Oral mesalamine formulations are widely used for the
|
|
|
- Bertha Parker
- 5 years ago
- Views:
Transcription
1 ORIGINAL ARTICLE Direct Comparison of Two Different Mesalamine Formulations for the Induction of Remission in Patients with Ulcerative Colitis: A Double-blind, Randomized Study Hiroaki Ito, MD,* Mitsuo Iida, MD, Takayuki Matsumoto, MD, Yasuo Suzuki, MD, Hidetaka Sasaki, PhD, k Toyomitsu Yoshida, BSc, k Yuichi Takano, BSc, k and Toshifumi Hibi, MD Background: Mesalamine is the first-line drug for the treatment of ulcerative colitis (UC). We directly compared the efficacy and safety of two mesalamine formulations for the induction of remission in patients with UC. Methods: In a multicenter, double-blind, randomized study, 229 patients with mild-to-moderate active UC were assigned to 4 groups: 66 and 65 received a ph-dependent release formulation of 2.4 g/day (ph-2.4 g) or 3.6 g/day (ph-3.6 g), respectively; 65 received a time-dependent release formulation of 2.25 g/day (Time g), and 33 received placebo (Placebo). The drugs were administered three times daily for eight weeks. The primary endpoint was a decrease in the UC disease activity index (UC-DAI). Results: In the full analysis set (n ¼ 225) the decrease in UC- DAI in each group was 1.5 in ph-2.4 g, 2.9 in ph-3.6 g, 1.3 in Time-2.25 g and 0.3 in Placebo, respectively. These results demonstrate the superiority of ph-3.6 g over Time-2.25 g (P ¼ 0.003) and the noninferiority of ph-2.4 g to Time-2.25 g. Among the patients with proctitis-type UC, a significant decrease in UC-DAI was observed in ph-2.4 g and ph-3.6 g as compared to Placebo, but not in Time-2.25 g. No differences were observed in the safety profiles. Received for publication September 9, 2009; Accepted November 3, From the *Digestive Disease Center of Excellence, Kitano Hospital, The Tazuke Kofukai Medical Research Institute, Osaka, Japan, Department of Medicine and Clinical Science, Graduate School of Medical Science, Kyushu University, Fukuoka, Japan, Division of Lower Gastroenterology, Department of Internal Medicine, Hyogo College of Medicine, Hyogo, Japan, Department of Internal Medicine, Toho University Sakura Medical Center, Chiba, Japan, k Clinical Research, ZERIA Pharmaceutical Co., Ltd., Tokyo, Japan, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. Supported by ZERIA Pharmaceutical Co., Ltd., Research and Development Division. Reprints: Toshifumi Hibi, MD, Department of Internal Medicine, Keio University School of Medicine, 35 Shinano-machi, Shinjuku-ku, Tokyo , Japan ( thibi@sc.itc.keio.ac.jp) Copyright VC 2010 Crohn s & Colitis Foundation of America, Inc. DOI /ibd Published online 4 January 2010 in Wiley Online Library (wileyonlinelibrary.com). Conclusions: Higher dose of the ph-dependent release formulation was more effective for induction of remission in patients with mild-to-moderate active UC. Additionally, the ph-dependent release formulation was preferable to the time-dependent release formulation for patients with proctitis-type UC (UMIN Clinical Trials Registry, no. C ). (Inflamm Bowel Dis 2010;16: ) Key Words: mesalamine, ulcerative colitis, randomized controlled trial, colonoscopy Oral mesalamine formulations are widely used for the treatment of mild-to-moderate active ulcerative colitis (UC) because they have shown excellent efficacy and safety, especially with long-term use. 1 3 Mesalamine is absorbed in the upper gastrointestinal tract; 3 5 whereas, it exerts antiinflammatory activity directly on the inflamed mucosa in colon and rectum. Thus, many types of release-controlled oral formulations of mesalamine have been developed to enhance its effect. The most widely-used formulation of mesalamine, the ph-dependent release formulation coated with Eudragit-S (Asacol), has been designed so that the coating film dissolves at a ph of 7 or higher. 3,6,7 The release of mesalamine starts in the terminal ileum due to its coating. The time-dependent release formulation of mesalamine coated with ethyl cellulose (Pentasa), on the other hand, gradually releasing mesalamine in the stomach. 3,7 Both formulations are designed to increase drug delivery to the inflamed areas of the colon and rectum as compared with the unmodified formulation. Since there have been no double-blind, randomized studies comparing the therapeutic efficacy of these formulations, physicians select these drugs with little guidance as to their proper use. Therefore, we conducted a double-blind, randomized trial in patients with active UC comparing the two formulations in terms of their efficacy and safety (UMIN Clinical Trials Registry, no. C ). 1567
2 Ito et al MATERIALS AND METHODS Patient Selection We conducted the study on patients with mild-tomoderate active UC on the basis of two inclusion criteria: 1) outpatients who were years of age at the time of informed consent, and 2) patients who had mild-to-moderate active UC defined by UC-DAI of 3 8 and a bloody stool score of 1 or greater. The UC-DAI was originally developed by Sutherland et al. 8 The patients were excluded according to the following criteria: 1) severe UC, chronic continuous type UC or acute fulminating type UC; 2) oral mesalamine more than 2.25 g daily, oral salazosulfapyridine more than 4.5 g daily, mesalamine enemas, salazosulfapyridine suppositories, corticosteroids (oral preparations, enemas, suppositories, injections and/or remedies for hemorrhoidal diseases) and/or cytapheresis within 14 days before the start of the investigational drug; 3) immunosuppressants within 90 days before the start of the investigational drug; 4) any other investigational drug within six months before informed consent; 5) a history of hypersensitivity to mesalamine or salicylate drugs, severe cardiac disease, severe pulmonary disease and/or severe hematological diseases; 6) severe hepatopathy, severe nephropathy and/or malignant tumors; and 7) pregnant or lactating. Ethical Considerations This study was conducted according to the principles of the Declaration of Helsinki after obtaining approvals from the Institutional Review Board at each of the participating medical centers. Written informed consent was obtained from all participants. Study Drugs The ph-dependent release mesalamine formulation used in this study is a tablet coated with Eudragit-S (Asacol 400 mg tablet, Tillotts Pharma AG, Ziefen, Switzerland, supplied by ZERIA Pharmaceutical, Tokyo, Japan). The time-dependent release mesalamine formulation used in this study is a tablet coated with ethyl cellulose (Pentasa 250 mg tablet, Nisshin Kyorin Pharmaceutical, Japan). This study was conducted using a double-dummy method. Study Design This double-blind, randomized, controlled study was conducted at 53 centers in Japan. Treatment assignments were balanced according to the inflamed areas (proctitistype or others) and the severity of the disease (range of UC-DAI at initial assessment: 3 5 or 6 8) with the use of a biased-coin minimization algorithm. Balance within each medical center was also taken into consideration. A person independent from the study was in charge of the random allocation. Seven patients were assigned as a block as follows: 2 to a group given the ph-dependent release formulation at 2.4 g/day (ph-2.4 g), 2 to a group given the ph-dependent release formulation at 3.6 g/day (ph-3.6 g), 2 to a group given the time-dependent release formulation at 2.25 g/day (Time-2.25 g), and 1 to a group given placebo (Placebo). The randomization code was sealed and stored until the blind was removed. At the time of informed consent, investigators evaluated the background characteristics of patients. After an observation period of 3 14 days from the time of informed consent, investigators assessed patients for their eligibility for enrolment according to criteria previously described. At the time of the eligibility assessment the UC-DAI was calculated using a previously reported method. 9,10 The UC- DAI is the sum of the mucosal appearance score (based on the colonoscopy findings by reference to atlases of mucosal appearance), stool frequency score, bloody stool score, and physician s global assessment score (stage 0, 1, 2, or 3). Each score was based on the patients diary for the last three days. The area of the inflammation was also determined by colonoscopy. Patients who were judged as eligible were enrolled and assigned to investigational drugs by a central registration center, and then administration was started. The investigational drugs were administered three times daily for eight weeks. During the study, each patient recorded the condition of their bloody stools, stool frequency and drug compliance in their diary and visited the medical center every two weeks. Each component of UC-DAI, except the mucosal appearance score, was assessed at each visit. Colonoscopy was performed at eight weeks or at withdrawal from the study, and UC-DAI was calculated at that time. To evaluate safety, clinical laboratory data and vital signs were checked at the time of informed consent and four weeks and eight weeks after enrolment (or upon withdrawal). The presence or absence of adverse events (AEs) and adverse drug reactions (ADRs) were recorded by investigators at each visit. Statistical Analysis In the statistical analysis, the primary endpoint was the decrease in UC-DAI at the final assessment. The principal aim of this study was to demonstrate two hypotheses with closed procedure; the first was the superiority of ph- 3.6 g over Time-2.25 g, and the second was the noninferiority of ph-2.4 g to Time-2.25 g. In the primary endpoint, a closed procedure was adopted. Individual hypotheses were verified by the following methods: 1) verification of the superiority of ph-3.6 g over Time-2.25 g: the superiority was demonstrated if the lower limit of the 95% confidence interval (CI) was more than 0.0 in the difference of the decrease in UC-DAI 1568
