Very low calorie diets and hypokalaemia: the importance of ammonium excretion
|
|
|
- Jeffry Melton
- 6 years ago
- Views:
Transcription
1 Nephrol Dial Transplant (2005) 20: doi: /ndt/gfh582 Teaching Point (Section Editor: K. Ku hn) Supported by an educational grant from Very low calorie diets and hypokalaemia: the importance of ammonium excretion Tane Liu 1,2, Glenn T. Nagami 1,2, Marcia L. Everett 1 and Barton S. Levine 1,2 1 Nephrology Section and Departments of Medicine VAGLAHS West Los Angeles and 2 The David Geffen School of Medicine at UCLA, Los Angeles, CA, USA Keywords: acute renal failure; ammonia; fasting; hypokalaemia; ketoacidosis; metabolic alkalosis Introduction besity has become a worldwide epidemic and is associated with significant morbidity and mortality. More than two-thirds of adults in the United States are either trying to lose weight or prevent weight gain. Very low calorie diets, diets with energy levels between 200 and 800 kcal/day, are used commonly for rapid weight loss. These diets are generally safe and well-tolerated and have been reported to improve blood pressure, glycaemic control and lipid profiles. Minor side effects, including fatigue, constipation, nausea and diarrhoea, often occur, but serious adverse events, including death, may result if the very low calorie diets are not used appropriately [1]. Hypokalaemia may occur with very low calorie diets but is usually mild [1,2]. Below, we present a case of severe hypokalaemia, which developed after 2 weeks of a very low calorie diet. The pathophysiological mechanisms that lead to the development of significant potassium depletion are discussed. Case A 61-year-old man was admitted to the inpatient service of the VAGLAHS for hypokalaemia. A serum potassium of 2.8 meq/l was obtained during a Correspondence and offprint requests to: Barton S. Levine, Nephrology Section (111L), VAGLAHS West Los Angeles, Wilshire Boulevard, Los Angeles, CA 90073, USA. barton.levine@med.va.gov routine outpatient visit [Table 1 (18 November 2002) and Figure 1]. He was instructed to return to the emergency room where he received 100 meq KCl over an 8 h period. His serum potassium remained at 2.9 meq/l, prompting his admission. The patient was morbidly obese with a body mass index of 43.6 kg/m 2. He had several medical problems associated with obesity, including hypertension, diabetes mellitus, hyperlipidaemia, obstructive sleep apnoea, microalbuminuria and osteoarthritis. ther past history included bipolar disorder and benign prostatic hypertrophy. In an attempt to lose weight, he self-initiated a low calorie, high protein diet 15 days prior to admission (Medifast Inc, winop Mills, Maryland, USA; see Table 2 for contents). He was ingesting three to six servings of Medifast as his sole energy source and denied eating other food. He achieved a weight loss of 13.6 kg during the 2 week period. The patient was asymptomatic at the time of his outpatient visit. He denied a history of vomiting, diarrhoea or excessive sweating. His daily medications included fosinopril (20 mg), hydrochlorothiazide (25 mg), potassium chloride (20 meq), felodipine (10 mg), lovastatin (40 mg) and aspirin (325 mg). The patient had been on the same therapeutic regimen for >1 year and before this episode of hypokalaemia, the serum potassium had been within or near the normal range (Figure 1). n physical examination, vital signs were normal and, aside from his morbid obesity and 2þ oedema of the lower extremities, the remainder of his examination was unremarkable. His serum and urine chemistries and an arterial blood gas are shown in Table 1. f note, he was hypokalaemic, his anion gap was 19 but his bicarbonate was elevated and he had a mild alkalaemia. His calculated transtubular potassium gradient (TTKG) was 16 and his serum ketones were positive the day after admission. Random plasma renin activity was ß The Author [2005]. Published by xford University Press on behalf of ERA-EDTA. All rights reserved. For Permissions, please journals.permissions@oupjournals.org
2 Very low calorie diets and hypokalaemia 643 Table 1. Serum and urine chemistries and arterial blood gas (ABG) 16 months prior to admission 12 months prior to admission admission a after admission Serum Na (meq/l) K (meq/l) Cl (meq/l) C 2 (mmol/l) BUN (mg/dl) Creatinine (mg/dl) Glucose (mg/dl) smolality (mosm/kg) 296 Anion gap (meq/l) Urine Na (meq/l) <10 K (meq/l) 83 Cl (meq/l) 22 Creatinine (mg/dl) 377 smolality (mosm/kg) 538 TTKG 16 ABG ph/pc 2 (U/mmHg) 7.45/49.2 HC 3 /P 2 (mmol/l/mmhg) 34.3/58.1 Lactate (mmol/l) 0.9 a Date of admission. Admission Table 2. Content of Medifast meq/l Serum HC3 Serum K 1 Pack 4.5 Packs Protein (g) Carbohydrate (g) Fat (g) Calories Na (mmol) K (mmol) ng/ml/h and serum aldosterone was 13.5 ng/dl. His electrocardiogram was notable for T-wave inversion in leads I, AVL and V4 V6 and Q waves in V1 and V2. ne day after admission, the patient agreed to start a regular diet and intravenous fluids were given to correct his volume depletion and hydrochlorothiazide was discontinued. The patient was given a total of 220 meq KCl for the first 24 h after admission and his serum potassium rose to 3.1 meq/l. The patient continued to require 120 meq of supplemental KCl daily for the next 3 days after which his serum potassium normalized (Figure 1). Days Fig. 1. Serial values for serum potassium and bicarbonate. Arrow indicates date of admission. Discussion The patient in this report, despite taking potassium supplements, developed severe hypokalaemia 2 weeks after initiating a very low calorie diet. The large amount of potassium required to correct the hypokalaemia suggested a large body deficit of potassium. Several factors probably contributed to potassium depletion in this patient. Hypokalaemia occurs commonly with the use of thiazide diuretics and is usually apparent during the first weeks of therapy. Thereafter, the serum potassium remains stable unless other disturbances of potassium homeostasis are superimposed. This patient had been taking hydrochlorothiazide for several years, but only manifested borderline hypokalaemia until the present admission. Mild hypokalaemia is also common after fasting or ingesting a low calorie diet. Significant renal losses of potassium occur during the first week of fasting due to the release of intracellular potassium resulting from tissue catabolism, in conjunction with obligatory losses from the urinary excretion of ketoacid anions generated during fasting; the excretion of ketoacid anions in the urine obligates the excretion of
