Treating Postprandial Hyperglycemia Does Not Appear to Delay Progression of Early Type 2 Diabetes
|
|
|
- Tracey Weaver
- 6 years ago
- Views:
Transcription
1 Cardiovascular and Metabolic Risk O R I G I N A L A R T I C L E Treating Postprandial Hyperglycemia Does Not Appear to Delay Progression of Early Type 2 Diabetes The Early Diabetes Intervention Program M. SUE KIRKMAN, MD 1,2 R. RAVI SHANKAR, MD 1 SUDHA SHANKAR, MD 1 CHANGYU SHEN, PHD 1 EDWARD BRIZENDINE, MS 1 ALAIN BARON, MD 1,3 JANET MCGILL, MD 4 OBJECTIVE Postprandial hyperglycemia characterizes early type 2 diabetes. We investigated whether ameliorating postprandial hyperglycemia with acarbose would prevent or delay progression of diabetes, defined as progression to frank fasting hyperglycemia, in subjects with early diabetes (fasting plasma glucose [FPG] 140 mg/dl and 2-h plasma glucose 200 mg/dl). RESEARCH DESIGN AND METHODS Two hundred nineteen subjects with early diabetes were randomly assigned to 100 mg acarbose t.i.d. or identical placebo and followed for 5 years or until they reached the primary outcome (two consecutive quarterly FPG measurements of 140 mg/dl). Secondary outcomes included measures of glycemia (meal tolerance tests, HbA 1c, annual oral glucose tolerance tests [OGTTs]), measures of insulin resistance (homeostasis model assessment [HOMA] of insulin resistance and insulin sensitivity index from hyperglycemic clamps), and secondary measures of -cell function (HOMA-, early- and latephase insulin secretion, and proinsulin-to-insulin ratio). RESULTS Acarbose significantly reduced postprandial hyperglycemia. However, there was no difference in the cumulative rate of frank fasting hyperglycemia (29% with acarbose and 34% with placebo; P 0.65 for survival analysis). There were no significant differences between groups in OGTT values, measures of insulin resistance, or secondary measures of -cell function. In a post hoc analysis of subjects with initial FPG 126 mg/dl, acarbose reduced the rate of development of FPG 126 mg/dl (27 vs. 50%; P 0.04). CONCLUSIONS Ameliorating postprandial hyperglycemia did not appear to delay progression of early type 2 diabetes. Factors other than postprandial hyperglycemia may be greater determinants of progression of diabetes. Alternatively, once FPG exceeds 126 mg/dl, -cell failure may no longer be remediable. Diabetes Care 29: , 2006 From the 1 Indiana University School of Medicine, Indianapolis, Indiana; the 2 Roudebush VA Medical Center, Indianapolis Indiana; 3 Amylin Pharmaceuticals, San Diego California; and the 4 Washington University School of Medicine, St. Louis, Missouri. Address correspondence and reprint requests to M. Sue Kirkman, MD, 545 Barnhill Dr., EH 421, Indianapolis, IN mkirkman@iupui.edu. Received for publication 9 January 2006 and accepted in revised form 25 May Abbreviations: AUC, area under the curve; DPP, Diabetes Prevention Program; EDIP, Early Diabetes Intervention Program; FPG, fasting plasma glucose; HOMA-, homeostasis model assessment of -cell function; HOMA-IR, homeostasis model assessment of of insulin resistance; IGT, impaired glucose tolerance; MPS, meal profile study; OGTT, oral glucose tolerance test; STOP-NIDDM, Study to Prevent NIDDM; UKPDS, U.K. Prospective Diabetes Study Group. A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances. DOI: /dc by the American Diabetes Association. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. Type 2 diabetes is a progressive disease, often with an inexorable rise in HbA1c (A1C) over time and the need for increasingly aggressive multidrug therapy (1). Insulin resistance is a fundamental abnormality in type 2 diabetes that appears to develop long before the development of hyperglycemia. However, many obese subjects never develop hyperglycemia despite significant insulin resistance. -Cell dysfunction, with progressive loss of the capacity to hypersecrete insulin to compensate for insulin resistance, seems to be required for the development of impaired glucose tolerance (IGT) or diabetes (2,3). Several abnormalities have been described as part of the syndrome of -cell dysfunction, including loss of first-phase insulin secretion, a defect in conversion of proinsulin to insulin, decreasing maximal capacity of glucose to potentiate nonglucose signals, and eventually loss of adequate basal insulin secretion (2 5). The causes of -cell dysfunction and failure are unknown but may include genetic factors, glucose toxicity, lipotoxicity, or some combination thereof. Most patients in whom type 2 diabetes is diagnosed in clinical practice do not actually have new-onset diabetes; on average, diabetes has been present for 9 12 years at the time of clinical diagnosis (6). During the pre-diabetic state of IGT and the early years of diabetes, hyperglycemia is primarily postprandial in nature (7 9), asymptomatic, and difficult to detect, leading to delay in diagnosis. With time, as the -cell defect becomes more severe, impaired basal insulin secretion leads to fasting hyperglycemia, more severe postprandial hyperglycemia, hyperglycemic symptoms, and a clinical diagnosis. At this point, the U.K. Prospective Diabetes Study Group (UKPDS) data suggest that -cell decline may not be remediable (1). Because postprandial hyperglycemia is an early abnormality in type 2 diabetes, the resultant glucose toxicity is a potential mediator of progressive -cell dysfunction (10 13). We hypothesized that ameliorating postprandial hyperglycemia in subjects with early type 2 diabetes (primarily postprandial hyperglycemia and found by screening asymptomatic individuals) would prevent or delay -cell failure, manifested by progression to frank fasting hyperglycemia. We used acarbose, a drug known to primarily af- DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER
2 Postprandial hyperglycemia in early diabetes fect postprandial hyperglycemia and to have little effect on fasting glucose (14), to test this hypothesis in a randomized, double-blind placebo-controlled clinical trial. RESEARCH DESIGN AND METHODS The Early Diabetes Intervention Program (EDIP) was a 5-year trial carried out at Indiana University School of Medicine and Washington University School of Medicine. The study was approved by the institutional review boards of both institutions, and all subjects provided informed consent for screening and for the trial. Recruitment for EDIP paralleled recruitment for the Diabetes Prevention Program (DPP) trial (15) at both institutions and has been described previously (16). Individuals at least 25 years of age with obesity, a history of gestational diabetes, or a family history of diabetes were targeted. After informed consent, subjects underwent fasting plasma glucose (FPG) measurement using a glucose analyzer (YSI, Yellow Springs, OH). Subjects with FPG measurements between 105 mg/dl (5.5 mmol/l) and 140 mg/dl (7.8 mmol/l, the diagnostic FPG cutoff for diabetes at the time of study initiation) underwent a 75-g oral glucose tolerance test (OGTT). Those with a 2-h postload plasma glucose measurement 200 mg/dl (11.1 mmol/l) along with FPG 140 mg/dl were considered to have early diabetes and screened for enrollment. Exclusion criteria included BMI 24 kg/m 2, cancer within 5 years, infectious disease including HIV infection, a cardiac event within the previous 6 months, uncontrolled hypertension or hypertension that could not be controlled with agents other than -blockers or thiazide diuretics, elevated aspartate aminotransferase or alanine aminotransferase, serum creatinine 1.4 mg/dl in men or 1.3 mg/dl in women, fasting plasma triglycerides 600 mg/dl despite treatment, any significant disease or medication that could interfere with medication tolerance or with outcomes, suspected inability to adhere to the protocol, or inability to give informed consent. Within 6 weeks of the qualifying OGTT, subjects were admitted for a 2-day study initiation visit. All subjects had a medical history taken and underwent physical examination, electrocardiogram, seven-field fundus photography, 24-h urine collection for creatinine clearance and microalbumin, and a 9-h meal profile study (MPS; described below). Half of the subjects were randomly assigned to undergo a hyperglycemic clamp procedure, described below. A registered dietitian counseled subjects on an appropriate diet for type 2 diabetes. At the end of the admission, subjects began either acarbose or an identical placebo based on a blinded randomization stratified by site and by randomization to the clamp. Study drug was initiated at a dose of 25 mg once daily with the evening meal, then titrated at weekly intervals by 25 mg daily to the maximum dose of 100 mg t.i.d. with meals. Study drug was down-titrated as needed in subjects who complained of gastrointestinal side effects. Efforts were made to reach a daily dosage of at least 50 mg t.i.d. The primary outcome was development of frank fasting hyperglycemia (defined a priori as two consecutive quarterly FPG measurements 140 mg/dl or 7.8 mmol/l). Subjects visited the study clinic at 3-month intervals and had FPG measured by the YSI glucose analyzer, among other measurements. Subjects whose FPG was 140 mg/dl had intensified dietary counseling. If the next quarterly FPG was 140 mg/dl, the subject continued in the study. If the next FPG again was 140 mg/dl, the subject was considered to have reached the primary end point and was asked to complete a closeout visit; this visit was equivalent to what would have been the next annual visit. Annually, each subject completed a visit at which multiple measures were done (OGTT, history, and physical examination, seven-field fundus photographs, electrocardiogram, and laboratory tests for renal and hepatic function). At years 1 and 2, MPS studies and hyperglycemic clamps were repeated, as detailed below. Meal profile studies Subjects underwent MPS at the initiation visit (before beginning the study drug) and at years 1 and 2, when the study drug was taken with the MPS. For each subject, a standardized isocaloric meal plan composed of 55% carbohydrate, 30% fat, and 15% protein was provided, with onethird of calories at each of three meals. Each breakfast or lunch consisted of the same food items from subject