Diabetic gastroparesis (DGP), defined as symptomatic delayed
|
|
|
- Katherine McLaughlin
- 6 years ago
- Views:
Transcription
1 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2010;8: Gastric Electrical Stimulation With Enterra Therapy Improves Symptoms From Diabetic Gastroparesis in a Prospective Study RICHARD W. MCCALLUM,* WILLIAM SNAPE, FREDRICK BRODY, JOHN WO, HENRY P. PARKMAN, and THOMAS NOWAK # *University of Kansas Medical Center, Kansas City, Kansas; California Pacific Medical Center, San Francisco, California; George Washington University Medical Center, Washington, District of Columbia; University of Louisville, Louisville, Kentucky; Temple University Hospital, Philadelphia, Pennsylvania; and # Saint John s Research Institute, Anderson, Indiana Podcast interview: see Editorial on page 908. BACKGROUND & AIMS: Gastric electrical stimulation (GES) treats refractory gastroparesis by delivering electric current, via electrodes, to gastric smooth muscle. Enterra therapy (Medtronic, Inc, Minneapolis, MN) uses an implantable neurostimulator with a high-frequency, low-energy output. We performed a controlled, multicenter, prospective study to evaluate the safety and efficacy of Enterra therapy in patients with chronic intractable nausea and vomiting from diabetic gastroparesis (DGP). METHODS: Patients with refractory DGP (n 55; mean age, 38 y; 66% female, 5.9 years of DGP) were given implants of the Enterra gastric stimulation system. After surgery, all patients had the stimulator turned on for 6 weeks and then they randomly were assigned to groups that had consecutive 3-month, cross-over periods with the device on or off. After this period, the device was turned on in all patients and they were followed up, unblinded, for 4.5 months. RE- SULTS: The median reduction in weekly vomiting frequency (WVF) at 6 weeks, compared with baseline, was 57% (P.001). There was no difference in WVF between patients who had the device turned on or off during the cross-over period (median reduction, 0%; P.215). At 1 year, the WVF of all patients was significantly lower than baseline values (median reduction, 67.8%; P.001). Patients also had significant improvements in total symptom score, gastric emptying, quality of life, and median days in the hospital. CONCLUSIONS: In patients with intractable DGP, 6 weeks of GES therapy with Enterra significantly reduced vomiting and gastroparetic symptoms. Patients had improvements in subjective and objective parameters with chronic stimulation after 12 months of GES, compared with baseline. Keywords: Enterra Therapy; Gastric Electrical Stimulation; Gastroparesis. Diabetic gastroparesis (DGP), defined as symptomatic delayed gastric emptying (GE) in the absence of mechanical obstruction, 1-4 affects 20% to 50% of the diabetic population. A majority of these individuals have long-standing, poorly controlled diabetes. 5,6 The most common symptoms of DGP are nausea and vomiting accompanied by early satiety, postprandial fullness, abdominal pain, and weight loss. 2 The standard treatment of symptomatic DGP consists of glycemic control (glucose levels, 180 mg/dl), dietary manipulation, and medications. Medications include antiemetic therapy combined with prokinetic agents, such as metoclopramide, erythromycin, and domperidone. However, only metoclopramide and erythromycin are commercially available in the United States and both have side effects that make them intolerable for more than 40% of patients. 7 If those nonsurgical therapies fail, palliative endoscopic or surgical therapies are required, including placement of a jejunostomy for nutrition and/or administering medications. Recently, gastric electrical stimulation (GES) has been investigated as a new approach for treating medically refractory gastroparesis. 8 GES delivers electric current via electrodes to gastric smooth muscle. Two methods of GES have been reported. The first uses electrical stimulation using an implantable neurostimulator with a high-frequency (12 cpm), lowenergy output (pulse width, 330 s) (Enterra therapy; Medtronic, Inc, Minneapolis, MN). This device is implanted into the abdomen via laparotomy or laparoscopy. The Food and Drug Administration approved Enterra therapy under the Humanitarian Device Exemption in April 2000 for patients with diabetic and idiopathic gastroparesis. A second method of GES (known as gastric pacing), still under investigation, uses a high-energy/low-frequency (similar to or slightly higher than that of the native slow wave, 3 cpm) and longer pulse duration (300 ms) system. Studies in both animals and human beings have suggested that this method entrains the gastric slow wave, normalizes gastric dysrhythmias, 9 11 and significantly improves GE and dyspeptic symptoms in patients with refractory gastroparesis. 11 The clinical applications of high-frequency GES for gastroparesis were based on experimental work in animals and human beings, showing that electrical stimulation with a higher frequency than the intrinsic gastric slow wave frequency (3 cycles/ min in human beings) and shorter pulse duration (300 s) improved nausea and vomiting and enhanced gastric emptying. 12 Abbreviations used in this paper: DGP, diabetic gastroparesis; GE, gastric emptying; GES, gastric electrical stimulation; HbA1c, gycated hemoglobin A1c; ITT, intent-to-treat; SF-36, Short Form 36; TSS, total symptom score; WAVESS, Worldwide Antivomiting Electrical Stimulation Study; WVF, weekly vomiting frequency by the AGA Institute /$36.00 doi: /j.cgh
2 948 MCCALLUM ET AL CLINICAL GASTROENTEROLOGY AND HEPATOLOGY Vol. 8, No. 11 To date, only one double-blind study, the Worldwide Antivomiting Electrical Stimulation Study (WAVESS), has evaluated the efficacy of GES in patients with gastroparesis. That study included 33 patients (17 diabetic and 16 idiopathic) who, after surgery to implant the device, immediately underwent 2 consecutive months of a randomized, placebo-controlled, doubleblind, cross-over trial followed by a 10-month, open-label period. During the blinded phase of the study, GES achieved a significant reduction in weekly vomiting frequency (WVF) and the majority of patients preferred the ON month. At 12 months, 70% of diabetic patients and 77% of idiopathic patients reported more than 50% improvement in median vomiting frequency. At 12 months, 73% of patients experienced improvements in quality of life. The 4-hour gastric retention decreased significantly from 34% at baseline to 22% at 12 months. 13 Apart from the WAVESS double-blind trial, all of the published literature on the efficacy of GES consists of open-label studies, mainly from centers with substantial experience with this device. Follow-up data for a period of 1 to 10 years postimplant uniformly show consistent improvement in symptoms. 14,15 Usually, early (3 6 mo) improvement in gastroparesis symptoms correlates with long-term sustained control of symptoms. Long-term GES also has been associated with improved quality of life, reduced health care use, improved glycemic control in diabetic patients, reduced dependence on enteral or parenteral nutrition, and improved nutritional status. 16 In addition, patients with diabetic gastroparesis may become candidates for renal and pancreas transplant because symptom control may improve the bioavailability of immunosuppressants. Because of the paucity of data from well-controlled GES studies, this trial was undertaken to evaluate the safety and efficacy of Enterra gastric electric stimulation therapy in the treatment of chronic and intractable (drug-refractory) nausea and vomiting secondary to DGP. Materials and Methods Study Design This was a prospective, multicenter, double-blinded, randomized, 2-period, cross-over study conducted at 8 centers in the United States under Institutional Review Board approval. The device was turned ON for the first 1.5 months after implant to allow for recovery from surgery before randomization. At 1.5 months, each subject was randomized in a masked fashion to 1 of 2 treatment arms: 3 months ON followed by 3 months OFF, or 3 months OFF followed by 3 months ON. The subject, physician, and study coordinator were blinded to the stimulation status during the cross-over phase. At the end of the cross-over period, the device was programmed ON and followed up at a 12-month follow-up visit and annually thereafter until study closure. Follow-up office visits were scheduled at 1.5 months, 4.5 months, 7.5 months, and 12 months postimplant. Figure 1 shows the detailed study design. Study Objectives The purpose of this clinical evaluation was to show the safety and efficacy of Enterra therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis of diabetic etiology. The primary objective was to show that there was a reduction in WVF when the device was turned on, relative to when the device was turned off during the blinded cross-over phase. The secondary objectives were as follows: (1) to show that there was a reduction in symptom scores when the device is turned on relative to when the device was turned off, and (2) to show a long-term reduction in WVF at 12 months relative to baseline. Additional goals included: assessment of the safety of Enterra therapy; evaluation of the 12-month responder rate; evaluation of the 12- month change in symptom score; and evaluation of the 12- month change in quality of life, gastric emptying, number of days in the hospital, body mass index, hypoglycemic attacks, and gycated hemoglobin A1c (HbA1c). Study Subjects All patients were required to be 18 years of age or older and to have been symptomatic requiring treatment for longer than 1 year. Full inclusion/exclusion criteria are shown in Table 1. A full history, physical, and clinical data review were completed to confirm that patients did not meet any exclusion criteria. Pseudo-obstruction was ruled out by abdominal obstruction series, with careful attention to small-bowel distension and air fluid levels. Patients from this study were referred primarily from practices of the study physicians, and had been followed up for several months or years, helping to exclude an underlying malignancy or paraneoplastic syndrome. Patients on hemodialysis were allowed to enter the study only if they were undergoing regular dialysis visits. Implant Technique All qualified patients were implanted with the Enterra therapy system (model 7425G or model 3116; Medtronic, Inc). Two intramuscular leads (model 4351; Medtronic) were inserted into the muscularis propria of the stomach using either laparoscopy or laparotomy as previously described. 14 The leads were placed 10 cm from the pylorus on the greater curvature of the stomach, 1-cm apart, and were connected to the neuro- Figure 1. Study design.
