1. Original protocol, final protocol, summary of changes. (highlighted in. 2. Original statistical analysis plan, final statistical analysis plan,
|
|
|
- Adrian Roberts
- 5 years ago
- Views:
Transcription
1 Protocol
2 This supplement contains the following items: 1. Original protocol, final protocol, summary of changes. (highlighted in yellow track change in the final protocol). 2. Original statistical analysis plan, final statistical analysis plan, summary of changes.(highlighted in yellow track change in the final protocol).
3 CLINICAL TRIAL PROTOCOL Intravitreal Injection of raav2-nd4 Treatment for Leber s Hereditary Optic Neuropathy Protocol No.: Version: 1 PRINCIPAL INVESTIGATOR Prof. Bin Li Tongji Hospital Tongji Medical College, Huazhong University of Science and Technology Wuhan, Hubei, China
4 LIST OF ABBREVIATIONS AND DEFINITIONS The following abbreviations and specialist terms are used in this study protocol: Abbreviation AAV BCVA CF ERG HM IOP LHON LogMAR MD MtDNA Explanation Adeno-associated virus Best corrected visual acuity Counting fingers Electroretinogram Hand movement Intraocular pressure Leber s hereditary optic neuropathy Logarithm of the minimum angle of resolution Mean defect Mitochondrial DNA ND4 NADH-ubiquinone oxidoreductase, subunit 4 OCT PSD RNFL VEP VFI Optical coherence tomography; Pattern standard deviation; Retinal nerve fiber layer; Visual evoked potential. Visual field index.
5 STUDY CONTACT LIST For questions regarding the conduct of this study, please contact: Project Leader: Bin Li, PhD,MD Telephone: Mobile: Telefax: Clinical Trial Manager: Xing Wan,MM (Clinical investigator) or Telephone: Mobile: Telefax: For reporting a serious adverse event, please contact: Clinical Trial Manager: Shuo Yang young_ophthalmology@foxmail.com Telephone: Mobile: Telefax:
6 1.Background Leber s hereditary optic neuropathy (LHON), which was first reported by the German scholar Theodore Leber in 1871, is a neurodegenerative genetic eye disease. It is caused by a gene mutation. The most common gene mutation is a mitochondrial mutation located at locus Most LHON patients are male. Onset of LHON usually occurs at 15 to 20 years of age. The primary clinical symptoms are acute or sub-acute painless eyesight degradation simultaneously or successively in both eyes, accompanied by central vision field loss and malfunction of chromatic vision. There is no sufficient therapy for LHON currently available. Neurotrophic drugs and traditional Chinese medicine have both been used to treat LHON, There is no definitively effective treatment available for LHON. It has been reported that idebenone, a short chain derivative of coenzyme Q10, is a potential agent to treat LHON [1], but its treatment efficiency is controversial. Idebenone and vitamin B12 therapy is reported effective in some patients [2], but failed for others [3, 4]. The latest studies have shown that the para-benzoquinone analog of CoQ and idebenone, EPI-743, holds promise with improved pharmacologic properties; there was a genuine improvement in several tested parameters of visual function [5-7]. Although the preliminary results are encouraging, new methods in the treatment of LHON still need to be explored. 2.Trial Objectives The purpose of this study was to determine if, in humans, gene therapy for LHON caused by the mtdna mutation would be associated with immediately obvious adverse events, and whether efficacy could be demonstrated. 3. Study population Enrollees in the study were patients diagnosed with LHON who carried the mtdna mutation. Patients did not suffer from any other diseases, and the best corrected visual acuity (BCVA) of both eyes was below 0.3. Because 4% of LHON patients
7 with the mutation recover spontaneously, to ensure that improvements during the study were due to gene therapy alone all the enrollees had been diagnosed for more than one year, and their BCVA had not changed within the previous year. Gene therapy was administered to the eye with the poorer eyesight. If the optic function of both eyes was casually identical, the right eye was chosen to receive gene therapy. Criteria for inclusion 1) Patients carry the mitochondrial point mutation at locus 11778, which is consistent with the diagnostic criteria for LHON. 2) No apparent eye sight improvement in LHON patients or any other treatment within the past year. 3) Eyesight of both eyes is below ) Patients signed written informed consent. 5) Patients are between the ages of 8 and 60 years old and able to tolerate the gene therapy procedure which includes local anesthesia. 6) Patients are willing to follow the doctor s instructions and to consult the doctor at prescribed times. 7) Patient s physical examination results are all normal, including liver function, kidney function, routine blood test, routine urine test, complete immunological test, and humoral immune response. Criteria of exclusion 1) Patients who are wearing a cardiac pacemaker, suffering from severe heart, lung or kidney function failure, various hemorrhagic diseases, acute infectious diseases, high fever, or convalescing after heart surgery or who are pregnant are excluded. 2) Patients who are participating in other clinical studies are excluded. 3) Patients who suffer from a diagnosed mental problem are excluded. 4) Patients who suffer from chronic diseases such as diabetes and hypertension are excluded. 5) Patients who show abnormal test results such as positive AAV2 humoral immune response (positive means that the AAV2 neutralizing antibody assay of patient was
8 significant different when comparing free serum with 1:20 serum concentrations) and abnormal human T lymphocyte subsets CD3+, CD3+/CD4+ and CD3+/CD8+ prior to gene therapy surgery are excluded. 4. Trial Design The gene therapy consists of injecting adeno-associated virus 2-ND4 (AAV2-ND4) into the vitreous cavity of the eye of LHON patients caused by the mtdna mutation to induce the expression of ND4 protein at the diseased sites, Ophthalmologic examination Before gene therapy, all patients underwent detailed whole-body physical and ophthalmologic examinations. The ophthalmologic examinations consisted of BCVA, intraocular pressure (IOP), anterior segment examination by slit-lamp biomicroscopy, and direct opthalmoscopy of the fundus. Ocular fundus photographs, visual field test, OCT, VEP, and ERG were also performed. Whole-body physical examinations The whole-body examination included routine blood and urine, and liver, kidney, and immune function tests. The venous blood of patients was collected and cluster of differentiation (CD) human T lymphocyte subsets CD3+, CD3+ /CD4+, CD3+/CD8+, and the neutralizing antibody were analyzed by the Department of Laboratory Medicine, Tongji Hospital, Huazhong University of Science and Technology. In addition, we determined the concentration of serum AAV2, ND4, and interferon (IFN)-gamma of the patients via ELISA. Oral prednisolone Patients were given a 9-week course of oral prednisolone. This regimen consisted of 0. 5 mg/kg/day for the week before administration of the vector, 1 mg/kg/day during the first week afterward, 0.5 mg/kg/day for the second and third week, 0.25 mg/kg/day for the fourth and fifth week, and mg/kg/day during the last three of the nine weeks. To prevent potentially severe adverse events during and after treatment, we
9 established preventative measures for patients for whom emergencies were a possibility. Intravitreal injection Injecting drug: AAV2/2-ND4 Injecting dose: vg/0.05 ml Procedure:The surgery was carried out in the operating room. Tropicamide eye drops (0.5%) were administrated 3 times, 30 minutes before surgery to enlarge the pupils. After pupil enlargement the patients lay supine on the operating table and 0.1% oxybuprocaine hydrochloride eye drops were used to anaesthetize the appropriate eye. The conjunctival sac was washed with 0.3% norfloxacin eye drops. A 1 ml syringe was used, and the injecting pinhead was 30G. The injection site was 3.5 mm away from the temporal limbus. The injecting pinhead was inserted into the conjunctiva, slid for 3 mm along the subconjunctiva, and then entered vertically into the vitreous body. When the pinhead was visible through the pupil, raav2-nd4 was injected. The needle was removed from the vitreous cavity. The injection site was pressed with a cotton swab after the intravitreal injection. The injected eye was covered with gauze, and the patient was allowed to lie on the operating table for 30 minutes. Clinical observation At months 1, 3, and 6 after surgery whole-body examinations were repeated, and at months 1, 3, 6, after surgery ophthalmologic examinations were repeated. 5. Risks and Benefits to participants and community Risks 1) Accidents related to anesthesia, such as drug allergy and allergic shock. 2) The oculocardiac reflex may be induced when the eyeball is pressed and the patient s life may be endangered; 3) The lens may be injured during the surgery, and cataract may be a complication; 4) Retinal detachment;
10 5) Postsurgery infection may affect the healing of the wound; 6) Postsurgical endophthalmitis may be a complication; 7) Eyesight may not improve after surgery; 8) Other potential risks include an immune rejection response, or impairment of liver function, kidney function and functions of other organs which may induce cancer and even endanger life; 9) Prednisone will be administrated orally for eight weeks in the present study, which often leads to upset stomach, increased blood pressure, rash, or urticaria. 10) Other potential unexpected or unpreventable complications. Benefits to participants: 1 Accept the AAV2-ND4 Treatment free, 2 Ocular and systemic examination free at preoperative and postoperative. 3 Possible to improve vision. 6. Selection and Withdrawal of Subjects Your participation in the study is completely dependent on your willingness. You can refuse to participate in the study, or, you can also withdraw from the study at any time in the course of research. Such a decision will not affect your relationship with the doctor, nor will it affect your medical or other benefits in any way. To ensure your maximal benefits, if necessary the doctor or researcher may suspend your participation in the current study at any time during research. If you withdraw from the current study for any reason, you probably will be asked questions relevant to your medication. If the doctor thinks it is necessary, you will be asked to accept a laboratory test and physical examination. 7. Treatment of Subjects The treatment to be administered, including the dose, the dosing schedule, theroute/mode of administration, and the treatment periods, including follow-up: See above.
11 Procedures to monitor subject compliance: All interventions will be conducted by study personnel; the only subject compliance required is to abide by scheduled visits. 8. Assessment of Efficacy Visual acuity tests were conducted by an ophthalmologist. The eye chart was a 2.5-m standard ETDRS charts (Star Kang Medical Technology Co., Ltd. Wen Zhou China). We used two different eye charts to check patient's both eyes respectively. The patients were examined repeatedly to confirm their visual improvements, including tests at a different hospital performed by a completely uninformed oculist. VEP, the function of the optic nerve was examined; primarily the value of the P 100 wave was determined with a DV-100 (Shanghai Dikon Medical). The tested eyes were optically corrected, and the binocular viewing condition was adopted. The data acquisition and analysis were implemented with a connected computer. Check-reversal amplitude was the difference between the first major positive peak near 100 ms (P 100 ) and the preceding negative peak. The P 100 amplitude was recorded. Main outcome measures included a latency period of the P 100 wave and latency period of the reaction capacity of optic nerve conduction signals from the eye to the brain (the value <105 ms regarded as normal). A Humphrey field analyzer (Carl Zeiss 740i, Carl Zeiss, Shanghai) was used for the visual field test. The testing procedure included the 30-2 central threshold test and SITA fast. Major record parameters were VFI, MD and PSD. Electroretinogram(ERG):Retinal ganglion cell function was examined with a Roland Electronic GmbH electroretinogram (Keltern, Germany). OCT was examined with a Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany), and the retinal nerve fiber layer (RNFL) thickness of four quadrants (bottom, up, left and right quadrants) of the retinal vessel was analyzed. All data of OCT were automatically calculated using the existing software. The above examinations were conducted by two technicians of the Ophthalmology Department.
12 9. Assessment of Safety The tonometer used for determining intraocular pressure was a TOPCON-CT-80 Computerized Auto Tonometer (TOPCOCN, Tokyo, Japan). The mean value of three measurements was used. The fundus (retinal) photography used the NIDEK Autofocus Fundus Camera, AFC-230 (Nidek, Japan). Whole-body physical examinations:whole-body physical examinations consisted of routine blood and urine, and liver, kidney, and immune function tests analyzed by the Laboratory Department of Tongji Hospital. The specific examinations were those that are routine at Tongji Hospital. Routine blood test included 24 items, such as white blood cell count and hemoglobin et al. The routine urine test included 24 items such as red blood cells, white blood cells, and bilirubin in urine et al. Liver and kidney function tests covered 11 items, including alanine aminotransferase, aspartate aminotransferase, and creatinine et al. The immune function test consisted of IgA, IgG, IgM, complement 3 and complement 4. The blood sample was sent to the Laboratory Department of our hospital. The tests for human T lymphocyte subsets CD3+, CD3+/CD4+ and CD3+/CD8+ were conducted by the Central Laboratory of Tongji Hospital. Venous blood from the patients was collected and sent to the Central Laboratory of Tongji Hospital and tested by the staff. Neutralizing antibody assay:to detect neutralizing antibodies to AAV2, we incubated 1:20, 1:60, 1:180, 1:540, and 1:1620 mouse serum samples with 10 8 vg AAV2-GFP in 25 μl of PBS for 2 h at 4 C. This mix was added to each well containing HEK293 cells grown in 6-well plates (to achieve a multiplicity of infection of 1000). The cells were grown at 37 C in 5% CO 2, in Dulbecco s Modified Eagle s Medium (HyClone, Logan, UT) containing 5% FBS (HyClone). Green fluorescent protein expression was evaluated 48 h after infection by flow cytometry (BD Biosciences, NJ, USA). The percentage of inhibition was calculated with no-antibody control samples as a reference. Every experiment was repeated three times.
