Vol - 4, Issue - 3, Apr-Jul 2013 ISSN: Sarkhejiya et al PHARMA SCIENCE MONITOR
|
|
|
- Lenard Spencer
- 6 years ago
- Views:
Transcription
1 PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES REVIEW ON ORAL MUCOSAL DRUG DELIVERY SYSTEM Naimish A. Sarkhejiya*, Hiten P. Sodha, Yatin D. Kapdia, Vipul P. Patel Department of Pharmaceutics, School of Pharmacy, RK University, Rajkot , Gujarat, India. ABSTRACT Oral mucosal drug delivery is an alternative method of systemic drug delivery that offers several advantages over both injectable and enteral methods and also enhances drug bioavailability because the mucosal surfaces are usually rich in blood supply, providing the means for rapid drug transport to the systemic circulation and avoiding, in most cases, degradation by first-pass hepatic metabolism. The systems contact with the absorption surface resulting in a better absorption, and also prolong residence time at the site of application to permit once or twice daily dosing. In this review, attention is focused to give regarding physiology of oral mucosal including tissue permeability, barriers to permeation and route of permeation, biopharmaceutics of buccal and sublingual absorption, factors affecting drug absorption, detailed information of penetration enhancers, design of oral mucosal drug delivery system and role of mucoadhesion and various theories of bioadhesion. Evaluation techniques and selection of animal model for In-vivo studies are also discussed. Keywords: Oral mucosal, Buccal route, Sublingual route, Mucoadhesion, In-vitro and In-vivo model. INTRODUCTION The cost involved both in terms of money and time in the development of a single new chemical entity has made it mandatory for pharmaceutical companies to reconsider delivery strategies to improve the efficacy of drugs that have already been approved. However, despite the tremendous advances in drug delivery, the oral route remains the preferred route for the administration of therapeutic agents due to low cost, ease of administration and high level of patient compliance. However, significant barriers are imposed on the per oral administration of drugs, such as hepatic first pass metabolism and drug degradation within the gastrointestinal (GI) tract prohibiting the oral administration of certain classes of drugs especially biologics e.g. peptides and proteins. Consequently, other absorptive mucosa are being considered as potential sites for drug administration including the mucosal linings of the nasal, rectal, vaginal, ocular, and oral cavity. IC Value
2 These transmucosal routes of drug delivery offer distinct advantages over per oral administration for systemic drug delivery such as the possible bypass of the first pass effect and avoidance of presystemic elimination within the GI tract [1]. Amongst these, delivery of drugs to the oral cavity has attracted particular attention due to its potential for high patient compliance and unique physiological features. Within the oral mucosal cavity, the delivery of drugs is classified into two categories: (i) local delivery and (ii) systemic delivery either via the buccal or sublingual mucosa. This review examines the physiological considerations of the oral cavity in light of systemic drug delivery and provides an insight into the advances in oral transmucosal delivery systems. Oral mucosal drug delivery system is subdivided into buccal and sublingual in which buccal cavity is widely applicable for drug administration through mucosa in case of sublingual route mostly useful for fastest onset of action as in the case of Angina pectoris. Advantages of Oral Mucosal Drug Delivery system: [2] 1. Bypass of the gastrointestinal tract and hepatic portal system, increasing the bioavailability of orally administered drugs that otherwise undergo hepatic first-pass metabolism. In addition the drug is protected from degradation due to ph and digestive enzymes of the middle gastrointestinal tract. 2. Improved patient compliance due to the elimination of associated pain with injections; administration of drugs in unconscious or incapacitated patients; convenience of administration as compared to injections or oral medications. 3. Sustained drug delivery. 4. A relatively rapid onset of action can be achieved relative to the oral route, and the formulation can be removed if therapy is required to be discontinued. 5. Increased ease of drug administration. 6. Though less permeable than the sublingual area, the buccal mucosa is well vascularized, and drugs can be rapidly absorbed into the venous system underneath the oral mucosa. 7. In comparison to TDDS, mucosal surfaces do not have a stratum corneum. Thus, the major barrier layer to transdermal drug delivery is not a factor in Oral mucosal routes IC Value
3 of administration. Hence Oral mucosal systems exhibit a faster initiation and decline of delivery than do transdermal patches. 8. Oral mucosal delivery occurs is less variable between patients, resulting in lower intersubject variability as compared to transdermal patches. 9. The large contact surface of the oral cavity contributes to rapid and extensive drug absorption. Limitations of Oral Mucosal Drug Delivery System: Depending on whether local or systemic action is required the challenges faced while delivering drug via oral especially buccal drug delivery can be enumerated as follows. [2] 1. For local action the rapid elimination of drugs due to the flushing action of saliva or the ingestion of foods stuffs may lead to the requirement for frequent dosing. 2. The non-uniform distribution of drugs within saliva on release from a solid or semisolid delivery system could mean that some areas of the oral cavity may not receive effective levels. 3. For both local and systemic action, patient acceptability in terms of taste, irritancy and mouth feel is an issue. 4. For systemic delivery the relative impermeability of oral cavity mucosa with regard to drug absorption, especially for large hydrophilic biopharmaceuticals, is a major concern. Flow Chart for the Development of Oral Solid Dosage Form [3] Conventional oral solid dosage forms (tablets, capsules) Modified release tablets/capsules Fast action oral solid dosage form (fast dissolving tablets/capsules) Fast action solid dosage form (fast dissolving oral IC Value
4 Physiology of the Oral Mucosa [4,5,6,7] Structure The cheeks, lips, hard and soft palates and tongue form the oral cavity. The main difference between the oral mucosa and skin as compared to the gastrointestinal (GI) tract lining lies in the organization of the different epithelia. While the latter has a single layer of cells forming the simple epithelium, the skin and the oral cavity have several layers of cells with various degrees of differentiation. Within the oral cavity, the masticatory mucosa has a keratinized or cornified epithelium, and covers the stress-enduring regions such as the gingival and the hard palate, providing chemical resistance and mechanical strength. It is divided into four layers: keratinized, granular, prickle-cell, and basal layer (Figure 1). The lining mucosa, which provides elasticity, in contrast, is comprised of noncornified surface epithelium covering the rest of the regions including the lips, cheeks, floor of the mouth, and soft palate. It also can be further divided into superficial, intermediate, prickle-cell, and basal layers. The third type of mucosa is the specialized mucosa consisting of both keratinized and non-keratinized layers, and is restricted to the dorsal surface of the tongue. The intercellular spaces contain water, lipids, and proteins. Physiological Importance of Mucins and Saliva The mucosal tissues are further covered with mucus, which is negatively charged, and contains large glycoproteins termed mucins. These are thought to contribute significantly to the visco-elastic nature of saliva, and maintain a ph of Mucin consists of a protein core, rich in O-glycosylated serine and threonine, containing many helix-breaking proline residues. The salivary glands secreting mucus also synthesize saliva, which offers protection to the soft tissues from chemical and mechanical abrasions. The average thickness of the salivary film in the mouth varies between 0.07 and 0.10 mm. Sustained adhesion of the dosage form (tablet, patch) to the mucosa is an important first step to successful buccal delivery. The mucus plays an important role during this mucoadhesive process by buccal drug delivery systems. The interaction between the mucus and mucoadhesive polymers generally used in most dosage forms can be explained by theories summarized in Table 1. IC Value
5 Figure 1: Structure of the mucosa IC Value
6 TABLE 1: POSTULATED MECHANISM FOR POLYMER MUCOSAL Theory of Adhesion Adsorption Diffusion Electronic Wetting Fracture ADHESIVE PROPERTIES Mechanism of Adhesion Secondary chemical bonds such as van der waal forces, hydrophobic interactions, electrostatic attraction, and hydrogen bonds between mucus and polymer. Entanglements of the polymer chains in to mucus network. Attractive forces across electrical double layer formed due to electron transfer across polymer and mucus. Analyze the ability of past to spared over the biological surface and calculate the interfacial tension between the two. The tension is considered to proportional to X1 /2, where X is the polymer polymer interaction parameter. Low values of these parameters correspond to structural similarities between polymers and an increased miscibility. Relates to the force necessary to separate to surfaces to the adhesive bond strength and it is often used to calculate fracture strength of adhesive bonds. The mean total surface area of the mouth has been calculated to be cm2. The teeth, keratinized epithelium, and non-keratinized epithelium occupy about 20%, 50%, and 30% of this surface area, respectively. Drug delivery through the oral mucosa can be achieved via different pathways: sublingual (floor of the mouth), buccal (lining of the cheeks), and gingival (gums). The sublingual mucosa is the most permeable followed by the buccal and then the palatal. This is due to the presence of neutral lipids such as ceramides and acylceramides in the keratinized epithelia present on the palatal region, which are impermeable to water. The non-keratinized epithelia contain water-permeable ceramides and cholesterol sulfate. A comparison of the various mucosae is provided in Table 2.The thickness of the buccal epithelium varies from 10 to about 50 cell layers in different regions because of serrations in connective tissue. In fact, the thickness of buccal mucosa has been observed to be 580 micron meter, the hard palate 310 micron meter, the epidermis 120 micron meter, and the floor of mouth mucosa 190 micron meter. IC Value
