Investigation of the Anti-Inflammatory Potential of Aloe vera Gel (97.5%) in the Ultraviolet Erythema Test
|
|
|
- Anthony Waters
- 6 years ago
- Views:
Transcription
1 Original Paper Skin Pharmacol Physiol 2008;21: DOI: / Received: May 11, 2007 Accepted after revision: November 14, 2007 Published online: February 5, 2008 Investigation of the Anti-Inflammatory Potential of Aloe vera Gel (97.5%) in the Ultraviolet Erythema Test J. Reuter a A. Jocher a J. Stump a B. Grossjohann b G. Franke b C.M. Schempp a a Department of Dermatology, University Medical Center Freiburg, Freiburg, and b Riemser Arzneimittel AG, Insel-Riems, Germany Key Words Aloe vera gel Aloe vera barbadensis Minimal erythema dose Erythema index Hydrocortisone Prednicarbate Abstract Background: Aloe vera is a natural product that is frequently used in soothing skin care products such as aftersun lotions. In the present study we aimed to explore the anti-inflammatory potential of a highly concentrated A. vera gel in the UV erythema test in vivo. Methods: 40 volunteers with skin types II and III were included in the randomized, doubleblind, placebo-controlled, phase III monocenter study. Test areas on the back were irradiated with the 1.5-fold minimal erythema dose of UVB. Subsequently, the test areas were treated occlusively on 2 subsequent days with A. vera gel (97.5%), the positive controls (0.25% prednicarbate, 1% hydrocortisone in placebo gel and 1% hydrocortisone cream) and a placebo gel. Erythema values were determined photometrically after 24 and 48 h. Results: A. vera gel (97.5%) significantly reduced UV-induced erythema after 48 h, being superior to 1% hydrocortisone in placebo gel. In contrast, 1% hydrocortisone in cream was more efficient than A. vera gel. Conclusions: In this study after 48 h the A. vera gel (97.5%) displayed some anti-inflammatory effects superior to those of 1% hydrocortisone in placebo gel. The A. vera gel tested here might be useful in the topical treatment of inflammatory skin conditions such as UV-induced erythema. Copyright 2008 S. Karger AG, Basel Introduction Aloe barbadensis Miller (synonym: Aloe vera ) is a perennial succulent plant belonging to the Liliaceae plant family. The plant is the source of two products, gel and latex. The parenchymatous cells in the leaf pulp contain the mucilaginous gel which is extracted by squeezing the leaves. The peripheral bundle sheath cells of the plant produce the yellow bitter juice, Aloe latex, mainly containing anthraquinones and their derivatives [barbaloin (aloin A), isobarbaloin (aloin B), aloesin, aloresin A, aloeemodin]. Aloe latex on a dry-weight base additionally contains acid-insoluble resin, a significant amount of ash and essential oil. The most important ingredients of the A. vera gel are polysaccharides, mainly acemannan [1]. Although the scientific literature yields little to substantiate claims regarding systemic effects of A. vera [2], the consumption of A. vera leaf formulations has been shown to display anti-arthritic and antirheumatoid [3], anticancer [4] and antidiabetic [5] properties. A. vera gel is used topically for a number of therapeutic purposes including treatment of chronic wounds and thermal injury [6, 7], inflammation [8], treatment of oral ulcers [9], prevention of ultraviolet (UV)-induced immunosuppression [10] and treatment of psoriasis [11] and skin infections [12]. Since published results on the effects of A. vera gel on skin conditions are inconsistent [13 15], well-designed scientific studies are needed to address this topic, especially with respect to sunburn treatment. Fax karger@karger.ch S. Karger AG, Basel /08/ $24.50/0 Accessible online at: Prof. Dr. med. Dipl. Biol. C.M. Schempp Universitäts-Hautklinik Freiburg, Hauptstrasse 7 DE Freiburg im Breisgau (Germany) Tel , Fax christoph.schempp@uniklinik-freiburg.de
2 The present study aimed to explore the anti-inflammatory potential of a highly concentrated A. vera gel in vivo. The most frequently used method in preclinical studies and screenings for active constituents is the UV erythema test [16 18]. Recently we have shown that the back is the best localization to perform the test, and the optimal UVB dose is the 1.5-fold minimal erythema dose (MED) [19]. Here, we investigated a concentrated A. vera gel (97.5%) in comparison to 1% hydrocortisone in placebo gel as well as two commercially available corticosteroid preparations, one containing 1% hydrocortisone (Dermallerg-Ratiopharm cream 1%) and the other containing 0.25% prednicarbate (Dermatop cream). Irradiated Nonirradiated Material and Methods Forty patients were included in this prospective, randomized, double-blind, placebo-controlled study. The study was approved by the local ethics committee and was performed according to established Good Clinical Practice guidelines. All subjects enrolled in the study gave their written informed consent. Included were healthy nonsmoking persons of both sexes, aged at least 18 years, with skin types II and III according to the classification of Fitzpatrick [20]. Exclusion criteria were skin types I and VI, allergic disposition, inflammatory skin diseases, photosensitivity, sunbed tanning, metabolic diseases, use of any drugs (except for contraceptives), alcohol consumption, infections, pregnancy and breast-feeding, and participation in other clinical studies during the last 2 months. No topical corticosteroids were allowed 8 weeks before the onset of the study. The UV erythema test was performed as described [19, 21]. In brief, for the determination of the MED, gradually increasing doses of UVB were administered on the lower back. Twenty-four hours after irradiation, the first barely perceptible erythema with sharp borders was defined as MED. After the erythema index zero value (baseline) had been measured in the test areas with a Mexameter [19], half of the test areas were irradiated with the 1.5-fold MED of UVB. The other half of the test areas was not irradiated ( fig. 1 ). Immediately after irradiation, the test substances were applied in a blinded manner according to a randomization plan. The substances, each 20 mg per test area, were applied occlusively using extra large Finn chambers (1.8 cm 2, Hermal, Hamburg, Germany) on 6! 4 cm stripes of Fixomull (BSN Medical, Hamburg, Germany). The tapes were fixed with larger stripes of Fixomull. To prevent false measurements due to reactions to the tape, the tape was removed 23 h after substance application, i.e. 1 h before evaluation of the test areas. Subsequently, the Mexameter measurement of the erythema index of the test areas and a photographic documentation were performed followed by taping the test areas another time with the substances. Twenty-three hours later, the tapes were removed again, 1 h before the Mexameter measurement and the photographic documentation 48 h after UV irradiation. Each analysis was documented in the case report form. Fig. 1. Arrangement of test areas on the left and right sides of the back. The following test substances were applied to the irradiated and nonirradiated sites: A. vera gel (97.5%) was the primary test substance and was manufactured in May 2004 (batch No ). Dermallerg-Ratiopharm cream 1% (1% hydrocortisone), Dermatop cream (0.25% prednicarbate) and 1% hydrocortisone in placebo gel base were chosen as positive controls. Because the A. vera gel (97% v/v) also functions as a vehicle, no identical vehicle could be used as placebo. Therefore, as a negative control a conventional gel base was used (water, glycerol 85%, phenoxyethanol, carbopol ETD 2020, sodium edetate, sodium hydroxide and perfume oil). One untreated test area served as baseline value. Erythema measurements were performed 3 times in each test site directly before the UV irradiation at time point zero (T 0 ), after 24 h (T 1 ) and after 48 h (T 2 ). All data were recorded in 2 separate databases and compared with each other. The median of the 3 measurements after T 1 and T 2 was taken and averaged over the 40 subjects. The primary endpoint of the study was the difference of erythema between the test substance at time points T 1 and T 2 and the value at time point T 0 in comparison to the untreated area. Secondary endpoints were the comparison of the test substance with the controls, and the skin tolerance on the nonirradiated sites. Statistical analysis was performed by repeated-measures ANOVA (SpSS software package, version 11.0). Due to a significant variety of differences between the measurements T 1 and T 2 and the basic value, univariate analysis with 1 = T 1 T 0 and 2 = T 2 T 0 was performed. R e s u l t s Nineteen males and 21 females, aged between 20 and 56 years, participated in the study. Twelve of the females were taking contraceptives. All females were tested nega- Anti-Inflammatory Effect of Aloe vera Gel Skin Pharmacol Physiol 2008;21:
3 MED (mj/cm 2 ) Erythema measurement a b c d e f Treatment Nonirradiated Irradiated Number of volunteers Fig. 3. Erythema values 24 h after irradiation and treatment of test areas with different substances. a Untreated. b Placebo gel. c A. vera gel (97.5%). d 1% hydrocortisone in placebo gel. e Dermallerg-Ratiopharm 1% cream. f Dermatop cream. p! 0.05: significant difference compared to untreated area. Fig. 2. Distribution of the MED of the 40 volunteers. 50 tive for pregnancy. All subjects completed the study according to the protocol. The MED of the 40 subjects ranged between 50 and 125 mj/cm 2 ( fig. 2 ). Irradiation of the skin resulted in a highly significant increase in erythema values. After 24 h, only the treatment with prednicarbate (Dermatop cream) followed by Dermallerg-Ratiopharm cream 1% efficiently reduced the erythema. On the nonirradiated sites, a typical blanching effect of the glucocorticosteroids could be observed. The A. vera gel (97.5%), the placebo gel but also 1% hydrocortisone in placebo gel did not show a significant effect 24 h after irradiation ( fig. 3 ). Forty-eight hours after irradiation, all substances including the placebo gel displayed significant erythema-reducing effects ( fig. 4 ). The erythema reduction achieved by the A. vera gel (97.5%) was not significantly superior to the effect of the placebo gel. Interestingly, 1% hydrocortisone in placebo gel was not as efficient as the placebo gel or A. vera gel (97.5%) whereas 1% hydrocortisone incorporated in a cream (Dermallerg-Ratiopharm cream 1%) resulted in an erythema reduction that was superior to the results of all other test substances. All test substances were well tolerated. Seven out of 40 subjects showed skin reactions to the tape. The irritation lasted maximally for 84 h and subsided without therapy. One subject had concomitant itching. The adverse events were mild to moderate and appeared isolated. A correlation with the applied test substances was estimated as Erythema measurement a b c d e f Treatment Nonirradiated Irradiated Fig. 4. Erythema values 48 h after irradiation and treatment of test areas with different substances. a Untreated. b Placebo gel. c A. vera gel (97.5%). d 1% hydrocortisone in placebo gel. e Dermallerg-Ratiopharm 1% cream. f Dermatop cream. p! 0.05: significant difference compared to untreated area. possible in 2 subjects, but as a result of irritations on several test areas no correlation of the side effect to a single substance could be assessed. Apart from longer-lasting postinflammatory hyperpigmentations of the irradiated areas, no persistent disadvantages were notified. All subjects showed complete remittance. No allergic contact dermatitis attributed to A. vera gel (97.5%) or other test substances was observed. 108 Skin Pharmacol Physiol 2008;21: Reuter /Jocher /Stump /Grossjohann / Franke /Schempp
4 Discussion For the treatment of acute cutaneous inflammation, predominantly topical corticosteroids are used. In chronic inflammatory frequently relapsing skin conditions, long-term treatment with corticosteroids bears the risk of adverse events like skin atrophy, teleangiectasia and dyspigmentation. Therefore, there is a need for topical preparations containing active ingredients with a comparative anti-inflammatory potential but without the side effects of corticosteroids. On this background there is an increasing scientific interest for herbal ingredients as anti-inflammatory agents. For screening purposes in vivo, the UV erythema test is emerging as a suitable tool [18, 19, 22 24]. Previously, the results of several controlled clinical studies had been in favor of the anti-inflammatory properties of A. vera [6, 7, 25, 26]. However, in a small study with 12 volunteers no anti-inflammatory properties of A. vera gel were found using the UV erythema test [27]. During this study the assessment of erythema was performed 6 and 24 h after administration of the A. vera gel. In the study presented here, we could not detect any anti-inflammatory effect of A. vera gel after 24 h either. Forty-eight hours after application of the gel, a significant effect could be detected. Obviously, the onset of the effect of botanical ingredients is delayed. This observation was also made in other studies that tested topical herbal preparations [18, 19]. In the present study, 40 subjects were included in the trial, who were irradiated with the 1.5-fold MED on test areas on the back. This localization is the best test site of the body with the smallest fluctuations of the photometric measurements and the best match of the erythema of the light scale compared to that of the test sites [19]. The effect of A. vera gel (97.5%) was compared to Dermallerg- Ratiopharm cream 1% (1% hydrocortisone) and Dermatop cream (0.25% prednicarbate) as well as 1% hydrocortisone in placebo gel as positive controls. As expected, the commercially available potent corticosteroid preparations resulted in significant erythema suppression already after 24 h. Also, the well-known blanching effect of corticosteroids related to vasoconstriction of the superficial vasculature could be observed in nonirradiated test areas. A. vera gel (97.5%) also caused a significant inhibition of UVB-induced erythema but only 48 h after the first application. This effect was even stronger than that of 1% hydrocortisone in placebo gel, but weaker compared to that of the commercially available corticosteroids. It is striking that the hydrophilic placebo gel used as negative control also showed a significant anti-inflammatory effect comparable to the effect of A. vera gel (97.5%) 48 h after administration. However, the effect of the A. vera gel can hardly be attributed to placebo effects because additional constituents make up only 2.5% in the A. vera gel. The success of the gel base might be explained by a refrigerant effect or specific effects of the gel compounds, such as glycerol or phenoxyethanol. Interestingly, 1% hydrocortisone in placebo gel was less effective after 48 h than the placebo gel or A. vera gel (97.5%) whereas 1% hydrocortisone in the base of a cream efficiently reduced erythema after 48 h. Presumably, the gel base may