Quick Reference Sheet for Guideline Patient Blood Management prior to Surgery CPDI 063, v5.1 Pathway for investigation of anaemia (Flowchart 1)
|
|
|
- Molly Bailey
- 5 years ago
- Views:
Transcription
1 Quick Reference Sheet for Guideline Patient Blood Management prior to Surgery CPDI 063, v5.1 Pathway for investigation of anaemia (Flowchart 1) If neutrophils <1 x10 9 /L or Platelets <80 x10 9 /L Refer to Haematology Check FBC, Haematinics, U&E and CRP Is the patient anaemic? Hb <130 g/l in male Hb <120 g/l in female No Yes Ferritin <30 µg/l Ferritin µg/l Ferritin >100 µg/l CRP High Normal No anaemia Iron deficiency anaemia Consider cause and need for GI investigation, based on clinical findings Commence iron therapy: Oral/IV depending on Hb level, symptoms and timescale Ferritin > 100 µg/l: no action Ferritin < 100 µg/l give oral iron tds for 4 weeks Ferritin < 30 µg/l: Consider cause and need for GI investigation, based on clinical findings. Give oral iron tds for 4-6 weeks Possible iron deficiency Consider clinical context Consider need for GI investigations, based on timing in relation to surgery Commence iron therapy Oral/IV depending on Hb, symptoms and timescale Possible anaemia of chronic disease Consider clinical context Review renal function, blood film, B12/folate, reticulocyte count, LFTs and SEP if indicated and as appropriate Seek haematology advice or, in the presence of chronic kidney disease, renal advice Consider erythropoietin therapy, if egfr <30 ml/min
2 Guideline Patient Blood Management prior to Surgery This guidance clearly sets out the appropriate actions required for the investigation and management of patients requiring surgery that are at risk of anaemia and/or bleeding, in order to minimise their need for a blood component transfusion. All surgeons and GPs should familiarise themselves with the content of this guidance document and begin to implement the actions. Key words: Surgery, Anaemia, Blood, Iron, Ferritin, Cosmofer, Monofer Document Number: CPDI 063 Version: 5.1 Developed in Consultation with: Hospital Transfusion Team (HTT) Ratified by: Diagnostics & Clinical Support Quality and Performance Committee Date Ratified: 10 December 2014 Date Modified: 6 th March 2015 Next review due to start: 10 June 2017 Expiry Date: 10 December 2017 Document Author: Dr A Allameddine (Chair HTT), Dr J. Graham (ST7 Haematology) & C Porada (Transfusion Practitioner) On behalf of the Trust Transfusion Committee Expiry Date: 10/12/17 Page 2 of 29
3 Pennine Acute Hospitals NHS Trust Guideline Patient Blood Management prior to Surgery Name of Previous Document: Main Revisions from previous issue Document Number: CPDI 063 Version Number: 5.0 Guidelines Patient Blood Management prior to Surgery Reason for Revision: Asthma is no longer contraindicated for the administration of IV Iron (Monofer & Cosmofer) - Removal of word Asthma from page 9 Expiry Date: 10/12/17 Page 3 of 29
4 Contents Page 1. Introduction 4 2. Aims 4 3. Scope 4 4. Roles, Responsibilities & Accountabilities 5 5. Guidance for Patient Blood Management prior to Surgery Pre-operative assessment Management of anaemia Assessment post-treatment Implementation Monitoring arrangement Review Arrangements Supporting Documents & References Abbreviations Appendices 15 Appendix 1 Patient Information Leaflet for Receiving a Blood Transfusion 15 Appendix 2 Patient Information Leaflet for Iron In Your Diet 16 Appendix 3 Patient Information Leaflet Regarding Receiving Your Own Blood 17 Appendix 4 Pathway for investigation of anaemia (Flowchart 1) 18 Appendix 5 Standard GP/Referring Doctor Information Letter 19 Appendix 6 Standard Patient Information Letter for Anaemia 20 Appendix 7 Standard Patient Information Letter For Anticoagulation Drugs 21 Appendix 8 Referral Letter for Iron Infusion Therapy to PIU/HHDC 22 Appendix 9 Protocol for Administering Tranexamic Acid 23 Appendix 10 Maximum Surgical Blood Order Schedule - Crossmatch 24 Appendix 11 Maximum Surgical Blood Order Schedule G&S 25 Appendix 12 Summary of Monitoring Arrangements 26 Appendix 13 Equality Impact Assessment Proforma 27 Expiry Date: 10/12/17 Page 4 of 29
5 1. Introduction 1.1 Patient Blood Management is a multidisciplinary, evidence-based approach to optimising the care of patients in order to avoid or minimise the need for allogeneic transfusion. 1.2 Anaemic patients are at increased risk of transfusion, mortality and major morbidity, in proportion to the severity of anaemia. Even mild anaemia increases relative mortality risk by a third. 1.3 Pre-operative anaemia is common and prevalence varies from 5-75% depending on the population studied and the surgical procedure. Transfusion increases the risk of peri-operative mortality and major morbidity in a dose-dependent fashion. 1.4 Pre-operative anaemia substantially increases health care costs with significant additional cost incurred out of hospital. It further predisposes patients to requiring allogeneic blood transfusion, thereby increasing the burden on blood donors and donor services. 1.5 The World Health Organization (WHO) has defined anaemia as Hb < 130 g/l for men Hb < 120 g/l for women 1.6 This document should be read in conjunction with the following trust policies Education Training & Development Induction & Mandatory Training Policy, EDH 024 Accident and Incident reporting policy, EDQ 008 Medicines Policy, EDC 018 Policy on Consent to Examination or Treatment, EDQ 002 Guideline for the management of patients receiving antithrombotic drugs CPD Aims These policies are available via Trust Document Management System (DMS). 2.1 To identify and optimise all patients with anaemia prior to surgery to minimise the risk of requiring an allogeneic transfusion. 2.2 To provide health care professionals with clear and simple recommendations for the management of anaemia prior to surgery. 2.3 To reduce blood usage in adult elective surgery. 3. Scope This guideline is for all clinical staff involved in the care of patients prior to surgery and to facilitate safe and appropriate treatment of anaemia where necessary. 4. Roles, Responsibilities and Accountabilities 4.1 The Division of Diagnostics Quality and Performance Committee is responsible for authorising Expiry Date: 10/12/17 Page 5 of 29
6 the document and receiving assurance of compliance through review of documentation provided by the Trust Transfusion Committee 4.2 The Trust Transfusion Committee (TTC) is the sponsor group for this document. They are responsible for promoting the safe care of patients who require treatment for anaemia, through local policy, based on national guidelines. The TTC will ensure compliance with this document by review of adverse incident reports. The TTC will also provide reports to the Division of Diagnostics Quality and Performance Committee 4.3 The Hospital Transfusion Team (HTT) is responsible for review of national guidelines and local adverse incidents making recommendations to the TTC 4.4 The Transfusion Practitioner Team is responsible for review of any individual incidents of noncompliance, report externally to SHOT and MHRA when required and feed in to the HTT. The transfusion practitioner team provide training on the application of this document. 4.5 Ward and departmental managers are responsible for dissemination of this document and ensuring compliance of all staff within their sphere of responsibility. 4.6 All Staff are required to comply with this document and to bring to the attention of their immediate manager any difficulties they encounter in using this document. Report any adverse incidents via the Trust Accident & Incident Reporting Policy (EDQ 008). 5. Guidance for Patient Blood Management in Patients prior to Surgery 5.1 Pre-operative assessment: performed by GPs or referring physicians or Pre-Operative Assessment Clinics (POACs) The investigation and management of pre-operative anaemia should ideally be a collaborative process involving primary and secondary care. To avoid disruption to surgical schedules, anaemia screening should take place as early as possible in the referral pathway, ideally when referral is first made. Wherever possible, this should take place with sufficient time to allow investigation and correction if appropriate. Where surgery is urgent, whatever time is available before the operation should still be used for anaemia investigation and treatment initiation. Anaemia may be expected as part of the presenting complaint. However, surgery represents a sentinel event for many patients and work-up may reveal previously unsuspected disease. Anaemic patients will therefore fall into two groups: - Those who may safely proceed to surgery with anaemia treatment. - Those who require investigation may require a delay of surgery whilst more extensive investigation is carried out, to exclude previously undetected disease. The pre-operative assessment should include: - Identification, investigation and management of patients with or at risk of anaemia. Expiry Date: 10/12/17 Page 6 of 29
7 - Assessment of the adequacy of iron stores in patients undergoing planned procedures in which substantial blood loss is anticipated. - Awareness and assessment of medications and complementary medicines that might increase bleeding risk. - Awareness of and ability to discuss with patients, the possible risks associated with blood transfusion and to give information on the possibility of requiring when appropriate cell salvage, intra or post-operatively (See Appendix 3). All patients who are identified as at risk of requiring a blood transfusion should have FBC, haematinics, CRP, U&Es and clotting screen (INR, APTT and Fibrinogen). (Serum Ferritin is an acute phase protein and may be raised if CRP is elevated). All blood results should be reviewed within 2 working days. Abnormal results should be discussed with a member of the clinical team who has sufficient authority to commence treatment, refer for further investigation or delay surgery as necessary. Determine possible cause of anaemia based on history, examination and laboratory results (see appendix 4 for flowchart). Seek specialist advice as appropriate. For example seek Haematology advice if concomitant low platelet and white cell counts, Gastroenterology advice in GI bleeding, Nephrology advice in the presence of chronic kidney disease (egfr<30 ml/min). For anaemic patients requiring treatment, a clear and timely approach should be available without undue disruption to existing patient pathway. Inherited haemoglobin disorders (haemoglobinopathies) should be considered in all individuals with microcytic anaemia if there is no evidence of iron deficiency, or if red cell changes persist after adequate iron replacement. Send an EDTA blood sample for HPLC testing. All patients who are taking anticoagulant drugs such warfarin, aspirin, or other anti-platelet agents (e.g. dipyridamole or Clopidogrel), Non Steroidal Anti-Inflammatory Drugs (NSAIDS) and novel oral anticoagulants (Dabigatran, Apixaban, Rivaroxaban) should be identified and necessary arrangements made to stop the drug at a suitable interval preoperatively (see Guideline for the management of patients receiving antithrombotic drugs CPDI 201). Patients on such drugs should have a clear, written guidance on when to stop and restart these medications prior to surgery. Perioperative plans should be provided to the patient and clearly recorded in the medical notes for admitting teams. Table 1: Outlines the questions to consider when referring a patient for elective surgery Expiry Date: 10/12/17 Page 7 of 29
