Endoscopic bariatric and metabolic
|
|
|
- Mariah Fisher
- 5 years ago
- Views:
Transcription
1 Endoscopic Medical Devices for Primary Obesity Treatment in Patients With Diabetes Shelby Sullivan IN BRIEF Several new endoscopic bariatric therapies have been approved by the U.S. Food and Drug Administration for the treatment of obesity, with many more devices and procedures undergoing investigational studies. This article describes these devices and procedures and special considerations for their use in patients with diabetes. University of Colorado School of Medicine, Aurora, CO Corresponding author: Shelby Sullivan, by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. See creativecommons.org/licenses/by-nc-nd/3.0 for details. Endoscopic bariatric and metabolic therapies (EBMTs) are a new category of therapies that expand the options for effective obesity treatment. These therapies require flexible endoscopy for either placement, removal, or procedure performance. EBMTs for primary obesity treatment can be divided into two categories: gastric therapies and small-bowel therapies. Gastric EBMTs are placed or performed in the stomach, whereas small-bowel EBMTs are placed or performed in the small intestine. In general, gastric therapies result in weight loss, which is associated with improvements in metabolic outcomes, but do not have weight loss independent effects on metabolic outcomes. Small-bowel therapies likely have both weight loss dependent and weight loss independent effects on metabolic outcomes, including blood glucose control. Currently, the only EBMT devices or procedures that are approved for use by the U.S. Food and Drug Administration (FDA) are gastric therapies. These include intragastric balloons (IGBs), aspiration therapy, and a suturing device that has been approved for the general indication of tissue approximation in the gastrointestinal (GI) tract and is used for a procedure called endoscopic sleeve gastroplasty (ESG). Multiple smallbowel therapies are being developed but are still in the research phase. FDA-Approved EBMTs IGBs IGBs are space-occupying devices that are placed endoscopically or swallowed by patients. Although the IGBs currently available in the United States were approved in the past 2 years, they are the not the first balloons to have received approval. The Garren-Edwards Gastric Bubble was the first IGB approved for use in the United States in Complications from this balloon, including gastric mucosa injury; deflation with migration into the small bowel, causing obstruction; and lack of weight loss (1 4) resulted in its removal from the market in All of the currently approved IGBs were designed to address the issues that plagued the Garren-Edwards device (Table 1). Multicenter trials for FDA approval of both the ReShape and Obalon balloons were randomized, sham-controlled trials, whereas the multicenter trial for Orbera was an open-label randomized, controlled trial (RCT). All three trials demonstrated a significant percentage of total body weight loss (TBWL) compared 258 SPECTRUM.DIABETESJOURNALS.ORG
2 s u l l i va n ReShape dual balloon system (ReShape Medical, San Clemente, Calif.) Orbera intragastric balloon system (Apollo Endosurgery, Austin, Tex.) Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) TABLE 1. FDA-Approved Intragastric Balloons Images Characteristics FDA Status Two medical-grade silicone spheres joined by a flexible shaft Each balloon is filled with ml saline dyed with methylene blue Endoscopically placed and removed Medical-grade silicone sphere Filled with ml saline Endoscopically placed and removed Thin polymer ellipse shape Filled with 250 ml nitrogen mix gas Three balloons administered in an 8- to 12-week period Swallowed and endoscopically removed to control conditions in intention-totreat (ITT) analyses that included all subjects who underwent device or sham device placement (ReShape active 6.8% vs. control 3.3% TBWL; Obalon active 6.6 ± 5.1% vs. control 3.4 ± 5.0% TBWL; and Orbera active 10.2 ± 6.6% vs. control 3.3 ± 5.0% TBWL) (5 7). As expected, subjects in the open-label Orbera trial achieved higher weight loss than in the sham trials. Clinical case series from outside of the United States have demonstrated greater weight loss than in the U.S. RCTs (8,9). This discrepancy is likely multifactorial and may include increased patient baseline motivation to change, increased personalized lifestyle therapy, and patient self-payment, resulting in increased adherence to lifestyle changes. Clinical case series from the United States are not yet available, but it is possible that clinical experience within the United States will be similar to that from elsewhere. Moreover, in the U.S. multicenter trials, patients maintained % of their weight loss 6 months after balloon removal, and clinical case series demonstrate weight loss maintenance in up to 23% of patients at 5 years (10). Cardiometabolic changes were evaluated in the U.S. pivotal trials of the ReShape, Orbera, and Obalon balloon systems. It is important to note that baseline metabolic parameters (i.e., fasting blood glucose, lipids, A1C, and blood pressure) were normal on average, and very few participants had a diagnosis of diabetes at study entry. Compared to subjects receiving the control condition of lifestyle therapy only, those treated with the Obalon balloon system had small but significant improvements in systolic blood pressure, fasting glucose, LDL cholesterol, and triglycerides at 24 weeks (11). Metabolic outcomes for the ReShape dual balloon were Approved 28 July 2015 For patients with a BMI of kg/m 2 and one obesityrelated comorbidity Remains in place for 6 months Approved 5 August 2015 For patients with a BMI of kg/m 2 Remains in place for 6 months Approved 8 September 2016 For patients with a BMI of kg/m 2 Remains in place for 6 months from first administration not reported compared to the control condition (12). No differences were seen between subjects in the control group and those treated with the Orbera balloon (13). A clinic case series of 143 consecutive patients with a metabolic syndrome rate of 34.8% and a type 2 diabetes rate of 32.6% found a reduction in the incidence of metabolic syndrome to 14.5% and of diabetes to 20.9% at IGB removal, with 14.1 ± 5.7% TBWL (14). Moreover, 12 months after IGB removal, the TBWL remained high at 11.2 ± 4.6%, and incidence of metabolic syndrome and diabetes remained low at 11.6 and 21.3%, respectively (14). Serious adverse events (AEs) were higher in the ReShape and Orbera trials than in the Obalon trial (10.6, 10, and 0.5%, respectively). Rates of serious AEs for both ReShape and Orbera were driven by dehydration requiring intravenous fluids due FROM RESEARCH TO PRACTICE VOLUME 30, NUMBER 4, FALL
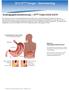
3 FROM RESEARCH TO PRACTICE / OBESITY TREATMENT IN DIABETES PATIENTS to nausea and vomiting after IGB placement or intolerability resulting in early device removal. The Obalon trial had only one serious AE due to a bleeding ulcer in a patient on protocol-prohibited nonsteroidal anti-inflammatory drugs. All serious AEs resolved without sequela. Nonserious AEs were common but varied slightly among the different IGBs. Vomiting was a more common AE in the ReShape and Orbera trials (86.7 and 86.8%, respectively) than in the Obalon trial (17.3%). Gastric ulceration occurred in 35% of ReShape subjects compared to 0 and 0.9% of Orbera and Obalon subjects, respectively; however, a design change during the ReShape trial reduced the ulceration rate to 10%. Incidence of gastroesophageal reflex disease was highest in the Orbera trial, at 30% (5 7). There are several management considerations for using IGB therapy for weight loss in patients with diabetes. First, IGBs are indicated for patients with a BMI of kg/m 2, with the ReShape dual balloon indications also requiring at least one obesity-related comorbidity. Use outside of this BMI range is considered off-label use, but evidence suggests that the percentage of TBWL in such instances is consistent with that in subjects with higher BMIs (15). Second, all patients initiating therapy with IGB and starting a low-calorie diet should have reductions in medications that can cause hypoglycemia. Moreover, the fluid-filled IGBs require a liquid and pureed diet for at least 2 weeks after IGB placement. Third, the fluid-filled IGBs can induce vomiting after placement, while a patient s stomach is adjusting to accommodate the IGB. This can be managed with antiemetic medication, but it is important to adjust antidiabetic medications in anticipation of significant vomiting. Fourth, data suggest that the fluid-filled IGBs alter gut physiology, resulting in delayed gastric emptying that may correlate with weight loss (16), and baseline FIGURE 1. AspireAssist system. A) Implanted components include the A-tube and skin-port. B) Components used only during aspiration include the connector, patient line, companion, reservoir, and drain tube. gastroparesis is considered a contraindication for IGB placement. Aspiration Therapy Aspiration therapy allows patients to remove a portion of their gastric contents after eating a meal with the use of the AspireAssist system (Aspire Bariatrics, King of Prussia, Pa.). The AspireAssist system contains both implanted components similar to a percutaneous endoscopic gastrostomy tube and components that are only used during aspiration (Figure 1). The implanted components include the A-tube, which is a silicone tube with a fenestrated 15-cm intragastric and extragastric portion separated by an intragastric bumper, and the skin-port, which is a <1-cm-high disc attached to the A-tube that can be opened to allow the flow of gastric contents. The components that are only used during aspiration include the connector, which attaches to and opens the skin-port and has a counter that locks and prevents further skinport opening after 115 openings; the patient line, which connects the connector to the companion; the companion, which is a siphon with a one-way valve allowing for passive flow of gastric contents