King s Research Portal
|
|
|
- Oliver Shaw
- 5 years ago
- Views:
Transcription
1 King s Research Portal DOI: /bjon S4 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Prichard, D., Norton, C., & Bharucha, A. E. (2016). Management of opioid-induced constipation. British Journal of Nursing, 25(10), S4-S /bjon S4 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact librarypure@kcl.ac.uk providing details, and we will remove access to the work immediately and investigate your claim. Download date: 18. Feb. 2017
2 Management of Opioid Induced Constipation Authors David Prichard, MB BCh, PhD. Division of Gastroenterology, Mayo Clinic Health System, La Crosse and Mayo Clinic, Rochester 800 West Avenue South, La Crosse, WI 54601, USA Christine Norton, PhD, MA, RN. Florence Nightingale Foundation Professor of Clinical Nursing Practice Research King s College, 57 Waterloo Road, London SE1 8WA, UK Christine.norton@kcl.ac.uk Adil E. Bharucha, MBBS, MD. (Corresponding Author) Professor of Medicine, Clinical Enteric Neuroscience Translational and Epidemiological Research Program, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 1st Street, Rochester, MN 55905, USA bharucha.adil@mayo.edu Word Count 3300 Keywords Chronic Pain, Constipation, Laxatives, Opioids, Naloxone, Methylnaltrexone Page 1 of 16
3 Abstract Up to 40% of patients taking opioids develop constipation. Opioid induced constipation (OIC) may limit the adequate dosing of opioids for pain relief and reduce quality of life. Hence, healthcare providers must inquire about bowel function in patients receiving opioids. The management of OIC includes carefully reevaluating the necessity, type, and dose of opioids at each visit. Lifestyle modification and alteration of aggravating factors, the use of simple laxatives, and when essential, the addition of newer laxatives or opioid antagonists (naloxone, naloxegol or methylnaltrexone) can be used to treat OIC. This review discusses the recent literature regarding the management of OIC and provides a rational approach to assessing and managing constipation in individuals receiving opioids. Abstract word count Page 2 of 16
4 Introduction Up to one third of the population in the western world report chronic pain (Johannes et al, 2010). For these individuals, effective analgesia alleviates suffering, promotes functionality and improves quality of life. In principle, the simplest and safest analgesic medication which provides adequate relief should be used. However, simple analgesics (e.g. acetaminophen [paracetamol] or non-steroidal anti-inflammatory drugs) provide insufficient relief for many individuals with chronic pain (Moore et al, 2013; Machado et al, 2015). For others, simple analgesics are avoided in view of their adverse gastrointestinal or cardiovascular risk profiles (Lanas et al, 2010). Consequently, opioid analgesia is prescribed. Although potentially effective in relieving pain (Mercadante, 1999) and improving quality of life (Klepstad et al, 2000), opioids are associated with different side effects compared to simple analgesics. Adverse effects associated with opioids are frequent and affect numerous organ systems. Within the gastrointestinal system constipation, nausea, vomiting, reflux, and bloating are reported (Bell et al, 2009). Among the opioid side effects, constipation is the most frequent. It may be more severe (i.e. less frequent bowel motions, harder stools, more straining and a greater sense of incomplete evacuation) than constipation unrelated to opioid use. Additionally, unlike many other opioid side effects (e.g. nausea), the severity of opioid induced constipation (OIC) does diminish over time with ongoing opioid administration (i.e. tolerance does not develop) (Bell et al, 2009). The severity of OIC, and other adverse effects, can limit effective dosing of opioid analgesics (Bell et al, 2009). Adverse effects can therefore negatively impact activities of daily living and quality of life. In general, there are two options for managing these side effects, i.e., reducing or discontinuing opioid administration or using other measures to treat side effects. As the prevalence of chronic pain and use of opioid medications increases in society (Zin et al, 2014), health care providers need to be versed in the appropriate use of opioids as well as preventing and managing OIC. Physiology of Opioid Induced Constipation A comprehensive description of the physiological effects of opioid agonists and antagonists is reviewed elsewhere.{wood, 2004 #167} Briefly, opioid receptors are the natural ligand for endogenously produced neurotransmitters. There are three types of receptors (μ, δ or κ receptors), distributed throughout the central, peripheral and enteric Page 3 of 16
5 nervous systems. Analgesia is achieved through stimulation of central μ opioid receptors. However, stimulation of μ receptors in the enteric nervous system results in adverse gastrointestinal effects, specifically because of i) increased pyloric, anal and biliary sphincter tone, ii) reduced enteric, biliary, and pancreatic secretions, iii) increased water absorption from the bowel and, iv) reduced gastric emptying and reduced propulsion of chyme through the intestine (De Schepper et al, 2004). Individually, and in combination, these physiological changes promote constipation. However, the acute effects of various opioids on gastrointestinal function and symptoms vary. For example, although codeine, oxycodone and tapentadol all cause constipation, only codeine delays colonic transit (Gonenne et al, 2005; Jeong et al, 2012). When compared to oxycodone, tapentadol appears to cause less constipation, nausea and vomiting (Stegmann et al, 2008). Assessment Clinical Some patients with constipation may not volunteer the symptom. Therefore, during each healthcare interaction with patients taking opioids, they should be questioned regarding bowel motion frequency, consistency, straining, completeness of evacuation and satisfaction with their bowel habit. The precise questions, which are detailed elsewhere {Longstreth, 2006 #168}, have been validated in ambulatory people but not in opioid-induced constipation. Responses to these questions should be documented to facilitate subsequent comparisons and treatment should be initiated if required. A systematic review of fifteen randomised, placebo-controlled trials of opioids observed that constipation (41%), nausea (32%) and somnolence (29%) were the most common side effects (Kalso et al, 2004). Although specific populations (e.g. older patients and those with cancer related pain) may have a greater risk of developing OIC (Rosti et al, 2010; Ishihara et al, 2012), constipation should not be attributed to opioid administration by default. OIC is defined as a change when initiating opioid therapy from baseline bowel habits that is characterised by any of the following: reduced bowel movement frequency, development or worsening of straining to pass bowel movements, a sense of incomplete rectal evacuation, or harder stool consistency (Camilleri et al, 2014). The temporal association between initiation or escalation of the dose of opioids and a change in bowel habit is required to establish the diagnosis. Without this clear association other structural, functional, pharmacological and biochemical causes (Table 1) should be excluded as Page 4 of 16
6 necessary (and as appropriate for the underlying condition and overall prognosis) as constipation is a common symptom in the general population (Bharucha et al, 2013). A meticulous examination of both the anal canal and rectum is mandatory during initial assessment to identify conditions causing, or contributing to, constipation. Within the anal canal, pathology affecting the mucosa (anal fissures) or poorly coordinated muscular function (dyssynergia) should be sought. The rectum should be evaluated for faeces (possibly suggesting faecal impaction) or neoplastic masses (Talley, 2008). Investigations Investigations are recommended when constipation does not respond to simple laxatives. When investigation of constipation is required, (i.e. not clearly associated with opioid use) the initial assessment should include a complete blood count and sodium, calcium, potassium and thyroid stimulating hormone levels (Bharucha et al, 2013). In individuals in whim adrenal failure is suspected, cortisol levels should be assessed. In general, endoscopic evaluation (colonoscopy or sigmoidoscopy) is probably unnecessary in patients with an advanced illness and OIC. However, these investigations should be considered when there is a strong index of suspicion for colorectal cancer, such as unexplained weight loss or significant rectal bleeding that cannot be explained by the underlying condition. A plain abdominal X-ray can be useful to identify faecal impaction and/or bowel obstruction. Cross sectional imaging (e.g., computed tomography or MRI) should be considered only if there is concern for sigmoid volvulus, malignant colonic obstruction or an intra-abdominal mass. In patients with constipation refractory to laxatives, anorectal manometry and a rectal balloon expulsion test are generally recommended to identify defecatory disorders such as functional outlet obstruction, which are treated with biofeedback therapy (Bharucha et al, 2013). Barium defecography can also be used for this purpose. Prevention of OIC Prophylactic laxatives are recommended at the time of, or before, opioid prescription to prevent OIC. While sensible, there is little research to support this practice. A single trial of 619 hospitalised patients from Japan reported that the use of laxatives before opioids were initiated was associated with a lower incidence of constipation, defined as more than 72 hours without a bowel motion (34% vs. 55%, Odds Ratio 0.43, 95% CI ) Page 5 of 16
