SUPPLEMENT ARTICLE. S62 JID 2014:210 (Suppl 1) Alleman et al
|
|
|
- Karen Hall
- 6 years ago
- Views:
Transcription
1 SUPPLEMENT ARTICLE Factors Contributing to Outbreaks of Wild Poliovirus Type 1 Infection Involving Persons Aged 15 Years in the Democratic Republic of the Congo, , Informed by a Pre-Outbreak Poliovirus Immunity Assessment Mary M. Alleman, 1 Kathleen A. Wannemuehler, 1 William C. Weldon, 2 Jean Pierre Kabuayi, 3 Felly Ekofo, 3 Samuel Edidi, 3 Audry Mulumba, 4 Albert Mbule, 5 Renée N. Ntumbannji, 5 Tiekoura Coulibaly, 5 Nadine Abiola, 6 Minlangu Mpingulu, 6 Kassim Sidibe, 6 and M. Steven Oberste 2 1 Global Immunization Division, and 2 Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia; 3 Programme National de Lutte contre les IST/SIDA, 4 Expanded Programme on Immunization, Ministry of Public Health, 5 Immunization, Vaccines, and Emergencies Cluster, World Health Organization, and 6 Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Kinshasa, Democratic Republic of the Congo Background. The Democratic Republic of the Congo (DRC) experienced atypical outbreaks of wild poliovirus type 1 (WPV1) infection during in that they affected persons aged 15 years in 4 (Bandundu, Bas Congo, Kasaï Occidental, and Kinshasa provinces) of the 6 provinces with outbreaks. Methods. Analyses of cases of WPV1 infection with onset during by province, age, polio vaccination status, and sex were conducted. The prevalence of antibodies to poliovirus (PV) types 1, 2, and 3 was assessed in sera collected before the outbreaks from women attending antenatal clinics in 3 of the 4 above-mentioned provinces. Results. Of 193 cases of WPV1 infection during , 32 (17%) occurred in individuals aged 15 years. Of these 32 cases, 31 (97%) occurred in individuals aged years; 9 (28%) were notified in Bandundu, 17 (53%) were notified in Kinshasa, and 22 (69%) had an unknown polio vaccination status. In the seroprevalence assessment, PV type 1 and 3 seroprevalence was lower among women aged years in Bandundu and Kinshasa, compared with those in Kasaï Occidental. Seropositivity to PVs was associated with increasing age, more pregnancies, and a younger age at first pregnancy. Conclusions. This spatiotemporal analysis strongly suggests that the outbreaks of WPV1 infection affecting young adults were caused by a PV type 1 immunity gap in Kinshasa and Bandundu due to insufficient exposure to PV type 1 through natural infection or vaccination. Poliovirus immunity gaps in this age group likely persist in DRC. Keywords. polio eradication; polio seroprevalence; neutralizing antibodies; Democratic Republic of the Congo; Africa; wild poliovirus outbreaks; polio in adults. Prior to 2001, wild poliovirus (WPV) transmission was endemic in the Democratic Republic of the Congo (DRC) [1 15]. Case-based acute flaccid paralysis Correspondence: Mary M. Alleman, PhD, Global Immunization Division, Centers for Disease Control and Prevention, 1600 Clifton Rd, NE, MS A-04, Atlanta, GA (mea4@cdc.gov). The Journal of Infectious Diseases 2014;210(S1):S62 73 Published by Oxford University Press on behalf of the Infectious Diseases Society of America This work is written by (a) US Government employee(s) and is in the public domain in the US. DOI: /infdis/jiu282 (AFP) surveillance for polio, in which each suspected polio case is investigated with collection and analysis of stool specimens, was not established nationwide in DRC until 1999 or later; however, historical reports of WPV transmission, cases of infection, and outbreaks of infection are available [1 16]. In those reports, most cases of WPV infection in which the age of the infected individual was documented occurred in individuals <5 years old; documentation of cases in individuals aged 15 years existed but was rare [4, 6, 7, 9,14]. WPV transmission persisted in DRC despite the inclusion of 3 S62 JID 2014:210 (Suppl 1) Alleman et al
2 doses of trivalent oral polio vaccine (topv) in the country s Expanded Programme on Immunization (EPI), which was initiated in 1977 (Figure 1) [1 12, 17 19]. National-level World Health Organization (WHO)/United Nations Children s Fund (UNICEF) coverage estimates for the third dose of topv (OPV3) during ranged from 15% in 1980 to 41% in 1988; other reports suggest that OPV3 coverage was poor and not uniform geographically across the DRC during these years (Figure 1)[1 7, 9, 11, 12, 17 20]. OPV3 coverage estimates remained <50% until 2004, when coverage began increasing, reaching 78% by 2011 [20]. In line with strategies of the Global Polio Eradication Initiative (GPEI), supplemental immunization activities (SIAs) with topv, in the form of subnational immunization days, began in 1996 in DRC (Figure 1) [1 3, 5, 11, 12, 21]. The first national immunization day (NID) was conducted in 1999, followed by others in ; after those years, NIDs were not conducted until 2011 [2 4, 12, 21 23]. These SIAs targeted children aged <5 years with topv through 2005, after which other poliovirus-containing vaccines became available and were used, and total populations were vaccinated (in 2011 and later) according to the particular epidemiological situation [2, 3, 21 23]. After the initiation of SIAs, there was a period ( ) with no confirmed cases of WPV infection in DRC, and the interruption of WPV transmission was assumed (Figure 1) [4, 23 32]. DRC s last case of WPV type 2 (WPV2) infection was documented in 1997 [4]. A series of WPV importations from Angola during led to outbreaks of WPV type 1 (WPV1) and WPV type 3 (WPV3) infections that lasted through 2011 [31 44]. In combined, there were 58 and 4 cases of WPV1 and WPV3 infection, respectively [45]. Of these 62 cases, 100% occurred in individuals aged <15 years. The last case of WPV3 Figure 1. Historical time line of implementation of polio eradication strategies in the Democratic Republic of the Congo, with national-level oral polio vaccine 3 (OPV3) coverage estimates (OPV3 WHO/UNICEF national-level coverage estimates are not available for years prior to 1980) and indication of birth years relative to the collection of blood ( ) used in the poliovirus sero-prevalence assessment and to the occurrence of wild poliovirus type 1 (WPV1) cases aged years during the WPV1 outbreaks. Abbreviations: AFP, acute flaccid paralysis; DRC, Democratic Republic of the Congo; EPI, Expanded Programme on Immunization; NID, national immunization day; WPV2, wild poliovirus type 2; WPV3, wild poliovirus type 3; WHO, World Health Organization; UNICEF, United Nations Children s Fund; y, year. Factors Contributing to Outbreaks of WPV1 Infection in DRC JID 2014:210 (Suppl 1) S63
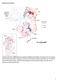
3 infection in the DRC had paralysis onset in June 2009 [45]. During the outbreaks of WPV1 infection, a higher than usual percentage of cases was notified among persons aged 15 years in Bandundu, Bas Congo, Kasaï Occidental, and Kinshasa provinces [46, 47]. To better understand the outbreaks and the susceptibility to WPV infection among individuals aged 15 years, the outbreak was analyzed, and a polio seroprevalence assessment was undertaken in Bandundu, Kasaï Occidental, and Kinshasa provinces. The results are presented in this report. METHODS Review of Epidemiological Data AFP cases reported to DRC s Ministry of Public Health and WHO-DRC with symptom onset during 1 January December 2011 were reviewed for WPV and compatible cases by geographic location, age, sex, and polio vaccination status. AFP cases were classified per definitions in the WHO African Region AFP surveillance guidelines [16]. Poliovirus Seroprevalence Assessments Titers of neutralizing antibodies against poliovirus (PV) types 1 (PV1), 2 (PV2), and 3 (PV3) were measured in sera prepared from venous blood collected during from 5942 pregnant women aged years from 7 different antenatal clinic (ANC) sites in Bandundu, Kasaï Occidental, and Kinshasa provinces (Figure 2) [48, 49]. The Binza Meteo, Boyambi, and Kingasani sites (urban sites in DRC s capital, Kinshasa) in Kinshasa province and the Vanga (rural site) and Kikwit (Kikwit city, urban site) sites in central Bandundu province were chosen because they were geographically near the location of WPV1 outbreaks in which a higher than usual percentage of cases occurred in persons aged 15 years. The remaining 2 sites, Mikalayi (rural site) and Tshikapa (Tshikapa city, urban site) both in Kasaï Occidental province, were Figure 2. Cases of wild poliovirus type 1 (WPV1) infection, and approximate location of antenatal clinic (ANC) sites where blood specimens were collected from persons in the seroprevalence assessment population, by zone de santé (health zone) and province, Democratic Republic of the Congo. One symbol represents 1 case of WPV1 infection. Symbols are drawn at random within the zones de santé. Boundaries of zones de santé are not shown except in the Kinshasa province inset. The geographic locations of the ANC sites are approximate and based on the zones de santé in which they are located. S64 JID 2014:210 (Suppl 1) Alleman et al
4 geographically near outbreaks of WPV1 infection in which there were cases in the older age group ( 15 years of age), but the percentage of cases in the older age group was relatively low compared to Kinshasa and Bandundu provinces. The seroprevalence assessment protocol was finalized prior to the onset of most WPV1 cases in persons aged 15 years in Bas Congo; thus, this province was not included. The sera were from 6615 original blood samples collected in the 7 ANC sites as part of nationwide sentinel site human immunodeficiency virus (HIV) seroprevalence surveys [48, 49]. From the 6615 samples, those for which the DRC National HIV Program laboratory had sufficient volume of sera (approximately 500 μl) were used for the PV antibody assessments; the 5942 sera analyzed for this report represent 90% of the 6615 samples. Delinked demographic attributes were available for each woman from whom blood was collected; only those attributes considered relevant to PV immunity were analyzed for associations with PV seroprevalence [48, 49]. Sera were shipped from Kinshasa to the Centers for Disease Control and Prevention (CDC-Atlanta) via air transport on dry ice and were thereafter stored at 20 C until antibody assessments were performed at CDC-Atlanta s Poliovirus and Picornavirus Laboratory according to previously described methods [50, 51]. Sera with 50% end point titers of 1:8 were considered seropositive [50, 51]. Data Analyses Seroprevalence for each PV type was calculated by dividing the number of seropositive sera in the stratum of interest by the total number of sera in that stratum. The seroprevalence estimates represent seroprevalence within the ANC assessment population only and are not representative of any DRC populations at large. Data analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, NC); Epi Info, version 7 (CDC-Atlanta, Atlanta, GA); and Excel, version 2010 (Microsoft, Redmond, WA). Figure 2 was created using ArcGIS, version 10.1 (Environmental Systems Research Institute, Redlands, CA). The Cochran-Armitage test for trend was used to test for linear trends in PV seroprevalence across substrata of increasing age, overall and by ANC site. The Cochran-Mantel-Haenszel test was used to assess the association between demographic attributes and PV seroprevalence while controlling for age or province, as indicated in the Results section. The test of binomial proportions was used to determine whether sex percentages among cases of WPV1 infection were different than 50%; the ratio of males to females is approximately 1:1 in DRC in the age groups of interest in this analysis [52]. Tests were considered statistically significant at P values of <.05. Ethical Approval The Human Subjects Research Coordinator at the CDC-Atlanta National Center for Immunization and Respiratory Diseases reviewed the seroprevalence assessment protocol. It was determinedtobeaprogramevaluationinresponsetothe outbreaks of WPV1 infection and was therefore exempt from requiring institutional review board approval. The protocol was reviewed and approved by DRC s Ministry of Public Health. RESULTS In 2010 and 2011, there were outbreaks of 100 (in 5 provinces) and 93 (in 6 provinces) cases of WPV1 infection, respectively, in DRC (Table 1 and Figure 2). Combining the 2 years, there were 32 cases among individuals aged 15 years in Bandundu, Bas Congo, Kasaï Occidental, and Kinshasa provinces; more