Modeling & Simulation in Pediatric Patients: Top down, Bottom Up, and What it All Means Clinically
|
|
|
- Kelly Jasmine Casey
- 5 years ago
- Views:
Transcription
1 Modeling & Simulation in Pediatric Patients: Top down, Bottom Up, and What it All Means Clinically Alexander A. Vinks, PharmD, PhD, FCP Professor, Pediatrics and Pharmacology University of Cincinnati, College of Medicine Director, Division of Clinical Pharmacology
2 AA Vinks, 2013, all rights reserved Outline Describe how growth and maturation of relevant processes are predictive of pediatric drug disposition Highlight how pharmacometrics can facilitate the development of informative pediatric clinical studies Describe examples of the application of modeling and simulation and Physiologically-Based Pharmacokinetics (PBPK)
3 Systems Pharmacology for Drug Discovery and Development: Paradigm Shift or Flash in the Pan? Vicini & van der Graaf. Clinical Pharmacology & Therapeutics (2013); 93 5,
4 Why Pediatric Pharmacometrics? To develop informative models based on pharmacology, physiology and disease for quantitative analysis of interactions between drugs and patients To collect informative PK, PD, PG data with a focus on variability across populations To better predict and control exposure and response in individual patients Achieve paradigm shift in way we perform clinical trials and individualized therapeutics in children Barrett J. et al., 2012 Clin Pharmacol Ther 92(1):40-9. Johnson T. et al., 2010 Pediatric Anesthesia. Edginton A. et al., 2006 Clin Pharmacokinet 45(10): ; Barrett J. et al., 2008 J Clin Pharmacol 48(5):632-49
5 Continuing Paradox of Drug Development Clinical trials provide evidence of efficacy and safety at usual doses in populations + = Efficacious & Safe Physicians treat individual patients who can vary widely in their response to drug therapy Adverse Drug Reaction + = Efficacious & Safe No Response Courtesy: Dr. Guido Filler, London, Ontario, Canada
6 Applying Pharmacometrics in Pediatrics Descriptive Population Analysis & Modeling Clinical data Population PK/PD & covariate exploration Top-down Prior Knowledge PK/PD Model Clinical Trial Simulation Scenario Analysis Dose Selection Learn & Confirm
7 Clinical Trial Simulation: Phase-I neonates Teduglutide dosing strategy to achieve optimal target attainment Percentages of patients with steady-state teduglutide exposure within the targeted window of efficacy Dose reductions of 55, 65, 75, and 85% in the 0 1-, 1 2-, 2 3-, and 3 6-month age groups, vs. the optimal dosing regimen in the 6 12-month age group. Mouksassi S. et al. Clin. Pharmacol. Ther. 2009, 86:667-71
8 MPA AUC (mg hr/l) MPA AUC (mg hr/l) MPA AUC (mg hr/l) Mycophenolates - One Dose Does Not Fit All Large variability at standard doses Kidney Target Heart Target Liver Dose, 1000 MMF mg Dose, twice 1 g BIDa day MPA: Mycophenolic acid AUC: Area under the concentration-time curve Shaw LM, et al, Am J Transplantation, 2003; Fukuda T. et al., J Clin Pharmacol, 2011 Consensus papers: Van Gelder et al. 2006; Kuypers et al.2011
9 Rationale for Developing of a Bayesian Estimator Large between patient variability Substantial within patient variability Complex absorption process Enterohepatic recycling Clearance changes first 3-6 months post-transplant Pre-dose concentration does not allow accurate estimation of drug exposure Fukuda T. et al J Clin Pharmacol 51: BCS Class II substance with strong ph-dependent solubility profile
10 Descriptive Modeling of PK variability Dong M. et al Population PK/PD of mycophenolic acid in pediatric renal transplant recipients. Br J Clin Pharmacol. Final model prediction: R²=0.27 Posthoc Bayesian estimates: R²=0.70
11
12 Mechanism based approach to MPA disposition Steroids Steroids MMF, mycophenolate mofetil MPA, mycophenolic acid MPAG, MPA-glucuronide CyA, cyclosporine
13 Predicting MPA Enterohepatic Recycling Hydrolysis G.I. Tract (MPAG->MPA) ECH Off MPAG_Obs MPA Obs MPAG_Sim MPA_Sim Enterohepatic Recycling (EHC) Circulation (MPA) Liver (MPA MPAG) GastroPlus TM Physiochemical properties with ADMET Predictor 6.0 Metabolism parameters based on in vitro UGT1A9 data - EHC process included Sherwin C. et al The evolution of models to describe the EHC of mycophenolic acid. Clin Pharmacokinet 50(1):1-24. Stemkens R. et al th International Congress IATDMCT, Salt Lake City. Data from: Burllingham et al. 1996: 1.5g MMF (IV infusion and oral administration in 12 healthy volunteers)
14 at (Sept 2013)
15 Cixutumumab MK-2206 Erlotinib Bevacizumab, Cediranib, Pazopanib Sunitinib Crizotinib Sorafenib Ruxolitinib mtor Bortezomib Vismodegib Obatoclax RO Sirolimus Temsirolimus
16 Phase I/II - Model-based concentration-controlled Studies Study of the mtor Inhibitor Sirolimus in Neurofibromatosis Type-1 Related Plexiform Neurofibromas Assessing Efficacy and Safety of the mtor Inhibitor Sirolimus in the Treatment of Complicated Vascular Anomalies patients 0-18 years of age Pilot study of sirolimus plus multiagent chemotherapy for relapsed/refractory acute lymphoblastic leukemia/lymphoma
17 Target Controlled Drug Management Participating Centers Patient visit Sample collection UPS shipment Web/ notification Centralized LC-MS/MS Bio-Analysis Confirmation Dose change Bayesian estimation Dosing recommendation Uploaded to web notification Results reported via Web portal notification Sent out
18 Allometrically Scaled CL Clinical evidence ontogeny - sirolimus Separation of size and maturation Age (years) Emoto C. et al submitted
19 Applying Pharmacometrics in Pediatrics Top-down Mechanistic approaches In vitro-in vivo extrapolation (IVIVE) & Physiologically-based pharmacokinetics (PBPK) Descriptive population analysis & modeling Dosing scenario 1 Drug data Dosing scenario 2 Dosing scenario 3 Pharmacogenomics Clinical data Empirical approaches Population PK-PD & covariate exploration Age appropriate Models & Algorithms Physiological Parameters Adults & Pediatric populations AAPS Translational Sciences 101 Bottom-up
20 IVIVC confirmation of ontogeny In vivo data p< 0.01 In vitro data p< 0.03 p< 0.03 N=2 N=8 N=4 N=3 Age group (years) Substrate depletion assay with human microsomes and recombinant CYP3A7: CYP3A4, CYP3A5, CYP3A7 and CYP2C8; montelukast and ketoconazole as inhibitors. Emoto C. et al submitted
21 Filler G. & Christians U. Pediatr Transplant. 2009;13(1):44-53 Emoto C. et al submitted. Sirolimus metabolic pathways in children
22 Pediatric PBPK model development Drug dependent parameters for Sirolimus Type Neutral MW 914 Log P o:w 4.3 B/P 35.6 f u,p 0.08 Caco cm/s CL int,3a µl/min/pmol CL int,3a µl/min/pmol CL int,2c µl/min/pmol f u,m 0.43 Simcyp Peds, Version 13 Drugdependent parameters Adult PBPK model Systemsdependent parameters (Adults) Develop and verify an adult PBPK model Pediatric PBPK model Drugdependent parameters Systemsdependent parameters (Pediatric) Perform sensitivity analysis to characterize residual clearance with available pediatric data Update ontogeny profiles for each CYP3A isoform Lipscomb JC et al Adapted from Leong et al with some modifications. Emoto C. et al Development of a PBPK Model for Sirolimus. CPT-PSP 2:e59
23 Vision of PBPK models in candidate selection and drug development Rowland, Momper and Peck, Wagner. and Tucker TDM as a component Physiologically-Based of personalized Pharmacokinetics medicine: applications in Drug Development in pediatric drug and Regulatory development. Science. Clin Pharmacol Annu. Rev. Ther Pharmacol. 2014, 95(2): Toxicol :45 73.