13 The levels of AAV2, IFN-γ and ND4 protein determined by ELISA assay:serum samples from the nine patients were obtained preoperatively and 1, 3, and 6 months after intravitreal injection, and screened by ELISA for immunoreactivity to AAV2, IFN-γ and ND4 protein. The ELISA kits for AAV2, IFN-γ and ND4 were purchased from BlueGene Biotech. Shanghai, China. ELISA was performed according to the manufacturer s protocol. 10. Statistics METHODS Determination of sample size Since this is exploratory study, we will chose six patients of LHON to received a single-dose intravitreal injection of raav2-nd4. Statistical analysis A single statistical analysis will be performed at the end of the study. The data are expressed as mean standard error. Record the value of treated eyes, including: BCVA in logmar, visual field record parameters were VFI, MD and PSD., VEP record parameters were the latency period and the amplitudes of the P100 wave, ERG record parameters were the latency period and the amplitudes of the P50 wave, thickness of the retinal nerve fiber layer, neutralizing antibody, and the concentrations of serum AAV2, ND4, and IFN-gamma were analyzed using the paired t-test. A probability (P) value of less than 0.05 was considered statistically significant. Baseline The data of treated yees at before treatment as baseline value, including BCVA, VFI, concentrations of serum AAV2, ND4, et al, These data were compared with month 1,3,6 after treatment of treated eyes. 11. ETHICS Ethics review The final study protocol, including the final version of the Subject Information and
14 Consent Forms, must be approved in writing by an Independent Ethics Committee (IEC). The Principle Investigator (Clinical Trial Manager) is responsible for informing the IEC of any serious adverse events (SAE) and amendment to the protocol as per regulatory requirement. Ethical conduct of the study The study will be performed in accordance with the ethical principles in the Declaration of Helsinki, and that are consistent with Good Clinical Practice and applicable regulatory requirements. Subject information and consent The Investigator will ensure that the subject is given full and adequate oral and written information about the nature, purpose, possible risk and benefit of the study. Subjects must also be notified that they are free to discontinue from the study at any time. The subject should be given the opportunity to ask questions and allowed time to consider the information provided. The subject s signed and dated informed consent must be obtained before conducting any study specific procedure. The investigator must store the original, signed Subject Informed Consent Form and a copy must be given to the subject. 11. STUDY TIME TABLE AND TERMINATION First subject in August 2011 Last patient out March 2012 Clean File March 2012 Study Report April References 1. Y. Mashima, K. Kigasawa, M.Wakakura, et al. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? Journal of Neuro-Ophthalmology. 2000; 20: V. Carelli, P. Barboni, A. Zacchini, et al. Leber s hereditary optic neuropathy (LHON) with 14484/ND6 mutation in a north African patient. Journal of the Neurological Sciences. 1998;160:
15 3. Barnils N, Mesa E, Muñoz S, et al. Response to idebenone and multivitamin therapy in Leber s hereditary optic neuropathy. Archivos de la Sociedad Espanola de Oftalmologia. 2007; 82: Angebault C, Gueguen N, Desquiret-Dumas V, et al. Idebenone increases mitochondrial complex I activity in fibroblasts from LHON patients while producing contradictory effects on respiration.bmc Res Notes. 2011, 22;4: Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011; 134: Carelli V, La Morgia C, Valentino ML, et al. Idebenone treatment in Leber's hereditary optic neuropathy. Brain. 2011; 134:e Sadun AA, Chicani CF, Ross-Cisneros FN, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol. 2012; 69:
16 APPENDIX 1
17 APPENDIX 2 DECLARATION OF HELSINKI WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 29th WMA General Assembly, Tokyo, Japan, October th WMA General Assembly, Venice, Italy, October st WMA General Assembly, Hong Kong, September th WMA General Assembly, Somerset West, Republic of South Africa, October 1996 and the 52nd WMA General Assembly, Edinburgh, Scotland, October 2000 Note of Clarification on Paragraph 29 added by the WMA General Assembly, Washington 2002 INTRODUCTION The World Medical Association has developed the Declaration of Helsinki as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving human subjects. Medical research involving human subjects includes research on identifiable human material or identifiable data. It is the duty of the physician to promote and safeguard the health of the people. The physician's knowledge and conscience are dedicated to the fulfillment of this duty.
18 The Declaration of Geneva of the World Medical Association binds the physician with the words, "The health of my patient will be my first consideration," and the International Code of Medical Ethics declares that, "A physician shall act only in the patient's interest when providing medical care which might have the effect of weakening the physical and mental condition of the patient." Medical progress is based on research, which ultimately must rest in part on experimentation involving human subjects. In medical research on human subjects, considerations related to the well-being of the human subject should take precedence over the interests of science and society. The primary purpose of medical research involving human subjects is to improve prophylactic, diagnostic and therapeutic procedures and the understanding of the aetiology and pathogenesis of disease. Even the best-proven prophylactic, diagnostic, and therapeutic methods must continuously be challenged through research for their effectiveness, efficiency, accessibility and quality. In current medical practice and in medical research, most prophylactic, diagnostic and therapeutic procedures involve risks and burdens. Medical research is subject to ethical standards that promote respect for all human beings and protect their health and rights. Some research populations are vulnerable and need special protection. The particular needs of the economically and medically disadvantaged must be recognized. Special attention is also required for those who cannot give or refuse consent for themselves, for those who may be subject to giving consent under duress, for those who will not benefit personally from the research and for those for whom the research is combined with care. Research Investigators should be aware of the ethical, legal and regulatory requirements for research on human subjects in their own countries as well as applicable international requirements. No national ethical, legal or regulatory requirement should be allowed to reduce or eliminate any of the protections for human subjects set forth in this Declaration.
19 B. BASIC PRINCIPLES FOR ALL MEDICAL RESEARCH 10. It is the duty of the physician in medical research to protect the life, health, privacy, and dignity of the human subject. 11. Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and on adequate laboratory and, where appropriate, animal experimentation. 12. Appropriate caution must be exercised in the conduct of research, which may affect the environment, and the welfare of animals used for research must be respected. 13. The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol. This protocol should be submitted for consideration, comment, guidance, and where appropriate, approval to a specially appointed ethical review committee, which must be independent of the investigator, the sponsor or any other kind of undue influence. This independent committee should be in conformity with the laws and regulations of the country in which the research experiment is performed. The committee has the right to monitor ongoing trials. The researcher has the obligation to provide monitoring information to the committee, especially any serious adverse events. The researcher should also submit to the committee, for review, information regarding funding, sponsors, institutional affiliations, other potential conflicts of interest and incentives for subjects. 14. The research protocol should always contain a statement of the ethical considerations involved and should indicate that there is compliance with the principles enunciated in this Declaration. 15. Medical research involving human subjects should be conducted only by scientifically qualified persons and under the supervision of a
20 clinically competent medical person. The responsibility for the human subject must always rest with a medically qualified person and never rest on the subject of the research, even though the subject has given consent. 16. Every medical research project involving human subjects should be preceded by careful assessment of predictable risks and burdens in comparison with foreseeable benefits to the subject or to others. This does not preclude the participation of healthy volunteers in medical research. The design of all studies should be publicly available. 17. Physicians should abstain from engaging in research projects involving human subjects unless they are confident that the risks involved have been adequately assessed and can be satisfactorily managed. Physicians should cease any investigation if the risks are found to outweigh the potential benefits or if there is conclusive proof of positive and beneficial results. 18. Medical research involving human subjects should only be conducted if the importance of the objective outweighs the inherent risks and burdens to the subject. This is especially important when the human subjects are healthy volunteers. 19. Medical research is only justified if there is a reasonable likelihood that the populations in which the research is carried out stand to benefit from the results of the research. 20. The subjects must be volunteers and informed participants in the research project. 21. The right of research subjects to safeguard their integrity must always be respected. Every precaution should be taken to respect the privacy of the subject, the confidentiality of the patient's information and to minimize the impact of the study on the subject's physical and mental integrity and on the personality of the subject.
21 22. In any research on human beings, each potential subject must be adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail. The subject should be informed of the right to abstain from participation in the study or to withdraw consent to participate at any time without reprisal. After ensuring that the subject has understood the information, the physician should then obtain the subject's freely given informed consent, preferably in writing. If the consent cannot be obtained in writing, the non-written consent must be formally documented and witnessed. 23. When obtaining informed consent for the research project the physician should be particularly cautious if the subject is in a dependent relationship with the physician or may consent under duress. In that case the informed consent should be obtained by a well-informed physician who is not engaged in the investigation and who is completely independent of this relationship. 24. For a research subject who is legally incompetent, physically or mentally incapable of giving consent or is a legally incompetent minor, the investigator must obtain informed consent from the legally authorized representative in accordance with applicable law. These groups should not be included in research unless the research is necessary to promote the health of the population represented and this research cannot instead be performed on legally competent persons. 25. When a subject deemed legally incompetent, such as a minor child, is able to give assent to decisions about participation in research, the investigator must obtain that assent in addition to the consent of the legally authorized representative. 26. Research on individuals from whom it is not possible to obtain consent, including proxy or advance consent, should be done only if the
22 physical/mental condition that prevents obtaining informed consent is a necessary characteristic of the research population. The specific reasons for involving research subjects with a condition that renders them unable to give informed consent should be stated in the experimental protocol for consideration and approval of the review committee. The protocol should state that consent to remain in the research should be obtained as soon as possible from the individual or a legally authorized surrogate. 27. Both authors and publishers have ethical obligations. In publication of the results of research, the investigators are obliged to preserve the accuracy of the results. Negative as well as positive results should be published or otherwise publicly available. Sources of funding, institutional affiliations and any possible conflicts of interest should be declared in the publication. Reports of experimentation not in accordance with the principles laid down in this Declaration should not be accepted for publication. C. ADDITIONAL PRINCIPLES FOR MEDICAL RESEARCH COMBINED WITH MEDICAL CARE 28. The physician may combine medical research with medical care, only to the extent that the research is justified by its potential prophylactic, diagnostic or therapeutic value. When medical research is combined with medical care, additional standards apply to protect the patients who are research subjects. 29. The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists. See footnote
23 30. At the conclusion of the study, every patient entered into the study should be assured of access to the best-proven prophylactic, diagnostic and therapeutic methods identified by the study. 31. The physician should fully inform the patient which aspects of the care are related to the research. The refusal of a patient to participate in a study must never interfere with the patient-physician relationship. 32. In the treatment of a patient, where proven prophylactic, diagnostic and therapeutic methods do not exist or have been ineffective, the physician, with informed consent from the patient, must be free to use unproven or new prophylactic, diagnostic and therapeutic measures, if in the physician's judgement it offers hope of saving life, re-establishing health or alleviating suffering. Where possible, these measures should be made the object of research, designed to evaluate their safety and efficacy. In all cases, new information should be recorded and, where appropriate, published. The other relevant guidelines of this Declaration should be followed. FOOTNOTE: NOTE OF CLARIFICATION ON PARAGRAPH 29 of the WMA DECLARATION OF HELSINKI The WMA hereby reaffirms its position that extreme care must be taken in making use of a placebo-controlled trial and that in general this methodology should only be used in the absence of existing proven therapy. However, a placebo-controlled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances: - Where for compelling and scientifically sound methodological reasons its use is necessary to determine the efficacy or safety of a prophylactic, diagnostic or therapeutic method; or - Where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition and the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm.
24 All other provisions of the Declaration of Helsinki must be adhered to, especially the need for appropriate ethical and scientific review. The Declaration of Helsinki (Document 17.C) is an official policy document of the World Medical Association, the global representative body for physicians. It was first adopted in 1964 (Helsinki, Finland) and revised in 1975 (Tokyo, Japan), 1983 (Venice, Italy), 1989 (Hong Kong), 1996 (Somerset-West, South Africa) and 2000 (Edinburgh, Scotland). Note of clarification on Paragraph 29 added by the WMA General Assembly, Washington h00
25 APPENDIX 3a PATIENT INFORMATION LEAFLET AND INFORMED CONSENT Intravitreal Injection of raav2-nd4 Treatment for Leber s Hereditary Optic Neuropathy Dear Patients: We sincerely invite you to participate in this study. Please read the following information carefully before deciding whether to participate. It will help you understand the schedule, duration, benefits, risks, and discomfort inherent in the present study and the reason why this study is being performed. If you would like to participate, please consult the doctors for detailed information, and the doctors will help you. Alternatively, you can also discuss your options with your relatives and friends. 1. Background of Leber s hereditary optic neuropathy Leber s hereditary optic neuropathy (LHON), which was first reported by the German scholar Theodore Leber in 1871, is a neurodegenerative genetic eye disease. It is caused by a gene mutation. The most common gene mutation is a mitochondrial mutation located at locus Most LHON patients are male. Onset of LHON usually occurs at 15 to 20 years of age. The primary clinical symptoms are acute or sub-acute painless eyesight degradation simultaneously or successively in both eyes, accompanied by central vision field loss and malfunction of chromatic vision. There is no sufficient therapy for LHON currently available. Neurotrophic drugs and traditional Chinese medicine have both been used to treat LHON, but no positive potency was observed. In Japan, some researchers have suggested that the vasodilator idebenone could treat acute LHON. However, it is still in the clinical research stage and its efficacy is uncertain. 2. Objective of the study The purpose of the current study was to assess the safety and potency of gene therapy
26 for LHON. The gene therapy consists of injecting adeno-associated virus 2-ND4 (AAV2-ND4) into the vitreous cavity of the eye of LHON patients to induce the expression of ND4 protein at the diseased sites 3. Criteria of inclusion and exclusion Only patients of the Department of Ophthalmology of Tongji Hospital Affiliated with Tongji Medical School of Huazhong University of Science and Technology are to be enrolled in the study. The criteria for inclusion are: 1) Patients carry the mitochondrial point mutation at locus 11778, which is consistent with the diagnostic criteria for LHON. 2) No apparent eye sight improvement in LHON patients or any other treatment within the past year. 3) Eyesight of both eyes is below ) Patients signed written informed consent. 5) Patients are between the ages of 8 and 60 years old and able to tolerate the gene therapy procedure which includes local anesthesia. 6) Patients are willing to follow the doctor s instructions and to consult the doctor at prescribed times. 7) Patient s physical examination results are all normal, including liver function, kidney function, routine blood test, routine urine test, complete immunological test, and humoral immune response. The criteria of exclusion are follows: 1) Patients who are wearing a cardiac pacemaker, suffering from severe heart, lung or kidney function failure, various hemorrhagic diseases, acute infectious diseases, high fever, or convalescing after heart surgery or who are pregnant are excluded. 2) Patients who are participating in other clinical studies are excluded. 3) Patients who suffer from a diagnosed mental problem are excluded. 4) Patients who suffer from chronic diseases such as diabetes and hypertension are excluded.
