DPP-4 Inhibitors: What Is Their Place in Therapy?
|
|
|
- Lilian Phillips
- 5 years ago
- Views:
Transcription
1 < Advertisement < Advertisement < DPP-4 Inhibitors: What Is Their Place in Therapy? Cori M. Brock, PharmD, CDE Clinical Assistant Professor of Pharmacy Xavier University of Louisiana College of Pharmacy Division of Clinical and Administrative Sciences New Orleans, Louisiana 5/20/2010 US Pharm. 2010;35(5)(Diabetes suppl):8-13. Diabetes is a complex metabolic disorder characterized by hyperglycemia that occurs from defects in insulin secretion, insulin action, or both. Approximately 95% of patients diagnosed with diabetes will have type 2 diabetes. 1 Type 2 diabetes occurs from progressive deterioration of insulin secretion from pancreatic beta cells coupled with insulin resistance. Most patients with type 2 diabetes are also obese, which further perpetuates insulin resistance. Furthermore, the chronic state of hyperglycemia worsens the continuous decline of beta-cell function. 1,2 In 2007, the estimated prevalence of diabetes was 23.6 million people in the United States alone. 3 Approximately 10% of non-hispanic whites have diabetes, and in non-hispanic blacks the prevalence exceeds 14%. In 2006, diabetes was the seventh leading cause of death, and it remains the leading cause of new cases of blindness and kidney failure. Diabetes is also responsible for approximately 60% of lower limb amputations. For every point drop in hemoglobin A1C (HbA 1C ) patients can reduce their risk for microvascular complications by 40%. 3 Unfortunately, approximately 36% of patients do not reach the goal A1C of <7% on either monotherapy or combination therapy. 4 Therefore, glycemic control is crucial to diabetes management and the reduction of diabetes-related complications. Page 1 of 7
2 Diagnosis Diabetes can be diagnosed using one of the following methods: HbA 1C 6.5%; fasting plasma glucose 126 mg/dl; 2-hour plasma glucose 200 mg/dl following an oral glucose tolerance test using 75 g of anhydrous glucose dissolved in water; or a random plasma glucose 200 mg/dl in patients with classic symptoms of hyperglycemia, such as polyuria, polyphagia, and polydipsia (TABLE 1). The first three criteria should be confirmed with repeat testing before a diagnosis of diabetes can be determined. Glycemic control is defined by an A1C of <7%. 5 Many available pharmacologic interventions effectively manage diabetes and help patients to achieve their glycemic goal. Several agents, however, are limited by side effects, such as weight gain and hypoglycemia. Incretin-based therapies represent a newer class of agents effective in lowering HbA 1C without the side effects of hypoglycemia and weight gain. 6,7 The Incretin Effect The incretin concept was identified from the difference in insulin response to an oral glucose load compared to an IV glucose load. 6 The incretin effect can be defined by the greater insulin response to an oral glucose load than that experienced with an IV glucose challenge. 4,6,8 There are two incretin hormones, glucosedependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). GIP, derived from purified porcine intestinal substance, was identified first and determined to have minimal effects on gastric acid secretion and a significant impact on insulin response in humans. GIP is produced from the duodenal and jejunal enteroendocrine K cells of the small bowel. GLP-1, synthesized from the enteroendocrine L cells of the distal ileum and colon, resulted from the cloning of complementary DNAs (cdnas) and genes that encoded proglucagon. GLP-1 exists as GLP-1(7-37) and GLP-1(7-36), with GLP-1(7-36) being more prevalent after food intake. 8 Both hormones, GIP and GLP-1, are secreted within minutes of the presence of food; however, GLP-1 is critical for glucose control. 8 While both GIP and GLP-1 promote beta-cell survival through cell proliferation at the site of G-proteincoupled receptor activation, GLP-1 is associated with additional glucose-lowering benefits. GLP-1 improves defective glucose-stimulated insulin secretion, slows insulin secretory response to meals, inhibits the secretion of glucagon, and increases the synthesis of proinsulin in the pancreatic beta cell. These effects are important to the pancreatic beta cell, whose progressive loss of cell mass and decline in function are characteristic of type 2 diabetes. 4 The time that incretin hormones are available in the circulation is, however, minimal. Page 2 of 7
3 Circulating GIP and GLP-1 are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4) and renal clearance. DPP-4 is an aminopeptidase found in the liver, lungs, kidneys, intestinal brush-border membranes, lymphocytes, and endothelial cells. 6,8 DPP-4 is essential to incretin inactivation, allowing only 10% to 20% of GLP-1 to be biologically active with a half-life of less than 2 minutes. The inhibition of DPP-4 produces actions similar to that of GLP-1; i.e., stimulation of insulin secretion, glucagon inhibition, and beta-cell mass preservation. 8 Therefore, newer diabetes agents have focused therapy development on creating GLP-1 analogs that are resistant to DPP-4 or agents that will inhibit DPP-4. 4 In type 2 diabetes, the incretin effect is diminished or lost. It is suspected that the defect occurs because of a weakened action of GIP and the significant decrease of circulating GLP-1 after food ingestion, resulting from diminished secretion of GLP-1. 9,10 Antidiabetic agents that mimic or prolong the action of GLP-1 are therefore important for glucose control. 8 There are multiple therapies available for the management of diabetes. Traditional therapies manage type 2 diabetes by increasing insulin secretion; decreasing insulin resistance by reducing lipotoxicity; decreasing the hepatic output of glucose; or blocking the absorption of glucose in the gastrointestinal (GI) tract. 4 Side effects of therapy (e.g., hypoglycemia, weight gain, and GI discomfort) are challenges associated with traditional pharmacologic agents and may be related to poor medication adherence and thus poorer patient outcomes. Newer agents seek to improve glycemic control while minimizing troublesome side effects. 11 The two classes of medications currently available to target the incretin system are incretin mimetics/enhancers and DPP-4 inhibitors. Incretin mimetics resemble the biologic GLP-1 and are associated with transient nausea and GI discomfort. In addition, GLP-1 agonists (i.e., mimetics) are peptides and must be administered subcutaneously. 9 DPP-4 inhibitors are another option to prolong the benefits of GLP-1 that also have proven efficacy, good bioavailability (i.e., 60%-90%), minimal side effects, and likely improved patient compliance from oral dosing. 8,10 In general, DPP-4 inhibitors offer 0.5% to 0.8% reduction in HbA 1C. 12 Currently, there are two marketed DPP-4 inhibitors, sitagliptin and saxagliptin. Dipeptidyl Peptidase-4 Inhibitors Sitagliptin: The first DPP-4 inhibitor to enter the U.S. market, Sitagliptin (Januvia), was approved in October 2006 as adjunctive therapy to diet and exercise in the treatment of type 2 diabetes. 13 It is a oncedaily, highly selective, potent inhibitor of DPP-4 enzymes. Sitagliptin is eliminated primarily by the kidneys, with approximately 79% of the drug unchanged in patients with normal renal function. 14 The dose must be adjusted in patients with both moderate and severe renal impairment (TABLE 2). Sitagliptin is not an inhibitor of cytochrome P isozymes or an inducer of CYP3A4, so drug interactions are minimal. Page 3 of 7