24 Concluding remarks Top-down statistics-based approaches alone are insufficient as they do not provide mechanistic basis for extrapolation Despite the promise of PBPK as a powerful tool, there still are significant knowledge gaps, and a great amount of work will need to go into developing fully predictive pediatric PBPK models DME and transporter ontogeny profiles Given the limited pool of experts in PBPK modeling and the lack of formal comparisons of existing PBPK software, it would be desirable to develop guidance or best practice documents for PBPK modeling using existing experience and databases But - It is here and it can only get better (Malcolm Rowland) With next steps toward PBPK-PD
25 Acknowledgements Clinical Pharmacology Shareen Cox, BS Tsuyoshi Fukuda, PhD Min Dong, PhD Tomoyuki Mizuno, PhD Kana Toyama-Mizuno, PhD Chie Emoto, PhD Raja Venkatasubramanian, PhD Tracy Glauser, MD Senthil Sadhasivam, MD, MPH Vidya Chidambaran, MD UC Biostatistics Siva Sivaganesan, PhD Supported by: NIH 5U10-HD037249, 1K24HD T32HD069054, 1U01 FD004573, HHSF C CCTST Translational Research Initiative
Modeling & Simulation in Pediatric Drug Development: Application of Pharmacometrics to Define the Right Dose for Children
 Modeling & Simulation in Pediatric Drug Development: Application of Pharmacometrics to Define the Right Dose for Children 小児医薬品開発におけるファーマコメトリックスの活用 Cincinnati, Ohio, USA Alexander A. Vinks, PhD, PharmD,
Modeling & Simulation in Pediatric Drug Development: Application of Pharmacometrics to Define the Right Dose for Children 小児医薬品開発におけるファーマコメトリックスの活用 Cincinnati, Ohio, USA Alexander A. Vinks, PhD, PharmD,
Drug-Disease Modeling Applied to Drug Development and Regulatory Decision Making in the Type 2 Diabetes Mellitus Arena
 Drug-Disease Modeling Applied to Drug Development and Regulatory Decision Making in the Type 2 Diabetes Mellitus Arena Tokyo, Japan, December 8, 2015 Stephan Schmidt, Ph.D. Assistant Professor Center for
Drug-Disease Modeling Applied to Drug Development and Regulatory Decision Making in the Type 2 Diabetes Mellitus Arena Tokyo, Japan, December 8, 2015 Stephan Schmidt, Ph.D. Assistant Professor Center for
Outline. How should we do TDM? Does the evidence support TDM outcomes
 Overcoming Barriers to TDM: Information and the TDM Renaissance How to integrate PK intelligence with routine clinical data Alexander A. Vinks, PharmD, PhD, FCP Professor, Director Pediatric Pharmacology
Overcoming Barriers to TDM: Information and the TDM Renaissance How to integrate PK intelligence with routine clinical data Alexander A. Vinks, PharmD, PhD, FCP Professor, Director Pediatric Pharmacology
Sub Population: The elderly
 Sub Population: The elderly How can Modelling and Simulation inform understanding of safety and efficacy? Amy S. Y. Cheung, Senior Clinical Pharmacometrician Early Clinical Development, AstraZeneca On
Sub Population: The elderly How can Modelling and Simulation inform understanding of safety and efficacy? Amy S. Y. Cheung, Senior Clinical Pharmacometrician Early Clinical Development, AstraZeneca On
Application of PBPK Modeling and Simulations in Drug Development
 Application of PBPK Modeling and Simulations in Drug Development Ping Zhao Division of Pharmacometrics Office of Clinical Pharmacology Office of Translational Sciences Center for Drug Evaluation and Research
Application of PBPK Modeling and Simulations in Drug Development Ping Zhao Division of Pharmacometrics Office of Clinical Pharmacology Office of Translational Sciences Center for Drug Evaluation and Research
DEVELOPMENTAL PK/PD: WHAT HAVE WE LEARNT? Geoff Tucker
 DEVELOPMENTAL PK/PD: WHAT HAVE WE LEARNT? Geoff Tucker UNDERSTANDING AND PREDICTING PK/PD IN JUVENILES PHARMACOKINETICS Can we scale from juvenile animals? Can we scale from allometry? Can we scale from
DEVELOPMENTAL PK/PD: WHAT HAVE WE LEARNT? Geoff Tucker UNDERSTANDING AND PREDICTING PK/PD IN JUVENILES PHARMACOKINETICS Can we scale from juvenile animals? Can we scale from allometry? Can we scale from
Altered GI absorption in special populations: An industry perspective
 Altered GI absorption in special populations: An industry perspective Cordula Stillhart and Neil J. Parrott F. Hoffmann La Roche Ltd, Basel (CH) UNGAP WG meeting, Leuven (B) 8 March 2018 Current challenges
Altered GI absorption in special populations: An industry perspective Cordula Stillhart and Neil J. Parrott F. Hoffmann La Roche Ltd, Basel (CH) UNGAP WG meeting, Leuven (B) 8 March 2018 Current challenges
Strategies for Developing and Validating PBPK Models for Extrapolation to Unstudied Population
 Strategies for Developing and Validating PBPK Models for Extrapolation to Unstudied Population Nina Isoherranen Department of Pharmaceutics University of Washington Processes driving drug disposition are
Strategies for Developing and Validating PBPK Models for Extrapolation to Unstudied Population Nina Isoherranen Department of Pharmaceutics University of Washington Processes driving drug disposition are
MODEL-BASED DOSE INDIVIDUALIZATION APPROACHES USING BIOMARKERS
 MODEL-BASED DOSE INDIVIDUALIZATION APPROACHES USING BIOMARKERS CATHERINE M SHERWIN PAGE 27 th Meeting, Montreux, Switzerland 30 th May 2018 Division of Clinical Pharmacology, Pediatrics, School of Medicine
MODEL-BASED DOSE INDIVIDUALIZATION APPROACHES USING BIOMARKERS CATHERINE M SHERWIN PAGE 27 th Meeting, Montreux, Switzerland 30 th May 2018 Division of Clinical Pharmacology, Pediatrics, School of Medicine
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/29755 holds various files of this Leiden University dissertation. Author: Moes, Dirk Jan Alie Roelof Title: Optimizing immunosuppression with mtor inhibitors
Cover Page The handle http://hdl.handle.net/1887/29755 holds various files of this Leiden University dissertation. Author: Moes, Dirk Jan Alie Roelof Title: Optimizing immunosuppression with mtor inhibitors
Viera Lukacova Director, Simulation Sciences
 Use of In Silico Mechanistic Models to Support Interspecies Extrapolation of Oral Bioavailability and Formulation Optimization: Model Example Using GastroPlus Viera Lukacova Director, Simulation Sciences
Use of In Silico Mechanistic Models to Support Interspecies Extrapolation of Oral Bioavailability and Formulation Optimization: Model Example Using GastroPlus Viera Lukacova Director, Simulation Sciences
Using virtual populations to solve drug development problems. Iain Gardner Head of Translational DMPK Science SIMCYP
 Using virtual populations to solve drug development problems Iain Gardner Head of Translational DMPK Science SIMCYP Prediction of human PK (PD) in virtual individuals In vitro system In vitro CLu int Scaling
Using virtual populations to solve drug development problems Iain Gardner Head of Translational DMPK Science SIMCYP Prediction of human PK (PD) in virtual individuals In vitro system In vitro CLu int Scaling
Practical Application of PBPK in Neonates and Infants, Including Case Studies
 Practical Application of PBPK in Neonates and Infants, Including Case Studies Presented at the conference : Innovative Approaches to Pediatric Drug Development and Pediatric Medical Countermeasures: A
Practical Application of PBPK in Neonates and Infants, Including Case Studies Presented at the conference : Innovative Approaches to Pediatric Drug Development and Pediatric Medical Countermeasures: A
TDM. Measurement techniques used to determine cyclosporine level include:
 TDM Lecture 15: Cyclosporine. Cyclosporine is a cyclic polypeptide medication with immunosuppressant effect. It has the ability to block the production of interleukin-2 and other cytokines by T-lymphocytes.