inhibit the effects of hydrocortisone. A. vera is known to cause hypersensitivity reactions in the form of contact dermatitis [7, 28]. The A. vera gel preparation (97.5%) used in our study was well tolerated without any side effects. No conclusion can be made about the sensitization potential of A. vera gel (97.5%) since the occurrence of an allergy after a single application is rather rare. We conclude that A. vera gel (97.5%) has some anti-inflammatory potential with regard to reducing skin inflammation caused by UVB irradiation. The effective use of A. vera gel (97.5%) for chronic inflammatory skin conditions cannot be predicted on the base of the present study. Because of the lack of corticosteroid side effects, it might be reasonable to use A. vera gel (97.5%) as an additive for skin care products such as aftersun lotions instead of a hydrocortisone-containing gel. References 1 Boudreau MD, Beland FA: An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2006; 24: Klein AD, Penneys NS: Aloe vera. J Am Acad Dermatol 1988; 18: Davis RH, Agnew PS, Shapiro E: Antiarthritic activity of anthraquinones found in aloe for podiatric medicine. J Am Podiatr Med Assoc 1986; 76: Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggioni A, Basso C, Diaspro A, Salvato B, Carli M, Palu G: Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res 2000; 60: Anti-Inflammatory Effect of Aloe vera Gel Skin Pharmacol Physiol 2008;21:
5 5 Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O: Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine 1996; 3: Avijgan M: Phytotherapy: an alternative treatment for non-healing ulcers. J Wound Care 2004; 13: Somboonwong J, Thanamittramanee S, Jariyapongskul A, Patumaj S: Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J Med Assoc Thai 2000; 83: Vázquez B, Avila G, Segura D, Escalante B: Antiinflammatory activity of extracts of Aloe vera gel. J Ethnopharmacol 1996; 55: Rees TD: Oral ulcers remedy gets FDA clearance. J Am Dent Assoc 1994; 125: Byeon SW, Pelley RP, Ullrich SE, Waller TA, Bucana CD, Strickland FM: Aloe barbadensis extracts reduce the production of interleukin-10 after exposure to ultraviolet radiation. J Invest Dermatol 1998; 110: Seyger MM, van de Kerkhof PC, van Vlijmen-Willems IM, de Bakker ES, Zwiers F, de Jong EM: The efficacy of a new topical treatment for psoriasis: Mirak. J Eur Acad Dermatol Venereol 1998; 11: Roesler J, Steinmuller C, Kiderlen A, Emmendorffer A, Wagner H, Lohmann-Matthes ML: Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int J Immunopharmacol 1991; 13: Vogler BK, Ernst E: Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract 1999; 49: Reynolds T, Dweck AC: Aloe vera leaf gel: a review update. J Ethnopharmacol 1999; 68: Capasso F, Borrelli F, Di Carlo G, Izzo AA, Pinto L, Mascolo N, Castaldo S, Longo R: Aloe and its therapeutic use. Phytother Res 1998; 12: Juhlin L, Shroot B: Effect of drugs on the early and late phase UV erythema. Acta Derm Venereol 1992; 72: Takiwaki H, Shirai S, Kohno H, Soh H, Arase S: The degrees of UVB-induced erythema and pigmentation correlate linearly and are reduced in a parallel manner by topical anti-inflammatory agents. J Invest Dermatol 1994; 103: Hughes-Formella BJ, Bohnsack K, Rippke F, Benner G, Rudolph M, Tausch I, Gassmueller J: Anti-inflammatory effect of hamamelis lotion in a UVB erythema test. Dermatology 1998; 196: Jocher A, Kessler S, Hornstein S, Schulte Monting J, Schempp CM: The UV erythema test as a model to investigate the anti-inflammatory potency of topical preparations reevaluation and optimization of the method. Skin Pharmacol Physiol 2005; 18: Fitzpatrick T: The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988; 124: Schempp CM, Muller K, Schulte-Monting J, Schopf E, Simon JC: Salt water bathing prior to UV-B irradiation leads to a decrease of the minimal erythema dose and an increased erythema index without affecting skin pigmentation. Photochem Photobiol 1999; 69: Krutmann J, Hönigsmann H: Handbuch der dermatologischen Phototherapie und Photodiagnostik. Berlin, Springer, 1997, pp Hughes-Formella BJ, Filbry A, Gassmueller J, Rippke F: Anti-inflammatory efficacy of topical preparations with 10% hamamelis destillate in a UVB erythema test. Skin Pharmacol Appl Skin Physiol 2002; 15: Rhodes LE, Friedman PS: A comparison of the ultraviolet B-induced erythemal response of back and buttock skin. Photoderm Photoimmunol Photomed 1992; 9: Hutter JA, Salman M, Stavinoha WB, Satsangi N, Williams RF, Streeper RT, Weintraub ST: Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J Nat Prod 1996; 59: Davis RH, Donato JJ, Hartman GM, Haas RC: Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J Am Podiatr Med Assoc 1994; 84: Crowell J, Hilsenbeck S, Penneys N: Aloe vera does not affect cutaneous erythema and blood flow following ultraviolet B exposure. Photodermatology 1989; 6: Morrow DM, Rapaport MJ, Strick RA: Hypersensitivity to aloe. Arch Dermatol 1980; 116: Skin Pharmacol Physiol 2008;21: Reuter /Jocher /Stump /Grossjohann / Franke /Schempp
6 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
ANTI-AGING. Aloe vera oil.
 ANTI-AGING Aloe vera oil www.provitalgroup.com Aloe vera oil BOTANY The genus Aloe, belonging to the family Asphodelaceae (formerly Liliaceae), comprises more than 350 species, which grow mainly in the
ANTI-AGING Aloe vera oil www.provitalgroup.com Aloe vera oil BOTANY The genus Aloe, belonging to the family Asphodelaceae (formerly Liliaceae), comprises more than 350 species, which grow mainly in the
HUMAN PHOTOTOXICITY AND PHOTOALLERGENICITY TEST. April, 2006
 HUMAN PHOTOTOXICITY AND PHOTOALLERGENICITY TEST April, 2006 Protocol Number: Title: Objective: Human Phototoxicity and Photoallergenicity Test The objective of the test is to assess the potential of a
HUMAN PHOTOTOXICITY AND PHOTOALLERGENICITY TEST April, 2006 Protocol Number: Title: Objective: Human Phototoxicity and Photoallergenicity Test The objective of the test is to assess the potential of a
An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma
 An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma Pearl E. Grimes, MD Melasma is a common disorder of hyperpigmentation typically characterized by relatively symmetric
An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma Pearl E. Grimes, MD Melasma is a common disorder of hyperpigmentation typically characterized by relatively symmetric
Clinical Study Report
 Clinical Study Report No. H 552 000-0902 / 290102BS page 1 of 40 Clinical Study Report Sponsor: Study No.: H 552 000-0902 / 290102BS EudraCT-No.: 2009-009948-23 Title: Study Preparation: A phase IIa single-center,
Clinical Study Report No. H 552 000-0902 / 290102BS page 1 of 40 Clinical Study Report Sponsor: Study No.: H 552 000-0902 / 290102BS EudraCT-No.: 2009-009948-23 Title: Study Preparation: A phase IIa single-center,
The Prevention and Management of Acute Skin Reactions Related to Radiation Therapy
 Evidence-Based Series 13-7 IN REVIEW A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Prevention and Management of Acute Skin Reactions Related to Radiation
Evidence-Based Series 13-7 IN REVIEW A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Prevention and Management of Acute Skin Reactions Related to Radiation
Effect of NPK fertilizers on chemical constituents of Aloe vera leaves
 Vol. 7/2 (2007) 258-262 JOURNAL OF NATURAL REMEDIES Effect of NPK fertilizers on chemical constituents of Aloe vera leaves V. S. P. Saradhi 1, Salma Khanam 1, B. G. Shivananda 1, T. Vasantha Kumar 2, T.