7 TABLE 2: SUITABILITY OF VARIOUS REGIONS OF THE ORAL MUCOSA FOR THE TRANSMUCOSAL DRUG DELIVERY BASED ON VARIOUS TISSUE Tissue Structure PROPERTIES Epithelial thickness, µm Permeability Blood flow Residence time Buccal Nonkeratinized Sublingual Nonkeratinized Gingival Keratinized Palatal Keratinized Note ++ means very suitable; -- means least suitable. Tissue Permeability In comparison to the skin, the buccal mucosa offers higher permeability and faster onset of drug delivery; whereas the key features which help it score over the other mucosal route, the nasal delivery system, include robustness, ease of use, and avoidance of drug metabolism and degradation. The buccal mucosa and the skin have similar structures with multiple cell layers at different degrees of maturation. The buccal mucosa, however, lacks the intercellular lamellar bilayer structure found in the stratum corneum, and hence is more permeable. An additional factor contributing to the enhanced permeability is the rich blood supply in the oral cavity. The lamina propia, an irregular dense connective tissue, supports the oral epithelium. Though the epithelium is avascular, the lamina propia is endowed with the presence of small capillaries. These vessels drain absorbed drugs along with the blood into three major veins-lingual, facial, and retro-mandibular, which open directly into the internal jugular vein, thus avoiding first-pass metabolism. Numerous studies have been conducted comparing the blood supply of the oral cavity to the skin in animals. A thicker epithelium has been associated with a higher blood flow probably due to the greater metabolic demands of such epithelia. Gingiva and anterior and posterior dorsum of tongue have significantly higher blood flows than all other regions; skin has a lower flow than the majority of oral regions; and palate has the lowest of all regions. In fact, the mean blood flow to the buccal mucosa in IC Value
8 the rhesus monkey was observed to be 20.3 ml/min/100 g tissue as compared to 9.4 ml/min/100 g in the skin. Barriers to Permeation [8-10] The main resistance to drug permeation is caused by the variant patterns of differentiation exhibited by the keratinized and non-keratinized epithelia. As mucosal cells leave the basal layer, they differentiate and become flattened. Accumulation of lipids and proteins also occurs. This further culminates in a portion of the lipid that concentrates into small organelles called membrane-coating granules (MCGs). In addition, the cornified cells also synthesize and retain a number of proteins such as profillagrin and involucrin, which contribute to the formation of a thick cell envelope. The MCGs then migrate further and fuse with the intercellular spaces to release the lipid lamellae. The lamellae then fuse from end to end to form broad lipid sheets in the extracellular matrix, forming the main barrier to permeation in the keratinized regions in the oral cavity. These lamellae were first observed in porcine buccal mucosa, and have been recently identified in human buccal mucosa. Though the non-keratinized epithelia also contain a small portion of these lamellae, the random placement of these lamellae in the non-cornified tissue vis-à-vis the organized structure in the cornified tissue makes the former more permeable. Also, the non-keratinized mucosa does not contain acylceramides, but has small amounts of ceramides, glucosylceramides, and cholesterol sulfate. The lack of organized lipid lamellae and the presence of other lipids instead of acylceramides make the nonkeratinized mucosa more water permeable as compared to the keratinized mucosa. Physicochemical properties and routes of permeation There are two possible routes of drug absorption through the squamous stratified epithelium of the oral mucosa: Transcellular (intracellular, passing through the cell) Paracellular (intercellular, passing around the cell) Permeation across the buccal mucosa has been reported to be mainly by the paracellular route through the intercellular lipids produced by membrane-coating granules. Although passive diffusion is the main mechanism of drug absorption, specialized transport mechanisms have been reported to exist in other oral mucosa (that of the tongue) for a few drugs and nutrients; glucose and cefadroxil were shown to be absorbed in this way. IC Value
9 Figure 2 shows the two routes of permeation that can be used by drugs to pass through the buccal mucosa. The buccal mucosa is a potential site for the controlled delivery of hydrophilic macromolecular therapeutic agents (biopharmaceuticals) such as peptides, oligonucleotides and polysaccharides. However, these high molecular weight drugs usually have low permeability leading to a low bioavailability, and absorption enhancers may be required to overcome this. The buccal mucosa also contains proteases that may degrade peptide-based drugs. In addition, the salivary enzymes may also reduce stability. FIG. 2: A, Ultrastructural features of oral buccal epithelium. MCGs become evident microscopically in the prickle cell layer, approximately at the midpoint of the epithelium. B, Routes of drug transport across oral epithelium. Disease states where the mucosa is damaged would also be expected to increase permeability. This would be particularly true in conditions that result in erosion of the mucosa such as lichen planus, pemphigus, viral infections and allergic reactions. Biopharmaceutics of Buccal and Sublingual Absorption [11-13] Principles of Drug Absorption The oral mucosa contains both hydrophilic and hydrophobic components and a combination of both keratinized and non-keratinized epithelia. Passive diffusion is the IC Value
10 most common route of permeation through the oral mucosa, and uses the Fick s first law of diffusion given by the general equation P = DK p h The amount of drug absorbed A is given by; DK p P = PCSt = CSt h Where, P is the permeability coefficient, C is the free drug concentration in the delivery medium, D is the diffusion coefficient of the drug in the oral mucosa, Kp is the partition coefficient of the drug between the delivery medium and the oral mucosa, h is the thickness of the oral mucosa, S is the surface area of the delivery or the absorption site on the mucosa, and t is the duration of time the drug stays in contact with the mucosa. The thickness of the tissue, partition coefficient, and the diffusion coefficient are properties of the mucosa and cannot be altered. Designing appropriate formulations that need the necessary conditions can vary the surface area for delivery of the drug, time of contact, and the free drug concentration. The partitioning of the drug into the membrane will depend on its ratio of hydrophilicity and lipophilicity. Studies performed with amines and acids showed that their absorptions were proportional to their partition coefficients, thus also establishing the fact that the transcellular route was the primary route of absorption of these drugs. Similar results were obtained for b-adrenoreceptor-blocking drugs. Since the drug will face different barriers through the paracellular and the transcellular routes, the flux of drug permeation through these routes will differ to some extent. The equation above can be modified to account for this difference. Hydrophilic compounds will tend to use the paracellular route and permeate through the intercellular spaces, which present a smaller surface area. IC Value
11 The flux of drug permeation through this pathway can be described as Where, DH is the diffusion coefficient, h is the length of the tortuous path followed in the paracellular route, CD is the concentration of the drug on the donor side is the fraction of the surface area of the paracellular route. A lipophilic drug will preferably use the transcellular route since it will be easier for it to partition into the lipophilic cell membrane. The path length here is shorter than for the paracellular route but the drug has to move through several types of barriers (cell membrane, the cytoplasm, as well as intercellular spaces). Factors affecting Drug Absorption Besides the biochemical characteristics of the buccal and sublingual membranes, which are responsible for the barrier function and permeability, various factors of the drug molecule influence the extent of permeation through the membranes. The lipid solubility, degree of ionization, pka of the drug, ph of the drug solution, presence of saliva and the membrane characteristics, molecular weight and size of the drug, various physicochemical properties of the formulation, and the presence or absence of permeation enhancers, all affect the absorption and the permeation of drugs through the oral mucosa. Degree of Ionization, ph, and Lipid Solubility The permeability of unionizable compounds is a function of their lipid solubilities, determined by their oil water partition coefficients demonstrated this dependence of water permeability on the lipid contents of keratinized and non-keratinized epithelia. The lipids present however contribute to this effect more in the keratinized epithelia (more total lipid content, non-polar lipids, ceramides) than in the non-keratinized epithelia where permeability seems to be related to the amount of glycosylceramides present. The absorption of drug through a membrane depends upon its lipophilicity, which in turn depends on its degree of ionization and partition coefficient. The higher the unionized fraction of a drug, the greater is its lipid solubility. The degree of ionization in turn depends on the ph of the mucosal membrane and the pka of the drug. Beckett and Triggs studied the buccal absorption of basic drugs over a range of concentration, ph, and the use of different drug combinations (alone and mixtures). The resultant ph absorption curves showed that the percentage of drug absorbed increased as the concentration of drug in the unionized form increased. Also, the shapes of the absorption curves were a function of the pka values and the lipid IC Value