3 644 T. Liu et al. Table 3. Equations and calculations for the UAG, UG, DPI, UUN and 24 h urine volume 1. UAG ¼ [Na þ ]u þ [K þ ]u [Cl ]u a. UAG ¼ (5 þ 83) 22 ¼ DPI a ¼ 6.25(UUN þ NUN) 3. UUN ¼ (DPI 6.25 NUN)/6.25 a. UUN ¼ (63 [ ])/6.25 ¼ UG ¼ [Uosm]m [Uosm]c 5. [Uosm]c ¼ 2([Na þ ]u þ [K þ ]u) þ Gu (mmol/l) þ Uu (mmol/l) a. [Uosm]c ¼ 2(5 þ 83) þ 0 þ 352 ¼ 528 b. UG ¼ ¼ Estimate of 24 h urine volume (UV) ¼ 100 (Cr) 24 h /(Cr) spot a. UV ¼ /377 ¼ 577 ml a Assuming there are no urinary or gastrointestinal losses of protein. [Na þ ]u, concentration of urine sodium (meq/l); [K þ ]u, concentration of urine potassium (meq/l); [Cl ]u, concentration of urine chloride (meq/l); [Uosm]m, measured urine osmolality (mosm/kg); [Uosm]c, calculated urine osmolality (mosm/kg); Gu, concentration of glucose in urine (mmol/l); Uu, concentration of urea in urine (mmol/l); UUN (g/day); NUN, non-urea nitrogen [estimated as bodyweight (g/day)]; (Cr) 24 h, 24 h urine creatinine excretion (mg/day); (Cr) spot, creatinine concentration in spot urine (mg/dl). an accompanying cation, such as sodium or potassium, to maintain electroneutrality. Consistent with the presence of ketosis in this patient was the elevated serum anion gap of 19 on admission, probably reflecting the presence of ketoacids (acetoacetate and/or b-hydroxybutyrate), and a positive test for urine ketones. In addition, the high urine anion gap (UAG; Table 3, equation 1) suggested the presence of unmeasured anions. With fasting, continued urinary excretion of potassium along with ketoacid anions could eventually lead to profound hypokalaemia. However, after the first week of fasting the renal losses of potassium are usually minimized as ketones are excreted with ammonium generated in response to the ketoacidosis, rather than with sodium or potassium [2,3]. As a result, serum potassium levels only fall slightly below normal. In the present case, severe hypokalaemia occurred after the initiation of the low caloric diet, even though the patient was ingesting 20 meq/day of supplemental KCl in addition to an average intake of 51 meq/day of potassium from the Medifast supplement (assuming an average intake of 4.5 packs of Medifast). Furthermore, the patient was on fosinopril, an agent that blunts diuretic-induced hypokalaemia through its effect on the renin angiotensin aldosterone system. Why then did this patient s serum potassium drop to 2.8 meq/l? From the data at hand it is clear that the severe hypokalaemia observed in this case resulted from excessive renal losses of potassium, as evidenced by a high TTKG [4] and the lack of any history of gastrointestinal losses. What is unusual in this case is that excessive renal potassium excretion continued after the initial week of the low caloric diet, when, as already mentioned, renal potassium losses are minimized with fasting and the TTKG is low [2,3]. Examination of the urine electrolytes reveals that potassium was being excreted at a high concentration with a non-chloride anion(s), reflecting the presence of ketoacid anions in the urine. Thus, a contributing factor to his continued renal loss of potassium was ketonuria, present 1 day after admission. But, as already mentioned, renal losses of potassium should have been minimal after 2 weeks of the low caloric diet, since at this stage the ketoacid anions should have been predominantly excreted with ammonium [2,3]. As evidenced by his urine electrolytes (Table 1), the patient was excreting minimal amounts of sodium due to volume contraction and a low salt intake, a typical finding after 2 weeks of fasting [2]. Therefore, the only cations that would have been available for excretion with the ketoacid anions were ammonium and potassium; any impairment in ammonium excretion would have obligated the continued renal excretion of potassium with the ketoacid anions and aggravated the hypokalaemia. Was urinary ammonium excretion impaired in this case? To implicate a lack of adequate urinary ammonium for the renal wasting of potassium in this instance, it would be helpful to know the approximate level of urinary ammonium excretion. Direct measurements of urinary ammonium excretion are not available readily in clinical practice. Therefore, means of estimating urinary ammonium excretion have been devised using specific urine indices, the UAG and urine osmolal gap (UG) [5]. The UAG is calculated from a spot urine sample by subtracting the concentration of urine chloride from the sum of the urine sodium and potassium concentrations obtained from the same specimen (Table 3, equation 1). A negative UAG indicates that chloride is being excreted with another cation besides sodium or potassium, namely ammonium. By contrast, a positive UAG in the presence of metabolic acidosis indicates that urinary ammonium excretion is impaired. Caution should be exercised in interpreting the UAG under certain circumstances [5]. If an anion other than chloride is present in the urine in significant amounts, it too must be accompanied by a cation. In this circumstance, ammonium may be excreted with the unmeasured anion, such as ketoacid anions, and the UAG would be positive despite the presence of substantial amounts of ammonium in the urine. Therefore, when an unmeasured anion is present in the urine in significant quantities the UAG cannot be used to accurately estimate ammonium excretion. The UG (Table 3, equation 4) is calculated by subtracting the calculated urine osmolality ([Uosm]c; Table 3, equation 5) from the measured urine osmolality ([Uosm]m). The UG/2 provides an estimate of the urine ammonium excretion, but is not subject to many of the vagaries that muddle the interpretation of the UAG, such as ketonuria. A drawback to the UG is that urine urea and glucose concentrations, necessary for the [Uosm]c, are not measured routinely. With both hypokalaemia and a high anion gap metabolic acidosis, a marked increase in urinary ammonium excretion would be expected. But the UAG was markedly positive in this case (Table 3, equation 1a),
