Clinical Development in the Treatment of Carcinoid Syndrome. Pablo Lapuerta, MD Executive Vice President and Chief Medical Officer
|
|
|
- Avice Marian Peters
- 6 years ago
- Views:
Transcription
1 Clinical Development in the Treatment of Carcinoid Syndrome Pablo Lapuerta, MD Executive Vice President and Chief Medical Officer
2 Carcinoid Syndrome Heart Pulmonic and tricuspid valve thickening Stenosis Endocardial fibrosis Gastrointestinal Diarrhea Cramps Nausea Vomiting Skin Flushing Tumoral release of serotonin and other vasoactive substances into the systemic circulation causes carcinoid syndrome 1 Treatment with SSAs is associated with improved symptom control, but patients may not maintain adequate control of symptoms 2,3 Inhibition of serotonin synthesis with PCPA was previously shown to provide symptom control, but its utility was limited by CNS side effects 4 CNS=central nervous system; PCPA=para-chlorophenylalanine; SSA=somatostatin analog. 1. Kulke MH, Mayer RJ. N Engl J Med. 1999;340: Rubin J, et al. J Clin Oncol. 1999;17: Strosberg J, et al. Gastrointest Cancer Res. 2013;6: Engelman K, et al. N Engl J Med. 1967;277:
3 Key Messages There are three keys to clinical development Study design Data analysis Patient focus 2
4 Telotristat Ethyl Background Telotristat ethyl is an investigational agent Top-line results of TELESTAR were recently reported at three scientific meetings the initial, placebo-controlled portion of TELESTAR part of the open-label extension portion of TELESTAR Top-line results of TELECAST were recently presented Evaluation of the long-term safety and efficacy of telotristat is ongoing TELEPATH
5 A Drug Development Timeline Pre-clinical Phase 1 Phase 2 Phase 3 FDA Review
6 Pre-Clinical Development Developing the mouse model Genetic technology Selecting the protein target Discovery biology Finding the compound Medicinal chemistry 5
7 Phase 1 Studies in Healthy Volunteers What is the broad dose range? Are there any early safety issues? Does the drug product inhibit the enzyme in clinic? Is it absorbed effectively? How long does it stay in the bloodstream? Do higher doses provide more inhibition of the enzyme? Telotristat Phase 1 Studies Initiated in
8 Phase 1 Progress Dosing 3 times daily is reasonable 500 mg was the maximal tolerated dose Monitor liver enzymes in Phase 2 7
9 Phase 2: Dose Ranging Studying carcinoid syndrome for the first time Key questions Is the 500 mg dose tolerated long-term? Could lower doses be effective? What safety monitoring will be needed for Phase 3? What are the best ways to identify efficacy in Phase 3? Bowel movement frequency Stool consistency 8
10 Making the Most of Phase 2 Study Design Data Analysis Sequential Dosing Patient Focus Identifying Responders Adequate Relief 9
11 Phase 2: A Placebo-Controlled Study 23 Patients 4 Weeks of Placebo-Controlled Treatment 10
12 Phase 2: An Open-Label Study 15 Patients Dose Titration in each Patient 12-Week Treatment 11
13 Lessons from Phase 2 Both 250 mg and 500 mg doses deserved further study Fewer visits and tests would be reasonable in Phase 3 Bowel movement frequency is a precise measure Daily reports provide a lot of information But we need to support the interpretation of results 12
14 Interviewing Patients from the Phase 2 Study A Reduction of 30% or more in Bowel Movement Frequency is Clinically Meaningful 13
15 Important Decisions: Advancing to Phase 3 How many studies? How big should they be? How can we recruit effectively? Telotristat Phase 3 Plan Initiated in
16 The Phase 3 Plan At least 120 patients TELESTAR At least 60 patients TELEPATH TELECAST 12 Weeks Placebo Control + 36 Weeks Open-Label Treatment PHASE 2 PATIENTS 15
17 TELESTAR: Phase 3 Study Design 1 3- to 4-week run-in (n=135) Run-in: Evaluation of BM frequency 1:1:1 R Placebo tid (n=45) Telotristat ethyl 250 mg tid (n=45) Telotristat ethyl 500 mg tid* (n=45) Telotristat ethyl 500 mg tid Evaluation of primary endpoint: Reduction in number of daily BMs from baseline (averaged over 12-week double-blind treatment phase)) All patients required to be on SSA at enrollment and continue SSA therapy throughout study period Antidiarrheal agent use (eg, loperamide) and rescue short-acting Sandostatin (octreotide) use were allowed while on study 2 BM=bowel movement; tid=three times daily. 1. TELESTAR (telotristat ethyl for somatostatin analogue not adequately controlled carcinoid syndrome). ClinicalTrials.gov website. Accessed 09/15. Kulke M, et al. Presented at: ESMO (abstr 37LBA). 16
18 Keys to Recruitment No required scans Optional home visits Support for travel All symptomatic treatments allowed All patients offered open-label treatment after 12 weeks If you did not qualify for TELESTAR, you might still qualify for TELECAST With the same randomized design 17
19 Recruitment Results Almost 1 year to initiate a significant number of sites Approximately 27 months from 1 st patient to last enrolled Sites in US, Canada, France, Italy, UK, Germany, Belgium, the Netherlands, Israel, and Australia 135 patients in TELESTAR 76 patients in TELECAST 18
20 Phase 3 Data Analyses Mean change in bowel movement frequency Proportions of patients with 30% reduction in bowel movement frequency Relationship between bowel movement frequency and other symptoms Urgency, consistency, and reports of overall symptom relief Different degrees of change (20%, 40%, 60% reductions) and different subgroups (men, women) Is the change real? Is it meaningful? Is it robust? 19
21 Patient Focus: Be Proactive Learn about the patient experience early in development Use the opportunity to prospectively define clinically meaningful change Who can you identify as a responder? Discuss responder analyses with FDA prior to phase 3 Interview again in Phase 3 Capture the patient voice Support your key efficacy measure Validate your responder analysis 20
22 TELESTAR Patient Exit Interviews: Methods 1 Clinical sites in 5 countries invited all TELESTAR patients to be interviewed Australia, Canada, England, Germany, and the United States The interview procedure was prespecified in the TELESTAR protocol and approved by ethics committees 2 Interview consent was obtained before initiation of blinded treatment 2 Interviews were conducted by phone between weeks 12 and 14 by an independent expert in patient-reported outcomes 1,2 Patients, clinical sites, and interviewers were blinded to treatment group assignment 1. Anthony L, et al. Poster. NANETS (abstr C1). 2. Lexicon Pharmaceuticals, Inc. Protocol LX CS