7 (Ishihara et al, 2012). Although the majority of patients received an osmotic laxative (magnesium oxide), with or without a stimulant laxative, the use of laxatives was not standardised. In addition, the trial was short, only hospitalised patients were included and neither other symptoms of constipation (e.g., excessive straining) nor the impact of this intervention on quality of life were assessed. Nonetheless, the high incidence of OIC and its impact on quality of life justifies this intervention. Management of OIC We recommend a stepwise approach to managing OIC commencing with modification of lifestyle factors and precipitants. Simple laxatives can be added simultaneously or sequentially as needed. Symptoms should be re-evaluated at intervals sufficient for the effects of laxatives to become apparent. The psychological aspects of the underlying disease should also be evaluated and managed. A careful evaluation of the patient s understanding regarding their constipation and underlying disease, with teaching, explanation and supportive reassurance, where required, can be of benefit in treating physical symptoms and influencing opioid use (Adams et al, 2006; Richardson et al, 2006). Exclusion and Treatment of Underlying Precipitants and Disorders The initial management of constipation should focus on treating underlying causes and reducing aggravating factors. For example, endocrine or metabolic disturbances should be treated and medications that exacerbate constipation should be discontinued or replaced with non-constipating alternatives where possible (Bharucha et al, 2013). Lifestyle choices which promote healthy bowel habits should be encouraged although limited evidence exists to support practice. Increasing dietary fibre content should be considered but can cause or increase abdominal bloating. Hydration and physical activity should be encouraged. One study suggested that immobility may have a greater constipating effect than opioid medications (Fallon, 1999). For patients with reduced mobility, the use of a bedpan is associated with constipation; hence this should be avoided when possible (Su et al, 2009). Optimisation of Opioid Administration In our opinion, the long term use of opioids in patients with non-cancer non-palliative pain (e.g. chronic abdominal pain, fibromyalgia) should be strongly discouraged. In these Page 6 of 16
8 patients, other multidisciplinary approaches to manage pain are often more effective and definitely safer (Chaparro et al, 2013). Where opioids are required, administration can frequently be optimised in patients experiencing adverse effects. To achieve this, at every visit, the requirement for opioids, the dose, and type of preparation should be reassessed. Patients who do not experience pain relief after an adequate trial of opioids are unlikely to achieve satisfaction with this class of medication (Stannard, 2013). In this situation, the risk of adverse effects outweighs the benefits. Opioids should be tapered and alternative approaches to pain management initiated (American Society of Anesthesiologists, 2010; Franklin, 2014). Limited evidence suggests that the incidence of OIC is associated with higher (Knezevic et al, 2014) and more frequent (Bell et al, 2009) opioid dosing. When opioids have relieved pain but caused adverse effects, the addition of analgesic adjuncts may facilitate opioid dose reduction. Non-opioid analgesics (acetaminophen or NSAIDs) are simple pharmacological adjuncts (Caraceni et al, 2012). Anti-depressant or anti-epileptic medications may benefit patients with neuropathic pain (Bennett, 2011). Nonpharmacological adjuncts (e.g. meditation, cognitive behavioral therapy [CBT], neurostimulators) may also be of benefit (Park and Hughes, 2012). CBT approaches such as The Pain Plan are increasingly being commissioned from pain teams in the UK (Cole et al, 2012). Where opioids have relieved pain and dose reduction is not possible, consideration should be given to opioid switching as adverse effects vary amongst opioids and by route of administration. Morphine is associated with a higher incidence of GI side effects than many other opioids (Cook et al, 2008; Ishihara et al, 2012). Tapentadol, which is a μ opioid agonist that also inhibits norepinephrine reuptake, was as effective as oxycodone for managing chronic moderate to severe skeletal pain (Lange et al, 2010), but has fewer side effects (Stegmann et al, 2008). The incidence of constipation is lower for transdermal fentanyl than an equianalgesic dose of oral morphine (Tassinari et al, 2008). Laxatives for the Treatment of OIC Simple laxatives are inexpensive and often effective for preventing and managing OIC. In our practice, treatment is initiated with regularly administered osmotic agents (e.g., polyethylene glycol or milk of magnesia). Additionally, a stimulant laxative (e.g. bisacodyl, senna, glycerine suppository or an enema) is administered on an as-needed basis if patients Page 7 of 16
9 do not have a bowel movement for 2 days. However, there is a limited amount of information regarding the efficacy of simple laxatives in OIC. One small study suggested that lactulose, senna, and codanthrusate were of comparable efficacy in treating opioid (loperamide) induced constipation in healthy volunteers (Sykes, 1996). In one survey, the prevalence of constipation was greater (40%) in 76 patients receiving opioids compared to a control group of 10,018 individuals (8%) from a different study (Pappagallo, 2001). Among these constipated individuals, a greater proportion of opioid users were taking laxatives (80% versus 55%). However, a smaller proportion of opioid users (46% versus 84%) reported a satisfactory response to laxatives more than 50% of the time (Pappagallo, 2001). In another study of 322 patients concurrently taking both laxatives and opioids, constipation was reported by 81%. Among the cohort, 45% reported fewer than 3 bowel motions per week, 58% reported straining, 50% reported too small/hard bowel movements and 45% reported incomplete evacuation. Use of more than one laxative was reported by 45% of the group (Bell et al, 2009). Additional studies comparing the response to simple laxatives in OIC and non-oic constipation would be useful. Where simple laxatives are insufficient to relieve OIC, consideration can be given to using lubiprostone or prucalopride. Lubiprostone stimulates intestinal fluid and electrolyte secretion by activating type 2 chloride channels. It is approved by the USA Food and Drug Administration (FDA) for treating refractory constipation and OIC in non-cancer pain. In the United Kingdom, it is only licensed for the treatment of refractory constipation. In one randomised double blind placebo controlled trial of patients taking stable doses of opioids for non-cancer pain, 24 micrograms of lubiprostone administered twice daily increased the number of bowel motions per week at 8 weeks (by 2.2 for lubiprostone versus 1.6 for placebo, P = 0.004). Lubiprostone was also more effective than placebo for reducing straining, abdominal discomfort, and stool consistency but not bloating (Cryer et al, 2014). However, there was no difference in the use of rescue medications for constipation between lubiprostone and placebo. Furthermore, at 12 weeks, differences between lubiprostone and placebo were not significant suggesting the possibility of tolerance. Prucalopride, a selective 5-HT4 agonist, is also effective for treating OIC. Among patients with chronic non cancer pain suffering from OIC, 69% of patients receiving 4mg of prucalopride passed 3 spontaneous bowel motions per week (compared to 43% of patients receiving placebo) after 4 weeks treatment (Sloots et al, 2010). However, it is not approved by the FDA and in the United Kingdom is only approved for the treatment of refractory constipation. Unfortunately, lubiprostone and prucalopride have only been compared to Page 8 of 16
10 placebo. Consequently, the incremental benefit of these drugs over simple laxatives is unknown. Additionally, their efficacy in OIC related to cancer pain is unknown. Treating OIC with Opioid Antagonists Naloxone is a central and peripheral acting opioid receptor antagonist that is efficacious for treating OIC (Meissner et al, 2000). This medication can be administered separately from opioid medications or as a premade fixed dose combination tablet. With fixed dose combinations of an opioid agonist and naloxone, it is not possible to individually titrate the analgesic effects of opioids and laxative effects of naloxone. The effects of naloxone are predominantly limited to the intestine due to a high first pass metabolism. Naloxone therefore inhibits stimulation of μ opioid receptors within the gastrointestinal tract without substantially altering the analgesic efficacy of opioids. However, in patients with impaired liver function (e.g. cirrhosis or metastatic burden), the first pass metabolism of naloxone is reduced and its bioavailability is increased. As naloxone can cross the blood brain barrier, opioid withdrawal and loss of analgesic effect may occur in this situation (Burns et al, 2014). To reduce the likelihood of withdrawal and loss of analgesic effect, naloxone has been conjugated with a polyethylene glycol moiety (Naloxegol), which reduces its ability to cross the blood brain barrier. Oral naloxegol (25 mg once daily) was effective for treating OIC in adults taking opioids for chronic non-cancer pain. In two large studies (total 1352 participants), the frequency of spontaneous bowel movements increased in 40% and 44% of patients. This was 10-15% greater than the response rate to placebo (Chey et al, 2014). Straining and stool consistency were also improved. Among the cohort of patients refractory to laxatives, the response rate was greater (47% and 49%). Given the stringent definition of response in these studies (three or more spontaneous bowel movements per week, without the use of bisacodyl or an enema in the previous 24 hours, and an increase of one or more spontaneous bowel movements over baseline for at least 9 of 12 treatment weeks and at least 3 of the final 4 treatment weeks), the benefit of this medication in clinical practice may be greater. However, adverse events were frequent in these trials, occurring in over two thirds of patients and resulting in naloxegol discontinuation in 10%. The most common were abdominal pain (19%), diarrhea (9%), nausea (9%) and flatulence (6%). Symptoms possibly consistent with opioid withdrawal (eg, hyperhidrosis, chills, diarrhea, abdominal pain, anxiety, irritability, and yawning) were reported in patients treated with naloxegol and may be more frequent in patients receiving methadone and among patients with disruptions to the Page 9 of 16