4 Very low calorie diets and hypokalaemia 645 consistent with an inappropriately low level of ammonium excretion. However, the patient was also excreting ketones when the spot urine electrolytes were obtained, making the interpretation of the UAG problematic for the reasons alluded to earlier. In this situation the UG would have provided more information, but no urine urea or glucose measurements were obtained. In calculating UG the glucose term can be ignored if the dipstick reading for glucose is negative. An estimate of urea excretion and 24 h urine volume can be made to obtain a urea concentration. Two independent methods were used to estimate urine urea excretion. In the first, his glomerular filtration rate (GFR) was assumed to be reduced given his pre-renal state. His blood urea nitrogen was 29 mg/dl or mmol/l of urea. If his GFR was decreased to 60 ml/min due to volume contraction (assuming his GFR declined 50% given his baseline serum creatinine was 0.9 mg/dl and his serum creatinine was mg/dl when the urine was collected), then over the 24 h period he would have filtered 86.4 l/day and 894 mmol/day of urea. Given his volume-contracted state and that he was on a diuretic, it can be assumed that he had a fractional excretion (FE) of urea of 30% [6] and, therefore, his total daily urea excretion was at 268 mmol; if his FE urea were 25% or 20% his total urea excretion would be mmol/day and mmol/day, respectively. Another estimate of his urea excretion can be obtained utilizing the formula for evaluating dietary protein intake (DPI; Table 3, equation 2) and rearranging it to solve for urine urea nitrogen (UUN; Table 3, equation 3). Since he was ingesting three to six packs of Medifast per day as his sole dietary source of protein it can estimated that he was ingesting, on average, 63 g of protein per day (4.5 packs of Medifast per day). Using his body weight of 142 kg, his estimated UUN is 5.7 g/day (Table 3, equation 3a), which is equivalent to a urine urea of 203 mmol/day. Since the patient was likely catabolic, the estimate using the DPI formula would tend to underestimate urea excretion. The 24 h urine output of the patient on the day the spot urine was obtained can be estimated using a 24 h urine creatinine of 2100 mg measured 2 days later. Since the creatinine excretion should be similar on any given day in the steady state, the urine output on the day of the spot urine can be estimated by dividing the creatinine in the 24 h urine by the spot urine value (Table 3, equation 6). Using this formula his estimated urine volume on the day in question was 577 ml/24 h (Table 3, equation 6a). This value is probably an overestimate of his urine output since his serum creatinine was still decreasing during the 24 h urine collection, which would result in a higher level of creatinine excretion. The low urine output would be consistent with his pre-renal state. Using the estimates of daily urea excretion and urine volume, the concentration of urine urea would be 268 mmol/0.577 l or 464 mmol/l urea if the FE urea were 30%, 387 mmol/l if the FE urea were 25% or 310 mmol/l if the FE urea were 20%. Using the DPI estimated urea, the concentration of urea would be 203 mmol/0.577 l or 352 mmol/l urea. The UG can now be calculated using the spot urine electrolytes (Table 2) and the estimated concentrations for urea. Assuming an FE urea of 25%, his estimated UG would be 538 (180 þ 387) or 29, while using the 20% estimate it would be 48. Using the data obtained from the DPI formula, the UG would be 10 (Table 3, equation 5a,5b). The urine ammonium excretion, calculated as the UG/2, would be low regardless of which estimate is used, leaving potassium as the sole cation available to accompany the excretion of the ketoacid anions. Why might this patient s ammonium excretion have been impaired? In this case several factors were present that could impair ammonia production and/or excretion, including pre-renal azotaemia resulting in a decrease in distal sodium delivery, ketosis, disruption of the angiotensin-ii system and metabolic alkalosis. f these, the most important factor was the development of metabolic alkalosis, as evidenced by his alkalaemia and elevated bicarbonate level during the admission in question [7]. Although his bicarbonate level had been elevated chronically, this appears to have been due to a chronic respiratory acidosis, as evidenced by an arterial blood gas obtained 1 month after the episode of severe hypokalaemia (ph 7.38; PC mmhg; P mmhg; HC meq/l). The acute metabolic alkalosis may have arisen after he initiated a low sodium chloride intake along with the continued use of a diuretic resulting in volume depletion. The acute metabolic alkalosis would have suppressed his ammonium excretion, hence aggravating potassium excretion and his hypokalaemia. In turn, the hypokalaemia may have further aggravated his metabolic alkalosis. Teaching points (i) Patients on very low calorie diets are predisposed to develop renal potassium wasting mediated, in part, by the need to excrete a cation with the increased production and excretion of ketoacid anions. (ii) In most individuals potassium wasting is limited by the excretion of ammonium instead of potassium. Conditions that impair ammonium excretion, such as metabolic alkalosis, may result in a higher degree of renal potassium loss. (iii) Pre-existing or concurrent conditions, such as the use of thiazide diuretics, which may cause potassium depletion in themselves, further predispose patients to more severe degrees of hypokalaemia. (iv) Therefore, patients who initiate a very low caloric diet require careful monitoring of their serum potassium, should avoid the use of concomitant
5 646 T. Liu et al. potassium wasting diuretics and should be given appropriate potassium supplementation so that potassium intake is meq/day. Acknowledgements. We wish to acknowledge Dr Arnold Felsenfeld for his careful review of the manuscript and his editorial assistance. Conflict of interest statement. None declared. References 1. Stevens A, Robinson DP, Turpin J, Groshong T, Tobias JD. Sudden cardiac death of an adolescent during dieting. South Med J 2002; 95: Lin SH, Cheema-Dhadli S, Wowrishankar M, Marliss EB, Kamel KS, Halperin ML. Control of excretion of potassium: lessons form studies during prolonged total fasting in human subjects. Potassium excretion in prolonged fasting. Am J Physiol 1997; 273: F796 F Kamel KS, Lin SH, Cheema-Dhadli S, Marliss EB, Halperin ML. Prolonged total fasting: a feast for the integrative physiologist. Kidney Int 1998; 53: Kamel KS, Quaggin S, Seich A, Halperin ML. Disorders of potassium homeostasis: an approach based on pathophysiology. Am J Kidney Dis 1994; 24: Halperin ML, Margolis BL, Robinson LA et al. The urine osmolal gap: clue to estimate urine ammonium in hybrid types of metabolic acidosis. Clin Invest Med 1988; 11: Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62: Lemann J, Jr, Lennon EJ, Goodman D, Litzow JR, Relman AS. The net balance of acid subjects given large loads of acid or alkali. J Clin Invest 1965; 44:
RENAL TUBULAR ACIDOSIS An Overview
 RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
Acid-Base Imbalance-2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD
 AcidBase Imbalance2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD Introduction Disturbance in acidbase balance are common clinical problem that range in severity from mild to life threatening, the acute
AcidBase Imbalance2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD Introduction Disturbance in acidbase balance are common clinical problem that range in severity from mild to life threatening, the acute