23 TELESTAR Patient Exit Interviews: Participant Demographics TELESTAR Interview Participants (N=35) TELESTAR Overall (N=135) Age, years Female, % White, % Baseline body mass index, kg/m Baseline BM frequency, BM/day Anthony L, et al. Poster. NANETS (abstr C1). 22
24 Phase 1: Patient Comments About Baseline Symptoms I was up over 10 [BMs] a day, which wasn't really acceptable... I felt like I was living in my bathroom all the time. Uncontrollable diarrhea where you have to go and there s no stopping it When all I had to do was try to move or walk, and I had to go running to the toilet Anthony L, et al. Poster. NANETS (abstr C1). 23
25 TELESTAR Patient Exit Interviews: Phase 2 Clinically Meaningful Change Participants who reported reductions in BM frequency described better enjoying life, leaving the house, and participating in social and other activities: I definitely feel like I'm not a prisoner in my house, staying 10 feet to the nearest bathroom. I can go out to activities But the biggest change is not having to run to the toilet constantly You can't live going 20 times a day. I was able to go out more often Most participants reported that a BM frequency reduction of at least 30% would be considered meaningful Anthony L, et al. Poster. NANETS (abstr C1). 24
26 TELESTAR Patient Exit Interviews: Phase 2 Satisfaction and Observed Reduction in BM Frequency at Week 12 1 Reduction in BM Frequency at Week 12, % No Satisfaction (n=14) Somewhat Satisfied (n=7) Very Satisfied (n=12) Note: Treatment satisfaction reported in question 3. BMs reported daily on electronic diaries during TELESTAR. N=33 from pooled treatment arms.* Patients who answered 3, 4, or 5 to the treatment satisfaction question were grouped into category no satisfaction. *Of 35 participants, 2 did not answer the satisfaction question due to early termination or scheduling issue Anthony L, et al. Poster. NANETS (abstr C1) 2. Data on file. Lexicon Pharmaceuticals, Inc. 25
27 TELESTAR Patient Exit Interviews: Conclusions The primary endpoint of TELESTAR, a reduction in BM frequency, was very meaningful to patients Effective reduction in BM frequency led to improvement in emotional well-being and social and physical function Anthony L, et al. Poster. NANETS (abstr C1). 26
28 Analyzing Safety TELESTAR provided placebo-controlled safety data on 135 TELECAST provided placebo-controlled safety data on 76 patients TELEPATH has open-label safety data for up to 6 years of treatment A recent safety update (based on continuing safety experience) was provided to FDA Control of carcinoid syndrome is relevant to the safety of patients 27
29 Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome Matthew H. Kulke 1, Dieter Hörsch 2, Martyn E. Caplin 3, Lowell B. Anthony 4, Emily Bergsland 5, Kjell Öberg 6,Staffan Welin 6, Richard R.P. Warner 7, Catherine Lombard-Bohas 8, Pamela L. Kunz 9, Enrique Grande 10, Juan W. Valle 11, Douglas Fleming 12, Pablo Lapuerta 13, Phillip Banks 13, Shanna Jackson 13, Brian Zambrowicz 13, Arthur T. Sands 13, and Marianne Pavel 14 1 Dana-Farber Cancer Institute, Boston, Massachusetts, USA, 2 Zentralklinik Bad Berka, Bad Berka, Germany, 3 Royal Free Hospital, London, UK, 4 University of Kentucky, Lexington, Kentucky, USA, 5 UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, California, USA, 6 Uppsala University, Uppsala, Sweden, 7 Icahn School of Medicine at Mount Sinai, New York, New York, USA, 8 Hôpital Edouard Herriot, Hospices Civils de Lyon, Lyon, France, 9 Stanford University, Palo Alto, California, USA, 10 Hospital Universitario Ramón y Cajal, Madrid, Spain, 11 The University of Manchester/ The Christie NHS Foundation Trust, Manchester, UK, 12 Ipsen BioScience, Cambridge, Massachusetts, USA, 13 Lexicon Pharmaceuticals, Inc., The Woodlands, Texas, USA, 14 Charitè Universitätsmedizin, Berlin, Germany Kulke M, et al. J Clin Oncol. 2016;34. DOI: /JCO
30 Filing for New Drug Approval TELESTAR TELECAST TELEPATH Two Phase 2 studies Compassionate use experience Several studies in healthy volunteers Animal safety data Important data on chemistry and manufacturing 29
31 Next Steps FDA is evaluating the efficacy and safety of telotristat ethyl FDA decision is anticipated by February 28 th, 2017 If approved, a product label will be provided Indications and Usage Dosage and Administration Contraindications Warnings and Precautions Adverse Reactions Drug Interactions Use in Specific Populations Clinical Pharmacology Nonclinical Toxicology Clinical Studies Storage and Handling Patient counseling 30
32 Example of an FDA Label: ARICEPT 31
33 Summary Clinical development of new treatment for carcinoid syndrome is an extensive process Its advancement is based on attention to three main areas Study Design Data Analysis Patient Focus 32
Discovering Breakthrough Treatments for Human Disease. Discovering Breakthrough Treatments for Human Disease
 Patient Reported Outcomes in Oncology Trials Pablo Lapuerta, MD Executive Vice President and Chief Medical Officer Discovering Breakthrough Treatments for Human Disease Discovering Breakthrough Treatments
Patient Reported Outcomes in Oncology Trials Pablo Lapuerta, MD Executive Vice President and Chief Medical Officer Discovering Breakthrough Treatments for Human Disease Discovering Breakthrough Treatments
Telotristat Ethyl in Carcinoid Syndrome: Safety and Efficacy in the TELECAST
 Telotristat Ethyl in Carcinoid Syndrome: Safety and Efficacy in the TELECAST Phase 3 Trial Authors: Marianne Pavel 1,2, David J. Gross 3, Marta Benavent 4,5, Petros Perros 6, Raj Srirajaskanthan 7, Richard
Telotristat Ethyl in Carcinoid Syndrome: Safety and Efficacy in the TELECAST Phase 3 Trial Authors: Marianne Pavel 1,2, David J. Gross 3, Marta Benavent 4,5, Petros Perros 6, Raj Srirajaskanthan 7, Richard
2015: Year in Review Results of Recent Trials
 2015: Year in Review Results of Recent Trials Pamela L. Kunz, MD Assistant Professor of Medicine / GI Oncology Director, Stanford NET Program Stanford University School of Medicine Disclosures Research