8 Questions to consider when referring a patient for elective surgery Is the surgery likely to result in significant blood loss? Does my patient have anaemia or are they at risk of anaemia? What are my patient s iron stores? Are there co-morbidities that may contribute to adverse outcomes if anaemia develops? If so, what steps are needed to optimise these conditions (e.g. cardiac disease)? Are there chronic conditions that may impede a haematopoietic response to anaemia (e.g. chronic kidney disease, inflammation or bone marrow pathology)? What medications is my patient taking that might increase their bleeding risk? Is my patient informed about the possible risks associated with blood transfusion and alternatives that may be available? Table 2: Outlines the risks and adverse outcomes associated with blood transfusion that the referring physician should be aware of and discuss with the patient (Printed patient leaflets are available to download from see appendices 1 and 2) Risks and adverse outcomes associated with blood transfusion Non-infectious risks Acute haemolytic reaction (e.g. incorrect blood component transfused) Allergic, including anaphylactic, reactions Transfusion associated circulatory overload (TACO) Transfusion related acute lung injury (TRALI) Delayed haemolytic transfusion reaction Transfusion associated graft versus host disease Adverse outcomes red blood cell transfusion has been associated with Increased morbidity and mortality Increased ICU and hospital length of stay Increased incidence of postoperative infection Transfusion related immunomodulation Infectious risks Blood transfusion is safe however there remains a very low risk of transmission of known infectious agents (HIV, hepatitis C and B). It is estimated that, the risk of getting hepatitis B is about 1 in 1.3 million, hepatitis C is about 1 in 28 million and HIV is about 1 in 6.5 million. The blood supply will always remain vulnerable to emerging infectious agents, such CJD Bacterial contamination is also low risk; however this is still an important consideration, particularly with platelet transfusion. Expiry Date: 10/12/17 Page 8 of 29
9 5.2 Management of anaemia The management option(s) appropriate for an anaemic patient depend on interplay between the following factors: The cause and severity of anaemia The anticipated peri-operative blood loss The timescale before surgery Whether surgery may safely be postponed Common causes of anaemia include iron, B12 or folate deficiency, anaemia of chronic disease and chronic kidney disease. Consider blood loss or haemolysis if reticulocyte count is increased. Iron Deficiency Anaemia (IDA) can be due to blood loss, impaired iron absorption or failure to utilise iron stores. Potential causes are: Menorrhagia, chronic gastro-intestinal symptoms or chronic bleeding from GI tract, acute or chronic inflammatory bowel disease or malabsorption, malignancy, pregnancy. Iron Therapy Both oral iron tablets and intravenous iron preparations are inexpensive products compared with the transfusion of red cells. Oral iron is indicated in iron deficient anaemic patients whose surgery is not urgent. While intravenous iron is indicated in patients intolerant or unresponsive to oral iron or when the timescale for surgery is predicted to be short. Iron therapy is indicated for non-anaemic iron deplete patients (ferritin <100ng/L) scheduled to undergo surgery with predicted total peri-operative erythrocyte loss >30g/L, to protect against postoperative IDA. Oral iron therapy: Commonly prescribed Ferrous Sulphate 200 mg TDS or Ferrous Fumarate 325 mg BD-TDS. Patients must be advised how to take oral iron effectively. Iron should be taken on an empty stomach with orange juice. Tea, coffee and calcium decrease the absorption of iron and should be avoided an hour either side. Oral iron can cause significant gastrointestinal side effects that result in poor compliance. A patient who fails to tolerate one preparation may tolerate another. Oral iron should be continued for 3 months after the haemoglobin and iron stores are replenished. Parenteral (IV iron): Intravenous iron should only be used when oral iron is not tolerated or there is a history of malabsorption or active inflammatory bowel disease, or the timescale before surgery is limited to <4 weeks. Dosing for intravenous iron is dependent on the patient s haemoglobin, ferritin level and weight. Facilities for cardiopulmonary resuscitation and personnel trained to handle anaphylactoid reactions must be available. Intravenous iron is contradicted in patients with a history of allergic eczema or other atopic allergy. Expiry Date: 10/12/17 Page 9 of 29
10 Table 3 Outlines the dosage of Intravenous Iron Dose IV iron recommended: Hb >80 g/l give intravenous iron 500 mg Hb < 80 g/l give intravenous iron 1000 mg Intravenous iron products available in the trust: Monofer 500 mg in 100 ml of sodium chloride (0.9%) over 10 min Monofer 1000 mg in 250 ml of sodium chloride (0.9%) over 30 min, max dose 20 mg/kg Cosmofer 500 mg in 500 ml of sodium chloride (0.9%) over 3-4 hours Cosmofer 1000 mg in 500 ml of sodium chloride (0.9%) over 3-4 hours, max dose 20 mg/kg NB: No test dose is required with IV iron but patients must be observed for 30 minutes post infusion. B12 and Folate therapy For B12 deficiency: Give Hydroxycobalamin by intramuscular injection1 mg 3 times a week first week (Monday-Wednesday - Friday) and 2 times a week second week (total of 5 injections). For Folate deficiency: Give Folic Acid orally 5 mg OD for 4 weeks. Erythrocytosis-stimulating agent (ESA) therapy In Anaemia of chronic kidney disease (egfr<30 ml/min or <45 ml/min in diabetics) consider ESA + iron therapy (if Ferritin < 100 µg/l) ESA therapy may be indicated in anaemic patients scheduled for surgery with significant predicted blood loss, in which iron deficiency has been excluded. Where the time before non-deferrable surgery is very short, combination therapy with an ESA and parenteral iron may be appropriate. Table 4 Outlines the dosage of erythropoietin: Dose recommended for patients with CKD: Erythropoietin alpha or beta: U s/c week For high risk patients: e.g. Jehovah s Witnesses: Erythropoietin alpha or beta: U s/c for 2 doses (one week interval) Intravenous iron: 500 mg Expiry Date: 10/12/17 Page 10 of 29
11 5.3 Assessment Post Treatment: Initiated by a surgeon and followed by GPs or referring physicians All patients who were found to be anaemic and were given treatment should be re-assessed before surgery. In patient with persistent anaemia, a decision to delay surgery is based on clinical circumstances For those at risk of requiring blood transfusion, ensure group and saved or cross-matched blood has been arranged, in accordance with the MSBOS (Maximum Surgical Blood Ordering Schedule). Consider the feasibility of intra-operative cell salvage or post-operative cell salvage (see policy Autologous Blood Transfusion CPDI 072), depending on the nature of procedure and the amount of blood likely to be lost. Consider peri-operative use of Tranexamic acid (1 gr tds started on day of surgery) if blood loss if likely and there are no contraindications. Continue Tranexamic acid for 72 hours post-surgery; if appropriate. (See Appendix 9). Where transfusion is required, consider accepting a lower post-operative haemoglobin before transfusing blood. A transfusion trigger could be as low as 70 g/l. Consider single unit transfusion when appropriate (see policy Indications for Red Cell Transfusion The Green Policy, EDC 007). Following surgery, all patients should have their FBC checked and management should be based depending on the clinical circumstances and level of haemoglobin (see 5.2 Management of anaemia). If following surgery there has been a significant blood loss, the patient should be given adequate iron replacement for a period of time to ensure iron stores are rapidly replenished and the haemoglobin rises to normal as rapidly as possible. Iron replacement may be more than adequate treatment for post-operative anaemia and may obviate the need for post-operative transfusion. It s expected a rise of haemoglobin by g/l in 4 weeks of treatment with oral iron and 2 weeks with intravenous iron. Arrangements should be made with the patients general practitioner to ensure that the treatment of post-operative iron deficiency is appropriately managed after discharge. 6. Implementation 6.1 Dissemination The Trust will demonstrate that this document has been issued, read and implemented as follows. A variety of dissemination methods are in place to ensure clinical staff are made aware of, have access to, and comply with this document, these include: Summary list of new documents published in the weekly bulletin, including a description of the document and its intended core audience. Inclusion on the Document Management System (DMS) on the Trust s intranet site, which all staff are encouraged to use. Information on the Trust intranet Blood Transfusion Web pages. Expiry Date: 10/12/17 Page 11 of 29