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Diabetic Ketoacidosis
 Diabetic Ketoacidosis Definition: Diabetic Ketoacidosis is one of the most serious acute complications of diabetes. It s more common in young patients with type 1 diabetes mellitus. It s usually characterized
Diabetic Ketoacidosis Definition: Diabetic Ketoacidosis is one of the most serious acute complications of diabetes. It s more common in young patients with type 1 diabetes mellitus. It s usually characterized
Acid-Base Physiology. Dr. Tamás Bense Dr. Alexandra Turi
 Acid-Base Physiology Dr. Tamás Bense Dr. Alexandra Turi What is a blood gas assessment? We get it from an arterial sample (a.radialis, a. brachialis, a. femoralis) Invasive technique If the patient is
Acid-Base Physiology Dr. Tamás Bense Dr. Alexandra Turi What is a blood gas assessment? We get it from an arterial sample (a.radialis, a. brachialis, a. femoralis) Invasive technique If the patient is
NATURAL HISTORY AND SURVIVAL OF PATIENTS WITH ASCITES. PATIENTS WHO DO NOT DEVELOP COMPLICATIONS HAVE MARKEDLY BETTER SURVIVAL THAN THOSE WHO DEVELOP
 PROGNOSIS Mortality rates as high as 18-30% are reported for hyponatremic patients. High mortality rates reflect the severity of underlying conditions and are not influenced by treatment of hyponatremia
PROGNOSIS Mortality rates as high as 18-30% are reported for hyponatremic patients. High mortality rates reflect the severity of underlying conditions and are not influenced by treatment of hyponatremia
Acid-Base Tutorial 2/10/2014. Overview. Physiology (2) Physiology (1)
 Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
CASE 27. What is the response of the kidney to metabolic acidosis? What is the response of the kidney to a respiratory alkalosis?
 CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
WATER, SODIUM AND POTASSIUM
 WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
Acid and Base Balance
 Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Dr. Suzana Voiculescu Discipline of Physiology and Fundamental Neurosciences Carol Davila Univ. of Medicine and Pharmacy
 Dr. Suzana Voiculescu Discipline of Physiology and Fundamental Neurosciences Carol Davila Univ. of Medicine and Pharmacy AB balance parameters Extracellular ph (plasmatic ph)= 7.35-7.45 < 7.35= acidosis
Dr. Suzana Voiculescu Discipline of Physiology and Fundamental Neurosciences Carol Davila Univ. of Medicine and Pharmacy AB balance parameters Extracellular ph (plasmatic ph)= 7.35-7.45 < 7.35= acidosis
URINARY NET CHARGE IN HYPERCHLOREMIC METABOLIC ACIDOSIS. Seema Kumar, Meera Vaswani*, R.N. Srivastava and Arvind Bagga
 INDIAN PEDIATRICS VOLUME 35-JANUARY 1998 Original Articles URINARY NET CHARGE IN HYPERCHLOREMIC METABOLIC ACIDOSIS Seema Kumar, Meera Vaswani*, R.N. Srivastava and Arvind Bagga From the Departments of
INDIAN PEDIATRICS VOLUME 35-JANUARY 1998 Original Articles URINARY NET CHARGE IN HYPERCHLOREMIC METABOLIC ACIDOSIS Seema Kumar, Meera Vaswani*, R.N. Srivastava and Arvind Bagga From the Departments of
INTRAVENOUS FLUIDS PRINCIPLES
 INTRAVENOUS FLUIDS PRINCIPLES Postnatal physiological weight loss is approximately 5-10% Postnatal diuresis is delayed in Respiratory Distress Syndrome (RDS) Preterm babies have limited capacity to excrete
INTRAVENOUS FLUIDS PRINCIPLES Postnatal physiological weight loss is approximately 5-10% Postnatal diuresis is delayed in Respiratory Distress Syndrome (RDS) Preterm babies have limited capacity to excrete
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
DIAGNOSIS AND MANAGEMENT OF DIURETIC RESISTANCE. Jules B. Puschett, M.D.
 DIAGNOSIS AND MANAGEMENT OF DIURETIC RESISTANCE Jules B. Puschett, M.D. Diuretic Resistance A clinical circumstance in which patients do not respond to a combination of salt restriction and even large
DIAGNOSIS AND MANAGEMENT OF DIURETIC RESISTANCE Jules B. Puschett, M.D. Diuretic Resistance A clinical circumstance in which patients do not respond to a combination of salt restriction and even large
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Dr. Suzana Voiculescu
 Dr. Suzana Voiculescu AB balance parameters Extracellular ph (plasmatic ph)= 7.35-7.45 < 7.35= acidosis >7.45= alkalosis Kassirer-Bleich equation [H+] = 24 PCO2/ [HCO3-] predicts that the ratio of dissolved
Dr. Suzana Voiculescu AB balance parameters Extracellular ph (plasmatic ph)= 7.35-7.45 < 7.35= acidosis >7.45= alkalosis Kassirer-Bleich equation [H+] = 24 PCO2/ [HCO3-] predicts that the ratio of dissolved
A case of DYSELECTROLYTEMIA. Dr. Prathyusha Dr. Lalitha janakiraman s unit
 A case of DYSELECTROLYTEMIA Dr. Prathyusha Dr. Lalitha janakiraman s unit CASE SUMMARY 4 month old, female infant 1 st born to NC parents, term, b.wt: 3.25kg No neonatal hospitalization Attained head control
A case of DYSELECTROLYTEMIA Dr. Prathyusha Dr. Lalitha janakiraman s unit CASE SUMMARY 4 month old, female infant 1 st born to NC parents, term, b.wt: 3.25kg No neonatal hospitalization Attained head control
Acid Base Disorders: Key Core Concepts. Thomas DuBose M.D., MACP, FASN ASN Board Review Course Online Resource Material 2014
 Acid Base Disorders: Key Core Concepts Thomas DuBose M.D., MACP, FASN ASN Board Review Course Online Resource Material 2014 Speaker Disclosure I, Thomas DuBose, M.D., have no financial relationships or
Acid Base Disorders: Key Core Concepts Thomas DuBose M.D., MACP, FASN ASN Board Review Course Online Resource Material 2014 Speaker Disclosure I, Thomas DuBose, M.D., have no financial relationships or
Mannitol-induced Metabolic Alkalosis
 Electrolyte & Blood Pressure :, 00 ) Mannitolinduced Metabolic Alkalosis Kyung Pyo Kang, M.D., Sik Lee, M.D., Kyung Hoon Lee, M.D., and Sung Kyew Kang, M.D. Department of Internal Medicine, Research Institute
Electrolyte & Blood Pressure :, 00 ) Mannitolinduced Metabolic Alkalosis Kyung Pyo Kang, M.D., Sik Lee, M.D., Kyung Hoon Lee, M.D., and Sung Kyew Kang, M.D. Department of Internal Medicine, Research Institute
The kidney. (Pseudo) Practical questions. The kidneys are all about keeping the body s homeostasis. for questions Ella
 The kidney (Pseudo) Practical questions for questions Ella (striemit@gmail.com) The kidneys are all about keeping the body s homeostasis Ingestion Product of metabolism H 2 O Ca ++ Cl - K + Na + H 2 O
The kidney (Pseudo) Practical questions for questions Ella (striemit@gmail.com) The kidneys are all about keeping the body s homeostasis Ingestion Product of metabolism H 2 O Ca ++ Cl - K + Na + H 2 O
Basic Fluid and Electrolytes
 Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
ACID/BASE. A. What is her acid-base disorder, what is her anion gap, and what is the likely cause?
