HORMONAL EFFECTS ON GLUCOSE REGULATION. Kenneth W. Sulston. William P. Ireland. Jeff C. Praught
|
|
|
- Cameron Bradford
- 5 years ago
- Views:
Transcription
1 Atlantic Electronic Journal of Mathematics Volume 1, Number 1, Summer 2006 pp HORMONAL EFFECTS ON GLUCOSE REGULATION Kenneth W. Sulston Department of Mathematics and Statistics University of Prince Edward Island Charlottetown, PEI, C1A 4P3, Canada William P. Ireland Department of Biomedical Sciences Atlantic Veterinary College University of Prince Edward Island Charlottetown, PEI, C1A 4P3, Canada Jeff C. Praught Department of Mathematics and Statistics University of Prince Edward Island Charlottetown, PEI, C1A 4P3, Canada Abstract. A dynamical-systems model of plasma glucose concentration, and its regulation by insulin and glucagon, is described, as pertains to types 1 and 2 diabetes. The hyperglycemic case is seen to be dependent only on insulin concentration, while the hypoglycemic case requires consideration of both insulin and glucagon concentrations. The role of healthy α cells in maintaining proper levels of glucose and the hormones is also highlighted. 1. Introduction. Glucose is a sugar that provides energy to all the cells in the body and consequently the regulation of its plasma levels is of the utmost importance. When glucose concentration in the bloodstream gets too high, the body reacts by storing excess glucose in the liver and muscles as glycogen, a starch made up of many glucose molecules. When blood glucose levels get too low, the liver then converts glycogen back to glucose so that a relatively constant glucose concentration is maintained. To achieve this equilibrium, the body relies primarily on two hormones, insulin and glucagon, which are produced in the β and α cells of the pancreas, respectively. Insulin and glucagon have effects opposing one another: when glucose levels are too high, the pancreas secretes insulin which lowers these levels, and when glucose levels are too low, glucagon is secreted to raise them. Insulin is required by almost all cells in the body, but has the greatest influence on liver cells, fat cells, and muscle cells. By targeting these cells, insulin causes liver and muscle cells to convert glucose to glycogen and fat cells to store fat from fatty acids in the body. Glucagon acts on the same cells as insulin, but has the opposite affect. Glucagon stimulates the liver cells to break down stored glycogen and release glucose into the bloodstream Mathematics Subject Classification. Primary: 58F15, 58F17; Secondary: 53C35. Key words and phrases. Diabetes, Glucose, Insulin, Glucagon, Mathematical Modelling. 31
2 32 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT Diabetes mellitus is a disorder characterized by abnormally high blood glucose levels. Diabetes is characterized by two types: insulin-dependent diabetes (type 1) and non-insulin-dependent diabetes (type 2). In type 1 diabetes, more than 90% of the β cells in the pancreas are destroyed, thereby causing the pancreas to produce little or no insulin. Type 1 diabetes is the rarer form and occurs in less than 10% of all cases, while type 2 diabetes accounts for about 90% of all diabetic patients. In type 2 diabetes, the pancreas continues to produce some insulin, sometimes even at the same levels as people without diabetes. However, the body develops a resistance to the insulin and the pancreas can not produce enough insulin to meet the body s requirements. As a result, glucose is not absorbed into muscle and fat cells at a normal rate, and the liver does not function properly, causing glucose levels to be too high. Mathematical modelling of diabetes is not a new area of research, as there has been an extensive amount of research done for more than 40 years. One of the earliest models was introduced by Bolie [8]; this model was a system of differential equations, with one variable for insulin and one for glucose. Although it was relatively simple, this model provided the foundation on which many of the pioneers of diabetes modelling, such as Cobelli and Ackerman, would base their work. Ackerman et al. ([1],[2],[3]) developed a model of the oral glucose tolerance test which measures the ability of a patient to utilize a specific amount of glucose. Much of the subsequent work that followed continued with the glucose-insulin model and variations of it. Bergman et al. [7] developed a model to study glucose disappearance and how it affects insulin sensitivity. Their model predicted two linearly connected pools in the pancreas; one pool for stored insulin and the other for promptly releasable insulin. Cobelli et al. [13] developed a complex and comprehensive non-linear model to study the short-term blood glucose regulation system. They include crucial processes of glucose, insulin, and glucagon dynamics and their interrelationships. There are limitations to comprehensive models like this one, which are intrinsic in nature, such as variations in the parameters among different individuals and the difficulty of finding data corresponding to all processes included in the model. Salzsieder et al. [19] argued that in order to control long-term glycemic regulation, it is necessary to estimate control parameters for each diabetic patient individually. Their model of glucose and insulin used optimal control theory to find the best estimate for these parameters. They considered physiological relevant processes such as endogenous glucose production, insulin-dependent glucose utilization, and insulin catabolism. Summers and Montani [22] presented a model of glucose homeostasis based on the bihormonal regulation of glucose by glucagon and insulin. Sturis et al. [21] developed a model to study oscillations in human insulin over two distinct time periods, rapid (10-15 minutes) and ultradian ( minutes). Their model used two major feedback loops to describe the effects of insulin on both glucose production and utilization. There have been numerous other models developed to study different aspects of diabetes, including those of Andreassen et al. [5], Boroujerdi et al. [10], and Cobelli et al. [12]. The focus of the model presented here is on the effect of glucagon, which has an important, albeit secondary, role in the regulation of glucose. At normal to high levels of plasma glucose, glucagon is secreted at a low rate, and its plasma concentration remains relatively constant. But when glucose levels are low, glucagon secretion increases and its plasma concentration rises significantly. Glucose levels
3 HORMONAL EFFECTS ON GLUCOSE REGULATION 33 in diabetics are usually in the above-normal range, so that it is the former situation that ordinarily applies, although hypoglycemia is a serious acute complication, with possible long-term effects on health. However, most models have been concerned with hyperglycemic situations, and for this reason, have not incorporated glucagon. Among those that did, Dobbins et al. [14] performed compartmental modelling of glucagon in dogs, as part of a theoretical and experimental study examining glucagon metabolism and distribution, although the study was not specifically aimed at diabetes. The main conclusion was that glucagon has a key role in the acute regulation of glucose homeostasis, due to glucagon s rapid equilibration in plasma and its rapid activation of hepatic glucose production. From a modelling perspective, this investigation settled the important question that glucagon kinetics are best described using a model with a single pool for glucagon. Celeste et al. [11] studied a (mostly) linear model involving glucagon, insulin, and glucose, which is an extension of the simple Ackerman model so as to include glucagon, with one pool for each substance. Insulin and glucagon were assumed to interact only with glucose, and not with each other. The goals of the study were to show the roles of both insulin and glucagon as co-regulators of glucose, and to simulate the oral glucose tolerance test and insulin infusion test. The simulation of the oral glucose tolerance test suggested that glucagon is not important at higher blood glucose levels, while the simulation of the insulin infusion test showed that glucagon plays a crucial role in recovery from hypoglycemia. They concluded by postulating that glucose levels can be regulated by a weighted combination of insulin and glucagon. Summers and co-workers ([22],[23]) also studied the bihormonal influence of insulin and glucagon on glucose homeostasis, with the aim of developing a broad-based model capable of examining various aspects of glucose metabolism. They adopted a model of three differential equations, one for each of glucose, insulin and glucagon, with the latter two interacting only with glucose, but not with each other, as was also done in Celeste et al. [11]. The resulting model was deemed useful for both theoretical analyses and experimental and clinical simulations. Other hormones, such as somatostatin and pancreatic polypeptide, play smaller roles in glucose dynamics, and have received virtually no attention in the modelling literature. In this paper, we develop a mathematical model of glucose dynamics which includes its interactions with both insulin and glucagon, and with an emphasis on the low-glucose-concentration regime. Novel features not included in other models are the direct influence of insulin and glucagon on each other, and the ingestion of glucose via a meal. 2. Physiological Description of the Model. There are a number of physiological factors that contribute to the concentrations of glucose, insulin, and glucagon in plasma. We develop a dynamic model for their levels, by constructing a differential equation to describe the changes with respect to time t (in minutes) of each of glucose G (in mg/dl), insulin I (in ng/dl), and glucagon E (in ng/dl). Parameter values are selected to be appropriate for a typical 70 kg man, with an assumed plasma volume of 35 dl. The change in the plasma glucose level can be described phenomenologically by dg/dt = (hepatic release/uptake) (uptake by brain and red blood cells) (uptake by peripherals) (uptake by kidneys) + (ingestion). (2.1)
4 34 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT The terms contributing to (2.1) are as follows. The hepatic uptake/release represents the central role played by the liver in controlling glucose levels. Glucose and insulin serve to increase uptake of glucose by the liver, while glucagon stimulates glucose s release. Uptake by brain and red blood cells represents glucose usage by these areas, and these processes are independent of insulin. Uptake by peripherals represents glucose storage by fat and muscle cells, with these processes being insulin-dependent. Uptake by the kidneys is due to the fact that when glucose levels get too high, excess amounts are passed out of the body in urine by the kidneys. This process is obviously dependent on the concentration of glucose in the blood, but not on that of insulin. Ingestion describes the amount of glucose obtained from a particular meal, and varies depending on the type and amount of food consumed by an individual. The change in the insulin level can be represented by di/dt = (secretion) (degradation) + (injection). (2.2) The first term in (2.2) describes the secretion of insulin, which is known to be stimulated by high levels of glucose and also, to a lesser extent, by glucagon. The degradation term determines how long insulin remains in the blood stream before it is cleared from the body. The injection term is for exogenous insulin externally administered into the bloodstream. The change in the glucagon level can similarly be described by de/dt = (secretion) (degradation). (2.3) The secretion of glucagon in (2.3) depends on both glucose and insulin levels. When glucose is at normal (or higher) levels, the secretion rate is minimal, but when the glucose level falls below a threshold value, glucagon secretion increases (so that the higher glucagon level will spur hepatic release of glucose, thereby raising that level towards the normal range). On the other hand, insulin suppresses the release of glucagon, so that as the insulin concentration rises, the secretion rate of glucagon decreases. The degradation term represents the clearance of glucagon from the bloodstream. 3. Mathematical Construction of the Model. In this section, we reshape the qualitative equations of the last section into differential equations, by modelling each term by an appropriate mathematical form, which mimics the known physiological function. Parameter values are selected, when possible, by performing a leastsquares fit of the chosen mathematical form to the corresponding experimental data Glucose. Conversion of glucose into glycogen, or vice versa, by the liver is one of the body s primary methods of regulating glucose concentrations in the bloodstream. High levels of either glucose or insulin serve to increase glucose uptake by the liver, while glucagon stimulates glucose release. To model the dependence of the hepatic release on the glucose concentration, a form that limits the amount of glucose absorbed is needed, because there is a saturation effect at high glucose levels. Thus an appropriate form is k 5 k 8G k 9 + G, (3.1) where k 5 represents the maximum release rate in the (hypothetical) absence of glucose and k 8 and k 9 are the saturation parameters.
