Improving drug safety decisions with more comprehensive searches
|
|
|
- Oscar Reynolds
- 5 years ago
- Views:
Transcription
1 FOR PHARMA AND LIFE SCIENCES Product Use Examples Improving drug safety decisions with more comprehensive searches PharmaPendium and PharmaPendium trademark are owned and protected by Reed Elsevier Properties SA. All rights reserved. Copyright Elsevier B.V. All rights reserved. Embase is a registered trademark of Elsevier B.V.
2 Example 1: Looking for additional indications beyond intended use of approved drug A pharmaceutical company is conducting preclinical testing on a drug candidate that is similar to Ezogabine, an antiepileptic drug that targets potassium channels. Their drug is being developed to treat epilepsy and experimental data suggest that it affects smooth muscles, an area they have been exploring as an additional indication. They want to find out what information, if any, was presented in the approval package on Ezogabine effects on smooth muscle cells in order to provide support for ongoing studies and, or, to provide insights into potential risks (adverse events) that may be seen in their clinical trials. Using the Quick Search bar in PharmaPendium, type in Ezogabine smooth muscle cells. Type in search query here. Of the fourteen results, line 6 provided insights on experimental findings regarding smooth muscle effects. Open file highlighted in orange 2
3 The Pharmacology Review discusses the effect of Ezogabine in a dog and pig study where the following observation was noted: decrease in blood pressure was not accompanied by the expected reflex tachycardia which may indicate other nonvascular effects such as on the heart or its nervous system regulation. This finding suggests that potential adverse effects of the heart may be minimal and is consistent with their in vitro data. This finding also informs them of which nonrodent model system to potentially use. Now they explore the new link Search in Embase to see if additional information has been published in the literature on Ezogabine and smooth muscle effects. Using this link, users can see if there is any additional information on potential adverse effects or if there is any insight into new indications. Click on Search in Embase The results in Embase will open in a new window with the same text query that was used in PharmaPendium. Sixteen results are retrieved that reference Ezogabine and smooth muscle cells. Filter categories 3
4 Some interesting findings suggest that Ezogabine could be useful in treating other potential disorders. For example, result #2 suggests that Ezogabine could be an effective treatment for asthma when used in combination with Formoterol. Result #6 suggests that Ezogabine could play a role in treating periadventitial (the membrane surrounding organs and blood vessels) vasoregulation and associated hypertension. This article suggests that Ezogabine could be a useful combination treatment for asthma. These literature findings, combined with the findings from PharmaPendium, may be useful to help optimize preclinical and clinical study designs for the new drug candidate. Evidence suggest that Ezogabine may play a role in treating periadventitial vasoregulation and associated hypertension. With this new functionality users have access to both precedent information and data from recently published preclinical/clinical models that could be used to test for new therapeutic indications and to help identify and develop mitigate plans for any unexpected adverse effects. 4
5 Example 2: Finding regulatory and literature evidence in a one session. A company is interested in finding post-market published information on Tafluprost, a drug in the same class as one of the company s drug candidates that is entering clinical trials. This information could offer additional insights into potential testing that may be required during clinical development. A Quick Search in PharmaPendium using the drug name followed by the term post-marketing found 18 entries. Type in search query here. The first entry contains a table summarizing all the on-going studies, including any Phase IV commitments. This investigator is specifically interested in looking at what Phase IV studies were required for Japanese or Asian patients at the time of drug approval. The study highlighted below is of particular interest is significantly large study that will be conducted over the course of 2 years. Click to open file. 5
6 To see if anything has been published on this study they can simply use the Search in Embase function from the results page in PharmaPendium. In Embase, one article was found. Click on Search in Embase The abstract indicates that Tafluprost provided greater efficacy than the two other drugs, latanoprost and travoprost, over a 2 month period in a study group of Japanese. As the study is over a 2 year period, information on long term use will emerge. Being better informed on the post-marketing requirements, as well as seeing some early stage results on the study, can help the company better understand how to mitigate any potential safety risks/concerns which impacts REMS planning and potentially their clinical trial designs for specific patient populations. For more information please visit, or 6
EMBASE Find quick, relevant answers to your biomedical questions
 EMBASE Find quick, relevant answers to your biomedical questions Piotr Golkiewicz Solution Sales Manager Life Sciences Central and Eastern Europe and Russia Embase is a registered trademark of Elsevier
EMBASE Find quick, relevant answers to your biomedical questions Piotr Golkiewicz Solution Sales Manager Life Sciences Central and Eastern Europe and Russia Embase is a registered trademark of Elsevier
Allergy Basics. This handout describes the process for adding and removing allergies from a patient s chart.
