Poliovirus Laboratory Survey. Introduction
|
|
|
- Erica Evans
- 5 years ago
- Views:
Transcription
1 Introduction The World Health Organization (WHO)-led Global Polio Laboratory Network (GPLN) continues to play an essential role in global polio eradication, and periodic efforts to quantify its overall value provide important information that helps to motivate financial support for GPLN laboratories. Assessing the value of the GPLN is of utmost importance at this stage of the GPEI, as the partners discuss the strategies to maintain polio laboratory functions pre- and post-certification of wild poliovirus eradication and global containment of live polioviruses. This GPLN survey aims to collect data on activities and costs of all of the GPLN laboratories to support an overall synthesis. The objectives of this survey include to: (1) update estimates of the total costs of the GPLN reported based on a similar 2003 survey, (2) better understand the different cost components, including environmental surveillance, and (3) characterize the extent to which the GPLN contributes to surveillance of other diseases. The survey form should take approximately 60 minutes to complete, and we expect that collecting data and calculating some of the costs may take an additional 1-4 hours, depending on the size and complexity of the laboratory. Please start the survey as soon as possible, so if you have any questions or if you need to compile data, you will have time to do so. The survey includes questions about acute flaccid paralysis (AFP) surveillance (i.e., stool samples from AFP cases and contacts) and environmental surveillance (i.e., sewage samples). Please note: we pre-filled some answers based on data collected in GPLNMS annual reports for 2016 as of June 2017, and we ask that you please check the pre-filled answers carefully and correct the information as appropriate. please do not leave any answers blank, because we cannot interpret these correctly, so please enter 0 for zero, unknown for unknown, not applicable for not applicable, or data not available or other appropriate text. If you find any question too difficult to answer, please do not quit the entire question or survey, but instead reply with unable to answer and please add any information that can help us understand the reason. We provided a glossary to promote consistent interpretation of survey language. If you have any questions, please contact Dr. Radboud Duintjer Tebbens (Kid Risk), at rdt@kidrisk.org and Dr. Ousmane Diop (WHO), diopo@who.int. Thank you very much for your time and effort to respond to the survey. We look forward to hearing from you - please complete your response by September 1, We will share the results with all polio laboratory directors for dissemination once they become available. 1
2 1. Please provide information about how to contact you and about your laboratory Laboratory Name Your Name Phone number address City Country WHO Region * Total employee full-time equivalents (FTEs) for poliovirus surveillance employed by the laboratory Please enter the percent (between 0-100, without the % sign) of FTEs reported for the line with the * above supported by National/internal funds Please enter the percent (between 0-100, without the % sign) of FTEs reported for the line with the * above supported by GPEI external funds Please enter the percent (between 0-100, without the % sign) of FTEs reported for the line with the * above supported by Other external funds (non GPEI-external funds, including bi-lateral support) - This line should total 100 minus the percents on the prior 2 lines. Laboratory characteristics 2
3 2. What role did your laboratory play in the global polio laboratory network in 2016? Subnational National Regional reference Specialized Other (please specify) 3. Please list the geographic areas (country, state, region) that your laboratory served in 2016 for each laboratory capacity (enter "None" for any you do not do and please note any special activities by including the word "Special" after the name of the geographic area indicated, for example to help with overflow from another lab, if applicable for 2016): Virus isolation Intratypic differentiation (ITD) Sequencing Serology Environmental surveillance 4. Please estimate what percentages (without including the "%" sign) of polio-supported staff time and equipment your laboratory spends on poliovirus surveillance and research activities (including methods development, serology, clinical trials, next generation or complete genome sequencing, etc.) versus surveillance and research activities for other diseases. Poliovirus activities (indicate 100 here and 0 on all other answers if your lab supports poliovirus surveillance activities exclusively) Non-polio enteroviruses Measles and/or rubella viruses Rotavirus Influenza Japanese encephalitis Yellow fever Other arboviruses (e.g., Zika, dengue) or hemorrhagic fever viruses Other (please provide percentage here and details about what this includes in Question 9) 3
4 5. Did your laboratory perform the following for poliovirus environmental surveillance in 2016 (if none indicate no for all)? Yes No Site selection Sample collection Sample transportation Concentration Virus isolation Intratypic differentiation Sequencing Other (please specify) 6. Please tell us about any poliovirus serology testing you did in 2016 (if none, then enter "None" for this question). How many serum samples did you test for poliovirus antibodies in 2016? Approximately how many employee hours did your laboratory spend in 2016 for poliovirus serum sample processing? What laboratory method do you use for poliovirus serology testing? Please indicate the purpose(s) for the poliovirus serology sampling (e.g., seroprevalence assessment, support for vaccine trials, etc.) 7. Please tell us the number of samples your laboratory processed in 2016 related to other activities (i.e., non-afp, non-poliovirus environmental surveillance, and non-poliovirus serology activities) for the following (please specify details about the methods used and your role in sample collection in Question 9) Non-polio enterovirus surveillance Healthy children / adult surveys (e.g., stool surveys) that are not part of AFP surveillance Clinical trial support Other (please specify the nature of these samples in Question 9) 4
5 8. What currency do you use to track laboratory costs and will you use to report costs in this survey? 9. Please specify details here if you answered "other" for Question 4 and/or 7, please also describe any research activities conducted by your laboratory in 2016 related to polioviruses, and please use this space to enter any other comments you would like to make related to the questions on this page. Acute flaccid paralysis (AFP) surveillance 10. How many samples/isolates from AFP cases and their contacts did you process in 2016? Virus isolation Intratypic differentiation Sequencing Other (please enter the number here and specify the type of processing in Question 14) 11. How many people (full-time equivalents) worked on the different steps of processing AFP samples in 2016? Cell culture Virus isolation Intratypic differentiation Sequencing Management (including supervisors, data management, analytics, recording, and reporting) Other (please enter number here and specify the type of processing in Question 14) 5
6 12. How much did your laboratory spend (in the currency you specified in Question 8) for analysis of AFP samples in 2016 for each cost category? Personnel (costs should correspond to number of people in Question 11 plus any staff not on payroll) Training (please exclude any costs counted in the personnel row above) Equipment, please estimate the amortized annual cost, see Excel worksheet Durable supplies, please estimate the amortized annual cost, see Excel worksheet Consumable supplies attributable to each sample Shared consumable supplies purchased by laboratory not easily attributable to each sample Donated supplies provided by your lab to other labs (please specify the other labs you provide these to in Question 14) Operations Shipping/transport Technical support (not otherwise captured) Other (please specify in Question 14) 6
7 13. Please indicate the approximate percents of the amounts spent in Question 12 for each cost category by contribution type: 1. National/internal; 2. GPEI external; and 3. Bilateral and non-gpei external. For example, if all support came from national sources then indicate "100; 0; 0" OR if all contributions came from the GPEI indicate "0; 100; 0" OR if approximately equal support came from each indicate "33.4; 33.3; 33.3" and please verify that the totals of all three components of the answer for each row add to 100) Personnel Training Equipment Durable supplies Consumable supplies Shared consumable supplies Donated supplies Operations Shipping/transport Technical support Other 14. Please specify details here about Questions for which you answered "other" or enter any comments you would like to make related to the questions on this page. Poliovirus environmental surveillance 15. Did your laboratory support any poliovirus environmental surveillance or research activities in 2016 (please verify)? No Yes Poliovirus environmental surveillance establishment timing 7