11 blood-brain barrier. Naloxegol is approved by regulatory agencies in the European Union (EU) and United States (US) for treating OIC in adult patients with chronic non-cancer pain. The peripherally acting μ opioid receptor antagonist (PAMORA) methylnaltrexone blocks only peripherally located μ opioid receptors. Therefore it restores gastrointestinal function with minimal effects on the centrally mediated analgesic properties of opioids. Methylnaltrexone is currently licensed in the US and EU for treating OIC in adults. In the EU, the license specifies that patients must have failed to respond to other laxatives. Approval was based on efficacy demonstrated in two double-blind randomised placebocontrolled trials (Thomas et al, 2008; Michna et al, 2011). During these trials, patients treated with methylnaltrexone reported less distress related to constipation and an improved quality of life. In the first trial, among patients with an advanced illness (60% had cancer), a single dose of methylnaltrexone (0.15mg/kg) was superior to placebo in inducing a bowel motion within 4 hours of administration (48% versus 15% of patients respectively) (Thomas et al, 2008). However, the proportion of patients with rescue-free laxation within 24 hours of dose administration was not significantly different between methlynaltrexone and placebo after the first four doses administered on alternate days. During the subsequent 12 week open label extension study, the response rate was approximately 50%. Patients who had a more pronounced initial response, as manifest by a response to 2 of the initial doses, were more likely to respond to subsequent doses over 12 weeks. Similar findings were reported in the second double-blind trial in patients with non-cancer chronic pain (Michna et al, 2011). In these trials there was only a minimal reduction in the use of simple laxatives. In these trials, mild to moderate abdominal pain (the most frequent side effect) was reported by up to 20% of patients (Thomas et al, 2008; Michna et al, 2011). Nausea, vomiting and flatulence were each reported in over 10% of patients. A reduction in opioid analgesic efficacy has been observed with supra-therapeutic doses (Jagla et al, 2014) but has not been identified in clinical trials of OIC. Although a review of the FDA Adverse Event Reporting System database identified 7 patients (from April October 2009) who had bowel perforation while receiving methylnaltrexone (Mackey et al, 2010), some of these patients had an underlying organic GI disorder or surgery (e.g., metastatic colon cancer, volvulus) and during the period approximately 14,000 patients were billed for the medication. Taken together, these findings do not demonstrate that methylnaltrexone leads to bowel perforation. In contrast to naloxone, methylnaltrexone is available only as a subcutaneous injection. Although an oral preparation of methylnaltrexone was efficacious in pilot studies, it is not available for clinical use (Rauck et al, 2012). Currently, dosing recommendations vary Page 10 of 16
12 slightly between the US and EU. In the US, the recommended dose, given subcutaneously every second day as needed, is 0.15 mg/kg for patients weighing less than 38 kg and more than 114 kg, 8 mg for patients weighing 38 to 62 kg, and 12 mg for patients weighing 62 to 114 kg. Dosing frequency can be increased to every 24 hours if required. The European Medicines (Evaluation) Agency Assessment Report recommends that methylnaltrexone can be given as two consecutive doses 24 hours apart, but thereafter, the dosing frequency should return to every 48 hours. Dosing at the 24 hour interval should be used only in exceptional circumstances. Although a dose response curve to methylnaltrexone has not been reported, there is a suggestion that patients with non-cancer pain and/or who require a morphine equivalent dose greater than 150mg daily at baseline may respond more favorably to 0.3mg/kg of methylnaltrexone than the 0.15mg/kg dose (Nalamachu et al, 2014). Additionally, some studies suggest that different doses of opioid antagonists may be required for treating acute and chronic OIC (Camilleri et al, 2014). In one study, 0.3 mg/kg methylnaltrexone (a dose greater than that effective for treating OIC in clinical trials) was ineffective in attenuating the gastrointestinal effects of acute codeine administration in healthy volunteers (Wong et al, 2010). Further research is needed to clarify these issues. To maximise its therapeutic benefit, methylnaltrexone should only be used only after trials of standard laxative therapies have failed. It is less effective in patients who have coexistent modifiable risk factors (e.g. medications, hypercalcemia) for constipation (Clark et al, 2012). Furthermore, in patients who respond to fewer than two of the initial four doses of methylnaltrexone, there may be little benefit in continuing the medication (Thomas et al, 2008). Management of Faecal Impaction Faecal impaction is a common cause of constipation, especially in patients receiving opioids. A rectal examination will usually reveal hard impacted stool in the rectal vault. A plain X-Ray of the abdomen also provides a qualitative estimate of the amount of stool in the colon. These patients are unlikely to respond to oral therapy. Per rectal measures (e.g. starting with sodium phosphate enemas and progressing to tap water enemas or manual disimpaction) are advisable. After resolution of the impaction, therapy should commence with simple laxatives with escalation as clinically warranted. Conclusion Page 11 of 16
13 Constipation is a common symptom among patients and may limit the ability to provide an adequate dose of opioids. Patients receiving opioids may not volunteer the symptom hence they should be asked about their bowel habits. Consideration should be given to identifying, as appropriate, other causes of constipation in patients taking opioids. Prophylactic laxatives should be administered in an attempt to prevent OIC. If OIC is diagnosed, lifestyle factors should be optimised and simple laxatives and/or per rectal measures (suppositories, enemas) should be used. When feasible, consideration should be given to discontinuing or altering the dose of opioids or switching to an alternative preparation. Adjunctive analgesia should be utilised. Re-evaluation should be performed after each intervention and therapy escalated as warranted by adding newer laxatives, naloxone or methylnaltrexone to a consistent regimen of simple laxatives. Where these medications are required, patients must be monitored for laxative associated side effects and loss of opioid analgesic efficacy. Page 12 of 16
14 References Adams N, Poole H and Richardson C (2006). "Psychological approaches to chronic pain management: part 1." Journal of Clinical Nursing 15(3): American Society of Anesthesiologists (2010). "Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine." Anesthesiology 112(4): Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T and Williamson R (2009). "The Prevalence, Severity, and Impact of Opioid-Induced Bowel Dysfunction: Results of a US and European Patient Survey (PROBE 1)." Pain Medicine 10(1): Bennett MI (2011). "Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review." Palliative Medicine 25(5): Bharucha AE, Pemberton JH and Locke GR, III (2013). "American Gastroenterological Association Technical Review on Constipation." Gastroenterology 144(1): Burns E, McWilliams K and Ross C (2014). "A Cautionary Tale of Oral Naloxone." Journal of Pain and Symptom Management 47(2): e1-e2. Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN and Mawe GM (2014). "Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation." Neurogastroenterology & Motility 26(10): Caraceni A, Hanks G, Kaasa Set al (2012). "Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC." The Lancet Oncology 13(2): e58-e68. Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S and Turk DC (2013). "Opioids compared to placebo or other treatments for chronic low-back pain." Cochrane Database Syst Rev 8: CD Chey W, Webster L, Sostek M, Lappalainen J, Barker PN and Tack J (2014). "Naloxegol for opioidinduced constipation in patients with noncancer pain." New England Journal of Medicine 370(25): Clark K, Byfieldt N, Dawe M and Currow DC (2012). "Treating Constipation in Palliative Care: The Impact of Other Factors Aside From Opioids." American Journal of Hospice and Palliative Medicine 29(2): Cole F, Ashworth P, Coote Let al (2012). "The Pain Management Plan." Pain News 10(2): Cook SF, Lanza L, Zhou Xet al (2008). "Gastrointestinal side effects in chronic opioid users: results from a population-based survey." Alimentary Pharmacology & Therapeutics 27(12): Cryer B, Katz S, Vallejo R, Popescu A and Ueno R (2014). "A Randomized Study of Lubiprostone for Opioid-Induced Constipation in Patients with Chronic Noncancer Pain." Pain Medicine 15(11): De Schepper HU, Cremonini F, Park MI and Camilleri M (2004). "Opioids and the gut: pharmacology and current clinical experience." Neurogastroenterology & Motility 16(4): Fallon MT (1999). "Constipation in cancer patients: prevalence, pathogenesis, and cost-related issues." European Journal of Pain 3, Supplement 1(0): 3-7. Franklin GM (2014). "Opioids for chronic noncancer pain: A position paper of the American Academy of Neurology." Neurology 83(14): Gonenne J, Camilleri M, Ferber Iet al (2005). "Effect of Alvimopan and Codeine on Gastrointestinal Transit: A Randomized Controlled Study." Clinical Gastroenterology and Hepatology 3(8): Ishihara M, Ikesue H, Matsunaga Het al (2012). "A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction." Clin J Pain 28(5): Page 13 of 16
