Andreea Ilinca Cotoi. A thesis submitted to the Centre for Neuroscience Studies. In conformity with the requirements for
|
|
|
- Cory Stevens
- 6 years ago
- Views:
Transcription
1 BEHAVIORAL AND NEURAL CORRELATES OF CONDITIONED PAIN MODULATION IN THE HUMAN BRAINSTEM AND CERVICAL SPINAL CORD USING FUNCTIONAL MAGNETIC RESONANCE IMAGING by Andreea Ilinca Cotoi A thesis submitted to the Centre for Neuroscience Studies In conformity with the requirements for the degree of Master of Science Queen s University Kingston, Ontario, Canada (August, 2015) Copyright Andreea Ilinca Cotoi, 2015
2 Abstract When one noxious stimulus is coupled with a second noxious stimulus applied to a remote region of the body relative to the first stimulus, the perception of pain is reduced. This phenomenon is termed conditioned pain modulation (CPM) and it s thought to engage descending inhibitory controls originating in the brainstem which modulate pain processing pathways, resulting in a decrease in pain. This thesis explores the CPM effect in humans, to further our understanding of pain modulation within the central nervous system (CNS). Previous research reveals that CPM evokes pain inhibition in the majority of healthy pain-free individuals, while recent studies have shown that a significant proportion of healthy individuals also demonstrate pain facilitatory responses similar to those exhibited by individuals with chronic pain. Currently, no imaging studies have exclusively explored subcortical changes in activity evoked by the CPM paradigm. Therefore, this thesis delves into uncovering the changes in the brainstem and spinal cord activity in response to the CPM paradigm by investigating the behavioral and neural responses of healthy females using functional magnetic resonance imaging (fmri). CPM was induced by delivering a heat test stimulus to the right thenar eminence and a cold conditioning stimulus to the left thenar eminence simultaneously, in 25 females aged 27.6 ± 7.82, while obtaining behavioral and imaging data. We found that the CPM paradigm evoked a significant reduction in perceived pain coupled with significantly greater blood oxygenation-level dependent (BOLD) responses in the PAG, RVM, DRt and the DH of the spinal cord, compared to the control condition, where only the heat test stimulus was delivered. Furthermore, the connectivity between these regions was greater during CPM than during the control condition. Lastly, we found significant differences in the behavioral and neural responses between individuals demonstrating pain inhibition and those demonstrating pain facilitation in response to CPM. Furthermore, connectivity findings suggest that different networks are engaged when experiencing an enhanced perception of pain compared to when the pain is reduced during CPM. These findings enhance our current knowledge of the subcortical network involved in CPM, and provide novel insights into the neural correlates of pain modulation. ii
3 Acknowledgements I would like to thank my supervisor Dr. Stroman, for the opportunity to work on this project and for the continued support and knowledge that that I ve gained throughout my two years while working on my Master s thesis. I feel privileged to have been given the opportunity to learn the ropes of research from an expert in the field of neuroimaging. A special thanks to all my colleagues, Rachael, Theresa, and Roxanne for the continued encouragement, help, and guidance. You have all been great mentors in different aspects of life whether personal or professional, and I am truly blessed to have worked alongside such caring, and charismatic colleagues. Don, thank you for helping me with the data collection and for making this project possible. To my dearest parents and family here in Canada and back home in Romania, thank you for your continuous love, support and prayers which have strengthened and encouraged me in every way. A huge thanks to my twin sister for believing in me and for finding the time in your busy schedule to visit me. I would like to thank my partner, Jon, for always trusting in me and encouraging me during the challenging times. Without fail, your humor has always brought a smile on my face regardless of my circumstances and I am so blessed to have you in my life. I love you dearly. Last but not least, I m grateful to God for being with me through every moment of this experience and always surrounding me with grace and peace. The strength I needed to work on this thesis has been so generously provided by You, Lord, and I am truly humbled by all the gifts You have bestowed on me. To anyone else who has been with me during this journey, thank you immensely! iii
4 Table of Contents Abstract... ii Acknowledgements... iii List of Figures... viii List of Tables... ix List of Abbreviations... x Chapter 1 Introduction Pain Nociceptors Spinal Cord Ascending Pain Pathways Lateral Spinothalamic Tract Spinoreticular Tract Spinomesencephalic Tract Gate Theory of Pain Emotional Modulation of Pain Descending Pain Modulation Descending Pathways of Pain Modulation Subcortical Origins of Descending Pathways Diffuse Noxious Inhibitory Controls (DNIC) DNIC in Animals DNIC in Humans Conditioned Pain Modulation (CPM) Inducing and Measuring CPM Applications of CPM in Clinical Settings Magnetic Resonance Imaging (MRI) The MR Signal MR Imaging Methods Functional MRI (fmri) Blood Oxygenation-Level Dependent (BOLD) Spinal Cord and Brainstem fmri Pain Processing and fmri Pain Processing and fmri: Regions in the Brain iv
5 1.4.2 Pain Processing and fmri: Regions in the Brainstem and Spinal Cord CPM and fmri Proposed Research Purpose Rationale Hypothesis Objectives Chapter 2 Investigating the neural correlates of conditioned pain modulation (CPM) in the human spinal cord and brainstem: an fmri study Introduction Methods Participants Questionnaires Pain rating scales Training session Test Stimulus (TS) for fmri studies Conditioning Stimulus (CS) for fmri studies Experimental design Functional MRI (fmri) session Set-up Data acquisition Data analysis Behavioral data Functional MRI data: Data preprocessing Functional MRI data: General Linear Model (GLM) analysis Functional MRI data: Structural Equation Modeling (SEM) analysis Results Questionnaires Behavioral results GLM results SEM results Discussion Conclusion v
6 Chapter 3 Differences in pain perception and in the brainstem and spinal cord neural responses to the conditioned pain modulation (CPM) paradigm in healthy controls: an fmri study Introduction Methods Participants Questionnaires Pain rating scales Training session Test Stimulus (TS) Conditioning Stimulus (CS) Experimental design Functional MRI (fmri) session Set-up Data acquisition Data analysis Behavioral data Questionnaires Functional MRI data: Data preprocessing Functional MRI data: General Linear Model (GLM) analysis Functional MRI data: Structural Equation Modeling (SEM) or Path analysis Results Behavioral results Questionnaires GLM results SEM results Discussion Conclusion Chapter 4 Discussion Research objectives Principal findings Interpretation Limitations Future directions Conclusion vi
7 References Appendix A MRI Safety Form Appendix B Participant Consent Form vii
8 List of Figures Figure 1. Pain rating scales used to rate pain sensations Figure 2. Schematic representation of the delivery locations for the test stimulus (TS) and the conditioning stimulus (CS) Figure 3. A schematic representation of the task paradigm used to assess the CPM effect in the fmri sessions Figure 4. Reported TS pain ratings for each condition obtained during the two fmri sessions Figure 5. BOLD signal changes that significantly match the stimulation paradigms, observed at different levels of the brainstem and spinal cord, during each experimental condition Figure 6. BOLD signal changes that that are significantly different between the CPM and the Control conditions Figure 7. Map illustrating network connections in the Control and the CPM conditions Figure 8. Distribution of the CPM effect across participants Figure 9. Reported TS pain ratings, for each condition, obtained during the two fmri sessions Figure 10. Correlation plots between questionnaire scores and the CPM effect Figure 11. BOLD signal changes that that are significantly different between the CPM and the Control conditions in the efficient CPM responders and in the less efficient CPM responders, observed at different levels of the brainstem and spinal cord Figure 12. BOLD signal changes that that are significantly different between the efficient CPM responders and the less efficient CPM responders, during the CPM condition Figure 13. Network connections detected during the CPM condition in both efficient and less efficient CPM responders viii
9 List of Tables Table 1. Descriptive statistics of demographic information and questionnaire scores from all participants Table 2. Descriptive statistics of pain 50 temperatures used to deliver the TS and the CS, and the TS pain ratings from the Control and CPM conditions obtained in the fmri runs Table 3. Descriptive statistics of pain 50 temperatures used to deliver the TS and the CS, and the TS pain ratings from the Control and CPM conditions obtained in the fmri runs, from the efficient CPM responders and the less efficient CPM responders Table 4. Descriptive statistics of demographic information and questionnaire scores from the efficient CPM responders and the less efficient CPM responders ix
10 List of Abbreviations ACC anterior cingulate cortex ANOVA analysis of variance BDI-II Beck s Depression Inventory BOLD blood oxygenation-level dependent C6 6 th cervical segment CBF cerebral blood flow CPM conditioned pain modulation CS conditioning stimulus CSF cerebral spinal fluid DH dorsal horn DLF dorsolateral funiculus DLPFC dorso-lateral prefrontal cortex DNIC diffuse noxious inhibitory controls DRt dorsal reticular nucleus EPI echo planar imaging fmri functional magnetic resonance imaging GLM general linear model HASTE half-fourier single-shot fast-spin echo HRF hemodynamic response function INS insula LC locus coeruleus MEG magnetoencephalography MRI magnetic resonance imaging NGC nucleus gigantocellularis NRM nucleus raphe magnus NTS nucleus tractus solitarius OFC orbito-frontal cortex PAG periaqueductal gray PBN parabrachial nucleus PCS pain catastrophizing scale PET positron emission tomography PFC prefrontal cortex RVM rostral ventromedial medulla S1 primary somatosensory cortex S2 secondary somatosensory cortex SEM structural equation modeling SG substantia gelatinosa STAI state-trait anxiety inventory TE echo time TR repetition time TRPM8 transient receptor potential cation channel M8 TRPV1 transient receptor potential cation channel vanilloid 1 TS test stimulus x
11 VLF WDR ventrolateral funiculus wide dynamic range xi
12 Chapter 1 Introduction Our understanding of pain has advanced greatly since 1664 when Descartes first theorized the processing of pain occurring in the central nervous system of man 1. We now know that pain is much more complex and that pain can be modulated at various subcortical sites prior to the signals reaching higher cortical regions for perception. The brain has the endogenous capacity to facilitate and inhibit the perception of pain to allow one to cope with pain appropriately. For instance, an athlete s pain perception may be highly reduced when engaged in extreme sports to allow the brain to focus its resources on avoiding further injury. On the other hand, enhanced pain sensitivity has been associated with promoting recovering rest after physical body insults 2-4. Most often, pain inhibition and pain facilitation are not exclusive and the overall experience of pain is the result of a balance between these two processes. Upsetting this balance by a dysregulation of one or both mechanisms, it can cause the pain perception to become maladaptive. For instance, a lack of inhibition and/or increased pain facilitation evokes an exaggerated pain sensation and even induces chronic pain if the dysregulation in neural networks becomes permanent. Growing interest in understanding the alterations in the neural networks that characterize chronic pain syndromes have led to numerous advancements in the research tools measuring the capacity of endogenous pain networks to modulate perceived pain. One tool often used to test the endogenous ability to inhibit pain is the conditioned pain modulation (CPM) paradigm, where the delivery of one noxious stimulus, the conditioning stimulus, reduces 1
13 the noxious perception of another, the test stimulus, in the majority of healthy individuals 5. Conversely, the delivery of this paradigm in individuals with chronic pain revealed a lack of changes in the perceived pain or a pain facilitatory response to CPM 6-8. There is an abundance of behavioral findings that may help understand the physiology of the neural networks involved in modulating pain via CPM. However, the neural correlates of this paradigm are still largely unknown. CPM is often employed in laboratory settings, but also has potential applications in clinical settings as well. For instance, behavioral findings revealed that CPM is one of the few dynamic tests that predicts the susceptibility of developing chronic post-operative pain 9, and to predict the efficacy of analgesics 10. Therefore, understanding the neural processes that mediate pain inhibition during CPM may provide understanding of the pain modulatory networks involved and how they are altered in chronic pain states. This thesis thus concentrates on investigating the neural networks underlying the analgesic effect of CPM by using brainstem and spinal cord functional MRI to further our understanding of the body s endogenous ability to modulate pain. This thesis consists of two studies. The goal of the first study was to use spinal cord and brainstem functional MRI to investigate the underlying neural correlates of pain inhibition in response to CPM. The findings from this study will not only improve our current understanding regarding the physiological mechanisms underlying efficient CPM, but it may also expand our knowledge about the endogenous processes involved in modulating and mitigating perceived pain. Early into the experiment, behavioral pilot data revealed that the CPM effect was not consistent across healthy individuals, but rather represented a gradient between pain 2
14 inhibition and pain facilitation. This prompted a more detailed investigation in the behavioral and neural characteristics that differentiate efficient CPM responders (participants demonstrating pain inhibition) from less efficient CPM responders (participants demonstrating pain facilitation), which became the second study of the thesis. Therefore the goal of this study was to use brainstem and spinal cord functional MRI to explore some of the behavioral and physiological characteristics that differentiate healthy participants experiencing an analgesic effect of CPM from those demonstrating hyperalgesia in response to CPM. Altogether, the findings from these two studies will greatly further our current understanding about the physiological processes mediating CPM in healthy participants and add to the existing body of knowledge regarding the endogenous processes implicated in modulating the experience of pain. Furthermore, findings may also reveal important insights into the use of CPM to further explore behavioral and neural characteristics that may serve as biomarkers for increased chronic pain development. Prior to delving into these two studies, an introduction that briefly outlines the topics discussed in this thesis is presented. 1.1 Pain The perception of pain is a complex experience that begins with noxious stimuli activating peripheral nociceptors whose afferent fibers terminate in the dorsal horn of the spinal cord. From there, the pain signals ascend to medullary sites for further processing prior to finally reaching cortical regions for conscious perception Nociceptors 3
15 The process of pain sensation begins with the activation of sensory receptors called nociceptors, which are free nerve endings that respond to painful stimuli. There are three types of nociceptors classified based on the noxious stimuli they respond to: mechanical nociceptors are activated by intense pressure applied to the skin, thermal nociceptors respond to extreme temperatures (>45 ºC or <5 ºC) and strong mechanical stimuli, and polymodal nociceptors are activated by a variety of high intensity chemical, mechanical, and thermal stimuli 11. Once a specific modality activates the appropriate nociceptor, the signal is propagated along the axon to the dorsal horn of the spinal cord. The speed of signal propagation depends largely on the diameter and the conducting velocity of the sensory axon. For instance, the Aδ fibers have conducting velocities of about 5-30 m/s, evoking the first sharp, acute pain that is experienced right after pain stimulus is delivered 12. Aδ fibers are characteristic of thermal and mechanical nociceptors. C fibers on the other hand, are smaller in diameter compared to the Aδ fibers and are also unmyelinated, resulting in slower conduction velocities ( m/s). Due to the slow transmission speed, the sensation evoked by activating C-fibers resembles that of a dull, throbbing pain that starts a second after the painful stimulus is delivered and lasts a few seconds after the cessation of the pain stimulus 13. C-fibers are often characteristic of polymodal nociceptors Spinal Cord The central nervous system (CNS) is comprised of the spinal cord and the brain. The spinal cord extends from the medulla oblongata at the inferior portion of the brain, in a rostral-caudal direction, and it is divided into 31 spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal). Each spinal nerve innervates a specific area of the skin 4
16 called a dermatome that corresponds to a spinal segment (i.e. the 6 th cervical spinal cord segment, receives sensory afferent input from the corresponding C6 dermatome). The sensory axons or first-order nerve afferents enter the dorsal side of the spinal cord via the dorsal roots and synapse on second-order neurons in the gray matter of the spinal cord for integration of sensory input. The gray matter consists of dendrites, cell bodies, and unmyelinated axons, and it forms a butterfly shape surrounded by white matter which consists largely of myelinated axons which form the tracts linking the spinal cord and the brain. The gray matter is subdivided into dorsal horns (DHs) which receive and direct the sensory input, and two ventral horns (VHs) which contain motoneurons whose axons innervate muscle fibers. The gray matter of the spinal cord is further subdivided into 10 cytoarchitectonic regions (laminae) arranged in parallel, where laminae I-VI correspond to the DHs, laminae VII-IX constitute the VHs, and laminae X surrounds the central canal 14. Sensory inputs mediating pain information of thermal origin arriving from first-order C fiber afferents terminate in laminae I and II of the dorsal horn, and pain signals mediated via primary Aδ fibers terminate in laminae II, III and V 15. Together, laminae II and III form the substantia gelatonosa (SG), which receives afferent input from both C and Aδ fibers 16. Laminae V corresponds to the wide dynamic range (WDR) of the DH containing cells responding to noxious inputs mediated via dorsal horn inhibitory interneurons Ascending Pain Pathways First-order Aδ and C fibers transmitting pain and temperature information synapse on second-order neurons in the dorsal horn of the spinal cord whose axons cross the midline and ascend to brainstem and thalamus. The primary ascending pain pathways are the lateral spinothalamic tract, the spinoreticular tract, and the spinomesencephalic tract 11. 5
17 Lateral Spinothalamic Tract The lateral spinothalamic tract is a major ascending pathway that conveys pain and temperature information from the spinal cord directly to the thalamus 11. Transmission of nociceptive information along this tract begins with the activation of various ion channels at the periphery of nociceptors which convey noxious inputs to the dorsal horn. Noxious heat is thought to activate the moderate heat threshold channel, the transient receptor potential cation channel vanilloid receptor 1 (TRPV1), which responds to temperatures ranging from 43 ºC to 51 ºC 17. Nociceptors also contain the transient receptor potential cation channel M8 (TRPM8) that responds to noxious cold temperatures below 20 ºC 18. Noxious heat evoked signals are propagated along Aδ and C fibers of first-order neurons, while the majority of noxious cold inputs are conveyed predominantly via C fibers with some inputs activating Aδ fibers 17,18. When the projecting axons of these neurons reach the dorsal horn of the spinal cord, they branch into ascending and descending collaterals, innervating the immediate corresponding spinal segment and just above and below the level of innervation, forming the dorsolateral tract of Lissauer 15. Once these axons enter the DHs of the gray matter they synapse on second-order neurons in laminae I and V. Animal studies suggest that both noxious heat and cold inputs terminate in laminae I, while a significant proportion of noxious heat inputs also synapse in laminae V of the dorsal horn The axonal fibers of second-order neurons decussate at the same spinal segment and ascend contralaterally through the spinal cord and the brainstem, and terminate in the ventral posterior lateral nucleus of the thalamus. Recent studies report that thermal and pain inputs are distributed somatotopically within the thalamus such that noxious cold inputs and noxious heat inputs converge on third-order neurons that located on separate 6
18 regions of the thalamus depending on the modality 22. These final neurons project ipsilaterally to cortical structures such as the somatosensory cortices (S1 and S2), the insula, and the cingulate cortex. Both noxious heat and noxious cold have been shown to activate the same cortical regions and much less is known with respect to the maintenance of the somatotopic representation within cortical activated structures 13, Spinoreticular Tract The spinoreticular tract is another major ascending pathway that mediates the transmission of temperature and nociception from the dorsal horn to the brainstem. As with the lateral spinothalamic tract, the axons of first-order neurons conveying nociceptive information enter the spinal cord and synapse on second-order neurons located deeper within the DHs, in laminae VII and VIII. The majority of the axons of these second-order neurons ascending through the spinal cord uncrossed and terminate on third-order neurons in the reticular formation, pons, and midbrain 24. The reticular formation integrates holistic patterns across the body and influences appropriate behavioral states for the entire body. The third-order neurons then relay nociceptive information to other structures such as the thalamus and the hypothalamus which are involved in processing the emotional and the autonomic aspects of pain. Due to the high level of convergence at each synapse, spatial localization is lost as information is propagated from the spinal cord to the reticular formation 16. Therefore, this pathway is largely practical in mediating the emotionalaffective component of pain rather than processing discriminative sensory information about noxious inputs Spinomesencephalic Tract 7
19 A third ascending pathway that mediates nociceptive information is the spinomesencephalic tract. As with the lateral spinothalamic tract and the spinoreticular tract, the first-order neuron synapse on second-order neurons originating in laminae I and V of the DH. These neurons ascend along the spinal cord and terminate in the mesencephalic reticular formation and in the periaqueductal gray matter (PAG). From here, second-order neurons synapse on third-order neurons located in the parabrachial nucleus (PBN). Although this tract does not have direct connections to cortical regions, the PBN is a major relay site for thermoreceptive and nociceptive information to the forebrain 25. The spinomesencephalic tract is thought to contribute to the maintenance of central sensitization and to play a major role in modulating descending controls via projections from the PAG Gate Theory of Pain Nociceptive inputs arriving at the DHs are subject to modulation prior to ascending to subcortical and cortical sites for further processing. The gate theory of pain suggests that nociceptive inputs arriving via myelinated Aδ and unmyelinated C fibers can be inhibited by the concomitant activation of low-threshold large myelinated Aβ fibers that carry innocuous pressure and touch information 30. Laminae II and III of the SG receive converging inputs from the Aδ and C fibers carrying noxious information and from the larger Aβ fibers conveying mechanoreceptive information. Activation of non-noxious Aβ fibers subsequently leads to the activation of inhibitory interneurons which ultimately block nociceptive transmission from Aδ and C fibers thus closing the gate 30. When the nociceptive information reaching the DHs surpasses a certain threshold, the inhibitory interneurons previously activated by the Aβ fibers carrying innocuous information are now 8
20 inhibited by incoming noxious inputs from Aδ and C fibers. This process opens the gate and promotes the nociceptive transmission to subcortical and cortical regions via ascending pathways 30. The gate theory of pain was the first to conceptualize the interaction of different sensory modalities and today we acknowledge that various higher cortical processes (i.e. emotions, memories, expectations, and mood states) have the capacity to modulate the perception of pain in similar ways to result in different expressions of pain Emotional Modulation of Pain Studies suggest that the perception of pain is not linearly related to the intensity of a noxious stimulus, but rather it can be strongly influenced by psychological factors. Positive mood is known to reduce the perception of pain 31,32, while negative mood such as depression and anxiety is known to facilitate the experience of pain 32,33. Studies demonstrate that pain-free depressed individuals have a higher risk of developing chronic musculoskeletal pain compared to non-depressed pain-free individuals 34,35. Similarly, anxiety disorders have been shown to mark the onset of hyperalgesia 36. A bidirectional relationship between pain and emotions has also been demonstrated in many clinical populations. For instance, individuals with chronic pain often experience feelings of depression or persistent mood disturbances 37. Conversely, clinically depressed individuals and those with post-traumatic stress disorders have reported heightened pain sensitivity or abnormal responses to pain when compared to healthy controls 38,39. Although the causal relationship between chronic pain and emotional disturbances in cases where both co-occur is often challenging to determine and not well understood, there is abundant 9