specifically, 21%, 16%, 3%, and 50% of all cases with known age in these provinces, respectively, were in this older age group [46].Theagerangeofthe32caseswas16 31 years, with 31 of 32 (97%) aged years; no deaths among these cases were documented. In 2011 alone, 29% of 93 cases of WPV1 infection nationally were aged 15 years. Comparing the provinces, Kinshasa had the highest annual percentage of cases of WPV1 infection in this older age group in 2011 (17 of 33 total cases [52%]; Table 1). Sixteen (94%) of the 17 cases were aged years (Figure 3A). All 17 presented in zones de santé (ZS; health zones) within Kinshasa city (Figure 2). Nine reported an unknown OPV vaccination status; of the 8 reporting vaccination, 75% had received 3 OPV doses. In addition to the confirmed cases of WPV1 infection in Kinshasa, there were 16 polio-compatible cases in , 7 (44%) of which involved individuals aged 15 years. Of these 7, 5 (71.4%) were aged years (data not shown). In Bandundu, among the 9 cases of WPV1 infection aged 15 years in , 100% were aged years (Table 1 and Figure 3B). Seven of 9 presented in ZS in the general vicinity of the Vanga and Kikwit ANC sites in central Bandundu, and 1 of the 9 had a known vaccination status reporting 0 OPV doses (Table 1 and Figure 2). In Bas Congo, the 4 cases of WPV1 infection aged 15 years in 2011 were all years of age (data not shown); 1 of the 4 had a known vaccination status (0 OPV doses reported), and the individuals presented in ZS scattered throughout the province (Table 1 and Figure 2). Two of 67 cases of WPV1 infection in Kasaï Occidental were aged 15 years. The adult cases involved individuals aged 18 (2010) and 25 years (2011), both had unknown vaccination status, with one presenting in a ZS in the same region of the province as the Mikalayi ANC site and the other presenting in a ZS relatively far from the Mikalayi and Tshikapa ANC sites (Table 1, Figure 2, and Figure 3C). At the national level, in , among cases of WPV1 infection aged <15 years and 15 years, 48% and 66% were males, respectively (Table 1). These percentages were not statistically significantly different from the expected percentage of males in these age groups in the population (ie, 50%) [52]. Factors Contributing to Outbreaks of WPV1 Infection in DRC JID 2014:210 (Suppl 1) S65
5 Table 1. Characteristics of Cases of Wild Poliovirus Type 1 (WPV1) Infection Nationally and by Province, Age Category, Oral Polio Vaccination Status, and Sex, , Democratic Republic of the Congo (DRC) Province Bas Kasaï Characteristic, by Year(s) National Bandundu Congo Occidental Katanga Kinshasa Maniema 2010 Cases, no. 100 a 23 a Cases in persons aged <15 y, no NA Cases in persons aged 15 y, no NA Cases in persons with unknown age, no. 2 a 2 a NA NA NA NA NA Cases in persons aged 15 y, % NA 2011 Cases, no Cases in persons aged <15 y, no Cases in persons aged 15 y, no Cases in persons aged 15 y, % combined Cases in persons aged 15 y, % 17 a 21 a Among cases in persons with age 15 y, those with NA 47 NA known vaccination status, % Among cases in persons with age 15 y and with known NA NA 75 NA vaccination status, those reported to have received 3 OPV doses, % Cases in persons aged <15 y who were male, % 48 b Cases in persons aged 15 y who were male, % 66 b NA 65 NA In , there were no cases of wild poliovirus infection in the DRC s remaining 5 provinces (ie, Equateur, Kasaï Oriental, Province Orientale, Nord Kivu, and Sud Kivu). Abbreviations: NA, not applicable; OPV, oral polio vaccine. a Two of the 23 cases from Bandundu had missing data on age. Of the 2, 1 was a female, and the other s sex was unknown. b P >.05, for the test of binomial proportions that the percentage is different than 50%. The percentages of males among cases in these age categories by province were too few to determine whether sex-specific percentages were statistically significantly different than 50% (Table 1 and Figures 3A C). Sera from blood specimens collected during from pregnant women attending ANC sites in Bandundu, Kasaï Occidental, and Kinshasa provinces were assessed for neutralizing antibodies against PV1, PV2, and PV3 (Figures 2 and 3). Selected demographic attributes of these women are described in Table 2. Of note, >99% of the women from each of the 3 sites in Kinshasa province declared residence in towns, consistent with the location of those ANC sites within Kinshasa city. For 6 of 7 sites, the highest percentage of women was in the years of age category at the time of blood collection; in Binza Meteo, the highest percentage was in the next highest age category. In all sites, 75% of women had their first pregnancy prior to age 24 years, and in all sites except those in Binza Meteo and Boyambi, 50% of women had been pregnant 3 times. HIV seroprevalence ranged from 0.8% to 3.2% in the various sites. The prevalence of neutralizing antibodies to PV1, PV2, and PV3 among the overall assessment population by age category is provided in Table 3; 73.8% of the women were seropositive for all 3 PV types (data not shown). For each PV type, there was a statistically significant increase in seroprevalence with increasing category of age. PV1 seroprevalence for all age categories was >95% in Tshikapa; >85% in Kingasani, Mikalayi, and Vanga; >75% in Boyambi and Kikwit; and >65% in Binza Meteo (Figures 3A C). In all sites, by age 30 years, PV1 seroprevalence was 94%, and, excepting Tshikapa, there was a statistically significant increase in seroprevalence with increasing age category (P <.05 for all sites except Tshikapa, where P =.12; Figures 3A C). PV2 seroprevalence was >90% for all age categories in all sites except Boyambi and Binza Meteo (Figures 3D F ). In all sites except Boyambi and Binza Meteo, by 30 years of age, seroprevalence was 96%, and in all sites except Boyambi and Kikwit, there was a statistically significant increase in seroprevalence with increasing age category (P <.05 for all sites except Boyambi, where P =.39, and Kikwit, where P =.06). PV3 seroprevalence was >70% for all age categories in all sites in Bandundu and Kasaï Occidental and in the Kingasani site S66 JID 2014:210 (Suppl 1) Alleman et al
6 Figure 3. Cases of wild poliovirus type 1 (WPV1) infection, , by age, sex, and province, and seroprevalence to polioviruses type 1 (PV1), 2 (PV2), and 3 (PV3) in the seroprevalence assessment population, by age and antenatal clinic site in Kinshasa (A, D, and G), Bandundu (B, E, and H ), and Kasaï Occidental (C, F, and I) provinces, Democratic Republic of the Congo. In all graphs, the secondary y-axis indicating seroprevalence is truncated and starts at 50%. Horizontal gridlines are associated with the secondary axis (seroprevalence values) and not the primary axis (cases of WPV1 infection). (Figures 3G I). In Boyambi, seroprevalence was 70% for all age categories except for years, and in Binza Meteo, it was 59% for all age categories. In Mikalayi and Vanga, by age 30 years, prevalence was 94%, whereas in Binza Meteo and Boyambi, by age 30 years, the seroprevalence was <80%. For all sites, there was a statistically significant increase in seroprevalence with increasing age category (P <.05). The associations of demographic attributes with seroprevalence to PV1, PV2, and PV3 are presented in Table 3. Inthe assessment population overall, after controlling for age at the time of blood collection, there was a statistically significant association between being of younger age during the first pregnancy and being seropositive for PV2 and PV3. Controlling for province, there was a statistically significant association between a greater number of pregnancies and a higher seroprevalence for all PV types in both the year and year age groups. There was no statistically significant association between seroprevalence for HIV and seroprevalence for any PV type. DISCUSSION In , DRC had historically atypical outbreaks of WPV1 infection in that they affected numerous persons aged 15 years in 4 (Bandundu, Bas Congo, Kasaï Occidental, and Kinshasa) of the 6 provinces with outbreaks. Prior to these 2 years, nearly all documented WPV cases had been in children <15 years of age [4, 6, 7, 9, 14]. Of the 4 provinces in the 2 years combined, Kinshasa had the highest percentage of cases of WPV1 infection in this older age group, followed by Bandundu, Bas Congo, and Kasaï Occidental. The analysis of poliocompatible cases from suggests that Kinshasa s outbreak might have been of greater magnitude and affected a larger number of persons aged 15 years than previously thought [46]. DRC s response to the outbreaks included numerous SIAs conducted during with monovalent oral polio vaccine targeting PV1 (mopv1), bivalent OPV targeting PV1 and PV3 (bopv1,3), and topv [21]. The campaigns generally targeted children aged <5 years; however, Factors Contributing to Outbreaks of WPV1 Infection in DRC JID 2014:210 (Suppl 1) S67
7 Table 2. Demographic Characteristics of the Poliovirus Seroprevalence Assessment Population, Overall and by 7 Antenatal Clinic Sites, in Bandundu, Kasaï Occidental, and Kinshasa Provinces, Democratic Republic of the Congo Bandundu Province, No. (%) Kasaï Occidental Province, No. (%) Kinshasa Province, No. (%) Characteristic Overall, No. (%) (n = 5942) Kikwit (n = 954) Vanga (n = 936) Mikalayi (n = 755) Tshikapa (n = 682) Binza Meteo (n = 805) Boyambi (n = 835) Kingasani (n = 975) Residence Town 4110 (69.2) 830 (87.0) 8 (0.9) 8 (1.1) 660 (96.8) 802 (99.6) 833 (99.8) 969 (99.4) Village 1830 (30.8) 124 (13.0) 928 (99.2) 747 (98.9) 22 (3.2) 3 (0.4) 0 (0.0) 6 (0.6) Unknown 2 (0.03) 2 (0.2) Age at time of blood collection, y (16.3) 154 (16.1) 128 (13.7) 170 (22.5) 164 (24.1) 99 (12.3) 120 (14.4) 131 (13.4) (27.6) 239 (25.1) 264 (28.2) 191 (25.3) 170 (24.9) 220 (27.3) 282 (33.8) 274 (28.1) (25.5) 232 (24.3) 251 (26.8) 164 (21.7) 155 (22.7) 222 (27.6) 229 (27.4) 263 (27.0) (18.0) 197 (20.7) 162 (17.3) 121 (16.0) 110 (16.1) 150 (18.6) 132 (15.8) 197 (20.2) (12.6) 132 (13.8) 131 (14.0) 109 (14.4) 83 (12.2) 114 (14.2) 72 (8.6) 110 (11.3) Age at first pregnancy, y (33.4) 225 (23.6) 312 (33.3) 490 (64.9) 375 (55.0) 162 (20.1) 185 (22.2) 236 (24.2) (52.8) 563 (59.0) 538 (57.5) 254 (33.6) 265 (38.9) 440 (54.7) 515 (61.7) 565 (58.0) (13.8) 166 (17.4) 86 (9.2) 11 (1.5) 42 (6.2) 203 (25.2) 135 (16.2) 174 (17.9) No. of pregnancies, including current pregnancy (25.7) 272 (28.5) 182 (19.4) 145 (19.2) 146 (21.4) 244 (30.3) 249 (29.8) 291 (29.9) (20.8) 206 (21.6) 185 (19.8) 105 (13.9) 100 (14.7) 189 (23.5) 253 (30.3) 197 (20.2) (53.5) 476 (49.9) 569 (60.8) 505 (66.9) 436 (63.9) 372 (46.2) 333 (39.9) 487 (50.0) HIV serologic test result Positive 113 (1.9) 8 (0.8) 22 (2.4) 9 (1.2) 19 (2.8) 13 (1.6) 11 (1.3) 31 (3.2) Negative 5829 (98.1) 946 (99.2) 914 (97.7) 746 (98.8) 663 (97.2) 792 (98.4) 824 (98.7) 944 (96.8) Abbreviation: HIV, human immunodeficiency virus. certain SIAs targeted all ages in relevant areas of Bandundu, Bas Congo, and Kinshasa [21, 46]. No cases of WPV infection were reported from Bandundu, Bas Congo, Kasaï Occidental, or Kinshasa after the third quarter of 2011; the last case of WPV infection (due to WPV1) in the DRC had paralysis onset in December 2011 (Maniema province) [43, 44, 46, 53]. The epidemiological data from the outbreak suggest that among those aged 15 years, the age group most susceptible to WPV1 infection were young adults (ie, those aged years). In 3 of the 4 provinces (Bandundu, Bas Congo, and Kasaï Occidental), 100% of cases aged 15 years were in persons years of age; in Kinshasa, it was 94%. Overall, of the 32 infected persons aged 15 years in , only 1 was >29 years old (age, 31 years). Outbreaks of WPV1 infection affecting adults have been reported recently in other countries, such as Albania, China, Namibia, the Republic of the Congo (ROC), and Tajikistan [54 60]. The analyses of infected persons aged 15 years from Namibia, the ROC, and Tajikistan yielded results similar to those of DRC, insofar as there was a high proportion/high incidence of cases in adults aged approximately years; in Albania and China the adults were approximately years of age [54 58, 60]. The high case-fatality ratios reported among infected young adults in Albania, Namibia, and the ROC and among WPV-infected adults in general were not documented among the cases notified in DRC [10, 54, 56 58, 61 63]. Moreover, a statistically significantly higher percentage of cases in one gender versus the other was not observed among the WPV1-infected individuals aged 15 years in DRC [62]. The investigators of the Albania, China, Namibia, and ROC outbreaks concluded that the young adults affected by those outbreaks were young children at a time when routine immunization coverage with topv was sufficiently high to reduce WPV transmission, and consequently opportunities for acquiring natural immunity, yet not high enough, leaving much of the cohort unvaccinated or incompletely vaccinated because of suboptimal routine immunization coverage coupled with age-based ineligibility for polio SIAs [54 58]. Other outbreaks, affecting adults, documented prior to these were explained similarly [10]. Persons aged years in DRC at the time of the outbreaks would have been born between approximately 1980 and 1996, a span when topv was being increasingly provided through the EPI; however, OPV3 coverage was low, only occasionally approaching 40% nationally, and the provision of S68 JID 2014:210 (Suppl 1) Alleman et al