Vol. 7/2 (2007) 258-262 JOURNAL OF NATURAL REMEDIES Effect of NPK fertilizers on chemical constituents of Aloe vera leaves V. S. P. Saradhi 1, Salma Khanam 1, B. G. Shivananda 1, T. Vasantha Kumar 2, T.
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
During the last 20 years, the number of topical
 THERAPEUTICS FOR THE CLINICIAN Cumulative Irritation Potential of Adapalene 0.1% Cream and Gel Compared With Tretinoin Microsphere 0.04% and 0.1% Jonathan S. Dosik, MD; Kenneth Homer, MS; Stéphanie Arsonnaud
THERAPEUTICS FOR THE CLINICIAN Cumulative Irritation Potential of Adapalene 0.1% Cream and Gel Compared With Tretinoin Microsphere 0.04% and 0.1% Jonathan S. Dosik, MD; Kenneth Homer, MS; Stéphanie Arsonnaud
An In-Depth Look at the Function and Efficacy of Oral Glutathione for Skin Care
 An In-Depth Look at the Function and Efficacy of Oral Glutathione for Skin Care Page 2 Page 3 Page 4 Relumins Advanced Nutrition 15x Glutathione Page 5 Page 6 Page 7 Page 8 Page 9 1.Matsuki, Mitsuo; Watanabe,
An In-Depth Look at the Function and Efficacy of Oral Glutathione for Skin Care Page 2 Page 3 Page 4 Relumins Advanced Nutrition 15x Glutathione Page 5 Page 6 Page 7 Page 8 Page 9 1.Matsuki, Mitsuo; Watanabe,
DOI /j x
 THERAPEUTICS DOI 10.1111/j.1365-2133.2007.08370.x The efficacy of aloe vera gel in the treatment of oral lichen planus: a randomized controlled trial C. Choonhakarn, P. Busaracome, B. Sripanidkulchai*
THERAPEUTICS DOI 10.1111/j.1365-2133.2007.08370.x The efficacy of aloe vera gel in the treatment of oral lichen planus: a randomized controlled trial C. Choonhakarn, P. Busaracome, B. Sripanidkulchai*
A comparative study between aloe vera gel dressing and conventional dressing in chronic wounds
 International Surgery Journal Athavale VS et al. Int Surg J. 2017 Oct;4(10):3427-3432 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174510
International Surgery Journal Athavale VS et al. Int Surg J. 2017 Oct;4(10):3427-3432 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174510
Atopic dermatitis (AD) is a common chronic skin. Phototherapy in the management of atopic dermatitis: a systematic review.
 Photodermatol Photoimmunol Photomed 2007; 23: 106 112 Blackwell Munksgaard r 2007 The Authors Journal compilation r 2007 Blackwell Munksgaard Review article Phototherapy in the management of atopic dermatitis:
Photodermatol Photoimmunol Photomed 2007; 23: 106 112 Blackwell Munksgaard r 2007 The Authors Journal compilation r 2007 Blackwell Munksgaard Review article Phototherapy in the management of atopic dermatitis:
Unofficial translation of the German package leaflet. Package leaflet: Information for the user
 Unofficial translation of the German package leaflet Package leaflet: Information for the user Soventol HydroCort 0.5% Spray 5 mg/g solution Active substance: Hydrocortisone For use in adults and children
Unofficial translation of the German package leaflet Package leaflet: Information for the user Soventol HydroCort 0.5% Spray 5 mg/g solution Active substance: Hydrocortisone For use in adults and children
This PDF is available for free download from a site hosted by Medknow Publications
 Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Original Policy Date
 MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
Clinical abstracts. Diabetes
 Clinical abstracts Diabetes Antidiabetic Activity Of Aloes: Preliminary Clinical & Experimental Observations Ghannam N; Kingston M; Al-Meshaal IA; Tariq M; Parman NS; Woodhouse N Horm Res 24(4):288-94
Clinical abstracts Diabetes Antidiabetic Activity Of Aloes: Preliminary Clinical & Experimental Observations Ghannam N; Kingston M; Al-Meshaal IA; Tariq M; Parman NS; Woodhouse N Horm Res 24(4):288-94
A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients
 Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP)
 CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
The Ointment is back!
 Now Available for Plaque Psoriasis The Ointment is back! Bioequivalent to Dovonex * Ointment Before prescribing, please see attached full Prescribing Information. The same Delivers the reliability you
Now Available for Plaque Psoriasis The Ointment is back! Bioequivalent to Dovonex * Ointment Before prescribing, please see attached full Prescribing Information. The same Delivers the reliability you
Pravit Asawanonda, MD, DSc, and Yaowalak Nateetongrungsak, MD Bangkok, Thailand
 Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque-type psoriasis: A randomized, placebo-controlled study Pravit Asawanonda, MD, DSc, and
Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque-type psoriasis: A randomized, placebo-controlled study Pravit Asawanonda, MD, DSc, and
Literature Scan: Topical Corticosteroids. Month/Year of Review: March 2015 Date of Last Review: March 2013 Source Document: OSU College of Pharmacy
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Skin Pigmentation Kinetics After UVB Exposure
 Acta Derm Venereol 2008; 88: 223 228 INVESTIGATIVE REPORT Skin Pigmentation Kinetics After UVB Exposure Mette H. Ravnbak, Peter A. Philipsen, Stine R. Wiegell and Hans C. Wulf Department of Dermatology,
Acta Derm Venereol 2008; 88: 223 228 INVESTIGATIVE REPORT Skin Pigmentation Kinetics After UVB Exposure Mette H. Ravnbak, Peter A. Philipsen, Stine R. Wiegell and Hans C. Wulf Department of Dermatology,
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
International Conference on Ayurveda Traditional Medicine and Medicinal Plant
 LITERARY REVIEW ON WOUND HEALING AND ANTI- INFLAMMATORY ACTIVITY OF ALOE VERA IN AYURVEDA & MODERN WAY. Dr.Sewwandi Darshika Kodituwakku Institute of Indigenous Medicine, University of Colombo, Rajagiriya,
LITERARY REVIEW ON WOUND HEALING AND ANTI- INFLAMMATORY ACTIVITY OF ALOE VERA IN AYURVEDA & MODERN WAY. Dr.Sewwandi Darshika Kodituwakku Institute of Indigenous Medicine, University of Colombo, Rajagiriya,
Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial
 Received: 10.7.2011 Accepted: 5.12.2011 Original Article Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial Fariba Iraji, 1
Received: 10.7.2011 Accepted: 5.12.2011 Original Article Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial Fariba Iraji, 1
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 January 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 January 2012 EPIDUO, gel Tube of 30 g (CIP code: 383 814-6) Tube of 60 g (CIP code: 383 816-9) Applicant: GALDERMA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 January 2012 EPIDUO, gel Tube of 30 g (CIP code: 383 814-6) Tube of 60 g (CIP code: 383 816-9) Applicant: GALDERMA
Public Assessment Report Scientific discussion. Gelistrol (estriol) SE/H/906/01/DC
 Public Assessment Report Scientific discussion Gelistrol (estriol) SE/H/906/01/DC This module reflects the scientific discussion for the approval of Gelistrol. The procedure was finalised at 2010-07-28.
