Therapeutic and Cosmetic uses of Botulinum Toxin
|
|
|
- Eugene Holt
- 5 years ago
- Views:
Transcription
1 Article 32 Therapeutic and Cosmetic uses of Botulinum Toxin Vinay Kant* 1, Rita Koshal 2, Pawan Kumar Verma 3 and Nrip Kishore Pankaj 3 KEY WORDS Botulinum toxin, Clostridium botulinum. INTRODUCTION From times unknown man has greatly been benefited from uncovering and utilizing the chemicals from the natural world. Living organisms, such as plants, animals, microorganisms, offer a huge source of pharmaceutically useful medicine and toxins. Depending upon their source, the toxins are categorized as phytotoxins, mycotoxins and, zootoxins including venoms and bacterial toxins. Botulinum toxin is neurotoxic protein produced by the gram-positive, rod shaped, spore forming, strictly anaerobic bacterium Clostridium botulinum. These bacteria are widely distributed in soil and water (Dowell, 1984). Botulinum toxin is one of the most acutely toxic naturally occurring substances in the world with a lethal dose of about pg/kg (100g could kill every human on earth. Botulinum toxin is odorless and tasteless, and shares many properties with the other bacterial toxins such as tetanospasmin and diphtheria toxin (Davis, 1993). Thousands of people in the world each year continue to be poisoned with botulinum toxin food-borne, infantile, or wound botulism but the neurotoxin is now sufficiently understood to allow 1 Corresponding author: M. V. Sc. Scholar Division of Pharmacology and Toxicology, Faculty of Veterinary Sciences & Animal Husbandry, SKUAST-J, R.S. Pura, Jammu M. V. Sc. Scholar, Department of Veterinary Public Health, GADVASU, Ludhiana, India. 3 Assistant Professor, Division of Pharmacology and Toxicology, SKUAST-J, R.S. Pura, Jammu it to be used as medicinal agent to paralyze specific muscles, giving temporary symptomatic relief from variety of neurologic disorders and for certain cosmetic purposes in minute doses. (Davis, 1993). The clostridia produce more protein toxins than any other bacterial genus and are a rich reservoir of toxins for research and medicinal uses. Research is underway to use these clostridial exotoxins or their toxin domains for drug delivery, prevention of food poisoning, and the treatment of cancer and other diseases. The remarkable success of botulinum toxin as a therapeutic agent has created a new field of investigation in microbiology. BOTULINUM TOXIN It constitutes a family of serologically closely related 7 neurotoxins i.e. A, B, C1, D, E, F, and G. The primary structure of most serotypes has been determined. Each toxin has a inhibitory chain (light chain) and binding chain (heavy chain) and the two chains are held together with a disulfide bond. These chains have three major types of domains, the binding and translocation domains present on heavy chain and the catalytic domain present on the light chain. Toxin types A, B, E and F are the main toxins that affect humans (Dowell, 1984) and toxin types C and D affects birds and mammals (Davis, 1993). For therapeutic purposes botulinum toxin A is preferred because of its long duration of action and ease of production. Botulinum toxin type A also has several advantages including relatively rare systemic side effects, lack of tissue destruction, graded therapeutic response by dosage adjustment and, above all, high patient acceptance (Dresseler et.al, 2000). The type A toxin is produced as a single chain polypeptide with a molecular weight ISSN
2 of Da and later on it is transformed into its active structure by protease which causes nicking of single chain polypeptide to di-chain polypeptide of about Da and Da molecular weight (Bandyopadhyay, et al.1987). Botulinum toxin B (BTX-B) is preffered to type A toxin for the treatment of cervical dystonia. BoNT/B is stable in solution at an acidic ph and is available as a solution containing 5000 units/ml. MECHANISM OF ACTION At neuromuscular (N-M) junction release of acetylcholine (Ach) is mediated by assembly of synaptic fusion complex that allows membrane of synaptic vesicle to fuse with neuronal cell membrane. Synaptic fusion complex is a set of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins, which include synaptobrevin, SNAP-25 (synaptosomal-associated protein of 25 kd), syntaxin. After membrane fusion, acetylcholine is released into synaptic cleft and then bound by receptors on the muscle cell. When there is exposure to botulinum toxin, it produces its action by involving several steps: systemic absorption, binding to the nerve terminal, internalization and synaptic poisoning. The toxin first reaches the lymphatic channels and then the blood stream either by absorption through the upper gastrointestinal tract (food borne and infantile botulism) or through tissue absorption (wound botulism) (Bonventre, 1979). Then the toxin circulates in the blood until it reaches cholinergic synapses in the peripheral nervous system. The toxin appears not to cross the blood brain barrier, so the cholinergic synapses of central nervous system are not involved (Sugiyama, 1980). The toxin binds with the help of binding domain to cholinergic neuronal cell membrane at nerve terminal and enters neuron by endocytosis (internalization). The light chain of botulinum toxin crosses the membrane of the endocytic vesicle and enters the cytoplasm of the pre synaptic terminal (Davis, 1993). Then it cleaves specific sites on SNARE proteins thus preventing complete assembly of synaptic fusion complex which in turn blocks docking, fusion and acetylcholine release. Blockage of acetylcholine release in turn blocks activation of muscarinic and nicotinic receptors due to which there will be decreased/no secretion in case of exocrine glands and paresis due to chemical denervation in case of a muscle. Botulinum toxins types B, D, F, and G cleave synaptobrevin; types A, C, and E cleave SNAP- 25; and type C cleaves syntaxin. HISTORY AND FDA (FOOD AND DRUG ADMINISTRATION) APPROVALS In 1944, Edward Schantz cultured Clostridium botulinum and isolated the toxin, and, in 1949, Burgen's group discovered that botulinum toxin blocks neuromuscular transmission thus blocks the release of acetylcholine at N-effector junctions. By 1973, Alan B Scott, MD, of Smith- Kettlewell Institute used botulinum toxin type A (BTX-A) in monkey experiments, and, in 1980, he officially used BTX-A for the first time in humans to treat strabismus. In December 1989, BTX-A (BOTOX) was approved by the US Food and Drug Administration (FDA) for the treatment of strabismus, blepharospasm, and hemifacial spasm in patients over 12 years old. (Dresseler, 2000) observed that the effects of botulinum toxin are fully reversible. Botulinum Toxin Type B (BTX-B) received FDA approval for treatment of cervical dystonia on December 21, On April 15, 2002, the FDA announced the approval of botulinum toxin type A to temporarily improve the appearance of moderate-to-severe frown lines between the eyebrows (glabellar lines). BTX-A has also been approved for the treatment of excessive underarm sweating. The acceptance of BTX-A use for the treatment of spasticity and muscle pain disorders is growing, with approvals pending in many European countries and studies on headaches (including migraine), prostatic symptoms, asthma, obesity and many other possible indications are ongoing. Botox is the only FDA-approved botulinum toxin A, but a potential competitor, ISSN