12 Staff should always consult the Document Management System (DMS) for the latest version of the document. 6.2 Training Arrangements This guideline will be highlighted at all education forums by the transfusion practitioner team and discussed in more detail where relevant. It is the responsibility of the Directorate Managers, Divisional Nurse Managers, Ward and departmental managers to ensure that staff are familiar with the content of this document and ensure that relevant training is made available as necessary. For further information on Mandatory Training requirements and updates, staff should refer to: The Mandatory Training Guidance Matrix and Schedule The Annual Training Prospectus & Plan The Bi-monthly Training Bulletin These are available on the Learning & Organisational Development Intranet page. Staff who do not attend Mandatory Training or Induction will be highlighted on the Mandatory Training Compliance Register and will be monitored via the process outlined in the Education Training & Development Induction & Mandatory Training Policy (EDH024). 6.3 Financial Impact There are resource implications associated with the introduction of this guideline. A business case has been submitted for the cost of implementing this document. Currently funding has been approved within the trust. 7. Monitoring Arrangements Please see Appendix 10 for a summary of arrangements. 8. Review Arrangements This guideline will be reviewed by the Hospital Transfusion Team and Trust Transfusion Committee every three years. It will then be approved by the Trust Transfusion Committee, before going to the Diagnostic Quality and Performance Committee for final approval. If there are any clinical indicators to review this guideline, this will be done on an ad-hoc basis. 9. Supporting Documents & References 9.1 Associated Trust Policies & Guidelines Education Training & Development Induction & Mandatory Training Policy, EDH 024 Accident and Incident reporting policy, EDQ 008 Policy on Consent to Examination or Treatment, EDQ 002 Expiry Date: 10/12/17 Page 12 of 29
13 Medicines Policy, EDC 018 The Administration of Blood Components Policy, EDC 006 Indications for Red Cell Transfusion The Green Policy, EDC 007 Autologous Blood Transfusion Policy, CPDI 072 The management of all patients who decline blood products including Jehovah s Witness patients policy, CPDI 064. Guideline for the management of patients receiving antithrombotic drugs, CPDI 201 Guidelines for Peri-operative Drug Administration, CPSU Supporting References Better blood transfusion Network iron. FACTSHEET- Transfusion guidelines version 2 February 2011 Anaemia management in people with chronic kidney disease (CG114 NICE 2011) McClelland. DBL. (2013) Handbook of Transfusion Medicine 5 th Edition. The Stationary Office. ISBN NHSBT & NBTC Patient Blood Management Document An Evidence based approach to patient care, 2014, Blood Transfusion and the Anaesthetists: Blood Component Therapy (2005). The Association of Anaesthetists of Great Britain and Ireland, London. Blood Transfusion and the Anaesthetist: Red Cell Transfusion 2 (2008). Association of Anaesthetist of Great Britain and Ireland, London. Department of Health Toolkit - Better Blood Transfusion: Appropriate use of Blood Preoperative Assessment: McClelland. DBL. (2007) Handbook of Transfusion Medicine 4 th Edition. The Stationary Office. ISBN NICE (2008) Inadvertent perioperative hypothermia: The management of inadvertent perioperative hypothermia in adults, NICE clinical guideline 65, London, April North West Region Transfusion Committee: Guidelines for the Management of Anaemia in Preoperative Assessment Clinics. (2008) 10. Abbreviations > Greater than BD BMS CRP ESA Fe Twice per day Biomedical Scientist C-Reactive Protein Erythropoiesis Stimulating Agents Iron Expiry Date: 10/12/17 Page 13 of 29
14 FBC Full blood count GI Gastrointestinal egfr Glomerular Filtration Rate EDTA Ethylenediaminetetraacetic acid EPO Erythropoietin g Grams g/l Grams per litre G&S Group & Save Hb Haemoglobin HPLC High Performance Liquid Chromatography HTT Hospital Transfusion Team IDA Iron deficiency anaemia IM Intramuscular IV Intravenous min minute mg Milligrams mg/l Milligrams per litre mg/kg Milligrams per kilogram ml/min Millilitres per minute µg Micrograms µg/l Micrograms per litre MSBOS Maximum Surgical Blood Order Schedule NBTC National Blood Transfusion Committee NHSBT National Blood Transfusion Service NSAIDS Nonsteroidal anti-inflammatory drugs OD Once per day PAHT Pennine Acute Hospitals Trust POACs Pre-Operative Assessment Clinics SEP Serum Electrophoresis SHOT Serious Hazards of Transfusion TDS To be taken three times a day TTC Trust Transfusion Committee U&E Urea and Electrolytes WHO World Health Organisation Expiry Date: 10/12/17 Page 14 of 29
15 Appendix 1 Patient Information Leaflet for Receiving a Blood Transfusion Information leaflet about blood transfusion Expiry Date: 10/12/17 Page 15 of 29
16 Appendix 2 Patient Information Leaflet for Iron In Your Diet Information leaflet about Iron In Your Diet Expiry Date: 10/12/17 Page 16 of 29
17 Appendix 3 Patient Information Leaflet Regarding Receiving Your Own Blood Information leaflet about Cell Salvage Expiry Date: 10/12/17 Page 17 of 29
18 Appendix 4 Pathway For Investigation of Anaemia (Flowchart 1) If neutrophils <1 x10 9 /L or Platelets <80 x10 9 /L Refer to Haematology Check FBC, Haematinics, U&E and CRP Is the patient anaemic? Hb <130 g/l in male Hb <120 g/l in female No Yes Ferritin <30 µg/l Ferritin µg/l Ferritin >100 µg/l CRP High Normal No anaemia Iron deficiency anaemia Consider cause and need for GI investigation, based on clinical findings Commence iron therapy: Oral/IV depending on Hb level, symptoms and timescale Ferritin > 100 µg/l: no action Ferritin < 100 µg/l give oral iron tds for 4 weeks Ferritin < 30 µg/l: Consider cause and need for GI investigation, based on clinical findings. Give oral iron tds for 4-6 weeks Possible iron deficiency Consider clinical context Consider need for GI investigations, based on timing in relation to surgery Commence iron therapy Oral/IV depending on Hb, symptoms and timescale Possible anaemia of chronic disease Consider clinical context Review renal function, blood film, B12/folate, reticulocyte count, LFTs and SEP if indicated and as appropriate Seek haematology advice or, in the presence of chronic kidney disease, renal advice Consider erythropoietin therapy, if egfr <30 ml/min Expiry Date: 10/12/17 Page 18 of 29
19 Appendix 5 Standard GP/Referring Doctor Information Letter Information for GP/referring doctor of the patient who is advised to take* oral iron/oral Folic Acid/ B12 injections supplement pre-operatively Dear Dr Name of Patient... NHS number... Your patient was seen in the Pre-operative Assessment Clinic on:... Planned surgical procedure:... Planned surgery date:... Full Blood Count Results have indicated that this patient is currently anaemic. FBC Results: Hb... Plts... WCC... Ferritin Folate... B12... We have decided to continue with the planned surgical procedure as detailed above. However, in order to help improve the haemoglobin prior to surgery, we initiated your patient a course of:...,as detailed below: Dosage:... Frequency:... Duration:... NOTE: the cause of this anaemia remains clear/unclear* and may warrant further investigation by yourself. Thank you Signed: Name (printed): Date: *Delete as appropriate Expiry Date: 10/12/17 Page 19 of 29
20 Appendix 6 Standard Patient Information Letter for Anaemia Information for patients who are advised to take an oral iron/folic Acid/B12 injections preoperatively Address, contact name and number Dear patient One of the routine blood tests taken when you came to the Pre- Operative Assessment Clinic has showed that you are mildly anaemic and/or iron and vitamin stores are low. This is not a serious problem but correction of this prior to surgery will reduce your need for transfusion either during or after your surgery, and will make you feel better and help speed up your recovery. Your treatment for this is to take a course of:.. for up to... weeks. After that, your response to this treatment will be assessed by a further blood test. The form for this blood test is supplied along with this letter and can be taken to your local GP or hospital phlebotomy area. Things you need to do are: 1. Arrange for a prescription to be obtained from your GP for the treatment listed above. Obtain a supply of this treatment. Arrange an appointment with your District Nurse if an injection is required. 2. After completion of the treatment, please take the blood sample request form to either your GP or local hospital phlebotomy service and have the repeat blood samples taken (form enclosed) 3. If you experience any problems or need any additional help or advise please contact your GP or the number above Please note: Iron tablets sometimes have side effects, which make it difficult to continue with the treatment. These are: sickness, some discomfort in the upper part of your stomach, diarrhoea or constipation. These are common and not serious but if it makes it difficult to continue with the course of tablets please contact either your GP or hospital on the above number; they may be able to suggest another type of treatment. Thank you Signed: Name (printed): Date: Expiry Date: 10/12/17 Page 20 of 29
21 Appendix 7 Standard Patient Information Letter For Anticoagulation Drugs Name of Patient... NHS number... Seen in the Pre-operative Assessment Clinic on:... Planned surgical procedure:... Planned surgery date:... Anticoagulation drugs:... Plan of action: Signed: Name (printed): Date: Expiry Date: 10/12/17 Page 21 of 29
22 Appendix 8 Referral Letter for Iron Infusion Therapy to PIU/HHDC Referral for iron infusion treatment Dear Matron I would be grateful if you can arrange for this patient the following treatment: Name of Patient... DOB Hospital number... Patient was seen in the Pre-operative Assessment Clinic on:... Planned surgical procedure:... Surgery date:... Full Blood Count Results have indicated that this patient is currently anaemic. Hb... Plts... WCC... Ferritin. Treatment required (tick the appropriate box): Monofer 500 mg in 100 ml of sodium chloride (0.9%) over 10 min Monofer 1000 mg in 250 ml of sodium chloride (0.9%) over 30 min, max dose 20 mg/kg Cosmofer 500 mg in 500 ml of sodium chloride (0.9%) over 3-4 hours Cosmofer 1000 mg in 500 ml of sodium chloride (0.9%) over 3-4 hours, max dose 20 mg/kg We have decided to continue with the planned surgical procedure as detailed above. Thank you Signed: Name (printed): Date: Dose IV iron recommended: Hb >80 g/l give intravenous iron 500 mg Hb < 80 g/l give intravenous iron 1000 mg Expiry Date: 10/12/17 Page 22 of 29
23 Appendix 9 Protocol for Administering Tranexamic Acid Tranexamic acid is an antifibrinolytic agent that has been widely used in the surgical settings to reduce blood loss. Tranexamic acid is an antifibrinolytic agent widely used in the non surgical setting to reduce expected blood loss (e.g. menorrhagia). It is also used to minimise the risk of bleeding in surgical patients with known bleeding disorders (e.g. haemophiliacs, von Willibrand disease). Licensed Indications: Tranexamic acid is licensed to treat excessive/life-threatening bleeding after antifibrinolytic therapy Tranexamic acid is licensed for the prevention of surgical blood loss in patients with bleeding disorders (see below). Use in the surgical setting Tranexamic acid is licensed for the prevention of surgical blood loss in patients with bleeding disorders, but is rarely used alone and other measures (such as use of DDAVP (Desmopressin), factor concentrates or other blood products) are usually more important. Advice on the peri-operative management of any patient with a known bleeding disorder must be sought in advance from a consultant haematologist. Apart from open heart surgery, the use of antifibrinolytics in all other surgical patients is unlicensed and should be used with caution, preferably after taking haematological advice. Adverse effects Tranexamic acid occasionally causes nausea, vomiting and diarrhoea (dose dependant) and disturbance of colour vision (stop drug). Dose of Tranexamic acid By mouth: 1g tds daily for up to 4 days usually commenced pre-operatively. By slow intravenous injection: Local Fibrinolysis, 0.5-1g 3 times daily. By continuous intravenous infusion: Local Fibrinolysis, following initial treatment by intravenous injection, 25-50mg/kg over 24 hours. Trauma patients, loading dose of 1g infused over 10 min within 3 hours of injury followed by maintenance dose of 1g over 8 hours. Expiry Date: 10/12/17 Page 23 of 29