 These fluid and electrolyte problems are modified from those in a previous textbook for this sequence, Renal Pathophysiology edited by James A. Shayman M.D., Professor of Internal Medicine, University
These fluid and electrolyte problems are modified from those in a previous textbook for this sequence, Renal Pathophysiology edited by James A. Shayman M.D., Professor of Internal Medicine, University
Slide 1. Slide 2. Slide 3. Learning Outcomes. Acid base terminology ARTERIAL BLOOD GAS INTERPRETATION
 Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
A case of hypokalemia MIHO TAGAWA FIRST DEPARTMENT OF MEDICINE NARA MEDICAL UNIVERSITY
 A case of hypokalemia MIHO TAGAWA FIRST DEPARTMENT OF MEDICINE NARA MEDICAL UNIVERSITY Case 57 y.o. male CC: Weakness HPI: About 20 years ago, he developed bilateral lower extremity weakness. Laboratory
A case of hypokalemia MIHO TAGAWA FIRST DEPARTMENT OF MEDICINE NARA MEDICAL UNIVERSITY Case 57 y.o. male CC: Weakness HPI: About 20 years ago, he developed bilateral lower extremity weakness. Laboratory
Electrolytes Solution
 Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
INTRAVENOUS FLUID THERAPY
 INTRAVENOUS FLUID THERAPY PRINCIPLES Postnatal physiological weight loss is approximately 5 10% in first week of life Preterm neonates have more total body water and may lose 10 15% of their weight in
INTRAVENOUS FLUID THERAPY PRINCIPLES Postnatal physiological weight loss is approximately 5 10% in first week of life Preterm neonates have more total body water and may lose 10 15% of their weight in
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis.
 Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
ELECTROLYTES RENAL SHO TEACHING
 ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
HHS Public Access Author manuscript Kidney Int. Author manuscript; available in PMC 2016 January 01.
 The Balance of the Evidence on Acid-Base Homeostasis and Progression of CKD Julia J. Scialla, MD, MHS 1,2 1 Division of Nephrology, Duke University School of Medicine, Durham, NC 2 Duke Clinical Research
The Balance of the Evidence on Acid-Base Homeostasis and Progression of CKD Julia J. Scialla, MD, MHS 1,2 1 Division of Nephrology, Duke University School of Medicine, Durham, NC 2 Duke Clinical Research
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
1.2 Synonyms There are several synonyms e.g. diaminomethanal, but in a medical context, this substance is always referred to as urea.
 Urea (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Urea 1.2 Synonyms There are several synonyms e.g. diaminomethanal, but in a medical context, this substance is always referred
Urea (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Urea 1.2 Synonyms There are several synonyms e.g. diaminomethanal, but in a medical context, this substance is always referred
Salicylate (Aspirin) Ingestion California Poison Control Background 1. The prevalence of aspirin-containing analgesic products makes
 Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Correction of hypervolaemic hypernatraemia by inducing negative Na + and K + balance in excess of negative water balance: a new quantitative approach
 Nephrol Dial Transplant (2008) 23: 2223 2227 doi: 10.1093/ndt/gfm932 Advance Access publication 18 February 2008 Original Article Correction of hypervolaemic hypernatraemia by inducing negative Na + and
Nephrol Dial Transplant (2008) 23: 2223 2227 doi: 10.1093/ndt/gfm932 Advance Access publication 18 February 2008 Original Article Correction of hypervolaemic hypernatraemia by inducing negative Na + and
keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests
 www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
Objectives. Blood Buffers. Definitions. Strong/Weak Acids. Fixed (Non-Volatile) Acids. Module H Malley pages
 Blood Buffers Module H Malley pages 120-126 Objectives Define a buffer system and differentiate between the buffering systems present in the body. Given an arterial blood-gas result, determine the degree
Blood Buffers Module H Malley pages 120-126 Objectives Define a buffer system and differentiate between the buffering systems present in the body. Given an arterial blood-gas result, determine the degree
There are many buffers in the kidney, but the main one is the phosphate buffer.
