V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM)
|
|
|
- Arlene Marsh
- 6 years ago
- Views:
Transcription
1 V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM) SAFETY INFORMATION ONLY FOR USE WITH THE KCI V.A.C.ULTA THERAPY SYSTEM Rx Only
2
3 TABLE OF CONTTS Important Information for Users... 4 Dressing Systems for Use with V.A.C.ULTA Therapy Unit... 4 Products not Intended for Use with V.A.C. VERAFLO Therapy (Instillation)... 5 Indications for Use... 5 Transitioning V.A.C. Therapy Into Home Care... 6 V.A.C.ULTA Therapy System Contraindications... 6 Additional Contraindications Specific to V.A.C. VERAFLO Therapy... 7 V.A.C.ULTA Therapy System Warnings... 7 Additional Warnings for V.A.C. VERAFLO Therapy V.A.C.ULTA Therapy System Precautions Additional Precautions for V.A.C. VERAFLO Therapy Additional Precautions for the PREVA Incision Management Dressing Additional Precautions for the ABTHERA SSAT.R.A.C. Open Abdomen Dressing Additional Precautions for V.A.C. GRANUFOAM SILVER Dressing Additional Precautions for the KCI Negative Pressure Wound Therapy Gauze Dressing
4 IMPORTANT INFORMATION FOR USERS The V.A.C.ULTA Negative Pressure Wound Therapy System (V.A.C.ULTA Therapy System) is an integrated wound therapy system that can be used for: V.A.C. VERAFLO Therapy (Instillation), which consists of negative pressure wound therapy (V.A.C. Therapy) coupled with controlled delivery and drainage of topical wound irrigation treatment solutions and suspensions over the wound bed. OR V.A.C. Therapy, which consists of negative pressure wound therapy alone. When using V.A.C. VERAFLO Therapy (Instillation), there are important Contraindications, Warnings, and Precautions that should be considered in addition to the Contraindications, Warnings and Precautions for V.A.C. Therapy. Contraindications, Warnings and Precautions specific to V.A.C. VERAFLO Therapy are highlighted in grey throughout the document and are identified by the V.A.C. VERAFLO Therapy symbol to the left of the text. When using V.A.C. Therapy alone, the V.A.C. VERAFLO Therapy Contraindications, Warnings and Precautions are not applicable. IMPORTANT: As with any prescription medical device, failure to consult a physician and carefully read and follow all safety information and application instructions provided with the therapy unit and dressing cartons prior to use may lead to improper product performance and the potential for serious or fatal injury. Do not adjust therapy unit settings or perform therapy application without directions from / or supervision by the clinical caregiver. DRESSING SYSTEMS FOR USE WITH V.A.C.ULTA THERAPY UNIT V.A.C. Therapy can be used with any of the following dressings: V.A.C. GRANUFOAM Dressings V.A.C. GRANUFOAM SILVER Dressings V.A.C. WHITEFOAM Dressings PREVA Incision Management Dressings ABTHERA SSAT.R.A.C. Open Abdomen Dressings KCI Negative Pressure Wound Therapy Gauze Dressing 4
5 V.A.C. VERAFLO Therapy should be delivered with V.A.C. VERAFLO or V.A.C. VERAFLO CLEANSE Dressings. PRODUCTS NOT INTDED FOR USE WITH V.A.C. VERAFLO THERAPY (INSTILLATION) Cellular or accellular bioengineered tissues. V.A.C. GRANUFOAM SILVER Dressings PREVA Incision Management Dressings The ABTHERA SSAT.R.A.C. Open Abdomen Dressing KCI Negative Pressure Wound Therapy Gauze Dressing Refer to the additional warnings and precautions for V.A.C. VERAFLO Therapy. INDICATIONS FOR USE The V.A.C.ULTA Negative Pressure Wound Therapy System is an integrated wound management system that provides Negative Pressure Wound Therapy (V.A.C. Therapy) with an instillation option (V.A.C. VERAFLO Therapy). V.A.C. Therapy in the absence of instillation is intended to create an environment that promotes wound healing by secondary or tertiary (delayed primary) intention by preparing the wound bed for closure, reducing edema, promoting granulation tissue formation and perfusion, and by removing exudate and infectious material. V.A.C. VERAFLO Therapy is indicated for patients who would benefit from vacuum assisted drainage and controlled delivery of topical wound treatment solutions and suspensions over the wound bed. The V.A.C.ULTA Negative Pressure Wound Therapy System with and without instillation is indicated for patients with chronic, acute, traumatic, sub-acute and dehisced wounds, partial-thickness burns, ulcers (such as diabetic, pressure and venous insufficiency), flaps and grafts. V.A.C. Therapy in the absence of instillation may also be used for: The temporary bridging of abdominal wall openings where primary closure is not possible and/or repeat abdominal entries are necessary The management of surgical incisions that continue to drain following closure. 5
6 TRANSITIONING V.A.C. THERAPY INTO HOME CARE The V.A.C.ULTA Therapy System is not intended for home use*. If there is a need to continue V.A.C. Therapy when a patient transitions home, consider using one of the KCI Therapy Systems approved for the post-acute environment, such as: PREVA 125 Therapy Unit ACTIV.A.C. Therapy Unit INFOV.A.C. Therapy Unit V.A.C. ATS Therapy Unit V.A.C. FREEDOM Therapy Unit V.A.C. SIMPLICITY Unit V.A.C.VIA Therapy System Refer to the safety information included with those devices for important information. V.A.C.ULTA THERAPY SYSTEM CONTRAINDICATIONS Do not place foam dressings of the V.A.C.ULTA Therapy System (including both V.A.C. Therapy and V.A.C. VERAFLO Therapy Dressings) directly in contact with exposed blood vessels, anastomotic sites, organs or nerves. NOTE: Refer to Warnings section for additional information concerning Bleeding. V.A.C. Therapy and V.A.C. VERAFLO Therapy are contraindicated for patients with: Malignancy in the wound Untreated osteomyelitis NOTE: Refer to Warnings section for Osteomyelitis information. Non-enteric and unexplored fistulas Necrotic tissue with eschar present NOTE: After debridement of necrotic tissue and complete removal of eschar, V.A.C. Therapy may be used. Sensitivity to silver (V.A.C. GRANUFOAM SILVER Dressing and PREVA Incision Management Dressings only) * In France, the V.A.C.ULTA Therapy System (P/N HCULTDEV01/FR) may be used in the HAD Healthcare System. 6