15 Jagla C, Martus P and Stein C (2014). "Peripheral opioid receptor blockade increases postoperative morphine demands A randomized, double-blind, placebo-controlled trial." PAIN 155(10): Jeong I, Camilleri M, Shin Aet al (2012). "A randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans." Alimentary Pharmacology & Therapeutics 35(9): Johannes CB, Le TK, Zhou X, Johnston JA and Dworkin RH (2010). "The Prevalence of Chronic Pain in United States Adults: Results of an Internet-Based Survey." The Journal of Pain 11(11): Kalso E, Edwards JE, Moore RA and McQuay HJ (2004). "Opioids in chronic non-cancer pain: systematic review of efficacy and safety." Pain 112(3): Klepstad P, Borchgrevink PC and Kaasa S (2000). "Effects on Cancer Patients' Health-Related Quality of Life After the Start of Morphine Therapy." Journal of Pain and Symptom Management 20(1): Knezevic NN, Candido KD and Sauer R (2014). "Is the Constipation Problem Adequately Addressed in Patients Using Opioid and Non-Opioid Medications for Chronic Noncancer Pain?. AAPM 2014 Annual Meeting Abstracts." Pain Medicine 15(3): Lanas A, Tornero J and Zamorano JL (2010). "Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study." Annals of the Rheumatic Diseases 69(8): Lange B, Kuperwasser B, Okamoto Aet al (2010). "Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain." Advances in Therapy 27(6): Machado GC, Maher CG, Ferreira PHet al (2015). "Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials." BMJ 350: h1225. Mackey AC, Green L, Greene P and Avigan M (2010). "Methylnaltrexone and Gastrointestinal Perforation." Journal of Pain and Symptom Management 40(1): e1-e3. Meissner W, Schmidt U, Hartmann M, Kath R and Reinhart K (2000). "Oral naloxone reverses opioidassociated constipation." Pain 84(1): Mercadante S (1999). "Pain treatment and outcomes for patients with advanced cancer who receive follow-up care at home." Cancer 85(8): Michna E, Blonsky ER, Schulman Set al (2011). "Subcutaneous Methylnaltrexone for Treatment of Opioid-Induced Constipation in Patients With Chronic, Nonmalignant Pain: A Randomized Controlled Study." The Journal of Pain 12(5): Moore A, Derry S, Eccleston C and Kalso E (2013). "Expect analgesic failure; pursue analgesic success." BMJ 346: f2690. Nalamachu SR, Pergolizzi J, Taylor R, Jr.et al (2014). "Efficacy and Tolerability of Subcutaneous Methylnaltrexone in Patients with Advanced Illness and Opioid-Induced Constipation: A Responder Analysis of 2 Randomized, Placebo-Controlled Trials." Pain Pract. Pappagallo M (2001). "Incidence, Prevalence, and Management of Opioid Bowel Dysfunction." The American Journal of Surgery 182(5, Supplement 1): S11-S18. Park J and Hughes AK (2012). "Nonpharmacological Approaches to the Management of Chronic Pain in Community-Dwelling Older Adults: A Review of Empirical Evidence." Journal of the American Geriatrics Society 60(3): Rauck RL, Peppin JF, Israel RJet al (2012). "943a Oral Methylnaltrexone for the Treatment of Opioid- Induced Constipation in Patients with Noncancer Pain." Gastroenterology 142(5): S-160. Richardson C, Adams N and Poole H (2006). "Psychological approaches for the nursing management of chronic pain: part 2." Journal of Clinical Nursing 15(9): Rosti G, Gatti A, Costantini A, Sabato AF and Zucco F (2010). "Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on Page 14 of 16
16 chronic treatment." European Review For Medical And Pharmacological Sciences 14(12): Sloots CJ, Rykx A, Cools M, Kerstens R and De Pauw M (2010). "Efficacy and Safety of Prucalopride in Patients with Chronic Noncancer Pain Suffering from Opioid-Induced Constipation." Digestive Diseases and Sciences 55(10): Stannard C (2013). "Opioids in the UK: what s the problem?" BMJ 347: f5108. Stegmann J-U, Weber H, Steup A, Okamoto A, Upmalis D and Daniels S (2008). "The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery." Current Medical Research & Opinion 24(11): Su Y, Zhang X, Zeng Jet al (2009). "New-Onset Constipation at Acute Stage After First Stroke: Incidence, Risk Factors, and Impact on the Stroke Outcome." Stroke 40(4): Sykes NP (1996). "A volunteer model for the comparison of laxatives in opioid-related constipation." Journal of Pain and Symptom Management 11(6): Talley NJ (2008). "How to do and interpret a rectal examination in gastroenterology." American Journal of Gastroenterology 103(4): Tassinari D, Sartori S, Tamburini Eet al (2008). "Adverse Effects of Transdermal Opiates Treating Moderate-Severe Cancer Pain in Comparison to Long-Acting Morphine: A Meta-Analysis and Systematic Review of the Literature." Journal of Palliative Medicine 11(3): Thomas J, Karver S, Cooney GAet al (2008). "Methylnaltrexone for opioid-induced constipation in advanced illness." N Engl J Med 358(22): Wong BS, Rao AS, Camilleri Met al (2010). "The effects of methylnaltrexone alone and in combination with acutely administered codeine on gastrointestinal and colonic transit in health." Alimentary Pharmacology & Therapeutics 32(7): Zin CS, Chen LC and Knaggs RD (2014). "Changes in trends and pattern of strong opioid prescribing in primary care." European Journal of Pain 18(9): Page 15 of 16
17 Cause Chronic constipation Constitutional factors Examples Slow transit constipation, defecatory disorders, constipation predominant irritable bowel syndrome Immobility, dehydration Medications Analgesics Opioids, Non-steroidal antiinflammatory drugs (NSAIDs) Anticholinergics Antihypertensives Antiemetics Minerals Dicyclomine, Benztropine, Diphenhydramine, Chlorpromazine, Amitriptyline Amlodipine, Atenolol, Verapamil, Nifedipine Ondansetron Aluminum, Calcium, Iron, Lithium Endocrine disorders Metabolic disturbances Neurological disorders Colonic strictures Diabetes, hypothyroidism Hypercalcemia, hypokalemia, hyponatraemia Parkinson s disease, multiple screrosis, autonomic neuropathies Colorectal cancer, post-radiotherapy, extrinsic compression from intra-abdominal tumor Table 1. Common causes of constipation Page 16 of 16
OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES
 OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES Introduction Introduction Mean faecal weight 128 g / cap / day Mean range 51-796 g Absolute range 15-1505 g Main factors affecting mass are caloric intake,
OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES Introduction Introduction Mean faecal weight 128 g / cap / day Mean range 51-796 g Absolute range 15-1505 g Main factors affecting mass are caloric intake,
daily; available as 10- mg g PO
 Overview of the PRN: The Pain and Palliative Care PRN of ACCP is an organization of pharmacy practitioners, clinical scientists, pharmacy educators, and others. Its mission is to advance pain and palliative
Overview of the PRN: The Pain and Palliative Care PRN of ACCP is an organization of pharmacy practitioners, clinical scientists, pharmacy educators, and others. Its mission is to advance pain and palliative
Elderly Man With Chronic Constipation
 Elderly Man With Chronic Constipation Linda Nguyen, MD Director, Neurogastroenterology and Motility Clinical Assistant Professor Stanford University Overview Normal bowel function Defining Constipation:
Elderly Man With Chronic Constipation Linda Nguyen, MD Director, Neurogastroenterology and Motility Clinical Assistant Professor Stanford University Overview Normal bowel function Defining Constipation:
Constipation an Old Friend. Presented by Dr. Keith Harris
 Constipation an Old Friend Presented by Dr. Keith Harris Irregularity and the Tricks of the Trade." CONSTIPATION What is constipation? INFREQUENT BOWEL MOVEMENTS DIFFICULTY DURING DEFECATION SENSATION
Constipation an Old Friend Presented by Dr. Keith Harris Irregularity and the Tricks of the Trade." CONSTIPATION What is constipation? INFREQUENT BOWEL MOVEMENTS DIFFICULTY DURING DEFECATION SENSATION
Opioid-Induced Constipation
 Objectives Opioid-Induced Constipation Brianna Jansma, PharmD Alex Smith, PharmD Megan Robinson, PharmD Summarize epidemiology of opioid-induced constipation (OIC) Understand opiates effects on the gastrointestinal
Objectives Opioid-Induced Constipation Brianna Jansma, PharmD Alex Smith, PharmD Megan Robinson, PharmD Summarize epidemiology of opioid-induced constipation (OIC) Understand opiates effects on the gastrointestinal
Horizon Scanning Technology Summary. Methylnaltrexone for opioid induced constipation in advanced illness and palliative care
 Horizon Scanning Technology Summary National Horizon Scanning Centre Methylnaltrexone for opioid induced constipation in advanced illness and palliative care April 2007 This technology summary is based
Horizon Scanning Technology Summary National Horizon Scanning Centre Methylnaltrexone for opioid induced constipation in advanced illness and palliative care April 2007 This technology summary is based
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 3Q17 July August
 BRAND NAME Symproic GENERIC NAME Naldemedine MANUFACTURER Shionogi Inc. DATE OF APPROVAL March 23, 2017 PRODUCT LAUNCH DATE Anticipated to launch mid-summer 2017 REVIEW TYPE Review type 1 (RT1): New Drug
BRAND NAME Symproic GENERIC NAME Naldemedine MANUFACTURER Shionogi Inc. DATE OF APPROVAL March 23, 2017 PRODUCT LAUNCH DATE Anticipated to launch mid-summer 2017 REVIEW TYPE Review type 1 (RT1): New Drug
Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment
 European Review for Medical and Pharmacological Sciences Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment
European Review for Medical and Pharmacological Sciences Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment
Understanding & Alleviating Constipation. Living (Well!) with Gastroparesis Program Warm-Up Class
 Understanding & Alleviating Constipation Living (Well!) with Gastroparesis Program Warm-Up Class Please Remember The information presented is for educational purposes only and is in no way intended as
Understanding & Alleviating Constipation Living (Well!) with Gastroparesis Program Warm-Up Class Please Remember The information presented is for educational purposes only and is in no way intended as
Constipation An Overview. Definition Physiology of GI tract Etiology Assessment Treatment
 CONSTIPATION Constipation An Overview Definition Physiology of GI tract Etiology Assessment Treatment Definition Constipation = the infrequent passage of hard feces Definition of Infrequent The meaning
CONSTIPATION Constipation An Overview Definition Physiology of GI tract Etiology Assessment Treatment Definition Constipation = the infrequent passage of hard feces Definition of Infrequent The meaning
Primary Care Constipation Guidelines. Version 1 November 2016
 Primary Care Constipation Guidelines Version 1 November 2016 VERSION CONTROL Version Date Amendments made Version 1 November 2016 New guideline Contents 1. Management of constipation in adults: acute and
Primary Care Constipation Guidelines Version 1 November 2016 VERSION CONTROL Version Date Amendments made Version 1 November 2016 New guideline Contents 1. Management of constipation in adults: acute and
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Constipation: management of idiopathic constipation in children in primary and secondary care 1.1 Short title Constipation
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Constipation: management of idiopathic constipation in children in primary and secondary care 1.1 Short title Constipation
754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011
 754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011 Brief Report Characterization of Abdominal Pain During Methylnaltrexone Treatment of Opioid-Induced Constipation in Advanced Illness:
754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011 Brief Report Characterization of Abdominal Pain During Methylnaltrexone Treatment of Opioid-Induced Constipation in Advanced Illness:
Is one of the most common chronic disorders. causing patients to seek medical treatment.
