Prof. of Neurology, Cairo University Vice President of the Egyptian Society of Neurology, Secretary General of Pan Arab union of Neurological
|
|
|
- Randell Sherman
- 5 years ago
- Views:
Transcription
1
2 Prof. of Neurology, Cairo University Vice President of the Egyptian Society of Neurology, Secretary General of Pan Arab union of Neurological Societies
3 The Egyptian Society of Neurology, Psychiatry and Neurosurgery (ESNPN)
4 Neurological Applications of BTX-A
5 Origin A B C1 D E F? G Botulinum Toxins
6 Botulism 1. Food Born 2.. Infant Bowel Infection 3. Wound Infection
7 Structure
8
9 FIRST SPECULATION ON THERAPEUTIC USE OF BTX In 1822, a German physician called Justinus Kerner was the 1st to give the idea of using Botulinum toxin in doses restricting its action to the sympathetic nervous system, to improve conditions he believed to originate from hyper excitation of this system such as st vitus dance, now known as Huntington's chorea.
10 Botulinum toxin type A: research The concept of using minute dosage of botulinum toxin to modify neuromuscular function focally in man has evolved by Alan Scott of the Smith Kettewell eye Research Foundation in San Francisco, CA 1960 s
11 Botulinum toxin type A: research In the late 1970 s Dr Scott formed his company, Oculinum Inc., where he continued research on botulinum toxin type A. In 1978 the FDA granted Dr Scott permission to study the use of toxin to treat strabismus in human volunteers s
12
13 Scott s first report of the technique in the treatment of strabismus in human appeared in Treatment began in the United Kingdom in 1982.
14 Clostridium botulinum unique molecular structures Non-toxic Associating Proteins Stabilize and protect the pure neurotoxin protein from degradation Influence the diffusion characteristics of the molecule Non-toxic, nonhemagglutinin (NTNH) Hemagglutinin (HA) 150 kda neurotoxin protein
15 Mechanism Of action
16 1. Binding BOTOX initially binds onto a distinct acceptor on the external membrane of cholinergic nerve terminals Light chain BOTOX Heavy chain ACh Synaptic vesicle The binding site is located on the heavy chain, which is highly selective for cholinergic nerve terminals Disulphide bond End-plate region
17 2. Internalisation Once bound to the nerve terminal, BOTOX is internalised by a process called endocytosis, to form a toxin-receptor vesicle inside the cell BOTOX ACh Synaptic vesicle The light chain of the molecule is then released into the centre of the cell (cytoplasm)
18 3. Blocking When inside the nerve terminal BOTOX cleaves SNAP-25 Heavy chain Light chai n SNARE complex does not form properly As the SNARE complex cannot form properly, exocytosis of cholinergic vesicles is prevented resulting in chemodenervation SNAP-25 Syntaxin VAMP Synaptic vesicle does not fuse with membrane, and ACh is not released
19 BTX-B BTX-D BTX-F BTX-G BTX-C1 BTX-A BTX-E BTX-C1
20 Serotypes and their targets The seven distinct neurotoxin serotypes have different: Biochemical structures Molecular weights Potencies SNARE protein targets Sero-type A B C1 D E F G Target protein SNAP-25 VAMP SNAP-25/Syntaxin VAMP SNAP-25 VAMP VAMP
21 Re-establishment of neuromuscular transmission BOTOX prevents affected terminals from stimulating muscle contraction, however, the synthesis and storage of ACh or the conduction of electrical signals along the nerve fibre is unaffected Collateral axonal sprout Evidence indicates that chemodenervation by BOTOX results in growth of the end-plate region, and the emergence of axonal sprouts. These sprouts may then begin to release ACh
22 Re-establishment of neuromuscular transmission (2) This development of a new neuromuscular junction means that muscle activity resumes usually after about three months Eventually, the original junction resumes activity, and sprouts regress Repeated injections are thus required to maintain therapeutic effects Functioning neuromuscular junction
23 Botulinum Toxin Mechanism Current Hypothesis depaiva et al., 1999
24 BOTOX duration of action The clinical effects are seen 2 to 4 days after initial injection Reasonable response after 2 weeks Studies indicate that it takes around 3 to 6 months for re-establishment of transmission, and thus re-emergence of symptoms
25 BOTOX duration of action Long-term treatment has been shown to be safe, and can be repeated as needed Number of months
26 Commercial available Botulinum Toxin Products BOTOX Allergan Dysport Ipsen Myobloc/ Neurobloc Elan Serotype A A B Complex MW 900kDA ~900kDA 700kDA* Package (units) ,500/5,000/ 10,000 Neurotoxin Protein per Vial(ng) ~ /50/100 Form Vacuum Dried Lyphollized Solution PH ~7 ~7 5.6 First Year of Approval
27
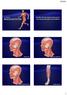
28
29
30 Dilutions of injection in case of Botox (each vial contains 100 units of Botulinum toxin type A) Saline added to vial 4 ml Final Dilution 2.5 u / 0.1 ml 2 ml 5.0 u / 0.1 ml 1 ml 10.0 u / 0.1ml Depending on: indication, size of muscles, treating physician preference.
31 Botulinum Toxin Type A use in medicine Belpharospasm Torticollis Hemifacial spasm Spasmodic dysphonia Writer s cramp Adult Spasticity Juvenile Spasticity Hyperhidrosis and glabellar lines
32 Botulinum Toxin Type A use in medicine Multiple Dystonias Tremors Tics Hypersalivation Headaches Tennis elbow Postoperative pain Detrusor-sphincter dessynergia Spastic bladder Achalasia Anal fissure Facial lines Bruxism
33 Blepharospasm (essential) A localised movement disorder that affects the muscles controlling the eyes (orbicularis oculi) Results in closure of the eyes
34 X X X X X
35 Neuromuscular disorder characterised by frequent involuntary contractions of the muscles on one side of the face Hemifacial spasm
36
37 A deviation in direction of one or both eyes that is uncontrollable by the patient, usually due to overexertion of a muscle or muscle group Results in eyes that are crossed or otherwise out of alignment Strabismus
38 Cervical Dystonia
39
40
41 Writer s Cramp
42 Laryngeal Dysphonia
43 Juvenile cerebral palsy A wide range of physical disabilities, caused by damage to the brain during its development Characterised by disorders of movement and posture
44
45
46 Adult Spasticity
47 Common Patterns Of Adult Spasticity: UPPER LIMB Adducted or internally rotated shoulder Flexion of the elbow Pronation of the forearm Wrist flexion Finger flexion with thumb in palm
48 Adult Spasticity: Upper Limb Deformities Adducted/ internally rotated shoulder Wrist-flexion
49 Adult Spasticity: Upper Limb Deformities Clenched fist Thumb-in-palm-deformity
50 Commen Patterns Of Adult Spasticity: LOWER LIMB Hip flexion Adducted thighs Knee flexion, knee extension Ankle plantar flexion Striatal toe Equinovarus foot
51 Adult Spasticity: Lower Limb Deformities Equinovarus Striatal toe
52 Adult Spasticity: Lower Limb Deformities Knee-extension Thigh-adduction
53 ASSESSMENT OF SPASTICITY Modified Ashworth Scale 0 No increase in muscle tone 1 Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved into flexion or extension. 1+ Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the range of motion. 2 More marked increase in muscle tone through most of the range of motion, but the affected part(s) is easily moved. 3 Considerable increase in muscle tone; passive movement is difficult. 4 Affected part(s) is rigid in flexion or extension
54 Sapsticity in Adult : Treatment Goals Improved mobility. Decreased pain. Decreased spasms. Increased range of motion. Improved orthotics fit. Improved positioning. Improved hygiene.
