same study, however, indicated that the anterior cingulate gyrus is not specifically involved in micturition control, but in more general control mechanisms, like goal directed attention and alertness. Thus, urge incontinence due to lesions in the anterior cingulate gyrus is the result of a general 93
94 Chapter 9 indifference of the patients, including indifference to a full bladder. Voluntary and unvoluntary pelvic floor control and micturition Although it can be concluded that the superomedial part of the precentral gyrus is involved in the control of the pelvic floor musculature, it should be emphasized that this cortical area is important for the conscious withholding of urine and subsequent suppression of the micturition reflex. This voluntary control mechanism is not involved in the control of micturition itself, because the micturition reflex is under unvoluntary control of structures belonging to the emotional motor system (Holstege, 1997). The previous PET study on micturition in human males has shown that the preoptic area of the hypothalamus and the periaqueductal gray (PAG) are activated during micturition (Blok et al., 1997). The same PET study demonstrated that another brain structure in the caudal brainstem, the so-called L-region or pontine storage center, is activated during unvoluntary and possibly also tonic contraction of the pelvic floor (Blok et al., 1997). Motor cortex involvement in micturition control Foerster (1936) reported that electrical stimulation of the pre- and postcentral gyrus just superior to the cingulate gyrus in humans resulted in an effect on the muscles of the bladder and rectum. One year later, Penfield and Boldrey (1937) found that electrical stimulation in the postcentral gyrus close to the cingulate gyrus evoked sensations of the genitalia, rectum, and buttock, but they could not evoke bladder or rectal contractions after stimulation in the corresponding precentral motor area. It seems that the cortical area close to the cingulate gyrus is primarily concerned with sensory information of the pelvic organs. This might explain why lesions in this area can result in urge incontinence (Kleist, 1918; Adler, 1919), since patients are not aware of a filled bladder or rectum. However, the micturition reflex itself is still intact in these patients. 94
95 PET study on micturition control in women Chapter 10 Brain Activation during Micturition in Women Bertil F.M. Blok, Leontien M. Sturms, and Gert Holstege Brain, in press ABSTRACT Experiments in the cat have led to a concept on how the central nervous system controls micturition. In a previous study (Brain 1997; 120: ) this concept was tested in a PET study in male volunteers. It was demonstrated that specific brainstem and forebrain areas are activated during micturition. It was unfortunate that this study did not involve women, because the results are important for understanding urge incontinence, which occurs more frequently in women than in men. Therefore, a similar study was done in eighteen right handed women, who were scanned during the following four conditions: (1) 15 minutes prior to micturition (urine withholding); (2) during micturition; (3) 15 minutes after micturition, and (4) 30 minutes after micturition. Of the eighteen volunteers ten were able to micturate during scanning, eight were not, despite trying vigorously. Micturition appeared to be associated with significantly increased blood flow in the right dorsal pontine tegmentum and the right inferior frontal gyrus. Decreased blood flow was found in the right anterior cingulate gyrus during urine withholding. The eight volunteers, who were not able to micturate during scanning, did not show significantly increased rcbf in the right dorsal, but in the right ventral pontine tegmentum. In the cat this region controls the motoneurons of the pelvic floor. In the same unsuccessful micturition group increased blood flow was also found in the right inferior frontal gyrus. In all eighteen volunteers, during the period they had to withhold their urine prior to the micturition condition, decreased blood flow in the right anterior cingulate gyrus was found. The results suggest that in men as well as in women the same specific nuclei exist in the pontine tegmentum responsible for the control of micturition. The results also indicate that the cortical and pontine micturition sites are more active on the right than on the left side. INTRODUCTION Micturition or urination is a coordinated action between the urinary bladder and its external urethral sphincter. When the bladder contracts, the sphincter relaxes. Although the motoneuronal cell groups of both bladder and sphincter are located in the sacral spinal cord, their coordination takes place in the pons. This brainstem organization is best shown in patients with spinal cord injuries above the sacral level. They have great difficulty emptying the bladder, because when their bladder contracts, their urethral sphincter contracts also, a disorder called detrusor-sphincter dyssynergia. Such disorders never occur in patients with neurologic lesions rostral to the pons, which indicates that the coordinating neurons are located in the pontine tegmentum (Blaivas, 1982). As early as 1925 Barrington in the cat showed that the neurons involved in micturition control are probably located in the dorsolateral part of the pontine tegmentum, because bilateral lesions in this area produced an inability to empty the bladder leading to urinary retention. Tracing studies in cat (Holstege et al., 1979) and rat (Loewy et al., 1979) revealed that a distinct cell group in the dorsal pontine tegmentum, called Barrington s area or pontine micturition center (PMC) or M-region, projects to the sacral cord intermediolateral cell col- 95
