1.Wilmer MJ, et al. Am J Physiol Renal Physiol 2010;299:F905-F916; 2. Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59.
|
|
|
- Derek Allen
- 6 years ago
- Views:
Transcription
1
2
3
4 The first report of a case of cystinosis appears to have been made in 1903 by a Swiss biochemist, Emil Abderhalden, 1 but the disorder was first described as a nosological entity in the 1930s by the distinguished European paediatrician Guido Fanconi, who categorized it as a cystine storage disease. 2 More precisely it is a lysosomal storage disease and was the first such disease to be recognized to be due to defective lysosomal membrane transport. The disease results in intracellular accumulation of cystine in the lysosomes of cells throughout the body, causing progressive dysfunction of multiple organs. 2 There are three forms of cystinosis: all are allelic recessive disorders caused by mutations in the CTNS gene encoding the lysosomal cystine transporter protein cystinosin. 2 1.Wilmer MJ, et al. Am J Physiol Renal Physiol 2010;299:F905-F916; 2. Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59.
5 Depending on the age at presentation and the degree of disease severity, three clinical forms of cystinosis are distinguished: (i) the nephropathic infantile form (MIM #219800), which is the most frequent and most severe form of the disease, (ii) the nephropathic juvenile form (MIM #219900), which is also called intermediate cystinosis, late-onset form, or adolescent form, and (iii) a non-nephropathic adult form (MIM #219750), which is also called benign non-nephropathic cystinosis or ocular non-nephropathic cystinosis. All three forms of the disease are caused by mutations of the CTNS gene and have some phenotypic overlap. Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
6
7 In North America, the incidence of infantile nephropathic cystinosis is 1 case per 100, ,000 live births. 1 The incidence of cystinosis is higher in certain subpopulations. The estimated incidence in the French province of Brittany is 1 case per 25,909 population, while the incidence in the rest of France is 1 case per 326,440 population. 2 Males are affected slightly more frequently than females, with a male-to-female ratio among cystinotic children having been reported to be 1.4:1. 3 Although cystinosis is often considered a disease of fair-skinned individuals of European descent, it is known to occur in other races Nesterova G & Gahl WA. Pediatr Nephrol 2013:28:51-59; 2. Kalazis V et al. J Am Soc Nephrol 2001;12: ; 3. Gahl WA, et al. Ann Intern Med 2007;147: ; 4.
8 Classic infantile nephropathic cystinosis accounts for perhaps 95% of cases of cystinosis. The forms with a later onset are much less frequent, accounting for approximately 5% of all cases of cystinosis. These forms have been divided into two basic phenotypes: nephropathic and non-nephropathic. The non-infantile nephropathic forms are associated with glomerular impairment but not necessarily Fanconi s syndrome. The disease in these patients may progress to cause chronic renal failure. In contrast, renal disease does not develop in the non-nephropathic (or ocular) form of cystinosis, in which crystal deposition is limited to the cornea and conjunctiva Servais A, et al. Clin J Am Soc Nephrol 2008;3(1):27-35.
9
10 Cystinosis is an inherited (autosomal recessive) metabolic disorder due to defective transport of the amino acid cystine out of lysosomes. It is therefore classified as a lysosomal storage disorder. The stored cystine is poorly soluble and crystallizes within the lysosomes of many cell types, leading to widespread tissue and organ damage. Patients store times the normal concentration of cystine in their cells. The impaired efflux of cystine out of lysosomes is the result of defective cystinosin, the cystine transporter protein on the lysosomal membrane, which is encoded for by the CTNS gene on the short arm of chromosome Harrison F, et al. Mol Ther 2013; ; 2. Nesterova G & Gahl WA. Pediatr Nephrol 2013:28:51-59; 3. Nesterova G et al. Pediatr Nephrol 2008;23: ; 4. Sonies BC et al. Medicine (Baltimore) 2005;84:
11 The name lysosome derives from the Greek words lysis, to separate, and soma, body. Lysosomes are intracellular organelles that contain digestive enzymes that act optimally at a ph of about 4.5. Lysosomes digest food particles, breaking down the complex molecules of lipids, proteins and carbohydrates into smaller molecules. Lysosomes also digest other intracellular organelles and can engulf viruses and bacteria before destroying them. Lysosomes are found in the cytosol. The lysosomal membrane protects the cytosol, and therefore the rest of the cell from the digestive enzymes within the lysosome. 11
12 Ingested protein enters the lysosome, where acid hydrolases degrade it into its component amino acids, including cysteine. Within the lysosome, cysteine is readily oxidised to cystine (a disulphide of the amino acid cysteine). In healthy individuals, cystine exits the lysosome through a transporter protein and enters the cytoplasm, where it is rapidly converted to cysteine by the reducing agent glutathione. Cytoplasmic cysteine is incorporated into newly synthesised proteins or degraded to inorganic sulphate for excretion. Wilmer M et al. Am J Physiol Renal Physiol 2010; 299: ; Patrick AD. Biochem J 1962;83:
13 In normal cells, the proteins that enter a lysosome are broken down into their constituent amino acids, which then exit the lysosome to enter the cytoplasm where they can be recycled for new protein synthesis. There are specific cell membrane transporters for various amino acids, including lysine and cystine. The transporter for cystine is called cystinosin. This transporter has seven transmembrane domains and is encoded by the CTNS gene. Levtchenko EN et al. Pediatr Nephrol 2006;21:
14 In cystinosis, the cystinosin transporter is defective, because of mutations in the gene encoding it. The cystine formed within the lysosomes is therefore unable to exit the organelle and, given its poor solubility, accumulates within the lysosomes in the form of birefringent, polyhedral crystals. 1 Patients with cystinosis can store 50 to 100 times the normal concentration of cystine in their cells Nesterova G & Gahl WA. Pediatr Nephrol 2013:28:51-59; 2. Sonies BC et al. Medicine (Baltimore) 2005;84:
15 CTNS is localized to the short arm of chromosome 17 (17p13) and is composed of 12 exons, the first two of which are non-coding. The remaining 10 exons encode cystinosin, with its seven transmembrane domains. The most common mutation associated with cystinosis is a 57.2 kb deletion that removes a region of the gene upstream of, and including, exon 10. This mutation is found in 76% of cystinotic patients of European origin and has been shown to be due to a founder effect that probably arose in Germany in about 500 AD. CTNS mutations can cause either an absence of cystinosin or a disruption of transmembrane domains and loss of the protein s function. More than 90 different CTNS mutations (missense, nonsense, splice-site, deletion, and promoter mutations) have been described. Goodyer P. Int J Nephrol. 2011;2011:929456;
16 While some CTNS mutations cause an absence of cystinosin or loss of its function, mutations of CTNS that affect functionally unimportant regions of cystinosin account for a milder clinical course. The various CTNS mutations can explain why patients have a wide spectrum of clinical symptomatology. Individuals with infantile cystinosis carry severe mutations on both alleles. Lateronset (intermediate) cystinosis appears to be due to the inheritance of a mutation known to cause infantile disease in one allele and a relatively less clinically severe mutation in the other or due to the inheritance of a relatively less severe mutation in both alleles. Servais A, et al. Clin J Am Soc Nephrol 2008;3: Wilmer M et al. Am J Physiol Renal Physiol 2010;299:
17 In cystinosis, the defective transport of cystine out of lysosomes leads to accumulation of this amino acid within lysosomes. The stored cystine is poorly soluble and crystallizes within the lysosomes of many cell types (but not all), leading to apoptotic cell death and widespread tissue damage and progressive organ dysfunction. Pastore A et al. Clin Chem 2000; 46: ; Nesterova G & Gahl WA. Pediatr Nephrol 2013:28:
18
19 Untreated infantile cystinosis presents with poor growth and proximal tubular Fanconi s syndrome at 6-12 months of age, glomerular failure by the age of 10 years, and various non-renal complications, including hypothyroidism and ocular disorders. Renal tubular damage presents at the time of diagnosis and is largely irreversible. The profound polyuria, polydipsia, dehydration and metabolic acidosis commonly result in life-threatening hypovolaemia. Failure of the proximal tubules to reabsorb phosphate and calcium leads to rickets in children; hypocalcaemia sometimes results in tetany. Other symptoms such as anorexia, vomiting, and feeding difficulties, combined with renal losses of nutrients, cause poor nutritional status and lead to failure to thrive. Cystine accumulates in virtually all organs and tissues and several complications accompany the tissue damage, including photophobia, hypothyroidism, heat prostration due to sweating impairment, and delayed puberty. Nesterova G & Gahl WA. Pediatr Nephrol 2013:28:
20 Late-onset (intermediate) nephropathic cystinosis is a more indolent form of the disease. The age at manifestation is later; most commonly in early adolescence. Symptoms are usually restricted to the kidneys (with a less severe form of Fanconi s syndrome and proteinuria) and eyes (e.g., photophobia). Although complete Fanconi s syndrome often does not develop in late-onset cystinosis, renal function deteriorates as in infantile nephropathic cystinosis, and patients often experience end-stage renal failure within a few years of diagnosis. Schneider JA, et al. Ped Nephrol 1990;4: ; Servais A, et al. Clin J Am Soc Nephrol 2008;3:
21 Non-nephropathic cystinosis is considered a benign variant and is usually diagnosed by an ophthalmologist treating patients for photophobia. Photophobia may not begin until middle age and is not usually as debilitating as in the nephropathic form of the disease. Slit-lamp examination reveals corneal crystal deposits. In addition to the eye, cystine crystals are present in the bone marrow and leucocytes but are absent from the kidney and the retina. Schneider JA, et al. Ped Nephrol 1990;4: ; Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
22 Without therapy, cystine accumulates in virtually all organs and tissues, causing a myriad of complications. Fanconi s syndrome is the main manifestation of damage to the kidney. Photophobia develops due to corneal cystine accumulation. Hypothyroidism is a common and early complication and contributes to growth failure. Insulin-dependent diabetes mellitus and pubertal delay are other endocrine disorders that may occur and male hypogonadism is particularly common. The musculoskeletal system may be involved with widespread muscle weakness and atrophy leading to motor problems and difficulty in swallowing and breathing. Rickets is part of Fanconi s syndrome. Heat prostration can occur due to sweating impairment. The liver, spleen and central nervous system can all be affected, with reports of encephalopathy and benign intracranial hypertension. Nesterova G et al. Pediatr Nephrol 2008;23: ; Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59.
23 Cystinosis is the most common inherited cause of Fanconi s syndrome. In the infantile nephropathic form of cystinosis, the kidney is affected early in life by cystine crystals deposited in proximal tubule cells, which are particularly susceptible to the adverse effects of cystine acccumulation. Fanconi s syndrome is characterized by the loss of substances not reabsorbed in the proximal tubule, including sodium, potassium, phosphate, calcium, magnesium, bicarbonate, and others. Metabolic acidosis and electrolyte disturbances ensue and contribute to the stunting of growth in children with cystinosis. 1 The deterioration of renal function is accompanied by progressive tubulo-interstitial and glomerular lesions, consisting of interstitial fibrosis, tubular atrophy, segmental or global collapsing of the glomerular tuft and an accumulation of mesangial matrix material Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59; 2. Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
24 Cystine crystals in the cornea are pathognomonic for cystinosis. Corneal cystine crystals are absent at birth but can generally be observed by an experienced ophthalmologist with slit lamp examination at the age of 1 year. These crystals cause reflections of light and result in photophobia with substantial discomfort. Untreated teenagers may develop painful corneal erosions, punctate, filamentous or band keratopathy, iris crystals, and peripheral corneal neovascularisation. The degeneration of the retinal pigment epithelium, resulting in patchy depigmentation of the retina (beginning in the periphery and extending over time), may cause visual impairment mostly starting from the second decade of life. However, retinal epithelium vacuolisation has been described to be present already in an 18-week-old foetus and fundoscopic retinal changes were observed in two siblings with cystinosis as early as 5 and 10 weeks of age. Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
25 The continuing multi-organ accumulation of cystine crystals leads to impairment of endocrine organs. Hypothyroidism is found in up to 70% of untreated cystinosis patients older than 10 years. Impaired insulin production can be exacerbated by steroid therapy after renal transplantation and results in insulin-dependent diabetes mellitus. Puberty generally proceeds normally in females, and several have given birth. Males may have primary hypogonadism and do not always complete pubertal development. So far, no male cystinotic patients are known to have induced a pregnancy, even while under adequate treatment. Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
26 Vacuolar myopathy, resulting from excessive cystine accumulation in the muscle, manifests generally after the 10th birthday. Patients suffer from progressive muscle wasting and reduced strength with a restrictive pattern of respiratory defects. There is also a high prevalence of difficulty in swallowing, with the severity of the swallowing dysfunction being directly related to the severity of the muscular disease. 1 Bone disease in cystinosis is multifactorial in nature and is attributed to losses of calcium, phosphate, and vitamin D, cystine accumulation in the bone, and uraemic osteodystrophy. Children can develop rickets, while the manifestation in adults is osteomalacia. 1 1.Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
27 As regards neurological effects, two major types of cystinotic encephalopathy, have been described: (i) encephalopathy presenting with cerebellar and pyramidal signs, mental deterioration and pseudo-bulbar palsy, and (ii) encephalopathy associated with stroke-like episodes. The most common imaging finding in these patients is cerebral cortical atrophy; however, non-absorptive hydrocephalus, demyelinisation and cerebral mineralisation have also been reported, as has benign intracranial hypertension presenting with headaches and papilloedema. Although general intelligence is normal in cystinosis patients, alterations of specific neurocognitive functions such as visual-motor integration and visual memory have been observed. Other reported symptoms include decreased skin and hair pigmentation, impaired sweating, hepatomegaly, portal hypertension, splenomegaly and hypersplenism. Nesterova G et al. Pediatr Nephrol 2008;23: ; Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
28 In the early 1960s, nephropathic cystinosis was considered a fatal renal disease of childhood; patients died of progressive renal failure before the age of 10 years. The natural history of the disease has changed dramatically since the introduction of cysteamine and renal transplantation. Patients with infantile cystinosis now survive into even the fifth decade of life. Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59.
29
30 Cystinosis should be suspected in all patients with failure to thrive and signs of renal Fanconi s syndrome, as it is the most common cause of inherited Fanconi s syndrome in children. The detection of elevated intracellular cystine content is the cornerstone for the diagnosis. The finding of typical corneal crystals is pathognomonic for cystinosis, but corneal cystine crystals may be absent before one year of age. Molecular analysis of the CTNS gene allows early diagnosis and can be used for prenatal diagnosis of the disease. Prenatal diagnosis of cystinosis can also be made by measuring 35 S-labelled cystine accumulation in cultured amniocytes or chorionic villi samples; and by a direct measurement of cystine in uncultured chorionic villi. Gahl WA et al. N Engl J Med 2002;347: ; Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
31 The threshold for bicarbonate reabsorption is greatly reduced in cystinosis and serum bicarbonate concentrations falls, creating hypochloraemic metabolic acidosis. The loss of sodium, not reabsorbed in the proximal tubule, causes hyponatraemia. The excess bicarbonate that reaches the distal tubule enhances potassium excretion, resulting in low serum potassium levels (hypokalaemia). Failure of the proximal tubules to reabsorb phosphate and calcium leads to hypophosphataemia and hypocalcaemia. As renal impairment progresses, the serum creatinine concentration increases. Children with Fanconi s syndrome may have plasma and muscle carnitine deficiency due to a failure to reabsorb carnitine. Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59.