27 5) Patients who show abnormal test results such as positive AAV2 humoral immune response (positive means that the AAV2 neutralizing antibody assay of patient was significant different when comparing free serum with 1:20 serum concentrations) and abnormal human T lymphocyte subsets CD3+, CD3+/CD4+ and CD3+/CD8+ prior to gene therapy surgery are excluded. 4. What is necessary for patient participation? 1) Before joining the present study, the doctor will inquire and record your medical history, and conduct examinations. 2) If you are qualified for inclusion, you will be asked whether you would like to participate voluntarily in the study, and to sign an informed consent form. 3) If you do not want to participate in the study, we will continue to treat you, as you wish. 4) If you would like to participate in the study, please expect to follow this procedure: a. Sign the informed consent; b. Allow pre-surgery examinations that include the eye and whole body; c. Dilate the pupils of both eyes before surgery; d. Submit to the operation, in which a single dose of approximately vg AAV2-ND4 in 0.05 ml will be injected into the vitreous cavity of one eye; e. Participate in the follow-up examinations, to be performed on days 1, 2, 3, and 7, and at the end of month 1, 3, and 6. The eye and whole-body examinations will be performed at every follow-up point and you can contact us at any time. 5. Potential adverse events, risks, discomfort and inconvenience 1) Accidents related to anesthesia, such as drug allergy and allergic shock. 2) The oculocardiac reflex may be induced when the eyeball is pressed and the patient s life may be endangered; 3) The lens may be injured during the surgery, and cataract may be a complication; 4) Retinal detachment; 5) Postsurgery infection may affect the healing of the wound;
28 6) Postsurgical endophthalmitis may be a complication; 7) Eyesight may not improve after surgery; 8) Other potential risks include an immune rejection response, or impairment of liver function, kidney function and functions of other organs which may induce cancer and even endanger life; 9) Prednisone will be administrated orally for eight weeks in the present study, which often leads to upset stomach, increased blood pressure, rash, or urticaria. 10) Other potential unexpected or unpreventable complications. 6. Is personal information confidential? Your medical record (patient history/case report form, laboratory test sheet) will be secured in the hospital. The doctor will record your laboratory test results in your patient history. The researcher, Hospital Ethics Committee, and Department of Drug Supervision and Administration are allowed to consult your medical record. Any public report relevant to the results of the present study will not reveal your personal information. Within the scope of legal permission, we try our best to keep secret your personal medical information. 7. How to get more information? You can ask any question about the current study at any time, and get corresponding answers. During the study, if there is any important new information that might affect your willingness to participate, your doctor will inform you in time. 8. To participate in or to withdraw from the study is voluntary. Your participation in the study is completely dependent on your willingness. You can refuse to participate in the study, or, you can also withdraw from the study at any time in the course of research. Such a decision will not affect your relationship with the doctor, nor will it affect your medical or other benefits in any way. To ensure your maximal benefits, if necessary the doctor or researcher may suspend your participation in the current study at any time during research. If you withdraw from the current study for any reason, you probably will be asked
29 questions relevant to your medication. If the doctor thinks it is necessary, you will be asked to accept a laboratory test and physical examination. 9. If you have any questions please contact Prof. Li Bin. Mobile phone number:
30 INFORMED CONSENT FORM Patient identification number:.... /..... Patient initials: Project title: Undertaker: Intravitreal Injection of raav2-nd4 Treatment for Leber s Hereditary Optic Neuropathy Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology By signing and dating this document, I confirm that I have had time to carefully read and understand the patient information sheet provided for this study. I confirm that I have had the opportunity to discuss the study and ask questions and I am satisfied with the answers and explanations that I have been provided. I give permission for my medical records to be reviewed by the Sponsor or designee, and/or representatives of the Medicines Control Council or Committee for Pharmaceutical Trials. I understand that my participation is voluntary and that I am free to withdraw at any time without giving any reason and without my medical care or legal rights being affected. I confirm that I have received a signed and dated copy of the Patient Information Sheet and Informed Consent Forms. VOLUNTEER: Name (capital letters) Signature Date WITNESS: (where required) Name (capital letters) Signature Date PERSON OBTAINING CONSENT: Name (capital letters) Signature Date CLINICAL INVESTIGATOR:
31 APPENDIX 3b 玻璃体腔注射 AAV2-ND4 治疗 Leber 遗传性视神经病变 亲爱的患者 : 研究 知情同意书 医生已经确诊您为 Leber 遗传性视神经病变 我们将邀请您参加这项 在您决定是否参加这项研究之前, 请尽可能仔细阅读以下内容 它可以帮助您 了解该项研究以及为何要进行这项研究, 研究的程序和期限, 参加研究后可能给您 带来的益处 风险和不适 如果您愿意, 您也可以和您的亲属 朋友一起讨论, 或 者请医生给予解释, 帮助您做出决定 一 研究背景和研究目的 1.1 疾病概况和治疗现况 Leber 遗传性视神经病变是一种主要累及黄斑乳头束纤维, 导致视神经退行 性变的遗传性疾病 由线粒体突变引起,11778 是最常见的位点 它是遗传性视 神经病变的常见类型, 由德国学者 Leber 于 1871 年首先报道 本病男性患者居 多, 常于 岁发病, 临床主要表现为双眼同时或先后急性或亚急性无痛性视 力减退, 同时可伴有中心视野缺失及色觉障碍 LHON 至今尚无有效的治疗方法 有日本研究者使用血管扩张剂艾地苯醌治 疗急性期病例, 尚在临床研究阶段, 疗效并不确切 临床有使用神经营养药物治 疗, 但并无肯定的疗效, 还有使用中医中药治疗, 但未进行科学的临床试验验证 1.2 本研究目的本次研究的目的在于评价基因治疗 Leber 遗传性视神经病变的安全性和疗效, 将重组腺相关病毒 2-ND4(AAV2-ND4)0.06ml 左右注射到患眼的玻璃体腔 ( 单次 ), 通过基因转染的方式在患眼病变部位表达正常的 ND4 蛋白, 达到治疗疾病的目的 1.3 研究参加单位和纳入患者例数 本研究的单位为华中科技大学同济医学院附属同济医院眼科, 拟纳入 7 名患者,
32 入选标准为 : 入选标准 : 1. 符合 Leber 遗传性视神经病变诊断标准, 并且为 位点突变, 近一年视力未提高者 2. 双眼视力均在 0.3 一下 3. 患者知情同意 自愿参加, 男女不限 4. 签署知情同意书 5. 8 岁 年龄 60 岁, 患者能耐受局麻手术 6. 遵从医生的指示, 能在规定的时间复诊 7. 我们选择单眼单次玻璃体腔注射治疗, 视力最差眼为注射眼, 两眼视力相同则选择右眼作为注射眼 8. 患者除了患有 Leber 遗传性视神经病变, 其他检查均正常 ( 肝肾功能 血常规 尿常规 免疫全套检测以及体液免疫反应等 ) 二 排除标准 1 佩带心脏起搏器者, 心肺及肾功能严重衰弱, 恶性肿瘤, 各种出血性疾病, 急性传感病, 高热, 高热性疾病, 妇女妊娠期, 心脏病手术后恢复期等 2 正参加其它临床研究的病人 3 有精神障碍的患者 4 患有糖尿病 高血压慢性疾病的患者 5 孕妇或在哺乳期妇女 6 术前检查项目异常, 如 AAV2 体液免疫反应阳性,CD3/CD8 异常等 三 如果参加研究将需要做什么? 1. 在您入选研究前, 医生将询问 记录您的病史, 并进行检查 2. 您是合格的纳入者, 您可自愿参加研究, 签署知情同意书 3. 如您不愿参加研究, 我们将按您的意愿施治 4. 若您自愿参加研究, 将按以下步骤进行 : 4.1. 签署知情同意书 ; 4.2. 进行术前检查, 包括眼部和全身检查 ;
33 4.3. 术前准备 ; 4.4. 手术, 单眼单次玻璃体腔注射 AAV2-ND4 0.05ml/ 左右 ; 4.5. 术后定期检查 : 术后 1 天 2 天 3 天 7 天,1 月 3 月 半年 4.6. 我们将长期追踪观察您的眼部和全身情况, 您可以随时与我们联系 四 参加研究可能的受益 1. 将免费获得 AAV2-ND4 治疗 2. 术前和术后免费进行眼部和全身检查 3. 可能缓解疾病, 提高视力 五 参加研究可能的不良反应 风险和不适 不方便 1. 麻醉以外, 药物过敏, 过敏性休克, 需进行抢救或暂停手术, 严重情况可危及生命 ; 2. 压迫眼球诱发眼心反射发生危及生命 ; 3. 术中损伤晶状体, 并发白内障 ; 4. 术中损伤视网膜, 导致视网膜脱离 ; 5. 术后感染, 影响伤口愈合 ; 6. 术后并发眼内炎 ; 7. 视力无提高 ; 8. 由于 AAV-ND4 是首次用于人体, 因此它对于人体的毒副作用, 目前尚未知晓, 可能的风险包括损伤眼睛, 严重的甚至失明, 引起免疫反应, 损伤患者肝肾及其他器官功能, 引起肿瘤危及生命等等 9. 本次研究将口服强的松 8 周, 可能导致患者胃部不适, 血压升高, 皮疹或荨麻疹等 10. 其他可能发生的无法预料或者不能防范的并发症等 六 有关费用 1. 将免费获得 AAV2-ND4 治疗 七 个人信息是保密的吗? 您的医疗记录 ( 研究病历 /CRF 化验单等 ) 将完整地保存在您所就诊的医
34 院 医生会将化验检查结果记录在您的病历上 研究者 伦理委员会和药品监督管理部门将被允许查阅您的医疗记录 任何有关本项研究结果的公开报告将不会披露您的个人身份 我们将在法律允许的范围内, 尽一切努力保护您个人医疗资料的隐私 八 怎样获得更多的信息? 您可以在任何时间提出有关本项研究的任何问题, 并得到相应的解答 如果在研究过程中有任何重要的新信息, 可能影响您继续参加研究的意愿时, 您的医生将会及时通知您 九 可以自愿选择参加研究和中途退出研究是否参加研究完全取决于您的意愿 您可以拒绝参加此项研究, 或在研究过程中的任何时间退出本研究, 这都不会影响您和医生间的关系, 都不会影响对您的医疗或有其他方面利益的损失 出于对您的最大利益考虑, 医生或研究者可能会在研究过程中随时中止您继续参加本项研究 如果您因为任何原因从研究中退出, 您可能被询问有关您使用试验药物的情况 如果医生认为需要, 您被要求进行实验室检查和体格检查 十. 您有任何疑问可与李斌教授联系, 联系电话
35 临床研究项目名称 : 玻璃体腔注射 AAV-ND4 治疗 Leber 遗传性视神经病变的研究课题承担单位 : 华中科技大学同济医学院附属同济医院同意声明我已经阅读了上述有关本研究的介绍, 而且有机会就此项研究与医生讨论并提出问题 我提出的所有问题都得到了满意的答复 我知道参加本研究可能产生的风险和受益 我知晓参加研究是自愿的, 我确认已有充足时间对此进行考虑, 而且明白 : 我可以随时向医生咨询更多的信息 我可以随时退出本研究, 而不会受到歧视或报复, 医疗待遇与权益不会受到影响 我同样清楚, 如果我中途退出研究, 特别是由于药物的原因使我退出研究时, 我若将我的病情变化告诉医生, 完成相应的体格检查和理化检查, 这将对整个研究十分有利 如果因病情变化我需要采取任何其他的药物治疗, 我会在事先征求医生的意见, 或在事后如实告诉医生 我同意药品监督管理部门伦理委员会或申办者代表查阅我的研究资料 我将获得一份经过签名并注明日期的知情同意书副本 最后, 我决定同意参加本项研究, 并保证尽量遵从医嘱 患者签名 : 患者监护人签名 联系电话 : 年 月 日 年 月 日 我确认已向患者解释了本实验的详细情况, 包括其权力以及可能的受益和风险, 并给其一份签署过的知情同意书副本 医生签名 : 年 月 日 医生的工作电话 :
36 APPENDIX 4 PRINCIPAL INVESTIGATOR Prof. Bin Li Published Literature in past 5 years 1. Han Pei, Xing Wan, Weikun Hu, xiaoyan Dong, Li B*. Constructing and detecting a new raav2/2-nd4, Eye Science, 2013, 28: Gao jing, Shi Hui,Pei Han, Li B*, Comparison of Immunosuppressive Effects and ND4 Expression among Different Immunosuppressive Strategies following AAV2-ND4 Gene Treatment for Leber Hereditary Optic Neuropathy, Acta Medicinae Universitatis Scientiae et Technologiae Huazhong, 2013,2, Cui G, Ding H, Xu Y, Li B*, Wang DW*. Applications of the method of high resolution melting analysis for diagnosis of Leber's disease and the three primary mutation spectrum of LHON in the Han Chinese population. Gene. Jan 1;512(1):108-12, Shi H, Gao J, Pei H, Liu R, Hu WK, Wan X, Li T, Li B*. AAV-mediated gene delivery of the human ND4 complex I subunit in rabbit eyes. Clin Experiment Ophthalmol. Dec;40 (9) : , Wei-Kun Hu,Rong Liu, Han Pei, Bin Li *, Endoplasmic Reticulum Stress-Related Factors Protect against Diabetic Retinopathy,Experimental Diabetes Research,2012,Epub 2011 Dec. 6. Shu Yan, Cui Zheng, Zhi-qi Chen, Rong Liu, Gui-gang Li, Wei-kun Hu, Han Pei, Bin Li *,Expression of Endoplasmic Reticulum Stress-Related Factors in the Retinas of Diabetic Rats,Experimental Diabetes Research, ID , 11 pages, Epub 2011 Aug 28, Hong Yang, Rong Liu, Zheng Cui, Zhi-qi Chen, Shu Yan, Han Pei, Bin Li*, Functional characterization of 58-kilodalton inhibitor of protein kinase in protecting against diabetic retinopathy via the endoplasmic reticulum stress pathway,molecular Vision,17:78-84, 2011;
37 8. Nan Xiang, Min-Jian Zhao, Xin-Yu Li, Hai-Hong Zheng, Gui-Gang Li, Li B*, Redundant Mechanisms for Vascular Growth Factors in Retinopathy of Prematurity in vitro. Ophthalmic Res.19;45(2):92-101, Li B*, Wang HS, Li GG, Zhao MJ, Zhao MH., The role of endoplasmic reticulum stress in the early stage of diabetic retinopathy. Acta Diabetol. 48(2):103-11, Bin Li, Hai-Qing Zhang, Yu Shi, Yun-Bing Min,Shao-Fen Lin, Kai-Li Wu,Jie Hu, Shi-Bo Tang, Overexpression of nuclear transport factor 2 may protect against diabetic retinopathy, Molecular Vision, 15: , Bin Li*, Dong Li, Gui-gang Li, Hao-wen Wang, Ai-xia Yu, P58 IPK inhibition of endoplasmic reticulum stress in human retinal capillary endothelial cells in vitro, Molecular Vision,4: , Bin Li*, Wei Yin, Xun Hong, Yu Shi, Hong-Sheng Wang, Shao-Fen Lin, and Shi-Bo Tang*, Remodeling Retinal Neovascularization by ALK1 Gene Transfection In Vitro, IOVS, 49: , 2008.
