Approval of Multiple Diabetes Management Products and Mesalazine proposals
|
|
|
- Victor Hamilton
- 5 years ago
- Views:
Transcription
1 8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic test meters, blood ketone, urine ketone, insulin pen, disposable insulin syringes, and mesalazine. Some of the proposals as originally consulted on in our letter dated 18 March are still under consideration and a notification will be issued when a decision is made. Summary of the decisions In relation to blood glucose and blood glucose diagnostic test meters Three new brands of blood glucose and blood glucose diagnostic test meters (in addition to those already listed) will be funded: o FreeStyle Lite (as supplied by Medica Pacifica); o CareSens II (as supplied by Pharmaco); and o CareSens P (as supplied by Pharmaco). SensoCard blood glucose (as supplied by Point of Care Diagnostics) will be funded for patients who are severely visually impaired; and Funded access to blood glucose diagnostic test meters will be widened to include people with gestational diabetes. A decision on the proposed funding of the On Call Plus brand of blood glucose and blood glucose diagnostic test meters (as supplied by Diabetes Supplies) is still under consideration. As a consequence, the related proposal to reduce the, through reference pricing, for the Accu-Chek Performa blood glucose brand (as supplied by Roche Diagnostics) is still under consideration. In relation to ketone testing Optium blood ketone (as supplied by Medica Pacifica) will be funded via an endorsement restriction for people with type 1 diabetes; Ketostix (foiled strips) urine ketone (as supplied by Bayer) will be funded; and The current, partially subsidised, glucose and/or ketones/urine testing brands will be delisted from the Pharmaceutical Schedule. A T of 9
2 In relation to insulin pen and disposable insulin syringes The for ABM insulin pen and disposable insulin syringes (as supplied by ABM Pharma) will reduce; SC Profi-Fine insulin pen (as supplied by Pharmaco) and DM Ject disposable insulin syringes (as supplied by Pharmaco) will be funded; The SC Profi-Fine brand of insulin pen and the DM Ject brand of disposable insulin syringes will be subject to a volume related adjustment, that will result in a reduction to match that of the ABM brand of insulin pen, should SC Profi-Fine and DM Ject (in any total combination) obtain approximately 30% market share; and The subsidies for the B-D (as supplied by Becton Dickinson) and NovoFine (as supplied by Novo Nordisk) brands of insulin pen and disposable insulin syringes will reduce, through reference pricing, to match that of ABM or SC Profi- Fine (where applicable). This will mean that a manufacturer s surcharge could apply if the s of B-D and NovoFine are not reduced. In relation to mesalazine The for Pentasa (as supplied by Pharmaco) will reduce; The current Retail pharmacy Specialist restrictions applying to mesalazine preparations will be removed. If you have any queries about this funding decision you can call our toll free number (9 am to 5 pm, Monday to Friday) on Details of the decision In relation to blood glucose s The following blood glucose will be listed (where applicable) in Section B of the Pharmaceutical Schedule from the following dates and at the following s and subsidies (ex-manufacturer, excluding GST): Subsidy Listing Date Optium 5 second test 25 test $.82 1 July Optium 5 second test 50 test $ July FreeStyle Lite 50 test $ July CareSens II 50 test $19.25 SensoCard 50 test $ July A T of 9
3 Note: The chemical description will be renamed from glucose dehydrogenase to blood glucose from 1 July. The above brands, excluding SensoCard, will be listed in Part II of Section H of the Pharmaceutical Schedule at the applicable dates s. The funding for SensoCard blood glucose will be restricted by the following note: SensoCard blood/glucose are subsidised only if prescribed for a patient who is severely visually impaired and is using a SensoCard Plus Talking Blood Glucose Monitor. In relation to blood glucose diagnostic test meters The following blood glucose diagnostic test meters will be listed in Section B and Part II of Section H of the Pharmaceutical Schedule from the following dates and at the following s and subsidies (ex-manufacturer, excluding GST): Subsidy Listing Date diagnostic test meter diagnostic test meter diagnostic test meter diagnostic test meter Meter Optium Xceed 1 $ July Meter FreeStyle Lite 1 $ July Meter CareSens II 1 $9.00 Meter CareSens P 1 $6.00 As previously noted, SensoCard Plus Talking Blood Glucose Monitor will not be listed on the Pharmaceutical Schedule. We understand this will continue to be distributed and managed by the Royal New Zealand Foundation for the Blind. The current endorsement restriction applying to blood glucose diagnostic test meters will be amended from 1 July to allow funding for people with gestational diabetes (changes are indicated by bold text): Subsidy by endorsement. a) Maximum of 1 meter per prescription. b) A diagnostic blood glucose test meter is subsidised for patients who begin insulin or sulphonylurea therapy after 1 March 2005 or is prescribed for a pregnant women with diabetes. c) Only one meter per patient. No further prescriptions will be subsidised. The prescription must be endorsed accordingly. Subsidy and delisting protection will apply to FreeStyle Lite, Optium, Optium Xceed, CareSens II and Care Sens P until 30 June 2011 in relation to blood glucose and blood glucose diagnostic test meters. A T of 9