 9 Yanal Obada Zalat Renal Control of AcidBase Balance The kidneys play three major roles in the maintenance of normal acidbase balance: 1excretion of H+ (fixed _non volatile H+) 2Reabsorption of filtrated
9 Yanal Obada Zalat Renal Control of AcidBase Balance The kidneys play three major roles in the maintenance of normal acidbase balance: 1excretion of H+ (fixed _non volatile H+) 2Reabsorption of filtrated
BIOL 2402 Renal Function
 BIOL 2402 Renal Function Dr. Chris Doumen Collin County Community College 1 Renal Clearance and GFR Refers to the volume of blood plasma from which a component is completely removed in one minute by all
BIOL 2402 Renal Function Dr. Chris Doumen Collin County Community College 1 Renal Clearance and GFR Refers to the volume of blood plasma from which a component is completely removed in one minute by all
Physiology questions review
 Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + )
 Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Wales Critical Care & Trauma Network (North) Management of Hyponatraemia in Intensive Care Guidelines
 Wales Critical Care & Trauma Network (North) Management of Hyponatraemia in Intensive Care Guidelines Author: Richard Pugh June 2015 Guideline for management of hyponatraemia in intensive care Background
Wales Critical Care & Trauma Network (North) Management of Hyponatraemia in Intensive Care Guidelines Author: Richard Pugh June 2015 Guideline for management of hyponatraemia in intensive care Background
PRODUCT INFORMATION UROCIT -K. Wax matrix tablets
 PRODUCT INFORMATION UROCIT -K Wax matrix tablets NAME OF DRUG Potassium Citrate CAS number- 6100-05-6 The empirical formula of potassium citrate is K 3 C 6 H 5 0 7.H 2 O, and its structural formula is:
PRODUCT INFORMATION UROCIT -K Wax matrix tablets NAME OF DRUG Potassium Citrate CAS number- 6100-05-6 The empirical formula of potassium citrate is K 3 C 6 H 5 0 7.H 2 O, and its structural formula is:
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
 COMPARISON OF TREATING METABOLIC ACIDOSIS IN CKD STAGE 4 HYPERTENSIVE KIDNEY DISEASE WITH FRUITS & VEGETABLES OR SODIUM BICARBONATE This was a 1-year, single-center, prospective, randomized, interventional
COMPARISON OF TREATING METABOLIC ACIDOSIS IN CKD STAGE 4 HYPERTENSIVE KIDNEY DISEASE WITH FRUITS & VEGETABLES OR SODIUM BICARBONATE This was a 1-year, single-center, prospective, randomized, interventional
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Studies to identify the basis for an alkaline urine ph in patients with calcium hydrogen phosphate kidney stones
 NDT Advance Access published November 1, 2006 Nephrol Dial Transplant (2006) 1 of 8 doi:10.1093/ndt/gfl588 Original Article Studies to identify the basis for an alkaline urine ph in patients with calcium
NDT Advance Access published November 1, 2006 Nephrol Dial Transplant (2006) 1 of 8 doi:10.1093/ndt/gfl588 Original Article Studies to identify the basis for an alkaline urine ph in patients with calcium
Inter Inter Pretation of Acid Base Disturbance in Critically ill Patients. By :-: Dr. Vinay Bhomia M.D.
 Inter Inter Pretation of Acid Base Disturbance in Critically ill Patients. By :-: Dr. Vinay Bhomia M.D. Normal Blood PH 7.35 to 7.45 Crucial importance to maintain homeostatic function of Body. Any Significant
Inter Inter Pretation of Acid Base Disturbance in Critically ill Patients. By :-: Dr. Vinay Bhomia M.D. Normal Blood PH 7.35 to 7.45 Crucial importance to maintain homeostatic function of Body. Any Significant
Metabolic Alkalosis: Vomiting
 RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
Acid-Base Balance Dr. Gary Mumaugh
 Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
TUBULOPATHY Intensive Care Unit Sina Hospital
 TUBULOPATHY Intensive Care Unit Sina Hospital A 13 years old female who is known case of Scoliosis. She was operated 2 months ago for spinal curve repair. PMH:EMG-MCV In 2 years old =>No Motoneuron Disease
TUBULOPATHY Intensive Care Unit Sina Hospital A 13 years old female who is known case of Scoliosis. She was operated 2 months ago for spinal curve repair. PMH:EMG-MCV In 2 years old =>No Motoneuron Disease
Renal Function and Associated Laboratory Tests
 Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
FLUIDS/ELECTROLYTES. Sahir Kalim, MD MMSc. Department of Medicine, Division of Nephrology, Massachusetts General Hospital Harvard Medical School
 FLUIDS/ELECTROLYTES Sahir Kalim, MD MMSc Department of Medicine, Division of Nephrology, Massachusetts General Hospital Harvard Medical School Dr. Kalim has no potential conflicts of interest to disclose.
FLUIDS/ELECTROLYTES Sahir Kalim, MD MMSc Department of Medicine, Division of Nephrology, Massachusetts General Hospital Harvard Medical School Dr. Kalim has no potential conflicts of interest to disclose.