Public Assessment Report Scientific discussion Gelistrol (estriol) SE/H/906/01/DC This module reflects the scientific discussion for the approval of Gelistrol. The procedure was finalised at 2010-07-28.
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology is intended for the treatment of: Steroid responsive dermatoses such as psoriasis and seborrheic dermatitis of the hairy regions,
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology is intended for the treatment of: Steroid responsive dermatoses such as psoriasis and seborrheic dermatitis of the hairy regions,
Non-invasive sun protection factor determination using LED light
 Non-invasive sun protection factor determination using LED light J. Lademann, S. Schanzer, S. Kobylinski, C. Reble, G. Khazaka, G. Wiora, H. Karrer, M. C. Meinke Outline 1. Introduction UV light and its
Non-invasive sun protection factor determination using LED light J. Lademann, S. Schanzer, S. Kobylinski, C. Reble, G. Khazaka, G. Wiora, H. Karrer, M. C. Meinke Outline 1. Introduction UV light and its
Activity of Different Desoximetasone Preparations Compared to Other Topical Corticosteroids in the Vasoconstriction Assay
 Original Paper DOI: 10.1159/000131082 Published online: June 3, 2008 Activity of Different Desoximetasone Preparations Compared to Other Topical Corticosteroids in the Vasoconstriction Assay C. Borelli
Original Paper DOI: 10.1159/000131082 Published online: June 3, 2008 Activity of Different Desoximetasone Preparations Compared to Other Topical Corticosteroids in the Vasoconstriction Assay C. Borelli
The Efficacy and Safety of 4-n-butylresorcinol 0.1% Cream for the Treatment of Melasma: A Randomized Controlled Split-face Trial
 Ann Dermatol Vol. 22, No. 1, 2010 DOI: 10.5021/ad.2010.22.1.21 ORIGINAL ARTICLE The Efficacy and Safety of 4-n-butylresorcinol 0.1% Cream for the Treatment of Melasma: A Randomized Controlled Split-face
Ann Dermatol Vol. 22, No. 1, 2010 DOI: 10.5021/ad.2010.22.1.21 ORIGINAL ARTICLE The Efficacy and Safety of 4-n-butylresorcinol 0.1% Cream for the Treatment of Melasma: A Randomized Controlled Split-face
Clinico Pathological Test SCPA605-Essential Pathology
 Clinico Pathological Test SCPA605-Essential Pathology Somphong Narkpinit, M.D. Department of Pathogbiology, Faculty of Science, Mahidol University e-mail : somphong.nar@mahidol.ac.th Pathogenesis of allergic
Clinico Pathological Test SCPA605-Essential Pathology Somphong Narkpinit, M.D. Department of Pathogbiology, Faculty of Science, Mahidol University e-mail : somphong.nar@mahidol.ac.th Pathogenesis of allergic
Bioavailability 55% - 77%
 Brand Name: Cosentyx Generic Name: secukinumab Manufacturer 1 : Novartis Pharmaceuticals Corporation Drug Class 2,3 : Antipsoriatic Agent, Interleukin-17A Receptor Antagonist Uses: Labeled Uses 1,2,3 :
Brand Name: Cosentyx Generic Name: secukinumab Manufacturer 1 : Novartis Pharmaceuticals Corporation Drug Class 2,3 : Antipsoriatic Agent, Interleukin-17A Receptor Antagonist Uses: Labeled Uses 1,2,3 :
New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
Patient Leaflet : Information for the user Locoid Cream 0.1% w/w Hydrocortisone butyrate
 Patient Leaflet : Information for the user Locoid Cream 0.1% w/w Hydrocortisone butyrate Read all of this leaflet carefully before you start using this medicine because it contains important information
Patient Leaflet : Information for the user Locoid Cream 0.1% w/w Hydrocortisone butyrate Read all of this leaflet carefully before you start using this medicine because it contains important information
SOME DOMINICAN MEDICINAL PLANTS IN THE ENID A. HAUPT CONSERVATORY AT THE NEW YORK BOTANICAL GARDEN
 SOME DOMINICAN MEDICINAL PLANTS IN THE ENID A. HAUPT CONSERVATORY AT THE NEW YORK BOTANICAL GARDEN Scientific Name: Costus spp. (Costaceae) Dominican common name: insulina English common name(s): crape
SOME DOMINICAN MEDICINAL PLANTS IN THE ENID A. HAUPT CONSERVATORY AT THE NEW YORK BOTANICAL GARDEN Scientific Name: Costus spp. (Costaceae) Dominican common name: insulina English common name(s): crape
PHOTOTHERAPY. With narrowband UVB, the light tubes produce a narrow part of the UVB spectrum. Two wavelengths
 Phototherapy (light therapy) refers to the use of ultraviolet (UV) light to treat moderate to severe eczema in children and adults. Phototherapy is a second-line treatment option that is available at specialist
Phototherapy (light therapy) refers to the use of ultraviolet (UV) light to treat moderate to severe eczema in children and adults. Phototherapy is a second-line treatment option that is available at specialist
Digoxin and aloe vera
 Digoxin and aloe vera Search What is aloe vera? What are the benefits of aloe vera? Get the scoop on this natural product, which is said to help with skin and digestive conditions. 15-10-2012 Aloe vera
Digoxin and aloe vera Search What is aloe vera? What are the benefits of aloe vera? Get the scoop on this natural product, which is said to help with skin and digestive conditions. 15-10-2012 Aloe vera
Update on emollients
 Update on emollients Amal Mhanna, MD Pediatric Dermatologist Clemenceau Medical Center Disclosure: I was a member of an advisory board y for J&J and received honoraria. Emollients and moisturizers are
Update on emollients Amal Mhanna, MD Pediatric Dermatologist Clemenceau Medical Center Disclosure: I was a member of an advisory board y for J&J and received honoraria. Emollients and moisturizers are
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Sunbed Use in Europe: Important Health Benefits and Minimal Health Risks
 Sunbed Use in Europe: Important Health Benefits and Minimal Health Risks William B. Grant, Ph.D. Director Sunlight, Nutrition and Health Research Center, San Francisco Outline Health Benefits of UV exposure
Sunbed Use in Europe: Important Health Benefits and Minimal Health Risks William B. Grant, Ph.D. Director Sunlight, Nutrition and Health Research Center, San Francisco Outline Health Benefits of UV exposure