96 Chapter 10 Fig. 1. Left: Significant differences in rcbf in the right dorsal pontine tegmentum (indicated by pmc = pontine micturition center) after the comparison between the conditions Successful micturition (scan 2) and Empty bladder (scan 3). Right: Significant differences in rcbf in the right ventral pontine tegmentum (indicated by L-region) after the comparison between the conditions Unsuccessful micturition (scan 2) and the condition Empty bladder (scan 3). Threshold used for display uncorrected P< The number -28 refers to the distance in millimeters relative to the horizontal plane through the anterior and posterior commissures (z-direction). The numbers on the color scale refer to the corresponding Z-scores. L = left side of the brain; R = right side. Fig. 2. Significant differences in rcbf in cortical areas after the comparison between the conditions Successful micturition (scan 2) and Urine withholding (scan 1). Note the activations in the right anterior cingulate gyrus (acg) in z planes +8 to +16, and the right inferior frontal gyrus (gfi) in the z- planes 0 to +12. For other details see Fig
The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten
 University of Groningen The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen. Neuronal control of micturition Kuipers, Rutger
 University of Groningen Neuronal control of micturition Kuipers, Rutger IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Neuronal control of micturition Kuipers, Rutger IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen. Neuronal control of micturition Kuipers, Rutger
 University of Groningen Neuronal control of micturition Kuipers, Rutger IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Neuronal control of micturition Kuipers, Rutger IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten
 University of Groningen The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten IMORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten IMORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen. Neuronal control of micturition Kuipers, Rutger
 University of Groningen Neuronal control of micturition Kuipers, Rutger IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Neuronal control of micturition Kuipers, Rutger IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
The central control of micturition and continence: implications for urology
 BJU International (1999), 83, Suppl. 2, 1 6 The central control of micturition and continence: implications for urology B.F.M. BLOK and G. HOLSTEGE Department of Anatomy and Embryology, Faculty of Medical
BJU International (1999), 83, Suppl. 2, 1 6 The central control of micturition and continence: implications for urology B.F.M. BLOK and G. HOLSTEGE Department of Anatomy and Embryology, Faculty of Medical
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther
 University of Groningen Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Neural Control of Lower Urinary Tract Function. William C. de Groat University of Pittsburgh Medical School
 Neural Control of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Medical School Disclosures Current funding: NIH Grants, DK093424, DK-091253, DK-094905, DK-090006. Other financial
Neural Control of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Medical School Disclosures Current funding: NIH Grants, DK093424, DK-091253, DK-094905, DK-090006. Other financial
Table of Contents: Chapter 1 The organization of the spinal cord Charles Watson and Gulgun Kayalioglu
 Table of Contents: Chapter 1 The organization of the spinal cord Charles Watson and Gulgun Kayalioglu The gross anatomy of the spinal cord Spinal cord segments Spinal nerves Spinal cord gray and white
Table of Contents: Chapter 1 The organization of the spinal cord Charles Watson and Gulgun Kayalioglu The gross anatomy of the spinal cord Spinal cord segments Spinal nerves Spinal cord gray and white
Spinal Cord Organization. January 12, 2011
 Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Micturition and the Soul
 THE JOURNAL OF COMPARATIVE NEUROLOGY 493:15 20 (2005) Micturition and the Soul GERT HOLSTEGE* Department of Anatomy and Embryology, University Medical Center Groningen, University of Groningen, 9713 AV
THE JOURNAL OF COMPARATIVE NEUROLOGY 493:15 20 (2005) Micturition and the Soul GERT HOLSTEGE* Department of Anatomy and Embryology, University Medical Center Groningen, University of Groningen, 9713 AV
The Nervous System: Sensory and Motor Tracts of the Spinal Cord
 15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
Chapter 16. APR Enhanced Lecture Slides
 Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
University of Groningen. Functional outcome after a spinal fracture Post, Richard Bernardus
 University of Groningen Functional outcome after a spinal fracture Post, Richard Bernardus IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
University of Groningen Functional outcome after a spinal fracture Post, Richard Bernardus IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
THE BACK. Dr. Ali Mohsin. Spinal Cord
 Spinal Cord THE BACK Dr. Ali Mohsin The spinal cord is the elongated caudal part of the CNS. It starts as the inferior continuation of the medulla oblongata at the level of foramen magnum, & ends as an
Spinal Cord THE BACK Dr. Ali Mohsin The spinal cord is the elongated caudal part of the CNS. It starts as the inferior continuation of the medulla oblongata at the level of foramen magnum, & ends as an
The Nervous System: Autonomic Nervous System Pearson Education, Inc.