Renal physiology D.HAMMOUDI.MD
 Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
CHAPTER 42 BLOOD GAS ANALYSIS AND ACID-BASE DISORDERS
 CHAPTER 42 BLOOD GAS ANALYSIS AND ACID-BASE DISORDERS STEVEN G. ACHINGER JUAN CARLOS AYUS Acid-base physiology is among the most complex topics in clinical medicine. Disturbances of this system are common
CHAPTER 42 BLOOD GAS ANALYSIS AND ACID-BASE DISORDERS STEVEN G. ACHINGER JUAN CARLOS AYUS Acid-base physiology is among the most complex topics in clinical medicine. Disturbances of this system are common
Managing Acid Base and Electrolyte Disturbances with RRT
 Managing Acid Base and Electrolyte Disturbances with RRT John R Prowle MA MSc MD MRCP FFICM Consultant in Intensive Care & Renal Medicine RRT for Regulation of Acid-base and Electrolyte Acid base load
Managing Acid Base and Electrolyte Disturbances with RRT John R Prowle MA MSc MD MRCP FFICM Consultant in Intensive Care & Renal Medicine RRT for Regulation of Acid-base and Electrolyte Acid base load
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
Total Body Potassium
 Potassium Kate Driver BMLSc MAACB Immunochemistry Product Manager ANZ PI Diasorin Australia kate.driver@diasorin.com.au AACB QLD Branch Education Representative Australasian Association of Clinical Biochemists
Potassium Kate Driver BMLSc MAACB Immunochemistry Product Manager ANZ PI Diasorin Australia kate.driver@diasorin.com.au AACB QLD Branch Education Representative Australasian Association of Clinical Biochemists
Stages of Chronic Kidney Disease (CKD)
 Early Treatment is the Key Stages of Chronic Kidney Disease (CKD) Stage Description GFR (ml/min/1.73 m 2 ) >90 1 Kidney damage with normal or GFR 2 Mild decrease in GFR 60-89 3 Moderate decrease in GFR
Early Treatment is the Key Stages of Chronic Kidney Disease (CKD) Stage Description GFR (ml/min/1.73 m 2 ) >90 1 Kidney damage with normal or GFR 2 Mild decrease in GFR 60-89 3 Moderate decrease in GFR
Virtual Mentor American Medical Association Journal of Ethics April 2007, Volume 9, Number 4:
 Virtual Mentor American Medical Association Journal of Ethics April 2007, Volume 9, Number 4: 295-299. Clinical pearl Hyperkalemia: newer considerations by Amar D. Bansal and David S. Goldfarb, MD Maintenance
Virtual Mentor American Medical Association Journal of Ethics April 2007, Volume 9, Number 4: 295-299. Clinical pearl Hyperkalemia: newer considerations by Amar D. Bansal and David S. Goldfarb, MD Maintenance
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
Fluids and electrolytes
 Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
Definition : Stages : ( RIFLE vs. AKIN ) Causes and classification : Pre-renal Renal Post- renal Clinical manifestations and Complication Management
 AKI Definition : Stages : ( RIFLE vs. AKIN ) Causes and classification : Pre-renal Renal Post- renal Clinical manifestations and Complication Management and indications for RRT Etiology prerenal causes
AKI Definition : Stages : ( RIFLE vs. AKIN ) Causes and classification : Pre-renal Renal Post- renal Clinical manifestations and Complication Management and indications for RRT Etiology prerenal causes
Composition: Each Tablet contains. Pharmacokinetic properties:
 Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Introduction to Clinical Diagnosis Nephrology
 Introduction to Clinical Diagnosis Nephrology I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Professor of Medicine and Physiology and Functional Genomics University of Florida College
Introduction to Clinical Diagnosis Nephrology I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Professor of Medicine and Physiology and Functional Genomics University of Florida College
NATIONAL KIDNEY MONTH
 NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
Distal and proximal RTA. Detlef Bockenhauer
 Distal and proximal RTA Detlef Bockenhauer acidosis: Is it real? H + + HCO 3 - > H2 O + CO 2 What do you need to assess an acid-base disorder Blood: blood gas, electrolytes including bicarbonate (measured)
Distal and proximal RTA Detlef Bockenhauer acidosis: Is it real? H + + HCO 3 - > H2 O + CO 2 What do you need to assess an acid-base disorder Blood: blood gas, electrolytes including bicarbonate (measured)
Outpatient Management of Chronic Kidney Disease for the Internist
 Outpatient Management of Chronic Kidney Disease for the Internist Annual Meeting of Maryland Chapter of the American College of Physicians February 3, 2018 MARY (TESSIE) BEHRENS, MD, FACP, FASN, FNKF MID-ATLANTIC
Outpatient Management of Chronic Kidney Disease for the Internist Annual Meeting of Maryland Chapter of the American College of Physicians February 3, 2018 MARY (TESSIE) BEHRENS, MD, FACP, FASN, FNKF MID-ATLANTIC
CDE Exam Preparation Presented by Wendy Graham RD CDE May 4, 2017
 CDE Exam Preparation Presented by Wendy Graham RD CDE May 4, 2017 DKA at organ level 3 Diabetic Ketoacidosis Characteristics Ketones positive Anion Gap > 12 (High) Blood Sugar > 14 (High) Bicarbonate
CDE Exam Preparation Presented by Wendy Graham RD CDE May 4, 2017 DKA at organ level 3 Diabetic Ketoacidosis Characteristics Ketones positive Anion Gap > 12 (High) Blood Sugar > 14 (High) Bicarbonate
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Acute Kidney Injury. APSN JSN CME for Nephrology Trainees May Professor Robert Walker
 Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
QUICK REFERENCE FOR HEALTHCARE PROVIDERS
 KEY MESSAGES 1 SCREENING CRITERIA Screen: Patients with DM and/or hypertension at least yearly. Consider screening patients with: Age >65 years old Family history of stage 5 CKD or hereditary kidney disease
KEY MESSAGES 1 SCREENING CRITERIA Screen: Patients with DM and/or hypertension at least yearly. Consider screening patients with: Age >65 years old Family history of stage 5 CKD or hereditary kidney disease
Water, Electrolytes, and Acid-Base Balance
 Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Lecture-2 Review of the previous lecture:
 Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
Guidelines for approach to a child with
 Guidelines for approach to a child with Metabolic acidosis (including RTA) Children s Kidney Centre University Hospital of Wales Cardiff CF14 4XW DISCLAIMER: These guidelines were produced in good faith
Guidelines for approach to a child with Metabolic acidosis (including RTA) Children s Kidney Centre University Hospital of Wales Cardiff CF14 4XW DISCLAIMER: These guidelines were produced in good faith
The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency *
 lournal of Clinical Investigation Vol. 45, No. 4, 1966 The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency * HOWARD L. BLEICH,t RICHARD L. TANNEN,t AND WILLIAM B. SCHWARTZ t (From
lournal of Clinical Investigation Vol. 45, No. 4, 1966 The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency * HOWARD L. BLEICH,t RICHARD L. TANNEN,t AND WILLIAM B. SCHWARTZ t (From
WEEK. MPharm Programme. Acute Kidney Injury. Alan M. Green MPHM13: Acute Kidney Injury. Slide 1 of 47