38 CLINICAL TRIAL PROTOCOL Intravitreal Injection of raav2-nd4 Treatment for Leber s Hereditary Optic Neuropathy Protocol No.: Version: 2 PRINCIPAL INVESTIGATOR Prof. Bin Li Tongji Hospital Tongji Medical College, Huazhong University of Science and Technology Wuhan, Hubei, China
39 LIST OF ABBREVIATIONS AND DEFINITIONS The following abbreviations and specialist terms are used in this study protocol: Abbreviation AAV BCVA CF ERG HM IOP LHON LogMAR MD MtDNA Explanation Adeno-associated virus Best corrected visual acuity Counting fingers Electroretinogram Hand movement Intraocular pressure Leber s hereditary optic neuropathy Logarithm of the minimum angle of resolution Mean defect Mitochondrial DNA ND4 NADH-ubiquinone oxidoreductase, subunit 4 OCT PSD RNFL VEP VFI Optical coherence tomography; Pattern standard deviation; Retinal nerve fiber layer; Visual evoked potential. Visual field index.
40 STUDY CONTACT LIST For questions regarding the conduct of this study, please contact: Project Leader: Bin Li, PhD,MD Telephone: Mobile: Telefax: Clinical Trial Manager: Xing Wan,MM (Clinical investigator) or Telephone: Mobile: Telefax: For reporting a serious adverse event, please contact: Clinical Trial Manager: Shuo Yang young_ophthalmology@foxmail.com Telephone: Mobile: Telefax:
41 1.Background Leber s hereditary optic neuropathy (LHON), which was first reported by the German scholar Theodore Leber in 1871, is a neurodegenerative genetic eye disease. It is caused by a gene mutation. The most common gene mutation is a mitochondrial mutation located at locus Most LHON patients are male. Onset of LHON usually occurs at 15 to 20 years of age. The primary clinical symptoms are acute or sub-acute painless eyesight degradation simultaneously or successively in both eyes, accompanied by central vision field loss and malfunction of chromatic vision. There is no sufficient therapy for LHON currently available. Neurotrophic drugs and traditional Chinese medicine have both been used to treat LHON, There is no definitively effective treatment available for LHON. It has been reported that idebenone, a short chain derivative of coenzyme Q10, is a potential agent to treat LHON [1], but its treatment efficiency is controversial. Idebenone and vitamin B12 therapy is reported effective in some patients [2], but failed for others [3, 4]. The latest studies have shown that the para-benzoquinone analog of CoQ and idebenone, EPI-743, holds promise with improved pharmacologic properties; there was a genuine improvement in several tested parameters of visual function [5-7]. Although the preliminary results are encouraging, new methods in the treatment of LHON still need to be explored. 2.Trial Objectives The purpose of this study was to determine if, in humans, gene therapy for LHON caused by the mtdna mutation would be associated with immediately obvious adverse events, and whether efficacy could be demonstrated. 3. Study population Enrollees in the study were patients diagnosed with LHON who carried the mtdna mutation. Patients did not suffer from any other diseases, and the best corrected visual acuity (BCVA) of both eyes was below 0.3. Because 4% of LHON patients
宫颈上皮内瘤变 ; IgG1 IgG2 亚类 ; 酶联免疫吸附试验 R A (2009)
 2009 年第 19 卷第 11 期 CHINA ONCOLOGY 2009 Vol.19 No.11 831 免疫球蛋白 G(immunoglobulin G,IgG) 是血清和细胞外液中含量最高的免疫球蛋白, 其某个亚类的升高, 可能与宫颈上皮内瘤变 (cervical intraepithelial neoplasia, CIN) 的发生 发展及转归有密切相关 然而对于 CIN 患者血清人乳头瘤病毒样颗粒
2009 年第 19 卷第 11 期 CHINA ONCOLOGY 2009 Vol.19 No.11 831 免疫球蛋白 G(immunoglobulin G,IgG) 是血清和细胞外液中含量最高的免疫球蛋白, 其某个亚类的升高, 可能与宫颈上皮内瘤变 (cervical intraepithelial neoplasia, CIN) 的发生 发展及转归有密切相关 然而对于 CIN 患者血清人乳头瘤病毒样颗粒
World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects
 Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the: 29th WMA General Assembly, Tokyo, Japan, October
Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the: 29th WMA General Assembly, Tokyo, Japan, October
读书报告 2015 年 月 唐之韵
 读书报告 2015 年 03-04 月 唐之韵 Mar. Apr. NEJM VOL.372 Kaukonen KM1, Bailey M, Pilcher D, et al. N Engl J Med. 2015 Apr 23;372(17):1629-38. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis.
读书报告 2015 年 03-04 月 唐之韵 Mar. Apr. NEJM VOL.372 Kaukonen KM1, Bailey M, Pilcher D, et al. N Engl J Med. 2015 Apr 23;372(17):1629-38. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis.
病毒基因组学与 病毒进化 刘翟博士研究员 中国科学院微生物研究所
 病毒基因组学与 病毒进化 刘翟博士研究员 中国科学院微生物研究所 2016-6-27 新一代测序技术带来了测序成本的大大降低!!! $100,000,000 $10,000,000 Moore s Law $1,000,000 $100,000 $10,000 $1,000 $1,000 $100 Pathogens Genomics Pathogen infections are among the
病毒基因组学与 病毒进化 刘翟博士研究员 中国科学院微生物研究所 2016-6-27 新一代测序技术带来了测序成本的大大降低!!! $100,000,000 $10,000,000 Moore s Law $1,000,000 $100,000 $10,000 $1,000 $1,000 $100 Pathogens Genomics Pathogen infections are among the
Health: Acupuncture in the UK 健康 : 针灸在英国
 Health: Acupuncture in the UK 健康 : 针灸在英国 1 Health: Acupuncture in the UK 健康 : 针灸在英国 A Better Way to Beat Back Pain? 腰疼有救吗? What is the single largest cause of sick leave in the UK? The answer is not the
Health: Acupuncture in the UK 健康 : 针灸在英国 1 Health: Acupuncture in the UK 健康 : 针灸在英国 A Better Way to Beat Back Pain? 腰疼有救吗? What is the single largest cause of sick leave in the UK? The answer is not the
A Case Report on Primary Ovarian Leiomyo-Sarcoma and Some Related Documents Review
 Asian Case Reports in Oncology 亚洲肿瘤科病例研究, 2013, 2, 11-15 http://dx.doi.org/10.12677/acrpo.2013.23004 Published Online July 2013 (http://www.hanspub.org/journal/acrpo.html) A Case Report on Primary Ovarian
Asian Case Reports in Oncology 亚洲肿瘤科病例研究, 2013, 2, 11-15 http://dx.doi.org/10.12677/acrpo.2013.23004 Published Online July 2013 (http://www.hanspub.org/journal/acrpo.html) A Case Report on Primary Ovarian
Part One-- Helicobacter Pylori. By Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd
 Part One-- Helicobacter Pylori (H. Pylori) By Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd. 2009.06 Helicobacter Pylori (H. Pylori) Helicobacter Pylori (H. Pylori) a spiral-shaped, flagellated, Gram-negative
Part One-- Helicobacter Pylori (H. Pylori) By Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd. 2009.06 Helicobacter Pylori (H. Pylori) Helicobacter Pylori (H. Pylori) a spiral-shaped, flagellated, Gram-negative
500 中国肺癌杂志2010年5月第13卷第5期 C h i n J L u n g C a n c e r, M ay , Vo l. 1 3, No. 5 临床研究 血清TPS CEA Pro-GRP和CYFRA21-1 水平在肺癌患者中的临床意义 王敬慧 时广利 张树才 王群慧
 5 临床研究 血清 和CYFRA- 水平在肺癌患者中的临床意义 王敬慧 时广利 张树才 王群慧 杨新杰 李曦 王海永 张卉 宋长兴 摘要 背景与目的 血清肿瘤标志物在肺癌的诊断 疗效 预后判断中起着重要作用 本研究探讨血清组 织多肽特异性抗原 tissue polypeptide specific antigen, 与癌胚抗原 carcinoembryonic antigen, 胃泌素释放 肽前体
5 临床研究 血清 和CYFRA- 水平在肺癌患者中的临床意义 王敬慧 时广利 张树才 王群慧 杨新杰 李曦 王海永 张卉 宋长兴 摘要 背景与目的 血清肿瘤标志物在肺癌的诊断 疗效 预后判断中起着重要作用 本研究探讨血清组 织多肽特异性抗原 tissue polypeptide specific antigen, 与癌胚抗原 carcinoembryonic antigen, 胃泌素释放 肽前体
中国 HIV 新发感染检测发展及未来 Development and Future of HIV-1 Incidence Assay in China
 中国 HIV 新发感染检测发展及未来 Development and Future of HIV-1 Incidence Assay in China Yan Jiang MD, PhD. National HIV Reference Laboratory, China CDC 中国 HIV 检测体系 HIV Testing in China Screening Confirmatory Recent
中国 HIV 新发感染检测发展及未来 Development and Future of HIV-1 Incidence Assay in China Yan Jiang MD, PhD. National HIV Reference Laboratory, China CDC 中国 HIV 检测体系 HIV Testing in China Screening Confirmatory Recent
Thyroid gland & Root of the neck OUTLINE. Thyroid gland
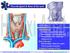 Dept. of Human Anatomy, Si Chuan University Zhou hongying eaglezhyxzy@163.com Thyroid gland & Root of the neck OUTLINE Thyroid gland Location & superficial feature Blood supply & Venous drainage Relationship
Dept. of Human Anatomy, Si Chuan University Zhou hongying eaglezhyxzy@163.com Thyroid gland & Root of the neck OUTLINE Thyroid gland Location & superficial feature Blood supply & Venous drainage Relationship
Thyroid gland & Root of neck. L o g o
 Thyroid gland & Root of neck Dept. of Human Anatomy Zhou Hong Ying L o g o Thyroid gland Outline Location & superficial feature Blood supply & Venous drainage Relationship of its vessels and related nerves
Thyroid gland & Root of neck Dept. of Human Anatomy Zhou Hong Ying L o g o Thyroid gland Outline Location & superficial feature Blood supply & Venous drainage Relationship of its vessels and related nerves
利用 PEN 项目网络教育 Making use of PEN project web-based education 发展聋人高等特殊教育 to develop higher education for the deaf
 利用 PEN 项目网络教育 Making use of PEN project web-based education 发展聋人高等特殊教育 to develop higher education for the deaf 长春大学副校长庄树范 Vice-president of Changchun University Zhuang Shufan 一 PEN 项目与我校的机缘 内容提要 Informative
利用 PEN 项目网络教育 Making use of PEN project web-based education 发展聋人高等特殊教育 to develop higher education for the deaf 长春大学副校长庄树范 Vice-president of Changchun University Zhuang Shufan 一 PEN 项目与我校的机缘 内容提要 Informative
Definition of Stroke ( 中风 )
 Definition of Stroke ( 中风 ) Sudden brain damage Lack of blood flow to the brain caused by a clot or rupture of a blood vessel Ischemia = Clot 缺血 (makes up approximately 87% of all strokes) Embolic Thrombotic
Definition of Stroke ( 中风 ) Sudden brain damage Lack of blood flow to the brain caused by a clot or rupture of a blood vessel Ischemia = Clot 缺血 (makes up approximately 87% of all strokes) Embolic Thrombotic
老年与中青年急性呼吸窘迫综合征患者的特点及预后相关危险因素分析
 794 中华危重病急救医学 2014 年 11 月第 26 卷第 11 期 Chin Crit Care Med,November 2014,Vol.26,No.11 论著 老年与中青年急性呼吸窘迫综合征患者的特点及预后相关危险因素分析 摘要 目的比较重症监护病房 (ICU) 内老年及中青年急性呼吸窘迫综合征 (ARDS) 患者的临床特点及预后相关危险因素 方法回顾性分析 2011 年 8 月至 2013
794 中华危重病急救医学 2014 年 11 月第 26 卷第 11 期 Chin Crit Care Med,November 2014,Vol.26,No.11 论著 老年与中青年急性呼吸窘迫综合征患者的特点及预后相关危险因素分析 摘要 目的比较重症监护病房 (ICU) 内老年及中青年急性呼吸窘迫综合征 (ARDS) 患者的临床特点及预后相关危险因素 方法回顾性分析 2011 年 8 月至 2013
Małgorzata Golik. Physiotherapist, certified, master s degree physiotherapist, neurological disorders specialist
 全身运动反馈系统在波兰和欧洲的应用 Małgorzata Golik Physiotherapist, certified, master s degree physiotherapist, neurological disorders specialist Graduation: Warsaw Academy of Physical Activity and Education, Poland Poland
全身运动反馈系统在波兰和欧洲的应用 Małgorzata Golik Physiotherapist, certified, master s degree physiotherapist, neurological disorders specialist Graduation: Warsaw Academy of Physical Activity and Education, Poland Poland
2015 年 4 月 25 日 GRE 考试 语文部分 真题回忆答案解析 小站教育独家出品 版权所有翻录必究
 2015 年 4 月 25 日 GRE 考试 语文部分 真题回忆答案解析 小站教育独家出品 版权所有翻录必究 [4.25GRE 填空部分 ]: 1.The subject who were engaged in more difficult tasks deterioration in iheir performance over times, and therefore the need to concentrate
2015 年 4 月 25 日 GRE 考试 语文部分 真题回忆答案解析 小站教育独家出品 版权所有翻录必究 [4.25GRE 填空部分 ]: 1.The subject who were engaged in more difficult tasks deterioration in iheir performance over times, and therefore the need to concentrate
Global Mental Health Challenges Facing China and the World
 Global Mental Health Challenges Facing China and the World 世界与中国面对的精神卫生挑战 Arthur Kleinman, M.D. Rabb Professor of, University and Professor of Medical and Psychiatry, Medical School; Fung Director, Asia
Global Mental Health Challenges Facing China and the World 世界与中国面对的精神卫生挑战 Arthur Kleinman, M.D. Rabb Professor of, University and Professor of Medical and Psychiatry, Medical School; Fung Director, Asia
Diseases of the Urinary System. Wang Yishu Department of Pathology Basic Medical School
 Diseases of the Urinary System Wang Yishu Department of Pathology Basic Medical School Introduction Anatomy and Histology of Urinary System Urinary System Kidney Bladder Ureter Urethra Functions of kidney
Diseases of the Urinary System Wang Yishu Department of Pathology Basic Medical School Introduction Anatomy and Histology of Urinary System Urinary System Kidney Bladder Ureter Urethra Functions of kidney
新型 DES 和 BVS 血栓发生现状及应对策略 钱菊英,MD, FACC,FESC 复旦大学附属中山医院上海市心血管病研究所
 新型 DES 和 BVS 血栓发生现状及应对策略 钱菊英,MD, FACC,FESC 复旦大学附属中山医院上海市心血管病研究所 OCC2016 Increase of LST and death for first generation DES after stop of 6m DAPT BASKET-LATE Study P
新型 DES 和 BVS 血栓发生现状及应对策略 钱菊英,MD, FACC,FESC 复旦大学附属中山医院上海市心血管病研究所 OCC2016 Increase of LST and death for first generation DES after stop of 6m DAPT BASKET-LATE Study P
Advances in Investigation and Management of Neurodegenerative Diseases in Aging
 Advances in Investigation and Management of Neurodegenerative Diseases in Aging Piu Chan, MD PhD Beijing Institute of Geriatrics Xuanwu Hospital of Capital Medical University 人口老龄化是世界发展的必然 (UN 2009 data)
Advances in Investigation and Management of Neurodegenerative Diseases in Aging Piu Chan, MD PhD Beijing Institute of Geriatrics Xuanwu Hospital of Capital Medical University 人口老龄化是世界发展的必然 (UN 2009 data)
How to Make the Choice?