3 Dysport, has an FDA approval application pending. THERAPEUTIC USES As the mechanism of action of the botulinum toxin was better understood, it was recognized that the toxin could be used selectively to paralyze muscles. Researchers discovered in the 1950s that injecting minute quantities of botulinum toxin type A in overactive muscles resulted in decreased muscle activity by blocking the release of acetylcholine at the neuromuscular junction, thereby rendering the muscle unable to contract for a period of 4-6 months. At that time, the botulinal toxin is often re-administered to the same muscles. Repeated muscle injection may provide relief for shorter periods than the initial administration. It is not yet clear if botulinum toxin can be re-administered indefinitely or whether the effectiveness eventually wears off. Clinical effect of the serotype A and B toxins begins within hours, peaks at 2-3 weeks and lasts for 3-4 months (Brin, 2002). FDA has recommended uses of botulinal toxins for: 1) Migraine Headaches: Botulinum toxin in headache prophylaxis was found by Serendipity whereby patients receiving BoNT/A for frown lines reported reduced frequency of their headaches. BoNT/A at the doses range between U is injected in the tender muscles of the frontal, temporal and cervical regions for the relief from tension-type and chronic daily headache. Studies have proven its efficacy in migraine prophylaxis (Silberstein et al., 2000). Though the exact mechanism is unknown, it has been postulated that BoNT/A reduces the afferent volley of pain impulses. The exact doses are still being worked out and it is best to begin with smaller doses and, if necessary, gradually increases the dose. 2) Dystonia: In general, more than 90% of treated cases of blepharospasm and laryngeal dystonia show a satisfactory result. Type B toxin, which has been carefully studied to date only in cervical dystonia, shows similar results as type A toxins. More than 75% of patients with cervical dystonia and jaw-closing oromandibular dystonias benefit significantly from type A toxins. The response in upper limb dystonias is less. a) Benign Essential Blepharospasm: Dystonic hyperactivity in the orbicularis oculi muscles causes blepharospasm. Orbicularis oculi, corrugator supercilii and procerus are targeted with botulinum toxin at the dose rate of 25 U for each side. Complications, namely ptosis, dryness of eyes, lateral rectus palsy, facial muscle paralysis and hematoma formation, are transient and reversible (Brin, 1998). Considerable improvement occurs in the quality of life measures in patients with blepharospasm. b) Meige's syndrome: Patients have dystonia of both upper and lower halves of face. The lower face muscles have a very narrow therapeutic window and thus, have substantially higher chances of functional impairment. The dose used varies between 50 to 100 units. c) Oromandibular Dystonia: In jaw closure dystonia, masseters are commonly targeted bilaterally by BoNT/A at the dose rate of 30U for each side. In jaw opening dystonia, lateral pterygoids together with anterior belly of omohyoid are targeted but the response is variable. It is advisable to avoid injecting tongue muscles in lingual dystonia as a weak tongue can choke a patient, requiring intubation (Brin, 1998). Oromandibular dystonias are difficult to treat without causing dysphagia and should only be done by well-trained and experienced clinicians with adequate experience in use of neurotoxins. d) Cervical dystonias: Botulinum toxin is the treatment of choice for cervical dystonias and they significantly improve the quality of life measures (Brin, 1998). Identification of the muscles responsible for torticollis, laterocollis, anterocollis and retrocollis is essential. Muscles are targeted specifically after observing the pattern of shift, tilt and rotation of the neck and through EMG assessment. Approximately U are needed for a muscle. Anterocollis with head protrusion has a poor response. Side effects include reduced head control and dysphagia. Injections into the sternocleidomastoid and ISSN
4 scalenes have a higher risk of dysphagia. Dysphagia can be minimized by avoiding bilateral sternocleidomastoid injections, targeting its insertion and ensuring that the muscle bulk is not penetrated. Patients in whom dysphagia might pose increased risk for aspiration pneumonia should be carefully assessed prior to injection. The effect of injections lasts for around 3-4 months. e) Pharyngolaryngeal dystonias: Botulinum toxin therapy is the treatment of choice for both abductor and adductor forms of pharyngolaryngeal dystonias. Amongst all the indications for BoNT/A therapy, spasmodic dysphonia shows the highest success rate (Dresseler, 2000). Botulinum toxin type A is injected either per orally under laryngoscopic guidance or transcutaneously through the cricothyroid membrane under EMG guidance. The vocalis muscle is targeted in the adductor form and posterior cricoarytenoid muscle in the abductor form. Approximately U are used and the effect begins within 2-3 days and lasts for 2-9 months. Complications include transient dysphagia, weak cough, hoarseness and hypophonia. Dyspnea and stridor can occur after treating the abductor form (Brin, 1998) and )(Dresseler, 2000). Quality of life measures improve significantly after BoNT/A injections. f) Writer's cramp and occupational cramps: Dystonic hyperactivity of forearm, hand and, occasionally, proximal arm muscles while writing occurs in writer's cramp. It is the most common occupational cramp. Response to BoNT/A is better if dystonia is limited to isolated muscles and if the initial hyperactive muscle trigger can be identified. Limitations to therapy include narrow therapeutic window of the wrist and finger flexors, large requirement for BoNT/A due to large number of muscles involved and difficulty to distinguish dystonic action from physiologic and compensatory action (Brin, 1998 and Dresseler, 2000). g) Non-action induced limb dystonias: Botulinum toxin type A is useful in pain reduction and improvement in function. 3) Hemifacial Spasms: Unilateral, involuntary, recurrent twitches of the eyelids and other muscles of face characterize hemifacial spasms. Periocular muscles, risorius, depressor anguli oris, depressor labii inferioris, zygomaticus and mentalis are targeted. Doses ranging from U are used. Therapy with BoNT/A has a high success rate and the effect is longer than for blepharospasm. Presently, BoNT is the first line treatment for hemifacial spasms and only those with a poor response may need surgical decompression of the facial nerve (Jankovic et al., 1997). 4) Strabismus or crossed eyes 5) Exocrine gland hyperactivity a) Focal hyperhidrosis: It is defined as excessive sweating of the palms, soles, axilla or face. The iodine-starch test delineates areas of hyperhidrosis and U/cm2 BoNT/A are injected intradermally. Approximately U are used at sites. The benefits last for 3-4 months and increased doses may extend this up to a year or more (Naumann et al., 1999). b) Relative sialorrhoea: Botulinum toxin type A injection into the parotid gland is effective for controlling drooling in conditions such as Parkinson's disease, motor neuron disease and bulbar/pseudobulbar palsy without causing xerostomia (Dresseler, 2000). c) Frey's syndrome: Areas of skin are targeted that show gustatory sweating due to aberrant innervation of facial nerve secretomotor fibers to sweat glands following parotidectomy (Dresseler, 2000). d) Crocodile tears syndrome: Lacrimal glands are targeted in gustatory lacrimation due to aberrant innervation of facial nerve secretomotor fibers (Dresseler, 2000). Other uses of botulinum toxin type A that are widely known but not specifically approved by FDA include treatment of: A) Prostate Hyperplasia: BTX- A injection induces prostate apotosis in dogs and relieves BOO (Bladder outlet obstruction) due to benign prostatic hyperplasia (BPH) in humans. Intraprostate BTX-A injection may be a ISSN