7 ADDITIONAL CONTRAINDICATIONS SPECIFIC TO V.A.C. VERAFLO THERAPY Do not use V.A.C. Dressings with Octenisept *, hydrogen peroxide or solutions that are alcohol-based or contain alcohol. Do not deliver fluids to the thoracic or abdominal cavity due to the potential risk to alter core body temperature and the potential for fluid retention within the cavity. Do not use V.A.C. VERAFLO Therapy unless the wound has been thoroughly explored due to the potential for inadvertent instillation of topical wound solutions to adjacent body cavities. *Not available in the United States. Brand name referenced is not a trademark of KCI, its affiliates and / or licensors. V.A.C.ULTA THERAPY SYSTEM WARNINGS Bleeding: With or without using V.A.C. Therapy or V.A.C. VERAFLO Therapy, certain patients are at high risk of bleeding complications. The following types of patients are at increased risk of bleeding, which, if uncontrolled, could be potentially fatal. Patients who have weakened or friable blood vessels or organs in or around the wound as a result of, but not limited to: Suturing of the blood vessel (native anastamoses or grafts) / organ Infection Trauma Radiation Patients without adequate wound hemostasis Patients who have been administered anticoagulants or platelet aggregation inhibitors Patients who do not have adequate tissue coverage over vascular structures. If V.A.C. Therapy or V.A.C. VERAFLO Therapy is prescribed for patients who have an increased risk of bleeding complications, they should be treated and monitored in a care setting deemed appropriate by the treating physician. If active bleeding develops suddenly or in large amounts during V.A.C. Therapy or V.A.C. VERAFLO Therapy, or if frank (bright red) blood is seen in the tubing or in the canister, immediately stop therapy, leave dressing in place, take measures to stop the bleeding, and seek immediate medical assistance. The V.A.C.ULTA Therapy Unit and dressings (both V.A.C. Therapy and V.A.C. VERAFLO Therapy) should not be used to prevent, minimize or stop vascular bleeding. 7
8 Protect Vessels and Organs: All exposed or superficial vessels and organs in or around the wound must be completely covered and protected prior to the administration of V.A.C. Therapy or V.A.C. VERAFLO Therapy. Always ensure that V.A.C. Foam Dressings and V.A.C. VERAFLO Foam Dressings do not come in direct contact with vessels or organs. Use of a thick layer of natural tissue should provide the most effective protection. If a thick layer of natural tissue is not available or is not surgically possible, multiple layers of fine-meshed, non-adherent material may be considered as an alternative, if deemed by the treating physician to provide a complete protective barrier. If using non-adherent materials, ensure that they are secured in a manner as to maintain their protective position throughout therapy. Consideration should also be given to the negative pressure setting and therapy mode used when initiating therapy. Caution should be taken when treating large wounds that may contain hidden vessels, which may not be readily apparent. The patient should be closely monitored for bleeding in a care setting deemed appropriate by the treating physician. Infected Blood Vessels: Infection may erode blood vessels and weaken the vascular wall which may increase susceptibility to vessel damage through abrasion or manipulation. Infected blood vessels are at risk of complications, including bleeding, which, if uncontrolled, could be potentially fatal. Extreme caution should be used when V.A.C. Therapy or V.A.C. VERAFLO Therapy is applied in close proximity to infected or potentially infected blood vessels. (Refer to Protect Vessels and Organs section.) Hemostasis, Anticoagulants and Platelet Aggregation Inhibitors: Patients without adequate wound hemostasis have an increased risk of bleeding, which, if uncontrolled, could be potentially fatal. These patients should be treated and monitored in a care setting deemed appropriate by the treating physician. Caution should be used in treating patients on doses of anticoagulants or platelet aggregation inhibitors thought to increase their risk for bleeding (relative to the type and complexity of the wound). Consideration should be given to the negative pressure setting and therapy mode used when initiating therapy. Hemostatic Agents Applied at the Wound Site: Non-sutured hemostatic agents (for example, bone wax, absorbable gelatin sponge or spray wound sealant) may, if disrupted, increase the risk of bleeding, which, if uncontrolled, could be potentially fatal. Protect against dislodging such agents. Consideration should be given to the negative pressure setting and therapy mode used when initiating therapy. (Refer to Additional Warnings for V.A.C. VERAFLO Therapy section). Sharp Edges: Bone fragments or sharp edges could puncture protective barriers, vessels or organs causing injury. Any injury could cause bleeding, which, if uncontrolled, could be potentially fatal. Beware of possible shifting in the relative position of tissues, vessels or organs within the wound that might increase the possibility of contact with sharp edges. Sharp edges or bone fragments must be eliminated from the wound area or covered to prevent them from puncturing blood vessels or organs before the application of V.A.C. Therapy or V.A.C. VERAFLO Therapy. Where possible, completely smooth and cover any residual edges to decrease the risk of serious or fatal injury, should shifting of structures occur. Use caution when removing dressing components from the wound so that wound tissue is not damaged by unprotected sharp edges. 8
9 1000 ml Canister: DO NOT USE the 1000 ml canister on patients with a high risk of bleeding or on patients unable to tolerate a large loss of fluid volume, including children and the elderly. Consider the size and weight of the patient, patient condition, wound type, monitoring capability and care setting when using this canister. This canister is recommended for acute care (hospital) use only. Infected Wounds: Infected wounds should be monitored closely and may require more frequent dressing changes than non-infected wounds, dependent upon factors such as wound conditions, treatment goals and V.A.C. VERAFLO Therapy parameters (for the V.A.C.ULTA Therapy System). Refer to dressing application instructions (found in V.A.C. Dressing and V.A.C. VERAFLO Dressing cartons) for details regarding dressing change frequency. As with any wound treatment, clinicians and patients / caregivers should frequently monitor the patient s wound, periwound tissue and exudate for signs of infection, worsening infection or other complications. Some signs of infection are fever, tenderness, redness, swelling, itching, rash, increased warmth in the wound or periwound area, purulent discharge or strong odor. Infection can be serious, and can lead to complications such as pain, discomfort, fever, gangrene, toxic shock, septic shock and / or fatal injury. Some signs or complications of systemic infection are nausea, vomiting, diarrhea, headache, dizziness, fainting, sore throat with swelling of the mucus membranes, disorientation, high fever, refractory and / or orthostatic hypotension, or erythroderma (a sunburn-like rash). If there are any signs of the onset of systemic infection or advancing infection at the wound site, contact a physician immediately to determine if V.A.C. Therapy or V.A.C. VERAFLO Therapy should be discontinued. For wound infections relating to blood vessels, please also refer to the section titled Infected Blood Vessels. Infected Wounds with V.A.C. GRANUFOAM SILVER Dressing: In the event of clinical infection, V.A.C. GRANUFOAM SILVER Dressing is not intended to replace the use of systemic therapy or other infection treatment regimens. V.A.C. GRANUFOAM SILVER Dressing may be used to provide a barrier to bacterial penetration. Refer to the section titled Additional Precautions for V.A.C. GRANUFOAM SILVER Dressing. Osteomyelitis: V.A.C. Therapy and V.A.C. VERAFLO Therapy should NOT be initiated on a wound with untreated osteomyelitis. Consideration