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
2 SYNOPSIS. Study code : MC 9308 FR.
 MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
Novan Announces Promising Clinical Results with SB414
 Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
CONSENT FOR LASER/LIGHT-BASED TREATMENT
 107 West 15th St. Hays, KS (785) 639-1873 A DAY & M E D SPA CONSENT FOR LASER/LIGHT-BASED TREATMENT I authorize David Lenser, MD or Terri Lenser, RN to perform laser/pulsed light cosmetic skin treatments
107 West 15th St. Hays, KS (785) 639-1873 A DAY & M E D SPA CONSENT FOR LASER/LIGHT-BASED TREATMENT I authorize David Lenser, MD or Terri Lenser, RN to perform laser/pulsed light cosmetic skin treatments
Psoriasis is a chronic, inflammatory, T-cell mediated
 Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis Seyamak Saleky, MD; Işıl Bulur, MD; Zeynep Nurhan Saraçoğlu, MD PRACTICE POINTS The 25-hydroxyvitamin
Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis Seyamak Saleky, MD; Işıl Bulur, MD; Zeynep Nurhan Saraçoğlu, MD PRACTICE POINTS The 25-hydroxyvitamin
A randomized, controlled study of the safety and efficacy of topical corticosteroid treatments of sunburn in healthy volunteers
 Experimental dermatology Original article A randomized, controlled study of the safety and efficacy of topical corticosteroid treatments of sunburn in healthy volunteers L. Duteil, C. Queille-Roussel,
Experimental dermatology Original article A randomized, controlled study of the safety and efficacy of topical corticosteroid treatments of sunburn in healthy volunteers L. Duteil, C. Queille-Roussel,
Psoriasis is a lifelong condition, with onset
 THERAPEUTICS FOR THE CLINICIAN Clobetasol Propionate Lotion in the Treatment of Moderate to Severe Plaque-Type Psoriasis Jacques Decroix, MD; Henrik Pres, MD; Nicolaï Tsankov, MD; Michel Poncet, PhD; Stéphanie
THERAPEUTICS FOR THE CLINICIAN Clobetasol Propionate Lotion in the Treatment of Moderate to Severe Plaque-Type Psoriasis Jacques Decroix, MD; Henrik Pres, MD; Nicolaï Tsankov, MD; Michel Poncet, PhD; Stéphanie
Yeast Essence Skin Care Actives. Yeast Essence C90 Yeast Essence E100 Yeast Essence N80 Yeast Essence Z20. Angel Yeast Co., Ltd.
 Yeast Essence Skin Care Actives Yeast Essence C90 Yeast Essence E100 Yeast Essence N80 Yeast Essence Z20 Angel Yeast Co., Ltd. Yeast Essence C90 Proposed INCI name: Sodium carboxymethyl beta glucan, water
Yeast Essence Skin Care Actives Yeast Essence C90 Yeast Essence E100 Yeast Essence N80 Yeast Essence Z20 Angel Yeast Co., Ltd. Yeast Essence C90 Proposed INCI name: Sodium carboxymethyl beta glucan, water
A STUDY OF EFFICACY RESULT BETWEEN TOPICAL HERBAL RHUBARB LIQUID AND LIQUID BASE FOR THE TREATMENT OF MELASMA IN THAI PEOPLE
 A STUDY OF EFFICACY RESULT BETWEEN TOPICAL HERBAL RHUBARB LIQUID AND LIQUID BASE FOR THE TREATMENT OF MELASMA IN THAI PEOPLE Zhang Hui Dermatology science, School of Anti Aging and Regenerative Medicine,
A STUDY OF EFFICACY RESULT BETWEEN TOPICAL HERBAL RHUBARB LIQUID AND LIQUID BASE FOR THE TREATMENT OF MELASMA IN THAI PEOPLE Zhang Hui Dermatology science, School of Anti Aging and Regenerative Medicine,
Unisooth PN-47 A complete reduction of pro-inflammatory factors for an instant soothing
 Unisooth PN-47 A complete reduction of pro-inflammatory factors for an instant soothing Skin irritation is amplified and controlled by various signaling pathways. By acting directly on the causes of skin
Unisooth PN-47 A complete reduction of pro-inflammatory factors for an instant soothing Skin irritation is amplified and controlled by various signaling pathways. By acting directly on the causes of skin
Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally
 University of Dundee Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally Published in: Photodermatology, Photoimmunology & Photomedicine DOI: 10.1111/phpp.12252
University of Dundee Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally Published in: Photodermatology, Photoimmunology & Photomedicine DOI: 10.1111/phpp.12252
New Medicines Profile
 New Medicines Profile August 2008 Issue. 08/07 Lidocaine 70mg/tetracaine 70mg medicated plaster (Rapydan ) Concise evaluated information to support the managed entry of new medicines in the NHS Summary
New Medicines Profile August 2008 Issue. 08/07 Lidocaine 70mg/tetracaine 70mg medicated plaster (Rapydan ) Concise evaluated information to support the managed entry of new medicines in the NHS Summary
Steroid use in managing your child s Atopic Eczema
 Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
CHAPTER 2. Is severity eruption assessment possible? in polymorphous light
 CHAPTER 2 Is severity assessment in polymorphous light Is severity eruption assessment possible? in polymorphous light Ines Schornagel, eruption Kees Guikers, possible? Huib van Weelden, Carla Bruijnzeel-Koomen
CHAPTER 2 Is severity assessment in polymorphous light Is severity eruption assessment possible? in polymorphous light Ines Schornagel, eruption Kees Guikers, possible? Huib van Weelden, Carla Bruijnzeel-Koomen
Psoriasis: Causes, Symptoms, And Treatment
 Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
EFFICACY AND SAFETY OF CLOBETASOL PROPIONATE SHAMPOO IN THERAPY OF PSORIASIS OF THE SCALP
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Platonova et al. SJIF Impact Factor 2.786 Volume 4, Issue 04, 238-246. Research Article ISSN 2278 4357 EFFICACY AND SAFETY OF CLOBETASOL PROPIONATE
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Platonova et al. SJIF Impact Factor 2.786 Volume 4, Issue 04, 238-246. Research Article ISSN 2278 4357 EFFICACY AND SAFETY OF CLOBETASOL PROPIONATE
Chemical structure of calcipotriol
 PRODUCT INFORMATION DAIVONEX CREAM AUST R 57354 Calcipotriol 50 microgram/g NAME OF THE MEDICINE: CALCIPOTRIOL DESCRIPTION Calcipotriol is a white or almost white crystalline substance. It is a vitamin
PRODUCT INFORMATION DAIVONEX CREAM AUST R 57354 Calcipotriol 50 microgram/g NAME OF THE MEDICINE: CALCIPOTRIOL DESCRIPTION Calcipotriol is a white or almost white crystalline substance. It is a vitamin
DermaPep A530. Multifunctional anti-inflammatory peptide for irritated and sensitive skin. Experience The Magic of Science
 Experience The Magic of Science DermaPep A53 Multifunctional anti-inflammatory peptide for irritated and sensitive skin DermaP ep Experience the magic of science Anti-aging DermaPep A35 DermaPep A42 DermaPep
Experience The Magic of Science DermaPep A53 Multifunctional anti-inflammatory peptide for irritated and sensitive skin DermaP ep Experience the magic of science Anti-aging DermaPep A35 DermaPep A42 DermaPep
Aloe vera induced oral mucositis: a case report
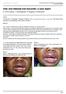 ISPUB.COM The Internet Journal of Pediatrics and Neonatology Volume 9 Number 2 K Chinnusamy, T Nandagopal, K Nagaraj, S Sridharan Citation K Chinnusamy, T Nandagopal, K Nagaraj, S Sridharan.. The Internet
ISPUB.COM The Internet Journal of Pediatrics and Neonatology Volume 9 Number 2 K Chinnusamy, T Nandagopal, K Nagaraj, S Sridharan Citation K Chinnusamy, T Nandagopal, K Nagaraj, S Sridharan.. The Internet
SENSITIVE SKINS. Aloe vera gel eco.