 MPharm Programme Acute Kidney Injury Alan M. Green 2017 Slide 1 of 47 Overview Renal Function What is it? Why does it matter? What causes it? Who is at risk? What can we (Pharmacists) do? How do you recognise
MPharm Programme Acute Kidney Injury Alan M. Green 2017 Slide 1 of 47 Overview Renal Function What is it? Why does it matter? What causes it? Who is at risk? What can we (Pharmacists) do? How do you recognise
Filtration and Reabsorption Amount Filter/d
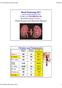 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Acid-Base Balance Workshop. Dr. Najla Al Kuwaiti Dr. Abdullah Al Ameri Dr. Amar Al Shibli
 Acid-Base Balance Workshop Dr. Najla Al Kuwaiti Dr. Abdullah Al Ameri Dr. Amar Al Shibli Objectives Normal Acid-Base Physiology Simple Acid-Base Disorders Compensations and Disorders The Anion Gap Mixed
Acid-Base Balance Workshop Dr. Najla Al Kuwaiti Dr. Abdullah Al Ameri Dr. Amar Al Shibli Objectives Normal Acid-Base Physiology Simple Acid-Base Disorders Compensations and Disorders The Anion Gap Mixed
New aspects of acid-base disorders
 New aspects of acid-base disorders I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Division of Nephrology, Hypertension and Transplantation University of Florida College of Medicine
New aspects of acid-base disorders I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Division of Nephrology, Hypertension and Transplantation University of Florida College of Medicine
Case TWO. Vital Signs: Temperature 36.6degC BP 137/89 HR 110 SpO2 97% on Room Air
 Mr N is a 64year old Chinese gentleman who is a heavy drinker, still actively drinking, and chronic smoker of >40pack year history. He has a past medical history significant for Hypertension, Hyperlipidemia,
Mr N is a 64year old Chinese gentleman who is a heavy drinker, still actively drinking, and chronic smoker of >40pack year history. He has a past medical history significant for Hypertension, Hyperlipidemia,
ADMIT DIABETIC KETOACIDOSIS (DKA) PLAN - Phase: Begin Immediately/Emergency Center
 - Phase: Begin Immediately/Emergency Center Weight PHYSICIAN S Allergies Admit/Discharge/Transfer Patient Status Requested Location: MICU, Pt Status: Inpatient (LOS > 2 midnights) Requested Location: 5E
- Phase: Begin Immediately/Emergency Center Weight PHYSICIAN S Allergies Admit/Discharge/Transfer Patient Status Requested Location: MICU, Pt Status: Inpatient (LOS > 2 midnights) Requested Location: 5E
Faculty version with model answers
 Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
3/19/2009. The task of the kidney in acid-base balance Excretion of the daily acid load. Buffering of an acid load. A o B - + H + B - A o +OH - C +
 The task of the kidney in acid-base balance Excretion of the daily acid load Buffering of an acid load Oxidation of amino acids, fats and carbohydrates often lead to acid production. On an average American
The task of the kidney in acid-base balance Excretion of the daily acid load Buffering of an acid load Oxidation of amino acids, fats and carbohydrates often lead to acid production. On an average American
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
Arterial blood gas analysis
 perioperativecpd.com continuing professional development Arterial blood gas analysis Article based on original by the Resuscitation Council U.K. Introduction Interpreting the analysis of an arterial blood
perioperativecpd.com continuing professional development Arterial blood gas analysis Article based on original by the Resuscitation Council U.K. Introduction Interpreting the analysis of an arterial blood
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES
 Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Arterial Blood Gas Interpretation: The Basics
 http://www.medicine-on-line.com ABG Basics: Page 1/10 Arterial Blood Gas Interpretation: The Basics Author: David C Chung MD, FRCPC Affiliation: The Chinese University of Hong Kong Sampling of arterial
http://www.medicine-on-line.com ABG Basics: Page 1/10 Arterial Blood Gas Interpretation: The Basics Author: David C Chung MD, FRCPC Affiliation: The Chinese University of Hong Kong Sampling of arterial
The relationship between H+,PaCO₂ and HCO₃ are expressed in the equation of:
 [Acid-Base Balance] [Dr. Bashir Khasawneh] [5 th February 2012] Acid-Base Basic Concepts: The relationship between H+,PaCO₂ and HCO₃ are expressed in the equation of: Which is modified from Henderson-Hasselbach
[Acid-Base Balance] [Dr. Bashir Khasawneh] [5 th February 2012] Acid-Base Basic Concepts: The relationship between H+,PaCO₂ and HCO₃ are expressed in the equation of: Which is modified from Henderson-Hasselbach
Nephrology / Urology. Hyperkalemia Causes and Definition Lecturio Online Medical Library. Definition. Epidemiology of Hyperkalemia.
 Nephrology / Urology Hyperkalemia Causes and Definition Lecturio Online Medical Library See online here Hyperkalemia is defined by the serum potassium level when it is higher than 5.5mEq/L. It is usually
Nephrology / Urology Hyperkalemia Causes and Definition Lecturio Online Medical Library See online here Hyperkalemia is defined by the serum potassium level when it is higher than 5.5mEq/L. It is usually
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
UNIT 9 INVESTIGATION OF ACID-BASE DISTURBANCES
 UNIT 9 INVESTIGATION OF ACIDBASE DISTURBANCES LEARNING OBJECTIVES At the end of this chapter, students must be able to: 1. Describe the main parametres that define the acidbase equilibrium 2. Identify
UNIT 9 INVESTIGATION OF ACIDBASE DISTURBANCES LEARNING OBJECTIVES At the end of this chapter, students must be able to: 1. Describe the main parametres that define the acidbase equilibrium 2. Identify
Module : Clinical correlates of disorders of metabolism Block 3, Week 2
 Module : Clinical correlates of disorders of metabolism Block 3, Week 2 Department of Paediatrics and Child Health University of Pretoria Tutor : Prof DF Wittenberg : dwittenb@medic.up.ac.za Aim of this
Module : Clinical correlates of disorders of metabolism Block 3, Week 2 Department of Paediatrics and Child Health University of Pretoria Tutor : Prof DF Wittenberg : dwittenb@medic.up.ac.za Aim of this
SUMMARY OF PRODUCT CHARACTERISTICS. Potassium Chloride 0.15% w/v & Sodium Chloride 0.9% w/v Solution for Infusion
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Potassium Chloride 0.15% w/v & Sodium Chloride 0.9% w/v Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Potassium Chloride
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Potassium Chloride 0.15% w/v & Sodium Chloride 0.9% w/v Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Potassium Chloride
Electrolytes by case examples. Graham Bilbrough, European Medical Affairs Manager
 Electrolytes by case examples Graham Bilbrough, European Medical Affairs Manager 1 Acid-bases disturbances Generally result from one of the following: 1. damage to an organ such as the kidneys or lungs
Electrolytes by case examples Graham Bilbrough, European Medical Affairs Manager 1 Acid-bases disturbances Generally result from one of the following: 1. damage to an organ such as the kidneys or lungs