8 16. Did your laboratory first establish its capacity to process poliovirus environmental samples between 2010 and 2016 (i.e., relatively recently)? (If yes, the survey will ask you to estimate set up costs. If your laboratory established its capacity to process environmental samples before 2010, but made significant investments in 2016 to expand its capacity, then answer yes and estimate the costs for expanding the capacity in 2016 in Question 18). No Yes 17. Please enter the dates your laboratory first began to develop the capacity to support poliovirus environmental surveillance efforts and became fully operational (if exact date unknown, please estimate month and enter "14" for day)? MM DD YYYY Date laboratory began to develop the poliovirus ES capacity / / Date your lab became fully operational to support poliovirus environmental surveillance / / Environmental surveillance SET UP (for capacity established AFTER 2009 OR expanded during 2016 ONLY) 8
9 18. Please estimate the costs your laboratory spent to SET UP poliovirus ES capacity between the dates you reported in Question 17 (in the currency you specified in Question 8) for each cost category. Facility (purchase/renovation of physical facility) New personnel for laboratory set up Training New equipment for concentration (e.g., centrifuge, refrigerators, funnels, filtration devices, etc.) New equipment for expanded poliovirus processing capacity Durable supplies for start up Consumable supplies for start up Operations for start up Technical support for start up Other (please specify in Question 20) 19. If you included estimates of SET UP costs in Question 18, please indicate the approximate percents of the amounts for each cost category by contribution type: 1. National/internal; 2. GPEI external; and 3. Bilateral and non-gpei external. For example, if all support came from national sources then indicate "100; 0; 0" OR if all contributions came from the GPEI indicate "0; 100; 0" OR if approximately equal support came from each indicate "33.4; 33.3; 33.3" and please verify that the totals of all three components of the answer for each row add to 100) Facility New personnel for laboratory set up Training for start up New equipment for concentration New equipment for expanded poliovirus processing capacity Durable supplies for start up Consumable supplies for start up Operations for start up Technical support for start up Other (please specify in Question 20) 9
10 20. Please specify details here about Questions for which you answered "other" or enter any comments you would like to make related to the questions on this page. Poliovirus environmental surveillance (ES) activities 21. Which organization(s) collect the poliovirus environmental samples that your laboratory receives? 22. Please enter the total number of environmental samples your laboratory received in 2016 from each of the following types of water source(s) sampled (if known). If only unknown water source(s) sampled, then please indicate the total number of environmental samples for 2016 in the second-to-last row. Wastewater treatment plant Pumping station Open drains or canals Streams, rivers, or other flowing surface water Lakes, ponds or other standing surface water Access point from sewage system Unknown Other (please indicate type in Question 27) 10
11 23. Please enter the number of environmental samples for which your laboratory took the indicated number of days between the time of sample collection and starting the process of virus isolation. Your internal data for all poliovirus ES samples should provide the sample collection date and the date your lab started sample processing. Less than 2 days 3 to 5 days 6 to 10 days 11 to 15 days 16 to 20 days 21 to 25 days 26 to 30 days 31 to 35 days More than 35 days 24. How many environmental samples did your laboratory process in 2016 for each of the following? Concentration using WHO-recommended twophase separation Concentration using other methods (please specify method(s) used in Question 27) Virus isolation Intratypic differentiation Sequencing Research Direct detection Other (please specify type of processing in the comment field at the bottom of this page in Question 27) 11
12 25. How much did your laboratory spend (in the currency you specified in Question 8) for analysis of environmental samples in 2016 (excluding any costs for SET UP that occurred in 2016, which you should have reported in Question 18) and excluding any costs already reported in Question 12 related to AFP processing that applied to processing environmental samples. Personnel (FTEs for environmental surveillance activities) Training Equipment, please estimate the amortized annual cost, see Excel worksheet Durable supplies, please estimate the amortized annual cost, see Excel worksheet Consumable supplies Shared consumable supplies Donated supplies (please specify the other labs you provide these to in Question 27) Operations Shipping/transport Technical support Other (please specify in Question 27) 26. Please indicate the approximate percent of the amounts spent in Question 25 for each cost category by contribution type: 1. National/internal; 2. GPEI external; and 3. Other external. For example, if all support came from national sources then indicate "100; 0; 0" OR if all contributions came from the GPEI indicate "0; 100; 0" OR if approximately equal support came from each indicate "33.4; 33.3; 33.3" and please verify that the totals of all three components of the answer for each row add to 100) Personnel Training Equipment Durable supplies Consumable supplies Shared consumable supplies Donated supplies Operations Shipping/transport Technical support Other (please specify in Question 27) 12
13 27. Please specify details here about Questions for which you answered "other" or enter any comments you would like to make related to the questions on this page. Closing questions 28. Please list and indicate the nature and source of all in-kind contributions your laboratory receives that support AFP and/or ES sample processing (please provide a brief description that includes the amount, source, and purpose of the in-kind support). If your laboratory provides in-kind support to other laboratories, please provide details about this. 29. Did your laboratory experience any significant changes in its workload/workflow in 2016 compared to 2015, if so please describe reasons (e.g., increased/decreased AFP, contact samples, special surveys, serology or clinical trials, introduction of environmental surveillance, implementation of polio laboratory containment and GAP III requirements or other activities, and impacts of changes in financials support, etc.)? No Yes (please specify) 13
14 30. Does your laboratory expect to make any significant changes in its workload/workflow in the future compared to 2016, if so please describe reasons (e.g., increased/decreased AFP, contact samples, special surveys, serology or clinical trials, or other activities, introduction of environmental surveillance)? No Yes (please specify) 31. What other costs or issues related to poliovirus laboratories do you think we should consider? What questions should we ask that we did not ask? Please use this space to make any final comments on the survey. Thank you very much for your responses. Final Page - Ready to Submit? 32. Are you ready to submit your completed survey? No (if not, please make sure to select "Prev" below to go back to the prior questions) Yes (if so, and only if so, select "Done" below, because you will not be able to make any changes after selecting "Done") 14
Polio Environmental Surveillance Enhancement Following Detection of Vaccine-Related Type-2 Poliovirus
 1. Goal and Objective: Although acute flaccid paralysis surveillance is the gold standard of polio surveillance, supplemental surveillance methods (which includes environmental surveillance (ES)) provide
1. Goal and Objective: Although acute flaccid paralysis surveillance is the gold standard of polio surveillance, supplemental surveillance methods (which includes environmental surveillance (ES)) provide
Polio Transition Planning: Risks and opportunities of transitioning resources to non-polio public health interventions
 Polio Transition Planning: Risks and opportunities of transitioning resources to non-polio public health interventions Global Polio Partners Group (PPG) meeting June 26, 2017 Underlying premise: Transition