out or water flush into the stomach; the reservoir, which is a 600-mL soft water bottle filled with tap water used for flushing the system; and the drain tube, which is a silicone tube attached to the companion that directs the flow of gastric contents into the toilet or sink. In addition to reducing the amount of food entering the small bowel, aspiration therapy also results in a decrease in food consumed at meals. This is caused by multiple factors, including the need to increase chewing to reduce food particle size to <5 mm to reliably fit through the A-tube, the requirement for increased water consumption during a meal, and an increased awareness of negative food choices due to the ability to see food contents exit through the clear drain tube. Analyses of the gastric aspirate in a U.S. pilot study revealed that only 80% of the weight loss was explained by aspiration of calories if the patient aspirated all meals and snacks optimally. Because subjects in that study only aspirated two meals on average and did not aspirate snacks, weight loss due to aspiration of gastric contents was likely significantly less than 80%, with reduced food intake responsible for significantly more than 20% of the weight loss (17). Weight loss in trials for aspiration therapy have resulted in % TBWL in subjects with a BMI of kg/m 2 who completed 12 months of therapy (17 19) and 21.4% TBWL in 11 subjects with a BMI of >55 kg/m 2 who completed 12 months of therapy (20). In the U.S. multicenter RCT (the PATHWAY study), the TBWL in the AspireAssist group was 12.1 ± 9.6% in the modified ITT analysis (n = 111) and 14.2 ± 9.8% in the completer analysis (n = 82) at 12 months compared to 3.5 ± 6.0% in the control group modified ITT analysis (n = 60) and 4.9 ± 7.0% in the control group completer analysis (n = 31) at 12 months (19). Two-year 260 SPECTRUM.DIABETESJOURNALS.ORG
4 s u l l i va n weight loss was reported in the U.S. pilot trial (n = 7) and a Swedish singlearm prospective study (n = 15), with 20.1 ± 3.5% TBWL and 61.5 ± 28.5% excess body weight loss (EBWL), respectively (17,18). As with the IGB trials, few subjects had abnormal cardiometabolic risk factors at baseline in the PATHWAY study, and only nine subjects had a diagnosis of diabetes. The study was not powered to detect changes in cardiometabolic risk factors; however, despite these limitations, A1C was decreased significantly more in the AspireAssist group than in the control group. Significant improvements were also seen in triglycerides and HDL cholesterol in the AspireAssist group compared to baseline (19). Five serious AEs occurred in four subjects in the PATHWAY study. One subject had severe abdominal pain after A-tube placement and required two separate overnight hospitalizations for pain control; one had mild peritonitis 2 days after A-tube placement requiring a 2-day hospitalization with intravenous antibiotics; one had gastric ulceration from the A-tube that was treated with removal of the A-tube; and one experienced a product malfunction that required replacement of an A-tube. All serious AEs resolved without sequela. The most common nonserious AEs were similar to those of percutaneous endoscopic gastrostomy tubes, including peristomal granulation tissue, abdominal pain after A-tube placement, and peristomal irritation. Only four subjects in the AspireAssist group developed hypokalemia (potassium meq/l), which was treated with oral potassium supplementation (19). Aspiration therapy management considerations for patients with diabetes are similar in principle to those for patients with a percutaneous endoscopic gastrostomy tube with regard to A-tube care. It is important to keep the A-tube site clean and dry. Patients can also use skin barriers that contain zinc oxide to prevent skin irritation. FDA indications for treatment with the AspireAssist system include patients with a BMI of kg/m 2. As with IGBs, use outside of this BMI range is considered off-label use. Patients will also have an altered diet after initiation of aspiration, with pureed food and a reduction in food intake. As with IGBs, medications that cause hypoglycemia should be reduced with the initiation of therapy. Patients also need to be instructed to take their oral medications after aspiration to ensure consistent absorption of the medications. Finally, as with all patients who initiate aspiration therapy, patients need to be instructed about how to adequately chew their food to ensure successful aspiration. Suturing and Plicating Two devices have received FDA clearance for the generic indication of tissue approximation in the GI tract. The Overstitch endoscopic suturing system (Overstitch, Apollo Endosurgery, Austin, Tex.) is a suturing device that attaches to the end of a double-channel upper endoscope. The Incisionless Operating Platform (IOP; USGI Medical, San Clemente, Calif.) is a 54 French flexible tube with a control handle like an endoscope and four channels with specialized instruments for placing plications in the GI tract. The Overstitch has been used to perform ESG, a new technique initially described in 2013 that uses the Overstitch to reduce gastric volume by suturing along the greater curvature of the stomach to create a tube shape along the lesser curve of the stomach (21). Although the Overstitch device does have FDA approval, it does not have a specific indication for ESG, and no RCTs have been performed with this therapy. A study on mechanisms of action suggests that this procedure may cause a delay in gastric emptying that correlates with increased satiety; however, only four participants were studied (22). The largest case series of ESG was reported in 2017 and included 248 patients at three centers. TBWL 6 months after ESG was 15.2% (95% CI %), with 13% of patients lost to follow-up. Fifty-seven of the 92 patients who were eligible for the 24-month follow-up visit completed it, yielding a follow-up rate of 62%. TBWL in the patients who completed the 24-month visit was 18.6% (95% CI %) (23). Cardiometabolic outcomes were not reported in this case series but were reported in a case series of a subset of the patients (n = 91) included in the original 248-case series. Follow-up at 12 months was conducted in 53 of the 91 patients with significant reductions in A1C, systolic blood pressure, fasting serum triglycerides, and serum alanine aminotransferase (24). Five serious AEs occurred in the 248-patient case series. These included two perigastric fluid collections requiring drainage and intravenous antibiotics, one case of extra-gastric bleeding requiring blood transfusion, one pulmonary embolism, and one pneumoperitoneum with pneumothorax requiring chest tube placement. It is important to note that the number of patients who required hospitalization for dehydration, nausea, or pain was not recorded. Nonserious AEs also were not recorded (23). The IOP has been used for the primary obesity surgery endoluminal (POSE) procedure. Case series of the POSE procedure in Europe in 147 patients (BMI 38.0 ± 4.8 kg/m 2 ) demonstrated a TBWL of 15.1 ± 7.8% with 21% lost to follow-up at 12 months (25). The POSE procedure was also studied in a multicenter, open-label RCT in Europe that demonstrated significantly more weight loss in the active group than in the control group (TBWL of 13.0 ± 1.4% [SE] vs. 5.3 ± 2.5%, respectively, P = 0.01) (26). The POSE procedure was also studied in a U.S. multicenter, ran- FROM RESEARCH TO PRACTICE VOLUME 30, NUMBER 4, FALL
5 FROM RESEARCH TO PRACTICE / OBESITY TREATMENT IN DIABETES PATIENTS domized, sham-controlled, doubleblind study with 332 subjects (active group n = 221, BMI 36.0 ± 2.4 kg/m 2 ; sham control group n = 111, BMI 36.2 ± 2.2 kg/m 2 ) (27). Serious AEs occurred in 4.7% of subjects, with 10 of the 12 serious AEs related to post-procedure symptoms that resolved with medical therapy. Although subjects in the active group achieved significantly more weight loss than sham control subjects at 12 months (4.94 ± 7.04 vs ± 5.58%, respectively, P <0.0001), the predefined study endpoints were not met, and the FDA did not approve the IOP for the specific indication of the POSE procedure. Of note, the trial design only used a low-intensity lifestyle therapy program (6 visits in the first 12 months of therapy), which led to lowerthan-expected weight loss in both the active and sham control groups. Only 26 patients who completed the trial had diabetes; however, resolution of diabetes, defined as cessation of antidiabetic medications, was seen in 9 of 16 patients in the active group and 1 of 10 in the sham group (P = ) (27). It is likely that, with more intensive lifestyle therapy, both the active and control groups weight loss would have been closer to the weight loss seen in the European multicenter trial. It is possible that future studies may lead to FDA approval, but currently the IOP is not available in the United States for the POSE procedure. Considerations for management of patients with diabetes after ESG or POSE are similar to those for IGB, with the exception of BMI indications, since ESG in particular has not been specifically approved by the FDA. Patients will have altered diet, including a liquid diet for 2 3 weeks, a pureed diet for 2 weeks, and then transition to a regular diet. Patients may also have significant nausea and vomiting after the procedure. These features require adjustments to antihyperglycemic medications. This procedure may also result in delayed gastric emptying and may not be appropriate for patients who have delayed gastric emptying from diabetic gastroparesis. Investigational EBMTs Two gastric EBMTs are currently being studied in U.S. multicenter RCTs, including one adjustable intragastric balloon (Spatz3 adjustable balloon system; Spatz FGIA, Great Neck, N.Y.) and the Transplyoric Shuttle (TPS; BAROnova, Inc., Goleta, Calif.). The Spatz3 system is a fluid-filled intragastric balloon with a catheter that allows for fill volume adjustment during an endoscopy after the initial placement. The TPS is a 56-mm outer silicone skin filled with a coiled cord of silicone tethered by