TDM Lecture 15: Cyclosporine. Cyclosporine is a cyclic polypeptide medication with immunosuppressant effect. It has the ability to block the production of interleukin-2 and other cytokines by T-lymphocytes.
Muhammad Fawad Rasool Feras Khalil Stephanie Läer
 Clin Pharmacokinet (2015) 54:943 962 DOI 10.1007/s40262-015-0253-7 ORIGINAL RESEARCH ARTICLE A Physiologically Based Pharmacokinetic Drug Disease Model to Predict Carvedilol Exposure in Adult and Paediatric
Clin Pharmacokinet (2015) 54:943 962 DOI 10.1007/s40262-015-0253-7 ORIGINAL RESEARCH ARTICLE A Physiologically Based Pharmacokinetic Drug Disease Model to Predict Carvedilol Exposure in Adult and Paediatric
Pharmacometric Modelling to Support Extrapolation in Regulatory Submissions
 Pharmacometric Modelling to Support Extrapolation in Regulatory Submissions Kristin Karlsson, PhD Pharmacometric/Pharmacokinetic assessor Medical Products Agency, Uppsala,Sweden PSI One Day Extrapolation
Pharmacometric Modelling to Support Extrapolation in Regulatory Submissions Kristin Karlsson, PhD Pharmacometric/Pharmacokinetic assessor Medical Products Agency, Uppsala,Sweden PSI One Day Extrapolation
Caveat: Validation and Limitations of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters
 Caveat: Validation and Limitations of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 How to Safeguard that Metrics Reflect E/T Activity? in healthy
Caveat: Validation and Limitations of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 How to Safeguard that Metrics Reflect E/T Activity? in healthy
Pharmacokinetic Modeling & Simulation in Discovery and non-clinical Development
 Pharmacokinetic Modeling & Simulation in Discovery and non-clinical Development Where do we stand? Disclaimer I am not a bioinformatician, mathematician or biomedical engineer. I am a simple minded pharmacist,
Pharmacokinetic Modeling & Simulation in Discovery and non-clinical Development Where do we stand? Disclaimer I am not a bioinformatician, mathematician or biomedical engineer. I am a simple minded pharmacist,
Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with. childhood-onset systemic lupus erythematosus
 British Journal of Clinical Pharmacology DOI:10.1111/j.1365-2125.2011.04140.x Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with childhood-onset systemic
British Journal of Clinical Pharmacology DOI:10.1111/j.1365-2125.2011.04140.x Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with childhood-onset systemic
Use of PBPK in simulating drug concentrations in pediatric populations: Case studies of Midazolam and Gabapentin
 Use of PBPK in simulating drug concentrations in pediatric populations: Case studies of Midazolam and Gabapentin WORKSHOP ON MODELLING IN PAEDIATRIC MEDICINES April 2008 Viera Lukacova, Ph.D. Walter Woltosz,
Use of PBPK in simulating drug concentrations in pediatric populations: Case studies of Midazolam and Gabapentin WORKSHOP ON MODELLING IN PAEDIATRIC MEDICINES April 2008 Viera Lukacova, Ph.D. Walter Woltosz,
FDA Use of Big Data in Modeling and Simulations
 FDA Use of Big Data in Modeling and Simulations Jeffry Florian, Ph.D., Division of Pharmacometrics, CDER/OTS/OCP/DPM DISCLAIMER: The views expressed in this presentation are that of the author and do not
FDA Use of Big Data in Modeling and Simulations Jeffry Florian, Ph.D., Division of Pharmacometrics, CDER/OTS/OCP/DPM DISCLAIMER: The views expressed in this presentation are that of the author and do not
HTPK: Conducting PK modeling and
 HTPK: Conducting PK modeling and simulations at high speed November 5, 2018 Robert Fraczkiewicz, David Miller, Marvin Waldman, Robert D. Clark Slide 1 Session Description and Objectives HTPK lightens the
HTPK: Conducting PK modeling and simulations at high speed November 5, 2018 Robert Fraczkiewicz, David Miller, Marvin Waldman, Robert D. Clark Slide 1 Session Description and Objectives HTPK lightens the
Introduction to Pharmacokinetics (PK) Anson K. Abraham, Ph.D. Associate Principal Scientist, PPDM- QP2 Merck & Co. Inc., West Point, PA 5- June- 2017
 Introduction to Pharmacokinetics (PK) Anson K. Abraham, Ph.D. Associate Principal Scientist, PPDM- QP2 Merck & Co. Inc., West Point, PA 5- June- 2017 1 Outline Definition & Relevance of Pharmacokinetics
Introduction to Pharmacokinetics (PK) Anson K. Abraham, Ph.D. Associate Principal Scientist, PPDM- QP2 Merck & Co. Inc., West Point, PA 5- June- 2017 1 Outline Definition & Relevance of Pharmacokinetics
Modeling and Simulation to Support Development and Approval of Complex Products
 Modeling and Simulation to Support Development and Approval of Complex Products Mathangi Gopalakrishnan, MS, PhD Research Assistant Professor Center for Translational Medicine, School of Pharmacy, UMB
Modeling and Simulation to Support Development and Approval of Complex Products Mathangi Gopalakrishnan, MS, PhD Research Assistant Professor Center for Translational Medicine, School of Pharmacy, UMB
Physiologically-Based Simulation of Daclatasvir Pharmacokinetics With Antiretroviral Inducers and Inhibitors of Cytochrome P450 and Drug Transporters
 Physiologically-Based Simulation of Daclatasvir Pharmacokinetics With Antiretroviral Inducers and Inhibitors of Cytochrome P450 and Drug Transporters Qi Wang, Wenying Li, Ming Zheng, Timothy Eley, Frank
Physiologically-Based Simulation of Daclatasvir Pharmacokinetics With Antiretroviral Inducers and Inhibitors of Cytochrome P450 and Drug Transporters Qi Wang, Wenying Li, Ming Zheng, Timothy Eley, Frank
Original Article. This PDF is available for free download from a site hosted by Medknow Publications. (
 Original Article www.jpgmonline.com A six-hour extrapolated sampling strategy for monitoring mycophenolic acid in renal transplant patients in the Indian subcontinent Fleming DH, Mathew BS, John GT*, Chandy
Original Article www.jpgmonline.com A six-hour extrapolated sampling strategy for monitoring mycophenolic acid in renal transplant patients in the Indian subcontinent Fleming DH, Mathew BS, John GT*, Chandy
Development and Evaluation of Bayesian Software for Improving Therapeutic Drug Monitoring of Gentamicin in Neonates
 Development and Evaluation of Bayesian Software for Improving Therapeutic Drug Monitoring of Gentamicin in Neonates Eva Germovšek, Alison Kent, Nigel Klein, Mark A Turner, Mike Sharland, Elisabet Nielsen,
Development and Evaluation of Bayesian Software for Improving Therapeutic Drug Monitoring of Gentamicin in Neonates Eva Germovšek, Alison Kent, Nigel Klein, Mark A Turner, Mike Sharland, Elisabet Nielsen,
CiPA, Pre-clinical Testing. Derek Leishman PhD DSP & ICH S7B
 CiPA, Pre-clinical Testing Derek Leishman PhD DSP & ICH S7B Outline Introduction Scenarios Conclusion Expressing an opinion What is not covered? Analysis/critique of the components too little time to try
CiPA, Pre-clinical Testing Derek Leishman PhD DSP & ICH S7B Outline Introduction Scenarios Conclusion Expressing an opinion What is not covered? Analysis/critique of the components too little time to try
When does exposure matching require additional consideration?