4 In relation to ketone testing Blood ketone will be listed in Section B and Part II of Section H of the Pharmaceutical Schedule at the following (ex-manufacturer, excluding GST) from 1 July : Subsidy Ketone blood beta-ketone electrodes Test strip Optium Blood Ketone Test Strips strip $8.50 Optium Blood Ketone Test Strips will be subject to the following restriction: Patient has type 1 diabetes and has had one or more episodes of ketoacidosis (excluding first presentation). Maximum quantity of 2 packs per annum. No further prescriptions will be subsidised. Note that the Optium Blood Ketone Test Strips are only compatible with Optium Xceed blood glucose meters. The Optium Xceed meter is capable of performing both blood glucose and blood ketone testing without the need for repeated calibrations however funding is restricted to one meter per patient; therefore, in cases where patients had initially received a funded Accu-Chek meter, a second Optium Xceed meter will not be funded. Urine ketone individually foil wrapped will be listed in Section B of the Pharmaceutical Schedule at the following (ex-manufacturer, excluding GST) from 1 August : Subsidy Sodium nitroprusside Test strip Ketostix 20 strip $14.14 Subsidy and delisting protection will apply to Optium Blood Ketone Test Strips until 30 June The following partially subsidised urine ketone will be delisted from Section B of the Pharmaceutical Schedule from 1 December : Subsidy (Price) Glucose oxidase Urine diagnostic test with peroxidase, sodium nitroprusside and aminoacetic acid Keto- Diabur stick $4.53 ($8.00) Glucose oxidase Urine diagnostic test with peroxidase, potassium iodide, sodium nitroprusside and aminoacetic acid Keto- Diastix 50 strip $4.53 ($7.50) A T of 9
5 Sodium nitroprusside Urine diagnostic strips, buffered Ketur- Test Ketostix 50 strip 50 strip Subsidy (Price) $3.39 ($6.00) $3.40 ($7.15) The current Glucose and/or Ketone/Urine Testing therapeutic subgroup will be renamed from 1 July to Ketone Testing. Both Optium Blood Ketone Test Strips and Ketostix will be listed in this therapeutic subgroup. In relation to insulin pen and disposable insulin syringes The following insulin pen will be listed in Section B and Part II of Section H of the Pharmaceutical Schedule from the following dates and at the following s and subsidies (ex-manufacturer, excluding GST): Subsidy and Date of Listing 29 g x 12.7 mm ABM 0 $.50 1 July SC Profi-Fine 0 $ g x 5 mm SC Profi-Fine 0 $ g x 6 mm 31 g x 8 mm ABM 0 $.50 1 July SC Profi-Fine 0 $11.75 ABM 0 $.50 1 July SC Profi-Fine 0 $11.75 The following disposable insulin syringes will be listed in Section B and Part II of Section H of the Pharmaceutical Schedule from the following dates and at the following s and subsidies (ex-manufacturer, excluding GST): Subsidy and Date of Listing ABM 0 $ July Syringe 0.3 ml with 29 g x 12.7 ABM 0 $ July Syringe 0.3 ml with 31 g x 8 A T of 9
6 Subsidy and Date of Listing ABM 0 $ July Syringe 0.5 ml with 29 g x 12.7 ABM 0 $ July Syringe 0.5 ml with 31 g x 8 ABM 0 $ July Syringe 1 ml with 29 g x 12.7 ABM 0 $ July Syringe 1 ml with 31 g x 8 The subsidies for B-D Micro-Fine and NovoFine will be reduced to the same level as ABM or SC Profi-Fine (where applicable) through the application of reference pricing, from the following dates as follows: Current New Change date 29 g x 12.7 mm B-D Micro- 30 $3.93 $3.15 Fine 0 $13.09 $.50 1 July 31 g x 5 mm B-D Micro- Fine 0 $13.09 $ g x 6 mm NovoFine 0 $26.00 $.50 1 July 31 g x 8 mm B-D Micro- 30 $3.93 $3.15 Fine 0 $13.09 $.50 1 July The subsidies for Fine and Fine II will be reduced to the same level as ABM and DM Ject through the application of reference pricing, from 1 July as follows: Current New Syringe 0.3 ml with 29 g x 12.7 mm needle Fine 0 $15.92 $13.00 Syringe 0.3 ml with 31 g x 8 mm needle Fine II 0 0 $15.92 $13.00 Syringe 0.5 ml with 29 g x 12.7 mm needle Fine 0 $15.92 $13.00 A T of 9
7 Current New Syringe 0.5 ml with 31 g x 8 mm needle Fine II 0 $15.92 $13.00 Syringe 1 ml with 29 g x 12.7 mm needle Fine 0 $15.92 $13.00 Syringe 1 ml with 31 g x 8 mm needle Fine II 0 $15.92 $13.00 Any supplier affected by the application of reference pricing above needs to notify PHARMAC by 12 June if it intends on reducing the of its brand using the relevant product notification form on our website. The following volume adjustment (ex-manufacturer, excluding GST) will apply to SC Profi-Fine and DM Ject based on the products (in any total combination) obtaining 30% market share (at Pharmaco s s, estimated to equal $200,000): Subsidy and 29 g x 12.7 mm SC Profi-Fine 0 $ g x 5 mm SC Profi-Fine 0 $ g x 6 mm SC Profi-Fine 0 $ g x 8 mm SC Profi-Fine 0 $.50 disposable with disposable with disposable with disposable with disposable with disposable with Syringe 0.3 ml with 29 g x 12.7 mm needle Syringe 0.3 ml with 31 g x 8 mm needle Syringe 0.5 ml with 29 g x 12.7 mm needle Syringe 0.5 ml with 31 g x 8 mm needle Syringe 1 ml with 29 g x 12.7 mm needle Syringe 1 ml with 31 g x 8 mm needle DM Ject 0 $.50 DM Ject 0 $.50 DM Ject 0 $.50 DM Ject 0 $.50 DM Ject 0 $.50 DM Ject 0 $.50 The for Fine and Fine II will be further reduced to match the lower DM Ject s in the event that its s are reduced on obtaining 30% market share. An announcement will be made on any such changes via the Pharmaceutical Schedule. Subsidy and delisting protection will apply to ABM, SC Profi-Fine and DM Ject until 30 June A T of 9