 ILOs After this lecture you should be able to : Define IBS Identify causes and risk factors of IBS Determine the appropriate therapeutic options for IBS Is one of the most common chronic disorders causing
ILOs After this lecture you should be able to : Define IBS Identify causes and risk factors of IBS Determine the appropriate therapeutic options for IBS Is one of the most common chronic disorders causing
Horizon Scanning Technology Briefing. Alvimopan (Entrareg ) for opioid-induced bowel disfunction. National Horizon Scanning Centre.
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Alvimopan (Entrareg ) for opioid-induced bowel disfunction August 2006 This technology briefing is based on information available at
Horizon Scanning Technology Briefing National Horizon Scanning Centre Alvimopan (Entrareg ) for opioid-induced bowel disfunction August 2006 This technology briefing is based on information available at
TRANSPARENCY COMMITTEE OPINION. 10 December 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
Constipation and bowel obstruction
 Constipation and bowel obstruction Constipation Infrequent or difficult defecation with reduced number of bowel movements, which may or may not be abnormally hard with increased difficulty or discomfort
Constipation and bowel obstruction Constipation Infrequent or difficult defecation with reduced number of bowel movements, which may or may not be abnormally hard with increased difficulty or discomfort
THE EAPC OPIOID GUIDELINES: PROCESS, RESULTS AND FUTURE DEVELOPMENT
 1 THE EAPC OPIOID GUIDELINES: PROCESS, RESULTS AND FUTURE DEVELOPMENT Jaegtvolden 4-5 June 2012 14. 12. 2012 2 1 3 WHO ANALGESIC LADDER (1996) NSAID +/- Adjuvant STEP II OPIODS Opids for mild to moderate
1 THE EAPC OPIOID GUIDELINES: PROCESS, RESULTS AND FUTURE DEVELOPMENT Jaegtvolden 4-5 June 2012 14. 12. 2012 2 1 3 WHO ANALGESIC LADDER (1996) NSAID +/- Adjuvant STEP II OPIODS Opids for mild to moderate
Constipation. What is constipation? What is the criteria for having constipation? What are the different types of constipation?
 What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
OCTOBER 7-10 PHILADELPHIA, PENNSYLVANIA
 OMED 17 OCTOBER 7-10 PHILADELPHIA, PENNSYLVANIA 29.5 Category 1-A CME credits anticipated ACOFP / AOA s 122 nd Annual Osteopathic Medical Conference & Exposition Joint Session with ACOFP and Cleveland
OMED 17 OCTOBER 7-10 PHILADELPHIA, PENNSYLVANIA 29.5 Category 1-A CME credits anticipated ACOFP / AOA s 122 nd Annual Osteopathic Medical Conference & Exposition Joint Session with ACOFP and Cleveland
What Is Constipation?
 CONSTIPATION What Is Constipation? Constipation is when you have infrequent or hard-to-pass bowel movements (meaning they are painful or you have to strain), have hard stools or feel like your bowel movements
CONSTIPATION What Is Constipation? Constipation is when you have infrequent or hard-to-pass bowel movements (meaning they are painful or you have to strain), have hard stools or feel like your bowel movements
GG&C Chronic Non Malignant Pain Opioid Prescribing Guideline
 GG&C Chronic Non Malignant Pain Opioid Prescribing Guideline Background Persistent pain is common, affecting around five million people in the UK. For many sufferers, pain can be frustrating and disabling,
GG&C Chronic Non Malignant Pain Opioid Prescribing Guideline Background Persistent pain is common, affecting around five million people in the UK. For many sufferers, pain can be frustrating and disabling,
Opioid Induced Constipation Lisa Clince Acute Pain CNS Palmerston North Hospital
 Opioid Induced Constipation Lisa Clince Acute Pain CNS Palmerston North Hospital 21-04-2016 MidCentral District Health Board 1 Overview Patient Experience Opioid Collaborative Constipation defined Opioid
Opioid Induced Constipation Lisa Clince Acute Pain CNS Palmerston North Hospital 21-04-2016 MidCentral District Health Board 1 Overview Patient Experience Opioid Collaborative Constipation defined Opioid
Summary of the risk management plan (RMP) for Moventig (naloxegol)
 EMA/611606/2014 Summary of the risk management plan (RMP) for Moventig (naloxegol) This is a summary of the risk management plan (RMP) for Moventig, which details the measures to be taken in order to ensure
EMA/611606/2014 Summary of the risk management plan (RMP) for Moventig (naloxegol) This is a summary of the risk management plan (RMP) for Moventig, which details the measures to be taken in order to ensure
Opioid Use in Palliative Care
 Opioid Use in Palliative Care Relief of pain is one of the core components of palliative care 1,2 Up to 69% of patients with advanced cancer experience pain 3 ~65% of patients dying from nonmalignant disease
Opioid Use in Palliative Care Relief of pain is one of the core components of palliative care 1,2 Up to 69% of patients with advanced cancer experience pain 3 ~65% of patients dying from nonmalignant disease
Guideline scope Diverticular disease: diagnosis and management
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary
 Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
FOOT OFF THE BRAKES. Kerri Novak MD MSc FRCPC. Chronic Constipation: Taking the Foot off the Brakes Dr. Kerri Novak
 CHRONIC CONSTIPATION: TAKING THE FOOT OFF THE BRAKES Kerri Novak MD MSc FRCPC www.seacourses.com 1 OUTLINE Epidemiology i Quality of life Approach Therapies www.seacourses.com 2 DEFINING CHRONIC CONSTIPATION
CHRONIC CONSTIPATION: TAKING THE FOOT OFF THE BRAKES Kerri Novak MD MSc FRCPC www.seacourses.com 1 OUTLINE Epidemiology i Quality of life Approach Therapies www.seacourses.com 2 DEFINING CHRONIC CONSTIPATION
The Use of Antidepressants in the Treatment of Irritable Bowel Syndrome and Other Functional GI Disorders What are functional GI disorders?