to subject and from year to year. Test meals were consumed within 20 min starting at 8:00 A.M. and 12:00 P.M., with the final (nontest) meal consumed after the 5:00 P.M. sampling. Blood samples for plasma glucose and insulin were obtained at 7:30 A.M. and then hourly from 8:00 A.M. through 5:00 P.M. Fasting samples for proinsulin were obtained at 7:30 and 8:00 A.M. Hyperglycemic clamp Of the subjects at each site, 50% were randomly assigned to undergo hyperglycemic clamp testing at the initiation visit and at years 1 and 2. Studies were done in the fasting state with no study medication. At 10 and 0 min, samples were obtained for plasma glucose and insulin. A priming and then a maintenance rate of glucose, calculated by modification of the Andres method (17), was infused to rapidly bring the plasma glucose to 200 mg/dl and clamp it there for 4 h. To prevent hypokalemia, potassium phosphate was infused. Samples for plasma glucose and insulin were obtained every 2 min for the first 10 min of glucose infusion, then at 15 and 30 min, and then every 30 min for 4 h. Other measurements A1C was measured every 6 months by immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN). Total and HDL cholesterol and triglycerides were measured annually by the enzymatic end point assay (Roche Diagnostics). LDL cholesterol was calculated by the Friedewald calculation (18) if the value for triglycerides was 400 mg/dl. Insulin and proinsulin were measured by radioimmunoassay (Linco Research), with fasting values being the mean of 30 and 0 min samples. Early insulin secretion in the clamp was defined as the incremental area under the curve (AUC) for insulin over the first 10 min, with late-phase insulin secretion defined as incremental AUC for insulin for min. All AUC measurements were calculated using the trapezoid rule. The insulin sensitivity index was assessed from the clamp by calculating (glucose infusion rate over final 30 min) divided by (mean insulin level over final 30 min). Peak postprandial glucose was calculated as the highest plasma glucose measurement after each test meal in the MPS, and glucose incremental AUC was calculated for each meal and for the entire 9 h. Homeostasis model assessments of insulin resistance (HOMA-IR) and -cell function (HOMA- ) were calculated as described by Matthews et al. (19). All laboratory assays for both sites, other than the YSI plasma glucose measurements, were done at the central study laboratory at the Indiana University School of Medicine DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER 2006
3 Kirkman and Associates Table 1 Baseline characteristics Overall Acarbose Placebo n Sex Male 74 (33.8) 36 (33.0) 38 (34.6) Female 145 (66.2) 73 (67.0) 72 (65.4) Race White 171 (78.1) 84 (77.1) 87 (79.1) African American 41 (18.7) 21 (19.3) 20 (18.2) Asian 3 (1.4) 2 (1.8) 1 (0.9) Hispanic 4 (1.8) 2 (1.8) 2 (1.8) Family history of diabetes 153 (79.3) 74 (77.9) 79 (80.6) Women with history of gestational 17 (11.7) 14 (19.2) 3 (4.2) diabetes mellitus Age (years) Weight (kg) BMI (kg/m 2 ) Waist circumference (inches) % body fat by dual-energy X-ray absorptiometry FPG (mg/dl) h plasma glucose (dl) A1C (%) Diagnosis of hypertension 107 (49.8) 54 (50.5) 53 (49.1) Systolic blood pressure (mmhg) Diastolic blood pressure (mmhg) Lipids (mg/dl) Total cholesterol HDL cholesterol LDL cholesterol Triglycerides Retinopathy present 25 (12.7) 11 (11.7) 14 (13.6) Microalbuminuria present 6 (2.8) 3 (2.8) 3 (2.8) Sensory neuropathy present 33 (15.5) 15 (14.2) 18 (16.7) Data are n (%) or means SD. Sample size The conversion rate from postprandial diabetes to frank fasting hyperglycemia is unknown but was estimated to be from 4 to 14% per year. We assumed a 10% annual rate of reaching the primary end point in the placebo arm. A sample size of 100 per group would provide 80% power to detect a decrease from 34% at 4 years to 18% and a difference of 7 mg/dl in FPG. With allowance for a 10% drop-out rate, the target sample size was 220 subjects. Analyses The primary outcome, time to development of frank fasting hyperglycemia, was assessed using Kaplan-Meier productlimit estimation. Cumulative probability curves were compared using the log-rank test. Repeated-measures ANOVA and random-effects models were used to analyze other longitudinal outcomes, with assumptions of normally distributed errors and subject-specific random effects. Tukey s correction was used to adjust significance levels of multiple tests. Results are presented as means SD. RESULTS To screen for the study, 454 subjects underwent OGTT testing. Of these, 246 had early diabetes by OGTT criteria, met entry criteria, and provided informed consent. Twenty-seven subjects subsequently did not complete the baseline testing, which took place during an initiation admission up to 6 weeks after the OGTT. In total, 219 subjects (160 at Indiana University and 59 at Washington University) completed the initiation visit, received the study drug, returned for at least one follow-up visit, and are included in the intention-to-treat analysis. (More details on subject flow are available in an online appendix at journals.org.) After study drug titration, 91% of subjects receiving acarbose had achieved the full dose (97% of placebo subjects). Compliance was assessed by pill counts; mean pill consumption was 79.5% of prescribed dose in the acarbose group at year 1 and 84.6% in the placebo group, with little change in compliance over time (year 5 means 79.0 and 83.8%, respectively). Table 1 shows demographic and clinical characteristics of the study population. On average, subjects were obese with a high prevalence of hypertension and diabetic dyslipidemia. Race and ethnicity reflected the populations of Indianapolis and St. Louis in the late 1990s. Retinopathy (Early Treatment of Diabetic Retinopathy Study [ETDRS] score 20 on fundus photographs) was present in 12% of subjects, whereas 14% had evidence of peripheral neuropathy (inability to sense a 10-g monofilament on two or more sites on the foot). There were no significant differences in any baseline variable between subjects randomly assigned to acarbose and those randomly assigned to placebo. Over the course of the 5-year trial, a total of 95 subjects terminated follow-up prematurely. In the acarbose group (53 subjects), reasons for early termination included drug side effects (13), loss to follow-up (14), withdrawal of consent (11), prescription of other diabetes medications by outside physicians (5), and other (10). In the placebo group (42 subjects), reasons included drug side effects (5), loss to follow-up (10), withdrawal of consent (12), prescription of other diabetes medications (9), and other (6). As shown in Table 2, acarbose significantly reduced peak postprandial glucose after both meals at year 1 and at year 2 compared with baseline, whereas there was no significant reduction with placebo; the difference between groups was highly significant at both time points. In addition, compared with placebo, acarbose significantly reduced the AUC curve for glucose after the second meal at year 1 and year 2 and for the entire 9-h MPS at year 2. Acarbose lowered AIC slightly at year 1 and significantly at year 2, whereas there was no significant improvement with placebo. For the OGTT, there was improvement in both fasting and 2-h glucose over the first 2 3 years in both groups, with a subsequent increase in both variables back to baseline. The difference from baseline was significant for FPG at year 1 and at year 2 for acarbose, whereas the 2-h values were significantly reduced from baseline in both groups at DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER
4 Postprandial hyperglycemia in early diabetes Table 2 Postprandial glucose, glucose tolerance, measures of insulin resistance, and -cell function by treatment group and visit Visit Acarbose Placebo n Mean SD n Mean SD Peak postprandial glucose, first meal (mg/dl) Baseline Year * Year * Peak postprandial glucose, second meal (mg/dl) Baseline Year * Year * AUC plasma glucose, first meal (mg h 1 dl 1 ) Baseline Year Year AUC plasma glucose, second meal (mg h 1 dl 1 ) Baseline Year * Year * AUC plasma glucose, both meals (mg h 1 dl 1 ) Baseline Year Year A1C (%) Baseline Year Year Year Year Year FPG in OGTT (mg/dl) Baseline Year Year Year Year Year h PG in OGTT (mg/dl) Baseline Year Year Year Year Year HOMA-IR Baseline Year Year Year Year Year HOMA- Baseline Year Year Year Year Year Continued on following page 2098 DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER 2006
5 Kirkman and Associates Table 2 Continued Visit Acarbose Placebo n Mean SD n Mean SD Insulin sensitivity index Baseline Year Year Insulin AUC early phase ( U min 1 ml 1 ) Baseline Year Year Insulin AUC late phase ( U min 1 ml 1 ) Baseline 43 7,264 4, ,722 15,547 Year ,031 5, ,383 4,248 Year ,696 6, ,622 10,341 Insulin AUC during MPS ( U h 1 ml 1 ) Baseline Year Year Proinsulin-to-insulin ratio Baseline Year Year *P 0.01, P 0.05 for difference from placebo at time point. P 0.05, P 0.01 for difference from baseline. years 1 3. However, there were no differences between acarbose and placebo for either variable at any time point. Figure 1 shows the cumulative probability curves for the primary outcome, progression to frank fasting hyperglycemia (two consecutive quarterly FPG measures of at least 140 mg/dl). Subjects with at least two consecutive quarterly visits after the baseline visit (n 196) were included in the analysis. The outcome was reached by 28 of 96 acarbose subjects (29%) and by 34 of 100 placebo subjects (34%), with no difference between groups in the survival analysis (P 0.65). The results were no different when adjusted for body weight; there were no significant changes in mean weight in either Figure 1 Cumulative probability of reaching the end point of frank fasting hyperglycemia (two consecutive quarterly FPG measurements 140 mg/dl or 7.8 mmol/l). For each group, the first number (*) represents the number of subjects not at the end point at that time period, and the second number (**) represents the number of subjects at the end point at that time period. DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER
6 Postprandial hyperglycemia in early diabetes group. During the study, some subjects were treated for diabetes with other medications by outside physicians and therefore withdrawn from the study. Assuming that these subjects had more significant hyperglycemia, we did an analysis defining progression to frank fasting hyperglycemia as either reaching the primary end point or starting diabetes medication by another physician. There was no difference between groups in this analysis (33 of 96 acarbose subjects and 43 of 100 placebo subjects; P 0.41 for the difference in survival curves). Although the survival curves appear to diverge until month 42, there was no statistical difference between groups at any time point, whether frank fasting hyperglycemia was defined as initially planned or whether we included the subjects treated by their primary care physicians. In a post hoc analysis of subjects with FPG measurements 7.0 mmol/l (126 mg/dl) at randomization, significantly fewer acarbose subjects (13 of 48) than placebo subjects (31 of 62) reached a FPG of 126 mg/dl (P 0.04 for survival analysis). As shown in Table 2, there were no significant differences in measures of insulin resistance (HOMA-IR or insulin sensitivity index) over time or between groups. Despite the differences in postprandial hyperglycemia between treatment groups, at least over the first 2 years, there were no significant differences over time or between groups for other measures of -cell function: HOMA-, early- or late-phase insulin secretion during hyperglycemic clamps, proinsulin-to-insulin ratio, or AUC for insulin during the MPS. CONCLUSIONS We assessed whether ameliorating postprandial hyperglycemia in subjects with early diabetes might delay or prevent -cell failure, defined as progression to frank fasting hyperglycemia. We used acarbose, a drug known to directly reduce postprandial glucose without direct effects on -cell function, insulin resistance, or fasting glucose. During the course of our study, the Study to Prevent NIDDM (STOP- NIDDM) trial showed that acarbose delayed the progression of IGT to diabetes (20). Because IGT is characterized primarily by mild postchallenge hyperglycemia, these findings seemed to strengthen our hypothesis. As expected, acarbose significantly reduced postprandial hyperglycemia when compared with placebo, at least over the first 2 years of therapy. Despite this amelioration of postprandial hyperglycemia, we found no difference in the rate of progression to frank fasting hyperglycemia in acarbose-treated subjects compared with those treated with placebo. We also found no differences between treatment groups in other measures of -cell function, including HOMA-, early and late insulin secretion, proinsulin-to-insulin ratio, or AUC for insulin during the MPS. There are several possible explanations for our results and for the discrepancy between our results in early diabetes and those of STOP-NIDDM for IGT. One must always consider whether a negative study was underpowered. In fact, the rate of reaching the primary outcome in the placebo arm was lower than predicted (34% over 5 years instead of 41%), and the drop-out rate was higher than expected. The latter was probably due to the length and complexity of our trial; additionally, the changing definition of fasting hyperglycemia and treatment standards since 1997 led to more aggressive treatment of diabetes by some subjects primary care physicians. However, the 34% cumulative incidence rate observed in the placebo arm is a conservative estimate, because it assumes that no subject who terminated early would have reached the end point. In addition, the results are no different if we include subjects who began diabetes therapy outside of the protocol in the primary end point, and results of all secondary outcomes related to -cell function were similarly negative. It is possible that the dietary suggestions provided to both groups were potent and overwhelmed any effects of acarbose on -cell function. However, postprandial glucose was significantly lower in the acarbose group, and acarbose lowered A1C slightly but significantly over the first several years, suggesting that the drug itself had a superior effect on glycemia. In addition, the dietary intervention was no more potent than that provided to the placebo arms in studies such as the DPP and STOP-NIDDM. Another possible explanation is that our subjects, like those in the UKPDS and in clinical practice, were too far gone along the path of -cell failure for the intervention to affect progression. This would require us to believe that early diabetes is quite different from IGT, a condition in which controlling postprandial hyperglycemia seems to preserve -cell function or at least delay onset of diabetes (20). Our post hoc analysis of subjects who entered with FPG 126 mg/dl (presumably with even earlier diabetes) suggested that the rate of progression to FPG 126 mg/dl may have been reduced by acarbose. This would support the argument that once FPG exceeds 126 mg/dl, it may be too late to significantly affect -cell function. It is also possible that EDIP truly disproved our hypothesis and that postprandial hyperglycemia is not the primary driver of -cell failure. Other factors such as genetics, lifestyle factors, or postprandial free fatty acids (lipotoxicity) may be more important. Examining the determinants of progression to frank fasting hyperglycemia in our population may help answer these questions. The results of our study would seem to support those of a smaller trial in which acarbose or placebo was given for 6 weeks to subjects with IGT. Despite significant reductions in postprandial hyperglycemia with acarbose in that trial, there were no differences in insulin secretion rates by glucose ramp clamp nor in the acute insulin response to intravenous glucose by frequently sampled intravenous glucose tolerance tests (21). Our results lend further support to efforts to prevent diabetes. It is unlikely that diabetes could be easily diagnosed in stages earlier than that of our subjects, a group in whom treating postprandial hyperglycemia did not seem to delay -cell failure. Subjects at high risk for diabetes should be offered interventions known to delay the onset of early diabetes, such as acarbose (20), metformin (15), or intensive lifestyle interventions (15,22). Acknowledgments This work was supported by an investigator-initiated grant from Bayer with additional support from the National Institutes of Health (grants P60 DK20542, P60 DK20579, GCRC M01RR00750, and M01RR00036). Parts of this work were presented in abstract form at the 65th annual scientific sessions of the American Diabetes Association, San Diego, California, June The authors express their gratitude to the following individuals: the late Julio Santiago, MD; Mark Deeg, MD, PhD; Naomi Fineberg, PhD; Ed Fineberg, MD; Kieren Mather, MD; Clark Perry, DO; Cristina Lazar, MD; Jessica Cronin, RN; Kristen Crowder, RN; Ginger Hook, RN; Lora Risley, RN; Lyla Christner, LPN; Stacy Hurst, RN; Sara Kissel, RN; and the nursing staff of the General Clinical Research Centers at Indiana University and Barnes Hospitals DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER 2006
7 References 1. UK Prospective Diabetes Study Group: U.K. Prospective Diabetes Study 16: overview of 6 years therapy of type II diabetes: a progressive disease. Diabetes 44: , Polonsky KS, Sturis J, Bell G: Non-insulin-dependent diabetes mellitus: a genetically programmed failure of the cell to compensate for insulin resistance. N Engl J Med 334: , Kahn SE: The importance of -cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86: , Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104: , Porte DL, Kahn SE: -Cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes 50:S160 S163, Harris MI, Klein R, Welborn TA, Knuiman MW: Onset of NIDDM occurs at least 4 7 years before clinical diagnosis. Diabetes Care 15: , Kanauchi M, Nakajima M, Saito Y, Kanauchi K: Pancreatic -cell function and insulin sensitivity in Japanese subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes mellitus. Metabolism 52: , Harris M, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS: Comparison of diabetes diagnostic categories in the U.S. population according to the 1997 American Diabetes Association and World Health Organization diagnostic criteria. Diabetes Care 20: , Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20: , Weir GC, Laybutt DR, Kaneto H, Bonner- Weir S, Sharma A: -Cell adaptation and decompensation during progression of diabetes. Diabetes 50:S154 S159, Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H: Glucose toxicity in -cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52: , Ryan EA, Imes S, Wallace C: Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 27: , Park S, Choi SB: Induction of long-term normoglycemia without medication in Korean type 2 patients after continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev 19: , Precose package insert. West Haven, CT, Bayer, The Diabetes Prevention Program Research Group: The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22: , Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD: HbA 1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the Kirkman and Associates Early Diabetes Intervention Program (EDIP). Diabetes Care 24: , DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 273:E214 E223, Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: , Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and -cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: , Chaisson J-L, Josse RG, Gomis R, Hanefield M, Karasik A, Laakso M, the STOP- NIDDM Trial Research Group: Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM trial. Lancet , Chang AM, Smith MJ, Bloem CJ, Galecki AT, Halter JB: Effect of lowering postprandial hyperglycemia on older people with impaired glucose tolerance. Am J Physiol 287:E906 E911, Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, the Finnish Diabetes Prevention Study Group: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: , 2001 DIABETES CARE, VOLUME 29, NUMBER 9, SEPTEMBER
Diabetes: Staying Two Steps Ahead. The prevalence of diabetes is increasing. What causes Type 2 diabetes?