5 promising, reversible and alternative treatment for refractory BOO due to BPH. Furthermore, previous studies in the rats have shown that intraprostatic injection of BTX-A induces selective denervation and subsequent atrophy of the glands. B) Smooth Muscle Disorder: Direct injection of botulinum toxin is disclosed as an effective, safe and simple method of treatment for disorders of gastrointestinal muscle or smooth muscles elsewhere in the body, with results that appear to be sustained for several months. Muscle disorders which are suitable for such treatment include achalasia, isolated disorders of the lower esophageal sphincter, gastroparesis, hypertrophic pyloric stenosis, sphincter of Oddi dysfunction, short-segment Hirschsprung's, anal fissure, hemorrhoids, proctalgia fugax, irritable bowel syndrome, disorders of the upper esophageal sphincter, vasospastic disorders, and disorders of uterine and bladder spasm. Devices suitable for delivering this therapy are also disclosed. C) Overactive Bladder Syndrome with or without incontinence. D) Spastic Disorders associated with injury or disease of the central nervous system including trauma, stroke, multiple sclerosis, or cerebral palsy. E) Anal Fissure F) Diabetic neuropathy G) Wound healing H) Excessive salivation I) Parkinson Disease J) Depression COSMETIC USES OF BOTULINUM TOXIN A (BTX-A) The cosmetic effect of BTX-A was initially described by the Carruthers, a dermatologist/ophthalmologist husband and wife team working in Vancouver, Canada, although the effect had been observed by a number of independent groups. Cosmetically desirable effects of Botox were quickly discovered thereafter when the frown lines between the eyebrows were observed to soften following treatment for eye muscle disorders, leading to clinical trials and subsequent FDA approval for cosmetic use in April As of 2006, Botox injection is the most common cosmetic operation in the United States. 1) Hyperkinetic Facial Lines: The main application of botulinum toxin in facial plastic surgery is in the effacement of dynamic or hyperkinetic facial lines i.e. Frown/glabellar lines, squint lines /eye crows feet lines, forehead lines and the muscle bands on platysma muscles often visible on the neck, commonly known as "turkey neck" or platysma banding. The granting of US Food and Drug Administration approval for the use of Botulinum Toxin type A in the treatment of glabellar lines for people with age years marks a major milestone for the more widespread usage of this product in cosmetic settings. The use of botulinum toxin in treatment of hyperkinetic conditions is well established and enjoys an excellent safety profile (Batniji, 2004). Either local injection or creams are available for local use and typically, no anesthetic is necessary. The muscle relaxant effect lasts about three to four months and can be repeated as needed. 2) Eyebrow Lifting: A study has been carried out on 22 patients desiring a cosmetic enhancement and injection of botulinum toxin A (7-10 units) directed to brow depressor muscle (lateral orbicularis oculi) bilaterally. No patients withdrew for adverse effects. All patients were evaluated 2 weeks after treatment. The average brow elevation from the mid-pupil observed after selected injection of brow depressors with botulinum toxin A was 1.02 mm. The average brow elevation from the lateral canthus observed after selected injection of brow depressors with botulinum toxin A was 4.83 mm. Significant temporal brow elevation occurs as the result of paralysis of brow depressors by using botulinum toxin A injection. This procedure may be considered an alternative to surgical brow elevation. 3) Frontalis muscle hyperactivity. 4) Dimpling of the chin from overactive muscles. 5) Raise drooping of the corners of the mouth. ISSN
6 6) Fine lines around the lips. 7) Fine wrinkles under the eye. TREATMENT FAILURE Treatment failure can be either primary, where failure occurs in a BoNT-naïve patient, or secondary, where failure occurs after an initial successful use. Treatment failure may result from misplaced toxin, sub-optimal dosing, or administration of toxin that has been inactivated by improper storage or handling. Antibodies to the toxin are presumed to be responsible for most remaining cases of resistance. Antibodies against the toxin have developed in patients receiving large doses of the toxin, and these patients do not benefit from repeated toxin injections. Treatment failure for antigenicity is assessed when there is either absent or reduced response to BoNT. It is assessed clinically using the Frontalis type A antibody test (FTAT) wherein U of BoNT/A are injected into two sites of the muscle and ability to raise the brow at 2 weeks is studied. If eyebrows can be raised, it indicates resistance (Brin, 1998). Persistence of motor activity with ability to raise the eyebrow during electromyography (EMG) of injected muscles gives electrophysiological evidence of resistance. Antibodies are also detectable using ELISA, sphere-linked immunodiagnostic assay, western blot assay and combined fluorescein- and enzyme-linked assays (Brin, 1998). Unfortunately, correlation between clinical resistance and detection of antibodies by assays, especially ELISA, has not been established. Mouse neutralization assay /mouse protection assay is considered the gold-standard assay, whereby injected mice will not die if antibodies are present in the test serum, though the meaning of the test in clinical practice is controversial. Early reports indicated that neutralizing antibodies occurred in 3-10% of cervical dystonia patients treated with BoNT/A. The more recent preparations (available after 1997) have lower protein content, andare believed not to produce neutralizing antibodies possibly due to lower protein load. Patients with resistance to BoNT/A may benefit from botulinum toxins B or F (commercially BoNT/F is not available presently), as their immunogenicity profiles are different (Dresseler, 2000). Care must be taken when the toxin is administered because it can diffuse from the inoculation site through tissue to paralyze neuromuscular junctions of adjacent muscles, causing unwanted side effects such as muscle weakness and dysphagia. Immune responses to BoNT/A and presumably other BoNT preparations can be minimized by using the least required dose and avoiding frequent repeat injections. Current labeling in most countries suggests 12-week separation between doses of BoNT. REFERENCES 1. Bandyopadhyay S, Clark AW, DasGupta BR, Sathyamoorthy V. Role of the heavy and light chains of botulinum neurotoxin in neuromuscular paralysis. J Biol Chem 1987;262: Batniji RK, Falk AN. Update on botulinum toxin use in facial plastic surgery. Curr Opin Otolaryngol Head Neck Surg 2004;12(4): Bonventre PF. Absorption of botulinal toxin from the gastrointestinal tract. Rev Infect Dis 1979;1: Brin MF. Treatment of dystonias. In: Jankovic, J., Tolosa, E., editors. Parkinson's isease and movement disorders. Baltimore: Williams & Wilkins., 1998, pp Davis LE. Botulinum toxin-from poison to medicine. West J Med 1993;158: Dowell VR Jr. Botulinum and tetanus: Selected epidemiological and microbiological aspects. Rev Infect Dis 1984;11: Dressler D. Botulinum Toxin Therapy. Stuttgart: Georg Thieme Verlag., 2000, pp Jankovic J, Brin MF. Botulinum toxin: historical perspective and potential new indications. Muscle And Nerve 1997;20: Middlebrook JL.. Cell surface receptors for protein toxins. In: Simpson, L.L.(Ed): Botulinum Neurotoxin and Tetanus Toxin San ISSN