 Allergy Basics This handout describes the process for adding and removing allergies from a patient s chart. Accessing Allergy Information Page 1 Recording No Known Medication Allergies Page 2 Recording
Allergy Basics This handout describes the process for adding and removing allergies from a patient s chart. Accessing Allergy Information Page 1 Recording No Known Medication Allergies Page 2 Recording
Elsevier ClinicalKey TM FAQs
 Elsevier ClinicalKey FAQs Table of Contents What is ClinicalKey? Where can I access ClinicalKey? What medical specialties are covered in ClinicalKey? What information is available through ClinicalKey?
Elsevier ClinicalKey FAQs Table of Contents What is ClinicalKey? Where can I access ClinicalKey? What medical specialties are covered in ClinicalKey? What information is available through ClinicalKey?
February 23, Q4 and Year-End 2016 Financial Results
 February 23, 2017 Q4 and Year-End 2016 Financial Results 2 RETHINKING CNS Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Jim Doherty, Ph.D.,
February 23, 2017 Q4 and Year-End 2016 Financial Results 2 RETHINKING CNS Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Jim Doherty, Ph.D.,
MedDRA Overview A Standardized Terminology
 MedDRA Overview A Standardized Terminology Patrick Revelle Director, MedDRA MSSO 6 May 2010 MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations
MedDRA Overview A Standardized Terminology Patrick Revelle Director, MedDRA MSSO 6 May 2010 MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations
Vitic Nexus Excipients database Industry (Pfizer) perspective
 Vitic Nexus Excipients database Industry (Pfizer) perspective Mazin Derzi 1, Nigel Greene 2, Michelle Marra 3 1 Drug Safety Research & Development, Pfizer Inc 2 Compound Safety Prediction, Pfizer Inc 3
Vitic Nexus Excipients database Industry (Pfizer) perspective Mazin Derzi 1, Nigel Greene 2, Michelle Marra 3 1 Drug Safety Research & Development, Pfizer Inc 2 Compound Safety Prediction, Pfizer Inc 3
Sleep Apnea Therapy Software User Manual
 Sleep Apnea Therapy Software User Manual Page ii Notices Revised Notice Trademark Copyright 103392 Rev B Published February 8, 2013 and supersedes all previous versions. The information contained in this
Sleep Apnea Therapy Software User Manual Page ii Notices Revised Notice Trademark Copyright 103392 Rev B Published February 8, 2013 and supersedes all previous versions. The information contained in this
introductory guide mdconsult.com Journal Summaries Patient Handouts CME Student Union POCKET Consult
 introductory guide Reference Books Journals MEDLINE Practice Guidelines Drug Information Personalized Updates Journal Summaries Patient Handouts CME Student Union POCKET Consult mdconsult.com welcome WELCOME
introductory guide Reference Books Journals MEDLINE Practice Guidelines Drug Information Personalized Updates Journal Summaries Patient Handouts CME Student Union POCKET Consult mdconsult.com welcome WELCOME
Cell Transport and Homeostasis
 Cell Transport and Homeostasis CK12 Editor Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive
Cell Transport and Homeostasis CK12 Editor Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive
ProScript User Guide. Pharmacy Access Medicines Manager
 User Guide Pharmacy Access Medicines Manager Version 3.0.0 Release Date 01/03/2014 Last Reviewed 11/04/2014 Author Rx Systems Service Desk (T): 01923 474 600 Service Desk (E): servicedesk@rxsystems.co.uk
User Guide Pharmacy Access Medicines Manager Version 3.0.0 Release Date 01/03/2014 Last Reviewed 11/04/2014 Author Rx Systems Service Desk (T): 01923 474 600 Service Desk (E): servicedesk@rxsystems.co.uk
GEX Recommended Procedure Eff. Date: 09/21/10 Rev.: D Pg. 1 of 7
 GEX Recommended Procedure Eff. Date: 09/21/10 Rev.: D Pg. 1 of 7 NOTICE: This document is version controlled and was produced as a part of the GEX Information Program which requires that all Series 100
GEX Recommended Procedure Eff. Date: 09/21/10 Rev.: D Pg. 1 of 7 NOTICE: This document is version controlled and was produced as a part of the GEX Information Program which requires that all Series 100
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach
 Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
ELSTER A3 ALPHA METER ESM Configuration
 ELSTER A3 ALPHA METER ESM Configuration EUG-10001-01 ii Revision History Rev No. Date Description Rev 01 10-MAY-2013 Initial Release Rev 02 20-JUN-2013 Minor updates. Copyright This document contains proprietary
ELSTER A3 ALPHA METER ESM Configuration EUG-10001-01 ii Revision History Rev No. Date Description Rev 01 10-MAY-2013 Initial Release Rev 02 20-JUN-2013 Minor updates. Copyright This document contains proprietary
Regulatory Status FDA approved indication: Migranal Nasal Spray is indicated for the acute treatment of migraine headaches with or without aura (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: November 30, 2018 Migranal Nasal Spray
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: November 30, 2018 Migranal Nasal Spray
hmhco.com National GO Math! K 6 USER GUIDE Personal Math Trainer Powered by Knewton
 hmhco.com National GO Math! K 6 USER GUIDE Personal Math Trainer Powered by Knewton Version.0 August 015 Contents I. OVERVIEW AND MODES OF THE PMT...3 II. LOCATING THE PMT TO MAKE ASSIGNMENTS...5 III.