21 evidence from human and animal studies that suggest that emotional factors play a large role in modulating nociceptive processing Descending Pain Modulation As alluded to previously, descending pain modulating controls from supraspinal regions (i.e. brainstem and cortical structures) can influence the perception of pain by exerting top-down controls to either facilitate or inhibit the transmission of nociceptive inputs. Several cortical origins of descending processes include the primary and the secondary somatosensory cortices (S1 and S2) the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate cortex (ACC), the amygdala, and the anterior insula, which together form the pain neuromatrix 40,41. Most of these regions project to midbrain structures such as the PAG and the rostral ventromedial medulla (RVM), which have direct and indirect connections through pontine and medullary regions to modulate the transmission of nociceptive information at the spinal level Descending Pathways of Pain Modulation Descending controls originating largely from subcortical regions access the DH via the dorsolateral funiculus (DLF) and the ventrolateral funiculus (VLF) which function differentially to facilitate or inhibit the transmission of nociceptive signals 42. It has been suggested that a prominent focus of the DLF is to mediate descending inhibition while the VLF is thought to contribute to descending modulation by facilitating transmission of nociceptive signals 43,44. Although these influences travel by different pathways, the engagement of descending inhibition and descending facilitation can occur simultaneously by activating several brainstem regions that have dual roles in modulating nociceptive transmission
22 Subcortical Origins of Descending Pathways A major player in the descending pain modulatory axis is the PAG. This medullary region is considered to be part of the endogenous pain control system, known to elicit generalized analgesia in both humans and animals when stimulated 46,47. It is highly interconnected with the hypothalamus, the prefrontal cortex (PFC), the amygdala, and the ACC, receiving inputs regarding emotional and cognitive states 42. Although it receives both direct and indirect input from the DH, the descending projections from the PAG to the spinal cord are largely indirect 48. Its antinociceptive effects are conveyed by activating brainstem regions, such as the RVM, the nucleus tractus solitarius (NTS), the PBN, and the locus coeruleus (LC) which have direct influence over spinal nociceptive inputs 15. The link between the PAG and the RVM is particularly important to the descending modulation of pain, as ascending sensory inputs from the DH activate upon arrival to the PAG descending controls that are conveyed through the RVM The RVM is subdivided into the nucleus raphe magnus (NRM) and the nucleus reticularis gigantocellularis (NGC), both receiving direct connections from the PAG 53. The PBN, NTS, and other supraspinal regions also send afferents targeting the RVM which then exerts inhibitory or facilitatory influences on the neurons of the DH 48,54. The ability to bimodally influence nociception has been attributed to the heterogeneity in the classes of neurons 42. The RVM consists of ON cells, OFF cells, and Neutral cells 55,56. Noxious stimulation activates ON cells, while opioids released from the PAG inhibit their activity 42. Conversely, OFF cells are inhibited by noxious inputs and activated by opioidergic inputs from the PAG 42. Findings suggest that activation of ON cells triggers 11
23 descending facilitation, while OFF cells participate in descending inhibition 43,54,57,58. Neutral cells do not respond to nociceptive inputs 59. Descending autonomic and somatosensory information regarding the emotional and the cognitive aspects of pain are integrated within the hypothalamus, the PBN, and the NTS via projections from higher cortical structures 42,48,60. These subcortical regions are highly interlinked and communicate both directly and indirectly with the DH to modulate nociceptive processing 32,38,42. A large portion of indirect projections to the DH are mediated through the RVM and the PAG, playing important roles in triggering the descending modulatory controls Studies have shown that stimulation of the lateral hypothalamus, PBN, and NTS suppressed DH neuron response to nociceptive inputs, while stimulation of the medial portion of the hypothalamus elicited hyperalgesia 61, Another structure within the caudal medulla that facilitates the nociceptive response of DH neurons is the dorsal reticular nucleus (DRt) 67. It is largely interconnected with various subcortical regions such as the LC, PAG, PBN, and the hypothalamus, and it receives bilateral projections from laminae I and IV-V of the DH 53,68,69. Neurons within the DRt are activated mainly by noxious stimulation conveyed by the Aδ and C fibers 70, and they respond proportionally to the intensity of the stimulus 71. The descending pathways to the DH are ipsilateral and contralateral to the site of stimulation and act to promote the spinal transmission of nociceptive inputs to other subcortical and cortical regions. It has also been postulated that the DRt plays a primary role in the expression of diffuse noxious inhibitory controls (DNIC), the activation of which by noxious stimulation can evoke an analgesic response in other regions of the body 72. As such, the DRt can mediate both descending facilitation by amplifying the nociceptive response of 12
24 DH neurons responding to noxious stimulation from primary afferent projections, and descending inhibition by reducing the activity of neurons receiving inputs from other regions of the body 67. Although much of the work investigating the involvement of the DRt in modulating the spinal neuron response to noxious stimulation during DNIC has been extensively studied in animal models, little is known of its contribution to the analgesic response in humans. 1.2 Diffuse Noxious Inhibitory Controls (DNIC) Although it seems contradictory at first, noxious stimuli can reduce the pain evoked by stimulation of a remote region of the body 73,74. This phenomenon has been employed in earlier years to suppress the pain induced by caudectomies on domestic animals by the use of nasal forceps 75. The hypothesis behind this phenomenon suggested that DH neurons transmitting nociceptive information can be inhibited by nociceptive stimulation applied to another region of the body with distinct receptive fields 76,77. This effect became known as the diffuse noxious inhibitory controls or DNIC DNIC in Animals Studies in animals revealed that DH neurons are inhibited by a noxious conditioning stimulus applied outside of the excitatory receptive field of the neuron under study 75, In rats, the C-fiber mediated nociceptive reflex evoked by electrical stimulation of the sural nerve and recorded in the biceps femoris muscle was inhibited by noxious thermal stimulation to the paw or tail 81. Interestingly, DNIC was not observed in decerebrated rats suggesting that the mechanisms underlying this phenomenon requires the involvement of supraspinal structures 77,78,82. Since the thalamus is an important structure 13
25 in the ascending spinothalamic pathway conveying nociceptive information, lesioning the thalamus was proposed to reduce the DNIC effects. Surprisingly, ablation of the thalamus had no effect on DNIC. This suggested that other brainstem structures known to mediate descending inhibition to have involvement in DNIC. However, as with thalamic lesions, ablation of the PAG, PBN, LC, and the RVM did not reduce DNIC Conversely, lesioning the DRt strongly reduced DNIC, thus suggesting that the DRt is a key structure in mediating the effects of DNIC DNIC in Humans Early human studies investigating the DNIC effect combined the use of psychophysical measures with recordings of the nociceptive reflex to test the inhibitory effect of DNIC previously demonstrated in animals As in animals, stimulation of the sural nerve in humans induced a nociceptive reflex in the biceps femoris muscle (the RIII reflex) of the thigh and evoked a painful sensation 90. However, when a noxious cutaneous conditioning stimulus was delivered concomitantly with the sural nerve electrical stimulation, the RIII reflex and the associated pain were significantly reduced 90. These findings parallel results from animal studies and demonstrate that in humans, a painful conditioning stimulus can also inhibit the transmission of nociceptive information induced by the activation of a nociceptive spinal reflex. Studies on tetraplegic patients revealed that the RIII reflex and the pain sensation remained unchanged when a noxious conditioning stimulus was delivered to the contralateral leg 91. These findings demonstrate that in humans, as in animals, the inhibitory effects induced by noxious conditioning stimuli likely involve supraspinal structures. Determining the specific regions involved in mediating DNIC is highly challenging in 14
26 humans since the majority of conclusions are based on observational studies in humans with injuries that caused cerebral or brainstem lesions and associated hemianalgesia Conditioned Pain Modulation (CPM) Although lesion case studies in humans provide the opportunity to explore the structures involved in mediating DNIC, the lesions are not often restricted to the area under investigation and may include surrounding regions or white matter tracts that may affect the interpretation of the symptoms or results. For this reason, the focus of exploring other non-invasive methods and testing similar DNIC evoking paradigms that better predict the mechanism underlying DNIC has greatly advanced in recent years. Different laboratories have employed a variety of approaches and experimental settings to study DNIC-like effects. One psychophysical paradigm often used to test the endogenous circuits of pain modulation in humans is conditioned pain modulation or CPM. This paradigm is based on the same underlying principle as DNIC, where one noxious conditioning stimulus modulates the perception of another noxious stimulus Inducing and Measuring CPM Currently, there is great variability in the experimental design used to test CPM in laboratory settings. Typically, the CPM effect is measured by obtaining subjective pain scores based on numerical scales. However, objective measures of pain evoked potentials recorded from electroencephalogram devices have also been used. Variability in the methodology is also found in the modality of the test stimulus which often depends on the experimental setting. The test stimulus can be delivered thermally via a contact-heat thermode, mechanically by using a pressure based device, or electrically by stimulation of the sural nerve and measuring the reflex response. The conditioning stimulus is often 15
27 delivered by a cold pressor test involving the immersion of a limb in cold water, but other methods such as a hot water bath and/or a second contact-heat thermode have also been employed. CPM methods also differ with respect to the stimulus parameters. Typically, the stimuli are delivered contralaterally however, several studies have also stimulated the same ipsilateral side of the body in different locations and evoked a similar inhibitory effect. The temporal parameters of the stimuli are also variable given that both sequential and simultaneous administration of stimuli have been shown to induce the CPM effect. Additionally, the test stimulus duration and intensity can also vary depending on the experimental set-up and on whether the stimulus follows a tonic or phasic delivery. Due to such variation in the CPM methodology it is challenging to generalize the results and to compare the effects observed between studies Therefore, to advance the translation of laboratory findings to the clinical field, replicable and consistent study designs are necessary Applications of CPM in Clinical Settings In healthy populations, the delivery of a noxious conditioning stimulus reduced the perceived pain of the test stimulus, thus demonstrating efficient CPM. Conversely, CPM was shown to be less efficient in populations with idiopathic pain syndromes such as fibromyalgia 95, tension-type headache 96, irritable bowel syndrome 97, and temporomandibular joint disorder 98. Furthermore, patients with depression experienced less efficient pain inhibition in response to the CPM paradigm, indicating that psychological factors may have a top-down effect on modulating the activity of brainstem regions involved in activating endogenous pain controls in response to CPM. 16
28 Given the promising applications of CPM as a clinical tool used to assess the endogenous analgesia capabilities of various clinical populations, the association between CPM efficiency and pain development has been recently further explored. In an attempt to discern whether low CPM efficiency is a causal factor in the development of chronic pain or the result of persistent pain, the efficiency of CPM in healthy pain-free patients was tested prior and post thoracotomy. Findings reveal that individuals demonstrating less efficient pain inhibition via CPM prior to surgery expressed a higher risk of developing chronic pain post-surgery, suggesting that CPM may have a pathophysiological role in predicting the development of clinical pain. Another clinical application of CPM is its relevance in determining the outcome of a drug treatment for pain. It is assumed that a dysfunction in the pain modulatory network that induces abnormal pain can be corrected by drugs capable of correcting the dysfunction 5. Therefore, patients with less efficient CPM should benefit more from drugs that increase the descending inhibition pathways compared to patients that show efficient CPM 99. As predicted, the less efficient CPM responders benefited more from the administration of drugs by demonstrating reduced perceived pain and an improvement in CPM efficiency, than participants already demonstrating efficient CPM 99. Thus far, there is a large body of behavioral evidence to support the integration of CPM in clinical settings as a tool to assess the capacity to modulate pain and to predict drug efficacy. Unfortunately, less attention has been given to the mechanism underlying CPM, and much of the research has been restricted to animals and patients with different forms of brain and spinal cord injuries 100. With recent advancements in the neuroimaging field, functional magnetic resonance imaging (fmri) has become a widely used non- 17
29 invasive method to study the central processing of pain. As with all other neuroimaging methods, it is crucial to first understand the basic principles behind fmri prior to its application to studying various pain modulating processes within the CNS. The following section will briefly cover the topics necessary to understand and interpret fmri data. 1.3 Magnetic Resonance Imaging (MRI) With the emergence of various neuroimaging techniques, magnetic resonance imaging (MRI) has quickly become a preferred non-invasive techniques used to study the anatomical structure and the neural function of the CNS. Although using MRI to obtain anatomical images differs from using functional MRI (fmri) to investigate neural processing, the basic concepts that underlie both applications are the same. To fully appreciate how to apply MRI techniques and interpret the data, a basic understanding about the fundamental concepts is necessary The MR Signal The source of the magnetic resonance (MR) signal stems from the magnetic properties of hydrogen nuclei (i.e. protons) which are found in abundance in water and lipid containing tissues within the body. Like magnets, hydrogen nuclei have a south and a north pole and have an inherent magnetic field called a magnetic moment. In addition, hydrogen nuclei spin about the same axis that runs between the north and south poles, and have an inherent angular momentum. Typically, hydrogen nuclei are oriented randomly and their magnetic moments cancel out when no external field is applied. However, when a body is placed within a stronger magnetic field such as that of an MR system, the hydrogen within the tissues in the body become weakly magnetized. This causes the 18
30 hydrogen nuclei to spin in alignment with the strong magnetic field (B0), and orient parallel or antiparallel to B0. Each orientation has different energies, where the parallel orientation is the low energy state while the antiparallel is the high energy state. A greater proportion of hydrogen nuclei orient in the lower energy state parallel to B0 than antiparallel to B0, which is considered the higher energy state. The alignment of the hydrogen nuclei to B0 does not occur instantaneously, rather it takes roughly 1-2 seconds for hydrogen nuclei to come into alignment with the static field, much like a spinning top, until reaching equilibrium. The net steady-state magnetization that is aligned to B0 is known as the equilibrium magnetization, denoted by M0. In the equilibrium state, the MR signal cannot be detected. To measure the MR signal, the net magnetization M0 must first be disturbed out of alignment with B0. This is achieved through the application of a second brief magnetic field that has the same frequency as the precession of the hydrogen nuclei. This brief pulse of magnetic field is called a radio frequency or an RF pulse, since the frequency of rotation is often in the radio-frequency ranges. The RF pulse is applied 90 to B0, producing a small magnetic field (B1) rotating at the same frequency of as the hydrogen nucleus precession, known as the Larmor frequency. This tips the magnetization out of alignment with B0 at an angle called the flip angle. Once the RF pulse is turned off and the magnetization M0 has been pushed away from alignment, the hydrogen nuclei begin to relax back to equilibrium in a process called relaxation. During relaxation, the energy that was provided by the RF pulse is now being lost largely by the interaction between hydrogen nuclei in the surrounding environment. Relaxation consists of two components, the longitudinal component and the 19
31 transverse component. The longitudinal relaxation, characterized by T1, is parallel to B0 and grows exponentially to its equilibrium value of M0 as hydrogen nuclei in a higher energy state flip to a lower energy state. The longitudinal relaxation rates vary depending on tissue composition and structure and are influenced by the movement of hydrogen nuclei. Immediately after the RF pulse, the nuclei that are in phase, begin to dephase in the transverse plane. Transverse relaxation is the second component characterized by the time T2. The transverse relaxation is perpendicular to B0 and decays exponentially to its equilibrium value of zero. The transverse relaxation rates also depend on the movement of hydrogen nuclei. If a second RF pulse is applied after the magnetization has completely recovered to equilibrium, the signal will depend on T2. Conversely, if an RF pulse is applied before the magnetization has fully recovered to equilibrium, the signal is dependent on T1. Both relaxation components, longitudinal and transverse (T1 and T2), are highly influenced by the mobility of hydrogen nuclei in their surrounding water and lipid environments. Hydrogen nuclei tend to interact more frequently with other hydrogen nuclei that are in close proximity, thus the relaxation rates are much shorter in lipids than in mobile water. Given that different tissues have different composition and structures, they create different relaxation environments and thus the resulting T1 and T2 relaxation times will differ between various tissues. In reality, there are also subtle changes in the magnetic field when transitioning between different biological tissues (i.e. from bone to air) which contribute to spatial variations within the magnetic field B0. These magnetic susceptibilities cause the transverse magnetization to decay to equilibrium much faster but have no effect on the longitudinal magnetization. Therefore, the effect of the transverse magnetization in 20
32 combination with the effects produced by the spatial variations in the magnetic field is characterized as T2* 101, MR Imaging Methods When in phase, the hydrogen nuclei produce the largest MR signal. Soon after the RF pulse is administered, the hydrogen nuclei dephase quickly due to the spatial variations in the magnetic field and the transverse relaxation, resulting in decay in the MR signal. To bring back the MR signal, it is necessary to reverse the dephasing of the hydrogen nuclei and produce an echo in the signal. In MRI, the fundamental principle of producing echoes to measure the MR signal is at the core of every imaging method. The two imaging methods that use this principle are the spin-echo method and the gradient-echo method. In the spin echo method, a second RF pulse is applied shortly (i.e. less time than the T1value) after the first RF pulse which rotates the magnetization at 90º. The second RF pulse is applied at a flip angle of 180º, causing the hydrogen nuclei to return back in phase, resulting in a brief peak in the signal, or an echo, and the MR signal can be detected. This second RF pulse also cancels out the inhomogeneties in the static magnetic field however, the transverse relaxation is not affected. Therefore, the signal obtained from the spin echo method is T2-weighted. As mentioned above, the gradient echo method also creates echoes to return the MR signal however it does so by applying a magnetic field gradient in one direction and then reversing the gradient to bring the transverse magnetization back into phase. Since the gradients are applied in opposite directions, their effect is cancelled out but the spatial variations in the static magnetic field are not affected and therefore, the peak of the echo is T2*-weighted Functional MRI (fmri) 21
33 Functional MRI (fmri) is one of the many applications of MRI, and is used to study the activation of different brain areas during task performance, or a sensory stimulus. The principle behind fmri is to detect changes in the MR signal over time such that the changes observed in the images correspond to the different neural states during task performance. Both imaging methods, gradient-echo and spin-echo can be applied to fmri however, the gradient-echo method offers the greatest blood oxygenation-level dependent (BOLD) sensitivity when the TE equals the T2* value of the tissues in the region under study 101, Blood Oxygenation-Level Dependent (BOLD) Typically, fmri uses the blood oxygenation-level dependent (BOLD) contrast to detect changes in the magnetic field that are dependent on the changes in neural activity. Because neurons, as with all other cells, do not have internal stores for oxygen and thus rely heavily on oxygen delivered from the immediate vasculature. During periods of increased neural activity, local oxygen consumption increases to meet the increased energy demand. To sustain the increase in activity, the cerebral blood flow (CBF) to the active region is also increased such that the supply of oxygenated hemoglobin exceeds the demand of the active neurons. As such, the levels of oxyhemoglobin and deoxyhemoglobin are altered at the site of increased activity. These changes can be detected by the MR scanner since deoxyhemoglobin and oxyhemoglobin differ in their magnetic properties. The oxyhemoglobin arriving at the active site is diamagnetic and has little effect on the magnetic field however, once it delivers the oxygen for metabolic consumption the deoxyhemoglobin becomes paramagnetic and introduces inhomogeneities in the nearby magnetic field. Distortions in the net magnetic field due to the different oxygenation levels 22
34 lead to changes in the T2 and T2* relaxation times which reveal differences in signal intensities in response to the increased neural activity. In this way, the MR signals can be made to be blood oxygenation-level dependent (BOLD) and reflect the state of metabolic activity of the neural tissues. By acquiring images with BOLD contrast, the data obtained from the images will reveal information about the neural function during task performance 101,102. Following the delivery of a brief stimulus, there is a delay of approximately 2 seconds in the MR signal before any change can be detected. This delay is followed by a gradual increase, reaching a peak at about 6 seconds. With the cessation of the stimulus, the MR signal decreases to a minimum, often dipping below baseline levels before recovering. It takes about 20 seconds for the signal to recover back to baseline levels after the stimulus ended. The resulting shape of the response is depends on the hemodynamic properties of the tissues such as oxygen consumption and changes in blood flow and volume and therefore, the response function is called the hemodynamic response function (HRF). This response function convolved with the stimulation pattern produces the expected BOLD MR signal change which is further used in the analysis of the MR data 101. One important aspect in interpreting the neural activity obtained from using BOLD contrast fmri, is to acknowledge that the activity observed in response to the task reflects the change in presynaptic input and not necessarily the neural firing rate or the postsynaptic output. Additionally, BOLD contrast fmri does not discriminate between types of input signals (i.e. inhibitory or excitatory), but rather the change in activity represents the net effect of a combination of presynaptic inputs 101, Spinal Cord and Brainstem fmri 23
35 Functional MRI is not restricted to the brain and can be applied to other areas of the body such as the spinal cord to view activity related to task performance in the gray matter of the cord. Although many of the imaging parameters used for brain imaging are similar to those applied to the spinal cord, the major differences come from the fact that the spinal cord is structurally different than the brain. For instance, the small size of the gray matter in the cord makes it highly susceptible to body movement which can cause false positive results. Furthermore, the spinal cord is located close to the lungs and heart and thus physiological noise arising from cardiac pulsations and respiration can generate signal intensity fluctuations that can also increase the rate of false positive activations. The spinal cord lies within the spinal canal and is surrounded by cerebrospinal fluid (CSF) which moves in a pulsatile manner with each heart beat, resulting in small movements of the spinal cord as well. The spinal cord is also surrounded by several meningeal layers (i.e. the pia mater, the arachnoid, and the dura mater) and encased in the bony vertebrae. The boundaries between different tissues cause magnetic field distortions which contribute to spatial distortions in MR images, and reductions in T2* values, and reduce the signal intensity in the vicinity of those regions. Often, brain fmri studies use the eco-planar imaging (EPI) method to spatially encode the MR signal because of its fast acquisition speed. On the other hand, employing EPI for spinal cord and brainstem imaging will not produce the same quality images as in the brain due to the different tissue interfaces that are proximal to the spinal cord which subsequently cause distortions in the images. One encoding method that was suggested to overcome these apparent distortions in the brainstem and spinal cord while maintaining a fast acquisition time and a high image quality is the fast spin echo method referred to as a 24