Polio Transition Planning: Risks and opportunities of transitioning resources to non-polio public health interventions Global Polio Partners Group (PPG) meeting June 26, 2017 Underlying premise: Transition
Sanofi Pasteur: A partner in eradicating vaccine preventable diseases and improving access to vaccines
 Sanofi Pasteur: A partner in eradicating vaccine preventable diseases and improving access to vaccines 1 Vaccines: the single most effective medical intervention 2 Vaccines save lives Millions of cases
Sanofi Pasteur: A partner in eradicating vaccine preventable diseases and improving access to vaccines 1 Vaccines: the single most effective medical intervention 2 Vaccines save lives Millions of cases
Why still Polio Donato Greco ECDC polio consultant 14 April 2016
 Why still Polio Donato Greco ECDC polio consultant 14 April 2016 A medical student with the last texbook on Communicable diseases! Where there is no chapter on Poliomielitis!!! What can I say o Background
Why still Polio Donato Greco ECDC polio consultant 14 April 2016 A medical student with the last texbook on Communicable diseases! Where there is no chapter on Poliomielitis!!! What can I say o Background
Polio Eradication and Surveillance. December
 Polio Eradication and Surveillance December 2017 1 Acute Flaccid Paralysis (AFP) surveillance for polio eradication: >100,000 reported AFP per year December 2017 2 Global Laboratory Network for Polio Eradication
Polio Eradication and Surveillance December 2017 1 Acute Flaccid Paralysis (AFP) surveillance for polio eradication: >100,000 reported AFP per year December 2017 2 Global Laboratory Network for Polio Eradication
All About Vaccines and How They Get to Those Who Need Them Most. Elesha Kingshott
 All About Vaccines and How They Get to Those Who Need Them Most Elesha Kingshott Shot@Life Four Priority Disease Areas 1. Polio 2. Measles 3. Diarrheal Disease 4. Pneumonia Polio is caused by a virus that
All About Vaccines and How They Get to Those Who Need Them Most Elesha Kingshott Shot@Life Four Priority Disease Areas 1. Polio 2. Measles 3. Diarrheal Disease 4. Pneumonia Polio is caused by a virus that
How to present the European Vaccine Action Plan (EVAP)
 How to present the European Vaccine Action Plan 2015-2020 () HOW TO USE THIS DOCUMENT By adopting the European Vaccine Action Plan 2015-2020 () in September 2014, all Member States of the WHO European
How to present the European Vaccine Action Plan 2015-2020 () HOW TO USE THIS DOCUMENT By adopting the European Vaccine Action Plan 2015-2020 () in September 2014, all Member States of the WHO European
SURVEILLANCE TECHNICAL
 CHAPTER 5 SURVEILLANCE TECHNICAL ASPECTS 55 Protect - detect - protect Polio eradication strategies can be summed up as protect and detect protect children against polio by vaccinating them, and detect
CHAPTER 5 SURVEILLANCE TECHNICAL ASPECTS 55 Protect - detect - protect Polio eradication strategies can be summed up as protect and detect protect children against polio by vaccinating them, and detect
The Global Commission for the Certification of Polio Eradication (GCCPE)
 The Global Commission for the Certification of Polio Eradication (GCCPE) In 1995, the Director-General of WHO charged the GCC with three tasks: 1. defining the parameters and processes by which polio eradication
The Global Commission for the Certification of Polio Eradication (GCCPE) In 1995, the Director-General of WHO charged the GCC with three tasks: 1. defining the parameters and processes by which polio eradication
Total population 24,759,000. Live births (LB) 342,458. Children <1 year 337,950. Children <5 years 1,698,664. Children <15 years 5,233,093
 DPR Korea 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering -5. A standing national technical advisory group on immunization (NTAGI) with formal
DPR Korea 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering -5. A standing national technical advisory group on immunization (NTAGI) with formal
3. CONCLUSIONS AND RECOMMENDATIONS
 3. CONCLUSIONS AND RECOMMENDATIONS 3.1 Polio Endgame Strategy Conclusions 1. The TAG welcomes the RCC conclusion that Western Pacific Region maintains its polio-free status, and commends China for the
3. CONCLUSIONS AND RECOMMENDATIONS 3.1 Polio Endgame Strategy Conclusions 1. The TAG welcomes the RCC conclusion that Western Pacific Region maintains its polio-free status, and commends China for the
Poliomyelitis eradication in the WHO European Region
 Provisional agenda item 6(i) EUR/RC60/16 (+EUR/RC60/Conf.Doc./9) 23 July 2010 101614 ORIGINAL: ENGLISH Poliomyelitis eradication in the WHO European Region WHO Regional Committee for Europe Sixtieth session
Provisional agenda item 6(i) EUR/RC60/16 (+EUR/RC60/Conf.Doc./9) 23 July 2010 101614 ORIGINAL: ENGLISH Poliomyelitis eradication in the WHO European Region WHO Regional Committee for Europe Sixtieth session
Ensuring the quality of polio outbreak response activities: A rationale and guide for 3 month, quarterly and 6 month independent assessments
 Ensuring the quality of polio outbreak response activities: A rationale and guide for 3 month, quarterly and 6 month independent assessments Introduction While polio exists anywhere, countries with low
Ensuring the quality of polio outbreak response activities: A rationale and guide for 3 month, quarterly and 6 month independent assessments Introduction While polio exists anywhere, countries with low
ANNEX Page. AFR/RC61/11 4 July 2011 ORIGINAL: ENGLISH REGIONAL COMMITTEE FOR AFRICA
 4 July 2011 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-first session Yamoussoukro, Côte d Ivoire, 29 August 2 September 2011 Provisional agenda item 16 PROGRESS REPORT ON POLIOMYELITIS ERADICATION
4 July 2011 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-first session Yamoussoukro, Côte d Ivoire, 29 August 2 September 2011 Provisional agenda item 16 PROGRESS REPORT ON POLIOMYELITIS ERADICATION
Sudan EPI Benefits From Polio Eradication Program
 Federal Ministry of Health Primary Health Care Maternal and Child Health Expanded Program on Immunization Sudan EPI Benefits From Polio Eradication Program Polio Legacy Planning and Implementation Workshop
Federal Ministry of Health Primary Health Care Maternal and Child Health Expanded Program on Immunization Sudan EPI Benefits From Polio Eradication Program Polio Legacy Planning and Implementation Workshop
KURUKSHETRA JULY 2017 RURAL HEALTH
 KURUKSHETRA JULY 2017 RURAL HEALTH Jagat Prakash Nadda is the Union Minister of Health and Family Welfare. Immunization, or immunisation, is the process by which an individual's immune system becomes
KURUKSHETRA JULY 2017 RURAL HEALTH Jagat Prakash Nadda is the Union Minister of Health and Family Welfare. Immunization, or immunisation, is the process by which an individual's immune system becomes
How would a comprehensive surveillance look like (with ball park costing?)
 1 How would a comprehensive surveillance look like (with ball park costing?) 2-3 Nov 2017, London, UK Zainul Abedin Khan, WHO HQ POL Polio surveillance and the (bumpy) road to transition 2 Tool Goals Overall
1 How would a comprehensive surveillance look like (with ball park costing?) 2-3 Nov 2017, London, UK Zainul Abedin Khan, WHO HQ POL Polio surveillance and the (bumpy) road to transition 2 Tool Goals Overall
Responding to a poliovirus event and outbreak
 Standard Operating Procedures Responding to a poliovirus event and outbreak Updated according to WHO guidelines from May 2017 This is a version of the original two-part document drawn up by the WHO and
Standard Operating Procedures Responding to a poliovirus event and outbreak Updated according to WHO guidelines from May 2017 This is a version of the original two-part document drawn up by the WHO and
D.A.Henderson, MD, MPH. Professor of Medicine, University of Pittsburgh Honorary Fellow, London School of Hygiene and Tropical Medicine
 Polio Eradication a reconsideration of strategy D.A.Henderson, MD, MPH Professor of Medicine, University of Pittsburgh Honorary Fellow, London School of Hygiene and Tropical Medicine Baltimore, Maryland
Polio Eradication a reconsideration of strategy D.A.Henderson, MD, MPH Professor of Medicine, University of Pittsburgh Honorary Fellow, London School of Hygiene and Tropical Medicine Baltimore, Maryland
Expanded Programme on Immunization
 1 Expanded Programme on Immunization Strategic issues Immunization is a cost-effective public health intervention that has dramatically reduced disease, disability and death in the Western Pacifi c Region.
1 Expanded Programme on Immunization Strategic issues Immunization is a cost-effective public health intervention that has dramatically reduced disease, disability and death in the Western Pacifi c Region.