2015: Year in Review Results of Recent Trials Pamela L. Kunz, MD Assistant Professor of Medicine / GI Oncology Director, Stanford NET Program Stanford University School of Medicine Disclosures Research
Impact of Functioning Metastatic Neuroendocrine Tumors
 Clinical Overview and Treatment Advances in Carcinoid Syndrome Sponsored by Lexicon Pharmaceuticals Impact of Functioning Metastatic Neuroendocrine Tumors Evaluation & Management 1 Presentation objectives
Clinical Overview and Treatment Advances in Carcinoid Syndrome Sponsored by Lexicon Pharmaceuticals Impact of Functioning Metastatic Neuroendocrine Tumors Evaluation & Management 1 Presentation objectives
Teresa Alonso Gordoa Servicio Oncología Médica Hospital Universitario Ramón y Cajal
 Teresa Alonso Gordoa Servicio Oncología Médica Hospital Universitario Ramón y Cajal Incidence per 100,000 EPIDEMIOLOGY Incidence rates of neuroendocrine tumors by primary tumor site 1.4 1.2 1.0 0.8 0.6
Teresa Alonso Gordoa Servicio Oncología Médica Hospital Universitario Ramón y Cajal Incidence per 100,000 EPIDEMIOLOGY Incidence rates of neuroendocrine tumors by primary tumor site 1.4 1.2 1.0 0.8 0.6
PRESS RELEASE. Beaumont et al. Pancreas Journal 2012 ; 41(3) : / 5
 PRESS RELEASE Ipsen receives approval from European Commission for Xermelo (telotristat ethyl) for the treatment of carcinoid syndrome diarrhea in patients inadequately controlled by somatostatin analogue
PRESS RELEASE Ipsen receives approval from European Commission for Xermelo (telotristat ethyl) for the treatment of carcinoid syndrome diarrhea in patients inadequately controlled by somatostatin analogue
Telotristat Ethyl (etiprate) : a new kid on the block Dr. Christos G. Toumpanakis MD PhD FRCP
 Telotristat Ethyl (etiprate) : a new kid on the block Dr. Christos G. Toumpanakis MD PhD FRCP Consultant in Gastroenterology/Neuroendocrine Tumours Hon. Senior Lecturer University College of London Neuroendocrine
Telotristat Ethyl (etiprate) : a new kid on the block Dr. Christos G. Toumpanakis MD PhD FRCP Consultant in Gastroenterology/Neuroendocrine Tumours Hon. Senior Lecturer University College of London Neuroendocrine
Clinical Trial Exit Interviews
 Clinical Trial Exit Interviews Presented at the Clinical Outcome Assessments: Establishing and Interpreting Meaningful Within-Patient Change Meeting The Duke-Margolis Center for Health Policy, Washington,
Clinical Trial Exit Interviews Presented at the Clinical Outcome Assessments: Establishing and Interpreting Meaningful Within-Patient Change Meeting The Duke-Margolis Center for Health Policy, Washington,
CLICK TO GO BACK TO KIOSK MENU
 Clinical outcomes in patients with baseline renal dysfunction in the NETTER- Study: 77 Lu-DOTATATE vs. high dose octreotide in progressive midgut neuroendocrine tumors J. Strosberg, E. Wolin 2, B. Chasen
Clinical outcomes in patients with baseline renal dysfunction in the NETTER- Study: 77 Lu-DOTATATE vs. high dose octreotide in progressive midgut neuroendocrine tumors J. Strosberg, E. Wolin 2, B. Chasen
WHAT TO EXPECT IN 2015? - Renuka Iyer, MD Associate Professor of Medicine, University at Buffalo Associate Professor of Oncology, Roswell Park Cancer
 WHAT TO EXPECT IN 2015? - Renuka Iyer, MD Associate Professor of Medicine, University at Buffalo Associate Professor of Oncology, Roswell Park Cancer Institute Overview Diagnosis: Gallium scan Biomarkers
WHAT TO EXPECT IN 2015? - Renuka Iyer, MD Associate Professor of Medicine, University at Buffalo Associate Professor of Oncology, Roswell Park Cancer Institute Overview Diagnosis: Gallium scan Biomarkers
Recent developments of oncology in neuroendocrine tumors (NETs)
 Recent developments of oncology in neuroendocrine tumors (NETs) Marc Peeters MD, PhD Coordinator Multidisciplinary Oncological Center Antwerpen (MOCA) Head of the Oncology Department UZA, Professor in
Recent developments of oncology in neuroendocrine tumors (NETs) Marc Peeters MD, PhD Coordinator Multidisciplinary Oncological Center Antwerpen (MOCA) Head of the Oncology Department UZA, Professor in
Review of Gastrointestinal Carcinoid Tumors: Latest Therapies
 Review of Gastrointestinal Carcinoid Tumors: Latest Therapies Arvind Dasari, MD, MS Department of Gastrointestinal Medical Oncology The University of Texas MD Anderson Cancer Center Houston, TX, USA Neuroendocrine
Review of Gastrointestinal Carcinoid Tumors: Latest Therapies Arvind Dasari, MD, MS Department of Gastrointestinal Medical Oncology The University of Texas MD Anderson Cancer Center Houston, TX, USA Neuroendocrine
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xermelo (telotristat) Page 1 of 5 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xermelo (telotristat) Prime Therapeutics will review Prior Authorization requests
Xermelo (telotristat) Page 1 of 5 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xermelo (telotristat) Prime Therapeutics will review Prior Authorization requests
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xermelo (telotristat) Page 1 of 5 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xermelo (telotristat) Prime Therapeutics will review Prior Authorization requests
Xermelo (telotristat) Page 1 of 5 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xermelo (telotristat) Prime Therapeutics will review Prior Authorization requests
Selection of Appropriate Treatment
 Expert Review in Metastatic Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): Selection of Appropriate Treatment Reference Slide Deck Neuroendocrine Tumors (NETs): A Diverse Group of Malignancies
Expert Review in Metastatic Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): Selection of Appropriate Treatment Reference Slide Deck Neuroendocrine Tumors (NETs): A Diverse Group of Malignancies
OPTIMISING OUTCOMES IN GASTROINTESTINAL NEUROENDOCRINE TUMOURS
 OPTIMISING OUTCOMES IN GASTROINTESTINAL NEUROENDOCRINE TUMOURS Dr Mairéad McNamara Senior lecturer, University of Manchester & Honorary Consultant in Medical Oncology, The Christie NHS Foundation Trust
OPTIMISING OUTCOMES IN GASTROINTESTINAL NEUROENDOCRINE TUMOURS Dr Mairéad McNamara Senior lecturer, University of Manchester & Honorary Consultant in Medical Oncology, The Christie NHS Foundation Trust
QOL Improvements in NETTER-1 Phase III Trial in Patients With Progressive Midgut Neuroendocrine Tumors