36 half-fourier single-shot fast spin echo (HASTE) 101,104. In a recent study by Bosma and Stroman (2014), HASTE was coupled with a parallel imaging method referred to as generalized autocorrelating partially parallel acquisition (GRAPPA) to determine the optimal imaging methods for spinal cord fmri. Findings from this study indicate that GRAPPA reduced the signal-to-noise ratio and thus consequently led to a reduction in sensitivity to BOLD signal changes. With respect to determining the ideal parameters for spinal cord fmri, this study also concluded that a TE of 75 msec produces the optimal BOLD contrast-to-noise ratio when the HASTE method is employed 104. As with brain fmri data, spinal cord data must follow several preprocessing steps prior to analysis. Bosma and Stroman (2014) have also investigated the method that best reduces the rate of false positives by analyzing spinal cord fmri data using a GLM model without preprocessing steps, with co-alignment only, and with a combination of coalignment and a model-free principal component method with temporal filtering. The findings of this study indicate that only accurate co-alignment steps can reduce the occurrence of false positives 104. Although, the use of model-free principal component method with temporal filtering is shown to increase the number of active voxels in the task data, it consequently also increases the rate of false positives 104. Often temporal filtering is applied as a preprocessing step to brain fmri data however when applied to the spinal cord data, there was no significant improvement in the quality of the signal 104. Overall, the work by the Stroman lab has provided findings that substantially advance the field of spinal cord fmri. 1.4 Pain Processing and fmri Pain Processing and fmri: Regions in the Brain 25
37 Despite the fact that pain perception is variable across individuals due to its subjective component, the neural correlates of thermal pain processing are highly consistent among individuals. Furthermore, fmri studies conducted on a large cohort of healthy volunteers indicated that both noxious cold and noxious heat evoked comparable BOLD responses in the brain 105. The regions of the brain activated in response to the noxious thermal stimulation include the thalamus, the PFC, basal ganglia, S1 and S2, insula, the posterior parietal cortex, and the anterior cingulate cortex (ACC) 106,107. Collectively, these cortical and limbic regions form the pain neuromatrix. During noxious cold and hot stimulation, bilateral activation of these regions was noted, with stronger activation corresponding to the contralateral side 107. The S1 and S2 cortices are known to mediate the sensory discriminative aspect of pain, while the insular cortex has been shown to participate in encoding temperature sensation 108,109. Conversely, the PFC, amygdala, and the thalamus have been suggested to process the affective components of pain 110, while the ACC has been activated in during attentional focus and distraction from painful stimuli 111. These cortical regions are also involved in modulating the perception of pain by projecting to brainstem structures which subsequently activate descending controls that modulate the transmission of nociceptive inputs Pain Processing and fmri: Regions in the Brainstem and Spinal Cord Prior to the application of fmri to the brainstem and the spinal cord, studies were limited to the use of animal models and case studies of injury and disease in humans to study the function of subcortical structures in the processing of pain. With advancements in the fmri field, several studies have shown the importance of the brainstem and spinal 26
38 cord in modulating the transmission and the processing of pain. For instance, a recent study conducted in a group of healthy participants demonstrated that administration of a noxious heat stimulus evoked changes in functional activity observed in the gray matter of the spinal cord at the dermatome level being stimulated 113. Interestingly, heat allodynia which is characterized as the perception of pain evoked by an innocuous stimulus was shown to induce similar functional responses in the dorsal horn as the delivery of noxious heat while innocuous heat did not induce significant changes in the dorsal horn region 113. Noxious cold was also found to strongly activate the superficial layers of the dorsal horn (i.e. laminae I-III) 114. In healthy individuals, a change in temperature from 29 ºC to 15 ºC resulted in a steady fluctuation of percent BOLD signal change between 2% and 3% however, when the temperature was further decreased to 10 ºC, the signal change increased substantially to roughly 8% 115. Interestingly, patients with spinal cord injuries at the lumbar level demonstrated similar percent signal changes as healthy controls however, the distribution of activity differed between the two groups of individuals 115. For instance, the noxious cold stimulation evoked a neural response localized to the ipsilateral side of the dorsal horn in healthy controls while the same stimulation elicited a heightened contralateral ventral horn response and absent or reduced activity in the dorsal horn in patients with spinal cord injuries whom had no sensation of the stimulus 115. The pain-related functional activity within the brainstem region of the CNS has also been recently investigated by the Stroman lab. In one study by Dobek et al. (2014), healthy pain-free participants were presented with a noxious heat stimulus coupled with music, and with another condition consisting of noxious heat stimulation without music, to explore the effect of music modulation on pain. Results indicate that the PAG and the RVM 27
39 demonstrate increased BOLD responses in conjunction with decreased pain perception to the condition with music compared the condition without music 116. This study demonstrates important functional changes occurring in the brainstem in response to pain and further supports the use of fmri as a method of characterizing the subcortical neural pain network participating in the music modulation of pain. A similar study conducted by the Stroman lab further explored the roles of the PAG and RVM in processing pain by evoking enhanced pain perception by means of the temporal summation paradigm. Imaging findings demonstrate greater BOLD responses in the RVM and PAG in response to temporal summation paralleled by an increase in perceived pain compared to the control condition where temporal summation was absent. These enhanced functional responses observed are suggested to demonstrate an engagement of the pain modulatory networks involving the PAG and the RVM, which may ultimately participate in the overall perception of pain. Altogether, these findings provide valuable evidence and more importantly promote the use of brainstem and spinal cord fmri for exploring the neural correlates of pain modulation and pain processing CPM and fmri Current studies using fmri to investigate the neural correlates of CPM have revealed changes in cortical regions involved in pain processing during CPM. In one fmri study, a reduction in pain evoked by the test stimulus was shown to parallel a decrease in activity in pain related regions such as the S1, PFC, ACC, and the anterior insula following the delivery of a conditioning stimulus 100. Similarly, another imaging study revealed reduced PFC, ACC, and S1 activity during analgesia evoked by CPM 40,41. 28
40 Furthermore, activity within the thalamus, insula, S2, medulla, and the amygdala decreased concurrently with the perceived pain evoked by a heat test stimulus upon delivery of a cold conditioning stimulus 117. The reduction in perceived pain correlated with the decrease in activity in the insula, thalamus, the dorsal medulla, and the PFC. When an opioid receptor blocker such as naloxone was administered, the CPM-related activation in the midbrain, OFC, S2, and the amygdala was decreased but the perceived pain was not affected. This suggests that opioidergic system originating in the PAG may not be directly related in mediating the CPM effects. Altogether, neuroimaging studies indicate decreased activity in the PFC, S1, insula, amygdala, and thalamus in response to the CPM paradigm, while brainstem regions such as the PAG and the RVM are suggested to play a more indirect role during CPM. Currently, the majority of fmri studies investigating CPM focus on identifying the cortical structures involved in mediating the analgesic effects of CPM. Few studies have attempted to explore the subcortical regions underlying CPM. Given that less cortical influences and rather activation of brainstem structures has been shown to facilitate the DNIC effect in animals, it is therefore necessary to investigate the brainstem and spinal cord neural correlates during CPM in order to gain a greater understanding regarding the mechanism involved in mediating pain inhibition via CPM. 1.5 Proposed Research Purpose The purpose of this Master s project was to use brainstem and spinal cord functional MRI (fmri) to investigate the neural correlates underlying the conditioned pain 29
41 modulation (CPM) paradigm in healthy participants that demonstrate different behavioral responses to CPM Rationale Studies suggest that when a noxious conditioning stimulus is applied to an area of the body that is remote from that of a test stimulus, the magnitude of the test-pain is reduced. This conditioned pain modulation (CPM) phenomenon has been used extensively to probe the capacity of the endogenous pain network to inhibit perceived pain in humans. Behavioral findings reveal that a large proportion of healthy individuals demonstrate a reduction in perceived pain in response to the CPM paradigm, while populations with various chronic pain syndromes experience a pain facilitatory response to the paradigm. More recently, several studies revealed that healthy pain-free groups have also reported facilitatory responses evoked by CPM which resemble those of patients with chronic pain. To understand the differences in the behavioral responses evoked by CPM in healthy populations, non-invasive methods such a functional MRI (fmri) have been employed, with a large proportion of these studies focusing mainly on cortical activity. Conversely, animal studies suggest that cortical structures are not directly involved in mediating this analgesic effect but rather subcortical regions involved in pain processing such as those located within the brainstem and spinal cord are suggested to underlie this phenomenon. Therefore, to gain a better understanding regarding the differences observed in the behavioral responses in healthy pain-free individuals, the neural responses in subcortical regions must be investigated. This thesis focuses on attempting to determine the mechanism behind CPM by comparing the neural differences in healthy pain-free 30
42 participants responding differentially to the paradigm while using brainstem and spinal cord fmri Hypothesis We hypothesize that the CPM paradigm will evoke a decrease in perceived pain in healthy pain-free participants. Furthermore, we expect significantly greater BOLD responses in the dorsal horn of the spinal cord, and at various levels of the brainstem known to be involved in pain modulation (i.e. PAG, RVM, DRt, PAG, LC, and NTS) during the CPM condition compared to a control condition with no CPM. Lastly, we predict that the connectivity between these regions will be greater during the CPM condition compared to the control condition Objectives The objectives of the study are the following: 1) To determine the spinal cord and brainstem neural correlates of pain inhibition in response to the CPM paradigm. 2) To investigate the differences in the behavioral and neural correlates of CPM in participants experiencing pain inhibition compared to healthy participants demonstrating altered behavioral responses (i.e. pain facilitation) evoked by the CPM paradigm. 31
43 Chapter 2 Investigating the neural correlates of conditioned pain modulation (CPM) in the human spinal cord and brainstem: an fmri study 2.1 Introduction Conditioned pain modulation (CPM) provides a valuable method for investigating a person s capacity to regulate their pain by means of a noxious conditioning stimulus to reduce the pain perceived from a test stimulus 9,10,92,93, Despite the extensive behavioral studies of CPM in humans, our understanding of the related neuronal processes is largely based on analogous animal studies investigating diffuse noxious inhibitory controls (DNIC) 123. Animal studies reveal that dorsal horn pain nociception is strongly influenced by descending inhibitory modulation from supraspinal structures; primarily those that comprise the DNIC system 124. The neuronal processes that underlie the CPM effect in humans may therefore be important to our understanding of endogenous pain regulation and how it can be altered in chronic pain conditions, or as a result of injury or disease. CPM may also involve cognitive and emotional factors that cannot be investigated in animal models. Studies using functional MRI have provided evidence linking the neuronal processes and the behavioral effects associated with CPM, but have been thus far limited to cortical regions 117,121,125,126. Because of the role of the spinal cord and brainstem in the neuronal processes underlying DNIC, we aim to use functional MRI to identify the neural responses in the spinal cord and brainstem that are associated with CPM in healthy human participants. 32
44 Studies in rats and cats have shown that dorsal horn neurons can be inhibited when a noxious stimulus is applied to a body region that is distant to the neurons excitatory receptive fields 74,127. Sectioning the spinal cord can cause a reduction in the DNIC effect suggesting the importance of supraspinal structures in DNIC 76,78,82. Furthermore, DNIC was found to be mediated via the dorsal reticular nucleus (DRt) while brainstem regions such as the periaqueductal grey (PAG) and the rostral ventral medulla (RVM) were suggested to have an indirect role in supporting DNIC 69,83,128. In humans, several studies using functional MRI have demonstrated CPM-related responses in the prefrontal cortex (PFC), the primary and the secondary somatosensory cortices (S1, S2), the anterior cingulate cortex (ACC), the insula (INS) and the PAG, in response to CPM 117,121,125,126. Increased activity in cortical regions such as the PFC, ACC, thalamus, and the insula during CPM was associated with descending pain modulating activity to subcortical structures 126,129,130. Upon recent advancements in spinal cord/brainstem fmri data acquisition and analysis we can now investigate descending inhibition of spinal cord nociception in key areas of the spinal cord and brainstem that were previously inaccessible 104, We hypothesize that a decrease in pain perception will correspond to an increased BOLD signal change (% change) in the dorsal horn of the spinal cord, the PAG, the RVM, and the DRt in the CPM condition compared to the Control condition. This is based on the expectation of increased descending inhibition of spinal cord responses. As the regions outlined above are known to participate in descending pain modulation and in DNIC, we further hypothesize that the connectivity between these regions will be greater in response to the CPM effect. 33
45 2.2 Methods Participants Eighteen healthy, right handed females (mean age 26.8 ± 6.21) with no history of chronic pain, psychiatric or neurological disease, were recruited from the local Kingston community to participate in the study. All subjects provided written consent, and were free to withdraw from the study at any time. The study included three study visits (one training session, and two imaging sessions). Six participants did not show the CPM effect during the first stage of behavioral training and were excluded from this study. From our final sample of twelve participants, two participants completed only the training and the first imaging sessions, and were thus excluded from the behavioral analysis. Only women were enrolled in the study to avoid gender confounds and sex differences in the CPM effect as discussed in other studies 94, Questionnaires All participants completed a set of questionnaires at the training session. The questionnaires included the Beck Depression Inventory-II (BDI-II) 139, the State/Trait Anxiety Questionnaire 140, the Social Desirability Scale 141, and the Pain Catastrophizing Scale 142. The BDI-II consists of 21 items where participants rated their experience with depressive symptoms on a scale from 0 to 3, with possible scores ranging from 0 to 63 (0-10 = normal ups/downs in mood, = mild mood disturbances, = borderline clinical depression, = moderate depression, = severe depression, and = extreme depression). The State/Trait Anxiety Questionnaire consists of two surveys measuring the transient state anxiety, and the trait anxiety, each section having 20 items. The participants rated their feelings based on a 1 to 4 Likert scale (1 = not at all, 2 = 34
46 somewhat, 3 = moderately so, 4 = very much so), for a possible range of scores from 20 to 80 (20-39 = low anxiety, = moderate anxiety, and = high anxiety). The Social Desirability Scale consists of a personal reaction inventory based on True/False statements. There are a total of 33 items for a total score of 0 to 33 (0-8 = low, 9-19 = average, and = high). Lastly, the Pain Catastrophizing Scale measures the participants level of rumination, magnification, and helplessness, based on 13 items ranging from 0 (not at all) to 4 (all the time) on a Likert scale. The total score ranges from 0 to 52, where a score higher than 30 is considered clinical relevant catastrophizing. Questionnaires were scored and descriptive statistics were calculated for each Pain rating scales Participants used a heat and a cold pain scale to rate their sensations to the test stimulus (TS) and to the conditioning stimulus (CS), respectively (Figure 1.). Both pain rating scales range in pain intensity from 0 (No sensation) to 100 (Intolerable pain) in increments of 10. A rating of 10 is considered a Warm sensation on the heat pain rating scale and a Cool sensation on the cold pain rating scale, 20 is a Barely painful sensation, 30 is Very weak pain, 40 is Weak pain, 50 is Moderate pain, 60 is Slightly strong pain, 70 is Strong pain, 80 is Very strong pain, and 90 is Nearly intolerable pain. Participants were instructed to give numerical values to rate their sensations. A rating of 20 on both pain scales indicates the pain threshold for heat and cold pain, and a rating of 50, which indicates moderate pain, was the target rating used to calibrate the temperatures applied for each participant during the experimental trials. 35
47 Figure 1. Pain rating scales used to rate pain sensations. The top scale is the heat pain rating scale used to rate the TS, and the bottom scale is the cold pain rating scale used to rate the CS. A rating of 20 on both scales represents the pain thresholds, while a rating of 50 is indicative of the target temperature used during the stimulation period of the experimental runs Training session All participants underwent a training session in a mock fmri environment to become familiar with the heat and cold sensations, the pain rating scales and the scanning environment. Once the pain rating scales were explained, participants were exposed to heat and cold sensations to test their pain thresholds via a Medoc TSA-II thermal sensory analyzer (Medoc Ltd, Ramat Yishai, Israel). The thermode delivering the heat sensations (TS) was always applied to the right thenar eminence and the thermode that delivered the cold sensation (CS) was always applied to the left thernar eminence (Figure 2.). 36
48 Figure 2. Schematic representation of the delivery locations for the test stimulus (TS) and the conditioning stimulus (CS). Heat and cold pain thresholds were both determined using the following procedure. By pressing the buttons on a mouse connected to the Medoc system, participants either decreased or increased the temperature of the heat/cold pain until they reached a pain rating of 20 on the pain rating scales. Afterwards, sensory discrimination was assessed and participants rated their pain when the thermode was applied to three locations on the forearm at three different temperatures (45 C, 46 C, and 47 C). Participants were then positioned supine on the bed inside the mock MRI system and underwent temperature calibrations and the CPM protocol as it would be presented in subsequent MRI sessions. They viewed a rear-projection screen (via a mirror) which enabled them to see the pain rating scales. While lying in the mock-mri, the heat pain was calibrated first, followed by the cold pain, to reach a pain rating of 50 with each type of stimulus. During the heat pain calibrations, the cold thermode remained at baseline (32 ºC), and while the cold pain was calibrated, the heat thermode also maintained the baseline temperature. The calibration step followed a block design where the thermode started at 32 C for 5 seconds, then ramped up (for the heat pain calibrations) or ramped down (for the cold pain calibrations) 37
49 to a new temperature which was maintained for 33 seconds (for the TS) or 43 seconds (for the CS). The participants were asked at the beginning, at the middle, and at the end of the stimulation period to rate their pain to the TS and/or to the CS to give an indication of how their sensation progressed during the stimulation period. A 2 minute break was given between each run to prevent sensitization of the nociceptive afferents and a total of 6 runs were employed. If the participants ratings were below or above 50, the temperatures were adjusted (increased or decreased) accordingly, and additional trials were run until the ratings during the stimulation period averaged to 50. Temperatures for the TS and the CS that elicited ratings of 50 were used in the experimental runs for the remainder of the study Test Stimulus (TS) for fmri studies The test stimulus (TS) used for fmri studies was the same as described above for the training sessions, and was applied to the skin overlaying the thenar eminence on the right hand, corresponding to the C6 dermatome. The stimulation paradigm was administered in a block design, where the test stimulus was flanked by two baseline periods set at an innocuous temperature of 32 C. The test stimulus began at the baseline for 51 seconds, and then increased to a predefined temperature calibrated at moderate pain for 33 seconds, followed by a decrease in temperature to the baseline which was maintained for 64 seconds. The entire run lasted 155 seconds (Figure 3.). Between each run, a 2 minute break was given to prevent sensitization of nociceptive afferents 143 and to let the participants sensation return to baseline prior to the next stimulation run. 38
50 Figure 3. A schematic representation of the task paradigm used to assess the CPM effect in the fmri sessions. Both TS and the CS stimuli begin and end at a baseline (32 C) temperature, flanking a stimulation period of 33 seconds for the TS and 43 seconds for the CS. One entire run is 155 seconds. In the Control condition, only the TS is delivered while the CS remains at a baseline temperature. In the CPM condition, both the TS and the CS are delivered simultaneously as indicated. Each condition consists of 6 total runs per participant Conditioning Stimulus (CS) for fmri studies The conditioning stimulus (CS) was again as used in the training sessions and was delivered using a second Medoc TSA-II thermal sensory analyzer. This thermode was secured to the thenar eminence of the left hand. The CS was applied simultaneously with the TS in order to generate the CPM effect however, the stimulation period (43 seconds) was preceded by a baseline period (41 seconds) and followed by a second baseline period (56 seconds). The CS change in temperature rates were longer than those used to deliver the TS because of hardware limitations in the rate of temperature change. The entire run lasted 155 seconds. The stimulation period for the CS started 5 seconds earlier and lasted 5 seconds longer than that of the TS to enable us to distinguish between the BOLD responses corresponding to the CS paradigm and those corresponding to the TS paradigm in the imaging data (Figure 3.) Experimental design 39
51 There were 2 experimental conditions that were tested in the study: 1) Control, and 2) CPM. In the Control condition, only the TS was delivered and the thermode inducing the CS remained at baseline during the entire run. In the CPM condition, both CS and the TS were employed to produce the pain modulation effect. In each experimental run, the participants viewed a set of instructions at the beginning of the run which indicated which pain (TS on the right hand or CS on the left hand) should be the focus of their attention, followed by the appropriate rating scale. The participants were blinded to which pain they would rate until after the stimulation period, when a set of instructions prompted the participants to rate their pain to either the TS or the CS. The study included runs in which participants rated the cold CS to further blind the participant to the study design and to prevent the participants from guessing which pain to rate. For each participant, both conditions were repeated three times for a total of twelve runs over the course of the two study visits. Out of the 6 runs in the CPM condition, two runs involved rating the CS. Only the ratings for the TS from all conditions were included in the analysis. All study sessions including the training session were conducted on different days to avoid participant fatigue and nociceptor over-sensitization over the course of the three visits Functional MRI (fmri) session Set-up At the start of each imaging session, the pain rating scales, the instructions, and the experimental design were once again explained to the participants. Participants were then positioned supine on the MRI bed and were given ear plugs as hearing protection from the scanner noise. The thermodes were then secured to the participants left and right hands. Once inside the scanner, two practice runs (without image acquisition) with the TS alone 40