Total population 1,212,110. Live births (LB) 43,924. Children <1 year 40,351. Children <5 years 192,340. Children <15 years 510,594
 Draft version for the SEAR-ITAG 5-9 June 5 All data provisional as of May 5 Timor Leste 4 Immunization system highlights There is a comprehensive multi-year plan (cmyp) for immunization covering 3-5. 8%
Draft version for the SEAR-ITAG 5-9 June 5 All data provisional as of May 5 Timor Leste 4 Immunization system highlights There is a comprehensive multi-year plan (cmyp) for immunization covering 3-5. 8%
Professor Michael Kossove
 Professor Michael Kossove Is this a new disease? No, but doctors only began noticing it in 2014. That s when dozens of children began showing up in hospitals unable to move their arms or legs. Ultimately,
Professor Michael Kossove Is this a new disease? No, but doctors only began noticing it in 2014. That s when dozens of children began showing up in hospitals unable to move their arms or legs. Ultimately,
Polio (Paralytic and Non-paralytic
 Polio (Paralytic and Non-paralytic Infection) rev Jan 2018 Infectious Agent Poliovirus (genus Enterovirus) types, 1, 2, and 3. BASIC EPIDEMIOLOGY Transmission Poliovirus is transmitted by person-to-person
Polio (Paralytic and Non-paralytic Infection) rev Jan 2018 Infectious Agent Poliovirus (genus Enterovirus) types, 1, 2, and 3. BASIC EPIDEMIOLOGY Transmission Poliovirus is transmitted by person-to-person
Expanded Programme on Immunization (EPI)
 Sri Lanka 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Sri Lanka 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Polio post-certification strategy
 1 Polio post-certification strategy SAGE Meeting, Geneva, 17 April 2018 Michel Zaffran, Director, Polio Eradication, WHO On Behalf o the GPEI Polio Eradication and Endgame Strategy 1. Poliovirus detection
1 Polio post-certification strategy SAGE Meeting, Geneva, 17 April 2018 Michel Zaffran, Director, Polio Eradication, WHO On Behalf o the GPEI Polio Eradication and Endgame Strategy 1. Poliovirus detection
Report. 10 th Meeting of the Expert Review Committee (ERC) on Polio Eradication in Nigeria
 Report 10 th Meeting of the Expert Review Committee (ERC) on Polio Eradication in Nigeria Kano, Nigeria 12-13 July 2006 Executive Summary The 10 th Expert Review Committee (ERC) met in Kano on 12-13 July
Report 10 th Meeting of the Expert Review Committee (ERC) on Polio Eradication in Nigeria Kano, Nigeria 12-13 July 2006 Executive Summary The 10 th Expert Review Committee (ERC) met in Kano on 12-13 July
Total population 20,675,000. Live births (LB) 349,715. Children <1 year 346,253. Children <5 years 1,778,050. Children <15 years 5,210,100
 Sri Lanka 4 Immunization system highlights There is a comprehensive multi-year plan (cmyp) for immunization system strengthening covering -6. A national policy on immunization has been developed. A standing
Sri Lanka 4 Immunization system highlights There is a comprehensive multi-year plan (cmyp) for immunization system strengthening covering -6. A national policy on immunization has been developed. A standing
A tool for investigation of Sabin Like 2 (SL2) poliovirus isolation in human or in the environment
 A tool for investigation of Sabin Like 2 (SL2) poliovirus isolation in human or in the environment WHO Geneva 8 March 2017 COUNTRY: Investigation of Sabin2 Positive AFP Case, Healthy Person or Environmental
A tool for investigation of Sabin Like 2 (SL2) poliovirus isolation in human or in the environment WHO Geneva 8 March 2017 COUNTRY: Investigation of Sabin2 Positive AFP Case, Healthy Person or Environmental
Expanded Programme on Immunization (EPI)
 Bhutan 2017 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles containing
Bhutan 2017 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles containing
Total population 1,265,308,000. Live births (LB) 27,016,000. Children <1 year 25,928,200. Children <5 years 23,818,000. Children <15 years 25,639,000
 India 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering 3-7. National technical advisory group on immunization (NTAGI) exists and formal written
India 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering 3-7. National technical advisory group on immunization (NTAGI) exists and formal written
Expanded Programme on Immunization (EPI)
 Timor-Leste 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Timor-Leste 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Appendix for Managing population immunity to reduce or eliminate the risks of circulation following the importation of live polioviruses
 Appendix for Managing population immunity to reduce or eliminate the risks of circulation following the importation of live polioviruses Authors: Kimberly M. Thompson, Dominika A. Kalkowska, Radboud J.
Appendix for Managing population immunity to reduce or eliminate the risks of circulation following the importation of live polioviruses Authors: Kimberly M. Thompson, Dominika A. Kalkowska, Radboud J.
Expanded Programme on Immunization (EPI)
 Indonesia 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Indonesia 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
USAID Progress: The U.S. National Vaccine Plan of 1994
 USAID Progress: The U.S. National Vaccine Plan of 1994 Ellyn Ogden Neal Brandes Angela Weaver Washington DC March 3, 2008 Objective 1.1/1.5 (New Vaccines) Support to GAVI for introduction of new vaccines
USAID Progress: The U.S. National Vaccine Plan of 1994 Ellyn Ogden Neal Brandes Angela Weaver Washington DC March 3, 2008 Objective 1.1/1.5 (New Vaccines) Support to GAVI for introduction of new vaccines
Integrated Disease Surveillance and Response Bulletin
 Republic of Liberia INTEGRATED DISEASE SURVEILLANCE AND RESPONSE BULLETIN Week-33 August 15-21, 2016 Data Source: CSOs from 15 Counties and Lab Highlights during the reporting week Eleven neonatal deaths
Republic of Liberia INTEGRATED DISEASE SURVEILLANCE AND RESPONSE BULLETIN Week-33 August 15-21, 2016 Data Source: CSOs from 15 Counties and Lab Highlights during the reporting week Eleven neonatal deaths
Polio Eradication in India. Dr Sunil Bahl Deputy Project Manager WHO Country Office for India 28 October 2014
 Polio Eradication in India Dr Sunil Bahl Deputy Project Manager WHO Country Office for India 28 October 2014 History of Polio in India Number of Cases 200,000 200,000 OPV Introduced in RI - 1978 150,000
Polio Eradication in India Dr Sunil Bahl Deputy Project Manager WHO Country Office for India 28 October 2014 History of Polio in India Number of Cases 200,000 200,000 OPV Introduced in RI - 1978 150,000
Integrated Disease Surveillance and Response Bulletin INTEGRATED DISEASE SURVEILLANCE AND RESPONSE BULLETIN
 Republic of Liberia INTEGRATED DISEASE SURVEILLANCE AND RESPONSE BULLETIN Week-23 June 6-12. 2016 Data Source: CSOs from 15 Counties Highlights during the reporting week Six maternal deaths reported from
Republic of Liberia INTEGRATED DISEASE SURVEILLANCE AND RESPONSE BULLETIN Week-23 June 6-12. 2016 Data Source: CSOs from 15 Counties Highlights during the reporting week Six maternal deaths reported from
RESPONDING TO A POLIOVIRUS EVENT OR OUTBREAK
 STANDARD OPERATING PROCEDURES RESPONDING TO A POLIOVIRUS Version 3 December 2018 STANDARD OPERATING PROCEDURES RESPONDING TO A POLIOVIRUS Version 3 December 2018 Published by the World Health Organization
STANDARD OPERATING PROCEDURES RESPONDING TO A POLIOVIRUS Version 3 December 2018 STANDARD OPERATING PROCEDURES RESPONDING TO A POLIOVIRUS Version 3 December 2018 Published by the World Health Organization
Table 1: Basic information ,000, (per 1,000 LB) 34.6 (per 1,000 LB) 174 (per 100,000 LB) District 712.
