Bisphosphonate Step Therapy Criteria
|
|
|
- Bartholomew Harrison
- 6 years ago
- Views:
Transcription
1 ϯ ϯ ϯ A Division of Health Care Service Corporation, a Mutual Legal Reserve Company, an Independent Licensee of the Blue Cross and Blue Shield Association Bisphosphonate Step Therapy Criteria Program may be implemented with the following options: 1) Option 1: generic before brand bisphosphonate OR 2) Option 2: generic before preferred brand before nonpreferred brand bisphosphonate For BlueCross BlueShield of Illinois, BlueCross BlueShield of New Mexico, BlueCross BlueShield of Oklahoma and BlueCross BlueShield of Texas, Option 1 (1-step step therapy) will apply. Boniva Injection will NOT be included in this step therapy program for BlueCross BlueShield of Illinois, BlueCross BlueShield of New Mexico, BlueCross BlueShield of Oklahoma and BlueCross BlueShield of Texas because these plans do not cover injectable bisphosphonates under the pharmacy benefit. Brand Generic Dosage Form Actonel risedronate oral tablets Actonel with Calcium risedronate/calcium carbonate oral tablets Boniva ibandronate oral tablets, injection Fosamax alendronate oral tablets a, oral solution Fosamax Plus D alendronate/cholecalciferol oral tablets a generic available HCSC_CS_S_Bisphosphonates_ST_0809_r0909.doc Page 1 of 6
2 FDA APPROVED INDICATIONS The following information is taken from individual drug prescribing information and is provided here as background information only. Not all FDA-approved indications may be considered medically necessary. All criteria are found in the section Prior Authorization Criteria for Approval. Table 1: FDA Approved Indications 1-6 Available Products Prevention of in Postmenopausal women Treatment of Postmenopausal women FDA Indications 1-6 Treatment to Increase Bone Mass in Men with Treatment of Paget s Disease Prevention of Glucocorticoi d Induced Treatment of Glucocorticoid Induced Actonel (risedronate Actonel w/ Calcium (risedronate tablets/ calcium carbonate Boniva (ibandronate Boniva (ibandronate injection) Fosamax (alendronate tablets, solution) Fosamax Plus D (alendronate/ cholecalciferol IU=International Units, mg=milligrams, ml=milliliters RATIONALE FOR STEP THERAPY The intent of the step therapy option of the bisphosphonate program is to encourage the use of generic alendronate while accommodating for the use of brand bisphosphonate agents for the treatment of osteoporosis when generic alendronate cannot be used due to allergy, intolerance, or contraindication. The bisphosphonates included in the step edit are those agents approved for treatment or prevention of osteoporosis in men or women. Generic alendronate is available in all tablet strengths. Fosamax Plus D and Actonel with Calcium may be included as target agents in the step edit because both calcium and vitamin D are available over-the-counter. The step therapy program for bisphosphonate agents has been developed with the opportunity to implement one of two options. (option 1): A one step edit that requires therapy with a generic bisphosphonate before therapy with brand agent. (option 2): A two step edit that requires therapy with a generic bisphosphonate before a preferred brand and both a generic and a preferred brand agent before use of a nonpreferred brand bisphosphonate agent. Guidelines for the treatment of osteoporosis recommend a bisphosphonate agent as first-line treatment (see Formulary Chapter 4.9A /Calcium Regulators/ Agents). 7 Alendronate and risedronate are both consistently mentioned as appropriate choices for initial treatment. 7 None of the available guidelines include the newer bisphosphonate agent, ibandronate, which was approved for marketing after development of the guidelines. Outcome studies evaluating the efficacy of alendronate and risedronate in post-menopausal women have been published 7,8 A summary of the outcomes for alendronate and risedronate are provided in Table 2. Data in this table was published before the approval of ibandronate. HCSC_CS_S_Bisphosphonates_ST_0809_r0909.doc Page 2 of 6
3 Table 2. Fracture Outcomes 7,8 Condition Primary Prevention of Vertebral Fractures Secondary Prevention of Vertebral Fractures Risedronate (Actonel) No (ARR 5%-7.4%) Alendronate (Fosamax) (ARR 1.7%) (ARR 8.0%) Primary Prevention of Hip Fractures No No Secondary Prevention of Hip Fractures Prevention of Glucocorticoid Induced Vertebral Fractures ARR=absolute risk reduction (ARR 1.4%) (ARR 11%) (ARR 1.1%) (ARR 6.1%) Approval of ibandronate for the treatment of osteoporosis by the Food and Drug Administration (FDA) was based on a three-year phase III trial (BONE). 9 Data from the BONE study demonstrated that daily ibandronate, relative to placebo, significantly reduced the 3-year risk for vertebral fracture from 9.6% to 4.7% (p=0.0001). 9 Approval for the prevention of osteoporosis was based on a two-year trial. 9 Data from the osteoporosis prevention study submitted to the FDA demonstrated ibandronate 2.5 mg relative to placebo, increased BMD of the lumbar spine and hip by 3.0% and 2.0% respectively. 