 How to Make the Choice? Translational Approach for Cancer Treatment 闻丹忆 Danyi.wen@lidebiotech.com 181 9229 52 Nov 23, 214 1 Tumor volume (mm 3 ) Tumor growth (Efficacy study in LIX4-FP1+2) 15 Vehiclel,
How to Make the Choice? Translational Approach for Cancer Treatment 闻丹忆 Danyi.wen@lidebiotech.com 181 9229 52 Nov 23, 214 1 Tumor volume (mm 3 ) Tumor growth (Efficacy study in LIX4-FP1+2) 15 Vehiclel,
博士后学位论文. Importin 13: 一个新的角膜上皮前体细胞标志物. Importin 13:a Novel Potential Marker for Corneal Epithelial Progenitor Cells 指导教师 : 刘祖国 专业名称 : 眼科学
 学校编码 :10384 学号 :BH17000206 博士后学位论文 Importin 13: 一个新的角膜上皮前体细胞标志物 Importin 13:a Novel Potential Marker for Corneal Epithelial Progenitor Cells 王华 指导教师 : 刘祖国 专业名称 : 眼科学 答辩日期 :2009 年 9 月 厦门大学学位论文原创性声明 本人呈交的学位论文是本人在导师指导下,
学校编码 :10384 学号 :BH17000206 博士后学位论文 Importin 13: 一个新的角膜上皮前体细胞标志物 Importin 13:a Novel Potential Marker for Corneal Epithelial Progenitor Cells 王华 指导教师 : 刘祖国 专业名称 : 眼科学 答辩日期 :2009 年 9 月 厦门大学学位论文原创性声明 本人呈交的学位论文是本人在导师指导下,
1) 有哪些方法, 为什么需要采用这些方法? 2) 有哪些参数, 这些参数的生理学意义是什么? 3) 功能的研究如何提示机制的改变?
 心脏泵功能的 测量方法 1 1) 有哪些方法, 为什么需要采用这些方法? 2) 有哪些参数, 这些参数的生理学意义是什么? 3) 功能的研究如何提示机制的改变? 2 心功能测量方法 In vivo 1. 超声法 2. 超声 + 导管法 In vitro 1. Langendorff mode 2. Working heart mode 3. Papillary muscle 4. Skinned fibers
心脏泵功能的 测量方法 1 1) 有哪些方法, 为什么需要采用这些方法? 2) 有哪些参数, 这些参数的生理学意义是什么? 3) 功能的研究如何提示机制的改变? 2 心功能测量方法 In vivo 1. 超声法 2. 超声 + 导管法 In vitro 1. Langendorff mode 2. Working heart mode 3. Papillary muscle 4. Skinned fibers
Doing Business in China
 Doing Business in China 1 Doing Business in China 在中国经商 How to Win in Business in China 怎么在中国赢取商机 Read the text below and do the activity that follows. 阅读下面的短文, 然后完成练习 China has changed enormously over
Doing Business in China 1 Doing Business in China 在中国经商 How to Win in Business in China 怎么在中国赢取商机 Read the text below and do the activity that follows. 阅读下面的短文, 然后完成练习 China has changed enormously over
Troubleshooting & Maintenance with PROFIBUS 故障排除及维护
 Troubleshooting & Maintenance with PROFIBUS 故障排除及维护 Mr. Ing. Dennis van Booma PROCENTEC dbooma@procentec.com PROFIBUS International 2006.ppt About the presenter 演讲者简介 Name: Dennis van Booma Company: PROCENTEC
Troubleshooting & Maintenance with PROFIBUS 故障排除及维护 Mr. Ing. Dennis van Booma PROCENTEC dbooma@procentec.com PROFIBUS International 2006.ppt About the presenter 演讲者简介 Name: Dennis van Booma Company: PROCENTEC
PowerPoint Slides English Text Mandarin Chinese Translation Palliative Care, Part 1. 姑息性护理, 第 1 部分 VideoTranscript
 PowerPoint Slides English Text Mandarin Chinese Translation Palliative Care, Part 1 姑息性护理, 第 1 部分 VideoTranscript Professional Oncology Education Palliative Care, Part 1 Time: 26:42 Donna S. Zhukovsky,
PowerPoint Slides English Text Mandarin Chinese Translation Palliative Care, Part 1 姑息性护理, 第 1 部分 VideoTranscript Professional Oncology Education Palliative Care, Part 1 Time: 26:42 Donna S. Zhukovsky,
Add Your Company Slogan 损伤的修复. Repair of the injury 白求恩医学院病理教研室 Logo
 Add Your Company Slogan 损伤的修复 白求恩医学院病理教研室 李 伟 2010.9.26 tissue and cellular injury Normal cells Adaptation irreversible injury reversible injury Adaptation hypertrophy hyperplasia atrophy metaplasia cellular
Add Your Company Slogan 损伤的修复 白求恩医学院病理教研室 李 伟 2010.9.26 tissue and cellular injury Normal cells Adaptation irreversible injury reversible injury Adaptation hypertrophy hyperplasia atrophy metaplasia cellular
Cross-sectional study on the relationship between life events and mental health of secondary school students in Shanghai, China
 162 Shanghai Archives of Psychiatry, 2012, Vol.24, No.3 Cross-sectional study on the relationship between life events and mental health of secondary school students in Shanghai, China Linlin ZHOU, Juan
162 Shanghai Archives of Psychiatry, 2012, Vol.24, No.3 Cross-sectional study on the relationship between life events and mental health of secondary school students in Shanghai, China Linlin ZHOU, Juan
Hypertension and cardiac arrhythmias
 CardioPulse 223 doi:10.1093/eurheartj/ehw664 Hypertension and cardiac arrhythmias A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Council
CardioPulse 223 doi:10.1093/eurheartj/ehw664 Hypertension and cardiac arrhythmias A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Council
病理学. Pathology 白求恩医学院病理学系 李伟
 病理学 Pathology 白求恩医学院病理学系 李伟 2010.9.21 The main duty of medicine Patients just want to know:? What disease am I suffering from?? How can you cure it?? How could you prevent me from having the illness in
病理学 Pathology 白求恩医学院病理学系 李伟 2010.9.21 The main duty of medicine Patients just want to know:? What disease am I suffering from?? How can you cure it?? How could you prevent me from having the illness in
Unit1 What s the matter? 东乌旗蒙中 --- 苏丽雅
 Unit1 What s the matter? 东乌旗蒙中 --- 苏丽雅 body feet tooth teeth ear eye head face nose mouth head hand knee t leg foot finger healthy neck shoulder arm back sing a song 1a Look at the picture. Write the
Unit1 What s the matter? 东乌旗蒙中 --- 苏丽雅 body feet tooth teeth ear eye head face nose mouth head hand knee t leg foot finger healthy neck shoulder arm back sing a song 1a Look at the picture. Write the
Author Academy: Effectively Communicating your Research
 Author Academy: Effectively Communicating your Research Chinese Academy of Sciences, Shanghai Branch& ShanghaiTech University April 2015 Jeffrey Robens, PhD Senior Research Consultant Education Group Leader
Author Academy: Effectively Communicating your Research Chinese Academy of Sciences, Shanghai Branch& ShanghaiTech University April 2015 Jeffrey Robens, PhD Senior Research Consultant Education Group Leader
ESCMID Online Lecture Library. by author
 Diagnosing common and novel respiratory virus infections in the clinical setting 常见和新型呼吸道病毒感染的临床 / 病理诊断 Prof. Nelson Lee 李礼舜教授 MD (CUHK), FRCP (Lond), FRCP (Edin) FHKCP (ID), FHKAM (Med) 香港中文大学内科及药物治疗学系感染及传染病科主管威爾斯親王醫院及香港新界東聯網醫院名譽顧問醫生香港中文大學何鴻燊防治傳染病研究中心執行委員會委員
Diagnosing common and novel respiratory virus infections in the clinical setting 常见和新型呼吸道病毒感染的临床 / 病理诊断 Prof. Nelson Lee 李礼舜教授 MD (CUHK), FRCP (Lond), FRCP (Edin) FHKCP (ID), FHKAM (Med) 香港中文大学内科及药物治疗学系感染及传染病科主管威爾斯親王醫院及香港新界東聯網醫院名譽顧問醫生香港中文大學何鴻燊防治傳染病研究中心執行委員會委員
HealthNews Inspiring and informative stories for patients MICA (P) No. 054/02/2010
 FREE October 2010 HealthNews Inspiring and informative stories for patients MICA (P) No. 054/02/2010 Conquering cancer at... 84 A wonderful testimony for elderly cancer patients, U Gyan fought and won
FREE October 2010 HealthNews Inspiring and informative stories for patients MICA (P) No. 054/02/2010 Conquering cancer at... 84 A wonderful testimony for elderly cancer patients, U Gyan fought and won
Metabolism Of Calcium and Phosphorus
 Metabolism Of Calcium and Phosphorus Three hormones crucially involved: Vitamin D (1,25- (OH)2-D3) Parathyroid hormone (PTH) Calcitonin (CT) Calcium Is Needed For: Bone Matrix (70% CaOH in form of Hydroxyapatite)
Metabolism Of Calcium and Phosphorus Three hormones crucially involved: Vitamin D (1,25- (OH)2-D3) Parathyroid hormone (PTH) Calcitonin (CT) Calcium Is Needed For: Bone Matrix (70% CaOH in form of Hydroxyapatite)
Uncertainty of Measurement Application to Laboratory Medicine 鏡檢組 蔡雅雯 2014/09/09
 Uncertainty of Measurement Application to Laboratory Medicine 鏡檢組 蔡雅雯 2014/09/09 Objectives Definitions Methodology Equation of Measurement Uncertainty Measurement Uncertainty Goal Examples Uncertainty
Uncertainty of Measurement Application to Laboratory Medicine 鏡檢組 蔡雅雯 2014/09/09 Objectives Definitions Methodology Equation of Measurement Uncertainty Measurement Uncertainty Goal Examples Uncertainty
Qingdao chronic disease management Comprehensive prevention and treatment integrated care system development. Qingdao HFPC Renmin Wei
 Qingdao chronic disease management Comprehensive prevention and treatment integrated care system development Qingdao HFPC Renmin Wei 2016-07-28 Qingdao China middle east area and south of Shandong Important
Qingdao chronic disease management Comprehensive prevention and treatment integrated care system development Qingdao HFPC Renmin Wei 2016-07-28 Qingdao China middle east area and south of Shandong Important
The Diseases of Lymphoid & Hematopoietic system ( 淋巴和造血系统疾病 )
 The Diseases of Lymphoid & Hematopoietic system ( 淋巴和造血系统疾病 ) Wei Zhang ( 张伟 ) Ph.D Associate Professor Department of Pathology, Zhejiang University School of Medicine zwei72@zju.edu.cn Tel: 13819103401
The Diseases of Lymphoid & Hematopoietic system ( 淋巴和造血系统疾病 ) Wei Zhang ( 张伟 ) Ph.D Associate Professor Department of Pathology, Zhejiang University School of Medicine zwei72@zju.edu.cn Tel: 13819103401
Obtaining phenotype and outcome data from health records: China Kadoorie Biobank experience
 Obtaining phenotype and outcome data from health records: China Kadoorie Biobank experience Zhengming CHEN CKB Principal Investigator Professor of Epidemiology Nuffield Dept. of Population Health University
Obtaining phenotype and outcome data from health records: China Kadoorie Biobank experience Zhengming CHEN CKB Principal Investigator Professor of Epidemiology Nuffield Dept. of Population Health University
Background. Background. Acupuncture Protocol
 Background The Achilles tendon is a problematic structure in the general population and in competitive and recreational athletes Specifically affecting middle aged runners (Paavola et al, 2002; Woo et
Background The Achilles tendon is a problematic structure in the general population and in competitive and recreational athletes Specifically affecting middle aged runners (Paavola et al, 2002; Woo et
Management Skills. Management Skills Syllabus 2017/11/2. 北校区 Wed. 18:30-20:40. Course Introduction. Mission, Vision, Values and Principles
 Management Skills Sherman Chui For 2017-2018-1 GUANGDONG UNIVERSITY OF FOREIGN STUDIES Management Skills Syllabus 北校区 2-223 Wed. 18:30-20:40 Week 1 Week 2 Week 3 Week 4 Course Introduction Mission, Vision,
Management Skills Sherman Chui For 2017-2018-1 GUANGDONG UNIVERSITY OF FOREIGN STUDIES Management Skills Syllabus 北校区 2-223 Wed. 18:30-20:40 Week 1 Week 2 Week 3 Week 4 Course Introduction Mission, Vision,
CU and CIS states UZBEKISTAN. (3.5 mill) (4.5 mill) (BELARUS 10.5 mill) (KAZAKHSTAN 17 mill) Yes Yes Yes 3) No Yes. (27 mill)
 CU and CIS states for - -City mark Requirements *) Sample needed for safety for reports accepted CU(Custom Union) UKRAINE UZBEKISTAN ARMENIA MOLDOVA (Russia 150 mill) (52 mill) (27 mill) (3.5 mill) (4.5
CU and CIS states for - -City mark Requirements *) Sample needed for safety for reports accepted CU(Custom Union) UKRAINE UZBEKISTAN ARMENIA MOLDOVA (Russia 150 mill) (52 mill) (27 mill) (3.5 mill) (4.5
Pulmonary Rehabilitation 肺康复 许灵玲
 Pulmonary Rehabilitation 肺康复 许灵玲 Contents 目录 What s pulmonary rehabilitation? Who are the members of the Interdisciplinary Team? Who benefits from pulmonary rehabilitation? What s the goals of pulmonary
Pulmonary Rehabilitation 肺康复 许灵玲 Contents 目录 What s pulmonary rehabilitation? Who are the members of the Interdisciplinary Team? Who benefits from pulmonary rehabilitation? What s the goals of pulmonary
Ling Zhao Huazhong Agricultural University Sep. 21, 2015
 Rabies vaccine development Ling Zhao Huazhong Agricultural University Sep. 21, 2015 Outlines 1. Rabies 2. Rabies virus 3. Rabies epidemiology 4. Rabies vaccine development 5. Perspective Rabies-one of
Rabies vaccine development Ling Zhao Huazhong Agricultural University Sep. 21, 2015 Outlines 1. Rabies 2. Rabies virus 3. Rabies epidemiology 4. Rabies vaccine development 5. Perspective Rabies-one of
Journal of Acupuncture and Tuina Science, 2012, v. 10 n. 2, p The original publication is available at
 Title What is the origin of acupoint Author(s) Li, L; Yau, T; Yau, C Citation Journal of Acupuncture and Tuina Science, 2012, v. 10 n. 2, p. 125-127 Issued Date 2012 URL http://hdl.handle.net/10722/160714
Title What is the origin of acupoint Author(s) Li, L; Yau, T; Yau, C Citation Journal of Acupuncture and Tuina Science, 2012, v. 10 n. 2, p. 125-127 Issued Date 2012 URL http://hdl.handle.net/10722/160714
Allergies the clinical picture, diagnosis, and treatments. 1. What is an allergy? 什么是过敏?
 Allergies the clinical picture, diagnosis, and treatments 1. What is an allergy? 什么是过敏? An allergy is an immunologic response to foreign substances in foods or in the environment. These substances, allergens,
Allergies the clinical picture, diagnosis, and treatments 1. What is an allergy? 什么是过敏? An allergy is an immunologic response to foreign substances in foods or in the environment. These substances, allergens,
Interferon-gamma and interleukin-10 levels in serum and saliva are related to different types of oral lichen planus
 中国组织工程研究第 19 卷第 2 期 2015 01 08 出版 Chinese Journal of Tissue Engineering Research January 8, 2015 Vol.19, No.2 Interferon-gamma and interleukin-10 levels in serum and saliva are related to different types
中国组织工程研究第 19 卷第 2 期 2015 01 08 出版 Chinese Journal of Tissue Engineering Research January 8, 2015 Vol.19, No.2 Interferon-gamma and interleukin-10 levels in serum and saliva are related to different types
Different firing patterns induced by veratridine and aconitine in injured dorsal root ganglion neurons
 Acta Physiologica Sinica, April 25, 2005, 57 (2): 169-174 http://www.actaps.com.cn 169 Research Paper Different firing patterns induced by veratridine and aconitine in injured dorsal root ganglion neurons
Acta Physiologica Sinica, April 25, 2005, 57 (2): 169-174 http://www.actaps.com.cn 169 Research Paper Different firing patterns induced by veratridine and aconitine in injured dorsal root ganglion neurons
Uric acid status and its correlates in Hangzhou urban population
 102 Asia Pac J Clin Nutr 2006;15 (1):102-106 Original Article Uric acid status and its correlates in Hangzhou urban population Duo Li PhD 1, Xiaomei Yu MB 2, Xiaoqun Zhou MB 2, Sirithon Siriamornpun PhD
102 Asia Pac J Clin Nutr 2006;15 (1):102-106 Original Article Uric acid status and its correlates in Hangzhou urban population Duo Li PhD 1, Xiaomei Yu MB 2, Xiaoqun Zhou MB 2, Sirithon Siriamornpun PhD
Testing the acceptability of liquid fish oil in older adults
 Asia Pac J Clin Nutr 2011;20 (2):175-179 175 Short Communication Testing the acceptability of liquid fish oil in older adults Alison Yaxley BSc(Hons) 1, Michelle D Miller PhD 2, Robert J Fraser PhD 3,
Asia Pac J Clin Nutr 2011;20 (2):175-179 175 Short Communication Testing the acceptability of liquid fish oil in older adults Alison Yaxley BSc(Hons) 1, Michelle D Miller PhD 2, Robert J Fraser PhD 3,
间歇性低氧运动对大鼠骨骼肌线粒体自由基代谢的影响
 第 14 卷第 7 期 2007 年 10 月 体育学刊 Journal of Physical Education Vol.14 No.7 Oct.2007 间歇性低氧运动对大鼠骨骼肌线粒体自由基代谢的影响 1 1 弢 2, 2 13 (1. 华南师范大学体育科学学院, 广东广州 510631; 广州体育学院运动人体科学系, 广东广州 510500) 摘要 : 100 SD (40 )(60 )
第 14 卷第 7 期 2007 年 10 月 体育学刊 Journal of Physical Education Vol.14 No.7 Oct.2007 间歇性低氧运动对大鼠骨骼肌线粒体自由基代谢的影响 1 1 弢 2, 2 13 (1. 华南师范大学体育科学学院, 广东广州 510631; 广州体育学院运动人体科学系, 广东广州 510500) 摘要 : 100 SD (40 )(60 )
学前 Screening 筛选 of Children Worthwhile?
 Is Pre-school 学前 Screening 筛选 of Children Worthwhile? Dr. Dorothy FAN Consultant Ophthalmologist Department of Ophthalmology Hong Kong Sanatorium & Hospital Amblyopia ( 弱视 ) Amblyopia results from degradation
Is Pre-school 学前 Screening 筛选 of Children Worthwhile? Dr. Dorothy FAN Consultant Ophthalmologist Department of Ophthalmology Hong Kong Sanatorium & Hospital Amblyopia ( 弱视 ) Amblyopia results from degradation
2009 甲流疫情发生概况 3 例确诊病例
 outline 2009 甲流疫情发生概况 2009 年 4 月 24 日 : 墨西哥出现 猪流感 患者约 800 例, 包括 60 例死亡病例 美国出现 7 例确诊病例 世卫组织要求各国卫生部门提高警惕 4 月 25 日 : 世卫组织确认该病毒属 H1N1 毒株族, 具有 潜在大流行 能力 4 月 27 日 : 世卫组织宣布将警戒级别从 3 级提升至 4 级 欧洲证实出现最早 3 例确诊病例 4
outline 2009 甲流疫情发生概况 2009 年 4 月 24 日 : 墨西哥出现 猪流感 患者约 800 例, 包括 60 例死亡病例 美国出现 7 例确诊病例 世卫组织要求各国卫生部门提高警惕 4 月 25 日 : 世卫组织确认该病毒属 H1N1 毒株族, 具有 潜在大流行 能力 4 月 27 日 : 世卫组织宣布将警戒级别从 3 级提升至 4 级 欧洲证实出现最早 3 例确诊病例 4
ISO TC34/SC19 Bee Products Secretariat
 ISO TC34/SC19 Bee Products Secretariat 1. The Establishment of ISO TC 34/SC 19 Royal Jelly Standard (WG13) 2008 TC 34/WG 13 Royal Jelly China, France, Japan, Turkey, Italy, Germany United States, Argentina,
ISO TC34/SC19 Bee Products Secretariat 1. The Establishment of ISO TC 34/SC 19 Royal Jelly Standard (WG13) 2008 TC 34/WG 13 Royal Jelly China, France, Japan, Turkey, Italy, Germany United States, Argentina,
乙型肝炎疫苗初次免疫成年正常应答和高应答者 3 年抗体持久性观察
 478 中华预防医学杂志 216 年 6 月第 5 卷第 6 期 Chin J Prev Med,June 216, Vol. 5, No. 6 乙型肝炎疫苗初次免疫成年正常应答和高应答者 3 年抗体持久性观察 吕静静 张丽 颜丙玉 刘甲野 冯艺 宋立志 陈士玉 周立波 梁晓峰 崔富强 王富珍 徐爱强 摘要 目的探讨乙型肝炎疫苗 (HepB) 初次免疫成年正常应答和高应答者 3 年的抗体持久性及其影响因素
478 中华预防医学杂志 216 年 6 月第 5 卷第 6 期 Chin J Prev Med,June 216, Vol. 5, No. 6 乙型肝炎疫苗初次免疫成年正常应答和高应答者 3 年抗体持久性观察 吕静静 张丽 颜丙玉 刘甲野 冯艺 宋立志 陈士玉 周立波 梁晓峰 崔富强 王富珍 徐爱强 摘要 目的探讨乙型肝炎疫苗 (HepB) 初次免疫成年正常应答和高应答者 3 年的抗体持久性及其影响因素
The Internal Structure of Spinal Cord
 The Internal Structure of Spinal Cord OUTLINE Gray Matter White Matter Conscious Sensory & Motor Tracts Chief Functions of Spinal Cord signal transmitting spinal reflex Internal Structure of Spinal Cord
The Internal Structure of Spinal Cord OUTLINE Gray Matter White Matter Conscious Sensory & Motor Tracts Chief Functions of Spinal Cord signal transmitting spinal reflex Internal Structure of Spinal Cord
吉林大学 教师教案 (2010 ~2011 学年第 1 学期 ) 课程名称 : 病理学年级 :2008 级七年制教研室 : 病理学系任课教师 : 王琳 吉林大学教务处制
 吉林大学 教师教案 (2010 ~2011 学年第 1 学期 ) 课程名称 : 病理学年级 :2008 级七年制教研室 : 病理学系任课教师 : 王琳 吉林大学教务处制 教案 课程名称 : 病理学 授课教师王琳所在单位白求恩医学院 课程类型理论授课时间 2010.10.29 (7-8) 授课对象 2008 级七年制 教学内容提要 时间分配及备注 Respiratory System-1 Normal
吉林大学 教师教案 (2010 ~2011 学年第 1 学期 ) 课程名称 : 病理学年级 :2008 级七年制教研室 : 病理学系任课教师 : 王琳 吉林大学教务处制 教案 课程名称 : 病理学 授课教师王琳所在单位白求恩医学院 课程类型理论授课时间 2010.10.29 (7-8) 授课对象 2008 级七年制 教学内容提要 时间分配及备注 Respiratory System-1 Normal
Bosworth, /1966. Clemmer, 1966 Driscoll McCorkle & Korn 1954 Ohlin 1956 Wheeler 1961 U U-shaped curve Wheeler
 关于服刑人员适应监狱生活的经验性研究起源于 20 世纪的美国之后, 社 会学家开始用人类学方法研究, 然而在这些研究中, 关于女性服刑人员监狱适应的研究较 少, 并被当做男性服刑人员 附属物 来比较, 此后有学者开始关注监狱环境以及社会文化背 景对于女性服刑人员监狱适应性状况的影响本文通过回顾与反思认为, 对女性应从两个维 度即适应监狱制度环境和适应服刑人员群体或服刑人员亚文化入手并从中探讨两者间关
关于服刑人员适应监狱生活的经验性研究起源于 20 世纪的美国之后, 社 会学家开始用人类学方法研究, 然而在这些研究中, 关于女性服刑人员监狱适应的研究较 少, 并被当做男性服刑人员 附属物 来比较, 此后有学者开始关注监狱环境以及社会文化背 景对于女性服刑人员监狱适应性状况的影响本文通过回顾与反思认为, 对女性应从两个维 度即适应监狱制度环境和适应服刑人员群体或服刑人员亚文化入手并从中探讨两者间关
芬美意对嗅觉受体的研究和应用 - 服务社会, 创造商机
 芬美意对嗅觉受体的研究和应用 - 服务社会, 创造商机 Firmenich Olfaction Receptor Biology: Understanding the human nose and its applications on social responsibility programs and business opportunities in the space of malodor
芬美意对嗅觉受体的研究和应用 - 服务社会, 创造商机 Firmenich Olfaction Receptor Biology: Understanding the human nose and its applications on social responsibility programs and business opportunities in the space of malodor
Iodine excess or not: analysis on the necessity of reducing the iodine content in edible salt based on the national monitoring results
 Asia Pac J Clin Nutr 2011;20 (4):501-506 501 Review Iodine excess or not: analysis on the necessity of reducing the iodine content in edible salt based on the national monitoring results Sumei Li 1, Qingsi
Asia Pac J Clin Nutr 2011;20 (4):501-506 501 Review Iodine excess or not: analysis on the necessity of reducing the iodine content in edible salt based on the national monitoring results Sumei Li 1, Qingsi
the micro level, only by hard work can we form the bedrock of good performance in school. 模块 4: 正反论述 第一句 : 引出争议
 四六级写作 魔 板 模块 1: 描图 第一部分 : 描述图片 (1) As is humorously/vividly/clearly/subtly depicted/described/portrayed/illustrated in the picture/drawing/cartoon,. ( 句子 ) (2) The picture/cartoon displays/portrays a very
四六级写作 魔 板 模块 1: 描图 第一部分 : 描述图片 (1) As is humorously/vividly/clearly/subtly depicted/described/portrayed/illustrated in the picture/drawing/cartoon,. ( 句子 ) (2) The picture/cartoon displays/portrays a very
通过将课程内容大纲和职业素养与现有课程进行对照, 找出差距或需要改进的地方 鼓励卫生科学领域的课程开发人员评价现有的教学内容, 采纳并测试课程内容大纲和职业素养 将疼痛课程和管理疼痛职业素养贯穿于学生健康教育和培训形成阶段的学习机会 活动以及未来的专业发展中
 FACT SHEET No. 4 将管理疼痛的职业素养和 IASP 课程大纲纳入职业教育 IASP 课程大纲为药学 心理学 物理治疗 职业治疗 护理 医学 牙医学 社会工作以及跨专业等教育提供相关推荐课程 该大纲有利于为本科生和研究生塑造一个关于急性 慢性和癌性疼痛的教学课程, 并在 2017 年已全部更新 欧洲疼痛协会出版的 欧洲疼痛医学文凭核心课程 (2016) 阐明了学员获取学习成果主要通过
FACT SHEET No. 4 将管理疼痛的职业素养和 IASP 课程大纲纳入职业教育 IASP 课程大纲为药学 心理学 物理治疗 职业治疗 护理 医学 牙医学 社会工作以及跨专业等教育提供相关推荐课程 该大纲有利于为本科生和研究生塑造一个关于急性 慢性和癌性疼痛的教学课程, 并在 2017 年已全部更新 欧洲疼痛协会出版的 欧洲疼痛医学文凭核心课程 (2016) 阐明了学员获取学习成果主要通过
Film: Harry Potter Premiere 电影 : 哈里 波特首映式
 Film: Harry Potter Premiere 电影 : 哈里 波特首映式 1 Film: Harry Potter Premiere 电影 : 哈里 波特首映式 Harry Potter Stars Drenched at Premiere 哈里 波特班底明星首映式遇倾盆大雨 Harry Potter's magic spells might be able to fight the forces
Film: Harry Potter Premiere 电影 : 哈里 波特首映式 1 Film: Harry Potter Premiere 电影 : 哈里 波特首映式 Harry Potter Stars Drenched at Premiere 哈里 波特班底明星首映式遇倾盆大雨 Harry Potter's magic spells might be able to fight the forces
图解脑疝. 北京天坛医院神经内科 杜万良 (reflexhammer)
 图解脑疝 北京天坛医院神经内科 杜万良 (reflexhammer) 脑疝 是指在颅内压增高的情况下, 脑组织通过某些脑池向压力相对较低的部位移位的结果, 即脑组织由其原来正常的位置而进入了一个异常的位置 脑疝的类型 : a. 大脑镰疝 : 一侧大脑半球占位病变可使同侧扣带回经大脑镰下缘疝入对侧, 胼胝体受压下移 小脑幕切迹疝 b. 前疝 : 也称颞叶沟回疝, 是颞叶沟回疝于脚间池及环池的前部 ;2
图解脑疝 北京天坛医院神经内科 杜万良 (reflexhammer) 脑疝 是指在颅内压增高的情况下, 脑组织通过某些脑池向压力相对较低的部位移位的结果, 即脑组织由其原来正常的位置而进入了一个异常的位置 脑疝的类型 : a. 大脑镰疝 : 一侧大脑半球占位病变可使同侧扣带回经大脑镰下缘疝入对侧, 胼胝体受压下移 小脑幕切迹疝 b. 前疝 : 也称颞叶沟回疝, 是颞叶沟回疝于脚间池及环池的前部 ;2
Acute Glomerular Nephritis. Mao Jianhua, Department of Nephrology, The Children Hospital of Zhejiang University,
 Acute Glomerular Nephritis Mao Jianhua, Department of Nephrology, The Children Hospital of Zhejiang University, maojh88@gmail.com DEFINITION APSGN is a immune-mediated inflammation disease with the mechanism
Acute Glomerular Nephritis Mao Jianhua, Department of Nephrology, The Children Hospital of Zhejiang University, maojh88@gmail.com DEFINITION APSGN is a immune-mediated inflammation disease with the mechanism
High-pressure balloon dilation for male anterior urethral stricture: single-center experience *
 722 Yu et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016 17(9):722-727 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus;
722 Yu et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016 17(9):722-727 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus;
!Mating! Shiping Tang , 2 nd Semester
 11A: From (Social) Psychology to Social Evolutionary Psychology!Mating! Shiping Tang Fudan University 2014 15, 2 nd Semester Two key tasks: Survival and Reproduction The majority of our psychology can
11A: From (Social) Psychology to Social Evolutionary Psychology!Mating! Shiping Tang Fudan University 2014 15, 2 nd Semester Two key tasks: Survival and Reproduction The majority of our psychology can
Study on current situation and development trends of domestic and foreign lead maximum level standards in food
 5 1 Vol. 5 No. 1 2014 1 Journal of Food Safety and Quality Jan., 2014 邵 1, 懿 2, 王君 1, 吴永宁 1* (1., 100022; 2., 100021) 摘要 : 目的, 方法 结果,, 结论,, 关键词 : ; ; ; Study on current situation and development trends
5 1 Vol. 5 No. 1 2014 1 Journal of Food Safety and Quality Jan., 2014 邵 1, 懿 2, 王君 1, 吴永宁 1* (1., 100022; 2., 100021) 摘要 : 目的, 方法 结果,, 结论,, 关键词 : ; ; ; Study on current situation and development trends
MACC1 upregulation promotes gastric cancer tumor cell metastasis and predicts a poor prognosis *
 Xie et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016 17(5):361-366 361 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus;
Xie et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016 17(5):361-366 361 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus;
Information for the examinees:
 试卷代号 : 1354 国家开放大学 ( 中央广播电视大学 )2016 年春季学期 开放本科 期末考试 高级英语阅读 (2) 试题 2016 年 7 月 注意事项一 将你的学号 姓名及分校 ( 工作站 ) 名称填写在答题纸的规定栏内 考试结束后, 把试卷和答题纸放在桌子上 试卷和答题纸均不得带出考场 监考人收完考卷和答题纸后才可离开考场 二 仔细读懂题目的说明, 并按题目要求和答题示例答题 答案一定要写在答题纸的指定位置上,
试卷代号 : 1354 国家开放大学 ( 中央广播电视大学 )2016 年春季学期 开放本科 期末考试 高级英语阅读 (2) 试题 2016 年 7 月 注意事项一 将你的学号 姓名及分校 ( 工作站 ) 名称填写在答题纸的规定栏内 考试结束后, 把试卷和答题纸放在桌子上 试卷和答题纸均不得带出考场 监考人收完考卷和答题纸后才可离开考场 二 仔细读懂题目的说明, 并按题目要求和答题示例答题 答案一定要写在答题纸的指定位置上,
Mental disorder: What do we need to know?
 Mental disorder: What do we need to know? Dr. Vincent Wong Choong Wai 王忠威心理精神专科医生 MBBS, M.Med(Psych),CMIA Consultant Psychiatrist www.brainmindspecialist.com Dr. Vincent Wong, Consultant Psychiatrist 1
Mental disorder: What do we need to know? Dr. Vincent Wong Choong Wai 王忠威心理精神专科医生 MBBS, M.Med(Psych),CMIA Consultant Psychiatrist www.brainmindspecialist.com Dr. Vincent Wong, Consultant Psychiatrist 1
The key techniques to improve reproductive performance of sows
 The key techniques to improve reproductive performance of sows 提高母猪繁殖性能的关键技术 Institute of Animal Nutrition Sichuan Agricultural University De Wu 吴德 Vice president of Sichuan Agricultural University, from
The key techniques to improve reproductive performance of sows 提高母猪繁殖性能的关键技术 Institute of Animal Nutrition Sichuan Agricultural University De Wu 吴德 Vice president of Sichuan Agricultural University, from
human umbilical blood-derived mesenchymal stem cells into nerve-like cells*
 中国组织工程研究与临床康复第 14 卷第 19 期 2010 05 07 出版 Journal of Clinical Rehabilitative Tissue Engineering Research May 7, 2010 Vol.14, No.19 Brain-derived neurotrophic factor, ciliary neurotrophic factor and their
中国组织工程研究与临床康复第 14 卷第 19 期 2010 05 07 出版 Journal of Clinical Rehabilitative Tissue Engineering Research May 7, 2010 Vol.14, No.19 Brain-derived neurotrophic factor, ciliary neurotrophic factor and their
Dietary Guidelines for Chinese Residents (2016): comments and comparisons
 Wang et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016 17(9):649-656 649 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus;
Wang et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016 17(9):649-656 649 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus;
不对称社会困境中的决策 : 行为的双重模式
 2015, Vol. 23, No. 1, 1 10 Advances in Psychological Science DOI: 10.3724/SP.J.1042.2015.00001 决策心理学专栏 (Special Column for Decision-making Psychology) * 不对称社会困境中的决策 : 行为的双重模式 刘长江 1,2 郝 芳 1 ( 1, 210097)
2015, Vol. 23, No. 1, 1 10 Advances in Psychological Science DOI: 10.3724/SP.J.1042.2015.00001 决策心理学专栏 (Special Column for Decision-making Psychology) * 不对称社会困境中的决策 : 行为的双重模式 刘长江 1,2 郝 芳 1 ( 1, 210097)
Predictors of long - term cataract surgical patient satisfaction found in cell - phone follow - up in a primarily Tibetan region of China
 Original article Predictors of long - term cataract surgical patient satisfaction found in cell - phone follow - up in a primarily Tibetan region of China Danba Jiachu, Hong Zheng, Ji Duo Int Eye Sci,
Original article Predictors of long - term cataract surgical patient satisfaction found in cell - phone follow - up in a primarily Tibetan region of China Danba Jiachu, Hong Zheng, Ji Duo Int Eye Sci,
第四节 切 诊 Pulse taking
 第四节切诊 Pulse taking 一 脉诊 Pulse taking 切脉部位 Location of the pulse 切脉方法 Method of pulse taking 正常脉象 Normal pulse 常见病脉 Common diseased pulses 相兼脉的主病规律 Compound pulses 脉症顺逆与从舍 Contradiction or correspondence
第四节切诊 Pulse taking 一 脉诊 Pulse taking 切脉部位 Location of the pulse 切脉方法 Method of pulse taking 正常脉象 Normal pulse 常见病脉 Common diseased pulses 相兼脉的主病规律 Compound pulses 脉症顺逆与从舍 Contradiction or correspondence
课程四 : 药物共晶 - 药物晶型开发的 新热点
 晶云药物 2011 年第一届晶型专题技术培训 课程四 : 药物共晶 - 药物晶型开发的 新热点 主讲人 : 陈敏华博士, 首席执行官 Crystal Pharmatech 苏州晶云药物科技有限公司 Email:sales@crystalpharmatech.com 电话 :0512-69561921 1 提纲 药物共晶简介 为何要开发药物共晶? 共晶发展历史 共晶的定义和表征 形成共晶的方法 药物共晶的案例分析
晶云药物 2011 年第一届晶型专题技术培训 课程四 : 药物共晶 - 药物晶型开发的 新热点 主讲人 : 陈敏华博士, 首席执行官 Crystal Pharmatech 苏州晶云药物科技有限公司 Email:sales@crystalpharmatech.com 电话 :0512-69561921 1 提纲 药物共晶简介 为何要开发药物共晶? 共晶发展历史 共晶的定义和表征 形成共晶的方法 药物共晶的案例分析
OTC Triptans in New Zealand
 OTC Triptans in New Zealand Customer Service Hotline:400-666-1917 Page 1 of 9 一 调研说明中商情报网全新发布的 OTC Triptans in New Zealand 主要依据国家统计局 国家发改委 商务部 中国海关 国务院发展研究中心 行业协会 工商 税务 海关 国内外相关刊物的基础信息以及行业研究单位等公布和提供的大量资料,
OTC Triptans in New Zealand Customer Service Hotline:400-666-1917 Page 1 of 9 一 调研说明中商情报网全新发布的 OTC Triptans in New Zealand 主要依据国家统计局 国家发改委 商务部 中国海关 国务院发展研究中心 行业协会 工商 税务 海关 国内外相关刊物的基础信息以及行业研究单位等公布和提供的大量资料,
大舜政策研究中心 Dashun Policy Research Centre Ltd Annual Conference 2012
 香港樂健會社 Happy Health Society in association with 大舜政策研究中心 Dashun Policy Research Centre Ltd Annual Conference 2012 人人健康由我做起 中西結合預防癌症 Prevention of Cancer East Meets West Approaches 2012 年 12 月 22 日 ( 星期六
香港樂健會社 Happy Health Society in association with 大舜政策研究中心 Dashun Policy Research Centre Ltd Annual Conference 2012 人人健康由我做起 中西結合預防癌症 Prevention of Cancer East Meets West Approaches 2012 年 12 月 22 日 ( 星期六
Fractures of Extremities (Upper Limbs) Dr. Zhong gang. Department of Orthopaedic Surgery West China Hospital of Sichuan University
 Fractures of Extremities (Upper Limbs) Dr. Zhong gang Department of Orthopaedic Surgery West China Hospital of Sichuan University 七 上肢骨折和手外伤 (Upper Limbs Fracture and Hand Injury) 一. 知识点与教学要求掌握 : 1. 肱骨干骨折,
Fractures of Extremities (Upper Limbs) Dr. Zhong gang Department of Orthopaedic Surgery West China Hospital of Sichuan University 七 上肢骨折和手外伤 (Upper Limbs Fracture and Hand Injury) 一. 知识点与教学要求掌握 : 1. 肱骨干骨折,
A Sustainable Hospitalcommunity. Programme for Orthopaedic Patients with Chronic Pain Syndrome
 A Sustainable Hospitalcommunity Partnership Programme for Orthopaedic Patients with Chronic Pain Syndrome Cheung KK 1 Chan PH 1 Chow YY 1 Hung ATF 2 Ho JPS 2 Tse A 1 Chan I 1 Ip A 1 Wong C 1 Fung C 1 Kwok
A Sustainable Hospitalcommunity Partnership Programme for Orthopaedic Patients with Chronic Pain Syndrome Cheung KK 1 Chan PH 1 Chow YY 1 Hung ATF 2 Ho JPS 2 Tse A 1 Chan I 1 Ip A 1 Wong C 1 Fung C 1 Kwok
中国糖网病防治现状和挑战. Status and Challenges in DR,China. 张丰生 CEO Steve Zhang, MD 朝聚眼科医院集团. Chaoju Eye Hospitals Group
 1 中国糖网病防治现状和挑战 Status and Challenges in DR,China 张丰生 CEO Steve Zhang, MD 朝聚眼科医院集团 Chaoju Eye Hospitals Group Lecture for IAPB COUNCIL OF MEMBERS,BEIJING,2015-10-13 Outline 2 The summary of DR The prevention
1 中国糖网病防治现状和挑战 Status and Challenges in DR,China 张丰生 CEO Steve Zhang, MD 朝聚眼科医院集团 Chaoju Eye Hospitals Group Lecture for IAPB COUNCIL OF MEMBERS,BEIJING,2015-10-13 Outline 2 The summary of DR The prevention
DNA-EGS1386 in cells induced RNase P inhibits the expression of human cytomegalovirus UL49 gene
 生物工程学报 Chin J Biotech 2009, November 25; 25(11): 1690-1696 journals.im.ac.cn Chinese Journal of Biotechnology ISSN 1000-3061 cjb@im.ac.cn 2009 Institute of Microbiology, CAS & CSM, All rights reserved
生物工程学报 Chin J Biotech 2009, November 25; 25(11): 1690-1696 journals.im.ac.cn Chinese Journal of Biotechnology ISSN 1000-3061 cjb@im.ac.cn 2009 Institute of Microbiology, CAS & CSM, All rights reserved
The ankle joint: MR sectional anatomy, anatomic variation and pathology. Part II: variation and pathology (coutinuous)
 海外来稿 Overseas Papers 磁共振成像 2010 年第 1 卷第 6 期 Chin J Magn Reson Imaging, 2010, Vol 1, No 6 踝关节 MR 断层解剖 解剖变异和病理 第二部分 : 解剖变异和病理 ( 续 ) 殷玉明 作者单位 : Radiology Associates, LLP, Corpus Christi, TX, USA 通讯作者 : Yuming
海外来稿 Overseas Papers 磁共振成像 2010 年第 1 卷第 6 期 Chin J Magn Reson Imaging, 2010, Vol 1, No 6 踝关节 MR 断层解剖 解剖变异和病理 第二部分 : 解剖变异和病理 ( 续 ) 殷玉明 作者单位 : Radiology Associates, LLP, Corpus Christi, TX, USA 通讯作者 : Yuming
办公电话 : 电子邮箱 研究方向 : 动物及人兽共患疫病病原学 细菌及病毒致病机理 病原与宿主互作关系 动物疾病防控
 姓名 : 姚火春性别 : 男毕业院校 : 南京农业大学最高学位 : 博士办公地址 : 逸夫楼 4053 办公电话 :025-84395328 电子邮箱 :yaohch@njau.edu.cn 研究方向 : 动物及人兽共患疫病病原学 细菌及病毒致病机理 病原与宿主互作关系 动物疾病防控 个人简介 : 姚火春 : 男,1963 年 2 月出生, 籍贯浙江湖州 研究工作经历 : 2009/12- 至今,
姓名 : 姚火春性别 : 男毕业院校 : 南京农业大学最高学位 : 博士办公地址 : 逸夫楼 4053 办公电话 :025-84395328 电子邮箱 :yaohch@njau.edu.cn 研究方向 : 动物及人兽共患疫病病原学 细菌及病毒致病机理 病原与宿主互作关系 动物疾病防控 个人简介 : 姚火春 : 男,1963 年 2 月出生, 籍贯浙江湖州 研究工作经历 : 2009/12- 至今,
Population coding/vector Coding Distributed representing 群体编码 / 向量编码 / 分布式表征
 Neural coding Population coding/vector Coding Distributed representing 群体编码 / 向量编码 / 分布式表征 Theory:Single Cells as Feature Detector 外界世界的信息 ( 甚至运动输出和复杂的认知过程 ) 的编码可以由单个神经元来完成的 Running-out-of-neurons problem?
Neural coding Population coding/vector Coding Distributed representing 群体编码 / 向量编码 / 分布式表征 Theory:Single Cells as Feature Detector 外界世界的信息 ( 甚至运动输出和复杂的认知过程 ) 的编码可以由单个神经元来完成的 Running-out-of-neurons problem?
Update on AIDS in China
 Update on AIDS in China Kong-Lai Zhang, MD Professor, Department of Epidemiology, Peking Union Medical College Beijing, CHINA March, 2013 1 HIV epidemic in Asia General Population Family Bridge Population
Update on AIDS in China Kong-Lai Zhang, MD Professor, Department of Epidemiology, Peking Union Medical College Beijing, CHINA March, 2013 1 HIV epidemic in Asia General Population Family Bridge Population
Pharmacokinetics of ibuprofen enantiomers in rats after intravenous and oral administration of ibuprofen arginate 布洛芬精氨酸注射及口服给药在大鼠体内的立体选择性药代动力学
 88 药学学报 Acta Pharmaceutica Sinica 2012, 47 (1): 88 93 Pharmacokinetics of ibuprofen enantiomers in rats after intravenous and oral administration of ibuprofen arginate WANG Xiao-lin, HAN Jing, ZHANG Dan,
88 药学学报 Acta Pharmaceutica Sinica 2012, 47 (1): 88 93 Pharmacokinetics of ibuprofen enantiomers in rats after intravenous and oral administration of ibuprofen arginate WANG Xiao-lin, HAN Jing, ZHANG Dan,
Two-Year Intensive Acupuncture Diploma Program
 Two-Year Intensive Acupuncture Diploma Program The Two Year Intensive Acupuncture Diploma Program and the Three Year Acupuncture Diploma Program share the same curriculum. The major difference is the timelines.
Two-Year Intensive Acupuncture Diploma Program The Two Year Intensive Acupuncture Diploma Program and the Three Year Acupuncture Diploma Program share the same curriculum. The major difference is the timelines.
小窝蛋白 -1/ 血红素加氧酶 -1 信号链轴对机械通气所致肺损伤调控效应的研究
 568 Chin Crit Care Med,July 2015,Vol.27,No.7 小窝蛋白 -1/ 血红素加氧酶 -1 信号链轴对机械通气所致肺损伤调控效应的研究 论著 摘要 目的探讨在活体动物用酪氨酸激酶抑制剂 (PP2) 阻断小窝蛋白酪氨酸残基 14(Cav-1-Y14) 磷酸化, 是否会上调血红素加氧酶 -1(HO-1) 活性, 以对抗机械通气所致的肺损伤 方法 54 只雄性 SD 大鼠按随机数字表法分为
568 Chin Crit Care Med,July 2015,Vol.27,No.7 小窝蛋白 -1/ 血红素加氧酶 -1 信号链轴对机械通气所致肺损伤调控效应的研究 论著 摘要 目的探讨在活体动物用酪氨酸激酶抑制剂 (PP2) 阻断小窝蛋白酪氨酸残基 14(Cav-1-Y14) 磷酸化, 是否会上调血红素加氧酶 -1(HO-1) 活性, 以对抗机械通气所致的肺损伤 方法 54 只雄性 SD 大鼠按随机数字表法分为
结构性心脏病介入治疗进展 周达新 复旦大学附属中山医院上海市心血管病研究所. Approaches to structural Heart Disease of Intervention
 结构性心脏病介入治疗进展 Approaches to structural Heart Disease of Intervention 周达新 复旦大学附属中山医院上海市心血管病研究所 Interventional Cath for CHD In 1967 Porstmann helped develop the Ivalon plug and was the first to use it clinically.
结构性心脏病介入治疗进展 Approaches to structural Heart Disease of Intervention 周达新 复旦大学附属中山医院上海市心血管病研究所 Interventional Cath for CHD In 1967 Porstmann helped develop the Ivalon plug and was the first to use it clinically.
I am glad to share my knowledge with you, and I want you to become a master of the subject, pharmacology Antiarrhythmic drugs 抗心律失常药 朱玲 四川大学华西医学中心 zhu
 I am glad to share my knowledge with you, and I want you to become a master of the subject, pharmacology Antiarrhythmic drugs 抗心律失常药 朱玲 四川大学华西医学中心 zhuling529@163.com 教学要求 1. 了解心脏的电生理特性 2. 熟悉心律失常的发生机制 3.
I am glad to share my knowledge with you, and I want you to become a master of the subject, pharmacology Antiarrhythmic drugs 抗心律失常药 朱玲 四川大学华西医学中心 zhuling529@163.com 教学要求 1. 了解心脏的电生理特性 2. 熟悉心律失常的发生机制 3.
Assessment of muscle mass and its association with protein intake in a multi-ethnic Asian population: relevance in chronic kidney disease
 Asia Pac J Clin Nutr 2014;23(4):619-625 619 Original Article Assessment of muscle mass and its association with protein intake in a multi-ethnic Asian population: relevance in chronic kidney disease Boon
Asia Pac J Clin Nutr 2014;23(4):619-625 619 Original Article Assessment of muscle mass and its association with protein intake in a multi-ethnic Asian population: relevance in chronic kidney disease Boon
联邦制药 [3933.HK] 收盘价 : 5.53 港元 (2015 年 6 月 10 日 ) 目标价 : 7.25 港元 (+31.1%) 首予覆盖 : 胰岛素业务将带领公司从谷底复苏
![联邦制药 [3933.HK] 收盘价 : 5.53 港元 (2015 年 6 月 10 日 ) 目标价 : 7.25 港元 (+31.1%) 首予覆盖 : 胰岛素业务将带领公司从谷底复苏 联邦制药 [3933.HK] 收盘价 : 5.53 港元 (2015 年 6 月 10 日 ) 目标价 : 7.25 港元 (+31.1%) 首予覆盖 : 胰岛素业务将带领公司从谷底复苏](/thumbs/95/126015785.jpg) 联邦制药 [3933.HK] 首予覆盖 : 胰岛素业务将带领公司从谷底复苏 虽然市场仍关注联邦制药的中间体和原料药业务表现下滑, 但我们倾向将焦点放在公司具有前途的胰岛素 ( 用于治疗糖尿病 ) 业务 联邦制药作为该市场的先行者, 其很可能在 2015 下半年推出第三代高端胰岛素, 成为第二家能生产该产品的国内制药商 公司的产品组合改善导致其毛利率上升, 加上财务费用率下降, 在这情况下, 我们预测公司的
联邦制药 [3933.HK] 首予覆盖 : 胰岛素业务将带领公司从谷底复苏 虽然市场仍关注联邦制药的中间体和原料药业务表现下滑, 但我们倾向将焦点放在公司具有前途的胰岛素 ( 用于治疗糖尿病 ) 业务 联邦制药作为该市场的先行者, 其很可能在 2015 下半年推出第三代高端胰岛素, 成为第二家能生产该产品的国内制药商 公司的产品组合改善导致其毛利率上升, 加上财务费用率下降, 在这情况下, 我们预测公司的
Effect of feeding practices on dental caries among preschool children: a hospital based analytical cross sectional study
 272 Asia Pac J Clin Nutr 2014;23(2):272-277 Original Article Effect of feeding practices on dental caries among preschool children: a hospital based analytical cross sectional study Priyantha Julian Perera
272 Asia Pac J Clin Nutr 2014;23(2):272-277 Original Article Effect of feeding practices on dental caries among preschool children: a hospital based analytical cross sectional study Priyantha Julian Perera
2017 年同等学力申硕考试 英语考前辅导 英语词汇 考点提炼班讲义 11.9 地址 : 北京市海淀区中关村南大街 27 号中扬大厦 2 层学习服务电话 :
 2017 年同等学力申硕考试 英语考前辅导 英语词汇 考点提炼班讲义 11.9 地址 : 北京市海淀区中关村南大街 27 号中扬大厦 2 层学习服务电话 :010-51658769 课程咨询电话 :010-51653340 学习网址 :www.geedu.com 二维码 : 扫描二维码获取免费增值课程 同等学力基础班词汇复习测验 (30 分钟 ) 1. 写出下列词根词缀的含义, 并给出一个例子 pre-
2017 年同等学力申硕考试 英语考前辅导 英语词汇 考点提炼班讲义 11.9 地址 : 北京市海淀区中关村南大街 27 号中扬大厦 2 层学习服务电话 :010-51658769 课程咨询电话 :010-51653340 学习网址 :www.geedu.com 二维码 : 扫描二维码获取免费增值课程 同等学力基础班词汇复习测验 (30 分钟 ) 1. 写出下列词根词缀的含义, 并给出一个例子 pre-
Received Accepted This work was supported by the National Natural Science Foundation of China (No ).
 Acta Physiologica Sinica, August 25, 2012, 64(4): 449 454 http://www.actaps.com.cn 449 Research Paper Involvement of protein kinase A activation and phospholipase A 2 inhibition in the adenosine-activated
Acta Physiologica Sinica, August 25, 2012, 64(4): 449 454 http://www.actaps.com.cn 449 Research Paper Involvement of protein kinase A activation and phospholipase A 2 inhibition in the adenosine-activated