32 Hyperaminoaciduria is a hallmark of Fanconi s syndrome; in normal children over 98% of the filtered load of amino acids is reabsorbed in the proximal tubules, whereas in patients with Fanconi s syndrome, the loss of amino acids is 6-16 fold normal. Another hallmark of Fanconi s syndrome is glycosuria with normal serum glucose concentrations, indicating that the renal threshold for glucose is abnormally low. Many different low-molecular weight proteins are excreted by cystinosis patients, with major losses of alpha1-microglobulin, retinol-binding protein and beta2- microglobulin (tubular proteinuria). In addition, enzymes, immunoglobulins, and hormones are frequently found in the urine of patients with cystinosis. Larger molecular weight proteins are found in the urine during the later stages of cystinosis as glomerular dysfunction becomes more evident (glomerular proteinuria). Hyperphosphaturia, hypercalciuria and high levels of bicarbonate in the urine are present. Nesterova G & Gahl WA. Pediatr Nephrol 2013;28:51-59.
33 The diagnosis of cystinosis is confirmed by the amount of cystine in white blood cells (WBC). The amount of cystine is measured relative to the amount of protein in the WBC, and results are expressed as nmol of hemicystine (or half-cystine) per mg of protein (half-cystine is the convention used, reflecting cystine as a disulphide of cysteine). In healthy humans, mixed leukocytes contain less than 0.2 nmol of half-cystine per mg of protein, whereas in patients with nephropathic cystinosis the values exceed 2 nmol half-cystine/mg of protein. Samples should be collected in tubes containing either heparin or acid-citrate dextrose (ACD), should be dispatched by first class-post to arrive in the testing laboratory within hours of sampling and should not be frozen during shipment. 33
34
35
36 Patients with cystinosis have a poor appetite, crave salty and hot and spicy foods, and prefer specific food textures. Each patient has specific food preferences that may already be evident by the age of 2 years. Dietary recommendations should follow daily Dietary Reference Intake requirements (i.e., 60% carbohydrate, 10% protein, 30% lipids), and calorie intake should aim to achieve weight gain. If the patient is a poor eater and oral feeding is unsuccessful, high-calorie oral supplements should be administered. If the patient does not take supplements or has an inappropriately low intake because of poor appetite or vomiting, gastric tube placement can help. Total parenteral nutrition is indicated if a cystinotic patient cannot tolerate any form of enteral feeding. Some patients may need additional therapy with agents improving gastrointestinal kinetics or antagonists of acid production.
37 Urinary losses must be carefully monitored and replaced. The cystinotic individual must be well-hydrated and administered supplements of potassium and bicarbonate, as needed. Rickets should be prevented or treated with vitamin D and phosphate supplementation. Patients with cystinosis should have free access to water or other fluids because they easily become dehydrated. Indomethacin may limit water losses in patients with nephropathic cystinosis by both reducing the glomerular filtration rate (GFR) and by sensitizing the collecting duct to the effects of antidiuretic hormone. In uncontrolled clinical reports, indomethacin has been shown to relieve polyuria and polydipsia and to improve appetite, energy, and general well-being by reducing urinary losses of water and other various substances. Indomethacin therapy may, however, worsen kidney function and its ulcerogenic potential is a major drawback in the treatment of cystinosis.
38 Treatment with recombinant human growth hormone improves growth velocity. Long-term recombinant human growth hormone treatment in young children with nephropathic cystinosis prior to renal replacement therapy is safe and efficient. Growth hormone treatment is less effective for peripubertal or adolescent patients on renal replacement therapy. Patients with diabetes mellitus require insulin therapy. Thyroid replacement therapy with thyroxine is indicated in patients diagnosed with hypothyroidism. Testosterone may be administered to induce secondary sexual characteristics in males with primary hypogonadism. Severe cystine crystal accumulation can damage the cornea, resulting in serious vision difficulties. Oral cysteamine is not distributed into the eye and thus has no impact on corneal crystal accummulation. An ophthalmic solution is available in the USA. Corneal crystals reaccumulate if cysteamine ophthalmic solution is discontinued.
39 In the past, the treatment of cystinosis was limited to treating metabolic acidosis and, often, replacing electrolytes lost in the urine; later during the course of the disease, the chronic kidney disease was treated. Today, the wide availability of an effective orally administered cystine-depleting drug, cysteamine, and kidney replacement therapy with transplantation has dramatically improved the outlook for patients and altered management strategies. Cystine-depleting treatment should be continued even after dialysis or kidney transplantation as it may delay complications in extra-renal tissues. Oral cysteamine is not distributed into the eye and thus has no impact on corneal crystal accumulation, which can, however, be treated with a cysteamine ophthalmic solution.
40 Children with cystinosis should be examined frequently (at least four times a year) to monitor growth, nutritional state, renal function, and white blood cell cystine content. Ocular symptoms should be controlled at least yearly. Starting from the end of the first decade of life, special attention should be paid to the possible appearance of extra-renal complications (thyroid function, glucose tolerance, gonadal function). The measurement of WBC cystine levels is an essential part of the follow-up of patients, since it indicates the effectiveness of treatment. Values should be maintained below 1 nmol half-cystine/mg of protein. Samples for WBC cystine analysis should always be collected just before administration of cysteamine treatment (i.e. 6 hours after the previous dose) and never immediately after administration. Wilmer JM & Schoeber JP. Pediatr Nephrol 2011;26:
41 41
42
43 Cysteamine is a drug which has been known for years. The first therapeutic use of the drug dates back to The cysteamine salt then used was cysteamine hydrochloride, in solution, which had several major disadvantages: a very unpleasant odour and taste, and a very marked hygroscopic character necessitating preparation immediately before use. Cysteamine has also been used in the form of the phosphothioester of cysteamine (or phosphocysteamine). When pure this salt is tasteless and odourless. However, when it is manufactured on a sufficient scale for therapeutic use, it contains about 0.5% of cysteamine hydrochloride, rendering it as difficult to use as cysteamine hydrochloride. Moreover the salt is particularly difficult to manufacture in a reproducible manner. The foregoing explains why neither of those two salts have ever been developed as a medicinal product. Cysteamine bitartrate, the active substance of Cystagon, is a salt that has the basic advantage of being relatively stable. Moreover it can be reproducibly manufactured in quantities compatible with therapeutic use.
44 Cysteamine was first used in the treatment of cystinosis in 1976 and was approved by the US Food and Drug Administration in Cystagon obtained a European marketing authorisation for use in all European countries through the centralised procedure in June The Marketing Authorisation Holder is Orphan Europe (France). Cherqui S. Kidney Int. 2012;81(2): ; 44
45 Cystagon is supplied as hard, white capsules of two different strengths of cysteamine: 50 mg and 150 mg. Individual bottles of the product contain 100 capsules of one or other of the strengths. Given the hygroscopic nature of the product, storage conditions designed to protect the hard capsules from humidity are required. It is therefore recommended to keep the product container tightly closed in order to protect from light and moisture and to store it at a temperature below 25 C. Under these storage conditions, Cystagon has an approved shelf-life of 2 years. Cystagon Summary of Product Characteristics.
46 The active principle of Cystagon is cysteamine bitartrate (INN mercaptamine bitartrate). The excipients are microcrystalline cellulose (filler), pregelatinised starch (binder), magnesium stearate/sodium lauryl sulfate (lubricants), colloidal silicon dioxide (glidant) and croscarmellose sodium (a disintegrant).
47 The manufacture of Cystagon is conducted in two stages: (i) manufacture of the intermediate mix by dry granulation and (ii) capsule filling with the intermediate mix. In the first stage cysteamine bitartrate is mixed, screened, milled and compacted with the various excipients required in order to obtain a uniform bulk intermediate product. This intermediate product is of identical composition for both Cystagon 50 mg and Cystagon 150 mg batches. The in-process controls consist in control of mass, mixing duration and screen mesh size at each stage of preparation. In the second stage, the intermediate mix is filled into size 3 hard capsules for the 50 mg strength and size 0 hard capsules for the 150 mg strength. The hard capsules are made of white gelatin. Filling weight is controlled. The full manufacture of Cystagon is made in strict compliance with the requirements of the European Good Manufacturing Practices (GMP).