hmhco.com National GO Math! K 6 USER GUIDE Personal Math Trainer Powered by Knewton Version.0 August 015 Contents I. OVERVIEW AND MODES OF THE PMT...3 II. LOCATING THE PMT TO MAKE ASSIGNMENTS...5 III.
NASDAQ: ZGNX. Company Presentation. October 2017
 NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
AVEO and Astellas Announce TAURUS Patient Preference Clinical Study Comparing Tivozanib with Sunitinib in First-Line Kidney Cancer
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce TAURUS Patient Preference Clinical Study Comparing Tivozanib with Sunitinib in First-Line Kidney Cancer Study designed to build upon safety profile demonstrated
FOR IMMEDIATE RELEASE AVEO and Astellas Announce TAURUS Patient Preference Clinical Study Comparing Tivozanib with Sunitinib in First-Line Kidney Cancer Study designed to build upon safety profile demonstrated
Clinical Audit Tool. Release Notes
 Clinical Audit Tool Version 1.0.46 (July 2013) Release Notes These Release Notes contain important information for all Medtech Users. Please ensure that they are circulated amongst all your staff. We suggest
Clinical Audit Tool Version 1.0.46 (July 2013) Release Notes These Release Notes contain important information for all Medtech Users. Please ensure that they are circulated amongst all your staff. We suggest
Decentralised Procedure. Public Assessment Report. Memantin AbZ 10 mg/20 mg Filmtabletten ; Starterpack
 Decentralised Procedure Public Assessment Report Memantin AbZ 10 mg/20 mg Filmtabletten ; Starterpack Memantin-ratiopharm Starterpackung 5 mg /10 mg /15 mg /20 mg Filmtabletten ;10 mg/20 mg Filmtabletten
Decentralised Procedure Public Assessment Report Memantin AbZ 10 mg/20 mg Filmtabletten ; Starterpack Memantin-ratiopharm Starterpackung 5 mg /10 mg /15 mg /20 mg Filmtabletten ;10 mg/20 mg Filmtabletten
NTT DOCOMO Technical Journal
 Tweet Analysis Place-name Identification Technology Location Information Twitter* 1 is an SNS for sharing real-time information, and this information can be analyzed to reveal trends around the world;
Tweet Analysis Place-name Identification Technology Location Information Twitter* 1 is an SNS for sharing real-time information, and this information can be analyzed to reveal trends around the world;
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
Combining Electronic Diary Seizure Data with Visit-Based Seizure Data in the MONEAD Study
 PharmaSUG 2018 - Paper RW-02 Combining Electronic Diary Seizure Data with Visit-Based Seizure Data in the MONEAD Study Julia Skinner and Ryan May, Emmes Corporation ABSTRACT Electronic diary (ediary) mobile
PharmaSUG 2018 - Paper RW-02 Combining Electronic Diary Seizure Data with Visit-Based Seizure Data in the MONEAD Study Julia Skinner and Ryan May, Emmes Corporation ABSTRACT Electronic diary (ediary) mobile
Clinical Policy: Clozapine orally disintegrating tablet (Fazaclo) Reference Number: CP.PMN.12 Effective Date: Last Review Date: 02.