8 The restriction applying to NovoFine pen 31 g x 6 mm (subsidised for children under 12 years of age) will be removed from 1 July. In relation to mesalazine The of mesalazine (Pentasa) as listed in Section B of the Pharmaceutical Schedule will be reduced as follows from 1 November (ex manufacturer and ex GST): Mesalazine Tab long-acting 500 mg Current and Proposed Pentasa 0 $69.06 $59.05 Pentasa will be listed in Part II of Section H of the Pharmaceutical Schedule at the applicable from 1 November. A rebate will apply to Pentasa for the period from 1 March until 30 October. The rebate has the effect of obtaining the lower for Pentasa for the duration of this period. All mesalazine presentations (tablets and enemas) will no longer be subject to a Retail pharmacy-specialist restriction, meaning that prescriptions written by other types of practitioners will no longer require a specialist endorsement for. Subsidy and delisting protection will apply to Pentasa until 30 June What feedback was received? We appreciate the feedback we received and acknowledge the time people took to respond. A variety of consultation responses were received in relation to the proposals and all responses were considered in the decisions on the proposed changes. Some concerns were raised with specific aspects of the proposal and we have outlined those we are able to publicise below, along with our response: Theme Support was provided for listing of gestational meters, listing the Sensocard test strip brand, and removing the mesalazine restrictions. Support noted. Response Requests were made that PHARMAC review the funding of lancets. Requests were made for funding 0.5 unit insulin syringes for paediatric patients. As mentioned in consultation PHARMAC intends to continue to search for an acceptable method for funding. PHARMAC was not aware of this issue and intends to investigate. A T of 9
9 Theme Queries were made regarding the suitability and testing of the new brands of insulin pen and insulin syringes. Requests that the restriction on the number of blood ketone strips be increased especially for those patients who are using insulin pumps and that ketone are available from diagnosis and not as proposed on presentation of DKA after diagnosis. Additional meters would dilute the training and resources for people with diabetes. Requests that more are available for patients with type 2 diabetes. Requests were made for the funding of insulin pump consumables. One pharmacist suggested that all pharmacists should be able to endorse the blood ketone test strip restriction prescriptions. Response We note that ABM has already been listed for 12 months and that this brand and the Pharmaco brands are standardised to fit the existing insulin pens. Limited field testing has occurred without any significant concerns raised. Clinical advice from the Diabetes Subcommittee of PTAC was that 2 boxes annually was clinically appropriate (including those patients using insulin pumps) and that they should be restricted to patients that have had one or more episodes of ketoacidosis. We intend to ask the Diabetes Subcommittee to review the restriction at its meeting in August, to ensure that this is still appropriate. We are not proposing to delist the current brands and healthcare professionals can continue to decide to use the current brands only. We note that some responders were positive towards a greater choice of meters. The Diabetes Subcommittee considered the restriction last year and considered that it is clinically appropriate. Funding of pumps and consumables is a related issue. Insulin pumps are not currently funded on the Pharmaceutical Schedule. We note that the Ministry of Health already provides some funding for insulin pumps to DHBs and is currently reviewing the funding availability. There is a wider issue about entitlements to authorise payments that this proposal was not seeking to address. We did not consider this change appropriate or necessary at this time. The Christchurch Diabetes Centre has undertaken a meter evaluation for the new brands of blood glucose and blood glucose diagnostic test meters. The independent study evaluated the accuracy and precision of the meters and showed good performance for all meters under controlled conditions. The performance of these brands appears similar to that of the two meters currently on the New Zealand market (namely the Accu- Chek Performa and Optium Xceed). Finally, we note that the field testing for the SensoCard Plus blood glucose and blood glucose diagnostic test meter provided positive results. A T of 9
Guidance for the choice of blood glucose testing meters, strips and lancets
 Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Proposals regarding the Cholesterol Lowering Pharmaceuticals Atorvastatin and Ezetimibe
 26 April 2010 Proposals regarding the Cholesterol Lowering Pharmaceuticals Atorvastatin and Ezetimibe PHARMAC is seeking feedback on the following proposals relating to the cholesterol lowering pharmaceuticals
26 April 2010 Proposals regarding the Cholesterol Lowering Pharmaceuticals Atorvastatin and Ezetimibe PHARMAC is seeking feedback on the following proposals relating to the cholesterol lowering pharmaceuticals
TENDER RESULTS. Notification of Product Changes (NOPC) forms and Pharmacodes. 19 December 2014
 19 December 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2012/13 Invitation to Tender, dated 31 October 2012
19 December 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2012/13 Invitation to Tender, dated 31 October 2012
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Approval of proposals for various pharmaceuticals
 9 August 2010 Approval of proposals for various pharmaceuticals PHARMAC is pleased to announce the approval of proposals for pharmaceuticals in several therapeutic groups which aim to address some issues
9 August 2010 Approval of proposals for various pharmaceuticals PHARMAC is pleased to announce the approval of proposals for pharmaceuticals in several therapeutic groups which aim to address some issues
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Proposal on subsidy changes for some respiratory inhalation products and access restrictions to combination inhalers.