5 HORMONAL EFFECTS ON GLUCOSE REGULATION 35 The next fact to consider is that rising insulin inhibits the release of glucose from the liver (via glycogen breakdown) up to a threshold point; beyond this point, insulin causes the liver to actually absorb glucose through the synthesis of glycogen. This effect can be modelled conveniently by multiplying k 5 by a damping factor to revise (3.1) to k 5 k 6 k 6 + I k 8G k 9 + G, (3.2) where the new factor has a value close to 1 (0) at low (high) insulin concentrations. Lastly, the stimulative effect of glucagon on hepatic release is thought to be linear (Dobbins et al., [14]). This is due to the fact that glucagon acts by interacting with liver hepatocytes, causing them to secrete glucose [15], suggesting that glucose production increases proportionally to the glucagon concentration. Thus the glucagon effect is modelled as a linear relationship, so that the final form for the hepatic uptake/release (the first term on the right-hand side of (2.1)) is dg hep /dt = k 5k 6 k 6 + I k 8G k 9 + G + k 7E. (3.3) The values of the parameters appearing in (3.3) were determined by reference to experiment. The value of k 7 is calculated from the work of Dobbins et al. [14], specifically a plot of the net hepatic glucose balance versus glucagon concentration (their fig. 4). This yields a value of k 7 = (mg/dl/min of G) per (ng/dl of E). The other parameters (k 5, k 6, k 8, k 9 ) were estimated by doing a least-squares fit to fig. 2 of Guyton et al. [16] of the right-hand side of (3.3) with E set equal to the calculated glucagon fasting level of 8.2 ng/dl (see section 3.3). The uptake of glucose by the brain and red blood cells is independent of insulin (and glucagon) concentration, but not of glucose concentration. Although some workers have suggested that the uptake rate should be constant, Andreassen et al. [5] and Boroujerdi et al. [10] argue that it should more realistically be a function of glucose concentration with a saturation limit. Following the latter perspective, the form for this contribution to (2.1) is taken to be dg brain /dt = k 4G k 3 + G. (3.4) Eq. (3.4) indicates that the uptake is effectively 0 at low glucose concentrations, and approaches a limiting value of k 4 at high concentrations. The parameter values appearing in (3.4) were determined, using the data in fig. 4A of Andreassen et al. [5], to be k 3 = mg/dl and k 4 = mg/dl/min. The uptake of glucose by the peripherals (primarily fat and muscle) is dependent not only on glucose, but also on insulin. Following Andreassen et al. [5] this insulin-dependent utilization is close to 0 when I = 0, and increases linearly with increasing blood glucose levels, suggesting proportionality to both G and I. Thus a suitable form is dg per /dt = (k 1 G + k 2 )I. (3.5) The values for the parameters in (3.5) were estimated using the data in fig. 5B of Andreassen et al. [5] to be k 1 = min 1 per (ng/dl of I) and k 2 = (mg/dl/min of G) per (ng/dl of I). The uptake rate by the kidneys is independent of insulin and glucagon, but not glucose. When G is below the renal threshold level G r, the uptake rate by the
6 36 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT kidneys is 0. When G is greater that G r, the rate of uptake increases linearly with increasing G. Thus we have dg kid /dt = k r (G G r )u(g G r ), (3.6) where the parameter k r controls the rate of uptake, and u(g G r ) is the Heaviside step function. The value of the renal threshold is well-established as G r = 180 mg/dl [15]. To calculate k r, we use the fact quoted by Summers and Montani [22] that the uptake by the kidneys increases 230 mg/min for every 180 mg/dl rise in glucose concentration, based on a 70 kg person with an estimated plasma volume of 35 dl. This results in a value of k r = min 1. The exogenous glucose is represented by an input function that describes the amount of glucose ingested and how it is processed in the body. Unlike many models which assume glucose as being given intravenously, we use an approach that models its absorption via a meal. We adopt here the model of Yates and Fletcher [25], which treats this situation as a two-compartment process. The first compartment models the stomach via a trapezoidal function, that describes the glucose flux as the stomach empties into the small intestine, and which has the form (V max /T asc )t, t < T asc V max, T asc < t < T asc + T max G empt = V max (V max /T desc )(t T asc T max ), T asc + T max < t < T asc + T max + T desc 0, otherwise (3.7) In (3.7), V max is the maximum rate of gastric emptying, and T asc (T desc ) is the duration of the ascending (descending) branch of the gastric emptying curve. We let G total represent the total amount of glucose contained in a meal, which is thus a control parameter that depends on the size of the meal and its glucose content. Then T max = G total V max (T asc + T desc )/2. V max The second compartment in the Yates/Fletcher model describes how glucose is processed in the gut, and is governed by the differential equation dg gut /dt = G empt K gabs G gut. (3.8) From here the exogenous glucose is determined, by virtue of its being proportional to G gut ; namely G exg = K gabs G gut. (3.9) Eq (3.8) describes the amount of glucose in the gut, namely G gut, while (3.9) calculates the rate of glucose input into the bloodstream via the gut wall. K gabs is the absorption rate constant of glucose from the gut. The set of equations (3.7) to (3.9) can be solved symbolically (via Maple, for example) to give an explicit, albeit lengthy, expression for G exg (t). The parameter V max is the maximum rate of gastric emptying, for which Yates and Fletcher [25] give a value of 360 mg/min. The parameters T asc and T desc vary with the individual, and are here both assumed to have a value of 15 minutes. G total is a control parameter which can be varied to simulate different glucose contents of a meal. The rate constant K gabs is taken to be 1/60 min 1 [25].
7 HORMONAL EFFECTS ON GLUCOSE REGULATION 37 Inserting the results of eqs. (3.3) to (3.6), and (3.9) into (2.1) gives the final form for the glucose equation to be dg/dt = k 5k 6 k 6 + I k 8G k 9 + G + k 7E k 4G k 3 + G (k 1G + k 2 )I k r (G G r )u(g G r ) + G exg (t). (3.10) 3.2. Insulin. Insulin is secreted by the β cells in the pancreas. It is well-known that both glucose and glucagon have stimulative effects on insulin release, and it is thought that either can act in the absence of the other. Thus the secretion term in (2.2) is modelled as having two terms, one for each of glucose and glucagon dependence. The rate of insulin release is considered to increase proportionally with the glucagon concentration in the blood, suggesting a simple linear relationship. On the other hand, the glucose dependence is more complicated; the rate of release rises with glucose concentration, but with a saturation effect. Consequently, an approach equivalent to that of Sturis et al. [21] is adopted, whereby the dependence on glucose is described by a hyperbolic tangent function. Thus the secretion term in (2.2) is modelled by di sec /dt = (a 1 /2)[tanh(a 2 (G a 3 )) + 1] + b 1 E, (3.11) where a 1 is the maximum secretion rate in the absence of glucagon. The parameter b 1 in (3.11) is estimated using the work of Kawai et al. [17], and specifically their fig. 1 wherein they plot the dependence of insulin on glucagon. Thus is obtained a value of b 1 = (ng/dl/min of I) per (ng/dl of E). The other parameters are taken from the work of Sturis et al. [21], considering that the tanh term in (3.11) is equivalent to the exponential-type form used therein, yielding a 1 = ng/dl/min, a 2 = (mg/dl) 1, and a 3 = 198 mg/dl. The degradation rate for insulin is clinically known to be proportional to the insulin concentration, so the second term in (2.2) has the form di deg /dt = b 2 I. (3.12) A value of b 2 is estimated from the work of Cobelli et al. [13], where their multi-pool submodel for insulin is treated as one effective pool with a decay constant b 2 = min 1. The injection term in (2.2), the so-called exogenous insulin, is modelled by an input function that varies according to the type of insulin administered, the duration of its effectiveness, the size of the dosage, and the time at which the dosage is given. Here, the mathematical form for the exogenous insulin is taken from the work of Basov et al. [6], which appears to be satisfactory for longer-acting insulin types (such as NPH and Lente), but less so for shorter term types (such as Regular). The Basov approach models a series of m insulin injections as m I exg (t) = i j (t), (3.13) with { Bj sin[π(t t i j (t) = j )/T j ], t j t t j + T j, (3.14) 0, otherwise, where t j corresponds to the time at which dosage j is given, T j is the duration of its effectiveness, and B j = (πi 0 )/(2T j ), with I 0 being the actual size of the dosage. Typical values of T j are 24 hours for Lente and 20 hours for NPH. I 0 and t j are control parameters, whose chosen values are discussed in section 4. j=1
8 38 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT Substituting eqs. (3.11) to (3.13) into (2.2) produces the final form for the insulin equation, viz., di/dt = (a 1 /2)[tanh(a 2 (G a 3 )) + 1] + b 1 E b 2 I + I exg (t). (3.15) 3.3. Glucagon. The degradation rate of glucagon in plasma is known from clinical experiments to be proportional to the glucagon concentration, so that the second term in (2.3) has the form de deg /dt = c 3 E. (3.16) The value of the degradation constant is well-known to be c 3 = 0.08 min 1 [13]. Glucagon is secreted in the α cells of the pancreas, with the secretion increasing at low glucose levels but being suppressed by high insulin levels. At normal fasting levels of glucose, there is a basal level of glucagon secretion, taken here to be a constant c 0. At higher glucose concentrations, additional secretion is suppressed, but when the glucose concentration falls below a threshold level G E, glucagon secretion should increase linearly with decreasing G. Thus an initial form for the secretion term in (2.3) is c 0 + c 1 (G E G)u(G E G), (3.17) with the Heaviside step function u serving to switch the secretion on or off at the threshold value G E. When I = 0, the glucagon secretion rate is at its maximum, and as I increases, the secretion rate should decrease. This fact suggests that (3.17) should be modified to de sec /dt = c 0 + c 1 c 2 + I (G E G)u(G E G). (3.18) The fasting level of glucagon is known to be Ē = 8.2 ng/dl [9], from which c 0 is determined from the relationship Ē = c 0/c 3 to be c 0 = ng/dl/min. The threshold value of glucose below which glucagon secretion occurs is well-established to be G E = 75 mg/dl [15]. The values of c 1 and c 2 were determined by fitting to data extracted from fig. 1 of Bolli et al. [9], yielding c 1 = (ng/dl/min of E)(ng/dl of I) per (mg/dl of G) and c 2 = ng/dl (of I). Substituting (3.16) and (3.18) into (2.3) produces the final form of the glucagon equation as de/dt = c 0 + c 1 c 2 + I (G E G)u(G E G) c 3 E. (3.19) Note that when G > G E, the Heaviside function is switched off, setting the middle term of (3.19) to 0, resulting in an equilibrium value of glucagon being achieved when de/dt = 0, i.e., at Ē = c 0/c Diabetes. The model of glucose utilization represented by equations (3.10), (3.15), and (3.19) pertains to a healthy individual. To simulate the different forms of diabetes requires modification of certain terms, which we discuss here. Type 1 diabetes is characterized by the destruction of most of the β cells, with the result that little insulin is produced. Although this effect could be modelled in various ways, we do so in a simple manner whereby the insulin secretion terms (3.11) are replaced by a single constant term b 3 so that the insulin equation (3.15) in the model is replaced by di/dt = b 3 b 2 I + I exg (t). (3.20) The value of b 3 can be expected to vary with the severity of the individual s condition, but here we choose b 3 = ng/dl/min, corresponding to an equilibrium level of insulin of 19 ng/dl. Type 1 diabetes is known to have complications, such