 Focus on CME at the University of University Manitoba of Manitoba : Staying Two Steps Ahead By Shagufta Khan, MD; and Liam J. Murphy, MD The prevalence of diabetes is increasing worldwide and will double
Focus on CME at the University of University Manitoba of Manitoba : Staying Two Steps Ahead By Shagufta Khan, MD; and Liam J. Murphy, MD The prevalence of diabetes is increasing worldwide and will double
Objectives. Objectives. Alejandro J. de la Torre, MD Cook Children s Hospital May 30, 2015
 Alejandro J. de la Torre, MD Cook Children s Hospital May 30, 2015 Presentation downloaded from http://ce.unthsc.edu Objectives Understand that the obesity epidemic is also affecting children and adolescents
Alejandro J. de la Torre, MD Cook Children s Hospital May 30, 2015 Presentation downloaded from http://ce.unthsc.edu Objectives Understand that the obesity epidemic is also affecting children and adolescents
In the past 6 years, several randomized controlled
 Perspectives in Diabetes (How) Can We Prevent Type 2 Diabetes? Thomas A. Buchanan In the past 6 years, several randomized controlled trials have been conducted to test the impact of behavioral and pharmacological
Perspectives in Diabetes (How) Can We Prevent Type 2 Diabetes? Thomas A. Buchanan In the past 6 years, several randomized controlled trials have been conducted to test the impact of behavioral and pharmacological
Discussion points. The cardiometabolic connection. Cardiometabolic Risk Management in the Primary Care Setting
 Session #5 Cardiometabolic Risk Management in the Primary Care Setting Sonja Reichert, MD MSc FCFP FACPM Betty Harvey, RNEC BScN MScN Amanda Mikalachki, RN BScN CDE S Discussion points Whom should we be
Session #5 Cardiometabolic Risk Management in the Primary Care Setting Sonja Reichert, MD MSc FCFP FACPM Betty Harvey, RNEC BScN MScN Amanda Mikalachki, RN BScN CDE S Discussion points Whom should we be
Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus. Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre
 Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre Outline How big is the problem? Natural progression of type 2 diabetes What
Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre Outline How big is the problem? Natural progression of type 2 diabetes What
The Efficacy and Cost of Alternative Strategies for Systematic Screening for Type 2 Diabetes in the U.S. Population Years of Age
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E The Efficacy and Cost of Alternative Strategies for Systematic Screening for Type 2 Diabetes in the U.S. Population 45 74
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E The Efficacy and Cost of Alternative Strategies for Systematic Screening for Type 2 Diabetes in the U.S. Population 45 74
Supplementary Online Content
 Supplementary Online Content Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia
Supplementary Online Content Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia
Diabetes Mellitus Type 2 Evidence-Based Drivers
 This module is supported by an unrestricted educational grant by Aventis Pharmaceuticals Education Center. Copyright 2003 1 Diabetes Mellitus Type 2 Evidence-Based Drivers Driver One: Reducing blood glucose
This module is supported by an unrestricted educational grant by Aventis Pharmaceuticals Education Center. Copyright 2003 1 Diabetes Mellitus Type 2 Evidence-Based Drivers Driver One: Reducing blood glucose
Energy Balance Equation
 Energy Balance Equation Intake Expenditure Hunger Satiety Nutrient Absorption Metabolic Rate Thermogenesis Activity Eat to Live! Live to Eat! EAT TO LIVE Intake = Expenditure Weight Stable LIVE TO EAT
Energy Balance Equation Intake Expenditure Hunger Satiety Nutrient Absorption Metabolic Rate Thermogenesis Activity Eat to Live! Live to Eat! EAT TO LIVE Intake = Expenditure Weight Stable LIVE TO EAT
Disclosures. Diabetes and Cardiovascular Risk Management. Learning Objectives. Atherosclerotic Cardiovascular Disease
 Disclosures Diabetes and Cardiovascular Risk Management Tony Hampton, MD, MBA Medical Director Advocate Aurora Operating System Advocate Aurora Healthcare Downers Grove, IL No conflicts or disclosures
Disclosures Diabetes and Cardiovascular Risk Management Tony Hampton, MD, MBA Medical Director Advocate Aurora Operating System Advocate Aurora Healthcare Downers Grove, IL No conflicts or disclosures
Obesity and Insulin Resistance According to Age in Newly Diagnosed Type 2 Diabetes Patients in Korea
 https://doi.org/10.7180/kmj.2016.31.2.157 KMJ Original Article Obesity and Insulin Resistance According to Age in Newly Diagnosed Type 2 Diabetes Patients in Korea Ju Won Lee, Nam Kyu Kim, Hyun Joon Park,
https://doi.org/10.7180/kmj.2016.31.2.157 KMJ Original Article Obesity and Insulin Resistance According to Age in Newly Diagnosed Type 2 Diabetes Patients in Korea Ju Won Lee, Nam Kyu Kim, Hyun Joon Park,
Diabetes: Definition Pathophysiology Treatment Goals. By Scott Magee, MD, FACE
 Diabetes: Definition Pathophysiology Treatment Goals By Scott Magee, MD, FACE Disclosures No disclosures to report Definition of Diabetes Mellitus Diabetes Mellitus comprises a group of disorders characterized
Diabetes: Definition Pathophysiology Treatment Goals By Scott Magee, MD, FACE Disclosures No disclosures to report Definition of Diabetes Mellitus Diabetes Mellitus comprises a group of disorders characterized
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine (HOE901) Insulin Glulisine (HMR1964)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Associations among Body Mass Index, Insulin Resistance, and Pancreatic ß-Cell Function in Korean Patients with New- Onset Type 2 Diabetes
 ORIGINAL ARTICLE korean j intern med 2012;27:66-71 pissn 1226-3303 eissn 2005-6648 Associations among Body Mass Index, Insulin Resistance, and Pancreatic ß-Cell Function in Korean Patients with New- Onset
ORIGINAL ARTICLE korean j intern med 2012;27:66-71 pissn 1226-3303 eissn 2005-6648 Associations among Body Mass Index, Insulin Resistance, and Pancreatic ß-Cell Function in Korean Patients with New- Onset
Insulin Secretion and Sensitivity during Oral Glucose Tolerance Test in Korean Lean Elderly Women
 J Korean Med Sci 2001; 16: 592-7 ISSN 1011-8934 Copyright The Korean Academy of Medical Sciences Insulin Secretion and Sensitivity during Oral Glucose Tolerance Test in Korean Lean Elderly Women Impaired
J Korean Med Sci 2001; 16: 592-7 ISSN 1011-8934 Copyright The Korean Academy of Medical Sciences Insulin Secretion and Sensitivity during Oral Glucose Tolerance Test in Korean Lean Elderly Women Impaired
Prevention of diabetes and its associated
 Metabolic Syndrome/Insulin Resistance Syndrome/Pre-Diabetes O R I G I N A L A R T I C L E Identifying Individuals at High Risk for Diabetes The Atherosclerosis Risk in Communities study MARIA INÊS SCHMIDT,
Metabolic Syndrome/Insulin Resistance Syndrome/Pre-Diabetes O R I G I N A L A R T I C L E Identifying Individuals at High Risk for Diabetes The Atherosclerosis Risk in Communities study MARIA INÊS SCHMIDT,
Why Do We Care About Prediabetes?