 SENSITIVE SKINS Aloe vera gel eco www.provitalgroup.com Aloe vera gel eco BOTANY Aloe vera L. (= Aloe barbadensis Mill.) is a perennial succulent plant, member of the Asphodelaceae family (former Liliaceae
SENSITIVE SKINS Aloe vera gel eco www.provitalgroup.com Aloe vera gel eco BOTANY Aloe vera L. (= Aloe barbadensis Mill.) is a perennial succulent plant, member of the Asphodelaceae family (former Liliaceae
Follow this and additional works at: Part of the Medicine and Health Sciences Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2012 Is High Dose Intramuscular Alefacept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2012 Is High Dose Intramuscular Alefacept
Journal of Food Science and Agricultural Technology
 Journal of Food Science and Agricultural Technology (2015) 1(1): 111-115 Journal of Food Science and Agricultural Technology International peer-reviewed scientific online journal Published online: http://jfat.mfu.ac.th
Journal of Food Science and Agricultural Technology (2015) 1(1): 111-115 Journal of Food Science and Agricultural Technology International peer-reviewed scientific online journal Published online: http://jfat.mfu.ac.th
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis
 Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
CHAPTER 3. Diagnostic phototesting in polymorphous. Diagnostic phototesting in polymorphous light eruption: the optimal number of irradiations
 CHAPTER 3 Diagnostic phototesting in polymorphous light eruption: the optimal number of irradiations Diagnostic phototesting in polymorphous light eruption: Ines Schornagel, Edward the optimal Knol, Huib
CHAPTER 3 Diagnostic phototesting in polymorphous light eruption: the optimal number of irradiations Diagnostic phototesting in polymorphous light eruption: Ines Schornagel, Edward the optimal Knol, Huib
Treating dermatomyositis
 Treating dermatomyositis David Fiorentino, MD, PhD Stanford University School of Medicine Department of Dermatology Department of Medicine (Rheumatology) September 25, 2010 DM affects many organs Approaching
Treating dermatomyositis David Fiorentino, MD, PhD Stanford University School of Medicine Department of Dermatology Department of Medicine (Rheumatology) September 25, 2010 DM affects many organs Approaching
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis for Public Disclosure
 Clinical Study Synopsis for Public Disclosure These results are supplied for informational purposes only in the interest of scientific disclosure. The synopsis may include approved and non-approved uses,
Clinical Study Synopsis for Public Disclosure These results are supplied for informational purposes only in the interest of scientific disclosure. The synopsis may include approved and non-approved uses,
Patients who achieved the primary criterion for response i.e.: complete clearance or a reduction
 MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
Lack of Prophylactic Effects of Aloe Vera Gel on Radiation Induced Dermatitis in Breast Cancer Patients
 DOI:10.22034/APJCP.2017.18.4.1139 RESEARCH ARTICLE Lack of Prophylactic Effects of Aloe Vera Gel on Radiation Induced Dermatitis in Breast Cancer Patients Niloofar Ahmadloo 1, Behnam Kadkhodaei 2, Shapour
DOI:10.22034/APJCP.2017.18.4.1139 RESEARCH ARTICLE Lack of Prophylactic Effects of Aloe Vera Gel on Radiation Induced Dermatitis in Breast Cancer Patients Niloofar Ahmadloo 1, Behnam Kadkhodaei 2, Shapour
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis A phase 2a, proof of concept trial, testing twice daily application of LEO 124249 ointment 30 mg/g in the treatment of mild to moderate inverse psoriasis Design of trial:
Clinical Trial Report Synopsis A phase 2a, proof of concept trial, testing twice daily application of LEO 124249 ointment 30 mg/g in the treatment of mild to moderate inverse psoriasis Design of trial:
Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis
 Aliment Pharmacol Ther 2004; 19: 739 747. doi: 10.1111/j.1365-2036.2004.01902.x Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis L. LANGMEAD*, R. M.
Aliment Pharmacol Ther 2004; 19: 739 747. doi: 10.1111/j.1365-2036.2004.01902.x Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis L. LANGMEAD*, R. M.
Hydrocortisyl Skin Cream 1% Hydrocortisone
 PACKAGE LEAFLET: INFORMATION FOR THE USER Hydrocortisyl Skin Cream 1% Hydrocortisone Is this leaflet hard to see or read? Phone 01403 5600 for help Read all of this leaflet carefully before you start using
PACKAGE LEAFLET: INFORMATION FOR THE USER Hydrocortisyl Skin Cream 1% Hydrocortisone Is this leaflet hard to see or read? Phone 01403 5600 for help Read all of this leaflet carefully before you start using
Public Assessment Report Scientific discussion. Ovixan (mometasone furoate) SE/H/1088/01/DC
 Public Assessment Report Scientific discussion Ovixan (mometasone furoate) SE/H/1088/01/DC This module reflects the scientific discussion for the approval of Ovixan. The procedure was finalised at 2012-12-14.
Public Assessment Report Scientific discussion Ovixan (mometasone furoate) SE/H/1088/01/DC This module reflects the scientific discussion for the approval of Ovixan. The procedure was finalised at 2012-12-14.