7 Diego, Calif. AcademicPress., 1989, pp Naumann M, Hamm H, Kinkelin I, Reiners K. Botulinum toxin type A in thetreatment of focal, axillary and palmar hyperhidrosis and other hyperhidrotic conditions. Eur J Neurol 1999;6: Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type a as a migraine preventive treatment. Headache 2000; 40: Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev 1980; 44: *Address for correspondence: Dr. Vinay Kant, Division of Pharmacology and Toxicology, Faculty of Veterinary Sciences & Animal Husbandry, SKUAST-J, R.S. Pura, Jammu drvinaykant2001@yahoo.co.in ISSN
THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN
 THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN Spasmodic torticollis (cervical dystonia), blepharospasm, and writer s cramp are specific types
THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN Spasmodic torticollis (cervical dystonia), blepharospasm, and writer s cramp are specific types
Clare Gaduzo BSc RMN Registered Aesthetics Practitioner (qualified with Medics Direct)
 Clare Gaduzo BSc RMN Registered Aesthetics Practitioner (qualified with Medics Direct) 07935567067 cjg.aesthetics@yahoo.co.uk www.cjgaesthetics.co.uk http://www.facebook.com/cjgaesthetics @CJGAesthetics
Clare Gaduzo BSc RMN Registered Aesthetics Practitioner (qualified with Medics Direct) 07935567067 cjg.aesthetics@yahoo.co.uk www.cjgaesthetics.co.uk http://www.facebook.com/cjgaesthetics @CJGAesthetics
Dr. F Didar CEO Cosmetic Facial UK(CFUK) Limited
 Dr. F Didar CEO Cosmetic Facial UK(CFUK) Limited www.cosmeticfacial.co.uk, info@cosmeticfacial.co.uk 1. 1895:professor Emile Pierre Van Ermengem discovered(identified) bacterium bacillus botulinum. It
Dr. F Didar CEO Cosmetic Facial UK(CFUK) Limited www.cosmeticfacial.co.uk, info@cosmeticfacial.co.uk 1. 1895:professor Emile Pierre Van Ermengem discovered(identified) bacterium bacillus botulinum. It
Botox. Botox (onabotulinum toxin A) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 8 Last Review Date: September 15, 2017 Botox Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 8 Last Review Date: September 15, 2017 Botox Description Botox (onabotulinum
Botulinum Toxin Application
 Botulinum Toxin Application Clostridium botulinum: rod-shaped bacterium producing the neurotoxin botulin Gram-positive anaerobic bacterium Seven serotypes - A, B, C, D, E, F, G http://standeyo.com/news/08_health/081202.biological.weapons.html
Botulinum Toxin Application Clostridium botulinum: rod-shaped bacterium producing the neurotoxin botulin Gram-positive anaerobic bacterium Seven serotypes - A, B, C, D, E, F, G http://standeyo.com/news/08_health/081202.biological.weapons.html
BOTOX. Description. Section: Prescription Drugs Effective Date: January 1, 2013 Subsection: CNS Original Policy Date: December 7, 2011
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.12.01 Subject: Botox Page: 1 of 6 Last Review Status/Date: December 6, 2012 BOTOX Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.12.01 Subject: Botox Page: 1 of 6 Last Review Status/Date: December 6, 2012 BOTOX Description Botox (onabotulinum
Botulinum toxins: Pharmacology and its current therapeutic evidence for use
 Review Article Botulinum toxins: Pharmacology and its current therapeutic evidence for use J. N. Panicker, U. B. Muthane Department of Neurology, NIMHANS Hosur Road, Bangalore - 560029, India. Botulinum
Review Article Botulinum toxins: Pharmacology and its current therapeutic evidence for use J. N. Panicker, U. B. Muthane Department of Neurology, NIMHANS Hosur Road, Bangalore - 560029, India. Botulinum
Botox. Botox (onabotulinum toxin A) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 10 Last Review Date: November 30, 2018 Botox Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 10 Last Review Date: November 30, 2018 Botox Description Botox (onabotulinum
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb)
 Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date:
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date:
Botuli focal d THERAPEUTIC INTERVENTION
 30 PRACTICAL NEUROLOGY THERAPEUTIC INTERVENTION The Little Milkmaid by Amedeo Modigliani (1884 1920). Does this represent artistic licence or cervical dystonia? Nigel Hyman Department of Neurology, Radcliffe
30 PRACTICAL NEUROLOGY THERAPEUTIC INTERVENTION The Little Milkmaid by Amedeo Modigliani (1884 1920). Does this represent artistic licence or cervical dystonia? Nigel Hyman Department of Neurology, Radcliffe
Botox , The Patient Education Institute, Inc. rxf60101 Last reviewed: 01/11/2018 1
 Botox Introduction Botox is a well known brand name for a medicinal form of a toxin, or poison. When injected in small doses into specific muscles, Botox doesn't poison you. Instead, it acts as a muscle
Botox Introduction Botox is a well known brand name for a medicinal form of a toxin, or poison. When injected in small doses into specific muscles, Botox doesn't poison you. Instead, it acts as a muscle
Prof. of Neurology, Cairo University Vice President of the Egyptian Society of Neurology, Secretary General of Pan Arab union of Neurological
 Prof. of Neurology, Cairo University Vice President of the Egyptian Society of Neurology, Secretary General of Pan Arab union of Neurological Societies The Egyptian Society of Neurology, Psychiatry and
Prof. of Neurology, Cairo University Vice President of the Egyptian Society of Neurology, Secretary General of Pan Arab union of Neurological Societies The Egyptian Society of Neurology, Psychiatry and
Disclaimer. All information shared in this presentation is in the public domain.
 THE BOTOX STORY Disclaimer All information shared in this presentation is in the public domain. Hence, no trade secret related to drug formulation, manufacturing process, commercial practice, etc. The
THE BOTOX STORY Disclaimer All information shared in this presentation is in the public domain. Hence, no trade secret related to drug formulation, manufacturing process, commercial practice, etc. The
Botulinum toxin: Getting started with cosmetic treatment Learning Objectives The Aging Face The Aging Face The Aging Face
 1 2 Botulinum toxin: Getting started with cosmetic treatment Suzanne K. Freitag, M.D. Director, Ophthalmic Plastic Surgery Service Massachusetts Eye and Ear Infirmary Harvard Medical School 3 I have no
1 2 Botulinum toxin: Getting started with cosmetic treatment Suzanne K. Freitag, M.D. Director, Ophthalmic Plastic Surgery Service Massachusetts Eye and Ear Infirmary Harvard Medical School 3 I have no
Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc )
 Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc ) These services may or may not be covered by your HealthPartners
Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc ) These services may or may not be covered by your HealthPartners
Subject Index /6/2018 2:06:06 AM. Downloaded by:
 Subject Index Acrosyringium, metal salt interactions 32 34, 40 Aluminum chloride hexahydrate antimicrobial effect 40 antiperspirant efficacy in hyperhidrosis 32, 38, 39 application mode 38 clinical indications