silicone to a small weight. Unlike the intragastric balloons, this device causes intermittent gastric outlet obstruction by intermittently blocking the pylorus. In addition, a U.S. multicenter RCT is being initiated on a completely procedureless intragastric balloon (Ellipse, Allurion Technologies, Natick, Mass.). This balloon is made of a thin film that is filled with 550 ml saline. It is swallowed by the patient and inflated after confirmation of the capsule in the stomach. The balloon has a release valve that catastrophically opens and deflates the balloon at 4 months. The balloon is then passed naturally through the GI tract. Pilot studies of these devices have been performed outside of the United States, but whether these devices will become available in the United States will depend on the outcomes of the current trials. Investigational Small-Bowel EBMTs Multiple small-bowel EBMTs are being studied for the management of obesity and diabetes. These technologies were developed to mimic the effects on diabetes of proximal small-bowel exclusion seen with bariatric surgery. Both animal and human data support the role of the proximal small bowel in glucose absorption, incretin secretion, and insulin resistance. Rat studies suggest that glucose sensing is decreased in the setting of diabetes (28), and high-fat feeding may stimulate duodenal proliferation of enteroendocrine cells that differentiate into K cells, producing glucose-dependent insulinotropic polypeptide (29). Moreover, proteins extracted from the duodenum and jejunum of diabetic mice and insulinresistant humans caused insulin resistance in cultured muscle cells, suggesting that the duodenum and jejunum may also secrete a protein that causes skeletal muscle insulin resistance (30). Humans with diabetes have also been shown to have mucosal hypertrophy and increased enteroendocrine cells in the proximal small bowel (31), and nutrient infusion into the jejunum with a feeding tube in patients with diabetes led to increases in the glucose absorption rate and insulin sensitivity (32). Endoluminal Bypass Liners The most well studied of these devices is the duodenal-jejunal bypass liner (EndoBarrier, GI Dynamics, Lexington, Mass.). This bypass liner is made from a 60-cm-long, ultra-thin film with a self-expanding nitinol ring with 10 barbs to anchor the device in the duodenal bulb. The ultra-thin film blocks nutrient interaction with the small-bowel mucosa until the film ends at 60 cm. A meta-analysis found a percentage of EBWL of 35.4% (95% CI %) at 12 months in three studies, with a decrease in A1C of 1.5% (95% CI 2.2 to 0.8) (9). However, the device had a pooled early removal rate of 18.4%, a migration rate of 4.9%, bleeding in 3.9%, and liver abscess in 0.13% (9). A U.S. multicenter, randomized, sham-controlled trial was stopped early after enrolling 325 of a planned 500 subjects because of a 3.5% incidence of hepatic abscess. All patients recovered after intravenous antibiot- 262 SPECTRUM.DIABETESJOURNALS.ORG
6 s u l l i va n ics and percutaneous drainage (33). Despite the reduced number of subjects, the study still demonstrated a significant decrease in A1C in the active group compared to the sham control group ( 1.0 and 0.3%, respectively). It is important to note that the average A1C of the study population was 8.8%, and they were already on oral antihyperglycemic agents (33). It is unclear why the rate of hepatic abscess was higher in this trial than in studies performed outside of the United States. The device is not available with the United States but is still available elsewhere. Another endoluminal bypass liner, a gastro-duodeno-jejunal bypass liner (ValenTx, Maple Grove, Minn.), has been developed to bypass both the stomach and proximal small bowel. The device is a 120-cm-long fluoropolymer that is anchored to the gastroesophageal junction and deployed through the pylorus into the small bowel. Endoscopic placement initially required laparoscopic assistance (34) and was successfully performed in 22 subjects, with 17 subjects completing 12 weeks of implantation. Baseline BMI was 42 kg/m 2 (range kg/m 2 ), and weight loss was 16.8 kg (range kg) (34). The study reported on seven subjects with diabetes on oral antihyperglycemic medications who did not need the medications at the end of the study, but no further detail was provided about this. An additional single-arm trial was performed with planned implantation duration of 12 months. Twelve patients underwent successful implantation, 10 completed 12 months of testing, and 6 patients had attached functional devices at 12 months (35). Weight loss was 14.9 kg at 12 months. Of four subjects with diabetes, three had a 1% decrease in A1C, and one stopped oral antihyperglycemic medications (35). Duodenal Mucosal Resurfacing Duodenal mucosal resurfacing (DMR) employs hydrothermal ablation to destroy the superficial mucosal layer and stimulate regrowth of normal mucosal tissue (Revita DMR system, Fractyl Laboratories, Lexington, Mass.). The device is a catheter capable of circumferential saline lift to protect the submucosa, followed by inflation of a 2-cm-long balloon filled with fluid heated to 90 C. Duodenal mucosa distal to the papilla and up to the ligament of Treitz are ablated under direct visualization in 2-cm increments. Similar principles are used in clinical practice for the ablation of Barrett s esophagus mucosal tissue (36), with regrowth of normal squamous epithelium. One human, open-label trial in 39 subjects (BMI 30.8 ± 3.5 kg/m 2, weight 84.4 ± 11.9 kg, and A1C 9.6 ± 1.4%) has been published (37). Subjects had 3 15 cm of ablation in the duodenum, with a decrease in A1C of 1.2 ± 0.3% at 6 months with only a 3% TBWL. In a subanalysis of subjects who had at least 9 cm of ablation and did not stop antihyperglycemic medications, the decrease in A1C was 1.8 ± 0.5% (37). Dual-Path Enteral Bypass The Incisionless Magnet Anastomotic System (GI Windows, Bridgewater, Mass.) is a new endoscopic therapy that requires the simultaneous deployment of self-assembling magnets in the proximal jejunum and ileum using pediatric colonoscopes under fluoroscopic guidance. The magnets attract each other and cause necrosis of the tissue compressed between the magnets, which leads to the creation of an anastomosis. The new dual-path enteral bypass allows for nutrients to flow through both the native and newly created anastomosis. The first human study was performed in 10 subjects (BMI 41 kg/m 2 ), with data reported for 6-month outcomes. TBWL was 10.6%, and in four subjects who had diabetes (baseline A1C 7.8%), A1C decreased by 1.8%. Conclusion EBMTs are a new class of treatment for obesity and include gastric and small-bowel therapies. In contrast to gastric therapies, which have metabolic effects related to weight loss, small-bowel therapies have both weight loss dependent and weight loss independent effects on glucose homeostasis. Several gastric EBMTs are approved or available in the United States. The FDA-approved devices, including three intragastric balloons and aspiration therapy, have demonstrated safety and efficacy in RCTs. A large case series is available in support of the ESG procedure, which does not have specific FDA approval. Additional gastric and small-bowel devices and procedures are being studied for the treatment of obesity and diabetes and may become available in the United States soon. EBMTs offer needed options to improve obesity and diabetes treatment in the United States and abroad. Duality of Interest S.S. is a member of the American Gastroenterological Association Institute s council section for Obesity, Metabolism, and Nutrition, an advisory board member for the Association for Bariatric Endoscopy, and vice president of The Obesity Society s Bariatric Section. She is also currently a consultant for Obalon Therapeutics, Aspire Bariatrics, USGI Medical, and Spatz FGIA. In the past 12 months, she has performed contracted research through her institution for Aspire Bariatrics, Obalon Therapeutics, and Allurion Technologies. No other potential conflicts of interest relevant to this article were reported. References 1. Benjamin SB. Small bowel obstruction and the Garren-Edwards gastric bubble: an iatrogenic bezoar. Gastrointest Endosc 1988;34: Benjamin SB, Maher KA, Cattau EL Jr, et al. Double-blind controlled trial of the Garren-Edwards gastric bubble: an adjunctive treatment for exogenous obesity. Gastroenterology 1988;95: Kirby DF, Wade JB, Mills PR, et al. A prospective assessment of the Garren- Edwards Gastric Bubble and bariatric surgery in the treatment of morbid obesity. Am Surg 1990;56: Zeman RK, Benjamin SB, Cunningham MB, et al. Small bowel obstruction due to Garren gastric bubble: radiographic diagnosis. AJR Am J Roentgenol 1988;150: U.S. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA intragastric balloon FROM RESEARCH TO PRACTICE VOLUME 30, NUMBER 4, FALL