Public Assessment Report Scientific discussion. Echinacea Friggs, effervescent tablet (Echinacea purpurea fresh herb, dried expressed juice (20-28:1))
 Public Assessment Report Scientific discussion Echinacea Friggs, effervescent tablet (Echinacea purpurea fresh herb, dried expressed juice (20-28:1)) Asp. no: 2008-0557 This module reflects the scientific
Public Assessment Report Scientific discussion Echinacea Friggs, effervescent tablet (Echinacea purpurea fresh herb, dried expressed juice (20-28:1)) Asp. no: 2008-0557 This module reflects the scientific
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
Summary. DOI /j x
 PHOTOBIOLOGY DOI 10.1111/j.1365-2133.2005.06533.x Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis K. Köllner, M.B. Wimmershoff,
PHOTOBIOLOGY DOI 10.1111/j.1365-2133.2005.06533.x Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis K. Köllner, M.B. Wimmershoff,
A comparison of the leaf gel extracts of Aloe ferox and Aloe vera in the topical treatment of atopic dermatitis in Balb/c mice
 A comparison of the leaf gel extracts of Aloe ferox and Aloe vera in the topical treatment of atopic dermatitis in Balb/c mice M. J. Finberg 1 ; G. L. Muntingh 1 ; C. E. J. van Rensburg 2* * C. E. J. van
A comparison of the leaf gel extracts of Aloe ferox and Aloe vera in the topical treatment of atopic dermatitis in Balb/c mice M. J. Finberg 1 ; G. L. Muntingh 1 ; C. E. J. van Rensburg 2* * C. E. J. van
ALOE VERA A Step-By-Step Guide to Understanding the Health Benefits of Premium Aloe Vera Juice
 Education Article ALOE VERA A Step-By-Step Guide to Understanding the Health Benefits of Premium Aloe Vera Juice Aloe vera has been used for health and medicinal purposes for centuries with Ancient Egyptians
Education Article ALOE VERA A Step-By-Step Guide to Understanding the Health Benefits of Premium Aloe Vera Juice Aloe vera has been used for health and medicinal purposes for centuries with Ancient Egyptians
Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis
 Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis Syed Shamsuddin, *Tahir Saeed Haroon Department of Dermatology, Bolan Medical Complex, Quetta * Department
Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis Syed Shamsuddin, *Tahir Saeed Haroon Department of Dermatology, Bolan Medical Complex, Quetta * Department
Daw Muyar Nyo Demonstrator Botany Department University of Medicine 2, Yangon
 Daw Muyar Nyo Demonstrator Botany Department University of Medicine 2, Yangon Aloe vera Linn. Shar-Zaung-Let-pat &Sm;apmif;vufywf (2). Source plant a. Botanical Name b. Myanmar Name c. English Name d.
Daw Muyar Nyo Demonstrator Botany Department University of Medicine 2, Yangon Aloe vera Linn. Shar-Zaung-Let-pat &Sm;apmif;vufywf (2). Source plant a. Botanical Name b. Myanmar Name c. English Name d.
LUMACIP PLUS Cream (Fluocinolone acetonide 0.01% + Hydroquinone 4% + Tretinoin 0.05%)
 Published on: 10 Jul 2014 LUMACIP PLUS Cream (Fluocinolone acetonide 0.01% + Hydroquinone 4% + Tretinoin 0.05%) Composition LUMACIP PLUS Cream Each gram contains: Fluocinolone acetonide IP.. 0.01% w/w
Published on: 10 Jul 2014 LUMACIP PLUS Cream (Fluocinolone acetonide 0.01% + Hydroquinone 4% + Tretinoin 0.05%) Composition LUMACIP PLUS Cream Each gram contains: Fluocinolone acetonide IP.. 0.01% w/w
KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata
 Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
A broad spectrum high-spf photostable sunscreen with a high UVA-PF can protect against cellular damage at high UV exposure doses
 Photodermatology, Photoimmunology & Photomedicine ORIGINAL ARTICLE A broad spectrum high-spf photostable sunscreen with a high UVA-PF can protect against cellular damage at high UV exposure doses Curtis
Photodermatology, Photoimmunology & Photomedicine ORIGINAL ARTICLE A broad spectrum high-spf photostable sunscreen with a high UVA-PF can protect against cellular damage at high UV exposure doses Curtis
Dr Ravi C. Ratnavel DM (Oxon) FRCP (UK)
 Dr Ravi C. Ratnavel DM (Oxon) FRCP (UK) TREATMENT OF SKIN CONDITIONS BY UVB PHOTHERAPY Ultraviolet radiation from artificial light sources (UV therapy) has been used by Dermatologists for almost 100 years
Dr Ravi C. Ratnavel DM (Oxon) FRCP (UK) TREATMENT OF SKIN CONDITIONS BY UVB PHOTHERAPY Ultraviolet radiation from artificial light sources (UV therapy) has been used by Dermatologists for almost 100 years
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
Public Assessment Report Scientific discussion
 Public Assessment Report Scientific discussion Hypermin, tablet [Hypericum perforatum L., (St. John s wort) herba recens, dry extract (3.1-4.0:1) ethanol 60 %] Asp. no: 2008-1034 This module reflects the
Public Assessment Report Scientific discussion Hypermin, tablet [Hypericum perforatum L., (St. John s wort) herba recens, dry extract (3.1-4.0:1) ethanol 60 %] Asp. no: 2008-1034 This module reflects the
PRESCRIBING INFORMATION. ratio-topisone. Betamethasone dipropionate USP. 0.5 mg Cream, Ointment and Lotion. Topical corticosteroid
 PRESCRIBING INFORMATION ratio-topisone Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court January 21, 2013 Toronto,
PRESCRIBING INFORMATION ratio-topisone Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court January 21, 2013 Toronto,
STUDY. Topical Corticosteroids in the Treatment of Acute Sunburn. process in the skin, most commonly encountered
 STUDY Topical Corticosteroids in the Treatment of Acute Sunburn A Randomized, Double-blind Clinical Trial Annesofie Faurschou, MD; Hans C. Wulf, MD, DSc Objective: To examine the effect of topical corticosteroid
STUDY Topical Corticosteroids in the Treatment of Acute Sunburn A Randomized, Double-blind Clinical Trial Annesofie Faurschou, MD; Hans C. Wulf, MD, DSc Objective: To examine the effect of topical corticosteroid
The effect of jewel weed in preventing poison ivy dermatitis
 Journalof Wilderness Medicine 2, 178-182 (1991) ORIGINAL ARTICLE The effect of jewel weed in preventing poison ivy dermatitis B.J. ZINK, E.J. orren, M. ROSENTHAL and B. SINGAL Department of Emergency Medicine,
Journalof Wilderness Medicine 2, 178-182 (1991) ORIGINAL ARTICLE The effect of jewel weed in preventing poison ivy dermatitis B.J. ZINK, E.J. orren, M. ROSENTHAL and B. SINGAL Department of Emergency Medicine,
ALOE VERA COULD IT HEAL THE GUT BOOST BRAIN POWER IN AUTISM
 page 1 / 5 page 2 / 5 aloe vera could it pdf Aloe vera (/? æ l o? i? / or /? æ l o? /) is a succulent plant species of the genus Aloe.An evergreen perennial, it originates from the Arabian Peninsula but
page 1 / 5 page 2 / 5 aloe vera could it pdf Aloe vera (/? æ l o? i? / or /? æ l o? /) is a succulent plant species of the genus Aloe.An evergreen perennial, it originates from the Arabian Peninsula but
IJPRD, 2011; Vol 4(03): May-2012 ( ) International Standard Serial Number
 IJPRD, 2011; Vol 4(03): May-2012 (024-028) International Standard Serial Number 0974 9446 --------------------------------------------------------------------------------------------------------------------------------------------------
IJPRD, 2011; Vol 4(03): May-2012 (024-028) International Standard Serial Number 0974 9446 --------------------------------------------------------------------------------------------------------------------------------------------------
Developing the next generation of dermatology products to treat serious skin diseases
 Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Page: Pre-test screening for eligible subjects was performed during the 28 days before the anticipated study start (Day 1).
 This document has been downloaded from www.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been downloaded from www.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