4 Phase III trials of sitagliptin revealed that it was well tolerated at doses of 100 mg once daily, either alone or in combination. 11 Sitagliptin was studied in participants with an average A1C of 8% in combination with metformin at doses >1,500 mg/day. After 24 weeks, more patients achieved goal A1C <7% in the sitagliptin group (47%), with only 18.3% in the metformin alone group. The reduction in A1C from baseline was also statistically significant in the sitagliptin group (P <.001). Only 4.5% of participants required rescue therapy with pioglitazone for inadequate glycemic control (P <.001). 15 Despite proven efficacy, there are recent safety concerns regarding sitagliptin use. In a 24-week study, patients were randomized to receive either glimepiride alone or in combination with metformin, or glimepiride and sitagliptin or both agents also in combination with metformin. 16 Rescue therapy was available with pioglitazone. Groups that included sitagliptin did achieve a lower A1C from baseline compared to the placebo groups (P <.001); however, there was an increased incidence of hypoglycemia in the sitagliptin group compared to the placebo groups. Patients within both sitagliptin groups had 27 events of hypoglycemia (12.2%), with the most occurring in the group receiving both glimepiride and metformin. The placebo groups experienced four events of hypoglycemia (1.8%) (P <.001). 16 It is now recommended that when using agents that promote insulin secretion, such as insulin secretagogues, or insulin in combination with sitagliptin, those doses be reduced. 12 Furthermore, postmarketing reports of acute pancreatitis, both fatal and nonfatal hemorrhagic and necrotizing, and rare hypersensitivity reactions such as angioedema and Stevens-Johnson syndrome may limit use in certain populations. 13 The hypersensitivity reactions usually occurred within the first 3 months of therapy. Sitagliptin is contraindicated in patients with a history of hypersensitivity reactions to sitagliptin. 13 Saxagliptin: Saxagliptin (Onglyza) was approved by the FDA in July Like sitagliptin, saxagliptin is also indicated as an adjunct to diet and exercise for the management of type 2 diabetes in adults. 17 Saxagliptin is a reversible, potent inhibitor that is selective for DPP-4 by more than 4,000-fold, compared with sitagliptin, whose selectivity is more than 2,600-fold. 6,18,19 Similar to sitagliptin, saxagliptin is excreted by the kidneys, but it also undergoes hepatic metabolism. Dosage adjustments are only required in patients with severe renal insufficiency. 11 Saxagliptin is metabolized by CYP isozymes, resulting in an increase in the concentration of saxagliptin when used in combination with 3A4 inhibitors. Therefore, a dosage reduction to 2.5 mg is required. Common adverse reactions include headache, upper respiratory infection, and urinary tract infection. 17 Saxagliptin has been studied both alone and in combination with metformin, pioglitazone, and glyburide. The efficacy of saxagliptin as add-on therapy with metformin was evaluated in 743 patients with an A1C Page 4 of 7
5 between 7% and 10% for a 24-week period, compared to metformin alone. 20 Participants were randomized to saxagliptin groups at 2.5 mg, 5 mg, and 10 mg in combination with metformin doses ranging from 500 to 2,550 mg/day at study entry. At 24 weeks, a statistically significant reduction in A1C occurred in all treatment groups (P.0001), compared to the metformin plus placebo group. Also in each treatment group, a significant number of participants achieved a goal A1C <7% at 37.1%, 43.5%, and 44.4% in saxagliptin/metformin groups 2.5 mg, 5 mg, and 10 mg, respectively (P.0001). 20 Similar results were obtained in a phase III trial including saxagliptin 5 mg and 10 mg in combination with maximal doses of metformin compared to saxagliptin and metformin alone. The participants in this study had a higher A1C (8%-12%). At 24 weeks, the combination groups had a statistically significant reduction in A1C (-2.5% and -2.5% vs. -1.7% and 2.0%; P <.0001). 20 Advantages and Disadvantages to DPP-4 Inhibitor Use DPP-4 inhibitors offer the option for improvement in both HbA 1C and beta-cell survival. In addition, risks associated with hypoglycemia are minimal and usually only occur in combination therapy with insulin secretagogues or insulin. Weight gain is also of concern in diabetes patients. Though DPP-4 inhibitors do not promote weight loss, they are weight neutral and can prevent further weight gain. Other therapies that target the incretin system cause GI discomfort and may worsen patient compliance. This effect does not occur within the DPP-4 inhibitor class of agents. Finally, convenient once-daily oral dosing may promote patient adherence and improve health outcomes. 8 Although DPP-4 inhibitors have proven to be efficacious in reducing HbA 1C and improving beta-cell function, long-term clinical trial data are not yet available to assess the sustainability of glycemic control and protection of beta-cell mass. 4,8,10 With several DPP-4 inhibitors in clinical development, their long-term safety profile as well as cardiovascular morbidity and mortality has become of significant interest. Therefore, further long-term studies with cardiovascular end points are warranted. Each agent is unique, however, and will likely differ in adverse effects. Additional concerns surround DPP-4 inhibitors and their interference with immune function. That too is poorly understood and warrants further research. 12 Place in Therapy The American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) developed an algorithm to assist providers in managing type 2 diabetes. When developing the algorithm, the AACE and ACE identified minimizing hypoglycemia and weight gain as their top priorities in drug therapy selection. As a result of that, initial recommendations focus on the use of metformin or a thiazolidinedione if metformin is contraindicated, followed by a GLP-1 agonist or a DPP-4 inhibitor. 11 In January 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) presented a medical management consensus statement that included a treatment algorithm to manage diabetes somewhat different from that of the AACE and ACE. 12 The algorithm is divided into two tiers and suggests a stepwise treatment approach. Tier 1 is well-validated core therapies consisting of lifestyle interventions, metformin, insulin, and second-generation sulfonylureas. Tier 2 is less well-validated therapies and includes agents from tier 1 with the addition of pioglitazone and GLP-1 agonists. DPP-4 Page 5 of 7