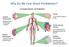 Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis
 CLINICAL RESEARCH STUDY Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis Gregory A. Nichols, PhD, Teresa A. Hillier, MD, MS, Jonathan B. Brown, PhD, MPP Center for Health Research, Kaiser
CLINICAL RESEARCH STUDY Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis Gregory A. Nichols, PhD, Teresa A. Hillier, MD, MS, Jonathan B. Brown, PhD, MPP Center for Health Research, Kaiser
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery
Design: EDIP was a randomized, placebo-controlled trial. Setting: Two university diabetes centers.
 ORIGINAL ARTICLE Changes in Weight and Glucose Can Protect Against Progression in Early Diabetes Independent of Improvements in -Cell Function Y. R. Patel, M. S. Kirkman, R. V. Considine, T. S. Hannon,
ORIGINAL ARTICLE Changes in Weight and Glucose Can Protect Against Progression in Early Diabetes Independent of Improvements in -Cell Function Y. R. Patel, M. S. Kirkman, R. V. Considine, T. S. Hannon,
Metabolic Syndrome. Shon Meek MD, PhD Mayo Clinic Florida Endocrinology
 Metabolic Syndrome Shon Meek MD, PhD Mayo Clinic Florida Endocrinology Disclosure No conflict of interest No financial disclosure Does This Patient Have Metabolic Syndrome? 1. Yes 2. No Does This Patient
Metabolic Syndrome Shon Meek MD, PhD Mayo Clinic Florida Endocrinology Disclosure No conflict of interest No financial disclosure Does This Patient Have Metabolic Syndrome? 1. Yes 2. No Does This Patient
The oral meal or oral glucose tolerance test. Original Article Two-Hour Seven-Sample Oral Glucose Tolerance Test and Meal Protocol
 Original Article Two-Hour Seven-Sample Oral Glucose Tolerance Test and Meal Protocol Minimal Model Assessment of -Cell Responsivity and Insulin Sensitivity in Nondiabetic Individuals Chiara Dalla Man,
Original Article Two-Hour Seven-Sample Oral Glucose Tolerance Test and Meal Protocol Minimal Model Assessment of -Cell Responsivity and Insulin Sensitivity in Nondiabetic Individuals Chiara Dalla Man,
Diabetes and the Heart
 Diabetes and the Heart Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 6, 2012 Outline Screening for diabetes in patients with CAD Screening for CAD in patients with
Diabetes and the Heart Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 6, 2012 Outline Screening for diabetes in patients with CAD Screening for CAD in patients with
Prediction of Homeostasis Model Assessment of Insulin Resistance in Japanese Subjects
 Tokai J Exp Clin Med., Vol. 37, No. 4, pp. 12-16, 212 Prediction of Homeostasis Model Assessment of Insulin Resistance in Japanese Subjects Masako NEGAMI, Eiko TAKAHASHI, Hiroki OTSUKA and Kengo MORIYAMA
Tokai J Exp Clin Med., Vol. 37, No. 4, pp. 12-16, 212 Prediction of Homeostasis Model Assessment of Insulin Resistance in Japanese Subjects Masako NEGAMI, Eiko TAKAHASHI, Hiroki OTSUKA and Kengo MORIYAMA
Modified version focused on CCNC Quality Measures and Feedback Processes
 Executive Summary: Standards of Medical Care in Diabetes 2010 Modified version focused on CCNC Quality Measures and Feedback Processes See http://care.diabetesjournals.org/content/33/supplement_1/s11.full
Executive Summary: Standards of Medical Care in Diabetes 2010 Modified version focused on CCNC Quality Measures and Feedback Processes See http://care.diabetesjournals.org/content/33/supplement_1/s11.full
The Metabolic Syndrome: Is It A Valid Concept? YES
 The Metabolic Syndrome: Is It A Valid Concept? YES Congress on Diabetes and Cardiometabolic Health Boston, MA April 23, 2013 Edward S Horton, MD Joslin Diabetes Center Harvard Medical School Boston, MA
The Metabolic Syndrome: Is It A Valid Concept? YES Congress on Diabetes and Cardiometabolic Health Boston, MA April 23, 2013 Edward S Horton, MD Joslin Diabetes Center Harvard Medical School Boston, MA
Strategies for the prevention of type 2 diabetes and cardiovascular disease
 European Heart Journal Supplements (2005) 7 (Supplement D), D18 D22 doi:10.1093/eurheartj/sui025 Strategies for the prevention of type 2 diabetes and cardiovascular disease Jaakko Tuomilehto 1,2,3 *, Jaana
European Heart Journal Supplements (2005) 7 (Supplement D), D18 D22 doi:10.1093/eurheartj/sui025 Strategies for the prevention of type 2 diabetes and cardiovascular disease Jaakko Tuomilehto 1,2,3 *, Jaana
Type 2 Diabetes Mellitus 2011
 2011 Michael T. McDermott MD Director, Endocrinology and Diabetes Practice University of Colorado Hospital Michael.mcdermott@ucdenver.edu Diabetes Mellitus Diagnosis 2011 Diabetes Mellitus Fasting Glucose
2011 Michael T. McDermott MD Director, Endocrinology and Diabetes Practice University of Colorado Hospital Michael.mcdermott@ucdenver.edu Diabetes Mellitus Diagnosis 2011 Diabetes Mellitus Fasting Glucose
Dr Aftab Ahmad Consultant Diabetologist at Royal Liverpool University Hospital Regional Diabetes Network Lead
 Dr Aftab Ahmad Consultant Diabetologist at Royal Liverpool University Hospital Regional Diabetes Network Lead Today s Presentation HbA1c & diagnosing Diabetes What is Impaired Glucose & IGR? Implications
Dr Aftab Ahmad Consultant Diabetologist at Royal Liverpool University Hospital Regional Diabetes Network Lead Today s Presentation HbA1c & diagnosing Diabetes What is Impaired Glucose & IGR? Implications
American Academy of Insurance Medicine
 American Academy of Insurance Medicine October 2012 Dr. Alison Moy Liberty Mutual Dr. John Kirkpatrick Thrivent Financial for Lutherans 1 59 year old male, diagnosed with T2DM six months ago Nonsmoker
American Academy of Insurance Medicine October 2012 Dr. Alison Moy Liberty Mutual Dr. John Kirkpatrick Thrivent Financial for Lutherans 1 59 year old male, diagnosed with T2DM six months ago Nonsmoker
SYNOPSIS OF RESEARCH REPORT (PROTOCOL BC20779)
 TITLE OF THE STUDY / REPORT No. / DATE OF REPORT INVESTIGATORS / CENTERS AND COUNTRIES Clinical Study Report Protocol BC20779: Multicenter, double-blind, randomized, placebo-controlled, dose ranging phase
TITLE OF THE STUDY / REPORT No. / DATE OF REPORT INVESTIGATORS / CENTERS AND COUNTRIES Clinical Study Report Protocol BC20779: Multicenter, double-blind, randomized, placebo-controlled, dose ranging phase
IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS
 IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS Dr Bidhu Mohapatra, MBBS, MD, FRACP Consultant Physician Endocrinology and General Medicine Introduction 382 million people affected by diabetes
IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS Dr Bidhu Mohapatra, MBBS, MD, FRACP Consultant Physician Endocrinology and General Medicine Introduction 382 million people affected by diabetes
Induction of Long-term Glycemic Control in Newly Diagnosed Type 2 Diabetic Patients Is Associated With Improvement of -Cell Function
 Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Induction of Long-term Glycemic Control in Newly Diagnosed Type 2 Diabetic Patients Is Associated With Improvement of -Cell Function YANBING
Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Induction of Long-term Glycemic Control in Newly Diagnosed Type 2 Diabetic Patients Is Associated With Improvement of -Cell Function YANBING
23-Aug-2011 Lixisenatide (AVE0010) - EFC6014 Version number: 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top of metformin
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top of metformin
Eugene Barrett M.D., Ph.D. University of Virginia 6/18/2007. Diagnosis and what is it Glucose Tolerance Categories FPG