55 BOTOX in Adult Spasticity: PATIENT SELECTION Particularly good candidates should : Experience moderate to severe muscle tone limiting their ability to benefit from physiotherapy. Likely to experience a clear functional benefit from a localized reduction in Spasticity (e.g.: improvement in movement, positioning, hygienic care, or pain). Be at risk of developing fixed deformities. Experience intolerable side-effects from systemic antispasmodic medications.
56 Examples of suggested injection sites
57 Upper Limb injection sites
58 Pectoralis Major
59 Biceps Brachii
60 Brachialis
61 Brachioradialis
62 Flexor Carpi Radialis
63 Flexor Carpi Ulnaris
64 Flexor Digitorum Profundus
65 Flexor Digitorum Superficialis
66 Lower Limb injection sites
67 Adductor Longus
68 Semi tendenosus
69 Semi Membrenosus
70 Gatsrocnemius
71 Tibialis Posterior
72 Soleus
73
74 BOTOX IN ADULT SPASTICITY: TREATMENT GOALS ACHEIVEMENT Improved wound healing after BOTOX
75 BOTOX IN ADULT SPASTICITY: TREATMENT GOALS ACHEIVEMENT Before BOTOX After BOTOX
76 BOTOX IN ADULT SPASTICITY: TREATMENT GOALS ACHEIVEMENT Before BOTOX After BOTOX
77 Botulinum Toxin Type A Egyptian History
78 First introduction to Egypt We were very close to Europe in using BTX-A in medicine First experience was on 1989 with Prof. James Toole
79 First introduction to Egypt-Cont. First injection in clinic with Dr. Marian Sominetta Moro ( France) in 1992
80 First introduction to Egypt-Cont. First one presented BTX-A in Egypt Dr. James A Temlett ( South Africa) in Neurology Department- Cairo University
81 First introduction to Egypt-Cont. First Egyptian trial on 12 cases Hemifacial spasm and Blepharospasm. Meeting of ESNPN in Hurghada on April 1994
82 Building Experience We start with Movement Disorders like Hemifacial spasm, Blepharospasms and Cervical Dystonia. Adult Spasticity, JCP Recently writer s cramp, oromandibular Collaborate with PT for patient best outcomes of the treatment
83
84
85 Advantages Effective in high percent of cases. Easily injectable (out patient procedure) Repeated easily, patient awake. Minimal side effects and if they happen they are : - Site specific (not systemic like oral medications). - Transient (unlike surgery). No anaphylactic reactions have been recorded. Could be combined with other measures.
86 Difficulties!!!
87 Cost? Benefit is transient Right dose Re-injection is mandatory (though could be in a modified smaller dose depending on degree of recurrence of symptoms) Injection tools guidance is sometimes a must Good patient selection
88 The problem of non response
89 Non- Response to Botulinum Toxin therapy Primary non-responder: no response to first injection of toxin. Secondary non-responder: a relative or complete loss of efficacy at subsequent injections.
90 Reasons of Non-response Causes in the toxin: - expired - Badly stored. - Inappropriate reconstitution.
91 Causes in the treating physician: Dose may be too low. Wrongly injected muscles (injection technique). Bad selection of patients e.g. : In cases of spasticity: the presence of contractures, joint deformities, weakness or atrophy present with no functional benefit.
92 Causes in patient himself: Presence of neutralizing antibodies: confirmed by in-vivo mouse neutralizing assay.
93 Recommendations to avoid antibodies Formation Wait as long as possible between injections (at least 3 months). Avoid boosters. Use smallest clinically effective dose. Use the lowest protein load product? Use another strain e.g. type B. (different serotype).
94 BOTOX IN ADULT-ONSET SPASTICITY: DOSE RECOMMENDATIONS Total maximum dose per visit = units BOTOX Total maximum dose per limb = units Botox Maximum dose per injection site = 50 units BOTOX Reinjection intervals 3 months
95
96
97
98 Before Botox After Botox
99 Before Botox After Botox
100 Before Botox After Botox
101 What is better for patient future
102 Objective Patient Passport Increase identification of spasticity patients by providing education, motivation and information for patients and their families Education about spasticity and treatments Motivation to request spasticity assessment and treatment Information provided to patients and their families about how to access specialist spasticity services Ideally given to patients 2 weeks after stroke Place of distribution: [stroke unit/rehabilitation unit/neurology ward/geriatric ward/other medical ward/community.