12 solubility of their unionized form. A study conducted with fentanyl, a weak base with a pka of 8.2, further demonstrated the relationship between the ph and the absorption across oral mucosa. When the ph of the delivery solution was increased, more of the drug was present in the unionized form, with the drug being 2.45% unionized at ph 6.6, 9.1% unionized at ph 7.2, and 24% unionized at ph 7.7. The fentanyl solutions with a ph range of 6.6 to 7.7 showed a three- to fivefold increase in peak plasma concentration, bioavailability, and permeability coefficients. Similar studies conducted with sublingual administration of opioids such as buprenorphine, methadone, and fentanyl showed increased absorption with increase in ph, where the drug was predominantly present in the unionized form. However, absorption of other opioids such as levorphanol, hydromorphone, oxycodone, and heroin under similar conditions did not improve. These drugs, however, were more hydrophilic as compared to the earlier set of opioids. Thus, ph modifiers can be used to adjust the ph of the saliva prior to drug administration to increase the absorption of such drugs through the mucosal membranes. However, the nature of the buccal and sublingual membrane complicates the above condition since the ph may vary depending on the area of the membrane and also on the layer of the membrane that is considered. The ph of the mucosal surface may be different from that of buccal and sublingual surfaces throughout the length of the permeation pathway. Thus, the drug in its unionized form may be well absorbed from the surface of the membrane, but the ph in the deeper layers of the membrane may change the ionization and thus the absorption. Also, the extent of ionization of a drug reflects the partitioning into the membrane, but may not reflect the permeation through the lipid layers of the mucosa. Henry et al., studied the buccal absorption of propranolol followed by repeated rinsing of the mouth with buffer solutions and recovered much of this drug in the rinsing. In addition, the effect of lipophilicity, ph, and pka will depend on the transport pathway used by the drug. Studies conducted with busiprone showed that the unionized form of the drug used the more lipophilic pathway, the transcellular route, but an increase in the ph increased the ionization of the drug and subsequently the absorption. It was concluded that this transport of the ionized form of the drug was through the more hydrophilic paracellular pathway. Therefore, at neutral ph the preferred pathway was found to be IC Value
13 transcellular, but at acidic ph, the ionized species of the drug also contributed to the absorption across the membrane. Molecular Size and Weight The permeability of a molecule through the mucosa is also related to its molecular size and weight, especially for hydrophilic substances. Molecules that are smaller in size appear to traverse the mucosa rapidly. The smaller hydrophilic molecules are thought to pass through the membrane pores, and larger molecules pass extracellularly. Increases in molar volume to greater than 80 ml/mol produced a sharp decrease in permeability. Due to the advantages offered by the buccal and the sublingual route, delivery of various proteins and peptides through this route has been investigated. It is difficult for the peptide molecules with high molecular weights to make passage through the mucosal membrane. Also, peptides are usually hydrophilic in nature. Thus, they would be traversing the membrane by the paracellular route, between cells through the aqueous regions next to the intercellular lipids. In addition, peptides often have charges associated with their molecules, and thus their absorption would depend on the amount of charge associated with the peptide, ph of the formulation and the membrane, and their isoelectric point. Permeability Coefficient To compare the permeation of various drugs, a standard equation calculating the permeability coefficient can be used. One form of this equation is P = % permeated V d A t 100 Where P is the permeability coefficient (cm/s), A is the surface area for permeation, Vd is the volume of donor compartment, and t is the time. This equation assumes that the concentration gradient of the drug passing through the membrane remains constant with time, as long as the percent of drug absorbed is small. IC Value
14 Formulation Factor The permeation of drugs across mucosal membranes also depends to an extent on the formulation factors. These will determine the amount and rate of drug released from the formulation, its solubility in saliva, and thus the concentration of drug in the tissues. In addition, the formulation can also influence the time the drug remains in contact with the mucosal membrane. After release from the formulation, the drug dissolves in the surrounding saliva, and then partitions into the membrane, thus the flux of drug permeation through the oral mucosa will depend on the concentration of the drug present in the saliva. This concentration can be manipulated by changing the amount of drug in the formulation, its release rate, and its solubility in the saliva. The first two factors vary in different types of formulations, and the last can be influenced by changing the properties of the saliva that affect the solubility (e.g., ph). Penetration Enhancers [14-16] To increase the absorption of poorly soluble drugs especially large hydrophilic molecules, permeation enhancers have become of increasing interest in recent years. Properties of Penetration Enhancers: 1. Safe and effective 2. Pharmacologically inactive 3. Chemically inert 4. Reversible effect Majority of the most widely investigated permeation enhancer have surfactant like properties and those that are water soluble seem to be most active at concentrations above the critical micelle concentration. The following have been investigated as a means of enhancing buccal permeability. Mechanism of Absorption Enhancement: Permeation enhancers in general act by following ways (figure 3): 1. Increasing the fluidity of the cell membrane 2. Extracting inter and intracellular lipids 3. Disrupting lipid structure e.g., solubilization by formation of micelles to create aqueous channels 4. Altering cellular proteins IC Value
15 5. Increasing the thermodynamic activity of the drug 6. Overcoming enzymatic barriers, particularly for peptide and protein drugs 7. Altering surface mucin rheology FIG. 3: MECHANISM OF ABSORPTION ENHANCEMENT Types of Penetration Enhancers: Bile salts Fatty acids and their salts and Esters Azones Surfactants Complexing Agents Co-solvents Miscellaneous Transmucosal drug delivery system Pharmaceutical consideration and formulation design for successful transmucosal drug delivery system Drug selection for oral transmucosal delivery is limited by the physicochemical properties of the drugs themselves. To be delivered transmucosally, drugs must have unique physicochemical properties, i.e. a proper balance between solubility and lipophilicity. Moreover, generally only a few milligrams of drug can cross the oral IC Value
16 mucosa, even if the drug has a favorable profile for oral mucosal delivery. Presently, new classes of drugs are typically not developed specifically for oral transmucosal delivery. Fig. 4: Oral transmucosal technology. It is also important to consider factors influencing drug release from a system. The release kinetics of a given drug from a system could be governed predominantly by the polymer morphology and excipients present in the system. Finally, ideal formulation and its degradation products should be non-toxic, non irritant and free from leachable impurities. It should not aid in development of secondary infections and prevent the effects of local drug irritation at the site of application. An ideal transmucosal drug delivery system must meet several prerequisites to be successful. The first prerequisite for a transmucosal drug delivery system is that it should rapidly attach to the mucosal surface and maintain a strong interaction to prevent displacement. Spontaneous adhesion of the system at the target site is critical and can be achieved through bioadhesion promoters that use tethered polymers. Contact time should also be sufficiently long at the target site, normally longer than that needed for complete drug release. The second prerequisite for a successful and effective transmucosal drug delivery system is that the bioadhesion performance should not be impacted by surrounding environmental ph. Other desirable characteristics of a transmucosal drug delivery system include high drug loading, complete drug release, and convenient administration. Drug release from a IC Value
17 polymeric material takes place either by the diffusion or by polymer degradation or by their combination. Polymer degradation usually takes place by the enzymes or hydrolysis. This may happen in the form of bulk erosion or surface erosion [17, 18]. It is also important to consider factors influencing drug release from a polymer. The release kinetics of a given drug from a polymeric matrix could be governed predominantly by the polymer morphology and excipients present in the system [19] Oral transmucosal dosage forms To improve oral transmucosal delivery of drugs, several new dosage forms have been developed: solutions, tablets/lozenges (including lyophilized and bioadhesive), chewing gum, solution sprays, laminated systems and patches, hydrogels, adhesive films, hollow fibres and microspheres [20]. Advances in oral mucosal drug delivery have focused on the development of drug delivery systems that not only achieve the therapeutic aims of delivery but also overcome the unfavorable environmental conditions found in the oral cavity. Modern formulations have used creative approaches that incorporate a combination of these strategies to create a balance between patient convenience and clinical benefits. Mucoadhesive carrier is a viable option to develop a non-invasive carrier platform for the controlled release of bioactive. Solid forms Several solid lozenges formulations have been developed and are commercially available, including nitroglycerin sublingual tablet, fentanyl lozenge on a handle and prochlorperazine buccal tablets. Although these formulations vary in shape and size, they share many common characteristics. This method of delivery is simple for patients to use. The solid formulations dissolve in the oral cavity. The drugs are released and exposed to the entire mucosa and the top third of the esophageal mucosa. The limitation of this delivery form is the short residence time. Depending on the size and formulation, the lozenge or tablet is usually dissolved within 30 min, thus limiting the total amount of drug that can be delivered. The dissolution or disintegration is usually controlled by the patient, i.e. how hard they suck the unit. Increased sucking and saliva production causes swallowing and loss of drug down the esophagus and the gastrointestinal tract. Thus, solid dosage forms generally have a much higher inter- and intraindividual variation in absorption and bioavailability. In addition, IC Value