 The Use of Antidepressants in the Treatment of Irritable Bowel Syndrome and Other Functional GI Disorders Christine B. Dalton, PA-C Douglas A. Drossman, MD and Kellie Bunn, PA-C What are functional GI
The Use of Antidepressants in the Treatment of Irritable Bowel Syndrome and Other Functional GI Disorders Christine B. Dalton, PA-C Douglas A. Drossman, MD and Kellie Bunn, PA-C What are functional GI
The Road to Opioid-Induced Constipation: Pathophysiology and Impact Kenneth C. Jackson, II, PharmD, CPE
 Disclaimer This slide deck in its original and unaltered format is for educational purposes and is current as of March 2015. The content and views presented in this educational activity are those of the
Disclaimer This slide deck in its original and unaltered format is for educational purposes and is current as of March 2015. The content and views presented in this educational activity are those of the
SYMPROIC (naldemedine tosylate) oral capsule
 SYMPROIC (naldemedine tosylate) oral capsule Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
SYMPROIC (naldemedine tosylate) oral capsule Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
Pharmacy Benefit Determination Policy
 Policy Subject: Opioid Induced Constipation Policy Number: SHS PBD11 Category: GI Agents Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS
Policy Subject: Opioid Induced Constipation Policy Number: SHS PBD11 Category: GI Agents Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS
Chronic constipation in the elderly
 Chronic constipation in the elderly 1 Dec,2011 R 2 Natta Asanaleykha Epidemiology Definition Scope The impact of chronic constipation in the elderly Pathophysiology Evaluation the elderly patient with
Chronic constipation in the elderly 1 Dec,2011 R 2 Natta Asanaleykha Epidemiology Definition Scope The impact of chronic constipation in the elderly Pathophysiology Evaluation the elderly patient with
MOVICOL HALF PI December MOVICOL-Half. Powder for Solution (macrogol 3350) Potassium 5.4 mmol/l. Bicarbonate 17 mmol/l
 MOVICOL -Half Powder for Solution (macrogol 3350) Product Name: Product Description: MOVICOL-Half Each sachet of MOVICOL-Half contains: Macrogol 3350 6.563 g Sodium chloride 175.4 mg Sodium bicarbonate
MOVICOL -Half Powder for Solution (macrogol 3350) Product Name: Product Description: MOVICOL-Half Each sachet of MOVICOL-Half contains: Macrogol 3350 6.563 g Sodium chloride 175.4 mg Sodium bicarbonate
Authors and Disclosures
 Role of Carbon Dioxide-Releasing Suppositories in the Treatment of Chronic Functional Constipation A Double-Blind, Randomised, Placebo-Controlled Trial M. Lazzaroni; V. Casini; G. Bianchi Porro Authors
Role of Carbon Dioxide-Releasing Suppositories in the Treatment of Chronic Functional Constipation A Double-Blind, Randomised, Placebo-Controlled Trial M. Lazzaroni; V. Casini; G. Bianchi Porro Authors
Slide #43. Functional Disorders - An Update 11/8/ MA ACP Annual Scientific Meeting. Functional Disorders: An Update
 Functional Disorders: An Update Anthony Lembo, M.D. Associate Professor of Medicine Beth Israel Deaconess Medical Center Harvard Medical School Boston, MA Disclosure of Financial Relationships Anthony
Functional Disorders: An Update Anthony Lembo, M.D. Associate Professor of Medicine Beth Israel Deaconess Medical Center Harvard Medical School Boston, MA Disclosure of Financial Relationships Anthony
Movantik (naloxegol), Relistor (methylnaltrexone bromide), Symproic (naldemedine)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 7 Last Review Date: November 30, 2018 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 7 Last Review Date: November 30, 2018 Opioid Antagonist
Antidiarrheals Antidiarrheal
 Antidiarrheals Major factors in diarrhea Increased motility of the GI tract. Decreased absorption of fluid. Antidiarrheal drugs include: Antimotility agents. Adsorbents. Drugs that modify fluid and electrolyte
Antidiarrheals Major factors in diarrhea Increased motility of the GI tract. Decreased absorption of fluid. Antidiarrheal drugs include: Antimotility agents. Adsorbents. Drugs that modify fluid and electrolyte
Management of Neurogenic Bowel Dysfunction. Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders
 Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Movantik (naloxegol), Relistor (methylnaltrexone bromide), Symproic (naldemedine)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: June 22, 2017 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: June 22, 2017 Opioid Antagonist
Drug Evaluation. Use of lubiprostone in constipating disorders and its potential for opioid-induced bowel dysfunction
 Use of lubiprostone in constipating disorders and its potential for opioid-induced bowel dysfunction Lubiprostone is a novel medication, approved by the US FDA for the treatment of chronic idiopathic constipation
Use of lubiprostone in constipating disorders and its potential for opioid-induced bowel dysfunction Lubiprostone is a novel medication, approved by the US FDA for the treatment of chronic idiopathic constipation
Why does my stomach hurt? Exploring irritable bowel syndrome
 Why does my stomach hurt? Exploring irritable bowel syndrome By Flavio M. Habal, MD, PhD, FRCPC Case In this article: 1. What is IBS? A 45-year-old female is referred to your office with recurrent 2. How
Why does my stomach hurt? Exploring irritable bowel syndrome By Flavio M. Habal, MD, PhD, FRCPC Case In this article: 1. What is IBS? A 45-year-old female is referred to your office with recurrent 2. How
Drugs in Development for Opioid-Induced Constipation
 7 Drugs in Development for Opioid-Induced Constipation Kelly S. Sprawls, Egilius L.H. Spierings and Dustin Tran MedVadis Research Corporation USA 1. Introduction Opioid-induced bowel dysfunction (OIBD)
7 Drugs in Development for Opioid-Induced Constipation Kelly S. Sprawls, Egilius L.H. Spierings and Dustin Tran MedVadis Research Corporation USA 1. Introduction Opioid-induced bowel dysfunction (OIBD)
MOVICOL Junior Powder for Solution (macrogol 3350)
 MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
ACTIVITY DESCRIPTION FACULTY. Opioid Use in Palliative Care. Opioid Analgesic Use in Chronic, Non-cancer Pain
 ACTIVITY DESCRIPTION Target Audience This continuing pharmacy education activity is planned to meet the needs of pharmacists in a variety of practice settings, including large and small healthcare systems,
ACTIVITY DESCRIPTION Target Audience This continuing pharmacy education activity is planned to meet the needs of pharmacists in a variety of practice settings, including large and small healthcare systems,
Efficacy and Safety of Lubiprostone. Laura Wozniak February 23, 2010 K30 Monthly Journal Club
 Efficacy and Safety of Lubiprostone Laura Wozniak February 23, 2010 K30 Monthly Journal Club Objectives Brief overview of constipation Review of article Discussion Constipation in Children 3-5% of all
Efficacy and Safety of Lubiprostone Laura Wozniak February 23, 2010 K30 Monthly Journal Club Objectives Brief overview of constipation Review of article Discussion Constipation in Children 3-5% of all
Irritable Bowel Syndrome. Mustafa Giaffer March 2017
 Irritable Bowel Syndrome Mustafa Giaffer March 2017 Introduction First described in 1771. 50% of patients present
Irritable Bowel Syndrome Mustafa Giaffer March 2017 Introduction First described in 1771. 50% of patients present
National Institute for Health and Clinical Excellence. Health Technology Appraisal. Prucalopride for the treatment of chronic constipation in women
 Health Technology Appraisal Summary form Prucalopride for the treatment of chronic constipation in women Comment 1: the draft remit Appropriateness Movetis Movetis entirely welcomes the opportunity to
Health Technology Appraisal Summary form Prucalopride for the treatment of chronic constipation in women Comment 1: the draft remit Appropriateness Movetis Movetis entirely welcomes the opportunity to
OIC, opioid-induced constipation.
 Disclosures Charles E. Argoff, MD Speakers Bureau for Allergan, Inc., AstraZeneca plc, Depomed, Inc., Iroko Pharmaceuticals LLC, Janssen Pharmaceuticals, Inc., Millenium Laboratories, and Xenoport Inc.
Disclosures Charles E. Argoff, MD Speakers Bureau for Allergan, Inc., AstraZeneca plc, Depomed, Inc., Iroko Pharmaceuticals LLC, Janssen Pharmaceuticals, Inc., Millenium Laboratories, and Xenoport Inc.
ALVIMOPAN 0.0 OVERVIEW
 ALVIMOPAN 0.0 OVERVIEW A. Alvimopan is a peripherally restricted mu-opioid receptor antagonist. B. DOSING INFORMATION : For the treatment of opioid bowel dysfunction, oral alvimopan doses between 0.5 milligrams
ALVIMOPAN 0.0 OVERVIEW A. Alvimopan is a peripherally restricted mu-opioid receptor antagonist. B. DOSING INFORMATION : For the treatment of opioid bowel dysfunction, oral alvimopan doses between 0.5 milligrams
Berkshire West Area Prescribing Committee Guidance
 Guideline Name Berkshire West Area Prescribing Committee Guidance Date of Issue: September 2015 Review Date: September 2017 Date taken to APC: 2 nd September 2015 Date Ratified by GP MOC: Guidelines for
Guideline Name Berkshire West Area Prescribing Committee Guidance Date of Issue: September 2015 Review Date: September 2017 Date taken to APC: 2 nd September 2015 Date Ratified by GP MOC: Guidelines for
7/13/16. Pharmaceuticals that alter GI motility. Financial disclosures. Common pharmaceuticals that alter GI Motility (an incomplete list) None
 Pharmaceuticals that alter GI motility Megan Taylor, ND AANP 2016 Motility Bootcamp Financial disclosures None Common pharmaceuticals that alter GI Motility (an incomplete list) Analgesics Opioids NSAIDs
Pharmaceuticals that alter GI motility Megan Taylor, ND AANP 2016 Motility Bootcamp Financial disclosures None Common pharmaceuticals that alter GI Motility (an incomplete list) Analgesics Opioids NSAIDs