 30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
Diabetes Subcommittee of PTAC meeting held 3 March (minutes for web publishing)
 Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics
Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Nutritional products (Special Foods) supplied by Nutricia
 2 April 2012 Nutritional products (Special Foods) supplied by Nutricia PHARMAC is pleased to announce the funding of a number of nutritional products (Special Foods) supplied by Nutricia. These products
2 April 2012 Nutritional products (Special Foods) supplied by Nutricia PHARMAC is pleased to announce the funding of a number of nutritional products (Special Foods) supplied by Nutricia. These products
REQUEST FOR PROPOSALS SUPPLY OF NICOTINE REPLACEMENT THERAPY
 16 October 2013 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF NICOTINE REPLACEMENT THERAPY PHARMAC invites proposals for the supply of nicotine replacement therapy (including, but not limited to, nicotine
16 October 2013 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF NICOTINE REPLACEMENT THERAPY PHARMAC invites proposals for the supply of nicotine replacement therapy (including, but not limited to, nicotine
Decision relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin)
 9 September 2015 Decision relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) The PHARMAC Board has approved the proposal relating to the funding of the TNF-inhibitor
9 September 2015 Decision relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) The PHARMAC Board has approved the proposal relating to the funding of the TNF-inhibitor
HYDROXYBUTERATE) Gluco Rx Evolve KetoneTest Strips
 Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Notification of Product Changes (NOPC) forms, Containered Trade Product Pack (CTPP) codes and Pharmacodes
 31 August 2016 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Hospital Supply Status for some products included in the 2015/16 Invitation to Tender, d 6 November
31 August 2016 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Hospital Supply Status for some products included in the 2015/16 Invitation to Tender, d 6 November
REQUEST FOR PROPOSALS SUPPLY OF NICOTINE REPLACEMENT THERAPY
 9 November 2016 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF NICOTINE REPLACEMENT THERAPY PHARMAC invites proposals for the supply of nicotine replacement therapy (NRT) (including, but not limited to,
9 November 2016 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF NICOTINE REPLACEMENT THERAPY PHARMAC invites proposals for the supply of nicotine replacement therapy (NRT) (including, but not limited to,
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st March 2016.
 PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st March 2018
 This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st July 2015.
 PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st October 2017
 This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Changes to the National Diabetes Services Scheme (NDSS)
 Changes to the National Diabetes Services Scheme (NDSS) June 2016 FREQUENTLY ASKED QUESTIONS 1. What are the changes from 1 July 2016? 2. Why are the supply changes happening? 3. What is the role of Diabetes
Changes to the National Diabetes Services Scheme (NDSS) June 2016 FREQUENTLY ASKED QUESTIONS 1. What are the changes from 1 July 2016? 2. Why are the supply changes happening? 3. What is the role of Diabetes
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Type 2 Diabetes Recommended SMBG
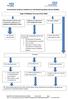 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin)
 14 July 2015 Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) PHARMAC is seeking feedback on a proposal relating to the funding of the TNF-inhibitor medicines
14 July 2015 Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) PHARMAC is seeking feedback on a proposal relating to the funding of the TNF-inhibitor medicines
How to Access A Free Blood Glucose Meter
 How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
TENDER RESULTS. Notification of Product Changes (NOPC) forms and Pharmacodes. 31 October 2014
 31 October 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2012/13 Invitation to Tender, dated 31 October 2012 and
31 October 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2012/13 Invitation to Tender, dated 31 October 2012 and
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER
 DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
RETAIL BULK DIABETIC SUPPLY TOOL BOX
 RETAIL BULK DIABETIC SUPPLY TOOL BOX 1 ADVOCATE Brand Advantage Employers Can Save 52-72% On Diabetic Supplies Free Talking Meter - No Coding Required One of the Most Accurate Blood Glucose Meters on the
RETAIL BULK DIABETIC SUPPLY TOOL BOX 1 ADVOCATE Brand Advantage Employers Can Save 52-72% On Diabetic Supplies Free Talking Meter - No Coding Required One of the Most Accurate Blood Glucose Meters on the
Frequently Used PINs for Billing Purposes November 2018
 Diabetic Test Strips PIN Description 97799824 ACCU-CHEK ADVANTAGE STRIPS (100) 97799823 ACCU-CHEK ADVANTAGE STRIPS (50) 97799814 ACCU-CHEK AVIVA TEST STRIPS (100) 97799815 ACCU-CHEK AVIVA TEST STRIPS (50)
Diabetic Test Strips PIN Description 97799824 ACCU-CHEK ADVANTAGE STRIPS (100) 97799823 ACCU-CHEK ADVANTAGE STRIPS (50) 97799814 ACCU-CHEK AVIVA TEST STRIPS (100) 97799815 ACCU-CHEK AVIVA TEST STRIPS (50)
Prescription Refill List Insulin and Related Supplies
 Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
New hepatitis C medicines Frequently Asked Questions
 New hepatitis C medicines Frequently Asked Questions The Minister for Health, the Hon Sussan Ley MP, announced on 20 December 205, that a number of new medicines would be made available through the Pharmaceutical
New hepatitis C medicines Frequently Asked Questions The Minister for Health, the Hon Sussan Ley MP, announced on 20 December 205, that a number of new medicines would be made available through the Pharmaceutical
Notification of Product Changes (NOPC) forms, Containered Trade Product Pack (CTPP) codes and Pharmacodes
 29 February 2016 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2014/15 Invitation to Tender, d 6 November 2014, and
29 February 2016 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2014/15 Invitation to Tender, d 6 November 2014, and