 When does exposure matching require additional consideration? Jeffrey S Barrett, PhD, FCP Vice-President, Interdisciplinary Pharmacometrics Program Global Head, Pediatric Clinical Pharmacology Sanofi Pharmaceuticals
When does exposure matching require additional consideration? Jeffrey S Barrett, PhD, FCP Vice-President, Interdisciplinary Pharmacometrics Program Global Head, Pediatric Clinical Pharmacology Sanofi Pharmaceuticals
Building innovative drug discovery alliances. Hepatic uptake and drug disposition o in vitro and in silico approaches
 Building innovative drug discovery alliances Hepatic uptake and drug disposition o in vitro and in silico approaches Dr Beth Williamson Evotec AG, 2017 Outline Importance of predicting clearance In vitro
Building innovative drug discovery alliances Hepatic uptake and drug disposition o in vitro and in silico approaches Dr Beth Williamson Evotec AG, 2017 Outline Importance of predicting clearance In vitro
Monica Edholm Medica Medic l a Pr oducts Agency
 EMA EFPIA workshop Break-out session no. 2 Case Study Title: M&S for dose adjustment in impaired patients M&S for dose adjustment in renally Monica Edholm MedicalProducts Agency Disclaimer: The views expressed
EMA EFPIA workshop Break-out session no. 2 Case Study Title: M&S for dose adjustment in impaired patients M&S for dose adjustment in renally Monica Edholm MedicalProducts Agency Disclaimer: The views expressed
Montpellier and Nimes University Hospital. 2nd International Workshop on Clinical Pharmacology of Anticancer Drugs Madrid, September the 13th and 14th
 Association of NR1I2, CYP3A5 and ABCB1 genetic polymorphisms with variability of temsirolimus pharmacokinetics and toxicity in patients with metastatic bladder cancer Litaty MBATCHI, Matthieu GASSIOT,
Association of NR1I2, CYP3A5 and ABCB1 genetic polymorphisms with variability of temsirolimus pharmacokinetics and toxicity in patients with metastatic bladder cancer Litaty MBATCHI, Matthieu GASSIOT,
Itraconazole and Clarithromycin as Ketoconazole Alternatives for Clinical CYP3A Inhibition Studies to Quantify Victim DDI Potential
 Itraconazole and Clarithromycin as Ketoconazole Alternatives for Clinical CYP3A Inhibition Studies to Quantify Victim DDI Potential Alice Ban Ke, Ph.D. Consultant & Scientific Advisor Simcyp Limited Alice.Ke@certara.com
Itraconazole and Clarithromycin as Ketoconazole Alternatives for Clinical CYP3A Inhibition Studies to Quantify Victim DDI Potential Alice Ban Ke, Ph.D. Consultant & Scientific Advisor Simcyp Limited Alice.Ke@certara.com
Modeling & Simulation to support evaluation of Safety and Efficacy of Drugs in Older Patients
 Modeling & Simulation to support evaluation of Safety and Efficacy of Drugs in Older Patients Eva Bredberg, Director Global Clinical Pharmacology, AstraZeneca On behalf of EFPIA EMA Geriatrics Workshop
Modeling & Simulation to support evaluation of Safety and Efficacy of Drugs in Older Patients Eva Bredberg, Director Global Clinical Pharmacology, AstraZeneca On behalf of EFPIA EMA Geriatrics Workshop
USING PBPK MODELING TO SIMULATE THE DISPOSITION OF CANAGLIFLOZIN
 USING PBPK MODELING TO SIMULATE THE DISPOSITION OF CANAGLIFLOZIN Christophe Tistaert PDMS Pharmaceutical Sciences Preformulation & Biopharmaceutics AAPS 2015, FLORIDA (USA) Canagliflozin An orally active
USING PBPK MODELING TO SIMULATE THE DISPOSITION OF CANAGLIFLOZIN Christophe Tistaert PDMS Pharmaceutical Sciences Preformulation & Biopharmaceutics AAPS 2015, FLORIDA (USA) Canagliflozin An orally active
Supporting a Pediatric Investigational Plan for Everolimus - Defining the extrapolation plan
 Supporting a Pediatric Investigational Plan for Everolimus - Defining the extrapolation plan Thomas Dumortier, Mick Looby Novartis Pharma AG, Basel, Switzerland EMA public workshop on extrapolation of
Supporting a Pediatric Investigational Plan for Everolimus - Defining the extrapolation plan Thomas Dumortier, Mick Looby Novartis Pharma AG, Basel, Switzerland EMA public workshop on extrapolation of
TDM of Targeted Therapies in Oncology: where do we stand? Dr. Joseph Ciccolini SMARTc - Pharmacokinetics Unit AMU/APHM Inserm U1068 CRCM
 TDM of Targeted Therapies in Oncology: where do we stand? Dr. Joseph Ciccolini SMARTc - Pharmacokinetics Unit AMU/APHM Inserm U1068 CRCM Disclosures Roche Merck Serono Novartis How to improve clinical
TDM of Targeted Therapies in Oncology: where do we stand? Dr. Joseph Ciccolini SMARTc - Pharmacokinetics Unit AMU/APHM Inserm U1068 CRCM Disclosures Roche Merck Serono Novartis How to improve clinical
Model-based dose adjustment in treatment personalization of immunosuppressive drugs
 Model-based dose adjustment in treatment personalization of immunosuppressive drugs Pierre Marquet, MD, PhD INSERM Unit 850 University Hospital Limoges, France Treatment personalization «The right patient,
Model-based dose adjustment in treatment personalization of immunosuppressive drugs Pierre Marquet, MD, PhD INSERM Unit 850 University Hospital Limoges, France Treatment personalization «The right patient,
Reflection paper on the use of extrapolation in the development of medicines for paediatrics
 7 October 2018 EMA/189724/2018 Reflection paper on the use of extrapolation in the development of medicines for paediatrics Final Draft agreed by Biostatistics Working Party, Modelling and Simulation September
7 October 2018 EMA/189724/2018 Reflection paper on the use of extrapolation in the development of medicines for paediatrics Final Draft agreed by Biostatistics Working Party, Modelling and Simulation September
Physiologically Based Pharmacokinetic Model of the CYP2D6 Probe Atomoxetine: Extrapolation to Special Populations and Drug-Drug Interactions s
 Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2017/08/31/dmd.117.076455.dc1 1521-009X/45/11/1156 1165$25.00 https://doi.org/10.1124/dmd.117.076455 DRUG
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2017/08/31/dmd.117.076455.dc1 1521-009X/45/11/1156 1165$25.00 https://doi.org/10.1124/dmd.117.076455 DRUG
Dr. Nele Plock. Dr. N. Plock, March 11, 2008, American Conference on Pharmacometrics
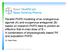 Parallel PKPD modeling of an endogenous agonist (A) and exogenous antagonist (B) based on research PKPD data to predict an effective first-in-man dose of B A combination of physiologically based PK and
Parallel PKPD modeling of an endogenous agonist (A) and exogenous antagonist (B) based on research PKPD data to predict an effective first-in-man dose of B A combination of physiologically based PK and
Target Concentration Intervention
 1 Target Concentration Intervention Dose Individualization using Monitoring of Patient Response Nick Holford University of Auckland 2 Objectives 1) Appreciate how a target concentration (TC) strategy is
1 Target Concentration Intervention Dose Individualization using Monitoring of Patient Response Nick Holford University of Auckland 2 Objectives 1) Appreciate how a target concentration (TC) strategy is
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/29755 holds various files of this Leiden University dissertation. Author: Moes, Dirk Jan Alie Roelof Title: Optimizing immunosuppression with mtor inhibitors
Cover Page The handle http://hdl.handle.net/1887/29755 holds various files of this Leiden University dissertation. Author: Moes, Dirk Jan Alie Roelof Title: Optimizing immunosuppression with mtor inhibitors
Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization
 Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization Nico Holmstock Scientist, Janssen R&D M CERSI 2017, BALTIMORE (USA) Canagliflozin An
Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization Nico Holmstock Scientist, Janssen R&D M CERSI 2017, BALTIMORE (USA) Canagliflozin An
DRUG LEVEL MONITORING AND ADJUSTMENT Silvio Sandrini, Brescia, Italy Chairs: Ryszard Grenda, Warsaw, Poland Julio Pascual, Barcelona, Spain
 DRUG LEVEL MONITORING AND ADJUSTMENT Silvio Sandrini, Brescia, Italy Chairs: Ryszard Grenda, Warsaw, Poland Julio Pascual, Barcelona, Spain Prof. Silvio Sandrini Division and Chair of Nephrology University
DRUG LEVEL MONITORING AND ADJUSTMENT Silvio Sandrini, Brescia, Italy Chairs: Ryszard Grenda, Warsaw, Poland Julio Pascual, Barcelona, Spain Prof. Silvio Sandrini Division and Chair of Nephrology University
Children are small adults and babies are young children
 1 Children are small adults and babies are young children Nick Holford Dept Pharmacology & Clinical Pharmacology Brian Anderson Dept Anaesthesia & Starship Hospital University of Auckland, New Zealand
1 Children are small adults and babies are young children Nick Holford Dept Pharmacology & Clinical Pharmacology Brian Anderson Dept Anaesthesia & Starship Hospital University of Auckland, New Zealand
Simulation of the Nonlinear PK of Gabapentin and Midazolam in. Adult and Pediatric Populations. Population Approach Group in Europe (PAGE)
 Simulation of the Nonlinear PK of Gabapentin and Midazolam in Adult and Pediatric Populations Population Approach Group in Europe (PAGE) Meeting June 2006 Brugge, Belgium Walter Woltosz, M.S., M.A.S. Viera
Simulation of the Nonlinear PK of Gabapentin and Midazolam in Adult and Pediatric Populations Population Approach Group in Europe (PAGE) Meeting June 2006 Brugge, Belgium Walter Woltosz, M.S., M.A.S. Viera
PK-PD modelling to support go/no go decisions for a novel gp120 inhibitor
 PK-PD modelling to support go/no go decisions for a novel gp120 inhibitor Phylinda LS Chan Pharmacometrics, Pfizer, UK EMA-EFPIA Modelling and Simulation Workshop BOS1 Pharmacometrics Global Clinical Pharmacology
PK-PD modelling to support go/no go decisions for a novel gp120 inhibitor Phylinda LS Chan Pharmacometrics, Pfizer, UK EMA-EFPIA Modelling and Simulation Workshop BOS1 Pharmacometrics Global Clinical Pharmacology
MODULE PHARMACOKINETICS WRITTEN SUMMARY
 MODULE 2.6.4. PHARMACOKINETICS WRITTEN SUMMARY m2.6.4. Pharmacokinetics Written Summary 2013N179518_00 TABLE OF CONTENTS PAGE 1. BRIEF SUMMARY...4 2. METHODS OF ANALYSIS...5 3. ABSORPTION...6 4. DISTRIBUTION...7
MODULE 2.6.4. PHARMACOKINETICS WRITTEN SUMMARY m2.6.4. Pharmacokinetics Written Summary 2013N179518_00 TABLE OF CONTENTS PAGE 1. BRIEF SUMMARY...4 2. METHODS OF ANALYSIS...5 3. ABSORPTION...6 4. DISTRIBUTION...7
The Role of Simulation in Assessing Extrapolation Assumptions
 Quantitative Assessment of Assumptions to Support Extrapolation of Efficacy in Pediatrics: FDA-U Maryland CERSI Cosponsored Workshop. FDA White Oak Campus. June 1, 2016 The Role of Simulation in Assessing
Quantitative Assessment of Assumptions to Support Extrapolation of Efficacy in Pediatrics: FDA-U Maryland CERSI Cosponsored Workshop. FDA White Oak Campus. June 1, 2016 The Role of Simulation in Assessing
BASIC PHARMACOKINETICS
 BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
Pharmacokinetics in Drug Development. Edward P. Acosta, PharmD Professor & Director Division of Clinical Pharmacology Director, CCC PK/PD Core
 Pharmacokinetics in Drug Development Edward P. Acosta, PharmD Professor & Director Division of Clinical Pharmacology Director, CCC PK/PD Core Finding new drugs: A crap shoot Clinical Development Phase
Pharmacokinetics in Drug Development Edward P. Acosta, PharmD Professor & Director Division of Clinical Pharmacology Director, CCC PK/PD Core Finding new drugs: A crap shoot Clinical Development Phase
DMPK. APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology
 DMPK APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology What I learned is a good DMPK profile have acceptable water solubility for development be completely absorbed, preferably via
DMPK APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology What I learned is a good DMPK profile have acceptable water solubility for development be completely absorbed, preferably via
Pediatric Extrapolation in FDA Submissions Sources of Data
 Pediatric Extrapolation in FDA Submissions Sources of Data Gilbert Burckart, Pharm.D. Associate Director of Pediatrics Office of Clinical Pharmacology Office of Translational Sciences, CDER 1 Pediatric
Pediatric Extrapolation in FDA Submissions Sources of Data Gilbert Burckart, Pharm.D. Associate Director of Pediatrics Office of Clinical Pharmacology Office of Translational Sciences, CDER 1 Pediatric
MODELING MECHANISM BASED INACTIVATION USING PBPK JAN WAHLSTROM DIRECTOR, PRECLINICAL
 MODELING MECHANISM BASED INACTIVATION USING PBPK JAN WAHLSTROM DIRECTOR, PRECLINICAL ABSTRACT Quantitative prediction of the magnitude of drug-drug interactions (DDI) is critical to underwriting patient
MODELING MECHANISM BASED INACTIVATION USING PBPK JAN WAHLSTROM DIRECTOR, PRECLINICAL ABSTRACT Quantitative prediction of the magnitude of drug-drug interactions (DDI) is critical to underwriting patient
PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the University of Virginia Children s Hospital
 PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the University of Virginia Children s Hospital Volume 15 Number 3 March 2009 Advances in Pediatric Pharmacokinetics Marcia
PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the University of Virginia Children s Hospital Volume 15 Number 3 March 2009 Advances in Pediatric Pharmacokinetics Marcia
A pharmacometric framework for dose individualisation of sunitinib in GIST
 A pharmacometric framework for dose individualisation of sunitinib in GIST Maddalena Centanni, Sreenath M. Krishnan, Lena E. Friberg Department of Pharmaceutical Biosciences, Uppsala University, Sweden
A pharmacometric framework for dose individualisation of sunitinib in GIST Maddalena Centanni, Sreenath M. Krishnan, Lena E. Friberg Department of Pharmaceutical Biosciences, Uppsala University, Sweden
Current Approaches and Applications of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters
 Current Approaches and Applications of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 Definition Phenotyping is quantifying the in vivo activity
Current Approaches and Applications of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 Definition Phenotyping is quantifying the in vivo activity
Culture Hepatocytes in Human Plasma to Count the free Concentration of Drug in Evaluation of Drug-drug Interaction. Chuang Lu
 Culture Hepatocytes in Human Plasma to Count the free Concentration of Drug in Evaluation of Drug-drug nteraction Chuang Lu Millennium, The Takeda Oncology Company Cambridge, MA, USA DD 205, Seattle, 6/29/205
Culture Hepatocytes in Human Plasma to Count the free Concentration of Drug in Evaluation of Drug-drug nteraction Chuang Lu Millennium, The Takeda Oncology Company Cambridge, MA, USA DD 205, Seattle, 6/29/205
How to use prior knowledge and still give new data a chance?