48 CYSTAGON February 2008 For children up to age 12 years, Cystagon dosing should be on the basis of body surface area (g/m 2 /day). The recommended dose is 1.30 g/m 2 /day of the free base divided four times daily. For patients over age 12 and over 50 kg weight, the recommended Cystagon dose is 2 g/day, divided four times daily. Starting doses should be 1/4 to 1/6 of the expected maintenance dose, and increased gradually over 4-6 weeks to avoid intolerance. The dose should be raised if there is adequate tolerance and the leucocyte cystine level remains >1 nmol hemicystine/mg protein. The use of doses higher than 1.95 g/m²/day is not recommended. Cystagon Summary of Product Characteristics. 48
49 CYSTAGON February 2008 Digestive tolerance of cysteamine is improved when the medicinal product is taken just after or with food. In children who are at risk of aspiration, aged approximately 6 years and under, the hard capsules should be opened and the content sprinkled on food. Experience suggests that foods such as milk, potatoes and other starch-based products seem to be appropriate for mixing with the powder. However, acidic drinks, such as orange juice, should generally be avoided as the powder tends not to mix not well and may precipitate out. The levels of cystine in white blood cells should be measured 5 to 6 hours after dosing and should be checked frequently when initiating therapy (e.g. monthly) and every 3-4 months when on a stable dose. Cystagon Summary of Product Characteristics. 49
50 The use of Cystagon is contraindicated in patients who are hypersensitive to the active substance, to any of the excipients, or to penicillamine. Cystagon is also contraindicated during breast-feeding and pregnancy, particularly during the first trimester. A few cases of Ehlers-Danlos like syndrome on elbows have been reported in children treated with high doses of different cysteamine preparations (cysteamine chlorhydrate or cystamine or cysteamine bitartrate) mostly above the maximal dose 1.95 g/m 2 /day. These skin lesions were associated with skin striae and bone lesions first seen during an X-ray examination. The skin should, therefore, be monitored regularly and X-ray examinations of the bone considered as necessary. Selfexamination of the skin by the patient or the parents is also advised. If any similar skin or bone abnormalities appear, the dose of Cystagon should be reduced. Doses should not exceed 1.95 g/m 2 /day. Intact Cystagon hard capsules should not be administered to children < 6 years old because of the risk of aspiration. Cystagon Summary of Product Characteristics.
51
52 The mechanism of lysosomal cystine depletion involves entry of cysteamine into the lysosomal compartment through a specific transporter, reaction with cystine to form cysteine and a mixed disulphide of cysteamine-cysteine, exit of that compound from the lysosomes through an intact lysine transporter, and reduction to cysteamine and cysteine by glutathione in the cytoplasm. This process permits the cycling of cysteamine between lysosomes and cytoplasm, with each cycle removing 1 mole of half-cystine per mole of cysteamine. Gahl WA et al. Biochem J 1985;228:
53 In normal lysosomes, cystine and lysine freely traverse the lysosomal membrane. In cystinotic lysosomes, lysine can freely traverse the lysosomal membrane, but cystine cannot, and cystine therefore accumulates inside the lysosome. In cysteaminetreated lysosomes, cysteamine combines with half-cystine (i.e., cysteine) to form the mixed disulphide cysteine-cysteamine, which uses the lysine transporter to exit the lysosome since its structure is similar to that of lysine. Gahl WA et al. N Engl J Med 2002;347: ; Levtchenko EN et al. Pediatr Nephrol 2006;21:
54
55 The steady-state pharmacokinetics of cysteamine bitartrate and correlation with the pharmacodynamic response were studied in 11 patients who had been treated with Cystagon for 12 months. The patients had confirmed cystinosis, had not undergone a kidney transplant, weighed over 10 kg, and had WBC cystine levels less than 2 nmol hemicystine/mg of protein. The study showed that there was a correlation between cysteamine absorption and the changes in WBC cystine levels, although there was a lag between the drug concentration and effect (the peak cysteamine plasma level occurred at 1.4 h postdosing, while the trough WBC cystine level was observed 1.8 h post-dosing), and confirmed that 6 hourly dosing was appropriate in steady state. Belldina EB et al. Br J Clin Pharmacol 2003;56:
56 In the absence of treatment, the renal function of patients with cystinosis deteriorates, necessitating dialysis or kidney transplant at about 10 years of age. A retrospective study compared three groups of children presenting with cystinosis: (i) 27 untreated children; (ii) 17 children receiving adequate treatment with cysteamine, i.e. treatment initiated before the age of 2 years and inducing WBC cystine levels of less than 2 nmol hemicystine/mg of protein; and (iii) 32 children receiving treatment termed 'partial', i.e. initiated after the age of 2 years or noncompliant. The children treated sufficiently early with an adequate dosage showed an increase in renal function over the first 5 years of extra-uterine life, and delayed progression to kidney failure from the end of the first decade to the mid-part of the third decade. Markello TC et al. N Engl J Med 1993;328:
57 Bordin-Sartorius studied the clinical outcomes of 86 French patients with nephropathic cystinosis and the impact of long-term oral cysteamine administration. Cysteamine therapy was administered to 75 (87%) patients, initiated at a mean age of 9.9 years (range ) and given during a mean period of 15.6 years (range ). Compliance was reported to be good in 27 patients and quite good in 31 patients. Twenty-one patients had extended periods without treatment (7 missing data). At the last follow-up, 91% of all 86 patients had end-stage renal disease (ESRD), 72% had hypothyroidism, 56% had diabetes and 37% had neuromuscular disorders. Only four patients (5%, mean age 21.4 ± 5.9 years, all treated with cysteamine) had not developed extra-renal complications. Twenty-four patients had died. The most frequent sequence of complications was: ESRD, hypothyroidism, diabetes, neuromuscular disorder and death. Brodin-Sartorius A et al. Kidney Int 2012;81: ; Servais A et al. Eur Nephrol 2012;6:42-46.