 17 The Nervous System: Autonomic Nervous System Introduction The autonomic nervous system: Functions outside of our conscious awareness Makes routine adjustments in our body s systems The autonomic nervous
17 The Nervous System: Autonomic Nervous System Introduction The autonomic nervous system: Functions outside of our conscious awareness Makes routine adjustments in our body s systems The autonomic nervous
Biology 218 Human Anatomy
 Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
CHAPTER 15 LECTURE OUTLINE
 CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
Brain Stem and cortical control of motor function. Dr Z Akbari
 Brain Stem and cortical control of motor function Dr Z Akbari Brain stem control of movement BS nuclear groups give rise to descending motor tracts that influence motor neurons and their associated interneurons
Brain Stem and cortical control of motor function Dr Z Akbari Brain stem control of movement BS nuclear groups give rise to descending motor tracts that influence motor neurons and their associated interneurons
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD
 AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
ParasymPathetic Nervous system. Done by : Zaid Al-Ghnaneem
 ParasymPathetic Nervous system Done by : Zaid Al-Ghnaneem In this lecture we are going to discuss Parasympathetic, in the last lecture we took sympathetic and one of the objectives of last lecture was
ParasymPathetic Nervous system Done by : Zaid Al-Ghnaneem In this lecture we are going to discuss Parasympathetic, in the last lecture we took sympathetic and one of the objectives of last lecture was
Sympathetic Nervous System
 Sympathetic Nervous System Lecture Objectives Review the subdivisions of the nervous system. Review the general arrangement and compare the sympathetic and parasympathetic parts. Describe the following
Sympathetic Nervous System Lecture Objectives Review the subdivisions of the nervous system. Review the general arrangement and compare the sympathetic and parasympathetic parts. Describe the following
Spinal Cord H. Ruth Clemo, Ph.D.
 Spinal Cord H. Ruth Clemo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be familiar with: 1. Surface anatomy of the spinal cord. 2. Internal structure and organization
Spinal Cord H. Ruth Clemo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be familiar with: 1. Surface anatomy of the spinal cord. 2. Internal structure and organization
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Apoptosis in (pre-) malignant lesions in the gastro-intestinal tract Woude, Christien Janneke van der
 University of Groningen Apoptosis in (pre-) malignant lesions in the gastro-intestinal tract Woude, Christien Janneke van der IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Apoptosis in (pre-) malignant lesions in the gastro-intestinal tract Woude, Christien Janneke van der IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Lecturer. Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014
 Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Systems Neuroscience November 21, 2017 The autonomic nervous system
 Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Composed by Natalia Leonidovna Svintsitskaya, Associate professor of the Chair of Human Anatomy, Candidate of Medicine
 Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
The Nervous System: Autonomic Nervous System
 17 The Nervous System: Autonomic Nervous System PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction The autonomic nervous system functions
17 The Nervous System: Autonomic Nervous System PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction The autonomic nervous system functions
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
Neural control of the lower urinary tract in health and disease
 Neural control of the lower urinary tract in health and disease Jalesh N. Panicker MD, DM, FRCP Consultant Neurologist and Clinical lead in Uro-Neurology The National Hospital for Neurology and Neurosurgery
Neural control of the lower urinary tract in health and disease Jalesh N. Panicker MD, DM, FRCP Consultant Neurologist and Clinical lead in Uro-Neurology The National Hospital for Neurology and Neurosurgery
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Orthotic interventions to improve standing balance in somatosensory loss Hijmans, Juha
 University of Groningen Orthotic interventions to improve standing balance in somatosensory loss Hijmans, Juha IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
University of Groningen Orthotic interventions to improve standing balance in somatosensory loss Hijmans, Juha IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
Neural control of the lower urinary tract
 Neural control of the lower urinary tract Jalesh N. Panicker Consultant Neurologist and Honorary Senior Lecturer The National Hospital for Neurology and Neurosurgery and UCL Institute of Neurology Queen
Neural control of the lower urinary tract Jalesh N. Panicker Consultant Neurologist and Honorary Senior Lecturer The National Hospital for Neurology and Neurosurgery and UCL Institute of Neurology Queen
University of Groningen. Symptomatic and asymptomatic airway hyperresponsiveness Jansen, Desiree
 University of Groningen Symptomatic and asymptomatic airway hyperresponsiveness Jansen, Desiree IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
University of Groningen Symptomatic and asymptomatic airway hyperresponsiveness Jansen, Desiree IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
Citation for published version (APA): Portman, A. T. (2005). Parkinson's Disease: deep brain stimulation and FDOPA-PET Groningen: s.n.