REQUEST FOR INFORMATION - HEPATITIS C TREATMENTS
 10 August 2015 REQUEST FOR INFORMATION - HEPATITIS C TREATMENTS PHARMAC is seeking information from suppliers of novel direct-acting antiviral agents (DAAs) for the treatment of hepatitis C. This Request
10 August 2015 REQUEST FOR INFORMATION - HEPATITIS C TREATMENTS PHARMAC is seeking information from suppliers of novel direct-acting antiviral agents (DAAs) for the treatment of hepatitis C. This Request
TENDER RESULTS. Notification of Product Changes (NOPC) forms and Pharmacodes. 28 February 2014
 28 February 2014 TENDER RESULTS PHARMAC has resolved to award tenders for for Sole Subsidised Supply Status and Status for some products included in the Invitation to Tender, dated 07 November 2013. Some
28 February 2014 TENDER RESULTS PHARMAC has resolved to award tenders for for Sole Subsidised Supply Status and Status for some products included in the Invitation to Tender, dated 07 November 2013. Some
TENDER RESULTS. Notification of Product Changes (NOPC) forms and Pharmacodes. 30 May 2014
 30 May 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Hospital Supply Status for some products included in the 2012/13 Invitation to Tender, dated 31 October
30 May 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Hospital Supply Status for some products included in the 2012/13 Invitation to Tender, dated 31 October
Release of the 2017/18 Invitation to Tender
 2 November 2017 Release of the 2017/18 Invitation to Tender The 2017/18 Invitation to Tender (2017/18 ITT) has been distributed today. If you have already registered your e-mail address with PHARMAC s
2 November 2017 Release of the 2017/18 Invitation to Tender The 2017/18 Invitation to Tender (2017/18 ITT) has been distributed today. If you have already registered your e-mail address with PHARMAC s
Changes to the National Immunisation Schedule
 17 December 2013 Changes to the National Immunisation Schedule PHARMAC is pleased to announce decisions related to the National Immunisation Schedule (NIS) that will take effect from 1 July 2014. This
17 December 2013 Changes to the National Immunisation Schedule PHARMAC is pleased to announce decisions related to the National Immunisation Schedule (NIS) that will take effect from 1 July 2014. This
NHS Greater Glasgow and Clyde Adult Blood Glucose Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
PHARMAC invites proposals for the supply of various vaccines in New Zealand.
 28 June 2013 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF VARIOUS VACCINES PHARMAC invites proposals for the supply of various vaccines in New Zealand. This request for proposals (RFP) letter incorporates
28 June 2013 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF VARIOUS VACCINES PHARMAC invites proposals for the supply of various vaccines in New Zealand. This request for proposals (RFP) letter incorporates
TENDER RESULTS. Notification of Product Changes (NOPC) forms and Pharmacodes. 30 April 2014
 30 April 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2012/13 Invitation to Tender, dated 31 October 2012 and
30 April 2014 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Status for some products included in the 2012/13 Invitation to Tender, dated 31 October 2012 and
Respiratory Subcommittee of PTAC meeting held 5 February (minutes for web publishing)
 Respiratory Subcommittee of PTAC meeting held 5 February 2010 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Respiratory Subcommittee of PTAC meeting held 5 February 2010 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Diabetes Newly Diagnosed with NO evidence of DKA
 1. DIAGNOSIS confirmed by doctor using below guidelines: (a) History of Polyuria (usually nocturia ± enuresis) Polydipsia. ± Weight Loss (b) Glycosuria (c) Blood Glucose (BG) > 11 mmol/l (confirm from
1. DIAGNOSIS confirmed by doctor using below guidelines: (a) History of Polyuria (usually nocturia ± enuresis) Polydipsia. ± Weight Loss (b) Glycosuria (c) Blood Glucose (BG) > 11 mmol/l (confirm from
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
Essential advice for people with diabetes from Accu-Chek. The inside story on diabetes
 Essential advice for people with diabetes from Accu-Chek The inside story on diabetes What is diabetes? Glucose is a form of sugar that is found in food you eat. It is a vital energy source for your body
Essential advice for people with diabetes from Accu-Chek The inside story on diabetes What is diabetes? Glucose is a form of sugar that is found in food you eat. It is a vital energy source for your body
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system
 NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
Endocrinology Subcommittee of PTAC Meeting held 29 May (minutes for web publishing)
 Endocrinology Subcommittee of PTAC Meeting held 29 May 2012 (minutes for web publishing) Endocrinology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Endocrinology Subcommittee of PTAC Meeting held 29 May 2012 (minutes for web publishing) Endocrinology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
DIABETES PATIENT STRATIFICATION AND TESTING SAVING MONEY, SAVING LIVES
 DIABETES PATIENT STRATIFICATION AND TESTING SAVING MONEY, SAVING LIVES Contents 2 The Rising Cost of Diabetes 5 Implementation 3 P atient Stratification is the Key to Saving Lives and Money 6 M eeting
DIABETES PATIENT STRATIFICATION AND TESTING SAVING MONEY, SAVING LIVES Contents 2 The Rising Cost of Diabetes 5 Implementation 3 P atient Stratification is the Key to Saving Lives and Money 6 M eeting
February Special Foods - Notification of Funding and Access Changes from 1 April
 February 2011 Special Foods - Notification of Funding and Access Changes from 1 April Changes to the Access and Funding of Special Foods from 1 April 2011 The PHARMAC Board has made a number of decisions
February 2011 Special Foods - Notification of Funding and Access Changes from 1 April Changes to the Access and Funding of Special Foods from 1 April 2011 The PHARMAC Board has made a number of decisions
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations
 Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
medicines_management/correspondence/pathway-for-the-managed- Access-of-FreeStyle-Libre.
 Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Decision to amend access criteria for some vaccines
 3 July 2015 Decision to amend access criteria for some vaccines PHARMAC is pleased to announce the following changes to the funding access criteria for a number of vaccines. The changes in criteria follow
3 July 2015 Decision to amend access criteria for some vaccines PHARMAC is pleased to announce the following changes to the funding access criteria for a number of vaccines. The changes in criteria follow
WELL CARD HEALTH TOOL BOX
 WELL CARD HEALTH TOOL BOX 1 ADVOCATE Brand Advantage Well Card Health Members Will Save 52-70% On Diabetic Supplies Free Talking Meter Or Dash Meter Programs To Well Card Health Members 3 to 5 day UPS
WELL CARD HEALTH TOOL BOX 1 ADVOCATE Brand Advantage Well Card Health Members Will Save 52-70% On Diabetic Supplies Free Talking Meter Or Dash Meter Programs To Well Card Health Members 3 to 5 day UPS
This information is intended for those with Type 1 Diabetes
 This information is intended for those with Type 1 Diabetes Get yourself sorted Planning ahead is a good idea if you are going away. Here are some things to consider. Time changes and travel meals Talk
This information is intended for those with Type 1 Diabetes Get yourself sorted Planning ahead is a good idea if you are going away. Here are some things to consider. Time changes and travel meals Talk
Raising the Standard
 DIABETES ACTION PLAN (Editable document) Directions: 1. To input data, click on the first blank line, type in information 2. Use tab key to advance to the next field 3. Check mark fields, use tab to advance
DIABETES ACTION PLAN (Editable document) Directions: 1. To input data, click on the first blank line, type in information 2. Use tab key to advance to the next field 3. Check mark fields, use tab to advance
TRAVELLING ndss.com.au AND TYPE 1 DIABETES
 TRAVELLING AND TYPE 1 DIABETES 1300 136 588 ndss.com.au The National Diabetes Services Scheme (NDSS) is an initiative of the Australian Government administered by Diabetes Australia. Contents Topic Page
TRAVELLING AND TYPE 1 DIABETES 1300 136 588 ndss.com.au The National Diabetes Services Scheme (NDSS) is an initiative of the Australian Government administered by Diabetes Australia. Contents Topic Page
Switch protocol: Insulin pen needles to cost effective brand
 Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
Switch protocol: Insulin pen needles to cost effective brand
 Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
Monitoring in Type 2 Diabetes. Learning Outcomes. Type 2 Diabetes. Senga Hunter Community Diabetes Specialist Nurse
 Monitoring in Type 2 Diabetes Senga Hunter Community Diabetes Specialist Nurse Learning Outcomes Understand why blood monitoring is necessary Understand the blood tests for monitoring diabetes Understand
Monitoring in Type 2 Diabetes Senga Hunter Community Diabetes Specialist Nurse Learning Outcomes Understand why blood monitoring is necessary Understand the blood tests for monitoring diabetes Understand
ACCU-CHEK AVIVA TEST STRIPS ACCU-CHEK COMPACT TEST STRIP ACCU-CHEK COMPACT TEST STRIP ACCU-CHEK FASTCLIX 6 LANCET DRUM (102S)
 Appendix H Product Identification Number (PIN) DIN Code Brand Name Canadian Product Name 00999997 99 EXTEMPOR. CPDS - NFLD- 00977062 ACCU-CHEK ADVANTAGE TEST ACCU-CHEK ADVANTAGE TEST 00977124 ACCU-CHEK
Appendix H Product Identification Number (PIN) DIN Code Brand Name Canadian Product Name 00999997 99 EXTEMPOR. CPDS - NFLD- 00977062 ACCU-CHEK ADVANTAGE TEST ACCU-CHEK ADVANTAGE TEST 00977124 ACCU-CHEK
Continuous Glucose Monitoring
 Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME
 STANDARD OPERATING PROCEDURE ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME First Issued July 08 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote
STANDARD OPERATING PROCEDURE ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME First Issued July 08 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote
Title Management of Children and Young People with Newly Diagnosed Type 1 Diabetes Mellitus Guideline
 Document Control Title Management of Children and Young People with Newly Diagnosed Type 1 Diabetes Mellitus Guideline Author Directorate Version Author s job title Consultant Paediatrician Department
Document Control Title Management of Children and Young People with Newly Diagnosed Type 1 Diabetes Mellitus Guideline Author Directorate Version Author s job title Consultant Paediatrician Department
Software Version 2.0. User s Guide
 Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Petition 2014/15 of Anthony Roberts and 40 others
 Petition 2014/15 of Anthony Roberts and 40 others Report of the Health Committee Contents Recommendation 2 Background 2 The petitioner s concerns 3 Best practice and international benchmarks 4 Ministry
Petition 2014/15 of Anthony Roberts and 40 others Report of the Health Committee Contents Recommendation 2 Background 2 The petitioner s concerns 3 Best practice and international benchmarks 4 Ministry
KEEPING SAFE WITH INSULIN THERAPY
 KEEPING SAFE WITH INSULIN THERAPY kk WHY IS THIS LEAFLET FOR YOU? Insulin treatment improves the quality of life in many people and saves the lives of others. It is used to lower blood glucose levels.