52 and then the CS alone were conducted to confirm that the target temperatures used in the training session still induced a moderate pain (i.e. pain rating of 50) in the fmri sessions. If the previously determined target temperatures for the two stimuli evoked different ratings than those obtained during the training session, the participants were recalibrated by adjusting the temperature until a pain rating of 50 was reached, as detailed in the Training session above Data acquisition All fmri data were acquired in 3 Tesla whole-body MRI system (Siemens Magnetom Trio; Siemens, Erlangen, Germany) equipped with a spine-array receiver coil, a 3-channel posterior neck coil, and a body coil to deliver the radio-frequency (RF) pulses. A T2-weighted half-fourier single-shot fast spin-echo (HASTE) imaging sequence was used to provide blood oxygenation-level dependent (BOLD) contrast. Imaging parameters included a repetition time (TR) of 4.77 sec and an echo time of 76 msec to produce optimal T2-weighted BOLD contrast. Nine contiguous slices in the sagittal orientation spanning from above the corpus callosum to just below the T1/T2 intervertebral disc were acquired per run. A total of 32 volumes/ run were generated and each run was repeated 3 times for each of the 2 conditions for a total of 6 functional runs were acquired in each fmri session. Each complete set fmri data set for each condition consisted of a total of 192 volumes. The image resolution was 1.5 mm x 1.5 mm, with a slice thickness of 2 mm. The field of view was 280 mm x 140 mm and the matrix size was 192 x 96. During each acquisition, a spatial suppression pulse was applied anterior to the spinal cord to reduce motion artifacts resulting from physiological movement such as swallowing, breathing, and heart beating. 41
53 Lastly, the first volume of each run was discarded to reduce the effects of variable T1- weighting Data analysis Behavioral data A two factor repeated-measures ANOVA was conducted to test for significant differences in pain ratings between the two imaging sessions, and between the two conditions. Significant effects were followed up by 2-tailed paired Student s t-tests. The descriptive statistics which include the mean, standard deviation (SD), and median were calculated at the group level to provide quantitative information regarding the TS pain ratings for each condition. The CPM effect size was calculated based on the difference in TS pain ratings obtained in the CPM condition and the Control condition Functional MRI data: Data preprocessing Custom written software in MatLab (MathWorks, Natick, MA) was used to analyze all brainstem and spinal cord fmri data. The image data were first converted from DICOM format to NIfTI format, followed by several preprocessing steps. These included coregistration of the data to correct for bulk movement using a non-rigid 3D registration tool, the MIRT toolbox 144,145, which applied a non-linear 3D adjustment to align each volume of time series to the third volume of the set. A spatial normalization step was also applied to the data to automatically calculate the parameters necessary to transform the original image data into a shape and size that matches a predefined target template. This step was completed in two steps, where the first step generated the rough normalization parameters as a close estimate to the template, followed by a fine-tuning step to improve the match to 42
54 the template. The resulting normalized data spanned the entire cervical spinal cord and brainstem with 1 mm cubic voxels Functional MRI data: General Linear Model (GLM) analysis The normalized image data from each experimental condition were combined and spatially smoothed with a 3 mm x 3 mm x 5 mm (R/L x A/P x S/I) boxcar kernel 104,146. One large time-series was created by averaging all the individual runs. This process maintained the benefits of acquiring large volumes of data by lowering the physiological noise in the data that was not associated with the stimulation paradigms to nearly zero, and improving the BOLD signal change that is correlated with the stimulation paradigms. This analysis method consisted of a set of basis functions that included the TS and the CS pain paradigms convolved with the BOLD hemodynamic response function (HRF) 104, the first two principle components of the time-series data from all voxels representing the global signal variance within the data, and a constant function. Statistical significance for BOLD signal changes was inferred at p < Functional MRI data: Structural Equation Modeling (SEM) analysis Structural equation modelling (SEM) or path analysis was used to determine hypothetical connections modelled between regions within the brainstem, spinal cord, thalamus, and hypothalamus. This analysis method is based on the assumption that the BOLD signal change in one region is predominantly related to signaling input from other sources. Therefore, the BOLD response observed in a certain region is modelled as the weighted sum of responses from other regions that are anatomically connected and may provide significant inputs in response to the stimulation. Each connection was assigned a weighting factor that best fitted the measured data. 43
55 The preprocessed data was subjected to SEM analysis made available through custom written software in MatLab developed based on methods described by McArdle and McDonald and also in Craggs et al. 147,148. The connectivity model that was used to test possible connections between brainstem, thalamic, and spinal cord regions was composed based on anatomical descriptions from Millan 42. The data from regions identified by the model were extracted from all participants and runs, and concatenated into one time-series of functional responses for each voxel. The temporal properties of the time-series data were then used to divide the normalized data into sub-regions by means of k-means clustering. This step allowed for the active voxels that corresponded to significant BOLD signal changes to be grouped separately from voxels which may have introduced false positive activity in the data due to the physiological movement at the edge of the CSF and cord. All sub-regions were analyzed, and the combination of region that generated the greatest fit was then used to determine the linear weighting factors calculated for 35 different network connections. Significant connections were determined by means of onesample t-tests that calculated the ratio of the magnitude of each weighting factor to its standard-error. The threshold for significance was inferred at T > 2 which corresponded roughly with p < Results Questionnaires Descriptive statistics of the questionnaire scores from all participants are summarized in Table 1. Based on normative data, all questionnaire scores fall within the normal ranges. 44
56 Table 1. Descriptive statistics of demographic information and questionnaire scores from all participants Behavioral results We conducted a two factor repeated-measures ANOVA to compare the TS pain rating scores between the two fmri sessions and between the conditions (CPM and Control). The results indicate that there was no significant effect between imaging sessions and therefore the TS pain ratings obtained during the first and second imaging session did not differ (F(1,9) = 1.05, p =.33). Figure 4. Reported TS pain ratings for each condition obtained during the two fmri sessions. The ratings are averaged TS pain ratings with standard error bars. The asterisk (*) indicates significance of p <
57 There was however, a significant difference in the TS pain ratings between the CPM and the Control conditions (F(1,9) = 16.25, p <.005). A paired-sample Student s t- test showed that the TS pain ratings in the Control condition were significantly higher than the ratings in the CPM condition (t(8) = 2.31, p <.005) (Figure 4). Descriptive statistics of the TS pain scores obtained in the two fmri sessions are summarized in Table 2. Table 2. Descriptive statistics of pain50 temperatures used to deliver the TS and the CS, and the TS pain ratings from the Control and CPM conditions obtained in the fmri runs GLM results GLM analysis conducted on fmri data from the Control and CPM conditions reveals BOLD signal changes in brainstem and spinal cord regions that significantly match the stimulation paradigms (p < 0.001) (Figure 5). In the Control condition, the BOLD signal change observed is in response to the TS paradigm, while the BOLD signal in the CPM condition is the response to both the TS and the CS paradigms. Results revealed active voxels in the vicinity of the PAG, Drt, RVM (NRM and NGc), and in the right DH of the C6 segment during both Control and CPM conditions, and in the left DH during the CPM condition only. Contrast analyses comparing the BOLD activity in the two conditions were calculated by subtracting the time-course data on a voxel-by-voxel basis between the two conditions, to determine regions of the brainstem and cervical spinal cord that respond 46
58 significantly different (i.e. more and/or less) during the CPM condition than during the Control condition. Figure 5. BOLD signal changes that significantly match the stimulation paradigms, observed at different levels of the brainstem and spinal cord, during each experimental condition. The location of the selected slices are illustrated in the left reference template. Regions of interest in each slice are marked in red and include the periaqueductal grey (PAG), rostral ventromedial medulla (RVM) which encompasses the nucleus raphe magnus (NRM) and the nucleus gigantocellularis (NGc), dorsal reticular nucleus (DRt), and the C6 spinal cord sections indicating the left and right dorsal horns (DH). The Control condition represents BOLD responses to the TS paradigm, and the BOLD response observed in the CPM condition represents activity to both the TS and the CS paradigms. The BOLD activity observed represents group results that are consistent across all participants. The functional activity is overlaid on anatomical scans of the selected levels of the brainstem and spinal cord. Cool colors indicate decreased BOLD signal activity from baseline, and warm colors show an increase in BOLD signal activity from baseline. The threshold for statistical significance is at p < Results reveal that the PAG, RVM (NRM and NGc), and the left and right dorsal horn at the C6 segment were more responsive, while the DRt was less responsive in the CPM condition compared to the Control condition (Figure 6). 47
59 Figure 6. BOLD signal changes that that are significantly different between the CPM and the Control conditions. The functional activity in the PAG, RVM, DRt, and the dorsal horn of the spinal cord is overlaid on anatomical templates. Cool colors represent negative difference in activity between two conditions, and warm colors indicate positive difference in activity between two conditions. The threshold for statistical significance is at p < SEM results SEM analysis revealed a network of significant connections within the brainstem and the right dorsal horn of the C6 segment, and included connections that were common across the conditions, and connections that were unique to each condition (Figure 7). The common connections exhibited in both conditions were bidirectional between the DRt and the C6 dorsal horn, and between the RVM and the C6 dorsal horn (indicated by the black lines). Results also revealed several connection connections that were unique to the CPM condition, including between the thalamus PAG, PAG RVM, PAG DRt, and hypothalamus RVM. 48
60 Figure 7. Map illustrating network connections in the Control and the CPM conditions. Red lines represent connections that are unique to the Control condition, blue lines represent connections that are unique to the CPM condition, and black lines illustrate connections that are common to both Control and CPM conditions. Arrows indicate the direction of input, solid lines represent positive connections and dashed lines represent negative connections. The line thickness indicates the strength of the connection. The RVM consists of the NRM and the NGc, outlined in yellow. All path coefficients have a significance of T > Discussion The objective of this study was to characterize the neural correlates of CPM in the human brainstem and spinal cord by means of functional MRI. The behavioral data demonstrate a significant reduction in the TS pain ratings when the CS was applied simultaneously, confirming the CPM effect. Furthermore, we observe changes in BOLD activity corresponding to the CPM effect in regions of the brainstem such as the PAG, RVM, and the DRt, which are known to be involved in the descending modulation of pain 42,149,150. Our connectivity data parallel these results and demonstrate effective 49
61 connectivity between Thal PAG, PAG RVM, PAG DRt which are present only during the CPM condition and absent during the Control condition. Altogether, these results indicate that the neural correlates of CPM involve the activation of brainstem regions that coordinate descending inhibition of the spinal cord which subsequently reduce pain perception. We found BOLD responses in the PAG, RVM, DRt, and the right dorsal horn of the C6 segment that are similar during both conditions (Figure 5). These results are further supported by our SEM analysis which indicate significant connections between the RVM and the right dorsal horn of the C6 segment that are bidirectional and common to both the CPM and the Control conditions (Figure 7). As both conditions involved a heat pain stimulus applied to the right hand, it is expected that similar areas would be engaged in both conditions, however the magnitudes and extent of these responses is expected to change with the addition of the cold stimulus in the CPM condition. The areas that responded to the heat stimulus are also consistent with previous findings which have shown increased BOLD responses in these areas upon noxious heat stimulation 113,132. The PAG and the RVM are well known to be implicated in descending pain modulation and thus the activity in these regions is believed to arise from incoming nociceptive inputs that trigger descending inhibitory controls to the dorsal horn 151,152. Likewise, the DRt is mainly activated by noxious stimulation and it is also an important structure involved in pain processing and descending modulation 70. Retrograde tracing has established that the DRt receives bilateral connections from the spinal cord 67,69,153,154. Our GLM results parallel these findings as the fmri data suggests an increase in BOLD activity in the DRt in response to noxious input ascending from the stimulated region of the spinal cord. The 50
62 relationship between the DRt and the dorsal horn of the spinal cord is further illustrated in our connectivity analysis which indicates significant connections between these two regions during both conditions. The GLM results together with the SEM results thus provide evidence that ascending nociceptive inputs from the dorsal horn activate the descending modulation network involving the PAG, RVM, and the DRt, which subsequently alter the dorsal horn neuronal activity. To date, this is the first imaging study conducted in humans that reveals changes in brainstem and spinal cord BOLD activity in response to the CPM effect. The results of our contrast analysis show more BOLD activity in the PAG, RVM, and in the left and right dorsal horn, in response to CPM (Figure 6). The addition of the conditioning stimulus results in greater input into these structures and/or greater local processing which suggests activation of the descending inhibitory network. In agreement with these results, connectivity analysis also demonstrates the existence of significant connections between the PAG and the RVM that are unique to the CPM condition. Sprenger et al. (2011) reported a similar increase in connectivity within the PAG-RVM system when a cold pressor test was administered on the right leg against a phasic test heat delivered to the left forearm. The PAG is known to mediate descending inhibition indirectly to the spinal cord by stimulating the RVM which projects to the cord and modulates the transmission of nociceptive signals This network is illustrated in our connectivity results, where the BOLD signals from the PAG RVM C6 right dorsal horn co-vary in response to CPM (Figure 7). Because the intensity and the duration of the test stimulus delivered to the right hand did not change between the two conditions, the increase in BOLD activity in the right dorsal horn observed in response to the CPM effect likely reflects increased descending 51
63 inhibition from the PAG and the RVM as suggested above. Descending inhibition leads to a reduction in spinal neuron activity and ultimately to less noxious transmission to cortical regions involved in pain processing 46,152,155. This assumption agrees with the behavioral results which indicate that the pain ratings to the test stimulus are lower during the CPM condition compared to the Control condition due to an increase in descending inhibition in response to CPM. Conversely, we cannot draw the same conclusion regarding the BOLD response in the left dorsal horn seen in our contrast data. This is because the CPM condition includes the additional input of the conditioning stimulus which is not present in the Control condition. Therefore, the difference in BOLD activity in the left dorsal horn may be attributed largely to the incoming noxious stimulation from the conditioning stimulus. Based on animal studies, the DRt is suggested to be the primary brainstem region known to mediate DNIC 71,72,156,157. Currently, there are no studies in humans that have looked at the participation of the DRt in CPM, presumably due to its small size and the acknowledged difficulty of imaging in this region 126. With recent advancements in functional imaging in the spinal cord and brainstem, this is the first study to investigate the activity of the DRt during CPM in humans. Our fmri results reveal that the DRt responds less in the CPM condition than in the Control condition (Figure 6). In animals, it was suggested that the DRt plays a role in exerting excitatory descending connections on the dorsal horn neurons that receive noxious inputs 153. Therefore, a reduction of the DRt activity, as shown in our imaging results, reflects a reduction in its facilitatory action which may contribute to a decrease in perceived pain during CPM. Additionally, our connectivity results show that the BOLD responses in the DRt co-vary with the BOLD responses in the PAG during CPM but not during the Control condition. This indicates that the DRt 52
64 receives additional inputs from brainstem regions during CPM which may modulate its activity and reduce evoked excitation in the dorsal horn neurons that are receiving the noxious input. Although our results provide valuable insight into the neural correlates of the CPM effect, it is important to note that fmri results must be interpreted with caution. The BOLD signal corresponds to changes in local field potentials, which are the electric potentials in the extracellular space generated by the summed current from multiple nearby neurons 103. Based on the fmri data, we cannot differentiate between facilitatory or inhibitory circuits because a change in BOLD activity can arise from either or both. For instance, the RVM is known to be an important relay site whose descending influences have a dual role in pain control 158. This structure can inhibit and facilitate nociceptive input, depending on the input it receives from other surrounding regions. In the case where the descending facilitatory and inhibitory influences are engaged simultaneously, the resultant BOLD signal changes can only provide information regarding the overall input to that region. There are also several study limitations that may restrict the generalizability of our current findings. Firstly, this study was limited to female participants to avoid gender differences that have previously been reported Obtaining data from male subjects may provide valuable information that could facilitate our understanding of the behavioral differences in the CPM effect observed between genders. Secondly, including a greater sample size may have yielded more generalizable results. Lastly, this study included only participants demonstrating the behavioral CPM effect for the purpose of investigating the neural correlates of the analgesic effect evoked by CPM. Collecting data from subjects 53
65 demonstrating an altered behavioral effect to the CPM paradigm would expand our understanding of the neural processes underlying CPM. 2.5 Conclusion This is the first study that demonstrates the neural correlates of CPM in the brainstem and spinal cord in humans. The results show increased BOLD activity in the PAG and in the RVM contributing to descending pain modulation during CPM. Connectivity results also reveal significant connections in the CPM condition between the PAG and the RVM that are not observed during the Control condition. We have also demonstrated decreased BOLD responses during CPM in the DRt region of the brainstem known to underlie the mechanism behind DNIC in animals 72. Collectively, the action of each of these brainstem regions induced the analgesic effect that characterizes CPM. This study helps understand the CPM response in humans and can be used as a tool to determine how altered CPM induces changes in descending modulation in chronic pain populations. 54
66 Chapter 3 Differences in pain perception and in the brainstem and spinal cord neural responses to the conditioned pain modulation (CPM) paradigm in healthy controls: an fmri study 3.1 Introduction Conditioned pain modulation (CPM) is a frequently employed laboratory method used to measure the ability of a conditioning noxious stimulus to reduce the pain of a noxious test stimulus, when applied simultaneously on distant regions of the body 92. CPM has been shown to induce pain inhibition in healthy controls 5 and also pain facilitation in several chronic pain syndromes as in irritable-bowel syndrome 6, fibromyalgia 7,8, and temporomandibular disorder 159. Although healthy pain-free individuals often demonstrate efficient CPM resulting in pain inhibition, several studies have also indicated variability in responses, with some healthy individuals demonstrating less efficient CPM with pain facilitation 117,125,126. Furthermore, pain-free patients demonstrating less efficient CPM prior to thoracotomy were found to have a higher risk of developing chronic pain postsurgery 9. Given the behavioral evidence suggesting the association of altered CPM responses with the development of chronic pain, investigating the neural correlates that reflect these altered behavioral responses to the CPM paradigm may deepen our understanding of the changes in the descending inhibitory controls that underlie chronic pain conditions. 55
67 In humans, only a limited number of studies have investigated the neural components involved in mediating the analgesic effect of CPM. Functional MRI studies reveal increased activity in the prefrontal cortex (PFC), thalamus, insula, and in the anterior cingulate cortex (ACC) associated with efficient CPM 117,121,125,126. Activation in these cortical regions has also been suggested to reflect increased descending modulation to brainstem structures such as the periaqueductal gray (PAG), the rostral ventral medulla (RVM), and the dorsal reticular nucleus (DRt), which are known to influence pain processing and perception 126,129,130 and have been suggested to underlie the diffuse noxious inhibitory controls (DNIC) equivalent of CPM in animals 46,72,83,85,86,156,160,161. Given the importance of these brainstem regions to modulate the transmission of noxious inputs during DNIC, the aim of this study was to use functional MRI to investigate brainstem and spinal cord responses to the CPM paradigm in separate groups of healthy participants who demonstrate efficient CPM, and less efficient CPM. The purpose of this study is to further our understanding of pain processing and the differences in descending pain networks that contribute to altered behavioral responses to the CPM paradigm. We hypothesize that efficient CPM responders will demonstrate greater BOLD changes (% change) in the PAG, the RVM, the DRt, and in the right DH of the spinal cord corresponding to pain inhibition during CPM, compared to participants demonstrating less efficient CPM. Furthermore, we also hypothesize that during CPM the connectivity between the regions outlined above will also differ between responders. 3.2 Methods The methods of this study are similar to those described in the previous chapter. 56
68 3.2.1 Participants Twenty-five right handed healthy females (mean 27.6 ± 7.82) with no history of chronic pain, psychiatric conditions, or neurological disease, were recruited from the local community to participate in the study. All subjects provided written consent, and were free to withdraw from the study which included three study visits (one training session, and two imaging sessions), at any time. Two participants completed only the training and the first imaging sessions, and were thus excluded from the behavioral analysis. Only women were enrolled in the study to avoid gender confounds and sex differences in the CPM response as discussed in other studies 94,136, Questionnaires At the training session, a set of four questionnaires were administered to all participants. These included the Beck Depression Inventory-II (BDI-II) 139, the State/Trait Anxiety Questionnaire 140, the Social Desirability Scale 141, and the Pain Catastrophizing Scale 142. The BDI-II evaluates one s experience with depressive symptoms using 21 items. Each item ranges in score from 0 to 3, for a total possible score of 0 to 63 (0-10 = normal ups/downs in mood, = mild mood disturbances, = borderline clinical depression, = moderate depression, = severe depression, and = extreme depression). The State/Trait Anxiety Questionnaire evaluates the transient state anxiety and the long standing trait anxiety using two surveys, each consisting of 20 items. Items are rated based on a 1 to 4 Likert scale (1 = not at all, 2 = somewhat, 3 = moderately so, 4 = very much so), for a possible total score ranging from 20 to 80 (20-39 = low anxiety, = moderate anxiety, and = high anxiety). The Social Desirability Scale assesses whether responders are truthful or misinterpreting their self-presentation. The scale 57
69 consists of 33 items that are scored based on a True/False response format, for possible scores ranging from 0 to 33 (0-8 = low, 9-19 = average, and = high). Lastly, the Pain Catastrophizing Scale evaluates the responder s level of rumination, magnification, and helplessness, based on 13 items ranging from 0 (not at all) to 4 (all the time) on a Likert scale. The total score ranges from 0 to 52, where a score higher than 30 is considered clinical relevant catastrophizing Pain rating scales Participants used a heat and a cold pain scale to rate their sensations to the test stimulus (TS) and to the conditioning stimulus (CS) respectively (Figure 1.). Both pain rating scales range in pain intensity from 0 to 100 in increments of 10 (0 = No sensation, 10 = Warm sensation/cool sensation, 20 = Barely painful sensation, 30 = Very weak pain, 40 = Weak pain, 50 = Moderate pain, 60 = Slightly strong pain, 70 = Strong pain, 80 = Very strong pain, 90 = Nearly intolerable pain, 100 = Intolerable pain). Participants were instructed to give numerical values to rate their pain. A rating of 20 on both pain scales indicates the pain threshold for heat and cold pain, and a rating of 50, was the target rating used to calibrate the temperatures applied for each participant Training session All participants underwent an initial training session in a mock fmri environment to become familiar with the heat and cold sensations, the pain rating scales, and the scanning environment. Once the pain rating scales were explained, participants were exposed to heat and cold sensations to test their pain thresholds via a Medoc TSA-II thermal sensory analyzer (Medoc Ltd, Ramat Yishai, Israel). The thermode delivering the heat sensations (TS) was always applied to the right thenar eminence and the thermode that 58