103 Patient Passport Page 1 Passport developed by an independent multi-disciplinary working group of experts in the area of spasticity management and rehabilitation Developed via collaboration during and after the Stroke Rehabilitation Advisory Board 27 November 2006, London, UK Multidisciplinary meeting of Injectors, Referrers and Patient Groups
104 Spasticity Patient Passport Page 2 : Information about the patient and aims of Passport Name of the patient, information about their stroke and explains aims of the Passport and why it should be kept by patient Page 3 : Health Care Team Contact Details Space to add contact details for the specialist Spasticity service Should be filled in before it is given to the patient so that the patient knows who to contact if Spasticity develops Page 4 : What is Spasticity? Provides explanation of disease Page 5 : Spasticity checklist and what to do next Provides patient (and their family) with checklist of Spasticity symptoms. If Spasticity is suspected, patient should request access to specialist Spasticity services Page 6 : Questions you should ask your family practitioner Questions to confirm Spasticity symptoms
105 Patient Passport Page 7 and 8 Year planner Encourages the patient to retain the passport
106 Thank you
Clare Gaduzo BSc RMN Registered Aesthetics Practitioner (qualified with Medics Direct)
 Clare Gaduzo BSc RMN Registered Aesthetics Practitioner (qualified with Medics Direct) 07935567067 cjg.aesthetics@yahoo.co.uk www.cjgaesthetics.co.uk http://www.facebook.com/cjgaesthetics @CJGAesthetics
Clare Gaduzo BSc RMN Registered Aesthetics Practitioner (qualified with Medics Direct) 07935567067 cjg.aesthetics@yahoo.co.uk www.cjgaesthetics.co.uk http://www.facebook.com/cjgaesthetics @CJGAesthetics
Botox. Botox (onabotulinum toxin A) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 8 Last Review Date: September 15, 2017 Botox Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 8 Last Review Date: September 15, 2017 Botox Description Botox (onabotulinum
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb)
 Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date:
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date:
BOTOX. Description. Section: Prescription Drugs Effective Date: January 1, 2013 Subsection: CNS Original Policy Date: December 7, 2011
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.12.01 Subject: Botox Page: 1 of 6 Last Review Status/Date: December 6, 2012 BOTOX Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.12.01 Subject: Botox Page: 1 of 6 Last Review Status/Date: December 6, 2012 BOTOX Description Botox (onabotulinum
Albert C. Clairmont, MD Associate Professor-Clinical Department of PM & R The Ohio State University
 Botulinum Toxin for Movement Disorders: Physiology, Pharmacology and Evaluation of Patients Albert C. Clairmont, MD Associate Professor-Clinical Department of PM & R The Ohio State University OBJECTIVES
Botulinum Toxin for Movement Disorders: Physiology, Pharmacology and Evaluation of Patients Albert C. Clairmont, MD Associate Professor-Clinical Department of PM & R The Ohio State University OBJECTIVES
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb)
 Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective
Botox. Botox (onabotulinum toxin A) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 10 Last Review Date: November 30, 2018 Botox Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 10 Last Review Date: November 30, 2018 Botox Description Botox (onabotulinum
Scientific Update Laxman Bahroo, MD
 Scientific Update Laxman Bahroo, MD Associate Professor Director; Botulinum Toxin Clinic Director; Neurology Residency Program Medstar Georgetown University Hospital Washington, D.C. Disclosures Advisory
Scientific Update Laxman Bahroo, MD Associate Professor Director; Botulinum Toxin Clinic Director; Neurology Residency Program Medstar Georgetown University Hospital Washington, D.C. Disclosures Advisory
Clinical Policy: IncobotulinumtoxinA (Xeomin) Reference Number: CP.PHAR.231
 Clinical Policy: (Xeomin) Reference Number: CP.PHAR.231 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Xeomin) Reference Number: CP.PHAR.231 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Treatment Framework patient tracker
 Treatment Framework patient tracker Utilize the BOTOX Treatment Framework when evaluating your Focal Spasticity patients to establish The right goals The right muscles/dose The right plan Use the following
Treatment Framework patient tracker Utilize the BOTOX Treatment Framework when evaluating your Focal Spasticity patients to establish The right goals The right muscles/dose The right plan Use the following
Ultrasonography guided dystonia & spasticity control Part I: trunk, upper extremity
 Ultrasonography guided dystonia & spasticity control Part I: trunk, upper extremity Don Kyu Kim, M.D. Department of PM & R Chung Ang University College of Medicine Spasticity Vs Dystonia Velocity dependent
Ultrasonography guided dystonia & spasticity control Part I: trunk, upper extremity Don Kyu Kim, M.D. Department of PM & R Chung Ang University College of Medicine Spasticity Vs Dystonia Velocity dependent
THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN
 THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN Spasmodic torticollis (cervical dystonia), blepharospasm, and writer s cramp are specific types
THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN Spasmodic torticollis (cervical dystonia), blepharospasm, and writer s cramp are specific types
USE OF BOTOX IN THE CHILD WITH CEREBRAL PALSY. Andrew Redfern Senior Registrar, Paed Neurodevelopment Red Cross Children s Hospital
 USE OF BOTOX IN THE CHILD WITH CEREBRAL PALSY Andrew Redfern Senior Registrar, Paed Neurodevelopment Red Cross Children s Hospital WHAT IS BOTOX? BOTOX is a purified form of Botulinum toxin Neurotoxin
USE OF BOTOX IN THE CHILD WITH CEREBRAL PALSY Andrew Redfern Senior Registrar, Paed Neurodevelopment Red Cross Children s Hospital WHAT IS BOTOX? BOTOX is a purified form of Botulinum toxin Neurotoxin
Dystonias Peter McAllister, MD
 Dystonias Peter McAllister, MD Medical Director, New England Institute for Neurology and Headache Chief Medical Officer, New England Institute for Clinical Research Clinical Assistant Professor, Neurology,
Dystonias Peter McAllister, MD Medical Director, New England Institute for Neurology and Headache Chief Medical Officer, New England Institute for Clinical Research Clinical Assistant Professor, Neurology,
Prior Authorization Update: Botulinum Toxins
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Management of upper limb in cerebral palsy. Dr Sameer Desai Pediatric Orthopedic Surgeon KEM, Ruby Hall, Sahyadri Hospital, Unique Childrens Hospital
 Management of upper limb in cerebral palsy Dr Sameer Desai Pediatric Orthopedic Surgeon KEM, Ruby Hall, Sahyadri Hospital, Unique Childrens Hospital Importance of upper limb in CP Activities of daily living
Management of upper limb in cerebral palsy Dr Sameer Desai Pediatric Orthopedic Surgeon KEM, Ruby Hall, Sahyadri Hospital, Unique Childrens Hospital Importance of upper limb in CP Activities of daily living
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Spasticity. Disclosure. Objectives
 Spasticity Noon, November 5, 2013 Dr. Bill Black Auditorium Glenrose Rehabilitation Hospital VC#515992 REMOTE SITES: PLEASE MUTE YOUR AUDIO AT ALL TIME EXCEPT WHEN ASKING QUESTION/COMMENTING AT Q/A. This
Spasticity Noon, November 5, 2013 Dr. Bill Black Auditorium Glenrose Rehabilitation Hospital VC#515992 REMOTE SITES: PLEASE MUTE YOUR AUDIO AT ALL TIME EXCEPT WHEN ASKING QUESTION/COMMENTING AT Q/A. This
Scottish Medicines Consortium
 Scottish Medicines Consortium clostridium botulinum neurotoxin type A, 100 unit powder for solution for injection (Xeomin ) No. (464/08) Merz Pharma UK Ltd 09 May 2008 The Scottish Medicines Consortium
Scottish Medicines Consortium clostridium botulinum neurotoxin type A, 100 unit powder for solution for injection (Xeomin ) No. (464/08) Merz Pharma UK Ltd 09 May 2008 The Scottish Medicines Consortium
NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (50U, 100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition
 NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (50U, 100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 50 units (U),
NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (50U, 100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 50 units (U),
Neuromuscular Blocking Agents
 Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
BOTOX Billing & Coding for NeuroRehab
 S T R E A M L I N E Billing & Coding for NeuroRehab Cervical Dystonia, Blepharospasm, and Focal Spasticity Indications Spasticity: Upper Limb Spasticity for injection is indicated for the treatment of
S T R E A M L I N E Billing & Coding for NeuroRehab Cervical Dystonia, Blepharospasm, and Focal Spasticity Indications Spasticity: Upper Limb Spasticity for injection is indicated for the treatment of
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 Local Coverage Determination (LCD): Botulinum Toxins (L33274) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME
Local Coverage Determination (LCD): Botulinum Toxins (L33274) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME
NAME OF THE MEDICINE. BOTOX purified neurotoxin complex injection (100 U or 200 U) (botulinum toxin, type A) DESCRIPTION.
 NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 100 units (U) or
NAME OF THE MEDICINE BOTOX purified neurotoxin complex injection (100 U or 200 U) (botulinum toxin, type A) DESCRIPTION Composition Active ingredient: Each vial of BOTOX contains either 100 units (U) or
For each of your Focal Spasticity patients, establish... The right goals. The right muscles/dose
 FOCAL SPASTICITY Treatment Framework For each of your Focal Spasticity patients, establish... The right goals The right plan The right muscles/dose Indications Spasticity: Upper Limb Spasticity for injection
FOCAL SPASTICITY Treatment Framework For each of your Focal Spasticity patients, establish... The right goals The right plan The right muscles/dose Indications Spasticity: Upper Limb Spasticity for injection
Aetna Better Health of Virginia
 Botox (onabotulinumtoxina) Myobloc (rimabotulinumtoxinb) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Indications: (use if necessary): Overactive Bladder BOTOX indicated for the treatment
Botox (onabotulinumtoxina) Myobloc (rimabotulinumtoxinb) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Indications: (use if necessary): Overactive Bladder BOTOX indicated for the treatment
DYSrupt Your Patients Spasticity Symptoms
 The Dysport Profiles RALPH PATRICIA In the treatment of upper limb spasticity and lower limb spasticity in adults DYSrupt Your Patients Spasticity Symptoms Not actual patients In clinical trials with Dysport,
The Dysport Profiles RALPH PATRICIA In the treatment of upper limb spasticity and lower limb spasticity in adults DYSrupt Your Patients Spasticity Symptoms Not actual patients In clinical trials with Dysport,
Nerve Injury. 1) Upper Lesions of the Brachial Plexus called Erb- Duchene Palsy or syndrome.
 Nerve Injury - Every nerve goes to muscle or skin so if the nerve is injured this will cause paralysis in the muscle supplied from that nerve (paralysis means loss of function) then other muscles and other
Nerve Injury - Every nerve goes to muscle or skin so if the nerve is injured this will cause paralysis in the muscle supplied from that nerve (paralysis means loss of function) then other muscles and other
2/4/2018. Identify the two reasons why muscle cells may go through muscle fatigue. Ch.7 Review. Sternocleidomastoid.
 Ch.7 Review Identify the two reasons why muscle cells may go through muscle fatigue Temporalis Depressor anguli oris Sternocleidomastoid Tibialis anterior 1 Gluteus medius Deltoid Adducts & rotates scapula
Ch.7 Review Identify the two reasons why muscle cells may go through muscle fatigue Temporalis Depressor anguli oris Sternocleidomastoid Tibialis anterior 1 Gluteus medius Deltoid Adducts & rotates scapula
Elements of Provider Documentation Consistent with Medicare Guidelines
 S T R E A M L I N E Elements of Provider Documentation Consistent with Medicare Guidelines 1 Document medical necessity Include a statement that BOTOX (onabotulinumtoxina) treatment is reasonable and necessary
S T R E A M L I N E Elements of Provider Documentation Consistent with Medicare Guidelines 1 Document medical necessity Include a statement that BOTOX (onabotulinumtoxina) treatment is reasonable and necessary
Injection insights and considerations for Focal Spasticity
 Learn how patients can save on treatment costs. Ask your representative for details. 1 1 2 2 Injection insights and considerations for Focal Spasticity 3 1 3 1 1 2 2 3 3 1 injection procedure suggestions
Learn how patients can save on treatment costs. Ask your representative for details. 1 1 2 2 Injection insights and considerations for Focal Spasticity 3 1 3 1 1 2 2 3 3 1 injection procedure suggestions
Key Points for Success:
 SELF WRIST & HAND 1 2 All of the stretches described in this chapter are detailed to stretch the right side. Key Points for Success: Sit comfortably in a position where you can straighten or fully extend
SELF WRIST & HAND 1 2 All of the stretches described in this chapter are detailed to stretch the right side. Key Points for Success: Sit comfortably in a position where you can straighten or fully extend
PRODUCT MONOGRAPH. abobotulinumtoxina for injection Ph. Eur. Sterile lyophilized powder for solution for injection. 300 and 500 Units per vial
 PRODUCT MONOGRAPH Pr DYSPORT THERAPEUTIC abobotulinumtoxina for injection Ph. Eur. Sterile lyophilized powder for solution for injection 300 and 500 Units per vial Neuromuscular Blocking Agent Manufactured
PRODUCT MONOGRAPH Pr DYSPORT THERAPEUTIC abobotulinumtoxina for injection Ph. Eur. Sterile lyophilized powder for solution for injection 300 and 500 Units per vial Neuromuscular Blocking Agent Manufactured
Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc )
 Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc ) These services may or may not be covered by your HealthPartners
Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc ) These services may or may not be covered by your HealthPartners
Clinical Policy: OnabotulinumtoxinA (Botox) Reference Number: ERX.SPMN.216
 Clinical Policy: (Botox) Reference Number: ERX.SPMN.216 Effective Date: 01/17 Last Review Date: Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Botox) Reference Number: ERX.SPMN.216 Effective Date: 01/17 Last Review Date: Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
USE OF BOTULINUM TOXIN-A IN PAIN ASSOCIATED WITH NEUROMUSCULAR DISORDERS July 2001 Executive Summary
 Page 1 of 34 Search Search Health Technology Advisory Committee Home Publications Links Search USE OF BOTULINUM TOXIN-A IN PAIN ASSOCIATED WITH NEUROMUSCULAR DISORDERS July 2001 Executive Summary The strains
Page 1 of 34 Search Search Health Technology Advisory Committee Home Publications Links Search USE OF BOTULINUM TOXIN-A IN PAIN ASSOCIATED WITH NEUROMUSCULAR DISORDERS July 2001 Executive Summary The strains
CLINICAL MEDICATION POLICY
 CLINICAL MEDICATION POLICY Policy Name: Botox (onabotulinumtoxina) Policy Number: Approved By: Medical Management, Clinical Pharmacy Revision Date: 12/11/2017 Products: Highmark Health Options Application:
CLINICAL MEDICATION POLICY Policy Name: Botox (onabotulinumtoxina) Policy Number: Approved By: Medical Management, Clinical Pharmacy Revision Date: 12/11/2017 Products: Highmark Health Options Application:
Medication Prior Authorization Form
 (OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 7/7/2016 Next Review:
(OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 7/7/2016 Next Review:
Clinical Policy: Botulinum Toxins Reference Number: NE.PHAR.15
 Clinical Policy: Reference Number: NE.PHAR.15 Effective Date: 01/01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: Reference Number: NE.PHAR.15 Effective Date: 01/01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Pharmacy Medical Necessity Guidelines: Botulinum Toxins
 Pharmacy Medical Necessity Guidelines: Effective: January 1, 2018 Effective: Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical
Pharmacy Medical Necessity Guidelines: Effective: January 1, 2018 Effective: Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical
XEOMIN in Dystonia* and Spasticity** Treatment Educational Material for Physicians 31 May 2012
 XEOMIN in Dystonia* and Spasticity** Treatment Educational Material for Physicians 31 May 2012 * XEOMIN is indicated for the symptomatic treatment of blepharospasm, cervical dystonia of a predominantly
XEOMIN in Dystonia* and Spasticity** Treatment Educational Material for Physicians 31 May 2012 * XEOMIN is indicated for the symptomatic treatment of blepharospasm, cervical dystonia of a predominantly
Chemodenervation With Botulinum Toxins & Phenol For Spasticity & Dystonia
 Chemodenervation With Botulinum Toxins & Phenol For Spasticity & Dystonia Orthopedic Rehabilitation Annual Meeting Cooper Medical School of Rowan University October 6, 2018 Abraham Alfaro, Ph.D., D.O.