6 inhibitors were not included in the two tiers because their effectiveness on glycemic control is lower than or equivalent to first- and second-tier agents, as well as for less clinical evidence and relative expense. The consensus statement does suggest, however, that DPP-4 inhibitors may be appropriate in select populations based on concerns associated with weight gain and hypoglycemia, similar to the AACE and ACE recommendations. 12 Summary DPP-4 inhibitors offer improved glycemic control in the management of type 2 diabetes, both alone and in combination with other antihyperglycemic agents, by reducing HbA 1C and improving beta-cell function. Agents such as sitagliptin and saxagliptin are available for first-line or adjunctive therapy. DPP-4 inhibitors have an attractive side effect profile that encourages the prevention of weight gain without hypoglycemia, making this class of agents an option for early intervention in the management of type 2 diabetes. REFERENCES 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S Gallwitz B. Saxagliptin, a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. IDrugs. 2008;11: National diabetes fact sheet: general information and national estimates on diabetes in the United States, CDC. ndfs_2007.pdf. Accessed April 5, Crepaldi G, Carruba M, Comaschi M, et al. Dipeptidyl peptidase 4 (DPP-4) inhibitors and their role in type 2 diabetes management. J Endocrinol Invest. 2007;30: American Diabetes Association. Standards of medical care in diabetes Diabetes Care. 2010;33(suppl 1):S11-S Tahrani AA, Piya MK, Barnett AH. Saxagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Adv Ther. 2009;26: Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63: Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368: Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2007;9(suppl 1): Halimi S. DPP-4 inhibitors and GLP-1 analogues: for whom? Which place for incretins in the management of type 2 diabetic patients? Diabetes Metab. 2008;34(suppl):S91-S Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an AACE/ACE consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15: Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and European Association for the Study of Diabetes. Diabetes Care. 2009;32: Januvia (sitagliptin) package insert. Whitehouse Station, NJ: Merck & Co., Inc; February Bergman AJ, Cote J, Yi B, et al. Effects of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30: Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor Page 6 of 7
7 sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29: Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9: Onglyza (saxagliptin) package insert. Princeton, NJ: Bristol-Myers Squibb; July Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25: Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11: DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32: To comment on this article, contact rdavidson@uspharmacist.com. Advertisement < U.S. Pharmacist is a monthly journal dedicated to providing the nation's pharmacists with up-to-date, authoritative, peer-reviewed clinical articles relevant to contemporary pharmacy practice in a variety of settings, including community pharmacy, hospitals, managed care systems, ambulatory care clinics, home care organizations, long-term care facilities, industry and academia. The publication is also useful to pharmacy technicians, students, other health professionals and individuals interested in health management. Pharmacists licensed in the U.S. can earn Continuing Education credits through Postgraduate Healthcare Education, LLC, accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education. Copyright Jobson Medical Information LLC unless otherwise noted. All rights reserved. Reproduction in whole or in part without permission is prohibited. Page 7 of 7
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP
 Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Chief of Endocrinology East Orange General Hospital
 Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
T2DM is a global epidemic with
 : a new option for the management of type 2 diabetes Marc Evans MRCP, MD, Consultant Diabetologist, Llandough Hospital, Cardiff Incretin-based therapies for the treatment of diabetes mellitus (T2DM) present
: a new option for the management of type 2 diabetes Marc Evans MRCP, MD, Consultant Diabetologist, Llandough Hospital, Cardiff Incretin-based therapies for the treatment of diabetes mellitus (T2DM) present
Drug Class Monograph
 Drug Class Monograph Class: Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drugs: alogliptin, alogliptin/metformin, Januvia (sitagliptin), Janumet (sitagliptin/metformin), Janumet XR (sitagliptin/metformin),
Drug Class Monograph Class: Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drugs: alogliptin, alogliptin/metformin, Januvia (sitagliptin), Janumet (sitagliptin/metformin), Janumet XR (sitagliptin/metformin),
Drug Class Monograph
 Class: Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Monograph Drugs: alogliptin, Januvia (sitagliptin), Janumet (sitagliptin/metformin), Janumet XR (sitagliptin/metformin), Jentadueto (linagliptin/metformin),
Class: Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Monograph Drugs: alogliptin, Januvia (sitagliptin), Janumet (sitagliptin/metformin), Janumet XR (sitagliptin/metformin), Jentadueto (linagliptin/metformin),
Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE. CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010
 Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE Robert R. Henry, MD Authors and Disclosures CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010 Introduction Type 2 diabetes
Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE Robert R. Henry, MD Authors and Disclosures CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010 Introduction Type 2 diabetes
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Single Technology Appraisal. Canagliflozin in combination therapy for treating type 2 diabetes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Canagliflozin in combination therapy for Final scope Remit/appraisal objective To appraise the clinical and cost effectiveness
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Canagliflozin in combination therapy for Final scope Remit/appraisal objective To appraise the clinical and cost effectiveness
Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Prior Authorization Protocol
 Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Newer and Expensive treatment of diabetes. Endocrinology Visiting Associate Professor Institute of Medicine TUTH
 Newer and Expensive treatment of diabetes Jyoti Bhattarai MD Endocrinology Visiting Associate Professor Institute of Medicine TUTH Four out of every five people with diabetes now live in developing countries.
Newer and Expensive treatment of diabetes Jyoti Bhattarai MD Endocrinology Visiting Associate Professor Institute of Medicine TUTH Four out of every five people with diabetes now live in developing countries.
Disclosure. Learning Objectives. Case. Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare
 Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors In the Management of Diabetes
 DRUG CLASS REVIEW Dipeptidyl Peptidase-4 (DPP-4) Inhibitors In the Management of Diabetes Rolee Pathak, PharmD, RPh, BCPS; and Mary Barna Bridgeman, PharmD, RPh INTRODUCTION Type-2 diabetes mellitus is
DRUG CLASS REVIEW Dipeptidyl Peptidase-4 (DPP-4) Inhibitors In the Management of Diabetes Rolee Pathak, PharmD, RPh, BCPS; and Mary Barna Bridgeman, PharmD, RPh INTRODUCTION Type-2 diabetes mellitus is
Incretin-based Therapies for Type 2 Diabetes Comparisons Between Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors
 Incretin-based Therapies for Type 2 Diabetes Comparisons Between Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors Timothy Bailey, MD, FACE, CPI Director, AMCR Institute,
Incretin-based Therapies for Type 2 Diabetes Comparisons Between Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors Timothy Bailey, MD, FACE, CPI Director, AMCR Institute,