70 delivered the cold sensation (CS) was always applied to the left thenar eminence (Figure 2.). Heat and cold pain thresholds were both determined using a computer mouse, where the participants pressed buttons to either decrease or increase the temperature of the heat/cold pain until they reached a pain rating of 20 on the pain rating scales. Afterwards, sensory discrimination was assessed and participants rated their pain when the thermode was applied to three locations on the right forearm at three different temperatures (45 C, 46 C, and 47 C). Participants were then positioned supine on the bed inside the mock MRI and underwent temperature calibrations and the CPM protocol as it would be presented in subsequent MRI sessions. They viewed a rear-projected screen (via a mirror) which enabled them to see the pain rating scales. While lying in the mock MRI, the heat pain was calibrated first, followed by the cold pain, to reach a pain rating of 50 with each. During the heat pain calibrations, the cold thermode remained at baseline (32 ºC), and while the cold pain was calibrated, the heat thermode also maintained the baseline temperature. The calibration step followed a block design where the thermode started at 32 C for 5 seconds, then ramped up (for the heat pain calibrations) or ramped down (for the cold pain calibrations) to a new temperature which was maintained for 33 seconds (for the TS) or 43 seconds (for the CS). The participants were asked at the beginning, at the middle, and at the end of the stimulation period to rate their pain to the TS and/or to the CS, to give an indication of how their sensation progressed during the stimulation period. A 2 minute break was given between each run to prevent sensitization of the nociceptive afferents and a total of 6 runs were employed. If the participants ratings were below or above 50, the temperatures were adjusted (increased or decreased) accordingly, and additional trials were run until the ratings during the stimulation period averaged to 50. Temperatures for the TS 59
71 and the CS that elicited ratings of 50 were used in the experimental runs for the remainder of the study Test Stimulus (TS) This study used a noxious thermal heat stimulus as the test stimulus (TS), applied to the skin overlaying the thenar eminence (corresponding to the C6 dermatome). The stimulation paradigm was administered in a block design, where the test stimulus was flanked by two baseline periods set at an innocuous temperature of 32 C. The test stimulus began at the baseline for 51 seconds, and then increased to a predefined temperature calibrated at moderate pain for 33 seconds, followed by a decrease in temperature to the baseline which was maintained for 64 seconds. The entire run lasted 155 seconds (Figure 3.). Between each run, a 2 minute break was given to prevent sensitization of nociceptive afferents and to let the participants sensation return to baseline prior to the next stimulation run Conditioning Stimulus (CS) The conditioning stimulus (CS) was a noxious thermal cold stimulus applied to the thenar eminence of the left hand. The CS was delivered simultaneously with the TS in order to generate the CPM effect however, the stimulation period started 5 seconds earlier and lasted 5 seconds longer than that of the TS (totaling to 43 seconds) to enable us to distinguish between the BOLD responses corresponding to the CS paradigm and those corresponding to the TS paradigm in the imaging data. As with the TS, the CS stimulation block was preceded by a baseline period of 41 seconds and followed by a second baseline period of 56 seconds. The CS change in temperature rates were longer than those used to 60
72 deliver the TS because of hardware limitations in the rate of temperature change. The entire run lasted 155 seconds (Figure 3.) Experimental design There were 2 experimental conditions that were tested in the study: 1) Control, and 2) CPM. In the Control condition, only the TS was delivered and the thermode inducing the CS remained at the baseline temperature (32 C) during the entire run. In the CPM condition, both CS and the TS were employed to produce the pain modulation effect. In each experimental run, the participants viewed a set of instructions at the beginning of the run which indicated which pain (TS on the right hand or CS on the left hand) should be the focus of their attention, followed by the appropriate rating scale. The participants were blinded to which sensation they would rate until after the stimulation period, when a set of instructions prompted the participants to rate their pain to either the TS or the CS. The study included runs in which participants rated the cold CS to further blind the participant to the study design and to prevent the participants from guessing which sensation to rate. Each condition was repeated three times during a study visit, for a total of six runs of each condition in the full study per participant. Out of the six runs in the CPM condition, two run involved rating the CS. Only the ratings for the TS from all conditions were included in the behavioral analysis. The MRI study sessions were conducted on separate days in order to avoid participant fatigue and nociceptor over-sensitization over the course of the three visits Functional MRI (fmri) session Set-up 61
73 At the start of each imaging session, the pain rating scales, the instructions, and the experimental design were once again explained to the participants. Participants were then positioned supine on the MRI bed and were given ear plugs as hearing protection from the scanner noise. The thermodes were then secured to the participants left and right hands. Once inside the scanner, two practice runs (without image acquisition) with the TS alone and then the CS alone were conducted to confirm that the target temperatures used in the training session still induced a moderate pain in the fmri sessions. If the previously determined target temperatures for the two stimuli evoked different ratings than those obtained during the training session, the participants were recalibrated by adjusting the temperature until a pain rating of 50 was reached, as detailed in the Training session above Data acquisition A 3 Tesla whole-body MRI system (Siemens Magnetom Trio; Siemens, Erlangen, Germany) was used to acquire all fmri data. The system included a spine-array coil to receive the MR signal, and a body coil to deliver the radio-frequency (RF) pulses. To avoid spatial distortions in the images caused by field inhomogeneities while providing blood oxygenation-level dependent (BOLD) contrast to acquire the functional data, a T2- weighted half-fourier single-shot fast spin-echo (HASTE) imaging sequence was used. All images were obtained in the sagittal orientation encompassing the region between the top of the corpus callosum and the T1/T2 intervertebral discs. The repetition time (TR) and the echo time (TE) used to produce optimal T2-weighted BOLD contrast in the spinal cord was 4.77 seconds and 76 msec respectively. Nine slices were acquired per run and each run consisted of 32 volumes repeated over a total of 6 functional runs per condition. A complete fmri data thus set consisted of 192 volumes. The field of view was and the 62
74 matrix size were set at 280 x 140 mm 2 and 192 x 96 respectively, yielding an image resolution of 1.5 mm x 1.5 mm with a slice thickness of 2 mm. A spatial suppression pulse was applied anterior to the cord to reduce motion artifacts evoked by breathing, swallowing, and heart beating. Lastly, the first volume of each run was discarded to reduce the effects of variable T1-weighting across the time-series data Data analysis Behavioral data The CPM effect was calculated by taking the difference in TS pain ratings between the CPM condition and the Control condition. Participants who reported a positive difference in TS pain ratings were identified as efficient CPM responders. Conversely, those who reported a negative difference in TS pain ratings were identified as the less efficient CPM responders. A two factor repeated-measures ANOVA was conducted to test for significant differences in pain ratings between the two imaging sessions, and between the two conditions, for both groups of CPM responders. Significant differences were followed up by 2-tailed paired Student t-tests. The descriptive statistics which include the mean, standard deviation (SD), and median were calculated at the group level to provide quantitative information regarding the TS pain ratings for each condition, for the efficient CPM responders and the less efficient CPM responders Questionnaires All questionnaires were scored and descriptive statistics were calculated for each group of CPM responders. To test for differences in the questionnaire scores between the two groups of responders, we conducted 2-tailed paired Student s t-tests. Pearson 63
75 Correlation analysis was conducted to test for significant correlations between the questionnaire scores and the CPM effect Functional MRI data: Data preprocessing Analysis of all fmri data was performed using custom-made software written in MatLab (The MathWorks Inc., Natick, MA). The brainstem and spinal cord data were converted from DICOM format to NIFTI format prior to preprocessing. The data was then corrected for bulk movement by means of a non-rigid 3 dimensional registration tool (MIRT toolbox) which applied a non-linear 3 dimensional adjustment to co-register each volume of a time-series to the third volume of a set 144,145. The fmri data was also spatially normalized to match a predefined target template. This process automatically calculated the parameters needed to convert the raw image data into a size and shape that matched the template, in two steps. The first step generated a set of rough image parameters as a close match to the template, and the second step fine-tuned the rough image parameters to enhance the match to the target template. The resulting data set consists of 1 mm cubic voxels Functional MRI data: General Linear Model (GLM) analysis Once the preprocessing steps were completed, the brainstem and spinal cord functional data was analyzed using the general linear model (GLM) analysis method. The image data was spatially smoothed using a 3 mm x 3 mm x 5 mm boxcar kernel 104,146 (R/L x A/P x S/I), followed by an averaging of all individual runs to create on large time-series. Averaging the data allows for the reduction in physiological noise arising from body movement that is uncorrelated across the runs, and strengthens the functional signal that is consistent across runs and individuals. The model includes a set of basis functions 64
76 consisting of the TS pain paradigm, and the CS pain paradigm, both convolved with the BOLD hemodynamic response function 104, the first two principal components of the timeseries data from all voxels representing the global signal variance within the data, and a constant function. The threshold for significance of the BOLD signal change observed is inferred at p < Functional MRI data: Structural Equation Modeling (SEM) or Path analysis This study uses Structural Equation Modelling (SEM) to test the plausibility of hypothetical connections between regions of the thalamus, brainstem and spinal cord. SEM calculates connectivity between regions based on the principle that multiple regions contribute to the signaling input to one region. BOLD signal changes are most closely related to input signaling and thus the BOLD time-series response is modelled as a weighted sum of BOLD responses in other regions. The functional data is fitted to a predefined relational model of possible network connections described by Millan 42, and the weighting factors are calculated as the best fit to the measured data. SEM analysis was conducted using custom made software written in MatLab based on the analyses described by Craggs et al., and by McArdle and McDonald 147,148. The regions in the normalized data identified by the model were extracted from all individual runs, and concatenated into one large time-series of responses for each voxel. The normalized data was sub-divided into sub-regions based on the time-series properties of the data using k-means clustering. This step groups voxels of activity that correspond to significant BOLD responses separately from those that are non-responding or whose activity corresponds to motion artifacts arising at the CSF/cord boundary. Analysis was further conducted on all of the sub-regions to test for the combination of regions that best 65
77 fit the network model. Linear weighting factors for 35 different connections were calculated and considered significant at T > 2 (corresponds roughly to p < 0.05) as determined by a one-sample t-test comparing the magnitude of the weighting factor to its standard error. 3.3 Results Behavioral results Figure 8 shows the distribution of the observed CPM effect across all participants. Twelve subjects demonstrated efficient CPM, one subject showed no change in pain ratings between experimental conditions, and twelve subjects demonstrated less efficient CPM. A two factor repeated-measures ANOVA was conducted to compare the TS pain rating scores between the two fmri sessions and between the conditions (CPM and Control) for each of the two groups of CPM responders. The results indicate that there was no significant difference between the TS pain ratings obtained during the first and second imaging sessions for the efficient CPM group (F(1,11) = 1.04, p =.33), or the less efficient CPM group (F(1,11) = 1.05, p =.33). There was however, a significant difference in the TS pain ratings between the Control and the CPM conditions in both the efficient CPM responders (F(1,11) = 12, p <.01), and in the less efficient CPM responders (F(1,11) = 16.25, p <.01). Paired-sample t tests were conducted to test the difference in the perceived pain ratings between the two experimental conditions in each group of CPM responders, and between the CPM responder groups within each condition. We found that the efficient CPM responders reported significantly lower TS pain ratings in the CPM condition compared to the Control condition (t(10) = 2.23, p <.01), while the less efficient CPM responders 66
78 demonstrated higher pain ratings in the CPM condition compared to the Control condition (t(10) = 2.23, p <.01) (Figure 9.). Figure 8. Distribution of the CPM effect across participants. The CPM effect was calculated by subtracting the averaged pain ratings during the CPM condition from the pain ratings obtained during the Control condition. Negative values indicate pain inhibition or efficient CPM, and positive values indicate pain facilitation or less efficient CPM. Twelve participants demonstrate efficient CPM, twelve participants demonstrate less efficient CPM, and one participant had no change in perceived pain. There was no significant difference in the TS pain ratings during the Control condition between the two groups of responders however, the pain ratings differed significantly during the CPM condition between the efficient CPM group and the less efficient CPM group (t(10) = 2.23, p <.05) (Figure 9.). The descriptive statistics of the TS pain scores obtained from the two groups of CPM responders are summarized in Table 3. 67
79 Figure 9. Reported TS pain ratings, for each condition, obtained during the two fmri sessions. The ratings are averaged TS pain ratings with standard error bars. The asterisk (*) indicates significance of p <.05, and the double asterisk (**) indicates significance of p <.01. Table 3. Descriptive statistics of pain50 temperatures used to deliver the TS and the CS, and the TS pain ratings from the Control and CPM conditions obtained in the fmri runs, from the efficient CPM responders and the less efficient CPM responders Questionnaires 68
80 Descriptive statistics of the questionnaire scores from the efficient CPM responders and the less efficient CPM responders are summarized in Table 4. Based on normative data, all questionnaire scores fall within the normal ranges Table 4. Descriptive statistics of demographic information and questionnaire scores from the efficient CPM responders and the less efficient CPM responders. We conducted 2-tailed paired Student s t-tests to test for differences between the questionnaire scores from the participants in the two study groups. Results reveal that the State Anxiety scores were significantly higher in the participants demonstrating less efficient CPM compared to the participants expressing efficient CPM (t(10) = 2.23, p <.05). Correlation analyses were conducted to test for significant trends between the questionnaire scores and the CPM effect. We found that both the state anxiety scores and the depression scores positively correlated with the CPM effect in participants demonstrating less efficient CPM however, the correlations did not reach significance (r = 0.53, p = 0.074; and r = 0.53, p = 0.079, respectively). When combining the data from all participants, the analysis revealed that state anxiety scores and the depression scores were positively correlated with the CPM effect (r = 0.56, p <.01; r = 0.42, p <.05) (Figure 10.). 69
81 Figure 10. Correlation plots between questionnaire scores and the CPM effect. The questionnaire scores from all 25 participants were included in the analysis. (A) State Anxiety scores correlate positively with the CPM effect (p = 0.005, r = 0.555). (B) Depression scores correlate positively with the CPM effect (p = 0.042, r = 0.418) GLM results The BOLD response to the CPM effect was calculated by subtracting the time course data on a voxel-by-voxel basis between the two conditions. Imaging results reveal 70
82 greater responses during the CPM condition compared to the Control condition in the vicinity of the PAG, RVM, and the left and right DH of the C6 segment in both groups of CPM responders (Figures 11.). Figure 11. BOLD signal changes that that are significantly different between the CPM and the Control conditions in the efficient CPM responders and in the less efficient CPM responders, observed at different levels of the brainstem and spinal cord. The location of the selected slices are illustrated in the left reference template. Regions of interest in each slice are marked in red and include the periaqueductal grey (PAG), rostral ventromedial medulla (RVM) which encompasses the nucleus raphe magnus (NRM) and the nucleus gigantocellularis (NGc), dorsal reticular nucleus (DRt), and the C6 spinal cord sections indicating the left and right dorsal horns (DH). The functional activity in the PAG, RVM, DRt, and the dorsal horn of the spinal cord is overlaid on anatomical templates. Cool colors represent negative difference in activity between two conditions, and warm colors indicate positive difference in activity between two conditions. The threshold for statistical significance is at p < Conversely, the region in the expected location of the DRt is less responsive during the CPM condition than in the Control condition in the efficient CPM responders, while in the participants demonstrating less efficient CPM, results show no difference in responsiveness between the two conditions at the level of the DRt (Figure 11.). We also found that the PAG, RVM, and the DRt were less responsive in the efficient CPM 71
83 responders compared to the less efficient CPM responders, during the CPM condition (Figure 12.). Conversely, the DH of the C6 spinal segment showed greater BOLD activity in the efficient CPM responders than in the less efficient CPM group, in response to the CPM condition (Figure 12.). Figure 12. BOLD signal changes that that are significantly different between the efficient CPM responders and the less efficient CPM responders, during the CPM condition. Transverse slices are displayed at selected levels of the PAG, RVM, DRt, and the DH of the C6 segment. Functional activity is overlaid on anatomical scans. The activity observed represents GLM results that are consistent across all participants. Cool colors represent negative difference in activity, and warm colors indicate positive difference in activity between the two groups of CPM responders. Significance is inferred at p < SEM results Connectivity analyses revealed few connections that are common across all participants, while the majority of connections are unique to the types of CPM responders (Figure 13.). Common connections across participants include the PBN RVM, and reciprocally between the RVM and the right DH of C6. We observed significant connections between the PAG RVM, PAG DRt, and bidirectionally between the DRt and the C6 DH of the cord, which are unique to the efficient CPM responders. Conversely, 72
84 results reveal significant connections between the PAG PBN, and bidirectionally between the hypothalamus and the NTS, and between the PBN and the NTS, that are present only in the less efficient CPM group. Figure 13. Network connections detected during the CPM condition in both efficient and less efficient CPM responders. Red lines represent connections that are unique to the efficient CPM responders, blue lines represent connections that are unique to the less efficient CPM responders, and black lines illustrate connections that are common to both types of responders. Arrows indicate the direction of input, solid lines represent positive connections and dashed lines represent negative connections. The line thickness indicates the strength of the connection. The RVM consists of the NRM and the NGc, outlined in yellow. The asterisk (*) indicates a change in sign for path coefficients. A significance of T > 2 is inferred for all path coefficients. 3.4 Discussion The purpose of this study was to identify the differences in BOLD responses in the brainstem and spinal cord between healthy participants demonstrating efficient CPM and those demonstrating less efficient CPM. Behavioral results confirmed that the TS pain 73
85 ratings during the CPM condition were significantly lower in the efficient CPM responders, and significantly higher in the less efficient CPM responders, when compared to the ratings obtained from the Control condition. Correlation analyses further indicate that state anxiety and depression scores were positively correlated with the CPM efficiency. Imaging data revealed greater BOLD responses in the PAG, RVM, and the right and left DH of the C6 segment in both groups of CPM responders, in the CPM condition compared to the Control condition. Results also showed differences in BOLD activity at the DRt, where negative BOLD signal changes observed in the efficient CPM responders were absent in the less efficient CPM responders. Lastly, our connectivity data revealed that the majority of connections were unique to the responder types, and few common connections were shared among all participants, such as PBN RVM, and RVM DH of C6. Altogether, these results demonstrate for the first time differences in the neural correlates of CPM in the brainstem and spinal cord that reflect differences in pain perception in response to the CPM paradigm. In the present study we observed individual differences in pain ratings during CPM, ranging from efficient CPM to less efficient CPM. In our population sample, 48% of participants responded efficiently to CPM, demonstrating significant test pain inhibition when the conditioning stimulus was applied. Conversely, 48% of the remaining population sample demonstrated less efficient CPM, indicating that the application of a conditioning stimulus induced a facilitatory pain response. Lastly, the remaining 4% of our subjects (1 participant) showed no change in perceived test pain during CPM. As with our sample of healthy participants, other studies have also reported varied responses to CPM 117,125,126. For instance, Bogdanov et. al (2015) indicated that only 29% of their population sample 74
86 expressed efficient CPM, while 29% experienced no change in pain perception, and 42% indicated increased test pain during CPM. Furthermore, in a study by Piché et al. (2009), only 4 out of the 12 participants (33%) reported decreased sural nerve (RIII) reflex amplitude during CPM, and the rest of the sample group showed unchanged or increased reflex responses to CPM. Based on these findings, it is evident that differences in the CPM responses exist among healthy controls. Potential sources of individual variability in the CPM effect may be attributed to psychobehavioral factors that can contribute to differences in the way pain is perceived. It is well known that a range of emotions such as depression and anxiety can influence and modulate pain perception 39,100,162. Previous findings suggest that depressed individuals experience pain differently 163, while anxiety can enhance the experience of pain 164. Using several questionnaires, we tested for preexisting states of anxiety, depression, and for factors such as pain catastrophizing, and social desirability. In the efficient CPM group we found no correlations between the CPM effect and the questionnaire scores. Conversely, the CPM effect correlated positively with the state anxiety and the depression scores in participants demonstrating less efficient CPM, however, the correlations were just below the significance threshold. When combining the data from all participants, the depression and the state anxiety scores were found to positively correlate with the CPM effect. These results indicate that with an increase in depression and state anxiety, the CPM effect is not only diminished but the pain perception is enhanced. This is consistent with previous findings by Cormer et al. (2013), where depressive symptoms were found to significantly correlate with the CPM effect, indicating that weaker or reversed CPM was related to higher depressive scores 165. Similarly, the trait anxiety scores also correlated with the CPM 75