3 November 2010 GASTRIC ELECTRICAL STIMULATION 949 Table 1. Inclusion/Exclusion Criteria Inclusion Patient has signed and dated the informed consent document Patient is at least 18 years of age Symptomatic requiring treatment for at least 1 year before baseline for diagnosed gastroparesis Etiology is idiopathic or diabetes mellitus Unresponsive or intolerant to prokinetic and antiemetic drug classes trialed over a minimum of 1 month, unless otherwise contraindicated Patients must experience at least 7 episodes of vomiting during a 7-consecutive-day period as captured on the 28-day diary Gastric retention of 10% at 4 hours, or 60% at 2 hours if the patient cannot complete the 4-hour gastric emptying test test Patient is willing and able to completely and accurately fill out the diary and questionnaires throughout the study Patients must be on a stable dose of prokinetic agents for a minimum of 30 days before baseline and be willing to continue that dose through the completion of the crossover period unless otherwise contraindicated Exclusion Diagnosis of any underlying illness that affects gastrointestinal motility such as mechanical obstruction, pseudo-obstruction, scleroderma, amyloidosis, Parkinson disease, muscular sclerosis, paraneoplastic syndromes, current parathyroid and adrenal disorders; if the patient has a history of a thyroid disorder, then he or she must currently show euthyroidism to be eligible for entry Current primary disorders such as psychogenic vomiting, eating disorder, or diagnosed swallowing disorder including achalasia Previous gastric surgery for total or partial gastric resection, fundoplication, and vagotomy Peritoneal dialysis or unstable hemodialysis Daily narcotic analgesia for abdominal pain Diagnosis of drug or alcohol dependency within 12 months of baseline visit Life expectancy 1 year Patients with other implantable neurostimulators, pacemakers, or defibrillators Current participation in other investigational device or drug studies with the exception of cisapride, which is currently available under a compassionate use protocol Pregnancy or planned pregnancy Patients who are planning to receive diathermy treatment Patients who have undergone radiation therapy of the upper abdomen Patients who are planning on having magnetic resonance imaging Study materials are not available in a language understood by the patient stimulator device placed subcutaneously in the abdominal wall. The device was programmed to standardized parameters (5 ma, 14 Hz, 330 s, cycle on 0.1 s, cycle off 5 s) using the clinician programmer (model 7432 or model 8840; Medtronic). Before the 7.5-month visit the programming parameters were not changed, with the exception that voltage was adjusted based on impedance to maintain a 5-mA current and the voltage was set to 0 during the off period. After the 7.5-month visit, programming parameters could be adjusted at the investigator s discretion. Measures Subjects were required to record daily vomiting episodes in a 28-day diary to assess WVF before each visit. The diary also was used to collect daily hypoglycemic attacks. GE was evaluated after a solid meal at baseline, at 12 months, and annually using a standardized scintigraphy method and a lowfat test meal. 17 The frequency and severity of gastroparesis symptoms (vomiting, nausea, early satiety, bloating, postprandial fullness, epigastric pain, and epigastric burning) were assessed using a 5-point symptom interview questionnaire at baseline and each follow-up visit. Scores were rated by the study coordinator or investigator based on an interview with the subject. The frequency symptom scores were rated as 0, absent; 1, rare (1/wk); 2, occasional (2 3/wk); 3, frequent (4 6/wk); or 4, extremely frequent ( 7/wk). The severity symptom scores were rated as 0, absent; 1, mild (not influencing the normal activities); 2, moderate (diverting from, but not urging modifications, of usual activities); 3, severe (influencing usual activities, severely enough to urge modifications); or 4, extremely severe (requiring bed rest). The sums of the frequency or severity ratings of the 7 symptoms were used as an overall frequency or severity total symptom score (TSS). Health-related quality of life was assessed at baseline and follow-up visits using the previously validated Short Form-36 (SF-36) Health Status Survey questionnaire, version HbA1c and type of nutritional support also were collected at baseline and follow-up visits. Type of nutritional support (oral, jejunostomy, G-tube, total parenteral nutrition) and whether or not it was continuous or intermittent was specified on the follow-up form. Adverse events were collected throughout the study. All events were classified using the Medical Dictionary for Regulatory Activities. Cause and severity of adverse events were classified by the principal investigator and adjudicated by an Adverse Events Committee. The cause of the adverse event was classified as being device-related (the event is caused by a suspected device malfunction), therapy-related (the event is directly or indirectly caused by the surgical implantation procedure; or is associated with the presence and/or use of the device), or patient-related (the event is associated with the subject s underlying diagnosis or a new diagnosis, unrelated to the device). Serious adverse events were considered as such when they resulted in death, were life-threatening, required in-patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or resulted in a congenital anomaly/birth defect. Sample Size, Randomization, and Blinding A 25% reduction in WVF when the device was on relative to when the device was off was considered clinically significant, and based on 80% power to detect a significant difference (P.05, 2-sided), 32 subjects were required for analysis. To compensate for nonevaluable subjects, a maximum of 75 subjects were allowed to be implanted. Subjects were randomized at a 1:1 ratio stratified by center in a block size of 4 to have therapy turned on or off at the beginning of the cross-over periods.
4 950 MCCALLUM ET AL CLINICAL GASTROENTEROLOGY AND HEPATOLOGY Vol. 8, No. 11 Randomization assignments were generated, put into sealed envelopes, and sent to an authorized unblinded person at the study site before the randomization visit. The authorized unblinded person was responsible for checking the device status and programming the device at follow-up visits during the cross-over period. The subjects, the investigators, and the study coordinators were blinded to the device settings during the cross-over period. Statistical Analysis The primary objective was assessed by the percentage reduction of WVF during the on period relative to the off period. The secondary objective of WVF was assessed by the percentage reduction of WVF at 12 months relative to baseline. Both objectives used completed cases and were analyzed using a Wilcoxon signed rank test. Probability values were deemed significant at a level of Subjects with a 50% or greater reduction in WVF at 12 months were defined as responders. A one-sided binomial test with a significance level of was used to test that the responder rate at 12 months was greater than 50%. Symptom scores (individual and TSS) and SF-36 scores (8 subscores, Physical Component Summary and Mental Component Summary) were analyzed using either a paired t test or the Wilcoxon signed rank test. Probability values were deemed significant at a level of No adjustments were made for multiple hypothesis testing. A Wilcoxon signed rank test was used and a significance level of 0.05 was applied for analyses of additional study measurements. Statistical analysis was performed with SAS version 9.1 (SAS Institute, Inc, Cary, NC). Results Baseline Demographics Fifty-five subjects (65.5% females) with a mean age of 38.3 years (range, y) and a mean body mass index of 26.4 kg/m 2 (range, kg/m 2 ) underwent implantation of Enterra therapy between December 2002 and August Subjects had symptoms of gastroparesis for a mean of 5.9 years (range, 1 38 y) before enrollment and a median vomiting frequency of 16.8 episodes per week. All subjects had delayed gastric emptying with a median gastric retention of 75.5% at 2 hours and 46.5% at 4 hours. A majority of the subjects were insulin dependent (n 52; 94.5%) with an average HbA1c of 7.95% (range, 4.6% 12.4%). Some subjects required nutritional support (23.6% oral, eg, nutritional supplement shake; 14.8% enteral; and 3.6% parenteral). There were no significant differences between the randomized groups at baseline. Patient Disposition Among the 55 subjects enrolled and implanted, 10 were not randomized (see explanation in Supplementary Figure 1). There were 43 subjects who completed the cross-over phase and 39 subjects who completed the 12-month visit. Supplementary Figure 1 shows the full details on patient flow. Initial 6-Week Results To assess the impact of the initial ON period before randomization, a post hoc analysis of WVF at 6 weeks compared with baseline was completed for subjects who finished the 12-month follow-up period and provided diary data (n 35). Figure 2. WVF at baseline and follow-up evaluations. The median reduction in WVF at 6 weeks compared with baseline was 57% (P.001), with a median WVF of 19.5 episodes at baseline and 4.75 episodes at 6 weeks (Figure 2). Cross-Over Phase Of those patients completing the cross-over phase, 32 provided diary data to assess the WVF during on and off states. The data analyses are provided in Table 2 and Figure 2. Most subjects showed a large reduction in WVF from baseline to either on or off states. The differences in WVF between on and off states during the double-blind period was not statistically significant (median % reduction, 0%; P.215). During the on state, the median WVF was 3.81 episodes, compared with a median WVF of 4.25 during the off state. The frequency and severity of the TSS also did not show a statistical difference between on and off states (P.903 and.302, respectively). Although there were no statistical differences observed during the cross-over period, WVF was somewhat better controlled during the on state than the off state (Table 2). 