Management of Type 2 Diabetes
 Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) (908)
 News Release FOR IMMEDIATE RELEASE Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) 423-6537 (908) 423-5185 Tracy Ogden (267) 305-0960 FDA Approves Once-Daily JANUVIA, the First and Only DPP-4
News Release FOR IMMEDIATE RELEASE Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) 423-6537 (908) 423-5185 Tracy Ogden (267) 305-0960 FDA Approves Once-Daily JANUVIA, the First and Only DPP-4
Reviewing Diabetes Guidelines. Newsletter compiled by Danny Jaek, Pharm.D. Candidate
 Reviewing Diabetes Guidelines Newsletter compiled by Danny Jaek, Pharm.D. Candidate AL AS KA N AT IV E DI AB ET ES TE A M Volume 6, Issue 1 Spring 2011 Dia bet es Dis pat ch There are nearly 24 million
Reviewing Diabetes Guidelines Newsletter compiled by Danny Jaek, Pharm.D. Candidate AL AS KA N AT IV E DI AB ET ES TE A M Volume 6, Issue 1 Spring 2011 Dia bet es Dis pat ch There are nearly 24 million
Scottish Medicines Consortium
 Scottish Medicines Consortium saxagliptin, 5mg film-coated tablet (Onglyza ) No. (603/10) Bristol-Myers Squibb Pharmaceuticals Ltd 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium saxagliptin, 5mg film-coated tablet (Onglyza ) No. (603/10) Bristol-Myers Squibb Pharmaceuticals Ltd 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
3/8/2011. Julie M. Sease, Pharm D, BCPS, CDE Associate Professor of Pharmacy Practice Presbyterian College School of Pharmacy
 Summarize revisions to the 2011 American Diabetes Association clinical practice guidelines. Evaluate bromocriptine as a therapeutic option in the management of type 2 diabetes. Compare and contrast the
Summarize revisions to the 2011 American Diabetes Association clinical practice guidelines. Evaluate bromocriptine as a therapeutic option in the management of type 2 diabetes. Compare and contrast the
National Horizon Scanning Centre. Saxagliptin (BMS ) for type 2 diabetes. April 2008
 Saxagliptin (BMS 477118) for type 2 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
Saxagliptin (BMS 477118) for type 2 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
Multiple Factors Should Be Considered When Setting a Glycemic Goal
 Multiple Facts Should Be Considered When Setting a Glycemic Goal Patient attitude and expected treatment effts Risks potentially associated with hypoglycemia, other adverse events Disease duration Me stringent
Multiple Facts Should Be Considered When Setting a Glycemic Goal Patient attitude and expected treatment effts Risks potentially associated with hypoglycemia, other adverse events Disease duration Me stringent
The Many Faces of T2DM in Long-term Care Facilities
 The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
Merck & Co, Inc. Announced Approval of JANUVIA TM (INN: sitagliptin), a new oral treatment of diabetes, by the US FDA
 October 23, 2006 Ono Pharmaceutical Co., Ltd., Public Relations Phone: +81-6-6263-5670 Banyu Pharmaceutical Co., Ltd., Public Relations Phone: +81-3-6272-1001 Merck & Co, Inc. Announced Approval of JANUVIA
October 23, 2006 Ono Pharmaceutical Co., Ltd., Public Relations Phone: +81-6-6263-5670 Banyu Pharmaceutical Co., Ltd., Public Relations Phone: +81-3-6272-1001 Merck & Co, Inc. Announced Approval of JANUVIA
Scope. History. History. Incretins. Incretin-based Therapy and DPP-4 Inhibitors
 Plasma Glucose (mg/dl) Plasma Insulin (pmol/l) Incretin-based Therapy and Inhibitors Scope Mechanism of action ผศ.ดร.นพ.ว ระเดช พ ศประเสร ฐ สาขาว ชาโภชนว ทยาคล น ก ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล
Plasma Glucose (mg/dl) Plasma Insulin (pmol/l) Incretin-based Therapy and Inhibitors Scope Mechanism of action ผศ.ดร.นพ.ว ระเดช พ ศประเสร ฐ สาขาว ชาโภชนว ทยาคล น ก ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Dapagliflozin in combination therapy for the Final scope Remit/appraisal objective To appraise the clinical and
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Dapagliflozin in combination therapy for the Final scope Remit/appraisal objective To appraise the clinical and
Abstract. Effect of sitagliptin on glycemic control in patients with type 2 diabetes. Introduction. Abbas Mahdi Rahmah
 Effect of sitagliptin on glycemic control in patients with type 2 diabetes Abbas Mahdi Rahmah Correspondence: Dr. Abbas Mahdi Rahmah Consultant Endocrinologist, FRCP (Edin) Director of Iraqi National Diabetes
Effect of sitagliptin on glycemic control in patients with type 2 diabetes Abbas Mahdi Rahmah Correspondence: Dr. Abbas Mahdi Rahmah Consultant Endocrinologist, FRCP (Edin) Director of Iraqi National Diabetes
Early treatment for patients with Type 2 Diabetes
 Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
Modulating the Incretin System: A New Therapeutic Strategy for Type 2 Diabetes
 Modulating the Incretin System: A New Therapeutic Strategy for Type 2 Diabetes Geneva Clark Briggs, PharmD, BCPS Adjunct Professor at University of Appalachia College of Pharmacy Clinical Associate, Medical
Modulating the Incretin System: A New Therapeutic Strategy for Type 2 Diabetes Geneva Clark Briggs, PharmD, BCPS Adjunct Professor at University of Appalachia College of Pharmacy Clinical Associate, Medical
New Treatment Options for Type 2 Diabetes: Incretin-Based Therapy
 New Treatment Options for Type 2 Diabetes: Incretin-Based Therapy New Treatment Options for Type 2 Diabetes: Incretin-Based Therapy is supported by an educational grant from Novo Nordisk Inc. This program
New Treatment Options for Type 2 Diabetes: Incretin-Based Therapy New Treatment Options for Type 2 Diabetes: Incretin-Based Therapy is supported by an educational grant from Novo Nordisk Inc. This program
Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol
 Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
la prise en charge du diabète de
 N21 XIII Congrès National de Diabétologie, 29 mai 2011, Alger Intérêt et place des Anti DPP4 dans la prise en charge du diabète de type 2 Nicolas PAQUOT, MD, PhD CHU Sart-Tilman, Université de Liège Belgique
N21 XIII Congrès National de Diabétologie, 29 mai 2011, Alger Intérêt et place des Anti DPP4 dans la prise en charge du diabète de type 2 Nicolas PAQUOT, MD, PhD CHU Sart-Tilman, Université de Liège Belgique
Type 2 DM in Adolescents: Use of GLP-1 RA. Objectives. Scope of Problem: Obesity. Background. Pathophysiology of T2DM
 Type 2 DM in Adolescents: Use of GLP-1 RA Objectives Identify patients in the pediatric population with T2DM that would potentially benefit from the use of GLP-1 RA Discuss changes in glycemic outcomes
Type 2 DM in Adolescents: Use of GLP-1 RA Objectives Identify patients in the pediatric population with T2DM that would potentially benefit from the use of GLP-1 RA Discuss changes in glycemic outcomes
Initial combination therapy for patients with type 2 diabetes mellitus: considerations for metformin plus linagliptin
 The journal of interventions in clinical practice www.drugsincontext.com CLINICAL COMMENTARY FULL TEXT ARTICLE Initial combination therapy for patients with type 2 diabetes mellitus: considerations for
The journal of interventions in clinical practice www.drugsincontext.com CLINICAL COMMENTARY FULL TEXT ARTICLE Initial combination therapy for patients with type 2 diabetes mellitus: considerations for
GLP-1. GLP-1 is produced by the L-cells of the gut after food intake in two biologically active forms It is rapidly degraded by DPP-4.