10 Data from the MOBILE study, submitted to the FDA for approval of once-monthly dosing, demonstrated that monthly ibandronate had at least an equal increase in BMD of the lumbar spine and hip compared to the daily administration of ibandronate. 11 Women treated with the 150 mg/month dose compared to Boniva 2.5 mg daily had the greatest increase in BMD. 11 ELECTRONIC EDITS The bisphosphonate program may be implemented as one of two step therapy options: Option 1: a one step edit that requires therapy with a generic bisphosphonate agent within 90 days prior to a brand claim Option 2: a two step edit that requires prior use of a generic bisphosphonate agent within 90 days prior to payment for a preferred brand and use of both a generic and a preferred brand bisphosphonate agent within 180 days prior to a nonpreferred brand claim. The overall process for a step therapy edit requires that another drug or drugs be tried within a specified time period prior to the claim drug. If the patient has met any of the requirements outlined below, the requested step therapy medication will be paid under the patient s current prescription benefit. If the patient does not meet the step therapy criteria, then the system will reject with the message indicating that prior authorization is necessary. The prior authorization (PA) criteria for approval would then be applied to requests submitted by the patient s practitioner for evaluation. Option 1: Generic Before Brand Edit The implementation of this option encourages the use of cost-effective generic bisphosphonate agent prior to use of any brand bisphosphonate. If the patient has a medication history including generic alendronate with a days supply beginning or ending within the 90 day look back parameter, the claim will automatically pay. Claims for brand bisphosphonates will also automatically pay if the patient s medication history contains evidence of the identical brand bisphosphonate within 90 days prior to the new claim. Approval of these agents if previous use is identified assures no disruption of therapy for those patients already stabilized on the medication. The 90-day search period was chosen to capture the most recent or current therapy for one preferred agent. The claims system is designed to identify and count any prerequisite drug claim with a days supply that begins or ends in the 90-day look-back parameter. If a generic bisphosphonate is not found, the Point of Sale message will be returned to the pharmacy stating the step is not met and PA is required. The Prior Authorization Criteria for Approval would then be applied to requests submitted by the patient s practitioner for evaluation. HCSC_CS_S_Bisphosphonates_ST_0809_r0909.doc Page 3 of 6
4 Option 2: Generic before Preferred Brand before Nonpreferred Brand The implementation of this option encourages the use of a cost-effective generic bisphosphonate prior to use of preferred brand agents, and the use of both a generic bisphosphonate and a preferred brand bisphosphonate prior to use of a nonpreferred brand bisphosphonate. This step edit option has been designed as two edits; one for preferred brand bisphosphonates and one for nonpreferred brand bisphosphonates. In order for a claim for a preferred brand bisphosphonate to pay automatically, the patient must have medication history of a previous claim for a generic bisphosphonate within 90 days prior to the current brand bisphosphonate claim. In order for a claim for nonpreferred brand bisphosphonate to pay automatically, the patient must have medication history of both a previous claim for a generic bisphosphonate and a claim for a preferred brand bisphosphonate within 180 days prior to the new claim for a nonpreferred brand bisphosphonate. Claims for either preferred or nonpreferred brand bisphosphonates will also automatically pay if the patient s medication history contains evidence of the identical brand bisphosphonate within 90 days prior to the new claim. Approval of these agents if previous use is identified assures no disruption of therapy for those patients already stabilized on the medication. The 90-day search period was chosen to capture the most recent or current therapy for one preferred agent; the 180-day search period is longer to review claims history for two preferred therapies. If the step therapy edit is not met, a Point of Sale message will be returned to the pharmacy stating the step is not met and prior authorization is required. The Prior Authorization (PA) Criteria for Approval would then be applied to requests submitted by the patient s practitioner for evaluation. Table 3: Summary of Bisphosphonate Step Therapy OPTION 1 BRAND OPTION 2 PREFERRED BRAND Targeted Agent(s) Brand bisphosphonate or Preferred brand bisphosphonate - Determined by client GPI ******** multisource code M,N, or O Is auto-grandfathering implemented? (lookback time frame) Prerequisite Agent(s) set up at drug or GPI 10 level (90 days a ) Generic bisphosphonate GPI ******** and MSC Y Number of 1 2 prerequisites required Prerequisite look-back 