8 Table 3. Seroprevalence to Poliovirus Type 1 (PV1), PV2, and PV3, by Demographic Characteristic, for the Assessment Population Overall and Associations Controlling for Age and Province, Democratic Republic of the Congo Characteristic PV1 Seropositivity PV2 Seropositivity PV3 Seropositivity Age at time of blood collection /966 (84.7) 883/966 (91.4) 699/966 (72.4) /1640 (89.7) 1527/1640 (93.1) 1247/1640 (76.0) /1516 (93.8) 1445/1516 (95.3) 1247/1516 (82.3) /1069 (96.6) 1028/1069 (96.2) 917/1069 (85.8) /751 (97.6) 732/851 (97.5) 670/751 (89.2) Overall 5477/5942 (92.2) 5615/5942 (94.5) 4780/5942 (80.4) Cochran-Armitage trend test (for age) statistic (P) (<.0001) 6.52 (<.0001) (<.0001) Age at first pregnancy, y /1985 (92.5) 1892/1985 (95.3) 1637/1985 (82.5) /3140 (91.4) 2968/3140 (94.5) 2511/3140 (80.0) /817 (94.4) 755/817 (92.4) 632/817 (77.4) CMH statistic (P), controlling for 2 categories of age at time 4.54 (.10) (<.0001) (<.0001) of blood collection a No. of pregnancies, including current pregnancy, by age y /1304 (85.2) 1179/1304 (90.4) 917/1304 (70.3) 2 675/763 (88.5) 724/763 (94.9) 585/763 (76.7) 3 503/539 (93.3) 507/539 (94.1) 444/539 (82.4) CMH statistic (P), controlling for province (.0002) (.001) (.0001) y 1 201/218 (92.2) 193/218 (88.5) 156/218 (71.6) 2 419/451 (92.9) 419/451 (92.9) 342/451 (75.8) /1916 (95.8) 1861/1916 (97.1) 1666/1916 (87.0) CMH statistic (P), controlling for province 6.95 (.031) (<.0001) (<.0001) HIV serologic test result Positive 108/113 (95.6) 106/113 (93.8) 91/113 (80.5) Negative 5369/5829 (92.1) 5509/5829 (94.5) 4689/5829 (80.4) CMH statistic (P), controlling for 2 categories of age at time of blood collection a 1.61 (.20) 0.15 (.69) (.93) Data are no. of women seropositive for the specified PV type/total no. in stratum (%), unless otherwise indicated. Trends and associations are considered statistically significant at a P value of <.05. Abbreviations: CMH, Cochran-Mantel-Haenszel; HIV, human immunodeficiency virus. a Ages y and 25 y. routine health services was not uniform geographically across DRC (Figure 1)[1, 4 7, 9, 11, 12, 17 20]. While WPV transmission remained endemic during those years and outbreaks were documented, it is possible that the increasing use of topv, despite low coverage, had the impact of gradually reducing transmission, leading to fewer opportunities for immunity through natural infection [1, 6 12]. The only additional opportunity for immunization for the majority of these persons would have been through exposure to topv excreted by vaccinated contacts [63]. Only the very youngest persons in this cohort (those born during 1991 or later) would have been eligible for polio subnational immunization days and NIDs and only until they were aged 5 years (Figure 1) [1 3, 5, 11, 12, 21]. It is thus plausible, based on the country s history of WPV transmission and polio vaccination, that the young adult population in certain of DRC s provinces was susceptible to WPV1 infection in because of lack of exposure to WPV transmission combined with insufficient exposure to OPV. The results of the PV seroprevalence assessment in Bandundu, Kasaï Occidental, and Kinshasa support this hypothesis and suggest that susceptibility might have differed geographically. More specifically, in the assessment population, in the Binza Meteo and Boyambi sites in Kinshasa city and in the Kikwit site in Kikwit city, PV1 seroprevalence was <80% in year-olds and <90% in year olds, notably lower than in the same age groups in the other 4 sites. Of the 4, of note are Factors Contributing to Outbreaks of WPV1 Infection in DRC JID 2014:210 (Suppl 1) S69
9 the 2 sites in Kasaï Occidental where the PV1 seroprevalence was nearly 90% in all age groups. The spatiotemporal overlap of low PV1 seroprevalence in the young adult women attending ANC clinics and numerous cases of WPV1 infection in persons in the same age range strongly suggests the presence of a subpopulation of young adults susceptible to WPV1 infection in Kinshasa and Bandundu provinces at the time of the outbreaks. Similarly, a spatiotemporal relationship between low polio vaccination coverage and high numbers of cases of WPV1 infection in the young adult population was observed in Pointe Noire, ROC, when a cross-sectional vaccination survey was conducted among young adults just after that outbreak [58]. In contrast are findings from Kasaï Occidental, where there was a spatiotemporal overlap of high PV1 seroprevalence among young adult women attending ANC clinics and few cases of WPV1 infection among young adults. Unfortunately, the polio vaccination status of 69% of the cases of WPV1 aged 15 years in the DRC s outbreaks was unknown, preventing analysis of individual susceptibility; this is a limitation of the analysis. In developing countries, it is estimated that immunity to PV1 needs to be at least 97% to prevent outbreaks [6, 63]. The seroprevalence assessment also provides an indication of PV1 immunity in women attending ANC clinics aged 30 years and of PV2 and PV3 immunity in all age groups. For the older women, born between approximately 1960 and 1978, seroprevalence to PV1 and PV3 was, with few exceptions, significantly higher statistically than in the younger women. Since only the youngest among the women aged 30 years (those born 1977 or later) would have been eligible for topv as infants through the EPI, the vast majority of these older women obtained PV immunity through natural infection in childhood, when PV was highly endemic in DRC, and/or through up to 30 years of contact exposure to OPV, boosted once polio SIAs were initiated (Figure 1) [1 5, 11, 12, 17, 21, 63]. The concept of obtaining PV immunity either through asymptomatic exposure to WPV or through OPV contact exposure is supported by the findings from this seroprevalence assessment in that, for all 3 PVs, seropositivity was associated with having had a greater number of pregnancies (a potential proxy for having more living children and, thus, greater doses and frequency of contact exposure) and that, for PV2 and PV3, seropositivity was associated with younger age at first pregnancy (a potential proxy for a longer duration of contact exposure) [63]. PV2 seroprevalence was uniformly high across all ages and most ANC sites (excepting Binza Meteo and Boyambi), consistent with exposure to WPV2 by older women and with high transmissibility and the high rate of seroconversion after exposure to PV2-containing OPV, even in the context of DRC s low vaccination coverage [63, 64]. Thus, there can be high PV2 seropositivity in a population simultaneous with lower seroprevalence to PV1 and PV3 [63]. Relative to PV1 and PV2, seroprevalence to PV3 was lower across most age groups in the assessment population; particularly noteworthy was the lowest PV3 seroprevalence in the 3 Kinshasa ANC sites and the Kikwit ANC site. The PV3 seroprevalence results reflect the lower immunogenicity of PV3 and could be used as a proxy indicator of exposure in the assessment population to topv, mopv3, or WPV3 [21, 45, 60, 63]. The assessment showed no differences in PV seroprevalence in women with HIV-positive results of serological tests, compared with women with HIV-negative results. There are few publications describing PV seroprevalence in HIV-seropositive African adults. A recent polio seroprevalence assessment in women attending ANC clinics during 2010 in Namibia, where the HIV prevalence among adults is higher than that in DRC, reports that among HIV-seropositive women there was lower seroprevalence for PV1 and PV3 [65]. Additional studies are needed in countries with high HIV seroprevalence to fully assess the relationship and to inform the GPEI. This analysis has a number of limitations. First, it is possible that in years prior to the outbreak, although cases of WPV infection occurred in persons aged 15 years, they were less likely to be identified, notified, and documented than cases in younger persons. It is only since 2011 that the DRC s AFP surveillance case definition was expanded from its focus on persons <15 years of age to specifically mention clinical signs in adults [66]. However it should be noted that the previous AFP case definition had always accounted for persons of any age in which a clinician suspected polio and that cases in persons aged 15 years, although few, had been documented in 2000, indicating a certain level of awareness that polio could occur in this older age group [4, 16]. Further, community-based active WPV case search conducted just after a 1995 outbreak of nearly 500 cases of WPV infection in the city of Mbuji Mayi revealed no adult cases [9]. Second, it is assumed that during the outbreaks, AFP surveillance was being conducted with equal rigor across all geographic areas of DRC and that the cases of WPV1 infection notified were a reflection of the true trends of cases in terms of absolute numbers, age, sex, and other relevant epidemiologic factors. Finally, the sera used in the seroprevalence assessment were from pregnant women voluntarily seeking antenatal care in certain geographic areas and were not representative of DRC s population at large; moreover, the women might have been more likely than males to be seropositive for PV because of greater contact with children excreting WPV or OPV [63]. Unfortunately, sera from adult males from this assessment period were not available. Data regarding the lifetime residential history of the women in the seroprevalence assessment would have been useful for more in-depth analyses of the lower PV seroprevalence in Kinshasa and Bandundu relative to that in Kasaï Occidental. In summary, this report describes a rare opportunity to obtain an indication of PV immunity in a large young adult S70 JID 2014:210 (Suppl 1) Alleman et al