3 Mesalamine for Induction of Remission in UC between the groups (ph-3.6 g minus Time-2.25 g); and 2) verification of the noninferiority of ph-2.4 g to Time-2.25 g: the noninferiority and superiority would be demonstrated if the lower limits of the 95% CI were more than 1.0 and 0.0, respectively, in the difference between the groups (ph-2.4 g minus Time-2.25 g). The secondary endpoints were the proportion of remission and the proportion of efficacy. Remission was defined as patients with a UC- DAI of 2 or less and a bloody stool score of 0 at the final assessment. Efficacy was defined as remission or improvement. Improvement was defined as patients with the decrease in UC-DAI by two points or more, except patients who experienced a remission. In the safety endpoints the numbers of patients with AEs and patients with ADRs were analyzed by Fisher s exact test. Unless otherwise specified, differences at a ¼ 0.05 (two-sided) and P < 0.05 were considered statistically significant. Differences were considered statistically significant when the 95% CI did not include zero in the difference between two groups. Multiplicity of these analyses was not taken into consideration. The statistical analyses were conducted by ZERIA Pharmaceutical, Japan, based on statistical advice of an expert independent of this study. The number of patients required to demonstrate the superiority of ph-3.6 g over Time-2.25 g was estimated to be 55 at a ¼ 0.05 (two-sided) and b ¼ 0.1 when the difference between the decreases in UC-DAI of the two groups was 2.0 and the standard deviation (SD) was 3.2. The number of the patients required to demonstrate the noninferiority of ph-2.4 g to Time-2.25 g was estimated to be 54 at a ¼ 0.05 (two-sided), b ¼ 0.1 and D ¼ 1 when the difference between the decreases in UC-DAI of the two groups was 1.0 and the SD was 3.2. According to the above estimations, we decided to enroll at least 60 patients in each active-drug group considering the patients excluded from the analysis set. Placebo was used as the reference in the analysis for efficacy, and the number of patients in the placebo group was half of that in each of the active drug groups. The full analysis set (FAS) consisted of all participants except those who had not taken even one tablet of the investigational drugs, those who did not comply with Good Clinical Practice (GCP), those who met exclusion criteria 1) and those whose data were missing. The per protocol set (PPS) consisted of the FAS except those who did not fulfill the inclusion criteria, those who met the exclusion criteria 2) 7), those who received forbidden drugs and those whose drug compliance was less than 75%. Concerning the withdrawal cases, their adoption was to be decided before the blind was removed. The statistical analysis of efficacy was performed primarily based on data from the FAS followed by comparison with those from the PPS. The dataset for safety consisted of all participants except those who had not taken even one tablet of the investigational drug and those who did not comply with the GCP. Independent Image Assessment Committee We established an image assessment committee independent from the investigators to ensure the reliability of the mucosal appearance scores, and each of the three members of the committee blindly and independently scored mucosal appearance by examining photos provided by the investigators. When the scores obtained from all three members was the same, that score was regarded as a judgment by the committee. If the scores were different, the committe members discussed the case until they reached a consensus. When the judgment by the committee and the evaluation by the investigators were the same, it was defined as an agreement case. RESULTS Patient Demographics Investigators obtained informed consent from 263 patients during the period from November 2005 to July 2007 and completed the final follow-up in September 2007 (Fig. 1). Of the 263 patients, 229 patients were assigned to all the groups (ph-2.4 g, 66; ph-3.6 g, 65; Time-2.25 g, 65; Placebo, 33). All 229 patients took the investigational drugs at least once. Drug compliance was greater than 75% in every patient except for 2 patients (Time-2.25 g, 1; Placebo, 1). A total of 47 patients (ph-2.4 g, 16; ph-3.6 g, 7; Time-2.25 g, 14; Placebo, 10) were withdrawn from the study. The most frequent reason for withdrawal was aggravation of UC (ph-2.4 g, 9; ph-3.6 g, 1; Time-2.25 g, 7; Placebo, 7). There were 225 patients in the FAS (ph-2.4 g, 66; ph-3.6 g, 64; Time-2.25 g, 63; Placebo, 32) and 222 patients in the PPS (ph-2.4 g, 65; ph-3.6 g, 62; Time-2.25 g, 63; Placebo, 32). The results were very similar when the data were analyzed according to the FAS or PPS. Therefore, the results analyzed according to the FAS will be shown at the following. We did not perform adjustments for the demographic factor because patient demographics in all groups were similar (Table 1). Efficacy The decrease in UC-DAI as the primary endpoint was most pronounced in ph-3.6 g (Table 2). The decrease in UC-DAI was greater by 1.6 (95% CI: 0.6, 2.6) in ph- 3.6 g compared to Time-2.25 g, demonstrating the superiority of ph-3.6 g over Time-2.25 g (P ¼ 0.003). The difference between ph-2.4 g and Time-2.25 g was 0.2 (95% CI: 0.8, 1.2), demonstrating the noninferiority of ph-2.4 g to 1569
4 Ito et al FIGURE 1. Enrolment, randomization, and follow-up of the study patients. TABLE 1. Patient Demographics ph-2.4 g (n ¼ 66) ph-3.6 g (n ¼ 64) Time-2.25 g (n ¼ 63) Placebo (n ¼ 32) Sex (male/female) 38/28 36/28 37/26 16/16 Age (years) Mean SD Weight (kg) Mean SD Years of disease duration (no. of patients) < < < < < Inflamed areas (no. of patients) Proctitis-type Others Clinical course (no. of patients) Initial Relapsed UC-DAI at initial assessment Mean SD
5 Mesalamine for Induction of Remission in UC TABLE 2. Decrease in the UC-DAI ph-2.4 g (n ¼ 66) ph-3.6 g (n ¼ 64) Time-2.25 g (n ¼ 63) Placebo (n ¼ 32) Decrease in the UC-DAI No. of patients Mean (95% CI ) 1.5 (0.7, 2.3) 2.9 (2.3, 3.5) 1.3 (0.6, 2.1) 0.3 ( 0.7, 1.2) 0.2 ( 0.8, 1.2) 1.6 (0.6, 2.6) Time-2.25 g (95% CI ) Placebo (95% CI ) 1.2 (0.0, 2.5) 2.7 (1.4, 3.9) 1.1 ( 0.1, 2.3) Decrease in the UC-DAI was calculated from the scores at the initial and final assessments. The data of 13 patients (ph-2.4 g, 8; ph-3.6 g, 2; Time-2.25 g, 3) had to be excluded from the analysis because the mucosal appearance data were missing. Time-2.25 g. The secondary endpoints, specifically the proportions of the remission and efficacy, were the highest in ph-3.6 g (Fig. 2). In the subgroup analyses, a significant difference in the decrease in UC-DAI was found between ph-3.6 g and placebo in all subgroups (Table 3). Among the patients with proctitis-type UC, there were significant differences both in ph-2.4 g and in ph-3.6 g as compared with placebo, but not in Time-2.25 g as compared with placebo. Among the patients in which UC-DAI at initial assessment was 3 5, the results showed a trend similar to the subgroup of patients with proctitis-type UC. On the other hand, among the cases where UC-DAI was 6 8, there was a significant difference only in ph-3.6 g as compared with placebo, but not in ph-2.4 g and Time-2.25 g as compared with placebo. DISCUSSION Previous randomized controlled studies have stated that the ph- and time-dependent release formulations of mesalamine used in the present study were superior to placebo. 