9 HORMONAL EFFECTS ON GLUCOSE REGULATION 39 as renal failure and liver disease, which occur over the long term; such factors are not incorporated into the model. In type 2 diabetes, the β cells continue to produce insulin, although at a reduced level. Moreover, the body develops a resistance to insulin thus reducing its effectiveness. The former effect can be modelled by multiplying the insulin secretion terms (3.11) by a reduction factor 0 InsRed 1. The value of InsRed would vary with the individual, so here we choose an intermediate value of InsRed = 0.5. The latter effect can be modelled by multiplying usage-related occurrences of I(t) by an effectiveness factor. Specifically we change the peripheral uptake (3.5) to and the hepatic uptake/release (3.3) to dg per /dt = (k 1 G + k 2 )I(InsEff), (3.21) dg hep /dt = k 5 k 6 k 6 + I(InsEff2) k 8G k 9 + G + k 7E, (3.22) where the effectiveness factors are such that 0 InsEf f, InsEf f 2 1. The actual values of the factors will depend upon the individual, and here we take them to be InsEff = InsEff2 = 0.2. The final situation that we simulate is that of damage to the α cells (which secrete glucagon), which is believed to be of possible importance in many diabetic cases. This is due to the fact that the sensitivity of the α cells to glucose is apparently diminished over time, so that more glucose is needed in order for suppression of glucagon secretion to occur, resulting in an increase in the value of G E. For our purposes here, we take that revised value to be G E = 125 mg/dl. In addition, the α cells may be desensitized to insulin, which may be modelled by multiplying I(t) in (3.19) by an effectiveness factor InsEf f 3 so that the glucagon equation becomes c 1 de/dt = c 0 + c 2 + I(InsEff3) (G E G)u(G E G) c 3 E. (3.23) We take InsEff3 = Results and Discussion. In this section, we look at some representative results for the model, for both healthy and diabetic individuals. The starting point is the set of equations (3.10), (3.15) and (3.19) for a healthy individual, with parameter values as indicated in sections These equations form a dynamical system, which can be solved numerically for arbitrary initial conditions. It can be shown that the system has just one equilibrium (i.e., steady-state solution), occurring at Ḡ = 93.6, Ī = 41.6, Ē = 8.2. The equilibrium can be shown to be stable, which indicates physiologically that, at least for modest deviations from the steady-state values, the system returns itself to equilibrium, as would be expected in a healthy individual; i.e., when the plasma concentration of one or more of the substances deviates from equilibrium, the system readjusts itself so as to drive the concentrations back to equilibrium. This behaviour is illustrated in Figure 1, where neither food nor insulin is given, while the initial values are taken to be G 0 = 60, I 0 = Ī, E 0 = Ē (i.e., glucose initially below equilibrium, insulin and glucagon at equilibrium). The graphs show that the glucose level very quickly (within 15 minutes) returns to the equilibrium level, due to a combination of a short-term drop in the insulin level (by about 25%) and a rise in the glucagon level (of about 20%). This behaviour is expected because of the known effect of glucagon to raise glucose levels and of insulin to lower them. When the calculation was repeated, but with insulin and glucagon levels held constant at their equilibrium values, it took about
10 40 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT Glucose (mg/dl) Insulin (ng/dl) Glucagon x 10 (ng/dl) 80 concentration time (minutes) Figure 1. G(t), I(t), and E(t) 10 for a healthy individual, given no food (G total = 0) and no insulin (I total = 0). 2-3 times longer for the glucose level to return to equilibrium. However, with just glucagon kept constant, the equilibration process is only modestly slower than when glucagon is allowed to vary. This is indicative of the fact (Unger [24], Kruger et al. [18]) that insulin is the primary regulator of the bloodstream s glucose levels, with glucagon acting in a secondary role as one (but not the only) counterbalance to insulin s actions. The role of glucagon becomes more pronounced when the glucose level falls even further below the secretion threshold of G E = 75 mg/dl, than the G 0 = 60 mg/dl of Figure 1. The distinction between holding glucagon constant, versus letting it vary, becomes greater with lower glucose levels. The importance of glucagon in correcting such situations of hypoglycemia is thus emphasized. The situation that is normal for healthy individuals is ingestion of a meal, but with no administration of exogenous insulin. This case is illustrated in Figure 2, for a meal with a content of G total = mg of glucose, and with all levels initially at their equilibrium values. As might be anticipated, the glucose level rises to a maximum of over 125 mg/dl and remains elevated over a period of several hours, before dropping back to equilibrium level. Correspondingly, the insulin level rises over the same time period, acting to bring the glucose levels down. The glucagon level (not shown) remains constant at Ē = 8.2 ng/dl because the glucose concentration always remains above the threshold value G E = 75 mg/dl, below which additional glucagon secretion commences (c.f. (3.17)). This circumstance would be the usual one for hyperglycemia wherein glucose levels are high resulting in glucagon levels remaining constant. The modellistic implication is that glucagon as a dynamic variable can be removed from the model, in such cases. Such is not the case for hypoglycemia, where glucagon levels may change significantly. We turn now to type 1 diabetes, with the model modifications outlined in section 3.4. Figure 3 illustrates a typical situation where a meal with G total = mg
11 HORMONAL EFFECTS ON GLUCOSE REGULATION Glucose (mg/dl) Insulin (ng/dl) 100 concentration time (minutes) Figure 2. G(t) and I(t) for a healthy individual, with ingestion of a meal (G total = ) but no insulin (I total = 0). E(t) = constant is not shown. glucose content is taken (at t = 0) followed an hour later by an administration of ng/dl Lente insulin, corresponding to 1 3 U per kg bodyweight. (The standard clinical unit for measurement of insulin is 1 U which corresponds to 1/24 mg of insulin.) The initial values are taken to be the new equilibrium levels of equations (3.10), (3.19), and (3.20), namely Ḡ = 120, Ī = 19, and Ē = 8.2. As expected, the meal causes the modelled glucose level to rise quickly to almost 200 mg/dl. The insulin injection an hour later immediately raises the plasma insulin level I(t), which the model responds with a steep decline in G(t). In this case, the insulin serves to decrease the glucose to a very low level (eventually around 50 mg/dl), thus causing the glucagon secretion to increase dramatically at t = 600 in order to drive G(t) back up to equilibrium. Thus the model predicts that glucagon plays a crucial role in correcting this hypoglycemic situation. Qualitatively similar results are obtained for other meal sizes and insulin doses and types. For example, a larger meal with higher glucose content (G total ) would cause more glucose to be in the body, and the predicted duration of any glucagon secretion would be considerably decreased. Figure 3 can be compared with Figure 2 (for a healthy person), where it can be noted that the return to equilibrium takes approximately twice as long for a diabetic as for a healthy person, and with noticeably more variation in the function values, especially G(t). Type 2 diabetes differs from type 1 because more significant amounts of insulin are produced, but the insulin s effectiveness is reduced, thus leading to the model implications discussed in section 3.4. Figure 4 displays a typical scenario wherein a meal with glucose content G total = mg is ingested at t = 0, but no insulin is administered (because type 2 diabetics usually do not take insulin). After the meal, the plasma glucose level G(t) increases rapidly to over 190 mg/dl and
12 42 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT 200 Glucose (mg/dl) Insulin (ng/dl) Glucagon x 10 (ng/dl) concentration time (minutes) Figure 3. G(t), I(t), and E(t) 10 for a type 1 diabetic, given both food (G total = ) and insulin (I total = 27778) Glucose (mg/dl) Insulin (ng/dl) 200 concentration time (minutes) Figure 4. G(t) and I(t) for a type 2 diabetic, given food (G total = ) but no insulin (I total = 0). E(t) = constant is not shown. remains high over the course of several hours. As a consequence, the plasma insulin level I(t) rises to very high levels (albeit more slowly than the rise in G(t)), and eventually lowers G(t) to its equilibrium level (around 140 mg/dl) after about 10
13 HORMONAL EFFECTS ON GLUCOSE REGULATION 43 hours. Comparison with Figure 2 shows that the equilibration process here takes about the same length of time as in a healthy person, but that the glucose levels go much higher. Due to the reduced effectiveness of the insulin, I(t) has its maximum value at over 250 ng/dl, much higher than in the corresponding situation for healthy subjects (Figure 2, where I(t) has a maximum of about 125 ng/dl), or even for type 1 diabetics (Figure 3, where the maximum of I(t) is about 160 ng/dl). Nonetheless, the individual s natural insulin is sufficient to control hyperglycemia (although at elevated equilibrium levels of glucose), without resorting to administration of exogenous insulin. The glucagon level (not shown in figure) remains constant at the equilibrium value Ē = 8.2 ng/dl, because the glucose levels always remain above G E = 75 mg/dl. Below this level, additional glucagon secretion occurs. The relevance of glucagon to type 2 diabetes can be seen by examining a hypoglycemic situation (induced by a period of fasting, for instance). This situation is shown in Figure 5, where the initial plasma glucose concentration is taken to be G 0 = 60 mg/dl. This is below G E, and leads to a sharp rise in the glucagon level, and a Glucose (mg/dl) Insulin (ng/dl) Glucagon x 10 (ng/dl) 120 concentration time (minutes) Figure 5. G(t), I(t), and E(t) 10 for a type 2 diabetic, given no food (G total = 0) and no insulin (I total = 0). corresponding drop in the insulin level, thus producing the desired increase in the glucose level to its equilibrium level, over a reasonably short time period of about 30 minutes. Figure 5 shows a strong qualitative resemblance to the corresponding situation for a healthy person (Figure 1), differing primarily in that the return to equilibrium in the diabetic case takes about times as long as it does in the healthy case. The equilibria for the two cases are obviously different, most importantly in that the equilibrium for the diabetic case has a significantly elevated level of glucose compared to that for a healthy person. The well-known counterbalancing effects of the two hormones (Unger [24], Kruger et al. [18]) are again illustrated (in both Figures 1 and 5), where glucagon acts to help correct the hypoglycemic
14 44 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT situation, but the rise in glucose levels is restricted by the counter-effect of insulin, which acts to prevent hyperglycemia. The importance of healthy α cells, with their complicated dependence on glucose and insulin concentrations determining the glucagon secretion rate, is demonstrated by looking at the consequences of the improper functioning of damaged α cells. This situation is shown in Figure 6, using the model modifications discussed in section 3.4, and with initial glucose concentration being very low, G 0 = 60 mg/dl. As Glucose (mg/dl) Insulin (ng/dl) Glucagon x 10 (ng/dl) 250 concentration time (minutes) Figure 6. G(t), I(t), and E(t) 10 for an individual with damaged α cells, given no food (G total = 0) and no insulin (I total = 0). expected, the model predicts that a glucagon concentration increase occurs, and in fact this increase happens quickly and sharply. Because of the reduced sensitivity to glucose (higher G E ), glucagon secretion continues even when glucose levels are in the range mg/dl. As a result, the glucose concentration increases as high as 180 mg/dl, before dropping to a new, elevated equilibrium value of about 120 mg/dl. The equilibrium values of insulin and glucose are now elevated to several times their values in the healthy case (c.f. Figure 1), and once again the return to equilibrium takes about twice as long as in a healthy person. Figure 6 emphasizes the role of the α cells, and their production of glucagon, in the regulation of plasma glucose concentration. Damage to the α cells, often believed to be a long-term effect of diabetes, has a significant impact on the glucose levels. There is experimental support for these theoretical results. Shah et al. [20] showed that in type 2 diabetics, a lack of suppression of glucagon is a contributory factor to hyperglycemia after a meal. Ahren and Larsson [4] reached a similar, and perhaps stronger, conclusion that impaired glucose tolerance is indeed associated with reduced suppression of glucagon secretion, which is insulin-induced and possibly caused by α cell resistance to insulin. In both cases, they emphasized that treatment of diabetes would be aided by the development of agents to inhibit glucagon secretion or counteract glucagon action.