Subject Index Acrosyringium, metal salt interactions 32 34, 40 Aluminum chloride hexahydrate antimicrobial effect 40 antiperspirant efficacy in hyperhidrosis 32, 38, 39 application mode 38 clinical indications
Neuromuscular Blocking Agents
 Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
BOTOX (onabotulinumtoxina) for Therapeutic Use
 BOTOX (onabotulinumtoxina) for Therapeutic Use BOTOX (onabotulinumtoxina) & BOTOX Cosmetic (onabotulinumtoxina) Important Information IMPORTANT SAFETY INFORMATION BOTOX and BOTOX Cosmetic may cause serious
BOTOX (onabotulinumtoxina) for Therapeutic Use BOTOX (onabotulinumtoxina) & BOTOX Cosmetic (onabotulinumtoxina) Important Information IMPORTANT SAFETY INFORMATION BOTOX and BOTOX Cosmetic may cause serious
ALTERNATIVE TREATMENTS
 Botox Consent INSTRUCTIONS This is an informed- consent document which has been prepared to help your plastic surgeon inform you concerning BOTOX (Botulina Toxin Type A, Allergan) injection, its risks,
Botox Consent INSTRUCTIONS This is an informed- consent document which has been prepared to help your plastic surgeon inform you concerning BOTOX (Botulina Toxin Type A, Allergan) injection, its risks,
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Clostridium botulinum neurotoxin type A haemagglutinin complex Pharmaceutical form(s)/strength: powder for solution for injection, 300 unit and 500 unit P-RMS:
M0BCore Safety Profile Active substance: Clostridium botulinum neurotoxin type A haemagglutinin complex Pharmaceutical form(s)/strength: powder for solution for injection, 300 unit and 500 unit P-RMS:
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb)
 Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Policy. Medical Policy Manual Approved Revised: Do Not Implement Until 4/2/19. IncobotulinumtoxinA
 IncobotulinumtoxinA NDC CODE(S) 00259-1605-XX Xeomin 50 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1610-XX Xeomin 100 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1620-XX Xeomin 200 UNIT SOLR (MERZ PHARMACEUTICAL)
IncobotulinumtoxinA NDC CODE(S) 00259-1605-XX Xeomin 50 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1610-XX Xeomin 100 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1620-XX Xeomin 200 UNIT SOLR (MERZ PHARMACEUTICAL)
Hubert H. Fernandez, MD
 Hubert H. Fernandez, MD Associate Professor Co-Director, Movement Disorders Center Director, Clinical Trials for Movement Disorders Program Director, Neurology Residency and Movement Disorders Fellowship
Hubert H. Fernandez, MD Associate Professor Co-Director, Movement Disorders Center Director, Clinical Trials for Movement Disorders Program Director, Neurology Residency and Movement Disorders Fellowship
Informed Consent Form
 Informed Consent Form Botox Cosmetic (onabotulinumtoxin A) Dysport (abobotulinumtoxin A) Xeomin (incobotulinumtoxin A) INSTRUCTIONS This is an informed-consent document that has been prepared to help inform
Informed Consent Form Botox Cosmetic (onabotulinumtoxin A) Dysport (abobotulinumtoxin A) Xeomin (incobotulinumtoxin A) INSTRUCTIONS This is an informed-consent document that has been prepared to help inform
Medication Prior Authorization Form
 (OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 7/7/2016 Next Review:
(OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 7/7/2016 Next Review:
Prior Authorization Update: Botulinum Toxins
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization AETA BETTER HEALTH MICHIGA Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Pharmacy Prior Authorization AETA BETTER HEALTH MICHIGA Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Botulinum toxin A (Dysport) for hyperhidrosis of the axillae
 April 2016 Horizon Scanning Research & Intelligence Centre Botulinum toxin A (Dysport) for hyperhidrosis of the axillae LAY SUMMARY This briefing is based on information available at the time of research
April 2016 Horizon Scanning Research & Intelligence Centre Botulinum toxin A (Dysport) for hyperhidrosis of the axillae LAY SUMMARY This briefing is based on information available at the time of research
BOTOX Cosmetic (onabotulinumtoxina) before-and-after
 BOTOX Cosmetic (onabotulinumtoxina) before-and-after Moderate to severe crow s feet lines Before After (Day 7) Actual patient. Results may vary. Photos taken at full smile before and after treatment with
BOTOX Cosmetic (onabotulinumtoxina) before-and-after Moderate to severe crow s feet lines Before After (Day 7) Actual patient. Results may vary. Photos taken at full smile before and after treatment with
Informed Consent Botulina Toxins Botox, Dysport, Xeomin Neurotoxins
 Informed Consent Botulina Toxins - Botox Neurotoxins Informed Consent Botulina Toxins Botox, Dysport, Xeomin Neurotoxins INSTRUCTIONS This is an informed-consent document that has been prepared to help
Informed Consent Botulina Toxins - Botox Neurotoxins Informed Consent Botulina Toxins Botox, Dysport, Xeomin Neurotoxins INSTRUCTIONS This is an informed-consent document that has been prepared to help
CONSUMER MEDICINE INFORMATION
 CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
Medication Prior Authorization Form
 (OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 07/07/2017 Next Review:
(OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 07/07/2017 Next Review:
Is OnabotulinumtoxinA Good for Other Head and Face Pain? Disclosures BoNT/A for non- CM Botulinum neurotoxin (BoNT) in clinical use for headache >20
 1 2 3 4 5 6 Is OnabotulinumtoxinA Good for Other Head and Face Pain? Disclosures BoNT/A for non- CM Botulinum neurotoxin (BoNT) in clinical use for headache >20 years Efficacy of BoNT type A (onabotulinumtoxina,
1 2 3 4 5 6 Is OnabotulinumtoxinA Good for Other Head and Face Pain? Disclosures BoNT/A for non- CM Botulinum neurotoxin (BoNT) in clinical use for headache >20 years Efficacy of BoNT type A (onabotulinumtoxina,
BOTOX Cosmetic (onabotulinumtoxina) before-and-after photo files
 BOTOX Cosmetic (onabotulinumtoxina) before-and-after photo files INSTRUCTIONS FOR USE This zip file contains PDF files with images and Important Safety Information to copy and paste into your practice
BOTOX Cosmetic (onabotulinumtoxina) before-and-after photo files INSTRUCTIONS FOR USE This zip file contains PDF files with images and Important Safety Information to copy and paste into your practice
Clinical Policy: IncobotulinumtoxinA (Xeomin) Reference Number: ERX.SPA.194 Effective Date:
 Clinical Policy: (Xeomin) Reference Number: ERX.SPA.194 Effective Date: 01.11.17 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xeomin) Reference Number: ERX.SPA.194 Effective Date: 01.11.17 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Parkinson's Disease Center and Movement Disorders Clinic
 Parkinson's Disease Center and Movement Disorders Clinic 7200 Cambridge Street, 9th Floor, Suite 9A Houston, Texas 77030 713-798-2273 phone www.jankovic.org Botulinum Toxin Botulinum toxin (BTX) has been
Parkinson's Disease Center and Movement Disorders Clinic 7200 Cambridge Street, 9th Floor, Suite 9A Houston, Texas 77030 713-798-2273 phone www.jankovic.org Botulinum Toxin Botulinum toxin (BTX) has been
BOTULINUM TOXINS A AND B
 DRUG POLICY BOTULINUM TOXINS A B Policy Number: 2014D0017N Effective Date: 9/1/2014 Table of Contents Page COVERAGE RATIONALE... 1 BENEFIT CONSIDERATIONS... 7 BACKGROUND... 8 CLINICAL EVIDENCE... 8 U.S.
DRUG POLICY BOTULINUM TOXINS A B Policy Number: 2014D0017N Effective Date: 9/1/2014 Table of Contents Page COVERAGE RATIONALE... 1 BENEFIT CONSIDERATIONS... 7 BACKGROUND... 8 CLINICAL EVIDENCE... 8 U.S.