10 population prior to outbreaks of WPV infection affecting young adults in the same geographic area. The results strongly suggest that the outbreaks of WPV1 infection affecting young adults in Kinshasa and Bandundu provinces could be explained by a PV1 immunity gap caused by lack of exposure to WPV and suboptimal exposure to OPV. The outbreak and the consequent vaccination response with mopv1, bopv1,3, and topv targeting all ages in certain areas might have narrowed the gap. However, susceptibility likely remains in young adults in DRC s other provinces. Except under exceptional circumstances, adults are rarely targeted for vaccination through polio eradication activities. Thus, the next best strategy to prevent future outbreaks in any age group is to attain and maintain the highest polio vaccination coverage possible among children, which will simultaneously boost immunity in contacts when OPV is used. While OPV3 coverage has improved in DRC during recent years and SIAs have been prevalent, not all children receive the minimum required doses. Thus, until OPV3 coverage is consistently sufficiently high across DRC, SIAs will be needed to build and maintain population immunity and to reduce the risk of widespread PV circulation should an introduction occur. A Demographic and Health Survey has just been conducted in DRC, during which blood specimens were obtained from a representative sample of children aged 6 months to 5 years from all provinces for PV neutralizing antibody assessments. The results of this survey will be extremely informative for guiding future polio eradication strategies in DRC. Moreover, the country should continue to include adults in AFP surveillance. These should be considerations for all countries with a history of polio epidemiology and vaccination similar to that of DRC. Notes Acknowledgments. We thank Luca Flamigni and Hypolite Sadiki, formerly, and Rogers Ngalamulume, currently, of the Division of Global HIV/ AIDS, CDC (Kinshasa, DRC), for facilitating the collaboration with DRC s Programme National de Lutte contre les IST/SIDA (PNLS); the technical staff of the PNLS laboratory who prepared the serum samples used in the seroprevalence assessment and shipped them to CDC-Atlanta; various staff of the Poliovirus and Picornavirus Laboratory at CDC-Atlanta, specifically, Michael McDonough, for assistance with the formalities for shipping the serum samples from Kinshasa to Atlanta, and Deborah Moore, Sharla McDonald, Yiting Zhang, and William Hendley, for working determinedly to perform the neutralizing antibody assessments in a short period; Brian Kaplan and Gina Marie Perleoni of the Geospatial Research Analysis and Services Program at the Agency for Toxic Substances and Disease Registry of CDC-Atlanta, for preparing the map in Figure 2; Kim Porter and Kristin Brown of CDC-Atlanta s Global Immunization Division, for assisting with the cleaning and merging of the various databases that were used in the analysis of the seroprevalence assessment; Steve Cochi, Allen Craig, Olen Kew, Melinda Mailhot, Mark Pallansch, Steve Wassilak, and Karen Wilkins, of CDC-Atlanta, and various staff of the DRC s Expanded Programme on Immunization, for invaluable comments and suggestions about the seroprevalence assessment protocol, analyses, interpretation of results, and the original version of the manuscript; and all staff of the DRC s Ministry of Public Health who work in HIV/AIDS surveillance and toward polio eradication, for their tireless efforts, often under difficult working conditions. Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Financial support. This work was supported by the Ministry of Public Health, Democratic Republic of the Congo (to J. P. K., F. E., S. E., and A. M.); the World Health Organization, Democratic Republic of the Congo (to A. M., R. N. N., and T. C.); and the Centers for Disease Control and Prevention (to M. M. A., K. A. W., W. C. W., N. A., M. M., K. S., and M. S. O.). Supplement sponsorship. This article is part of a supplement entitled The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era, which was sponsored by the Centers for Disease Control and Prevention. Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. References 1. Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication Africa, MMWR Morb Mortal Wkly Rep 1997; 46: Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication Democratic Republic of Congo, MMWR Morb Mortal Wkly Rep 2000; 49: World Health Organization. Progress towards poliomyelitis eradication, Democratic Republic of the Congo, Wkly Epidemiol Rec 2000; 75: Kabue JP, Mushiya F, Pukuta E, et al. Surveillance virologique des paralysies flasques aiguës en République Démocratique du Congo (RDC) [Acute flaccid paralysis virologic surveillance in the Democratic Republic of the Congo ]. Med Trop 2004; 64: Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication African Region, MMWR Morb Mortal Wkly Rep 1998; 47: Patriarca PA, Sutter RW, Oostvogel PM. Outbreaks of paralytic poliomyelitis, J Infect Dis 1997; 175(suppl 1):S World Health Organization. Expanded Programme on Immunization (EPI), poliomyelitis surveillance. Wkly Epidemiol Rec 1991; 66: World Health Organization. Expanded Programme on Immunization (EPI), Poliomyelitis in Wkly Epidemiol Rec 1993; 68: Lambert ML, Doussantousse S, Onadikondo L, et al. Poliomyelitis outbreak in Zaire. Lancet 1995; 346: Crawford C. Epidemics of poliomyelitis in Africa since Clin Infect Dis 2009; 48: Okwo-Bele JM, Lobanov A, Biellik RJ, et al. Overview of poliomyelitis in the African region and current regional plan of action. J Infect Dis 1997; 175(suppl 1):S Tangermann RH, Hull HF, Jafari H, et al. Eradication of poliomyelitis in countries affected by conflict. Bull World Health Organ 2000; 78: Barski G, Lépine P, Cornefert F. Recherche des anticorps neutralisants de la poliomyélite chez les Africains (noirs et pygmées) du Congo Belge [Neutralizing antibodies against poliomyelitis in pygmy and black Africans in the Belgian Congo]. Bull World Health Organ 1956; 14: De Moor J. Evolution de la poliomyélite à Léopoldville de 1951 à 1963 [Occurrence of poliomyelitis in Leopoldville, ]. Ann Soc Belg Med Trop 1965; 45: Pattyn SR, Delville JP, DeBont AF. Etude des anticorps antipoliomyélitiques dans la population congolaise de Léopoldville [Study of antipoliomyelitis antibodies in the Congolese population in Leopoldville]. Ann Soc Belg Med Trop 1957; 37: World Health Organization Regional Office for Africa. Acute flaccid paralysis surveillance field guide, January World Health Organization Regional Office for Africa. 17. ma-disu M. Role of nongovernmental agencies in vaccine delivery. Rev Infect Dis 1989; 11(suppl 3):S Factors Contributing to Outbreaks of WPV1 Infection in DRC JID 2014:210 (Suppl 1) S71
11 18. Mashako LMN, Kapongo CN, Nsibu CN, et al. Evaluation de la couverture vaccinale des enfants de moins de 2 ans à Kinshasa (Zaïre) [Evaluation of vaccination coverage among infants aged less than 2 years in Kinshasa (Zaïre)]. Arch Fr Pediatr 1992; 49: Tonglet R, Soron gane M, Lembo M, et al. Evaluation of immunization coverage at local level. World Health Forum 1993; 14: World Health Organization. WHO/UNICEF estimates of national immunization coverage, WHO-UNICEF estimates of POL3 coverage. timeseries/tscoveragepol3.html. Accessed 23 March World Health Organization, Democratic Republic of the Congo Country Office. Database of supplementary immunization activities for polio, January 1996 April World Health Organization, Democratic Republic of the Congo Country Office. 22. World Health Organization, Democratic Republic of the Congo Country Office. Activités d éradication de la poliomyélite, République Démocratique du Congo, 20 mai 2011 (Polio Eradication Activities, the Democratic Republic of the Congo, 20 May 2011). World Health Organization, Democratic Republic of the Congo Country Office. 23. Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication Angola, Democratic Republic of Congo, Ethiopia, and Nigeria, January 2000 July MMWR Morb Mortal Wkly Rep 2001; 50: Centers for Disease Control and Prevention. Progress toward global eradication of poliomyelitis, MMWR Morb Mortal Wkly Rep 2002; 51: Centers for Disease Control and Prevention. Progress toward global eradication of poliomyelitis, MMWR Morb Mortal Wkly Rep 2003; 52: Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication Angola and the Democratic Republic of Congo, January 2002 June MMWR Morb Mortal Wkly Rep 2003; 52: Centers for Disease Control and Prevention. Progress toward global eradication of poliomyelitis, January 2003 April MMWR Morb Mortal Wkly Rep 2004; 53: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, January 2004 March MMWR Morb Mortal Wkly Rep 2005; 54: Centers for Disease Control and Prevention. Conclusions and recommendations of the Advisory Committee on Poliomyelitis Eradication Geneva Switzerland, October MMWR Morb, Mortal Wkly Rep 2005; 54: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, January 2005 March MMWR Morb Mortal Wkly Rep 2006; 55: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, January 2007 April MMWR Morb Mortal Wkly Rep 2008; 57: Centers for Disease Control and Prevention. Wild poliovirus type 1 and type 3 importations 15 countries, Africa MMWR Morb Mortal Wkly Rep 2009; 58: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, January 2006 May MMWR Morb Mortal Wkly Rep 2007; 56: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, MMWR Morb Mortal Wkly Rep 2009; 58: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, MMWR Morb Mortal Wkly Rep 2010; 59: Centers for Disease Control and Prevention. Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission Africa, MMWR Morb Mortal Wkly Rep 2011; 60: Centers for Disease Control and Prevention. Tracking progress toward global polio eradication worldwide, MMWR Morb Mortal Wkly Rep 2011; 60: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, January 2010 March MMWR Morb Mortal Wkly Rep 2011; 60: Centers for Disease Control and Prevention. Progress toward global polio eradication Africa, MMWR Morb Mortal Wkly Rep 2012; 61: Centers for Disease Control and Prevention. Tracking progress toward global polio eradication, MMWR Morb Mortal Wkly Rep 2012; 61: Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission worldwide, January 2011 March MMWR Morb Mortal Wkly Rep 2012; 61: Centers for Disease Control and Prevention. Progress toward eradication of polio worldwide, January 2011 March MMWR Morb Mortal Wkly Rep 2013; 62: Global Polio Eradication Initiative. List of wild polio virus by country, wild polio virus Document/Data&Monitoring/Wild_poliovirus_list_2008_2013_16Jul. pdf. Accessed 23 March World Health Organization, Democratic Republic of the Congo Country Office. Activités d éradication de la poliomyélite (IEP), République Démocratique du Congo, 2 août 2013 [Polio eradication activities (GPEI), the Democratic Republic of the Congo, 2 August 2013]. World Health Organization, Democratic Republic of the Congo Country Office. 45. World Health Organization, Democratic Republic of the Congo Country Office. AFP surveillance databases for World Health Organization, Democratic Republic of the Congo Country Office. 46. Nsambu MN, Bazira L, Coulibaly T, et al. Investigation et riposte à une épidémie de poliovirus sauvage à Kinshasa [Investigation of and response to a wild poliovirus outbreak in Kinshasa]. Pan Afr Med J 2013; 15: World Health Organization, Democratic Republic of the Congo Country Office. Activités d éradication de la poliomyélite, République Démocratique du Congo, Semaine 43, 28 octobre 2011 [Polio eradication activities, the Democratic Republic of the Congo, week 43, 28 October 2011]. World Health Organization, Democratic Republic of the Congo Country Office. 48. Ministry of Public Health-Democratic Republic of the Congo. Rapport épidémiologique de surveillance du VIH chez les femmes enceintes fréquentant les structures de CPN 2008 [Epidemiological report of HIV surveillance in pregnant women attending prenatal consultations 2008]. National HIV/AIDS Program, Ministry of Public Health-Democratic Republic of the Congo. 49. Ministry of Public Health-Democratic Republic of the Congo. Rapport épidémiologique de surveillance du VIH/SIDA chez les femmes enceintes fréquentant les structures de CPN 2009 [Epidemiological report of HIV/AIDS surveillance in pregnant women attending prenatal consultations 2009]. National HIV/AIDS Program, Ministry of Public Health-Democratic Republic of the Congo. 50. WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. J Infect Dis 1997; 175:S Expanded Progamme on Immunization. Report of a WHO informal consultation on polio neutralizing antibody assays. Nashville, 5 6 December Geneva: World Health Organization, Index Mundi. Congo, Democratic Republic of the, Demographics Profile congo/demographics_profile.html. Accessed 23 March World Health Organization, Democratic Republic of the Congo Country Office. Activités d éradication de la poliomyélite, République Démocratique du Congo, Semaine 52, 30 décembre 2011 [Polio eradication activities, the Democratic Republic of the Congo, week 52, 30 December 2011). World Health Organization, Democratic Republic of the Congo Country Office. 54. Prevots DR, Ciofi degli Atti ML, Sallabanda A, et al. Outbreak of paralytic poliomyelitis in Albania, 1996: High attack rate among adults and S72 JID 2014:210 (Suppl 1) Alleman et al
SUPPLEMENT ARTICLE. Geneva, Switzerland. India Polio Seroprevalence Survey, 2007 JID 2014:210 (Suppl 1) S225
 SUPPLEMENT ARTICLE Assessing Population Immunity in a Persistently High-Risk Area for Wild Poliovirus Transmission in India: A Serological Study in Moradabad, Western Uttar Pradesh Jagadish M. Deshpande,
SUPPLEMENT ARTICLE Assessing Population Immunity in a Persistently High-Risk Area for Wild Poliovirus Transmission in India: A Serological Study in Moradabad, Western Uttar Pradesh Jagadish M. Deshpande,
Poliomyelitis eradication in the WHO European Region
 Provisional agenda item 6(i) EUR/RC60/16 (+EUR/RC60/Conf.Doc./9) 23 July 2010 101614 ORIGINAL: ENGLISH Poliomyelitis eradication in the WHO European Region WHO Regional Committee for Europe Sixtieth session