18 since these formulations are open systems, the delivery medium is not well controlled. Although the formulation offers some control, it is difficult to control drug or other ingredient concentrations because the media is constantly diluted by saliva. This makes it difficult to effectively use permeation enhancers in this type of system. Taste of the drug is another hurdle for this delivery system. Unless the drug is tasteless or the taste can be masked by sweetening and flavorings agents, it is difficult to achieve high patient acceptability of this type of product. Gum Chewing gum is one of the modern approaches to oral transmucosal drug delivery and is a useful means for systemic drug delivery. The advantages of chewing gum over other oral mucosal drug delivery systems are the possibility of controlled drug release over an extended time and the potential to improve the variability in drug release and retention times. One of the advantages of chewing gum convenience. Furthermore, an individual may be able to control the drug intake by simply changing the rate and vigour of chewing, or expelling the gum altogether. Since chewing gum is also an open system, it shares many of the same limitations of the other solid formulations. Patches Flexible adhesive patches have been developed in an effort to overcome some of the drawbacks of other dosage forms. Transmucosal delivery patches have unique characteristics, including relatively rapid onset of drug delivery, sustained drug release and rapid decline in the serum drug concentration when the patch is removed. Also, a buccal patch is confined to the buccal area over which it is attached and therefore the absorption profile may have less inter- and intraindividual variability. In general, oral mucosal patches can be classified into three categories: patches with a dissolvable matrix, patches with a non-dissolvable backing, and patches with a dissolvable backing. Patches with a dissolvable matrix are designed to release drug into the oral cavity. They work similarly to, and share many of the limitations of, the solid dos e form. The mucoadhesive layer, either in the drug matrix or attached to drug matrix as an additional layer, prolongs the duration of drug matrix in the oral cavity. Therefore, compared with other open dosage forms, these types of patches are longer acting and can potentially deliver more drug. They also use the entire oral cavity mucosa as compared with other closed systems IC Value
19 that typically use smaller areas. These types of patches are also suitable for treating local diseases such as candidiasis or mucositis. Patches with non-dissolvable backing are usually designed for systemic delivery. Since they are closed systems and the formulations are protected from saliva, the drug concentrations are controlled and drug is continuously delivered for 10 to 15 h. The disadvantages of these systems are that they use only a small mucosal area and the backings have to be removed by the patient after drug administration. Patches with dissolvable backing share many characteristics of patches with non-dissolvable backing, but they have the advantage of the entire patch dissolving in the oral cavity. Patches with dissolvable backings are shorter acting than patches with non-dissolvable backing. Oral mucosal dosage forms are convenient, easy to use, and have the potential to offer a lowcost and painless alternative to more invasive routes of administration. Each delivery form offers very distinct delivery characteristics that can be used in a broad range of therapies. The majority of patches provide a longer period over which to deliver the formulated as either solvent cast mucoadhesive polymer discs or drug to and through the buccal mucosa. Solution, suspension, and gel-forming liquids Viscous liquids have been investigated primarily to coat the mucosa to act as a protectant or a vehicle for drug delivery for the treatment of local disorders, including motility dysfunction, fungal infections. Using sodium alginate suspension as a novel bioadhesive liquid, researchers showed that the esophageal surface can be coated to protect against reflux and can deliver therapeutic agents to the damaged mucosa [20, 21]. The retention behavior of various bioadhesive formulations was evaluated on the esophageal surface under conditions mimicking the salivary flow. Both polycarbophil and xanthum gum demonstrated excellent bioadhesive potential, and carmellose sodium and theromo sensitive poloxamer (Lutrol 407) demonstrated poor retention. A thermo sensitive hydrogel of poloxamer covalently linked to polyacrylic acid and carbopol. This esophageal bandage, upon oral administration, demonstrated significant retention within the esophagus. Multiparticulates, microparticles, and nanoparticles IC Value
20 Oral delivery systems based on multiparticulates, microparticles, and nanoparticles often exhibit improved performance in comparison with monolithic matrix tablets. 22 By diffusing into the mucous gel layer by virtue of their relatively small size, these small immobilized carriers show a prolonged gastrointestinal residence time. Recent work has shown that, in addition to size and chemistry, shape is also a critical feature of transmucosal drug delivery particles and can dictate particle velocity, diffusion and adhesion to the mucus surface in a complex manner [23-24]. In- vitro and in- vivo Study Methods [25-28] Animal Models for Studies The limited available tissue area in the human buccal cavity has encouraged the use of animal models that may mimic human oral mucosal absorption. Rats, hamsters, dogs, rabbits, guinea pigs, and rhesus monkeys have all been used in buccal studies. As with any animal model, these all have their advantages and disadvantages. Almost all animals have a completely keratinized epithelium. The hamster cheek pouch offers a large surface area but is not flushed with saliva. The oral mucosa of the monkey, a primate, has been widely used but the high cost of procurement as well as challenging handling are disadvantages when it comes to selecting these animals. Rabbit mucosa is similar to human mucosa since it has regions of non-keratinized tissue. However, the small surface area and difficulty in accessing the required tissue make it an impractical choice. The animal of choice remains the pig because of comparable permeability to human buccal mucosa and a large surface area enabling reduced variability in the data. The methods used for measuring the amount of drug absorbed have to be designed in such a way as to account for local delivery of the drug to the mucosa as well as systemic delivery through the mucosa into the circulation. A selection of in vivo and in vitro techniques has been developed and tested over the years. In- vivo Methods Both human and animal models have been used for in- vivo testing of oral mucosal drug delivery. Choices of animal models depend on how closely the mucosal membrane reflects the structure and properties of human mucosa. An important in vivo technique using human test subjects, the buccal absorption test was developed and established by Beckett and Triggs. They adjusted solutions of several basic drugs to various ph IC Value
21 values with buffer, and placed the solution in the subject s mouth. The solution was circulated about times by the movement of the cheeks and tongue for a contact time of 5 min. The solution was then expelled, and the subject s mouth was rinsed with 10 ml distilled water for 10 s. The rinsing was collected, and combined with the earlier expelled solution, and the fraction of the drug remaining in this solution was measured by gas liquid chromatography. It was observed that the absorption of drug from the oral cavity was dependent on ph. Though this technique is easy to perform, noninvasive, and gives relatively consistent results with little intra and inter subject variation, limitations for the method do exist. It does not provide information concerning the varying permeabilities of different regions in the oral cavity. Also, the continuous flow of saliva affects the ph of the applied solution as well as the overall volume. In addition, the test analyzes the amount of drug that has been transported from the sample into the oral cavity and does not provide information on the actual systemic absorption of the drugs. Some of the drugs could be swallowed or accumulated, and redistributed into the epithelium or biotransformed in the mucosa. Simultaneous measurement of appearance of the drug in the systemic circulation could further validate this test. Disk methods for assessing absorption have also been studied where the drug-loaded disk is kept in contact with certain area of the mucosal membrane to allow for absorption. One such polytef disk was used by Anders et al., for the buccal absorption of protirelin. The disk had an area of ~10 cm 2 and a central circular depression containing the drug. It was removed after 30 min of contact with the buccal mucosa, and blood samples were taken to determine the amount of drug absorbed from the mucosa. The disk method provides information about absorption from a specific area of the mucosa. Interference from salivary secretions, difficulties in keeping the disk adhered, and loss of drug permeating due to leakage of the drug from the disk is some disadvantages with this method. Another method has been the use of perfusion cells. These cells have certain specific area and can contain a drug solution that is stirred continuously. The closed cell isolates the solution from the surroundings, thus negating the effects of the environmental factors such as saliva and ph. Solution under test can be passed through the mucosal membrane once or it can be re-circulated. The solution in the IC Value