90 days a 180 days b time frame Age-related edit? No No Additional comments OPTION 2 NONPREFERRED BRAND Nonpreferred brand bisphosphonate - Determined by client GPI ******** multisource code M,N, or O set up at drug or GPI 10 level (90 days a ) Generic bisphosphonate GPI ******** and MSC Y AND Preferred brand bisphosphonate - Determined by client GPI ******** multisource code M,N, or O set up at drug or GPI 10 level The injectable (IV) dosage form of Boniva is not included in this step therapy program. a - The system searches for a claim with a days supply that begins or ends in the past 90 days. For claims with a 30 day supply the system would be able to identify a claim processed for payment between 1 and 120 days prior to the new claim. For claims that are dispensed as an extended days supply (90 days), the system would identify a claim processed between 1 and 180 days. b - The system searches for two claims with a days supply that begins or ends in the past 180 days. For claims with a 30 day supply the system would be able to identify a claim processed for payment between 1 and 210 days prior to the new claim. For claims that are dispensed as an extended days supply (90 days), the system would identify a claim processed between 1 and 270 days prior to the new claim. HCSC_CS_S_Bisphosphonates_ST_0809_r0909.doc Page 4 of 6
5 PRIOR AUTHORIZATION CRITERIA FOR APPROVAL The intent of the PA Criteria for Approval for step therapy is to allow for approval of brand bisphosphonate agents under step therapy option 1 when the patient s medication history indicates prior generic alendronate tablets not identified by the electronic claims history edit. The PA criteria also allows for use of brand agents if the patient has allergies, intolerance, or contraindication to the use of generic alendronate. The PA Criteria for Approval for step therapy implementing option 2 allows approval of preferred brand agents when the patient s medication history indicates prior generic alendronate tablets not identified by the electronic claims history. The PA criteria also allows for use of preferred brand agents if the patient has allergies, intolerance, or contraindication to the use of generic alendronate. The PA Criteria for Approval for step therapy option 2 also allows approval of nonpreferred brand agents when there is claims history evidence or medical history evidence that both generic and preferred agents have been previously tried. The PA criteria also allows for use of nonpreferred brand agents if the patient has allergies, intolerance, or contraindication to the use of generic alendronate or brand preferred agents. The PA Criteria for Approval will also approve any brand bisphosphonate when the patient is currently receiving and stabilized on the brand agent. The PA length of approval has been set at twelve months to allow for changes in preferred and nonpreferred formulary status. Step Therapy PA Criteria for Approval Bisphosphonate Step Therapy Option 1 Initial and Renewal Evaluation 1. Has the Bisphosphonate Step Therapy criteria been implemented with a 1-step option (Option 1)? If yes, continue to 2. If no, go to appropriate criteria question set. 2. Is the patient currently being treated with the requested brand bisphosphonate? If yes, approve for 12 months. If no, continue to Does the patient s medication history indicate previous use of the generic bisphosphonate alendronate? If yes, approve for 12 months. If no, continue to Does the patient have an allergy, contraindication, or intolerance to generic alendronate? If yes, approve for 12 months. If no, deny. Bisphosphonate Step Therapy Option 2 Initial and Renewal Evaluation 1. Has the Bisphosphonate Step Therapy criteria been implemented with a 2-step option (Option 2)? If yes, continue to 2. If no, go to appropriate criteria question set. 2. Is the patient currently being treated with the requested brand bisphosphonate? If yes, approve for 12 months. If no, continue to What drug is requested? a. generic bisphosphonate b. preferred brand bisphosphonate c. nonpreferred brand bisphosphonate If a, review is not applicable. Claim will adjudicate. If b, continue to 4. If c, continue to Has the patient tried and failed the generic bisphosphonate alendronate? If yes, approve requested preferred brand for 12 months. If no, continue to Does the patient have an allergy, contraindication, or intolerance to the generic bisphosphonate alendronate? If yes, approve requested preferred brand for 12 months. If no, deny. 6. Has the patient tried and failed the generic bisphosphonate alendronate? If yes, continue to 8. If no, continue to 7. HCSC_CS_S_Bisphosphonates_ST_0809_r0909.doc Page 5 of 6