Provisional agenda item 6(i) EUR/RC60/16 (+EUR/RC60/Conf.Doc./9) 23 July 2010 101614 ORIGINAL: ENGLISH Poliomyelitis eradication in the WHO European Region WHO Regional Committee for Europe Sixtieth session
ANNEX Page. AFR/RC61/11 4 July 2011 ORIGINAL: ENGLISH REGIONAL COMMITTEE FOR AFRICA
 4 July 2011 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-first session Yamoussoukro, Côte d Ivoire, 29 August 2 September 2011 Provisional agenda item 16 PROGRESS REPORT ON POLIOMYELITIS ERADICATION
4 July 2011 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-first session Yamoussoukro, Côte d Ivoire, 29 August 2 September 2011 Provisional agenda item 16 PROGRESS REPORT ON POLIOMYELITIS ERADICATION
The Eradication of Poliomyelitis in Egypt: Critical Factors Affecting Progress to Date
 S56 The Eradication of Poliomyelitis in Egypt: Critical Factors Affecting Progress to Date R. Bruce Aylward, Esmat Mansour, EI Said Aly Don, Ahmed Haridi, Abdulla Abu EI Kheir, and AtefHassan World Health
S56 The Eradication of Poliomyelitis in Egypt: Critical Factors Affecting Progress to Date R. Bruce Aylward, Esmat Mansour, EI Said Aly Don, Ahmed Haridi, Abdulla Abu EI Kheir, and AtefHassan World Health
Wild Poliovirus Weekly Update
 1 of 5 12/07/2010 12:09 PM Google search this site contact links donate Home > Global Situation Wild Poliovirus Weekly Update 7 July 2010 Data as at 6 July 2010 Map of polio cases worldwide Wild poliovirus
1 of 5 12/07/2010 12:09 PM Google search this site contact links donate Home > Global Situation Wild Poliovirus Weekly Update 7 July 2010 Data as at 6 July 2010 Map of polio cases worldwide Wild poliovirus
Preparing for the withdrawal of all oral polio vaccines (OPVs): Replacing trivalent OPV with bivalent OPV
 Preparing for the withdrawal of all oral polio vaccines (OPVs): Replacing trivalent OPV with bivalent OPV Frequently Asked Questions February 2015 Table of Contents Rationale for OPV cessation... 2 About
Preparing for the withdrawal of all oral polio vaccines (OPVs): Replacing trivalent OPV with bivalent OPV Frequently Asked Questions February 2015 Table of Contents Rationale for OPV cessation... 2 About
Global and Regional update on Polio Eradication Activities. Kenya Paediatric Association Pride Inn, Mombasa April 26, 2018
 Global and Regional update on Polio Eradication Activities Kenya Paediatric Association Pride Inn, Mombasa April 26, 2018 Presentation Outline 1. Background information 2. Poliovirus detection & interruption
Global and Regional update on Polio Eradication Activities Kenya Paediatric Association Pride Inn, Mombasa April 26, 2018 Presentation Outline 1. Background information 2. Poliovirus detection & interruption
CDC ASSESSMENT OF RISKS TO THE GLOBAL POLIO ERADICATION INITIATIVE (GPEI) STRATEGIC PLAN
 CDC ASSESSMENT OF RISKS TO THE GLOBAL POLIO ERADICATION INITIATIVE (GPEI) STRATEGIC PLAN 2010-2012 14 Sept-10 2010 First and Second Quarters (January June) Geographic distribution of wild poliovirus (WPV)
CDC ASSESSMENT OF RISKS TO THE GLOBAL POLIO ERADICATION INITIATIVE (GPEI) STRATEGIC PLAN 2010-2012 14 Sept-10 2010 First and Second Quarters (January June) Geographic distribution of wild poliovirus (WPV)
POLIOMYELITIS ERADICATION: PROGRESS REPORT. Information Document CONTENTS BACKGROUND PROGRESS MADE NEXT STEPS... 12
 5 August 9 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, August 4 September 9 POLIOMYELITIS ERADICATION: PROGRESS REPORT Information Document CONTENTS
5 August 9 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, August 4 September 9 POLIOMYELITIS ERADICATION: PROGRESS REPORT Information Document CONTENTS
Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Published in WER 4 June, 2010
 Polio vaccines and polio immunization in the pre-eradication era: WHO position paper Published in WER 4 June, 2010 Epidemiology & Background Poliomyelitis (polio) is an acute communicable disease of humans
Polio vaccines and polio immunization in the pre-eradication era: WHO position paper Published in WER 4 June, 2010 Epidemiology & Background Poliomyelitis (polio) is an acute communicable disease of humans
Revisiting Old and Addressing Current Issues on Vaccines: POLIO
 Revisiting Old and Addressing Current Issues on Vaccines: POLIO Maria Carmen B. Nievera MD Fellow, Pediatric Infectious Disease Society of the Philippines Fellow, Philippine Pediatric Society Regional
Revisiting Old and Addressing Current Issues on Vaccines: POLIO Maria Carmen B. Nievera MD Fellow, Pediatric Infectious Disease Society of the Philippines Fellow, Philippine Pediatric Society Regional
Could a combination of OPV & IPV accelerate wild type poliovirus eradication? Nicholas Grassly
 Could a combination of OPV & IPV accelerate wild type poliovirus eradication? Nicholas Grassly SAGE working group on IPV (est. Aug 2008) SAGE Members Elizabeth Miller, Chair Hyam Bashour Peter Figueroa
Could a combination of OPV & IPV accelerate wild type poliovirus eradication? Nicholas Grassly SAGE working group on IPV (est. Aug 2008) SAGE Members Elizabeth Miller, Chair Hyam Bashour Peter Figueroa
Expanded Programme on Immunization (EPI)
 Indonesia 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Indonesia 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Expanded Programme on Immunization (EPI)
 Timor-Leste 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Timor-Leste 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
AIDA M. SALONGA, MD, FPNA, FCNSP CHAIR, AFP EXPERT PANEL, DOH MEMBER, REGIONAL CERTIFICATION COMMITTEE, WHO
 POLIO FAQs on ENDGAME Tuberculosis AIDA M. SALONGA, MD, FPNA, FCNSP CHAIR, AFP EXPERT PANEL, DOH MEMBER, REGIONAL CERTIFICATION COMMITTEE, WHO OBJECTIVES Brief history of poliomyelitis Polio eradication:
POLIO FAQs on ENDGAME Tuberculosis AIDA M. SALONGA, MD, FPNA, FCNSP CHAIR, AFP EXPERT PANEL, DOH MEMBER, REGIONAL CERTIFICATION COMMITTEE, WHO OBJECTIVES Brief history of poliomyelitis Polio eradication:
Expanded Programme on Immunization (EPI)
 Bhutan 2017 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles containing
Bhutan 2017 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles containing
poliomyelitis; community transmission; India; enhanced surveillance.
 SUPPLEMENT ARTICLE Prevalence of Asymptomatic Poliovirus Infection in Older Children and Adults in Northern India: Analysis of Contact and Enhanced Community Surveillance, 2009 Ondrej Mach, 1,a Harish
SUPPLEMENT ARTICLE Prevalence of Asymptomatic Poliovirus Infection in Older Children and Adults in Northern India: Analysis of Contact and Enhanced Community Surveillance, 2009 Ondrej Mach, 1,a Harish
Expanded Programme on Immunization (EPI)
 Sri Lanka 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Sri Lanka 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Poliomyelitis: successful and critical issues in the eradication process
 Vaccine Preventable Diseases in the Mediterranean Basin and Black Sea: immunization strategies and coverage in the general population and the newly arrived migrants the ProVacMed project Poliomyelitis:
Vaccine Preventable Diseases in the Mediterranean Basin and Black Sea: immunization strategies and coverage in the general population and the newly arrived migrants the ProVacMed project Poliomyelitis:
Progress Towards Global Polio Eradication by 2012: Progress and Ongoing Challenges
 Progress Towards Global Polio Eradication by 2012: Progress and Ongoing Challenges European Conference on Post Polio Syndrome 31 August 2 September 2011, Copenhagen, Denmark Dr Rebecca Martin Vaccine Preventable
Progress Towards Global Polio Eradication by 2012: Progress and Ongoing Challenges European Conference on Post Polio Syndrome 31 August 2 September 2011, Copenhagen, Denmark Dr Rebecca Martin Vaccine Preventable
Why still Polio Donato Greco ECDC polio consultant 14 April 2016
 Why still Polio Donato Greco ECDC polio consultant 14 April 2016 A medical student with the last texbook on Communicable diseases! Where there is no chapter on Poliomielitis!!! What can I say o Background
Why still Polio Donato Greco ECDC polio consultant 14 April 2016 A medical student with the last texbook on Communicable diseases! Where there is no chapter on Poliomielitis!!! What can I say o Background
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1: Map of the ten countries included in the analysis. Showing the first sub-national administrative boundaries in light grey, and DHS survey locations representing
Supplementary Figures Supplementary Fig. 1: Map of the ten countries included in the analysis. Showing the first sub-national administrative boundaries in light grey, and DHS survey locations representing
Table 1: Basic information Total population 25,030, (per 100,000 LB) Division/Province/State/Region 11. District 210
 EPI history EPI launched in 980 HepB vaccine introduced in 003 AD syringes introduced in 003 HepB birth dose introduced in 004 DTP-HepB vaccine introduced in 006 MCV introduced in 008 DTP-Hib-HepB vaccine
EPI history EPI launched in 980 HepB vaccine introduced in 003 AD syringes introduced in 003 HepB birth dose introduced in 004 DTP-HepB vaccine introduced in 006 MCV introduced in 008 DTP-Hib-HepB vaccine
POLIO ERADICATION AND POST-CERTIFICATION STRATEGY
 POLIO ERADICATION AND POST-CERTIFICATION STRATEGY PRE-BOARD MEETING 5 June 2018, Geneva Reach every child www.gavi.org Agenda Topic Presenter 1. Polio eradication update WHO 10 minutes 2. IPV supply, demand
POLIO ERADICATION AND POST-CERTIFICATION STRATEGY PRE-BOARD MEETING 5 June 2018, Geneva Reach every child www.gavi.org Agenda Topic Presenter 1. Polio eradication update WHO 10 minutes 2. IPV supply, demand
CONTENTS. Paragraphs I. BACKGROUND II. PROGRESS REPORT ON THE AFRICAN REGIONAL IMMUNIZATION STRATEGIC PLAN
 23 September 2013 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-third session Brazzaville, Republic of Congo, 2 6 September, 2013 Agenda item 14 IMMUNIZATION IN THE AFRICAN REGION: PROGRESS REPORT
23 September 2013 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-third session Brazzaville, Republic of Congo, 2 6 September, 2013 Agenda item 14 IMMUNIZATION IN THE AFRICAN REGION: PROGRESS REPORT
Task Force on Immunization (TFI) in Africa 14 th Annual Meeting. And. Africa Regional Inter-Agency Coordination Committee (ARICC) 13 th Annual Meeting
 Task Force on Immunization (TFI) in Africa 14 th Annual Meeting And Africa Regional Inter-Agency Coordination Committee (ARICC) 13 th Annual Meeting Meeting Report Maputo, Mozambique 27 29 November 2006
Task Force on Immunization (TFI) in Africa 14 th Annual Meeting And Africa Regional Inter-Agency Coordination Committee (ARICC) 13 th Annual Meeting Meeting Report Maputo, Mozambique 27 29 November 2006
Global Polio Eradication & Endgame Strategy. December 2015
 Global Polio Eradication & Endgame Strategy December 2015 Poliovirus detection & interruption OPV withdrawal, IPV introduction, RI strengthening Containment & Global Certification Legacy Planning Polio
Global Polio Eradication & Endgame Strategy December 2015 Poliovirus detection & interruption OPV withdrawal, IPV introduction, RI strengthening Containment & Global Certification Legacy Planning Polio
Vaccination Recommendations for Travellers from Polio-infected Countries: Report of the SAGE Polio Working Group
 Vaccination Recommendations for Travellers from Polio-infected Countries: Report of the SAGE Polio Working Group Peter Figueroa, Chair SAGE Polio Working Group April 1, 2014 1 Overview Context for the
Vaccination Recommendations for Travellers from Polio-infected Countries: Report of the SAGE Polio Working Group Peter Figueroa, Chair SAGE Polio Working Group April 1, 2014 1 Overview Context for the
Responding to a poliovirus event and outbreak
 Standard Operating Procedures Responding to a poliovirus event and outbreak Updated according to WHO guidelines from May 2017 This is a version of the original two-part document drawn up by the WHO and
Standard Operating Procedures Responding to a poliovirus event and outbreak Updated according to WHO guidelines from May 2017 This is a version of the original two-part document drawn up by the WHO and
GOAL 2: ACHIEVE RUBELLA AND CRS ELIMINATION. (indicator G2.2) Highlights
 GOAL 2: ACHIEVE RUBELLA AND CRS ELIMINATION (indicator G2.2) Highlights As of December 2014, 140 Member States had introduced rubella vaccines; coverage, however, varies from 12% to 94% depending on region.