9,11 However, exposure periods and dosage differed in the individual studies. These differences made it difficult to compare drug efficacies. Reliability of the Mucosal Appearance Scores The proportion of agreement between the judges by the image assessment committee and the evaluations by the investigators are summarized in Table 4. The proportion was 67.9%, and Cohen s j coefficient was In all the disagreement cases there was one degree of difference in the scores between the judges by the committee and the evaluations by the investigators. Safety In patients with AEs and ADRs, there were no significant differences between each of the active drug groups and placebo (Table 5). Serious adverse events included aggravation of UC in 2 patients in ph-2.4 g, malaise in 1 patient in ph-3.6 g, abdominal abscess in 1 patient in ph- 3.6 g and aggravation of UC in 3 patients in Time-2.25 g. A causal relationship to the drug could not be ruled out in 3 patients with serious AEs (1 patient in ph-2.4 g and 2 patients in Time-2.25 g). FIGURE 2. Proportion of remission and efficacy. The graphs show proportions of remission and efficacy in each group within 95% CIs. Each graph includes 225 patients for analyses (ph-2.4 g, 66; ph-3.6 g, 64; Time-2.25 g, 63; Placebo, 32). A: The proportion of patients who experienced a remission was 30.3% (CI, ) in ph-2.4 g, 45.3% (CI, ) in ph-3.6 g, 28.6% (CI, ) in Time-2.25 g, and 9.4% (CI, ) in Placebo. There were statistically significant differences from Placebo in all active-drug groups. B: Efficacy was archived in 45.5% (CI, ) in ph-2.4 g, 64.1% (CI, ) in ph-3.6 g, 49.2% (CI, ) in Time-2.25 g, and 28.1% (CI, ) in Placebo. There were significant differences from Placebo in both ph-3.6 g and Time-2.25 g. 1571
6 Ito et al TABLE 3. Subgroup Analysis: Inflamed Areas and Severity ph-2.4 g (n ¼ 66) ph-3.6 g (n ¼ 64) Time-2.25 g (n ¼ 63) Placebo (n ¼ 32) Decrease in UC-DAI Inflamed areas Proctitis-type No. of patients Mean (95% CI) 1.8 (0.7, 2.8) 1.7 (0.7, 2.6) 1.1 (-0.2, 2.3) 0.4 (-1.8, 1.1) 0.7 (-0.8, 2.1) 0.6 (-0.9, 2.0) Time-2.25 g (95% CI) 2.1 (0.3, 4.0) 2.0 (0.2, 3.8) 1.5 (-0.4, 3.3) Placebo (95% CI) Others No. of patients Mean (95% CI) 1.3 (0.2, 2.4) 3.6 (2.9, 4.4) 1.5 (0.5, 2.5) 0.6 (-0.8, 1.9) 0.2 (-1.5, 1.2) 2.2 (0.8, 3.5) Time-2.25 g (95% CI) 0.7 (-0.9, 2.4) 3.1 (1.5, 4.7) 0.9 (-0.7, 2.5) Placebo (95% CI) UC-DAI at initial assessment 3-5 No. of patients Mean (95% CI) 1.7 (0.8, 2.6) 1.8 (0.9, 2.7) 1.5 (0.3, 2.6) 0.1 (-1.5, 1.3) 0.2 (-1.1, 1.6) 0.4 (-1.0, 1.7) Time-2.25 g (95% CI) 1.8 (0.1, 3.4) 1.9 (0.3, 3.5) 1.5 (-0.1, 3.2) Placebo (95% CI) 6-8 No. of patients Mean (95% CI) 1.3 (0.1, 2.5) 3.7 (2.9, 4.6) 1.2 (0.2, 2.3) 0.5 (-0.9, 1.9) 0.1 (-1.3, 1.6) 2.5 (1.1, 4.0) Time-2.25 g (95% CI) Placebo (95% CI) 0.9 (-0.9, 2.6) 3.3 (1.5, 5.0) 0.7 (-1.0, 2.5) Decrease in the UC-DAI was calculated from the scores at the initial and final assessments. The data of 13 patients (ph-2.4 g, 8; ph-3.6 g, 2; Time-2.25 g, 3) had to be excluded from the analysis because the mucosal appearance data were missing. In the present study the ph-3.6 g group showed the highest efficacy at every endpoint. In general, it has been proposed that mesalamine exerts a greater therapeutic effect at higher doses. 1,2,12 Schroeder et al 13 demonstrated that a higher dose of the ph-dependent release formulation of 4.8 g/day showed greater efficacy than a dose of 1.6 g/ TABLE 4. Agreement Between Evaluations by the Investigators and Judgments by the Image Assessment Committee n ¼ 193 Evaluations by the Investigators Total Judgments by committee Proportion of agreement (%) Total Cohen s j coefficient Proportion of agreement (%) ¼ (number of agreement cases) / (number of cases confirmed by colonoscopy) x 100 In this trial, 229 patients were allocated to an intervention. The data of 35 patients had to be excluded from the analysis because the mucosal appearance score was missing, and the data of one patient had to be excluded from the analysis because of a GCP violation. 1572
7 Mesalamine for Induction of Remission in UC TABLE 5. Adverse Events and Adverse Drug Reactions ph-2.4 g (n ¼ 66) ph-3.6 g (n ¼ 64) Time-2.25 g (n ¼ 65) Placebo (n ¼ 33) No. of Patients (%) No. of Patients (%) No. of Patients (%) No. of Patients (%) Adverse events* 56 (84.8) 53 (82.8) 55 (84.6) 22 (66.7) Nasopharyngitis 11 (16.7) 10 (15.6) 7 (10.8) 2 (6.1) C-reactive protein increased 13 (19.7) 14 (21.9) 18 (27.7) 6 (18.2) Beta-N-acetyl-D-glucosaminidase 13 (19.7) 12 (18.8) 13 (20.0) 6 (18.2) increased Eosinophil count increased 12 (18.2) 9 (14.1) 14 (21.5) 4 (12.1) Lymphocyte count decreased 10 (15.2) 5 (7.8) 11 (16.9) 1 (3.0) Blood bilirubin increased 6 (9.1) 8 (12.5) 5 (7.7) 4 (12.1) White blood cell count 4 (6.1) 4 (6.3) 7 (10.8) 2 (6.1) increased Blood lactate dehydrogenase 2 (3.0) 0 (0.0) 6 (9.2) 4 (12.1) increased Adverse drug reactions 27 (40.9) 31 (48.4) 28 (43.1) 10 (30.3) *Events that occurred in more than 10% of the patients in at least one group. day. In addition, when another formulation of ph-dependent release mesalamine (Rowasa) was administered to patients with active UC, UC-DAI after six weeks of treatment decreased from 8.5 to 4.8 at the 4 g/day dose, from 9.0 to 7.7 at the 2 g/day dose, and from 8.2 to 7.7 in the placebo group. 14 In this report, we also showed that the ph-dependent release formulation at a higher dose significantly decreased UC-DAI (Table 2). Patients with proctitis-type UC account for 40 50% of the patients with UC. 12 Physicians often use mesalamine suppositories and enemas to treat these patients, but compliance is poorer than with oral formulations. We found significant differences both in the ph-2.4 g and in the ph- 3.6 g group as compared with placebo in patients with proctitis-type disease, but not in the Time-2.25 g group (Table 3). These findings suggest that the ph-dependent release formulation is preferable for patients with inflammation of the distal intestine. Until now, mesalamine formulations have been chosen without adequate supporting evidence. The results of the present study provide scientific evidence for the proper use of the different mesalamine formulations, especially when the location of the inflammation is taken into consideration. In every subgroup of disease characteristics, only the ph-3.6 g group showed significant differences as compared with placebo (Table 3). Especially in subgroups classified as others and as UC-DAI of 6 8, the ph-3.6 g group showed greater a decrease in UC-DAI compared to the other groups. We presume that patients who are classified as UC-DAI of 6 8 (i.e., the patients with more severe disease) can only achieve an adequate level of mesalamine at the dosage of 3.6 g/day. As a result, a substantial change in the UC-DAI score may occur. Patients with more extensive disease generally have more severe disease. 12 Therefore, because the patients classified as others had more severe disease than the patients with the proctitis-type UC, a substantial change in UC-DAI might be also observed in patients classified as others. In the primary endpoint, neither the ph-2.4 g group nor Time-2.25 g group showed significant differences when compared with placebo (Table 2). These results were in contrast to the findings from previous studies. 9,11 This discrepancy was likely related to the lack of power; i.e., the number of patients in placebo was half of that in the active drug groups because the present study was not designed mainly for comparing the active drug groups with placebo. The proportion of agreement between the judges by committee and the evaluations by the investigators was 67.9% (Cohen s j coefficient: 0.497) (Table 4). Mucosal appearance in patients with UC is often evaluated using the Baron score, 15 which is similar to the index used in our study. Hirai and Matsui 16 reported a proportion of agreement between the scores of the two raters that used the Baron score. In their study, the proportion of agreement and j coefficient between two raters were 51% and 0.31, respectively, but their coefficient was lower compared to our study. In the Hirai and Matsui study, 8.7% of all patients observed two degrees of difference in the scores between two raters. On the other hand, in our study, there were no cases showing two degrees of difference. Thus, we 1573