This guide describes some of the important facts about Neurobloc that you need to be aware of, however, it does not replace the advice given to you
 This guide describes some of the important facts about Neurobloc that you need to be aware of, however, it does not replace the advice given to you by a healthcare professional. Further very important
This guide describes some of the important facts about Neurobloc that you need to be aware of, however, it does not replace the advice given to you by a healthcare professional. Further very important
Scottish Medicines Consortium
 Scottish Medicines Consortium clostridium botulinum neurotoxin type A, 100 unit powder for solution for injection (Xeomin ) No. (464/08) Merz Pharma UK Ltd 09 May 2008 The Scottish Medicines Consortium
Scottish Medicines Consortium clostridium botulinum neurotoxin type A, 100 unit powder for solution for injection (Xeomin ) No. (464/08) Merz Pharma UK Ltd 09 May 2008 The Scottish Medicines Consortium
Botulinum Neuromodulators: The Basics. Karol A Gutowski, MD, FACS
 Botulinum Neuromodulators: The Basics Karol A Gutowski, MD, FACS Disclosures AxcelRx Pharmacuticals - Advisory Board Suneva Medical - Instructor Will discuss off-label uses Will use brand names for ease
Botulinum Neuromodulators: The Basics Karol A Gutowski, MD, FACS Disclosures AxcelRx Pharmacuticals - Advisory Board Suneva Medical - Instructor Will discuss off-label uses Will use brand names for ease
BOTULINUM TOXIN IN LOWER URINARY TRACT
 BOTULINUM TOXIN IN LOWER URINARY TRACT Selcuk Yucel, MD Professor in Urology and Pediatric Urology Acibadem University School of Medicine Department of Uroloy and Pediatric Urology Acibadem University
BOTULINUM TOXIN IN LOWER URINARY TRACT Selcuk Yucel, MD Professor in Urology and Pediatric Urology Acibadem University School of Medicine Department of Uroloy and Pediatric Urology Acibadem University
Sierra Smith Bio 205 Extra Credit Essay. My Face. Growing up I was always told that it takes 43 muscles to frown but only 17
 Sierra Smith Bio 205 Extra Credit Essay My Face Growing up I was always told that it takes 43 muscles to frown but only 17 muscles to smile and I should just smile because it's easier. It wasn't until
Sierra Smith Bio 205 Extra Credit Essay My Face Growing up I was always told that it takes 43 muscles to frown but only 17 muscles to smile and I should just smile because it's easier. It wasn't until
BOTULINUM TOXIN POLICY TO INCLUDE:
 BOTULINUM TOXIN POLICY TO INCLUDE: Blepharospasm in adults, Hemi facial spasm in adults, spasmodic torticollis (cervical dystonia), focal spasticity treatment of dynamic equinus foot deformity, focal spasticity
BOTULINUM TOXIN POLICY TO INCLUDE: Blepharospasm in adults, Hemi facial spasm in adults, spasmodic torticollis (cervical dystonia), focal spasticity treatment of dynamic equinus foot deformity, focal spasticity
Office Phone: * Cigna ID: * Date of Birth: Office Street Address: City: State: Zip:
 Phone: (800) 244-6224 Fax: (855) 840-1678 Botox (botulinum toxin type A) Notice: Please be sure to complete this form in its entirety. Missing information makes it difficult to approve requests and creates
Phone: (800) 244-6224 Fax: (855) 840-1678 Botox (botulinum toxin type A) Notice: Please be sure to complete this form in its entirety. Missing information makes it difficult to approve requests and creates
Crow's Feet Treatment with Botulinum Toxin Type A. I Petropoulos, G Noussios, P Chouridis, G Kontzoglou, K Karagiannidis
 ISPUB.COM The Internet Journal of Aesthetic and Antiaging Medicine Volume 1 Number 2 Crow's Feet Treatment with Botulinum Toxin Type A I Petropoulos, G Noussios, P Chouridis, G Kontzoglou, K Karagiannidis
ISPUB.COM The Internet Journal of Aesthetic and Antiaging Medicine Volume 1 Number 2 Crow's Feet Treatment with Botulinum Toxin Type A I Petropoulos, G Noussios, P Chouridis, G Kontzoglou, K Karagiannidis
Drug Name (select from list of drugs shown / provide drug information) Patient Information. Prescribing Physician
 Texas Standard Prior Authorization Form Addendum MOLINA TX MARKETPLACE Botulinum Toxins CL Molina Universal This fax machine is located in a secure location as required by HIPAA Regulations. Complete /
Texas Standard Prior Authorization Form Addendum MOLINA TX MARKETPLACE Botulinum Toxins CL Molina Universal This fax machine is located in a secure location as required by HIPAA Regulations. Complete /
DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex)
 DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex) CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some common questions about Dysport. It does not contain all
DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex) CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some common questions about Dysport. It does not contain all
MEDICATION GUIDE BOTOX BOTOX Cosmetic (Boe-tox) (onabotulinumtoxina) for Injection
 17.2 Ability to Operate Machinery or Vehicles Advise patients that if loss of strength, muscle weakness, blurred vision, or drooping eyelids occur, they should avoid driving a car or engaging in other
17.2 Ability to Operate Machinery or Vehicles Advise patients that if loss of strength, muscle weakness, blurred vision, or drooping eyelids occur, they should avoid driving a car or engaging in other
Muscles of the Face, Head, and Neck
 Muscles of the Face, Head, and Neck 1 How Muscles Are Named Many muscles named using such features as Location Function Shape Direction of fibers Number of heads or divisions Points of attachment Size
Muscles of the Face, Head, and Neck 1 How Muscles Are Named Many muscles named using such features as Location Function Shape Direction of fibers Number of heads or divisions Points of attachment Size
DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex)
 DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex) CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some common questions about Dysport. It does not contain all
DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex) CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some common questions about Dysport. It does not contain all
Scientific Update Laxman Bahroo, MD
 Scientific Update Laxman Bahroo, MD Associate Professor Director; Botulinum Toxin Clinic Director; Neurology Residency Program Medstar Georgetown University Hospital Washington, D.C. Disclosures Advisory
Scientific Update Laxman Bahroo, MD Associate Professor Director; Botulinum Toxin Clinic Director; Neurology Residency Program Medstar Georgetown University Hospital Washington, D.C. Disclosures Advisory
Botulinum Toxin: Applications in Urology
 Botulinum Toxin: Applications in Urology Dr. Lee Jonat, PGY-4 Department of Urologic Sciences University of British Columbia Outline Mechanism of Action Technical Considerations Adverse Events Neurogenic
Botulinum Toxin: Applications in Urology Dr. Lee Jonat, PGY-4 Department of Urologic Sciences University of British Columbia Outline Mechanism of Action Technical Considerations Adverse Events Neurogenic
This policy addresses only the type B formulation rimabotulinumtoxinb marketed as Myobloc.
 RimabotulinumtoxinB NDC CODE(S) 10454-0710-XX Myobloc 2500 UNIT/0.5ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0711-XX Myobloc 5000 UNIT/ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0712-XX Myobloc 10000 UNIT/2ML
RimabotulinumtoxinB NDC CODE(S) 10454-0710-XX Myobloc 2500 UNIT/0.5ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0711-XX Myobloc 5000 UNIT/ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0712-XX Myobloc 10000 UNIT/2ML
USE OF BOTOX IN THE CHILD WITH CEREBRAL PALSY. Andrew Redfern Senior Registrar, Paed Neurodevelopment Red Cross Children s Hospital
 USE OF BOTOX IN THE CHILD WITH CEREBRAL PALSY Andrew Redfern Senior Registrar, Paed Neurodevelopment Red Cross Children s Hospital WHAT IS BOTOX? BOTOX is a purified form of Botulinum toxin Neurotoxin
USE OF BOTOX IN THE CHILD WITH CEREBRAL PALSY Andrew Redfern Senior Registrar, Paed Neurodevelopment Red Cross Children s Hospital WHAT IS BOTOX? BOTOX is a purified form of Botulinum toxin Neurotoxin