Chemodenervation With Botulinum Toxins & Phenol For Spasticity & Dystonia Orthopedic Rehabilitation Annual Meeting Cooper Medical School of Rowan University October 6, 2018 Abraham Alfaro, Ph.D., D.O.
Clinical Policy: OnabotulinumtoxinA (Botox) Reference Number: CP.PHAR.232
 Clinical Policy: (Botox) Reference Number: CP.PHAR.232 Effective Date: 07/16 Last Review Date: 05/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Botox) Reference Number: CP.PHAR.232 Effective Date: 07/16 Last Review Date: 05/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the Children s Medical Center at the University of Virginia
 PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the Children s Medical Center at the University of Virginia Volume 9 Number 3 March 2003 W Clinical Applications for Botulinum
PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the Children s Medical Center at the University of Virginia Volume 9 Number 3 March 2003 W Clinical Applications for Botulinum
Advanced Treatment of Spasticity 14 th Jan 2017
 About the Society of Rehabilitation Medicine (Singapore) Advanced Treatment of Spasticity 14 th Jan 2017 Dr Geoffrey S Samuel Consultant Department of Rehabilitation Medicine Singapore General Hospital
About the Society of Rehabilitation Medicine (Singapore) Advanced Treatment of Spasticity 14 th Jan 2017 Dr Geoffrey S Samuel Consultant Department of Rehabilitation Medicine Singapore General Hospital
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Botox (onabotulinumtoxina) Injections MP-024-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date: 07/15/2018;
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Botox (onabotulinumtoxina) Injections MP-024-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date: 07/15/2018;
Human Anatomy Lab #7: Muscles of the Cadaver
 Human Anatomy Lab #7: Muscles of the Cadaver Table of Contents: Expected Learning Outcomes.... 1 Introduction...... 1 Identifying Muscles on Yourself.... 2 Muscles of the Anterior Trunk and Arm.. 2 Muscles
Human Anatomy Lab #7: Muscles of the Cadaver Table of Contents: Expected Learning Outcomes.... 1 Introduction...... 1 Identifying Muscles on Yourself.... 2 Muscles of the Anterior Trunk and Arm.. 2 Muscles
200 meter or yard event. However, it is also the lead off event in the individual medley,
 Butterfly is one of four competitive strokes in swimming. Usually it is raced, in a 100 or 200 meter or yard event. However, it is also the lead off event in the individual medley, meaning it is part of
Butterfly is one of four competitive strokes in swimming. Usually it is raced, in a 100 or 200 meter or yard event. However, it is also the lead off event in the individual medley, meaning it is part of
Appendix Section 1: Evaluation Section 2: Surgical planning Section 3: Children
 Appendix In order to ease the evaluation and decision-making processes and the surgical planning, the following provides commonly used scales, schematic drawings, and forms. Important, scales are not conceived
Appendix In order to ease the evaluation and decision-making processes and the surgical planning, the following provides commonly used scales, schematic drawings, and forms. Important, scales are not conceived
Circle Yes or No Y N. [If yes, no further questions.]
![Circle Yes or No Y N. [If yes, no further questions.] Circle Yes or No Y N. [If yes, no further questions.]](/thumbs/83/88529512.jpg) 02/18/2016 Prior Authorization AETA BETTER HEALTH PE MEDICAID & AETA BETTER HEALTH KIDS Botulinum Toxins (PA88) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
02/18/2016 Prior Authorization AETA BETTER HEALTH PE MEDICAID & AETA BETTER HEALTH KIDS Botulinum Toxins (PA88) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Botulinum Toxins. Length of Authorization: From 90 days to 12 months
 Botulinum Toxins Goal(s): Approve botulinum toxins for funded OHP conditions supported by evidence of benefit (eg, dystonia or spasticity associated with certain neurological diseases). Require positive
Botulinum Toxins Goal(s): Approve botulinum toxins for funded OHP conditions supported by evidence of benefit (eg, dystonia or spasticity associated with certain neurological diseases). Require positive
Botulinum Toxins (BOTOX, DYSPORT, MYOBLOC and XEOMIN)
 Botulinum Toxins (BOTOX, DYSPORT, MYOBLOC and XEOMIN) Policy Number: Original Effective Date: MM.04.004 03/14/2006 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Prescription
Botulinum Toxins (BOTOX, DYSPORT, MYOBLOC and XEOMIN) Policy Number: Original Effective Date: MM.04.004 03/14/2006 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Prescription
Developing a Patient Welcome Letter BOTOX (onabotulinumtoxina) for appropriate patients
 S T R E A M L I N E Developing a Patient Welcome Letter (onabotulinumtoxina) for appropriate patients Indications Chronic Migraine for injection is indicated for the prophylaxis of headaches in adult patients
S T R E A M L I N E Developing a Patient Welcome Letter (onabotulinumtoxina) for appropriate patients Indications Chronic Migraine for injection is indicated for the prophylaxis of headaches in adult patients
Restoration of Reaching and Grasping Functions in Hemiplegic Patients with Severe Arm Paralysis
 Restoration of Reaching and Grasping Functions in Hemiplegic Patients with Severe Arm Paralysis Milos R. Popovic* 1,2, Vlasta Hajek 2, Jenifer Takaki 2, AbdulKadir Bulsen 2 and Vera Zivanovic 1,2 1 Institute
Restoration of Reaching and Grasping Functions in Hemiplegic Patients with Severe Arm Paralysis Milos R. Popovic* 1,2, Vlasta Hajek 2, Jenifer Takaki 2, AbdulKadir Bulsen 2 and Vera Zivanovic 1,2 1 Institute
Ratified by: Care and Clinical Policies Date: 17 th February 2016
 Clinical Guideline Reference Number: 0803 Version 5 Title: Physiotherapy guidelines for the Management of People with Multiple Sclerosis Document Author: Henrieke Dimmendaal / Laura Shenton Date February
Clinical Guideline Reference Number: 0803 Version 5 Title: Physiotherapy guidelines for the Management of People with Multiple Sclerosis Document Author: Henrieke Dimmendaal / Laura Shenton Date February
Headache and Migraine Treatment
 Many of us think of Botox primarily as a cosmetic treatment for lines and wrinkles on the face, but the botulinum toxin that Botox is derived from has a long history of medically therapeutic uses. In fact,
Many of us think of Botox primarily as a cosmetic treatment for lines and wrinkles on the face, but the botulinum toxin that Botox is derived from has a long history of medically therapeutic uses. In fact,
This policy addresses only the type B formulation rimabotulinumtoxinb marketed as Myobloc.