KEEPING SAFE WITH INSULIN THERAPY kk WHY IS THIS LEAFLET FOR YOU? Insulin treatment improves the quality of life in many people and saves the lives of others. It is used to lower blood glucose levels.
Ask about your diabetes medicines
 Ask about your diabetes medicines This guide is to help you get the best from your diabetes medicines. This guide is to enable you to get the best from your diabetes medicines. We know that many people
Ask about your diabetes medicines This guide is to help you get the best from your diabetes medicines. This guide is to enable you to get the best from your diabetes medicines. We know that many people
Summary of healthcare professional feedback of CareSens blood glucose meters for PHARMAC
 Summary healthcare pressional feedback CareSens blood glucose meters for PHARMAC Background In October 2015, PHARMAC ran focus groups around the country with primary and secondary care diabetes health
Summary healthcare pressional feedback CareSens blood glucose meters for PHARMAC Background In October 2015, PHARMAC ran focus groups around the country with primary and secondary care diabetes health
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations
 Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
TRAVEL & TYPE 1 DIABETES ndss.com.au
 TRAVEL & TYPE 1 DIABETES 1300 136 588 ndss.com.au The National Diabetes Services Scheme (NDSS) is an initiative of the Australian Government administered by Diabetes Australia. CONTENTS Introduction 3
TRAVEL & TYPE 1 DIABETES 1300 136 588 ndss.com.au The National Diabetes Services Scheme (NDSS) is an initiative of the Australian Government administered by Diabetes Australia. CONTENTS Introduction 3
PHARMAC invites proposals for the supply of various vaccines in New Zealand.
 15 February 2016 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF VARIOUS VACCINES PHARMAC invites proposals for the supply of various vaccines in New Zealand. This request for proposals (RFP) letter incorporates
15 February 2016 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF VARIOUS VACCINES PHARMAC invites proposals for the supply of various vaccines in New Zealand. This request for proposals (RFP) letter incorporates
Cost-Utility Analysis (CUA) Explained
 Pharmaceutical Management Agency Cost-Utility Analysis (CUA) Explained Cost-Utility Analysis (CUA) at PHARMAC Questions and Answers go to page 9 >> This document explains the process that PHARMAC generally
Pharmaceutical Management Agency Cost-Utility Analysis (CUA) Explained Cost-Utility Analysis (CUA) at PHARMAC Questions and Answers go to page 9 >> This document explains the process that PHARMAC generally
January Special Foods Consultation Document. Proposals regarding the funding and access arrangements for a number of Special Foods
 January 2010 Special Foods Consultation Document Proposals regarding the funding and access arrangements for a number of Special Foods Funding and access for a number of Special Foods Summary Foods are
January 2010 Special Foods Consultation Document Proposals regarding the funding and access arrangements for a number of Special Foods Funding and access for a number of Special Foods Summary Foods are
Introduction. Methods
 A New Perspective On A Difficult Discharge: Ensuring the newly diagnosed diabetic patient gets the proper prescriptions when it s time to go home. Ashley Carter, PGY 3 Introduction A hospitalized patient
A New Perspective On A Difficult Discharge: Ensuring the newly diagnosed diabetic patient gets the proper prescriptions when it s time to go home. Ashley Carter, PGY 3 Introduction A hospitalized patient
Diabetes, Ketone testing in Type 1 Diabetes
 CLINICAL GUIDELINE Diabetes, Ketone testing in Type 1 Diabetes A guideline is intended to assist healthcare professionals in the choice of disease-specific treatments. Clinical judgement should be exercised
CLINICAL GUIDELINE Diabetes, Ketone testing in Type 1 Diabetes A guideline is intended to assist healthcare professionals in the choice of disease-specific treatments. Clinical judgement should be exercised
Insulin detemir (Levemir)... 2 Leuprorelin... 4 Levetiracetam (Keppra)... 5 Budesonide/eformoteral (Symbicort) widening access...