12-Month Follow-Up Phase There were 39 subjects who finished the 12-month follow-up visit and provided data. Three of the 39 subjects had missing diary data for WVF. The analysis results are provided in Tables 3 and 4. The WVF at 12 months decreased significantly when compared with baseline, with a median reduction of 67.8% (P.001). The median WVF was 19.5 episodes at baseline and 4.25 episodes at 12 months. Defining a responder as having a 50% or greater reduction in WVF from baseline to 12 months, there were 25 responders (69.4%; P.014). Two sensitivity analyses, intent-to-treat (ITT) and per-protocol, were performed to address the missing WVF data at 12 months. The ITT analysis included all the subjects who were randomized. Per-protocol analysis included all subjects who finished 12 months of follow-up evaluation, including 3 subjects missing diary data. The imputation method of last-observation-carried-forward was applied to adjust for the missing data for the per-protocol and ITT analyses. Only those observations made while the device was turned on were carried forward. Both per-protocol and ITT analyses revealed a median reduction of 66.5% (P.001) from baseline to 12 months. The frequency and severity of the TSS was decreased significantly from baseline to 12 months (P.001). Six individual symptom scores, including vomiting, nausea, early satiety, bloating, postprandial fullness, and epigastric pain, also were decreased significantly from baseline to 12 months for both
5 November 2010 GASTRIC ELECTRICAL STIMULATION 951 Table 2. Results During Cross-Over Phase Variable N On state Off state P value WVF, median (interquartile range) ( ) 4.25 ( ).215 Frequency symptom score, mean SD a Vomiting Nausea Early satiety Bloating Postprandial fullness Epigastric pain Epigastric burning TSS Severity symptom score, mean SD b Vomiting Nausea Early satiety Bloating Postprandial fullness Epigastric pain Epigastric burning TSS SD, standard deviation. a For each individual symptom frequency score, 0 absent and 4 extremely frequent ( 7/wk), the total symptom frequency score is the sum of all the individual symptom scores. b For each individual symptom severity score, 0 absent and 4 extremely severe (requiring bed rest), the total symptom severity score is the sum of all the individual symptom scores. frequency and severity symptom scores (P.005). There was no significant reduction in the frequency or severity symptom scores of epigastric burning at 12 months (P.205 and.356, respectively). Quality-of-life scores at 12 months also were improved from baseline. Statistically significant improvements were observed in the Physical Component Summary and Mental Component Summary scores (P.001 and.009, respectively). Improvements in subscores were observed in all 8 domains of the SF-36 survey, with statistically significant improvements in the physical functioning, role physical, bodily pain, general health, vitality, and social functioning domains (P.005). Gastric emptying was significantly improved at 12 months with a median retention at 4 hours of 20.5% compared with 46.5% at baseline (P.001). Only 6 patients had the same or worsening of their GE based on the 4-hour data; the other 22 patients had an improved rate of emptying. Seven of the 28 subjects (25%) who completed the GE test at 12 months normalized their GE ( 10% retention at 4 hours). Annualized median days in the hospital decreased from 40 days at baseline to 10 days at 12 months (P.001). The measurements of body mass index, HbA1c, and hypoglycemic attack did not show significant improvements. Adverse Events A total of 732 adverse events were collected in the study. Of these, 687 were patient-related (93.9%). There were 45 (6.1%) therapy- or device-related events, of which 15 were serious. Among these 15 serious events, there were 3 lead migration/ dislodgements, 2 device migrations, 1 implant site hematoma, and 1 implant site infection. The remaining 8 serious events were not directly related to the device; however, they were coded as therapy-related because they occurred within 2 weeks of the implant procedure. Overall, 3 of 55 patients (5.6%) required surgical intervention, but the implant site infection was the only event that resulted in device explant. Among the 687 patient-related events, 438 were serious. Signs and symptoms of gastroparesis, such as nausea and vomiting, were considered an adverse event only when they resulted in hospitalization. Gastroparesis-related hospitalizations (coded as impaired gastric emptying in the Medical Dictionary for Regulatory Activities) occurred 225 times in 40 patients, comprising 32.8% of all serious patient-related adverse events. Other frequently reported serious patient-related adverse events were ketoacidosis (21), vomiting (10), hematemesis (8), hypoglycemia (7), and hypertension (7). Table 3. Results of WVF at the 12-Month Follow-Up Evaluation Variable Analysis method N Baseline 12 Months Median percentage reduction P value WVF Completed case ( ) 4.25 ( ) 67.8% (23.7% 92.4%).001 Per-protocol ( ) 5.5 ( ) 66.5% (17.7% 90.7%).001 ITT ( ) 5.5 ( ) 66.5% (17.7% 90.7%).001 NOTE. Results are presented as median (interquartile range).
6 952 MCCALLUM ET AL CLINICAL GASTROENTEROLOGY AND HEPATOLOGY Vol. 8, No. 11 Table 4. Other Study Results at the 12-Month Follow-Up Evaluation Variable N Baseline 12 Months P value Frequency symptom score, mean SD Vomiting Nausea Early satiety Bloating Postprandial fullness Epigastric pain Epigastric burning TSS Severity symptom score, mean SD Vomiting Nausea Early satiety Bloating Postprandial fullness Epigastric pain Epigastric burning TSS SF-36 Health Survey, mean SD Physical Functioning Role Physical Bodily Pain General Health Vitality Social Functioning Role Emotional Mental Health Physical Component Summary Mental Component Summary Percentage of gastric retention, median (interquartile range) At 2 hours ( ) 51 (37 69).001 At 4 hours ( ) 20.5 (9.5 33).001 Days in hospital, median (interquartile range) (13 84) 10 (0 25).001 BMI, median (interquartile range) ( ) 25.1 ( ).213 HbA1c, median (interquartile range) ( ) 8.35 ( ).260 Weekly hypoglycemic attack, median (interquartile range) ( ) 0.25 (0 3.75).258 BMI, body mass index. There was a mortality rate of 12.7% (7 of 55 patients) at 1 year. The causes of death were cardiovascular (5), Staphylococcus infection of knee/septicemia (1), and cerebral aneurysm (1). None of the deaths was related to the device or therapy. Discussion In the present study, we confirmed that GES by an implanted system (Enterra therapy) significantly reduced the severity and frequency of 6 upper GI symptoms assessed including nausea, vomiting, early satiety, bloating, postprandial fullness, and epigastric pain. This significant reduction in symptoms was highlighted by the substantial and significant reduction in WVF that was first appreciated within 6 weeks of initiating Enterra therapy. The median reduction at 6 weeks was 57% compared with the baseline status for the subjects who finished the 12-month follow-up period (P.001). This significant reduction in WVF was sustained throughout the crossover period and until the 12-month follow-up evaluation (Figure 2). However, the study did not meet the primary end point because there was no difference observed between the on and off state in the cross-over period. The data also documented improvements in quality of life and symptom scores. The SF-36 quality-of-life instrument documented a significant improvement in both physical and mental aspects. These findings are consistent with those observed in the initial double-blind study. 13 In addition, the annualized median days in the hospital decreased significantly from 40 to 10, suggesting a significant economic impact of Enterra therapy. These savings likely are magnified because of the pharmaceutical requirements of this diabetic cohort. Similar to the initial double-blind study, 13 GE results at 12 months showed a significant improvement in the median retention isotope at 4 hours (20.5%) versus the baseline retention (46.5%) using the standardized scintigraphic low-fat egg-substitute meal. Normal GE for this method is defined as less than 10% retention at 4 hours. This improvement in GE was not associated with an improvement in HbA1c, as might be expected. One explanation may be that improved GE resulted in increased carbohydrate consumption and poorer glucose control. Although 75% of patients had improved GE from baseline, GE only normalized in 25%.