7 FROM RESEARCH TO PRACTICE / OBESITY TREATMENT IN DIABETES PATIENTS system. Available from pdf. Accessed 22 September U.S. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape integrated dual balloon system. Available from accessdata.fda.gov/cdrh_docs/pdf14/ P140012b.pdf. Accessed 22 September U.S. Food and Drug Administration. Summary of safety and effectiveness data (SSED) Obalon balloon system. Available from cdrh_docs/pdf16/p160001b.pdf. Accessed 22 September Lopez-Nava G, Bautista-Castaño I, Jimenez-Baños A, Fernandez-Corbelle JP. Dual intragastric balloon: single ambulatory center Spanish experience with 60 patients in endoscopic weight loss management. Obes Surg 2015;25: Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015;82: e5 10. Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg 2012;22: Sullivan S, Swain JM, Woodman G, et al. The Obalon swallowable 6-month balloon system is more effective than moderate intensity lifestyle therapy alone: results from a 6-month randomized sham controlled trial [Abstract 812d]. Gastroenterology 2016;150(Suppl. 1):S Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis 2015;11: Courcoulas A, Abu Dayyeh BK, Eaton L, et al. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes 2017;41: Crea N, Pata G, Della Casa D, et al. Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg 2009;19: ASGE Technical Committee; Abu Dayyeh BK, Thosani N, Konda V, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015;81:502.e1 502.e Gómez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity (Silver Spring) 2016;24: Sullivan S, Stein R, Jonnalagadda S, Mullady D, Edmundowicz S. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology 2013;145: e5 18. Norén E, Forssell H. Aspiration therapy for obesity; a safe and effective treatment. BMC Obes 2016;3: Thompson CC, Abu Dayyeh BK, Kushner R, et al. Percutaneous gastrostomy device for the treatment of class II and class III obesity: results of a randomized controlled trial. Am J Gastroenterol 2017;112: Machytka E, Turro R, Huberty V, et al. Aspiration therapy in super obese patients: pilot trial [Abstract No. 1944]. Gastroenterology 2016;150(Suppl. 1):S822 S Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc 2013;78: Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol 2017;15:37 43.e1 23. Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg. Epub ahead of print on 27 April 2017 (DOI: /s ) 24. Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol 2017;15: López-Nava G, Bautista-Castaño I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The primary obesity surgery endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis 2015;11: Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC. MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after POSESM vs. medical therapy. Obes Surg 2017;27: Sullivan S, Swain JM, Woodman G, et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: the ESSENTIAL trial. Obesity (Silver Spring) 2017;25: Lee J, Cummings BP, Martin E, et al. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 2012;302:R657 R Gniuli D, Calcagno A, Dalla Libera L, et al. High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia 2010;53: Salinari S, Debard C, Bertuzzi A, et al. Jejunal proteins secreted by db/db mice or insulin-resistant humans impair the insulin signaling and determine insulin resistance. PLoS One 2013;8:e Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006;290:E550 E Salinari S, Carr RD, Guidone C, et al. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab 2013;305:E59 E Kaplan LM, Buse JB, Mullin C, et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral antihyperglycemic agents. Presented at the American Diabetes Association 76th Scientific Sessions, New Orleans, La., Sandler BJ, Rumbaut R, Paul Swain C, et al. Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc 2011;25: Sandler BJ, Rumbaut R, Swain CP, et al. One-year human experience with a novel endoluminal, endoscopic gastric bypass sleeve for morbid obesity. Surg Endosc 2015;29: Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low-grade dysplasia in Barrett s esophagus: expert review from the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151: Rajagopalan H, Cherrington AD, Thompson CC, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care 2016;39: SPECTRUM.DIABETESJOURNALS.ORG
Endoscopic Interventions
 Endoscopic Interventions Shelby Sullivan, MD Director of the Gastroenterology Metabolic and Bariatric Program University of Colorado School of Medicine Disclosures Shelby Sullivan, M.D. has financial interests
Endoscopic Interventions Shelby Sullivan, MD Director of the Gastroenterology Metabolic and Bariatric Program University of Colorado School of Medicine Disclosures Shelby Sullivan, M.D. has financial interests
Obesity Management Workshop for Health Professionals
 Obesity Management Workshop for Health Professionals 17 th November 2017 Dr Graeme Rich Gastroenterologist Director of Bariatrics Australia Is a procedure the magic bullet? Energy in >> Energy out Accepted
Obesity Management Workshop for Health Professionals 17 th November 2017 Dr Graeme Rich Gastroenterologist Director of Bariatrics Australia Is a procedure the magic bullet? Energy in >> Energy out Accepted
Mustafa W. Aman, M.D. Director, Bariatric Surgery Program Guthrie Robert Packer Hospital
 09/16/2017 presented by: Mustafa W. Aman, M.D. Director, Bariatric Surgery Program Guthrie Robert Packer Hospital I have no financial disclosures pertaining to any commercial interests Describe the role
09/16/2017 presented by: Mustafa W. Aman, M.D. Director, Bariatric Surgery Program Guthrie Robert Packer Hospital I have no financial disclosures pertaining to any commercial interests Describe the role
Role of Malabsorptive Endoscopic Procedures in Obesity Treatment
 FOCUSED REVIEW SERIES: Roles of Bariatric Endoscopy in Obesity Treatment Clin Endosc 2017;50:26-30 https://doi.org/10.5946/ce.2017.004 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Role of Malabsorptive
FOCUSED REVIEW SERIES: Roles of Bariatric Endoscopy in Obesity Treatment Clin Endosc 2017;50:26-30 https://doi.org/10.5946/ce.2017.004 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Role of Malabsorptive
Endoscopie bariatrique et métabolique. Prof Guido Costamagna. DIGESTIVE ENDOSCOPY UNIT - Foundation Policlinico A. Gemelli - Rome
 XVI ème Congrès National de la SMED Endoscopie bariatrique et métabolique Prof Guido Costamagna DIGESTIVE ENDOSCOPY UNIT - Foundation Policlinico A. Gemelli - Rome European Endoscopy Training Centre -
XVI ème Congrès National de la SMED Endoscopie bariatrique et métabolique Prof Guido Costamagna DIGESTIVE ENDOSCOPY UNIT - Foundation Policlinico A. Gemelli - Rome European Endoscopy Training Centre -
Recent Clinical Results of Endoscopic Bariatric Therapies as an Obesity Intervention
 FOCUSED REVIEW SERIES: Roles of Bariatric Endoscopy in Obesity Treatment Clin Endosc 2017;50:42-50 https://doi.org/10.5946/ce.2017.013 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Recent Clinical
FOCUSED REVIEW SERIES: Roles of Bariatric Endoscopy in Obesity Treatment Clin Endosc 2017;50:42-50 https://doi.org/10.5946/ce.2017.013 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Recent Clinical
Endoscopic Advances for the Management of Obesity. Obesity
 Endoscopic Advances for the Management of Obesity Gregory G. Ginsberg, MD, FACG, FASGE Professor of Medicine University of Pennsylvania Perelman School of Medicine Gastroenterology Division Director of
Endoscopic Advances for the Management of Obesity Gregory G. Ginsberg, MD, FACG, FASGE Professor of Medicine University of Pennsylvania Perelman School of Medicine Gastroenterology Division Director of
Bariatric Surgery: How complex is this? Pradeep Pallati, MD, FACS, FASMBS
 Bariatric Surgery: How complex is this? Pradeep Pallati, MD, FACS, FASMBS Nothing to Disclose Types of Bariatric Surgery Restrictive Malabsorptive Combination Restrictive and Malabsorptive Newer Endoluminal
Bariatric Surgery: How complex is this? Pradeep Pallati, MD, FACS, FASMBS Nothing to Disclose Types of Bariatric Surgery Restrictive Malabsorptive Combination Restrictive and Malabsorptive Newer Endoluminal
Itemized Billing and Procedure Description for the AspireAssist
 Itemized Billing and Procedure Description for the AspireAssist The following describes the recommended therapy course for the first year for a patient undergoing AspireAssist therapy. Although providers
Itemized Billing and Procedure Description for the AspireAssist The following describes the recommended therapy course for the first year for a patient undergoing AspireAssist therapy. Although providers
WEIGHT LOSS SURGERY A Primer on Current Options and Outcomes. Caitlin A. Halbert DO, MS, FACS, FASMBS April 5, 2018
 WEIGHT LOSS SURGERY A Primer on Current Options and Outcomes Caitlin A. Halbert DO, MS, FACS, FASMBS April 5, 2018 A Little Bit About Me Bariatric Surgical Services Reflux Surgery General Surgery Overview
WEIGHT LOSS SURGERY A Primer on Current Options and Outcomes Caitlin A. Halbert DO, MS, FACS, FASMBS April 5, 2018 A Little Bit About Me Bariatric Surgical Services Reflux Surgery General Surgery Overview
Metabolic Interventions and the GI Tract: Issues
 Metabolic Interventions and the GI Tract: Issues Michael L. Kochman, M.D., AGAF Wilmott Family Professor of Medicine Vice-Chair of Medicine for Clinical Affairs University of Pennsylvania Health System
Metabolic Interventions and the GI Tract: Issues Michael L. Kochman, M.D., AGAF Wilmott Family Professor of Medicine Vice-Chair of Medicine for Clinical Affairs University of Pennsylvania Health System
A Bariatric Patient in my Waiting Room: Choosing the Right Patient for the Right Operation: Bariatric Surgery Indications
 A Bariatric Patient in my Waiting Room: Choosing the Right Patient for the Right Operation: Bariatric Surgery Indications Shahzeer Karmali MD FRCSC FACS Associate Professor Surgery University of Alberta
A Bariatric Patient in my Waiting Room: Choosing the Right Patient for the Right Operation: Bariatric Surgery Indications Shahzeer Karmali MD FRCSC FACS Associate Professor Surgery University of Alberta
Gastric Balloon for Weight Loss. Karol A Gutowski, MD, FACS Hot Topics
 Gastric Balloon for Weight Loss Karol A Gutowski, MD, FACS Hot Topics Disclosures None related to this topic Will use brand names due to lack of distinguishing generic names Presentation Level of Evidence
Gastric Balloon for Weight Loss Karol A Gutowski, MD, FACS Hot Topics Disclosures None related to this topic Will use brand names due to lack of distinguishing generic names Presentation Level of Evidence
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER AUGMENTATION FOR THE TREATMENT OF GASTROESOPHAGEAL REFLUX DISEASE (GERD)
 MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
Endoscopic Bariatric/Metabolic Surgery
 375 Daanish Kazi, DO 1 Leena Khaitan, MD, MPH, FASMBS 1 1 University Hospitals, Cleveland Medical Center, Cleveland, Ohio Dig Dis Interv 2018;2:375 382. Address for correspondence Leena Khaitan, MD, MPH,
375 Daanish Kazi, DO 1 Leena Khaitan, MD, MPH, FASMBS 1 1 University Hospitals, Cleveland Medical Center, Cleveland, Ohio Dig Dis Interv 2018;2:375 382. Address for correspondence Leena Khaitan, MD, MPH,
Apollo Endosurgery, Inc.