8 Ito et al assumed that the interobserver variation among the investigators was well controlled in our study. In the present study, the proportion of cases showing disagreement was 30%. This disagreement was predominately ascribed to a difference in the approach to the patients mucosa, because the investigators evaluated the actual mucous membrane through colonoscopy; whereas the committee used photos of the mucous membrane taken by the investigators. We provided atlases of mucosal appearance to the investigators in order to minimize interobserver variation (both investigator investigator and investigator committee). However, as mentioned by Hirai and Matsui, 16 further improvement in the agreement between raters could be accomplished by better training of the raters, the use of good photos, and so on. Mesalamine has generally been found to be a safe drug, 1,2 but the transfer of mesalamine from the upper gastrointestinal tract to the plasma should be minimized considering its nephrotoxicity. We expect the ph-dependent release formulation to reduce the frequency of AEs because it suppresses the transfer of mesalamine to plasma due to its release mechanism. 3,4 However, no difference between the two formulations in the proportion of the patients with AEs was observed (Table 5). The wide safety margin of mesalamine may blur the differences between the two formulations. In summary, this is the first study to directly compare the efficacy and safety of two different mesalamine formulations for the induction of remission in patients with UC. The results of our study clearly showed superior efficacy of the ph-dependent release formulation administered at a dose of 3.6 g/day and superior characteristics of this formulation to treat patients with proctitis-type UC. However, it is unknown whether the formulation is also efficacious in patients with more severe UC because the subjects in this study were patients with mild-to-moderate active UC. Mesalamine is considered safer than corticosteroids. Accordingly, further research will be necessary to fully evaluate the role of mesalamine formulations for the treatment of severe UC. If this is accomplished, it is likely that mesalamine contributes to the improvement in the quality of life of patients with UC. ACKNOWLEDGMENTS Drs. Ito, Iida and Suzuki received consulting fees from ZERIA Pharmaceutical. Drs. Matsumoto and Hibi received consulting fees and grant support from ZERIA Pharmaceutical. No other potential conflicts of interest related to this article were reported. This study was made possible by the cooperation of the many participants in the study, the physicians in charge and associated staff members. We thank all of those involved in supporting this study. In addition, the authors thank Dr. Fumiaki Ueno of Ofuna Chuo Hospital, Dr. Masahiro Igarashi of the Cancer Institute Hospital of JFCR and Dr. Haruhiko Ogata of Keio University for the members of independent image assessment committee, Prof. Hideki Origasa of the University of Toyama for statistical advice, Prof. Ulrich Mittmann and the staff of Tillotts Pharma AG for scientific advice regarding the article and the planning of the study, and Hayato Koyama of ZERIA Pharmaceutical for review of article and writing assistance. REFERENCES 1. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99: Carter MJ, Lobo AJ, Travis SP, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(suppl 5): Brogden RN, Sorkin EM. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 1989;38: Myers B, Evans DN, Rhodes J, et al. Metabolism and urinary excretion of 5-amino salicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut. 1987;28: Mardini HA, Lindsay DC, Deighton CM, et al. Effect of polymer coating on faecal recovery of ingested 5-amino salicylic acid in patients with ulcerative colitis. Gut. 1987;28: Riley SA, Tavares IA, Bennett A, et al. Delayed-release mesalazine (5-aminosalicylic acid): coat dissolution and excretion in ileostomy subjects. Br J Clin Pharmacol. 1988;26: Sinha A, Ball DJ, Connor AL, et al. Intestinal performance of two mesalamine formulations in patients with active ulcerative colitis as assessed by gamma scintigraphy. Pract Gastroenterol. 2003;27: Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92: Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115: Farup PG, Hinterleitner TA, Lukas M, et al. Mesalazine 4 g daily given as prolonged-release granules twice daily and four times daily is at least as effective as prolonged-release tablets four times daily in patients with ulcerative colitis. Inflamm Bowel Dis. 2001;7: Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsule for treatment of active ulcerative colitis: results of a controlled trial. Am J Gastroenterol. 1993;88: Friedman S, Blumberg RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Hauser SL, et al, eds. Harrison s Principles of Internal Medicine, 16th ed. New York: McGraw-Hill; 2004: Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317: Sutherland LR, Robinson M, Onstad G, et al. A double-blind, placebo controlled, multicentre study of the efficacy and safety of 5-aminosalicylic acid tablets in the treatment of ulcerative colitis. Can J Gastroenterol. 1990;4: Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J. 1964;1: Hirai F, Matsui T. A critical review of endoscopic indices in ulcerative colitis: inter-observer variation of the endoscopic index. Clin J Gastroenterol. 2008;1:
Review article: induction therapy for patients with active ulcerative colitis
 Alimentary Pharmacology & Therapeutics Review article: induction therapy for patients with active ulcerative colitis S. P. L. TRAVIS John Radcliffe Hospital and Linacre College, Oxford, UK Correspondence
Alimentary Pharmacology & Therapeutics Review article: induction therapy for patients with active ulcerative colitis S. P. L. TRAVIS John Radcliffe Hospital and Linacre College, Oxford, UK Correspondence
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 20 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
Month/Year of Review: September 2012 Date of Last Review: September 2010
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
How do I choose amongst medicines for inflammatory bowel disease. Maria T. Abreu, MD
 How do I choose amongst medicines for inflammatory bowel disease Maria T. Abreu, MD Overview of IBD Pathogenesis Bacterial Products Moderately Acutely Inflamed Chronic Inflammation = IBD Normal Gut Mildly
How do I choose amongst medicines for inflammatory bowel disease Maria T. Abreu, MD Overview of IBD Pathogenesis Bacterial Products Moderately Acutely Inflamed Chronic Inflammation = IBD Normal Gut Mildly
Doncaster & Bassetlaw Medicines Formulary
 Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Synopsis. Clinical Report Synopsis for Protocol Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc.
 Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease
 5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease David T. Rubin, MD Associate Professor of Medicine Co-Director, Inflammatory Bowel Disease Center University it of Chicago Medical
5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease David T. Rubin, MD Associate Professor of Medicine Co-Director, Inflammatory Bowel Disease Center University it of Chicago Medical
Crohn's disease. Appendix J. Clinical Guideline < > 10 October Review of Cochrane ASA review
 Crohn's disease Clinical Guideline < > Review of Cochrane ASA review 0 October 202 Commissioned by the National Institute for Health and Clinical Excellence Review of Cochrane 5-ASA review Contents Published
Crohn's disease Clinical Guideline < > Review of Cochrane ASA review 0 October 202 Commissioned by the National Institute for Health and Clinical Excellence Review of Cochrane 5-ASA review Contents Published
Literature Review: CORE I & II: COlonic RElease Budesonide for the Induction of Remission for Mild- Moderate Ulcerative Colitis
 VOLUME 13, ISSUE 1, YEAR 2014 Literature Review: CORE I & II: COlonic RElease Budesonide for the Induction of Remission for Mild- Moderate Ulcerative Colitis Peter R. McNally, DO, MACG, MSRF Center for
VOLUME 13, ISSUE 1, YEAR 2014 Literature Review: CORE I & II: COlonic RElease Budesonide for the Induction of Remission for Mild- Moderate Ulcerative Colitis Peter R. McNally, DO, MACG, MSRF Center for
FERRING PHARMACEUTICALS. Enjoy The simple COR/939/2014/CH3
 Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Ulcerative colitis (UC) is a chronic disease that is commonly
 ORIGINAL ARTICLE Voting with Their Feet (VWF) Endpoint: A Meta-Analysis of an Alternative Endpoint in Clinical Trials, Using 5-ASA Induction Studies in Ulcerative Colitis Sujal C. Rangwalla, DO,* Akbar