GOAL 2: ACHIEVE RUBELLA AND CRS ELIMINATION (indicator G2.2) Highlights As of December 2014, 140 Member States had introduced rubella vaccines; coverage, however, varies from 12% to 94% depending on region.
Progress report on eradication of poliomyelitis: regional implications of the endgame strategy
 Regional Committee for the Eastern Mediterranean Sixtieth session Provisional agenda item 3 (a) August 2013 Progress report on eradication of poliomyelitis: regional implications of the endgame strategy
Regional Committee for the Eastern Mediterranean Sixtieth session Provisional agenda item 3 (a) August 2013 Progress report on eradication of poliomyelitis: regional implications of the endgame strategy
International PolioPlus Committee PolioPlus Facts and Figures June Rotary s financial contribution to the polio eradication effort:
 International PolioPlus Committee PolioPlus Facts and Figures June 2018 1. The goal of the PolioPlus program: The goal of the PolioPlus program is the global certification of polio eradication. By eradication,
International PolioPlus Committee PolioPlus Facts and Figures June 2018 1. The goal of the PolioPlus program: The goal of the PolioPlus program is the global certification of polio eradication. By eradication,
Responding to a poliovirus event and outbreak Part 2: Protocol for poliovirus type 2
 Standard Operating Procedures Responding to a poliovirus event and outbreak Part 2: Protocol for poliovirus type 2 April 20, 2016 Effective 1 st May 2016 till 30 April 2017 Contents Tables and Figures...
Standard Operating Procedures Responding to a poliovirus event and outbreak Part 2: Protocol for poliovirus type 2 April 20, 2016 Effective 1 st May 2016 till 30 April 2017 Contents Tables and Figures...
India s last polio case was reported on January 13,
 P E R S P E C T I V E Polio Eradication and Endgame Plan Victory within Grasp MANISH PATEL, * LISA MENNING AND # PANKAJ BHATNAGAR From Task Force for Global Health, Atlanta, GA, USA; * World Health Organization,
P E R S P E C T I V E Polio Eradication and Endgame Plan Victory within Grasp MANISH PATEL, * LISA MENNING AND # PANKAJ BHATNAGAR From Task Force for Global Health, Atlanta, GA, USA; * World Health Organization,
Modeling Population Immunity to Support Efforts to End the Transmission of Live Polioviruses
 Risk Analysis DOI: 10.1111/j.1539-6924.2012.01891.x Modeling Population Immunity to Support Efforts to End the Transmission of Live Polioviruses Kimberly M. Thompson, 1,4 Mark A. Pallansch, 2 Radboud J.
Risk Analysis DOI: 10.1111/j.1539-6924.2012.01891.x Modeling Population Immunity to Support Efforts to End the Transmission of Live Polioviruses Kimberly M. Thompson, 1,4 Mark A. Pallansch, 2 Radboud J.
Table 1: Basic information 2017
 EPI history EPI launched in 978 OPV and MCV introduced in 987 AD syringes introduced in 00 HepB vaccine introduced in 003 MCV introduced partially in 008 and made available nationwide in 0 DTP-Hib-HepB
EPI history EPI launched in 978 OPV and MCV introduced in 987 AD syringes introduced in 00 HepB vaccine introduced in 003 MCV introduced partially in 008 and made available nationwide in 0 DTP-Hib-HepB
KEY ISSUES IN IMMUNIZATION IN AFRICA
 SAGE Meeting April 2011 KEY ISSUES IN IMMUNIZATION IN AFRICA Deo NSHIMIRIMANA IVD / ARD / AFRO 1 Outline Efforts to reduce un-immunized children in Africa Attaining Global Polio Eradication Initiative
SAGE Meeting April 2011 KEY ISSUES IN IMMUNIZATION IN AFRICA Deo NSHIMIRIMANA IVD / ARD / AFRO 1 Outline Efforts to reduce un-immunized children in Africa Attaining Global Polio Eradication Initiative
Estimating the Extent of Vaccine-Derived Poliovirus Infection
 Estimating the Extent of Vaccine-Derived Poliovirus Infection Alison Wringe 1 *, Paul E. M. Fine 1, Roland W. Sutter 2, Olen M. Kew 3 1 Department of Epidemiology and Population Health, London School of
Estimating the Extent of Vaccine-Derived Poliovirus Infection Alison Wringe 1 *, Paul E. M. Fine 1, Roland W. Sutter 2, Olen M. Kew 3 1 Department of Epidemiology and Population Health, London School of
Table 1: Basic information (per 1,000 LB) 42.4 (per 1,000 LB) 49.7 (per 1,000 LB) 215 (per 100,000 LB)
 EPI history EPI started in 978 EPI re-structured in March 000 DTP-HepB vaccine introduced in 007 DTP-Hib-HepB)vaccine introduced in 0 MR vaccine introduced in Feb 06 Second dose of MR introduced in Feb
EPI history EPI started in 978 EPI re-structured in March 000 DTP-HepB vaccine introduced in 007 DTP-Hib-HepB)vaccine introduced in 0 MR vaccine introduced in Feb 06 Second dose of MR introduced in Feb
D.A.Henderson, MD, MPH. Professor of Medicine, University of Pittsburgh Honorary Fellow, London School of Hygiene and Tropical Medicine
 Polio Eradication a reconsideration of strategy D.A.Henderson, MD, MPH Professor of Medicine, University of Pittsburgh Honorary Fellow, London School of Hygiene and Tropical Medicine Baltimore, Maryland
Polio Eradication a reconsideration of strategy D.A.Henderson, MD, MPH Professor of Medicine, University of Pittsburgh Honorary Fellow, London School of Hygiene and Tropical Medicine Baltimore, Maryland
Total population 24,759,000. Live births (LB) 342,458. Children <1 year 337,950. Children <5 years 1,698,664. Children <15 years 5,233,093
 DPR Korea 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering -5. A standing national technical advisory group on immunization (NTAGI) with formal
DPR Korea 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering -5. A standing national technical advisory group on immunization (NTAGI) with formal
Modeling undetected live poliovirus circulation after apparent interruption of transmission: implications for surveillance and vaccination
 Kalkowska et al. BMC Infectious Diseases (2015) 15:66 DOI 10.1186/s12879-015-0791-5 RESEARCH ARTICLE Open Access Modeling undetected live poliovirus circulation after apparent interruption of transmission:
Kalkowska et al. BMC Infectious Diseases (2015) 15:66 DOI 10.1186/s12879-015-0791-5 RESEARCH ARTICLE Open Access Modeling undetected live poliovirus circulation after apparent interruption of transmission:
Considerations for the Full Global Withdrawal of Oral Polio Vaccine After Eradication of Polio
 The Journal of Infectious Diseases SUPPLEMENT ARTICLE Considerations for the Full Global Withdrawal of Oral Polio Vaccine After Eradication of Polio Lee M. Hampton, 1 Gaël Maufras du Châtellier, 2 Jacqueline
The Journal of Infectious Diseases SUPPLEMENT ARTICLE Considerations for the Full Global Withdrawal of Oral Polio Vaccine After Eradication of Polio Lee M. Hampton, 1 Gaël Maufras du Châtellier, 2 Jacqueline
Radboud J. Duintjer Tebbens 1*, Lee M. Hampton 2 and Kimberly M. Thompson 1
 Duintjer Tebbens et al. BMC Infectious Diseases (2018) 18:165 https://doi.org/10.1186/s12879-018-3074-0 RESEARCH ARTICLE Open Access Planning for globally coordinated cessation of bivalent oral poliovirus
Duintjer Tebbens et al. BMC Infectious Diseases (2018) 18:165 https://doi.org/10.1186/s12879-018-3074-0 RESEARCH ARTICLE Open Access Planning for globally coordinated cessation of bivalent oral poliovirus
Status of Outbreak Vulnerable Countries
 Status of Outbreak Vulnerable Countries Hamid Jafari, WHO Robert Linkins, CDC Independent Monitoring Board Meeting 7-8 May, 2013 Outline Non-Endemic Vulnerable & At-Risk Countries Recently infected countries
Status of Outbreak Vulnerable Countries Hamid Jafari, WHO Robert Linkins, CDC Independent Monitoring Board Meeting 7-8 May, 2013 Outline Non-Endemic Vulnerable & At-Risk Countries Recently infected countries
The Polio Endgame
 The Polio Endgame 2013-2018 context the Endgame Plan implications for DCVMN Context 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007
The Polio Endgame 2013-2018 context the Endgame Plan implications for DCVMN Context 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007
Oral Polio Vaccine Supply Outlook. UNICEF Supply Division
 Oral Polio Vaccine Supply Outlook UNICEF Supply Division May 2017 Oral Polio Vaccine Supply Outlook May 2017 This note provides updated information on 2016 and 2017 oral polio vaccine supply and demand,
Oral Polio Vaccine Supply Outlook UNICEF Supply Division May 2017 Oral Polio Vaccine Supply Outlook May 2017 This note provides updated information on 2016 and 2017 oral polio vaccine supply and demand,
Outbreak of Type 2 Vaccine-Derived Poliovirus in Nigeria: Emergence and Widespread Circulation in an Underimmunized Population
 MAJOR ARTICLE Outbreak of Type 2 Vaccine-Derived Poliovirus in Nigeria: Emergence and Widespread Circulation in an Underimmunized Population Steven Wassilak, 1 Muhammad Ali Pate, 2 Kathleen Wannemuehler,
MAJOR ARTICLE Outbreak of Type 2 Vaccine-Derived Poliovirus in Nigeria: Emergence and Widespread Circulation in an Underimmunized Population Steven Wassilak, 1 Muhammad Ali Pate, 2 Kathleen Wannemuehler,
TECHNICAL ADVISORY GROUP ON POLIO ERADICATION IN THE HORN OF AFRICA. 8-9 February 2012 in Nairobi, Kenya. Executive Summary
 TECHNICAL ADVISORY GROUP ON POLIO ERADICATION IN THE HORN OF AFRICA 7 th Meeting Report 8-9 February 2012 in Nairobi, Kenya Executive Summary The 7 th meeting of the Technical Advisory Group on Polio Eradication
TECHNICAL ADVISORY GROUP ON POLIO ERADICATION IN THE HORN OF AFRICA 7 th Meeting Report 8-9 February 2012 in Nairobi, Kenya Executive Summary The 7 th meeting of the Technical Advisory Group on Polio Eradication
PART 1: General SOPs RESPONDING TO A POLIOVIRUS EVENT OR OUTBREAK STANDARD OPERATING PROCEDURES
 STANDARD OPERATING PROCEDURES A POLIOVIRUS EVENT OR OUTBREAK PART 1: General SOPs V2.1 20 April 2016 V2.2 15 August 2016 V2.3 01 May 2017 EFFECTIVE 1 MAY 2017 UNTIL 31 OCTOBER 2017 Published by the World
STANDARD OPERATING PROCEDURES A POLIOVIRUS EVENT OR OUTBREAK PART 1: General SOPs V2.1 20 April 2016 V2.2 15 August 2016 V2.3 01 May 2017 EFFECTIVE 1 MAY 2017 UNTIL 31 OCTOBER 2017 Published by the World
Summary of the October 2017 meeting of the Strategic Advisory Group of Experts on Immunization
 Summary of the October 2017 meeting of the Strategic Advisory Group of Experts on Immunization The Strategic Advisory Group of Experts (SAGE) on Immunization 1 met on 17-19 October 2017 in Geneva, Switzerland.
Summary of the October 2017 meeting of the Strategic Advisory Group of Experts on Immunization The Strategic Advisory Group of Experts (SAGE) on Immunization 1 met on 17-19 October 2017 in Geneva, Switzerland.