 August 2005 PTAC Meeting PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002: Minute means that part of the record of a PTAC or Sub-committee meeting (including
August 2005 PTAC Meeting PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002: Minute means that part of the record of a PTAC or Sub-committee meeting (including
Notification of Product Changes (NOPC) forms, Containered Trade Product Pack (CTPP) codes and Pharmacodes
 28 April 2017 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Hospital Supply Status for some products included in the 2016/17 Invitation to Tender, d 3 November
28 April 2017 TENDER RESULTS PHARMAC has resolved to award tenders for Sole Subsidised Supply Status and Hospital Supply Status for some products included in the 2016/17 Invitation to Tender, d 3 November
Insulin Pump and Supplies Policy and Administration Manual
 Ministry of Health & Long-Term Care Insulin Pump and Supplies Policy and Administration Manual Assistive Devices Program Ministry of Health & Long-Term Care www.health.gov.on.ca/adp Table of Amendments
Ministry of Health & Long-Term Care Insulin Pump and Supplies Policy and Administration Manual Assistive Devices Program Ministry of Health & Long-Term Care www.health.gov.on.ca/adp Table of Amendments
Guideline on the Regulation of Therapeutic Products in New Zealand
 Guideline on the Regulation of Therapeutic Products in New Zealand Part 10: Requirements for information for prescribers and consumers Edition 7.0 January 2016 Section 1: Legislation Section summary This
Guideline on the Regulation of Therapeutic Products in New Zealand Part 10: Requirements for information for prescribers and consumers Edition 7.0 January 2016 Section 1: Legislation Section summary This
This is a licensed product of Ken Research and should not be copied
 1 TABLE OF CONTENTS 1. The US Diabetes Care Devices Market Introduction 1.1. What is Diabetes and it s Types? 2. The US Diabetes Care Devices Market Size, 2007-2013 3. The US Diabetes Care Devices Market
1 TABLE OF CONTENTS 1. The US Diabetes Care Devices Market Introduction 1.1. What is Diabetes and it s Types? 2. The US Diabetes Care Devices Market Size, 2007-2013 3. The US Diabetes Care Devices Market
Effective Shared Care Agreement for the treatment of severe motor complications in people with Parkinson Disease with apomorphine (APO-go )
 Effective Shared Care Agreement for the treatment of severe motor complications in people with Parkinson Disease with apomorphine (APO-go ) This shared care agreement outlines the ways in which the responsibilities
Effective Shared Care Agreement for the treatment of severe motor complications in people with Parkinson Disease with apomorphine (APO-go ) This shared care agreement outlines the ways in which the responsibilities
Inhalers containing CFCs. CFC-free inhalers
 Propellants used in medical metered dose inhalers and aerosol-based breath activated devices in New Zealand August to October 2002 (most recent period for which data is available) (February 2003) Inhalers
Propellants used in medical metered dose inhalers and aerosol-based breath activated devices in New Zealand August to October 2002 (most recent period for which data is available) (February 2003) Inhalers
MORE HEALTHY RECIPES Volume 2
 MORE HEALTHY RECIPES Volume 2 brought to you by Healthy Recipes Healthy Recipes Healthy Recipes Healthy Recipes RESEARCH. TESTING. HIGH STANDARDS. A lot goes into these vitamins. Don t brush it off use
MORE HEALTHY RECIPES Volume 2 brought to you by Healthy Recipes Healthy Recipes Healthy Recipes Healthy Recipes RESEARCH. TESTING. HIGH STANDARDS. A lot goes into these vitamins. Don t brush it off use
Proposal to fund a number of Nutritional products (Special Foods) supplied by Nutricia
 16 February 2012 Proposal to fund a number of Nutritional products (Special Foods) supplied by Nutricia PHARMAC is seeking feedback on a proposal to fund a number of nutritional products (Special Foods)
16 February 2012 Proposal to fund a number of Nutritional products (Special Foods) supplied by Nutricia PHARMAC is seeking feedback on a proposal to fund a number of nutritional products (Special Foods)
Access Points: Frequently Asked Questions 15 June 2016
 Access Points: Frequently Asked Questions 15 June 2016 Access Point Interim Agreement Q. Why have I received an Interim Agreement? The Commonwealth and Diabetes Australia have entered into a new NDSS Agreement
Access Points: Frequently Asked Questions 15 June 2016 Access Point Interim Agreement Q. Why have I received an Interim Agreement? The Commonwealth and Diabetes Australia have entered into a new NDSS Agreement
Northumbria Healthcare NHS Foundation Trust. Sick Day Rules for People with Diabetes. Issued by the Diabetes Service
 Northumbria Healthcare NHS Foundation Trust Sick Day Rules for People with Diabetes Issued by the Diabetes Service www.northumbria.nhs.uk Background: How illness may affect your diabetes 3 What to do for
Northumbria Healthcare NHS Foundation Trust Sick Day Rules for People with Diabetes Issued by the Diabetes Service www.northumbria.nhs.uk Background: How illness may affect your diabetes 3 What to do for
SENSOR ELECTRODE/PRECISION (Also Medisense Companion)
 DIABETIC PSEUDO-DIN LIST - Revised Mar 2017 For Provinces not listed, please submit using the diabetic pseudo DIN listed in your provincial formulary or the AB/ON pseudo DIN (except for insulin). Pricing
DIABETIC PSEUDO-DIN LIST - Revised Mar 2017 For Provinces not listed, please submit using the diabetic pseudo DIN listed in your provincial formulary or the AB/ON pseudo DIN (except for insulin). Pricing
Setting The setting was primary care. The economic study was carried out in the USA.
 Preference and resource utilization in elderly patients: InnoLet (R) versus vial/syringe Shelmet J, Schwartz S, Cappleman J, Peterson G, Skovlund S, Lytzen L, Nicklasson L, Liang J, Lyness W Record Status
Preference and resource utilization in elderly patients: InnoLet (R) versus vial/syringe Shelmet J, Schwartz S, Cappleman J, Peterson G, Skovlund S, Lytzen L, Nicklasson L, Liang J, Lyness W Record Status
How can I access flash glucose monitoring if I need it? Support pack. This pack will help you to find out more about flash and how you can access it.
 How can I access flash glucose monitoring if I need it? Support pack This pack will help you to find out more about flash and how you can access it. Reviewed March 2019 Introduction Following several major
How can I access flash glucose monitoring if I need it? Support pack This pack will help you to find out more about flash and how you can access it. Reviewed March 2019 Introduction Following several major
URGENT MEDICINE RECALL
 Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Description of the technology
 Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol
 Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team
Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team