22 cells is then analyzed for drug content. However, the surface area for absorption is low, and the tissue has a tendency to become erythematous. This method, like the buccal absorption test, measures the loss of drug from the cell, but not the actual absorption of the drug through the buccal mucosa. These methods have been used to analyze different types of dosage forms (composite films, patches, and bioadhesive tablets) and their mucosal drug absorption and have been used to assess both buccal and sublingual absorptions across the respective mucosa. A glass perfusion cell was developed and used by Yamahara et al., for the measurement of drug absorption through mucosal membranes of anesthetized male beagle dogs. The cell contained a biocompatible bioadhesive polymer O-ring that adhere the cell to the oral mucosal membrane. This type of cell can be used to measure buccal and sublingual absorption as well as perfusion through the surface of the tongue. In- vitro Methods These methods have proven to be important tools in the study of Oral mucosal absorption, since they can facilitate studies of drug permeation under controlled experimental conditions. Oral mucosal tissue can be surgically removed from the oral cavity of animals. These tissues contain a fair amount of connective tissue, which is separated from the mucosal membrane. This connective tissue, if not removed, may contribute to the permeability barrier. This separation can be carried out with the aid of heat where tissues are separated at 60oC, or chemically by the use of various enzymes or EDTA. These tissues are then stored in buffer solution (usually Krebs). This storage step is important in preserving the viability and integrity of the tissue. The tissue is then placed in a side-by-side diffusion cell, where the placement of the tissue is in between the donor and the receptor chambers. The donor contains the drug solution, whereas the receptor usually contains a buffer solution to emulate the body fluids. The chambers can be stirred continuously to ensure even distribution of the drug and are maintained at a desired temperature. The epithelial side of the tissue faces the donor chamber, allowing the drug to pass from the donor chamber through the tissue into the receptor chamber from where samples can be withdrawn at specific time intervals and replaced with fresh receptor solution. IC Value
23 Cell and Tissue Culture Systems The advantages of the in vitro approaches described above also apply to buccal cell culture systems. In addition, other aspects such as cell growth and differentiation can be studied in these systems in detail. Also, once the source is established, a continuous supply of cell lines can be obtained, which obviates the need for expensive animal or human tissues that are often difficult to obtain in large quantities. On the other hand, the established cell line must simulate, as closely as possible, the physical and biochemical properties of the buccal or sublingual tissues in- vivo. These properties such as the growth, differentiation, biological barrier effectiveness, permeability levels, and metabolic pathways are crucial to the permeation studies. Current and future development of transmucosal drug delivery Many dosage forms have been developed and include toothpastes, mouthwashes, lozenges, gels, chewing gums, lollipops, films, patches, tablets and some specialized devices [29]. Conventional dosage forms, however, exhibit some drawbacks, for example, low bioavailability, because of the washing effect of saliva and mechanical stresses. Formulations that prolong the drug release in the mouth offer great advantages in preventing and treating local diseases or in promoting transmucosal delivery of drugs for systemic therapies [30]. Despite these obstacles for transmucosal drug delivery mentioned above, the buccal mucosa remains an attractive site for the delivery of systemic drugs, in particular for those who are prone to a high level of degradation inside the gastrointestinal tract. Various buccal delivery applications have thus been marketed or proposed in treatment of systemic and chronic diseases among them are trigeminal neuralgia, Meiniere's disease, diabetes, addiction and so on [31-37]. Similar to the treatment of diseases affecting the oral cavity, intraoral systemic drug delivery would benefit from sustained drug release, without the need for the patient to intervene. This would raise the patient's compliance particularly of chronically ill. Acharya et al. patented a device and method for oral transmucosal delivery of drugs or any other constituents via the inner buccal cavity. The device is applied and adheres to the mucosa of the oral cavity without causing adverse effects. It consists of a bilayer tablet: a mucoadhesive layer and an overlying active substance containing layer. The mucoadhesive layer can contain polyvinylpyrrolidone (PVP) as the only adhesive or can IC Value
24 be combined with other hydrophilic polymeric substances. It was claimed that this nonplasticized PVP mucoadhesive has sufficient adhesion not only for mucosal membranes but also to a variety of materials, such as polyacrylic denture material. The active layer also contains a hydrophilic polymer carrier. The layers in the device dissolve and release the active substance into the oral cavity and are particularly suitable for delivering substances active in the oral cavity such as breath fresheners and substances to combat dry mouth. It is also useful for the delivery of ionic drugs such as peptides. Krumme et al. patented a device and a method of multi-layer transmucosal therapeutic film, comprising at least two layers connected with each other, for transmucosal administration of active substances [38]. The therapeutic systems which are suitable, in particular, for transmucosal administration (entering through or across the mucous membrane) of active substances have a structure of at least two layers that are connected with each other. The mucoadhesive layer is capable of swelling in an aqueous medium, although it is insoluble or only poorly soluble in such media. One of the two sides of the inventive system is limited by a mucoadhesive layer which optionally contains active substance or is free of active substance. The mucoadhesive layer of the system is connected with a backing layer that is monolayered or double-layered and which may serve as an active substance reservoir. The insolubility or reduced solubility of the adhesive layer increases the period of adhesion to the mucosa, thereby enabling an active substance release that lasts for a prolonged period. Since the inventive systems are film-shaped and may have a thickness of less than 1 mm, they do not cause a foreign body sensation and are not unpleasant for patients thus contributing to improved compliance. Clinical application of oral transmucosal drug delivery Oral transmucosal delivery of analgesics has received considerable attention. Oral transmucosal fentanyl is designed to deliver rapid analgesia for breakthrough pain, providing patients with a noninvasive, easy to use and non-intimidating option. For analgesics that are used to treat mild to moderate pain, rapid onset has relatively little benefit and oral mucosal delivery is a poor option. Oral mucosal deliveries of sedatives such as midazolam, triazolam and etomidate have shown favorable results with clinical advantages over other routes of administration. Oral mucosal delivery of the antinausea IC Value
25 drugs scopolamine and prochlorperazine has received some attention, as has oral mucosal delivery of drugs for erectile dysfunction. Oral transmucosal formulations of testosterone and estrogen have been developed. In clinical studies, sublingual testosterone has been shown to result in increase in the lean muscle mass and muscle strength, improvement in positive mood parameters, and increases in genital responsiveness in women. Short-term administration of estrogen to menopausal women with cardiovascular disease has been shown to produce coronary and peripheral vasodilatation, reduction of vascular resistance and improvement in endothelial function. Studies of sublingual administration of estrogen are needed to clarify the most beneficial regimen. Although many drugs have been evaluated for oral transmucosal delivery, few are commercially available. The clinical need for oral transmucosal delivery of a drug must be high enough to offset the high costs associated with developing this type of product. Several cardiovascular drugs administered transmucosally have been studied extensively. Nitroglycerin is one of the most common drugs delivered through the oral mucosa. Transmucosal absorption of nitroglycerin from solutions through the oral cavity was demonstrated in the midnineteenth century. Research on other cardiovascular drugs, such as captopril, verapamil and propafenone, has proven promising. Oral transmucosal delivery of analgesics has received considerable attention. These drugs include potent analgesics such as oral transmucosal fentanyl citrate and buprenorphine. Oral transmucosal fentanyl is designed to deliver rapid analgesia for breakthrough pain, providing patients with a non-invasive, easy to use and non-intimidating option. For analgesics that are used to treat mild to moderate pain, rapid onset has relatively little benefit and oral mucosal delivery is a poor option. Oral mucosal delivery of sedatives such as midazolam, triazolam and etomidate has shown favorable results with clinical advantages over other routes of administration. Oral mucosal drug delivery offers several advantages over both injectable and enteral delivery. Drugs absorbed via the oral mucosa to avoid the fate of enterically administered drugs: low gastric ph and proteases, and first-pass hepatic degradation. One early study of the hypoglycaemic effects of sublingual insulin indicated that absorption of human insulin through the oral mucosa is possible [39]. Oral transmucosal fentanyl is one such example. IC Value
Topical Preparations
 Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
BUCCAL DRUG DELIVERY SYSTEM
 BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
D9G : Oro-Mucosal Dosage Forms Development Background Paper
 D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
Journal of Controlled Release
 Journal of Controlled Release 140 (2009) 2 11 Contents lists available at ScienceDirect Journal of Controlled Release journal homepage: www.elsevier.com/locate/jconrel Review Orotransmucosal drug delivery
Journal of Controlled Release 140 (2009) 2 11 Contents lists available at ScienceDirect Journal of Controlled Release journal homepage: www.elsevier.com/locate/jconrel Review Orotransmucosal drug delivery
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p.
 Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Pharmacokinetics I. Dr. M.Mothilal Assistant professor
 Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
OVERVIEW OF ORAL MUCOSA 1 : A] Structure:
![OVERVIEW OF ORAL MUCOSA 1 : A] Structure: OVERVIEW OF ORAL MUCOSA 1 : A] Structure:](/thumbs/95/123509578.jpg) INTRODUCTION Drug delivery systems that can precisely control the release rate of drug to specific body sites have an enormous potential in the healthcare world. The last two decades in the pharmaceutical
INTRODUCTION Drug delivery systems that can precisely control the release rate of drug to specific body sites have an enormous potential in the healthcare world. The last two decades in the pharmaceutical
Enhanced delivery methods for greater efficacy
 On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester
 University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
Routes of drug administration
 Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Fundamentals of Pharmacology for Veterinary Technicians Chapter 4
 (A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
(A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
Determination of bioavailability
 Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION
 PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
School of Pharmacy Faculty of Medicine The Chinese University of Hong Kong. Transdermal Drug Delivery System
 Workshop in Celebration of 25 th Anniversary of the School of Pharmacy Biopharmaceutics of Modified Release Products and Challenging Drug Molecules Design and Regulatory Assessment of Transdermal Drug
Workshop in Celebration of 25 th Anniversary of the School of Pharmacy Biopharmaceutics of Modified Release Products and Challenging Drug Molecules Design and Regulatory Assessment of Transdermal Drug
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Development of Nutrient Delivery Systems: Ingredients & Challenges
 Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Suppository Chapter Content
 10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
PHAR 7632 Chapter 7. Table Market and Share of Pharmaceuticals by ROA Data from Viswanathan, 2004
 Student Objectives for this Chapter After completing the material in this chapter each student should:- be able to describe various routes of drug administration including the concentration versus time
Student Objectives for this Chapter After completing the material in this chapter each student should:- be able to describe various routes of drug administration including the concentration versus time
Concepts for the talk. Poisoning by Topical Medications The Toxicology of Transdermal Drug Delivery. Early patches. The transdermal patch
 Concepts for the talk Poisoning by Topical Medications The Toxicology of Transdermal Drug Delivery Lewis Nelson, M.D. New York University School of Medicine New York City Poison Control Center Understand
Concepts for the talk Poisoning by Topical Medications The Toxicology of Transdermal Drug Delivery Lewis Nelson, M.D. New York University School of Medicine New York City Poison Control Center Understand
5 Application of the ESR online-method for the monitoring of nanocapsule digestion
 5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
Delivery of proteins (Routes of administration and absorption enhancement) The parenteral route of administration:
 Delivery of proteins (Routes of administration and absorption enhancement) 1 The parenteral route of administration: Parenteral administration is here defined as administration via those routes where a
Delivery of proteins (Routes of administration and absorption enhancement) 1 The parenteral route of administration: Parenteral administration is here defined as administration via those routes where a
Compounding Options in Women s Health: Dosage Forms
 Compounding Options in Women s Health: Dosage Forms Ranel A. Larsen, RPh, PharmD HRT Symposium Las Vegas, NV February 16 18, 2017 Routes of Administration Common: Topical Oral Sublingual Vaginal Other:
Compounding Options in Women s Health: Dosage Forms Ranel A. Larsen, RPh, PharmD HRT Symposium Las Vegas, NV February 16 18, 2017 Routes of Administration Common: Topical Oral Sublingual Vaginal Other:
NANO 243/CENG 207 Course Use Only
 L6. Drug Administration & Transport by Fluid Motion April 19, 2018 Part I: Drug Administration Routes of Drug Administration Topical: local effect, substance is applied directly where its action is desired.
L6. Drug Administration & Transport by Fluid Motion April 19, 2018 Part I: Drug Administration Routes of Drug Administration Topical: local effect, substance is applied directly where its action is desired.
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more
 Lecture-4 Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more easily than less lipid-soluble or more water-soluble
Lecture-4 Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more easily than less lipid-soluble or more water-soluble
FENTANYL CITRATE TRANSMUCOSAL UTILIZATION MANAGEMENT CRITERIA
 FENTANYL CITRATE TRANSMUCOSAL UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: BRAND (generic) NAMES: HICL = H3AT Fentanyl citrate transmucosal Actiq (fentanyl citrate) lozenge on a handle 200, 400, 600, 800,
FENTANYL CITRATE TRANSMUCOSAL UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: BRAND (generic) NAMES: HICL = H3AT Fentanyl citrate transmucosal Actiq (fentanyl citrate) lozenge on a handle 200, 400, 600, 800,
FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels
 Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Lecture 1: Physicochemical Properties of Drugs and Drug Disposition
 Lecture 1: Physicochemical Properties of Drugs and Drug Disposition Key objectives: 1. Be able to explain the benefits of oral versus IV drug administration 2. Be able to explain the factors involved in
Lecture 1: Physicochemical Properties of Drugs and Drug Disposition Key objectives: 1. Be able to explain the benefits of oral versus IV drug administration 2. Be able to explain the factors involved in
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Transdermal Delivery of Newer Atypical Antipsychotics ABSTRACT
 Transdermal Delivery of Newer Atypical Antipsychotics ABSTRACT Abstract Risperidone and olanzapine, newer atypical antipsychotics are highly effective and safer in the treatment of psychosis. A low dose
Transdermal Delivery of Newer Atypical Antipsychotics ABSTRACT Abstract Risperidone and olanzapine, newer atypical antipsychotics are highly effective and safer in the treatment of psychosis. A low dose
A review on Mucoadhesive Buccal Patches
 June - July 2017; 6(4):2654-2660 International Journal of Research and Development in Pharmacy & Life Science An International Open access peer reviewed journal ISSN (P): 2393-932X, ISSN (E): 2278-0238
June - July 2017; 6(4):2654-2660 International Journal of Research and Development in Pharmacy & Life Science An International Open access peer reviewed journal ISSN (P): 2393-932X, ISSN (E): 2278-0238
BUCCAL DRUG DELIVERY SYSTEM
 BUCCAL DRUG DELIVERY SYSTEM INTRODUCTION: 1. Sublingual delivery, consisting of administration through the membrane of the ventral surface of the tongue and the floor of the mouth. 2. Buccal delivery,
BUCCAL DRUG DELIVERY SYSTEM INTRODUCTION: 1. Sublingual delivery, consisting of administration through the membrane of the ventral surface of the tongue and the floor of the mouth. 2. Buccal delivery,
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS. Pharmaceutical Manufacturing-4
 CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
Introduction to. Pharmacokinetics. University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 Introduction to 1 Pharmacokinetics University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 2 Learning objectives Understand compartment models and how they effects
Introduction to 1 Pharmacokinetics University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 2 Learning objectives Understand compartment models and how they effects
Physical Pharmacy. Interfacial phenomena. Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department
 Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE
 UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE FACULTY OF MEDICAL SCIENCES SCHOOL OF PHARMACY BACHELOR OF SCIENCE IN PHARMACY DEGREE COURSE SYLLABUS COURSE TITLE: COURSE CODE: BIOPHARMACEUTICS, NEW DRUG DELIVERY
UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE FACULTY OF MEDICAL SCIENCES SCHOOL OF PHARMACY BACHELOR OF SCIENCE IN PHARMACY DEGREE COURSE SYLLABUS COURSE TITLE: COURSE CODE: BIOPHARMACEUTICS, NEW DRUG DELIVERY
Emulsions. Purpose of emulsions and of emulsification:
 Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
DRUG DISTRIBUTION. Distribution Blood Brain Barrier Protein Binding
 DRUG DISTRIBUTION Distribution Blood Brain Barrier Protein Binding DRUG DISTRIBUTION Drug distribution is a reversible transport of drug through the body by the systemic circulation The drug molecules
DRUG DISTRIBUTION Distribution Blood Brain Barrier Protein Binding DRUG DISTRIBUTION Drug distribution is a reversible transport of drug through the body by the systemic circulation The drug molecules
REVISION OF MONOGRAPH ON TABLETS. Tablets
 March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Chapter 2 Transport Systems
 Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
An introduction to Liposomal Encapsulation Technology
 An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
Right time, right place: bioactive delivery systems
 Right time, right place: bioactive delivery systems Zhigao Niu, Alejandra Acevedo-Fani & Ali Rashidinejad Science of Food Team Riddet Institute, Massey University Developing High-Value Foods Food Systems
Right time, right place: bioactive delivery systems Zhigao Niu, Alejandra Acevedo-Fani & Ali Rashidinejad Science of Food Team Riddet Institute, Massey University Developing High-Value Foods Food Systems
Membrane Structure and Function - 1
 Membrane Structure and Function - 1 The Cell Membrane and Interactions with the Environment Cells interact with their environment in a number of ways. Each cell needs to obtain oxygen and other nutrients
Membrane Structure and Function - 1 The Cell Membrane and Interactions with the Environment Cells interact with their environment in a number of ways. Each cell needs to obtain oxygen and other nutrients
Pharmacokinetic Phase
 RSPT 2217 Principles of Drug Action Part 2: The Pharmacokinetic Phase Gardenhire Chapter 2; p. 14-25 From the Text Common Pathways for Drug Box 2-3; page 18 Plasma Half-lives of Common Drugs Table 2-4;
RSPT 2217 Principles of Drug Action Part 2: The Pharmacokinetic Phase Gardenhire Chapter 2; p. 14-25 From the Text Common Pathways for Drug Box 2-3; page 18 Plasma Half-lives of Common Drugs Table 2-4;
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Cell membrane & Transport. Dr. Ali Ebneshahidi Ebneshahidi
 Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
Chimica Farmaceutica. Pharmacokinetics and related topics
 Chimica Farmaceutica Pharmacokinetics and related topics INTRODUCTION In order to produce its intended effect, a drug must be present at an appropriate concentration in the fluid surrounding the effect
Chimica Farmaceutica Pharmacokinetics and related topics INTRODUCTION In order to produce its intended effect, a drug must be present at an appropriate concentration in the fluid surrounding the effect
Cell Membranes and Signaling
 5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS
 CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS by: j. jayasutha lecturer department of pharmacy practice Srm college of pharmacy srm university DRUG CHARACTERIZATION: Pre-formulation studies will attempt
CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS by: j. jayasutha lecturer department of pharmacy practice Srm college of pharmacy srm university DRUG CHARACTERIZATION: Pre-formulation studies will attempt
7. SUMMARY, CONCLUSION AND RECOMMENDATIONS
 211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
Chemical Level Of Organization
 Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
Is the science that study relation of physicochemical properties of drug, dosage form, & route of administration on rate and extent of drug
 Chapter 5 Is the science that study relation of physicochemical properties of drug, dosage form, & route of administration on rate and extent of drug absorption. It is the study of the kinetics of absorption,
Chapter 5 Is the science that study relation of physicochemical properties of drug, dosage form, & route of administration on rate and extent of drug absorption. It is the study of the kinetics of absorption,
Tongue In the buccal cavity of the digestive system
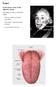 Tongue In the buccal cavity of the digestive system same layers as those of tubular organs Mucosa, submucosa, and muscularis muscularis = the muscularis externa no muscularis mucosa 1 Tongue ling = tongue
Tongue In the buccal cavity of the digestive system same layers as those of tubular organs Mucosa, submucosa, and muscularis muscularis = the muscularis externa no muscularis mucosa 1 Tongue ling = tongue
INFORMATION TOPIC: II-5 OR DEMONSTRATION: II-5. DOSAGE, MEASUREMENTS, AND DRUG FORMS (Lesson Title) OBJECTIVES THE STUDENT WILL BE ABLE TO:
 LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
Cell Membranes Valencia college
 6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
Membrane transport. Pharmacy Dr. Szilvia Barkó
 Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
4. ABSORPTION. Transport mechanisms. Absorption ABSORPTION MECHANISMS. Active transport. Active transport uses metabolic energy
 4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
Biopharmaceutics. Lec: 4
 64 Biopharmaceutics Physicochemical Properties of Drugs Affecting Bioavailability Lec: 4 1 Assist. Lecturer Ali Yaseen Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School
64 Biopharmaceutics Physicochemical Properties of Drugs Affecting Bioavailability Lec: 4 1 Assist. Lecturer Ali Yaseen Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School
Saliva. Introduction. Salivary Flow. Saliva and the Plaque Biofilm. The Minerals in Saliva
 Saliva Introduction Saliva is like a bloodstream to the mouth. As does blood, saliva helps build and maintain the health of the soft and hard tissues. Saliva removes waste products and provides disease-fighting
Saliva Introduction Saliva is like a bloodstream to the mouth. As does blood, saliva helps build and maintain the health of the soft and hard tissues. Saliva removes waste products and provides disease-fighting
Digestive System. How your body obtains nutrients. Wednesday, March 2, 16
 Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Molecular Cell Biology. Prof. D. Karunagaran. Department of Biotechnology. Indian Institute of Technology Madras
 Molecular Cell Biology Prof. D. Karunagaran Department of Biotechnology Indian Institute of Technology Madras Module 4 Membrane Organization and Transport Across Membranes Lecture 1 Cell Membrane and Transport
Molecular Cell Biology Prof. D. Karunagaran Department of Biotechnology Indian Institute of Technology Madras Module 4 Membrane Organization and Transport Across Membranes Lecture 1 Cell Membrane and Transport
B4 NUTRITION 4.3 Animal Nutrition
 B4 NUTRITION 4.3 Animal Nutrition 1. State the term balanced diet & describe how balanced diet is related to age, sex & activity of an individual. Balanced diet: A diet that contains all the main nutrients
B4 NUTRITION 4.3 Animal Nutrition 1. State the term balanced diet & describe how balanced diet is related to age, sex & activity of an individual. Balanced diet: A diet that contains all the main nutrients
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
Pharmaceutics I صيدالنيات 1. Unit 2 Route of Drug Administration
 Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
Assem Al Refaei. Sameer Emeish. Dr.Alia. Hodaifa Ababneh & Abdullah Shurafa
 8 Assem Al Refaei Sameer Emeish Hodaifa Ababneh & Abdullah Shurafa Dr.Alia Sheet Checklist Bioequivalence and Therapeutic equivalence. Factors Influencing Absorption. Revising Bioavailability. Factors
8 Assem Al Refaei Sameer Emeish Hodaifa Ababneh & Abdullah Shurafa Dr.Alia Sheet Checklist Bioequivalence and Therapeutic equivalence. Factors Influencing Absorption. Revising Bioavailability. Factors
Pharmacokinetic Phase