15 HORMONAL EFFECTS ON GLUCOSE REGULATION 45 In summary, the model developed in this paper demonstrates the strong dependence of plasma glucose levels on the concentrations of the key hormones, insulin and glucagon. In the hyperglycemic situation that is most important to diabetics, the glucagon concentration stays constant and can be removed from consideration, as is done by most models. For the hypoglycemic case, which is a serious acute complication, consideration of the role of glucagon is vital to an accurate calculation of the glucose level. The oft-ignored α cells are shown to play an essential role in glucose regulation, and their malfunction can have a significant effect by raising glucose levels. Acknowledgements. We have benefited from discussions with Profs. Cathy Chan and Sherri Ihle, both of the Atlantic Veterinary College. REFERENCES [1] E. Ackerman, J.W. Rosevar and W.F. McGuckin, A mathematical model of the glucosetolerance test, Phys. Med. Biol., 9 (1964), [2] E. Ackerman, L.C. Gatewood, J.W. Rosevar and G.D. Molnar, Model studies of blood-glucose regulation, Bull. Math. Biophys., 27 (1965), [3] E. Ackerman, L.C. Gatewood, J.W. Rosevar and G.D. Molnar, Blood Glucose Regulation and Diabetes. In: Concepts and Models of Biomathematics, Chapter 4, F. Heinmets, ed., Marcel Dekker, (1969) [4] B. Ahren and H. Larsson, Impaired glucose tolerance (IGT) is associated with reduced insulininduced suppression of glucagon concentrations, Diabetologia, 44 (2001), [5] S. Andreassen, J.J. Benn, R. Hovorka, K.G. Olesen and E.R. Carson, A probabilistic approach to glucose prediction and insulin dose adjustment: description of metabolic model and pilot evaluation study, Comp. Meth. Prog. Biomed., 41 (1994), [6] I. Basov, M. Meilunas and D. Svitra, Glycemia monitoring: the problem of exogenous insulin input, Math. Model. Anal., 4 (1999), [7] R.N. Bergman, G. Bortolan, C. Cobelli and G. Toffolo, Identification of a minimal model of glucose disappearance for estimating insulin sensitivity. In: Identification and System Parameter Estimation, (Proc. 5th IFAC Symposium, Darmstadt, 1979) R. Isermann, ed., Pergamon Press, Oxford (1979), vol. 2, [8] V.W. Bolie, Coefficients of normal blood glucose regulation, J. Appl. Physiol., 16 (1961), [9] G. Bolli, P. DeFeo, G. Perriello, S. DeCosmo, P. Compagnucci, F. Santeusanio, P. Brunetti and R.H. Unger, Mechanisms of glucagon secretion during insulin-induced hypoglycemia in man: role of the beta cell and arterial hyperinsulinemia, J. Clin. Invest., 73 (1984), [10] M.A. Boroujerdi, A.M. Umpleby, R.H. Jones and P.H. Sonksen, A simulation model for glucose kinetics and estimates of glucose utilization rate in type 1 diabetic patients, Am. J. Physiol., 268 (1995), E766 E774. [11] R. Celeste, E. Ackerman, L.C. Gatewood, C. Reynolds and G.D. Molnar, The role of glucagon in the regulation of blood glucose model studies, Bull. Math. Biol., 40 (1978), [12] C. Cobelli, G. Pacini and A. Salvan, On a simple model of insulin secretion, Med. & Biol. Eng. & Comput., 18 (1980), [13] C. Cobelli, G. Federspil, G. Pacini, A. Salvan and C. Scandellari, An integrated mathematical model of the dynamics of blood glucose and its hormonal control, Math. Bioscien., 58 (1982), [14] R.L. Dobbins, S.N. Davis, D.W. Neal, C. Cobelli, J. Jaspan and A.D. Cherrington, Compartmental modelling of glucagon kinetics in the conscious dog, Metabolism, 44 (1995), [15] W.F. Ganong, Review of medical physiology, 21st edition, McGraw-Hill, New York, [16] J.R. Guyton, R.O. Foster, J.S. Soeldner, M.H. Tan, C.B. Kahn, L. Konez and R.E. Gleason, A model of glucose-insulin homeostasis in man that incorporates the heterogeneous fast pool theory of pancreatic insulin release, Diabetes, 27 (1978), [17] K. Kawai, C. Yokota, S. Ohashi, Y. Watanabe and K. Yamashita, Evidence that glucagon stimulates insulin secretion through its own receptor in rats, Diabetologia, 38 (1995),
16 46 KENNETH W. SULSTON, WILLIAM P. IRELAND, JEFF C. PRAUGHT [18] D.F. Kruger, C.L. Martin and C.E. Sadler, New insights into glucose regulaton, The Diabetes Educator, 32 (2006), [19] E. Salzsieder, G. Albrecht, E. Jutzi and U. Fischer, Estimation of individually adapted control parameters for an artificial beta cell, Biomed. Biochim., 5 (1984), [20] P. Shah, A. Vella, A. Basu, R. Basu, W.F. Schwenk and R.A. Rizza, Lack of suppression of glucagon contributes to postpandrial hyperglycemia in subjects with type 2 diabetes mellitus, J. Clin. Endocrinol. Metab., 85 (2000), [21] J. Sturis, K.S. Polonsky, E. Mosekilde and E.V. Cauter, Computer model for mechanisms underlying ultradian oscillations of insulin and glucose, Am. J. Physiol., 260 (1991), E801 E809. [22] R.L. Summers and J-P. Montani, Mathematical model of glucose homeostasis for the study of metabolic states, J. Mississippi Acad. Sci., 34 (1989), [23] R.L. Summers, J-P. Montani, L.H. Woodward, T.G. Coleman and J.E. Hall, Theoretical analysis of the mechanisms of chronic hyperinsulinemia, Comput. Biol. Med., 27 (1997), [24] R.H. Unger, Insulin-glucagon relationships in the defense against hypoglycemia, Diabetes, 32 (1983), [25] T.L. Yates and L.R. Fletcher, Prediction of a glucose appearance function from foods using deconvolution, IMA J. Appl. Math. Med. Biol., 17 (2000), Received November 2005; revised May address: sulston@upei.ca; ireland@upei.ca; jpraught@dal.ca
A mathematical model of glucose-insulin interaction
 Science Vision www.sciencevision.org Science Vision www.sciencevision.org Science Vision www.sciencevision.org Science Vision www.sciencevision.org the alpha and beta cells of the pancreas respectively.
Science Vision www.sciencevision.org Science Vision www.sciencevision.org Science Vision www.sciencevision.org Science Vision www.sciencevision.org the alpha and beta cells of the pancreas respectively.
Analysis of glucose-insulin-glucagon interaction models.
 C.J. in t Veld Analysis of glucose-insulin-glucagon interaction models. Bachelor thesis August 1, 2017 Thesis supervisor: dr. V. Rottschäfer Leiden University Mathematical Institute Contents 1 Introduction
C.J. in t Veld Analysis of glucose-insulin-glucagon interaction models. Bachelor thesis August 1, 2017 Thesis supervisor: dr. V. Rottschäfer Leiden University Mathematical Institute Contents 1 Introduction
Alternative insulin delivery systems: how demanding should the patient be?
 Diabetologia (1997) 4: S97 S11 Springer-Verlag 1997 Alternative insulin delivery systems: how demanding should the patient be? K.S. Polonsky, M. M. Byrne, J. Sturis Department of Medicine, The University
Diabetologia (1997) 4: S97 S11 Springer-Verlag 1997 Alternative insulin delivery systems: how demanding should the patient be? K.S. Polonsky, M. M. Byrne, J. Sturis Department of Medicine, The University
28 Regulation of Fasting and Post-
 28 Regulation of Fasting and Post- Prandial Glucose Metabolism Keywords: Type 2 Diabetes, endogenous glucose production, splanchnic glucose uptake, gluconeo-genesis, glycogenolysis, glucose effectiveness.
28 Regulation of Fasting and Post- Prandial Glucose Metabolism Keywords: Type 2 Diabetes, endogenous glucose production, splanchnic glucose uptake, gluconeo-genesis, glycogenolysis, glucose effectiveness.