 EPI history EPI launched in 978 with DPT, OPV, BCG and typhoid vaccines TT immunization of pregnant women introduced in 983 MCV introduced in 985 HepB piloted in 00 and made universal in 0 MCV introduced
EPI history EPI launched in 978 with DPT, OPV, BCG and typhoid vaccines TT immunization of pregnant women introduced in 983 MCV introduced in 985 HepB piloted in 00 and made universal in 0 MCV introduced
Global and Regional update on Polio Eradication Activities. Kenya Paediatric Association Pride Inn, Mombasa April 26, 2018
 Global and Regional update on Polio Eradication Activities Kenya Paediatric Association Pride Inn, Mombasa April 26, 2018 Presentation Outline 1. Background information 2. Poliovirus detection & interruption
Global and Regional update on Polio Eradication Activities Kenya Paediatric Association Pride Inn, Mombasa April 26, 2018 Presentation Outline 1. Background information 2. Poliovirus detection & interruption
POLIO POST-CERTIFICATION STRATEGY. Summary overview 8 August 2017
 POLIO POST-CERTIFICATION STRATEGY Summary overview 8 August 2017 Introduction This PowerPoint is intended to provide a summary overview of the polio Post- Certification Strategy (PCS) 2021-2030, at its
POLIO POST-CERTIFICATION STRATEGY Summary overview 8 August 2017 Introduction This PowerPoint is intended to provide a summary overview of the polio Post- Certification Strategy (PCS) 2021-2030, at its
Fact sheet. Poliomyelitis. WHO Media centre. ADAPTED FOR ADDITION TO THE RHIZOME POLIOK.IT PLATFORM 16 Jan 2017 INFORMATION FOR ERADICATION FROM
 Fact sheet Poliomyelitis WHO Media centre ADAPTED FOR ADDITION TO THE RHIZOME POLIOK.IT PLATFORM 16 Jan 2017 INFORMATION FOR ERADICATION FROM Media centre Poliomyelitis Fact sheet Updated April 2016 Key
Fact sheet Poliomyelitis WHO Media centre ADAPTED FOR ADDITION TO THE RHIZOME POLIOK.IT PLATFORM 16 Jan 2017 INFORMATION FOR ERADICATION FROM Media centre Poliomyelitis Fact sheet Updated April 2016 Key
INTEGRATED DISEASE SURVEILLANCE AND RESPONSE (IDSR)
 INTEGRATED DISEASE SURVEILLANCE AND RESPONSE (IDSR) Surveillance Report Republic Of Zambia (Ministry Of Health) Reporting Period: 11/11/218 to 17/11//218. Epidemiology Bulletin: Week 46 Issue: 46 Issue
INTEGRATED DISEASE SURVEILLANCE AND RESPONSE (IDSR) Surveillance Report Republic Of Zambia (Ministry Of Health) Reporting Period: 11/11/218 to 17/11//218. Epidemiology Bulletin: Week 46 Issue: 46 Issue
Medical Information Form
 Fill in form with a computer Medical Information Form Child s Name: first name, last name Date of Birth: month, day, year Health Insurance information o Insurance group number, policy number, contact information:
Fill in form with a computer Medical Information Form Child s Name: first name, last name Date of Birth: month, day, year Health Insurance information o Insurance group number, policy number, contact information:
CHAPTER ONE: EXECUTIVE SUMMARY. The Global Vaccine Industry CHAPTER TWO: INTRODUCTION TO VACCINES
 CHAPTER ONE: EXECUTIVE SUMMARY The Global Vaccine Industry o Scope and Methodology o Overview o Pediatric Preventative Vaccines o The Market o Adult Preventative Vaccines o The Market o Total Market o
CHAPTER ONE: EXECUTIVE SUMMARY The Global Vaccine Industry o Scope and Methodology o Overview o Pediatric Preventative Vaccines o The Market o Adult Preventative Vaccines o The Market o Total Market o
Ex Post-Evaluation Brief India: Polio Immunisation Programme, Phases I to VII
 Ex Post-Evaluation Brief India: Polio Immunisation Programme, Phases I to VII Polio Immunisation Programme Phases I VII (1996 Programme/Client 66 140; 1999 65 906; 2000 65 581; 2002 66 213; 2002 66 858;
Ex Post-Evaluation Brief India: Polio Immunisation Programme, Phases I to VII Polio Immunisation Programme Phases I VII (1996 Programme/Client 66 140; 1999 65 906; 2000 65 581; 2002 66 213; 2002 66 858;
WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC
 WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC REPORT FIFTH MEETING ON LABORATORY SURVEILLANCE FOR POLIOMYELITIS ERADICATION IN THE WESTERN PACIFIC REGION Manila, Philippines 16-17 August
WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC REPORT FIFTH MEETING ON LABORATORY SURVEILLANCE FOR POLIOMYELITIS ERADICATION IN THE WESTERN PACIFIC REGION Manila, Philippines 16-17 August
Table 1: Basic information 2017
 EPI history EPI launched in 978 OPV and MCV introduced in 987 AD syringes introduced in 00 HepB vaccine introduced in 003 MCV introduced partially in 008 and made available nationwide in 0 DTP-Hib-HepB
EPI history EPI launched in 978 OPV and MCV introduced in 987 AD syringes introduced in 00 HepB vaccine introduced in 003 MCV introduced partially in 008 and made available nationwide in 0 DTP-Hib-HepB
Responding to a poliovirus event and outbreak Part 2: Protocol for poliovirus type 2
 Standard Operating Procedures Responding to a poliovirus event and outbreak Part 2: Protocol for poliovirus type 2 April 20, 2016 Effective 1 st May 2016 till 30 April 2017 Contents Tables and Figures...
Standard Operating Procedures Responding to a poliovirus event and outbreak Part 2: Protocol for poliovirus type 2 April 20, 2016 Effective 1 st May 2016 till 30 April 2017 Contents Tables and Figures...
Table 1: Basic information (per 1,000 LB) 42.4 (per 1,000 LB) 49.7 (per 1,000 LB) 215 (per 100,000 LB)
 EPI history EPI started in 978 EPI re-structured in March 000 DTP-HepB vaccine introduced in 007 DTP-Hib-HepB)vaccine introduced in 0 MR vaccine introduced in Feb 06 Second dose of MR introduced in Feb
EPI history EPI started in 978 EPI re-structured in March 000 DTP-HepB vaccine introduced in 007 DTP-Hib-HepB)vaccine introduced in 0 MR vaccine introduced in Feb 06 Second dose of MR introduced in Feb
The Global Polio Laboratory Network as a Platform for the Viral Vaccine-Preventable and Emerging Diseases Laboratory Networks
 The Journal of Infectious Diseases SUPPLEMENT ARTICLE The Global Polio Laboratory Network as a Platform for the Viral Vaccine-Preventable and Emerging Diseases Laboratory Networks Ousmane M. Diop, 1 Olen
The Journal of Infectious Diseases SUPPLEMENT ARTICLE The Global Polio Laboratory Network as a Platform for the Viral Vaccine-Preventable and Emerging Diseases Laboratory Networks Ousmane M. Diop, 1 Olen
3-4 September 2015 // Antwerp, Belgium. Meeting of the Global Polio Laboratory Network (GPLN) in the WHO European Region
 3-4 September 2015 // Antwerp, Belgium Meeting of the Global Polio Laboratory Network (GPLN) in the WHO European Region KEYWORDS POLIOMYELITIS diagnosis, epidemiology, prevention and control POLIOVIRUS
3-4 September 2015 // Antwerp, Belgium Meeting of the Global Polio Laboratory Network (GPLN) in the WHO European Region KEYWORDS POLIOMYELITIS diagnosis, epidemiology, prevention and control POLIOVIRUS
3/25/2018. End Polio Now an Update. Type 3 has not been seen since The Polio Virus. Polio What is it and Where did it Originate?