 QOL Improvements in NETTER-1 Phase III Trial in Patients With Progressive Midgut Neuroendocrine Tumors Abstract C-33 Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Kunz P, Hobday
QOL Improvements in NETTER-1 Phase III Trial in Patients With Progressive Midgut Neuroendocrine Tumors Abstract C-33 Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Kunz P, Hobday
Nuevas alternativas en el manejo de TNE avanzados
 Nuevas alternativas en el manejo de TNE avanzados Jaume Capdevila Hospital Universitari Vall d Hebron Barcelona Coordinación científica: Dr. Fernando Rivera Hospital Universitario Marqués de Valdecilla,
Nuevas alternativas en el manejo de TNE avanzados Jaume Capdevila Hospital Universitari Vall d Hebron Barcelona Coordinación científica: Dr. Fernando Rivera Hospital Universitario Marqués de Valdecilla,
OTEZLA (Apremilast) Showed Meaningful Improvements in Clinical and Quality of Life Measures of Psoriasis Beyond Those Captured by Assessing Skin Alone
 OTEZLA (Apremilast) Showed Meaningful Improvements in Clinical and Quality of Life Measures of Psoriasis Beyond Those Captured by Assessing Skin Alone Patients with moderate to severe plaque psoriasis
OTEZLA (Apremilast) Showed Meaningful Improvements in Clinical and Quality of Life Measures of Psoriasis Beyond Those Captured by Assessing Skin Alone Patients with moderate to severe plaque psoriasis
AUSTRALIAN PRODUCT INFORMATION 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse
This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse
Alpelisib (ALP) + Fulvestrant (FUL) for Advanced Breast Cancer (ABC): Phase 3 SOLAR-1 Trial Results
 Alpelisib (ALP) + Fulvestrant (FUL) for Advanced Breast Cancer (ABC): Phase 3 SOLAR-1 Trial Results Dejan Juric, 1* Eva Maria Ciruelos, 2 Gabor Rubovszky, 3 Mario Campone, 4 Sibylle Loibl, 5 Hope S. Rugo,
Alpelisib (ALP) + Fulvestrant (FUL) for Advanced Breast Cancer (ABC): Phase 3 SOLAR-1 Trial Results Dejan Juric, 1* Eva Maria Ciruelos, 2 Gabor Rubovszky, 3 Mario Campone, 4 Sibylle Loibl, 5 Hope S. Rugo,
Phase II Study of Subcutaneous Injection Depot of Octreotide in Patients With Acromegaly and Ne uroe ndocrine Tumours (NETs)
 A service of the U.S. National Institutes of Health Trial record 1 of 4 for: CAMURUS Previous Study Return to List Next Study Phase II Study of Subcutaneous Injection Depot of Octreotide in Patients With
A service of the U.S. National Institutes of Health Trial record 1 of 4 for: CAMURUS Previous Study Return to List Next Study Phase II Study of Subcutaneous Injection Depot of Octreotide in Patients With
TRACTAMENT ONCOLÒGIC DELS TUMORS NEUROENDOCRINS METASTÀSICS
 TRACTAMENT ONCOLÒGIC DELS TUMORS NEUROENDOCRINS METASTÀSICS Jaume Capdevila Unitat de Tumors GI i Endocrins Hospital Universitari Vall d Hebron Barcelona Experts, acollidors i solidaris OUTLINE BACKGROUND
TRACTAMENT ONCOLÒGIC DELS TUMORS NEUROENDOCRINS METASTÀSICS Jaume Capdevila Unitat de Tumors GI i Endocrins Hospital Universitari Vall d Hebron Barcelona Experts, acollidors i solidaris OUTLINE BACKGROUND
Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome
 Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome Primary Endpoint Achieved - Statistically Significant Convulsive Seizure Reduction for ZX008
Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome Primary Endpoint Achieved - Statistically Significant Convulsive Seizure Reduction for ZX008
Epidemiology and cost of chronic pain
 Epidemiology and cost of chronic pain Dr Beverly Collett Consultant in Pain Medicine University Hospitals of Leicester Regional Advisor Treasurer of IASP Epidemiology Chronic pain is defined differently,
Epidemiology and cost of chronic pain Dr Beverly Collett Consultant in Pain Medicine University Hospitals of Leicester Regional Advisor Treasurer of IASP Epidemiology Chronic pain is defined differently,
Phase 2b/3 Topline Trial Results
 Phase 2b/3 Topline Trial Results RP-G28 For the Treatment of Lactose Intolerance March 2017 Forward - Looking Statement To the extent that statements contained in this presentation are not descriptions
Phase 2b/3 Topline Trial Results RP-G28 For the Treatment of Lactose Intolerance March 2017 Forward - Looking Statement To the extent that statements contained in this presentation are not descriptions
The MOVE Trial Summary. Palovarotene FAQs. 1. What is the MOVE Trial?
 MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
MEDICAL MANAGEMENT OF METASTATIC GEP-NET
 MEDICAL MANAGEMENT OF METASTATIC GEP-NET Jeremy Kortmansky, MD Associate Professor of Clinical Medicine Yale Cancer Center DISCLOSURES: NONE Introduction Gastrointestinal and pancreatic neuroendocrine
MEDICAL MANAGEMENT OF METASTATIC GEP-NET Jeremy Kortmansky, MD Associate Professor of Clinical Medicine Yale Cancer Center DISCLOSURES: NONE Introduction Gastrointestinal and pancreatic neuroendocrine
Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With
 VOLUME 36 NUMBER 25 SEPTEMBER 1, 218 JOURNAL OF CLINICAL ONCOLOGY R A P I D C O M M U N I C A T I O N Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With
VOLUME 36 NUMBER 25 SEPTEMBER 1, 218 JOURNAL OF CLINICAL ONCOLOGY R A P I D C O M M U N I C A T I O N Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With
Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer
 Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer Soledad Gallego, 1 Valentina Boni, 2 Ulrik Lassen, 3 Anna Farago, 4 Wafik El-Deiry, 5 David Hong, 6 Blanca López-Ibor, 2
Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer Soledad Gallego, 1 Valentina Boni, 2 Ulrik Lassen, 3 Anna Farago, 4 Wafik El-Deiry, 5 David Hong, 6 Blanca López-Ibor, 2
Migraine: Developing Drugs for Acute Treatment Guidance for Industry
 Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
INSPIRE: A Phase 3 Open-Label, Multicenter Study to Evaluate the Safety and Tolerability of LIQ861 in Pulmonary Arterial Hypertension (PAH)
 INSPIRE: A Phase 3 Open-Label, Multicenter Study to Evaluate the Safety and Tolerability of LIQ861 in Pulmonary Arterial Hypertension (PAH) N. S. Hill; J. P. Feldman; S. Sahay; D. J. Levine; R. F. Roscigno;
INSPIRE: A Phase 3 Open-Label, Multicenter Study to Evaluate the Safety and Tolerability of LIQ861 in Pulmonary Arterial Hypertension (PAH) N. S. Hill; J. P. Feldman; S. Sahay; D. J. Levine; R. F. Roscigno;
CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
Cutting Edge Treatment of Neuroendocrine Tumors
 Cutting Edge Treatment of Neuroendocrine Tumors Daneng Li, MD Assistant Clinical Professor Department of Medical Oncology & Therapeutics Research City of Hope Click to edit Master Presentation Date DISCLOSURE
Cutting Edge Treatment of Neuroendocrine Tumors Daneng Li, MD Assistant Clinical Professor Department of Medical Oncology & Therapeutics Research City of Hope Click to edit Master Presentation Date DISCLOSURE
Cutting Edge Treatment of Neuroendocrine Tumors
 Cutting Edge Treatment of Neuroendocrine Tumors Daneng Li, MD Assistant Clinical Professor Department of Medical Oncology & Therapeutics Research City of Hope Click to edit Master Presentation Date DISCLOSURE
Cutting Edge Treatment of Neuroendocrine Tumors Daneng Li, MD Assistant Clinical Professor Department of Medical Oncology & Therapeutics Research City of Hope Click to edit Master Presentation Date DISCLOSURE
Crisis Investing: Profits From a Diabetes Drug About to Get Approved Crisis Investing: Profits From a Diabetes Drug About to Get Approved
 Crisis Investing: Profits From a Diabetes Drug About to Get Approved A Special Research Report from Bret Jensen's Biotech Gems Lexicon Pharmaceuticals (Nasdaq: LXRX): tackling one of America s biggest
Crisis Investing: Profits From a Diabetes Drug About to Get Approved A Special Research Report from Bret Jensen's Biotech Gems Lexicon Pharmaceuticals (Nasdaq: LXRX): tackling one of America s biggest
7/14/2014. SOLIRIS (eculizumab) SOLIRIS PNH Clinical Studies. SOLIRIS Blocks Terminal Complement. 86% Reduction in LDH: TRIUMPH and SHEPHERD
 Proximal Terminal Lactate Dehydrogenase (U/L) 7/1/1 SOLIRIS (eculizumab) Humanized First in Class Anti - C5 Antibody SOLIRIS (eculizumab) Human Framework Regions No mutations Germline SOLIRIS is a Complement
Proximal Terminal Lactate Dehydrogenase (U/L) 7/1/1 SOLIRIS (eculizumab) Humanized First in Class Anti - C5 Antibody SOLIRIS (eculizumab) Human Framework Regions No mutations Germline SOLIRIS is a Complement
NET εντέρου Τι νεότερο/ Νέες μελέτες. Μαντώ Νικολαΐδη παθολόγος-ογκολόγος ΜΗΤΕΡΑ
 NET εντέρου Τι νεότερο/ Νέες μελέτες Μαντώ Νικολαΐδη παθολόγος-ογκολόγος ΜΗΤΕΡΑ NET: A Diverse Group of Malignancies 1-3 Wide spectrum of malignancies arising in neuroendocrine cells throughout the body
NET εντέρου Τι νεότερο/ Νέες μελέτες Μαντώ Νικολαΐδη παθολόγος-ογκολόγος ΜΗΤΕΡΑ NET: A Diverse Group of Malignancies 1-3 Wide spectrum of malignancies arising in neuroendocrine cells throughout the body
Pembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Phase 2 KEYNOTE-087 Study
 Pembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Phase 2 KEYNOTE-087 Study Craig H. Moskowitz, 1 Pier Luigi Zinzani, 2 Michelle A. Fanale, 3 Philippe Armand, 4 Nathalie Johnson, 5 John
Pembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Phase 2 KEYNOTE-087 Study Craig H. Moskowitz, 1 Pier Luigi Zinzani, 2 Michelle A. Fanale, 3 Philippe Armand, 4 Nathalie Johnson, 5 John
Systemic Therapy for Gastroenteropancreatic (GEP) Neuroendocrine Tumors and Lung Carcinoid
 Systemic Therapy for Gastroenteropancreatic (GEP) Neuroendocrine Tumors and Lung Carcinoid The Medical Oncology Perspective Nevena Damjanov, MD Associate professor Abramson Cancer Center of the University
Systemic Therapy for Gastroenteropancreatic (GEP) Neuroendocrine Tumors and Lung Carcinoid The Medical Oncology Perspective Nevena Damjanov, MD Associate professor Abramson Cancer Center of the University
Jefferies Healthcare Conference
 Jefferies Healthcare Conference June 7, 2016 NASDAQ: CHMA Forward-Looking Statements These slides contain forward-looking statements and information. The use of words such as may, might, will, should,
Jefferies Healthcare Conference June 7, 2016 NASDAQ: CHMA Forward-Looking Statements These slides contain forward-looking statements and information. The use of words such as may, might, will, should,
SOLIRIS is a Complement Inhibitor Indicated for the Treatment of Patients With PNH to Reduce Hemolysis
 SOLIRIS (eculizumab) SOLIRIS is a Complement Inhibitor Indicated for the Treatment of Patients With PNH to Reduce Hemolysis SOLIRIS is the First and Only Approved Therapy for PNH SOLIRIS (eculizumab) [package
SOLIRIS (eculizumab) SOLIRIS is a Complement Inhibitor Indicated for the Treatment of Patients With PNH to Reduce Hemolysis SOLIRIS is the First and Only Approved Therapy for PNH SOLIRIS (eculizumab) [package
-- Edasalonexent Substantially Slowed Duchenne Muscular Dystrophy Disease Progression through 36 Weeks --
 Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
William D Chey, 1 Anthony J Lembo, 2 James A Phillips, 3 David P Rosenbaum 4
 Efficacy and safety of tenapanor in patients with constipationpredominant irritable bowel syndrome: a 12-week, double-blind, placebocontrolled, randomized phase 2b trial William D Chey, 1 Anthony J Lembo,
Efficacy and safety of tenapanor in patients with constipationpredominant irritable bowel syndrome: a 12-week, double-blind, placebocontrolled, randomized phase 2b trial William D Chey, 1 Anthony J Lembo,
(direct) (609) (mobile)
 MEDIA Sanofi-aventis Bristol-Myers Squibb Ingrid Görg-Armbrecht Laura Hortas + 33 153-774-625 (direct) (609) 252-4587 +33 686-056-688 (mobile) laura.hortas@bms.com ingrid.goerg-armbrecht@sanofi-aventis.com
MEDIA Sanofi-aventis Bristol-Myers Squibb Ingrid Görg-Armbrecht Laura Hortas + 33 153-774-625 (direct) (609) 252-4587 +33 686-056-688 (mobile) laura.hortas@bms.com ingrid.goerg-armbrecht@sanofi-aventis.com
Lu 177-Dotatate (Lutathera) Therapy Information
 Lu 177-Dotatate (Lutathera) Therapy Information Information for Lu 177-dotatate therapy also known as Lutathera, for the treatment of metastatic midgut neuroendocrine tumor and other metastatic neuroendocrine
Lu 177-Dotatate (Lutathera) Therapy Information Information for Lu 177-dotatate therapy also known as Lutathera, for the treatment of metastatic midgut neuroendocrine tumor and other metastatic neuroendocrine
Infographic (right): ESMO 2014 record breaking Congress
 ESMO 2014 Congress Scientific Meeting Report Supportive and Palliative Care Extract 26-30 September 2014 Madrid, Spain Summary The European Society for Medical Oncology (ESMO) Congress, held September