Abbreviated Class Review: Botulinum toxins
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
INSTRUCTIONS INTRODUCTION RISKS OF BOTULINUM TOXIN / DERMAL FILLER INJECTIONS
 INSTRUCTIONS This is an informed consent document that has been prepared to help inform you concerning botulinum toxin / dermal filler injections and the risks involved. It is important that you read this
INSTRUCTIONS This is an informed consent document that has been prepared to help inform you concerning botulinum toxin / dermal filler injections and the risks involved. It is important that you read this
Drug Class Update: Botulinum Toxins
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
BOTULINUM TOXINS IN NEUROLOGICAL DISEASE
 INVITED REVIEW ABSTRACT: Botulinum toxins are among the most potent neurotoxins known to humans. In the past 25 years, botulinum toxin has emerged as both a potential weapon of bioterrorism and as a powerful
INVITED REVIEW ABSTRACT: Botulinum toxins are among the most potent neurotoxins known to humans. In the past 25 years, botulinum toxin has emerged as both a potential weapon of bioterrorism and as a powerful
Ultrasonography guided dystonia & spasticity control Part I: trunk, upper extremity
 Ultrasonography guided dystonia & spasticity control Part I: trunk, upper extremity Don Kyu Kim, M.D. Department of PM & R Chung Ang University College of Medicine Spasticity Vs Dystonia Velocity dependent
Ultrasonography guided dystonia & spasticity control Part I: trunk, upper extremity Don Kyu Kim, M.D. Department of PM & R Chung Ang University College of Medicine Spasticity Vs Dystonia Velocity dependent
Botulinum Toxin Type A for Injection Ph.Eur. Brand Name: BOTO GENIE
 Summary of Product Characteristics of Botulinum Toxin Type A for Injection Ph.Eur. Brand Name: BOTO GENIE Manufactured By: BIO-MED (P) LTD. C-96, Site No. 1, Bulandshahr Road Industrial Area, Ghaziabad
Summary of Product Characteristics of Botulinum Toxin Type A for Injection Ph.Eur. Brand Name: BOTO GENIE Manufactured By: BIO-MED (P) LTD. C-96, Site No. 1, Bulandshahr Road Industrial Area, Ghaziabad
Table of Contents. 1.0 Description of the Procedure, Product, or Service Safety and Provider Compliance... 1
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
INFORMED CONSENT BOTOX INJECTION
 . Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
PRE- READING COURSE MATERIAL ADVANCED BOTOX Module 2
 PRE- READING COURSE MATERIAL ADVANCED BOTOX Module 2 Unit Overview Storage and shelf-life Cautions and contra-indications of treatment Reconstitution The treatment process Cosmetic indications for use
PRE- READING COURSE MATERIAL ADVANCED BOTOX Module 2 Unit Overview Storage and shelf-life Cautions and contra-indications of treatment Reconstitution The treatment process Cosmetic indications for use
NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (50U, 100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition
 NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (50U, 100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 50 units (U),
NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (50U, 100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 50 units (U),
CONSUMER MEDICINE INFORMATION
 CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
Botulinum Neuromodulators: Clinical Uses. Karol A Gutowski, MD, FACS
 Botulinum Neuromodulators: Clinical Uses Karol A Gutowski, MD, FACS Disclosures Angiotech/Surgical Specialties - Advisory Board AxcelRx Pharmacuticals - Advisory Board Suneva Medical - Instructor Will
Botulinum Neuromodulators: Clinical Uses Karol A Gutowski, MD, FACS Disclosures Angiotech/Surgical Specialties - Advisory Board AxcelRx Pharmacuticals - Advisory Board Suneva Medical - Instructor Will
Frequently Asked Questions
 Frequently Asked Questions What are the causes of wrinkles and facial expression lines? As noted above, lines and wrinkles develop, at least in part, from the action of facial muscles under the skin. After
Frequently Asked Questions What are the causes of wrinkles and facial expression lines? As noted above, lines and wrinkles develop, at least in part, from the action of facial muscles under the skin. After
NAME OF THE MEDICINE. BOTOX purified neurotoxin complex injection (100 U or 200 U) (botulinum toxin, type A) DESCRIPTION.
 NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 100 units (U) or
NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 100 units (U) or
Botulinum Toxin
 Botulinum Toxin Botulinum toxin type A injections are one of the most popular minimally-invasive cosmetic procedures performed to treat facial lines and wrinkles, called frown lines, on the forehead. Botulinum
Botulinum Toxin Botulinum toxin type A injections are one of the most popular minimally-invasive cosmetic procedures performed to treat facial lines and wrinkles, called frown lines, on the forehead. Botulinum
Overview of Botulinum Toxin
 Chapter 1 Overview of Botulinum Toxin Berthold Rzany, Hendrik Zielke 1 Contents 1.1 Introduction............. 1 1.2 Different Subtypes of Botulinum Toxin. 1 1.3 Mode of Action............ 1 1.4 Antidote...............
Chapter 1 Overview of Botulinum Toxin Berthold Rzany, Hendrik Zielke 1 Contents 1.1 Introduction............. 1 1.2 Different Subtypes of Botulinum Toxin. 1 1.3 Mode of Action............ 1 1.4 Antidote...............
Headache and Migraine Treatment
 Many of us think of Botox primarily as a cosmetic treatment for lines and wrinkles on the face, but the botulinum toxin that Botox is derived from has a long history of medically therapeutic uses. In fact,
Many of us think of Botox primarily as a cosmetic treatment for lines and wrinkles on the face, but the botulinum toxin that Botox is derived from has a long history of medically therapeutic uses. In fact,
Antibody-Induced Failure of Botulinum Toxin A Therapy in Cosmetic Indications
 Antibody-Induced Failure of Botulinum Toxin A Therapy in Cosmetic Indications DIRK DRESSLER, MD, PHD, KAI WOHLFAHRT, MD, PHD, y ELLEN MEYER-ROGGE, MD, z LUITGARD WIEST, MD, y AND HANS BIGALKE, MD, PHD
Antibody-Induced Failure of Botulinum Toxin A Therapy in Cosmetic Indications DIRK DRESSLER, MD, PHD, KAI WOHLFAHRT, MD, PHD, y ELLEN MEYER-ROGGE, MD, z LUITGARD WIEST, MD, y AND HANS BIGALKE, MD, PHD
The Scalp and Face Protocol. Julie Goodwin, BA, LMT
 The Scalp and Face Protocol Julie Goodwin, BA, LMT The Scalp and Face Protocol Julie Goodwin, BA, LMT Julie Goodwin 2014 2 Agenda Pertinent Anatomy and Physiology Treatment Planning Strokes, Techniques
The Scalp and Face Protocol Julie Goodwin, BA, LMT The Scalp and Face Protocol Julie Goodwin, BA, LMT Julie Goodwin 2014 2 Agenda Pertinent Anatomy and Physiology Treatment Planning Strokes, Techniques
Head and Face Anatomy
 Head and Face Anatomy Epicranial region The Scalp The soft tissue that covers the vault of skull. Extends from supraorbital margin to superior nuchal line. Layers of the scalp S C A L P = skin = connective
Head and Face Anatomy Epicranial region The Scalp The soft tissue that covers the vault of skull. Extends from supraorbital margin to superior nuchal line. Layers of the scalp S C A L P = skin = connective
This lab activity is aligned with Visible Body s Anatomy and Physiology app. Learn more at visiblebody.com/professors
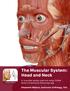 1 This lab activity is aligned with Visible Body s Anatomy and Physiology app. Learn more at visiblebody.com/professors 2 PRE-LAB EXERCISES A. Watch the video 13.1 Muscular System Overview and observe
1 This lab activity is aligned with Visible Body s Anatomy and Physiology app. Learn more at visiblebody.com/professors 2 PRE-LAB EXERCISES A. Watch the video 13.1 Muscular System Overview and observe
CONSUMER MEDICINE INFORMATION
 CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
Original Policy Date
 MP 5.01.03 Botulinum Toxin Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical Policy Index
MP 5.01.03 Botulinum Toxin Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical Policy Index
What it looks like 2. WHAT BOTOX INJECTION IS USED FOR. How BOTOX injection works