 Apollo Endosurgery, Inc. August 2017 Apollo Endosurgery Overview 2 Market share leader in less invasive devices that treat obesity Total Revenue Large target market of more than 600 million obese people
Apollo Endosurgery, Inc. August 2017 Apollo Endosurgery Overview 2 Market share leader in less invasive devices that treat obesity Total Revenue Large target market of more than 600 million obese people
DON T LET OBESITY SPOIL YOUR HEALTH AND YOUR LIFE
 July 2015 Issue No.17 DON T LET OBESITY SPOIL YOUR HEALTH AND YOUR LIFE www.sghgroup.com JEDDAH RIYADH MEDINA ASEER HAIL SANAA DUBAI CAIRO Definitions Over View and General Facts General Key facts! Worldwide
July 2015 Issue No.17 DON T LET OBESITY SPOIL YOUR HEALTH AND YOUR LIFE www.sghgroup.com JEDDAH RIYADH MEDINA ASEER HAIL SANAA DUBAI CAIRO Definitions Over View and General Facts General Key facts! Worldwide
LOSE WEIGHT WITHOUT SURGERY
 THE THE 100% 100% NON-SURGICAL NON-SURGICAL SOLUTION SOLUTION LOSE WEIGHT WITHOUT SURGERY O V E R 2 2 0 B A L L O O N S P L A C E D, 0 0 0 #1 GASTRIC BALLOON I D E W O R L D W ORBERA Lose helped 3x patients
THE THE 100% 100% NON-SURGICAL NON-SURGICAL SOLUTION SOLUTION LOSE WEIGHT WITHOUT SURGERY O V E R 2 2 0 B A L L O O N S P L A C E D, 0 0 0 #1 GASTRIC BALLOON I D E W O R L D W ORBERA Lose helped 3x patients
Bariatric Surgery MM /11/2001. HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient; Inpatient
 Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
Disclosure Statement. Covidien: Consultant, Grants
 Disclosure Statement Covidien: Consultant, Grants Non-Invasive Bariatric Procedures Michel M. Murr, MD, FACS Director of Bariatric Surgery Metabolic and Bariatric Surgery Outline for Non-Invasive Bariatrics
Disclosure Statement Covidien: Consultant, Grants Non-Invasive Bariatric Procedures Michel M. Murr, MD, FACS Director of Bariatric Surgery Metabolic and Bariatric Surgery Outline for Non-Invasive Bariatrics
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of implantation of a duodenal jejunal bypass sleeve for managing obesity Inserting
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of implantation of a duodenal jejunal bypass sleeve for managing obesity Inserting
type 2 diabetes is a surgical disease
 M. Lannoo, MD, University Hospitals Leuven Walter Pories claimed in 1992 type 2 diabetes is a surgical disease Buchwald et al. conducted a large meta-analysis THE FIRST OBSERVATIONS W. Pories 500 patients
M. Lannoo, MD, University Hospitals Leuven Walter Pories claimed in 1992 type 2 diabetes is a surgical disease Buchwald et al. conducted a large meta-analysis THE FIRST OBSERVATIONS W. Pories 500 patients
SESSION 6 LONG TERM OUTCOMES AND THE IMPORTANCE OF FOLLOW UP OUTCOMES OF ENDOSCOPIC PROCEDURES. Kiron Bhatia MMedSci(Surg) FRACS
 SESSION 6 LONG TERM OUTCOMES AND THE IMPORTANCE OF FOLLOW UP OUTCOMES OF ENDOSCOPIC PROCEDURES Kiron Bhatia MMedSci(Surg) FRACS DISCLAIMER Consultancy for Apollo Endosurgery there is a prevalent reluctance
SESSION 6 LONG TERM OUTCOMES AND THE IMPORTANCE OF FOLLOW UP OUTCOMES OF ENDOSCOPIC PROCEDURES Kiron Bhatia MMedSci(Surg) FRACS DISCLAIMER Consultancy for Apollo Endosurgery there is a prevalent reluctance
Here are some types of gastric bypass surgery:
 Gastric Bypass- Definition By Mayo Clinic staff Weight-loss (bariatric) surgeries change your digestive system, often limiting the amount of food you can eat. These surgeries help you lose weight and can
Gastric Bypass- Definition By Mayo Clinic staff Weight-loss (bariatric) surgeries change your digestive system, often limiting the amount of food you can eat. These surgeries help you lose weight and can
Commonly Performed Bariatric Procedures in Singapore. Lin Jinlin Associate Consultant General, Upper GI and Bariatric Surgery Changi General Hospital
 Commonly Performed Bariatric Procedures in Singapore Lin Jinlin Associate Consultant General, Upper GI and Bariatric Surgery Changi General Hospital Scope 1. Introduction 2. Principles of bariatric surgery
Commonly Performed Bariatric Procedures in Singapore Lin Jinlin Associate Consultant General, Upper GI and Bariatric Surgery Changi General Hospital Scope 1. Introduction 2. Principles of bariatric surgery
Imaging of gastric bands and their complications: an educational pictorial review
 Imaging of gastric bands and their complications: an educational pictorial review Poster No.: C-1142 Congress: ECR 2014 Type: Educational Exhibit Authors: F. Moloney, M. Twomey, C. Bogue ; Cork/IE, IE,
Imaging of gastric bands and their complications: an educational pictorial review Poster No.: C-1142 Congress: ECR 2014 Type: Educational Exhibit Authors: F. Moloney, M. Twomey, C. Bogue ; Cork/IE, IE,
MULTI-CENTER, PROSPECTIVE, CONTROLLED TRIAL OF THE DUODENAL JEJUNAL BYPASS LINER FOR THE TREATMENT OF TYPE 2 DIABETES IN OBESE PATIENTS
 Bariatric endoscopy MULTI-CENTER, PROSPECTIVE, CONTROLLED TRIAL OF THE DUODENAL JEJUNAL BYPASS LINER FOR THE TREATMENT OF TYPE 2 DIABETES IN OBESE PATIENTS Marek Benes, Tomas Hucl, Pavel Drastich, Julius
Bariatric endoscopy MULTI-CENTER, PROSPECTIVE, CONTROLLED TRIAL OF THE DUODENAL JEJUNAL BYPASS LINER FOR THE TREATMENT OF TYPE 2 DIABETES IN OBESE PATIENTS Marek Benes, Tomas Hucl, Pavel Drastich, Julius
CME Post Test. D. Treatment with insulin E. Age older than 55 years
 CME Post Test Translational Endocrinology & Metabolism: Metabolic Surgery Update Please select the best answer to each question on the online answer sheet. Go to http://www.endojournals.org/translational/
CME Post Test Translational Endocrinology & Metabolism: Metabolic Surgery Update Please select the best answer to each question on the online answer sheet. Go to http://www.endojournals.org/translational/
Endoscopic sleeve gastroplasty: the learning curve
 Endoscopic sleeve gastroplasty: the learning curve Authors Christine Hill 1, Mohamad El Zein 2, Abhishek Agnihotri 3, Margo Dunlap 2, Angela Chang 2, Alison Agrawal 2, Sindhu Barola 2, Saowanee Ngamruengphong
Endoscopic sleeve gastroplasty: the learning curve Authors Christine Hill 1, Mohamad El Zein 2, Abhishek Agnihotri 3, Margo Dunlap 2, Angela Chang 2, Alison Agrawal 2, Sindhu Barola 2, Saowanee Ngamruengphong
NEUE CHIRURGISCH- ENDOSKOPISCHE THERAPIEN
 NEUE CHIRURGISCH- ENDOSKOPISCHE THERAPIEN FÜR ADIPOSITAS UND DIABETES Ricardo Zorron, MD, PhD Adipositaszentrum Charité Center for Innovative Surgery- ZIC Department of Surgery Campus Charité Mitte, Campus
NEUE CHIRURGISCH- ENDOSKOPISCHE THERAPIEN FÜR ADIPOSITAS UND DIABETES Ricardo Zorron, MD, PhD Adipositaszentrum Charité Center for Innovative Surgery- ZIC Department of Surgery Campus Charité Mitte, Campus
Adipocytes, Obesity, Bariatric Surgery and its Complications
 Adipocytes, Obesity, Bariatric Surgery and its Complications Daniel C. Morris, MD, FACEP, FAHA Senior Staff Physician Department of Emergency Medicine Objectives Basic science of adipocyte Adipocyte tissue
Adipocytes, Obesity, Bariatric Surgery and its Complications Daniel C. Morris, MD, FACEP, FAHA Senior Staff Physician Department of Emergency Medicine Objectives Basic science of adipocyte Adipocyte tissue
Technique. Matthew Bettendorf, MD Essentia Health Duluth Clinic. Laparoscopic approach One 12mm port, Four 5mm ports
 Matthew Bettendorf, MD Essentia Health Duluth Clinic Technique Laparoscopic approach One 12mm port, Four 5mm ports Single staple line with no anastamosis 85% gastrectomy Goal to remove
Matthew Bettendorf, MD Essentia Health Duluth Clinic Technique Laparoscopic approach One 12mm port, Four 5mm ports Single staple line with no anastamosis 85% gastrectomy Goal to remove
BARIATRIC SURGERY. Weight Loss Surgery. A variety of surgical procedures to reduce weight performed on people who have obesity. Therapy Male & Female
 BARIATRIC SURGERY Weight Loss Surgery A variety of surgical procedures to reduce weight performed on people who have obesity. Therapy Male & Female About Bariatric surgery Bariatric surgery offers a treatment
BARIATRIC SURGERY Weight Loss Surgery A variety of surgical procedures to reduce weight performed on people who have obesity. Therapy Male & Female About Bariatric surgery Bariatric surgery offers a treatment
The Surgical Management of Obesity
 The Surgical Management of Obesity Omar al noubani MD,MRCS وك ل وا و اش ز ب وا و ال ت س رف وا األعراف ما مأل ابن آدم وعاء شر ا من بطنه Persons who are naturally fat are apt to die earlier than those who
The Surgical Management of Obesity Omar al noubani MD,MRCS وك ل وا و اش ز ب وا و ال ت س رف وا األعراف ما مأل ابن آدم وعاء شر ا من بطنه Persons who are naturally fat are apt to die earlier than those who
Benefits of Bariatric Surgery
 Benefits of Bariatric Surgery Dr Tan Bo Chuan Registrar, Department of Surgery GP Forum 27 May 2017 Improvements of Co-morbidities Type 2 diabetes mellitus Hypertension Hyperlipidemia Degenerative joint
Benefits of Bariatric Surgery Dr Tan Bo Chuan Registrar, Department of Surgery GP Forum 27 May 2017 Improvements of Co-morbidities Type 2 diabetes mellitus Hypertension Hyperlipidemia Degenerative joint
6/23/2011. Bariatric Surgery: What the Primary Care Provider Should Know. Case Presentation: Rachelle
 Bariatric Surgery: What the Primary Care Provider Should Know 2,000 B.C. 2,000 A.D. Case Presentation: Rachelle 35 year-old woman with morbid obesity. 5 1 236 lbs BMI 44.5 PMHx: mild depression obstructive
Bariatric Surgery: What the Primary Care Provider Should Know 2,000 B.C. 2,000 A.D. Case Presentation: Rachelle 35 year-old woman with morbid obesity. 5 1 236 lbs BMI 44.5 PMHx: mild depression obstructive
Dr Alasdair Patrick. Ms Miriam Mullard. 8:30-9:25 WS #95: The Weight Loss Menu 9:35-10:30 WS #107: The Weight Loss Menu (Repeated)
 Ms Miriam Mullard Lead Dietitian MacMurray Centre Auckland Dr Alasdair Patrick Gastroenterologist and General Physician Middlemore Hospital Auckland 8:30-9:25 WS #95: The Weight Loss Menu 9:35-10:30 WS
Ms Miriam Mullard Lead Dietitian MacMurray Centre Auckland Dr Alasdair Patrick Gastroenterologist and General Physician Middlemore Hospital Auckland 8:30-9:25 WS #95: The Weight Loss Menu 9:35-10:30 WS
Laparoscopic Gastric Bypass Information
 1441 Constitution Boulevard, Salinas, CA 93906 (831) 783-2556 www.natividad.com/weight-loss (Roux-en-Y Gastric Bypass) What is gastric bypass surgery? Gastric bypass surgery, a type of bariatric surgery
1441 Constitution Boulevard, Salinas, CA 93906 (831) 783-2556 www.natividad.com/weight-loss (Roux-en-Y Gastric Bypass) What is gastric bypass surgery? Gastric bypass surgery, a type of bariatric surgery
Surgery for Obesity and Related Diseases 12 (2016) ASMBS Guidelines/Statements
 Surgery for Obesity and Related Diseases 12 (2016) 462 467 ASMBS Guidelines/Statements American Society for Metabolic and Bariatric Surgery position statement on intragastric balloon therapy endorsed by
Surgery for Obesity and Related Diseases 12 (2016) 462 467 ASMBS Guidelines/Statements American Society for Metabolic and Bariatric Surgery position statement on intragastric balloon therapy endorsed by
Managing Complications of Bariatric Surgery. Objectives
 Managing Complications of Bariatric Surgery John J. Vargo, II, MD, MPH, FACG Chair, Department of Gastroenterology and Hepatology Digestive Disease and Surgery Institute Cleveland Clinic Cleveland, OH
Managing Complications of Bariatric Surgery John J. Vargo, II, MD, MPH, FACG Chair, Department of Gastroenterology and Hepatology Digestive Disease and Surgery Institute Cleveland Clinic Cleveland, OH
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abdominal pain, enteral therapy in acute pancreatitis and, 812 Abscess(es), pancreatic, nutritional support for, 814 815 Acute Physiology and
Note: Page numbers of article titles are in boldface type. A Abdominal pain, enteral therapy in acute pancreatitis and, 812 Abscess(es), pancreatic, nutritional support for, 814 815 Acute Physiology and
Treating obesity with endoscopic bariatric therapy
 Treating obesity with endoscopic bariatric therapy Nitin Kumar MD Developmental Endoscopy Lab, BWH Abby Lowe MS RD LD Washington University School of Medicine How to Submit a Question Your Participation
Treating obesity with endoscopic bariatric therapy Nitin Kumar MD Developmental Endoscopy Lab, BWH Abby Lowe MS RD LD Washington University School of Medicine How to Submit a Question Your Participation
Endobarrier: a viable alternative to gastric bypass surgery?