 End Polio Now an Update The Polio Virus Presentation to the Northern California Chapter American Microbiology Association Spring Meeting, March 2, 2018 Polio What is it and Where did it Originate? Polio
End Polio Now an Update The Polio Virus Presentation to the Northern California Chapter American Microbiology Association Spring Meeting, March 2, 2018 Polio What is it and Where did it Originate? Polio
GAVI Role in IPV Introductions
 GAVI Role in IPV Introductions Melissa Malhame 12 th WHO/UNICEF Consultation with OPV/IPV Manufacturers and National Regulatory Authorities Geneva, Switzerland, 10 GAVI vaccine support Currently supported
GAVI Role in IPV Introductions Melissa Malhame 12 th WHO/UNICEF Consultation with OPV/IPV Manufacturers and National Regulatory Authorities Geneva, Switzerland, 10 GAVI vaccine support Currently supported
Article Title : The Global Polio Laboratory Network as a Platform for the Viral Vaccine-Preventable and Emerging Diseases Laboratory Networks
 Journal : INFDIS Article Doi : 10.1093/infdis/jix092 Article Title : The Global Polio Laboratory Network as a Platform for the Viral Vaccine-Preventable and Emerging Diseases Laboratory Networks INSTRUCTIONS
Journal : INFDIS Article Doi : 10.1093/infdis/jix092 Article Title : The Global Polio Laboratory Network as a Platform for the Viral Vaccine-Preventable and Emerging Diseases Laboratory Networks INSTRUCTIONS
Meeting Report. Fourth Meeting on Vaccine Preventable Diseases Laboratory Networks in the Western Pacific Region March 2013 Manila, Philippines
 Meeting Report Fourth Meeting on Vaccine Preventable Diseases Laboratory Networks in the Western Pacific Region 11 15 March 2013 Manila, Philippines Participants of the Fourth Meeting on Vaccine-Preventable
Meeting Report Fourth Meeting on Vaccine Preventable Diseases Laboratory Networks in the Western Pacific Region 11 15 March 2013 Manila, Philippines Participants of the Fourth Meeting on Vaccine-Preventable
Modeling Population Immunity to Support Efforts to End the Transmission of Live Polioviruses
 Risk Analysis DOI: 10.1111/j.1539-6924.2012.01891.x Modeling Population Immunity to Support Efforts to End the Transmission of Live Polioviruses Kimberly M. Thompson, 1,4 Mark A. Pallansch, 2 Radboud J.
Risk Analysis DOI: 10.1111/j.1539-6924.2012.01891.x Modeling Population Immunity to Support Efforts to End the Transmission of Live Polioviruses Kimberly M. Thompson, 1,4 Mark A. Pallansch, 2 Radboud J.
Our Mission. To promote healthy and safe travel by providing medicines, preventive vaccines and health counseling to a diverse group of travelers
 Our Mission To promote healthy and safe travel by providing medicines, preventive vaccines and health counseling to a diverse group of travelers The Travel Clinic utilizes national and international travel
Our Mission To promote healthy and safe travel by providing medicines, preventive vaccines and health counseling to a diverse group of travelers The Travel Clinic utilizes national and international travel
Table Of Contents Executive Summary Introduction to Vaccines Pediatric Preventive Vaccines
 Table Of Contents Executive Summary THE GLOBAL VACCINES INDUSTRY Scope and Methodology Overview Pediatric Preventative Vaccines THE MARKET Adult Preventative Vaccines THE MARKET TOTAL MARKET ISSUES AND
Table Of Contents Executive Summary THE GLOBAL VACCINES INDUSTRY Scope and Methodology Overview Pediatric Preventative Vaccines THE MARKET Adult Preventative Vaccines THE MARKET TOTAL MARKET ISSUES AND
Union Theological Seminary Measles, Mumps & Rubella Form
 Union Theological Seminary Measles, Mumps & Rubella Form Please return this form by fax: (212) 202-4667) or by mail/in person: Office of Student Affairs, Union Theological Seminary, 3041 Broadway, New
Union Theological Seminary Measles, Mumps & Rubella Form Please return this form by fax: (212) 202-4667) or by mail/in person: Office of Student Affairs, Union Theological Seminary, 3041 Broadway, New
INTERNATIONAL CERTIFICATION COMMISSION FOR POLIO ERADICATION IN THE SOUTH-EAST ASIA REGION PLAN OF ACTION
 WORLD HEALTH ORGANIZATION Regional Office for South-East Asia New Delhi SEA/Polio/11 12 February 1998 RESTRICTED INTERNATIONAL CERTIFICATION COMMISSION FOR POLIO ERADICATION IN THE SOUTH-EAST ASIA REGION
WORLD HEALTH ORGANIZATION Regional Office for South-East Asia New Delhi SEA/Polio/11 12 February 1998 RESTRICTED INTERNATIONAL CERTIFICATION COMMISSION FOR POLIO ERADICATION IN THE SOUTH-EAST ASIA REGION
Draft Programme (05 May 2016)
 Innovations in sustainable Draft Programme (05 May 2016) Draft 7 th Meeting of the South-East Asia Region Immunization Technical Advisory Group (SEAR ITAG) 6-10 June 2016, New Delhi (India) Day 1, Monday,
Innovations in sustainable Draft Programme (05 May 2016) Draft 7 th Meeting of the South-East Asia Region Immunization Technical Advisory Group (SEAR ITAG) 6-10 June 2016, New Delhi (India) Day 1, Monday,
Quarterly Epidemiological Bulletin
 WHO - ERITREA Quarterly Epidemiological Bulletin 3rd Quarter Year 2014 Introduction Contents Introduction Epidemic prone disease Disease for elimination and eradication Emerging and re - emerging disease
WHO - ERITREA Quarterly Epidemiological Bulletin 3rd Quarter Year 2014 Introduction Contents Introduction Epidemic prone disease Disease for elimination and eradication Emerging and re - emerging disease
EURO POLIO PAGE Data as of 04 October 2005 (Week 38)
 World Health Organization Regional Office for Europe EURO POLIO PAGE Data as of 04 October 2005 (Week 38) Vaccine-preventable Diseases and Immunization programme, Division of Technical Support website:
World Health Organization Regional Office for Europe EURO POLIO PAGE Data as of 04 October 2005 (Week 38) Vaccine-preventable Diseases and Immunization programme, Division of Technical Support website:
TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT. RAJEEV VENKAYYA, MD President, Global Vaccine Business Unit
 TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT RAJEEV VENKAYYA, MD President, Global Vaccine Business Unit OUR MISSION Develop and deliver innovative vaccines that tackle the toughest problems in public
TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT RAJEEV VENKAYYA, MD President, Global Vaccine Business Unit OUR MISSION Develop and deliver innovative vaccines that tackle the toughest problems in public
Immunizations for Children and Teens with Suppressed Immune Systems
 Immunizations for Children and Teens with Suppressed Immune Systems Your child is starting treatment that will suppress the immune system. This will affect how your child s body responds to routine immunizations
Immunizations for Children and Teens with Suppressed Immune Systems Your child is starting treatment that will suppress the immune system. This will affect how your child s body responds to routine immunizations
Sample Collections and Poliovirus Containment: What s the Connection
 Centers for Disease Control and Prevention Center for Preparedness and Response Sample Collections and Poliovirus Containment: What s the Connection October 15, 2018 Christy Myrick, PhD, RBP What s in
Centers for Disease Control and Prevention Center for Preparedness and Response Sample Collections and Poliovirus Containment: What s the Connection October 15, 2018 Christy Myrick, PhD, RBP What s in
Clinical Preparedness Permit (Revised June 2018)
 (Please ensure student name appears on each page) For Collaborative Students only: College Student Number College Student Email All Students to indicate: York Student Number York Student E-mail Students