7 November 2010 GASTRIC ELECTRICAL STIMULATION 953 During the 3-month, double-blind, cross-over component of our trial, there was no significant difference in vomiting status between on and off states, although during the off state patients experienced slightly more vomiting episodes compared with the on state. These observations suggest that the rapid and significant induction of symptom improvement in the first 6 weeks was sustained despite a period of up to 3 months with the device off. Therefore, the carryover affects of neurostimulation may linger and remain effective over a number of weeks to months. The design concept for this study originated from observations in the double-blind WAVESS study 13 in which randomization began at the time of surgery for 1 month either on or off. Although there was a reduction in vomiting for the whole group of 33 diabetic and idiopathic gastroparetic patients during the cross-over in that trial, it was thought that the postoperative recovery time, as well as pain medication needs, resetting of glucose control, and the potential placebo effect of surgery, warranted a period of time postoperatively for the patient s condition to stabilize before starting the double-blind assessment of electrical stimulation. Therefore, for the current study, the Enterra device was activated for a 6-week period of time before randomization to the cross-over periods. The marked and rapid decline in symptoms was unexpected during these first 6 weeks. When subsequently turned off for up to 12 weeks the entrainment of central nausea and control mechanisms or other pathways that were activated or sensitized apparently remained effective, thus preventing a significant relapse of symptoms during the off period. It is therefore possible that the initial 6-week stimulation period rendered the subsequent double-blind data null. Data from the original WAVESS study suggest a similar sustained effect. In that study, patients who began with a 4-week on period showed sustained symptom reduction even after beginning the blinded off period. Other potential explanations for the study results that were considered include placebo effect or regression to the mean. The patients entered into the trial were diabetics with severe gastroparesis who had failed all medical therapies for a mean of 5.9 years with a mean of 40 days in the hospital during the year preceding the trial. In addition, 12.7% of patients died during the first year of this protocol with complications from diabetes. Although possible, it seems unlikely that the Enterra device could have induced a placebo effect extending for 12 months in this advanced and complicated patient set who experienced a significant and sustained symptom reduction as well as improvements in quality of life, GE, and hospitalizations. A follow-up study with a different design is suggested to address these other potential explanations for the current studies results. In light of data suggesting the possibility of longterm carryover effects of the therapy, a cross-over design probably should be avoided. Therefore, the most meaningful design is a parallel study design with subjects randomized at the time of surgery to implant the Enterra device to either on or off for a 3- to 6-month time period. This would address previous study concerns. The current results represent only patients with diabetic gastroparesis. Caution should be used when interpreting these results because they may not apply to the broader gastroparesis population of nondiabetic etiology. The major adverse event of this study related to GES therapy requiring explant and discontinuation of therapy was infection. Patients with long-term diabetes are at a higher risk for postoperative infection. In addition, once the implanted GES system is infected, it may not be possible to resolve the infection without removing the device. In the present study, 1.8% (1 of 55) of patients had the GES system removed because of infection. Two other patients also required surgical intervention because of lead dislodgement or device migration. In this study, there was a 12.7% (7 of 55) mortality rate at 1 year. A review of the literature provides insight into the longterm mortality in DGP. A recent study showed the natural history of a patient with diabetic gastroparesis has a mortality rate of 24% after 9 years of follow-up evaluation. 19 The major cause of death in that study was cardiac arrest or renal failure. There was a significant relation between the risk of death and duration of diabetes in that study (P.02). This re-emphasizes the fact that DGP patients who have failed medical therapy often have significant comorbidities including subclinical cardiovascular and renal disease. Therefore, despite the efficacy of Enterra therapy on upper GI symptoms, overall prognosis still has to be guarded. These results support the efficacy of Enterra therapy for severe diabetic gastroparesis patients failing medical therapy but also indicate that more research is necessary to address remaining questions about study results. Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at and at doi: / j.cgh References 1. Nilsson PH. Diabetic gastroparesis: a review. J Diabet Complications 1996;10: Kinsley BT, Gramm HF, Rolla AR. Diabetic gastroparesis: a review. J Diabet Complications 1991;5: Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum). Ann Intern Med 1958;48: Rundles RW. Diabetic neuropathy: general review of 125 cases. Medicine 1945;24: Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med 1983;98: Horowitz M, Harding PE, Maddox A, et al. Gastric and esophageal emptying in insulin-dependent diabetes mellitus. J Gastroenterol Hepatol 1986;1: Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion 1999;60: Lin Z, Forster J, Sarosiek I, et al. Treatment of gastroparesis with electrical stimulation. Dig Dis Sci 2003;48: McCallum RW, Chen JD, Lin Z, et al. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology 1998;114: Lin ZY, McCallum RW, Schirmer BD, et al. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol 1998;274:G186 G Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology 1992;103: Familoni BO, Abell TL, Voeller G, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci 1997;42: Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003;125:
8 954 MCCALLUM ET AL CLINICAL GASTROENTEROLOGY AND HEPATOLOGY Vol. 8, No Forster J, Sarosiek I, Delcore R, et al. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg 2001;182: Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion 2002;66: Lin Z, Sarosiek I, Forster J, et al. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil 2006;18: Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95: Ware JE, Kosinski M. SF-36 physical & mental health summary scales: a manual for users of version 1. QualityMetric, Kong MF, Horowitz M, Jones KL, et al. Natural history of diabetic gastroparesis. Diabetes Care 1999;22: Reprint requests Address requests for reprints to: Richard W. McCallum, MD, Founding Chair, Department of Internal Medicine, Texas Tech University Health Sciences Center, Paul L. Foster School of Medicine, 4800 Alberta Avenue, El Paso, Texas richard.mccallum@ ttuhsc.edu; fax: (915) Acknowledgments The authors would like to especially thank Lisa Ruehlow for overall study management and Ye Tan for statistical analysis, as well as their colleagues at Medtronic Neuromodulation. A special acknowledgement is given to the late Dr Gordon Eckerling from Western Gastroenterologists, who was a principal investigator on the study. The authors also would like to thank the following individuals for their contributions: Dr Irene Sarosiek, Dr Jameson Forster, Katherine Roeser, Z. Lin, Dr R. Dusig, and Chris McMillan (Kansas University Medical Center); Shelley Gray, Eve Pillor, Doug Raggett, Nata DeVole, and Dr Gregg Jossart (California Pacific Medical Center); Dr Christine Carter and Brook Hanna (George Washington University Medical Center); Jennifer Eversmann, Dr Robert Cacchione, and Dr Jeffrey Allen (University of Louisville); Vanessa Lytes, Dr John Meilahn, and Dr Sean Harbison (Temple University); and Lisa Ruehr, Laura Sylvester, and Dr Joel Hammond (St. John s Research). Writing assistance was provided by Lisa Ruehlow and Ye Tan; both individuals are employees of Medtronic, Inc, Minneapolis, MN. identifier: NCT Conflicts of interest The authors disclose the following: All authors received funding from Medtronic for the completion of this research. Medtronic was involved in the study design in conjunction with the study investigators. Data were collected at investigational sites and compiled by Medtronic. Medtronic conducted the statistical analysis. Data interpretation was completed by the study investigators in conjunction with Medtronic statisticians and data managers. Potential conflicts of interest have been disclosed to study participants. Funding This clinical study was sponsored by Medtronic, Inc.
9 954.e1 MCCALLUM ET AL CLINICAL GASTROENTEROLOGY AND HEPATOLOGY Vol. 8, No. 11 Supplementary Figure 1. Patient disposition.