58 The patients were separated into three groups: patients who started cysteamine therapy before 5 years of age (n=40, 46.5%), after 5 years of age (n=8, 9.3%), and those who were not treated before ESRD (n=38, 44.2%). The incidence of ESRD was lower in cysteamine-treated patients than in untreated patients. However, starting cysteamine therapy after 5 years old did not significantly decrease the incidence of ESRD in comparison with the absence of treatment. The median age at onset of ESRD was 12.2 and 9.5 years in the groups starting treatment before and after 5 years old, respectively. Eight patients had chronic kidney disease but did not reach ESRD. All of them had been treated with cysteamine before 2.5 years of age (median 1.5). Kaplan-Meier survival curves indicated that starting cysteamine therapy before the age of 5 years significantly delayed the onset of ESRD (p<0.0001). When cysteamine therapy is started after 5 years of age, ESRD is not significantly delayed in comparison with the absence of treatment. Brodin-Sartorius A et al. Kidney Int 2012;81:
59 The incidence of hypothyroidism, diabetes, and neuromuscular disorders was significantly reduced when cysteamine was started before 5 years of age in comparison with the absence of treatment. Starting therapy after 5 years still decreased the incidence of hypothyroidism and diabetes when compared with no treatment. Survival curves indicated that treatment started before 5 years of age was associated with significant delay, compared with untreated patients, in the occurrence of hypothyroidism, diabetes, and neuromuscular disorders. For diabetes and hypothyroidism, a statistically significant delay of the event was still noticed between patients who started treatment after the age of 5 years compared with the absence of treatment. Brodin-Sartorius A et al. Kidney Int 2012;81:
60 Twenty-four (27.9%) patients died: 29.2% (7/24) had never been treated, 8.3% (2/24) had been treated before 5 years old, and 62.5% (15/24) started treatment after the age of 5 years of age. Survival curves show that life expectancy is significantly improved in patients treated before 5 years old compared to untreated patients. Starting cysteamine after 5 years of age still significantly improves the life expectancy in comparison with the untreated patients. Causes of death were linked to cystinosis or related to renal failure. Patients treated before 5 years of age died from infection (n=1) and neurological causes (n=1). In the group treated after 5 years of age, death occurred because of pulmonary oedema (n=2), infections (n=2), neurological causes (n=4), suicide (n=1), respiratory distress due to swallowing impairment (n=4), trauma (n=1), and unknown (n=1). The non-treated patients died from pulmonary oedema (n=1), infections (n=2), digestive tract haemorrhage (n=1), and neurological causes (n=3). Brodin-Sartorius A et al. Kidney Int 2012;81:
61 Gahl et al. collected historical data on 100 adults (58 men and 42 women) aged 18 to 45 years with nephropathic cystinosis examined between January 1985 and May Of the 100 adults with nephropathic cystinosis, 92 had received a renal allograft and 33 had died. Of the 100 patients studied, all eight with functioning native kidneys had received cysteamine for at least 8 years; the 31 adequately treated ( 8 years of oral cysteamine treatment) patients who had undergone kidney transplantation received their allografts, on average, 3.8 years later than the 61 inadequately treated (<8 years of treatment) patients. Gahl WA et al. Ann Intern Med 2007;147:
62 To evaluate the influence of oral cysteamine therapy, the frequency of a complication for each 10-year span that a patient either lived without adequate cysteamine treatment or continued cysteamine treatment diligently (0 to 10 years, 11 to 20 years, and so forth) was determined. The frequency of diabetes mellitus increased dramatically from 4% to 50% as the time off oral cysteamine therapy increased from less than 10 years to more than 30 years. In contrast, the frequency of diabetes decreased from 28% to 0% as the time on therapy increased to greater than 20 years. Similarly, the frequency of myopathy increased from 12% to 80% as time off cysteamine increased and decreased from 60% to 0% as time on therapy increased to greater than 20 years. Gahl WA et al. Ann Intern Med 2007;147:
63 Pulmonary dysfunction increased in frequency and severity with time off cysteamine therapy and decreased with time on cysteamine therapy. For the 21 patients who lived without cysteamine therapy for 10 years or fewer, the mean forced vital capacity (FVC) was 86% (SD, 20%) of predicted, compared with 56% (SD, 17%) for the 10 patients who lived without cysteamine therapy for more than 30 years. For the 53 patients who received cysteamine therapy for 10 years or fewer, the mean FVC was 54% (SD, 19%) of predicted, compared with 83% (SD, 22%) for the 24 patients who received cysteamine therapy for more than 10 years. The frequency of death also increased with time off cysteamine therapy and decreased with time on cysteamine therapy. Gahl WA et al. Ann Intern Med 2007;147:
64 Before 1960, all people with cystinosis died in infancy, because of renal Fanconi s syndrome, or in the first decade of life, because of chronic glomerular failure. In the late 1960s, renal allograft procedures for children dramatically increased the longevity of these patients, but long-term cystine accumulation continued to damage non-renal tissues. Organs previously thought to be spared by cystinosis, such as the brain, the liver, and muscle, were seen to be affected. Now, as children with cystinosis survive into adulthood, the true burden of cystinosis has become clear. The only therapeutic option is oral cysteamine, which substantial evidence indicates is safe and effective in preventing late complications. This finding has two major implications: (i) cysteamine therapy should be considered for all patients with cystinosis, regardless of age and transplantation status and (ii) the registration for cysteamine bitartrate should be re-evaluated to include among its indications posttransplantation cystinosis and its associated non-renal organ damage. Gahl WA et al. Ann Intern Med 2007;147:
65
66 Genotoxicity studies have been performed: specific studies with cysteamine bitartrate did not show any mutagenic effects in the Ames test or any clastogenic effect in the mouse micronucleus test. High doses of cysteamine, either by oral or parenteral routes, produce duodenal ulcers in rats and mice but not in monkeys. Experimental administration of the drug causes depletion of somatostatin in several animal species. The consequence of this for the clinical use of the drug is unknown. No carcinogenic studies have been conducted with Cystagon. Cystagon Summary of Product Characteristics.
67 Reproduction studies showed embryofoetotoxic effects (resorptions and postimplantation losses) in rats at a dose of 100 mg/kg/day and in rabbits receiving cysteamine 50 mg/kg/day. Teratogenic effects have been described in rats when cysteamine is administered over the period of organogenesis at a dose of 100 mg/kg/day which is equivalent to less than half the recommended clinical maintenance dose of cysteamine. A reduction of fertility was observed in rats at 375 mg/kg/day, a dose at which body weight gain was retarded. At this dose, weight gain and survival of the offspring during lactation was also reduced. High doses of cysteamine impair the ability of lactating mothers to feed their pups. Single doses of the drug inhibit prolactin secretion in animals. Cystagon should not be used during pregnancy, particularly during the first trimester, unless clearly necessary and the patient must be advised of the possible teratogenic risk of the drug. Breast-feeding is contraindicated in women taking Cystagon. Cystagon Summary of Product Characteristics.
68 Approximately 35% of patients can be expected to experience adverse reactions. These mainly involve the gastrointestinal and central nervous systems. When these effects appear at the initiation of cysteamine therapy, temporary suspension and gradual reintroduction of treatment may be effective in improving tolerance. The most frequent reactions include vomiting, nausea, dyspepsia and abdominal pain and are generally reversible. These disorders can lead to anorexia. They can be managed by symptomatic treatment (proton pump inhibitor) or dosage adjustment. The drug is best tolerated if taken just after or with food. Halitosis is frequently reported and is due to the expiratory elimination of the sulphur group of cysteamine. Products that correct or improve the breath should be tried.
69 Cystagon was granted a European Marketing Approval for all EU countries on 23 June A voluntary safety report covering the period from June 1997 to March 2005 described almost 8 years of experience with Cystagon given to 507 patients, i.e. approximately 92% of the population so far treated with Cystagon in the EU. Most of the adverse reactions involved the gastrointestinal system and were consistent with the known safety profile of Cystagon. However four serious cases of elbow skin lesions (Ehler-Danlos like) were reported in patients treated with doses above the recommended one. Due to the detection of these new safety signals in patients treated with a dose above the recommended one, the safety profile has been reassessed. However, the benefit-risk ratio for Cystagon in the treatment of nephropathic cystinosis remains favourable.