 Diabetes Mellitus: Update 7 What is the unifying basis of this vascular disease? Eugene J. Barrett, MD, PhD Professor of Internal Medicine and Pediatrics Director, Diabetes Center and GCRC Health System
Diabetes Mellitus: Update 7 What is the unifying basis of this vascular disease? Eugene J. Barrett, MD, PhD Professor of Internal Medicine and Pediatrics Director, Diabetes Center and GCRC Health System
Clinical Practice Guideline Key Points
 Clinical Practice Guideline Key Points Clinical Practice Guideline 2008 Key Points Diabetes Mellitus Provided by: Highmark Endocrinology Clinical Quality Improvement Committee In accordance with Highmark
Clinical Practice Guideline Key Points Clinical Practice Guideline 2008 Key Points Diabetes Mellitus Provided by: Highmark Endocrinology Clinical Quality Improvement Committee In accordance with Highmark
Clinical Recommendations: Patients with Periodontitis
 The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. Friedewald VE, Kornman KS, Beck JD, et al. J Periodontol 2009;
The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. Friedewald VE, Kornman KS, Beck JD, et al. J Periodontol 2009;
Implementing Type 2 Diabetes Prevention Programmes
 Implementing Type 2 Diabetes Prevention Programmes Jaakko Tuomilehto Department of Public Health University of Helsinki Helsinki, Finland FIN-D2D Survey 2004 Prevalence of previously diagnosed and screen-detected
Implementing Type 2 Diabetes Prevention Programmes Jaakko Tuomilehto Department of Public Health University of Helsinki Helsinki, Finland FIN-D2D Survey 2004 Prevalence of previously diagnosed and screen-detected
Helpful Hints for Taking Care of Your Diabetes. Farahnaz Joarder, MD and Don Kain, MA, RD,CDE Harold Schnitzer Diabetes Health Center
 Helpful Hints for Taking Care of Your Diabetes Farahnaz Joarder, MD and Don Kain, MA, RD,CDE Harold Schnitzer Diabetes Health Center Objectives How big of a problem is diabetes? What is diabetes? How is
Helpful Hints for Taking Care of Your Diabetes Farahnaz Joarder, MD and Don Kain, MA, RD,CDE Harold Schnitzer Diabetes Health Center Objectives How big of a problem is diabetes? What is diabetes? How is
Challenges in type 2 diabetes control: slipping control and weight gain
 control Earn 3 CPD Points online Case study Challenges in type 2 diabetes control: slipping control and weight gain Presenter Dr Sedeshan Govender Specialist Physician, Endocrinologist and Diabetologists
control Earn 3 CPD Points online Case study Challenges in type 2 diabetes control: slipping control and weight gain Presenter Dr Sedeshan Govender Specialist Physician, Endocrinologist and Diabetologists
Diabetes Care 24: , 2001
 Pathophysiology/Complications O R I G I N A L A R T I C L E Prevalence and Significance of Retinopathy in Subjects With Type 1 Diabetes of Less Than 5 Years Duration Screened for the Diabetes Control and
Pathophysiology/Complications O R I G I N A L A R T I C L E Prevalence and Significance of Retinopathy in Subjects With Type 1 Diabetes of Less Than 5 Years Duration Screened for the Diabetes Control and
A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Hypotheses: Among individuals with type 2 diabetes, the risks of major microvascular
A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Hypotheses: Among individuals with type 2 diabetes, the risks of major microvascular
Diabetes Overview. How Food is Digested
 Diabetes Overview You are The Teacher, The Coach and the Fan Pathophysiology of Diabetes Complications Know the Numbers Treatment Can Good Control Make a Difference? Can Tight Control Be too Tight? How
Diabetes Overview You are The Teacher, The Coach and the Fan Pathophysiology of Diabetes Complications Know the Numbers Treatment Can Good Control Make a Difference? Can Tight Control Be too Tight? How
Objectives 10/11/2013. Diabetes- The Real Cost of Sugar. Diabetes 101: What is Diabetes. By Ruth Nekonchuk RD CDE LMNT
 Diabetes- The Real Cost of Sugar By Ruth Nekonchuk RD CDE LMNT Objectives To explain diabetes To explain the risks of diabetes To enumerate the cost of diabetes to our country To enumerate the cost of
Diabetes- The Real Cost of Sugar By Ruth Nekonchuk RD CDE LMNT Objectives To explain diabetes To explain the risks of diabetes To enumerate the cost of diabetes to our country To enumerate the cost of
Ischemic Heart and Cerebrovascular Disease. Harold E. Lebovitz, MD, FACE Kathmandu November 2010
 Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
SYNOPSIS. Administration: subcutaneous injection Batch number(s):
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top
Metformin should be considered in all patients with type 2 diabetes unless contra-indicated
 November 2001 N P S National Prescribing Service Limited PPR fifteen Prescribing Practice Review PPR Managing type 2 diabetes For General Practice Key messages Metformin should be considered in all patients
November 2001 N P S National Prescribing Service Limited PPR fifteen Prescribing Practice Review PPR Managing type 2 diabetes For General Practice Key messages Metformin should be considered in all patients
Keywords: Type 2 DM, lipid profile, metformin, glimepiride ABSTRACT
 Human Journals Research Article September 2015 Vol.:4, Issue:2 All rights are reserved by K. Saravanan et al. Effects of Monotherapy and Combination Therapy Involving Metformin and Glimepiride on HbA1c
Human Journals Research Article September 2015 Vol.:4, Issue:2 All rights are reserved by K. Saravanan et al. Effects of Monotherapy and Combination Therapy Involving Metformin and Glimepiride on HbA1c
Pathogenesis of Type 2 Diabetes
 9/23/215 Multiple, Complex Pathophysiological Abnmalities in T2DM incretin effect gut carbohydrate delivery & absption pancreatic insulin secretion pancreatic glucagon secretion HYPERGLYCEMIA? Pathogenesis
9/23/215 Multiple, Complex Pathophysiological Abnmalities in T2DM incretin effect gut carbohydrate delivery & absption pancreatic insulin secretion pancreatic glucagon secretion HYPERGLYCEMIA? Pathogenesis
associated with serious complications, but reduce occurrences with preventive measures
 Wk 9. Management of Clients with Diabetes Mellitus 1. Diabetes Mellitus body s inability to metabolize carbohydrates, fats, proteins hyperglycemia associated with serious complications, but reduce occurrences
Wk 9. Management of Clients with Diabetes Mellitus 1. Diabetes Mellitus body s inability to metabolize carbohydrates, fats, proteins hyperglycemia associated with serious complications, but reduce occurrences
Diabetes mellitus. Treatment
 Diabetes mellitus Treatment Recommended glycemic targets for the clinical management of diabetes(ada) Fasting glycemia: 80-110 mg/dl Postprandial : 100-145 mg/dl HbA1c: < 6,5 % Total cholesterol: < 200
Diabetes mellitus Treatment Recommended glycemic targets for the clinical management of diabetes(ada) Fasting glycemia: 80-110 mg/dl Postprandial : 100-145 mg/dl HbA1c: < 6,5 % Total cholesterol: < 200
Non-insulin treatment in Type 1 DM Sang Yong Kim
 Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Copyright 2017 by Sea Courses Inc.
 Pre-Diabetes: Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any means graphic, electronic, or mechanical,
Pre-Diabetes: Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any means graphic, electronic, or mechanical,
Alternative insulin delivery systems: how demanding should the patient be?