 Clinical Policy: Clozapine orally disintegrating tablet (Fazaclo) Reference Number: CP.PMN.12 Effective Date: 08.01.15 Last Review Date: 02.18 Line of Business: Medicaid Revision Log See Important Reminder
Clinical Policy: Clozapine orally disintegrating tablet (Fazaclo) Reference Number: CP.PMN.12 Effective Date: 08.01.15 Last Review Date: 02.18 Line of Business: Medicaid Revision Log See Important Reminder
MOVICOL Launched in Japan -The First Polyethylene Glycol Preparation for Chronic Constipation in Japan-
 MOVICOL Launched in Japan -The First Polyethylene Glycol Preparation for Chronic Constipation in Japan- This material is an English translation of the press release issued on November 29, 2018 in Japanese,
MOVICOL Launched in Japan -The First Polyethylene Glycol Preparation for Chronic Constipation in Japan- This material is an English translation of the press release issued on November 29, 2018 in Japanese,
Kofax VRS. Installation Guide
 Kofax VRS Installation Guide 2013-06-27 1999-2013 Kofax, Inc., 15211 Laguna Canyon Road, Irvine, California 92618, U.S.A. All rights reserved. Use is subject to license terms. Third-party software is copyrighted
Kofax VRS Installation Guide 2013-06-27 1999-2013 Kofax, Inc., 15211 Laguna Canyon Road, Irvine, California 92618, U.S.A. All rights reserved. Use is subject to license terms. Third-party software is copyrighted
MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES
 MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES PUSHING BOUNDARIES Terumo Interventional Systems is committed to your success with innovative procedural solutions and ongoing support for your most challenging
MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES PUSHING BOUNDARIES Terumo Interventional Systems is committed to your success with innovative procedural solutions and ongoing support for your most challenging
Red Light - Green Light
 iworx Physiology Lab Experiment Experiment HN-9 Red Light - Green Light Background Setup Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
iworx Physiology Lab Experiment Experiment HN-9 Red Light - Green Light Background Setup Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
GST: Step by step Build Diary page
 GST: At A Glance The home page has a brief overview of the GST app. Navigate through the app using either the buttons on the left side of the screen, or the forward/back arrows at the bottom right. There
GST: At A Glance The home page has a brief overview of the GST app. Navigate through the app using either the buttons on the left side of the screen, or the forward/back arrows at the bottom right. There
Tamsulosin Hydrochloride 0.4 mg Capsule
 Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
Health Care Mitigation Grants Program Final Report
 Health Care Mitigation Grants Program Final Report Grant Recipient: LB Memorial Med Ctr dba Miller Children s Hospital LB, LBACA Contract Number: HD-7870 Award Amount: 710,660 Date Submitted: 9/29/2016
Health Care Mitigation Grants Program Final Report Grant Recipient: LB Memorial Med Ctr dba Miller Children s Hospital LB, LBACA Contract Number: HD-7870 Award Amount: 710,660 Date Submitted: 9/29/2016
Lionbridge Connector for Hybris. User Guide
 Lionbridge Connector for Hybris User Guide Version 2.1.0 November 24, 2017 Copyright Copyright 2017 Lionbridge Technologies, Inc. All rights reserved. Published in the USA. March, 2016. Lionbridge and
Lionbridge Connector for Hybris User Guide Version 2.1.0 November 24, 2017 Copyright Copyright 2017 Lionbridge Technologies, Inc. All rights reserved. Published in the USA. March, 2016. Lionbridge and
Getting Started.
 Getting Started www.scientificbraintrainingpro.com Summary 1. First steps... 2 2. Log in... 2 3. Create an account for a patient... 3 4. Access an exercise with this patient... 4 5. Viewing the results
Getting Started www.scientificbraintrainingpro.com Summary 1. First steps... 2 2. Log in... 2 3. Create an account for a patient... 3 4. Access an exercise with this patient... 4 5. Viewing the results
Disclaimer. The following report contains a description of the request, request specifications, and results from the modular program run(s).
 Disclaimer The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product safety
Disclaimer The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product safety
Migranal Nasal Spray. Migranal Nasal Spray (dihydroergotamine) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: June 22, 2017 Migranal Nasal Spray
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: June 22, 2017 Migranal Nasal Spray
User Manual. RaySafe i2 dose viewer