 GLP-1 GLP-1 is produced by the L-cells of the gut after food intake in two biologically active forms It is rapidly degraded by DPP-4 Food intake éinsulin Gut églucose uptake Pancreas Beta cells Alpha cells
GLP-1 GLP-1 is produced by the L-cells of the gut after food intake in two biologically active forms It is rapidly degraded by DPP-4 Food intake éinsulin Gut églucose uptake Pancreas Beta cells Alpha cells
Diabetes Management DPP-4 Inhibitors
 Drug Profile Saxagliptin, a Novel Dipeptidyl Peptidase-4 Inhibitor for the Treatment of Type 2 Diabetes Baptist Gallwitz, MD, PhD Professor of Medicine, Department of Medicine IV, Eberhard Karls University
Drug Profile Saxagliptin, a Novel Dipeptidyl Peptidase-4 Inhibitor for the Treatment of Type 2 Diabetes Baptist Gallwitz, MD, PhD Professor of Medicine, Department of Medicine IV, Eberhard Karls University
DOI: /jemds/2014/2044 ORIGINAL ARTICLE
 AN OBSERVATIONAL STUDY COMPARING SITAGLIPTIN TO METFORMIN AS A INITIAL MONOTHERAPY IN TYPE 2 DIABETES MELLITUS PATIENTS Mohd. Riyaz 1, Imran 2, Rinu Manuel 3, Nidhisha K. Joseph 4 HOW TO CITE THIS ARTICLE:
AN OBSERVATIONAL STUDY COMPARING SITAGLIPTIN TO METFORMIN AS A INITIAL MONOTHERAPY IN TYPE 2 DIABETES MELLITUS PATIENTS Mohd. Riyaz 1, Imran 2, Rinu Manuel 3, Nidhisha K. Joseph 4 HOW TO CITE THIS ARTICLE:
Current evidence on the effect of DPP-4 inhibitor drugs on mortality in type 2 diabetic (T2D) patients: A meta-analysis
 Current evidence on the effect of DPP-4 inhibitor drugs on mortality in type 2 diabetic (T2D) patients: A meta-analysis Raja Chakraverty Assistant Professor in Pharmacology Bengal College of Pharmaceutical
Current evidence on the effect of DPP-4 inhibitor drugs on mortality in type 2 diabetic (T2D) patients: A meta-analysis Raja Chakraverty Assistant Professor in Pharmacology Bengal College of Pharmaceutical
Clinical Overview of Combination Therapy with Sitagliptin and Metformin
 Clinical Overview of Combination Therapy with Sitagliptin and Metformin 1 Contents Pathophysiology of type 2 diabetes and mechanism of action of sitagliptin Clinical data overview of sitagliptin: Monotherapy
Clinical Overview of Combination Therapy with Sitagliptin and Metformin 1 Contents Pathophysiology of type 2 diabetes and mechanism of action of sitagliptin Clinical data overview of sitagliptin: Monotherapy
What s New on the Horizon: Diabetes Medication Update. Michael Shannon, MD Providence Endocrinology, Olympia WA
 What s New on the Horizon: Diabetes Medication Update Michael Shannon, MD Providence Endocrinology, Olympia WA 1 Outline of Talk Newly released and upcoming medications: the incretins, DPP-IV inhibitors,
What s New on the Horizon: Diabetes Medication Update Michael Shannon, MD Providence Endocrinology, Olympia WA 1 Outline of Talk Newly released and upcoming medications: the incretins, DPP-IV inhibitors,
What s New on the Horizon: Diabetes Medication Update
 What s New on the Horizon: Diabetes Medication Update Outline of Talk Newly released and upcoming medications: the incretins, DPP-IV inhibitors, and what s coming Revised ADA/EASD and AACE guidelines:
What s New on the Horizon: Diabetes Medication Update Outline of Talk Newly released and upcoming medications: the incretins, DPP-IV inhibitors, and what s coming Revised ADA/EASD and AACE guidelines:
Efficacy and Safety of Sitagliptin in Various Clinical Settings of T2DM
 Efficacy and Safety of Sitagliptin in arious Clinical Settings of T2DM Young Min Cho, MD, PhD Division of Endocrinology and Metabolism Department of Internal Medicine Seoul National University College
Efficacy and Safety of Sitagliptin in arious Clinical Settings of T2DM Young Min Cho, MD, PhD Division of Endocrinology and Metabolism Department of Internal Medicine Seoul National University College
DPP-4/SGLT2 inhibitor combined therapy for type 2 diabetes
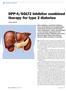 THERAPY REVIEW DPP-4/SGLT2 inhibitor combined therapy for type 2 diabetes STEVE CHAPLIN SPL DPP-4 inhibitors and SGLT2 inhibitors lower blood glucose by complementary mechanisms of action, and two fixeddose
THERAPY REVIEW DPP-4/SGLT2 inhibitor combined therapy for type 2 diabetes STEVE CHAPLIN SPL DPP-4 inhibitors and SGLT2 inhibitors lower blood glucose by complementary mechanisms of action, and two fixeddose
Effect of macronutrients and mixed meals on incretin hormone secretion and islet cell function
 Effect of macronutrients and mixed meals on incretin hormone secretion and islet cell function Background. Following meal ingestion, several hormones are released from the gastrointestinal tract. Some
Effect of macronutrients and mixed meals on incretin hormone secretion and islet cell function Background. Following meal ingestion, several hormones are released from the gastrointestinal tract. Some
GLP-1 (glucagon-like peptide-1) Agonists (Byetta, Bydureon, Tanzeum, Trulicity, Victoza ) Step Therapy and Quantity Limit Criteria Program Summary
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
GLYXAMBI (empagliflozin-linagliptin) oral tablet
 GLYXAMBI (empagliflozin-linagliptin) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
GLYXAMBI (empagliflozin-linagliptin) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
Current Status of Incretin Based Therapies in Type 2 Diabetes
 Current Status of Incretin Based Therapies in Type 2 Diabetes DR.M.Mukhyaprana Prabhu Professor of Internal Medicine Kasturba Medical College, Manipal, Manipal University, India 2 nd International Endocrine
Current Status of Incretin Based Therapies in Type 2 Diabetes DR.M.Mukhyaprana Prabhu Professor of Internal Medicine Kasturba Medical College, Manipal, Manipal University, India 2 nd International Endocrine
TREATMENTS FOR TYPE 2 DIABETES. Susan Henry Diabetes Specialist Nurse
 TREATMENTS FOR TYPE 2 DIABETES Susan Henry Diabetes Specialist Nurse How can we improve outcomes in Type 2 diabetes? Earlier diagnosis Better patient education Stress central role of lifestyle management
TREATMENTS FOR TYPE 2 DIABETES Susan Henry Diabetes Specialist Nurse How can we improve outcomes in Type 2 diabetes? Earlier diagnosis Better patient education Stress central role of lifestyle management
Sitagliptin. Agreed by Clinical Priorities Group
 New Medicine Report Document Status Sitagliptin Agreed by Clinical Priorities Group Traffic Light Decision Blue- Primary Care Prescriber s Rating Offers an advantage - The product has some value but does
New Medicine Report Document Status Sitagliptin Agreed by Clinical Priorities Group Traffic Light Decision Blue- Primary Care Prescriber s Rating Offers an advantage - The product has some value but does
IDF Regions and global projections of the number of people with diabetes (20-79 years), 2013 and Diabetes Atlas -sixth Edition: IDF 2013
 IDF Regions and global projections of the number of people with diabetes (20-79 years), 2013 and 2035 Diabetes Atlas -sixth Edition: IDF 2013 Diabetes Atlas -sixth Edition: IDF 2013 Chronic complications
IDF Regions and global projections of the number of people with diabetes (20-79 years), 2013 and 2035 Diabetes Atlas -sixth Edition: IDF 2013 Diabetes Atlas -sixth Edition: IDF 2013 Chronic complications
GLP-1 agonists. Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK
 GLP-1 agonists Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK What do GLP-1 agonists do? Physiology of postprandial glucose regulation Meal ❶ ❷ Insulin Rising plasma
GLP-1 agonists Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK What do GLP-1 agonists do? Physiology of postprandial glucose regulation Meal ❶ ❷ Insulin Rising plasma
COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP Policy agreed by (Vale of York CCG/date)
 Drug, Treatment, Device name ( Vipidia; Takeda) COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP Policy agreed by (Vale of York CCG/date) Licensed indication To improve glycaemic control in
Drug, Treatment, Device name ( Vipidia; Takeda) COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP Policy agreed by (Vale of York CCG/date) Licensed indication To improve glycaemic control in
Type 2 Diabetes: Where Do We Start with Treatment? DIABETES EDUCATION. Diabetes Mellitus: Complications and Co-Morbid Conditions
 Diabetes Mellitus: Complications and Co-Morbid Conditions ADA Guidelines for Glycemic Control: 2016 Retinopathy Between 2005-2008, 28.5% of patients with diabetes 40 years and older diagnosed with diabetic
Diabetes Mellitus: Complications and Co-Morbid Conditions ADA Guidelines for Glycemic Control: 2016 Retinopathy Between 2005-2008, 28.5% of patients with diabetes 40 years and older diagnosed with diabetic
Volume : 05 Issue : 03 July-Sept Pages:
 Middle East Journal of Applied Sciences Volume : 05 Issue : 03 July-Sept. 2015 Pages: 695-699 Efficacy and Safety of Vildagliptin as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Poorly Controlled
Middle East Journal of Applied Sciences Volume : 05 Issue : 03 July-Sept. 2015 Pages: 695-699 Efficacy and Safety of Vildagliptin as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Poorly Controlled
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.18 Line of Business: Health Insurance Marketplace See Important
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.18 Line of Business: Health Insurance Marketplace See Important
Side Effects of: GLP-1 agonists DPP-4 inhibitors SGLT-2 inhibitors. Bryce Fukunaga PharmD April 25, 2018
 Side Effects of: GLP-1 agonists DPP-4 inhibitors SGLT-2 inhibitors Bryce Fukunaga PharmD April 25, 2018 Objectives For each drug class: Identify the overall place in therapy Explain the mechanism of action
Side Effects of: GLP-1 agonists DPP-4 inhibitors SGLT-2 inhibitors Bryce Fukunaga PharmD April 25, 2018 Objectives For each drug class: Identify the overall place in therapy Explain the mechanism of action
Thiazolidinedione Step Therapy Program
 Thiazolidinedione Step Therapy Program Policy Number: 5.01.580 Last Review: 7/2018 Origination: 07/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Thiazolidinedione Step Therapy Program Policy Number: 5.01.580 Last Review: 7/2018 Origination: 07/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Management of Type 2 Diabetes. Why Do We Bother to Achieve Good Control in DM2. Insulin Secretion. The Importance of BP and Glucose Control
 Insulin Secretion Management of Type 2 Diabetes DG van Zyl Why Do We Bother to Achieve Good Control in DM2 % reduction 0-5 -10-15 -20-25 -30-35 -40 The Importance of BP and Glucose Control Effects of tight
Insulin Secretion Management of Type 2 Diabetes DG van Zyl Why Do We Bother to Achieve Good Control in DM2 % reduction 0-5 -10-15 -20-25 -30-35 -40 The Importance of BP and Glucose Control Effects of tight
Modulating the Incretin System: A New Therapeutic Strategy for Type 2 Diabetes. Overview. Prevalence of Overweight in the U.S.