 Diabetologia (1997) 4: S97 S11 Springer-Verlag 1997 Alternative insulin delivery systems: how demanding should the patient be? K.S. Polonsky, M. M. Byrne, J. Sturis Department of Medicine, The University
Diabetologia (1997) 4: S97 S11 Springer-Verlag 1997 Alternative insulin delivery systems: how demanding should the patient be? K.S. Polonsky, M. M. Byrne, J. Sturis Department of Medicine, The University
Presenter Disclosure Information
 Prediabetes & Type 2 Diabetes Prevention Cari Ritter, PA-C Presenter Disclosure Information In compliance with the accrediting board policies, the American Diabetes Association requires the following disclosure
Prediabetes & Type 2 Diabetes Prevention Cari Ritter, PA-C Presenter Disclosure Information In compliance with the accrediting board policies, the American Diabetes Association requires the following disclosure
Diabetes and Hypertension
 Diabetes and Hypertension M.Nakhjvani,M.D Tehran University of Medical Sciences 20-8-96 Hypertension Common DM comorbidity Prevalence depends on diabetes type, age, BMI, ethnicity Major risk factor for
Diabetes and Hypertension M.Nakhjvani,M.D Tehran University of Medical Sciences 20-8-96 Hypertension Common DM comorbidity Prevalence depends on diabetes type, age, BMI, ethnicity Major risk factor for
American Diabetes Association: Standards of Medical Care in Diabetes 2015
 American Diabetes Association: Standards of Medical Care in Diabetes 2015 Synopsis of ADA standards relevant to the 11 th Scope of Work under Task B.2 ASSESSMENT OF GLYCEMIC CONTROL Recommendations: Perform
American Diabetes Association: Standards of Medical Care in Diabetes 2015 Synopsis of ADA standards relevant to the 11 th Scope of Work under Task B.2 ASSESSMENT OF GLYCEMIC CONTROL Recommendations: Perform
Pre-diabetes. Pharmacological Approaches to Delay Progression to Diabetes
 Pre-diabetes Pharmacological Approaches to Delay Progression to Diabetes Overview Definition of Pre-diabetes Risk Factors for Pre-diabetes Clinical practice guidelines for diabetes Management, including
Pre-diabetes Pharmacological Approaches to Delay Progression to Diabetes Overview Definition of Pre-diabetes Risk Factors for Pre-diabetes Clinical practice guidelines for diabetes Management, including
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Pramlintide & Weight. Diane M Karl MD. The Endocrine Clinic & Oregon Health & Science University Portland, Oregon
 Pramlintide & Weight Diane M Karl MD The Endocrine Clinic & Oregon Health & Science University Portland, Oregon Conflict of Interest Speakers Bureau: Amylin Pharmaceuticals Consultant: sanofi-aventis Grant
Pramlintide & Weight Diane M Karl MD The Endocrine Clinic & Oregon Health & Science University Portland, Oregon Conflict of Interest Speakers Bureau: Amylin Pharmaceuticals Consultant: sanofi-aventis Grant
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
DIABETES MEASURES GROUP OVERVIEW
 2014 PQRS OPTIONS F MEASURES GROUPS: DIABETES MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN DIABETES MEASURES GROUP: #1. Diabetes: Hemoglobin A1c Poor Control #2. Diabetes: Low Density Lipoprotein (LDL-C)
2014 PQRS OPTIONS F MEASURES GROUPS: DIABETES MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN DIABETES MEASURES GROUP: #1. Diabetes: Hemoglobin A1c Poor Control #2. Diabetes: Low Density Lipoprotein (LDL-C)
TYPE 2 DIABETES MELLITUS (T2DM) is a heterogeneous
 0021-972X/04/$15.00/0 The Journal of Clinical Endocrinology & Metabolism 89(6):2846 2851 Printed in U.S.A. Copyright 2004 by The Endocrine Society doi: 10.1210/jc.2003-032044 Pharmacological Treatment
0021-972X/04/$15.00/0 The Journal of Clinical Endocrinology & Metabolism 89(6):2846 2851 Printed in U.S.A. Copyright 2004 by The Endocrine Society doi: 10.1210/jc.2003-032044 Pharmacological Treatment
Diabetes. Health Care Disparities: Medical Evidence. A Constellation of Complications. Every 24 hours.
 Health Care Disparities: Medical Evidence Diabetes Effects 2.8 Million People in US 7% of the US Population Sixth Leading Cause of Death Kenneth J. Steier, DO, MBA, MPH, MHA, MGH Dean of Clinical Education
Health Care Disparities: Medical Evidence Diabetes Effects 2.8 Million People in US 7% of the US Population Sixth Leading Cause of Death Kenneth J. Steier, DO, MBA, MPH, MHA, MGH Dean of Clinical Education
Cardiovascular Complications of Diabetes
 VBWG Cardiovascular Complications of Diabetes Nicola Abate, M.D., F.N.L.A. Professor and Chief Division of Endocrinology and Metabolism The University of Texas Medical Branch Galveston, Texas Coronary
VBWG Cardiovascular Complications of Diabetes Nicola Abate, M.D., F.N.L.A. Professor and Chief Division of Endocrinology and Metabolism The University of Texas Medical Branch Galveston, Texas Coronary
Chronic Benefit Application Form Cardiovascular Disease and Diabetes
 Chronic Benefit Application Form Cardiovascular Disease and Diabetes 19 West Street, Houghton, South Africa, 2198 Postnet Suite 411, Private Bag X1, Melrose Arch, 2076 Tel: +27 (11) 715 3000 Fax: +27 (11)
Chronic Benefit Application Form Cardiovascular Disease and Diabetes 19 West Street, Houghton, South Africa, 2198 Postnet Suite 411, Private Bag X1, Melrose Arch, 2076 Tel: +27 (11) 715 3000 Fax: +27 (11)
Treating Type 2 Diabetes by Treating Obesity. Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition
 Treating Type 2 Diabetes by Treating Obesity Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition 2 Center Stage Obesity is currently an epidemic in the United States, with
Treating Type 2 Diabetes by Treating Obesity Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition 2 Center Stage Obesity is currently an epidemic in the United States, with
Managing Diabetes for Improved Health and Economic Outcomes
 Managing Diabetes for Improved Health and Economic Outcomes Based on a presentation by David McCulloch, MD Presentation Summary The contribution of postprandial glucose to diabetes progression and diabetes-related
Managing Diabetes for Improved Health and Economic Outcomes Based on a presentation by David McCulloch, MD Presentation Summary The contribution of postprandial glucose to diabetes progression and diabetes-related
Know Your Number Aggregate Report Single Analysis Compared to National Averages
 Know Your Number Aggregate Report Single Analysis Compared to National s Client: Study Population: 2242 Population: 3,000 Date Range: 04/20/07-08/08/07 Version of Report: V6.2 Page 2 Study Population Demographics
Know Your Number Aggregate Report Single Analysis Compared to National s Client: Study Population: 2242 Population: 3,000 Date Range: 04/20/07-08/08/07 Version of Report: V6.2 Page 2 Study Population Demographics
Serum uric acid levels improve prediction of incident Type 2 Diabetes in individuals with impaired fasting glucose: The Rancho Bernardo Study
 Diabetes Care Publish Ahead of Print, published online June 9, 2009 Serum uric acid and incident DM2 Serum uric acid levels improve prediction of incident Type 2 Diabetes in individuals with impaired fasting
Diabetes Care Publish Ahead of Print, published online June 9, 2009 Serum uric acid and incident DM2 Serum uric acid levels improve prediction of incident Type 2 Diabetes in individuals with impaired fasting
Standards of Medical Care in Diabetes 2016
 Standards of Medical Care in Diabetes 2016 Care Delivery Systems 33-49% of patients still do not meet targets for A1C, blood pressure, or lipids. 14% meet targets for all A1C, BP, lipids, and nonsmoking
Standards of Medical Care in Diabetes 2016 Care Delivery Systems 33-49% of patients still do not meet targets for A1C, blood pressure, or lipids. 14% meet targets for all A1C, BP, lipids, and nonsmoking
Janice Lazear, DNP, FNP-C, CDE DIAGNOSIS AND CLASSIFICATION OF DIABETES
 Janice Lazear, DNP, FNP-C, CDE DIAGNOSIS AND CLASSIFICATION OF DIABETES Objectives u At conclusion of the lecture the participant will be able to: 1. Differentiate between the classifications of diabetes
Janice Lazear, DNP, FNP-C, CDE DIAGNOSIS AND CLASSIFICATION OF DIABETES Objectives u At conclusion of the lecture the participant will be able to: 1. Differentiate between the classifications of diabetes
Prevention of diabetes in the modern era of affluent society and economic constraints
 Prevention of diabetes in the modern era of affluent society and economic constraints KONSTANTINOS MAKRILAKIS, MD, MPH, PhD ASSOCIATE PROFESSOR IN INTERNAL MEDICINE NATIONAL AND KAPODISTRIAN UNIVERSITY
Prevention of diabetes in the modern era of affluent society and economic constraints KONSTANTINOS MAKRILAKIS, MD, MPH, PhD ASSOCIATE PROFESSOR IN INTERNAL MEDICINE NATIONAL AND KAPODISTRIAN UNIVERSITY
Diabetes Mellitus (DM) - Dr Hiren Patt
 Diabetes Mellitus (DM) - Dr Hiren Patt What is DM? FPG 2-Hour PG on OGTT Diabetes Mellitus Diabetes Mellitus 126 mg/dl 100 mg/dl Impaired Fasting Glucose 200 mg/dl 140 mg/dl Impaired Glucose Tolerance