(Please ensure student name appears on each page) For Collaborative Students only: College Student Number College Student Email All Students to indicate: York Student Number York Student E-mail Students
Poliomyelitis: successful and critical issues in the eradication process
 Vaccine Preventable Diseases in the Mediterranean Basin and Black Sea: immunization strategies and coverage in the general population and the newly arrived migrants the ProVacMed project Poliomyelitis:
Vaccine Preventable Diseases in the Mediterranean Basin and Black Sea: immunization strategies and coverage in the general population and the newly arrived migrants the ProVacMed project Poliomyelitis:
Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Published in WER 4 June, 2010
 Polio vaccines and polio immunization in the pre-eradication era: WHO position paper Published in WER 4 June, 2010 Epidemiology & Background Poliomyelitis (polio) is an acute communicable disease of humans
Polio vaccines and polio immunization in the pre-eradication era: WHO position paper Published in WER 4 June, 2010 Epidemiology & Background Poliomyelitis (polio) is an acute communicable disease of humans
Report of the 23 rd Informal Consultation of the Global Polio Laboratory Network (GPLN)
 Report of the 23 rd Informal Consultation of the Global Polio Laboratory Network (GPLN) Venue and dates: WHO Headquarters, Geneva, Switzerland, 15-16 March 2017 List of abreviations used AFP acute flaccid
Report of the 23 rd Informal Consultation of the Global Polio Laboratory Network (GPLN) Venue and dates: WHO Headquarters, Geneva, Switzerland, 15-16 March 2017 List of abreviations used AFP acute flaccid
Table 1: Basic information Total population 25,030, (per 100,000 LB) Division/Province/State/Region 11. District 210
 EPI history EPI launched in 980 HepB vaccine introduced in 003 AD syringes introduced in 003 HepB birth dose introduced in 004 DTP-HepB vaccine introduced in 006 MCV introduced in 008 DTP-Hib-HepB vaccine
EPI history EPI launched in 980 HepB vaccine introduced in 003 AD syringes introduced in 003 HepB birth dose introduced in 004 DTP-HepB vaccine introduced in 006 MCV introduced in 008 DTP-Hib-HepB vaccine
Proposed Recommendations. Terry Nolan
 Proposed Recommendations CYD-TDV Denvaxia, Dengue Vaccine Terry Nolan SAGE, Co-Chair Dengue Vaccine WG WG Considerations A number of dimensions: Population benefit versus individual risk Ethical considerations
Proposed Recommendations CYD-TDV Denvaxia, Dengue Vaccine Terry Nolan SAGE, Co-Chair Dengue Vaccine WG WG Considerations A number of dimensions: Population benefit versus individual risk Ethical considerations
Surveillance for encephalitis in Bangladesh: preliminary results
 Surveillance for encephalitis in Bangladesh: preliminary results In Asia, the epidemiology and aetiology of encephalitis remain largely unknown, particularly in Bangladesh. A prospective, hospital-based
Surveillance for encephalitis in Bangladesh: preliminary results In Asia, the epidemiology and aetiology of encephalitis remain largely unknown, particularly in Bangladesh. A prospective, hospital-based
67% 65% 91% Borno State Integrated Disease Surveillance and Response (IDSR) Nigeria Emergency Response. Cumulative Low risk Moderate risk
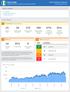 Borno State Integrated Disease Surveillance and Response (IDSR) Nigeria Emergency Response W34 218 (Aug 2-Aug 26) Table of Contents A. Key indicators B. Indicator-based surveillance C. System performance
Borno State Integrated Disease Surveillance and Response (IDSR) Nigeria Emergency Response W34 218 (Aug 2-Aug 26) Table of Contents A. Key indicators B. Indicator-based surveillance C. System performance
IMMUNIZATION & PHYSICAL FORM CELOP
 Boston University Student Health Services 881 Commonwealth Ave 1 st floor WEST Boston, MA 02215 Phone: (617) 353 3575 IMMUNIZATION & PHYSICAL FM CELOP BU Student ID #: Necessary for all students U PLEASE
Boston University Student Health Services 881 Commonwealth Ave 1 st floor WEST Boston, MA 02215 Phone: (617) 353 3575 IMMUNIZATION & PHYSICAL FM CELOP BU Student ID #: Necessary for all students U PLEASE
WHO Polio Eradication Programme UK National Survey of Polioviruses
 E WHO Polio Eradication Programme UK National Survey of Polioviruses A Paper submitted to the Health and Safety Committee Meeting, 17 th April 2008, by Alastair Reid. In September 2001, the University
E WHO Polio Eradication Programme UK National Survey of Polioviruses A Paper submitted to the Health and Safety Committee Meeting, 17 th April 2008, by Alastair Reid. In September 2001, the University
Zika Virus Guidance for Medical Providers. Denise Smith, PHN, MPA Director of Disease Control Kern County Public Health Services Department
 Zika Virus Guidance for Medical Providers Denise Smith, PHN, MPA Director of Disease Control Kern County Public Health Services Department Kern Perinatal Symposium March 3, 2017 CME DISCLOSURE The Planners,
Zika Virus Guidance for Medical Providers Denise Smith, PHN, MPA Director of Disease Control Kern County Public Health Services Department Kern Perinatal Symposium March 3, 2017 CME DISCLOSURE The Planners,
The Polio Endgame
 The Polio Endgame 2013-2018 context the Endgame Plan implications for DCVMN Context 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007
The Polio Endgame 2013-2018 context the Endgame Plan implications for DCVMN Context 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007
New Indian Model School Al Garhoud Dubai
 New Indian Model School Al Garhoud Dubai 1]. Section-A Name of Policy Writer Dr.Hala Najib Designation School Doctor Date of Policy Revision 27/3/18 Date of Next Revision 28/3/18 Policy Code Clinic/IM/1819/01
New Indian Model School Al Garhoud Dubai 1]. Section-A Name of Policy Writer Dr.Hala Najib Designation School Doctor Date of Policy Revision 27/3/18 Date of Next Revision 28/3/18 Policy Code Clinic/IM/1819/01
Quantitative Immunization and Vaccines Related Research Advisory Committee (QUIVER) Alan R. Hinman, MD, MPH SAGE meeting October 27, 2009
 Quantitative Immunization and Vaccines Related Research Advisory Committee (QUIVER) Alan R. Hinman, MD, MPH SAGE meeting October 27, 2009 Outline QUIVER Composition Draft recommendations from QUIVER meeting
Quantitative Immunization and Vaccines Related Research Advisory Committee (QUIVER) Alan R. Hinman, MD, MPH SAGE meeting October 27, 2009 Outline QUIVER Composition Draft recommendations from QUIVER meeting
PLANNING & EVALUATION WORKBOOK
 25 April-2 May 2015 PLANNING & EVALUATION WORKBOOK WEB: www.paho.org/vwa FACEBOOK: https://www.facebook.com/pahowho TWITTER: https://twitter.com/pahowho VWA 2015 PLANNING INTRODUCTION Originally launched
25 April-2 May 2015 PLANNING & EVALUATION WORKBOOK WEB: www.paho.org/vwa FACEBOOK: https://www.facebook.com/pahowho TWITTER: https://twitter.com/pahowho VWA 2015 PLANNING INTRODUCTION Originally launched
PLANNING & EVALUATION WORKBOOK. WEB: FACEBOOK:
 PLANNING & EVALUATION WORKBOOK WEB: www.paho.org/vwa FACEBOOK: https://www.facebook.com/paho.im TWITTER: http://twitter.com/opspaho_vac INTRODUCTION Originally launched in 2003, Vaccination Week in the
PLANNING & EVALUATION WORKBOOK WEB: www.paho.org/vwa FACEBOOK: https://www.facebook.com/paho.im TWITTER: http://twitter.com/opspaho_vac INTRODUCTION Originally launched in 2003, Vaccination Week in the
Achievements in Public Health, Impact of Vaccines Universal... Children -- United States,
 1 of 6 2/10/2005 7:40 PM Weekly April 02, 1999 / 48(12);243-248 Achievements in Public Health, 1900-1999 Impact of Vaccines Universally Recommended for Children -- United States, 1990-1998 At the beginning