 How to use prior knowledge and still give new data a chance? Kristina Weber1, Rob Hemmings2, Armin Koch 19.12.18 1 now with Roche, 1 2 MHRA, London, UK Part I Background Extrapolation and Bayesian methods:
How to use prior knowledge and still give new data a chance? Kristina Weber1, Rob Hemmings2, Armin Koch 19.12.18 1 now with Roche, 1 2 MHRA, London, UK Part I Background Extrapolation and Bayesian methods:
Supplemental Information
 Supplemental Information Article Title:The Proton Pump Inhibitor, Omeprazole, but not Lansoprazole or Pantoprazole, is a Metabolism-Dependent Inhibitor of CYP2C19: Implications for Co-Administration with
Supplemental Information Article Title:The Proton Pump Inhibitor, Omeprazole, but not Lansoprazole or Pantoprazole, is a Metabolism-Dependent Inhibitor of CYP2C19: Implications for Co-Administration with
BK Virus (BKV) Management Guideline: July 2017
 BK Virus (BKV) Management Guideline: July 2017 BK virus has up to a 60-80% seroprevalence rate in adults due to a primary oral or respiratory exposure in childhood. In the immumocompromised renal transplant
BK Virus (BKV) Management Guideline: July 2017 BK virus has up to a 60-80% seroprevalence rate in adults due to a primary oral or respiratory exposure in childhood. In the immumocompromised renal transplant
Public Assessment Report. EU worksharing project paediatric data. Valcyte. Valganciclovir
 Public Assessment Report EU worksharing project paediatric data Valcyte Valganciclovir Currently approved indication(s): Pharmaceutical form(s) affected by this project: Strength(s) affected by this variation:
Public Assessment Report EU worksharing project paediatric data Valcyte Valganciclovir Currently approved indication(s): Pharmaceutical form(s) affected by this project: Strength(s) affected by this variation:
FDA s Clinical Drug Interaction Studies Guidance (2017 Draft Guidance)
 FDA s Clinical Drug Interaction Studies Guidance (2017 Draft Guidance) Kellie S. Reynolds, Pharm.D. Deputy Director, Division of Clinical Pharmacology IV Office of Clinical Pharmacology (OCP) Office of
FDA s Clinical Drug Interaction Studies Guidance (2017 Draft Guidance) Kellie S. Reynolds, Pharm.D. Deputy Director, Division of Clinical Pharmacology IV Office of Clinical Pharmacology (OCP) Office of
Mycophénolate mofétil
 Mycophénolate mofétil O OH CH 3 O O-desmethyl O glucosides OH CH 3 OCH 3 CH 3 CYP 3A UGT2B7 C O HO O HO AcMPAG (acyl-glucuronide) ACTIF TOXIQUE O O CH 3 OCH 3 Mycophenolate (MPA) OH COOH UGT enzymes COOH
Mycophénolate mofétil O OH CH 3 O O-desmethyl O glucosides OH CH 3 OCH 3 CH 3 CYP 3A UGT2B7 C O HO O HO AcMPAG (acyl-glucuronide) ACTIF TOXIQUE O O CH 3 OCH 3 Mycophenolate (MPA) OH COOH UGT enzymes COOH
Mycophenolate Blood Level Monitoring: Recent Progress
 American Journal of Transplantation 2009; 9: 1495 1499 Wiley Periodicals Inc. Minireview C 2009 The Author Journal compilation C 2009 The American Society of Transplantation and the American Society of
American Journal of Transplantation 2009; 9: 1495 1499 Wiley Periodicals Inc. Minireview C 2009 The Author Journal compilation C 2009 The American Society of Transplantation and the American Society of
Click to edit Master title style
 A Short Course in Pharmacokinetics Chris Town Research Pharmacokinetics Outline Pharmacokinetics - Definition Ideal Pharmacokinetic Parameters of a New Drug How do we optimize PK for new compounds Why
A Short Course in Pharmacokinetics Chris Town Research Pharmacokinetics Outline Pharmacokinetics - Definition Ideal Pharmacokinetic Parameters of a New Drug How do we optimize PK for new compounds Why
Optimizing Drug Exposure
 Optimizing Drug Exposure All Patients Are Not Average Michael Neely, MD, MSc Associate Professor, Pediatrics Keck School of Medicine, University of Southern California Laboratory of Applied Pharmacokinetics
Optimizing Drug Exposure All Patients Are Not Average Michael Neely, MD, MSc Associate Professor, Pediatrics Keck School of Medicine, University of Southern California Laboratory of Applied Pharmacokinetics
A unified in vivomodelingapproach for quantitative prediction of the impact of gene polymorphism and drug interactions on drug exposure
 A unified in vivomodelingapproach for quantitative prediction of the impact of gene polymorphism and drug interactions on drug exposure Sylvain Goutelle, Michel Tod, Laurent Bourguignon, Nathalie Bleyzac,
A unified in vivomodelingapproach for quantitative prediction of the impact of gene polymorphism and drug interactions on drug exposure Sylvain Goutelle, Michel Tod, Laurent Bourguignon, Nathalie Bleyzac,
The Importance of Early Interaction with FDA: EOP2A Experiences
 The Importance of Early Interaction with FDA: EOP2A Experiences Yaning Wang, Ph.D. Office of Clinical Pharmacology Center of Drug Evaluation and Research Food and Drug Administration Schematic of Development
The Importance of Early Interaction with FDA: EOP2A Experiences Yaning Wang, Ph.D. Office of Clinical Pharmacology Center of Drug Evaluation and Research Food and Drug Administration Schematic of Development
Evaluation of Drug-Drug Interactions FDA Perspective
 Evaluation of Drug-Drug Interactions FDA Perspective Kellie Schoolar Reynolds, Pharm.D. Deputy Director Division of Clinical Pharmacology IV Office of Clinical Pharmacology Office of Translational Sciences
Evaluation of Drug-Drug Interactions FDA Perspective Kellie Schoolar Reynolds, Pharm.D. Deputy Director Division of Clinical Pharmacology IV Office of Clinical Pharmacology Office of Translational Sciences
Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program November 4, 2010 National
 Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program November 4, 2010 National Institutes of Health Clinical Center GOALS of Liver Disease
Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program November 4, 2010 National Institutes of Health Clinical Center GOALS of Liver Disease
Model Informed Drug Development: Is there a need to repaint the canvas?