 RimabotulinumtoxinB NDC CODE(S) 10454-0710-XX Myobloc 2500 UNIT/0.5ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0711-XX Myobloc 5000 UNIT/ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0712-XX Myobloc 10000 UNIT/2ML
RimabotulinumtoxinB NDC CODE(S) 10454-0710-XX Myobloc 2500 UNIT/0.5ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0711-XX Myobloc 5000 UNIT/ML SOLN (SOLSTICE NEUROSCIENCES) 10454-0712-XX Myobloc 10000 UNIT/2ML
AUSTRALIAN PRODUCT INFORMATION DYSPORT clostridium botulinum type A toxin - haemagglutinin complex powder for injection vial
 AUSTRALIAN PRODUCT INFORMATION DYSPORT clostridium botulinum type A toxin - haemagglutinin complex powder for injection vial 1 NAME OF THE MEDICINE clostridium botulinum type A toxin - haemagglutinin complex
AUSTRALIAN PRODUCT INFORMATION DYSPORT clostridium botulinum type A toxin - haemagglutinin complex powder for injection vial 1 NAME OF THE MEDICINE clostridium botulinum type A toxin - haemagglutinin complex
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization AETA BETTER HEALTH MICHIGA Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Pharmacy Prior Authorization AETA BETTER HEALTH MICHIGA Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Botulinum Toxins Reference Number: TCHP.PHAR.1812 Effective Date: 07.01.2018 Last Review Date: 04.13.2018 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the
Clinical Policy: Botulinum Toxins Reference Number: TCHP.PHAR.1812 Effective Date: 07.01.2018 Last Review Date: 04.13.2018 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the
Considerations in the selection of patients for Selective Dorsal Rhizotomy
 Considerations in the selection of patients for Selective Dorsal Rhizotomy The best and the worst surgery I have ever been associated with. A therapist's perspective Dean Morgan, PT Disclosure Statement
Considerations in the selection of patients for Selective Dorsal Rhizotomy The best and the worst surgery I have ever been associated with. A therapist's perspective Dean Morgan, PT Disclosure Statement
The Human Muscular System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5th ed (2017) Chapters 10, 11, 12 INTRODUCTION
 Biology 322: Human Anatomy The Human Muscular System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapters 10, 11, 12 INTRODUCTION We will use a number of lab periods
Biology 322: Human Anatomy The Human Muscular System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapters 10, 11, 12 INTRODUCTION We will use a number of lab periods
Botulinum toxin A (Dysport) for hyperhidrosis of the axillae
 April 2016 Horizon Scanning Research & Intelligence Centre Botulinum toxin A (Dysport) for hyperhidrosis of the axillae LAY SUMMARY This briefing is based on information available at the time of research
April 2016 Horizon Scanning Research & Intelligence Centre Botulinum toxin A (Dysport) for hyperhidrosis of the axillae LAY SUMMARY This briefing is based on information available at the time of research
Developing a Patient Welcome Letter BOTOX (onabotulinumtoxina) for appropriate patients
 S T R E A M L I N E Developing a Patient Welcome Letter (onabotulinumtoxina) for appropriate patients Indications Bladder Dysfunction: Overactive Bladder for injection is indicated for the treatment of
S T R E A M L I N E Developing a Patient Welcome Letter (onabotulinumtoxina) for appropriate patients Indications Bladder Dysfunction: Overactive Bladder for injection is indicated for the treatment of
In which arm muscle are intramuscular injections most often given? (not in text)
 AP1 Lab 9 - Muscles of the Arms and Legs Locate the following muscles on the models and on yourself. Recall anatomical position. Directional terms such as anterior, posterior, lateral, etc. all assume
AP1 Lab 9 - Muscles of the Arms and Legs Locate the following muscles on the models and on yourself. Recall anatomical position. Directional terms such as anterior, posterior, lateral, etc. all assume
Match the types of muscle tissues with the words and phrases. 1) Skeletal 2) Smooth 3) Cardiac 2 Walls of blood vessels. 2 Walls of digestive tract
 S T U D Y G U I D E. Types of Muscle Tissues Match the types of muscle tissues with the words and phrases. ) Skeletal ) Smooth ) Cardiac, Striated Walls of blood vessels, Single nucleus Heart muscle, Involuntary
S T U D Y G U I D E. Types of Muscle Tissues Match the types of muscle tissues with the words and phrases. ) Skeletal ) Smooth ) Cardiac, Striated Walls of blood vessels, Single nucleus Heart muscle, Involuntary
BOTULINUM TOXIN POLICY TO INCLUDE:
 BOTULINUM TOXIN POLICY TO INCLUDE: Blepharospasm in adults, Hemi facial spasm in adults, spasmodic torticollis (cervical dystonia), focal spasticity treatment of dynamic equinus foot deformity, focal spasticity
BOTULINUM TOXIN POLICY TO INCLUDE: Blepharospasm in adults, Hemi facial spasm in adults, spasmodic torticollis (cervical dystonia), focal spasticity treatment of dynamic equinus foot deformity, focal spasticity
XEOMIN (incobotulinumtoxina) for injection, for intramuscular use Initial U.S. Approval: 2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. (incobotulinumtoxina) for injection,
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. (incobotulinumtoxina) for injection,
BOTULINUM TOXINS A AND B
 DRUG POLICY BOTULINUM TOXINS A B Policy Number: 2014D0017N Effective Date: 9/1/2014 Table of Contents Page COVERAGE RATIONALE... 1 BENEFIT CONSIDERATIONS... 7 BACKGROUND... 8 CLINICAL EVIDENCE... 8 U.S.