Amitiza. Amitiza (lubiprostone) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.22 Subject: Amitiza Page: 1 of 5 Last Review Date: March 16, 2018 Amitiza Description Amitiza (lubiprostone)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.22 Subject: Amitiza Page: 1 of 5 Last Review Date: March 16, 2018 Amitiza Description Amitiza (lubiprostone)
Movantik (naloxegol), Relistor (methylnaltrexone bromide)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: December 2, 2016 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: December 2, 2016 Opioid Antagonist
Opioids and the Gastroenterologist: A Painful Issue
 Opioids and the Gastroenterologist: A Painful Issue Wendell K. Clarkston, MD Professor of Medicine GI Program Director UMKC School of Medicine Disclosures: No financial relationships to disclose 1 Outline
Opioids and the Gastroenterologist: A Painful Issue Wendell K. Clarkston, MD Professor of Medicine GI Program Director UMKC School of Medicine Disclosures: No financial relationships to disclose 1 Outline
Constipation Information Leaflet THE DIGESTIVE SYSTEM. gutscharity.org.uk
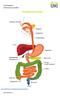 THE DIGESTIVE SYSTEM http://healthfavo.com/digestive-system-for-kids.html This factsheet is about Constipation Constipation is a symptom that can mean different things to different people but the usual
THE DIGESTIVE SYSTEM http://healthfavo.com/digestive-system-for-kids.html This factsheet is about Constipation Constipation is a symptom that can mean different things to different people but the usual
Full details and resource documents available:
 Clinical & Regulatory News by Pharmerica Urinary Tract Infection (UTI) Second Most Common Cause of Hospital Readmission within 30 days UTIs are prevalent and account for up to 22% of infections in LTC,
Clinical & Regulatory News by Pharmerica Urinary Tract Infection (UTI) Second Most Common Cause of Hospital Readmission within 30 days UTIs are prevalent and account for up to 22% of infections in LTC,
Oral Methylnaltrexone for the. Constipation in Patients with Chronic Non-cancer Pain
 Oral Methylnaltrexone for the Treatment of Opioid-induced Constipation in Patients with Chronic Non-cancer Pain Richard L. Rauck, 1 John F. Peppin, 2 Robert J.Israel, 3 Jennifer Carpenito, 3 Jeffrey Cohn,
Oral Methylnaltrexone for the Treatment of Opioid-induced Constipation in Patients with Chronic Non-cancer Pain Richard L. Rauck, 1 John F. Peppin, 2 Robert J.Israel, 3 Jennifer Carpenito, 3 Jeffrey Cohn,
Constipation. H. David Vargas, MD. Overview
 Constipation H. David Vargas, MD Overview Constipation is a very common complaint affecting upwards of 15% of all Americans. Fortunately, constipation usually is simple to avoid and easy to treat when
Constipation H. David Vargas, MD Overview Constipation is a very common complaint affecting upwards of 15% of all Americans. Fortunately, constipation usually is simple to avoid and easy to treat when
IBS Irritable Bowel syndrome Therapeutics II PHCL 430
 Salman Bin AbdulAziz University College Of Pharmacy IBS Irritable Bowel syndrome Therapeutics II PHCL 430 Email:- ahmedadel.pharmd@gmail.com Ahmed A AlAmer PharmD R.S is 32-year-old woman experiences intermittent
Salman Bin AbdulAziz University College Of Pharmacy IBS Irritable Bowel syndrome Therapeutics II PHCL 430 Email:- ahmedadel.pharmd@gmail.com Ahmed A AlAmer PharmD R.S is 32-year-old woman experiences intermittent
Effectiveness and Tolerability of Amidotrizoate for the Treatment of Constipation Resistant to Laxatives in Advanced Cancer Patients
 Vol. 41 No. 2 February 2011 Journal of Pain and Symptom Management 421 Original Article Effectiveness and Tolerability of Amidotrizoate for the Treatment of Constipation Resistant to Laxatives in Advanced
Vol. 41 No. 2 February 2011 Journal of Pain and Symptom Management 421 Original Article Effectiveness and Tolerability of Amidotrizoate for the Treatment of Constipation Resistant to Laxatives in Advanced
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Relistor) Reference Number: CP.CPA.274 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Relistor) Reference Number: CP.CPA.274 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Constipation: pathophysiology and management
 REVIEW C URRENT OPINION Constipation: pathophysiology and management Arnold Wald Purpose of review Continuing advances in pharmaceutical development are providing an expanding array of treatment approaches
REVIEW C URRENT OPINION Constipation: pathophysiology and management Arnold Wald Purpose of review Continuing advances in pharmaceutical development are providing an expanding array of treatment approaches
Improving the Diagnosis and Management of Opioid-induced Constipation to Optimize Outcomes of Patients with Chronic Pain
 Improving the Diagnosis and Management of Opioid-induced Constipation to Optimize Outcomes of Patients with Chronic Pain Sponsored by Integrity Continuing Education, Inc. Supported by an educational grant
Improving the Diagnosis and Management of Opioid-induced Constipation to Optimize Outcomes of Patients with Chronic Pain Sponsored by Integrity Continuing Education, Inc. Supported by an educational grant
Opioid constipation treatment dulcolax
 P ford residence southampton, ny Opioid constipation treatment dulcolax Posts about how to relieve constipation instantly written by gbrese1958. Official Web site for MOVANTIK (naloxegol) tablets, for
P ford residence southampton, ny Opioid constipation treatment dulcolax Posts about how to relieve constipation instantly written by gbrese1958. Official Web site for MOVANTIK (naloxegol) tablets, for
Opioid-induced constipation a preventable problem
 www.veteransmates.net.au Opioid-induced a preventable problem One of the most common adverse effects of chronic opioid therapy is. 1-4 Up to 95% of patients prescribed an opioid report as a side effect,
www.veteransmates.net.au Opioid-induced a preventable problem One of the most common adverse effects of chronic opioid therapy is. 1-4 Up to 95% of patients prescribed an opioid report as a side effect,
BJF Acute Pain Team Formulary Group
 Title Analgesia Guidelines for Acute Pain Management (Adults) in BGH Document Type Issue no Clinical guideline Clinical Governance Support Team Use Issue date April 2013 Review date April 2015 Distribution
Title Analgesia Guidelines for Acute Pain Management (Adults) in BGH Document Type Issue no Clinical guideline Clinical Governance Support Team Use Issue date April 2013 Review date April 2015 Distribution
Multimodal Approach for Managing Postoperative Ileus: Role of Health- System Pharmacists (ACPE program H01P)
 1. In the normal gastrointestinal tract, what percent of nutrient absorption occurs in the jejunum? a. 20%. b. 40%. c. 70%. d. 90%. 2. According to Dr. Erstad, the four components of gastrointestinal control
1. In the normal gastrointestinal tract, what percent of nutrient absorption occurs in the jejunum? a. 20%. b. 40%. c. 70%. d. 90%. 2. According to Dr. Erstad, the four components of gastrointestinal control
MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350)
 MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Chocolate Flavour Product Description: Each sachet of MOVICOL Junior Chocolate contains: Macrogol 3350
MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Chocolate Flavour Product Description: Each sachet of MOVICOL Junior Chocolate contains: Macrogol 3350
Irritable Bowel Syndrome Now. George M. Logan, MD Friday, May 5, :35 4:05 PM
 Irritable Bowel Syndrome Now George M. Logan, MD Friday, May 5, 2017 3:35 4:05 PM Dr. Logan indicated no potential conflict of interest to this presentation. He does not intend to discuss any unapproved/investigative
Irritable Bowel Syndrome Now George M. Logan, MD Friday, May 5, 2017 3:35 4:05 PM Dr. Logan indicated no potential conflict of interest to this presentation. He does not intend to discuss any unapproved/investigative
Gastrointestinal obstruction Dr Iain Lawrie
 Gastrointestinal obstruction Dr Iain Lawrie Consultant and Honorary Clinical Senior Lecturer in Palliative Medicine The Pennine Acute Hospitals NHS Trust / The University of Manchester iain.lawrie@pat.nhs.uk
Gastrointestinal obstruction Dr Iain Lawrie Consultant and Honorary Clinical Senior Lecturer in Palliative Medicine The Pennine Acute Hospitals NHS Trust / The University of Manchester iain.lawrie@pat.nhs.uk
Drugs Affecting the Gastrointestinal System. Antidiarrheal and Laxatives
 Drugs Affecting the Gastrointestinal System Antidiarrheal and Laxatives Diarrhea Abnormal frequent passage of loose stools or Abnormal passage of stools with increased frequency, fluidity, and weight,
Drugs Affecting the Gastrointestinal System Antidiarrheal and Laxatives Diarrhea Abnormal frequent passage of loose stools or Abnormal passage of stools with increased frequency, fluidity, and weight,
Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345
 Naloxegol for treating opioid-induced constipation Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Naloxegol for treating opioid-induced constipation Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Active ingredients per ml: Docusate sodium 1 mg/sorbitol solution (70%) (crystallising) 357 mg Structural formula: Docusate.
 NAME OF THE MEDICINE KLYX Enema Active ingredients per ml: Docusate sodium 1 mg/sorbitol solution (70%) (crystallising) 357 mg Structural formula: Docusate Sorbitol C20H37NaO7S MW: 444.56 CAS no: 577-11-7
NAME OF THE MEDICINE KLYX Enema Active ingredients per ml: Docusate sodium 1 mg/sorbitol solution (70%) (crystallising) 357 mg Structural formula: Docusate Sorbitol C20H37NaO7S MW: 444.56 CAS no: 577-11-7
Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS OVERVIEW
 Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS A major medical conditions that commonly is seen among persons with IDD and may lead to serious complications is constipation.
Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS A major medical conditions that commonly is seen among persons with IDD and may lead to serious complications is constipation.