 University of Groningen Parkinson's Disease Portman, Axel Tiddo IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Parkinson's Disease Portman, Axel Tiddo IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
The psychophysiology of selective attention and working memory in children with PPDNOS and/or ADHD Gomarus, Henriette Karin
 University of Groningen The psychophysiology of selective attention and working memory in children with PPDNOS and/or ADHD Gomarus, Henriette Karin IMPORTANT NOTE: You are advised to consult the publisher's
University of Groningen The psychophysiology of selective attention and working memory in children with PPDNOS and/or ADHD Gomarus, Henriette Karin IMPORTANT NOTE: You are advised to consult the publisher's
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Regulation of the Urinary Bladder Chapter 26
 Regulation of the Urinary Bladder Chapter 26 Anatomy 1. The urinary bladder is smooth muscle lined internally by transitional epithelium and externally by the parietal peritoneum. Contraction of the smooth
Regulation of the Urinary Bladder Chapter 26 Anatomy 1. The urinary bladder is smooth muscle lined internally by transitional epithelium and externally by the parietal peritoneum. Contraction of the smooth
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Major role of the extracellular matrix in airway smooth muscle phenotype plasticity Dekkers, Bart
 University of Groningen Major role of the extracellular matrix in airway smooth muscle phenotype plasticity Dekkers, Bart IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Major role of the extracellular matrix in airway smooth muscle phenotype plasticity Dekkers, Bart IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Citation for published version (APA): Brinkman, J. W. (2007). Albuminuria as a laboratory risk marker: Methods evaluated s.n.
 University of Groningen Albuminuria as a laboratory risk marker Brinkman, Jacoline Willijanne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
University of Groningen Albuminuria as a laboratory risk marker Brinkman, Jacoline Willijanne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
Physiologic Anatomy and Nervous Connections of the Bladder
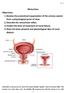 Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
Neurodevelopmental outcome of children born following assisted reproductive technology Middelburg, Karin Janette
 University of Groningen Neurodevelopmental outcome of children born following assisted reproductive technology Middelburg, Karin Janette IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Neurodevelopmental outcome of children born following assisted reproductive technology Middelburg, Karin Janette IMPORTANT NOTE: You are advised to consult the publisher's version
Autonomic Nervous System (the visceral motor system) Steven McLoon Department of Neuroscience University of Minnesota
 Autonomic Nervous System (the visceral motor system) Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Monday, Nov 6, 9:00-10:00am Surdyk s Café in Northrop Auditorium
Autonomic Nervous System (the visceral motor system) Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Monday, Nov 6, 9:00-10:00am Surdyk s Café in Northrop Auditorium
Proteinuria-associated renal injury and the effects of intervention in the renin-angiotensinaldosterone
 University of Groningen Proteinuria-associated renal injury and the effects of intervention in the renin-angiotensinaldosterone system Kramer, Andrea Brechtsje IMPORTANT NOTE: You are advised to consult
University of Groningen Proteinuria-associated renal injury and the effects of intervention in the renin-angiotensinaldosterone system Kramer, Andrea Brechtsje IMPORTANT NOTE: You are advised to consult
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy
 By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
skilled pathways: distal somatic muscles (fingers, hands) (brainstem, cortex) are giving excitatory signals to the descending pathway
 L15 - Motor Cortex General - descending pathways: how we control our body - motor = somatic muscles and movement (it is a descending motor output pathway) - two types of movement: goal-driven/voluntary
L15 - Motor Cortex General - descending pathways: how we control our body - motor = somatic muscles and movement (it is a descending motor output pathway) - two types of movement: goal-driven/voluntary
Arterial Blood Supply
 Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Insulin sensitivity of hepatic glucose and lipid metabolism in animal models of hepatic steatosis Grefhorst, Aldo
 University of Groningen Insulin sensitivity of hepatic glucose and lipid metabolism in animal models of hepatic steatosis Grefhorst, Aldo IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Insulin sensitivity of hepatic glucose and lipid metabolism in animal models of hepatic steatosis Grefhorst, Aldo IMPORTANT NOTE: You are advised to consult the publisher's version
Citation for published version (APA): Bijl, M. (2001). Apoptosis and autoantibodies in systemic lupus erythematosus Groningen: s.n.