ORIGINAL ARTICLE Voting with Their Feet (VWF) Endpoint: A Meta-Analysis of an Alternative Endpoint in Clinical Trials, Using 5-ASA Induction Studies in Ulcerative Colitis Sujal C. Rangwalla, DO,* Akbar
Clinical Medicine Insights: Gastroenterology. Once-Daily MMX Mesalamine in the Management of Ulcerative Colitis
 Clinical Medicine Insights: Gastroenterology Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Once-Daily MMX Mesalamine in the Management of Ulcerative
Clinical Medicine Insights: Gastroenterology Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Once-Daily MMX Mesalamine in the Management of Ulcerative
Evaluation of the severity of ulcerative colitis using endoscopic dual red imaging targeting deep vessels
 Evaluation of the severity of ulcerative colitis using endoscopic dual red imaging targeting deep vessels Authors Makoto Naganuma 1, 2, Naohisa Yahagi 3,RiekoBessho 1, Keiko Ohno 1, Mari Arai 1, Makoto
Evaluation of the severity of ulcerative colitis using endoscopic dual red imaging targeting deep vessels Authors Makoto Naganuma 1, 2, Naohisa Yahagi 3,RiekoBessho 1, Keiko Ohno 1, Mari Arai 1, Makoto
Medical therapies and IBD
 Medical therapies and IBD Although there is no cure for IBD, there are many treatment options available. There is no standard treatment for IBD that is effective in all situations or for all patients,
Medical therapies and IBD Although there is no cure for IBD, there are many treatment options available. There is no standard treatment for IBD that is effective in all situations or for all patients,
Ulcerative colitis (UC) is a chronic relapsing and remitting inflammatory
 TREATMENT UPDATE The Efficacy of Oral 5-ASAs in the Treatment of Active Ulcerative Colitis: A Systematic Review Sunanda V. Kane, MD, MSPH,* David J. Bjorkman, MD, MSPH, SM (Epid) *University of Chicago,
TREATMENT UPDATE The Efficacy of Oral 5-ASAs in the Treatment of Active Ulcerative Colitis: A Systematic Review Sunanda V. Kane, MD, MSPH,* David J. Bjorkman, MD, MSPH, SM (Epid) *University of Chicago,
Moderately to severely active ulcerative colitis
 Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Oral mesalamine formulations are first-line treatments
 COCHRANE REVIEW Once Daily Oral Mesalamine Compared to Conventional Dosing for Induction and Maintenance of Remission in Ulcerative Colitis: A Systematic Review and Meta-Analysis Brian G. Feagan, MD, and
COCHRANE REVIEW Once Daily Oral Mesalamine Compared to Conventional Dosing for Induction and Maintenance of Remission in Ulcerative Colitis: A Systematic Review and Meta-Analysis Brian G. Feagan, MD, and
Once Daily Dosing for Induction and Maintenance of Remission in Ulcerative Colitis
 Once Daily Dosing for Induction and Maintenance of Remission in Ulcerative Colitis John K. Marshall MD MSc FRCPC AGAF Division of Gastroenterology McMaster University JKM 2014 Svartz N. Acta Med Scand
Once Daily Dosing for Induction and Maintenance of Remission in Ulcerative Colitis John K. Marshall MD MSc FRCPC AGAF Division of Gastroenterology McMaster University JKM 2014 Svartz N. Acta Med Scand
INFLAMMATORY BOWEL DISEASE
 1. Medical Condition INFLAMMATORY BOWEL DISEASE (IBD) specifically includes Crohn s disease (CD) and ulcerative colitis (UC) but also includes IBD unclassified (IBDu), seen in about 10% of cases. These
1. Medical Condition INFLAMMATORY BOWEL DISEASE (IBD) specifically includes Crohn s disease (CD) and ulcerative colitis (UC) but also includes IBD unclassified (IBDu), seen in about 10% of cases. These
Novel Formulations and Dosing Strategies for 5-ASA
 D e c e m b e r 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e 1 2, S u p p l e m e n t 2 8 Novel Formulations and Dosing Strategies for 5-ASA A Summary of Selected Recent
D e c e m b e r 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e 1 2, S u p p l e m e n t 2 8 Novel Formulations and Dosing Strategies for 5-ASA A Summary of Selected Recent
Clinical Study Clinical Study of the Relation between Mucosal Healing and Long-Term Outcomes in Ulcerative Colitis
 Hindawi Publishing Corporation Gastroenterology Research and Practice Volume 2013, Article ID 192794, 6 pages http://dx.doi.org/10.1155/2013/192794 Clinical Study Clinical Study of the Relation between
Hindawi Publishing Corporation Gastroenterology Research and Practice Volume 2013, Article ID 192794, 6 pages http://dx.doi.org/10.1155/2013/192794 Clinical Study Clinical Study of the Relation between
vedolizumab (Entyvio )
 vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
Public Assessment Report Scientific discussion. Mesalazin ESPL (mesalazine) SE/H/1654/01/DC
 Public Assessment Report Scientific discussion Mesalazin ESPL (mesalazine) SE/H/1654/01/DC This module reflects the scientific discussion for the approval of Mesalazin ESPL. The procedure was finalised
Public Assessment Report Scientific discussion Mesalazin ESPL (mesalazine) SE/H/1654/01/DC This module reflects the scientific discussion for the approval of Mesalazin ESPL. The procedure was finalised
Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various ph Levels
 Adv Ther (2015) 32:477 484 DOI 10.1007/s12325-015-0206-4 ORIGINAL RESEARCH Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various ph Levels Adeyinka Abinusawa. Srini Tenjarla
Adv Ther (2015) 32:477 484 DOI 10.1007/s12325-015-0206-4 ORIGINAL RESEARCH Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various ph Levels Adeyinka Abinusawa. Srini Tenjarla
Ulcerative Colitis. ulcerative colitis usually only affects the colon.
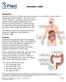 Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Mild-moderate Ulcerative Colitis Sequential & Combined treatments need to be tested. Philippe Marteau, Paris, France
 Mild-moderate Ulcerative Colitis Sequential & Combined treatments need to be tested Philippe Marteau, Paris, France Sequential vs combined treatments When should one switch? Sequential vs combined treatments
Mild-moderate Ulcerative Colitis Sequential & Combined treatments need to be tested Philippe Marteau, Paris, France Sequential vs combined treatments When should one switch? Sequential vs combined treatments
5-ASA for the treatment of Crohn s disease DR. STEPHEN HANAUER FEINBERG SCHOOL OF MEDICINE, NORTHWESTERN UNIVERSITY, CHICAGO, IL, USA
 5-ASA for the treatment of Crohn s disease DR. STEPHEN HANAUER FEINBERG SCHOOL OF MEDICINE, NORTHWESTERN UNIVERSITY, CHICAGO, IL, USA Background RCTs investigating the efficacy of aminosalicylates for
5-ASA for the treatment of Crohn s disease DR. STEPHEN HANAUER FEINBERG SCHOOL OF MEDICINE, NORTHWESTERN UNIVERSITY, CHICAGO, IL, USA Background RCTs investigating the efficacy of aminosalicylates for
Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis
 Alimentary Pharmacology and Therapeutics Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis T. Yamamoto, T. Shimoyama
Alimentary Pharmacology and Therapeutics Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis T. Yamamoto, T. Shimoyama
Efficacy and Safety of Treatment for Pediatric IBD
 Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Associate Professor of Clinical Pediatrics Division of Gastroenterology,
Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Associate Professor of Clinical Pediatrics Division of Gastroenterology,
National Institute for Health and Care Excellence
 National Institute for Health and Care Excellence 4-year surveillance (2017) Ulcerative colitis: management (2013) NICE guideline CG166 Appendix A.2: Summary of new evidence from surveillance Patient information
National Institute for Health and Care Excellence 4-year surveillance (2017) Ulcerative colitis: management (2013) NICE guideline CG166 Appendix A.2: Summary of new evidence from surveillance Patient information
Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis
 CLINICAL AND SYSTEMATIC S nature publishing group 601 Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis Alexander C. Ford, MBChB, MD, MRCP 1, 2, Je an - Pau l Ach
CLINICAL AND SYSTEMATIC S nature publishing group 601 Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis Alexander C. Ford, MBChB, MD, MRCP 1, 2, Je an - Pau l Ach
New treatment options in UC. Rob Bryant IBD Consultant Royal Adelaide Hospital
 New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
AVX-470, an Orally Delivered Anti-TNF Antibody for Treatment of Acute Ulcerative Colitis: Results of a First-in- Human Trial
 AVX-470, an Orally Delivered Anti-TNF Antibody for Treatment of Acute Ulcerative Colitis: Results of a First-in- Human Trial M Scott Harris 1, Deborah Hartman 1, Sharon Spence 1, Sally Kennedy 1, Theodore