Diabetes Mellitus (DM) - Dr Hiren Patt What is DM? FPG 2-Hour PG on OGTT Diabetes Mellitus Diabetes Mellitus 126 mg/dl 100 mg/dl Impaired Fasting Glucose 200 mg/dl 140 mg/dl Impaired Glucose Tolerance
Early diagnosis, early treatment and the new diagnostic criteria of diabetes mellitus
 British Journal of Nutrition (2000), 84, Suppl. 2, S177±S181 S177 Early diagnosis, early treatment and the new diagnostic criteria of diabetes mellitus Takeshi Kuzuya* JA Shioya General Hospital, Tomita
British Journal of Nutrition (2000), 84, Suppl. 2, S177±S181 S177 Early diagnosis, early treatment and the new diagnostic criteria of diabetes mellitus Takeshi Kuzuya* JA Shioya General Hospital, Tomita
Type 2 Diabetes in Adolescents
 Type 2 Diabetes in Adolescents Disclosures Paid consultant, Eli Lilly, Inc, Pediatric Type 2 Diabetes Clinical Trials Outline The burden of diabetes Treatment and Prevention Youth Diabetes Prevention Clinic
Type 2 Diabetes in Adolescents Disclosures Paid consultant, Eli Lilly, Inc, Pediatric Type 2 Diabetes Clinical Trials Outline The burden of diabetes Treatment and Prevention Youth Diabetes Prevention Clinic
The American Diabetes Association estimates
 DYSLIPIDEMIA, PREDIABETES, AND TYPE 2 DIABETES: CLINICAL IMPLICATIONS OF THE VA-HIT SUBANALYSIS Frank M. Sacks, MD* ABSTRACT The most serious and common complication in adults with diabetes is cardiovascular
DYSLIPIDEMIA, PREDIABETES, AND TYPE 2 DIABETES: CLINICAL IMPLICATIONS OF THE VA-HIT SUBANALYSIS Frank M. Sacks, MD* ABSTRACT The most serious and common complication in adults with diabetes is cardiovascular
Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes
 Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes Genetics, environment, and lifestyle (obesity, inactivity, poor diet) Impaired fasting glucose Decreased β-cell
Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes Genetics, environment, and lifestyle (obesity, inactivity, poor diet) Impaired fasting glucose Decreased β-cell
Risks and benefits of weight loss: challenges to obesity research
 European Heart Journal Supplements (2005) 7 (Supplement L), L27 L31 doi:10.1093/eurheartj/sui083 Risks and benefits of weight loss: challenges to obesity research Donna Ryan* Pennington Biomedical Research
European Heart Journal Supplements (2005) 7 (Supplement L), L27 L31 doi:10.1093/eurheartj/sui083 Risks and benefits of weight loss: challenges to obesity research Donna Ryan* Pennington Biomedical Research
Established Risk Factors for Coronary Heart Disease (CHD)
 Getting Patients to Make Small Lifestyle Changes That Result in SIGNIFICANT Improvements in Health - Prevention of Diabetes and Obesity for Better Health Maureen E. Mays, MD, MS, FACC Director ~ Portland
Getting Patients to Make Small Lifestyle Changes That Result in SIGNIFICANT Improvements in Health - Prevention of Diabetes and Obesity for Better Health Maureen E. Mays, MD, MS, FACC Director ~ Portland
Executive Summary: Standards of Medical Care in Diabetes 2010
 E X E C U T I V E S U M M A R Y Executive Summary: Standards of Medical Care in Diabetes 2010 Current criteria for the diagnosis of diabetes A1C 6.5%: The test should be performed in a laboratory using
E X E C U T I V E S U M M A R Y Executive Summary: Standards of Medical Care in Diabetes 2010 Current criteria for the diagnosis of diabetes A1C 6.5%: The test should be performed in a laboratory using
Francesca Porcellati
 XX Congresso Nazionale AMD Razionali e Benefici dell Aggiunta del GLP-1 RA Short-Acting all Insulina Basale Francesca Porcellati Dipartimento di Medicina Interna, Sezione di Medicina Interna, Endocrinologia
XX Congresso Nazionale AMD Razionali e Benefici dell Aggiunta del GLP-1 RA Short-Acting all Insulina Basale Francesca Porcellati Dipartimento di Medicina Interna, Sezione di Medicina Interna, Endocrinologia
Adult Diabetes Clinician Guide NOVEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Adult Diabetes Clinician Guide Introduction NOVEMBER 2017 This evidence-based guideline summary is based on the 2017 KP National Diabetes Guideline.
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Adult Diabetes Clinician Guide Introduction NOVEMBER 2017 This evidence-based guideline summary is based on the 2017 KP National Diabetes Guideline.
Chapter 37: Exercise Prescription in Patients with Diabetes
 Chapter 37: Exercise Prescription in Patients with Diabetes American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Chapter 37: Exercise Prescription in Patients with Diabetes American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Hormonal Regulations Of Glucose Metabolism & DM
 Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
PREVALENCE OF METABOLİC SYNDROME İN CHİLDREN AND ADOLESCENTS
 PREVALENCE OF METABOLİC SYNDROME İN CHİLDREN AND ADOLESCENTS Mehmet Emre Atabek,MD,PhD Necmettin Erbakan University Faculty of Medicine, Department of Pediatrics, Division of Pediatric Endocrinology and
PREVALENCE OF METABOLİC SYNDROME İN CHİLDREN AND ADOLESCENTS Mehmet Emre Atabek,MD,PhD Necmettin Erbakan University Faculty of Medicine, Department of Pediatrics, Division of Pediatric Endocrinology and
RISK FACTORS OR COMPLICATIONS AND RECOMMENDED TREATMENT GOALS AND FREQUENCY OF EVALUATION FOR ADULTS WITH DIABETES
 RISK FACTORS OR COMPLICATIONS AND RECOMMENDED TREATMENT GOALS AND FREQUENCY OF EVALUATION FOR ADULTS WITH DIABETES Risk Factors or Complications Glycemic Control Fasting & Capillary Plasma Glucose Anti-platelet
RISK FACTORS OR COMPLICATIONS AND RECOMMENDED TREATMENT GOALS AND FREQUENCY OF EVALUATION FOR ADULTS WITH DIABETES Risk Factors or Complications Glycemic Control Fasting & Capillary Plasma Glucose Anti-platelet
Rehabilitation and Research Training Center on Secondary Conditions in Individuals with SCI. James S. Krause, PhD
 Disclosure The contents of this presentation were developed with support from educational grants from the Department of Education, NIDRR grant numbers H133B090005, H133B970011 and H133G010160. However,
Disclosure The contents of this presentation were developed with support from educational grants from the Department of Education, NIDRR grant numbers H133B090005, H133B970011 and H133G010160. However,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
American Diabetes Association 2018 Guidelines Important Notable Points
 American Diabetes Association 2018 Guidelines Important Notable Points The Standards of Medical Care in Diabetes-2018 by ADA include the most current evidencebased recommendations for diagnosing and treating
American Diabetes Association 2018 Guidelines Important Notable Points The Standards of Medical Care in Diabetes-2018 by ADA include the most current evidencebased recommendations for diagnosing and treating
The Finnish Diabetes Prevention Study
 British Journal of Nutrition (2000), 83, Suppl. 1, S137 S142 S137 The Finnish Diabetes Prevention Study Matti Uusitupa 1 *, Anne Louheranta 2, Jaana Lindström 2, Timo Valle 2, Jouko Sundvall 2, Johan Eriksson
British Journal of Nutrition (2000), 83, Suppl. 1, S137 S142 S137 The Finnish Diabetes Prevention Study Matti Uusitupa 1 *, Anne Louheranta 2, Jaana Lindström 2, Timo Valle 2, Jouko Sundvall 2, Johan Eriksson
The National Diabetes Prevention Program in Washington State March 2012
 The National Diabetes Prevention Program in Washington State March 2012 Session Objectives 1. Overview of pre-diabetes. 2. Describe the Diabetes Prevention Program (DPP). 3. Eligibility for the DPP. 4.
The National Diabetes Prevention Program in Washington State March 2012 Session Objectives 1. Overview of pre-diabetes. 2. Describe the Diabetes Prevention Program (DPP). 3. Eligibility for the DPP. 4.
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response
Cedars Sinai Diabetes. Michael A. Weber
 Cedars Sinai Diabetes Michael A. Weber Speaker Disclosures I disclose that I am a Consultant for: Ablative Solutions, Boston Scientific, Boehringer Ingelheim, Eli Lilly, Forest, Medtronics, Novartis, ReCor
Cedars Sinai Diabetes Michael A. Weber Speaker Disclosures I disclose that I am a Consultant for: Ablative Solutions, Boston Scientific, Boehringer Ingelheim, Eli Lilly, Forest, Medtronics, Novartis, ReCor
Application of the Diabetes Algorithm to a Patient
 Application of the Diabetes Algorithm to a Patient Apply knowledge gained from this activity to improve disease management and outcomes for patients with T2DM and obesity Note: The cases in this deck represent
Application of the Diabetes Algorithm to a Patient Apply knowledge gained from this activity to improve disease management and outcomes for patients with T2DM and obesity Note: The cases in this deck represent
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy
 Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
ClinialTrials.gov Identifier: HOE901_4020 Insulin Glargine Date: Study Code: This was a multicenter study that was conducted at 59 US sites
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