70
71
CYSTARAN (cysteamine hydrochloride) ophthalmic solution
 CYSTARAN (cysteamine hydrochloride) ophthalmic solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
CYSTARAN (cysteamine hydrochloride) ophthalmic solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CYSTAGON 50 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 50 mg of cysteamine (as
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CYSTAGON 50 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 50 mg of cysteamine (as
BMT for Cystinosis? K30 Journal Club Jan 26, 2009
 BMT for Cystinosis? K30 Journal Club Jan 26, 2009 Cystine Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues Cystinosis is a lysosomal storage disorder QuickTime and a TIFF
BMT for Cystinosis? K30 Journal Club Jan 26, 2009 Cystine Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues Cystinosis is a lysosomal storage disorder QuickTime and a TIFF
Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis
 Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis Elena N. Levtchenko 1, Carin M. van Dael 2, Addy C. de Graaf-Hess 3, Martijn J.G. Wilmer 3, Lambertus
Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis Elena N. Levtchenko 1, Carin M. van Dael 2, Addy C. de Graaf-Hess 3, Martijn J.G. Wilmer 3, Lambertus
Nephropathic cystinosis is a rare, inherited autosomal
 A SUPPLEMENT TO NEPHROPATHIC CYSTINOSIS: DIAGNOSIS, MANAGEMENT, AND CHALLENGES IN LONG-TERM TREATMENT Larry A. Greenbaum, MD, PhD Chief, Pediatric Nephrology Children s Healthcare of Atlanta and Emory
A SUPPLEMENT TO NEPHROPATHIC CYSTINOSIS: DIAGNOSIS, MANAGEMENT, AND CHALLENGES IN LONG-TERM TREATMENT Larry A. Greenbaum, MD, PhD Chief, Pediatric Nephrology Children s Healthcare of Atlanta and Emory
Renal Tubular Acidosis
 1 Renal Tubular Acidosis Mohammad Tariq Ibrahim 6 th Grade Diyala College Of Medicine supervisor DR. Sabah Almaamoory 2 *Renal Tubular Acidosis:- RTA:- is a disease state characterized by a normal anion
1 Renal Tubular Acidosis Mohammad Tariq Ibrahim 6 th Grade Diyala College Of Medicine supervisor DR. Sabah Almaamoory 2 *Renal Tubular Acidosis:- RTA:- is a disease state characterized by a normal anion
cysteamine Delayed Release Capsules (Procysbi )
 cysteamine Delayed Release Capsules (Procysbi ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred
cysteamine Delayed Release Capsules (Procysbi ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcium Sandoz 500 mg, effervescent tablets Calcium Sandoz 1000 mg, effervescent tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcium Sandoz 500 mg, effervescent tablets Calcium Sandoz 1000 mg, effervescent tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Colecalciferol Meda 800 IU tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 800 IU
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Colecalciferol Meda 800 IU tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 800 IU
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
Chronic kidney disease in cats
 Chronic kidney disease in cats What is chronic kidney disease (CKD)? Chronic kidney disease (CKD) is the name now used to refer to cats with kidney failure (or chronic kidney failure). CKD is one of the
Chronic kidney disease in cats What is chronic kidney disease (CKD)? Chronic kidney disease (CKD) is the name now used to refer to cats with kidney failure (or chronic kidney failure). CKD is one of the
Infantile Nephropathic Cystinosis Standards of Care A reference for people with infantile nephropathic cystinosis, their families, and medical team
 Infantile Nephropathic Cystinosis Standards of Care A reference for people with infantile nephropathic cystinosis, their families, and medical team Galina Nesterova, MD, nesterovag@mail.nih.gov and William
Infantile Nephropathic Cystinosis Standards of Care A reference for people with infantile nephropathic cystinosis, their families, and medical team Galina Nesterova, MD, nesterovag@mail.nih.gov and William
ANNEX 1 SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX 1 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT PROCYSBI 25 mg gastro-resistant hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 25 mg
ANNEX 1 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT PROCYSBI 25 mg gastro-resistant hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 25 mg
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
Description Cysteamine delayed-release (DR) capsule (Procysbi) is an oral medication used for the chronic management of nephropathic cystinosis.
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru311 Topic: Procysbi, cysteamine delayed-release Date of Origin: August 1, 2013 Committee Approval
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru311 Topic: Procysbi, cysteamine delayed-release Date of Origin: August 1, 2013 Committee Approval
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ANCOTIL 500 mg, tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flucytosine 500 mg Per tablet. 3. PHARMACEUTICAL FORM Tablet. 4. CLINICAL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ANCOTIL 500 mg, tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flucytosine 500 mg Per tablet. 3. PHARMACEUTICAL FORM Tablet. 4. CLINICAL
STANDARDS OF CARE. Infantile Nephropathic Cystinosis. A Reference for People with Infantile Nephropathic Cystinosis, their Families, and Medical Team
 Dedicated to a Cure. Committed to Our Community. Infantile Nephropathic Cystinosis STANDARDS OF CARE A Reference for People with Infantile Nephropathic Cystinosis, their Families, and Medical Team Galina
Dedicated to a Cure. Committed to Our Community. Infantile Nephropathic Cystinosis STANDARDS OF CARE A Reference for People with Infantile Nephropathic Cystinosis, their Families, and Medical Team Galina
Cystinosis. Transition Management Tool
 Cystinosis Transition Management Tool Table of Contents Genetics and Pathophysiology................ 3 Treatment with Cysteine-depleting Agents.......... 3 Nephrology........................... 3 Transplant............................
Cystinosis Transition Management Tool Table of Contents Genetics and Pathophysiology................ 3 Treatment with Cysteine-depleting Agents.......... 3 Nephrology........................... 3 Transplant............................
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University
 RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure
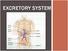 EXCRETORY SYSTEM WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure These wastes include: Carbon dioxide Mostly through breathing
EXCRETORY SYSTEM WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure These wastes include: Carbon dioxide Mostly through breathing
Grand Rounds. CYSTINOSIS Denis Jusufbegovic, M.D. University of Louisville Department of Ophthalmology and Visual Sciences 10/05/12
 Grand Rounds CYSTINOSIS Denis Jusufbegovic, M.D. University of Louisville Department of Ophthalmology and Visual Sciences 10/05/12 Subjective CC: bilateral ocular opacities x yrs HPI: : 11 yo WF referred
Grand Rounds CYSTINOSIS Denis Jusufbegovic, M.D. University of Louisville Department of Ophthalmology and Visual Sciences 10/05/12 Subjective CC: bilateral ocular opacities x yrs HPI: : 11 yo WF referred
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
Refer to the figure below, a diagram of a renal tubule, to answer the following questions.
 1. The digestion and utilization of which nutrient creates the greatest need for osmoregulation by the kidneys? a. protein b. starch c. fat d. oil e. cellulose 2. Which of the following is true of urea?
1. The digestion and utilization of which nutrient creates the greatest need for osmoregulation by the kidneys? a. protein b. starch c. fat d. oil e. cellulose 2. Which of the following is true of urea?
SUMMARY OF PRODUCT CHARACTERISTICS. One chewable tablet contains 1250 mg calcium carbonate (equivalent to 500 mg calcium).
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [XXX] 500 mg chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One chewable tablet contains 1250 mg calcium carbonate (equivalent
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [XXX] 500 mg chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One chewable tablet contains 1250 mg calcium carbonate (equivalent
PACKAGE LEAFLET: Information for the user. METFORMINE Film-coated tablets 500 mg, 850 mg or 1000 mg (Metformin hydrochloride)
 PACKAGE LEAFLET: Information for the user METFORMINE Film-coated tablets 500 mg, 850 mg or 1000 mg (Metformin hydrochloride) Read this leaflet carefully before you start taking this medicine. - Keep this
PACKAGE LEAFLET: Information for the user METFORMINE Film-coated tablets 500 mg, 850 mg or 1000 mg (Metformin hydrochloride) Read this leaflet carefully before you start taking this medicine. - Keep this
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Kidneys and Homeostasis
 16 The Urinary System The Urinary System OUTLINE: Eliminating Waste Components of the Urinary System Kidneys and Homeostasis Urination Urinary Tract Infections Eliminating Waste Excretion Elimination of
16 The Urinary System The Urinary System OUTLINE: Eliminating Waste Components of the Urinary System Kidneys and Homeostasis Urination Urinary Tract Infections Eliminating Waste Excretion Elimination of
Package Insert. Constipeg
 Package Insert Constipeg Product Summary 1. Name of the medicinal product Constipeg sachets 2. Qualitative and quantitative composition Each 13.7 g sachet contains PEG polyethylene glycol (macrogol) USNF
Package Insert Constipeg Product Summary 1. Name of the medicinal product Constipeg sachets 2. Qualitative and quantitative composition Each 13.7 g sachet contains PEG polyethylene glycol (macrogol) USNF
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
PROCYSBI (CYSTEAMINE BITARTRATE) DELAYED-RELEASE CAPSULES PRESCRIPTION ENROLLMENT FORM INSTRUCTIONS
 PROCYSBI (CYSTEAMINE BITARTRATE) PRESCRIPTION ENROLLMENT FORM INSTRUCTIONS The Prescription Enrollment Form is required to initiate treatment with Horizon Pharma s prescription medicine, PROCYSBI. Instructions:
PROCYSBI (CYSTEAMINE BITARTRATE) PRESCRIPTION ENROLLMENT FORM INSTRUCTIONS The Prescription Enrollment Form is required to initiate treatment with Horizon Pharma s prescription medicine, PROCYSBI. Instructions:
SUMMARY OF PRODUCT CHARACTERISTICS. Synthamin 14, 8.5% Amino Acid Intravenous Infusion
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Synthamin 14, 8.5% Amino Acid Intravenous Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION L-Leucine Ph. Eur 0.620% w/v L-Isoleucine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Synthamin 14, 8.5% Amino Acid Intravenous Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION L-Leucine Ph. Eur 0.620% w/v L-Isoleucine
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ketosteril film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: (RS)-3-methyl-2-oxovaleric
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ketosteril film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: (RS)-3-methyl-2-oxovaleric
SUMMARY OF PRODUCT CHARACTERISTICS. A 2.5ml single-dose bottle containing IU Cholecalciferol (equivalent to 625 micrograms vitamin D 3 )
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fultium 25 000 IU Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION A 2.5ml single-dose bottle containing 25 000 IU Cholecalciferol
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fultium 25 000 IU Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION A 2.5ml single-dose bottle containing 25 000 IU Cholecalciferol
2. QUALITATIVE AND QUANTITATIVE COMPOSITION. Each capsule contains PARACETAMOL 500mg For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS PRODUCT SUMMARY 1. NAME OF THE MEDICINAL PRODUCT Sterile Potassium Chloride Concentrate 15%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15% of Potassium Chloride in
SUMMARY OF PRODUCT CHARACTERISTICS PRODUCT SUMMARY 1. NAME OF THE MEDICINAL PRODUCT Sterile Potassium Chloride Concentrate 15%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15% of Potassium Chloride in
Package Insert. Elkar
 Package Insert Elkar Product Summary 1. Name of the medicinal product Elkar 500 mg tablets 2. Qualitative and quantitative composition Each tablet contains levocarnitine 500 mg. 3. Pharmaceutical form
Package Insert Elkar Product Summary 1. Name of the medicinal product Elkar 500 mg tablets 2. Qualitative and quantitative composition Each tablet contains levocarnitine 500 mg. 3. Pharmaceutical form
1.2 Synonyms There are several synonyms e.g. diaminomethanal, but in a medical context, this substance is always referred to as urea.