6 7. Does the patient have an allergy, contraindication, or intolerance to the generic bisphosphonate alendronate? If yes, continue to 8. If no, deny. 8. Has the patient tried and failed a preferred brand bisphosphonate? If yes, approve requested nonpreferred brand agent for 12 months. If no, continue to Does the patient have an allergy, contraindication, or intolerance to a preferred brand bisphosphonate? If yes, approve requested nonpreferred brand agent for 12 months. If no, deny. SUMMARY The intent of the step therapy edit for the Bisphosphonate Step Therapy Criteria is to encourage use of the generic bisphosphonate alendronate before brand products and to accommodate for the use of brand agents when generic alendronate cannot be used due to allergy, intolerance, or contraindication. The criteria have been designed with the option of implementing a one step edit requiring generic alendronate before brand agents or a two step edit requiring a generic before a preferred brand and both generic alendronate and preferred brand before a nonpreferred brand agent. The edit also allows automatic payment of claims for a brand bisphosphonate when a patient is already stabilized on the agent. The bisphosphonates included in the step edit are those agents approved for treatment or prevention of osteoporosis in men or women. Generic alendronate is available in all tablet strengths. Fosamax Plus D and Actonel with Calcium will be included as target agents in the step edit because both calcium and vitamin D are available over-the-counter. REFERENCES 1. Actonel prescribing information. Sanofi-Aventis U.S. LLC/Proctor & Gamble Pharmaceuticals, Inc. April Actonel with Calcium prescribing information. Sanofi-Aventis U.S. LLC/Proctor & Gamble Pharmaceuticals, Inc. February Boniva prescribing information. Roche Laboratories, Inc. November Boniva Injection prescribing information. Roche Laboratories, Inc. February Fosamax prescribing information. Merck & Co., Inc. February Fosamax Plus D prescribing information. Merck & Co., Inc. February Prime Therapeutics Formulary Chapter 4.9A Calcium Regulators/ Agents July Wells G, Cranney A, Peterson J, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Review 2008 Jan 23(1):CD Cooper C, Emkey RD, McDonald, et al. Oral ibandronate osteoporosis vertebral fracture trial in North America and Europe (BONE). [Study #4411 in FDA Medical Review]. FDA Medical Review Application # Boniva Oct Accessed also published in J Bone Miner Res 2004;19(8): McClung MR, Wasnich RD, Recker R, et al. Oral daily ibandronate prevents bone loss in early menopausal women without osteoporosis. J Bone Miner Res 2004;19: Miller PD, McClung, Macovei L, et al. Monthly oral ibandronate therapy in post-menopausal osteoporosis: 1 year results from the MOBILE study. J Bone Miner Res 2005;20(8): Document History Criteria developed from PS_Fosamax_ST/QL_0208, PS_Bisphosphonate_QL_AR1107 approved by P & T UM Committee 08/2008 Criteria changed to Bisphosphonate Step Therapy, approved by P & T: UM Committee 02/2009 Initial Client Review, Client Specific Criteria, approved by HCSC Corporate Clinical Committee, 08/2009 Administrative Addition, revision to textbox to indicate Boniva injection is not included in program 09/2009 HCSC_CS_S_Bisphosphonates_ST_0809_r0909.doc Page 6 of 6
Clinical Policy: Abaloparatide (Tymlos) Reference Number: CP.CPA.306 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE
 CASES AUTH/1803/2/06 and AUTH/1804/2/06 PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE Bonviva Once Monthly slide kits Procter & Gamble and Sanofi-Aventis complained jointly about two
CASES AUTH/1803/2/06 and AUTH/1804/2/06 PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE Bonviva Once Monthly slide kits Procter & Gamble and Sanofi-Aventis complained jointly about two
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Lipid Management Step Therapy Criteria with Medical Diagnoses Option*
 Lipid Management Step Therapy Criteria with Medical Diagnoses Option* * Medical diagnoses are required for implementation of this option. Program may be implemented with the following options: Option One
Lipid Management Step Therapy Criteria with Medical Diagnoses Option* * Medical diagnoses are required for implementation of this option. Program may be implemented with the following options: Option One
4.7 Studies of Quality Holy Cross Hospital Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017
 4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: HIM.PA.51 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this policy for
Clinical Policy: Reference Number: HIM.PA.51 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this policy for
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 ACTONEL 5 mg, film-coated tablet B/14 (CIP code: 354 362-3) ACTONEL 30 mg, film-coated tablet B/28 (CIP
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 ACTONEL 5 mg, film-coated tablet B/14 (CIP code: 354 362-3) ACTONEL 30 mg, film-coated tablet B/28 (CIP
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Actonel, Atelvia) Reference Number: CP.PMN.100 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the
Clinical Policy: (Actonel, Atelvia) Reference Number: CP.PMN.100 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
FORTEO (teriparatide) INJECTION
 FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
PROLIA: Medical Coverage Policy Denosumab (Prolia and. Xgeva) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS
 New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
Adherence with Oral Bisphosphonate Therapy for Osteoporosis Among Patients in Canadian Clinical Practice. Not for Sale or Commercial Distribution
 Adherence with Oral Bisphosphonate Therapy for Osteoporosis Among Patients in Canadian Clinical Practice Nader Habib, MD Heather McDonald-Blumer, MD Michele Moss, MBChB, MCFP Angèle Turcotte, MD Copyright
Adherence with Oral Bisphosphonate Therapy for Osteoporosis Among Patients in Canadian Clinical Practice Nader Habib, MD Heather McDonald-Blumer, MD Michele Moss, MBChB, MCFP Angèle Turcotte, MD Copyright
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES. SEDATIVE HYPNOTIC AGENTS Generic Brand HICL GCN Exception/Other ZOLPIDEM
 Generic Brand HICL GCN Exception/Other ZOLPIDEM AMBIEN 07842 GENERIC IS UNRESTRICTED TARTRATE AMBIEN CR EDLUAR INTERMEZZO ZOLPIMIST ESZOPICLONE LUNESTA 26791 GENERIC IS UNRESTRICTED RAMELTEON ROZEREM 33126
Generic Brand HICL GCN Exception/Other ZOLPIDEM AMBIEN 07842 GENERIC IS UNRESTRICTED TARTRATE AMBIEN CR EDLUAR INTERMEZZO ZOLPIMIST ESZOPICLONE LUNESTA 26791 GENERIC IS UNRESTRICTED RAMELTEON ROZEREM 33126
Tymlos (abaloparatide)
 Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
Bisphosphonate treatment break
 Bulletin 110 December 2015 Bisphosphonate treatment break Bisphosphonates have been widely used in the treatment of osteoporosis with robust data demonstrating efficacy in fracture risk reduction over
Bulletin 110 December 2015 Bisphosphonate treatment break Bisphosphonates have been widely used in the treatment of osteoporosis with robust data demonstrating efficacy in fracture risk reduction over
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
Clinician s Guide to Prevention and Treatment of Osteoporosis
 Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis
 Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis Miriam Silverberg A. Study Purpose and Rationale More than 70% of fractures in people after the age of
Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis Miriam Silverberg A. Study Purpose and Rationale More than 70% of fractures in people after the age of
Prior Authorization Required: Yes as shown below
 PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Osteoporosis in Men Wendy Rosenthal PharmD. This program has been brought to you by PharmCon
 Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Comparison of the efficacy of three once-weekly bisphosphonates on bone mineral density gains in Korean women
 Original Article Obstet Gynecol Sci 2013;56(3):176-181 http://dx.doi.org/10.5468/ogs.2013.56.3.176 pissn 2287-8572 eissn 2287-8580 Comparison of the efficacy of three once-weekly bisphosphonates on bone
Original Article Obstet Gynecol Sci 2013;56(3):176-181 http://dx.doi.org/10.5468/ogs.2013.56.3.176 pissn 2287-8572 eissn 2287-8580 Comparison of the efficacy of three once-weekly bisphosphonates on bone
Osteoporosis medication prescribing in British Columbia and Ontario: impact of public drug coverage
 Osteoporos Int (212) 23:1475 148 DOI 1.17/s198-11-1771-2 ORIGINAL ARTICLE Osteoporosis medication prescribing in British Columbia and Ontario: impact of public drug coverage S. M. Cadarette & G. Carney
Osteoporos Int (212) 23:1475 148 DOI 1.17/s198-11-1771-2 ORIGINAL ARTICLE Osteoporosis medication prescribing in British Columbia and Ontario: impact of public drug coverage S. M. Cadarette & G. Carney
Pharmacy Management Drug Policy
 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Prevention of Osteoporotic Hip Fracture
 Prevention of Osteoporotic Hip Fracture Dr Law Sheung Wai 8th July 2007 Associate Consultant Spine team / Orthopedic Rehabilitation Department of Orthopedics and Traumatology NTE Cluster 1 Objectives Problems
Prevention of Osteoporotic Hip Fracture Dr Law Sheung Wai 8th July 2007 Associate Consultant Spine team / Orthopedic Rehabilitation Department of Orthopedics and Traumatology NTE Cluster 1 Objectives Problems
Guidelines for the Pharmaceutical Management of Osteoporosis in Adult WA Public Hospitals
 WA.DRUG EVALUATION PANEL Guidelines for the Pharmaceutical Management of Osteoporosis in Adult WA Public Hospitals Introduction Osteoporotic fracture-related hospitalisations impose a substantial financial
WA.DRUG EVALUATION PANEL Guidelines for the Pharmaceutical Management of Osteoporosis in Adult WA Public Hospitals Introduction Osteoporotic fracture-related hospitalisations impose a substantial financial
Improving Compliance and Persistence with Bisphosphonate Therapy for Osteoporosis
 The American Journal of Medicine (2006) Vol 119 (4A), 18S-24S Improving Compliance and Persistence with Bisphosphonate Therapy for Osteoporosis Ronald D. Emkey, MD, a Mark Ettinger, MD a,b a Radiant Research,
The American Journal of Medicine (2006) Vol 119 (4A), 18S-24S Improving Compliance and Persistence with Bisphosphonate Therapy for Osteoporosis Ronald D. Emkey, MD, a Mark Ettinger, MD a,b a Radiant Research,
[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.