should be given to thorough debridement of all necrotic, nonviable tissue, including infected bone (if necessary), and appropriate antibiotic therapy. Protect Tendons, Ligaments and Nerves: Tendons, ligaments and nerves should be protected to avoid direct contact with V.A.C. Foam Dressings or V.A.C. VERAFLO Therapy Foam Dressings. These structures may be covered with natural tissue or meshed non-adherent material to help minimize risk of desiccation or injury. Foam Placement: Always use V.A.C. Dressings or V.A.C. VERAFLO Therapy Dressings from sterile packages that have not been opened or damaged. Do not place any foam dressing into blind / unexplored tunnels. The V.A.C. WHITEFOAM Dressing may be more appropriate for use with explored tunnels. The V.A.C. VERAFLO CLEANSE Dressing System may be more appropriate for use with explored tunnels when using V.A.C. VERAFLO Therapy where robust granulation tissue formation is not desired. Do not force foam dressings into any area of the wound, as this may damage tissue, alter the delivery of negative pressure, or hinder exudate and foam removal. Always count the total number of pieces of foam used in the wound and the dressing change date and document that number on the drape, in the patient s chart and on the foam quantity label attached to the pad tubing (if provided). 9
10 Foam Removal: V.A.C. Foam Dressings and V.A.C. VERAFLO Therapy Foam Dressings are not bioabsorbable. Always count the total number of pieces of foam removed from the wound and ensure the same number of foam pieces was removed as placed. Foam left in the wound for greater than the recommended time period may foster ingrowth of tissue into the foam, create difficulty in removing foam from the wound, or lead to infection or other adverse events. If significant bleeding develops, immediately discontinue the use of the V.A.C.ULTA Therapy System, take measures to stop the bleeding, and do not remove the foam dressing until the treating physician or surgeon is consulted. Do not resume the use of the V.A.C. Therapy or V.A.C. VERAFLO Therapy until adequate hemostasis has been achieved and the patient is not at risk for continued bleeding. Keep V.A.C. Therapy and V.A.C. VERAFLO Therapy On: Never leave a V.A.C. Dressing or V.A.C. VERAFLO Therapy Dressing in place without active V.A.C. Therapy or V.A.C. VERAFLO Therapy for more than two hours. If therapy is off for more than two hours, remove the old dressing and irrigate the wound. Either apply a new V.A.C. Dressing or V.A.C. VERAFLO Therapy Dressing from an unopened sterile package and restart therapy; or apply an alternative dressing at the direction of the treating clinician. Acrylic Adhesive: The V.A.C. Drape (supplied with V.A.C. Dressings) and the V.A.C. Advanced Drape (supplied with V.A.C. VERAFLO Therapy Dressings) have an acrylic adhesive coating, which may present a risk of an adverse reaction in patients who are allergic or hypersensitive to acrylic adhesives. If a patient has a known allergy or hypersensitivity to such adhesives, do not use the V.A.C.ULTA Therapy System. If any signs of allergic reaction or hypersensitivity develop, such as redness, swelling, rash, urticaria or significant pruritus, discontinue use and consult a physician immediately. If bronchospasm or more serious signs of allergic reaction appear, seek immediate medical assistance. Defibrillation: Remove the V.A.C. Dressing or V.A.C. VERAFLO Therapy Dressing if defibrillation is required in the area of dressing placement. Failure to remove the dressing may inhibit transmission of electrical energy and / or patient resuscitation. Magnetic Resonance Imaging (MRI) Therapy Unit: The V.A.C.ULTA Therapy Unit is MR Unsafe. Do not take the V.A.C.ULTA Therapy Unit into the MR environment. Magnetic Resonance Imaging (MRI) V.A.C. Dressings: V.A.C. Dressings and V.A.C. VERAFLO Therapy Dressings can typically remain on the patient with minimal risk in an MR environment, assuming that use of the V.A.C.ULTA Therapy System is not interrupted for more than two hours (refer to Keep V.A.C. Therapy and V.A.C. VERAFLO Therapy On above). NOTE: If using V.A.C. VERAFLO Therapy ensure that irrigation fluid or treatment solutions are fully removed from the dressing prior to stopping negative pressure wound therapy. The V.A.C. GRANUFOAM SILVER Dressing has been shown to pose no known hazards in an MR environment with the following conditions of use: Static magnetic field of 3 Tesla or less, Spatial gradient field of 720 Gauss / cm or less, and Maximum whole-body-averaged specific absorption rate (SAR) of 3 W / kg for 15 minutes of scanning 10
11 Non-clinical testing under these same conditions produced a temperature rise of <0.4 C. MR image quality may be compromised if the area of interest is in the same area or relatively close to the position of the V.A.C. GRANUFOAM SILVER Dressing. Hyperbaric Oxygen Therapy (HBO): Do not take the V.A.C.ULTA Therapy Unit into a hyperbaric oxygen chamber. The V.A.C.ULTA Therapy Unit is not designed for this environment and should be considered a fire hazard. After disconnecting the V.A.C.ULTA Therapy Unit, either (i) replace the V.A.C. Dressing or V.A.C. VERAFLO Therapy Dressing with another HBO compatible material during the hyperbaric treatment, or (ii) cover the unclamped end of the V.A.C. Tubing with dry gauze. For HBO therapy, the V.A.C. Tubing or V.A.C. VERAFLO Therapy Tubing must not be clamped. Never leave a V.A.C. Dressing in place without active V.A.C. Therapy for more than two hours; please refer to the Keep V.A.C. Therapy On section. 11
12 NOTE: If using V.A.C. VERAFLO Therapy ensure that irrigation fluid or treatment solutions are fully removed from the dressing prior to stopping negative pressure wound therapy. ADDITIONAL WARNINGS FOR V.A.C. VERAFLO THERAPY Topical Wound Solutions: Topical wound solutions or suspensions may enter internal body cavities if the wound is open to such cavities. They should not be infused into wounds with unexplored tunnels or unexplored undermining as they may enter into unintended cavities. Pauses in Negative Pressure: Application of V.A.C. VERAFLO Therapy will result in pauses of negative pressure wound therapy, which is not recommended on wounds requiring continuous V.A.C. Therapy. Do not use V.A.C. VERAFLO Therapy over unstable structures, such as unstable chest wall or non-intact fascia, on patients at increased risk of bleeding, on flaps, grafts or wounds with acute enteric fistulae. Bioengineered Tissue: V.A.C. VERAFLO Therapy is not intended for use with cellular or accellular bioengineered tissues. Hemostasis: Patients with difficult or fragile wound hemostasis are at increased risk of bleeding associated with V.A.C. VERAFLO Therapy due to the potential for disruption of clots or dilution of clotting factors. Do not use V.A.C. VERAFLO Therapy where hemostatic agents have been used in the wound bed. Closed Surgical Incisions: DO NOT use V.A.C. VERAFLO Therapy with PREVA Dressings over closed surgical incisions. Instillation may result in pooling of fluid which may result in maceration. Open Abdomen: DO NOT use V.A.C. VERAFLO Therapy with the ABTHERA SSAT.R.A.C. Open Abdomen Dressing over an open abdomen. Potential risks of instillation into the open abdomen include: Instillation of fluid in the abdomen without sufficient fluid recovery may lead to abdominal compartment syndrome. Instillation of fluids in the abdomen that are untested for safety and efficacy with this application could lead to severe hollow viscus and solid organ damage. Instillation of unwarmed fluid in large quantities may lead to hypothermia. 12