DRUG POLICY BOTULINUM TOXINS A B Policy Number: 2014D0017N Effective Date: 9/1/2014 Table of Contents Page COVERAGE RATIONALE... 1 BENEFIT CONSIDERATIONS... 7 BACKGROUND... 8 CLINICAL EVIDENCE... 8 U.S.
Lever system. Rigid bar. Fulcrum. Force (effort) Resistance (load)
 Lever system lever is any elongated, rigid (bar) object that move or rotates around a fixed point called the fulcrum when force is applied to overcome resistance. Force (effort) Resistance (load) R Rigid
Lever system lever is any elongated, rigid (bar) object that move or rotates around a fixed point called the fulcrum when force is applied to overcome resistance. Force (effort) Resistance (load) R Rigid
Location Terms. Anterior and posterior. Proximal and Distal The term proximal (Latin proximus; nearest) describes where the appendage joins the body.
 HUMAN ANAT OMY Location Terms Anterior and posterior In human anatomical usage, anterior refers to the front of the individual. Similarly, posterior refers to the back of the subject. In standard anatomical
HUMAN ANAT OMY Location Terms Anterior and posterior In human anatomical usage, anterior refers to the front of the individual. Similarly, posterior refers to the back of the subject. In standard anatomical
Year 2 MBChB Clinical Skills Session Examination of the Motor System
 Year 2 MBChB Clinical Skills Session Examination of the Motor System Reviewed & ratified by: o o o o Dr D Smith Consultant Neurologist Dr R Davies Consultant Neurologist Dr B Michael Neurology Clinical
Year 2 MBChB Clinical Skills Session Examination of the Motor System Reviewed & ratified by: o o o o Dr D Smith Consultant Neurologist Dr R Davies Consultant Neurologist Dr B Michael Neurology Clinical
BOTULINUM TOXINS IN NEUROLOGICAL DISEASE
 INVITED REVIEW ABSTRACT: Botulinum toxins are among the most potent neurotoxins known to humans. In the past 25 years, botulinum toxin has emerged as both a potential weapon of bioterrorism and as a powerful
INVITED REVIEW ABSTRACT: Botulinum toxins are among the most potent neurotoxins known to humans. In the past 25 years, botulinum toxin has emerged as both a potential weapon of bioterrorism and as a powerful
This policy addresses only onabotulinumtoxina, commercially available as Botox.
 OnabotulinumtoxinA DESCRIPTION Botulinum toxin, produced by the bacterium Clostridium botulinum, is one of the most potent naturally occurring neurotoxins known. It induces chemodenervation by first binding
OnabotulinumtoxinA DESCRIPTION Botulinum toxin, produced by the bacterium Clostridium botulinum, is one of the most potent naturally occurring neurotoxins known. It induces chemodenervation by first binding
Your Spasticity Management Service: Managing spasticity with Botulinum Toxin A in children with cerebral palsy
 Paediatric Unit information for parents and carers Your Spasticity Management Service: Managing spasticity with Botulinum Toxin A in children with cerebral palsy This leaflet is for children and young
Paediatric Unit information for parents and carers Your Spasticity Management Service: Managing spasticity with Botulinum Toxin A in children with cerebral palsy This leaflet is for children and young
AUSTRALIAN PRODUCT INFORMATION DYSPORT clostridium botulinum type A toxin - haemagglutinin complex powder for injection vial
 AUSTRALIAN PRODUCT INFORMATION DYSPORT clostridium botulinum type A toxin - haemagglutinin complex powder for injection vial 1 NAME OF THE MEDICINE clostridium botulinum type A toxin - haemagglutinin complex
AUSTRALIAN PRODUCT INFORMATION DYSPORT clostridium botulinum type A toxin - haemagglutinin complex powder for injection vial 1 NAME OF THE MEDICINE clostridium botulinum type A toxin - haemagglutinin complex
CONSUMER MEDICINE INFORMATION
 CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
CONSUMER MEDICINE INFORMATION BOTOX (botulinum toxin, type A) purified neurotoxin complex The information in this leaflet is ONLY a summary and is not a complete statement about BOTOX injection. Your doctor
Best practices for implementing a BOTOX patient-involved approach to Focal Spasticity treatment planning
 Best practices for implementing a patient-involved approach to Focal Spasticity treatment planning Establishing appropriate goals Communicating treatment expectations Developing a goal-oriented treatment
Best practices for implementing a patient-involved approach to Focal Spasticity treatment planning Establishing appropriate goals Communicating treatment expectations Developing a goal-oriented treatment
The Muscular System. Chapter 10 Part C. PowerPoint Lecture Slides prepared by Karen Dunbar Kareiva Ivy Tech Community College
 Chapter 10 Part C The Muscular System Annie Leibovitz/Contact Press Images PowerPoint Lecture Slides prepared by Karen Dunbar Kareiva Ivy Tech Community College Table 10.9: Muscles Crossing the Shoulder
Chapter 10 Part C The Muscular System Annie Leibovitz/Contact Press Images PowerPoint Lecture Slides prepared by Karen Dunbar Kareiva Ivy Tech Community College Table 10.9: Muscles Crossing the Shoulder
The Muscular System PART C. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Muscular System 6 PART C Five Golden Rules of Skeletal Muscle Activity Table 6.2 Muscles and Body
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Muscular System 6 PART C Five Golden Rules of Skeletal Muscle Activity Table 6.2 Muscles and Body
For adults with spasticity or cervical dystonia How long does your patients symptom * relief last?