 User Manual RaySafe i2 dose viewer 2012.03 Unfors RaySafe 5001048-A All rights are reserved. Reproduction or transmission in whole or in part, in any form or by any means, electronic, mechanical or otherwise,
User Manual RaySafe i2 dose viewer 2012.03 Unfors RaySafe 5001048-A All rights are reserved. Reproduction or transmission in whole or in part, in any form or by any means, electronic, mechanical or otherwise,
Laboratory Report, APA Style (Psychology)
 Laboratory Report, APA Style (Psychology) Running head: REACTION TIMES IN TWO VISUAL SEARCH TASKS 1 The header consists of a shortened title (no more than 50 characters) in all capital letters at the left
Laboratory Report, APA Style (Psychology) Running head: REACTION TIMES IN TWO VISUAL SEARCH TASKS 1 The header consists of a shortened title (no more than 50 characters) in all capital letters at the left
Boehringer Ingelheim Pharmaceuticals, Inc. & Univision. Making Diabetic Hispanics Healthier
 Boehringer Ingelheim Pharmaceuticals, Inc. & Univision Making Diabetic Hispanics Healthier Agenda BIPI and the Hispanic segment Organizing and Executing to Win The Proof is in the Results Making Hispanics
Boehringer Ingelheim Pharmaceuticals, Inc. & Univision Making Diabetic Hispanics Healthier Agenda BIPI and the Hispanic segment Organizing and Executing to Win The Proof is in the Results Making Hispanics
PROUS SCIENCE INTEGRITY. Leukemia Therapy: Bcr-Abl Kinase Inhibitors: Selective vs Nonselective
 PROUS SCIENCE INTEGRITY Leukemia Therapy: Bcr-Abl Kinase Inhibitors: Selective vs Nonselective SUMMARY In this use case, active compounds for Leukemia will be found and filtered by mechanisms of action
PROUS SCIENCE INTEGRITY Leukemia Therapy: Bcr-Abl Kinase Inhibitors: Selective vs Nonselective SUMMARY In this use case, active compounds for Leukemia will be found and filtered by mechanisms of action
CENTER FOR DRUG EVALUATION AND RESEARCH. APPLICATION NUMBER: Orig1s000 APPROVAL LETTER
 CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202107Orig1s000 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202107 NDA APPROVAL
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202107Orig1s000 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202107 NDA APPROVAL
What s New MedDRA Version 13.1
 What s New MedDRA Version 13.1 Acknowledgements ACKNOWLEDGEMENTS MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Adobe is a registered
What s New MedDRA Version 13.1 Acknowledgements ACKNOWLEDGEMENTS MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Adobe is a registered
Provider Dashboard User's Manual
 Provider Dashboard User's Manual CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. IMPORTANT: READ THE SAEBOVR GETTING STARTED GUIDE (BAI-VOTA-LBL- 001) IN ITS ENTIRETY
Provider Dashboard User's Manual CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. IMPORTANT: READ THE SAEBOVR GETTING STARTED GUIDE (BAI-VOTA-LBL- 001) IN ITS ENTIRETY
User Guide for Classification of Diabetes: A search tool for identifying miscoded, misclassified or misdiagnosed patients
 User Guide for Classification of Diabetes: A search tool for identifying miscoded, misclassified or misdiagnosed patients For use with isoft Premiere Synergy Produced by André Ring 1 Table of Contents
User Guide for Classification of Diabetes: A search tool for identifying miscoded, misclassified or misdiagnosed patients For use with isoft Premiere Synergy Produced by André Ring 1 Table of Contents
Welcome to CareLink Pro
 Reference Guide Welcome to CareLink Pro This guide was developed to serve as a reference for obtaining patient data and reviewing CareLink Pro reports. Getting Started with CareLink Pro Adding New Patients
Reference Guide Welcome to CareLink Pro This guide was developed to serve as a reference for obtaining patient data and reviewing CareLink Pro reports. Getting Started with CareLink Pro Adding New Patients
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
Scopus. Cancer Research and Treatment Activity Report
 Scopus Cancer Research and Treatment Activity Report Scopus offers researchers an extensive global and interdisciplinary research database of abstracts and citations from over 5,000 international publishers,
Scopus Cancer Research and Treatment Activity Report Scopus offers researchers an extensive global and interdisciplinary research database of abstracts and citations from over 5,000 international publishers,
The Role of Systematic Reviews
 The Role of Systematic Reviews Can Systematic Review of Preclinical Data Assist with Replacement, Refinement, and Reduction of Animal Use in Neuroscience Research? Anne N. Murphy, Ph.D. Dept of Pharmacology