AVX-470, an Orally Delivered Anti-TNF Antibody for Treatment of Acute Ulcerative Colitis: Results of a First-in- Human Trial M Scott Harris 1, Deborah Hartman 1, Sharon Spence 1, Sally Kennedy 1, Theodore
Review article: the long-term management of ulcerative colitis
 Aliment Pharmacol Ther 2004; 20 (Suppl. 4): 97 101. Review article: the long-term management of ulcerative colitis S. B. HANAUER Section of Gastroenterology, University of Chicago, Chicago, IL, USA SUMMARY
Aliment Pharmacol Ther 2004; 20 (Suppl. 4): 97 101. Review article: the long-term management of ulcerative colitis S. B. HANAUER Section of Gastroenterology, University of Chicago, Chicago, IL, USA SUMMARY
ENTYVIO (VEDOLIZUMAB)
 ENTYVIO (VEDOLIZUMAB) UnitedHealthcare Community Plan Medical Benefit Drug Policy Policy Number: CS2017D0053F Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
ENTYVIO (VEDOLIZUMAB) UnitedHealthcare Community Plan Medical Benefit Drug Policy Policy Number: CS2017D0053F Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Positioning Biologics in Ulcerative Colitis
 Positioning Biologics in Ulcerative Colitis Bruce E. Sands, MD, MS Acting Chief, Gastrointestinal Unit Massachusetts General Hospital Associate Professor of Medicine Harvard Medical School Sequential Therapies
Positioning Biologics in Ulcerative Colitis Bruce E. Sands, MD, MS Acting Chief, Gastrointestinal Unit Massachusetts General Hospital Associate Professor of Medicine Harvard Medical School Sequential Therapies
As clinicians we would all agree that the goal for our
 CURRENT CONTROVERSIES: PRO, CON, AND BALANCE Controversies in Mucosal Healing in Ulcerative Colitis Sunanda Kane, MD,* Frances Lu, MD, Asher Kornbluth, MD, Dahlia Awais, MD, and Peter D.R. Higgins, MD,
CURRENT CONTROVERSIES: PRO, CON, AND BALANCE Controversies in Mucosal Healing in Ulcerative Colitis Sunanda Kane, MD,* Frances Lu, MD, Asher Kornbluth, MD, Dahlia Awais, MD, and Peter D.R. Higgins, MD,
There is a well-established association between inflammatory
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:1346 1350 Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis DAVID T. RUBIN, ANDELKA LOSAVIO, NICOLE YADRON,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:1346 1350 Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis DAVID T. RUBIN, ANDELKA LOSAVIO, NICOLE YADRON,
Full title of guideline: Ulcerative colitis: the management of ulcerative colitis
 Economic Plan This document identifies the priorities for economic analysis and the proposed methods for addressing these questions as described in section 7.1.3 of the Guidelines Manual (2009). 1 Guideline
Economic Plan This document identifies the priorities for economic analysis and the proposed methods for addressing these questions as described in section 7.1.3 of the Guidelines Manual (2009). 1 Guideline
SYNOPSIS (PROTOCOL WX17796)
 TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
Clinical Policy: Budesonide (Uceris) Reference Number: CP.PCH.11 Effective Date: Last Review Date: Line of Business: Commercial, HIM*
 Clinical Policy: (Uceris) Reference Number: CP.PCH.11 Effective Date: 08.14.18 Last Review Date: 11.18 Line of Business: Commercial, HIM* Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Uceris) Reference Number: CP.PCH.11 Effective Date: 08.14.18 Last Review Date: 11.18 Line of Business: Commercial, HIM* Revision Log See Important Reminder at the end of this policy for
Endpoints for Stopping Treatment in UC
 Endpoints for Stopping Treatment in UC Jana G. Hashash, MD Assistant Professor of Medicine Inflammatory Bowel Disease Center Division of Gastroenterology, Hepatology, and Nutrition University of Pittsburgh
Endpoints for Stopping Treatment in UC Jana G. Hashash, MD Assistant Professor of Medicine Inflammatory Bowel Disease Center Division of Gastroenterology, Hepatology, and Nutrition University of Pittsburgh
Efficacy and Safety of Treatment for Pediatric IBD
 Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Assistant Professor of Clinical Pediatrics Division of Gastroenterology,
Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Assistant Professor of Clinical Pediatrics Division of Gastroenterology,
Definitions. Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency)
 CROHN S DISEASE Definitions Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency) Recurrence: The reappearance of lesions after surgical resection Endoscopic remission:
CROHN S DISEASE Definitions Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency) Recurrence: The reappearance of lesions after surgical resection Endoscopic remission:
FOR UK NURSING MEDIA Embargoed until: 00:01 GMT, Friday 13 March 2015
 Contact: Ross Selby Takeda UK Ltd Email ross.selby@takeda.com News Release FOR UK NURSING MEDIA Embargoed until: 00:01 GMT, Friday 13 March 2015 World s first gut-selective treatment for ulcerative colitis
Contact: Ross Selby Takeda UK Ltd Email ross.selby@takeda.com News Release FOR UK NURSING MEDIA Embargoed until: 00:01 GMT, Friday 13 March 2015 World s first gut-selective treatment for ulcerative colitis
1. Comparative effectiveness of vedolizumab
 Cost-effectiveness of vedolizumab (Entyvio ) for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were
Cost-effectiveness of vedolizumab (Entyvio ) for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were
Clinical Trials in IBD. Bruce Yacyshyn MD Professor of Medicine Division of Digestive Diseases
 Clinical Trials in IBD Bruce Yacyshyn MD Professor of Medicine Division of Digestive Diseases Objectives Today s discussion will address the following topics: Similarities and differences between Crohn
Clinical Trials in IBD Bruce Yacyshyn MD Professor of Medicine Division of Digestive Diseases Objectives Today s discussion will address the following topics: Similarities and differences between Crohn
Dr. Elmer Schabel, MD. Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn, Germany (No conflicts of interest)
 EMA workshop on the development of new medicinal products for the treatment of ulcerative colitis and Crohn s disease Overview of authorised medicines for IBD in Europe - previous regulatory positions
EMA workshop on the development of new medicinal products for the treatment of ulcerative colitis and Crohn s disease Overview of authorised medicines for IBD in Europe - previous regulatory positions
Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy
 Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy Stephen B. Hanauer, MD University of Chicago Potential Conflicts: Centocor/Schering, Abbott, UCB, Elan, Berlex, PDL Goals of Treatment
Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy Stephen B. Hanauer, MD University of Chicago Potential Conflicts: Centocor/Schering, Abbott, UCB, Elan, Berlex, PDL Goals of Treatment
Mucosal healing: does it really matter?
 Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does it really matter? Professor Jean-Frédéric Colombel, New York, USA Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does
Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does it really matter? Professor Jean-Frédéric Colombel, New York, USA Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
Makoto Naganuma 1 Nobuo Aoyama. Fumihito Hirai 5 Kenji Watanabe
 J Gastroenterol (2018) 53:494 506 https://doi.org/10.1007/s00535-017-1376-4 ORIGINAL ARTICLE ALIMENTARY TRACT Complete mucosal healing of distal lesions induced by twice-daily budesonide 2-mg foam promoted
J Gastroenterol (2018) 53:494 506 https://doi.org/10.1007/s00535-017-1376-4 ORIGINAL ARTICLE ALIMENTARY TRACT Complete mucosal healing of distal lesions induced by twice-daily budesonide 2-mg foam promoted
Article: Min, T and Ford, AC (2016) Efficacy of mesalazine in IBS. Gut, 65 (1). pp ISSN
 This is a repository copy of Efficacy of mesalazine in IBS. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/97338/ Version: Accepted Version Article: Min, T and Ford, AC (2016)
This is a repository copy of Efficacy of mesalazine in IBS. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/97338/ Version: Accepted Version Article: Min, T and Ford, AC (2016)
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Sat, 01 Dec 2018 00:07:49 GMT) CTRI Number CTRI/2010/091/000387 [Registered on: 20/05/2010] - Last Modified On 15/03/2013 Post Graduate Thesis Type of Trial
Clinical Trial Details (PDF Generation Date :- Sat, 01 Dec 2018 00:07:49 GMT) CTRI Number CTRI/2010/091/000387 [Registered on: 20/05/2010] - Last Modified On 15/03/2013 Post Graduate Thesis Type of Trial
Dr David Epstein Vincent Pallotti Hospital and University of Cape Town
 Inflammatory Bowel Disease Management in South Africa in 2016 Pharmaceutical Care Management Association Dr David Epstein Vincent Pallotti Hospital and University of Cape Town Inflammatory Bowel Disease