Public Statement: Medical Policy
 ARBenefits Approval: 10/12/11 Medical Policy Title: Gastric Neurostimulation Effective Date: 01/01/2012 Document: ARB0166 Revision Date: Code(s): 0155T Laparoscopy, surgical; implantation or replacement
ARBenefits Approval: 10/12/11 Medical Policy Title: Gastric Neurostimulation Effective Date: 01/01/2012 Document: ARB0166 Revision Date: Code(s): 0155T Laparoscopy, surgical; implantation or replacement
Postsurgical gastroparesis (PSG), identified as a. Clinical Response to Gastric Electrical Stimulation in Patients With Postsurgical Gastroparesis
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:49 54 Clinical Response to Gastric Electrical Stimulation in Patients With Postsurgical Gastroparesis RICHARD MCCALLUM,* ZHIYUE LIN,* PAUL WETZEL,* IRENE
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:49 54 Clinical Response to Gastric Electrical Stimulation in Patients With Postsurgical Gastroparesis RICHARD MCCALLUM,* ZHIYUE LIN,* PAUL WETZEL,* IRENE
Treatment of Diabetic Gastroparesis by High-Frequency Gastric Electrical Stimulation
 Emerging Treatments and Technologies O R I G I N A L A R T I C L E Treatment of Diabetic Gastroparesis by High-Frequency Gastric Electrical Stimulation ZHIYUE LIN, MS 1 JAMESON FORSTER, MD 2 1 IRENE SAROSIEK,
Emerging Treatments and Technologies O R I G I N A L A R T I C L E Treatment of Diabetic Gastroparesis by High-Frequency Gastric Electrical Stimulation ZHIYUE LIN, MS 1 JAMESON FORSTER, MD 2 1 IRENE SAROSIEK,
Original Policy Date
 MP 7.01.57 Gastric Electrical Stimulation Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy
MP 7.01.57 Gastric Electrical Stimulation Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy
Gastric Electrical Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Gastric Electrical Stimulation
 Gastric Electrical Stimulation Policy Number: 7.01.73 Last Review: 6/2017 Origination: 7/2002 Next Review: 6/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
Gastric Electrical Stimulation Policy Number: 7.01.73 Last Review: 6/2017 Origination: 7/2002 Next Review: 6/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
Gastric Electrical Stimulation. Description. Section: Surgery Effective Date: July 15, 2016
 Subject: Gastric Electrical Stimulation Page: 1 of 11 Last Review Status/Date: June 2016 Gastric Electrical Stimulation Description Gastric electrical stimulation is performed using an implantable device
Subject: Gastric Electrical Stimulation Page: 1 of 11 Last Review Status/Date: June 2016 Gastric Electrical Stimulation Description Gastric electrical stimulation is performed using an implantable device
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview gastroelectrical stimulation for gastroparesis Introduction This overview has been prepared
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview gastroelectrical stimulation for gastroparesis Introduction This overview has been prepared
Corporate Medical Policy Gastric Electrical Stimulation
 File name: Gastric Electrical Stimulation File code: UM.NS.06 Origination: 2007 Last Review: 02/2017 Next Review: 02/2018 Effective Date: 05/01/2017 Description/Summary Corporate Medical Policy Gastric
File name: Gastric Electrical Stimulation File code: UM.NS.06 Origination: 2007 Last Review: 02/2017 Next Review: 02/2018 Effective Date: 05/01/2017 Description/Summary Corporate Medical Policy Gastric
Gastric Electrical Stimulation: Who Are the Best Candidates? What Are the Results?
 NaminArticle 12/7/05 12:23 PM Page 23 : Who Are the Best Candidates? What Are the Results? by Farid Namin, Zhiyue Lin, Irene Sarosiek, and Richard W. McCallum Farid Namin Zhiyue Lin Irene Sarosiek Richard
NaminArticle 12/7/05 12:23 PM Page 23 : Who Are the Best Candidates? What Are the Results? by Farid Namin, Zhiyue Lin, Irene Sarosiek, and Richard W. McCallum Farid Namin Zhiyue Lin Irene Sarosiek Richard
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gastric_electrical_stimulation 9/2003 5/2017 5/2018 5/2017 Description of Procedure or Service Gastric electrical
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gastric_electrical_stimulation 9/2003 5/2017 5/2018 5/2017 Description of Procedure or Service Gastric electrical
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of gastroelectrical stimulation for gastroparesis Gastroparesis is a long-term condition
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of gastroelectrical stimulation for gastroparesis Gastroparesis is a long-term condition
Subject: Gastric Electric Simulation in Gastroparesis Patient. Review: HJ Hiew Dec Recommendation
 Subject: Gastric Electric Simulation in Gastroparesis Patient Review: HJ Hiew Dec 2014 Recommendation GES may be effective in reducing nausea and vomiting symptoms and need of enteral/parenteral nutrition
Subject: Gastric Electric Simulation in Gastroparesis Patient Review: HJ Hiew Dec 2014 Recommendation GES may be effective in reducing nausea and vomiting symptoms and need of enteral/parenteral nutrition
Gastroparesis, a disorder of gastric motility, is defined. Gastric Electrical Stimulation for Medically Refractory Gastroparesis
 GASTROENTEROLOGY 2003;125:421 428 Gastric Electrical Stimulation for Medically Refractory Gastroparesis THOMAS ABELL,* RICHARD MCCALLUM, MICHAEL HOCKING, KENNETH KOCH, HASSE ABRAHAMSSON, ISABELLE LEBLANC,
GASTROENTEROLOGY 2003;125:421 428 Gastric Electrical Stimulation for Medically Refractory Gastroparesis THOMAS ABELL,* RICHARD MCCALLUM, MICHAEL HOCKING, KENNETH KOCH, HASSE ABRAHAMSSON, ISABELLE LEBLANC,
Medical Policy Gastric Electrical Stimulation Section Effective Date Subsection Original Policy Date Next Review Date Description
 7.01.73 Gastric Electrical Stimulation Section 7.0 Surgery Subsection Effective Date 11/26/2014 Original Policy Date December 7, 2006 Next Review Date November 2015 Description Gastric electrical stimulation
7.01.73 Gastric Electrical Stimulation Section 7.0 Surgery Subsection Effective Date 11/26/2014 Original Policy Date December 7, 2006 Next Review Date November 2015 Description Gastric electrical stimulation
Effectiveness of Gastric Neurostimulation in Patients With Gastroparesis
 SCIENTIFIC PAPER Effectiveness of Gastric Neurostimulation in Patients With Gastroparesis Jeremy Ross, MS, Mario Masrur, MD, Raquel Gonzalez-Heredia, MD, E. Fernando Elli, MD ABSTRACT Background: Patients
SCIENTIFIC PAPER Effectiveness of Gastric Neurostimulation in Patients With Gastroparesis Jeremy Ross, MS, Mario Masrur, MD, Raquel Gonzalez-Heredia, MD, E. Fernando Elli, MD ABSTRACT Background: Patients
Clinical Policy: Gastric Electrical Stimulation Reference Number: CP.MP.40
 Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Gastric Electrical Stimulation
 Clinical Policy: Reference Number: CP.MP.40 Last Review Date: 08/18 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Clinical Policy: Reference Number: CP.MP.40 Last Review Date: 08/18 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
GASTROPARESIS. C. Prakash Gyawali, MD Professor of Medicine Washington University in St. Louis
 GASTROPARESIS C. Prakash Gyawali, MD Professor of Medicine Washington University in St. Louis Symptom Definitions Nausea: a subjective feeling of wanting to vomit Vomiting: forceful expulsion of gastroduodenal
GASTROPARESIS C. Prakash Gyawali, MD Professor of Medicine Washington University in St. Louis Symptom Definitions Nausea: a subjective feeling of wanting to vomit Vomiting: forceful expulsion of gastroduodenal
MEDICAL POLICY SUBJECT: GASTRIC ELECTRICAL STIMULATION
 MEDICAL POLICY SUBJECT: GASTRIC ELECTRICAL PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: GASTRIC ELECTRICAL PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
PAPER. the absence of a structural
 PAPER Gastric Electrical Stimulation An Alternative Surgical Therapy for Patients With Gastroparesis Rodney J. Mason, PhD, MD; John Lipham, MD; Gordon Eckerling, MD; Alan Schwartz, MD; Tom R. DeMeester,
PAPER Gastric Electrical Stimulation An Alternative Surgical Therapy for Patients With Gastroparesis Rodney J. Mason, PhD, MD; John Lipham, MD; Gordon Eckerling, MD; Alan Schwartz, MD; Tom R. DeMeester,
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Gastric Pacing/Gastric Electrical Stimulation (GES) Table of Contents Coverage Policy... 1 General Background... 1 Coding/Billing Information... 5 References... 6
Cigna Medical Coverage Policy Subject Gastric Pacing/Gastric Electrical Stimulation (GES) Table of Contents Coverage Policy... 1 General Background... 1 Coding/Billing Information... 5 References... 6
The Challenge of Gastroparesis
 The Challenge of Gastroparesis A Guide to Understanding and Management TABLE OF CONTENTS Gastroparesis: A Serious Digestive Problem Gastroparesis: A Serious Digestive Problem....3 How the Stomach Works.................................................................
The Challenge of Gastroparesis A Guide to Understanding and Management TABLE OF CONTENTS Gastroparesis: A Serious Digestive Problem Gastroparesis: A Serious Digestive Problem....3 How the Stomach Works.................................................................