 RSPT 2317 Principles of Drug Action Part 2: The Pharmacokinetic Phase Pharmacokinetic Phase This phase describes the time course and disposition of a drug in the body, based on its absorption, distribution,
RSPT 2317 Principles of Drug Action Part 2: The Pharmacokinetic Phase Pharmacokinetic Phase This phase describes the time course and disposition of a drug in the body, based on its absorption, distribution,
KING KHALID UNIVERSITY
 KING KHALID UNIVERSITY COLLEGE OF PHARMACY DEPARTMENT OF PHARMACEUTICS COURSE SCHEDULE MALE SECTION SOLID DOSAGE FORMS FOR PHARMACEUTICAL SCIENCES/CLINICAL PHARMACY BY PROF DR MOHAMED FATHY Academic Session
KING KHALID UNIVERSITY COLLEGE OF PHARMACY DEPARTMENT OF PHARMACEUTICS COURSE SCHEDULE MALE SECTION SOLID DOSAGE FORMS FOR PHARMACEUTICAL SCIENCES/CLINICAL PHARMACY BY PROF DR MOHAMED FATHY Academic Session
Microneedles for Drug Delivery via Gastrointestinal Tract. Maleeha Akram 08-arid-1772 Ph.D. Zoology
 Microneedles for Drug Delivery via Gastrointestinal Tract Maleeha Akram 08-arid-1772 Ph.D. Zoology 1 Contents Gastrointestinal tract Histology of GI tract Absorption through GI tract Drug delivery History
Microneedles for Drug Delivery via Gastrointestinal Tract Maleeha Akram 08-arid-1772 Ph.D. Zoology 1 Contents Gastrointestinal tract Histology of GI tract Absorption through GI tract Drug delivery History
Two main groups Alimentary canal continuous coiled hollow tube Accessory digestive organs
 Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Cells & Tissues. Chapter 3
 Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Pharmacokinetics Dr. Iman Lec. 3
 Pharmacokinetics r. Iman Lec. 3 Pharmacokinetics A dequate drug doses must be delivered to the target organ to get therapeutic but not toxic levels. So, pharmacokinetic examines the movement of drug over
Pharmacokinetics r. Iman Lec. 3 Pharmacokinetics A dequate drug doses must be delivered to the target organ to get therapeutic but not toxic levels. So, pharmacokinetic examines the movement of drug over
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
Liquid-Liquid Extraction Prof. Mukesh Doble Department Of Biotechnology Indian Institute Of Technology, Madras. Lecture - 19
 Liquid-Liquid Extraction Prof. Mukesh Doble Department Of Biotechnology Indian Institute Of Technology, Madras Lecture - 19 Liquid-Liquid Extraction Let us continue with the Liquid- Liquid Extraction.
Liquid-Liquid Extraction Prof. Mukesh Doble Department Of Biotechnology Indian Institute Of Technology, Madras Lecture - 19 Liquid-Liquid Extraction Let us continue with the Liquid- Liquid Extraction.
Dow Wolff Cellulosics. Using ingenuity and savvy. TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow
 Dow Wolff Cellulosics Pharmaceutical Excipients Using ingenuity and savvy to help design healthcare solutions TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Enhancing
Dow Wolff Cellulosics Pharmaceutical Excipients Using ingenuity and savvy to help design healthcare solutions TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Enhancing
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Ch 7 Nutrition in humans
 Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
WHY... 8/21/2013 LEARNING OUTCOMES PHARMACOKINETICS I. A Absorption. D Distribution DEFINITION ADME AND THERAPEUIC ACTION
 PHARMACOKINETICS I Absorption & Distribution LEARNING OUTCOMES By the end of the lecture students will be able to.. Dr Ruwan Parakramawansha MBBS, MD, MRCP(UK),MRCPE, DMT(UK) (2013/08/21) Define pharmacokinetics,
PHARMACOKINETICS I Absorption & Distribution LEARNING OUTCOMES By the end of the lecture students will be able to.. Dr Ruwan Parakramawansha MBBS, MD, MRCP(UK),MRCPE, DMT(UK) (2013/08/21) Define pharmacokinetics,
Scholars Research Library. Formulation and evaluation of buccoadhesive tablet of Atenolol
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(2): 34-38 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(2): 34-38 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
ORAL TRANSMUCOSAL AND NASAL FENTANYL UTILIZATION MANAGEMENT CRITERIA
 ORAL TRANSMUCOSAL AND NASAL FENTANYL UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: BRAND (generic) NAMES: Fentanyl by oral transmucosal and nasal delivery Actiq (fentanyl citrate) lozenge on a handle 200,
ORAL TRANSMUCOSAL AND NASAL FENTANYL UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: BRAND (generic) NAMES: Fentanyl by oral transmucosal and nasal delivery Actiq (fentanyl citrate) lozenge on a handle 200,
1 - Drug preparations and route of drug administration
 1 - Drug preparations and route of drug administration - There are many ways to administer drugs 1. Enteral > drugs taken into gastro-intestinal (GI) tract, e.g. swallowing a pill 2. Parenteral > drugs
1 - Drug preparations and route of drug administration - There are many ways to administer drugs 1. Enteral > drugs taken into gastro-intestinal (GI) tract, e.g. swallowing a pill 2. Parenteral > drugs
Summary and general discussion
 Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
8 Influence of permeation modulators on the behaviour of a SC lipid model mixture
 8 Influence of permeation modulators on the behaviour of a SC lipid model mixture 8.1 Introduction In the foregoing parts of this thesis, a model membrane system of SC lipids has been developed and characterized.
8 Influence of permeation modulators on the behaviour of a SC lipid model mixture 8.1 Introduction In the foregoing parts of this thesis, a model membrane system of SC lipids has been developed and characterized.
WHAT. Vademecum lens care
 WHAT Vademecum lens care HA Multipurpose solution with hyaluronic acid The most complete multipurpose solution on the market Hidro Health HA is a multipurpose solution with hyaluronic acid used to disinfect,
WHAT Vademecum lens care HA Multipurpose solution with hyaluronic acid The most complete multipurpose solution on the market Hidro Health HA is a multipurpose solution with hyaluronic acid used to disinfect,
Membrane Structure and Membrane Transport of Small Molecules. Assist. Prof. Pinar Tulay Faculty of Medicine
 Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Pharmacokinetics of Drugs. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Pharmacokinetics of Drugs Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Absorption Is the transfer of a drug from its site of administration to the bloodstream.
Pharmacokinetics of Drugs Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Absorption Is the transfer of a drug from its site of administration to the bloodstream.
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
a. parotid b. sublingual c. submandibular
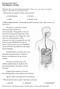 Bozeman Science/ Nature The Digestive System Watch the videos, and answer the questions below. Please write your answers in complete sentences, and explain all concepts thoroughly. 1. What are the four
Bozeman Science/ Nature The Digestive System Watch the videos, and answer the questions below. Please write your answers in complete sentences, and explain all concepts thoroughly. 1. What are the four
Includes mouth, pharynx, esophagus, stomach, small intestine, large intestine, rectum, anus. Salivary glands, liver, gallbladder, pancreas
 Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
International Journal of Pharmacy
 International Journal of Pharmacy Journal Homepage: http://www.pharmascholars.com Review Article CODEN: IJPNL6 SUBLINGUAL SPRAY: A BOOST TO NOVEL DRUG DELIVERY SYSTEM Karishma Yogeshkumar Halatwala*, Devarshi
International Journal of Pharmacy Journal Homepage: http://www.pharmascholars.com Review Article CODEN: IJPNL6 SUBLINGUAL SPRAY: A BOOST TO NOVEL DRUG DELIVERY SYSTEM Karishma Yogeshkumar Halatwala*, Devarshi
Cell Membrane Structure (1.3) IB Diploma Biology
 Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Pharmacodynamics & Pharmacokinetics 1
 PCTH 325 Pharmacodynamics & Pharmacokinetics 1 Dr. Shabbits jennifer.shabbits@ubc.ca September 9, 2014 Learning objectives 1. Describe the categories of intended drug action 2. Compare and contrast agonists
PCTH 325 Pharmacodynamics & Pharmacokinetics 1 Dr. Shabbits jennifer.shabbits@ubc.ca September 9, 2014 Learning objectives 1. Describe the categories of intended drug action 2. Compare and contrast agonists