 For adults with spasticity or cervical dystonia How long does your patients symptom * relief last? Consider Dysport because a long duration of response should matter 1 In clinical trials, retreatment was
For adults with spasticity or cervical dystonia How long does your patients symptom * relief last? Consider Dysport because a long duration of response should matter 1 In clinical trials, retreatment was
Past, Present and Future of Spasmodic Dysphonia and the use of Botulinum toxin therapy
 Past, Present and Future of Spasmodic Dysphonia and the use of Botulinum toxin therapy ANDREW BLITZER, MD, DDS Professor of Clinical Otolaryngology Columbia University College Director, New York Center
Past, Present and Future of Spasmodic Dysphonia and the use of Botulinum toxin therapy ANDREW BLITZER, MD, DDS Professor of Clinical Otolaryngology Columbia University College Director, New York Center
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
ORTHOPEDIC PHYSIOTHERAPY EVALUATION FORM. Age: Gender: M/F IP/OP
 ORTHOPEDIC PHYSIOTHERAPY EVALUATION FORM NAME: DATE: Age: Gender: M/F IP/OP Occupation: Referred by: Address: Phone Number: Registration Number: Civil Status: Diagnosis: Chief Complaints: Past Medical
ORTHOPEDIC PHYSIOTHERAPY EVALUATION FORM NAME: DATE: Age: Gender: M/F IP/OP Occupation: Referred by: Address: Phone Number: Registration Number: Civil Status: Diagnosis: Chief Complaints: Past Medical
Policy. Medical Policy Manual Approved Revised: Do Not Implement Until 4/2/19. IncobotulinumtoxinA
 IncobotulinumtoxinA NDC CODE(S) 00259-1605-XX Xeomin 50 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1610-XX Xeomin 100 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1620-XX Xeomin 200 UNIT SOLR (MERZ PHARMACEUTICAL)
IncobotulinumtoxinA NDC CODE(S) 00259-1605-XX Xeomin 50 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1610-XX Xeomin 100 UNIT SOLR (MERZ PHARMACEUTICAL) 00259-1620-XX Xeomin 200 UNIT SOLR (MERZ PHARMACEUTICAL)
Treatment of the Child with Cerebral Palsy Post Surgical Rehabilitation
 Treatment of the Child with Cerebral Palsy Post Surgical Rehabilitation Sheelah Cochran OTR/L, CPAM, CKTP Objectives Perform an initial evaluation of post-surgical conditions Identify appropriate splinting
Treatment of the Child with Cerebral Palsy Post Surgical Rehabilitation Sheelah Cochran OTR/L, CPAM, CKTP Objectives Perform an initial evaluation of post-surgical conditions Identify appropriate splinting
Clinical Policy: OnabotulinumtoxinA (Botox) Reference Number: CP.PHAR.232
 Clinical Policy: (Botox) Reference Number: CP.PHAR.232 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Botox) Reference Number: CP.PHAR.232 Effective Date: 07/16 Last Review Date: 07/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Medication Prior Authorization Form
 (OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 07/07/2017 Next Review:
(OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 07/07/2017 Next Review:
Human Anatomy and Physiology I Laboratory
 Human Anatomy and Physiology I Laboratory Gross Anatomy of the Muscular System (Two weeks) 1 This lab involves study of the laboratory exercise Gross Anatomy of the Muscular System. Complete the Review
Human Anatomy and Physiology I Laboratory Gross Anatomy of the Muscular System (Two weeks) 1 This lab involves study of the laboratory exercise Gross Anatomy of the Muscular System. Complete the Review
Fight spasticity with everything you ve got Does your everything include BOTOX?
 Fight spasticity with everything you ve got Does your everything include? Indications is a prescription medicine that is injected into muscles to treat increased muscle stiffness in elbow, wrist, finger,
Fight spasticity with everything you ve got Does your everything include? Indications is a prescription medicine that is injected into muscles to treat increased muscle stiffness in elbow, wrist, finger,
GLOSSARY. Active assisted movement: movement where the actions are assisted by an outside force.
 GLOSSARY The technical words used in this guide are listed here in alphabetic order. The first time one of these words is used in the guide, it is written in italics. Sometimes there is reference to a
GLOSSARY The technical words used in this guide are listed here in alphabetic order. The first time one of these words is used in the guide, it is written in italics. Sometimes there is reference to a
A. All movements require muscle which are organs using chemical energy to contract.
 Ch 8 Muscles Introduction: A. All movements require muscle which are organs using chemical energy to contract. B. The three types of muscle in the body are skeletal, smooth, and cardiac muscle. C. This
Ch 8 Muscles Introduction: A. All movements require muscle which are organs using chemical energy to contract. B. The three types of muscle in the body are skeletal, smooth, and cardiac muscle. C. This
Epicranius (frontal belly) Zygomaticus minor. Zygomaticus major Buccinator
 Epicranius (frontal belly) Zygomaticus minor Zygomaticus major Buccinator Masseter Digastric (posterior belly) Stylohyoid Sternocleidomastoid Trapezius Scalenus Omohyoid (inferior belly) Orbicularis oris
Epicranius (frontal belly) Zygomaticus minor Zygomaticus major Buccinator Masseter Digastric (posterior belly) Stylohyoid Sternocleidomastoid Trapezius Scalenus Omohyoid (inferior belly) Orbicularis oris
Table of Contents. 1.0 Description of the Procedure, Product, or Service Safety and Provider Compliance... 1
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
New Zealand Datasheet
 New Zealand Datasheet Name of Medicine Dysport Clostridium botulinum type toxin A toxin-haemagglutinin complex Presentation Dysport Powder for Injection contains 300 or 500 IPSEN UNITS* per vial of Clostridium
New Zealand Datasheet Name of Medicine Dysport Clostridium botulinum type toxin A toxin-haemagglutinin complex Presentation Dysport Powder for Injection contains 300 or 500 IPSEN UNITS* per vial of Clostridium
BOTOX (onabotulinumtoxina) Treatment Record for Blepharospasm
 (onabotulinumtoxina) Record for Blepharospasm Patient name: Clinical rationale for injection: Response prior to treatment: Comments: Diluent to Add (0.9% Sodium Chloride Injection Only) 200-Unit Vial Resulting
(onabotulinumtoxina) Record for Blepharospasm Patient name: Clinical rationale for injection: Response prior to treatment: Comments: Diluent to Add (0.9% Sodium Chloride Injection Only) 200-Unit Vial Resulting
Clinical Policy: OnabotulinumtoxinA (Botox) Reference Number: ERX.SPA.192 Effective Date:
 Clinical Policy: (Botox) Reference Number: ERX.SPA.192 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Botox) Reference Number: ERX.SPA.192 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clostridium botulinum type A toxin-haemagglutinin complex has a molecular weight of about 900,000D and is a complex of proteins.
 DYSPORT: PRODUCT INFORMATION NAME OF THE MEDICINE:- Clostridium botulinum type A toxin-haemagglutinin complex DESCRIPTION:- Dysport Powder for Injection contains 125U, 300 or 500 IPSEN UNITS* per vial
DYSPORT: PRODUCT INFORMATION NAME OF THE MEDICINE:- Clostridium botulinum type A toxin-haemagglutinin complex DESCRIPTION:- Dysport Powder for Injection contains 125U, 300 or 500 IPSEN UNITS* per vial
NAVIGATING THE BOTOX REIMBURSEMENT LANDSCAPE
 S T R E A M L I N E NAVIGATING THE REIMBURSEMENT LANDSCAPE (onabotulinumtoxina) for appropriate patients Indications Chronic Migraine is indicated for the prophylaxis of headaches in adult patients with
S T R E A M L I N E NAVIGATING THE REIMBURSEMENT LANDSCAPE (onabotulinumtoxina) for appropriate patients Indications Chronic Migraine is indicated for the prophylaxis of headaches in adult patients with