87 evoked pain, suggesting that higher pain-related anxiety contributed to the reduction in the CPM efficiency. Furthermore, Bogdanov et al. (2015) found that high anxiety was associated with greater test stimulus-induced pain during CPM. Therefore, altered states of anxiety and depression could have contributed to the individual differences in the CPM effect, as observed in our study. To date, this is the first study that demonstrates differences in BOLD responses in the brainstem and spinal cord between pain-free efficient CPM responders and less efficient CPM responders. Results reveal greater BOLD responses in the PAG, RVM, and in the left and right DH of the cord in both groups of CPM responders, during the CPM condition compared to the Control condition. This data is consistent with our SEM results which demonstrate significant connections involving the PAG, RVM, and the right DH of the C6 segment across all participants. The PAG and the RVM are known to be highly involved in regulating nociceptive inputs by activating descending controls that reach and modulate spinal neuron activity 42,47,161. Our data therefore indicate that during CPM, ascending nociceptive inputs activate the PAG and the RVM, either directly or via other brainstem or cortical regions, which subsequently transmit descending controls to alter the dorsal horn activity and thus modulate the overall subjective experience of pain. A recent imaging study also reported increased activity in the PAG-RVM network when the conditioning stimulus was delivered during the CPM condition 117. However, when treated with naloxone to eliminate the pain inhibitory effect of CPM, the PAG and the RVM activity was reduced but the subjective pain experience remained unchanged. Furthermore, a similar study also revealed activation in the PAG during CPM, however the reported inhibitory CPM effect was not related to the PAG activity 126. Our findings suggest 76
88 that participants demonstrating pain facilitation in response to CPM showed greater PAG and RVM BOLD responses than participants expressing pain inhibition to the CPM paradigm. In line with these findings, Weich et al. (2009) also found that the PAG shows increased activity in response to increased pain perception 33. Several authors suggest that the activation of the PAG-RVM network during CPM may reflect the simultaneous activation of other pain control mechanisms that are not directly involved in CPM and difficult to control for during lab induced pain 166. One postulated pain modulating network that activates the PAG, RVM, and PBN during pain delivery paradigms is the anxietyinduced hyperalgesia network 117,167. The PAG is known to be highly implicated in emotional processing during pain anticipation 158, and thus increased PAG activity during lab induced pain paradigms may reflect higher states of anxiety which can contribute to the enhanced pain perception 167. Similarly, our correlation analyses suggest that CPM efficiency is reduced when anxiety is enhanced. Because greater PAG and RVM responses were observed in the less efficient CPM responders who also reported higher state anxiety scores, it is thus highly likely that the PAG-RVM top-down modulating network is highly influenced by altered psychological states that can be activated simultaneously during CPM. The DRt is known to have direct connections to the dorsal horn of the spinal cord 67 and based on animal studies, it is thought to be the primary region to sustain DNIC 72. Ablation of the DRt eliminated pain inhibition via DNIC 72 while stimulation of the DRt region facilitated acute nociception in rats 153. In humans, we found results that parallel findings from animal studies, evidencing the importance of the DRt in modulating the transmission of noxious inputs to the cord during CPM. In the participants demonstrating 77
89 pain inhibition to the CPM paradigm, our findings reveal that the DRt is less responsive during the CPM condition compared to the Control condition. Additionally, we also found effective connectivity between the PAG DRt and reciprocally between the DRt and the cord during the CPM condition, which is not demonstrated in the participants responding less efficiently to the CPM paradigm. The absence of such connections in the participants showing pain facilitation in response to CPM, further parallel the fmri results, which reveal no difference in the BOLD signal change between the CPM condition and the Control condition at the level of the DRt. Furthermore, we found that the DRt is less responsive in the efficient CPM responders compared to participants reporting less efficient CPM, during the CPM condition. Altogether, these results indicate that a reduction in DRt activity contributes to decreased perceived pain during CPM, while an absence of inhibition in the DRt activity may reinforce pain facilitation, resulting in hyperalgesia in response to the CPM paradigm. Therefore, the activity of the DRt during CPM plays an essential role in determining the ability to modulate the overall perception of pain. This study also has several limitations that may affect the generalization of these findings. Firstly, our study cohort included only females to avoid effects due to sex differences in pain perception. Previous studies have suggested that men demonstrate more efficient endogenous pain inhibition during CPM 118,168,169, and therefore the distribution of the CPM effect found in our study may not reflect the true proportions of individuals demonstrating efficient CPM in a larger sample size that includes both genders and just men. Secondly, we found that the depression and the state anxiety scores correlated positively with the CPM effect in the less efficient CPM responders, however the relationship was just below significance. Obtaining a larger sample size in the group 78
90 demonstrating less efficient CPM would have allowed us to determine if high scores in these psychobehavioral measures are possible markers of reduced ability to express the analgesic CPM effect. It has been previously suggested that individuals with chronic pain also experience depressive symptoms 34,170,171, thus investigating the neural correlates of CPM in individuals with chronic pain and depression compared against our findings from healthy individuals demonstrating less efficient CPM, may provide a crucial insight into understanding the development of chronic pain. 3.5 Conclusion In summary, this is the first study that demonstrates differences in BOLD responses at the levels of the brainstem and spinal cord, in response to the CPM paradigm in healthy pain-free females. We found large differences in the behavioral correlates of CPM ranging from pain inhibition illustrated by efficient CPM to pain facilitation as indicated by less efficient CPM. State anxiety and depression were found to positively correlate with the CPM effect, where greater anxiety and depression scores were indicative of less efficient CPM. Greater BOLD responses in the PAG and RVM were shown in the participants demonstrating less efficient CPM compared to those reporting efficient CPM, suggesting that these regions of the brainstem play a more indirect role in the descending modulation network that underlies CPM. We also found differences in the DRt activity between the responder types during CPM, which thus provide evidence that this structure is highly involved in mediating the analgesic effect of CPM, as it has been previously demonstrated to play an essential role in DNIC 72. Altogether, these findings expand our understanding regarding the individual differences in the behavioral and neural correlates of CPM among 79
91 healthy pain-free individuals, and further our prior knowledge of how altered descending pain networks can reflect the ability to modulate perceived pain. 80
92 Chapter 4 Discussion 4.1 Research objectives Evaluating one s ability to modulate pain in an experimental setting is a valuable technique that aids in characterizing one s pain profile and determining an individuals susceptibility of developing future chronic pain. One experimental paradigm that probes the efficiency of the descending pain inhibition system is conditioned pain modulation (CPM). CPM is a bottom-up process that engages the descending inhibitory controls which modulates pain processing pathways, resulting in an overall decrease in the subjective pain experience. Current studies focus largely on the behavioral effect of CPM, suggesting that healthy populations demonstrate the analgesic CPM effect, while chronic pain populations show a lack of pain inhibition and most demonstrate pain facilitation to the CPM paradigm 172. The mechanism behind CPM is largely based on animal studies, where a similar phenomenon labeled diffuse noxious inhibitory controls or DNIC demonstrates an inhibition in the spinal neuron response from one location of the body when another noxious stimulus is applied on remote location of the body 76. In rats, DNIC is thought to be mediated via a spinal-brainstem-spinal loop 77,82,83,127, while in humans, few studies have used non-invasive tools such as functional MRI to probe the CPM paradigm in healthy controls, and the majority of the findings focus on cortical structures 117,121,126,130. Therefore, the next step in understanding how CPM functions in humans is to investigate the brainstem and spinal cord activity during CPM. The goal of this research was to 81
93 provide insight into the neural processes occurring at the various levels of the brainstem and at the cervical spinal cord that underlie CPM in healthy adults by using functional MRI. Prior to delving into data collection and analysis, we first had to develop a study design that would yield reliable and comparable results with other human fmri studies on CPM. There is great variability in the experimental methods used to evoke the CPM effect in humans making it difficult to generalize findings across studies. Currently, there is no accepted standard for evoking and measuring the CPM effect and many studies vary with respect to the spatial design (i.e. contralateral vs. ipilateral regions of the body), duration and intensity of the stimuli, temporal characteristics (i.e. sequential or simultaneous stimulation), and evaluations (i.e. evoked potentials, RIII reflex amplitude, behavioral pain ratings). The reason for our choice in study design is largely based around the scope of our research and the limitations of having to induce experimental pain in an MRI environment. We chose to deliver the test stimulus on the thenar eminence of the right hand to be able to use the analysis software currently available in the lab and to be able to compare our results to previous findings from the lab. There is no agreed modality of delivering the test stimulus and so given the extensive research on the brainstem and spinal cord activity related to thermal pain, we chose thermal heat as our test stimulus. Conversely, the choice in delivering the conditioning stimulus to the contralateral hand stemmed from research indicating that the greatest behavioral CPM effect is observed when the two stimuli are delivered to contralateral body regions 173. The most commonly used paradigm to simulate the conditioning stimulus is the use of noxious cold via the cold pressor test (CPT) which involves the immersion of an extremity (i.e. foot or hand) in controlled cold water. This equipment is not MRI compatible, and the immersion of an entire extremity in cold water 82
94 increases the surface area stimulated which can produce attentional bias further leading to a skewed CPM effect size. Therefore, to control for these effects, we chose to use a second thermode to deliver controlled noxious cold stimulation. Lastly, we chose tonic stimulation for our paradigm since it has been suggested to better reflect clinical pain 174, and we measured the behavioral effect of CPM via subjective pain ratings. To test the efficiency of our study design in evoking the behavioral effect of CPM, we recruited several participants to take part in a pilot study that took place in the mock scanner. Analysis of these preliminary behavioral data suggested that not only were our methods evoking the analgesic response of CPM we were looking for to study, but the results were also comparable with previous studies. With that in mind, we resumed data collection from a larger sample of participants and introduced brainstem and spinal cord fmri sessions with the goal of investigating the neural correlates of CPM in healthy individuals. This work became the Chapter 2 of my thesis. Results from our pilot study also revealed that that roughly a third of our pilot sample (35%) reported a pain facilitatory response to CPM, which has been previously reported in chronic pain populations 172. Several fmri studies have also reported this enhanced pain effect in healthy individuals 125,126 ranging from 42% to 66% of the population samples however, this data was not further analyzed in these studies as it surpassed the intended research goals. Given that there is a lack of research investigating altered CPM effects in healthy participants, we decided to continue obtaining these data with the purpose of understanding how is this group of healthy individuals differed in their behavioral and neural responses to CPM against individuals demonstrating efficient CPM. This work would provide a crucial link in understanding the CPM mechanism in humans 83
95 and aid in contributing to our understanding of chronic pain development. Therefore, this became the purpose of the second part of this research, discussed in Chapter 3 of my thesis. 4.2 Principal findings Findings from both studies revealed that CPM evokes neural changes at various levels of the brainstem and at the cervical the spinal cord which are reflected by the changes in the overall experience of pain. Behaviorally, we found individual differences in pain perception during the CPM condition compared to the pain ratings obtained during the Control condition. Participants ranged in responses from evoked pain inhibition indicative of efficient CPM, to pain facilitation or less efficient CPM. The state anxiety scores were significantly higher in the less efficient CPM group compared to the scores reported by the efficient CPM responders. Furthermore, the CPM effect was positively correlated with state anxiety and depression scores, suggesting that psychological factors may play an important role in modulating pain perception during CPM. The imaging data also revealed significant findings that reflect the differences in the CPM response observed in the behavioral data. In the efficient CPM responders, we found active voxels in the vicinity of the PAG, RVM, DRt, and in the right DH of the C6 spinal segment corresponding to the test stimulus, in both the Control and the CPM conditions. Results further revealed that the PAG, RVM, and the right and left DH were more responsive, while the DRt was less responsive during the CPM condition compared to the Control condition. In the less efficient CPM responders, we found similar BOLD signal changes in the PAG, RVM, and in the left and right DH of the C6 segment as in the less efficient CPM responders when the two conditions were contrasted. On the other hand, 84
96 differences between the two groups of responders emerge at the level of the DRt, where the less efficient CPM responders show no difference in BOLD signal change between the CPM and the Control conditions, while the efficient CPM group demonstrates a negative difference in BOLD signal change between the experimental conditions. Furthermore, when the CPM fmri data from the two groups of responders were contrasted, results reveal that the PAG, RVM, and the DRt are less responsive, while the right DH is more responsive in the efficient CPM group compared to the less efficient CPM group. The results from the SEM analysis parallel these findings, illustrating significant differences and similarities in the connections within the brainstem and the right dorsal horn of the C6 segment, between conditions and between CPM responders. In the efficient CPM group, the bidirectional connections between the DRt and the C6 right DH were common to both the Control and the CPM conditions. Furthermore, we also observed similar connections between the RVM to the right dorsal horn of the C6 segment in both experimental conditions. We found additional connections between the thalamus PAG, PAG RVM, PAG DRt, and hypothalamus RVM that are unique to the CPM condition and to the efficient CPM responders. Common connections across all participants during CPM include PBN RVM, and reciprocally between the RVM and the right DH of C6, indicating strong covariance between these regions in both groups of CPM responders. Unlike in the efficient CPM group, we find connections between the PAG PBN, and reciprocally between the hypothalamus and the NTS, and between the PBN and the NTS that are unique among the less efficient CPM responders. Altogether, these findings provide the basis for several discussion topics which I have attempted to delve into by combining the behavioral and the fmri results, and by 85
97 considering findings and interpretations from previous studies investigating the CPM and the DNIC phenomena. 4.3 Interpretation Pain perception is a complex process and a highly subjective experience. It is thus not surprising to observe a large variability in the CPM effect given that CPM probes the inhibitory mechanism involved in modulating one s overall pain experience. Some participants demonstrate strong pain inhibition in response to CPM, which is indicative of an efficient pain inhibitory system while others, exhibit a bias towards pain facilitation during CPM, suggesting potential alterations in the inhibitory pain network that reduce one s ability to suppress pain. Given that all participants were healthy, pain-free, and subjected to the same paradigm, this raises the question regarding what factors may have contributed to the variability in the pain experience during CPM. To address this question, several psychobehavioral factors such as depression, anxiety, pain catastrophizing, and social desirability were briefly examined to test for differences in these states that could shed light on the differences observed in the CPM effect. Interestingly, higher states of anxiety were reported by the less efficient CPM responders, while the efficient CPM responders were found to have lower states of anxiety. Furthermore, correlation analysis revealed that higher states of anxiety reflect lower CPM efficiency, suggesting that altering one s state of anxiety can alter their ability to modulate pain. Another major contributor to the variation in the CPM effect is the state of depression, as indicated by the reported positive correlation between depression scores and the CPM efficiency. Low depression scores reflected lower pain scores during CPM, which was indicative of efficient CPM. As 86
98 the depression scores increased, so did the perception of pain, resulting in less efficient pain inhibition via CPM. These findings reinforce and support current literature that suggests the influence of psychological factors on the ability to modulate pain. Moreover, these findings indicate that although the CPM mechanism is suggested to be confined to the subcortical structures, top-down modulation from cortical regions involved in processing of psychological states can alter the CPM mechanism and reduce its efficiency. The results from the correlations found between the psychological states discussed above and the CPM efficiency, also shed light on the potential of applying these results in the context of chronic pain treatment. Often, individuals with chronic depression experience enhanced pain perception compared to healthy controls 163,164. Conversely, studies indicate that patients with chronic pain conditions such as migraine headache and fibromyalgia also experience high levels of depression 175,176. In our study, higher scores of depression were reported by participants who experienced more pain in response to the CPM paradigm, which under typical circumstances healthy individuals would elicit a pain inhibitory response. Although our group of participants was healthy and pain-free, the results parallel those obtained from populations afflicted with chronic pain and depression, revealing an important relationship between depression and enhanced pain perception. Moreover, our results also suggest that alleviating high states of depression may reflect more efficient CPM, thus a greater ability to suppress and manage pain. Therefore, these results reinforce the necessity of addressing comorbidities like depression when developing a treatment plan for alleviating chronic pain, as it may have the potential of increasing the efficiency of the pain-relief treatment. 87
99 The findings from our study may also have important implications in our understanding of the development of chronic pain. We observed that while a proportion of healthy participants respond favorably to CPM, others report less efficient CPM. Several studies have shown that less efficient CPM was also observed in chronic pain populations such as in patients afflicted with fibromyalgia 7,8 and irritable bowel syndrome 6, with results comparable to those obtained in our study. Furthermore, a recent study found that patients demonstrating less efficient CPM prior to thoracotomy, were round to have a higher risk of developing chronic pain post-surgery. While our study subjects were not followed-up to determine their risk of developing future chronic pain, the similarities in the CPM responses between the fibromyalgia group and the less efficient CPM responders from our study support the hypothesis that CPM efficiency can be an important predictor in the development of chronic pain. In addition to obtaining behavioral data, we obtained imaging data that evidences the variability in the CPM effect observed in the behavioral data and expands on the neural correlates of the CPM phenomenon in healthy pain-free adults. Results revealed more PAG, RVM, and right DH activity during the CPM condition than during the Control condition, in participants demonstrating reduced pain in response to CPM. Furthermore, the connectivity results revealed significant connections between the PAG RVM which were only observed during the CPM condition. The PAG and the RVM are brainstem structures known to be highly involved in descending modulatory controls 42. Electrical stimulation of the PAG was shown to induce generalized analgesia in rats 46 and in humans 47 through the RVM, which has direct inhibitory projections to the spinal cord, modulating the transmission of nociceptive inputs 161. Based on data obtained from 88
100 participants demonstrating efficient CPM, we found that the CPM phenomenon may share a common mechanism with the descending inhibitory controls that originate in the PAG and are mediated via the RVM, given the increased connectivity between these regions and the greater BOLD responses demonstrated in response to CPM. Similar to the results obtained from participants demonstrating pain inhibition during CPM, participants exhibiting pain facilitation also show greater BOLD responses in the PAG, RVM and in the right DH of the spinal cord in response to CPM compared to the Control condition. However, unlike the efficient CPM responders, the connectivity results revealed an absence of a direct PAG RVM connection, and rather demonstrate an indirect connection thought the PBN (i.e. PAG PBN RVM). In humans, the PAG is also found to be implicated in processing the affective component of pain during pain anticipation 158, suggesting that the intensity of the emotion associated with pain may reflect increased PAG activity. Another structure involved in emotional processing of pain is the PBN 177, which based on our connectivity analysis, is shown to receive significant inputs from the PAG in the less efficient CPM responders. Interestingly, a correlation analysis also revealed a strong but not significant trend between the depression scores and the CPM effect in the less efficient CPM responders, which became significant when all participants were included in the analysis. Research findings revealed that the emotional processing of pain is different in individuals with depression compared to control groups 178. When in a negative mood state participants demonstrated increased activation in the areas of the pain neuromatrix which include the insula, thalamus, hippocampus, dorsolateral PFC, OFC, and the ACC 179. These cortical regions have descending projections to the brainstem and specifically to the PAG, which is an essential component of the descending inhibitory pain 89
101 pathway 166. Thus, given these findings, it is possible that the affective elements of pain processing may be sensitive to altered mood states resulting in enhanced pain being perceived. Ultimately, less efficient CPM reflects differences not only behaviorally but also at the neural level, by activating a descending modulation network to target the DH spinal activity that is highly susceptible to altered psychological states. Amplifying the emotional components of pain by altering mood states therefore translates into an overall increase in pain perceived. Although a greater BOLD response in the PAG and the RVM was noted in both the efficient CPM responders and those demonstrating less efficient CPM, the contrast between the two groups of participants reveal more BOLD activity in the less efficient CPM responders during CPM compared to those exhibiting efficient CPM. This suggests that the role of the PAG in modulating pain through descending inhibitory controls is not be a critical region involved in mediating CPM. Several studies have also reported increased BOLD responses in the vicinity of the PAG during a CPM related paradigm, however treatment with naloxone reduced the PAG BOLD activity but it had no effect on the subjective pain experience 117. Additionally, Piché et al. (2009) found that the reduction in pain ratings was not related to the PAG activity during CPM. In animals, focal lesions to the PAG and to the RVM have been shown to have no effect on DNIC 85,86,128, suggesting that these structures are not directly involved in the mechanism mediating DNIC in animals. Several authors have suggested that the activation of the PAG-RVM network during CPM may reflect the simultaneous activation of other pain networks that are not directly involved in the mechanism underlying CPM but are difficult to control for during lab induced pain 166. One such mechanism is the anxiety-induced-hyperalgesia, which was 90
102 shown to activate among other cortical regions, the PAG, RVM, and the PBN in humans 180. Therefore, higher states of anxiety were shown to increase the sensitivity in brainstem regions such as the PAG, RVM and the PBN which evaluate and process the emotional components of pain. Based on these findings, it is not surprising that we find greater BOLD activity in the PAG and the RVM in the less efficient CPM responders which reported to have significantly higher state-anxiety scores compared to the efficient CPM responders. Thus, our results further support the hypothesis that the increased activation in the PAG- RVM network during CPM reflects the simultaneous recruitment of other descending mechanisms that are highly susceptible to changes in psychological states and can function to modulate other aspects of pain processing, but are likely not directly involved in the mechanism underlying CPM. The results discussed in the two middle chapters of this thesis are the first to show BOLD signal changes in the DRt during CPM. The DRt is known to be activated by noxious stimulation and is thought to play a facilitatory role in pain perception 153,154. In animals, lesioning the DRt abolished the DNIC effect, suggesting that this region of the brainstem plays a crucial role in maintaining DNIC in animals 72,157. Our results also reveal BOLD signal changes at the level of the DRt that are different between the two groups of CPM responders. In the efficient CPM responders, we found negative DRt BOLD signal changes in response to the CPM effect, which are absent in the less efficient CPM responders. The negative BOLD responses observed in the efficient CPM responders reflects a decrease in the DRt pain facilitatory activity which ultimately leads to a reduction in the perceived pain, as demonstrated in our behavioral data. Conversely, the lack of activity in the less efficient CPM responders suggests that there is no significant difference 91