24 Appendix 10 TRUSTWIDE MAXIMUM SURGICAL BLOOD ORDER SCHEDULE (MSBOS) ADULT ELECTIVE SURGERY ONLY VASCULAR SURGERY HEAD & NECK Aortic Surgery = 4 units Major Head & Neck Procedures = 2 units ENSURE FULL USE OF Reconstructions = 2 units INTRA-OPERATIVE CELL Le Fort = 2 units SALVAGE MACHINE GENERAL SURGERY OBSTETRICS & GYNAECOLOGY Liver Resection = 2 units Placenta praevia = Whipples = 2 units 2 units on standby A/P Resection = 2 units Placenta praevia for Oesophagectomy = 4 units caesarean section = 4 units ORTHOPAEDIC ALL OTHER MAJOR SURGICAL Femoral nailing = 2 units PROCEDURES WILL BE TREATED Revision Surgery = 2 units AS A GROUP AND SAVE Elective Pelvic surgery= 4 units ENSURE FULL USE OF INTRA- OPERATIVE CELL SALVAGE MACHINE AND POST-OPERATIVE CELL SALVAGE DRAINS *For individuals who have an increased risk of intra-operative haemorrhage, the responsible clinician will instruct the blood bank Accordingly. UROLOGY Cystectomy = 4 units Trust Transfusion Committee Nephrectomy = 3 units 06/07/09 Expiry Date: 10/12/17 Page 24 of 29
25 Appendix 11 TRUSTWIDE MAXIMUM SURGICAL BLOOD ORDER SCHEDULE (MSBOS) GROUP AND SAVE PROCEDURES ADULT ELECTIVE SURGERY ONLY VASCULAR SURGERY GENERAL SURGERY Amputation = Group & Save Cholecystectomy = Group & Save Endarterectomy = Group & Save Major breast surgery= Group & Save Fem Pop Bypass = Group & Save Hemicolectomy = Group & Save EVARs = Group & Save Colostomy = Group & Save Anterior resection = Group & Save ENSURE FULL USE OF INTRA- Sigmoid Colectomy = Group & Save OPERATIVE CELL SALVAGE Reversal Loop Ileostomy MACHINE = Group & Save Gastrectomy = Group & Save Laparoscopy = Group & Save HEAD & NECK UROLOGY All major procedures that are not TURP = Group & Save Included within the Trust s TURBT = Group & Save cross matching schedule. ORTHOPAEDIC OBSTETRIC & GYNAECOLOGY THR = Group & Save LSCS = Group & Save TKR = Group & Save TAH = Group & Save ENSURE FULL USE OF INTRA- OPERATIVE CELL SALVAGE AND POST OPERATIVE CELL SALVAGE DRAINS For patients who are listed for laparotomy procedures, the responsible clinician will instruct the Blood bank accordingly. Trust Transfusion Committee 06/07/09 Expiry Date: 10/12/17 Page 25 of 29
26 Standard/ Criterion Pennine Acute Hospitals NHS Trust Guideline Patient Blood Management prior to Surgery CPDI 063, v5.1 Appendix 12 - Arrangements for Monitoring Compliance The arrangements for monitoring compliance of these guidelines are summarised in the following table: Minimum requirement to be monitored Staff that care for elective surgical patients are aware of the appropriate treatments available to reduce the use of allogeneic blood. Appropriate use of Trust/NHS Resources (blood & blood components) Staff involved in the care of elective surgical patients are aware of their responsibilities under this guideline & act accordingly Allogeneic blood usage of patients involved in any of the 4 phases of patient care in elective surgery. Appropriate ordering and usage to minimise preventable wastage of blood & blood products Process for monitori ng e.g. audit Responsible individual/ group/ committee Frequency of monitoring Responsible individual/ group/ committee for review of results Responsible individual/ group/ committee for development of action plan Responsible individual/ group/ committee for monitoring of action plan Review of incident investigation reports as per Trust s Accident & Incident Reporting Policy (EDQ008) Staff who do not attend Mandatory Training or Induction will be highlighted on the Mandatory Training Compliance Register as per the Trust s Induction & Mandatory Training Policy (EDH024) Review of Incident forms where there has been a failure to follow this guideline. Review of Incident forms where there has been a failure to follow this guideline. All requests outside the guidance of the MSBOS are questioned Review of Blood Bank Usage & Wastage Report Review of incident investigation reports as per Trust s Accident & Incident Reporting Policy (EDQ008) Transfusion Practitioners Hospital Transfusion Team Laboratory BMS Hospital Transfusion Team All reported incidents At every 6 weekly meeting Hospital Trust Transfusion Transfusion Committee Team Hospital Trust Transfusion Transfusion Committee Team All requests Hospital Trust Transfusion for allogeneic Transfusion Committee blood for Team elective surgery At every 6 weekly meeting Hospital Hospital Transfusion Transfusion Team Team Trust Transfusion Committee Trust Transfusion Committee Trust Transfusion Committee Hospital Transfusion Team Expiry Date: 10/12/17 Page 26 of 29
27 Appendix 13 - Completed Equality Impact Assessment for these Guidelines Part One Name of Document Guideline For the Patient Blood Management prior to Surgery Date of assessment September 2014 Is the document new or for review? Review Area Pathology Blood Transfusion Name of Author(s) 1.1 Briefly describe the aims and objectives and the purpose of the document 1.2 Are there any associated objectives or directives of the document? i.e. Care Quality Commission (CQC), NHS Litigation Authority (NHSLA) Christopher Porada (On behalf of the Trust Transfusion Committee) The main aim of this guideline is to promote the safe and effective treatment for anaemia prior to surgery Yes - Patient Blood Management Document by the National Blood Transfusion Committee (NBTC) 1.3 Who is the document intended to benefit, and what are the expected outcomes? 1.4 What factors could influence the intended outcomes either positively or negatively? All trust staff involved in the diagnosis and treatment of anaemia prior to undergoing surgery Enhance the treatment and care of patients diagnosed with anaemia prior to surgery, in order to avoid or minimise the need for an allogeneic transfusion and instead promote alternatives to a red cell transfusion. Staff not adhering to UK guidelines and patient blood management guidelines 1.5 Who are the main stakeholders in relation to the document *Staff *Patients 1.6 Who implements and is responsible for the document? Trust Transfusion Committee Expiry Date: 10/12/17 Page 27 of 29
28 Human Rights Age Disability Ethnicity (Race) Religion Gender Sexual orientation Carers Social Deprivation Pennine Acute Hospitals NHS Trust Guideline Patient Blood Management prior to Surgery CPDI 063, v5.1 Part One (cont.) For each of the nine Equality Categories ask the question below: 1.7 From the evidence, does the document affect or have the potential to affect individuals or communities differently or disproportionately, either positively or negatively (including discrimination)? No Yes No No No No No No No 1.8 Is there potential for, or evidence that, the proposed document will promote equality of opportunity for all and promote good relations with different groups? N/A N/A N/A N/A N/A N/A N/A N/A N/A 1.9 Is there public concern (including media, academic, voluntary or sector specific interest) in the document area about No No No No No No No No No actual, perceived or potential discrimination about a particular community? 1.10 Is there any doubt about answers to any of the questions? No No No No No No No No No Part Two 2.1 In what way does the document impact on any particular group listed above? Include here what evidence you have collated, whether there are any gaps and what further information is required. This document is for the treatment of Adults. A separate document is available for paediatrics and neonates for the administration of blood products. 2.2 Adverse Impact - if you have identified potential or real direct or indirect discrimination? If so, can it be justified (e.g., legislation, clinical or social evidence)? N/A 2.3 Positive Impact - does the document actively promote equality of opportunity and/or good relations between different groups of people? N/A Expiry Date: 10/12/17 Page 28 of 29
29 Part Three Document Title: Guideline For the Patient Blood Management prior to Surgery Ratifying Committee Trust Transfusion Committee & The Division of Diagnostics & Clinical Support Divisional Governance Committee. Document Number CPDI 063 Date sent to Committee September 2014 This document has been assessed as having no or low equality impact. Part 1 is completed. This document has been assessed as having low to medium impact. Parts 1 and 2 have been completed. Full impact assessment is unnecessary. Yes This document has been assessed as having medium to high impact. Parts 1 and 2 have been completed. Full impact assessment is necessary. Assessors Name Christopher Porada on behalf of the Trust Transfusion Committee Equality Champion Designation Transfusion Practitioner Division Signed* Signed* Joanne Stephenson Date: 04/09/14 Diagnostics and Clinical Support Joanne Stephenson Expiry Date: 10/12/17 Page 29 of 29
A Guide To Safe Blood Transfusion Practice
 A Guide To Safe Blood Transfusion Practice Marie Browett, Pavlina Sharp, Fiona Waller, Hafiz Qureshi, Malcolm Chambers (on behalf of the UHL Blood Transfusion Team) A Guide To Safe Blood Transfusion Practice
A Guide To Safe Blood Transfusion Practice Marie Browett, Pavlina Sharp, Fiona Waller, Hafiz Qureshi, Malcolm Chambers (on behalf of the UHL Blood Transfusion Team) A Guide To Safe Blood Transfusion Practice
Blood Conservation. To introduce the learner to the basic concepts of blood conservation!! Learning Outcomes
 Section 4 Blood Conservation Aim To introduce the learner to the basic concepts of blood conservation Learning Outcomes Identify the principles of blood conservation Identify the areas where blood conservation
Section 4 Blood Conservation Aim To introduce the learner to the basic concepts of blood conservation Learning Outcomes Identify the principles of blood conservation Identify the areas where blood conservation
CAUTION: You must refer to the intranet for the most recent version of this procedural document.