Clinical Policy Title: Gastric electrical stimulation (GES)
 Clinical Policy Title: Gastric electrical stimulation (GES) Clinical Policy Number: 08.02.03 Effective Date: October 1, 2015 Initial Review Date: September 17, 2015 Most Recent Review Date: May 19, 2017
Clinical Policy Title: Gastric electrical stimulation (GES) Clinical Policy Number: 08.02.03 Effective Date: October 1, 2015 Initial Review Date: September 17, 2015 Most Recent Review Date: May 19, 2017
Does the Injection of Botulinum Toxin Improve Symptoms in Patients With Gastroparesis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Does the Injection of Botulinum Toxin
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Does the Injection of Botulinum Toxin
Clinical Commissioning Policy: Gastroelectrical stimulation for gastroparesis
 Clinical Commissioning Policy: Gastroelectrical stimulation for gastroparesis Reference: NHS England: 16025/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised
Clinical Commissioning Policy: Gastroelectrical stimulation for gastroparesis Reference: NHS England: 16025/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised
DIABETIC GASTROPARESIS OBESE VERSUS NON OBESE PATIENTS
 Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 4 (53) No. 2-2011 DIABETIC GASTROPARESIS OBESE VERSUS NON OBESE PATIENTS L. POANTA 1 D. L. DUMITRAŞCU 1 Abstract: Symptoms
Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 4 (53) No. 2-2011 DIABETIC GASTROPARESIS OBESE VERSUS NON OBESE PATIENTS L. POANTA 1 D. L. DUMITRAŞCU 1 Abstract: Symptoms
Severe Gastroparesis: Medical Therapy or Gastric Electrical Stimulation
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2010;8:117 124 EDUCATION PRACTICE Severe Gastroparesis: Medical Therapy or Gastric Electrical Stimulation SAVIO C. REDDYMASU, IRENE SAROSIEK, and RICHARD W. McCALLUM
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2010;8:117 124 EDUCATION PRACTICE Severe Gastroparesis: Medical Therapy or Gastric Electrical Stimulation SAVIO C. REDDYMASU, IRENE SAROSIEK, and RICHARD W. McCALLUM
ACG Clinical Guideline: Management of Gastroparesis
 ACG Clinical Guideline: Management of Gastroparesis Michael Camilleri, MD 1, Henry P. Parkman, MD 2, Mehnaz A. Shafi, MD 3, Thomas L. Abell, MD 4 and Lauren Gerson, MD, MSc 5 1 Department of Gastroenterology,
ACG Clinical Guideline: Management of Gastroparesis Michael Camilleri, MD 1, Henry P. Parkman, MD 2, Mehnaz A. Shafi, MD 3, Thomas L. Abell, MD 4 and Lauren Gerson, MD, MSc 5 1 Department of Gastroenterology,
Dr. Patsy Smyth, FNP-BC
 Dr. Patsy Smyth, FNP-BC Gastroparesis literally translated means stomach paralysis. Gastroparesis is a syndrome characterized by delayed gastric emptying in absence of mechanical obstruction of the stomach.
Dr. Patsy Smyth, FNP-BC Gastroparesis literally translated means stomach paralysis. Gastroparesis is a syndrome characterized by delayed gastric emptying in absence of mechanical obstruction of the stomach.
Gastric Electrical Stimulation Corporate Medical Policy
 File name: Gastric Electrical Stimulation File code: UM.NS.06 Origination: 2007 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary Gastric Electrical Stimulation Corporate
File name: Gastric Electrical Stimulation File code: UM.NS.06 Origination: 2007 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary Gastric Electrical Stimulation Corporate
Gastroparesis: Diagnosis and Management
 Gastroparesis: Diagnosis and Management Rodica Pop-Busui MD, PhD Professor of Internal Medicine, Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI Disclosures Astra Zeneca Research
Gastroparesis: Diagnosis and Management Rodica Pop-Busui MD, PhD Professor of Internal Medicine, Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI Disclosures Astra Zeneca Research
Medical Policy. MP Ingestible ph and Pressure Capsule
 Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Gastric Electrical Stimulation
 Ontario Health Technology Assessment Series 2006; Vol. 6, No. 16 Gastric Electrical Stimulation An Evidence-Based Analysis August 2006 Medical Advisory Secretariat Ministry of Health and Long-Term Care
Ontario Health Technology Assessment Series 2006; Vol. 6, No. 16 Gastric Electrical Stimulation An Evidence-Based Analysis August 2006 Medical Advisory Secretariat Ministry of Health and Long-Term Care
Sponsor / Company: Sanofi Drug substance(s): insulin glargine (HOE901) According to template: QSD VERSION N 4.0 (07-JUN-2012) Page 1
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
JNM Journal of Neurogastroenterology and Motility
 JNM Journal of Neurogastroenterology and Motility J Neurogastroenterol Motil, Vol. 18 No. 2 April, 2012 pissn: 2093-0879 eissn: 2093-0887 http://dx.doi.org/10.5056/jnm.2012.18.2.131 Review Gastric Electrical
JNM Journal of Neurogastroenterology and Motility J Neurogastroenterol Motil, Vol. 18 No. 2 April, 2012 pissn: 2093-0879 eissn: 2093-0887 http://dx.doi.org/10.5056/jnm.2012.18.2.131 Review Gastric Electrical
Fluoroscopic gastric peroral endoscopic pyloromyotomy (G-POEM) in patients with a failed gastric electrical stimulator
 Gastroenterology Report, 6(2), 2018, 122 126 doi: 10.1093/gastro/gox040 Advance Access Publication Date: 13 December 2017 Original article ORIGINAL ARTICLE Fluoroscopic gastric peroral endoscopic pyloromyotomy
Gastroenterology Report, 6(2), 2018, 122 126 doi: 10.1093/gastro/gox040 Advance Access Publication Date: 13 December 2017 Original article ORIGINAL ARTICLE Fluoroscopic gastric peroral endoscopic pyloromyotomy
Outline. Definition (s) Epidemiology Pathophysiology Management With an emphasis on recent developments
 Chronic Dyspepsia Eamonn M M Quigley MD FRCP FACP MACG FRCPI Lynda K and David M Underwood Center for Digestive Disorders Houston Methodist Hospital Houston, Texas Outline Definition (s) Epidemiology Pathophysiology
Chronic Dyspepsia Eamonn M M Quigley MD FRCP FACP MACG FRCPI Lynda K and David M Underwood Center for Digestive Disorders Houston Methodist Hospital Houston, Texas Outline Definition (s) Epidemiology Pathophysiology
ENABLE MRI CLINICAL STUDY SUMMARY
 CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
Gastric Pacing/Gastric Electrical Stimulation (GES)
 Medical Coverage Policy Effective Date...11/15/2017 Next Review Date...11/15/2018 Coverage Policy Number... 0103 Gastric Pacing/Gastric Electrical Stimulation (GES) Table of Contents Related Coverage Resources
Medical Coverage Policy Effective Date...11/15/2017 Next Review Date...11/15/2018 Coverage Policy Number... 0103 Gastric Pacing/Gastric Electrical Stimulation (GES) Table of Contents Related Coverage Resources
Home Total Parenteral Nutrition for Adults
 Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
What you really need to know about Gastroparesis?
 What you really need to know about Gastroparesis? John M. Wo, MD Division of Gastroenterology/Hepatology Director of GI Motility and Neurogastroenterology 8/3/2016 1/4/2017 1 What you really need to know
What you really need to know about Gastroparesis? John M. Wo, MD Division of Gastroenterology/Hepatology Director of GI Motility and Neurogastroenterology 8/3/2016 1/4/2017 1 What you really need to know
Pharmacodynamic Effects of Ghrelin Agonist Relamorelin (RM-131) in Patients with Type 1 and Type 2 Diabetes Mellitus and Delayed Gastric Emptying
 Pharmacodynamic Effects of Ghrelin Agonist Relamorelin (RM-131) in Patients with Type 1 and Type 2 Diabetes Mellitus and Delayed Gastric Emptying Andrea Shin Motility Conference 2/4/15 Disclosures No conflicts
Pharmacodynamic Effects of Ghrelin Agonist Relamorelin (RM-131) in Patients with Type 1 and Type 2 Diabetes Mellitus and Delayed Gastric Emptying Andrea Shin Motility Conference 2/4/15 Disclosures No conflicts
Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing
 Received: 16 July 2016 Accepted: 23 September 2016 DOI: 10.1111/nmo.12981 ORIGINAL ARTICLE Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying,
Received: 16 July 2016 Accepted: 23 September 2016 DOI: 10.1111/nmo.12981 ORIGINAL ARTICLE Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying,
Medical Policy. Description/Scope. Position Statement. Rationale
 Subject: Document#: Current Effective Date: 06/28/2017 Status: Reviewed Last Review Date: 05/04/2017 Description/Scope This document addresses occipital nerve stimulation (ONS), which involves delivering
Subject: Document#: Current Effective Date: 06/28/2017 Status: Reviewed Last Review Date: 05/04/2017 Description/Scope This document addresses occipital nerve stimulation (ONS), which involves delivering
EDUCATION PRACTICE. Management of Delayed Gastric Emptying. Clinical Scenario. The Problem. Management Strategies and Supporting Evidence
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:642 646 EDUCATION PRACTICE Management of Delayed Gastric Emptying FRANK K. FRIEDENBERG and HENRY P. PARKMAN Temple University Hospital, Philadelphia, Pennsylvania
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:642 646 EDUCATION PRACTICE Management of Delayed Gastric Emptying FRANK K. FRIEDENBERG and HENRY P. PARKMAN Temple University Hospital, Philadelphia, Pennsylvania
Our evidence. Your expertise. SmartPill : The data you need to evaluate motility disorders.