ESMO 2014 Congress Scientific Meeting Report Supportive and Palliative Care Extract 26-30 September 2014 Madrid, Spain Summary The European Society for Medical Oncology (ESMO) Congress, held September
XARACOLL Phase 3 Results Webcast. MATRIX 1 and MATRIX 2 Clinical Trials May 25, 2016
 XARACOLL Phase 3 Results Webcast MATRIX 1 and MATRIX 2 Clinical Trials May 25, 2016 Forward Looking Statements This presentation contains forward-looking statements about our ongoing development of XARACOLL
XARACOLL Phase 3 Results Webcast MATRIX 1 and MATRIX 2 Clinical Trials May 25, 2016 Forward Looking Statements This presentation contains forward-looking statements about our ongoing development of XARACOLL
The Treatment of Rett Syndrome with Trofinetide (NNZ-2566): Past, Present, Future. Daniel Glaze, MD Baylor College of Medicine
 The Treatment of Rett Syndrome with Trofinetide (NNZ-2566): Past, Present, Future Daniel Glaze, MD Baylor College of Medicine Multicenter Trials of Trofinetide Past: Neu-2566-RETT-001 Phase 2 in Adolescents
The Treatment of Rett Syndrome with Trofinetide (NNZ-2566): Past, Present, Future Daniel Glaze, MD Baylor College of Medicine Multicenter Trials of Trofinetide Past: Neu-2566-RETT-001 Phase 2 in Adolescents
Theravance Announces Positive Results from Phase 1 and Phase 2 Clinical Studies with TD-1211 in Development for Opioid-Induced Constipation
 Theravance Announces Positive Results from Phase 1 and Phase 2 Clinical Studies with TD-1211 in Development for Opioid-Induced Constipation TD-1211 Achieves Primary and Secondary Endpoints SOUTH SAN FRANCISCO,
Theravance Announces Positive Results from Phase 1 and Phase 2 Clinical Studies with TD-1211 in Development for Opioid-Induced Constipation TD-1211 Achieves Primary and Secondary Endpoints SOUTH SAN FRANCISCO,
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Antiangiogenics are effective treatments in NETs
 RENET: A randomized phase III trial comparing REgorafenib to placebo in patients with advanced, progressive, well-differentiated NEuroendocrine Tumors (NETs). Coordinators: Dr Julien Hadoux & Dr David
RENET: A randomized phase III trial comparing REgorafenib to placebo in patients with advanced, progressive, well-differentiated NEuroendocrine Tumors (NETs). Coordinators: Dr Julien Hadoux & Dr David
 Page 1 of 5 VERZENIO abemaciclib tablet Eli Lilly and Company AddendaIndex Summary View All Sections HIGHLIGHTS OF PRESCRIBING INFORMATION FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE
Page 1 of 5 VERZENIO abemaciclib tablet Eli Lilly and Company AddendaIndex Summary View All Sections HIGHLIGHTS OF PRESCRIBING INFORMATION FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE
Treatment Expectations and Priorities of People with MS
 Treatment Expectations and Priorities of People with MS Prepared by Spoonful of Sugar 97 Tottenham Court Road London W1T 4TP Date: October 2017 Spoonful of Sugar 2017 Contents Executive Summary.. 3 TaP-MS
Treatment Expectations and Priorities of People with MS Prepared by Spoonful of Sugar 97 Tottenham Court Road London W1T 4TP Date: October 2017 Spoonful of Sugar 2017 Contents Executive Summary.. 3 TaP-MS
Sunitinib Achieved Fast and Sustained Control of VIPoma. Symptoms
 Page 1 of 13 Accepted Preprint first posted on 10 October 2014 as Manuscript EJE-14-0682 1 1 Case report 2 3 4 Sunitinib Achieved Fast and Sustained Control of VIPoma Symptoms 5 6 7 Louis de Mestier 1,
Page 1 of 13 Accepted Preprint first posted on 10 October 2014 as Manuscript EJE-14-0682 1 1 Case report 2 3 4 Sunitinib Achieved Fast and Sustained Control of VIPoma Symptoms 5 6 7 Louis de Mestier 1,
Phase 2 placebo-controlled withdrawal study of the ASBT inhibitor maralixibat in children with Alagille syndrome 48-week efficacy analysis
 Phase 2 placebo-controlled withdrawal study of the ASBT inhibitor maralixibat in children with Alagille syndrome 48-week efficacy analysis ICONIC Study Emmanuel Gonzales, Ekkehard Sturm, Michael Stormon,
Phase 2 placebo-controlled withdrawal study of the ASBT inhibitor maralixibat in children with Alagille syndrome 48-week efficacy analysis ICONIC Study Emmanuel Gonzales, Ekkehard Sturm, Michael Stormon,
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
3/31/2014 PNH. Jack Goldberg MD FACP. Clinical Professor of Medicine University of Pennsylvania
 PNH Jack Goldberg MD FACP Clinical Professor of Medicine University of Pennsylvania 1 2 3 4 1 5 6 Historically Viewed as a Hemolytic Anemia Normal red blood cells are protected from complement attack by
PNH Jack Goldberg MD FACP Clinical Professor of Medicine University of Pennsylvania 1 2 3 4 1 5 6 Historically Viewed as a Hemolytic Anemia Normal red blood cells are protected from complement attack by
Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta471
 Eluxadoline for treating irritable bowel syndrome with diarrhoea Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta471 NICE 2017. All rights reserved. Subject to Notice of
Eluxadoline for treating irritable bowel syndrome with diarrhoea Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta471 NICE 2017. All rights reserved. Subject to Notice of
-- Single Global Phase 3 Trial Expected to Begin in First Half of
 Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C FORM 8-K
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
MANAGEMENT OF CHRONIC CONSTIPATION BEYOND LAXATIVES
 Enrique Rey Professor of Medicine Head. Department of Digestive Diseases Hospital Clínico San Carlos Complutense University Madrid, Spain MANAGEMENT OF CHRONIC CONSTIPATION BEYOND LAXATIVES CONSTIPATION:
Enrique Rey Professor of Medicine Head. Department of Digestive Diseases Hospital Clínico San Carlos Complutense University Madrid, Spain MANAGEMENT OF CHRONIC CONSTIPATION BEYOND LAXATIVES CONSTIPATION:
Evaluation and Management of Neuroendocrine Tumors
 Evaluation and Management of Neuroendocrine Tumors Jennifer Chan, MD, MPH Clinical Director, Program in Neuroendocrine and Carcinoid Tumors Dana-Farber/Brigham and Women's Cancer Center October 14, 2017
Evaluation and Management of Neuroendocrine Tumors Jennifer Chan, MD, MPH Clinical Director, Program in Neuroendocrine and Carcinoid Tumors Dana-Farber/Brigham and Women's Cancer Center October 14, 2017
*Bert Bakker was an employee of Novartis Pharmaceuticals Corporation until June 06, 2014.