 University of Groningen Apoptosis and autoantibodies in systemic lupus erythematosus Bijl, Marc IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
University of Groningen Apoptosis and autoantibodies in systemic lupus erythematosus Bijl, Marc IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
I. Neural Control of Involuntary Effectors. Chapter 9. Autonomic Motor Nerves. Autonomic Neurons. Autonomic Ganglia. Autonomic Neurons 9/19/11
 Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
University of Groningen. Depression in general practice Piek, Ellen
 University of Groningen Depression in general practice Piek, Ellen IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Depression in general practice Piek, Ellen IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Brain Stem. Nervous System (Part A-3) Module 8 -Chapter 14
 Nervous System (Part A-3) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
Nervous System (Part A-3) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
The Nervous System. Autonomic Division. C h a p t e r. PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas
 C h a p t e r 17 The Nervous System Autonomic Division PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson
C h a p t e r 17 The Nervous System Autonomic Division PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson
University of Groningen. Understanding negative symptoms Klaasen, Nicky Gabriëlle
 University of Groningen Understanding negative symptoms Klaasen, Nicky Gabriëlle IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Understanding negative symptoms Klaasen, Nicky Gabriëlle IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM
 Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
The role of the general practitioner in the care for patients with colorectal cancer Brandenbarg, Daan
 University of Groningen The role of the general practitioner in the care for patients with colorectal cancer Brandenbarg, Daan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen The role of the general practitioner in the care for patients with colorectal cancer Brandenbarg, Daan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
NERVOUS SYSTEM. Academic Resource Center. Forskellen mellem oscillator og krystal
 NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Autonomic Nervous System DR JAMILA EL MEDANY
 Autonomic Nervous System DR JAMILA EL MEDANY OBJECTIVES At the end of the lecture, students should be able to: Define the autonomic nervous system. Describe the structure of autonomic nervous system Trace
Autonomic Nervous System DR JAMILA EL MEDANY OBJECTIVES At the end of the lecture, students should be able to: Define the autonomic nervous system. Describe the structure of autonomic nervous system Trace
Posterior White Column-Medial Lemniscal Pathway
 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
PETER PAZMANY CATHOLIC UNIVERSITY Consortium members SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
 PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
Pain classifications slow and fast
 Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland
 AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
Neural Basis of Motor Control. Chapter 4
 Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Chapter 23. Micturition and Renal Insufficiency
 Chapter 23 Micturition and Renal Insufficiency Voiding Urine Between acts of urination, the bladder is filling. detrusor muscle relaxes urethral sphincters are tightly closed accomplished by sympathetic
Chapter 23 Micturition and Renal Insufficiency Voiding Urine Between acts of urination, the bladder is filling. detrusor muscle relaxes urethral sphincters are tightly closed accomplished by sympathetic
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Autonomic Nervous System and Hypothalamus
 Lu Chen LSA room 201 Phone: (510) 643-8163 Email: luchen@berkeley.edu Office hours: M,W,F, 10-11 am 1 Autonomic Nervous System and Hypothalamus Lu Chen, Ph.D. MCB, UC Berkeley 2 Hypothalamus Brain stem
Lu Chen LSA room 201 Phone: (510) 643-8163 Email: luchen@berkeley.edu Office hours: M,W,F, 10-11 am 1 Autonomic Nervous System and Hypothalamus Lu Chen, Ph.D. MCB, UC Berkeley 2 Hypothalamus Brain stem
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
The Motor Systems. What s the motor system? Plan