 (botulinum toxin, type A) purified neurotoxin complex Consumer Medicine Information The information in this leaflet is ONLY a summary and is not a complete statement about injection. Your doctor has more
(botulinum toxin, type A) purified neurotoxin complex Consumer Medicine Information The information in this leaflet is ONLY a summary and is not a complete statement about injection. Your doctor has more
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 4/1/2017 Section: MED Policy No.: 209 Medical Officer 4/1/2018 Date Technology Assessment Committee Approved Date: 4/09;6/10, 01/11; 5/13; 2/15 Medical Policy Committee Approved Date: 11/09;
Effective Date: 4/1/2017 Section: MED Policy No.: 209 Medical Officer 4/1/2018 Date Technology Assessment Committee Approved Date: 4/09;6/10, 01/11; 5/13; 2/15 Medical Policy Committee Approved Date: 11/09;
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 Local Coverage Determination (LCD): Drugs and Biologicals: Botulinum Toxins (L34253) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Drugs and Biologicals: Botulinum Toxins (L34253) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Xeomin) Reference Number: CP.PHAR.231 Effective Date: 07.01.16 Last Review Date 05.18 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Xeomin) Reference Number: CP.PHAR.231 Effective Date: 07.01.16 Last Review Date 05.18 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Injections, Vaccines, and Other Physician-Administered Drugs Codes Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Injections, Vaccines, and Other Physician-Administered Drugs Codes Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy
Long-term Efficacy of Botulinum Neurotoxin-A Treatment for Essential Blepharospasm
 pissn: 111-8942 eissn: 292-9382 Korean J Ophthalmol 218;32(1):1-7 https://doi.org/1.3341/kjo.217.3 Original Article Long-term Efficacy of Botulinum Neurotoxin-A Treatment for Essential Blepharospasm Seunghyun
pissn: 111-8942 eissn: 292-9382 Korean J Ophthalmol 218;32(1):1-7 https://doi.org/1.3341/kjo.217.3 Original Article Long-term Efficacy of Botulinum Neurotoxin-A Treatment for Essential Blepharospasm Seunghyun
Albert C. Clairmont, MD Associate Professor-Clinical Department of PM & R The Ohio State University
 Botulinum Toxin for Movement Disorders: Physiology, Pharmacology and Evaluation of Patients Albert C. Clairmont, MD Associate Professor-Clinical Department of PM & R The Ohio State University OBJECTIVES
Botulinum Toxin for Movement Disorders: Physiology, Pharmacology and Evaluation of Patients Albert C. Clairmont, MD Associate Professor-Clinical Department of PM & R The Ohio State University OBJECTIVES
200 SW 8 th St, STE A, Ocala, FL PH: FAX: Dr. Omar Garcia, Medical Director
 200 SW 8 th St, STE A, Ocala, FL 34471 PH: 352-369-0104 FAX: 352-369-0107 Dr. Omar Garcia, Medical Director BRIEF MEDICAL HISTORY Name Phone Age Height Weight Address City State Zip Current Physician's
200 SW 8 th St, STE A, Ocala, FL 34471 PH: 352-369-0104 FAX: 352-369-0107 Dr. Omar Garcia, Medical Director BRIEF MEDICAL HISTORY Name Phone Age Height Weight Address City State Zip Current Physician's
Clinical Policy: IncobotulinumtoxinA (Xeomin) Reference Number: CP.PHAR.231
 Clinical Policy: (Xeomin) Reference Number: CP.PHAR.231 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Xeomin) Reference Number: CP.PHAR.231 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
An infected insect bite? Dr Estée Török Honorary Consultant in Infectious Diseases & Microbiology Addenbrooke s Hospital, Cambridge
 An infected insect bite? Dr Estée Török Honorary Consultant in Infectious Diseases & Microbiology Addenbrooke s Hospital, Cambridge Case history 48 year old man Presented to A&E with 48 hour history double
An infected insect bite? Dr Estée Török Honorary Consultant in Infectious Diseases & Microbiology Addenbrooke s Hospital, Cambridge Case history 48 year old man Presented to A&E with 48 hour history double
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Botulinum toxin and its clinical aspects: An overview
 Review Article Botulinum toxin and its clinical aspects: An overview Shatavisa Mukherjee Department of Pharmacology, NSHM Knowledge Campus Kolkata Group of Institutions, Kolkata, West Bengal, India Botulinum
Review Article Botulinum toxin and its clinical aspects: An overview Shatavisa Mukherjee Department of Pharmacology, NSHM Knowledge Campus Kolkata Group of Institutions, Kolkata, West Bengal, India Botulinum
3-Deep fascia: is absent (except over the parotid gland & buccopharngeal fascia covering the buccinator muscle)
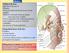 The Face 1-Skin of the Face The skin of the face is: Elastic Vascular (bleed profusely however heal rapidly) Rich in sweat and sebaceous glands (can cause acne in adults) It is connected to the underlying
The Face 1-Skin of the Face The skin of the face is: Elastic Vascular (bleed profusely however heal rapidly) Rich in sweat and sebaceous glands (can cause acne in adults) It is connected to the underlying
Botulinum Toxins. Length of Authorization: From 90 days to 12 months
 Botulinum Toxins Goal(s): Approve botulinum toxins for funded OHP conditions supported by evidence of benefit (eg, dystonia or spasticity associated with certain neurological diseases). Require positive
Botulinum Toxins Goal(s): Approve botulinum toxins for funded OHP conditions supported by evidence of benefit (eg, dystonia or spasticity associated with certain neurological diseases). Require positive
Circle Yes or No Y N. [If yes, no further questions.]
![Circle Yes or No Y N. [If yes, no further questions.] Circle Yes or No Y N. [If yes, no further questions.]](/thumbs/83/88529512.jpg) 02/18/2016 Prior Authorization AETA BETTER HEALTH PE MEDICAID & AETA BETTER HEALTH KIDS Botulinum Toxins (PA88) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
02/18/2016 Prior Authorization AETA BETTER HEALTH PE MEDICAID & AETA BETTER HEALTH KIDS Botulinum Toxins (PA88) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
A consumer s guide to Botox
 A consumer s guide to Botox In 2001, when Health Canada approved botulinum toxin type A for the treatment of frown lines, Botox suddenly became a household name. While you may know that the drug is great
A consumer s guide to Botox In 2001, when Health Canada approved botulinum toxin type A for the treatment of frown lines, Botox suddenly became a household name. While you may know that the drug is great
Hemifacial spasm. Parkinson's Disease Center and Movement Disorders Clinic
 Parkinson's Disease Center and Movement Disorders Clinic 7200 Cambridge Street, 9th Floor, Suite 9A Houston, Texas 77030 713-798-2273 phone www.jankovic.org Hemifacial spasm Diagnosis Hemifacial spasm
Parkinson's Disease Center and Movement Disorders Clinic 7200 Cambridge Street, 9th Floor, Suite 9A Houston, Texas 77030 713-798-2273 phone www.jankovic.org Hemifacial spasm Diagnosis Hemifacial spasm
Myriad Uses of Botulinum Toxin
 Myriad Uses of Botulinum Toxin 31 6 Myriad Uses of Botulinum Toxin BP SINGH, M BEHARI Introduction Botulinum toxin (BTX) is an endotoxin produced by Clostridium botulinum, a gram-positive anaerobic bacterium
Myriad Uses of Botulinum Toxin 31 6 Myriad Uses of Botulinum Toxin BP SINGH, M BEHARI Introduction Botulinum toxin (BTX) is an endotoxin produced by Clostridium botulinum, a gram-positive anaerobic bacterium
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: botulinum_toxin_injection 8/1985 10/2017 10/2018 10/2017 Description of Procedure or Service Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: botulinum_toxin_injection 8/1985 10/2017 10/2018 10/2017 Description of Procedure or Service Description
Treatment of facial palsy with Botulinum Toxin (Botox) Information for patients
 Treatment of facial palsy with Botulinum Toxin (Botox) Information for patients page 2 of 8 You have been recommended treatment with Botulinum Toxin. This leaflet aims to explain the treatment, what to
Treatment of facial palsy with Botulinum Toxin (Botox) Information for patients page 2 of 8 You have been recommended treatment with Botulinum Toxin. This leaflet aims to explain the treatment, what to