103 between the BOLD responses during the Control condition compared to the CPM condition. Since the DRt responds to noxious stimuli by facilitating the transmission of these inputs to DH regions, a lack of inhibition by CPM allows for the transmission of facilitatory pain signals to the cord. These results are reflected by the hyperalgesic responses to the CPM paradigm in the less efficient CPM responders. Lastly, when contrasting the CPM BOLD responses between the two groups of responders, we found that the efficient CPM group demonstrates less DRt activity compared to the less efficient CPM group of participants. Connectivity data also illustrates the significant bidirectional connections between the DRt and the right DH in the efficient CPM responders, which are absent in the participant reporting hyperalgesia in response to CPM. Given that less DRt activity may translate into less pain facilitation, our imaging results together with our behavioral data obtained, parallels findings from animal studies and suggest that the DRt plays a crucial role in evoking the analgesic effect of CPM in humans. 4.4 Limitations There are several limitations to this work that temper the interpretation of these data. First, since gender differences to the CPM effect have been reported 100,138, we only recruited healthy females for participation in this study. With that in mind, the results obtained in this study cannot be generalized over large populations since the gender is restricted to females. Secondly, we did not control for the use of oral contraceptives which have been reported to reduce the efficiency of the endogenous pain modulation system and to inhibit the CPM effect 181,182. Lastly, a lager sample size would have provided advantages to the behavioral and the imaging results. For instance, in regards to the 92
104 behavioral data, larger sample groups would have allowed for determining potential differences between the questionnaire scores from the different CPM responders. Furthermore, given that the state anxiety scores and the depression scores showed a positive trend with the CPM effect in the less efficient CPM group that was close to reaching significance, a larger group size would have provided for a more definite conclusion about the influence of psychological factors on the CPM effect in the hyperalgesia group. Additionally, studying more participants would have provided greater statistical power in the imaging and connectivity results. In humans, anticipatory effects have been found to strongly modulate the overall perceived pain. For instance, increased anticipation of pain led to higher pain being perceived compared to when the stimulus was not anticipated 180. Furthermore, expectations of a hyperalgesic response to CPM were found to block the endogenous analgesic effect of CPM 183, suggesting that the effect of expectation influences the brainstem networks involved in descending pain inhibition. Our study did not measure the effect of expectation and thus we are limited in determining other possible factors that may have induced pain facilitation in response to the CPM paradigm. Although the results from our two human studies reflect the findings from animal studies, the comparison between human and animal models must be interpreted with caution. Firstly, DNIC describes a specific lower brainstem mediated inhibitory mechanism while during CPM the specific mechanism cannot be discerned due to the limited non-invasive techniques that can be used to study the specific mechanism underlying CPM. As previous authors have suggested, BOLD related signal changes may reflect activation of other descending controls that are not directly involved in CPM but 93
105 can be activated in parallel with CPM and have the potential to influence pain processing during CPM. Secondly, animal studies often involve electrical recording from spinal neurons and invasive lesions for determining the exact structures mediating DNIC. During these procedures animals are anesthetized, paralyzed, and artificially ventilated and therefore, descending cortical controls which may have an effect on DNIC, are altered or even absent due to the invasiveness of the procedures. Conversely, human studies are often conducted in fully conscious participants using non-invasive techniques and therefore descending pain controls from cortical regions are intact. As alluded to previously, it is probable that emotional factors as well as expectations for instance, can influence the perception of pain, the CPM effect size, and the regions activated during CPM, and these effects may not be characterized in animal models. A major limitation to interpreting functional MRI data is that the BOLD activity observed is a combination of facilitatory and inhibitory signals and the overall change in activity is reflective of the prominent local field potentials. Therefore, we cannot determine just by observing the imaging data if the activity is inhibitory or facilitatory in nature, since the descending controls from brainstem regions may be a combination of both states. We are thus limited to draw conclusions regarding which pathways, whether inhibitory or facilitatory, are activated during CPM. Lastly, it is also important to acknowledge the limitations of spinal fmri as an imaging method itself. Physiological motion arising from heart beating, breathing, and cerebral spinal fluid (CSF) pulsations are difficult to control for and can reduce the quality of the MR signal by introducing confounds. Furthermore, spinal fmri offers limited spatial resolution and given the small cross-sectional dimensions and location of the spinal cord 94
106 within bony vertebral canal, this region can induce image distortions and lower the image quality if the appropriate imaging method is not employed. However, work done by the Stroman lab has successfully addressed many of these challenges by improving spinal fmri acquisition methods and optimizing data analysis methods that greatly advance the field of spinal cord fmri 101, Future directions These studies are the first to provide insight on the neural processes occurring in the brainstem and the spinal cord in response to CPM, in healthy pain-free females. These studies also provide novel evidence of altered subcortical neural responses that reflect less efficient descending inhibition via CPM. These findings provide essential data to characterize one s inhibitory pain profile, in addition to aiding our understanding of how altered inhibitory pain networks lead to the development of future chronic pain. Although there are several studies that have investigated cortical activity in the brain in response to CPM in healthy controls, there are no studies that have looked at the brain activity of healthy participants expressing less efficient CPM, or participants with chronic pain that have also been shown to have impaired CPM 94,184. Coupling the activity observed in the brainstem and the spinal cord with the activity of that in the brain would improve our understanding of the functional connectivity at the different levels of the pain modulation pathway during CPM. Moreover, it would allow us to determine how alterations in cortical regions affect brainstem and spinal cord activity during CPM. Our findings that high states of anxiety and depression correlate with less efficient CPM support the role of psychological modulators. Current studies indicate that negative 95
107 mood reduces the inhibitory effect of pain modulation, leading to an enhanced pain experience 179. Conversely, anxiety induced hyperalgesia has been recorded in animal and in humans 180. To better understand the effects of depression and anxiety on CPM efficiency, future studies should incorporate treatments for individuals with chronic depression and high states of anxiety to investigate how the CPM effect is altered by topdown influences. 4.6 Conclusion Findings from these studies revealed that probing the pain inhibitory system with the CPM paradigm resulted in variable behavioral and neural responses in healthy painfree female adults. At one end of the spectrum some individuals reported analgesia in response to CPM, while others did not benefit from CPM but rather experienced facilitated pain. The imaging results indicate that there are numerous brainstem regions that function collectively to induce the CPM effect. Several of these regions include the PAG, RVM, and the DRt, which are directly or indirectly involved in mediating the analgesic effect of CPM. Although efficient CPM seemed to engage the PAG-RVM network of descending controls to the spinal cord, altered CPM revealed that activation of these regions may suggest the simultaneous recruitment of other pain modulating networks such as those induced by changes in psychological mood states. On the other hand, reduced DRt activity reflects the evoked CPM effect, while an absence of BOLD signal changes at the DRt reflects a reduction in the CPM effect. These findings provide the first evidence in humans that parallels the direct recruitment of the DRt in the mechanism underlying CPM. By understanding the CPM mechanism, we gain an important insight into how pain is 96
108 processed and mitigated by the descending inhibitory networks. In this way, CPM has substantial applications to clinical populations as it can also reveal important findings regarding altered pain processing which are valuable in understanding the development of future chronic pain. 97
109 References 1 Descartes, R. & Hall, T. S. Treatise of man. (Harvard University Press, 1972). 2 Wall, P. D. On the relation of injury to pain. The John J. Bonica lecture. Pain 6, (1979). 3 McNally, G. P. Pain facilitatory circuits in the mammalian central nervous system: their behavioral significance and role in morphine analgesic tolerance. Neuroscience and Biobehavioral Reviews 23, (1999). 4 Bolles, R. C. & Fanselow, M. S. A Perceptual-Defensive-Recuperative Model of Fear and Pain. Behavioral Brain Science 3, (1980). 5 Yarnitsky, D., Granot, M. & Granovsky, Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain 155, (2014). 6 Chang, L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterology Clinics of North America 34, (2005). 7 Kosek, E. & Hansson, P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 70, (1997). 8 Lautenbacher, S. & Rollman, G. B. Possible deficiencies of pain modulation in fibromyalgia. The Clinical Jjournal of Pain 13, (1997). 9 Yarnitsky, D., Crispel, Y., Eisenberg, E., Granovsky, Y., Ben-Hun, A., Srecher, E., Best, L. A., Granot, M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 138, (2008). 98
110 10 Yarnitsky, D., Granot, M., Nahman-Averbuch, H., Khamaisi, M. & Granovsky, Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 153, (2012). 11 Kandel, E. R., Schwartz, J. H., Jessell, T. M. Principles of Neural Science. 4 edn, (McGraw-Hill Companies, 2000). 12 Dubin, A. E. & Patapoutian, A. Nociceptors: the sensors of the pain pathway. The Journal of clinical investigation 120, (2010). 13 Snell, R. S. Clinical Neuroanatomy. 7 edn, (Lippincott Williams & Wilkins, 2010). 14 Dostrovsky, J. O. Chapter 10 Brainstem and thalamic relays. Handbook of clinical neurology 81, (2006). 15 Purves, D., Augustine, G. J., Fitzpatrick, D., Hall, W. C., LaMantia, A. S., McNamara, J. O., White, L. E. Neuroscience. 4 edn, (Sinauer Associates, 2008). 16 MacKay, W. A. Neuro 101: Neurophysiology without tears. 5 edn, (Sefalotek Ltd., 2007). 17 Julius, D. & Basbaum, A. I. Molecular mechanisms of nociception. Nature 413, (2001). 18 Dostrovsky, J. O. & Craig, A. D. Cooling-specific spinothalamic neurons in the monkey. Journal of Neurophysiology 76, (1996). 19 Craig, A. D. & Bushnell, M. C. The thermal grill illusion: unmasking the burn of cold pain. Science 265, (1994). 20 Dostrovsky, J. O. & Hellon, R. F. The representation of facial temperature in the caudal trigeminal nucleus of the cat. The Journal of Physiology 277, (1978). 99
111 21 Eckert, W. A., 3rd, Julius, D. & Basbaum, A. I. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain 126, (2006). 22 Patel, S., Ohara, S., Dougherty, P. M., Gracely, R. H. & Lenz, F. A. Psychophysical elements of place and modality specificity in the thalamic somatic sensory nucleus (ventral caudal, vc) of awake humans. Journal of Neurophysiology 95, (2006). 23 Green, B. G. & Akirav, C. Individual differences in temperature perception: evidence of common processing of sensation intensity of warmth and cold. Somatosensory & Motor Research 24, (2007). 24 Rossi, G. F. & Brodal, A. Terminal distribution of spinoreticular fibers in the cat. A.M.A. Archives of Neurology and Psychiatry 78, (1957). 25 Benarroch, E. E. Pain-autonomic interactions. Neurological Sciences 27 Suppl 2, S (2006). 26 Ikeda, H., Stark, J., Fischer, H., Wagner, M., Drdla, R., Jager, T., Sandkuhler, J.. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312, (2006). 27 Ikeda, H., Heinke, B., Ruscheweyh, R. & Sandkuhler, J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299, (2003). 28 Hunt, S. P. & Mantyh, P. W. The molecular dynamics of pain control. Nature Reviews. Neuroscience 2, (2001). 100
112 29 Brooks, J. & Tracey, I. From nociception to pain perception: imaging the spinal and supraspinal pathways. Journal of Anatomy 207, (2005). 30 Melzack, R. & Wall, P. D. Pain mechanisms: a new theory. Science 150, (1965). 31 Zelman, D. C., Howland, E. W., Nichols, S. N. & Cleeland, C. S. The effects of induced mood on laboratory pain. Pain 46, (1991). 32 Meagher, M. W., Arnau, R. C. & Rhudy, J. L. Pain and emotion: effects of affective picture modulation. Psychosomatic Medicine 63, (2001). 33 Wiech, K. & Tracey, I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. NeuroImage 47, (2009). 34 Magni, G., Moreschi, C., Rigatti-Luchini, S. & Merskey, H. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. Pain 56, (1994). 35 Larson, S. L., Clark, M. R. & Eaton, W. W. Depressive disorder as a long-term antecedent risk factor for incident back pain: a 13-year follow-up study from the Baltimore Epidemiological Catchment Area sample. Psychological Medicine 34, (2004). 36 Roy-Byrne, P. P., Davidson, K.W., Kessler, R. C., Asmundson, G. J., Goodwin, R. D., Kubzansky, L., Lydiard, R. B., Massie, M. J., Katon, W., Laden, S. K., Stein, M. B. Anxiety disorders and comorbid medical illness. General hospital psychiatry 30, (2008). 37 Price, D. D. Psychological and neural mechanisms of the affective dimension of pain. Science 288, (2000). 101
113 38 Gureje, O. Comorbidity of pain and anxiety disorders. Current Psychiatry Reports 10, (2008). 39 Bair, M. J., Robinson, R. L., Katon, W. & Kroenke, K. Depression and pain comorbidity: a literature review. Archives of Internal Medicine 163, (2003). 40 Dubner, R. & Ren, K. Endogenous mechanisms of sensory modulation. Pain Suppl 6, S45-53 (1999). 41 Willer, J. C., Bouhassira, D. & Le Bars, D. Neurophysiological bases of the counterirritation phenomenon:diffuse control inhibitors induced by nociceptive stimulation]. Neurophysiologie clinique = Clinical neurophysiology 29, (1999). 42 Millan, M. J. Descending control of pain. Progress in Neurobiology 66, (2002). 43 Zhuo, M. & Gebhart, G. F. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. Journal of Neurophysiology 78, (1997). 44 Watkins, L. R. et al. Neurocircuitry of conditioned inhibition of analgesia: effects of amygdala, dorsal raphe, ventral medullary, and spinal cord lesions on antianalgesia in the rat. Behavioral Neuroscience 112, (1998). 45 Zhuo, M. & Gebhart, G. F. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Journal of Neurophysiology 67, (1992). 102
114 46 Reynolds, D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164, (1969). 47 Young, R. F., Kroening, R., Fulton, W., Feldman, R. A. & Chambi, I. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. Journal of Neurosurgery 62, (1985). 48 Millan, M. J. The induction of pain: an integrative review. Progress in Neurobiology 57, (1999). 49 Behbehani, M. M. & Fields, H. L. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Research 170, (1979). 50 Aimone, L. D. & Gebhart, G. F. Stimulation-produced spinal inhibition from the midbrain in the rat is mediated by an excitatory amino acid neurotransmitter in the medial medulla. The Journal of neuroscience 6, (1986). 51 Light, A. R., Casale, E. J. & Menetrey, D. M. The effects of focal stimulation in nucleus raphe magnus and periaqueductal gray on intracellularly recorded neurons in spinal laminae I and II. Journal of Neurophysiology 56, (1986). 52 Jones, S. L. & Light, A. R. Electrical stimulation in the medullary nucleus raphe magnus inhibits noxious heat-evoked fos protein-like immunoreactivity in the rat lumbar spinal cord. Brain Research 530, (1990). 53 Beitz, A. J. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7, (1982). 54 Fields, H. L., Basbaum, A. I.. Textbook of pain. 4 edn, (Churchill Livingston, 1999). 103
115 55 Suzuki, R., Rygh, L. J. & Dickenson, A. H. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends in Pharmacological Sciences 25, (2004). 56 Morgan, M. M., Whittier, K. L., Hegarty, D. M. & Aicher, S. A. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 140, (2008). 57 Fields, H. L. Pain modulation: expectation, opioid analgesia and virtual pain. Progress in Brain Research 122, (2000). 58 Mason, P. Central mechanisms of pain modulation. Current Opinion in Neurobiology 9, (1999). 59 Urban, M. O. & Gebhart, G. F. Supraspinal contributions to hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America 96, (1999). 60 Lumb, B. M. Hypothalamic influences on viscero-somatic neurones in the lower thoracic spinal cord of the anaesthetized rat. The Journal of Physiology 424, (1990). 61 Aimone, L. D., Bauer, C. A. & Gebhart, G. F. Brain-stem relays mediating stimulation-produced antinociception from the lateral hypothalamus in the rat. The Journal of Neuroscience 8, (1988). 62 Behbehani, M. M., Park, M. R. & Clement, M. E. Interactions between the lateral hypothalamus and the periaqueductal gray. The Journal of Neuroscience 8, (1988). 104
116 63 Holden, J. E. & Naleway, E. Microinjection of carbachol in the lateral hypothalamus produces opposing actions on nociception mediated by alpha(1)- and alpha(2)-adrenoceptors. Brain Research 911, (2001). 64 Aicher, S. A. & Randich, A. Antinociception and cardiovascular responses produced by electrical stimulation in the nucleus tractus solitarius, nucleus reticularis ventralis, and the caudal medulla. Pain 42, (1990). 65 Beitz, A. J., Clements, J. R., Ecklund, L. J. & Mullett, M. M. The nuclei of origin of brainstem enkephalin and cholecystokinin projections to the spinal trigeminal nucleus of the rat. Neuroscience 20, (1987). 66 Millan, M. J. Multiple opioid systems and pain. Pain 27, (1986). 67 Almeida, A., Cobos, A., Tavares, I. & Lima, D. Brain afferents to the medullary dorsal reticular nucleus: a retrograde and anterograde tracing study in the rat. The European Journal of Neuroscience 16, (2002). 68 Almeida, A., Storkson, R., Lima, D., Hole, K. & Tjolsen, A. The medullary dorsal reticular nucleus facilitates pain behaviour induced by formalin in the rat. The European Journal of Neuroscience 11, (1999). 69 Villanueva, L., de Pommery, J., Menetrey, D. & Le Bars, D. Spinal afferent projections to subnucleus reticularis dorsalis in the rat. Neuroscience Letters 134, (1991). 70 Villanueva, L., Bouhassira, D., Bing, Z. & Le Bars, D. Convergence of heterotopic nociceptive information onto subnucleus reticularis dorsalis neurons in the rat medulla. Journal of Neurophysiology 60, (1988). 105
117 71 Villanueva, L., Bing, Z., Bouhassira, D. & Le Bars, D. Encoding of electrical, thermal, and mechanical noxious stimuli by subnucleus reticularis dorsalis neurons in the rat medulla. Journal of Neurophysiology 61, (1989). 72 Bouhassira, D., Villanueva, L., Bing, Z. & le Bars, D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Research 595, (1992). 73 Calvino, B., Villanueva, L. & Le Bars, D. The heterotopic effects of visceral pain: behavioural and electrophysiological approaches in the rat. Pain 20, (1984). 74 Le Bars, D. & Villanueva, L. Electrophysiological evidence for the activation of descending inhibitory controls by nociceptive afferent pathways. Progress in Brain Research 77, (1988). 75 Le Bars, D. & Willer, J. C. Pain modulation triggered by high-intensity stimulation: implication for acupuncture analgesia? International Congress Series 1238, (2002). 76 Le Bars D, D. A., Besson JM. Diffuse noxious inhibitory controls (DNIC).I. Effects on dorsal horn convergent neurones in the rat. Pain 6, (1979). 77 Le Bars, D., Dickenson, A. H. & Besson, J. M. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6, (1979). 78 Morton, C. R., Maisch, B. & Zimmermann, M. Diffuse noxious inhibitory controls of lumbar spinal neurons involve a supraspinal loop in the cat. Brain Research 410, (1987). 106
118 79 Gerhart, K. D., Yezierski, R. P., Giesler, G. J., Jr. & Willis, W. D. Inhibitory receptive fields of primate spinothalamic tract cells. Journal of Neurophysiology 46, (1981). 80 Brennan, T. J., Oh, U. T., Hobbs, S. F., Garrison, D. W. & Foreman, R. D. Urinary bladder and hindlimb afferent input inhibits activity of primate T2-T5 spinothalamic tract neurons. Journal of Neurophysiology 61, (1989). 81 Falinower, S., Willer, J. C., Junien, J. L. & Le Bars, D. A C-fiber reflex modulated by heterotopic noxious somatic stimuli in the rat. Journal of Neurophysiology 72, (1994). 82 Cadden, S. W., Villanueva, L., Chitour, D. & Le Bars, D. Depression of activities of dorsal horn convergent neurones by propriospinal mechanisms triggered by noxious inputs; comparison with diffuse noxious inhibitory controls (DNIC). Brain Research 275, 1-11 (1983). 83 Bouhassira, D., Bing, Z. & Le Bars, D. Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. Journal of Neurophysiology 64, (1990). 84 Bouhassira, D., Bing, Z. & Le Bars, D. Effects of lesions of locus coeruleus/subcoeruleus on diffuse noxious inhibitory controls in the rat. Brain Research 571, (1992). 85 Bouhassira, D., Villanueva, L. & Le Bars, D. Effects of systemic morphine on diffuse noxious inhibitory controls: role of the periaqueductal grey. European Journal of Pharmacology 216, (1992). 107
119 86 Bouhassira, D., Chitour, D., Villanueva, L. & Le Bars, D. Morphine and diffuse noxious inhibitory controls in the rat: effects of lesions of the rostral ventromedial medulla. European Journal of Pharmacology 232, (1993). 87 Willer, J. C., Roby, A. & Le Bars, D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain : a Journal of Neurology 107 ( Pt 4), (1984). 88 Willer, J. C., De Broucker, T. & Le Bars, D. Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. Journal of Neurophysiology 62, (1989). 89 Sandrini, G., Milanov, I., Malaguti, S., Nigrelli, M. P., Moglia, A., Nappi, G. Effects of hypnosis on diffuse noxious inhibitory controls. Physiology & behavior 69, (2000). 90 Willer, J. C. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain 3, (1977). 91 Roby-Brami, A., Bussel, B., Willer, J. C. & Le Bars, D. An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli. Probable involvement of a supraspinal loop. Brain : a journal of neurology 110 ( Pt 6), (1987). 92 Pud, D., Granovsky, Y. & Yarnitsky, D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144, (2009). 93 Nir, R. R. & Yarnitsky, D. Conditioned pain modulation. Current Opinion in Supportive and Palliative Care (2015). 108
120 94 Van Wijk, G. & Veldhuijzen, D. S. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. Journal of Pain 11, (2010). 95 Potvin, S., Larouche, A., Normand, E., de Souza, J. B., Gaumond, I., Marchand, S., Grignon, S. No relationship between the ins del polymorphism of the serotonin transporter promoter and pain perception in fibromyalgia patients and healthy controls. European Journal of Pain 14, (2010). 96 Cathcart, S., Winefield, A. H., Lushington, K. & Rolan, P. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache 50, (2010). 97 Heymen, S., Maixner, W., Whitehead, W. E., Klatzkin, R. R., Mechlin, B., Light, K. C.. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. The Clinical Journal of Pain 26, (2010). 98 King, C. D., Wong, F., Currie, T., Mauderli, A. P., Fillingim, R., B., Riley, J. L. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain 143, (2009). 99 Granovsky, Y. & Yarnitsky, D. Personalized pain medicine: the clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Medical Journal 4, (2013). 109
121 100 Knudsen L, P. G., Norskov KN, Vase L, Finnerup N, Jensen TS, Svensson P. Review of neuroimaging studies related to pain modulation. Scandinavian Journal of Pain 2, (2011). 101 Stroman, P. W. Essentials of Functional MRI. (Taylor Francis, 2011). 102 Stroman, P. W., Bosma, R. L., Kornelsen., J., Lawrence-Dewar, J., Wheeler- Kingshott, C., Cadotte, D., Fehlings, M. G.. Advanced MR imaging techniques and characterization of residual anatomy. Clinical Neurological Neurosurgery 114, (2012). 103 Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fmri signal. Nature 412, (2001). 104 Bosma, R. L. & Stroman, P. W. Assessment of data acquisition parameters, and analysis techniques for noise reduction in spinal cord fmri data. Magnetic Resonance Imaging 32, (2014). 105 Jones, A. K., Kulkarni, B. & Derbyshire, S. W. Functional imaging of pain perception. Current Rheumatology Reports 4, (2002). 106 Davis, K. D., Kwan, C. L., Crawley, A. P. & Mikulis, D. J. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. Journal of Neurophysiology 80, (1998). 107 Casey, K. L., Minoshima, S., Morrow, T. J. & Koeppe, R. A. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. Journal of Neurophysiology 76, (1996). 110
122 108 Craig, A. D., Chen, K., Bandy, D. & Reiman, E. M. Thermosensory activation of insular cortex. Nature Neuroscience 3, (2000). 109 Maihofner, C., Kaltenhauser, M., Neundorfer, B. & Lang, E. Temporo-spatial analysis of cortical activation by phasic innocuous and noxious cold stimuli--a magnetoencephalographic study. Pain 100, (2002). 110 Wagner, G., Sinsel, E., Sobanski, T., Kohler, S., Marinou, V., Mentzel., H. J., Sauer, H., Schlosser, R. G. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological Psychiatry 59, (2006). 111 Dunckley, P. et al. Attentional modulation of visceral and somatic pain. Neurogastroenterology and Motility 19, (2007). 112 Wilder-Smith, C. H., Schindler, D., Lovblad, K., Redmond, S. M. & Nirkko, A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 53, (2004). 113 Rempe, T., Wolff, S., Riedel, C., Baron, R., Stroman, P. W., Jansen, O., Gierthumhlen, J. Spinal and supraspinal processing of thermal stimuli: An fmri study. Journal of Magnetic Resonance Imaging (2014). 114 Stroman, P. W., Tomanek, B., Krause, V., Frankenstein, U. N. & Malisza, K. L. Mapping of neuronal function in the healthy and injured human spinal cord with spinal fmri. NeuroImage 17, (2002). 111