13 V.A.C.ULTA THERAPY SYSTEM PRECAUTIONS Standard Precautions: To reduce the risk of transmission of bloodborne pathogens, apply standard precautions for infection control with all patients, per institutional protocol, regardless of their diagnosis or presumed infection status. In addition to gloves, use gown and goggles if exposure to body fluids is likely. Continuous Versus DPC (Dynamic Pressure Control) V.A.C. Therapy: Continuous V.A.C. Therapy is recommended over unstable structures, such as an unstable chest wall or non-intact fascia, in order to help minimize movement and stabilize the wound bed. Continuous therapy is also generally recommended for patients at increased risk of bleeding, fresh flaps and grafts, and wounds with acute enteric fistulae. NOTE: V.A.C. VERAFLO Therapy, due to the controlled delivery of wound irrigation and treatment solutions, provides intermittent V.A.C. Therapy and is not recommended in the above wound types or conditions. Patient Size and Weight: The size and weight of the patient should be considered when prescribing V.A.C. Therapy or V.A.C. VERAFLO Therapy. Infants, children, certain small adults and elderly patients should be closely monitored for fluid loss and dehydration. Also, patients with highly exudating wounds or large wounds in relation to the patient size and weight should be closely monitored, as they have a risk of excessive fluid loss and dehydration. When monitoring fluid output, consider the volume of fluid in both the tubing and canister. Spinal Cord Injury (SCI): In the event an SCI patient experiences autonomic dysreflexia (sudden changes in blood pressure or heart rate in response to stimulation of the sympathetic nervous system), discontinue V.A.C. Therapy or V.A.C. VERAFLO Therapy to help minimize sensory stimulation and seek immediate medical assistance. Bradycardia: To minimize the risk of bradycardia, V.A.C. Therapy and V.A.C. VERAFLO Therapy must not be placed in proximity to the vagus nerve. Enteric Fistulas: Wounds with enteric fistulas require special precautions to optimize V.A.C. Therapy. Refer to V.A.C. Therapy Clinical Guidelines for more detail. V.A.C. Therapy is not recommended if enteric fistula effluent management or containment is the sole goal of therapy. NOTE: V.A.C. VERAFLO Therapy should not be used in the presence of enteric fistula to prevent wound contamination. Protect Periwound Skin: Consider use of a skin preparation product to protect periwound skin. Do not allow foam to overlap onto intact skin. Protect fragile / friable periwound skin with additional V.A.C. Advanced Drape, skin protectant, hydrocolloid or other transparent film. Multiple layers of the V.A.C. Advanced Drape may decrease the moisture vapor transmission rate, which may increase the risk of maceration. If any signs of irritation or sensitivity to the drape, foam or tubing assembly appear, discontinue use and consult treating physician. To avoid trauma to the periwound skin, do not pull or stretch the drape over the foam dressing during drape application. Extra caution should be used for patients with neuropathic etiologies or circulatory compromise. 13
14 Circumferential Dressing Application: Avoid use of circumferential dressings except in the presence of anasarca or excessively weeping extremities, where a circumferential drape technique may be necessary to establish and maintain a seal. Consider using multiple small pieces of V.A.C. Advanced Drape rather than one continuous piece to minimize the risk of decreased distal circulation. Extreme care should be taken not to stretch or pull the drape when securing it, but let it attach loosely and stabilize the edges with an elastic wrap, if necessary. When using circumferential drape applications, it is crucial to systematically and recurrently palpate distal pulses, and assess distal circulatory status. If circulatory compromise is suspected, discontinue therapy, remove dressing and contact treating physician. Pressure Points: Periodically assess and monitor the location of tubing connectors, caps, clamps or other rigid components to ensure they do not create inadvertent pressure points in relation to patient position. V.A.C.ULTA Therapy Unit Pressure Excursions: In rare instances, tubing blockages with the V.A.C.ULTA Therapy Unit may result in brief vacuum excursions to more than 250 mmhg negative pressure. Resolve alarm conditions immediately. Refer to the V.A.C.ULTA Therapy System User Manual or contact your KCI representative for additional information. 14
15 ADDITIONAL PRECAUTIONS FOR V.A.C. VERAFLO THERAPY Suitable Solutions: V.A.C. VERAFLO Therapy is intended for use with V.A.C. VERAFLO Therapy disposables and topical wound treatment solutions and suspensions. Only use solutions or suspensions that are: Indicated for topical wound treatment according to solution manufacturer s instructions for use. Some topical agents may not be intended for extended tissue contact. If in doubt about the appropriateness of using a particular solution for V.A.C. VERAFLO Therapy, contact the solution s manufacturer about its suitability for saturated topical wound exposure. Compatible with V.A.C. Dressings and disposable components. Contact your KCI representative for a list of solutions shown to be compatible with V.A.C. Dressings and disposable components. NOTE: Hypochlorous acid solutions applied frequently at high concentrations can lead to significant material degradation. Consider utilizing concentrations and exposure durations as low as clinically relevant. NOTE: The V.A.C. GRANUFOAM SILVER Dressing is not intended to be used with V.A.C. VERAFLO Therapy because instillation solutions may negatively impact the benefits of the V.A.C. GRANUFOAM SILVER Dressing. KCI Negative Pressure Wound Therapy Gauze Dressing: The KCI Negative Pressure Wound Therapy Gauze Dressing is not intended for use with V.A.C. VERAFLO Therapy. Canister Changes: Monitor fluid level in canisters frequently during use of the V.A.C. VERAFLO Therapy. Frequent canister changes may be necessary depending on volume of fluid instilled and wound exudates. At a minimum the canister should be changed weekly and disposed of according to institutional protocol. 15