MODELING GLUCOSE-INSULIN METABOLIC SYSTEM AND INSULIN SECRETORY ULTRADIAN OSCILLATIONS WITH EXPLICIT TIME DELAYS. Yang Kuang
 MODELING GLUCOSE-INSULIN METABOLIC SYSTEM AND INSULIN SECRETORY ULTRADIAN OSCILLATIONS WITH EXPLICIT TIME DELAYS Yang Kuang (joint work with Jiaxu Li and Clinton C. Mason) Department of Mathematics and
MODELING GLUCOSE-INSULIN METABOLIC SYSTEM AND INSULIN SECRETORY ULTRADIAN OSCILLATIONS WITH EXPLICIT TIME DELAYS Yang Kuang (joint work with Jiaxu Li and Clinton C. Mason) Department of Mathematics and
The oral meal or oral glucose tolerance test. Original Article Two-Hour Seven-Sample Oral Glucose Tolerance Test and Meal Protocol
 Original Article Two-Hour Seven-Sample Oral Glucose Tolerance Test and Meal Protocol Minimal Model Assessment of -Cell Responsivity and Insulin Sensitivity in Nondiabetic Individuals Chiara Dalla Man,
Original Article Two-Hour Seven-Sample Oral Glucose Tolerance Test and Meal Protocol Minimal Model Assessment of -Cell Responsivity and Insulin Sensitivity in Nondiabetic Individuals Chiara Dalla Man,
Active Insulin Infusion Using Fuzzy-Based Closed-loop Control
 Active Insulin Infusion Using Fuzzy-Based Closed-loop Control Sh. Yasini, M. B. Naghibi-Sistani, A. Karimpour Department of Electrical Engineering, Ferdowsi University of Mashhad, Mashhad, Iran E-mail:
Active Insulin Infusion Using Fuzzy-Based Closed-loop Control Sh. Yasini, M. B. Naghibi-Sistani, A. Karimpour Department of Electrical Engineering, Ferdowsi University of Mashhad, Mashhad, Iran E-mail:
Mathematical Modeling of Diabetes
 Mathematical Modeling of Diabetes Dr. Shyam Kamal Department of Systems Design and Informatics Kyushu Institute of Technology Japan kamal@ces.kyutech.ac.jp 20 May 2016 Overview Role of Glucose Homeostasis
Mathematical Modeling of Diabetes Dr. Shyam Kamal Department of Systems Design and Informatics Kyushu Institute of Technology Japan kamal@ces.kyutech.ac.jp 20 May 2016 Overview Role of Glucose Homeostasis
Electronic Supplementary Material to the article entitled Altered pattern of the
 Electronic Supplementary Material to the article entitled Altered pattern of the incretin effect as assessed by modelling in individuals with glucose tolerance ranging from normal to diabetic Integrated
Electronic Supplementary Material to the article entitled Altered pattern of the incretin effect as assessed by modelling in individuals with glucose tolerance ranging from normal to diabetic Integrated
Control of Glucose Metabolism
 Glucose Metabolism Control of Glucose Metabolism The pancreas is both an exocrine and endocrine gland. It secretes digestive enzymes into the duodenum (exocrine) and 3 specific hormones into the bloodstream
Glucose Metabolism Control of Glucose Metabolism The pancreas is both an exocrine and endocrine gland. It secretes digestive enzymes into the duodenum (exocrine) and 3 specific hormones into the bloodstream
A Mathematical Model of Glucose - Insulin regulation under the influence of externally ingested glucose (G-I-E model)
 International Journal of Mathematics and Statistics Invention (IJMSI) -ISSN: 2321 4767 P-ISSN: 2321-4759 Volume 4 Issue 5 June. 2016 PP-54-58 A Mathematical Model of Glucose - Insulin regulation under
International Journal of Mathematics and Statistics Invention (IJMSI) -ISSN: 2321 4767 P-ISSN: 2321-4759 Volume 4 Issue 5 June. 2016 PP-54-58 A Mathematical Model of Glucose - Insulin regulation under
The effect of food on triggering type II diabetes: modeling a CBD Pancreas
 The effect of food on triggering type II diabetes: modeling a CBD Pancreas Mohamed Raef Smaoui School of Computer Science, McGill University, Montreal, QC, Canada Abstract Type II diabetes affects around
The effect of food on triggering type II diabetes: modeling a CBD Pancreas Mohamed Raef Smaoui School of Computer Science, McGill University, Montreal, QC, Canada Abstract Type II diabetes affects around
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
Chapter 3: Linear & Non-Linear Interaction Models
 Chapter 3: 155/226 Chapter develops the models above to examine models which involve interacting species or quantities. Models lead to simultaneous differential equations for coupled quantites due to the
Chapter 3: 155/226 Chapter develops the models above to examine models which involve interacting species or quantities. Models lead to simultaneous differential equations for coupled quantites due to the
CoreModels Glucose-Insulin Model A CORE LEARNING GOALS ACTIVITY FOR SCIENCE AND MATHEMATICS
 CoreModels Glucose-Insulin Model A CORE LEARNING GOALS ACTIVITY FOR SCIENCE AND MATHEMATICS Summary Students use two prebuilt STELLA models to investigate the relationship between the amount of glucose
CoreModels Glucose-Insulin Model A CORE LEARNING GOALS ACTIVITY FOR SCIENCE AND MATHEMATICS Summary Students use two prebuilt STELLA models to investigate the relationship between the amount of glucose
Outline Insulin-Glucose Dynamics a la Deterministic models Biomath Summer School and Workshop 2008 Denmark
 Outline Insulin-Glucose Dynamics a la Deterministic models Biomath Summer School and Workshop 2008 Denmark Seema Nanda Tata Institute of Fundamental Research Centre for Applicable Mathematics, Bangalore,
Outline Insulin-Glucose Dynamics a la Deterministic models Biomath Summer School and Workshop 2008 Denmark Seema Nanda Tata Institute of Fundamental Research Centre for Applicable Mathematics, Bangalore,
Hypoglycemia. When recognized early, hypoglycemia can be treated successfully.
 Hypoglycemia Introduction Hypoglycemia is a condition that causes blood sugar level to drop dangerously low. It mostly shows up in diabetic patients who take insulin. When recognized early, hypoglycemia
Hypoglycemia Introduction Hypoglycemia is a condition that causes blood sugar level to drop dangerously low. It mostly shows up in diabetic patients who take insulin. When recognized early, hypoglycemia
INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY
 INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY A PATH FOR HORIZING YOUR INNOVATIVE WORK A SLIDING MODE CONTROL ALGORITHM FOR ARTIFICIAL PANCREAS NIRLIPTA RANJAN MOHANTY
INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY A PATH FOR HORIZING YOUR INNOVATIVE WORK A SLIDING MODE CONTROL ALGORITHM FOR ARTIFICIAL PANCREAS NIRLIPTA RANJAN MOHANTY
Project for Math. 224 DETECTION OF DIABETES
 Project for Math. 224 DETECTION OF DIABETES Diabetes is a disease of metabolism which is characterized by too much sugar in the blood and urine. Because of the lack of insulin (a hormone), the patient
Project for Math. 224 DETECTION OF DIABETES Diabetes is a disease of metabolism which is characterized by too much sugar in the blood and urine. Because of the lack of insulin (a hormone), the patient
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
An integrated glucose-insulin model to describe oral glucose tolerance test data in healthy volunteers
 Title: An integrated glucose-insulin model to describe oral glucose tolerance test data in healthy volunteers Authors: Hanna E. Silber 1, Nicolas Frey 2 and Mats O. Karlsson 1 Address: 1 Department of
Title: An integrated glucose-insulin model to describe oral glucose tolerance test data in healthy volunteers Authors: Hanna E. Silber 1, Nicolas Frey 2 and Mats O. Karlsson 1 Address: 1 Department of
CLINICAL SIMULATION OF OGTT GLUCOSE RESPONSE MODEL FOR DIAGNOSIS OF DIABETIC PATIENT
 Journal of Mechanics in Medicine and Biology Vol. 5, No. 1 (2005) 123 138 c World Scientific Publishing Company CLINICAL SIMULATION OF OGTT GLUCOSE RESPONSE MODEL FOR DIAGNOSIS OF DIABETIC PATIENT DHANJOO
Journal of Mechanics in Medicine and Biology Vol. 5, No. 1 (2005) 123 138 c World Scientific Publishing Company CLINICAL SIMULATION OF OGTT GLUCOSE RESPONSE MODEL FOR DIAGNOSIS OF DIABETIC PATIENT DHANJOO
ENERGY FROM INGESTED NUTREINTS MAY BE USED IMMEDIATELY OR STORED
 QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
Pharmacokinetics Overview
 Pharmacokinetics Overview Disclaimer: This handout and the associated lectures are intended as a very superficial overview of pharmacokinetics. Summary of Important Terms and Concepts - Absorption, peak
Pharmacokinetics Overview Disclaimer: This handout and the associated lectures are intended as a very superficial overview of pharmacokinetics. Summary of Important Terms and Concepts - Absorption, peak
Pancreas Fox Chapter 18 part 2 (also Chapter 19.3 & 19.4)
 Vert Phys PCB3743 Pancreas Fox Chapter 18 part 2 (also Chapter 19.3 & 19.4) T. Houpt, Ph.D. Anatomy of Digestive System Peristalsis Stomach and Acid Secretion Liver and Bile Secretion Pancreas and pancreatic
Vert Phys PCB3743 Pancreas Fox Chapter 18 part 2 (also Chapter 19.3 & 19.4) T. Houpt, Ph.D. Anatomy of Digestive System Peristalsis Stomach and Acid Secretion Liver and Bile Secretion Pancreas and pancreatic
Hormones and Homeostasis
 Hormones and Homeostasis The endocrine system is a system of organs that releases chemical message molecules, called hormones, into the blood. Unlike the nervous system whose action helps the body react
Hormones and Homeostasis The endocrine system is a system of organs that releases chemical message molecules, called hormones, into the blood. Unlike the nervous system whose action helps the body react
Basic Concepts of TDM
 TDM Lecture 1 5 th stage What is TDM? Basic Concepts of TDM Therapeutic drug monitoring (TDM) is a branch of clinical pharmacology that specializes in the measurement of medication concentrations in blood.
TDM Lecture 1 5 th stage What is TDM? Basic Concepts of TDM Therapeutic drug monitoring (TDM) is a branch of clinical pharmacology that specializes in the measurement of medication concentrations in blood.