 Our evidence. Your expertise. SmartPill : The data you need to evaluate motility disorders. SmartPill benefits your practice: Convenient performed right in your office Test standardization Provides direct
Our evidence. Your expertise. SmartPill : The data you need to evaluate motility disorders. SmartPill benefits your practice: Convenient performed right in your office Test standardization Provides direct
Clinical Snapshot: Making sense of Autonomic Neuropathy. M S Kamaruddin Consultant Physician South Tees NHS Foundation Trust
 Clinical Snapshot: Making sense of Autonomic Neuropathy M S Kamaruddin Consultant Physician South Tees NHS Foundation Trust Diabetic autonomic neuropathy Classified as clinical or subclinical based on
Clinical Snapshot: Making sense of Autonomic Neuropathy M S Kamaruddin Consultant Physician South Tees NHS Foundation Trust Diabetic autonomic neuropathy Classified as clinical or subclinical based on
Medical Review Criteria Implantable Neurostimulators
 Medical Review Criteria Implantable Neurostimulators Subject: Implantable Neurostimulators Effective Date: April 14, 2017 Authorization: Prior authorization is required for covered implantable stimulators
Medical Review Criteria Implantable Neurostimulators Subject: Implantable Neurostimulators Effective Date: April 14, 2017 Authorization: Prior authorization is required for covered implantable stimulators
Efficacy/pharmacodynamics: 85 Safety: 89
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: Sanofi Drug substance:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: Sanofi Drug substance:
PARENTERAL NUTRITION THERAPY
 UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare West BENEFIT INTERPRETATION POLICY
UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare West BENEFIT INTERPRETATION POLICY
Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540
 Electrical stimulation of the lower oesophageal sphincter for treating gastro-oesophageal reflux disease Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540 Your
Electrical stimulation of the lower oesophageal sphincter for treating gastro-oesophageal reflux disease Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540 Your
Diabetic Gastroparesis. Evan M. Klass, MD, FACP February 16, 2017
 Diabetic Gastroparesis Evan M. Klass, MD, FACP February 16, 2017 Scope of the problem The disorder can affect any part of the GI tract Although 10-20% of the general population suffer from functional GI
Diabetic Gastroparesis Evan M. Klass, MD, FACP February 16, 2017 Scope of the problem The disorder can affect any part of the GI tract Although 10-20% of the general population suffer from functional GI
GASTRIC EMPTYING STUDY (SOLID)
 GASTRIC EMPTYING STUDY (SOLID) Aim To evaluate patients with symptoms of altered of gastric emptying and/or motility, and quantitatively measure the rate of gastric emptying. This study provides a physiologic,
GASTRIC EMPTYING STUDY (SOLID) Aim To evaluate patients with symptoms of altered of gastric emptying and/or motility, and quantitatively measure the rate of gastric emptying. This study provides a physiologic,
Gastroparesis: A chronic disorder
 Diagnosis and Management of Gastroparesis: The Stomach that Refuses to Empty Improving Symptoms or Gastric Emptying Henry P. Parkman, MD Professor of Medicine Director of GI Motility Laboratory Gastroenterology
Diagnosis and Management of Gastroparesis: The Stomach that Refuses to Empty Improving Symptoms or Gastric Emptying Henry P. Parkman, MD Professor of Medicine Director of GI Motility Laboratory Gastroenterology
Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity. Original Effective Date: 7/8/2015
 Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity Original Effective Date: 7/8/2015 Policy Number: MCP-243 Revision Date(s): Review Date: 12/16/15,
Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity Original Effective Date: 7/8/2015 Policy Number: MCP-243 Revision Date(s): Review Date: 12/16/15,
June By: Reza Gholami
 ACG/CAG guideline on Management of Dyspepsia June 2017 By: Reza Gholami DEFINITION OF DYSPEPSIA AND SCOPE OF THE GUIDELINE Dyspepsia was originally defined as any symptoms referable to the upper gastrointestinal
ACG/CAG guideline on Management of Dyspepsia June 2017 By: Reza Gholami DEFINITION OF DYSPEPSIA AND SCOPE OF THE GUIDELINE Dyspepsia was originally defined as any symptoms referable to the upper gastrointestinal
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine. Study Identifiers: NCT
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Gut involvement in PoTS an overview
 Gut involvement in PoTS an overview Qasim Aziz, PhD, FRCP Centre for Neuroscience and Trauma Wingate Institute of Neurogastroenterology Case Hx * 28 year old lady presents with a long hx of constipation
Gut involvement in PoTS an overview Qasim Aziz, PhD, FRCP Centre for Neuroscience and Trauma Wingate Institute of Neurogastroenterology Case Hx * 28 year old lady presents with a long hx of constipation
Sponsor / Company: Sanofi Drug substance(s): HOE901-U300 (insulin glargine) According to template: QSD VERSION N 4.0 (07-JUN-2012) Page 1
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
GASTROPARESIS 101: BEGINNERS GUIDE TO UNDERSTANDING GASTROPARESIS By: Dr. Josh Ferguson 2017
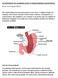 GASTROPARESIS 101: BEGINNERS GUIDE TO UNDERSTANDING GASTROPARESIS By: Dr. Josh Ferguson 2017 This tough looking word, pronounced gas-tro-par-ees-is, is simple enough in its meaning. Gastro means stomach.
GASTROPARESIS 101: BEGINNERS GUIDE TO UNDERSTANDING GASTROPARESIS By: Dr. Josh Ferguson 2017 This tough looking word, pronounced gas-tro-par-ees-is, is simple enough in its meaning. Gastro means stomach.
Objectives. Shocking The GI Tract: Electrical Stimulation From Top to Bottom. Steven Teich, M.D. Levine Children s Hospital.
 Shocking The GI Tract: Electrical Stimulation From Top to Bottom Steven Teich, M.D. Levine Children s Hospital Disclosures I have the following financial relationship to disclose: Endostim, Inc* * Products
Shocking The GI Tract: Electrical Stimulation From Top to Bottom Steven Teich, M.D. Levine Children s Hospital Disclosures I have the following financial relationship to disclose: Endostim, Inc* * Products
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine (HOE901) Insulin Glulisine (HMR1964)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Management of Gastroparesis
 Management of Gastroparesis Bible Class Jan Hendrik Niess As published in Am J Gastroenterol. 2013 Jan;108(1):18-37 What is the definition of gastroparesis? What are cardinal symptoms of gastroparesis?
Management of Gastroparesis Bible Class Jan Hendrik Niess As published in Am J Gastroenterol. 2013 Jan;108(1):18-37 What is the definition of gastroparesis? What are cardinal symptoms of gastroparesis?