![[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4. [If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.](/thumbs/86/94943423.jpg) Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
3. Has bone specific alkaline phosphatase level increased OR does the member have symptoms related to active Paget s?
 Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above
 Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above 2 or > vertebral fractures Low trauma fracture In past 5 years Risk Factors (table1)
Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above 2 or > vertebral fractures Low trauma fracture In past 5 years Risk Factors (table1)
Role of alendronate and risedronate in preventing and treating osteoporosis
 REVIEW MARGARET L. PETERS, PharmD Department of Pharmacy, The Cleveland Clinic MANDY LEONARD, PharmD Drug information specialist, Department of Pharmacy, The Cleveland Clinic ANGELO A. LICATA, MD, PhD
REVIEW MARGARET L. PETERS, PharmD Department of Pharmacy, The Cleveland Clinic MANDY LEONARD, PharmD Drug information specialist, Department of Pharmacy, The Cleveland Clinic ANGELO A. LICATA, MD, PhD
Application for Endorsement of CQI (Clinical Audit) Activities for MOPS Credits Allocation
 Application for Endorsement of CQI (Clinical Audit) Activities for MOPS Credits Allocation Below is the form you need to fill out. NAME Health Hawke s Bay DATE NOVEMBER 2012 TITLE OF ACTIVITY OSTEOPOROSIS
Application for Endorsement of CQI (Clinical Audit) Activities for MOPS Credits Allocation Below is the form you need to fill out. NAME Health Hawke s Bay DATE NOVEMBER 2012 TITLE OF ACTIVITY OSTEOPOROSIS
Fragile Bones and how to recognise them. Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey
 Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
3-Year Results of a Member and Physician Intervention to Reduce Risk Associated With Glucocorticoid-Induced Osteoporosis in a Health Plan
 RESEARCH 3-Year Results of a Member and Physician Intervention to Reduce Risk Associated With Glucocorticoid-Induced Osteoporosis in a Health Plan Mona M. Chitre, PharmD, CGP, and William Hayes, BS ABSTRACT
RESEARCH 3-Year Results of a Member and Physician Intervention to Reduce Risk Associated With Glucocorticoid-Induced Osteoporosis in a Health Plan Mona M. Chitre, PharmD, CGP, and William Hayes, BS ABSTRACT
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Common Drug Review Pharmacoeconomic Review Report
 Common Drug Review Pharmacoeconomic Review Report October 2015 Drug denosumab (Prolia) Indication Treatment to increase bone mass in men with osteoporosis at high risk for fracture; or who have failed
Common Drug Review Pharmacoeconomic Review Report October 2015 Drug denosumab (Prolia) Indication Treatment to increase bone mass in men with osteoporosis at high risk for fracture; or who have failed
Trends in Glucocorticoid-Induced Osteoporosis Management Among Seniors in Ontario,
 Trends in Glucocorticoid-Induced Osteoporosis Management Among Seniors in Ontario, 1996-2012 Jordan M Albaum 1, Linda E Lévesque 2, Andrea S Gershon 3, Yan Yun Liu 1, Suzanne M Cadarette 1 1 Leslie Dan
Trends in Glucocorticoid-Induced Osteoporosis Management Among Seniors in Ontario, 1996-2012 Jordan M Albaum 1, Linda E Lévesque 2, Andrea S Gershon 3, Yan Yun Liu 1, Suzanne M Cadarette 1 1 Leslie Dan
MOST Operations Manual page 1 MEDICATION INVENTORY
 MOST Operations Manual page 1 TABLE OF CONTENTS MEDICATION INVENTORY 1. Background and rationale... 2 2. Equipment and supplies... 2 3. Detailed measurement procedures... 2 3.1 Drug definition guidelines...