 Page 1 of 20 Accepted Preprint first posted on 15 September 2015 as Manuscript ERC-15-0314 1 2 Efficacy of Octreotide LAR in Neuroendocrine Tumors: RADIANT-2 Placebo Arm Post Hoc Analysis 3 4 Authors:
Page 1 of 20 Accepted Preprint first posted on 15 September 2015 as Manuscript ERC-15-0314 1 2 Efficacy of Octreotide LAR in Neuroendocrine Tumors: RADIANT-2 Placebo Arm Post Hoc Analysis 3 4 Authors:
IBS. Patient INFO. A Guide to Irritable Bowel Syndrome
 Patient INFO IBS A Guide to Irritable Bowel Syndrome The information provided by the AGA Institute is not medical advice and should not be considered a replacement for seeing a medical professional. About
Patient INFO IBS A Guide to Irritable Bowel Syndrome The information provided by the AGA Institute is not medical advice and should not be considered a replacement for seeing a medical professional. About
8:30-9:00 Increased dependence on glutamine in PTEN-deficient breast cancer Ramon E. Parsons, Icahn School of Medicine at Mount Sinai, New York, NY
 CONFERENCE PROGRAM SATURDAY, OCTOBER 7 3:00 p.m.-7:00 p.m. Salon 3 of the Terrace Welcome and Keynote Lectures 6:00 p.m.-7:30 p.m. Session Chair: Ramon E. Parsons, Icahn School of Medicine at Mount Sinai,
CONFERENCE PROGRAM SATURDAY, OCTOBER 7 3:00 p.m.-7:00 p.m. Salon 3 of the Terrace Welcome and Keynote Lectures 6:00 p.m.-7:30 p.m. Session Chair: Ramon E. Parsons, Icahn School of Medicine at Mount Sinai,
Fact Sheet. Zohydro ER (hydrocodone bitartrate) Extended-Release Capsules, CII
 Zohydro ER (hydrocodone bitartrate) Extended-Release Capsules, CII Fact Sheet Zohydro ER (hydrocodone bitartrate) Extended-Release Capsule, CII, is a long-acting (extendedrelease) type of pain medication
Zohydro ER (hydrocodone bitartrate) Extended-Release Capsules, CII Fact Sheet Zohydro ER (hydrocodone bitartrate) Extended-Release Capsule, CII, is a long-acting (extendedrelease) type of pain medication
 13TH INTERNATIONAL WORKSHOP ON CO-INFECTION HIV & HEPATITIS LISBON, PORTUGAL 24-26 MAY 2017 SPONSORSHIP REQUEST 2017 www.expertmedicalevents.com INTRODUCTION & SIGNIFICANCE BACKGROUND Support our efforts
13TH INTERNATIONAL WORKSHOP ON CO-INFECTION HIV & HEPATITIS LISBON, PORTUGAL 24-26 MAY 2017 SPONSORSHIP REQUEST 2017 www.expertmedicalevents.com INTRODUCTION & SIGNIFICANCE BACKGROUND Support our efforts
Global Irritable Bowel Syndrome (IBS) Market: Trends & Opportunities ( )
 Global Irritable Bowel Syndrome (IBS) Market: Trends & Opportunities (2012-17) Scope of the Report The report titled Global Irritable Bowel Syndrome Market: Trends and Opportunities (2012-17) provides
Global Irritable Bowel Syndrome (IBS) Market: Trends & Opportunities (2012-17) Scope of the Report The report titled Global Irritable Bowel Syndrome Market: Trends and Opportunities (2012-17) provides
Design and Analysis of a Cancer Prevention Trial: Plans and Results. Matthew Somerville 09 November 2009
 Design and Analysis of a Cancer Prevention Trial: Plans and Results Matthew Somerville 09 November 2009 Overview Objective: Review the planned analyses for a large prostate cancer prevention study and
Design and Analysis of a Cancer Prevention Trial: Plans and Results Matthew Somerville 09 November 2009 Overview Objective: Review the planned analyses for a large prostate cancer prevention study and
PRESS RELEASE. Lanreotide (Somatuline Autogel ) is featured in 17 presentations: ABSTRACTS SELECTED FOR ORAL PRESENTATIONS
 PRESS RELEASE Ipsen announces data presentations of lanreotide (Somatuline Autogel ), telotristat ethyl and the investigational compound 177 Lu-OPS201 at the European Neuroendocrine Tumor Society (ENETS)
PRESS RELEASE Ipsen announces data presentations of lanreotide (Somatuline Autogel ), telotristat ethyl and the investigational compound 177 Lu-OPS201 at the European Neuroendocrine Tumor Society (ENETS)
10 th Annual ENETS Conference
 10 th Annual ENETS Conference for the Diagnosis and Treatment of Neuroendocrine Tumor Disease 6-8 March 2013 Barcelona P R E L I M I N A R Y P R O G R A M 1 Scientific Organizing Committee Martyn Caplin,
10 th Annual ENETS Conference for the Diagnosis and Treatment of Neuroendocrine Tumor Disease 6-8 March 2013 Barcelona P R E L I M I N A R Y P R O G R A M 1 Scientific Organizing Committee Martyn Caplin,
Safety Pharmacology Post Meeting Survey 2014
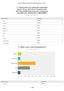 Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
Acasti Pharma Provides Business Update for the Third Quarter of Fiscal 2019
 Acasti Pharma Provides Business Update for the Third Quarter of Fiscal 2019 Planned Enrollment targets achieved in both TRILOGY studies with over 74% of patients randomized at more than 150 clinical sites
Acasti Pharma Provides Business Update for the Third Quarter of Fiscal 2019 Planned Enrollment targets achieved in both TRILOGY studies with over 74% of patients randomized at more than 150 clinical sites
Nuove terapie in ambito Nefrologico: Etelcalcetide (AMG-416)
 Nuove terapie in ambito Nefrologico: Etelcalcetide (AMG-416) Antonio Bellasi, MD, PhD U.O.C. Nefrologia & Dialisi ASST-Lariana, Ospedale S. Anna, Como, Italy Improvement of mineral and bone metabolism
Nuove terapie in ambito Nefrologico: Etelcalcetide (AMG-416) Antonio Bellasi, MD, PhD U.O.C. Nefrologia & Dialisi ASST-Lariana, Ospedale S. Anna, Como, Italy Improvement of mineral and bone metabolism
Peptide Receptor Radionuclide Therapy (PRRT) Lu-177
 PATIENT EDUCATION patienteducation.osumc.edu Peptide Receptor Radionuclide Therapy (PRRT) Lu-177 What is a neuroendocrine tumor? Neuroendocrine cells are mainly found in your bowel, pancreas and lungs.
PATIENT EDUCATION patienteducation.osumc.edu Peptide Receptor Radionuclide Therapy (PRRT) Lu-177 What is a neuroendocrine tumor? Neuroendocrine cells are mainly found in your bowel, pancreas and lungs.
3. Chantix [package insert]. New York, NY: Pfizer, Inc,; Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine
![3. Chantix [package insert]. New York, NY: Pfizer, Inc,; Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine 3. Chantix [package insert]. New York, NY: Pfizer, Inc,; Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine](/thumbs/87/96607870.jpg) How can there be a warning regarding concomitant use of varenicline with nicotine replacement therapy yet patients can be on varenicline and smoke concurrently? April 20, 2017 The United States (US) Preventive
How can there be a warning regarding concomitant use of varenicline with nicotine replacement therapy yet patients can be on varenicline and smoke concurrently? April 20, 2017 The United States (US) Preventive
Trial record 1 of 1 for: Previous Study Return to List Next Study
 1 von 6 14.01.2014 09:11 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 2012-001834-33 Previous Study Return to List Next Study Study of Cabozantinib (XL184) Versus Prednisone
1 von 6 14.01.2014 09:11 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 2012-001834-33 Previous Study Return to List Next Study Study of Cabozantinib (XL184) Versus Prednisone
ADVERSE REACTIONS The most common (>10%) adverse reactions are hypercalcemia, nausea, and diarrhea. (6.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
BMS for the Acute Treatment of Migraine: A Double-Blind, Randomized, Placebo Controlled, Dose-Ranging Trial
 BMS-927711 for the Acute Treatment of Migraine: A Double-Blind, Randomized, Placebo Controlled, Dose-Ranging Trial Ronald Marcus, MD Chief Medical Officer Spinifex Pharmaceuticals Migraine: Case Study