 Modulating the Incretin System: A New Therapeutic Strategy for Type 2 Diabetes Geneva Clark Briggs, PharmD, BCPS Overview Underlying defects with Type 2 diabetes Importance of managing postprandial glucose
Modulating the Incretin System: A New Therapeutic Strategy for Type 2 Diabetes Geneva Clark Briggs, PharmD, BCPS Overview Underlying defects with Type 2 diabetes Importance of managing postprandial glucose
Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: theoretical consideration and therapeutic potential
 REVIEW Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: theoretical consideration and therapeutic potential Nasser Mikhail Endocrinology Division, Olive View-UCLA Medical
REVIEW Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: theoretical consideration and therapeutic potential Nasser Mikhail Endocrinology Division, Olive View-UCLA Medical
Scottish Medicines Consortium
 Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Sodium-Glucose Co-Transporter 2 (SGLT-2) Inhibitors Drug Class Prior Authorization Protocol
 Sodium-Glucose Co-Transporter 2 (SGLT-2) Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has
Sodium-Glucose Co-Transporter 2 (SGLT-2) Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has
Diabetes: What is the scope of the problem?
 Diabetes: What is the scope of the problem? Elizabeth R. Seaquist MD Division of Endocrinology and Diabetes Department of Medicine Director, General Clinical Research Center Pennock Family Chair in Diabetes
Diabetes: What is the scope of the problem? Elizabeth R. Seaquist MD Division of Endocrinology and Diabetes Department of Medicine Director, General Clinical Research Center Pennock Family Chair in Diabetes
Newer Drugs in the Management of Type 2 Diabetes Mellitus
 Newer Drugs in the Management of Type 2 Diabetes Mellitus Dr. C. Dinesh M. Naidu Professor of Pharmacology, Kamineni Institute of Medical Sciences, Narketpally. 1 Presentation Outline Introduction Pathogenesis
Newer Drugs in the Management of Type 2 Diabetes Mellitus Dr. C. Dinesh M. Naidu Professor of Pharmacology, Kamineni Institute of Medical Sciences, Narketpally. 1 Presentation Outline Introduction Pathogenesis
A New Therapeutic Strategey for Type II Diabetes: Update 2008
 Live, One Hour Webinar A New Therapeutic Strategey for Type II Diabetes: Update 2008 Geneva Clark Briggs, PharmD, BCPS Adjunct Professor at University of Appalachia College of Pharmacy in Grundy, Virginia.
Live, One Hour Webinar A New Therapeutic Strategey for Type II Diabetes: Update 2008 Geneva Clark Briggs, PharmD, BCPS Adjunct Professor at University of Appalachia College of Pharmacy in Grundy, Virginia.
Diabetes 2013: Achieving Goals Through Comprehensive Treatment. Session 2: Individualizing Therapy
 Diabetes 2013: Achieving Goals Through Comprehensive Treatment Session 2: Individualizing Therapy Joshua L. Cohen, M.D., F.A.C.P. Professor of Medicine Interim Director, Division of Endocrinology & Metabolism
Diabetes 2013: Achieving Goals Through Comprehensive Treatment Session 2: Individualizing Therapy Joshua L. Cohen, M.D., F.A.C.P. Professor of Medicine Interim Director, Division of Endocrinology & Metabolism
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy
 Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
MOA: Long acting glucagon-like peptide 1 receptor agonist
 Alexandria Rydz MOA: Long acting glucagon-like peptide 1 receptor agonist Increases glucose dependent insulin secretion Decreases inappropriate glucagon secretion Increases β- cell growth and replication
Alexandria Rydz MOA: Long acting glucagon-like peptide 1 receptor agonist Increases glucose dependent insulin secretion Decreases inappropriate glucagon secretion Increases β- cell growth and replication
SGLT2 Inhibitors
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: November 30, 2018 SGLT2 Inhibitors Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: November 30, 2018 SGLT2 Inhibitors Description
Diabetes Oral Agents Pharmacology. University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 Diabetes Oral Agents Pharmacology University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 Learning Objectives Understand the role of the utilization of free
Diabetes Oral Agents Pharmacology University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 Learning Objectives Understand the role of the utilization of free
Utility of Saxagliptin in the Treatment of Type 2 Diabetes: Review of Efficacy and Safety
 Adv Ther (2015) 32:1065 1084 DOI 10.1007/s12325-015-0262-9 REVIEW Utility of Saxagliptin in the Treatment of Type 2 Diabetes: Review of Efficacy and Safety Rajeev Jain To view enhanced content go to www.advancesintherapy.com
Adv Ther (2015) 32:1065 1084 DOI 10.1007/s12325-015-0262-9 REVIEW Utility of Saxagliptin in the Treatment of Type 2 Diabetes: Review of Efficacy and Safety Rajeev Jain To view enhanced content go to www.advancesintherapy.com
Diabetes Management DPP-4 Inhibitors
 Dipeptidyl Peptidase-4 Inhibitors and the Management of Hyperglycemia Pamela M Katz, MD 1 and Lawrence A Leiter, MD 2 1. Resident, Division of Endocrinology and Metabolism, University of Toronto; 2. Head,
Dipeptidyl Peptidase-4 Inhibitors and the Management of Hyperglycemia Pamela M Katz, MD 1 and Lawrence A Leiter, MD 2 1. Resident, Division of Endocrinology and Metabolism, University of Toronto; 2. Head,
PROCEEDINGS CLINICAL RESEARCH AND EXPERIENCE WITH INCRETIN-BASED THERAPIES * Vivian A. Fonseca, MD, FRCP ABSTRACT
 CLINICAL RESEARCH AND EXPERIENCE WITH INCRETIN-BASED THERAPIES Vivian A. Fonseca, MD, FRCP ABSTRACT Despite proven lifestyle recommendations and the availability of a range of oral antidiabetic agents,
CLINICAL RESEARCH AND EXPERIENCE WITH INCRETIN-BASED THERAPIES Vivian A. Fonseca, MD, FRCP ABSTRACT Despite proven lifestyle recommendations and the availability of a range of oral antidiabetic agents,
Update on Diabetes Mellitus
 Update on Diabetes Mellitus Treatment: Targeting the Incretin System Overview Underlying defects with Type 2 diabetes Importance of managing postprandial glucose control Amylin Incretin Hormones New therapies
Update on Diabetes Mellitus Treatment: Targeting the Incretin System Overview Underlying defects with Type 2 diabetes Importance of managing postprandial glucose control Amylin Incretin Hormones New therapies