16 ADDITIONAL PRECAUTIONS FOR THE PREVA INCISION MANAGEMT DRESSING PREVA Incision Management Dressing: When using the V.A.C.ULTA Therapy Unit as the negative pressure source for the PREVA Incision Management Dressings, refer to the Instructions for Use provided with PREVA Incision Management System for complete safety information, dressing application instructions and the procedure for connection to the V.A.C.ULTA Therapy Unit. The PREVA Incision Management System is intended to manage the environment of surgical incisions that continue to drain following sutured or stapled closure by maintaining a closed environment and removing exudates via the application of negative pressure wound therapy. Before transitioning the patient to home care, the V.A.C.ULTA Therapy Unit must be replaced with one indicated for home care (refer to Transitioning V.A.C. Therapy Into Home Care). ADDITIONAL PRECAUTIONS FOR THE ABTHERA SSAT.R.A.C. OP ABDOM DRESSING ABTHERA SSAT.R.A.C. Open Abdomen Dressing: When using the V.A.C.ULTA Therapy Unit as the negative pressure source for the ABTHERA SSAT.R.A.C. Open Abdomen Dressing, refer to the Instructions for Use provided with the ABTHERA SSAT.R.A.C. Open Abdomen Dressing for complete safety information, dressing application instructions and the procedure for connection to the V.A.C.ULTA Therapy Unit. The ABTHERA SSAT.R.A.C. Open Abdomen Dressing is indicated for temporary bridging of abdominal wall openings where primary closure is not possible and/or repeat abdominal entries are necessary. The intended use of this dressing is in open abdominal wounds with exposed viscera. The intended care setting is a closely monitored area within the acute care hospital, such as the ICU. 16
17 ADDITIONAL PRECAUTIONS FOR V.A.C. GRANUFOAM SILVER DRESSING When using the V.A.C.ULTA Therapy Unit as the negative pressure source for the V.A.C. GRANUFOAM SILVER Dressing, refer to the Instructions for Use provided with the V.A.C. GRANUFOAM SILVER Dressing for complete safety information and dressing application instructions. V.A.C. GRANUFOAM SILVER Dressing may be used in the acute care as well as home settings with a therapy unit indicated for home care (refer to Transitioning V.A.C. Therapy Into Home Care). ADDITIONAL PRECAUTIONS FOR THE KCI NEGATIVE PRESSURE WOUND THERAPY GAUZE DRESSING When using the V.A.C.ULTA Therapy Unit as the negative pressure source for the KCI NPWT Gauze Dressing, refer to the Instructions for Use provided with the KCI NPWT Gauze Dressing for complete safety information and dressing application instructions. The KCI NPWT Gauze Dressing is not intended for use with V.A.C. VERAFLO Therapy. Before transitioning the patient to home care, the V.A.C.ULTA Therapy Unit must be replaced with one indicated for home care (refer to Transitioning V.A.C. Therapy Into Home Care). Additional warnings and precautions apply to certain V.A.C. specialty dressings and V.A.C. Therapy Units. Please refer to the specific product instructions for use prior to use. If there are any questions regarding the proper placement or usage of V.A.C. Therapy, please refer to the V.A.C. Therapy Clinical Guidelines for more detailed instructions or contact your local KCI representative. For additional and most current information, please see KCI s website at (US) or www. kci-medical.com (OUS). 17
18 18
19 19
20 KCI USA, Inc IH 10 West San Antonio, TX USA EC REP KCI Medical Products (UK), Ltd. 11 Nimrod Way Wimborne, Dorset BH21 7SH United Kingdom All trademarks designated herein are proprietary to KCI Licensing, Inc., its affiliates and / or licensors. Copyright 2016 KCI Licensing, Inc. All rights reserved Rev A 10/
V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM)
 V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM) SAFETY INFORMATION ONLY FOR USE WITH THE KCI V.A.C.ULTA THERAPY SYSTEM Rx Only TABLE OF CONTENTS Important Information for
V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM) SAFETY INFORMATION ONLY FOR USE WITH THE KCI V.A.C.ULTA THERAPY SYSTEM Rx Only TABLE OF CONTENTS Important Information for
CLINICAL GUIDE Genadyne A4 & Genadyne A4 XLR8
 CLINICAL GUIDE Genadyne A4 & Genadyne A4 XLR8 Table of Contents Indications for use... 3 Contraindications... 3 Warnings... 4 Bleeding... 4 1000mL Canister Size:... 6 Infected Wounds... 6 Infected Wounds
CLINICAL GUIDE Genadyne A4 & Genadyne A4 XLR8 Table of Contents Indications for use... 3 Contraindications... 3 Warnings... 4 Bleeding... 4 1000mL Canister Size:... 6 Infected Wounds... 6 Infected Wounds
V.A.C. Therapy Safety Information
 Bringing Safety Home V.A.C. Therapy Safety Information Bleeding Precautions Dressing Change Frequency Foam Removal Important Information 2 Prior to use of V.A.C. Therapy System it is important for the
Bringing Safety Home V.A.C. Therapy Safety Information Bleeding Precautions Dressing Change Frequency Foam Removal Important Information 2 Prior to use of V.A.C. Therapy System it is important for the
Introduction. Follow standard infection control precautions
 Patient Handbook 1 TABLE OF CONTENTS 1. Introduction. 2 2. Indications for Use. 3 3. Contraindications... 3 4. Warnings 4 5. Precautions.. 6 6. Patient Information Guide. 6 7. Key Pad Features.. 7 8. Battery
Patient Handbook 1 TABLE OF CONTENTS 1. Introduction. 2 2. Indications for Use. 3 3. Contraindications... 3 4. Warnings 4 5. Precautions.. 6 6. Patient Information Guide. 6 7. Key Pad Features.. 7 8. Battery
V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM)
 V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM) SAFETY INFORMATION AND V.A.C. VERAFLO CLEANSE CHOICE DRESSING SYSTEM APPLICATION INSTRUCTIONS ONLY FOR USE WITH THE KCI V.A.C.ULTA
V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM (V.A.C.ULTA THERAPY SYSTEM) SAFETY INFORMATION AND V.A.C. VERAFLO CLEANSE CHOICE DRESSING SYSTEM APPLICATION INSTRUCTIONS ONLY FOR USE WITH THE KCI V.A.C.ULTA
V.A.C. THERAPY SYSTEM SAFETY INFORMATION AND V.A.C. GRANUFOAM, V.A.C. GRANUFOAM SILVER AND V.A.C. WHITEFOAM DRESSING APPLICATION INSTRUCTIONS
 ENGLISH V.A.C. THERAPY SYSTEM SAFETY INFORMATION AND V.A.C. GRANUFOAM, V.A.C. GRANUFOAM SILVER AND V.A.C. WHITEFOAM DRESSING APPLICATION INSTRUCTIONS ONLY FOR USE WITH KCI V.A.C. THERAPY SYSTEMS V.A.C.
ENGLISH V.A.C. THERAPY SYSTEM SAFETY INFORMATION AND V.A.C. GRANUFOAM, V.A.C. GRANUFOAM SILVER AND V.A.C. WHITEFOAM DRESSING APPLICATION INSTRUCTIONS ONLY FOR USE WITH KCI V.A.C. THERAPY SYSTEMS V.A.C.
V.A.C. THERAPY CLINICAL GUIDELINES. Rx Only A REFERENCE SOURCE FOR CLINICIANS
 V.A.C. THERAPY CLINICAL GUIDELINES A REFERENCE SOURCE FOR CLINICIANS Rx Only This copy supercedes any previous revision. For revision level and contact information, refer to back cover of these guidelines.
V.A.C. THERAPY CLINICAL GUIDELINES A REFERENCE SOURCE FOR CLINICIANS Rx Only This copy supercedes any previous revision. For revision level and contact information, refer to back cover of these guidelines.
V.A.C. Therapy Patient Guide. Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you.
 V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
Introduction. Points to remember:
 Clinical Guidelines TABLE OF CONTENTS 1. Introduction... 3 2. Indications for Use.. 4 3. Contraindications. 5 4. Warnings. 5 5. Precautions 8 6. XLR8 System. 10 7. General Dressing Applications Guidelines..
Clinical Guidelines TABLE OF CONTENTS 1. Introduction... 3 2. Indications for Use.. 4 3. Contraindications. 5 4. Warnings. 5 5. Precautions 8 6. XLR8 System. 10 7. General Dressing Applications Guidelines..