 Endobarrier: a viable alternative to gastric bypass surgery? Dr G Longcroft-Wheaton MB, BS, MD, MRCP, Consultant Gastroenterologist, Queen Alexandra Hospital, Portsmouth, UK Professor P Bhandari MD, FRCP,
Endobarrier: a viable alternative to gastric bypass surgery? Dr G Longcroft-Wheaton MB, BS, MD, MRCP, Consultant Gastroenterologist, Queen Alexandra Hospital, Portsmouth, UK Professor P Bhandari MD, FRCP,
Endoscopic Treatment of Luminal Perforations and Leaks
 Endoscopic Treatment of Luminal Perforations and Leaks Ali A. Siddiqui, MD Professor of Medicine Director of Interventional Endoscopy Jefferson Medical College Philadelphia, PA When Do You Suspect a Luminal
Endoscopic Treatment of Luminal Perforations and Leaks Ali A. Siddiqui, MD Professor of Medicine Director of Interventional Endoscopy Jefferson Medical College Philadelphia, PA When Do You Suspect a Luminal
Bariatric Surgery. Overview of Procedural Options
 Bariatric Surgery Overview of Procedural Options The Obesity Epidemic In 1991, NO state had an obesity rate above 20% 1 As of 2010, more than two-thirds of states (38) now have adult obesity rates above
Bariatric Surgery Overview of Procedural Options The Obesity Epidemic In 1991, NO state had an obesity rate above 20% 1 As of 2010, more than two-thirds of states (38) now have adult obesity rates above
Protocol. Bariatric Surgery
 Protocol Bariatric Surgery (70147) Medical Benefit Effective Date: 04/01/18 Next Review Date: 11/18 Preauthorization No Review Dates: 04/07, 05/08, 05/09, 03/10, 03/11, 07/11, 07/12, 9/12, 05/13, 01/14,
Protocol Bariatric Surgery (70147) Medical Benefit Effective Date: 04/01/18 Next Review Date: 11/18 Preauthorization No Review Dates: 04/07, 05/08, 05/09, 03/10, 03/11, 07/11, 07/12, 9/12, 05/13, 01/14,
Other Ways to Achieve Metabolic Control
 Other Ways to Achieve Metabolic Control Nestor de la Cruz- Muñoz, MD, FACS Associate Professor of Clinical Surgery Chief, Division of Laparoendoscopic and Bariatric Surgery DeWitt Daughtry Family Department
Other Ways to Achieve Metabolic Control Nestor de la Cruz- Muñoz, MD, FACS Associate Professor of Clinical Surgery Chief, Division of Laparoendoscopic and Bariatric Surgery DeWitt Daughtry Family Department
Gastrointestinal Surgery for Severe Obesity 2.0 Contact Hours Presented by: CEU Professor
 Gastrointestinal Surgery for Severe Obesity 2.0 Contact Hours Presented by: CEU Professor 7 www.ceuprofessoronline.com Copyright 8 2007 The Magellan Group, LLC All Rights Reserved. Reproduction and distribution
Gastrointestinal Surgery for Severe Obesity 2.0 Contact Hours Presented by: CEU Professor 7 www.ceuprofessoronline.com Copyright 8 2007 The Magellan Group, LLC All Rights Reserved. Reproduction and distribution
Bariatric Surgery. Options & Outcomes
 Bariatric Surgery Options & Outcomes Obesity Obesity now leading cause of premature death & illness in Australia 67% of Australians are overweight or obese Australia 4 th fattest nation in OECD Obesity
Bariatric Surgery Options & Outcomes Obesity Obesity now leading cause of premature death & illness in Australia 67% of Australians are overweight or obese Australia 4 th fattest nation in OECD Obesity
Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity. Original Effective Date: 7/8/2015
 Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity Original Effective Date: 7/8/2015 Policy Number: MCP-243 Revision Date(s): Review Date: 12/16/15,
Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity Original Effective Date: 7/8/2015 Policy Number: MCP-243 Revision Date(s): Review Date: 12/16/15,
Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE. CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010
 Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE Robert R. Henry, MD Authors and Disclosures CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010 Introduction Type 2 diabetes
Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE Robert R. Henry, MD Authors and Disclosures CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010 Introduction Type 2 diabetes
Subject: Weight Loss Surgery Effective Date: 1/1/2000 Review Date: 8/1/2017
 Subject: Weight Loss Surgery Effective Date: 1/1/2000 Review Date: 8/1/2017 DESCRIPTION OSU Health Plans supports covered members with a spectrum of service for obesity and weight loss attempts. The coverage
Subject: Weight Loss Surgery Effective Date: 1/1/2000 Review Date: 8/1/2017 DESCRIPTION OSU Health Plans supports covered members with a spectrum of service for obesity and weight loss attempts. The coverage
Medicare Part C Medical Coverage Policy
 Morbid Obesity Surgery Origination: June 30, 1988 Review Date: October 18, 2017 Next Review: October, 2019 Medicare Part C Medical Coverage Policy DESCRIPTION OF PROCEDURE OR SERVICE Bariatric surgery
Morbid Obesity Surgery Origination: June 30, 1988 Review Date: October 18, 2017 Next Review: October, 2019 Medicare Part C Medical Coverage Policy DESCRIPTION OF PROCEDURE OR SERVICE Bariatric surgery
Endoscopic Therapies for Obesity: where we are and where we go?
 Endoscopic Therapies for Obesity: where we are and where we go? Gregory G. Ginsberg, M.D. Professor of Medicine University of Pennsylvania Perelman School of Medicine Gastroenterology Division Director
Endoscopic Therapies for Obesity: where we are and where we go? Gregory G. Ginsberg, M.D. Professor of Medicine University of Pennsylvania Perelman School of Medicine Gastroenterology Division Director
The Spiral Enteroscopy Experience in 101 Consecutive Patients: Safety and Efficacy Using the Discovery SB
 The Spiral Enteroscopy Experience in 101 Consecutive Patients: Safety and Efficacy Using the Discovery SB Akerman, Paul A. 1 ; Cantero, Daniel 2 ; Avila, Jose 2 ; Pangtay, Jesus 3 ; Agrawal, Deepak 1 Introduction:
The Spiral Enteroscopy Experience in 101 Consecutive Patients: Safety and Efficacy Using the Discovery SB Akerman, Paul A. 1 ; Cantero, Daniel 2 ; Avila, Jose 2 ; Pangtay, Jesus 3 ; Agrawal, Deepak 1 Introduction:
Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, :15 a.m. 11:00 a.m.
 Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, 2018 10:15 a.m. 11:00 a.m. Type 2 diabetes mellitus (T2DM) is closely associated with obesity, primarily through the link
Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, 2018 10:15 a.m. 11:00 a.m. Type 2 diabetes mellitus (T2DM) is closely associated with obesity, primarily through the link
See Policy CPT CODE section below for any prior authorization requirements
 Effective Date: 9/1/2018 Section: SUR Policy No: 139 Medical Officer 9/1/2018 Date Technology Assessment Committee Approved Date: 3/04; 3/05; 3/06; 4/12; 4/16 Medical Policy Committee Approved Date: 11/08;
Effective Date: 9/1/2018 Section: SUR Policy No: 139 Medical Officer 9/1/2018 Date Technology Assessment Committee Approved Date: 3/04; 3/05; 3/06; 4/12; 4/16 Medical Policy Committee Approved Date: 11/08;
Megan Lawless. Journal Club. January 20 th, 2011
 Megan Lawless Journal Club January 20 th, 2011 Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1 Proceedings of the National Academy of Sciences September 2007 Abstract
Megan Lawless Journal Club January 20 th, 2011 Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1 Proceedings of the National Academy of Sciences September 2007 Abstract
Tools of the Gastroenterologist: Introduction to GI Endoscopy
 Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Migration of endoluminal gastroesophageal stents: A case series
 www.edoriumjournals.com Case series PEER REVIEWED OPEN ACCESS Migration of endoluminal gastroesophageal stents: A case series Kent C. Sasse, David L. Warner, Jared Brandt, Ellen Ackerman ABSTRACT Introduction:
www.edoriumjournals.com Case series PEER REVIEWED OPEN ACCESS Migration of endoluminal gastroesophageal stents: A case series Kent C. Sasse, David L. Warner, Jared Brandt, Ellen Ackerman ABSTRACT Introduction:
Policy Specific Section: April 14, 1970 June 28, 2013
 Medical Policy Bariatric Surgery Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: April 14, 1970 June 28, 2013 Definitions
Medical Policy Bariatric Surgery Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: April 14, 1970 June 28, 2013 Definitions
New endoscopic procedures for diabetes mellitus type 2 and obesity treatment
 Original Article New endoscopic procedures for diabetes mellitus type 2 and obesity treatment Francesco Frattini 1, Giacomo Borroni 1, Matteo Lavazza 1, Xiaoli Liu 2, Hoon Yub Kim 3, Renbin Liu 4, Angkoon
Original Article New endoscopic procedures for diabetes mellitus type 2 and obesity treatment Francesco Frattini 1, Giacomo Borroni 1, Matteo Lavazza 1, Xiaoli Liu 2, Hoon Yub Kim 3, Renbin Liu 4, Angkoon
Bariatric Surgery. Policy Number: Last Review: 12/2018 Origination: 10/1988 Next Review: 12/2019
 Bariatric Surgery Policy Number: 7.01.47 Last Review: 12/2018 Origination: 10/1988 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for bariatric surgery
Bariatric Surgery Policy Number: 7.01.47 Last Review: 12/2018 Origination: 10/1988 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for bariatric surgery
Adult Trauma Feeding Access Guideline
 Adult Trauma Feeding Access Guideline Background: Enteral feeding access mode (NGT, NDT, PEG, PEG-J, Jejunostomy tube) dependent upon patient characteristics. Enteral feeding management guidelines aim
Adult Trauma Feeding Access Guideline Background: Enteral feeding access mode (NGT, NDT, PEG, PEG-J, Jejunostomy tube) dependent upon patient characteristics. Enteral feeding management guidelines aim
A tale of two LAMS: a report of benign tissue ingrowth resulting in recurrent gastric outlet obstruction
 A tale of two LAMS: a report of benign tissue ingrowth resulting in recurrent gastric outlet obstruction Authors Parth J. Parekh, Mohammad H. Shakhatreh, Paul Yeaton Institution Department of Internal
A tale of two LAMS: a report of benign tissue ingrowth resulting in recurrent gastric outlet obstruction Authors Parth J. Parekh, Mohammad H. Shakhatreh, Paul Yeaton Institution Department of Internal
ORBERA Intragastric Balloon System (ORBERA )
 ORBERA Intragastric Balloon System (ORBERA ) Directions for Use (DFU) Rx Only Apollo Endosurgery, Inc. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY Apollo Endosurgery GRF-00346-00R02
ORBERA Intragastric Balloon System (ORBERA ) Directions for Use (DFU) Rx Only Apollo Endosurgery, Inc. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY Apollo Endosurgery GRF-00346-00R02
Diagnosis and management of early gastric band slip after laparoscopic adjustable gastric banding
 Case report Videosurgery Diagnosis and management of early gastric band slip after laparoscopic adjustable gastric banding Mehmet Sertkaya, Arif Emre, Fatih Mehmet Yazar, Ertan Bülbüloğlu Department of
Case report Videosurgery Diagnosis and management of early gastric band slip after laparoscopic adjustable gastric banding Mehmet Sertkaya, Arif Emre, Fatih Mehmet Yazar, Ertan Bülbüloğlu Department of
Bariatric Surgery MM /11/2001. HMO; PPO; QUEST Integration 04/28/2017 Section: Surgery Place(s) of Service: Outpatient; Inpatient
 Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/28/2017 Section: Surgery Place(s) of Service:
Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/28/2017 Section: Surgery Place(s) of Service:
Marc Bessler, M.D.*, Amna Daud, M.D., M.P.H., Teresa Kim, M.D., Mary DiGiorgi, M.P.H.
 Surgery for Obesity and Related Diseases 3 (2007) 480 485 Original article Prospective randomized trial of banded versus nonbanded gastric bypass for the super obese: early results Marc Bessler, M.D.*,
Surgery for Obesity and Related Diseases 3 (2007) 480 485 Original article Prospective randomized trial of banded versus nonbanded gastric bypass for the super obese: early results Marc Bessler, M.D.*,
Bariatric Surgery Corporate Medical Policy
 Bariatric Surgery Corporate Medical Policy File name: Bariatric Surgery File code: UM.SURG.01 Origination: 07/2008 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary
Bariatric Surgery Corporate Medical Policy File name: Bariatric Surgery File code: UM.SURG.01 Origination: 07/2008 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary
ORBERA Intragastric Balloon System (ORBERA )
 ORBERA Intragastric Balloon System (ORBERA ) Directions for Use (DFU) Rx Only Apollo Endosurgery, Inc. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY Apollo Endosurgery GRF-00346-00R06
ORBERA Intragastric Balloon System (ORBERA ) Directions for Use (DFU) Rx Only Apollo Endosurgery, Inc. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY Apollo Endosurgery GRF-00346-00R06
Procedure. P/N Rev B
 Patient Information Guide for the ReShape Procedure Before your procedure, please review this important information P/N 03 0312 Rev B Table of Contents Why is it Important to Lose Weight?... 3 Why Do Doctors
Patient Information Guide for the ReShape Procedure Before your procedure, please review this important information P/N 03 0312 Rev B Table of Contents Why is it Important to Lose Weight?... 3 Why Do Doctors
Roux-and-Y Gastric Bypass and its Metabolic Effects
 Roux-and-Y Gastric Bypass and its Metabolic Effects Nicola Di Lorenzo President elect of SICOb Italian Society for Bariatric Surgery and Metabolic Diseases Dept. of General Surgery-Università di Roma Tor
Roux-and-Y Gastric Bypass and its Metabolic Effects Nicola Di Lorenzo President elect of SICOb Italian Society for Bariatric Surgery and Metabolic Diseases Dept. of General Surgery-Università di Roma Tor
BARIATRIC SURGERY: GLOBAL MARKETS FOR SERVICES AND DEVICES
 BARIATRIC SURGERY: GLOBAL MARKETS FOR SERVICES AND DEVICES HLC153B February 2016 Dr. Ritu Thakur Dangi Project Analyst ISBN: 1-62296-229-X BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
BARIATRIC SURGERY: GLOBAL MARKETS FOR SERVICES AND DEVICES HLC153B February 2016 Dr. Ritu Thakur Dangi Project Analyst ISBN: 1-62296-229-X BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
Information Technology Solutions
 2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
Nutritional Management in Enterocutaneous fistula Dr Deepak Govil
 Nutritional Management in Enterocutaneous fistula Dr Deepak Govil MS, PhD (GI Surgery) Senior Consultant Surgical Gastroenterology Indraprastha Apollo Hospital New Delhi What is enterocutaneous fistula
Nutritional Management in Enterocutaneous fistula Dr Deepak Govil MS, PhD (GI Surgery) Senior Consultant Surgical Gastroenterology Indraprastha Apollo Hospital New Delhi What is enterocutaneous fistula
Viriato Fiallo, MD Ursula McMillian, MD
 Viriato Fiallo, MD Ursula McMillian, MD Objectives Define obesity and effects on society and healthcare Define bariatric surgery Discuss recent medical management versus surgery research Evaluate different
Viriato Fiallo, MD Ursula McMillian, MD Objectives Define obesity and effects on society and healthcare Define bariatric surgery Discuss recent medical management versus surgery research Evaluate different
3 Things To Know About Obesity Surgery
 3 Things To Know About Obesity Surgery Dr Jon Armstrong 1st Edition Introduction... 3 1. Am I A Candidate?... 4 2. What Are The Options?... 5 3. How Does It Work?... 6 Conclusion... 9 Follow me here...
3 Things To Know About Obesity Surgery Dr Jon Armstrong 1st Edition Introduction... 3 1. Am I A Candidate?... 4 2. What Are The Options?... 5 3. How Does It Work?... 6 Conclusion... 9 Follow me here...