123 115 Stroman, P. W., Kornelsen, J., Bergman, A., Krause, V., Ethans, K., Malisza, K. L., Tomanek, B. Noninvasive assessment of the injured human spinal cord by means of functional magnetic resonance imaging. Spinal Cord 42, (2004). 116 Dobek, C. E., Beynon, M. E., Bosma, R. L. & Stroman, P. W. Music modulation of pain perception and pain-related activity in the brain, brain stem, and spinal cord: a functional magnetic resonance imaging study. Journal of Pain 15, (2014). 117 Sprenger, C., Bingel, U. & Buchel, C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain 152, (2011). 118 Granot, M., Weissman-Fogel, L.,Crispel, Y., Pud, D., Granovsky, Y., Sprecher, E., Yarnitsky, D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain 136, (2008). 119 Razavi, M., Hansson, P. T., Johansson, B. & Leffler, A. S. The influence of intensity and duration of a painful conditioning stimulation on conditioned pain modulation in volunteers. European Journal of Pain 18, (2014). 120 Yarnitsky, D., Arendt-Neilsen, L., Bouhassira, D., Edwards, R. R., Fillingim, R. B., Granot, M., Hansson, P., Lautenbacher, S., Marchand, S., Wilder-Smith, O. Recommendations on terminology and practice of psychophysical DNIC testing. European Journal of Pain 14, 339 (2010). 112
124 121 Nahman-Averbuch, H., Martucci, K. T., Granvosky, Y., Weissman-Fogel, I., Yarnitsky, D., Coghill, R. C. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain 155, (2014). 122 Tousignant-Laflamme, Y., Page, S., Goffaux, P. & Marchand, S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Research 1230, (2008). 123 Lewis, G. N., Heales, L., Rice, D. A., Rome, K. & McNair, P. J. Reliability of the conditioned pain modulation paradigm to assess endogenous inhibitory pain pathways. Pain Research & Management 17, (2012). 124 Lewis, G. N., Rice, D. A. & McNair, P. J. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. Journal of Pain 13, (2012). 125 Bogdanov, V. B., Vigano, A., Noirhomme, Q., Bogdanova, O. V., Guy, N., Laureys, S., Renshaw, P. F., Dallel, R., Phillips, C., Schoenen, J. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fmri study in healthy subjects. Behavioural Brain Research 281, (2015). 126 Piche, M., Arsenault, M. & Rainville, P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. The Journal of Neuroscience 29, (2009). 127 Dickenson, A. H., Le Bars, D. & Besson, J. M. Diffuse noxious inhibitory controls (DNIC). Effects on trigeminal nucleus caudalis neurones in the rat. Brain Research 200, (1980). 113
125 128 Bouhassira, D., Bing, Z. & Le Bars, D. Studies of brain structures involved in diffuse noxious inhibitory controls in the rat: the rostral ventromedial medulla. The Journal of Physiology 463, (1993). 129 Moont, R., Pud, D., Sprecher, E., Sharvit, G. & Yarnitsky, D. 'Pain inhibits pain' mechanisms: Is pain modulation simply due to distraction? Pain 150, (2010). 130 Valet, M., Sprenger, T., Boecker, H., Willoch, F., Rummeny, E., Conrad, B., Erhard, P, Tolle, T. R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fmri analysis. Pain 109, (2004). 131 Cadotte, D. W., Stroman, P. W., Mikulis, D. & Fehlings, M. G. A systematic review of spinal fmri research: outlining the elements of experimental design. Journal of Neurosurgery. Spine 17, (2012). 132 Cahill, C. M. & Stroman, P. W. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: a functional magnetic resonance imaging study. Magnetic Resonance Imaging 29, (2011). 133 Ghazni, N. F., Cahill, C. M. & Stroman, P. W. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR American Journal of Neuroradiology 31, (2010). 134 Lawrence, J. M., Kornelsen, J. & Stroman, P. W. Noninvasive observation of cervical spinal cord activity in children by functional MRI during cold thermal stimulation. Magnetic Resonance Imaging 29, (2011). 135 Leitch, J. K., Figley, C. R. & Stroman, P. W. Applying functional MRI to the spinal cord and brainstem. Magnetic Resonance Imaging 28, (2010). 114
126 136 Wiesenfeld-Hallin, Z. Sex differences in pain perception. Gender Medicine 2, (2005). 137 Tsao, J. C. I., Seidman, L. C., Evans, S., Lung, K. C., Zeltzer, L. K., Naliboff, B, D. Conditioned Pain Modulation in Children and Adolescents: Effects of Sex and Age. Journal of Pain 14, (2013). 138 Martel, M. O., Wasan, A. D. & Edwards, R. R. Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Medicine 14, (2013). 139 Beck, A. T., Steer, R. A., Ball, R. & Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment 67, (1996). 140 Spielberger, C., Gorsuch, RL, Lushene, R, Vagg, PR, Jacobs, GA. Manual for the State-Trait Anxiety Inventory (STAI) (Self Evaltuation Questionnaire). California Consulting Psychologists Press (1983). 141 Crowne, D. P. & Marlowe, D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology 24, (1960). 142 Sullivan, M. J. L., Bishop, S. R. & Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 7, (1995). 143 Price, D. D., Hu, J. W., Dubner, R. & Gracely, R. H. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain 3, (1977). 144 Myronenko, A., Song, XB. Image Registration by Minimization of Residual Complexity. Proc Cvpr Ieee, (2009). 115
127 145 Myronenko, A. & Song, X. Intensity-based image registration by minimizing residual complexity. IEEE transactions on medical imaging 29, (2010). 146 Stroman, P. W., Bosma, R. L. & Tsyben, A. Somatotopic arrangement of thermal sensory regions in the healthy human spinal cord determined by means of spinal cord functional MRI. Magnetic Resonance Medicine 68, (2012). 147 McArdle, J. J. & McDonald, R. P. Some algebraic properties of the Reticular Action Model for moment structures. The British Journal of Mathematical and Statistical Psychology 37 ( Pt 2), (1984). 148 Craggs, J. G., Staud, R., Robinson, M. E., Perlstein, W. M. & Price, D. D. Effective connectivity among brain regions associated with slow temporal summation of C- fiber-evoked pain in fibromyalgia patients and healthy controls. Journal of Pain 13, (2012). 149 Dafny, N., Dong, W. Q., Prieto-Gomez, C., Reyes-Vazquez, C., Stanford, J., Qiao, J. T. Lateral hypothalamus: site involved in pain modulation. Neuroscience 70, (1996). 150 Ab Aziz, C. B. & Ahmad, A. H. The role of the thalamus in modulating pain. The Malaysian Journal of Medical Sciences 13, (2006). 151 Zorman, G., Hentall, I. D., Adams, J. E. & Fields, H. L. Naloxone-reversible analgesia produced by microstimulation in the rat medulla. Brain Research 219, (1981). 152 Liebeskind, J. C., Guilbaud, G., Besson, J. M. & Oliveras, J. L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral 116
128 observations and inhibitory effects on spinal cord interneurons. Brain Research 50, (1973). 153 Almeida, A., Tjolsen, A., Lima, D., Coimbra, A. & Hole, K. The medullary dorsal reticular nucleus facilitates acute nociception in the rat. Brain Research Bulletin 39, 7-15 (1996). 154 Lima, D. & Almeida, A. The medullary dorsal reticular nucleus as a pronociceptive centre of the pain control system. Progress in Neurobiology 66, (2002). 155 Mayer, D. J., Wolfle, T. L., Akil, H., Carder, B. & Liebeskind, J. C. Analgesia from electrical stimulation in the brainstem of the rat. Science 174, (1971). 156 Bouhassira, D., Chitour, D., Villaneuva, L. & Le Bars, D. The spinal transmission of nociceptive information: modulation by the caudal medulla. Neuroscience 69, (1995). 157 Villanueva, L., Bouhassira, D. & Le Bars, D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain 67, (1996). 158 Dunckley, P., Wise, R. G., Fairhurst, M., Hobden, P., Aziz, Q., Chang, L., Tracey, I. A. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. Journal of Neuroscience 25, (2005). 159 Maixner, W., Fillingim, R., Booker, D. & Sigurdsson, A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain 63, (1995). 117
129 160 Besson, J. M. & Chaouch, A. Peripheral and spinal mechanisms of nociception. Physiological Rreviews 67, (1987). 161 Basbaum, A. I. & Fields, H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience 7, (1984). 162 Bar, K. J., Brehm, S., Boettger, M. K., Boettger, S., Wagner, G., Sauer, H. Pain perception in major depression depends on pain modality. Pain 117, (2005). 163 Strigo, I. A., Simmons, A. N., Matthews, S. C., Craig, A. D. & Paulus, M. P. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Archives of General Psychiatry 65, (2008). 164 Kain, Z. N., Sevarino, F., Alexander, G. M., Pincus, S. & Mayes, L. C. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeatedmeasures design. Journal of Psychosomatic Research 49, (2000). 165 Cormier, S., Piche, M. & Rainville, P. Expectations modulate heterotopic noxious counter-stimulation analgesia. The Journal of Pain 14, (2013). 166 Butler, R. K. & Finn, D. P. Stress-induced analgesia. Progress in Neurobiology 88, (2009). 167 Fairhurst, M., Wiech, K., Dunckley, P. & Tracey, I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 128, (2007). 168 Arendt-Nielsen, L., Sluka, K. A. & Nie, H. L. Experimental muscle pain impairs descending inhibition. Pain 140, (2008). 118
130 169 Staud, R., Robinson, M. E., Vierck, C. J., Jr. & Price, D. D. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain 101, (2003). 170 Carroll, L. J., Cassidy, J. D. & Cote, P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain 107, (2004). 171 Kashikar-Zuck, S., Goldschneider, K. R., Powers, S. W., Vaught, M. H. & Hershey, A. D. Depression and functional disability in chronic pediatric pain. The Clinical Journal of Pain 17, (2001). 172 Ossipov, M. H., Dussor, G. O. & Porreca, F. Central modulation of pain. The Journal of Clinical Investigation 120, (2010). 173 Defrin, R., Tsedek, I., Lugasi, I., Moriles, I. & Urca, G. The interactions between spatial summation and DNIC: effect of the distance between two painful stimuli and attentional factors on pain perception. Pain 151, (2010). 174 Rainville, P., Feine, J. S., Bushnell, M. C. & Duncan, G. H. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosensory & Motor Research 9, (1992). 175 Fiest, K. M., Currie, S. R., Williams, J. V. & Wang, J. Chronic conditions and major depression in community-dwelling older adults. Journal of Affective Disorders 131, (2011). 176 Goesling, J., Brummett, C. M., Meraj, T. S., Moser, S. E., Hassett, A. L., Ditre, J. W. Associations between Pain, Current Tobacco Smoking, Depression, and 119
131 Fibromyalgia Status Among Treatment-Seeking Chronic Pain Patients. Pain Medicine (2015). 177 Gauriau, C. & Bernard, J. F. Pain pathways and parabrachial circuits in the rat. Experimental Physiology 87, (2002). 178 Giesecke, T., Gracely, R. H., Williams, D. A., Geisser, M. E., Petzke, F. W., Clauw, D. J. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis and Rheumatism 52, (2005). 179 Berna, C., Leknes, S., Holmes, E. A., Edwards, R. R., Goodwin, G. M., Tracey, I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biological Psychiatry 67, (2010). 180 Fairhurst, M., Wiech, K., Dunckley, P. & Tracey, I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 128, (2007). 181 Rezaii, T. & Ernberg, M. Influence of oral contraceptives on endogenous pain control in healthy women. Experimental Brain Research 203, (2010). 182 Goubert, D., Danneels, L., Cagnie, B., Van Oosterwijck, J., Kolba, K., Noyez, H., Meeus, M. Effect of Pain Induction or Pain Reduction on Conditioned Pain Modulation in Adults: A Systematic Review. Pain Practice (2014). 183 Goffaux, P., Redmond, W. J., Rainville, P. & Marchand, S. Descending analgesia- -when the spine echoes what the brain expects. Pain 130, (2007). 184 Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory controllike effect): its relevance for acute and chronic pain states. Current Opinion in Anaesthesiology 23, (2010). 120
132 185 Liu, H., Chandler, S., Beitz, A. J., Shipley, M. T. & Behbehani, M. M. Characterization of the effect of cholecystokinin (CCK) on neurons in the periaqueductal gray of the rat: immunocytochemical and in vivo and in vitro electrophysiological studies. Brain Research 642, (1994). 186 Brack, K. E. & Lovick, T. A. Neuronal excitability in the periaqueductal grey matter during the estrous cycle in female Wistar rats. Neuroscience 144, (2007). 187 Heinricher, M. M. & Neubert, M. J. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. Journal of Neurophysiology 92, (2004). 121
133 Appendix A MRI Safety Form 122
134 Appendix B Participant Consent Form 123
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Chapter 16. Sense of Pain
 Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Pain classifications slow and fast
 Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
General Sensory Pathways of the Trunk and Limbs
 General Sensory Pathways of the Trunk and Limbs Lecture Objectives Describe gracile and cuneate tracts and pathways for conscious proprioception, touch, pressure and vibration from the limbs and trunk.
General Sensory Pathways of the Trunk and Limbs Lecture Objectives Describe gracile and cuneate tracts and pathways for conscious proprioception, touch, pressure and vibration from the limbs and trunk.
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Posterior White Column-Medial Lemniscal Pathway
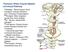 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
The Somatosensory System
 The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014
 UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Anatomical Substrates of Somatic Sensation
 Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D.
 Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
*Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord.
 *somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
*somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile
 Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
The anatomy and physiology of pain
 The anatomy and physiology of pain Charlotte E Steeds Abstract Pain is an unpleasant experience that results from both physical and psychological responses to injury. A complex set of pathways transmits
The anatomy and physiology of pain Charlotte E Steeds Abstract Pain is an unpleasant experience that results from both physical and psychological responses to injury. A complex set of pathways transmits
Pain Pathways. Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH
 Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Pharmacology of Pain Transmission and Modulation
 Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
Chapter 14: The Cutaneous Senses
 Chapter 14: The Cutaneous Senses Somatosensory System There are three parts Cutaneous senses - perception of touch and pain from stimulation of the skin Proprioception - ability to sense position of the
Chapter 14: The Cutaneous Senses Somatosensory System There are three parts Cutaneous senses - perception of touch and pain from stimulation of the skin Proprioception - ability to sense position of the
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Lecturer. Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014
 Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Our senses provide us with wonderful capabilities. If you had to lose one, which would it be?
 Our senses provide us with wonderful capabilities. If you had to lose one, which would it be? Neurological disorders take away sensation without a choice! http://neuroscience.uth.tmc.edu/s2/chapter04.html
Our senses provide us with wonderful capabilities. If you had to lose one, which would it be? Neurological disorders take away sensation without a choice! http://neuroscience.uth.tmc.edu/s2/chapter04.html
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Primary Functions. Monitor changes. Integrate input. Initiate a response. External / internal. Process, interpret, make decisions, store information
 NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus. MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive
 PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Laurie L. Wellman Ph.D.
 Laurie L. Wellman Ph.D. Theodore Tzavaras MD2015 Eastern Virginia Medical School Dr. Craig Goodmurphy Anatomy Guy 1. Discuss -- what is pain? 2. Outline the Anterolateral Quadrant (ALQ) pathway 3. Locate
Laurie L. Wellman Ph.D. Theodore Tzavaras MD2015 Eastern Virginia Medical School Dr. Craig Goodmurphy Anatomy Guy 1. Discuss -- what is pain? 2. Outline the Anterolateral Quadrant (ALQ) pathway 3. Locate
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. Pain is always subjective
 Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
San Francisco Chronicle, June 2001
 PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
Pain. Pain. Pain: One definition. Pain: One definition. Pain: One definition. Pain: One definition. Psyc 2906: Sensation--Introduction 9/27/2006
 Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Biomechanics of Pain: Dynamics of the Neuromatrix
 Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Spinal Cord Organization. January 12, 2011
 Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy
 By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
Chapter 6. Gathering information; the sensory systems
 Chapter 6 Gathering information; the sensory systems Gathering information the sensory systems The parts of the nervous system that receive and process information are termed sensory systems. There are
Chapter 6 Gathering information; the sensory systems Gathering information the sensory systems The parts of the nervous system that receive and process information are termed sensory systems. There are
Overview of Questions
 Overview of Questions What are the sensors in the skin, what do they respond to and how is this transmitted to the brain? How does the brain represent touch information? What is the system for sensing
Overview of Questions What are the sensors in the skin, what do they respond to and how is this transmitted to the brain? How does the brain represent touch information? What is the system for sensing
Last time we talked about the descending pathways of pain and the ALS. Today we will continue talking about these descending pathways.
 Last time we talked about the descending pathways of pain and the ALS. Today we will continue talking about these descending pathways. Each higher level will control the level under It. In controlling
Last time we talked about the descending pathways of pain and the ALS. Today we will continue talking about these descending pathways. Each higher level will control the level under It. In controlling
Skin types: hairy and glabrous (e.g. back vs. palm of hand)
 Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Chapter 14: Integration of Nervous System Functions I. Sensation.
 Chapter 14: Integration of Nervous System Functions I. Sensation A. General Organization 1. General senses have receptors a. The somatic senses provide information about & 1. Somatic senses include: a.
Chapter 14: Integration of Nervous System Functions I. Sensation A. General Organization 1. General senses have receptors a. The somatic senses provide information about & 1. Somatic senses include: a.
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Basic Neuroscience. Sally Curtis
 The Physiology of Pain Basic Neuroscience Sally Curtis sac3@soton.ac.uk The behaviour of humans is a result of the actions of nerves. Nerves form the basis of Thoughts, sensations and actions both reflex
The Physiology of Pain Basic Neuroscience Sally Curtis sac3@soton.ac.uk The behaviour of humans is a result of the actions of nerves. Nerves form the basis of Thoughts, sensations and actions both reflex
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
The Nervous System: Sensory and Motor Tracts of the Spinal Cord
 15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
How strong is it? What is it? Where is it? What must sensory systems encode? 9/8/2010. Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Somatosensory System. Steven McLoon Department of Neuroscience University of Minnesota
 Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Chapter 7. The Nervous System: Structure and Control of Movement
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7. Objectives
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
1 The Physiology of Pain
 1 The Physiology of Pain Rohit Juneja and Siân Jaggar Introduction Definitions Key Messages Pain is still underdiagnosed and undertreated. Pain is a subjective experience and may even be present in the
1 The Physiology of Pain Rohit Juneja and Siân Jaggar Introduction Definitions Key Messages Pain is still underdiagnosed and undertreated. Pain is a subjective experience and may even be present in the
PAIN MANAGEMENT in the CANINE PATIENT
 PAIN MANAGEMENT in the CANINE PATIENT Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT Part 1: Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT 1 Pain is the most common reason
PAIN MANAGEMENT in the CANINE PATIENT Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT Part 1: Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT 1 Pain is the most common reason
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Gross Morphology of Spinal Cord
 Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Introduction to Physiological Psychology
 Introduction to Physiological Psychology Review Kim Sweeney ksweeney@cogsci.ucsd.edu www.cogsci.ucsd.edu/~ksweeney/psy260.html Today n Discuss Final Paper Proposal (due 3/10) n General Review 1 The article
Introduction to Physiological Psychology Review Kim Sweeney ksweeney@cogsci.ucsd.edu www.cogsci.ucsd.edu/~ksweeney/psy260.html Today n Discuss Final Paper Proposal (due 3/10) n General Review 1 The article
Unit 3: The Biological Bases of Behaviour
 Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
Pathways of proprioception
 The Autonomic Nervous Assess Prof. Fawzia Al-Rouq Department of Physiology College of Medicine King Saud University Pathways of proprioception System posterior column& Spinocerebellar Pathways https://www.youtube.com/watch?v=pmeropok6v8
The Autonomic Nervous Assess Prof. Fawzia Al-Rouq Department of Physiology College of Medicine King Saud University Pathways of proprioception System posterior column& Spinocerebellar Pathways https://www.youtube.com/watch?v=pmeropok6v8
Thalamus and Sensory Functions of Cerebral Cortex
 Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
SOMATOSENSORY SYSTEMS AND PAIN
 SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for
 Chapter 13 Outline Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for what you need to know from Exhibits 13.1 13.4 I. INTRODUCTION A. The spinal cord and spinal nerves
Chapter 13 Outline Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for what you need to know from Exhibits 13.1 13.4 I. INTRODUCTION A. The spinal cord and spinal nerves
COGS 107B Week 1. Hyun Ji Friday 4:00-4:50pm
 COGS 107B Week 1 Hyun Ji Friday 4:00-4:50pm Before We Begin... Hyun Ji 4th year Cognitive Behavioral Neuroscience Email: hji@ucsd.edu In subject, always add [COGS107B] Office hours: Wednesdays, 3-4pm in
COGS 107B Week 1 Hyun Ji Friday 4:00-4:50pm Before We Begin... Hyun Ji 4th year Cognitive Behavioral Neuroscience Email: hji@ucsd.edu In subject, always add [COGS107B] Office hours: Wednesdays, 3-4pm in
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
fmri (functional MRI)
 Lesion fmri (functional MRI) Electroencephalogram (EEG) Brainstem CT (computed tomography) Scan Medulla PET (positron emission tomography) Scan Reticular Formation MRI (magnetic resonance imaging) Thalamus
Lesion fmri (functional MRI) Electroencephalogram (EEG) Brainstem CT (computed tomography) Scan Medulla PET (positron emission tomography) Scan Reticular Formation MRI (magnetic resonance imaging) Thalamus
Neural Basis of Motor Control. Chapter 4
 Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Psychophysical laws. Legge di Fechner: I=K*log(S/S 0 )
 Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Sensory information processing, somato-sensory systems
 mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
1. Somatosensory Pathways
 1. Somatosensory Pathways Objectives 1. Describe the general characteristics of sensory pathways 2. Understand the general organization and numbered areas of spinal cord gray matter 3. Understand dermatomes
1. Somatosensory Pathways Objectives 1. Describe the general characteristics of sensory pathways 2. Understand the general organization and numbered areas of spinal cord gray matter 3. Understand dermatomes
10/3/2016. T1 Anatomical structures are clearly identified, white matter (which has a high fat content) appears bright.
 H2O -2 atoms of Hydrogen, 1 of Oxygen Hydrogen just has one single proton and orbited by one single electron Proton has a magnetic moment similar to the earths magnetic pole Also similar to earth in that
H2O -2 atoms of Hydrogen, 1 of Oxygen Hydrogen just has one single proton and orbited by one single electron Proton has a magnetic moment similar to the earths magnetic pole Also similar to earth in that
Is mindfulness meditation an appropriate therapy in patients presenting with chronic pain?
 Is mindfulness meditation an appropriate therapy in patients presenting with chronic pain? Background Chronic pain affects between one-third and one-half of the UK adult 10.4% to 14.3% of cases being moderate-severely
Is mindfulness meditation an appropriate therapy in patients presenting with chronic pain? Background Chronic pain affects between one-third and one-half of the UK adult 10.4% to 14.3% of cases being moderate-severely
Organization of the nervous system. The withdrawal reflex. The central nervous system. Structure of a neuron. Overview
 Overview The nervous system- central and peripheral The brain: The source of mind and self Neurons Neuron Communication Chemical messengers Inside the brain Parts of the brain Split Brain Patients Organization
Overview The nervous system- central and peripheral The brain: The source of mind and self Neurons Neuron Communication Chemical messengers Inside the brain Parts of the brain Split Brain Patients Organization
Somatosensation. Recording somatosensory responses. Receptive field response to pressure
 Somatosensation Mechanoreceptors that respond to touch/pressure on the surface of the body. Sensory nerve responds propotional to pressure 4 types of mechanoreceptors: Meissner corpuscles & Merkel discs
Somatosensation Mechanoreceptors that respond to touch/pressure on the surface of the body. Sensory nerve responds propotional to pressure 4 types of mechanoreceptors: Meissner corpuscles & Merkel discs
PETER PAZMANY CATHOLIC UNIVERSITY Consortium members SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
 PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
b. The groove between the two crests is called 2. The neural folds move toward each other & the fuse to create a
 Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Organization of the nervous system. [See Fig. 48.1]
![Organization of the nervous system. [See Fig. 48.1] Organization of the nervous system. [See Fig. 48.1]](/thumbs/90/103926552.jpg) Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