Gamma Variate Analysis of Insulin Kinetics in Type 2 Diabetes
 Gamma Variate Analysis of Insulin Kinetics in Type 2 Diabetes Anthony Shannon Faculty of Engineering & IT, University of Technology Sydney, NSW 2007, Australia PO Box 314, Balgowlah, NSW 2093, Australia
Gamma Variate Analysis of Insulin Kinetics in Type 2 Diabetes Anthony Shannon Faculty of Engineering & IT, University of Technology Sydney, NSW 2007, Australia PO Box 314, Balgowlah, NSW 2093, Australia
Modellering av blodsukkerdynamikk
 Modellering av blodsukkerdynamikk Marianne Myhre Master i teknisk kybernetikk (2-årig) Innlevert: juli 2013 Hovedveileder: Steinar Sælid, ITK Norges teknisk-naturvitenskapelige universitet Institutt for
Modellering av blodsukkerdynamikk Marianne Myhre Master i teknisk kybernetikk (2-årig) Innlevert: juli 2013 Hovedveileder: Steinar Sælid, ITK Norges teknisk-naturvitenskapelige universitet Institutt for
Proceedings of the 5th WSEAS International Conference on Telecommunications and Informatics, Istanbul, Turkey, May 27-29, 2006 (pp )
 A Predictive Control Approach For Nonlinear Systems MALIK F. ALAMAIREH, MOHAMMAD R. HASSAN Department of Computer Science Amman University Assalt 19112 P. O. Box 5043 JORDAN Abstract: - The theory of artificial
A Predictive Control Approach For Nonlinear Systems MALIK F. ALAMAIREH, MOHAMMAD R. HASSAN Department of Computer Science Amman University Assalt 19112 P. O. Box 5043 JORDAN Abstract: - The theory of artificial
SIMULATIONS OF A MODEL-BASED FUZZY CONTROL SYSTEM FOR GLYCEMIC CONTROL IN DIABETES
 Bulletin of the Transilvania University of Braşov Vol. 8 (57) No. 2-2015 Series I: Engineering Sciences SIMULATIONS OF A MODEL-BASED FUZZY CONTROL SYSTEM FOR GLYCEMIC CONTROL IN DIABETES C. BOLDIȘOR 1
Bulletin of the Transilvania University of Braşov Vol. 8 (57) No. 2-2015 Series I: Engineering Sciences SIMULATIONS OF A MODEL-BASED FUZZY CONTROL SYSTEM FOR GLYCEMIC CONTROL IN DIABETES C. BOLDIȘOR 1
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM
 5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
Carbohydrate Metabolism
 Chapter 34 Carbohydrate Metabolism Carbohydrate metabolism is important for both plants and animals. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison,
Chapter 34 Carbohydrate Metabolism Carbohydrate metabolism is important for both plants and animals. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison,
Control of Blood Sugar Levels
 Why? Control of Sugar Levels What hormones are involved in the homeostasis of blood sugar? All living things use as a source of energy. In vertebrates it is critical that the levels of in the blood are
Why? Control of Sugar Levels What hormones are involved in the homeostasis of blood sugar? All living things use as a source of energy. In vertebrates it is critical that the levels of in the blood are
An integrated glucose homeostasis model of glucose, insulin, C-peptide, GLP-1, GIP and glucagon in healthy subjects and patients with type 2 diabetes
 An integrated glucose homeostasis model of glucose, insulin, C-peptide, GLP-1, GIP and glucagon in healthy subjects and patients with type 2 diabetes Oskar Alskär, Jonatan Bagger, Rikke Røge, Kanji Komatsu,
An integrated glucose homeostasis model of glucose, insulin, C-peptide, GLP-1, GIP and glucagon in healthy subjects and patients with type 2 diabetes Oskar Alskär, Jonatan Bagger, Rikke Røge, Kanji Komatsu,
The Chemostat: Stability at Steady States. Chapter 5: Linear & Non-Linear Interaction Models. So, in dimensional form, α 1 > 1 corresponds to
 Introduction & Simple Models Logistic Growth Models The Chemostat: Stability at Steady States 1 So, in dimensional form, α 1 > 1 corresponds to K max < V F. As K max is max bacterial repro rate with unlimited
Introduction & Simple Models Logistic Growth Models The Chemostat: Stability at Steady States 1 So, in dimensional form, α 1 > 1 corresponds to K max < V F. As K max is max bacterial repro rate with unlimited
6. The diagram below represents an interaction between parts of an organism.
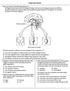 Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Quantitative indexes of -cell function during graded up&down glucose infusion from C-peptide minimal models
 Am J Physiol Endocrinol Metab 280: E2 E10, 2001. Quantitative indexes of -cell function during graded up&down glucose infusion from C-peptide minimal models GIANNA TOFFOLO, 1 ELENA BREDA, 1 MELISSA K.
Am J Physiol Endocrinol Metab 280: E2 E10, 2001. Quantitative indexes of -cell function during graded up&down glucose infusion from C-peptide minimal models GIANNA TOFFOLO, 1 ELENA BREDA, 1 MELISSA K.
History of Investigation
 Acini - Pancreatic juice (1º) (2º) Secretions- neuronal and hormonal mechanisms 1) Secretin - bicarbonate rich 2) Cholecystokinin - enzyme rich Islets of Langerhans (contain 4 cell types) Alpha cells (α)-
Acini - Pancreatic juice (1º) (2º) Secretions- neuronal and hormonal mechanisms 1) Secretin - bicarbonate rich 2) Cholecystokinin - enzyme rich Islets of Langerhans (contain 4 cell types) Alpha cells (α)-
Glucose-Insulin Pharmacodynamic Surface Modeling Comparison
 Glucose-Insulin Pharmacodynamic Surface Modeling Comparison J. Geoffrey Chase*, Steen Andreassen.**, Ulrike Pielmeier**, Christopher E. Hann * *Mechanical Eng, Centre for Bio-Engineering, University of
Glucose-Insulin Pharmacodynamic Surface Modeling Comparison J. Geoffrey Chase*, Steen Andreassen.**, Ulrike Pielmeier**, Christopher E. Hann * *Mechanical Eng, Centre for Bio-Engineering, University of
The Endocrine Pancreas (Chapter 10) *
 OpenStax-CNX module: m62118 1 The Endocrine Pancreas (Chapter 10) * Ildar Yakhin Based on The Endocrine Pancreas by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons
OpenStax-CNX module: m62118 1 The Endocrine Pancreas (Chapter 10) * Ildar Yakhin Based on The Endocrine Pancreas by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons
associated with serious complications, but reduce occurrences with preventive measures
 Wk 9. Management of Clients with Diabetes Mellitus 1. Diabetes Mellitus body s inability to metabolize carbohydrates, fats, proteins hyperglycemia associated with serious complications, but reduce occurrences
Wk 9. Management of Clients with Diabetes Mellitus 1. Diabetes Mellitus body s inability to metabolize carbohydrates, fats, proteins hyperglycemia associated with serious complications, but reduce occurrences
Causal Modeling of the Glucose-Insulin System in Type-I Diabetic Patients J. Fernandez, N. Aguilar, R. Fernandez de Canete, J. C.
 Causal Modeling of the Glucose-Insulin System in Type-I Diabetic Patients J. Fernandez, N. Aguilar, R. Fernandez de Canete, J. C. Ramos-Diaz Abstract In this paper, a simulation model of the glucoseinsulin
Causal Modeling of the Glucose-Insulin System in Type-I Diabetic Patients J. Fernandez, N. Aguilar, R. Fernandez de Canete, J. C. Ramos-Diaz Abstract In this paper, a simulation model of the glucoseinsulin
LESSON 2.4 WORKBOOK. Part two: Glucose homeostasis in the blood Un-Storing energy
 DEFINITIONS OF TERMS Fasting A state of abstinence from all food or drinks that provide calories. For a complete list of defined terms, see the Glossary. LESSON 2.4 WORKBOOK Part two: Glucose homeostasis
DEFINITIONS OF TERMS Fasting A state of abstinence from all food or drinks that provide calories. For a complete list of defined terms, see the Glossary. LESSON 2.4 WORKBOOK Part two: Glucose homeostasis
Fundamentals of Exercise Physiology and T1D
 COMPLIMENTARY CE Fundamentals of Exercise Physiology and T1D Jointly Provided by Developed in collaboration with 1 INTRODUCTION TO PHYSICAL ACTIVITY AND T1D 2 Many People with T1D Have Lower Levels of
COMPLIMENTARY CE Fundamentals of Exercise Physiology and T1D Jointly Provided by Developed in collaboration with 1 INTRODUCTION TO PHYSICAL ACTIVITY AND T1D 2 Many People with T1D Have Lower Levels of
Achieving Open-loop Insulin Delivery using ITM Designed for T1DM Patients
 I. J. Computer Network and Information Security, 2012, 1, 52-58 Published Online February 2012 in MECS (http://www.mecs-press.org/) DOI: 10.5815/ijcnis.2012.01.07 Achieving Open-loop Insulin Delivery using
I. J. Computer Network and Information Security, 2012, 1, 52-58 Published Online February 2012 in MECS (http://www.mecs-press.org/) DOI: 10.5815/ijcnis.2012.01.07 Achieving Open-loop Insulin Delivery using
PROCTOR VERSION. 2.9 B: Movement of Carbon, Nitrogen, Phosphorus and Water Quiz
 1. A person s blood glucose level is affected by the sugars contained in food. Blood glucose levels are controlled by the hormone insulin via a homeostatic feedback mechanism. A person eats a meal containing
1. A person s blood glucose level is affected by the sugars contained in food. Blood glucose levels are controlled by the hormone insulin via a homeostatic feedback mechanism. A person eats a meal containing
Glucoregulation 1 of 27 Boardworks Ltd 2012
 Glucoregulation 1 of 27 Boardworks Ltd 2012 2 of 27 Boardworks Ltd 2012 Glucose 3 of 27 Boardworks Ltd 2012 Glucose is a type of sugar. It is the basic fuel for aerobic respiration, which provides the
Glucoregulation 1 of 27 Boardworks Ltd 2012 2 of 27 Boardworks Ltd 2012 Glucose 3 of 27 Boardworks Ltd 2012 Glucose is a type of sugar. It is the basic fuel for aerobic respiration, which provides the
Pharmacokinetics of drug infusions
 SA Hill MA PhD FRCA Key points The i.v. route provides the most predictable plasma concentrations. Pharmacodynamic effects of a drug are related to plasma concentration. Both plasma and effect compartments
SA Hill MA PhD FRCA Key points The i.v. route provides the most predictable plasma concentrations. Pharmacodynamic effects of a drug are related to plasma concentration. Both plasma and effect compartments
Management of Type 2 Diabetes
 Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
18. PANCREATIC FUNCTION AND METABOLISM. Pancreatic secretions ISLETS OF LANGERHANS. Insulin
 18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
One-Compartment Open Model: Intravenous Bolus Administration:
 One-Compartment Open Model: Intravenous Bolus Administration: Introduction The most common and most desirable route of drug administration is orally by mouth using tablets, capsules, or oral solutions.
One-Compartment Open Model: Intravenous Bolus Administration: Introduction The most common and most desirable route of drug administration is orally by mouth using tablets, capsules, or oral solutions.