BMS-927711 for the Acute Treatment of Migraine: A Double-Blind, Randomized, Placebo Controlled, Dose-Ranging Trial Ronald Marcus, MD Chief Medical Officer Spinifex Pharmaceuticals Migraine: Case Study
Routine monitoring requirements for your mcrpc patients on Xofigo
 XOFIGO IS INDICATED for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bone metastases and no known visceral metastatic disease. Introduce Xofigo at the first sign
XOFIGO IS INDICATED for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bone metastases and no known visceral metastatic disease. Introduce Xofigo at the first sign
TUMORES NEUROENDOCRINOS. Miguel Navarro. Salamanca
 TUMORES NEUROENDOCRINOS Miguel Navarro. Salamanca Introduction to Neuroendocrine Tumours (NETs) NETs are relatively RARE At least 40 different entities are described arising in different organs. Different
TUMORES NEUROENDOCRINOS Miguel Navarro. Salamanca Introduction to Neuroendocrine Tumours (NETs) NETs are relatively RARE At least 40 different entities are described arising in different organs. Different
ALVIMOPAN 0.0 OVERVIEW
 ALVIMOPAN 0.0 OVERVIEW A. Alvimopan is a peripherally restricted mu-opioid receptor antagonist. B. DOSING INFORMATION : For the treatment of opioid bowel dysfunction, oral alvimopan doses between 0.5 milligrams
ALVIMOPAN 0.0 OVERVIEW A. Alvimopan is a peripherally restricted mu-opioid receptor antagonist. B. DOSING INFORMATION : For the treatment of opioid bowel dysfunction, oral alvimopan doses between 0.5 milligrams
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 2Q17 April May
 BRAND NAME Trulance GENERIC NAME Plecanatide MANUFACTURER Synergy Pharmaceuticals, Inc. DATE OF APPROVAL January 19, 2017 PRODUCT LAUNCH DATE Anticipated in 1Q2017 REVIEW TYPE Review type 1 (RT1): New
BRAND NAME Trulance GENERIC NAME Plecanatide MANUFACTURER Synergy Pharmaceuticals, Inc. DATE OF APPROVAL January 19, 2017 PRODUCT LAUNCH DATE Anticipated in 1Q2017 REVIEW TYPE Review type 1 (RT1): New
REFERENCE CODE GDHC518DFR PUBLICAT ION DATE DECEMBER 2014 LINZESS (IRRITABLE BOWEL SYNDROME) - FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC518DFR PUBLICAT ION DATE DECEMBER 2014 LINZESS (IRRITABLE BOWEL SYNDROME) - Executive Summary The Table below presents the key metrics of Ironwood/Actavis/Almirall/Astellas Linzess for
REFERENCE CODE GDHC518DFR PUBLICAT ION DATE DECEMBER 2014 LINZESS (IRRITABLE BOWEL SYNDROME) - Executive Summary The Table below presents the key metrics of Ironwood/Actavis/Almirall/Astellas Linzess for
Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) (908)
 News Release FOR IMMEDIATE RELEASE Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) 423-6537 (908) 423-5185 Tracy Ogden (267) 305-0960 FDA Approves Once-Daily JANUVIA, the First and Only DPP-4
News Release FOR IMMEDIATE RELEASE Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) 423-6537 (908) 423-5185 Tracy Ogden (267) 305-0960 FDA Approves Once-Daily JANUVIA, the First and Only DPP-4
Broad and clinically important benefits beyond the initial registrational endpoints are now reported.
 Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom
Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom
Pharmacy Prior Authorization Somatostatin Analogs Clinical Guideline
 Sandostatin LAR (octreotide) Signifor (pasireotide) Signifor LAR (pasireotide) Somatuline Depot (lanreotide) octreotide FDA Approved Indications: Acromegaly: Octreotide Injection is indicated to reduce
Sandostatin LAR (octreotide) Signifor (pasireotide) Signifor LAR (pasireotide) Somatuline Depot (lanreotide) octreotide FDA Approved Indications: Acromegaly: Octreotide Injection is indicated to reduce
Sandostatin LAR. Sandostatin LAR (octreotide acetate) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.09 Subject: Sandostatin LAR Page: 1 of 5 Last Review Date: March 16, 2018 Sandostatin LAR Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.09 Subject: Sandostatin LAR Page: 1 of 5 Last Review Date: March 16, 2018 Sandostatin LAR Description
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
New Developments in the Care and Management of Patients with Gastroenteropancreatic Neuroendocrine Tumors Dr. Tim Asmis The Ottawa Hospital Cancer
 New Developments in the Care and Management of Patients with Gastroenteropancreatic Neuroendocrine Tumors Dr. Tim Asmis The Ottawa Hospital Cancer Centre MD, FRCPC CAGPO September 2018 Disclosures Consultant
New Developments in the Care and Management of Patients with Gastroenteropancreatic Neuroendocrine Tumors Dr. Tim Asmis The Ottawa Hospital Cancer Centre MD, FRCPC CAGPO September 2018 Disclosures Consultant
Edith A. Perez, Ahmad Awada, Joyce O Shaughnessy, Hope Rugo, Chris Twelves, Seock-Ah Im, Carol Zhao, Ute Hoch, Alison L. Hannah, Javier Cortes
 BEACON: A Phase 3 Open-label, Randomized, Multicenter Study of Etirinotecan Pegol (EP) versus Treatment of Physician s Choice (TPC) in Patients With Locally Recurrent or Metastatic Breast Cancer Previously
BEACON: A Phase 3 Open-label, Randomized, Multicenter Study of Etirinotecan Pegol (EP) versus Treatment of Physician s Choice (TPC) in Patients With Locally Recurrent or Metastatic Breast Cancer Previously
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Ipilimumab ASCO Data Review and Discussion Webcast. Monday, June 2, 2008
 Ipilimumab ASCO Data Review and Discussion Webcast Monday, June 2, 2008 Slide 2 Forward Looking Statements Except for historical information, the matters contained in this slide presentation may constitute
Ipilimumab ASCO Data Review and Discussion Webcast Monday, June 2, 2008 Slide 2 Forward Looking Statements Except for historical information, the matters contained in this slide presentation may constitute
GSK Q&A For Patient Advocacy Groups: 04 October 2013 For reactive use in response to enquiries from patient groups only
 1. Will assessments and visits continue now that the patients are no longer receiving study treatment? Yes, while dosing of boys in the ongoing studies (DMD114349, DMD115501 and DMD114673) has been placed
1. Will assessments and visits continue now that the patients are no longer receiving study treatment? Yes, while dosing of boys in the ongoing studies (DMD114349, DMD115501 and DMD114673) has been placed
Conducting Successful Oncology Clinical Trials in Asia Pacific: PharmaNet experience using Case Studies
 Conducting Successful Oncology Clinical Trials in Asia Pacific: PharmaNet experience using Case Studies Emily Tan Executive Director Asia Pacific PharmaNet Conducting Successful Oncology Clinical Trials
Conducting Successful Oncology Clinical Trials in Asia Pacific: PharmaNet experience using Case Studies Emily Tan Executive Director Asia Pacific PharmaNet Conducting Successful Oncology Clinical Trials
Health Care Providers:
 Health Care Providers: 1. What is SERVE-HF? SERVE-HF is a multinational, multi-center, randomized controlled trial designed to assess whether treatment of predominantly Central Sleep Apnea with Adaptive
Health Care Providers: 1. What is SERVE-HF? SERVE-HF is a multinational, multi-center, randomized controlled trial designed to assess whether treatment of predominantly Central Sleep Apnea with Adaptive
Novartis announces Phase III STRIVE data published in NEJM demonstrating significant and sustained efficacy of erenumab in migraine prevention
 Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland http://www.novartis.com MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG Novartis announces Phase III STRIVE data
Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland http://www.novartis.com MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG Novartis announces Phase III STRIVE data