NEGATIVE PRESURE WOUND THERAPY PROGRAM
 NEGATIVE PRESURE WOUND THERAPY PROGRAM WWW.USTOMWOUNDCARE.COM 866-802-0006/901-619-8897 SUPPORT@USTOM.COM TABLE OF CONTENTS 1. 2. 3. 4. To place an order: 1. Please visit our website 2. Please email to
NEGATIVE PRESURE WOUND THERAPY PROGRAM WWW.USTOMWOUNDCARE.COM 866-802-0006/901-619-8897 SUPPORT@USTOM.COM TABLE OF CONTENTS 1. 2. 3. 4. To place an order: 1. Please visit our website 2. Please email to
V.A.C. Therapy for Veterinary Use USER MANUAL
 for veterinary use V.A.C. Therapy for Veterinary Use USER MANUAL TAbLE of CoNTENTS V.A.C. THERAPY RECOMMENDED GUIDELINES...3 INTRODUCTION...3 V.A.C. THERAPY SYSTEM SAFETY INFORMATION FOR VETERINARY USE...4
for veterinary use V.A.C. Therapy for Veterinary Use USER MANUAL TAbLE of CoNTENTS V.A.C. THERAPY RECOMMENDED GUIDELINES...3 INTRODUCTION...3 V.A.C. THERAPY SYSTEM SAFETY INFORMATION FOR VETERINARY USE...4
Vacuumed Assisted Closure
 Vacuumed Assisted Closure Louise Morris Lead Nurse in Tissue Viability Jackie Stephen-Haynes Consultant Nurse and senior Lecturer in Tissue Viability 2009 Aims and Objectives To develop an awareness of
Vacuumed Assisted Closure Louise Morris Lead Nurse in Tissue Viability Jackie Stephen-Haynes Consultant Nurse and senior Lecturer in Tissue Viability 2009 Aims and Objectives To develop an awareness of
Negative Pressure Wound Therapy(NPWT)
 Negative Pressure Wound Therapy(NPWT) Mark Goetcheus BSN, RN, CWON, CFCN, CDE Wound, Ostomy, Limb Preservation & Amputee Services Harborview Medical Center DISCLOSURES Mark Goetcheus, BSN, RN No relevant
Negative Pressure Wound Therapy(NPWT) Mark Goetcheus BSN, RN, CWON, CFCN, CDE Wound, Ostomy, Limb Preservation & Amputee Services Harborview Medical Center DISCLOSURES Mark Goetcheus, BSN, RN No relevant
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur
 VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
Management of Complex Wounds with Vacuum Assisted Closure
 Management of Complex Wounds with Vacuum Assisted Closure Wendy McInnes Vascular / Wound Nurse Practitioner The Queen Elizabeth Hospital, Adelaide, South Australia Treasurer ANZSVN wendy.mcinnes@health.sa.gov.au
Management of Complex Wounds with Vacuum Assisted Closure Wendy McInnes Vascular / Wound Nurse Practitioner The Queen Elizabeth Hospital, Adelaide, South Australia Treasurer ANZSVN wendy.mcinnes@health.sa.gov.au
POLYHEAL MICRO - INSTRUCTIONS FOR USE
 POLYHEAL MICRO - INSTRUCTIONS FOR USE PRODUCT DESCRIPTION PolyHeal Micro is a medical device indicated for the treatment of wounds. It is comprised of a suspension of polystyrene negatively charged microspheres
POLYHEAL MICRO - INSTRUCTIONS FOR USE PRODUCT DESCRIPTION PolyHeal Micro is a medical device indicated for the treatment of wounds. It is comprised of a suspension of polystyrene negatively charged microspheres
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Patient-Centric Wound Care: Case-Based Journeys with Leading-Edge Modalities
 Patient-Centric Wound Care: Case-Based Journeys with Leading-Edge Modalities Christopher L. Barrett, DPM, CWS, FAPWCA Director - The Centers for Wound Healing Springfield Hospital Springfield, PA Delaware
Patient-Centric Wound Care: Case-Based Journeys with Leading-Edge Modalities Christopher L. Barrett, DPM, CWS, FAPWCA Director - The Centers for Wound Healing Springfield Hospital Springfield, PA Delaware
For optimal incision management. The PREVENA Therapy portfolio of closed incision management solutions.
 For optimal incision management. The PREVENA Therapy portfolio of closed incision management solutions. The PREVENA Incision Management System manages the surgical incision by: Helping to hold incision
For optimal incision management. The PREVENA Therapy portfolio of closed incision management solutions. The PREVENA Incision Management System manages the surgical incision by: Helping to hold incision
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine
 Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine Dr. Julian Vitse, Montellier University Hospital, France Negative Pressure Wound
Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine Dr. Julian Vitse, Montellier University Hospital, France Negative Pressure Wound
Appropriate Dressing Selection For Treating Wounds
 Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
Electrodes (for TransAeris System)
 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used
 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
V.A.C. VeraFlo Therapy can help.
 WOUND CARE IS COMPLEX & COSTLY Therapy can help. PROBLEM Complex wounds pose challenges to clinicians and patients in terms of achieving desired outcomes while managing healthcare costs. 1-5 OUTCOMES &
WOUND CARE IS COMPLEX & COSTLY Therapy can help. PROBLEM Complex wounds pose challenges to clinicians and patients in terms of achieving desired outcomes while managing healthcare costs. 1-5 OUTCOMES &
11/5/2012. Ron Silverman, MD, FACS Clinical Associate Professor of Surgery University of Maryland Chief Medical Officer, KCI.
 V.A.C.Ulta Negative Pressure Wound Therapy System Ron Silverman, MD, FACS Clinical Associate Professor of Surgery University of Maryland Chief Medical Officer, KCI Chris Lessing, BS, PhD Sr. Scientist,
V.A.C.Ulta Negative Pressure Wound Therapy System Ron Silverman, MD, FACS Clinical Associate Professor of Surgery University of Maryland Chief Medical Officer, KCI Chris Lessing, BS, PhD Sr. Scientist,
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology
 The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Chapter 14 8/23/2016. Surgical Wound Care. Wound Classifications. Wound Healing. Classified According to. Phases
 Chapter 14 Surgical Wound Care All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Wound Classifications Classified According to Cause Incision
Chapter 14 Surgical Wound Care All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Wound Classifications Classified According to Cause Incision
FOR HOSPITAL PHARMACY USE ONLY
 V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM FOR HOSPITAL PHARMACY USE ONLY Use of Topical Wound Solutions with the V.A.C.ULTA Negative Pressure Wound Therapy System This information should be used
V.A.C.ULTA NEGATIVE PRESSURE WOUND THERAPY SYSTEM FOR HOSPITAL PHARMACY USE ONLY Use of Topical Wound Solutions with the V.A.C.ULTA Negative Pressure Wound Therapy System This information should be used
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
Basic Dressing Categories
 Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
Skin Integrity and Wound Care
 Skin Integrity and Wound Care By Dr. Amer Hasanien & Dr. Ali Saleh Skin Integrity and Wound Care Skin integrity: the presence of normal Skin & Uninterrupted skin layers by wounds. Factors affecting appearance
Skin Integrity and Wound Care By Dr. Amer Hasanien & Dr. Ali Saleh Skin Integrity and Wound Care Skin integrity: the presence of normal Skin & Uninterrupted skin layers by wounds. Factors affecting appearance
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types. Summary
 Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Wound Care per HHVNA Wound Product Formulary
 Venous Ulcers ABI of 0.9-1.2 = normal blood flow An ABI MUST be obtained prior to inititiation of compression therapy. Compression is the Gold Standard of care to promote wound of venous ulcers. Elevation
Venous Ulcers ABI of 0.9-1.2 = normal blood flow An ABI MUST be obtained prior to inititiation of compression therapy. Compression is the Gold Standard of care to promote wound of venous ulcers. Elevation