sitagliptin, 25mg, 50mg and 100mg film-coated tablets (Januvia ) SMC No. (1083/15) Merck Sharp and Dohme UK Ltd
 sitagliptin, 25mg, 50mg and 100mg film-coated tablets (Januvia ) SMC No. (1083/15) Merck Sharp and Dohme UK Ltd 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the
sitagliptin, 25mg, 50mg and 100mg film-coated tablets (Januvia ) SMC No. (1083/15) Merck Sharp and Dohme UK Ltd 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Types of Diabetes that the Dipeptidyl Peptidase-4 Inhibitor May Act Effectively and Safely
 The Open Diabetes Journal, 2011, 4, 1-5 1 Open Access Types of Diabetes that the Dipeptidyl Peptidase-4 Inhibitor May Act Effectively and Safely Hidekatsu Yanai * and Hiroki Adachi Department of Internal
The Open Diabetes Journal, 2011, 4, 1-5 1 Open Access Types of Diabetes that the Dipeptidyl Peptidase-4 Inhibitor May Act Effectively and Safely Hidekatsu Yanai * and Hiroki Adachi Department of Internal
Diabetes update - Diagnosis and Treatment
 Diabetes update - Diagnosis and Treatment Eugene J Barrett, MD,PhD Madge Jones Professor of Medicine Director, University of Virginia Diabetes Center Disclosures - None Case 1 - Screening for Diabetes
Diabetes update - Diagnosis and Treatment Eugene J Barrett, MD,PhD Madge Jones Professor of Medicine Director, University of Virginia Diabetes Center Disclosures - None Case 1 - Screening for Diabetes
Drug Class Review Newer Diabetes Medications and Combinations
 Drug Class Review Newer Diabetes Medications and Combinations Final Update 2 Report July 2016 The purpose reports is to make available information regarding the comparative clinical effectiveness and harms
Drug Class Review Newer Diabetes Medications and Combinations Final Update 2 Report July 2016 The purpose reports is to make available information regarding the comparative clinical effectiveness and harms
SGLT2 Inhibitors
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: June 22, 2018 SGLT2 Inhibitors Description Invokana
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: June 22, 2018 SGLT2 Inhibitors Description Invokana
New and Emerging Therapies for Type 2 DM
 Dale Clayton MHSc, MD, FRCPC Dalhousie University/Capital Health April 28, 2011 New and Emerging Therapies for Type 2 DM The science of today, is the technology of tomorrow. Edward Teller American Physicist
Dale Clayton MHSc, MD, FRCPC Dalhousie University/Capital Health April 28, 2011 New and Emerging Therapies for Type 2 DM The science of today, is the technology of tomorrow. Edward Teller American Physicist
Data from an epidemiologic analysis of
 CLINICAL TRIAL RESULTS OF GLP-1 RELATED AGENTS: THE EARLY EVIDENCE Lawrence Blonde, MD, FACP, FACE ABSTRACT Although it is well known that lowering A 1c (also known as glycated hemoglobin) is associated
CLINICAL TRIAL RESULTS OF GLP-1 RELATED AGENTS: THE EARLY EVIDENCE Lawrence Blonde, MD, FACP, FACE ABSTRACT Although it is well known that lowering A 1c (also known as glycated hemoglobin) is associated
Barbara Cadario, BSc(Hon), BScPhm., MSc Barbara Cadario SAXAGLIPTIN
 Volume 31 (1) 2011 Editor: Barbara Cadario, BSc(Hon), BScPhm., MSc Contents - Saxagliptin Barbara Cadario Chairman, Medical Review Laird Birmingham, MD, MHSc, FRCP(C) TRADE NAME: Onglyza CLASSIFICATION
Volume 31 (1) 2011 Editor: Barbara Cadario, BSc(Hon), BScPhm., MSc Contents - Saxagliptin Barbara Cadario Chairman, Medical Review Laird Birmingham, MD, MHSc, FRCP(C) TRADE NAME: Onglyza CLASSIFICATION
Improving Glycemic Control With Combination Therapy for Adult Patients With Type 2 Diabetes. Topic Highlights
 Improving Glycemic Control With Combination Therapy for Adult Patients With Type 2 Diabetes Featured Expert Topic Highlights CHARLES REASNER, MD Adjunct Professor of Medicine University of Texas Health
Improving Glycemic Control With Combination Therapy for Adult Patients With Type 2 Diabetes Featured Expert Topic Highlights CHARLES REASNER, MD Adjunct Professor of Medicine University of Texas Health
Pharmacy Drug Class Review. Type 2 Diabetes Mellitus Focus on DPP-4 inhibitors, Glp-1 Analogs, And Amylin Analogs
 Pharmacy Drug Class Review November 30, 2009 Disclaimer: Specific agents may have variations Authored By: Kirsten Butterfoss Pharm. D. Candidate 2010; Edited By: Richard J. Kraft, Pharm.D., Type 2 Diabetes
Pharmacy Drug Class Review November 30, 2009 Disclaimer: Specific agents may have variations Authored By: Kirsten Butterfoss Pharm. D. Candidate 2010; Edited By: Richard J. Kraft, Pharm.D., Type 2 Diabetes
What s New in Diabetes Treatment. Disclosures
 What s New in Diabetes Treatment Shiri Levy M.D. Henry Ford Hospital Senior Staff Physician Service Chief, West Bloomfield Hospital Endocrinology, Metabolism, Bone and Mineral Disorders Disclosures None
What s New in Diabetes Treatment Shiri Levy M.D. Henry Ford Hospital Senior Staff Physician Service Chief, West Bloomfield Hospital Endocrinology, Metabolism, Bone and Mineral Disorders Disclosures None
Horizon Scanning Technology Summary. Liraglutide for type 2 diabetes. National Horizon Scanning Centre. April 2007
 Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Update on Insulin-based Agents for T2D
 Update on Insulin-based Agents for T2D Injectable Therapies for Type 2 Diabetes Mellitus (T2DM) and Obesity This presentation will: Describe established and newly available insulin therapies for treatment
Update on Insulin-based Agents for T2D Injectable Therapies for Type 2 Diabetes Mellitus (T2DM) and Obesity This presentation will: Describe established and newly available insulin therapies for treatment
Society for Ambulatory Anesthesia Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery
 Society for Ambulatory Anesthesia Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery Girish P. Joshi, MB BS, MD, FFARCSI Anesthesia & Analgesia
Society for Ambulatory Anesthesia Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery Girish P. Joshi, MB BS, MD, FFARCSI Anesthesia & Analgesia
Sitagliptin: A component of incretin based therapy. Rezvan Salehidoost, M.D., Endocrinologist
 Sitagliptin: A component of incretin based therapy Rezvan Salehidoost, M.D., Endocrinologist Agenda Mode of Action Evidences for sitagliptine cardiovascular safety of sitagliptin Ramadan study Impact of
Sitagliptin: A component of incretin based therapy Rezvan Salehidoost, M.D., Endocrinologist Agenda Mode of Action Evidences for sitagliptine cardiovascular safety of sitagliptin Ramadan study Impact of
Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes
 Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes Genetics, environment, and lifestyle (obesity, inactivity, poor diet) Impaired fasting glucose Decreased β-cell
Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes Genetics, environment, and lifestyle (obesity, inactivity, poor diet) Impaired fasting glucose Decreased β-cell
Clinical study on the therapeutic efficacy of the dipeptidyl peptidase 4 inhibitors, in type 2 diabetes
 UNIVERSITY OF MEDICINE AND PHARMACY OF CRAIOVA FACULTY OF MEDICINE Clinical study on the therapeutic efficacy of the dipeptidyl peptidase 4 inhibitors, in type 2 diabetes PhD Thesis Abstract Key words:
UNIVERSITY OF MEDICINE AND PHARMACY OF CRAIOVA FACULTY OF MEDICINE Clinical study on the therapeutic efficacy of the dipeptidyl peptidase 4 inhibitors, in type 2 diabetes PhD Thesis Abstract Key words:
Role of incretins in the treatment of type 2 diabetes
 Role of incretins in the treatment of type 2 diabetes Jens Juul Holst Department of Medical Physiology Panum Institute University of Copenhagen Denmark Diabetes & Obesity Spanish Society of Internal Medicine
Role of incretins in the treatment of type 2 diabetes Jens Juul Holst Department of Medical Physiology Panum Institute University of Copenhagen Denmark Diabetes & Obesity Spanish Society of Internal Medicine
DPP-4 inhibitor. The new class drug for Diabetes
 DPP-4 inhibitor The new class drug for Diabetes 1 Cause of Death in Korea 1 st ; Neoplasm 2 nd ; Cardiovascular Disease 3 rd ; Cerebrovascular Disease Diabetes 2 Incidence of Fatal or Nonfatal MI During
DPP-4 inhibitor The new class drug for Diabetes 1 Cause of Death in Korea 1 st ; Neoplasm 2 nd ; Cardiovascular Disease 3 rd ; Cerebrovascular Disease Diabetes 2 Incidence of Fatal or Nonfatal MI During
Timely!Insulinization In!Type!2! Diabetes,!When!and!How
 Timely!Insulinization In!Type!2! Diabetes,!When!and!How, FACP, FACE, CDE Professor of Internal Medicine UT Southwestern Medical Center Dallas, Texas Current Control and Targets 1 Treatment Guidelines for
Timely!Insulinization In!Type!2! Diabetes,!When!and!How, FACP, FACE, CDE Professor of Internal Medicine UT Southwestern Medical Center Dallas, Texas Current Control and Targets 1 Treatment Guidelines for
My Journey in Endocrinology. Samuel Cataland M.D
 My Journey in Endocrinology Samuel Cataland M.D. 1968-2015 Drs Berson M.D. Yalow phd Insulin Radioimmunoassay Nobel Prize Physiology or Medicine 1977 Rosalyn Yalow: Radioimmunoassay Technology Andrew Schally
My Journey in Endocrinology Samuel Cataland M.D. 1968-2015 Drs Berson M.D. Yalow phd Insulin Radioimmunoassay Nobel Prize Physiology or Medicine 1977 Rosalyn Yalow: Radioimmunoassay Technology Andrew Schally
Pharmacology Update for the Adult Patient - Newer Oral Medications for Diabetes
 Pharmacology Update for the Adult Patient - Newer Oral Medications for Diabetes Brooke Hudspeth, PharmD, CDE, MLDE Director of Diabetes Prevention, Kroger Pharmacy Adjunct Assistant Professor, University
Pharmacology Update for the Adult Patient - Newer Oral Medications for Diabetes Brooke Hudspeth, PharmD, CDE, MLDE Director of Diabetes Prevention, Kroger Pharmacy Adjunct Assistant Professor, University
Treatment Options for Diabetes: An Update
 Treatment Options for Diabetes: An Update A/Prof. Marg McGill Manager, Diabetes Centre Dr. Ted Wu Staff Specialist Endocrinologist Diabetes Centre Centre of Health Professional Education Education Provider
Treatment Options for Diabetes: An Update A/Prof. Marg McGill Manager, Diabetes Centre Dr. Ted Wu Staff Specialist Endocrinologist Diabetes Centre Centre of Health Professional Education Education Provider
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: Last Review Date: 02.
 Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: HIM Revision Log See Important Reminder
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: HIM Revision Log See Important Reminder
Understanding the Mechanisms to Maintain Glucose
 n posttest n Understanding the Mechanisms to Maintain Glucose Homeostasis: A Review for Managed Care Instructions After reading Understanding the Mechanisms to Maintain Glucose Homeostasis: A Review for
n posttest n Understanding the Mechanisms to Maintain Glucose Homeostasis: A Review for Managed Care Instructions After reading Understanding the Mechanisms to Maintain Glucose Homeostasis: A Review for
INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES
 INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES ARSHNA SANGHRAJKA DIABETES SPECIALIST PRESCRIBING PHARMACIST OBJECTIVES EXPLORE THE TYPES OF INSULIN AND INJECTABLE DIABETES TREATMENTS AND DEVICES AVAILABLE
INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES ARSHNA SANGHRAJKA DIABETES SPECIALIST PRESCRIBING PHARMACIST OBJECTIVES EXPLORE THE TYPES OF INSULIN AND INJECTABLE DIABETES TREATMENTS AND DEVICES AVAILABLE
Bristol-Myers Squibb / AstraZeneca ADVICE dapagliflozin (Forxiga ) Indication under review: SMC restriction: Chairman, Scottish Medicines Consortium
 Re-Submission dapagliflozin 5mg and 10mg film-coated tablets (Forxiga ) SMC No. (799/12) Bristol-Myers Squibb / AstraZeneca 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
Re-Submission dapagliflozin 5mg and 10mg film-coated tablets (Forxiga ) SMC No. (799/12) Bristol-Myers Squibb / AstraZeneca 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus
 Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus You should be offering psychosocial care to all patients with diabetes, says the ADA. Here are the specific recommendations. Summary Recommendation
Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus You should be offering psychosocial care to all patients with diabetes, says the ADA. Here are the specific recommendations. Summary Recommendation
Combination treatment for T2DM
 Combination treatment for T2DM Date of approval: December 2016 SAGLB.DIA.16.08.0657 Abbreviations ADA: American Diabetes Association CVD: Cardiovascular disease DPP-4: Dipeptidyl Peptidase-4 EASD: European
Combination treatment for T2DM Date of approval: December 2016 SAGLB.DIA.16.08.0657 Abbreviations ADA: American Diabetes Association CVD: Cardiovascular disease DPP-4: Dipeptidyl Peptidase-4 EASD: European