Agus Kartono, Egha Sabila Putri, Ardian Arif Setiawan, Heriyanto Syafutra and Tony Sumaryada
 American Journal of Applied Sciences Original Research Paper Study of Modified Oral Minimal Model using n-order Decay Rate of Plasma Insulin for the Oral Glucose Tolerance Test in Subjects with Normal,
American Journal of Applied Sciences Original Research Paper Study of Modified Oral Minimal Model using n-order Decay Rate of Plasma Insulin for the Oral Glucose Tolerance Test in Subjects with Normal,
A Practical Approach to Prescribe The Amount of Used Insulin of Diabetic Patients
 A Practical Approach to Prescribe The Amount of Used Insulin of Diabetic Patients Mehran Mazandarani*, Ali Vahidian Kamyad** *M.Sc. in Electrical Engineering, Ferdowsi University of Mashhad, Iran, me.mazandarani@gmail.com
A Practical Approach to Prescribe The Amount of Used Insulin of Diabetic Patients Mehran Mazandarani*, Ali Vahidian Kamyad** *M.Sc. in Electrical Engineering, Ferdowsi University of Mashhad, Iran, me.mazandarani@gmail.com
Dynamic Modeling of Exercise Effects on Plasma Glucose and Insulin Levels
 Journal of Diabetes Science and Technology Volume 1, Issue 3, May 2007 Diabetes Technology Society SYMPOSIUM Dynamic Modeling of Exercise Effects on Plasma Glucose and Insulin Levels Anirban, M.S., and
Journal of Diabetes Science and Technology Volume 1, Issue 3, May 2007 Diabetes Technology Society SYMPOSIUM Dynamic Modeling of Exercise Effects on Plasma Glucose and Insulin Levels Anirban, M.S., and
New and Emerging Therapies for Type 2 DM
 Dale Clayton MHSc, MD, FRCPC Dalhousie University/Capital Health April 28, 2011 New and Emerging Therapies for Type 2 DM The science of today, is the technology of tomorrow. Edward Teller American Physicist
Dale Clayton MHSc, MD, FRCPC Dalhousie University/Capital Health April 28, 2011 New and Emerging Therapies for Type 2 DM The science of today, is the technology of tomorrow. Edward Teller American Physicist
Regulation of Metabolism
 Regulation of Metabolism Pratt and Cornely Chapter 19 Regulation by Compartmentalization Form of reciprocal regulation Degradation vs biosynthesis Requires transporters 1 Specialization of organs Fuel
Regulation of Metabolism Pratt and Cornely Chapter 19 Regulation by Compartmentalization Form of reciprocal regulation Degradation vs biosynthesis Requires transporters 1 Specialization of organs Fuel
Glucose Concentration Simulation for Closed-Loop Treatment in Type 1 Diabetes
 American Society for Engineering Education (ASEE), Northeast Section Annual Conference, April 27-28, 208 Glucose Concentration Simulation for Closed-Loop Treatment in Type Diabetes Marilyn Urrea, Nora
American Society for Engineering Education (ASEE), Northeast Section Annual Conference, April 27-28, 208 Glucose Concentration Simulation for Closed-Loop Treatment in Type Diabetes Marilyn Urrea, Nora
Module 2 Endocrine System
 Module 2 Endocrine System Student Name: 1 Lesson 1 Lesson 2 Lesson 3 Lesson 4 Lesson 5 Total Marks Total Possible Marks 10 8 14 21 16 69 Your Mark Teacher Comments: 2 (10 marks) Lesson 1: Structure and
Module 2 Endocrine System Student Name: 1 Lesson 1 Lesson 2 Lesson 3 Lesson 4 Lesson 5 Total Marks Total Possible Marks 10 8 14 21 16 69 Your Mark Teacher Comments: 2 (10 marks) Lesson 1: Structure and
A Diabetes minimal model for Oral Glucose Tolerance Tests
 arxiv:1601.04753v1 [stat.ap] 18 Jan 2016 A Diabetes minimal model for Oral Glucose Tolerance Tests J. Andrés Christen a, Marcos Capistrán a, Adriana Monroy b, Silvestre Alavez c, Silvia Quintana Vargas
arxiv:1601.04753v1 [stat.ap] 18 Jan 2016 A Diabetes minimal model for Oral Glucose Tolerance Tests J. Andrés Christen a, Marcos Capistrán a, Adriana Monroy b, Silvestre Alavez c, Silvia Quintana Vargas
BASIC PHARMACOKINETICS
 BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
The Small Intestine. The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine.
 The Small Intestine The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine. approximately six metres (the longest section of your digestive
The Small Intestine The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine. approximately six metres (the longest section of your digestive
EAT TO LIVE: THE ROLE OF THE PANCREAS. Felicia V. Nowak, M.D., Ph.D. Ohio University COM 22 January, 2008
 EAT TO LIVE: THE ROLE OF THE PANCREAS Felicia V. Nowak, M.D., Ph.D. Ohio University COM 22 January, 2008 THE ROLE OF THE PANCREAS Exocrine pancreas Endocrine pancreas THE ROLE OF THE PANCREAS EXOCRINE
EAT TO LIVE: THE ROLE OF THE PANCREAS Felicia V. Nowak, M.D., Ph.D. Ohio University COM 22 January, 2008 THE ROLE OF THE PANCREAS Exocrine pancreas Endocrine pancreas THE ROLE OF THE PANCREAS EXOCRINE
Physiological Simulations: Plasma Glucose Regulation 1 Physiology Biology 390
 Physiological Simulations: Plasma Glucose Regulation 1 Physiology Biology 390 I. An Introduction to this Lab and to Models 2 in General: The purpose of this exercise is to use a computer simulation to
Physiological Simulations: Plasma Glucose Regulation 1 Physiology Biology 390 I. An Introduction to this Lab and to Models 2 in General: The purpose of this exercise is to use a computer simulation to
abnormally high compared to those encountered when animals are fed by University of Iowa, Iowa City, Iowa, U.S.A.
 J. Phy8iol. (1965), 181, pp. 59-67 59 With 5 text-ftgure8 Printed in Great Britain THE ANALYSIS OF GLUCOSE MEASUREMENTS BY COMPUTER SIMULATION* BY R. G. JANES "D J. 0. OSBURN From the Departments of Anatomy
J. Phy8iol. (1965), 181, pp. 59-67 59 With 5 text-ftgure8 Printed in Great Britain THE ANALYSIS OF GLUCOSE MEASUREMENTS BY COMPUTER SIMULATION* BY R. G. JANES "D J. 0. OSBURN From the Departments of Anatomy
Insulin plays a key role in glucose homeostasis by
 Section 3: Phasic Insulin Release and Metabolic Control Physiological Consequences of Phasic Insulin Release in the Normal Animal Alan D. Cherrington, 1 Dana Sindelar, 2 Dale Edgerton, 1 Kurt Steiner,
Section 3: Phasic Insulin Release and Metabolic Control Physiological Consequences of Phasic Insulin Release in the Normal Animal Alan D. Cherrington, 1 Dana Sindelar, 2 Dale Edgerton, 1 Kurt Steiner,
Hormonal Regulations Of Glucose Metabolism & DM
 Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY PID CONTROLLER FOR BLOOD GLUCOSE CONTROL Ajmal M M *, C R Srinivasan *
 [Ajmal* et al., 6(3): March, 217] Impact Factor: 4.116 IC Value: 3. IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY PID CONTROLLER FOR BLOOD GLUCOSE CONTROL Ajmal M M *, C R
[Ajmal* et al., 6(3): March, 217] Impact Factor: 4.116 IC Value: 3. IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY PID CONTROLLER FOR BLOOD GLUCOSE CONTROL Ajmal M M *, C R
Diabetes Oral Agents Pharmacology. University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 Diabetes Oral Agents Pharmacology University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 Learning Objectives Understand the role of the utilization of free
Diabetes Oral Agents Pharmacology University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 Learning Objectives Understand the role of the utilization of free
A Virtual Glucose Homeostasis Model for Verification, Simulation and Clinical Trials
 A Virtual Glucose Homeostasis Model for Verification, Simulation and Clinical Trials Neeraj Kumar Singh INPT-ENSEEIHT/IRIT University of Toulouse, France September 14, 2016 Neeraj Kumar Singh A Perspective
A Virtual Glucose Homeostasis Model for Verification, Simulation and Clinical Trials Neeraj Kumar Singh INPT-ENSEEIHT/IRIT University of Toulouse, France September 14, 2016 Neeraj Kumar Singh A Perspective
Back Stepping SMC for Blood Glucose Control of Type-1 Diabetes Mellitus Patients
 Back Stepping SMC for Blood Glucose Control of Type-1 Diabetes Mellitus Patients Deirdre D. Sylvester and Ravindra K. Munje K.K. Wagh Institute of Engineering Education and Research, Nashik-4003, (M.S.),
Back Stepping SMC for Blood Glucose Control of Type-1 Diabetes Mellitus Patients Deirdre D. Sylvester and Ravindra K. Munje K.K. Wagh Institute of Engineering Education and Research, Nashik-4003, (M.S.),
What is Diabetes Mellitus?