V.A.C.Ulta Negative Pressure Wound Therapy System. Monograph
 V.A.C.Ulta Negative Pressure Wound Therapy System Monograph Table of Contents Preface... 3 Introduction... 3 Wound Management with NPWT and NPWTi... 5 Literature Review of NPWT... 6 Literature Review of
V.A.C.Ulta Negative Pressure Wound Therapy System Monograph Table of Contents Preface... 3 Introduction... 3 Wound Management with NPWT and NPWTi... 5 Literature Review of NPWT... 6 Literature Review of
Wound Jeopardy: Name That Wound Session 142 Saturday, September 10 th 2011
 Initial Wound Care Consult History Physical Examination Detailed examination of the wound Photographs Cultures Procedures TCOM ABI Debridement Management Decisions A Detailed History and Physical (wound)
Initial Wound Care Consult History Physical Examination Detailed examination of the wound Photographs Cultures Procedures TCOM ABI Debridement Management Decisions A Detailed History and Physical (wound)
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
The Use of the. in Clinical Practice
 The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Risk. Background / Bias. Integrating Wound Care into a Limb Preservation Initiative 4/24/2009
 Stimulating Wound Granulation: Advances in NPWT and other Measures (Wound Bed Preparation) Charles Andersen MD, FACS, FAPWCA Clinical Prof of Surgery UW, USUHS Chief Vascular/Endovascular/ Limb Preservation
Stimulating Wound Granulation: Advances in NPWT and other Measures (Wound Bed Preparation) Charles Andersen MD, FACS, FAPWCA Clinical Prof of Surgery UW, USUHS Chief Vascular/Endovascular/ Limb Preservation
Disclosures for Tarik Alam. Wound Bed Preparation. Wound Prognosis. Session Objectives. Debridement 4/26/2015
 Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
SKIN INTEGRITY & WOUND CARE
 SKIN INTEGRITY & WOUND CARE Chapter 34 1 skin integrity: intact skin refers to the presence of normal skin layer uninterrupted by wound 2 WOUNDS DISRUPTION IN THE INTEGRITY OF BODY TISSUE CLASSIFIED AS:
SKIN INTEGRITY & WOUND CARE Chapter 34 1 skin integrity: intact skin refers to the presence of normal skin layer uninterrupted by wound 2 WOUNDS DISRUPTION IN THE INTEGRITY OF BODY TISSUE CLASSIFIED AS:
How Wounds Heal: A Guide for the Wound-care Novice
 C L I N I C A L P R A C T I C E How Wounds Heal: A Guide for the Wound-care Novice BY Christine Pearson Christine Pearson, RN, IIWCC, is a wound clinician for Vancouver Coastal Health and has worked in
C L I N I C A L P R A C T I C E How Wounds Heal: A Guide for the Wound-care Novice BY Christine Pearson Christine Pearson, RN, IIWCC, is a wound clinician for Vancouver Coastal Health and has worked in
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Algovita. MRI Procedure Guidelines. Spinal Cord Stimulation System. 201x
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
INTRODUCTION TO WOUND DRESSINGS
 WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
Module 10 Troubleshooting Guide
 Module 10 Troubleshooting Guide Your safety and wellbeing are our priority. Issues can occur during your treatment and it is important that you recognize the symptoms. This guide will teach you how to
Module 10 Troubleshooting Guide Your safety and wellbeing are our priority. Issues can occur during your treatment and it is important that you recognize the symptoms. This guide will teach you how to
Potential Complications
 Potential Complications The risk of complications associated with wisdom teeth removal is less than 0.5 percent when performed by an experienced oral surgeons who uses contemporary techniques and surgical
Potential Complications The risk of complications associated with wisdom teeth removal is less than 0.5 percent when performed by an experienced oral surgeons who uses contemporary techniques and surgical
WHY WOUNDS FAIL TO HEAL SIMPLIFIED
 WHY WOUNDS FAIL TO HEAL SIMPLIFIED 10 Some of the common signs of failure to heal with possible causes and some interventions WHY WOUNDS FAIL TO HEAL There must be adequate supplies of nutrients and oxygen
WHY WOUNDS FAIL TO HEAL SIMPLIFIED 10 Some of the common signs of failure to heal with possible causes and some interventions WHY WOUNDS FAIL TO HEAL There must be adequate supplies of nutrients and oxygen
Afistula is an abnormal opening between two or. Colocutaneous Fistula Management in a Dehisced Wound: A Case Study FEATURE
 FEATURE Colocutaneous Fistula Management in a Dehisced Wound: A Case Study Terri Reed, BSN, WOCN; Diana Economon, RN, BSN, CWOCN; and Laurel Wiersema-Bryant, APRN, BC A fistula is an abnormal opening between
FEATURE Colocutaneous Fistula Management in a Dehisced Wound: A Case Study Terri Reed, BSN, WOCN; Diana Economon, RN, BSN, CWOCN; and Laurel Wiersema-Bryant, APRN, BC A fistula is an abnormal opening between
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
CLINICAL EVIDENCE Partial and Deep Partial Burns
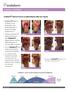 CLINICAL EVIDENCE Partial and Deep Partial Burns Endoform helps to improve re-epithelialization after burn injuries Endoform helps to facilitate tissue granulation and epithelialization in partial and
CLINICAL EVIDENCE Partial and Deep Partial Burns Endoform helps to improve re-epithelialization after burn injuries Endoform helps to facilitate tissue granulation and epithelialization in partial and
Hemostasis Inflammatory Phase Proliferative/rebuilding Phase Maturation Phase
 The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
Positioning System. Laparoscopic ventral hernia repair KEY BENEFITS SOFT TISSUE REPAIR
 Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Wound Dressing. Choosing the Right Dressing
 Wound Dressing Choosing the Right Dressing Benefits of using the correct Drsg Helps create the optimal wound environment Increases healing rates Reduces pain Decreases infection rates Cost effective Care
Wound Dressing Choosing the Right Dressing Benefits of using the correct Drsg Helps create the optimal wound environment Increases healing rates Reduces pain Decreases infection rates Cost effective Care
WOUNDS. Emergency Procedures in PT
 WOUNDS Emergency Procedures in PT Types of Wounds Abrasions uppermost layer scraped away, minor capillary bleeding occurs, nerve endings exposed Lacerations skin tear with edges jagged and uneven Incisions
WOUNDS Emergency Procedures in PT Types of Wounds Abrasions uppermost layer scraped away, minor capillary bleeding occurs, nerve endings exposed Lacerations skin tear with edges jagged and uneven Incisions
A step-by-step preparation guide
 A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
Vaxcel Implantable Ports Valved and Non-Valved. A Patient s Guide
 Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Pressure ulcer. Dressing Selection Guide, Protocol, & Procedure
 Pressure ulcer Dressing Selection Guide, Protocol, & Procedure Sponsored by: Ferris Mfg. Corp. 16W300 83rd Street, Burr Ridge, IL 60527 USA Toll Free U.S.A.:800-765-9636 International: +1 630-887-9797
Pressure ulcer Dressing Selection Guide, Protocol, & Procedure Sponsored by: Ferris Mfg. Corp. 16W300 83rd Street, Burr Ridge, IL 60527 USA Toll Free U.S.A.:800-765-9636 International: +1 630-887-9797
CASE 1: TYPE-II DIABETIC FOOT ULCER
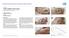 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
To standardize wound care and prevent infection in compromised patients who have a Berlin Heart Ventricular Assist Device (VAD).