The Role of Systematic Reviews Can Systematic Review of Preclinical Data Assist with Replacement, Refinement, and Reduction of Animal Use in Neuroscience Research? Anne N. Murphy, Ph.D. Dept of Pharmacology
ANALYSIS OF SELF-REPORTED HEALTH OUTCOMES DATA FROM WEB-BASED MEDIA SOURCES
 ANALYSIS OF SELF-REPORTED HEALTH OUTCOMES DATA FROM WEB-BASED MEDIA SOURCES Mark Wolff, Ph.D. Principal Industry Consultant Health & Life Sciences Global Practice SAS Institute Cary, North Carolina Copyright
ANALYSIS OF SELF-REPORTED HEALTH OUTCOMES DATA FROM WEB-BASED MEDIA SOURCES Mark Wolff, Ph.D. Principal Industry Consultant Health & Life Sciences Global Practice SAS Institute Cary, North Carolina Copyright
WOMEN OF THE PLEASURE QUARTERS BY LESLEY DOWNER DOWNLOAD EBOOK : WOMEN OF THE PLEASURE QUARTERS BY LESLEY DOWNER PDF
 Read Online and Download Ebook WOMEN OF THE PLEASURE QUARTERS BY LESLEY DOWNER DOWNLOAD EBOOK : WOMEN OF THE PLEASURE QUARTERS BY LESLEY Click link bellow and free register to download ebook: WOMEN OF
Read Online and Download Ebook WOMEN OF THE PLEASURE QUARTERS BY LESLEY DOWNER DOWNLOAD EBOOK : WOMEN OF THE PLEASURE QUARTERS BY LESLEY Click link bellow and free register to download ebook: WOMEN OF
BioMarin Pharmaceutical Inc. Conference Call to Discuss Approval of
 BioMarin Pharmaceutical Inc. Conference Call to Discuss Approval of For adult patients with PKU who have uncontrolled blood phenylalanine (Phe) concentrations > 600 µmol/l on existing management May 24,
BioMarin Pharmaceutical Inc. Conference Call to Discuss Approval of For adult patients with PKU who have uncontrolled blood phenylalanine (Phe) concentrations > 600 µmol/l on existing management May 24,
Qualys PC/SCAP Auditor
 Qualys PC/SCAP Auditor Getting Started Guide November 15, 2017 COPYRIGHT 2011-2017 BY QUALYS, INC. ALL RIGHTS RESERVED. QUALYS AND THE QUALYS LOGO ARE REGISTERED TRADEMARKS OF QUALYS, INC. ALL OTHER TRADEMARKS
Qualys PC/SCAP Auditor Getting Started Guide November 15, 2017 COPYRIGHT 2011-2017 BY QUALYS, INC. ALL RIGHTS RESERVED. QUALYS AND THE QUALYS LOGO ARE REGISTERED TRADEMARKS OF QUALYS, INC. ALL OTHER TRADEMARKS
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Krystexxa) Reference Number: CP.CPA.57 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Krystexxa) Reference Number: CP.CPA.57 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Almirall grants Invida commercial rights for Aclidinium in Australia and New ZealandAlmirall????? (Invida)??????????? Aclidinium?????
 Almirall grants Invida commercial rights for Aclidinium in Australia and New ZealandAlmirall????? (Invida)??????????? Aclidinium????? Almirall licenses Aclidinium to Invida in Australia and New Zealand
Almirall grants Invida commercial rights for Aclidinium in Australia and New ZealandAlmirall????? (Invida)??????????? Aclidinium????? Almirall licenses Aclidinium to Invida in Australia and New Zealand
Japanese Regulatory and Industry perspective. Kazuhiro Shimomura, Ph.D. Medicinal Safety Research Laboratories Daiichi Sankyo Co., Ltd.
 Japanese Regulatory and Industry perspective Kazuhiro Shimomura, Ph.D. Medicinal Safety Research Laboratories Daiichi Sankyo Co., Ltd. 1 Activities for Juvenile Study in Japan 2004.2 Repro. Tox. Tokyo
Japanese Regulatory and Industry perspective Kazuhiro Shimomura, Ph.D. Medicinal Safety Research Laboratories Daiichi Sankyo Co., Ltd. 1 Activities for Juvenile Study in Japan 2004.2 Repro. Tox. Tokyo
Modular Program Report
 Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Public Assessment Report. Mebeverine hydrochloride 50mg/5ml Oral Suspension. (Mebeverine hydrochloride) UK Licence No: PL 44710/0024
 Public Assessment Report Mebeverine hydrochloride 50mg/5ml Oral Suspension (Mebeverine hydrochloride) UK Licence No: PL 44710/0024 Kinedexe UK Limited 1 LAY SUMMARY Mebeverine hydrochloride 50mg/5ml Oral
Public Assessment Report Mebeverine hydrochloride 50mg/5ml Oral Suspension (Mebeverine hydrochloride) UK Licence No: PL 44710/0024 Kinedexe UK Limited 1 LAY SUMMARY Mebeverine hydrochloride 50mg/5ml Oral
Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome
 Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome Primary Endpoint Achieved - Statistically Significant Convulsive Seizure Reduction for ZX008
Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome Primary Endpoint Achieved - Statistically Significant Convulsive Seizure Reduction for ZX008
NCCN Chemotherapy Order Templates
 USER GUIDE NCCN Chemotherapy Order Templates (NCCN Templates ) Access to the NCCN Chemotherapy Order Templates (NCCN Templates ) for non-commercial users is available via subscription. Prior to accessing