1 of 6 2/10/2005 7:40 PM Weekly April 02, 1999 / 48(12);243-248 Achievements in Public Health, 1900-1999 Impact of Vaccines Universally Recommended for Children -- United States, 1990-1998 At the beginning
2016 NON FINANCIAL RESOURCE REQUIREMENTS GPEI DONOR CONTRIBUTIONS
 2016 NON FINANCIAL RESOURCE REQUIREMENTS GPEI DONOR CONTRIBUTIONS Background The Global Polio Eradication Initiative (GPEI) is financed through a range of public and private donations. The Financial Resource
2016 NON FINANCIAL RESOURCE REQUIREMENTS GPEI DONOR CONTRIBUTIONS Background The Global Polio Eradication Initiative (GPEI) is financed through a range of public and private donations. The Financial Resource
Acute neurological syndromes
 Acute neurological syndromes Assoc.Prof. Murat Sayan Kocaeli Üniversitesi, Rutin PCR Lab. Sorumlu Öğt.Üyesi Yakın Doğu Üniversitesi, DESAM Kurucu Öğrt. Üyesi sayanmurat@hotmail.com 0533 6479020 Medical
Acute neurological syndromes Assoc.Prof. Murat Sayan Kocaeli Üniversitesi, Rutin PCR Lab. Sorumlu Öğt.Üyesi Yakın Doğu Üniversitesi, DESAM Kurucu Öğrt. Üyesi sayanmurat@hotmail.com 0533 6479020 Medical
Cumulative vaccination coverage, Japanese encephalitis (JE), Stage I JE vaccination, Nationwide survey, Random sampling
 Research and Reviews Cumulative Vaccination Coverage for the 1st, 2nd, and Booster Doses of Stage I Japanese Encephalitis Vaccination in Japan: Results of Year 2009 Nationwide Survey JMAJ 54(3): 186 190,
Research and Reviews Cumulative Vaccination Coverage for the 1st, 2nd, and Booster Doses of Stage I Japanese Encephalitis Vaccination in Japan: Results of Year 2009 Nationwide Survey JMAJ 54(3): 186 190,
VERSION APPROVAL PROCESS NUMBER 1.0 Nina Schwalbe, Managing Director, Policy and Performance
 Version No.: 1.0 Page 1 / 5 DOCUMENT ADMINISTRATION VERSION APPROVAL PROCESS DATE NUMBER 1.0 Nina Schwalbe, Managing Director, and Performance Reviewed by: GAVI Programme 23 April 2012 and Committee Approved
Version No.: 1.0 Page 1 / 5 DOCUMENT ADMINISTRATION VERSION APPROVAL PROCESS DATE NUMBER 1.0 Nina Schwalbe, Managing Director, and Performance Reviewed by: GAVI Programme 23 April 2012 and Committee Approved
Title: Revision to the Standardized Surveillance and Case Definition for Acute Flaccid Myelitis
 17-ID-01 Committee: Infectious Disease Title: Revision to the Standardized Surveillance and Case Definition for Acute Flaccid Myelitis I. Statement of the Problem Acute flaccid myelitis (AFM) is a syndrome
17-ID-01 Committee: Infectious Disease Title: Revision to the Standardized Surveillance and Case Definition for Acute Flaccid Myelitis I. Statement of the Problem Acute flaccid myelitis (AFM) is a syndrome
CIDRAP Leadership Forum Infectious Disease BRIEFING
 Center for Infectious Disease Research and Policy University of Minnesota CIDRAP Leadership Forum Infectious Disease BRIEFING August 22 nd, 2018 CLF BRIEFING HOT TOPICS 1. Ebola 2. Foodborne diseases 3.
Center for Infectious Disease Research and Policy University of Minnesota CIDRAP Leadership Forum Infectious Disease BRIEFING August 22 nd, 2018 CLF BRIEFING HOT TOPICS 1. Ebola 2. Foodborne diseases 3.
Global Polio Eradication, Progress, Impact of Ebola, Risks & Opportunities. Polio Partners Group 8 December 2014
 Global Polio Eradication, Progress, Impact of Ebola, Risks & Opportunities Polio Partners Group 8 December 2014 Outline Progress Impact of Ebola Priorities Risks Wild Poliovirus type 1 (WPV1) Cases, 2013
Global Polio Eradication, Progress, Impact of Ebola, Risks & Opportunities Polio Partners Group 8 December 2014 Outline Progress Impact of Ebola Priorities Risks Wild Poliovirus type 1 (WPV1) Cases, 2013
Summary of the Meeting of the Strategic Advisory Group of Experts on immunization, April 2018
 Summary of the Meeting of the Strategic Advisory Group of Experts on immunization, 17-18 April 2018 The Strategic Advisory Group of Experts (SAGE) on Immunization 1 met on 17 18 April 2018. This full report
Summary of the Meeting of the Strategic Advisory Group of Experts on immunization, 17-18 April 2018 The Strategic Advisory Group of Experts (SAGE) on Immunization 1 met on 17 18 April 2018. This full report
Update on Status of Wild Poliovirus Outbreak in Kenya. 9 th Meeting of the IMB 1-3 October 2013 London, UK
 Update on Status of Wild Poliovirus Outbreak in Kenya 9 th Meeting of the IMB 1-3 October 2013 London, UK Contents Background Confirmation and Immediate Response High Risk Areas Coordination of Response
Update on Status of Wild Poliovirus Outbreak in Kenya 9 th Meeting of the IMB 1-3 October 2013 London, UK Contents Background Confirmation and Immediate Response High Risk Areas Coordination of Response
West Nile Virus in the Region of Peel 2002
 HUMAN CASE SURVEILLANCE Introduction Human illness caused by mosquito-borne WNV acquired in Peel occurred for the first time in 2002. In 1999, a Peel resident who had traveled to New York City acquired
HUMAN CASE SURVEILLANCE Introduction Human illness caused by mosquito-borne WNV acquired in Peel occurred for the first time in 2002. In 1999, a Peel resident who had traveled to New York City acquired
TFI Proceedings, Recommendations and implications for 2005
 January 2005 N 053 TFI 2004 - Proceedings, Recommendations and implications for 2005 The 12th meeting of the Task Force on Immunization (TFI) in Africa and the 11th meeting of the Africa Regional Inter-
January 2005 N 053 TFI 2004 - Proceedings, Recommendations and implications for 2005 The 12th meeting of the Task Force on Immunization (TFI) in Africa and the 11th meeting of the Africa Regional Inter-
Message from. Dr Samlee Plianbangchang Regional Director, WHO South-East Asia. At the. Regional Review Meeting on Immunization
 Message from Dr Samlee Plianbangchang Regional Director, WHO South-East Asia At the Regional Review Meeting on Immunization 19-23 July 2010 WHO/SEARO, New Delhi Regional Review Meeting on Immunization
Message from Dr Samlee Plianbangchang Regional Director, WHO South-East Asia At the Regional Review Meeting on Immunization 19-23 July 2010 WHO/SEARO, New Delhi Regional Review Meeting on Immunization
CIDRAP Leadership Forum Infectious Disease BRIEFING
 Center for Infectious Disease Research and Policy University of Minnesota CIDRAP Leadership Forum Infectious Disease BRIEFING December 13 th, 2017 CLF BRIEFING HOT TOPICS 1. Influenza (surveillance, vaccine,
Center for Infectious Disease Research and Policy University of Minnesota CIDRAP Leadership Forum Infectious Disease BRIEFING December 13 th, 2017 CLF BRIEFING HOT TOPICS 1. Influenza (surveillance, vaccine,
PART 1: General SOPs RESPONDING TO A POLIOVIRUS EVENT OR OUTBREAK STANDARD OPERATING PROCEDURES
 STANDARD OPERATING PROCEDURES A POLIOVIRUS EVENT OR OUTBREAK PART 1: General SOPs V2.1 20 April 2016 V2.2 15 August 2016 V2.3 01 May 2017 EFFECTIVE 1 MAY 2017 UNTIL 31 OCTOBER 2017 Published by the World
STANDARD OPERATING PROCEDURES A POLIOVIRUS EVENT OR OUTBREAK PART 1: General SOPs V2.1 20 April 2016 V2.2 15 August 2016 V2.3 01 May 2017 EFFECTIVE 1 MAY 2017 UNTIL 31 OCTOBER 2017 Published by the World
Modeling undetected live poliovirus circulation after apparent interruption of transmission: implications for surveillance and vaccination
 Kalkowska et al. BMC Infectious Diseases (2015) 15:66 DOI 10.1186/s12879-015-0791-5 RESEARCH ARTICLE Open Access Modeling undetected live poliovirus circulation after apparent interruption of transmission:
Kalkowska et al. BMC Infectious Diseases (2015) 15:66 DOI 10.1186/s12879-015-0791-5 RESEARCH ARTICLE Open Access Modeling undetected live poliovirus circulation after apparent interruption of transmission:
