VEGF-C shown to have major role in Age-Related Macular Degeneration (AMD)
|
|
|
- Lenard Montgomery
- 5 years ago
- Views:
Transcription
1 ASX and Media release 9 May 2013 VEGF-C shown to have major role in Age-Related Macular Degeneration (AMD) Data presented at the Association for Research in Vision and Ophthalmology (ARVO) 2013 conference in Seattle shows that circulating blood plasma levels of VEGF-C are markedly increased in AMD patients and that VGX-300, an inhibitor of VEGF-C, works comparably to the marketed agent EYLEA in the laser-induced mouse model of wet AMD. The data strongly implicates VEGF-C in the pathogenesis of wet AMD and confirms the potential of VGX-300 as a novel therapeutic for the disease either as a single agent or as an adjunct to existing agents targeting VEGF-A. Circadian Technologies Limited (ASX: CIR, OTCQX:CKDXY), through its 100% owned subsidiary Opthea Pty Ltd, announced today that its collaborator Dr. Kameran Lashkari of The Schepens Eye Research Institute at Harvard Medical School, publicly presented data overnight at the annual ARVO conference in Seattle showing a major link between Vascular Endothelial Growth Factor (VEGF)-C levels and AMD and that assessment of Opthea s drug development candidate, VGX-300 (a soluble form of VEGF Receptor-3 that blocks VEGF-C and VEGF-D) in animal models of wet AMD confirms its potential as a new therapy for the disease. The poster presentation entitled Expression of VEGF-C, VEGF-D and their cognate receptors in experimental and clinical choroidal neovascularisation (copy attached) showed for the first time, that circulating levels of VEGF-C are markedly elevated in AMD patients and that VGX-300 can inhibit the growth and leakage of new blood vessels in the retina, both hallmarks of wet AMD, in the internationally accepted laser-induced model of wet AMD disease in mice. The efficacy of VGX-300 in the study was comparable to that of EYLEA which is similar in structure to VGX-300 but has a different mechanism of action and blocks VEGF-A, but not VEGF-C and VEGF- D. Sales of EYLEA (Regeneron/Bayer), first marketed in November 2011 for the treatment of wet AMD, were $838M in 2012 and are forecast to reach $1.3BN in Opthea was created in 2012 to specifically develop Circadian s portfolio of VEGF-C/VEGF-D inhibitors for ophthalmic disease. Opthea is currently developing VGX-300 for the treatment of wet AMD. Wet AMD is the leading cause of blindness for people over the age of 50 in the US and Europe and is estimated to affect over 1.5 million people worldwide. Wet AMD is characterised by the growth of new blood vessels into the central region of the retina (the macula). This new vessel growth leads to severe and rapid vision loss, exacerbated by fluid and protein leakage, inflammation and scar formation in the retinal tissue.
2 Dr. Kameran Lashkari, M.D., PhD, stated, There continues to be an unmet medical need for improved treatment for wet AMD patients. Despite the recent advances made with the approval of agents targeting VEGF-A for this disease such as EYLEA and Lucentis, at least 40-50% of these treated patients will exhibit a sub-response, indicating that this persistent angiogenesis and vascular leakage in the retinal tissue of these patients is driven by more than just one growth factor, VEGF-A. Dr. Megan Baldwin, CEO of Opthea, said Our findings of the increased levels of VEGF-C in AMD patients as well as the results we have obtained with VGX-300 in an AMD disease model indicate that VEGF-C blockade could be a very important new therapeutic approach for wet AMD. The data highlights the potential of VGX-300 to improve vision in patients either when used alone, or as an adjunct therapy with existing anti-vegf-a agents. We expect clinical trials in wet AMD patients to commence in H Regeneron as reported by Reuters, Jan Company enquiries Megan Baldwin CEO Opthea Pty Ltd Tel: +61 (0) or megan.baldwin@circadian.com.au Robert Klupacs CEO & Managing Director Circadian Tel: +61 (0) or robert.klupacs@circadian.com.au Media enquiries international Lauren Glaser The Trout Group LLC 251 Post Street, Suite 412 San Francisco, CA Tel lglaser@troutgroup.com About Opthea Pty Ltd Opthea Pty Ltd is a private, 100% owned subsidiary of Circadian Technologies Limited based in Melbourne, Australia. Opthea is developing novel biologic inhibitors of angiogenesis (blood vessel growth), lymphangiogenesis (lymphatic vessel growth) and vascular leakage for the treatment of ophthalmic diseases. Opthea s compounds have broad utility in a range of eye diseases characterised by aberrant blood and/or lymphatic vessel growth, vascular leakage or edema, and inflammation, including wet AMD, diabetic macula edema, corneal neovascularisation and transplantation, and dry eye disease. Opthea s lead compound, VGX-300, is a soluble receptor that specifically and potently blocks the activity of two members of the vascular endothelial growth factor family, namely VEGF-C and VEGF-D that are involved in the progression of both retinal and corneal diseases. Opthea s lead program is the development of VGX-300 for the treatment of wet (neovascular) age-related macular degeneration (wet AMD).
3 Opthea has the option to expand its pipeline and preclinical and clinical programs through the development of VGX-300 for indications in addition to wet AMD and by progressing VGX-100, a fully human antibody targeting VEGF-C, for the treatment of ocular disorders. About Circadian Technologies Limited Circadian (ASX:CIR; OTCQX:CKDXY)) is a biologics drug developer focusing on cancer, cancer related and ophthalmic disease therapies. It controls exclusive worldwide rights to a significant intellectual property portfolio around Vascular Endothelial Growth Factor (VEGF)-C and D and VEGFR-3. The applications for the VEGF technology, which functions in regulating blood and lymphatic vessel growth, are substantial and broad. Circadian s internal product development programs are primarily focused on developing VGX-100 (a human antibody against VEGF-C) as a treatment for lymphedema resulting from breast cancer treatment and solid tumours, in particular glioblastoma and colorectal cancer, as well as developing VGX-300 (soluble VEGFR-3) for back of the eye disease such as wet Age Related Macular Degeneration through its subsidiary Opthea. Circadian has also licensed rights to some parts of its intellectual property portfolio for the development of other products to ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company, including the anti-lymphatic antibody-based drug IMC-3C5 targeting VEGFR-3. About wet AMD Wet (neovascular) age-related macular degeneration, or wet AMD, is a disease characterised by the loss of vision in the middle of the visual field caused by degeneration of the central portion of the retina (the macula). Abnormal growth of blood vessels below the retina, and the leakage of fluid and protein from the vessels, causes retinal degeneration and leads to severe and rapid loss of vision. Wet AMD typically affects individuals aged 50 years or older, and is the leading cause of blindness in the developed world. Sales of the drug Lucentis (Roche), which targets VEGF-A but not VEGF-C, were over $US3B in Sales of EYLEA (Regeneron/Bayer), which also targets VEGF-A but not VEGF-C first marketed in November 2011 for the treatment of wet AMD, were $US838M in 2012 and are forecast to reach $1.3BN in Approximately half of the people receiving Lucentis /Eylea are classified as non-responders or poor responders and experience no gain in vision and/or have persistent retinal vascular leakage. There is great opportunity to improve patient responses by targeting more than one factor involved in disease progression. Existing therapies, such as Lucentis /Eylea, target VEGF-A that promotes blood vessel growth and leakage through its receptor VEGFR-2. VEGF-C can also induce angiogenesis and vessel leakage through the same receptor. Combined inhibition of VEGF-A and VEGF-C, has the potential to improve patient response by more effective inhibition of the pathways involved in disease progression. A preclinical model of wet AMD has demonstrated that VEGF-C blockade can significantly inhibit disease progression and that low levels of VEGF-C during development affects formation of the retinal vessels. Like VEGF-A, VEGF-C can also induce vessel permeability that leads to vascular fluid and protein leakage. Inflammatory cytokines associated with wet AMD upregulate VEGF-C levels, and increased levels of the receptors for VEGF-C are detected in AMD tissue. VEGF-C is strongly implicated in the progression of wet AMD.
4 Inherent risks of Investment in Biotechnology Companies There are a number of inherent risks associated with the development of pharmaceutical products to a marketable stage. The lengthy clinical trial process is designed to assess the safety and efficacy of a drug prior to commercialisation and a significant proportion of drugs fail one or both of these criteria. Other risks include uncertainty of patent protection and proprietary rights, whether patent applications and issued patents will offer adequate protection to enable product development, the obtaining of necessary drug regulatory authority approvals and difficulties caused by the rapid advancements in technology. Companies such as Circadian are dependent on the success of their research and development projects and on the ability to attract funding to support these activities. Investment in research and development projects cannot be assessed on the same fundamentals as trading and manufacturing enterprises. Thus investment in companies specialising in drug development must be regarded as highly speculative. Circadian strongly recommends that professional investment advice be sought prior to such investments. Forward-looking statements Certain statements in this ASX announcement may contain forward-looking statements regarding Company business and the therapeutic and commercial potential of its technologies and products in development. Any statement describing Company goals, expectations, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those risks or uncertainties inherent in the process of developing technology and in the process of discovering, developing and commercialising drugs that can be proven to be safe and effective for use as human therapeutics, and in the endeavour of building a business around such products and services. Circadian undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Actual results could differ materially from those discussed in this ASX announcement.
5 Expression of VEGF-C, VEGF-D and their Cognate Receptors in Experimental and Clinical Choroidal Neovascularization Kameran Lashkari 1, Jie Ma 1, Gianna C. Teague 1, Megan E. Baldwin 2, Jorge Arroyo 3 1 Schepens Eye Research Institute, Massachusetts Eye & Ear, Department of Ophthalmology, Harvard Medical School, Boston, MA 2 Opthea Pty Ltd, Circadian Technologies, Level 1, 10 Wallace Avenue, Toorak, Victoria 3142, Australia. 3 Beth Israel-Deaconess Medical Center, Department of Ophthalmology, Harvard Medical School, Boston, MA PURPOSE and METHODS: Choroidal neovascularization (CNV) is the major cause of severe visual loss in subjects with age-related macular degeneration (AMD). At least 40% of subjects with wet AMD exhibit some degree of resistance to anti-vegf-a monotherapy. Persistent angiogenesis and vascular leakage in these sub-responsive subjects may be mediated by other members of the VEGF family. VEGF-C is implicated in mediating subresponse to anti-vegf-a therapies because it can promote angiogenesis and vascular leakage through VEGFR-2 and VEGFR- 3. We sought to investigate if circulating and tissue levels of VEGF- C are elevated in AMD subjects and determine if VEGF-C/-D blockade can inhibit choroidal neovascularization and leakage in a mouse model of wet AMD. Plasma VEGF-A, VEGF-C and VEGF-D levels in subjects with AMD and controls were quantitated using multiplex assay (Biorad). VEGF-C, VEGFR-2 and VEGFR-3 were localized in retinal tissues of C57BL/6 mice and AMD subjects by immunofluorescence using commercially available antibodies (Abcam). To determine whether VEGF-C and VEGF-D participate in choroidal neovascularization, we employed a mouse model of laser-induced CNV in which photocoagulation was induced at 8 9 positions of each mouse retina (50 μm, 700 mw, 100 ms). Mice (5/group) were administered non-specific IgG (20 mg/kg IP every 2 days), Eylea (aflibercept, 20 mg/kg IP every 2 days), or VGX-300 (VEGFR-3 Trap for VEGF- C & VEGF-D; single-intravitreal injection of 40 µg on day 1). CNV areas and extent of leakage were determined by fluorescein angiography (FA) followed by intracardiac perfusion of FITC-dextran in gelatin (10%) on day 14 after photocoagulation. RESULTS I. Circulating VEGF-A, -C and -D levels and tissue localization in CNV/AMD AMD II. Effects of VEGF-A and VEGF-C Inhibition on Laser-induced CNV FIG 2. Fundus images and angiography of laser-induced CNV membranes 14 days after photocoagulation and administration of (A B) IgG, (C D) Eylea, or (E F) VGX-300. III. CNV lesion sizes in choroidal flatmounts FIG 3. Anatomical areas of CNV lesions 14 days after photocoagulation in IgG-treated eyes (A) was much larger than those of Eylea and VGX-300-treated eyes (B C). Red circle, photocoagulation area; red arrow head, optic nerve head; white arrow, CNV leakage. FIG 1. (A) Plasma concentrations of VEGF-A (n = 142), VEGF-C (n = 125) and VEGF-D (n = 70) in controls and AMD subjects. (B) Co-localization of VEGF-C, VEGFR-2, & (C) VEGFR-3 in mouse retina 14 days after laser injury. (D) Co-localization of VEGF-C, VEGFR-2 & (E) VEGFR-3 in clinical specimens of wet AMD. (F, inset) OCT image of laser-induced bulb in choroidal layer. CNV area, as shown in Fig.5A, was determined from FA as shown in (G or H) (Area 2). Percentage of relative increase of CNV area, as shown in Fig. 5B, was determined by calculating [(Area 2 Area 1)/Area 1] x 100%. CL: choroidal layer. IV. Incidence of CNV and intensity of leakage FIG 4. (A) Incidence of laser-induced leaking spots (leakage spots/photocoagulated spots 100%). (B) Comparison of leakage intensity after FA. ACKNOWLEDGEMENTS V. Effects of treatment on CNV area FIG 5. (A) Mean size of laser-induced CNV membranes (total area of leakage spots/total photocoagulated spots). (B) Percentage of relative increase in CNV area (refer to Fig. 1 for calculation method). CONCLUSIONS 1. VEGF-C levels are significantly elevated in the plasma of AMD subjects compared to healthy volunteers (controls). 2. VEGF-C and its cognate receptors are expressed and co-localized in CNV membranes. 3. VGX-300 mediated blockade of VEGF-C/-D significantly inhibits choroidal neovascularization and vascular leakage comparably to Eylea in the laser-induced mouse model of wet AMD. 4. VGX-300 may be an effective monotherapy for the treatment of wet AMD by blocking VEGF-C/-D. 5. Used in combination with anti-vegf-a agents, VGX- 300 may have the potential to improve clinical responses in wet AMD by more effective inhibition of the pathways involved in disease progression. This study was funded by Opthea Pty Ltd, a 100% owned subsidiary of Circadian Technologies, Victoria, Australia. M.B. is an employee of Opthea. The authors KL, JM, GCT & JA have no financial interests in the products.
Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial
 ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
For personal use only
 ASX and Media release 6 April 211 Circadian s Inhibits Tumour Growth in Models of Lung, Ovarian and Prostate Cancer Data demonstrates efficacy of with other therapeutic agents in mouse models of lung,
ASX and Media release 6 April 211 Circadian s Inhibits Tumour Growth in Models of Lung, Ovarian and Prostate Cancer Data demonstrates efficacy of with other therapeutic agents in mouse models of lung,
CIRCADIAN TECHNOLOGIES LIMITED ASX:CIR, OTCQX:CKDXY
 1 AUSBIOTECH INVESTMENT SHOWCASE MELBOURNE NOVEMBER 2 2012 CIRCADIAN TECHNOLOGIES LIMITED ASX:CIR, OTCQX:CKDXY Robert Klupacs, CEO & Managing Director DISCLAIMER Investment in Circadian Technologies Limited
1 AUSBIOTECH INVESTMENT SHOWCASE MELBOURNE NOVEMBER 2 2012 CIRCADIAN TECHNOLOGIES LIMITED ASX:CIR, OTCQX:CKDXY Robert Klupacs, CEO & Managing Director DISCLAIMER Investment in Circadian Technologies Limited
Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration International
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration International
Ophthalmic VEGF Inhibitors. Eylea (aflibercept), Macugen (pegaptanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Treatment of visual impairment due to macular edema secondary to branch retinal vein occlusion (BRVO):
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Treatment of visual impairment due to macular edema secondary to branch retinal
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Treatment of visual impairment due to macular edema secondary to branch retinal
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
News Release - 1/5 - Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd.
 News Release Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd. Intravitreal VEGF Inhibitor EYLEA (aflibercept) Solution for Intravitreal Injection Released on the Market for the Treatment of Wet Age-Related
News Release Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd. Intravitreal VEGF Inhibitor EYLEA (aflibercept) Solution for Intravitreal Injection Released on the Market for the Treatment of Wet Age-Related
Bayer and Regeneron start clinical Phase III trial to extend ophthalmology research & development program for VEGF Trap- Eye in Asia
 Investor News Not intended for U.S. Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com VEGF Trap-Eye in myopic choroidal neovascularization: Bayer and Regeneron start clinical
Investor News Not intended for U.S. Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com VEGF Trap-Eye in myopic choroidal neovascularization: Bayer and Regeneron start clinical
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
VEGF Trap-Eye: New Data Confirm Successes in the Treatment of Age-related Macular Degeneration
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Final Phase 2 Results Presented at Retina Society Meeting VEGF Trap-Eye: New Data Confirm Successes in the Treatment
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Final Phase 2 Results Presented at Retina Society Meeting VEGF Trap-Eye: New Data Confirm Successes in the Treatment
Rising to the Challenge of Satisfying Unmet Medical Needs. Back-of-the-eye. Main Back-of-the-Eye Diseases. Uveitis. Behcet s disease.
 Feature Rising to the Challenge of Satisfying Unmet Medical Needs Eyeing Further Specialization Back-of-the-eye Diseases Main Back-of-the-Eye Diseases Age-related macular degeneration Uveitis Behcet s
Feature Rising to the Challenge of Satisfying Unmet Medical Needs Eyeing Further Specialization Back-of-the-eye Diseases Main Back-of-the-Eye Diseases Age-related macular degeneration Uveitis Behcet s
OPT-302: VEGF-C/D trap for Eye Diseases. Ophthalmology Innovation ASRS, August Megan Baldwin PhD, CEO & Managing Director
 OPT-32: VEGF-C/D trap for Eye Diseases Ophthalmology Innovation Summit @ ASRS, August 1 217 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment in Opthea Limited ( Opthea ) is subject to investment
OPT-32: VEGF-C/D trap for Eye Diseases Ophthalmology Innovation Summit @ ASRS, August 1 217 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment in Opthea Limited ( Opthea ) is subject to investment
Bayer to showcase latest Ophthalmology research at EURETINA 2017
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 17 th European Society of Retina Specialists (EURETINA) Congress Bayer to showcase
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 17 th European Society of Retina Specialists (EURETINA) Congress Bayer to showcase
EyeGene Inc. Contact Information Korea Health Industry Development Institute
 EG-Mirotin: First-in-Class recombinant polypeptide novel subcutaneous drug containing RGD-motif targeted to suppress edema and vascular leakage for Nonproliferative Diabetic Retinopathy (NPDR) EyeGene
EG-Mirotin: First-in-Class recombinant polypeptide novel subcutaneous drug containing RGD-motif targeted to suppress edema and vascular leakage for Nonproliferative Diabetic Retinopathy (NPDR) EyeGene
ThromboGenics Business Update Q1 2018
 Press release 9 May 2018 Regulated Information ThromboGenics Business Update Q1 2018 Advancing Diabetic Eye Disease Portfolio Positive Initial Topline Data from Phase 1/ 2a Clinical Study evaluating THR-
Press release 9 May 2018 Regulated Information ThromboGenics Business Update Q1 2018 Advancing Diabetic Eye Disease Portfolio Positive Initial Topline Data from Phase 1/ 2a Clinical Study evaluating THR-
Building a Major Ophthalmic Pharmaceutical Company. Aerie Pharmaceuticals, Inc. ASRS August 8, 2016
 Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. OIS @ ASRS August 8, 2016 1 Important Information Any discussion of the potential use or expected success of our product candidates
Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. OIS @ ASRS August 8, 2016 1 Important Information Any discussion of the potential use or expected success of our product candidates
Phase 1 Study Results
 The Novel Trap Inhibitor of Vascular Endothelial Growth Factors C/D, OPT-302, and Ranibizumab, an anti-vegf-a Agent, for Wet Age-Related Macular Degeneration: Phase 1 Study Results John A. Wells, III MD
The Novel Trap Inhibitor of Vascular Endothelial Growth Factors C/D, OPT-302, and Ranibizumab, an anti-vegf-a Agent, for Wet Age-Related Macular Degeneration: Phase 1 Study Results John A. Wells, III MD
New STAIRWAY study data shows potential for extended durability with Roche s faricimab in neovascular age-related macular degeneration (namd)
 Media Release New STAIRWAY study data shows potential for extended durability with Roche s faricimab in neovascular age-related macular degeneration (namd) Faricimab the first bispecific antibody designed
Media Release New STAIRWAY study data shows potential for extended durability with Roche s faricimab in neovascular age-related macular degeneration (namd) Faricimab the first bispecific antibody designed
Clinical Policy: Aflibercept (Eylea) Reference Number: CP.PHAR.184
 Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Retinal Disease Program
 Retinal Disease Program OIS @ ASRS Presentation August 2017 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates
Retinal Disease Program OIS @ ASRS Presentation August 2017 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates
Photodynamic Therapy (PDT) for agerelated
 Page 1 of 5 Photodynamic Therapy (PDT) for agerelated eye conditions Introduction This leaflet gives you information about Photodynamic Therapy (PDT) for the age--related eye conditions macular degeneration
Page 1 of 5 Photodynamic Therapy (PDT) for agerelated eye conditions Introduction This leaflet gives you information about Photodynamic Therapy (PDT) for the age--related eye conditions macular degeneration
RXi Pharmaceuticals. sd-rxrna Demonstrate Robust Efficacy in the Eye. Dr. Geert Cauwenbergh President & CEO OTCQX: RXII. Next Generation in RNAi
 RXi Pharmaceuticals sd-rxrna Demonstrate Robust Efficacy in the Eye Next Generation in RNAi Dr. Geert Cauwenbergh President & CEO OTCQX: RXII 2 Forward Looking Statements This presentation contains forward-looking
RXi Pharmaceuticals sd-rxrna Demonstrate Robust Efficacy in the Eye Next Generation in RNAi Dr. Geert Cauwenbergh President & CEO OTCQX: RXII 2 Forward Looking Statements This presentation contains forward-looking
Eye injection treatment costs and rebates
 Eye injection treatment costs and rebates This fact sheet provides general information on the bill you receive from your ophthalmologist for eye injections for the management of wet macular degeneration,
Eye injection treatment costs and rebates This fact sheet provides general information on the bill you receive from your ophthalmologist for eye injections for the management of wet macular degeneration,
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Applications of Sustained Release Delivery Systems in Ocular Disease
 Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
Introduction How the eye works
 1 Introduction Diabetic retinopathy is a condition that can cause permanent loss of eyesight and even blindness. It is a major cause of loss of vision. But if a person with diabetes receives proper eye
1 Introduction Diabetic retinopathy is a condition that can cause permanent loss of eyesight and even blindness. It is a major cause of loss of vision. But if a person with diabetes receives proper eye
Blockade of Prolymphangiogenic VEGF-C suppresses Dry Eye Disease. Sunali Goyal MD
 Blockade of Prolymphangiogenic VEGF-C suppresses Dry Eye Disease Sunali Goyal MD Mentor: Reza Dana, MD, MPH, MSc Claes Dohlman Chair in Ophthalmology Director, Cornea & Refractive Surgery Massachusetts
Blockade of Prolymphangiogenic VEGF-C suppresses Dry Eye Disease Sunali Goyal MD Mentor: Reza Dana, MD, MPH, MSc Claes Dohlman Chair in Ophthalmology Director, Cornea & Refractive Surgery Massachusetts
Arkadin Managed Calls
 Arkadin Managed Calls ThromboGenics Conference Call FY 2017 Business Update & Results Thursday 15.03.2018 18u30 CET Speakers: Patrik De Haes, MD, CEO and Dominique Vanfleteren, CFO Call Duration: 19:05
Arkadin Managed Calls ThromboGenics Conference Call FY 2017 Business Update & Results Thursday 15.03.2018 18u30 CET Speakers: Patrik De Haes, MD, CEO and Dominique Vanfleteren, CFO Call Duration: 19:05
The Foundation WHAT IS THE RETINA?
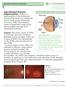 Age-Related Macular Degeneration (AMD) is a deterioration of the retina and choroid that leads to a substantial loss in visual acuity (sharpness of vision). AMD is the leading cause of significant visual
Age-Related Macular Degeneration (AMD) is a deterioration of the retina and choroid that leads to a substantial loss in visual acuity (sharpness of vision). AMD is the leading cause of significant visual
Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis)
 Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Eye injection treatment costs and rebates
 Eye injection treatment costs and rebates This fact sheet provides general information on the bill you receive from your ophthalmologist for eye injections for the management of wet macular degeneration,
Eye injection treatment costs and rebates This fact sheet provides general information on the bill you receive from your ophthalmologist for eye injections for the management of wet macular degeneration,
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at the
Spotlight on SF0166: topical eye droplet treatment for retinal diseases DME and wet-amd
 Spotlight on SF0166: topical eye droplet treatment for retinal diseases DME and wet-amd Disclaimer The following presentation, including any printed or electronic copy of these slides, the talks given
Spotlight on SF0166: topical eye droplet treatment for retinal diseases DME and wet-amd Disclaimer The following presentation, including any printed or electronic copy of these slides, the talks given
FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema
 Investor Update Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First Major Treatment Advance in More Than 25 Years for Sight-Threatening Condition
Investor Update Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First Major Treatment Advance in More Than 25 Years for Sight-Threatening Condition
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION
 THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
REFERENCE CODE GDHC482DFR PUBLICAT ION DATE OCTOBER 2014 EYLEA (MACULAR EDEMA AND MACULAR DEGENERATION) - FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC482DFR PUBLICAT ION DATE OCTOBER 2014 EYLEA (MACULAR EDEMA AND MACULAR DEGENERATION) - Executive Summary Table below presents the key metrics of Eylea for macular edema (ME) and age-related
REFERENCE CODE GDHC482DFR PUBLICAT ION DATE OCTOBER 2014 EYLEA (MACULAR EDEMA AND MACULAR DEGENERATION) - Executive Summary Table below presents the key metrics of Eylea for macular edema (ME) and age-related
Oxurion NV Business Update Q End Q cash position 95.1 million
 Oxurion NV Business Update Q3 2018 Shareholders Approval and Launch of Oxurion NV as new Company Name First patient enrolled in Phase 1 study evaluating THR-687, a Novel Pan-RGD Integrin Antagonist, for
Oxurion NV Business Update Q3 2018 Shareholders Approval and Launch of Oxurion NV as new Company Name First patient enrolled in Phase 1 study evaluating THR-687, a Novel Pan-RGD Integrin Antagonist, for
Anti-VEGF drugs & CATT Results. in AMD. Apollo Hospitals, Hyderabad. Mallika Goyal, MD
 Anti-VEGF drugs & CATT Results in AMD Mallika Goyal, MD Apollo Hospitals, Hyderabad Vascular Endothelial Growth Factor Napoleone Ferrara at Genentech 1989 identified & cloned the gene Protein that causes
Anti-VEGF drugs & CATT Results in AMD Mallika Goyal, MD Apollo Hospitals, Hyderabad Vascular Endothelial Growth Factor Napoleone Ferrara at Genentech 1989 identified & cloned the gene Protein that causes
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist
 Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist PRESENT PROFESSIONAL POSITION OPHTALMOLOGIST DOCTOR, Private practice Retinal medical Specialist, DMLA in particular Installation year
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist PRESENT PROFESSIONAL POSITION OPHTALMOLOGIST DOCTOR, Private practice Retinal medical Specialist, DMLA in particular Installation year
A Patient s Guide to Diabetic Retinopathy
 Diabetic Retinopathy A Patient s Guide to Diabetic Retinopathy 840 Walnut Street, Philadelphia PA 19107 www.willseye.org Diabetic Retinopathy 1. Definition Diabetic retinopathy is a complication of diabetes
Diabetic Retinopathy A Patient s Guide to Diabetic Retinopathy 840 Walnut Street, Philadelphia PA 19107 www.willseye.org Diabetic Retinopathy 1. Definition Diabetic retinopathy is a complication of diabetes
The Era of anti- - - VEGF Kirk L. Halvorson, OD
 The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
Presentation to AGM 9 November Deborah Rathjen CEO & Managing Director
 Presentation to AGM 9 November 2011 Deborah Rathjen CEO & Managing Director Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward looking" statements within the
Presentation to AGM 9 November 2011 Deborah Rathjen CEO & Managing Director Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward looking" statements within the
Management of Neovascular AMD
 Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Retinab for Diabetic Retinopathy
 Retinab for Diabetic Retinopathy Tarnhelm Therapeutics January 2014 2 2013 Tarnhelm Therapeutics Company Background Virtual company based in Bryn Mawr, PA Developing biologics for diabetic retinopathy
Retinab for Diabetic Retinopathy Tarnhelm Therapeutics January 2014 2 2013 Tarnhelm Therapeutics Company Background Virtual company based in Bryn Mawr, PA Developing biologics for diabetic retinopathy
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal. Aflibercept for treating diabetic macular oedema.
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
 measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lucentis) Reference Number: CP.PHAR.186 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Lucentis) Reference Number: CP.PHAR.186 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
AMD research at Moorfields
 Recruiting Research Studies AMD research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the opportunities for patients
Recruiting Research Studies AMD research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the opportunities for patients
Date approved: 04/18/18. Approved by: Pharmacy and Therapeutics Quality Management Subcommittee Effective Date: Department of Origin: Pharmacy
 Integrated Healthcare Services and Criteria Document: Reference #: PC/V001 Page: 1 of 9 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Integrated Healthcare Services and Criteria Document: Reference #: PC/V001 Page: 1 of 9 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Diabetic Retinopathy A Presentation for the Public
 Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
i-bodies a new class of protein therapeutics to treat fibrosis
 i-bodies a new class of protein therapeutics to treat fibrosis BIOSHARES JULY 2017 Sam Cobb, CEO and Managing Director AdAlta Limited (ASX:1AD) s.cobb@adalta.com.au Disclaimer Investment in AdAlta is subject
i-bodies a new class of protein therapeutics to treat fibrosis BIOSHARES JULY 2017 Sam Cobb, CEO and Managing Director AdAlta Limited (ASX:1AD) s.cobb@adalta.com.au Disclaimer Investment in AdAlta is subject
Bank of America Merrill Lynch HealthCare Conference Bayer HealthCare Dr. Jörg Reinhardt. September 14, 2011
 Bank of America Merrill Lynch HealthCare Conference Bayer HealthCare Dr. Jörg Reinhardt Chairman of the Board of Management of Bayer HealthCare AG and Chairman of the Bayer HealthCare Executive Committee
Bank of America Merrill Lynch HealthCare Conference Bayer HealthCare Dr. Jörg Reinhardt Chairman of the Board of Management of Bayer HealthCare AG and Chairman of the Bayer HealthCare Executive Committee
Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection
 Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection Kenalog/SS/ST/04.2012/v1.1 review 05.2013 Page 1 Introduction Your doctor has found that you have leakage of
Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection Kenalog/SS/ST/04.2012/v1.1 review 05.2013 Page 1 Introduction Your doctor has found that you have leakage of
Diabetic Retinopathy
 Diabetic Retinopathy Diabetes mellitus is one of the leading causes of irreversible blindness worldwide. In the United States, it is the most common cause of blindness in people younger than 65 years.
Diabetic Retinopathy Diabetes mellitus is one of the leading causes of irreversible blindness worldwide. In the United States, it is the most common cause of blindness in people younger than 65 years.
FDA approves Roche s Lucentis (ranibizumab injection) for treatment of diabetic retinopathy in people with diabetic macular edema
 Media Release Basel, 9 February 2015 FDA approves Roche s Lucentis (ranibizumab injection) for treatment of diabetic retinopathy in people with diabetic macular edema First eye medicine approved for treatment
Media Release Basel, 9 February 2015 FDA approves Roche s Lucentis (ranibizumab injection) for treatment of diabetic retinopathy in people with diabetic macular edema First eye medicine approved for treatment
Although photocoagulation and photodynamic PROCEEDINGS PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION *
 PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
Dr Dianne Sharp Ophthalmologist Retina Specialists, Parnell Greenlane Clinical Centre
 Dr Dianne Sharp Ophthalmologist Retina Specialists, Parnell Greenlane Clinical Centre 11:00-11:55 WS #115: The Revolution in Macular Degeneration Management 12:05-13:00 WS #127: The Revolution in Macular
Dr Dianne Sharp Ophthalmologist Retina Specialists, Parnell Greenlane Clinical Centre 11:00-11:55 WS #115: The Revolution in Macular Degeneration Management 12:05-13:00 WS #127: The Revolution in Macular
Merriman Investment Summit 2009
 Merriman Investment Summit 2009 10 November 2009 Robert Klupacs, CEO & Managing Director Circadian Technologies (ASX.CIR) Disclaimer Investment in Circadian Technologies Limited ( Circadian ) is subject
Merriman Investment Summit 2009 10 November 2009 Robert Klupacs, CEO & Managing Director Circadian Technologies (ASX.CIR) Disclaimer Investment in Circadian Technologies Limited ( Circadian ) is subject
2/1/2017. Agenda. David F. Williams, MD, MBA. History of Current Treatment. Current Treatment/Wet AMD Anti-VEGF Drugs. History of Current Treatment
 UPDATE ON CLINICAL TRIALS FOR AMD David F. Williams, MD, MBA Agenda Brief History of Current Rx Recently Completed/New CTs: Wet AMD Recently Completed/New CTs: Dry AMD Current Treatment/Wet AMD Anti-VEGF
UPDATE ON CLINICAL TRIALS FOR AMD David F. Williams, MD, MBA Agenda Brief History of Current Rx Recently Completed/New CTs: Wet AMD Recently Completed/New CTs: Dry AMD Current Treatment/Wet AMD Anti-VEGF
Major new Macular Degeneration Foundation research initiative
 Research Update 2010 Global research on the causes, genetics and treatment of Macular Degeneration continues at a hectic pace. In fact, no other area of ophthalmology is receiving as much attention at
Research Update 2010 Global research on the causes, genetics and treatment of Macular Degeneration continues at a hectic pace. In fact, no other area of ophthalmology is receiving as much attention at
Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection
 Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection What is age related macular degeneration (AMD)?
Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection What is age related macular degeneration (AMD)?
Dendrimer-Oxaliplatin shows better anti-cancer efficacy and less toxicity
 Dendrimer-Oxaliplatin shows better anti-cancer efficacy and less toxicity Melbourne, Australia; Wednesday 11 September 2013: Starpharma Holdings Ltd (ASX: SPL, OTCQX: SPHRY) today announced it had achieved
Dendrimer-Oxaliplatin shows better anti-cancer efficacy and less toxicity Melbourne, Australia; Wednesday 11 September 2013: Starpharma Holdings Ltd (ASX: SPL, OTCQX: SPHRY) today announced it had achieved
INTERIM MANAGEMENT STATEMENT Q3 2017
 INTERIM MANAGEMENT STATEMENT Q3 2017 Significant progress across all clinical programs New pre-clinical immunooncology data to be presented during R&D Day in New York Research & Development highlights:
INTERIM MANAGEMENT STATEMENT Q3 2017 Significant progress across all clinical programs New pre-clinical immunooncology data to be presented during R&D Day in New York Research & Development highlights:
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Retinal rejuvenation a restorative treatment breakthrough
 Retinal rejuvenation a restorative treatment breakthrough AGE-RELATED MACULAR DEGENERATION (AMD) CLINICALLY SIGNIFICANT MACULAR EDEMA (CSME) Helping the world see clearly 2 2RT FROM ELLEX Combating some
Retinal rejuvenation a restorative treatment breakthrough AGE-RELATED MACULAR DEGENERATION (AMD) CLINICALLY SIGNIFICANT MACULAR EDEMA (CSME) Helping the world see clearly 2 2RT FROM ELLEX Combating some
Company Overview. Investor Presentation November 2018
 Company Overview Investor Presentation November 2018 Important Information The information in this presentation does not contain all of the information that a potential investor should review before investing
Company Overview Investor Presentation November 2018 Important Information The information in this presentation does not contain all of the information that a potential investor should review before investing
Jefferies Global Life Sciences Conference June 2010
 Jefferies Global Life Sciences Conference June 2010 Cautionary Statement This presentation contains "forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act
Jefferies Global Life Sciences Conference June 2010 Cautionary Statement This presentation contains "forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act
GENERAL INFORMATION DIABETIC EYE DISEASE
 GENERAL INFORMATION DIABETIC EYE DISEASE WHAT IS DIABETIC EYE DISEASE? Diabetic eye disease is a term used to describe the common eye complications seen in people with diabetes. It includes: Diabetic retinopathy
GENERAL INFORMATION DIABETIC EYE DISEASE WHAT IS DIABETIC EYE DISEASE? Diabetic eye disease is a term used to describe the common eye complications seen in people with diabetes. It includes: Diabetic retinopathy
Diagnosis and treatment of diabetic retinopathy. Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City
 Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Outline. Preventing & Treating Diabetes Related Blindness. Eye Care Center Doctors. Justin Kanoff, MD. Eye Care Center of Northern Colorado
 Outline Preventing & Treating Diabetes Related Blindness Justin Kanoff, MD Eye Care Center of Northern Colorado 303 974 4302 Introduction to Eye Care Center of Northern Colorado How the eye works Eye problems
Outline Preventing & Treating Diabetes Related Blindness Justin Kanoff, MD Eye Care Center of Northern Colorado 303 974 4302 Introduction to Eye Care Center of Northern Colorado How the eye works Eye problems
Good News for The Macular Generation. Dr Andrew Thompson FRANZCO
 Good News for The Macular Generation Dr Andrew Thompson FRANZCO Anti-VEGFs for wet MD are one of the greatest advances in medicine in past decade Prof. Gary Brown Director Wills Eye Institue Retina Service
Good News for The Macular Generation Dr Andrew Thompson FRANZCO Anti-VEGFs for wet MD are one of the greatest advances in medicine in past decade Prof. Gary Brown Director Wills Eye Institue Retina Service
Two-stage study designed to evaluate tolerability and efficacy of pracinostat combined with azacitidine in patients with high and very high risk MDS
 Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy
 Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy Fawwaz Al Mamoori* Medical Retina Department, Eye Specialty Hospital,
Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy Fawwaz Al Mamoori* Medical Retina Department, Eye Specialty Hospital,
Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187
 Clinical Policy: (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Anti VEGF Agents in Retinal Disorders Current Scenario
 Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Growth Conference. October 19, May 2013
 Dawson Corporate James Securities Presentation 3rd Annual Small Cap Growth Conference October 19, 2017 May 2013 Safe Harbor This document contains forward-looking statements within the meaning of the "safe
Dawson Corporate James Securities Presentation 3rd Annual Small Cap Growth Conference October 19, 2017 May 2013 Safe Harbor This document contains forward-looking statements within the meaning of the "safe
Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283
 Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Andrew Rae, President & CEO / TSX-V: ICO
 ico Therapeutics Andrew Rae, President & CEO 604-602-9414602 www.icotherapeutics.com / TSX-V: ICO Forward Looking Statements Certain of the statements contained in this presentation are forward-looking
ico Therapeutics Andrew Rae, President & CEO 604-602-9414602 www.icotherapeutics.com / TSX-V: ICO Forward Looking Statements Certain of the statements contained in this presentation are forward-looking
Abicipar pegol for wet age related macular degeneration
 EVIDENCE BRIEFING AUGUST 2018 Abicipar pegol for wet age related macular degeneration NIHRIO ID 7084 NICE ID 9724 Developer/Company Allergan Ltd and Molecular Partners UKPS ID 646978 Licensing and market
EVIDENCE BRIEFING AUGUST 2018 Abicipar pegol for wet age related macular degeneration NIHRIO ID 7084 NICE ID 9724 Developer/Company Allergan Ltd and Molecular Partners UKPS ID 646978 Licensing and market
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Retinal Complications of Obstructive Sleep Apnea A Growing Concern!
 Retinal Complications of Obstructive Sleep Apnea A Growing Concern! Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl
Retinal Complications of Obstructive Sleep Apnea A Growing Concern! Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
Ophthalmologic Policy. Vascular Endothelial Growth Factor (VEGF) Inhibitors
 Ophthalmologic Policy UnitedHealthcare Commercial Drug Policy Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: 2016D0042H Effective Date: October 1, 2016 Table of Contents Page INSTRUCTIONS
Ophthalmologic Policy UnitedHealthcare Commercial Drug Policy Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: 2016D0042H Effective Date: October 1, 2016 Table of Contents Page INSTRUCTIONS
Inovio Pharmaceuticals, Inc. (Exact name of registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma
 PRESS RELEASE IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading
PRESS RELEASE IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading
Ophthalmology Macular Pathways
 Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England. Retinal Vein Occlusion (RVO) with Macular oedema (MO)
 Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
For personal use only
 Targeted DEP shows sustained superior performance in ovarian cancer model Melbourne, Australia; 2 February 2016: Starpharma (ASX: SPL, OTCQX: SPHRY) today announced the final results of the preclinical
Targeted DEP shows sustained superior performance in ovarian cancer model Melbourne, Australia; 2 February 2016: Starpharma (ASX: SPL, OTCQX: SPHRY) today announced the final results of the preclinical
Savolitinib Global Phase II Trial Initiated in EGFR Mutant Non-Small Cell Lung Cancer
 Savolitinib Global Phase II Trial Initiated in EGFR Mutant Non-Small Cell Lung Cancer Initiation of expanded Phase II trials in NSCLC triggers US$10 million milestone from AstraZeneca to Chi-Med New study
Savolitinib Global Phase II Trial Initiated in EGFR Mutant Non-Small Cell Lung Cancer Initiation of expanded Phase II trials in NSCLC triggers US$10 million milestone from AstraZeneca to Chi-Med New study
Bayer s Commitment in Ophthalmology. Zig Lang, MD, PhD Vice President and Head of Medical Affairs & Pharmacovigilance, Bayer HealthCare Company Ltd.
 Bayer s Commitment in Ophthalmology Zig Lang, MD, PhD Vice President and Head of Medical Affairs & Pharmacovigilance, Bayer HealthCare Company Ltd. Bayer s commitment to addressing unmet needs in ophthalmology
Bayer s Commitment in Ophthalmology Zig Lang, MD, PhD Vice President and Head of Medical Affairs & Pharmacovigilance, Bayer HealthCare Company Ltd. Bayer s commitment to addressing unmet needs in ophthalmology
Another Record Year for Bayer HealthCare Pharmaceuticals in Asia Pacific
 News Release Bayer (South East Asia) Pte Ltd Communications 63 Chulia Street OCBC Centre East, 14 th Floor Singapore 049514 Tel. +65 6496 1888 Co. No.: 197901344Z Another Record Year for Bayer HealthCare
News Release Bayer (South East Asia) Pte Ltd Communications 63 Chulia Street OCBC Centre East, 14 th Floor Singapore 049514 Tel. +65 6496 1888 Co. No.: 197901344Z Another Record Year for Bayer HealthCare
Since /01/2014 Private practice Ophthalmology, Retinal Medical Specialty and AMD, private office
 Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist SELARL CABINET DOCTEUR GONZALEZ 27, Boulevard des Minimes 31 200 Toulouse! 05 34 40 77 30 e-mail : cabinet.dr.gonzalez@wanadoo.fr Fax
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist SELARL CABINET DOCTEUR GONZALEZ 27, Boulevard des Minimes 31 200 Toulouse! 05 34 40 77 30 e-mail : cabinet.dr.gonzalez@wanadoo.fr Fax
Clinical Trials Related to Age Related Macular Degeneration
 Clinical Trials Related to Age Related Macular Degeneration Kirti Singh MD, DNB, FRCS Kirti Singh MD, DNB, FRCS, Pooja Jain MBBS, Nitasha Ahir MBBS, Divya Jain MD, DNB Guru Nanak Eye Centre, Maulana Azad
Clinical Trials Related to Age Related Macular Degeneration Kirti Singh MD, DNB, FRCS Kirti Singh MD, DNB, FRCS, Pooja Jain MBBS, Nitasha Ahir MBBS, Divya Jain MD, DNB Guru Nanak Eye Centre, Maulana Azad
Optical Coherence Tomography: Pearls for the Anterior Segment Surgeon Basic Science Michael Stewart, M.D.
 Optical Coherence Tomography: Pearls for the Anterior Segment Surgeon Basic Science Michael Stewart, M.D. Disclosure OCT Optical Coherence Tomography No relevant financial relationships I will refer to
Optical Coherence Tomography: Pearls for the Anterior Segment Surgeon Basic Science Michael Stewart, M.D. Disclosure OCT Optical Coherence Tomography No relevant financial relationships I will refer to
For personal use only
 Australian Securities Exchange Limited Companies Announcements Office SYDNEY 4 April, 2012 New Australian Drug Developer Lists on ASX Key points Growth-focused Australian drug and therapeutic development
Australian Securities Exchange Limited Companies Announcements Office SYDNEY 4 April, 2012 New Australian Drug Developer Lists on ASX Key points Growth-focused Australian drug and therapeutic development
DIABETIC RETINOPATHY
 THE UK GUIDE DIABETIC RETINOPATHY Everything you need to know about diabetic retinopathy Jaheed Khan BSc (Hons) MBBS MD FRCOphth Fellow of the Royal College of Ophthalmologists Association for Research
THE UK GUIDE DIABETIC RETINOPATHY Everything you need to know about diabetic retinopathy Jaheed Khan BSc (Hons) MBBS MD FRCOphth Fellow of the Royal College of Ophthalmologists Association for Research
Results Confirm and Extend 40-Week Findings that Treatment with Crysvita is Superior to Conventional Therapy
 Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
For personal use only
 ASX: LCT - OTCQX: LVCLY Diabetes Neurodegenerative Diseases Cell Encapsulation Consolidation & Acceleration CEO REPORT AGM 2012 SAFE HARBOR STATEMENT This document contains certain forward-looking statements,
ASX: LCT - OTCQX: LVCLY Diabetes Neurodegenerative Diseases Cell Encapsulation Consolidation & Acceleration CEO REPORT AGM 2012 SAFE HARBOR STATEMENT This document contains certain forward-looking statements,