USER GUIDE NCCN Chemotherapy Order Templates (NCCN Templates ) Access to the NCCN Chemotherapy Order Templates (NCCN Templates ) for non-commercial users is available via subscription. Prior to accessing
Public Assessment Report. Scientific discussion. Pentasa Compact 4 g, prolonged-release granules. (mesalazine) NL License RVG:
 Public Assessment Report Scientific discussion Pentasa Compact 4 g, prolonged-release granules (mesalazine) NL License RVG: 114015 Date: 30 March 2015 This module reflects the scientific discussion for
Public Assessment Report Scientific discussion Pentasa Compact 4 g, prolonged-release granules (mesalazine) NL License RVG: 114015 Date: 30 March 2015 This module reflects the scientific discussion for
ISR Process for Internal Service Providers
 ISR Process for Internal Service Providers Course Outline 1) Internal Service Request Process Overview Internal Service Requests (ISR) are created by the end user via the BUworks Central Portal Procurement
ISR Process for Internal Service Providers Course Outline 1) Internal Service Request Process Overview Internal Service Requests (ISR) are created by the end user via the BUworks Central Portal Procurement
Dr N T R UNIVERSITY OF HEALTH SCIENCES A.P., VIJAYAWADA
 FOR SECOND M.B.B.S., RESULTS VISIT Dr. NTRUHS WEBSITE http://ntruhs.ap.nic.in The following is the provisional list of registered numbers of all the successful candidates at SECOND M.B.B.S., Examinations
FOR SECOND M.B.B.S., RESULTS VISIT Dr. NTRUHS WEBSITE http://ntruhs.ap.nic.in The following is the provisional list of registered numbers of all the successful candidates at SECOND M.B.B.S., Examinations
ICD-10 Readiness (*9/14/15) By PracticeHwy.com, Inc.
 ICD-10 Readiness (*9/14/15) By PracticeHwy.com, Inc. Notice Information in this document is subject to change without notice and does not represent a commitment on the part of PracticeHwy.com, Inc. Companies,
ICD-10 Readiness (*9/14/15) By PracticeHwy.com, Inc. Notice Information in this document is subject to change without notice and does not represent a commitment on the part of PracticeHwy.com, Inc. Companies,
Data retrieval using the new SMQ Medication Errors
 Data retrieval using the new SMQ Medication Errors Christina Winter Medical Director Safety Evaluation and Risk Management, GSK Information Day on Medication Errors 20 October 2016 London, UK Disclaimer
Data retrieval using the new SMQ Medication Errors Christina Winter Medical Director Safety Evaluation and Risk Management, GSK Information Day on Medication Errors 20 October 2016 London, UK Disclaimer
The PlantFAdb website and database are based on the superb SOFA database (sofa.mri.bund.de).
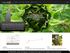 A major goal of PlantFAdb is to allow users to easily explore relationships between unusual fatty acid structures and the plant species that produce them. Clicking on Tree from the home page provides an
A major goal of PlantFAdb is to allow users to easily explore relationships between unusual fatty acid structures and the plant species that produce them. Clicking on Tree from the home page provides an
Annex. (Draft for Comments)
 Annex Announcement on the Matters related to filing and Joint Review & Approval of Active Pharmaceutical Ingredients, Pharmaceutical Excipients and Pharmaceutical Packaging Materials for Drug Products
Annex Announcement on the Matters related to filing and Joint Review & Approval of Active Pharmaceutical Ingredients, Pharmaceutical Excipients and Pharmaceutical Packaging Materials for Drug Products
November 2, Q Financial Results
 November 2, 2017 Q3 2017 Financial Results Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
November 2, 2017 Q3 2017 Financial Results Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
smk72+ Handbook Prof. Dr. Andreas Frey Dr. Lars Balzer Stephan Spuhler smk72+ Handbook Page 1
 smk72+ Handbook Prof. Dr. Andreas Frey Dr. Lars Balzer Stephan Spuhler Email: support@kompetenzscreening.de Page 1 Table of Contents HOME... 3 BASIC INFORMATION ON LOGGING IN... 4 LOGIN PROCESS FOR PARTICIPANTS...
smk72+ Handbook Prof. Dr. Andreas Frey Dr. Lars Balzer Stephan Spuhler Email: support@kompetenzscreening.de Page 1 Table of Contents HOME... 3 BASIC INFORMATION ON LOGGING IN... 4 LOGIN PROCESS FOR PARTICIPANTS...
ShadeVision v Color Map
 Kevin Aamodt Page 1 7/1/2004 ShadeVision v.3.01 Color Map The ShadeVision v.3.01 color map will allow the user to custom select the lookup regions of the Gingival, Middle, and Incisal areas of a tooth.
Kevin Aamodt Page 1 7/1/2004 ShadeVision v.3.01 Color Map The ShadeVision v.3.01 color map will allow the user to custom select the lookup regions of the Gingival, Middle, and Incisal areas of a tooth.