 The Motor Systems What s the motor system? Parts of CNS and PNS specialized for control of limb, trunk, and eye movements Also holds us together From simple reflexes (knee jerk) to voluntary movements
The Motor Systems What s the motor system? Parts of CNS and PNS specialized for control of limb, trunk, and eye movements Also holds us together From simple reflexes (knee jerk) to voluntary movements
University of Groningen. ADHD and atopic diseases van der Schans, Jurjen
 University of Groningen ADHD and atopic diseases van der Schans, Jurjen IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen ADHD and atopic diseases van der Schans, Jurjen IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
University of Groningen. A geriatric perspective on chronic kidney disease Bos, Harmke Anthonia
 University of Groningen A geriatric perspective on chronic kidney disease Bos, Harmke Anthonia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
University of Groningen A geriatric perspective on chronic kidney disease Bos, Harmke Anthonia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
Goal-oriented hemodynamic treatment in high-risk surgical patients Sonneveld, Johan Pieter Cornelis
 University of Groningen Goal-oriented hemodynamic treatment in high-risk surgical patients Sonneveld, Johan Pieter Cornelis IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Goal-oriented hemodynamic treatment in high-risk surgical patients Sonneveld, Johan Pieter Cornelis IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Role of brainstem in somatomotor (postural) functions
 Role of brainstem in somatomotor (postural) functions (vestibular apparatus) The muscle tone and its regulation VESTIBULAR SYSTEM (Equilibrium) Receptors: Otolith organs Semicircular canals Sensation (information):
Role of brainstem in somatomotor (postural) functions (vestibular apparatus) The muscle tone and its regulation VESTIBULAR SYSTEM (Equilibrium) Receptors: Otolith organs Semicircular canals Sensation (information):
Renal Physiology: Filling of the Urinary Bladder, Micturition, Physiologic Basis of some Renal Function Tests. Amelyn R.
 Renal Physiology: Filling of the Urinary Bladder, Micturition, Physiologic Basis of some Renal Function Tests Amelyn R. Rafael, MD 1 Functions of the Urinary Bladder 1. storage of urine 150 cc 1 st urge
Renal Physiology: Filling of the Urinary Bladder, Micturition, Physiologic Basis of some Renal Function Tests Amelyn R. Rafael, MD 1 Functions of the Urinary Bladder 1. storage of urine 150 cc 1 st urge
University of Groningen. Physical activity and cognition in children van der Niet, Anneke Gerarda
 University of Groningen Physical activity and cognition in children van der Niet, Anneke Gerarda IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
University of Groningen Physical activity and cognition in children van der Niet, Anneke Gerarda IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
The Spinal Cord. The Nervous System. The Spinal Cord. The Spinal Cord 1/2/2016. Continuation of CNS inferior to foramen magnum.
 The Nervous System Spinal Cord Continuation of CNS inferior to foramen magnum Simpler than the brain Conducts impulses to and from brain Two way conduction pathway Reflex actions Passes through vertebral
The Nervous System Spinal Cord Continuation of CNS inferior to foramen magnum Simpler than the brain Conducts impulses to and from brain Two way conduction pathway Reflex actions Passes through vertebral
Physical activity and physical fitness in juvenile idiopathic arthritis Lelieveld, Otto
 University of Groningen Physical activity and physical fitness in juvenile idiopathic arthritis Lelieveld, Otto IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
University of Groningen Physical activity and physical fitness in juvenile idiopathic arthritis Lelieveld, Otto IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
Autonomic Nervous System
 Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Clinical applications of positron emission tomography in coronary atherosclerosis Siebelink, Hans-Marc José
 University of Groningen Clinical applications of positron emission tomography in coronary atherosclerosis Siebelink, Hans-Marc José IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Clinical applications of positron emission tomography in coronary atherosclerosis Siebelink, Hans-Marc José IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