Inflammatory Bowel Disease Management in South Africa in 2016 Pharmaceutical Care Management Association Dr David Epstein Vincent Pallotti Hospital and University of Cape Town Inflammatory Bowel Disease
Clinical Policy: Vedolizumab (Entyvio) Reference Number: CP.PHAR.265
 Clinical Policy: (Entyvio) Reference Number: CP.PHAR.265 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Entyvio) Reference Number: CP.PHAR.265 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
How to Optimize Induction and Maintenance Responses: Definitions and Dosing Advances in Inflammatory Bowel Disease December 6, 2009
 How to Optimize Induction and Maintenance Responses: Definitions and Dosing 2009 Advances in Inflammatory Bowel Disease December 6, 2009 Fernando Velayos MD MPH University of California, San Francisco
How to Optimize Induction and Maintenance Responses: Definitions and Dosing 2009 Advances in Inflammatory Bowel Disease December 6, 2009 Fernando Velayos MD MPH University of California, San Francisco
INFLAMMATORY BOWEL DISEASE. Brittany Palasik, PharmD, BCPS University of North Texas System College of Pharmacy
 INFLAMMATORY BOWEL DISEASE Brittany Palasik, PharmD, BCPS University of North Texas System College of Pharmacy Pharmacist learning objectives By the end of this presentation, the pharmacist should be able
INFLAMMATORY BOWEL DISEASE Brittany Palasik, PharmD, BCPS University of North Texas System College of Pharmacy Pharmacist learning objectives By the end of this presentation, the pharmacist should be able
Medical Therapy for Pediatric IBD: Efficacy and Safety
 Medical Therapy for Pediatric IBD: Efficacy and Safety Betsy Maxwell, MD Assistant Professor of Clinical Pediatrics Division of Gastroenterology, Hepatology, and Nutrition Pediatric IBD: Defining Remission
Medical Therapy for Pediatric IBD: Efficacy and Safety Betsy Maxwell, MD Assistant Professor of Clinical Pediatrics Division of Gastroenterology, Hepatology, and Nutrition Pediatric IBD: Defining Remission
Randomised clinical trial: delayed-release oral mesalazine 4.8 g day vs. 2.4 g day in endoscopic mucosal healing ASCEND I and II combined analysis
 Alimentary Pharmacology and Therapeutics Randomised clinical trial: delayed-release oral mesalazine 4.8 g day vs. 2.4 g day in endoscopic mucosal healing ASCEND I and II combined analysis G. R. Lichtenstein*,
Alimentary Pharmacology and Therapeutics Randomised clinical trial: delayed-release oral mesalazine 4.8 g day vs. 2.4 g day in endoscopic mucosal healing ASCEND I and II combined analysis G. R. Lichtenstein*,
APDW 2016 Poster No. a90312
 APDW 2016 Poster No. a90312 SYN-010, a Proprietary Modified-Release Formulation of Lovastatin Lactone, Lowered Breath Methane and Improved Stool Frequency in Patients with IBS-C Results of a multi-center,
APDW 2016 Poster No. a90312 SYN-010, a Proprietary Modified-Release Formulation of Lovastatin Lactone, Lowered Breath Methane and Improved Stool Frequency in Patients with IBS-C Results of a multi-center,
CLINICAL TRIAL PROTOCOL
 CLINICAL TRIAL PROTOCOL Protocol Title: A randomised, open study of aminosalicylate withdrawal in patients with ulcerative colitis in established remission on combination treatment of azathioprine (or
CLINICAL TRIAL PROTOCOL Protocol Title: A randomised, open study of aminosalicylate withdrawal in patients with ulcerative colitis in established remission on combination treatment of azathioprine (or
Treating Crohn s and Colitis in the ASC
 Treating Crohn s and Colitis in the ASC Kimberly M Persley, MD Texas Digestive Disease consultants TASC Meeting Outline IBD 101 Diagnosis Treatment Burden of Disease Role of ASC Inflammatory Bowel Disease
Treating Crohn s and Colitis in the ASC Kimberly M Persley, MD Texas Digestive Disease consultants TASC Meeting Outline IBD 101 Diagnosis Treatment Burden of Disease Role of ASC Inflammatory Bowel Disease
Azathioprine for Induction and Maintenance of Remission in Crohn s Disease
 Azathioprine for Induction and Maintenance of Remission in Crohn s Disease William J. Sandborn, MD Chief, Division of Gastroenterology Director, UCSD IBD Center Objectives Azathioprine as induction and
Azathioprine for Induction and Maintenance of Remission in Crohn s Disease William J. Sandborn, MD Chief, Division of Gastroenterology Director, UCSD IBD Center Objectives Azathioprine as induction and
Treatment of Inflammatory Bowel Disease. Michael Weiss MD, FACG
 Treatment of Inflammatory Bowel Disease Michael Weiss MD, FACG What is IBD? IBD is an immune-mediated chronic intestinal disorder, characterized by chronic or relapsing inflammation within the GI tract.
Treatment of Inflammatory Bowel Disease Michael Weiss MD, FACG What is IBD? IBD is an immune-mediated chronic intestinal disorder, characterized by chronic or relapsing inflammation within the GI tract.
Understanding Inflammatory Bowel Diseases (IBD):
 Understanding Inflammatory Bowel Diseases (IBD): What Every Patient Needs to Know William H Holderman, MD Digestive Health Specialists Tacoma, WA Today s Objectives Define IBD, its potential causes and
Understanding Inflammatory Bowel Diseases (IBD): What Every Patient Needs to Know William H Holderman, MD Digestive Health Specialists Tacoma, WA Today s Objectives Define IBD, its potential causes and
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Title: Authors: Journal:
 IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
CLINICAL TRIAL PROTOCOL
 CLINICAL TRIAL PROTOCOL Azathioprine maintenance therapy in steroid-refractory Ulcerative Colitis responsive to i.v. Cyclosporine A: Is a therapeutic bridge with oral Cyclosporine A necessary? Miquel A.
CLINICAL TRIAL PROTOCOL Azathioprine maintenance therapy in steroid-refractory Ulcerative Colitis responsive to i.v. Cyclosporine A: Is a therapeutic bridge with oral Cyclosporine A necessary? Miquel A.
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017
 SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
5-aminosalicylic acid (5-ASA) is the mainstay of first-line therapy
 MMX Mesalamine for Induction and Maintenance Therapy in Mild-to-Moderate Ulcerative Colitis Stephen B. Hanauer, MD 1 ; Gary R. Lichtenstein, MD 2 ; Michael A. Kamm, MD 3 ; William J. Sandborn, MD 4 ; Kirstin
MMX Mesalamine for Induction and Maintenance Therapy in Mild-to-Moderate Ulcerative Colitis Stephen B. Hanauer, MD 1 ; Gary R. Lichtenstein, MD 2 ; Michael A. Kamm, MD 3 ; William J. Sandborn, MD 4 ; Kirstin
Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial
 Alimentary Pharmacology and Therapeutics Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial G. R. Lichtenstein*, G. L.
Alimentary Pharmacology and Therapeutics Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial G. R. Lichtenstein*, G. L.
The National Association of Crohn s and Colitis of Trinidad and Tobago CROHN S DISEASE AND ULCERATIVE COLITIS GENERAL PATIENT INFORMATION
 The National Association of Crohn s and Colitis of Trinidad and Tobago CROHN S DISEASE AND ULCERATIVE COLITIS GENERAL PATIENT INFORMATION You are reading this pamphlet because you or someone you known
The National Association of Crohn s and Colitis of Trinidad and Tobago CROHN S DISEASE AND ULCERATIVE COLITIS GENERAL PATIENT INFORMATION You are reading this pamphlet because you or someone you known
SYNOPSIS. Issue Date: 25 Oct 2011
 SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
BRL /RSD-101C0D/1/CPMS-704. Report Synopsis
 Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Crohn's disease CAUSES COURSE OF CROHN'S DISEASE TREATMENT. Sulfasalazine
 Crohn's disease Crohn's disease is an inflammatory condition of the digestive tract that affects children and adults. Common features of Crohn's disease include mouth sores, diarrhea, abdominal pain, weight
Crohn's disease Crohn's disease is an inflammatory condition of the digestive tract that affects children and adults. Common features of Crohn's disease include mouth sores, diarrhea, abdominal pain, weight
Review article: balsalazide therapy in ulcerative colitis
 Aliment Pharmacol Ther 2001; 15: 1549±1554. Review article: balsalazide therapy in ulcerative colitis K. RAGUNATH & J. G. WILLIAMS Centre for Digestive Diseases and Nutrition, Morriston Hospital, Swansea,
Aliment Pharmacol Ther 2001; 15: 1549±1554. Review article: balsalazide therapy in ulcerative colitis K. RAGUNATH & J. G. WILLIAMS Centre for Digestive Diseases and Nutrition, Morriston Hospital, Swansea,
Ulcerative Colitis. National Digestive Diseases Information Clearinghouse. What is ulcerative colitis (UC)?