MOST Operations Manual page 1 TABLE OF CONTENTS MEDICATION INVENTORY 1. Background and rationale... 2 2. Equipment and supplies... 2 3. Detailed measurement procedures... 2 3.1 Drug definition guidelines...
Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis
 Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis Balanced data about medications www.rxfacts.org Copyright 2010 by The Alosa Foundation. All rights reserved. www.rxfacts.org
Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis Balanced data about medications www.rxfacts.org Copyright 2010 by The Alosa Foundation. All rights reserved. www.rxfacts.org
Bone Health in Celiac Disease. Partha S. Sinha MD, PhD October 29 th, 2017
 Bone Health in Celiac Disease Partha S. Sinha MD, PhD October 29 th, 2017 No Disclosures Objectives Recognize the mechanisms by which celiac disease can affect bone health Review what diagnostic tests
Bone Health in Celiac Disease Partha S. Sinha MD, PhD October 29 th, 2017 No Disclosures Objectives Recognize the mechanisms by which celiac disease can affect bone health Review what diagnostic tests
New Osteoporosis Guidelines: What you and your health provider need to know QUESTION & ANSWER
 The first of the newsletters on these Qs and As should include a refresher on the Virtual Forums what they are, how they work, etc. The fact is that less than 5% of COPN members tune in for any given Forum.
The first of the newsletters on these Qs and As should include a refresher on the Virtual Forums what they are, how they work, etc. The fact is that less than 5% of COPN members tune in for any given Forum.
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer
 Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against
 This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against Juliet Compston Professor of Bone Medicine University of Cambridge School of Clinical
This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against Juliet Compston Professor of Bone Medicine University of Cambridge School of Clinical
Bone Mass Measurement BONE MASS MEASUREMENT HS-042. Policy Number: HS-042. Original Effective Date: 8/25/2008
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Disclosures. Diagnostic Challenges in Osteoporosis: Whom To Treat 9/25/2014
 Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Osteoporosis and Lupus. Andrew Ruthberg, MD University Rheumatologists
 Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection
 Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection Table of Contents Policy: Commercial Policy History References Coding Information Information Pertaining to All Policies
Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection Table of Contents Policy: Commercial Policy History References Coding Information Information Pertaining to All Policies
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures
 Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Osteoporosis Management
 Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Disclosures. Bisphosphonate Treatment for Osteoporosis: Do the Benefits Outweigh the Risks? Risks vs Benefits. Bisphosphonates: Benefits and Risks
 Bisphosphonate Treatment for steoporosis: Do the Benefits utweigh the Risks? 37 th Annual Advances in Internal Medicine May 18 and June 22, 29 hsponsored resentations Disclosures Eli Lilly & Company, GlaxoSmithKline,
Bisphosphonate Treatment for steoporosis: Do the Benefits utweigh the Risks? 37 th Annual Advances in Internal Medicine May 18 and June 22, 29 hsponsored resentations Disclosures Eli Lilly & Company, GlaxoSmithKline,
Osteoporosis Update. Greg Summers Consultant Rheumatologist
 Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Summary of results. Name of the sponsor. Name of the finished product Name of active ingredient Title of the study
 Summary of results Name of the sponsor Name of the finished product Name of active ingredient Title of the study Study centers: Related publications Study period (Date of obtaining first subject s informed
Summary of results Name of the sponsor Name of the finished product Name of active ingredient Title of the study Study centers: Related publications Study period (Date of obtaining first subject s informed
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents
 AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
Osteoporosis. Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective. Old Definition of Osteoporosis
 Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Biologic Immunomodulators Prior Authorization with Quantity Limit Program Summary
 Biologic Immunomodulators Prior Authorization with Quantity Limit Program Summary Biologic Immunomodulators Prior Authorization with Quantity Limit (with a preferred option) OBJECTIVE The intent of the