 Procedure for the use of Intravenous Iron Dextran (CosmoFer ) Sharepoint Location Sharepoint Index Directory Clinical Policies and Guidelines General Policies and Guidelines/ Haematology And blood transfusion
Procedure for the use of Intravenous Iron Dextran (CosmoFer ) Sharepoint Location Sharepoint Index Directory Clinical Policies and Guidelines General Policies and Guidelines/ Haematology And blood transfusion
Patient Blood Management and alternatives to transfusion
 Patient Blood Management and alternatives to transfusion Patient Blood Management and the alternatives to transfusion and when these should be used Learning Outcomes Explain techniques that can be used
Patient Blood Management and alternatives to transfusion Patient Blood Management and the alternatives to transfusion and when these should be used Learning Outcomes Explain techniques that can be used
Mr John Faulds Blood Conservation Co-ordinator Royal Cornwall Hospital
 Mr John Faulds Blood Conservation Co-ordinator Royal Cornwall Hospital Primary aim to reduce the need for red blood cell transfusion, in those patients where transfusion can be avoided, through the use
Mr John Faulds Blood Conservation Co-ordinator Royal Cornwall Hospital Primary aim to reduce the need for red blood cell transfusion, in those patients where transfusion can be avoided, through the use
GUIDELINES FOR MANAGEMENT OF BLEEDING AND EXCESSIVE ANTICOAGULATION WITH ORAL ANTICOAGULANTS
 GUIDELINES FOR MANAGEMENT OF BLEEDING AND EXCESSIVE ANTICOAGULATION WITH ORAL ANTICOAGULANTS This guideline covers the management of patients being treated with Vitamin K antagonists (VKA): Warfarin Acenocoumarol
GUIDELINES FOR MANAGEMENT OF BLEEDING AND EXCESSIVE ANTICOAGULATION WITH ORAL ANTICOAGULANTS This guideline covers the management of patients being treated with Vitamin K antagonists (VKA): Warfarin Acenocoumarol
Dr Charlie Baker Consultant Anaesthetist UHNM. Being a place our f amilies would choose
 Dr Charlie Baker Consultant Anaesthetist UHNM Being a place our f amilies would choose The story so far: Anaemia is associated with transfusion. The more anaemic you are pre op the more likely you are
Dr Charlie Baker Consultant Anaesthetist UHNM Being a place our f amilies would choose The story so far: Anaemia is associated with transfusion. The more anaemic you are pre op the more likely you are
Policy for the use of intravenous Iron Dextran (CosmoFer )
 Policy for the use of intravenous Iron Dextran (CosmoFer ) Sharepoint Location Clinical Policies and Guidelines Sharepoint Index Directory General Policies and Guidelines Sub Area Haematology and Blood
Policy for the use of intravenous Iron Dextran (CosmoFer ) Sharepoint Location Clinical Policies and Guidelines Sharepoint Index Directory General Policies and Guidelines Sub Area Haematology and Blood
West Midlands Regional Transfusion Committee (RTC) Guidelines for the management of anaemia in pre-operative assessment clinics
 West Midlands West Midlands Regional Transfusion Committee (RTC) Guidelines for the management of anaemia in pre-operative assessment clinics Blest, A. 1, Baker, C. 2, Taylor, C. 3, Brothwood, D. 4, Bramhall,
West Midlands West Midlands Regional Transfusion Committee (RTC) Guidelines for the management of anaemia in pre-operative assessment clinics Blest, A. 1, Baker, C. 2, Taylor, C. 3, Brothwood, D. 4, Bramhall,
Non-Medical Authorisation Course TRANSFUSION ALTERNATIVES. East Midlands Regional Transfusion Committee
 Non-Medical Authorisation Course TRANSFUSION ALTERNATIVES Janice Smith Matron Transfusion Specialist (Slides Leanne Hostler & Ant Jackson!) Aims Why we need to consider alternatives? What alternatives
Non-Medical Authorisation Course TRANSFUSION ALTERNATIVES Janice Smith Matron Transfusion Specialist (Slides Leanne Hostler & Ant Jackson!) Aims Why we need to consider alternatives? What alternatives
DETECTION, INVESTIGATION AND MANAGEMENT OF ANAEMIA
 From the Chief Medical Officer Dr Michael McBride HSS(MD) 22/2012 Circulation list: For Action: Chief Executives of HSC Trusts Medical Directors of HSC Trusts (for onward distribution to All Hospital Doctors)
From the Chief Medical Officer Dr Michael McBride HSS(MD) 22/2012 Circulation list: For Action: Chief Executives of HSC Trusts Medical Directors of HSC Trusts (for onward distribution to All Hospital Doctors)
DOAC and NOAC are terms for a novel class of directly acting oral anticoagulant drugs including Rivaroxaban, Apixaban, Edoxaban, and Dabigatran.
 Guideline for Patients on Direct Oral Anticoagulant Therapy Requiring Urgent Surgery for Hip Fracture Trust Ref:C10/2017 1. Introduction This guideline is for the clinical management of patients on direct
Guideline for Patients on Direct Oral Anticoagulant Therapy Requiring Urgent Surgery for Hip Fracture Trust Ref:C10/2017 1. Introduction This guideline is for the clinical management of patients on direct
NHS Dumfries & Galloway Ferrous Salt Review Protocol November 09
 Title of Project: NHS Dumfries & Galloway Ferrous Salt Review Protocol November 09 1 Reason for the review Choice of iron preparation is based on cost and incidence of side effects (BNF). There is little
Title of Project: NHS Dumfries & Galloway Ferrous Salt Review Protocol November 09 1 Reason for the review Choice of iron preparation is based on cost and incidence of side effects (BNF). There is little
Northern Treatment Advisory Group
 Northern Treatment Advisory Group Ferric Maltol (Feraccru ) for the treatment of iron deficiency Lead author: Daniel Hill Regional Drug & Therapeutics Centre (Newcastle) September 2018 2018 Summary Iron
Northern Treatment Advisory Group Ferric Maltol (Feraccru ) for the treatment of iron deficiency Lead author: Daniel Hill Regional Drug & Therapeutics Centre (Newcastle) September 2018 2018 Summary Iron
Pre-operative Anaemia Colorectal and Orthopaedic Surgery
 Pre-operative Anaemia Colorectal and Orthopaedic Surgery Dr Simon Rang Consultant Anaesthetist East Kent Hospitals NHS Trust Dreamland Pre-operative Anaemia Anaemia is a perioperative risk factor Perioperative
Pre-operative Anaemia Colorectal and Orthopaedic Surgery Dr Simon Rang Consultant Anaesthetist East Kent Hospitals NHS Trust Dreamland Pre-operative Anaemia Anaemia is a perioperative risk factor Perioperative
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc)
 Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Guideline on the management of excessive coumarin anticoagulation in adults
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Guideline on the management of excessive coumarin anticoagulation in adults
Pathology Service User Guide Haematology
 Pathology Service User Guide Haematology St Richard s This section of the Pathology Service User Guide includes: Anticoagulant Therapy Information about the Anticoagulant Clinic Low Molecular Weight Heparin
Pathology Service User Guide Haematology St Richard s This section of the Pathology Service User Guide includes: Anticoagulant Therapy Information about the Anticoagulant Clinic Low Molecular Weight Heparin
Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital
 Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Tranexamic acid and Iron in Haematology. Andy King-Venables Transfusion Practitioner Hinchingbrooke Hospital
 Tranexamic acid and Iron in Haematology Andy King-Venables Transfusion Practitioner Hinchingbrooke Hospital Why consider an alternative? Can t we just give blood? Why consider an alternative? Can t we
Tranexamic acid and Iron in Haematology Andy King-Venables Transfusion Practitioner Hinchingbrooke Hospital Why consider an alternative? Can t we just give blood? Why consider an alternative? Can t we
Blood Transfusions in Children with Haemoglobinopathies
 Blood Transfusions in Children with Haemoglobinopathies Version: 2 Date: 22 nd April 2010 Authors: Responsible committee or Director: Review date: Target audience: Stakeholders/ committees involved in
Blood Transfusions in Children with Haemoglobinopathies Version: 2 Date: 22 nd April 2010 Authors: Responsible committee or Director: Review date: Target audience: Stakeholders/ committees involved in
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Managing peri-operative anaemiathe Papworth way. Dr Andrew A Klein Royal Papworth Hospital Cambridge UK
 Managing peri-operative anaemiathe Papworth way Dr Andrew A Klein Royal Papworth Hospital Cambridge UK Conflicts of interest: Unrestricted educational grants/honoraria from CSL Behring, Brightwake Ltd,
Managing peri-operative anaemiathe Papworth way Dr Andrew A Klein Royal Papworth Hospital Cambridge UK Conflicts of interest: Unrestricted educational grants/honoraria from CSL Behring, Brightwake Ltd,
Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008
 Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008 Shared care agreement has been developed appropriately
Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008 Shared care agreement has been developed appropriately
NICE guideline Published: 18 November 2015 nice.org.uk/guidance/ng24
 Blood transfusion NICE guideline Published: 18 November 2015 nice.org.uk/guidance/ng24 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Blood transfusion NICE guideline Published: 18 November 2015 nice.org.uk/guidance/ng24 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
SHARED CARE PRESCRIBING GUIDELINE
 WORKING IN PARTNERSHIP East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, North East Hampshire & Farnham CCG, Crawley CCG, Horsham & Mid-Sussex CCG SHARED
WORKING IN PARTNERSHIP East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, North East Hampshire & Farnham CCG, Crawley CCG, Horsham & Mid-Sussex CCG SHARED
Trust Guideline for the Management and Administration of Intravenous Iron in Adults under the Gastroenterology Directorate
 A clinical guideline recommended for use For Use in: The Gastroenterology Directorate By: Registered nurses competent in the administration of intravenous therapy and medical staff For: Adult patients
A clinical guideline recommended for use For Use in: The Gastroenterology Directorate By: Registered nurses competent in the administration of intravenous therapy and medical staff For: Adult patients
Policy for the use of Cytomegalovirus (CMV) negative blood products
 Policy for the use of Cytomegalovirus (CMV) negative blood products SharePoint Location Clinical Policies and Guidelines SharePoint Index Directory Cancer and Specialist Care Sub Area Haematology and Blood
Policy for the use of Cytomegalovirus (CMV) negative blood products SharePoint Location Clinical Policies and Guidelines SharePoint Index Directory Cancer and Specialist Care Sub Area Haematology and Blood
pat hways Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16
 pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
Julie Ball SHOT Clinical Incidents Specialist
 Julie Ball SHOT Clinical Incidents Specialist Surveillance procedures from the collection of blood and its components to the follow up of the recipients To collect and assess information on unexpected
Julie Ball SHOT Clinical Incidents Specialist Surveillance procedures from the collection of blood and its components to the follow up of the recipients To collect and assess information on unexpected
Guidelines for slow loading of patients on warfarin for Atrial Fibrillation (AF) in the non acute setting
 ANTICOAGULANT SERVICE Guidelines for slow loading of patients on warfarin for Atrial Fibrillation (AF) in the non acute setting Introduction Fast loading of warfarin carries a risk of over anticoagulation
ANTICOAGULANT SERVICE Guidelines for slow loading of patients on warfarin for Atrial Fibrillation (AF) in the non acute setting Introduction Fast loading of warfarin carries a risk of over anticoagulation
Prescribing Framework for Methotrexate for Immunosuppression in ADULTS
 Hull & East Riding Prescribing Committee Prescribing Framework for Methotrexate for Immunosuppression in ADULTS Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
Hull & East Riding Prescribing Committee Prescribing Framework for Methotrexate for Immunosuppression in ADULTS Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
PREOPERATIVE ANAEMIA PATHWAY
 PREOPERATIVE ANAEMIA PATHWAY Surname: Patient ID No. Forename: DOB: / / Age: NHS Number: Likes to be called: Address: Tel. No. Religion/Spirituality: Next of Kin: Name GP Name: GP Practice: Planned Operation:
PREOPERATIVE ANAEMIA PATHWAY Surname: Patient ID No. Forename: DOB: / / Age: NHS Number: Likes to be called: Address: Tel. No. Religion/Spirituality: Next of Kin: Name GP Name: GP Practice: Planned Operation:
28 th September Author Jeremy Gilbert Bariatric Nurse Specialist
 POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
Transfusion Requirements and Management in Trauma RACHEL JACK
 Transfusion Requirements and Management in Trauma RACHEL JACK Overview Haemostatic resuscitation Massive Transfusion Protocol Overview of NBA research guidelines Haemostatic resuscitation Permissive hypotension
Transfusion Requirements and Management in Trauma RACHEL JACK Overview Haemostatic resuscitation Massive Transfusion Protocol Overview of NBA research guidelines Haemostatic resuscitation Permissive hypotension
EFFECTIVE SHARE CARE AGREEMENT
 Specialist details Patient identifier Name: Tel: EFFECTIVE SHARE CARE AGREEMENT For the specialist use of Erythropoietin Stimulating Agent (ESA) Therapy (formerly known as EPO) for the correction of Anaemia
Specialist details Patient identifier Name: Tel: EFFECTIVE SHARE CARE AGREEMENT For the specialist use of Erythropoietin Stimulating Agent (ESA) Therapy (formerly known as EPO) for the correction of Anaemia
SULFASALAZINE (Adults)
 Shared Care Guideline DRUG: Introduction: SULFASALAZINE (Adults) Indication: Disease modifying drug for rheumatoid arthritis, psoriatic arthritis, undifferentiated arthritis, spondyloarthropathies, Crohn
Shared Care Guideline DRUG: Introduction: SULFASALAZINE (Adults) Indication: Disease modifying drug for rheumatoid arthritis, psoriatic arthritis, undifferentiated arthritis, spondyloarthropathies, Crohn
Prevention of Contrast Induced Nephropathy (CIN) Guidelines
 Prevention of Contrast Induced Nephropathy (CIN) Guidelines This procedural document supersedes: PAT/T 48 v.1 - Guidelines for Prevention of Contrast Induced Nephropathy (CIN) Did you print this document
Prevention of Contrast Induced Nephropathy (CIN) Guidelines This procedural document supersedes: PAT/T 48 v.1 - Guidelines for Prevention of Contrast Induced Nephropathy (CIN) Did you print this document
GUIDELINES FOR ADMINISTRATION OF INTRAVENOUS IRON IN ADULTS WITH CHRONIC KIDNEY DISEASE
 GUIDELINES FOR ADMINISTRATION OF INTRAVENOUS IRON IN ADULTS WITH CHRONIC KIDNEY DISEASE Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target audience, state if
GUIDELINES FOR ADMINISTRATION OF INTRAVENOUS IRON IN ADULTS WITH CHRONIC KIDNEY DISEASE Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target audience, state if
Consensus Statement for Management of Anticoagulants and Antiplatelet drugs in Patients with Hip Fracture
 Consensus Statement for Management of Anticoagulants and Antiplatelet drugs in Patients with Hip Fracture Patients with hip fractures should be operated on within 36 hours of presentation wherever possible.
Consensus Statement for Management of Anticoagulants and Antiplatelet drugs in Patients with Hip Fracture Patients with hip fractures should be operated on within 36 hours of presentation wherever possible.
This guideline describes the care required for a patient receiving a red blood cell transfusion whilst undergoing extra corporeal therapies.
 Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guidelines for care of a patient receiving a red blood cell transfusion whilst undergoing extra corporeal therapies.
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guidelines for care of a patient receiving a red blood cell transfusion whilst undergoing extra corporeal therapies.
Haemophilia Suspected Bleed
 Haemophilia Suspected Bleed Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Directorate & Speciality Guideline for the management
Haemophilia Suspected Bleed Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Directorate & Speciality Guideline for the management
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens
 EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
Title Use of Pre-Operative Tests for Elective Surgery Guideline. Department. Anaesthetics. Comment / Changes / Approval
 Document Control Title Use of Pre-Operative Tests for Elective Surgery Guideline Author Consultant Anaesthetist Author s job title Consultant Anaesthetist Directorate Clinical Support Services Department
Document Control Title Use of Pre-Operative Tests for Elective Surgery Guideline Author Consultant Anaesthetist Author s job title Consultant Anaesthetist Directorate Clinical Support Services Department
PAEDIATRIC DOSAGE GUIDELINES For management of post-operative acute pain
 Index No: MMG43 PAEDIATRIC DOSAGE GUIDELINES For management of post-operative acute pain Version: 3.1 (Includes anti-emetics and naloxone) Date ratified: July 2013 Ratified by: (Name of Committee) Name
Index No: MMG43 PAEDIATRIC DOSAGE GUIDELINES For management of post-operative acute pain Version: 3.1 (Includes anti-emetics and naloxone) Date ratified: July 2013 Ratified by: (Name of Committee) Name
CAUTION: Refer to the Document Library for the most recent version of this document. Cryoprecipitate Transfusion Guideline for Practice.
 Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
Trust Guideline. for Ciclosporin Treatment & Monitoring for Adult* Patients with Acute, Severe Ulcerative Colitis. (*ie aged 16 years and over)
 Trust Guideline for Ciclosporin Treatment & Monitoring for Adult* Patients with Acute, Severe Ulcerative Colitis (*ie aged 16 years and over) abc A guideline recommended for use In: Gastroenterology/Medical
Trust Guideline for Ciclosporin Treatment & Monitoring for Adult* Patients with Acute, Severe Ulcerative Colitis (*ie aged 16 years and over) abc A guideline recommended for use In: Gastroenterology/Medical
University Hospitals Coventry & Warwickshire NHS Trust
 University Hospitals Coventry & Warwickshire NHS Trust Clinical Guideline (full) Anaemia management for chronic kidney disease, peritoneal dialysis, renal transplant and haemodialysis patients E-Library
University Hospitals Coventry & Warwickshire NHS Trust Clinical Guideline (full) Anaemia management for chronic kidney disease, peritoneal dialysis, renal transplant and haemodialysis patients E-Library
Laboratory Empowerment. Debbie Asher Adrian Ebbs Transfusion Laboratory Managers, Eastern Pathology Alliance
 Laboratory Empowerment Debbie Asher Adrian Ebbs Transfusion Laboratory Managers, Eastern Pathology Alliance Why? Electronic ICE requesting was in use for requesting red cells NBTC Indication Codes were
Laboratory Empowerment Debbie Asher Adrian Ebbs Transfusion Laboratory Managers, Eastern Pathology Alliance Why? Electronic ICE requesting was in use for requesting red cells NBTC Indication Codes were
Guidelines on Anaemia Management in Patients with Chronic Kidney Disease (CKD)
 Guidelines on Anaemia Management in Patients with Chronic Kidney Disease (CKD) This guideline is for use in adult patients with an estimated Glomerular Filtration Rate (egfr) of less than 60ml/min/1.73m
Guidelines on Anaemia Management in Patients with Chronic Kidney Disease (CKD) This guideline is for use in adult patients with an estimated Glomerular Filtration Rate (egfr) of less than 60ml/min/1.73m
Prescribing Framework for Mycophenolate Mofetil for Immunosuppression in ADULTs
 Hull & East Riding Prescribing Committee Prescribing Framework for Mycophenolate Mofetil for Immunosuppression in ADULTs Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker)
Hull & East Riding Prescribing Committee Prescribing Framework for Mycophenolate Mofetil for Immunosuppression in ADULTs Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker)
Alister Jones Patient Blood Management Practitioner NHS Blood and Transplant
 Alister Jones Patient Blood Management Practitioner NHS Blood and Transplant All medical RCC transfusions (but only 1 in 3 haematology or oncology cases) in 3 x one week periods Medical specialties include:
Alister Jones Patient Blood Management Practitioner NHS Blood and Transplant All medical RCC transfusions (but only 1 in 3 haematology or oncology cases) in 3 x one week periods Medical specialties include:
Patient Group Directions (PGDs)
 Patient Group Directions (PGDs) Document level: Trustwide (TW) Code: MP2 Issue number: 4 Lead executive Authors details Type of document Target audience Document purpose Medical Director Senior Clinical
Patient Group Directions (PGDs) Document level: Trustwide (TW) Code: MP2 Issue number: 4 Lead executive Authors details Type of document Target audience Document purpose Medical Director Senior Clinical
Shân Edwards Clinical Pharmacist Royal Devon & Exeter Hospital November 2012
 Shân Edwards Clinical Pharmacist Royal Devon & Exeter Hospital November 2012 Discontinuation of drugs that increase risk of bleeding Optimise patients haemoglobin pre-op Reducing blood loss peri-operatively
Shân Edwards Clinical Pharmacist Royal Devon & Exeter Hospital November 2012 Discontinuation of drugs that increase risk of bleeding Optimise patients haemoglobin pre-op Reducing blood loss peri-operatively
Specialised Services Commissioning Policy. CP29: Bariatric Surgery
 Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
National Comparative Audit of red cell transfusion in Medical Patients Part Two
 National Comparative Audit of red cell transfusion in Medical Patients Part Two Dr. Kate Pendry, Project Clinical Lead John Grant-Casey, Project Manager August 2013 Part One 9216 cases from 181 sites (90%
National Comparative Audit of red cell transfusion in Medical Patients Part Two Dr. Kate Pendry, Project Clinical Lead John Grant-Casey, Project Manager August 2013 Part One 9216 cases from 181 sites (90%
PATIENT GROUP DIRECTION PROCEDURE
 PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension Direct Line tadamson(anhs.
 NHS Grampian Westholme Woodend Hospital Queens Road ABERDEEN AB15 6LS NHS Grampian Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension 56689 Direct Line 01224 556689
NHS Grampian Westholme Woodend Hospital Queens Road ABERDEEN AB15 6LS NHS Grampian Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension 56689 Direct Line 01224 556689
GREATER MANCHESTER INTERFACE PRESCRIBING GROUP
 GREATER MANCHESTER INTERFACE PRESCRIBING GROUP On behalf of the GREATER MANCHESTER MEDICINES MANAGEMENT GROUP SHARED CARE GUIDELINE FOR THE PRESCRIBING OF SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs)
GREATER MANCHESTER INTERFACE PRESCRIBING GROUP On behalf of the GREATER MANCHESTER MEDICINES MANAGEMENT GROUP SHARED CARE GUIDELINE FOR THE PRESCRIBING OF SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs)
Guidance for management of bleeding in patients taking the new oral anticoagulant drugs: rivaroxaban, dabigatran or apixaban
 Guidance for management of bleeding in patients taking the new oral anticoagulant drugs: rivaroxaban, dabigatran or apixaban Purpose The aim of this guidance is to outline the management of patients presenting
Guidance for management of bleeding in patients taking the new oral anticoagulant drugs: rivaroxaban, dabigatran or apixaban Purpose The aim of this guidance is to outline the management of patients presenting
Management of haemorrhage in patients taking DOACs/ NOACs (direct/ novel oral anticoagulants) Guideline. Contents
 Management of haemorrhage in patients taking DOACs/ NOACs (direct/ novel oral anticoagulants) Guideline Classification: Clinical Guideline Lead Author: Dr Rowena Thomas-Dewing, Consultant Haematologist
Management of haemorrhage in patients taking DOACs/ NOACs (direct/ novel oral anticoagulants) Guideline Classification: Clinical Guideline Lead Author: Dr Rowena Thomas-Dewing, Consultant Haematologist
Developed By Name Signature Date
 Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
Update on the management of iron deficiency
 Update on the management of iron deficiency Outline Need to improve management & avoid transfusion Diagnosis & investigation Oral iron & IV iron Tools & resources No conflicts of interest All natural
Update on the management of iron deficiency Outline Need to improve management & avoid transfusion Diagnosis & investigation Oral iron & IV iron Tools & resources No conflicts of interest All natural
Western Locality Shared care Information ~ Penicillamine, Rheumatology April 2013
 Western Locality Shared care Information ~ Penicillamine, Rheumatology April 2013 Penicillamine Treatment of: Rheumatoid arthritis Specialist: Please complete the Shared Care letter sending a request to
Western Locality Shared care Information ~ Penicillamine, Rheumatology April 2013 Penicillamine Treatment of: Rheumatoid arthritis Specialist: Please complete the Shared Care letter sending a request to
Ketorolac injection. Supportive care
 Supportive care Ketorolac injection Supportive care: specialist medicines This leaflet provides information on a medicine called ketorolac which is used to treat pain that is difficult to control. It is
Supportive care Ketorolac injection Supportive care: specialist medicines This leaflet provides information on a medicine called ketorolac which is used to treat pain that is difficult to control. It is
Guidelines for the management of warfarin reversal in adults
 SharePoint Location Clinical Policies and Guidelines SharePoint Index Directory General Policies and Guidelines Sub Area Haematology and Transfusion Key words (for search purposes) Warfarin, Bleeding Central
SharePoint Location Clinical Policies and Guidelines SharePoint Index Directory General Policies and Guidelines Sub Area Haematology and Transfusion Key words (for search purposes) Warfarin, Bleeding Central
Preoperative anemia Common, consequential and correctable in non-emergent surgery By Kathrine Frey, MD
 Preoperative anemia Common, consequential and correctable in non-emergent surgery By Kathrine Frey, MD Preoperative anemia is common, especially in patients undergoing nonemergent high-blood-loss surgical
Preoperative anemia Common, consequential and correctable in non-emergent surgery By Kathrine Frey, MD Preoperative anemia is common, especially in patients undergoing nonemergent high-blood-loss surgical
Document Details. Ibuprofen 200mg tablets and Ibuprofen oral liquid 100mg in 5ml
 Title Document Details Patient Group Direction (PGD) Ibuprofen 200mg tablets and Ibuprofen oral liquid 100mg in 5ml Trust Ref No 1445-36348 Local Ref (optional) Main points the document The treatment of
Title Document Details Patient Group Direction (PGD) Ibuprofen 200mg tablets and Ibuprofen oral liquid 100mg in 5ml Trust Ref No 1445-36348 Local Ref (optional) Main points the document The treatment of
A Patient s guide to. Blood Transfusions and Human Tissue Transplant
 A Patient s guide to Blood Transfusions and Human Tissue Transplant Why might I need a blood transfusion? Blood transfusions are given to replace blood lost in surgery or to treat anaemia (lack of red
A Patient s guide to Blood Transfusions and Human Tissue Transplant Why might I need a blood transfusion? Blood transfusions are given to replace blood lost in surgery or to treat anaemia (lack of red
HYDROXYCARBAMIDE for Haematological conditions (Adults)