 Urea (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Urea 1.2 Synonyms There are several synonyms e.g. diaminomethanal, but in a medical context, this substance is always referred
Urea (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Urea 1.2 Synonyms There are several synonyms e.g. diaminomethanal, but in a medical context, this substance is always referred
PHYSICAL PROPERTIES AND DETECTION OF NORMAL CONSTITUENTS OF URINE
 PHYSICAL PROPERTIES AND DETECTION OF NORMAL CONSTITUENTS OF URINE - OBJECTIVES: 1- The simple examination of urine. 2- To detect some of the normal organic constituents of urine. 3- To detect some of the
PHYSICAL PROPERTIES AND DETECTION OF NORMAL CONSTITUENTS OF URINE - OBJECTIVES: 1- The simple examination of urine. 2- To detect some of the normal organic constituents of urine. 3- To detect some of the
RENAL TUBULAR ACIDOSIS An Overview
 RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
Diuretic Agents Part-2. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Diuretic Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Potassium-sparing diuretics The Ion transport pathways across the luminal and basolateral
Diuretic Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Potassium-sparing diuretics The Ion transport pathways across the luminal and basolateral
The Urinary System. BIOLOGY OF HUMANS Concepts, Applications, and Issues. Judith Goodenough Betty McGuire
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 16 The Urinary System Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 16 The Urinary System Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
Pediatric metabolic bone diseases
 Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR
 1 School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR URINARY (RENAL) STONE FORMATION An Overview What are Urinary (Renal)
1 School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR URINARY (RENAL) STONE FORMATION An Overview What are Urinary (Renal)
12/7/10. Excretory System. The basic function of the excretory system is to regulate the volume and composition of body fluids by:
 Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
Ketosteril. Total nitrogen content per tablet
 1. NAME OF THE MEDICINAL PRODUCT Ketosteril film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: (RS)-3-methyl-2-oxovaleric acid (α-ketoanalogue to DL-isoleucine),
1. NAME OF THE MEDICINAL PRODUCT Ketosteril film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: (RS)-3-methyl-2-oxovaleric acid (α-ketoanalogue to DL-isoleucine),
CEDIAMATE Metformin Tablets USP 500 mg
 CEDIAMATE Metformin Tablets USP 500 mg COMPOSITION: Cediamate Each un-coated tablet contains: Metformin Hydrochloride USP Excipients 500 mg Q.S PHARMACOLOGY: Pharmacotherapeutic group: Blood Glucose lowering
CEDIAMATE Metformin Tablets USP 500 mg COMPOSITION: Cediamate Each un-coated tablet contains: Metformin Hydrochloride USP Excipients 500 mg Q.S PHARMACOLOGY: Pharmacotherapeutic group: Blood Glucose lowering
Health Products Regulatory Authority IPAR. Public Assessment Report. Scientific discussion
 IPAR Public Assessment Report Scientific discussion Magnaspartate 243mg Powder for Oral Solution MAGNESIUM ASPARTATE DIHYDRATE IE/H/436/001/DC Date: 3 rd November 2014 This module reflects the scientific
IPAR Public Assessment Report Scientific discussion Magnaspartate 243mg Powder for Oral Solution MAGNESIUM ASPARTATE DIHYDRATE IE/H/436/001/DC Date: 3 rd November 2014 This module reflects the scientific
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Desunin 4000 IU Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 4000 IU (equivalent
1 NAME OF THE MEDICINAL PRODUCT Desunin 4000 IU Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 4000 IU (equivalent
Evaluation of Failure to Thrive in a Young Child: Case Example of Jeff. Andrew Hsi, MD, MPH Family Medicine Pediatric Grand Rounds, 8 August 2012
 Evaluation of Failure to Thrive in a Young Child: Case Example of Jeff Andrew Hsi, MD, MPH Family Medicine Pediatric Grand Rounds, 8 August 2012 Objectives for Presentation At the end of this talk; the
Evaluation of Failure to Thrive in a Young Child: Case Example of Jeff Andrew Hsi, MD, MPH Family Medicine Pediatric Grand Rounds, 8 August 2012 Objectives for Presentation At the end of this talk; the
The Urinary S. (Chp. 10) & Excretion. What are the functions of the urinary system? Maintenance of water-salt and acidbase
 10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
PROCYSBI (CYSTEAMINE BITARTRATE) DELAYED-RELEASE CAPSULES PRESCRIPTION ENROLLMENT FORM INSTRUCTIONS
 PRESCRIPTION ENROLLMENT FORM INSTRUCTIONS The Prescription Enrollment Form is required to initiate treatment with Horizon Pharma s prescription medicine, PROCYSBI. Instructions: 1. Fill out all patient
PRESCRIPTION ENROLLMENT FORM INSTRUCTIONS The Prescription Enrollment Form is required to initiate treatment with Horizon Pharma s prescription medicine, PROCYSBI. Instructions: 1. Fill out all patient
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Package leaflet: Information for the user PROCYSBI 75 mg gastro resistant hard capsules
 Package leaflet: Information for the user PROCYSBI 25 mg gastro resistant hard capsules PROCYSBI 75 mg gastro resistant hard capsules Cysteamine (mercaptamine bitartrate) Read all of this leaflet carefully
Package leaflet: Information for the user PROCYSBI 25 mg gastro resistant hard capsules PROCYSBI 75 mg gastro resistant hard capsules Cysteamine (mercaptamine bitartrate) Read all of this leaflet carefully
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
220 SUBJECT INDEX. D Diarrhea and sodium balance, 74 weanling, 161,179,208,212; see also Infection
 Subject Index Acid balance, see ph Allergy, food, see also Immunity and beikost, 143-144 and breast milk, 91,143 and formula, 89-90 Antidiuretic hormone, 66 67 Antigens, see also Immunity determinants,
Subject Index Acid balance, see ph Allergy, food, see also Immunity and beikost, 143-144 and breast milk, 91,143 and formula, 89-90 Antidiuretic hormone, 66 67 Antigens, see also Immunity determinants,
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
April 08, biology 2201 ch 11.3 excretion.notebook. Biology The Excretory System. Apr 13 9:14 PM EXCRETORY SYSTEM.
 Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Acute Kidney Injury. Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1
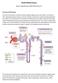 Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
The Endocrine System 2
 The Endocrine System 2 Continuing on from the previous instalment, we will now look at the adrenal glands, the pancreas and the gonads as parts of the endocrine system. Adrenal Glands The adrenal glands
The Endocrine System 2 Continuing on from the previous instalment, we will now look at the adrenal glands, the pancreas and the gonads as parts of the endocrine system. Adrenal Glands The adrenal glands
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fluoride 5000 ppm Toothpaste 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of toothpaste contains 5 mg fluoride (as sodium fluoride),
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fluoride 5000 ppm Toothpaste 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of toothpaste contains 5 mg fluoride (as sodium fluoride),
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion , version 1.1
 EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT SPASMAG, capsule 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Magnesium sulphate trihydrate... 423.5 mg quantity equivalent to elemental
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT SPASMAG, capsule 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Magnesium sulphate trihydrate... 423.5 mg quantity equivalent to elemental
KELFER Deferiprone. COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg
 KELFER Deferiprone COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg DOSAGE FORM Capsules PHARMACOLOGY Pharmacodynamics
KELFER Deferiprone COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg DOSAGE FORM Capsules PHARMACOLOGY Pharmacodynamics
1. remove: waste products: urea, creatinine, and uric acid foreign chemicals: drugs, water soluble vitamins, and food additives, etc.
 Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
9/11/2012. Chapter 11. Learning Objectives. Learning Objectives. Endocrine Emergencies. Differentiate type 1 and type 2 diabetes
 Chapter 11 Endocrine Emergencies Learning Objectives Differentiate type 1 and type 2 diabetes Explain roles of glucagon, glycogen, and glucose in hypoglycemia Learning Objectives Discuss following medications
Chapter 11 Endocrine Emergencies Learning Objectives Differentiate type 1 and type 2 diabetes Explain roles of glucagon, glycogen, and glucose in hypoglycemia Learning Objectives Discuss following medications
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Omnilax 10 g powder for oral solution, sachet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One sachet contains 10 g of macrogol 4000.
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Omnilax 10 g powder for oral solution, sachet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One sachet contains 10 g of macrogol 4000.
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
PACKAGE LEAFLET: INFORMATION FOR THE USER. Nutriflex plus Solution for Infusion Amino acids / Glucose / Electrolytes
 PACKAGE LEAFLET: INFORMATION FOR THE USER Nutriflex plus Solution for Infusion Amino acids / Glucose / Electrolytes Read all of this leaflet carefully. It provides a summary of information about your medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER Nutriflex plus Solution for Infusion Amino acids / Glucose / Electrolytes Read all of this leaflet carefully. It provides a summary of information about your medicine.
KELFER Capsules (Deferiprone)
 Published on: 22 Sep 2014 KELFER Capsules (Deferiprone) Composition KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg Dosage Form
Published on: 22 Sep 2014 KELFER Capsules (Deferiprone) Composition KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg Dosage Form
SUMMARY OF PRODUCT CHARACTERISTICS. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
MOVICOL Junior Powder for Solution (macrogol 3350)
 MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT ALVERINE Mayoly Spindler, 60 mg hard capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 60 mg of alverine citrate.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT ALVERINE Mayoly Spindler, 60 mg hard capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 60 mg of alverine citrate.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Zaditen 0.25 mg/ml, eye drops, solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains 0.345 mg ketotifen fumarate corresponding
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Zaditen 0.25 mg/ml, eye drops, solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains 0.345 mg ketotifen fumarate corresponding
Homeostatic Regulation
 Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Topic 1: Chemistry of Living Things
 1. Some processes that occur in a cell are listed below.1 utilize energy 2 detect changes in the environment 3 rearrange and synthesize chemical compounds 4. The diagram below represents a sequence of
1. Some processes that occur in a cell are listed below.1 utilize energy 2 detect changes in the environment 3 rearrange and synthesize chemical compounds 4. The diagram below represents a sequence of
Urinary System. Analyze the Anatomy and Physiology of the urinary system
 Urinary System Analyze the Anatomy and Physiology of the urinary system Kidney Bean-shaped Located between peritoneum and the back muscles (retroperitoneal) Renal pelvis funnelshaped structure at the beginning
Urinary System Analyze the Anatomy and Physiology of the urinary system Kidney Bean-shaped Located between peritoneum and the back muscles (retroperitoneal) Renal pelvis funnelshaped structure at the beginning
Physical Characteristics of
 Physical Characteristics of Urine Bởi: OpenStaxCollege The urinary system s ability to filter the blood resides in about 2 to 3 million tufts of specialized capillaries the glomeruli distributed more or
Physical Characteristics of Urine Bởi: OpenStaxCollege The urinary system s ability to filter the blood resides in about 2 to 3 million tufts of specialized capillaries the glomeruli distributed more or
DATA SHEET 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 DATA SHEET 1 PRODUCT NAME 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Sachet, each 5.1g sachet contains: Sodium chloride 470mg, potassium chloride 300mg, sodium acid citrate 530mg, glucose 3.56g. Tablet,
DATA SHEET 1 PRODUCT NAME 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Sachet, each 5.1g sachet contains: Sodium chloride 470mg, potassium chloride 300mg, sodium acid citrate 530mg, glucose 3.56g. Tablet,
SUMMARY OF PRODUCT CHARACTERISTICS
 MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
NATIONAL KIDNEY MONTH
 NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Libromide 325 mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 tablet contains: Active substance: Potassium
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Libromide 325 mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 tablet contains: Active substance: Potassium
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
Mouth. Digestion begins in the Mouth. Chewing begins the process of digestion. breakdown of large pieces of food into smaller pieces.
 Digestive System Mouth Digestion begins in the Mouth. Chewing begins the process of digestion - Mechanical digestion is the physical breakdown of large pieces of food into smaller pieces. - Chemical digestion
Digestive System Mouth Digestion begins in the Mouth. Chewing begins the process of digestion - Mechanical digestion is the physical breakdown of large pieces of food into smaller pieces. - Chemical digestion
Chapter 13 The Urinary System
 Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Ch17-18 Urinary System
 Ch17-18 Urinary System Main Function: Filter the blood Other Functions: maintain purity and consistency of internal fluids eliminates nitrogenous wastes, toxins, and drugs from the body regulates blood
Ch17-18 Urinary System Main Function: Filter the blood Other Functions: maintain purity and consistency of internal fluids eliminates nitrogenous wastes, toxins, and drugs from the body regulates blood
ART 50 Capsules. Symptomatic treatment of functional symptoms and signs of osteoarthritis.
 Doctor leaflet Art50-DL-March2012-01 ART 50 Capsules 1. NAME OF THE MEDICINAL PRODUCT Art 50 mg, capsule. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule of ART 50 contains Diacerein 50 mg. For
Doctor leaflet Art50-DL-March2012-01 ART 50 Capsules 1. NAME OF THE MEDICINAL PRODUCT Art 50 mg, capsule. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule of ART 50 contains Diacerein 50 mg. For
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Kalcipos-D mite 500 mg/200 IU film-coated tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 500 mg
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Kalcipos-D mite 500 mg/200 IU film-coated tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 500 mg
The Urinary System and Homeostasis *
 OpenStax-CNX module: m46446 1 The Urinary System and Homeostasis * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m46446 1 The Urinary System and Homeostasis * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Atrosan Devil's Claw film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 480 mg of extract
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Atrosan Devil's Claw film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 480 mg of extract
Salicylate (Aspirin) Ingestion California Poison Control Background 1. The prevalence of aspirin-containing analgesic products makes
 Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Excretion and Water Balance
 Excretion and Water Balance 1. Osmoregulation (water balance) a. Most marine invertebrates are osmoconformers in which the concentration of solutes in their body fluid is equal to that of their environment.
Excretion and Water Balance 1. Osmoregulation (water balance) a. Most marine invertebrates are osmoconformers in which the concentration of solutes in their body fluid is equal to that of their environment.