Biologic Immunomodulators Prior Authorization with Quantity Limit Program Summary Biologic Immunomodulators Prior Authorization with Quantity Limit (with a preferred option) OBJECTIVE The intent of the
ORILISSA (elagolix) oral tablet
 ORILISSA (elagolix) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
ORILISSA (elagolix) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
Osteoporosis. Definition
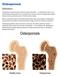 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Spring Understanding the potential of generic substitution
 Spring 2014 Understanding the potential of generic substitution Understanding the potential of generic substitution Generic pricing reforms and the availability of generics for blockbuster drugs coming
Spring 2014 Understanding the potential of generic substitution Understanding the potential of generic substitution Generic pricing reforms and the availability of generics for blockbuster drugs coming
Transmucosal Immediate Release Fentanyl (TIRF) Prior Authorization, (Through Generic), and Quantity Limit Program Summary
 Transmucosal Immediate Release Fentanyl (TIRF) Prior Authorization, (Through Generic), and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-5 Drug Indication Dosage Abstral (fentanyl sublingual
Transmucosal Immediate Release Fentanyl (TIRF) Prior Authorization, (Through Generic), and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-5 Drug Indication Dosage Abstral (fentanyl sublingual
Medication Policy Manual. Topic: Prolia, denosumab Date of Origin: August 11, 2010
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
Medication Prior Authorization Form
 Reclast (Zoledronic Acid) Policy Number: 1050 Policy History Approve Date: 12/11/2015 Revise Dates: 8/22/2016 Next Review: 12/11/2016 Review Dates: Preauthorization All Plans Benefit plans vary in coverage
Reclast (Zoledronic Acid) Policy Number: 1050 Policy History Approve Date: 12/11/2015 Revise Dates: 8/22/2016 Next Review: 12/11/2016 Review Dates: Preauthorization All Plans Benefit plans vary in coverage
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
Bone Health for Women: Current Research, Initiatives and Recommendations
 Page 1 BONE HEALTH FOR WOMEN: CURRENT RESEARCH, INITIATIVES AND RECOMMENDATIONS Dr. Melissa Kagarise This program has been brought to you by PharmCon PharmCon is accredited by the Accreditation Council
Page 1 BONE HEALTH FOR WOMEN: CURRENT RESEARCH, INITIATIVES AND RECOMMENDATIONS Dr. Melissa Kagarise This program has been brought to you by PharmCon PharmCon is accredited by the Accreditation Council
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
Calcium, Vitamin D and Bisphosphonates: Disclosures. Benefits, Risks and Drug Holiday. Calcium YES or NO? Calcium Bad News!!
 Calcium, Vitamin D and Bisphosphonates: Benefits, Risks and Drug Holiday Disclosures I am disclosing financial relationships as follows: Global Advisory Boards: Amgen, Lilly, Merck, Novartis Research grants:
Calcium, Vitamin D and Bisphosphonates: Benefits, Risks and Drug Holiday Disclosures I am disclosing financial relationships as follows: Global Advisory Boards: Amgen, Lilly, Merck, Novartis Research grants:
Osteoporosis Physician Performance Measurement Set. October 2006
 American Academy of Family Physicians/American Academy of Orthopaedic Surgeons/American Association of Clinical Endocrinologists/American College of Rheumatology/The Endocrine Society/Physician Consortium
American Academy of Family Physicians/American Academy of Orthopaedic Surgeons/American Association of Clinical Endocrinologists/American College of Rheumatology/The Endocrine Society/Physician Consortium
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 Metformin tablet SR 24-hour modified release oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
Metformin tablet SR 24-hour modified release oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
TREATING OSTEOPOROSIS IN 2018: WHAT'S OLD, WHAT'S NEW, WHAT'S UNPROVEN AND WHAT'S TRUE. Nelson B. Watts, MD
 TREATING OSTEOPOROSIS IN 2018: WHAT'S OLD, WHAT'S NEW, WHAT'S UNPROVEN AND WHAT'S TRUE Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO Honoraria: Amgen, Radius, Shire Consulting
TREATING OSTEOPOROSIS IN 2018: WHAT'S OLD, WHAT'S NEW, WHAT'S UNPROVEN AND WHAT'S TRUE Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO Honoraria: Amgen, Radius, Shire Consulting
OSTEOPOROSIS MEDICINES
 Bone Basics 2010. NOF. All rights reserved. National Osteoporosis Foundation 1150 17th Street, NW, Suite 850 Washington, DC 20036 (800) 223-9994 www.nof.org OSTEOPOROSIS MEDICINES Although there is no
Bone Basics 2010. NOF. All rights reserved. National Osteoporosis Foundation 1150 17th Street, NW, Suite 850 Washington, DC 20036 (800) 223-9994 www.nof.org OSTEOPOROSIS MEDICINES Although there is no
OSTEOPOROSIS IN MEN. Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO
 OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