 PURPOSE To standardize wound care and prevent infection in compromised patients who have a Berlin Heart Ventricular Assist Device (VAD). POLICY STATEMENTS Dressing change should be done no sooner than
PURPOSE To standardize wound care and prevent infection in compromised patients who have a Berlin Heart Ventricular Assist Device (VAD). POLICY STATEMENTS Dressing change should be done no sooner than
A step-by-step preparation guide
 A step-by-step preparation guide This guide provides detailed instruction on the reconstitution, dilution, and storage of Veletri (epoprostenol) for Injection. It is intended to be used after your healthcare
A step-by-step preparation guide This guide provides detailed instruction on the reconstitution, dilution, and storage of Veletri (epoprostenol) for Injection. It is intended to be used after your healthcare
Enluxtra Self-Adaptive Wound Dressing
 Enluxtra Self-Adaptive Wound Dressing Case Studies OSNovation Systems, Inc. www.anywound.com Enluxtra is the registered trademark of BASF, Inc. OSNovative Systems, Inc. 500 Laurelwood Rd, Ste 1 & 4, Santa
Enluxtra Self-Adaptive Wound Dressing Case Studies OSNovation Systems, Inc. www.anywound.com Enluxtra is the registered trademark of BASF, Inc. OSNovative Systems, Inc. 500 Laurelwood Rd, Ste 1 & 4, Santa
Durable Medical Equipment Providers
 August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
WOUND CARE. By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare
 WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Negative Pressure Wound Therapy Overlay Technique With Collagen Dressings for Nonhealing Wounds
 J Wound Ostomy Continence Nurs. 2010;37(5):549-553. Published by Lippincott Williams & Wilkins CHALLENGES IN PRACTICE Negative Pressure Wound Therapy Overlay Technique With Collagen Dressings for Nonhealing
J Wound Ostomy Continence Nurs. 2010;37(5):549-553. Published by Lippincott Williams & Wilkins CHALLENGES IN PRACTICE Negative Pressure Wound Therapy Overlay Technique With Collagen Dressings for Nonhealing
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
STEP-BY-STEP GUIDE TO SELF-INFUSION. Subcutaneous Administration of GAMMAGARD LIQUID
 STEP-BY-STEP GUIDE TO SELF-INFUSION Subcutaneous Administration of GAMMAGARD LIQUID This handy guide will help you manage your subcutaneous administration of GAMMAGARD LIQUID. If you have questions on
STEP-BY-STEP GUIDE TO SELF-INFUSION Subcutaneous Administration of GAMMAGARD LIQUID This handy guide will help you manage your subcutaneous administration of GAMMAGARD LIQUID. If you have questions on
Human experience with a biodegradable polyurethane scaffold I: Short-term implantation
 Human experience with a biodegradable polyurethane scaffold I: Short-term implantation A/Prof. John E Greenwood AM BSc(Hons), MBChB, MD, FRCS(Eng.), FRCS(Plast.), FRACS Director, Burns Unit, Royal Adelaide
Human experience with a biodegradable polyurethane scaffold I: Short-term implantation A/Prof. John E Greenwood AM BSc(Hons), MBChB, MD, FRCS(Eng.), FRCS(Plast.), FRACS Director, Burns Unit, Royal Adelaide
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
Warning: Patients should not be exposed to MRI environments until the surgical site following pump implantation is fully healed.
 Prometra and Prometra II Programmable Pumps Magnetic Resonance Imaging (MRI) Safety Information GENERAL MR Conditional WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT COULD RESULT
Prometra and Prometra II Programmable Pumps Magnetic Resonance Imaging (MRI) Safety Information GENERAL MR Conditional WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT COULD RESULT
LABETTE COMMUNITY COLLEGE BRIEF SYLLABUS. Please check with the LCC bookstore for the required texts for this class.
 LABETTE COMMUNITY COLLEGE BRIEF SYLLABUS SPECIAL NOTE: This brief syllabus is not intended to be a legal contract. A full syllabus will be distributed to students at the first class session. TEXT AND SUPPLEMENTARY
LABETTE COMMUNITY COLLEGE BRIEF SYLLABUS SPECIAL NOTE: This brief syllabus is not intended to be a legal contract. A full syllabus will be distributed to students at the first class session. TEXT AND SUPPLEMENTARY
Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System.
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Better Post-Op Pain Control Starts Here
 Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
We look forward to serving you.
 ADVANCED CARE GEMCORE360 offers healthcare professionals a simple, clear and cost-effective wound care range while ensuring excellent clinical outcomes for their patients. 1 At GEMCO Medical, we strive
ADVANCED CARE GEMCORE360 offers healthcare professionals a simple, clear and cost-effective wound care range while ensuring excellent clinical outcomes for their patients. 1 At GEMCO Medical, we strive
Scott E. Palmer, V.M.D. Diplomate, A.B.V.P., Eq. Practice New Jersey Equine Clinic
 Scott E. Palmer, V.M.D. Diplomate, A.B.V.P., Eq. Practice New Jersey Equine Clinic spalmer@njequine.com Hemostasis Inflammation Proliferation Remodeling Inflammation Matrix production Angiogenesis Epithelialization
Scott E. Palmer, V.M.D. Diplomate, A.B.V.P., Eq. Practice New Jersey Equine Clinic spalmer@njequine.com Hemostasis Inflammation Proliferation Remodeling Inflammation Matrix production Angiogenesis Epithelialization
Important Safety Information for Adlyxin (lixisenatide) injection
 A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
South West Regional Wound Care Toolkit F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE)
 F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE) F.5 SURGICAL WOUND (CLOSED AND OPEN) 5.1 Background to Etiology Closed surgical wounds are well-approximated with a palpable healing ridge
F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE) F.5 SURGICAL WOUND (CLOSED AND OPEN) 5.1 Background to Etiology Closed surgical wounds are well-approximated with a palpable healing ridge
Skin Protectant and Skin & Incontinent Cleanser
 Skin Protectant and Skin & Incontinent Cleanser NEW! Cleanser Comprehensive system for the treatment and prevention of IAD, pressure ulcers* and other moisture lesions Safe and skin-friendly Prevents moisture
Skin Protectant and Skin & Incontinent Cleanser NEW! Cleanser Comprehensive system for the treatment and prevention of IAD, pressure ulcers* and other moisture lesions Safe and skin-friendly Prevents moisture
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
1/5. Introduction. Primary endpoint Time to reach readiness for closure by surgical intervention or left for closure by secondary intention
 1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
WOUND DRESSING Daily Dressing Packets
 AMERIGEL WOUND DRESSING Daily Dressing Packets P R O D U C T I N F O R M AT I O N MSDS APPLICATION PROTOCOLS AmeriGel WOUND DRESSING Daily Dressing Packets A HYDROGEL WITH A UNIQUE AUTOLYTIC DEBRIDER Diabetic
AMERIGEL WOUND DRESSING Daily Dressing Packets P R O D U C T I N F O R M AT I O N MSDS APPLICATION PROTOCOLS AmeriGel WOUND DRESSING Daily Dressing Packets A HYDROGEL WITH A UNIQUE AUTOLYTIC DEBRIDER Diabetic
NEGATIVE PRESSURE WOUND THERAPY Wound Vac System
 NEGATIVE PRESSURE WOUND THERAPY Wound Vac System JASSIN M. JOURIA MD Dr. Jassin M. Jouria is a practicing Emergency Medicine physician, professor of academic medicine, and medical author. He graduated
NEGATIVE PRESSURE WOUND THERAPY Wound Vac System JASSIN M. JOURIA MD Dr. Jassin M. Jouria is a practicing Emergency Medicine physician, professor of academic medicine, and medical author. He graduated