 Ulcerative Colitis National Digestive Diseases Information Clearinghouse What is ulcerative colitis (UC)? Ulcerative colitis is a chronic, or long-lasting, disease that causes inflammation and sores, called
Ulcerative Colitis National Digestive Diseases Information Clearinghouse What is ulcerative colitis (UC)? Ulcerative colitis is a chronic, or long-lasting, disease that causes inflammation and sores, called
Latest Treatment Updates for Ulcerative Colitis: Evolving Treatment Goals
 Latest Treatment Updates for Ulcerative Colitis: Evolving Treatment Goals Stephen Hanauer, MD Professor of Medicine Medical Director, Digestive Disease Center Northwestern Medicine Chicago, Illinois Speaker
Latest Treatment Updates for Ulcerative Colitis: Evolving Treatment Goals Stephen Hanauer, MD Professor of Medicine Medical Director, Digestive Disease Center Northwestern Medicine Chicago, Illinois Speaker
A Case of Crohn s Disease with Mesalazine Allergy that was Difficult to Differentiate from Comorbid Ulcerative Colitis
 doi: 10.2169/internalmedicine.1607-18 http://internmed.jp CASE REPORT A Case of Crohn s Disease with Mesalazine Allergy that was Difficult to Differentiate from Comorbid Ulcerative Colitis Rumiko Tsuboi,
doi: 10.2169/internalmedicine.1607-18 http://internmed.jp CASE REPORT A Case of Crohn s Disease with Mesalazine Allergy that was Difficult to Differentiate from Comorbid Ulcerative Colitis Rumiko Tsuboi,
DENOMINATOR: All patients aged 18 and older with a diagnosis of inflammatory bowel disease
 Measure #270: Inflammatory Bowel Disease (IBD): Preventive Care: Corticosteroid Sparing Therapy National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Measure #270: Inflammatory Bowel Disease (IBD): Preventive Care: Corticosteroid Sparing Therapy National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
What is ulcerative colitis?
 What is ulcerative colitis? Ulcerative colitis is a disease that causes inflammation and sores, called ulcers, in the lining of the rectum and colon. Ulcers form where inflammation has killed the cells
What is ulcerative colitis? Ulcerative colitis is a disease that causes inflammation and sores, called ulcers, in the lining of the rectum and colon. Ulcers form where inflammation has killed the cells
Soleus 75 (6 ml) 0 (6 ml) 75 (6 ml. Tibialis posterior 75 (6 ml) 0 (6 ml) 75 (6 ml) Total 300 (24 ml) 0 (24 ml) 300 (24 ml) Dose: U (solution volume)
 Study No.: BTX108512 Title: A Multicenter Study to Evaluate the Efficacy and Safety in Patients with Post-Stroke lower Limb Spasticity Receiving a Double-Blind, -Controlled GSK1358820 Treatment Followed
Study No.: BTX108512 Title: A Multicenter Study to Evaluate the Efficacy and Safety in Patients with Post-Stroke lower Limb Spasticity Receiving a Double-Blind, -Controlled GSK1358820 Treatment Followed
Clinical Study Tacrolimus for Remission Induction and Maintenance Therapy in Patients with Ulcerative Colitis: A Retrospective Evaluation Study
 Gastroenterology Research and Practice Volume 2016, Article ID 5956316, 6 pages http://dx.doi.org/10.1155/2016/5956316 Clinical Study Tacrolimus for Remission Induction and Maintenance Therapy in Patients
Gastroenterology Research and Practice Volume 2016, Article ID 5956316, 6 pages http://dx.doi.org/10.1155/2016/5956316 Clinical Study Tacrolimus for Remission Induction and Maintenance Therapy in Patients
Oxford Inflammatory Bowel Disease MasterClass The mesalazine chamaeleon
 Oxford Inflammatory Bowel Disease MasterClass The mesalazine chamaeleon Ailsa Hart Lead IBD Unit, St Mark s Hospital Senior Clinical lecturer Imperial College, London Oxford Inflammatory Bowel Disease
Oxford Inflammatory Bowel Disease MasterClass The mesalazine chamaeleon Ailsa Hart Lead IBD Unit, St Mark s Hospital Senior Clinical lecturer Imperial College, London Oxford Inflammatory Bowel Disease
Treatment Goals. Current Therapeutic Pyramids Crohn s Disease Ulcerative Colitis 11/14/10
 Current Management of IBD: From Conventional Agents to Biologics Stephen B. Hanauer, M.D. University of Chicago Treatment Goals Induce and maintain response/ remission Prevent complications Improve quality
Current Management of IBD: From Conventional Agents to Biologics Stephen B. Hanauer, M.D. University of Chicago Treatment Goals Induce and maintain response/ remission Prevent complications Improve quality
WHAT IS ULCERATIVE COLITIS?
 235 60th Street, West New York, NJ 07093 T: (201) 854-4646 F: (201) 854-4647 810 Main Street, Hackensack, NJ 07601 T: (201) 488-0095 Ulcerative Colitis WHAT IS ULCERATIVE COLITIS? Ulcerative colitis is
235 60th Street, West New York, NJ 07093 T: (201) 854-4646 F: (201) 854-4647 810 Main Street, Hackensack, NJ 07601 T: (201) 488-0095 Ulcerative Colitis WHAT IS ULCERATIVE COLITIS? Ulcerative colitis is
Which is the Safest Strategy to Treat Moderate to Severe IBD?
 Which is the Safest Strategy to Treat Moderate to Severe IBD? David G. Binion, M.D. Co-Director, Inflammatory Bowel Disease Center Director, Translational Inflammatory Bowel Disease Research Visiting Professor
Which is the Safest Strategy to Treat Moderate to Severe IBD? David G. Binion, M.D. Co-Director, Inflammatory Bowel Disease Center Director, Translational Inflammatory Bowel Disease Research Visiting Professor
To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture,
 To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture, click Options in the Message Bar, and then click Enable
To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture, click Options in the Message Bar, and then click Enable
Title: Value of fecal calprotectin in the evaluation of patients with abdominal discomfort: an observational study
 Author's response to reviews Title: Value of fecal calprotectin in the evaluation of patients with abdominal discomfort: an observational study Authors: Michael Manz (michael.manz@claraspital.ch) Emanuel
Author's response to reviews Title: Value of fecal calprotectin in the evaluation of patients with abdominal discomfort: an observational study Authors: Michael Manz (michael.manz@claraspital.ch) Emanuel
Inflammatory bowel disease (IBD) Overview of the Paediatric investigation plans. Presented by: Richard Veselý. An agency of the European Union
 Inflammatory bowel disease (IBD) Overview of the Paediatric investigation plans Presented by: Richard Veselý An agency of the European Union Adalimumab - Crohn s disease Indication: Treatment of severe,
Inflammatory bowel disease (IBD) Overview of the Paediatric investigation plans Presented by: Richard Veselý An agency of the European Union Adalimumab - Crohn s disease Indication: Treatment of severe,
WHAT IF WE HAVE A FUN DAY WITHOUT UC GETTING IN THE WAY? INDICATIONS IMPORTANT RISK INFORMATION
 WHAT IF WE HAVE A FUN DAY WITHOUT UC GETTING IN THE WAY? Learn how DELZICOL can help you manage your ulcerative colitis INDICATIONS DELZICOL (mesalamine) delayed-release capsules is a prescription medication
WHAT IF WE HAVE A FUN DAY WITHOUT UC GETTING IN THE WAY? Learn how DELZICOL can help you manage your ulcerative colitis INDICATIONS DELZICOL (mesalamine) delayed-release capsules is a prescription medication
Ulcerative colitis (UC) is a chronic inflammatory
 Induction and Maintenance Therapy with Vedolizumab, a Novel Biologic Therapy for Ulcerative Colitis Feagan BG, Rutgeerts P, Sands BE, et al; GEMINI 1 Study Group. Vedolizumab as induction and maintenance
Induction and Maintenance Therapy with Vedolizumab, a Novel Biologic Therapy for Ulcerative Colitis Feagan BG, Rutgeerts P, Sands BE, et al; GEMINI 1 Study Group. Vedolizumab as induction and maintenance
The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. doi:10.
 Oral 5-Aminosalicylate, Suppository, and Enema as Initial Therapy for Ulcerative Proctitis in Clinical Practice with Quality of Care Implications The Harvard community has made this article openly available.
Oral 5-Aminosalicylate, Suppository, and Enema as Initial Therapy for Ulcerative Proctitis in Clinical Practice with Quality of Care Implications The Harvard community has made this article openly available.
Determinants of Topical 5-Aminosalicylic Acid Use in Veterans with Ulcerative Colitis
 Research Article imedpub Journals http://www.imedpub.com/ Abstract Determinants of Topical 5-Aminosalicylic Acid Use in Veterans with Ulcerative Colitis Aims: The aim of our study was to identify the determinants
Research Article imedpub Journals http://www.imedpub.com/ Abstract Determinants of Topical 5-Aminosalicylic Acid Use in Veterans with Ulcerative Colitis Aims: The aim of our study was to identify the determinants
Anti-TNF and cyclosporine are identical choices for severe ulcerative colitis refractory to steroid therapy CON Peter Laszlo LAKATOS Semmelweis
 Anti-TNF and cyclosporine are identical choices for severe ulcerative colitis refractory to steroid therapy CON Peter Laszlo LAKATOS Semmelweis University, 1st Department of Medicine Budapest June 13-15,
Anti-TNF and cyclosporine are identical choices for severe ulcerative colitis refractory to steroid therapy CON Peter Laszlo LAKATOS Semmelweis University, 1st Department of Medicine Budapest June 13-15,