 Model Informed Drug Development: Is there a need to repaint the canvas? Regulatory Perspectives Vikram Sinha, PhD Director, Division of Pharmacometrics Office of Clinical Pharmacology Office of Translational
Model Informed Drug Development: Is there a need to repaint the canvas? Regulatory Perspectives Vikram Sinha, PhD Director, Division of Pharmacometrics Office of Clinical Pharmacology Office of Translational
SYNOPSIS (PROTOCOL WX17796)
 TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
PK-UK Challenges and benefits of using PBPK to evaluate an IVIVC for drugs with nonideal solubility and/or permeability. Bath, November 2014
 PK-UK 2014 Challenges and benefits of using PBPK to evaluate an IVIVC for drugs with nonideal solubility and/or permeability Bath, November 2014 Prof. Dr. Jennifer Dressman Dressman Bath 2014 Why IVIVC
PK-UK 2014 Challenges and benefits of using PBPK to evaluate an IVIVC for drugs with nonideal solubility and/or permeability Bath, November 2014 Prof. Dr. Jennifer Dressman Dressman Bath 2014 Why IVIVC
Saião A, Chan B, Isbister GK Department of Clinical Toxicology and Pharmacology, Calvary Mater Newcastle, NSW Emergency Department, Prince of Wales
 Saião A, Chan B, Isbister GK Department of Clinical Toxicology and Pharmacology, Calvary Mater Newcastle, NSW Emergency Department, Prince of Wales Hospital, Sydney, NSW, Australia Paracetamol Poisoning
Saião A, Chan B, Isbister GK Department of Clinical Toxicology and Pharmacology, Calvary Mater Newcastle, NSW Emergency Department, Prince of Wales Hospital, Sydney, NSW, Australia Paracetamol Poisoning
ZIN EN ONZIN VAN ANTIBIOTICASPIEGELS BIJ NEONATEN
 ZIN EN ONZIN VAN ANTIBIOTICASPIEGELS BIJ NEONATEN Anne Smits Fellow neonatologie UZ Leuven Use of antibiotics in neonates 50 European hospitals 23 non-european hospitals Countries n = 14 n = 9 Pediatric
ZIN EN ONZIN VAN ANTIBIOTICASPIEGELS BIJ NEONATEN Anne Smits Fellow neonatologie UZ Leuven Use of antibiotics in neonates 50 European hospitals 23 non-european hospitals Countries n = 14 n = 9 Pediatric
BIOPHARMACEUTICS and CLINICAL PHARMACY
 11 years papers covered BIOPHARMACEUTICS and CLINICAL PHARMACY IV B.Pharm II Semester, Andhra University Topics: Absorption Distribution Protein binding Metabolism Excretion Bioavailability Drug Interactions
11 years papers covered BIOPHARMACEUTICS and CLINICAL PHARMACY IV B.Pharm II Semester, Andhra University Topics: Absorption Distribution Protein binding Metabolism Excretion Bioavailability Drug Interactions
Use of Target Tissue Concentrations in PK-PD Modeling of Inhaled Drugs Ramon Hendrickx, AstraZeneca, RIA IMed Gothenburg, Sweden
 Use of Target Tissue Concentrations in PK-PD Modeling of Inhaled Drugs Ramon Hendrickx, AstraZeneca, RIA IMed Gothenburg, Sweden IQ DMPK Leadership Group 31 May 2017 What s On? Lung as target organ Benefit
Use of Target Tissue Concentrations in PK-PD Modeling of Inhaled Drugs Ramon Hendrickx, AstraZeneca, RIA IMed Gothenburg, Sweden IQ DMPK Leadership Group 31 May 2017 What s On? Lung as target organ Benefit
Use of quantitative tools for study planning purposes and study design optimisation
 Use of quantitative tools for study planning purposes and study design optimisation Valeria Gigante AIFA - Italian Medicines Agency EMA MSWG 12.06.2016 Public Declaration of transparency/interests* The
Use of quantitative tools for study planning purposes and study design optimisation Valeria Gigante AIFA - Italian Medicines Agency EMA MSWG 12.06.2016 Public Declaration of transparency/interests* The
Pharmacokinetic and absolute bioavailability studies in early clinical development using microdose and microtracer approaches.
 Pharmacokinetic and absolute bioavailability studies in early clinical development using microdose and microtracer approaches. Lloyd Stevens PhD Senior Research Fellow Pharmaceutical Profiles Nottingham,
Pharmacokinetic and absolute bioavailability studies in early clinical development using microdose and microtracer approaches. Lloyd Stevens PhD Senior Research Fellow Pharmaceutical Profiles Nottingham,
Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling
 British Journal of Clinical Pharmacology DOI:.1111/bcp.12361 Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling Elisabet Størset,
British Journal of Clinical Pharmacology DOI:.1111/bcp.12361 Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling Elisabet Størset,
Effects of Liver Disease on Pharmacokinetics
 Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program October 31, 2013 National Institutes of Health Clinical Center 1 GOALS of Effects of Liver
Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program October 31, 2013 National Institutes of Health Clinical Center 1 GOALS of Effects of Liver
Strategy on Drug Transporter Investigation Why, How, Which & When. Jasminder Sahi
 Strategy on Drug Transporter Investigation Why, How, Which & When Jasminder Sahi Intestine Drug Absorption PEPT1 OATPs MCTs AE2 Epithelial Cell MCTs MRP3 Liver Excretion via Liver Kidney MRPs OATPs N PT1
Strategy on Drug Transporter Investigation Why, How, Which & When Jasminder Sahi Intestine Drug Absorption PEPT1 OATPs MCTs AE2 Epithelial Cell MCTs MRP3 Liver Excretion via Liver Kidney MRPs OATPs N PT1
Drugs Being Eliminated via the Same Pathway Will Not Always Require Similar Pediatric Dose Adjustments
 Citation: CPT Pharmacometrics Syst. Pharmacol. (2018) 7, 175 185; VC 2018 ASCPT All rights reserved doi:10.1002/psp4.12273 ARTICLE Drugs Being Eliminated via the Same Pathway Will Not Always Require Similar
Citation: CPT Pharmacometrics Syst. Pharmacol. (2018) 7, 175 185; VC 2018 ASCPT All rights reserved doi:10.1002/psp4.12273 ARTICLE Drugs Being Eliminated via the Same Pathway Will Not Always Require Similar
DRUG METABOLISM AND PHARMACOKINETICS (DMPK) Lena Gustavsson, H. Lundbeck A/S, November 2015
 DRUG METABOLISM AND PHARMACOKINETICS (DMPK), H. Lundbeck A/S, LEGU@lundbeck.com November 2015 DMPK in Drug Discovery and Development Agenda Introduction Optimizing pharmacokinetic properties Absorption
DRUG METABOLISM AND PHARMACOKINETICS (DMPK), H. Lundbeck A/S, LEGU@lundbeck.com November 2015 DMPK in Drug Discovery and Development Agenda Introduction Optimizing pharmacokinetic properties Absorption
Transplantation in Australia and New Zealand
 Transplantation in Australia and New Zealand Matthew D. Jose MBBS (Adel), FRACP, FASN, PhD (Monash), AFRACMA Professor of Medicine, UTAS Renal Physician, Royal Hobart Hospital Overview CKD in Australia
Transplantation in Australia and New Zealand Matthew D. Jose MBBS (Adel), FRACP, FASN, PhD (Monash), AFRACMA Professor of Medicine, UTAS Renal Physician, Royal Hobart Hospital Overview CKD in Australia
Population Pharmacokinetics and Pharmacodynamics as a Tool in Drug Development. Leon Aarons Manchester Pharmacy School University of Manchester
 Population Pharmacokinetics and Pharmacodynamics as a Tool in Drug Development Leon Aarons Manchester Pharmacy School University of Manchester Pharmacokinetics and Pharmacodynamics Clinical Pharmacokinetics
Population Pharmacokinetics and Pharmacodynamics as a Tool in Drug Development Leon Aarons Manchester Pharmacy School University of Manchester Pharmacokinetics and Pharmacodynamics Clinical Pharmacokinetics
Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program October 29, 2015 National
 Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program October 29, 2015 National Institutes of Health Clinical Center GOALS of Liver Disease
Effects of Liver Disease on Pharmacokinetics Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program October 29, 2015 National Institutes of Health Clinical Center GOALS of Liver Disease
The Future of In Vitro Systems for the Assessment of Induction and Suppression of Enzymes and Transporters
 The Future of In Vitro Systems for the Assessment of Induction and Suppression of Enzymes and Transporters AAPS, San Diego November 6 th, 2014 Andrew Parkinson, XPD Consulting Lisa Almond, Simcyp-Certara
The Future of In Vitro Systems for the Assessment of Induction and Suppression of Enzymes and Transporters AAPS, San Diego November 6 th, 2014 Andrew Parkinson, XPD Consulting Lisa Almond, Simcyp-Certara
Comparison Between the US FDA, Japan PMDA and EMA In Vitro DDI Guidance: Are we Close to Harmonization?