Individual Study Table Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: DUODOPA Intestinal Gel Name of Active Ingredient: Levodopa-carbidopa Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Synopsis Abbott Laboratories Name of Study Drug: DUODOPA Intestinal Gel Name of Active Ingredient: Levodopa-carbidopa Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine (HOE901) Insulin Glulisine (HMR1964)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Corporate Medical Policy
 Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Aalborg Universitet. Published in: Neurogastroenterology and Motility. DOI (link to publication from Publisher): /jnm16179
 Aalborg Universitet Early assessment of cost-effectiveness of gastric electrical stimulation for diabetic nausea and vomiting Klinge, Mette; Rask, Peter; Ejskjaer, Niels; Krogh, Klaus; Lassen, Kathrine;
Aalborg Universitet Early assessment of cost-effectiveness of gastric electrical stimulation for diabetic nausea and vomiting Klinge, Mette; Rask, Peter; Ejskjaer, Niels; Krogh, Klaus; Lassen, Kathrine;
original article Prospective evaluation of gastric neurostimulation for diabetic gastroparesis in Canada
 original article Prospective evaluation of gastric neurostimulation for diabetic gastroparesis in Canada Himanish Panda BHSc 1, Philip Mitchell MD 2, Michael Curley MD 1, Michelle Buresi MD 1, Lynn Wilsack
original article Prospective evaluation of gastric neurostimulation for diabetic gastroparesis in Canada Himanish Panda BHSc 1, Philip Mitchell MD 2, Michael Curley MD 1, Michelle Buresi MD 1, Lynn Wilsack
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Impaired Gastric Slow Wave Rhythmicity in Patients After Bone Marrow or Stem Cell Transplant
 Digestive Diseases and Sciences, Vol. 47, No. 8 (August 2002), pp. 1746 1751 ( 2002) Impaired Gastric Slow Wave Rhythmicity in Patients After Bone Marrow or Stem Cell Transplant XIAOHONG XU, MS, ROMEO
Digestive Diseases and Sciences, Vol. 47, No. 8 (August 2002), pp. 1746 1751 ( 2002) Impaired Gastric Slow Wave Rhythmicity in Patients After Bone Marrow or Stem Cell Transplant XIAOHONG XU, MS, ROMEO
Gastroparesis and other upper GI problems DR ANDREW DAVIES
 Gastroparesis and other upper GI problems DR ANDREW DAVIES Outline Gastroparesis Hiccoughs Gastroparesis Gastroparesis Definition: A syndrome of objectively delayed gastric emptying in the absence of mechanical
Gastroparesis and other upper GI problems DR ANDREW DAVIES Outline Gastroparesis Hiccoughs Gastroparesis Gastroparesis Definition: A syndrome of objectively delayed gastric emptying in the absence of mechanical
Achalasia is a rare disease with an annual incidence estimated REVIEWS. Erroneous Diagnosis of Gastroesophageal Reflux Disease in Achalasia
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:1020 1024 REVIEWS Erroneous Diagnosis of Gastroesophageal Reflux Disease in Achalasia BOUDEWIJN F. KESSING, ALBERT J. BREDENOORD, and ANDRÉ J. P. M. SMOUT
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:1020 1024 REVIEWS Erroneous Diagnosis of Gastroesophageal Reflux Disease in Achalasia BOUDEWIJN F. KESSING, ALBERT J. BREDENOORD, and ANDRÉ J. P. M. SMOUT
PEER REVIEW HISTORY ARTICLE DETAILS TITLE (PROVISIONAL)
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (see an example) and are provided with free text boxes to
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (see an example) and are provided with free text boxes to
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod
 Aliment Pharmacol Ther 2005; 22: 59 65. doi: 10.1111/j.1365-2036.2005.02528.x Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod G. TOUGAS*,,D.L.EARNESTà, Y. CHEN*,
Aliment Pharmacol Ther 2005; 22: 59 65. doi: 10.1111/j.1365-2036.2005.02528.x Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod G. TOUGAS*,,D.L.EARNESTà, Y. CHEN*,
Ingestible ph and Pressure Capsule
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Pocket Adaptor Kit FOR SPINAL CORD STIMULATION. Tip Cards
 Pocket Adaptor Kit FOR SPINAL CORD STIMULATION Tip Cards model 74001 (1 4) model 74002 (2 4) The Pocket Adaptor Tip Cards are to be used for educational purposes only. Please refer to the Pocket Adaptor
Pocket Adaptor Kit FOR SPINAL CORD STIMULATION Tip Cards model 74001 (1 4) model 74002 (2 4) The Pocket Adaptor Tip Cards are to be used for educational purposes only. Please refer to the Pocket Adaptor
Ingestible ph and Pressure Capsule
 Medical Policy Manual Medicine, Policy No. 117 Ingestible ph and Pressure Capsule Next Review: October 2018 Last Review: October 2017 Effective: December 1, 2017 IMPORTANT REMINDER Medical Policies are
Medical Policy Manual Medicine, Policy No. 117 Ingestible ph and Pressure Capsule Next Review: October 2018 Last Review: October 2017 Effective: December 1, 2017 IMPORTANT REMINDER Medical Policies are
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable
A review on enteral nutrition guidelines for traumatic brain injury
 A review on enteral nutrition guidelines for traumatic brain injury According to the Centers for Disease Control and Prevention, at least 1.7 million people suffer from traumatic brain injury (TBI) every
A review on enteral nutrition guidelines for traumatic brain injury According to the Centers for Disease Control and Prevention, at least 1.7 million people suffer from traumatic brain injury (TBI) every
Number of studies. Endoscopic finding. Number of subjects. Pooled prevalence 95% CI
 Clinical Approach to the Patient t with Dyspepsia William D. Chey, MD, FACG Professor of Medicine University of Michigan Prevalence of Endoscopic Findings in Individuals with Dyspepsia Systematic Review
Clinical Approach to the Patient t with Dyspepsia William D. Chey, MD, FACG Professor of Medicine University of Michigan Prevalence of Endoscopic Findings in Individuals with Dyspepsia Systematic Review
Functional Dyspepsia
 Functional Dyspepsia American College of Gastroenterology Boston Massachusetts, June 2015 Brian E. Lacy, PhD, MD, FACG Professor of Medicine Geisel School of Medicine at Dartmouth Chief, Section of Gastroenterology
Functional Dyspepsia American College of Gastroenterology Boston Massachusetts, June 2015 Brian E. Lacy, PhD, MD, FACG Professor of Medicine Geisel School of Medicine at Dartmouth Chief, Section of Gastroenterology
Gastroparesis. - Recent advances in the pathophysiology and treatment -
 ICDM 2015 Gastroparesis - Recent advances in the pathophysiology and treatment - Department of Internal Medicine, College of Medicine, St. Paul s hospital, The Catholic University of Korea, Seoul, Korea
ICDM 2015 Gastroparesis - Recent advances in the pathophysiology and treatment - Department of Internal Medicine, College of Medicine, St. Paul s hospital, The Catholic University of Korea, Seoul, Korea
STRATEGIES TO IMPROVE ENTERAL FEEDING TOLERANCE. IS IT WORTH IT? ENGELA FRANCIS RD(SA)
 STRATEGIES TO IMPROVE ENTERAL FEEDING TOLERANCE. IS IT WORTH IT? ENGELA FRANCIS RD(SA) DEFINITION OF ENTERAL FEEDING INTOLERANCE Gastrointestinal feeding intolerance are usually defined as: High gastric
STRATEGIES TO IMPROVE ENTERAL FEEDING TOLERANCE. IS IT WORTH IT? ENGELA FRANCIS RD(SA) DEFINITION OF ENTERAL FEEDING INTOLERANCE Gastrointestinal feeding intolerance are usually defined as: High gastric
2.0 Synopsis. Paricalcitol Capsules M Clinical Study Report R&D/15/0380. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
The Many Faces of T2DM in Long-term Care Facilities
 The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
Lawrence R. Schiller, MD, FACG. Digestive Health Associates of Texas Baylor University Medical Center Dallas, Texas.
 Chronic Nausea & Vomiting Lawrence R. Schiller, MD, FACG Digestive Health Associates of Texas Baylor University Medical Center Dallas, Texas Nausea & Vomiting Common symptoms Quite troubling to patients
Chronic Nausea & Vomiting Lawrence R. Schiller, MD, FACG Digestive Health Associates of Texas Baylor University Medical Center Dallas, Texas Nausea & Vomiting Common symptoms Quite troubling to patients
Gut complications in autonomic dysfunction Qasim Aziz, PhD, FRCP
 Gut complications in autonomic dysfunction Qasim Aziz, PhD, FRCP Centre for Neuroscience and Trauma Wingate Institute of Neurogastroenterology GI involvement in autonomic dysfunction Conditions Diabetes
Gut complications in autonomic dysfunction Qasim Aziz, PhD, FRCP Centre for Neuroscience and Trauma Wingate Institute of Neurogastroenterology GI involvement in autonomic dysfunction Conditions Diabetes
Individual Study Table Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Gastroparesis is a syndrome characterized by delayed
 GASTROENTEROLOGY 2009;136:1225 1233 The Incidence, Prevalence, and Outcomes of Patients With Gastroparesis in Olmsted County, Minnesota, From 1996 to 2006 HYE KYUNG JUNG,*, ROK SEON CHOUNG,* G. RICHARD
GASTROENTEROLOGY 2009;136:1225 1233 The Incidence, Prevalence, and Outcomes of Patients With Gastroparesis in Olmsted County, Minnesota, From 1996 to 2006 HYE KYUNG JUNG,*, ROK SEON CHOUNG,* G. RICHARD
OHTAC Recommendation
 OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
Gastric Electrical Stimulation With Short Pulses Reduces Vomiting but not Dysrhythmias in Dogs
 GASTROENTEROLOGY 2003;124:401 409 Gastric Electrical Stimulation With Short Pulses Reduces Vomiting but not Dysrhythmias in Dogs JIANDE D. Z. CHEN, LIWEI QIAN, HUI OUYANG, JIEYUN YIN, and ENTERIC NEUROMUSCULAR
GASTROENTEROLOGY 2003;124:401 409 Gastric Electrical Stimulation With Short Pulses Reduces Vomiting but not Dysrhythmias in Dogs JIANDE D. Z. CHEN, LIWEI QIAN, HUI OUYANG, JIEYUN YIN, and ENTERIC NEUROMUSCULAR