GUIDELINES AND AUDIT IMPLEMENTATION NETWORK
 GUIDELINES AND AUDIT IMPLEMENTATION NETWORK General Palliative Care Guidelines The Management of Pain at the End Of Life November 2010 Aim To provide a user friendly, evidence based guide for the management
GUIDELINES AND AUDIT IMPLEMENTATION NETWORK General Palliative Care Guidelines The Management of Pain at the End Of Life November 2010 Aim To provide a user friendly, evidence based guide for the management
IBS - Definition. Chronic functional disorder of GI generally characterized by:
 IBS - Definition Chronic functional disorder of GI generally characterized by: 3500 3000 No. of Publications 2500 2000 1500 1000 Irritable Bowel syndrome Irritable Bowel Syndrome 500 0 1968-1977 1978-1987
IBS - Definition Chronic functional disorder of GI generally characterized by: 3500 3000 No. of Publications 2500 2000 1500 1000 Irritable Bowel syndrome Irritable Bowel Syndrome 500 0 1968-1977 1978-1987
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
Advancing gastroenterology, improving patient care
 American College of Gastroenterology Advancing gastroenterology, improving patient care Note to Visitors: A fully updated ACG Systematic Review on the Management of Chronic Idiopathic Constipation and
American College of Gastroenterology Advancing gastroenterology, improving patient care Note to Visitors: A fully updated ACG Systematic Review on the Management of Chronic Idiopathic Constipation and
Scandinavian Journal of Pain
 Scandinavian Journal of Pain 11 (2016) 111 122 Contents lists available at ScienceDirect Scandinavian Journal of Pain journal homepage: www.scandinavianjournalpain.com Topical review Definition, diagnosis
Scandinavian Journal of Pain 11 (2016) 111 122 Contents lists available at ScienceDirect Scandinavian Journal of Pain journal homepage: www.scandinavianjournalpain.com Topical review Definition, diagnosis
Knock Out Opioid Abuse in New Jersey:
 Knock Out Opioid Abuse in New Jersey: A Resource for Safer Prescribing GUIDELINE FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN IMPROVING PRACTICE THROUGH RECOMMENDATIONS CDC s Guideline for Prescribing Opioids
Knock Out Opioid Abuse in New Jersey: A Resource for Safer Prescribing GUIDELINE FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN IMPROVING PRACTICE THROUGH RECOMMENDATIONS CDC s Guideline for Prescribing Opioids
SUPPLEMENTARY INFORMATION Associated with
 Table1: Rome III and Rome IV diagnostic criteria for IBS, functional constipation and functional dyspepsia. Rome III diagnostic criteria 1,2 Rome IV diagnostic criteria 3,4 Diagnostic criteria for IBS
Table1: Rome III and Rome IV diagnostic criteria for IBS, functional constipation and functional dyspepsia. Rome III diagnostic criteria 1,2 Rome IV diagnostic criteria 3,4 Diagnostic criteria for IBS
Constipation. (Medical Aspects)
 Constipation (Medical Aspects) By Dr. Ehab Abdel Khalik MD. Anatomy of the anorectum The rectum is 12-15 15 cm. long. It connects with the sigmoid colon by the rectosigmoid junction which is believed to
Constipation (Medical Aspects) By Dr. Ehab Abdel Khalik MD. Anatomy of the anorectum The rectum is 12-15 15 cm. long. It connects with the sigmoid colon by the rectosigmoid junction which is believed to
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
Opioid-Induced Constipation: Update on Prevention and Management EDUCATIONALPROGRAM
 EDUCATIONALPROGRAM Recognizing i the Growing Burden of OIC Bill H. McCarberg, MD Founder, Chronic Pain Management Program Kaiser Permanente San Diego Adjunct Assistant Clinical Professor University of
EDUCATIONALPROGRAM Recognizing i the Growing Burden of OIC Bill H. McCarberg, MD Founder, Chronic Pain Management Program Kaiser Permanente San Diego Adjunct Assistant Clinical Professor University of
Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211
 Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Learning Objectives. Perioperative goals. Acute Pain in the Chronic Pain Patient for Ambulatory Surgery 9/8/16
 Acute Pain in the Chronic Pain Patient for Ambulatory Surgery Danielle Ludwin, MD Associate Professor of Anesthesiology Division of Regional and Orthopedic Anesthesia Columbia University Medical Center
Acute Pain in the Chronic Pain Patient for Ambulatory Surgery Danielle Ludwin, MD Associate Professor of Anesthesiology Division of Regional and Orthopedic Anesthesia Columbia University Medical Center
Common Gastrointestinal Problems in the Elderly
 Common Gastrointestinal Problems in the Elderly Brian Viviano, D.O. Objectives Understand the pathophysiology, clinical manifestations, diagnosis and management of GI diseases of the elderly. Differentiate
Common Gastrointestinal Problems in the Elderly Brian Viviano, D.O. Objectives Understand the pathophysiology, clinical manifestations, diagnosis and management of GI diseases of the elderly. Differentiate
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350)
 MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350)
 MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
Constipation. Disease Review
 bowel. 1 Constipation is a symptom, not a disease. Almost everyone experiences Disease Review Constipation Introduction: Constipation is exceedingly common and is rarely associated with mortality in developed
bowel. 1 Constipation is a symptom, not a disease. Almost everyone experiences Disease Review Constipation Introduction: Constipation is exceedingly common and is rarely associated with mortality in developed
Biofeedback for Pelvic Floor Disorders and Incontinence
 The UNC Center for Functional GI & Motility Disorders www.med.unc.edu/ibs Biofeedback for Pelvic Floor Disorders and Incontinence Olafur S. Palsson, Psy.D. Associate Professor of Medicine UNC Center for
The UNC Center for Functional GI & Motility Disorders www.med.unc.edu/ibs Biofeedback for Pelvic Floor Disorders and Incontinence Olafur S. Palsson, Psy.D. Associate Professor of Medicine UNC Center for
2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Macrovic Junior powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Macrovic Junior powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution
Naloxegol for Opioid-Induced Constipation in Patients with Noncancer Pain
 The new england journal of medicine original article Naloxegol for Opioid-Induced Constipation in Patients with Noncancer Pain William D. Chey, M.D., Lynn Webster, M.D., Mark Sostek, M.D., Jaakko Lappalainen,
The new england journal of medicine original article Naloxegol for Opioid-Induced Constipation in Patients with Noncancer Pain William D. Chey, M.D., Lynn Webster, M.D., Mark Sostek, M.D., Jaakko Lappalainen,
TREATMENT SOCIETY GUIDELINES FOR CONSTIPATION: WHAT IS NEW? FUNCTIONAL CONSTIPATION
 SOCIETY GUIDELINES FOR CONSTIPATION: WHAT IS NEW? Samuel Nurko MD MPH Center for Motility and Functional Gastrointestinal Disorders FUNCTIONAL CONSTIPATION One of the most common functional GI disorders
SOCIETY GUIDELINES FOR CONSTIPATION: WHAT IS NEW? Samuel Nurko MD MPH Center for Motility and Functional Gastrointestinal Disorders FUNCTIONAL CONSTIPATION One of the most common functional GI disorders
PALLIATIVE TREATMENT BY DR. KHRONGKAMOL SIHABAN MEDICAL ONCOLOGIST
 PALLIATIVE TREATMENT BY DR. KHRONGKAMOL SIHABAN MEDICAL ONCOLOGIST TREATMENT IN ONCOLOGY Main treatment : surgery Neoadjuvant treatment : RT, CMT Adjuvant treatment : Tx micrometastatic disease -CMT,Targeted
PALLIATIVE TREATMENT BY DR. KHRONGKAMOL SIHABAN MEDICAL ONCOLOGIST TREATMENT IN ONCOLOGY Main treatment : surgery Neoadjuvant treatment : RT, CMT Adjuvant treatment : Tx micrometastatic disease -CMT,Targeted
HOPE. Considerations. Considerations ISING. Safe Opioid Prescribing Guidelines for ACUTE Non-Malignant Pain
 Due to the high level of prescription drug use and abuse in Lake County, these guidelines have been developed to standardize prescribing habits and limit risk of unintended harm when prescribing opioid
Due to the high level of prescription drug use and abuse in Lake County, these guidelines have been developed to standardize prescribing habits and limit risk of unintended harm when prescribing opioid
SUMMARY OF ARIZONA OPIOID PRESCRIBING GUIDELINES FOR THE TREATMENT OF CHRONIC NON-TERMINAL PAIN (CNTP)
 9 SUMMARY OF ARIZONA OPIOID PRESCRIBING GUIDELINES FOR THE TREATMENT OF CHRONIC NON-TERMINAL PAIN (CNTP) SUMMARY OF ARIZONA OPIOID PRESCRIBING GUIDELINES FOR THE TREATMENT OF ACUTE PAIN NONOPIOID TREATMENTS
9 SUMMARY OF ARIZONA OPIOID PRESCRIBING GUIDELINES FOR THE TREATMENT OF CHRONIC NON-TERMINAL PAIN (CNTP) SUMMARY OF ARIZONA OPIOID PRESCRIBING GUIDELINES FOR THE TREATMENT OF ACUTE PAIN NONOPIOID TREATMENTS
SYMPTOM MANAGEMENT GUIDANCE FOR PATIENTS RECEIVING PALLIATIVE CARE AT ROYAL DERBY HOSPITAL
 SYMPTOM MANAGEMENT GUIDANCE FOR PATIENTS RECEIVING PALLIATIVE CARE AT ROYAL DERBY HOSPITAL If a patient is believed to be approaching the end of their life, medication should be prescribed in anticipation
SYMPTOM MANAGEMENT GUIDANCE FOR PATIENTS RECEIVING PALLIATIVE CARE AT ROYAL DERBY HOSPITAL If a patient is believed to be approaching the end of their life, medication should be prescribed in anticipation
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 6 April 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 April 2011 METEOXANE, capsules B/60 (CIP code: 306 693-3) Applicant: IPRAD Simethicone Hydrated phloroglucinol ATC
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 April 2011 METEOXANE, capsules B/60 (CIP code: 306 693-3) Applicant: IPRAD Simethicone Hydrated phloroglucinol ATC