Bariatric Surgery Work Up, Patient Selection and Follow Up
 Bariatric Surgery Work Up, Patient Selection and Follow Up A/Professor Tania Markovic Metabolism & Obesity Services, RPAH Boden Institute of Obesity, Nutrition, Exercise & Eating Disorders SLHD Bariatric
Bariatric Surgery Work Up, Patient Selection and Follow Up A/Professor Tania Markovic Metabolism & Obesity Services, RPAH Boden Institute of Obesity, Nutrition, Exercise & Eating Disorders SLHD Bariatric
TUBES R US. Enteral Access & Management
 TUBES R US SHEILA BELL, MS, RN, CPNP PC Christine Gangi, RN, CPN Center for Gastrointestinal Motility and Functional Disorders Division of Pediatric Gastroenterology & Nutrition Enteral Access & Management
TUBES R US SHEILA BELL, MS, RN, CPNP PC Christine Gangi, RN, CPN Center for Gastrointestinal Motility and Functional Disorders Division of Pediatric Gastroenterology & Nutrition Enteral Access & Management
What is Metabolic About Metabolic Surgery? The New ADA Recommendations
 What is Metabolic About Metabolic Surgery? The New ADA Recommendations Obesity Symposium September 16, 2017 Timothy Howland, MD Lourdes Endocrinology Bariatric from the Greek root bar- ("weight" as in
What is Metabolic About Metabolic Surgery? The New ADA Recommendations Obesity Symposium September 16, 2017 Timothy Howland, MD Lourdes Endocrinology Bariatric from the Greek root bar- ("weight" as in
Classification and Management of Leaks after Gastric Bypass for Patients with Morbid Obesity: A Prospective Study of 60 Patients
 OBES SURG (2012) 22:855 862 DOI 10.1007/s11695-011-0519-6 CLINICAL REPORT Classification and Management of Leaks after Gastric Bypass for Patients with Morbid Obesity: A Prospective Study of 60 Patients
OBES SURG (2012) 22:855 862 DOI 10.1007/s11695-011-0519-6 CLINICAL REPORT Classification and Management of Leaks after Gastric Bypass for Patients with Morbid Obesity: A Prospective Study of 60 Patients
Bariatric surgery: has anything changed in the last few years?
 Bariatric surgery: has anything changed in the last few years? Mauro Toppino University of Turin Digestive and Colorectal Surgery Minimal Invasive Surgery Center (Head:Prof. Mario Morino) XIV Annual Conference
Bariatric surgery: has anything changed in the last few years? Mauro Toppino University of Turin Digestive and Colorectal Surgery Minimal Invasive Surgery Center (Head:Prof. Mario Morino) XIV Annual Conference
Developing an Endoscopic Bariatric Practice and Reimbursement. Shawn Garber, MD FACS FASMBS
 Developing an Endoscopic Bariatric Practice and Reimbursement Shawn Garber, MD FACS FASMBS www.stopobesityforlife.com www.bellyballoon.com Disclosures Companies with which I have a financial or other relationship(s):
Developing an Endoscopic Bariatric Practice and Reimbursement Shawn Garber, MD FACS FASMBS www.stopobesityforlife.com www.bellyballoon.com Disclosures Companies with which I have a financial or other relationship(s):
MB02 Inserting a Gastric Balloon
 MB02 Inserting a Gastric Balloon Expires end of May 2015 Issued December 2013 You can get information locally by contacting the Senior Nurse on duty at your local Ramsay Health Care hospital or treatment
MB02 Inserting a Gastric Balloon Expires end of May 2015 Issued December 2013 You can get information locally by contacting the Senior Nurse on duty at your local Ramsay Health Care hospital or treatment
Obesity and Weight Loss Surgery for the Primary Care Physician
 Saturday General Session Obesity and Weight Loss Surgery for the Primary Care Physician Nicole Basa, MD Bariatric and General Surgeon Cedar Park Surgeons, PA Cedar Park, Texas Educational Objectives By
Saturday General Session Obesity and Weight Loss Surgery for the Primary Care Physician Nicole Basa, MD Bariatric and General Surgeon Cedar Park Surgeons, PA Cedar Park, Texas Educational Objectives By
INFORMED CONSENT FOR LAPAROSCOPIC ADJUSTABLE GASTRIC BAND. Please read this form carefully and ask about anything you may not understand.
 Please read this form carefully and ask about anything you may not understand. I consent to undergo laparoscopic placement of a laparoscopic Adjustable Gastric Band for the purposes of weight loss. I met
Please read this form carefully and ask about anything you may not understand. I consent to undergo laparoscopic placement of a laparoscopic Adjustable Gastric Band for the purposes of weight loss. I met
MEDICAL POLICY SUBJECT: SURGICAL MANAGEMENT OF OBESITY. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: SURGICAL MANAGEMENT OF PAGE: 1 OF: 25 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: SURGICAL MANAGEMENT OF PAGE: 1 OF: 25 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Update on the Recent Advances in Obesity Management. Benjamin O Donnell, MD Oct 5 th, 2018
 Update on the Recent Advances in Obesity Management Benjamin O Donnell, MD Oct 5 th, 2018 Objectives Background Control of Energy Homeostasis Approach to Diet and Exercise Medications Recently Approved
Update on the Recent Advances in Obesity Management Benjamin O Donnell, MD Oct 5 th, 2018 Objectives Background Control of Energy Homeostasis Approach to Diet and Exercise Medications Recently Approved
Nutrition Intervention After Gastric Bypass Revision
 Nutrition Intervention After Gastric Bypass Revision With an Anastomotic Leak Ali Fox- Montana Dietetic Intern Objectives 1. Describe the etiology of anastomotic leak post Roux-en-Y gastric bypass (G.B.)
Nutrition Intervention After Gastric Bypass Revision With an Anastomotic Leak Ali Fox- Montana Dietetic Intern Objectives 1. Describe the etiology of anastomotic leak post Roux-en-Y gastric bypass (G.B.)
ENDOLUMINAL THERAPIES FOR GERD. University of Colorado Department of Surgery Grand Rounds March 31st, 2008
 ENDOLUMINAL THERAPIES FOR GERD University of Colorado Department of Surgery Grand Rounds March 31st, 2008 Overview GERD Healthcare significance Definitions Treatment objectives Endoscopic options Plication
ENDOLUMINAL THERAPIES FOR GERD University of Colorado Department of Surgery Grand Rounds March 31st, 2008 Overview GERD Healthcare significance Definitions Treatment objectives Endoscopic options Plication
NOTE: This policy is not effective until May 1, To view the current policy, click here. IMPORTANT REMINDER
 NOTE: This policy is not effective until May 1, 2018. To view the current policy, click here. Medical Policy Manual Surgery, Policy No. 58 Bariatric Surgery Next Review: December 2018 Last Review: January
NOTE: This policy is not effective until May 1, 2018. To view the current policy, click here. Medical Policy Manual Surgery, Policy No. 58 Bariatric Surgery Next Review: December 2018 Last Review: January
Appetite, Glycemia and Entero-Insular Hormone Responses Differ Between Oral, Gastric-Remnant and Duodenal Administration of a Mixed Meal Test After
 Appetite, Glycemia and Entero-Insular Hormone Responses Differ Between Oral, Gastric-Remnant and Duodenal Administration of a Mixed Meal Test After Roux-en-Y Gastric Bypass June 2018 How a surgical complication
Appetite, Glycemia and Entero-Insular Hormone Responses Differ Between Oral, Gastric-Remnant and Duodenal Administration of a Mixed Meal Test After Roux-en-Y Gastric Bypass June 2018 How a surgical complication
Diabesity. Metabolic dysfunction that ranges from mild blood glucose imbalance to full fledged Type 2 DM Signs
 Diabesity Metabolic dysfunction that ranges from mild blood glucose imbalance to full fledged Type 2 DM Signs Abdominal obesity Low HDL, high LDL, and high triglycerides HTN High blood glucose (F>100l,
Diabesity Metabolic dysfunction that ranges from mild blood glucose imbalance to full fledged Type 2 DM Signs Abdominal obesity Low HDL, high LDL, and high triglycerides HTN High blood glucose (F>100l,
ReShape Integrated Dual Balloon System Procedure Manual
 ReShape Integrated Dual Balloon System Procedure Manual 1 TABLE OF CONTENTS Introduction... 3 Product Description... 3 Overview... 3 Component description... 4 Intragastric Balloon... 4 Placement Catheter...
ReShape Integrated Dual Balloon System Procedure Manual 1 TABLE OF CONTENTS Introduction... 3 Product Description... 3 Overview... 3 Component description... 4 Intragastric Balloon... 4 Placement Catheter...
FDA Perspective. Carolyn Y. Neuland, Ph.D.
 FDA Perspective Carolyn Y. Neuland, Ph.D. Chief Gastroenterology and Renal Devices Branch Division of Reproductive, Gastro-Renal and Urological Devices Center for Devices and Radiological Health Device
FDA Perspective Carolyn Y. Neuland, Ph.D. Chief Gastroenterology and Renal Devices Branch Division of Reproductive, Gastro-Renal and Urological Devices Center for Devices and Radiological Health Device
OBESITY AND WEIGHT LOSS SURGERY FOR THE PRIMARY CARE PHYSICIAN
 OBESITY AND WEIGHT LOSS SURGERY FOR THE PRIMARY CARE PHYSICIAN Nicole Basa, M.D., F.A.C.S., F.A.S.M.B.S Assistant Professor of Surgery Texas A&M Medical School Bariatric Medical Director- Cedar Park Regional
OBESITY AND WEIGHT LOSS SURGERY FOR THE PRIMARY CARE PHYSICIAN Nicole Basa, M.D., F.A.C.S., F.A.S.M.B.S Assistant Professor of Surgery Texas A&M Medical School Bariatric Medical Director- Cedar Park Regional
It s More Than Surgery. It s a Life Changer. Scripps Clinic Center for Weight Management is the most comprehensive weight loss program in San Diego.
 It s More Than Surgery. It s a Life Changer. Scripps Clinic Center for Weight Management is the most comprehensive weight loss program in San Diego. Now that you have your guide, how about taking one more
It s More Than Surgery. It s a Life Changer. Scripps Clinic Center for Weight Management is the most comprehensive weight loss program in San Diego. Now that you have your guide, how about taking one more
Emerging Endoluminal Bariatric Techniques
 Session IV UGS-IV: Emerging but rarely Used Treatment Options in Asia Emerging Endoluminal Bariatric Techniques Jacques Devière, M.D., Ph.D. Department of Gastroenterology, Hepatopancreatology and Digestive
Session IV UGS-IV: Emerging but rarely Used Treatment Options in Asia Emerging Endoluminal Bariatric Techniques Jacques Devière, M.D., Ph.D. Department of Gastroenterology, Hepatopancreatology and Digestive