Switch From Oral to Inactivated Poliovirus Vaccine in Yogyakarta Province, Indonesia: Summary of Coverage, Immunity, and Environmental Surveillance
 SUPPLEMENT ARTICLE Switch From Oral to Inactivated Poliovirus Vaccine in Yogyakarta Province, Indonesia: Summary of Coverage, Immunity, and Environmental Surveillance Gendro Wahjuhono, 1 Revolusiana, 4
SUPPLEMENT ARTICLE Switch From Oral to Inactivated Poliovirus Vaccine in Yogyakarta Province, Indonesia: Summary of Coverage, Immunity, and Environmental Surveillance Gendro Wahjuhono, 1 Revolusiana, 4
We compute the incremental net benefits of an alternative option (alt) compared to each reference case (ref) as:
 TECHNICAL APPENDIX Main paper: Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Thompson KM: An economic analysis of poliovirus risk management policy options for 2013-2052. BMC Infect Dis 2015;
TECHNICAL APPENDIX Main paper: Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Thompson KM: An economic analysis of poliovirus risk management policy options for 2013-2052. BMC Infect Dis 2015;
International Measles Incidence and Immunization Coverage
 SUPPLEMENT ARTICLE International Measles Incidence and Immunization Coverage Robert Hall and Damien Jolley Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine,
SUPPLEMENT ARTICLE International Measles Incidence and Immunization Coverage Robert Hall and Damien Jolley Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine,
Update on Polio Eradication in the World Health Organization South-East Asia Region, 2013
 SUPPLEMENT ARTICLE Update on Polio Eradication in the World Health Organization South-East Asia Region, 2013 Patrick Michael O Connor, 1 Robert Allison, 1 Arun Thapa, 1 Sunil Bahl, 2 Supamit Chunsuittiwat,
SUPPLEMENT ARTICLE Update on Polio Eradication in the World Health Organization South-East Asia Region, 2013 Patrick Michael O Connor, 1 Robert Allison, 1 Arun Thapa, 1 Sunil Bahl, 2 Supamit Chunsuittiwat,
Polio Eradication: The Difficult Inclusion of IPV. Research, Policy and Product Development (RAP), Polio Operations & Research Department (POL)
 Polio Eradication: The Difficult Inclusion of IPV Research, Policy and Product Development (RAP), Polio Operations & Research Department (POL) Overview Historical context Early inequality in case burden
Polio Eradication: The Difficult Inclusion of IPV Research, Policy and Product Development (RAP), Polio Operations & Research Department (POL) Overview Historical context Early inequality in case burden
Polio Eradication in India. Dr Sunil Bahl Deputy Project Manager WHO Country Office for India 28 October 2014
 Polio Eradication in India Dr Sunil Bahl Deputy Project Manager WHO Country Office for India 28 October 2014 History of Polio in India Number of Cases 200,000 200,000 OPV Introduced in RI - 1978 150,000
Polio Eradication in India Dr Sunil Bahl Deputy Project Manager WHO Country Office for India 28 October 2014 History of Polio in India Number of Cases 200,000 200,000 OPV Introduced in RI - 1978 150,000
Endgame Issues for the Global Polio Eradication Initiative
 VIEWPOINTS Endgame Issues for the Global Polio Eradication Initiative Technical Consultative Group to the World Health Organization on the Global Eradication of Poliomyelitis a The polio eradication initiative,
VIEWPOINTS Endgame Issues for the Global Polio Eradication Initiative Technical Consultative Group to the World Health Organization on the Global Eradication of Poliomyelitis a The polio eradication initiative,
POLIO ERADICATION IN THE AFRICAN REGION: PROGRESS REPORT. Information document EXECUTIVE SUMMARY
 7 July 2006 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-sixth session Addis Ababa, Ethiopia, 28 August 1 September 2006 Provisional agenda item 10.1 POLIO ERADICATION IN THE AFRICAN REGION: PROGRESS
7 July 2006 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-sixth session Addis Ababa, Ethiopia, 28 August 1 September 2006 Provisional agenda item 10.1 POLIO ERADICATION IN THE AFRICAN REGION: PROGRESS
Polio Environmental Surveillance Enhancement Following Detection of Vaccine-Related Type-2 Poliovirus
 1. Goal and Objective: Although acute flaccid paralysis surveillance is the gold standard of polio surveillance, supplemental surveillance methods (which includes environmental surveillance (ES)) provide
1. Goal and Objective: Although acute flaccid paralysis surveillance is the gold standard of polio surveillance, supplemental surveillance methods (which includes environmental surveillance (ES)) provide
Global reductions in measles mortality and the risk of measles resurgence
 Global reductions in measles mortality 2000 2008 and the risk of measles resurgence Measles is one of the most contagious human diseases. In 1980 before the use of measles vaccine was widespread, there
Global reductions in measles mortality 2000 2008 and the risk of measles resurgence Measles is one of the most contagious human diseases. In 1980 before the use of measles vaccine was widespread, there
Wild poliovirus type 1 and Circulating vaccine-derived poliovirus cases
 1 of 5 4/8/2016 5:29 PM Polio this week as of 6 April 2016 The third Outbreak Response Assessment in Madagascar found that the surveillance system is not yet strong enough to conclude that polio transmission
1 of 5 4/8/2016 5:29 PM Polio this week as of 6 April 2016 The third Outbreak Response Assessment in Madagascar found that the surveillance system is not yet strong enough to conclude that polio transmission
Media centre Statement on the 7th IHR Emergency Committee meeting regarding the international spread of poliovirus
 Media centre Statement on the 7th IHR Emergency Committee meeting regarding the international spread of poliovirus WHO statement 26 November The 7th meeting of the Emergency Committee under the International
Media centre Statement on the 7th IHR Emergency Committee meeting regarding the international spread of poliovirus WHO statement 26 November The 7th meeting of the Emergency Committee under the International
Context 4.1 WHERE WE ARE TODAY. Figure 2: Countries with cases of wild poliovirus, 2011 and 2012
 POLIO ERADICATION & ENDGAME STRATEGIC PLAN 2013-2018 Context 4.1 WHERE WE ARE TODAY Figure 2: Countries with cases of wild poliovirus, 2011 and 2012 2011 2012 Data as of 19 February 2013 Countries with
POLIO ERADICATION & ENDGAME STRATEGIC PLAN 2013-2018 Context 4.1 WHERE WE ARE TODAY Figure 2: Countries with cases of wild poliovirus, 2011 and 2012 2011 2012 Data as of 19 February 2013 Countries with
Eradication of poliomyelitis
 EXECUTIVE BOARD EB142/37 142nd session 27 November 2017 Provisional agenda item 6.4 Eradication of poliomyelitis 1. This report provides an update on the status of the four objectives of the Polio Eradication
EXECUTIVE BOARD EB142/37 142nd session 27 November 2017 Provisional agenda item 6.4 Eradication of poliomyelitis 1. This report provides an update on the status of the four objectives of the Polio Eradication
Table 1: Basic information ,000, (per 1,000 LB) 34.6 (per 1,000 LB) 174 (per 100,000 LB) District 712.
 EPI history EPI launched in 978 with DPT, OPV, BCG and typhoid vaccines TT immunization of pregnant women introduced in 983 MCV introduced in 985 HepB piloted in 00 and made universal in 0 MCV introduced
EPI history EPI launched in 978 with DPT, OPV, BCG and typhoid vaccines TT immunization of pregnant women introduced in 983 MCV introduced in 985 HepB piloted in 00 and made universal in 0 MCV introduced
Report to the Board June 2015
 10-11 June 2015 SUBJECT: Report of: Authored by: Agenda item: Category: MEASLES SUPPLEMENTARY IMMUNISATION ACTIVITIES Hind Khatib-Othman, Managing Director, Country Programmes Khin Devi Aung, Senior Programme
10-11 June 2015 SUBJECT: Report of: Authored by: Agenda item: Category: MEASLES SUPPLEMENTARY IMMUNISATION ACTIVITIES Hind Khatib-Othman, Managing Director, Country Programmes Khin Devi Aung, Senior Programme
The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission
 Thompson and Duintjer Tebbens BMC Infectious Diseases (2015) 15:376 DOI 10.1186/s12879-015-1116-4 RESEARCH ARTICLE Open Access The differential impact of oral poliovirus vaccine formulation choices on
Thompson and Duintjer Tebbens BMC Infectious Diseases (2015) 15:376 DOI 10.1186/s12879-015-1116-4 RESEARCH ARTICLE Open Access The differential impact of oral poliovirus vaccine formulation choices on
IMMUNIZATION VACCINES & EMERGENCIES
 No report No data No data IMMUNIZATION VACCINES & EMERGENCIES ROUTINE IMMUNIZATION PERFORMANCE IN THE AFRICAN REGION October 2013 issue Number of children vaccinated with the 3 rd dose of DTPcontaining
No report No data No data IMMUNIZATION VACCINES & EMERGENCIES ROUTINE IMMUNIZATION PERFORMANCE IN THE AFRICAN REGION October 2013 issue Number of children vaccinated with the 3 rd dose of DTPcontaining
Ensuring the quality of polio outbreak response activities: A rationale and guide for 3 month, quarterly and 6 month independent assessments
 Ensuring the quality of polio outbreak response activities: A rationale and guide for 3 month, quarterly and 6 month independent assessments Introduction While polio exists anywhere, countries with low
Ensuring the quality of polio outbreak response activities: A rationale and guide for 3 month, quarterly and 6 month independent assessments Introduction While polio exists anywhere, countries with low
Meeting Report TWENTY-THIRD MEETING OF THE REGIONAL COMMISSION FOR THE CERTIFICATION OF POLIOMYELITIS ERADICATION IN THE WESTERN PACIFIC
 Meeting Report TWENTY-THIRD MEETING OF THE REGIONAL COMMISSION FOR THE CERTIFICATION OF POLIOMYELITIS ERADICATION IN THE WESTERN PACIFIC 14 16 November 2017 Vientiane, Lao People s Democratic Republic
Meeting Report TWENTY-THIRD MEETING OF THE REGIONAL COMMISSION FOR THE CERTIFICATION OF POLIOMYELITIS ERADICATION IN THE WESTERN PACIFIC 14 16 November 2017 Vientiane, Lao People s Democratic Republic
Total population 1,265,308,000. Live births (LB) 27,016,000. Children <1 year 25,928,200. Children <5 years 23,818,000. Children <15 years 25,639,000
 India 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering 3-7. National technical advisory group on immunization (NTAGI) exists and formal written
India 4 Immunization system highlights There is a comprehensive multiyear plan (cmyp) for immunization covering 3-7. National technical advisory group on immunization (NTAGI) exists and formal written
Maintaining Polio-Free Certification in the World Health Organization Western Pacific Region for Over a Decade
 SUPPLEMENT ARTICLE Maintaining Polio-Free Certification in the World Health Organization Western Pacific Region for Over a Decade Anthony Adams, 1 Liliane Boualam, 2 Sergey Diorditsa, 2 Christopher Gregory,
SUPPLEMENT ARTICLE Maintaining Polio-Free Certification in the World Health Organization Western Pacific Region for Over a Decade Anthony Adams, 1 Liliane Boualam, 2 Sergey Diorditsa, 2 Christopher Gregory,
Polio vaccines: WHO position paper, January 2014
 Polio vaccines: WHO position paper, January 2014 References with abstracts cited in the position paper Polio Eradication and Endgame Strategic Plan 2013 2018 http://www.polioeradication.org/resourcelibrary/strategyandwork.aspx
Polio vaccines: WHO position paper, January 2014 References with abstracts cited in the position paper Polio Eradication and Endgame Strategic Plan 2013 2018 http://www.polioeradication.org/resourcelibrary/strategyandwork.aspx
Achieving polio eradication in Nigeria: prospects and challenges
 Niger J Paed 2013; 40 (1):15 23 Tagbo BN COMMENTARY Achieving polio eradication in Nigeria: prospects and challenges DOI:http://dx.doi.org/10.4314/njp.v40i1,3 Accepted: 24th July 2012 Tagbo B ( ) Institute
Niger J Paed 2013; 40 (1):15 23 Tagbo BN COMMENTARY Achieving polio eradication in Nigeria: prospects and challenges DOI:http://dx.doi.org/10.4314/njp.v40i1,3 Accepted: 24th July 2012 Tagbo B ( ) Institute
DEMOCRATIC REPUBLIC OF THE CONGO (THE)
 Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Anaemia The database on Anaemia includes data by country on prevalence of anaemia and mean haemoglobin concentration Last
Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Anaemia The database on Anaemia includes data by country on prevalence of anaemia and mean haemoglobin concentration Last
Preeradication Vaccine Policy Options for Poliovirus Infection and Disease Control
 Risk Analysis, Vol. 33, No. 4, 2013 DOI: 10.1111/risa.12019 Preeradication Vaccine Policy Options for Poliovirus Infection and Disease Control Kimberly M. Thompson, 1,2, Mark A. Pallansch, 3 Radboud J.