 Shared Care Guideline DRUG: Introduction: Contraindications & Warnings: HYDROXYCARBAMIDE for Haematological conditions (Adults) Indication: Hydroxycarbamide is an established treatment in haematological
Shared Care Guideline DRUG: Introduction: Contraindications & Warnings: HYDROXYCARBAMIDE for Haematological conditions (Adults) Indication: Hydroxycarbamide is an established treatment in haematological
Pre-operative Assessment
 Pre-operative Assessment Dr Craig Taylor Andrea Harris On behalf of the WM RTC Audit Group A good example of an audit cycle...... or is it!!?? Identify Better Blood Transfusion 2002 2007 West Midlands
Pre-operative Assessment Dr Craig Taylor Andrea Harris On behalf of the WM RTC Audit Group A good example of an audit cycle...... or is it!!?? Identify Better Blood Transfusion 2002 2007 West Midlands
ferric carboxymaltose 50mg iron/ml solution for injection/infusion (Ferinject ) SMC No. (463/08) Vifor Pharmaceuticals
 ferric carboxymaltose 50mg iron/ml solution for injection/infusion (Ferinject ) SMC No. (463/08) Vifor Pharmaceuticals 17 December 2010 The Scottish Medicines Consortium (SMC) has completed its assessment
ferric carboxymaltose 50mg iron/ml solution for injection/infusion (Ferinject ) SMC No. (463/08) Vifor Pharmaceuticals 17 December 2010 The Scottish Medicines Consortium (SMC) has completed its assessment
patient group direction
 ASPIRIN v01 1/8 ASPIRIN PGD Details Version 1.0 Legal category P Staff grades Approved by Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
ASPIRIN v01 1/8 ASPIRIN PGD Details Version 1.0 Legal category P Staff grades Approved by Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
SHARED CARE PRESCRIBING GUIDELINE AZATHIOPRINE IN ADULT PATIENTS WITH RHEUMATOID ARTHRITIS DOCUMENT DETAILS
 SHARED CARE PRESCRIBING GUIDELINE AZATHIOPRINE IN ADULT PATIENTS WITH RHEUMATOID ARTHRITIS DOCUMENT DETAILS Document type Shared Care Prescribing Guideline Document name Shared Care Prescribing Guideline:
SHARED CARE PRESCRIBING GUIDELINE AZATHIOPRINE IN ADULT PATIENTS WITH RHEUMATOID ARTHRITIS DOCUMENT DETAILS Document type Shared Care Prescribing Guideline Document name Shared Care Prescribing Guideline:
ESCA: Cinacalcet (Mimpara )
 ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
Ambulatory Emergency Care Pathways. Painless Obstructive Jaundice
 Ambulatory Emergency Care Pathways Painless Obstructive Jaundice Effective Date: December 2011 Content Summary Ref Title Description 1 Condition Details Identifies pathway details and clinical sign-off
Ambulatory Emergency Care Pathways Painless Obstructive Jaundice Effective Date: December 2011 Content Summary Ref Title Description 1 Condition Details Identifies pathway details and clinical sign-off
CAUTION: Refer to the Document Library for the most recent version of this policy. Policy for the use of Cytomegalovirus (CMV) negative blood products
 Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
Factsheet LINACLOTIDE (Constella ) Irritable Bowel Syndrome constipation predominant (IBS-C)
 North Central London Joint Formulary Committee Factsheet LINACLOTIDE (Constella ) Irritable Bowel Syndrome constipation predominant (IBS-C) Start date: September 2018 Review date: September 2021 Document
North Central London Joint Formulary Committee Factsheet LINACLOTIDE (Constella ) Irritable Bowel Syndrome constipation predominant (IBS-C) Start date: September 2018 Review date: September 2021 Document
North East Essex Medicines Management Committee
 Colchester Hospital University NHS Foundation Trust North East Essex Clinical Commissioning Group North East Essex Medicines Management Committee ORAL ANTICOAGULANT (Vit K antagonist only) MANAGEMENT GUIDELINES
Colchester Hospital University NHS Foundation Trust North East Essex Clinical Commissioning Group North East Essex Medicines Management Committee ORAL ANTICOAGULANT (Vit K antagonist only) MANAGEMENT GUIDELINES
NICE Guidance. Suspected Cancer in Adults COLORECTAL (2WW)
 NICE Guidance Suspected Cancer in Adults COLORECTAL (2WW) Date of Referral: Short date letter merged Name: Full Name DOB: Date of Birth NHS No NHS Number Attach this form to the e-referral within 24 hours
NICE Guidance Suspected Cancer in Adults COLORECTAL (2WW) Date of Referral: Short date letter merged Name: Full Name DOB: Date of Birth NHS No NHS Number Attach this form to the e-referral within 24 hours
27/01/2019. Anaemia, Transfusion and TACO Lise Estcourt. Anaemia. What is anaemia?
 Anaemia, Transfusion and TACO Lise Estcourt 1 Anaemia 2 What is anaemia? 3 1 Anaemia according to WHO 4 Anaemia in palliative care Common (77% men 68% women) Symptoms often non-specific Some causes potentially
Anaemia, Transfusion and TACO Lise Estcourt 1 Anaemia 2 What is anaemia? 3 1 Anaemia according to WHO 4 Anaemia in palliative care Common (77% men 68% women) Symptoms often non-specific Some causes potentially
CLINICAL PROTOCOL THE PREVENTION OF FATALITIES FROM MEDICATION LOADING DOSES
 National Patient Safety Alert RRR018 Preventing Fatalities From Medication Loading Doses (November 2010) MMCP05 CLINICAL PROTOCOL THE PREVENTION OF FATALITIES FROM MEDICATION LOADING DOSES INTRODUCTION
National Patient Safety Alert RRR018 Preventing Fatalities From Medication Loading Doses (November 2010) MMCP05 CLINICAL PROTOCOL THE PREVENTION OF FATALITIES FROM MEDICATION LOADING DOSES INTRODUCTION
National Comparative Audit of Blood Transfusion Repeat Audit of Patient Blood Management in Adults undergoing elective, scheduled surgery
 National Comparative Audit of Blood Transfusion 2016 Repeat Audit of Patient Blood Management in Adults undergoing elective, scheduled surgery 1 Acknowledgements We wish to thank all those who have participated
National Comparative Audit of Blood Transfusion 2016 Repeat Audit of Patient Blood Management in Adults undergoing elective, scheduled surgery 1 Acknowledgements We wish to thank all those who have participated
Appendix IV - Prescribing Guidance for Apixaban
 Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Policy for the use of Irradiated blood products
 Policy for the use of Irradiated blood products SharePoint Location General Policies and Guidelines SharePoint Index Directory Haematology and Blood Transfusion Sub Area - Key words (for search purposes)
Policy for the use of Irradiated blood products SharePoint Location General Policies and Guidelines SharePoint Index Directory Haematology and Blood Transfusion Sub Area - Key words (for search purposes)
Developed By Name Signature Date
 Patient Group Direction 2156 version 2.0 Administration of Ipratropium 250mcg/ml Nebuliser Solution in Acute Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Date of
Patient Group Direction 2156 version 2.0 Administration of Ipratropium 250mcg/ml Nebuliser Solution in Acute Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Date of
Prescribing Framework for Mycophenolate Mofetil or Mycophenolic Acid (Myfortic ) Post Solid Organ Transplant
 Hull & East Riding Prescribing Committee Prescribing Framework for Mycophenolate Mofetil or Mycophenolic Acid (Myfortic ) Post Solid Organ Transplant Patient s Name:.. NHS Number: Patient s Address:...
Hull & East Riding Prescribing Committee Prescribing Framework for Mycophenolate Mofetil or Mycophenolic Acid (Myfortic ) Post Solid Organ Transplant Patient s Name:.. NHS Number: Patient s Address:...
2 Summary of NICE TA 249: Atrial fibrillation - Dabigatran Etexilate
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Rivaroxaban film coated tablets are available in 2 strengths for this indication: 15mg and 20mg.
 Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults
 Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
Major Haemorrhage Protocol. Commentary
 Hairmyres Hospital Monklands Hospital Wishaw General Hospital Major Haemorrhage Protocol Commentary N.B. There is a separate NHSL protocol for the Management of Obstetric Haemorrhage Authors Dr Tracey
Hairmyres Hospital Monklands Hospital Wishaw General Hospital Major Haemorrhage Protocol Commentary N.B. There is a separate NHSL protocol for the Management of Obstetric Haemorrhage Authors Dr Tracey
All institutions that transfuse blood components and products should implement national and local policies and written procedures for:
 5.0 GENERAL GUIDE TO GOOD TRANSFUSION PRACTICE Blood and the various components prepared or manufactured from it are biologic (in the case of blood cells, living human tissues) products intended for use
5.0 GENERAL GUIDE TO GOOD TRANSFUSION PRACTICE Blood and the various components prepared or manufactured from it are biologic (in the case of blood cells, living human tissues) products intended for use
Trust Protocol for the Administration of Intravenous Methylprednisolone for Thyroid Eye Disease A Protocol. The Clinical Investigation Unit (CIU)
 A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
StRs and CT doctors in haematology. September Folinic acid dose modified.
 High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
Title Use and monitoring of Low Molecular Weight Heparins (LMWHs) in community hospitals and community nursing Clinical Guidelines
 Document Control Title Use and monitoring of Low Molecular Weight Heparins (LMWHs) in community hospitals and community nursing Clinical Guidelines Author Team Directorate Medical Date Version Status Issued
Document Control Title Use and monitoring of Low Molecular Weight Heparins (LMWHs) in community hospitals and community nursing Clinical Guidelines Author Team Directorate Medical Date Version Status Issued
Jo Shorthouse. With thanks to Dr Kate Pendry. Consultant Haematologist Central Manchester Hospitals. and. Clinical Director for PBM NHSBT
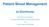 Jo Shorthouse With thanks to Dr Kate Pendry Consultant Haematologist Central Manchester Hospitals and Clinical Director for PBM NHSBT What is Patient Blood Management? Why is Patient Blood Management important?
Jo Shorthouse With thanks to Dr Kate Pendry Consultant Haematologist Central Manchester Hospitals and Clinical Director for PBM NHSBT What is Patient Blood Management? Why is Patient Blood Management important?