 Comparison Between the US FDA, Japan PMDA and EMA In Vitro DDI Guidance: Are we Close to Harmonization? Brian Ogilvie, Ph.D. VP Scientific Consulting XenoTech, LLC bogilvie@xenotechllc.com 14 Jun, 2018
Comparison Between the US FDA, Japan PMDA and EMA In Vitro DDI Guidance: Are we Close to Harmonization? Brian Ogilvie, Ph.D. VP Scientific Consulting XenoTech, LLC bogilvie@xenotechllc.com 14 Jun, 2018
Prediction of THC Plasma and Brain Concentrations following. Marijuana Administration: Approach and Challenges
 Prediction of THC Plasma and Brain Concentrations following Marijuana Administration: Approach and Challenges Contents: 1. Background and Significance 1.1. Introduction 1.2. THC - the primary chemical
Prediction of THC Plasma and Brain Concentrations following Marijuana Administration: Approach and Challenges Contents: 1. Background and Significance 1.1. Introduction 1.2. THC - the primary chemical
Biomath M263 Clinical Pharmacology
 Training Program in Translational Science Biomath M263 Clinical Pharmacology Spring 2013 www.ctsi.ucla.edu/education/training/webcasts Wednesdays 3 PM room 17-187 CHS 4/3/2013 Pharmacokinetics and Pharmacodynamics
Training Program in Translational Science Biomath M263 Clinical Pharmacology Spring 2013 www.ctsi.ucla.edu/education/training/webcasts Wednesdays 3 PM room 17-187 CHS 4/3/2013 Pharmacokinetics and Pharmacodynamics
Scientific And Regulatory Background For The Revised Bioequivalence Requirements For NTI, Steep Exposure-Response, And Drugs With Complex PK Profiles
 Scientific And Regulatory Background For The Revised Bioequivalence Requirements For NTI, Steep Exposure-Response, And Drugs With Complex PK Profiles Liang Zhao, Ph.D. Director, Division of Quantitative
Scientific And Regulatory Background For The Revised Bioequivalence Requirements For NTI, Steep Exposure-Response, And Drugs With Complex PK Profiles Liang Zhao, Ph.D. Director, Division of Quantitative
IOM Workshop on Comparative Oncology Pharmacokinetics in Companion Animal Cancer Studies
 IOM Workshop on Comparative Oncology Pharmacokinetics in Companion Animal Cancer Studies Daniel L. Gustafson, Ph.D. Professor Shipley University Chair in Comparative Oncology Director of Basic Research,
IOM Workshop on Comparative Oncology Pharmacokinetics in Companion Animal Cancer Studies Daniel L. Gustafson, Ph.D. Professor Shipley University Chair in Comparative Oncology Director of Basic Research,
Metformin: Mechanistic Absorption Modeling and IVIVC Development
 Metformin: Mechanistic Absorption Modeling and IVIVC Development Maziar Kakhi *, Ph.D. FDA Silver Spring, MD 20993 Maziar.kakhi@fda.hhs.gov Viera Lukacova, Ph.D. Simulations Plus Lancaster, CA 93534 viera@simulations-plus.com
Metformin: Mechanistic Absorption Modeling and IVIVC Development Maziar Kakhi *, Ph.D. FDA Silver Spring, MD 20993 Maziar.kakhi@fda.hhs.gov Viera Lukacova, Ph.D. Simulations Plus Lancaster, CA 93534 viera@simulations-plus.com
Drug Dosing in Renal Insufficiency. Coralie Therese D. Dimacali, MD College of Medicine University of the Philippines Manila
 Drug Dosing in Renal Insufficiency Coralie Therese D. Dimacali, MD College of Medicine University of the Philippines Manila Declaration of Conflict of Interest For today s lecture on Drug Dosing in Renal
Drug Dosing in Renal Insufficiency Coralie Therese D. Dimacali, MD College of Medicine University of the Philippines Manila Declaration of Conflict of Interest For today s lecture on Drug Dosing in Renal
3. Describe how variants in the CYP2C19 gene impact Plavix metabolism. 4. Compare molecular genetic technologies for pharmacogenomics testing
 UP! UNDERSTANDING PHARMACOGENOMICS (PGX) Robert Pyatt Ph.D., Director Sanford Medical Genetics and Genomics Laboratories and Associate Professor, Dept of Internal Medicine, University of South Dakota April
UP! UNDERSTANDING PHARMACOGENOMICS (PGX) Robert Pyatt Ph.D., Director Sanford Medical Genetics and Genomics Laboratories and Associate Professor, Dept of Internal Medicine, University of South Dakota April
Pharmacokinetic Optimization of Immunosuppressive Therapy in Thoracic Transplantation
 Pharmacokinetic Optimization of Immunosuppressive Therapy in Thoracic Transplantation Caroline Monchaud and Pierre Marquet INSERM Unit 850, CHU Limoges, Univ Limoges, France. Word count (text only): 25132
Pharmacokinetic Optimization of Immunosuppressive Therapy in Thoracic Transplantation Caroline Monchaud and Pierre Marquet INSERM Unit 850, CHU Limoges, Univ Limoges, France. Word count (text only): 25132
SYNOPSIS. The study results and synopsis are supplied for informational purposes only.
 SYNOPSIS INN : LEFLUNOMIDE Study number : HMR486/1037 et HMR486/3503 Study title : Population pharmacokinetics of A77 1726 (M1) after oral administration of leflunomide in pediatric subjects with polyarticular
SYNOPSIS INN : LEFLUNOMIDE Study number : HMR486/1037 et HMR486/3503 Study title : Population pharmacokinetics of A77 1726 (M1) after oral administration of leflunomide in pediatric subjects with polyarticular