 Normal Glucose Metabolism What is Diabetes Mellitus? When the amount of glucose in the blood increases, After a meal, it triggers the release of the hormone insulin from the pancreas. Insulin stimulates
Normal Glucose Metabolism What is Diabetes Mellitus? When the amount of glucose in the blood increases, After a meal, it triggers the release of the hormone insulin from the pancreas. Insulin stimulates
Normal Fuel Metabolism Five phases of fuel homeostasis have been described A. Phase I is the fed state (0 to 3.9 hours after meal/food consumption),
 Normal Fuel Metabolism Five phases of fuel homeostasis have been described A. Phase I is the fed state (0 to 3.9 hours after meal/food consumption), in which blood glucose predominantly originates from
Normal Fuel Metabolism Five phases of fuel homeostasis have been described A. Phase I is the fed state (0 to 3.9 hours after meal/food consumption), in which blood glucose predominantly originates from
Metabolic integration and Regulation
 Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Multicellular Organisms
 Multicellular Organisms Sub-Topic (2.2) Control and Communication Hormonal Control (b) On completion of this sub-topic I will be able to state that: Hormones are chemical messengers Hormones are released
Multicellular Organisms Sub-Topic (2.2) Control and Communication Hormonal Control (b) On completion of this sub-topic I will be able to state that: Hormones are chemical messengers Hormones are released
Osnove farmakokinetike. Aleš Mrhar. Prirejeno po. A First Course in Pharmacokinetics and Biopharmaceutics by David Bourne,
 Osnove farmakokinetike Aleš Mrhar Prirejeno po A First Course in Pharmacokinetics and Biopharmaceutics by David Bourne, College of Pharmacy, University of Oklahoma Pharmacokinetics/Pharmacodynamics Pharmacodynamics
Osnove farmakokinetike Aleš Mrhar Prirejeno po A First Course in Pharmacokinetics and Biopharmaceutics by David Bourne, College of Pharmacy, University of Oklahoma Pharmacokinetics/Pharmacodynamics Pharmacodynamics
Tutorial ADAPT Case study 1. Data sampling / error model. Yared Paalvast Yvonne Rozendaal
 Tutorial ADAPT 13.15 17.00 Yared Paalvast (t.paalvast@umcg.nl) Yvonne Rozendaal (y.j.w.rozendaal@tue.nl) Case study 1. Data sampling / error model 1.1 Visualize raw data (dataset1a.mat) time data: t concentration
Tutorial ADAPT 13.15 17.00 Yared Paalvast (t.paalvast@umcg.nl) Yvonne Rozendaal (y.j.w.rozendaal@tue.nl) Case study 1. Data sampling / error model 1.1 Visualize raw data (dataset1a.mat) time data: t concentration
Stability Analysis of Sorensen's Model for Controllability and Observability
 Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences 54 (2): 133 145 (2017) Copyright Pakistan Academy of Sciences ISSN: 2518-4261 (print), ISSN 2518-427X (online) Stability
Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences 54 (2): 133 145 (2017) Copyright Pakistan Academy of Sciences ISSN: 2518-4261 (print), ISSN 2518-427X (online) Stability
Mathematical model of standard oral glucose tolerance test for characterization of insulin potentiation in health
 Università Politecnica delle Marche Scuola di Dottorato di Ricerca in Scienze dell Ingegneria Curriculum in Elettromagnetismo e Bioingegneria ----------------------------------------------------------------------------------------
Università Politecnica delle Marche Scuola di Dottorato di Ricerca in Scienze dell Ingegneria Curriculum in Elettromagnetismo e Bioingegneria ----------------------------------------------------------------------------------------
9.3 Stress Response and Blood Sugar
 9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
Diabetes and Kidney Disease A Balancing Act. Patricia Davidson DCN, RDN, CDE, LDN West Chester University-PA
 Diabetes and Kidney Disease A Balancing Act Patricia Davidson DCN, RDN, CDE, LDN West Chester University-PA pdavidson@wcupa.edu Objectives 2 To be able to describe the multifactorial influences in the
Diabetes and Kidney Disease A Balancing Act Patricia Davidson DCN, RDN, CDE, LDN West Chester University-PA pdavidson@wcupa.edu Objectives 2 To be able to describe the multifactorial influences in the
Outline. Model Development GLUCOSIM. Conventional Feedback and Model-Based Control of Blood Glucose Level in Type-I Diabetes Mellitus
 Conventional Feedback and Model-Based Control of Blood Glucose Level in Type-I Diabetes Mellitus Barış Ağar, Gülnur Birol*, Ali Çınar Department of Chemical and Environmental Engineering Illinois Institute
Conventional Feedback and Model-Based Control of Blood Glucose Level in Type-I Diabetes Mellitus Barış Ağar, Gülnur Birol*, Ali Çınar Department of Chemical and Environmental Engineering Illinois Institute
10/27/2016. Processing in the Large Intestine. The colon of the large intestine is connected to the small intestine
 The hepatic portal vein carries nutrient-rich blood from the capillaries of the villi to the liver, then to the heart The liver regulates nutrient distribution, interconverts many organic molecules, and
The hepatic portal vein carries nutrient-rich blood from the capillaries of the villi to the liver, then to the heart The liver regulates nutrient distribution, interconverts many organic molecules, and
Part I. Boolean modelling exercises
 Part I. Boolean modelling exercises. Glucose repression of Icl in yeast In yeast Saccharomyces cerevisiae, expression of enzyme Icl (isocitrate lyase-, involved in the gluconeogenesis pathway) is important
Part I. Boolean modelling exercises. Glucose repression of Icl in yeast In yeast Saccharomyces cerevisiae, expression of enzyme Icl (isocitrate lyase-, involved in the gluconeogenesis pathway) is important
A Comparison of Leucine- and Acetoacetate-induced Hypoglycemia in Man *
 Journal of Clinical Investigation Vol. 43, No. 1, 1964 A Comparison of Leucine- and Acetoacetate-induced Hypoglycemia in Man * STEFAN S. FAJANS, JOHN C. FLOYD, JR., RALPH F. KNOPF, AND JEROME W. CONN (From
Journal of Clinical Investigation Vol. 43, No. 1, 1964 A Comparison of Leucine- and Acetoacetate-induced Hypoglycemia in Man * STEFAN S. FAJANS, JOHN C. FLOYD, JR., RALPH F. KNOPF, AND JEROME W. CONN (From
Client Information Sheet Copyright Bilton Veterinary Centre All rights Reserved. Diabetes Mellitus
 What is Diabetes Mellitus? Diabetes Mellitus Diabetes mellitus (DM) is often called sugar diabetes in order to distinguish it from a condition called Diabetes insipidus, which is a totally separate condition.
What is Diabetes Mellitus? Diabetes Mellitus Diabetes mellitus (DM) is often called sugar diabetes in order to distinguish it from a condition called Diabetes insipidus, which is a totally separate condition.
Research Article An Improved PID Algorithm Based on Insulin-on-Board Estimate for Blood Glucose Control with Type 1 Diabetes
 Computational and Mathematical Methods in Medicine Volume 215, Article ID 281589, 8 pages http://dx.doi.org/1.1155/215/281589 Research Article An Improved PID Algorithm Based on Insulin-on-Board Estimate
Computational and Mathematical Methods in Medicine Volume 215, Article ID 281589, 8 pages http://dx.doi.org/1.1155/215/281589 Research Article An Improved PID Algorithm Based on Insulin-on-Board Estimate
MODELING OF THE CARDIOVASCULAR SYSTEM AND ITS CONTROL MECHANISMS FOR THE EXERCISE SCENARIO
 MODELING OF THE CARDIOVASCULAR SYSTEM AND ITS CONTROL MECHANISMS FOR THE EXERCISE SCENARIO PhD Thesis Summary eng. Ana-Maria Dan PhD. Supervisor: Prof. univ. dr. eng. Toma-Leonida Dragomir The objective
MODELING OF THE CARDIOVASCULAR SYSTEM AND ITS CONTROL MECHANISMS FOR THE EXERCISE SCENARIO PhD Thesis Summary eng. Ana-Maria Dan PhD. Supervisor: Prof. univ. dr. eng. Toma-Leonida Dragomir The objective
INJECTABLE THERAPIES IN DIABETES. Barbara Ann McKee Diabetes Specialist Nurse
 INJECTABLE THERAPIES IN DIABETES Barbara Ann McKee Diabetes Specialist Nurse 1 Aims of the session Describe the different injectable agents for diabetes and when they would be used. Describe some common
INJECTABLE THERAPIES IN DIABETES Barbara Ann McKee Diabetes Specialist Nurse 1 Aims of the session Describe the different injectable agents for diabetes and when they would be used. Describe some common
How Diabetes Works by Craig C. Freudenrich, Ph.D.
 How Diabetes Works by Craig C. Freudenrich, Ph.D. Odds are that you know someone with diabetes mellitus, possibly even someone who has to take insulin each day to manage the disease. Diabetes is a growing
How Diabetes Works by Craig C. Freudenrich, Ph.D. Odds are that you know someone with diabetes mellitus, possibly even someone who has to take insulin each day to manage the disease. Diabetes is a growing
Understand the physiological determinants of extent and rate of absorption
 Absorption and Half-Life Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland, New Zealand Objectives Understand the physiological determinants of extent and rate of absorption
Absorption and Half-Life Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland, New Zealand Objectives Understand the physiological determinants of extent and rate of absorption
A Mathematical Model of the Human Metabolic System and Metabolic Flexibility
 Bull Math Biol manuscript No. (will be inserted by the editor) A Mathematical Model of the Human Metabolic System and Metabolic Flexibility T. Pearson J.A.D. Wattis J.R. King I.A. MacDonald D.J. Mazzatti
Bull Math Biol manuscript No. (will be inserted by the editor) A Mathematical Model of the Human Metabolic System and Metabolic Flexibility T. Pearson J.A.D. Wattis J.R. King I.A. MacDonald D.J. Mazzatti
VIRTUAL PATIENTS DERIVED FROM THE
 VIRTUAL PATIENTS DERIVED FROM THE CARELINK DATABASE Benyamin Grosman PhD Senior Principle Scientist Medtronic Diabetes WHY VIRTUAL PATIENT MODELING? Reduce the cost of clinical trials Use models in conjunction
VIRTUAL PATIENTS DERIVED FROM THE CARELINK DATABASE Benyamin Grosman PhD Senior Principle Scientist Medtronic Diabetes WHY VIRTUAL PATIENT MODELING? Reduce the cost of clinical trials Use models in conjunction
IDENTIFICATION OF LINEAR DYNAMIC MODELS FOR TYPE 1 DIABETES: A SIMULATION STUDY
 IDENTIFICATION OF LINEAR DYNAMIC MODELS FOR TYPE 1 DIABETES: A SIMULATION STUDY Daniel A. Finan Howard Zisser Lois Jovanovic Wendy C. Bevier Dale E. Seborg Department of Chemical Engineering University
IDENTIFICATION OF LINEAR DYNAMIC MODELS FOR TYPE 1 DIABETES: A SIMULATION STUDY Daniel A. Finan Howard Zisser Lois Jovanovic Wendy C. Bevier Dale E. Seborg Department of Chemical Engineering University
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion , version 1.1
 EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
Report Documentation Page
 Performance Monitoring Of Diabetic Patient Systems Camelia L. Owens and Francis J. Doyle III Department of Chemical Engineering, University of Delaware, Newark, DE 976 Email of corresponding author: fdoyle@udel.edu
Performance Monitoring Of Diabetic Patient Systems Camelia L. Owens and Francis J. Doyle III Department of Chemical Engineering, University of Delaware, Newark, DE 976 Email of corresponding author: fdoyle@udel.edu
The Many Faces of T2DM in Long-term Care Facilities
 The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
9/11/2012. Chapter 11. Learning Objectives. Learning Objectives. Endocrine Emergencies. Differentiate type 1 and type 2 diabetes
 Chapter 11 Endocrine Emergencies Learning Objectives Differentiate type 1 and type 2 diabetes Explain roles of glucagon, glycogen, and glucose in hypoglycemia Learning Objectives Discuss following medications
Chapter 11 Endocrine Emergencies Learning Objectives Differentiate type 1 and type 2 diabetes Explain roles of glucagon, glycogen, and glucose in hypoglycemia Learning Objectives Discuss following medications