Risk Analysis, Vol. 33, No. 4, 2013 DOI: 10.1111/risa.12019 Preeradication Vaccine Policy Options for Poliovirus Infection and Disease Control Kimberly M. Thompson, 1,2, Mark A. Pallansch, 3 Radboud J.
Global Overview Polio Partners Group, December Note: Gavi requirements of $122.2 million are not included in this slide
 Global Overview Polio Partners Group, December 2015 1 Note: Gavi requirements of $122.2 million are not included in this slide The Polio Endgame Plan 1. Poliovirus detection & interruption 2. OPV2 withdrawal,
Global Overview Polio Partners Group, December 2015 1 Note: Gavi requirements of $122.2 million are not included in this slide The Polio Endgame Plan 1. Poliovirus detection & interruption 2. OPV2 withdrawal,
Total population 1,212,110. Live births (LB) 43,924. Children <1 year 40,351. Children <5 years 192,340. Children <15 years 510,594
 Draft version for the SEAR-ITAG 5-9 June 5 All data provisional as of May 5 Timor Leste 4 Immunization system highlights There is a comprehensive multi-year plan (cmyp) for immunization covering 3-5. 8%
Draft version for the SEAR-ITAG 5-9 June 5 All data provisional as of May 5 Timor Leste 4 Immunization system highlights There is a comprehensive multi-year plan (cmyp) for immunization covering 3-5. 8%
Enterovirus D68-US Outbreak 2014 Laboratory Perspective
 Enterovirus D68-US Outbreak 2014 Laboratory Perspective Allan Nix Team Lead Picornavirus Laboratory Polio and Picornavirus Laboratory Branch PAHO / SARInet Webinar November 6, 2014 National Center for
Enterovirus D68-US Outbreak 2014 Laboratory Perspective Allan Nix Team Lead Picornavirus Laboratory Polio and Picornavirus Laboratory Branch PAHO / SARInet Webinar November 6, 2014 National Center for
Impact of Recurrent Epidemics of Hepatitis A Virus Infection on Population Immunity Levels: Bristol Bay, Alaska
 1081 Impact of Recurrent Epidemics of Hepatitis A Virus Infection on Population Immunity Levels: Bristol Bay, Alaska Dolly Peach, 1,a Brian J. McMahon, 1,2 Lisa Bulkow, 1 Elizabeth Funk, 3 Rafael Harpaz,
1081 Impact of Recurrent Epidemics of Hepatitis A Virus Infection on Population Immunity Levels: Bristol Bay, Alaska Dolly Peach, 1,a Brian J. McMahon, 1,2 Lisa Bulkow, 1 Elizabeth Funk, 3 Rafael Harpaz,
Update/Le point. Principles of measles control* R.H. Henderson,2 C.J. Clements,3 R.T. Chen,4 & P.A. Patriarca5
 Update/Le point Principles of measles control* F.T. Cutts,, R.H. Henderson, C.J. Clements,3 R.T. Chen,4 & P.A. Patriarca5 WHO's Expanded Programme on Immunization has significantly helped to reduce global
Update/Le point Principles of measles control* F.T. Cutts,, R.H. Henderson, C.J. Clements,3 R.T. Chen,4 & P.A. Patriarca5 WHO's Expanded Programme on Immunization has significantly helped to reduce global
Development of the Polio Eradication and Endgame Strategic plan
 Development of the Polio Eradication and Endgame Strategic plan SAGE Meeting 6 November 2012 Overview Process, Consultations, Development Eradication and Endgame Outcomes and Activities Legacy Planning
Development of the Polio Eradication and Endgame Strategic plan SAGE Meeting 6 November 2012 Overview Process, Consultations, Development Eradication and Endgame Outcomes and Activities Legacy Planning
Conclusions of the SAGE Working Group on Measles and Rubella June 2017, Geneva
 Conclusions of the SAGE Working Group on Measles and Rubella 21-22 June 2017, Geneva WHO Policy Recommendation on administration of MCV to infants
Conclusions of the SAGE Working Group on Measles and Rubella 21-22 June 2017, Geneva WHO Policy Recommendation on administration of MCV to infants
Report on MCSP Support for the Polio Switch in April 2016
 Report on MCSP Support for the Polio Switch in April 2016 www.mcsprogram.org Report date: September 1, 2016 This report is made possible by the generous support of the American people through the United
Report on MCSP Support for the Polio Switch in April 2016 www.mcsprogram.org Report date: September 1, 2016 This report is made possible by the generous support of the American people through the United
Progress Toward Rubella and Congenital Rubella Syndrome Elimination in the Western Hemisphere,
 1 Introduction: Progress Toward Rubella and Congenital Rubella Syndrome Elimination in the Western Hemisphere, 2003-2008 1 Enhanced measles elimination activities in the Region of the Americas during the
1 Introduction: Progress Toward Rubella and Congenital Rubella Syndrome Elimination in the Western Hemisphere, 2003-2008 1 Enhanced measles elimination activities in the Region of the Americas during the
Global Polio Eradication and Endgame
 P E R S P E C T I V E India s Preparedness for Introduction of IPV and Switch from topv to bopv PRADEEP HALDAR AND PANKAJ AGRAWAL From Immunization Division, Nirman Bhawan, Ministry of Health and Family
P E R S P E C T I V E India s Preparedness for Introduction of IPV and Switch from topv to bopv PRADEEP HALDAR AND PANKAJ AGRAWAL From Immunization Division, Nirman Bhawan, Ministry of Health and Family
Title: National Surveillance for Paralytic Poliomyelitis and Nonparalytic Poliovirus Infection
 09-ID-53 Committee: Infectious Title: National Surveillance for Paralytic Poliomyelitis and Nonparalytic Poliovirus Infection I. Statement of the Problem CSTE position statement 07-EC-02 recognized the
09-ID-53 Committee: Infectious Title: National Surveillance for Paralytic Poliomyelitis and Nonparalytic Poliovirus Infection I. Statement of the Problem CSTE position statement 07-EC-02 recognized the
3. CONCLUSIONS AND RECOMMENDATIONS
 3. CONCLUSIONS AND RECOMMENDATIONS 3.1 Polio Endgame Strategy Conclusions 1. The TAG welcomes the RCC conclusion that Western Pacific Region maintains its polio-free status, and commends China for the
3. CONCLUSIONS AND RECOMMENDATIONS 3.1 Polio Endgame Strategy Conclusions 1. The TAG welcomes the RCC conclusion that Western Pacific Region maintains its polio-free status, and commends China for the
keywords eradication, Madagascar, mass vaccination campaigns, poliomyelitis, seroprevalence
 Tropical Medicine and International Health volume 6 no 12 pp 1032±1039 december 2001 Mass vaccination campaigns to eradicate poliomyelitis in Madagascar: oral poliovirus vaccine increased immunity of children
Tropical Medicine and International Health volume 6 no 12 pp 1032±1039 december 2001 Mass vaccination campaigns to eradicate poliomyelitis in Madagascar: oral poliovirus vaccine increased immunity of children
topv to bopv FACTSHEET 25 APRIL 2016
 topv to bopv FACTSHEET th 25 APRIL 2016 POLIO FREE INDIA POLIO FREE WORLD Polio immunization is important Polio is a crippling disease that can permanently paralyze any part of the body especially the
topv to bopv FACTSHEET th 25 APRIL 2016 POLIO FREE INDIA POLIO FREE WORLD Polio immunization is important Polio is a crippling disease that can permanently paralyze any part of the body especially the
Report of the Polio WG Meeting February Dr. Peter Figueroa Co-Chair, SAGE Polio Working Group 17 April, 2018
 Report of the Polio WG Meeting 20-21 February 2018 Dr. Peter Figueroa Co-Chair, SAGE Polio Working Group 17 April, 2018 Polio WG Discussions: Objectives To review progress towards global polio eradication
Report of the Polio WG Meeting 20-21 February 2018 Dr. Peter Figueroa Co-Chair, SAGE Polio Working Group 17 April, 2018 Polio WG Discussions: Objectives To review progress towards global polio eradication
Sample Collections and Poliovirus Containment: What s the Connection
 Centers for Disease Control and Prevention Center for Preparedness and Response Sample Collections and Poliovirus Containment: What s the Connection October 15, 2018 Christy Myrick, PhD, RBP What s in
Centers for Disease Control and Prevention Center for Preparedness and Response Sample Collections and Poliovirus Containment: What s the Connection October 15, 2018 Christy Myrick, PhD, RBP What s in
Abstract. n engl j med 369;21 nejm.org november 21,
 The new england journal of medicine established in 1812 november 21, 2013 vol. 369 no. 21 Identification and Control of a Poliomyelitis Outbreak in Xinjiang, China Hui-Ming Luo, M.D., Yong Zhang, M.D.,
The new england journal of medicine established in 1812 november 21, 2013 vol. 369 no. 21 Identification and Control of a Poliomyelitis Outbreak in Xinjiang, China Hui-Ming Luo, M.D., Yong Zhang, M.D.,
Performance of National Measles Case-Based Surveillance Systems in The WHO African Region
 Research Article Open Access Performance of National Measles Case-Based Surveillance Systems in The WHO African Region. 2012 2016 Balcha Masresha 1 *, Reggis Katsande 1, Richard Luce 2, Amadou Fall 3,
Research Article Open Access Performance of National Measles Case-Based Surveillance Systems in The WHO African Region. 2012 2016 Balcha Masresha 1 *, Reggis Katsande 1, Richard Luce 2, Amadou Fall 3,
TECHNICAL ADVISORY GROUP ON POLIO ERADICATION IN THE HORN OF AFRICA
 TECHNICAL ADVISORY GROUP ON POLIO ERADICATION IN THE HORN OF AFRICA 6 th Meeting Report NAIROBI, KENYA, 4-5 MAY 2011 Executive Summary The 6 th meeting of the Technical Advisory Group on Polio Eradication
TECHNICAL ADVISORY GROUP ON POLIO ERADICATION IN THE HORN OF AFRICA 6 th Meeting Report NAIROBI, KENYA, 4-5 MAY 2011 Executive Summary The 6 th meeting of the Technical Advisory Group on Polio Eradication
Objective 2 Strengthening Routine Immunization, IPV introduction and the topv-bopv switch
 Objective 2 Strengthening Routine Immunization, IPV introduction and the topv-bopv switch Polio Partners Group meeting Geneva 8 December 2014 Michel Zaffran Coordinator, WHO/EPI, IMG Co-chair On behalf
Objective 2 Strengthening Routine Immunization, IPV introduction and the topv-bopv switch Polio Partners Group meeting Geneva 8 December 2014 Michel Zaffran Coordinator, WHO/EPI, IMG Co-chair On behalf
SITUATION REPORT YELLOW FEVER 26 MAY 2016 SUMMARY
 ZIKA VIRUS SITUATION REPORT YELLOW FEVER 26 MAY 2016 SUMMARY A yellow fever outbreak was detected in Luanda, Angola late in December 2015. The first cases were confirmed by the National Institute for Communicable
ZIKA VIRUS SITUATION REPORT YELLOW FEVER 26 MAY 2016 SUMMARY A yellow fever outbreak was detected in Luanda, Angola late in December 2015. The first cases were confirmed by the National Institute for Communicable
National Action Plan for Response to Poliovirus Importation
 Public Health Res Perspect 2011 2(1), 65e71 doi:10.1016/j.phrp.2011.04.003 pissn 2210-9099 eissn 2233-6052 - BRIEF REPORT - National Action Plan for Response to Poliovirus Importation Kyung Min Song a,
Public Health Res Perspect 2011 2(1), 65e71 doi:10.1016/j.phrp.2011.04.003 pissn 2210-9099 eissn 2233-6052 - BRIEF REPORT - National Action Plan for Response to Poliovirus Importation Kyung Min Song a,
Mapping for Health in Cameroon: Polio Legacy and Beyond
 The Journal of Infectious Diseases SUPPLEMENT ARTICLE Mapping for Health in Cameroon: Polio Legacy and Beyond Louie C. Rosencrans, 1 Gerald E. Sume, 2 Jean-Christian Kouontchou, 2 Arend Voorman, 3 Yaw
The Journal of Infectious Diseases SUPPLEMENT ARTICLE Mapping for Health in Cameroon: Polio Legacy and Beyond Louie C. Rosencrans, 1 Gerald E. Sume, 2 Jean-Christian Kouontchou, 2 Arend Voorman, 3 Yaw