Instil one or two drops in the conjunctival sac(s) every three to four hours as needed.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON FORTE Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon Forte contains naphazoline hydrochloride 1.0 mg in 1 ml (0.1%). Excipient with known
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON FORTE Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon Forte contains naphazoline hydrochloride 1.0 mg in 1 ml (0.1%). Excipient with known
Company Update with a Focus on Pipeline
 NASDAQ: Company Update with a Focus on Pipeline December 2014 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts
NASDAQ: Company Update with a Focus on Pipeline December 2014 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts
MB6810B-Regional Per-Dose Funding Report Report User Guide Modified: Document Version:
 Manitoba Panorama MB6810B-Regional Per-Dose Funding Report Report User Guide Modified: 2016-01-06 Manitoba ehealth Document Version: 0.05 Document Status: Final January 6, 2016 Document Author: Manitoba
Manitoba Panorama MB6810B-Regional Per-Dose Funding Report Report User Guide Modified: 2016-01-06 Manitoba ehealth Document Version: 0.05 Document Status: Final January 6, 2016 Document Author: Manitoba
Quality Management System Certification. Understanding Quality Management System (QMS) certification
 Quality Management System Certification Understanding Quality Management System (QMS) certification The medical device manufacturing sector is one of the most regulated sectors in which significant quality
Quality Management System Certification Understanding Quality Management System (QMS) certification The medical device manufacturing sector is one of the most regulated sectors in which significant quality
Cancer Decision Support Tool Walk through user guide
 These screenshots show exactly how a GP should use the CDS tool, covering the three ways in which it works, and how to log in to the software. Logging into the software It s really easy to log into the
These screenshots show exactly how a GP should use the CDS tool, covering the three ways in which it works, and how to log in to the software. Logging into the software It s really easy to log into the
Cloud Condensation Nuclei Counter (CCN) Module
 Particle Analysis and Display System (PADS): Cloud Condensation Nuclei Counter (CCN) Module Operator Manual DOC-0190 A-1 PADS 2.5.6, CCN Module 2.5.1 5710 Flatiron Parkway, Unit B Boulder, CO 80301 USA
Particle Analysis and Display System (PADS): Cloud Condensation Nuclei Counter (CCN) Module Operator Manual DOC-0190 A-1 PADS 2.5.6, CCN Module 2.5.1 5710 Flatiron Parkway, Unit B Boulder, CO 80301 USA
Basic Reporting in EvaluationWeb
 Basic ing in EvaluationWeb User Guide Luther Consulting, LLC March 2014 All rights reserved. Table of Contents Running s in EvaluationWeb 3 Running s... 3 Accessing the s Window... 5 Running the Data Quality
Basic ing in EvaluationWeb User Guide Luther Consulting, LLC March 2014 All rights reserved. Table of Contents Running s in EvaluationWeb 3 Running s... 3 Accessing the s Window... 5 Running the Data Quality
Regulatory Support for Tobacco Products. Feeling daunted by the regulatory process for tobacco products? Don t worry Battelle can help.
 Regulatory Support for Tobacco Products Feeling daunted by the regulatory process for tobacco products? Don t worry Battelle can help. PREMARKET TOBACCO (PMTA) HUMAN FACTORS STUDIES SUBSTANTIAL EQUIVALENCE
Regulatory Support for Tobacco Products Feeling daunted by the regulatory process for tobacco products? Don t worry Battelle can help. PREMARKET TOBACCO (PMTA) HUMAN FACTORS STUDIES SUBSTANTIAL EQUIVALENCE
AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
RADIESSE Volumizing Filler E-Media Kit Terms of Use PERMISSION TO USE MERZ AESTHETICS COPYRIGHTED MATERIAL AND TRADEMARKS
 RADIESSE Volumizing Filler E-Media Kit Terms of Use PERMISSION TO USE MERZ AESTHETICS COPYRIGHTED MATERIAL AND TRADEMARKS For good and valuable consideration, the receipt and sufficiency of which is hereby
RADIESSE Volumizing Filler E-Media Kit Terms of Use PERMISSION TO USE MERZ AESTHETICS COPYRIGHTED MATERIAL AND TRADEMARKS For good and valuable consideration, the receipt and sufficiency of which is hereby
BRITE R450. LightTrak Sensor Time/Date. Goal Progress Bar Activity Value Activity Type Notification Alert. Quick Start Guide
 BRITE R450 LightTrak Sensor Time/Date Goal Progress Bar Activity Value Activity Type Notification Alert Quick Start Guide Welcome to LifeTrak. Let s take a quick tour of the basic features. To turn on
BRITE R450 LightTrak Sensor Time/Date Goal Progress Bar Activity Value Activity Type Notification Alert Quick Start Guide Welcome to LifeTrak. Let s take a quick tour of the basic features. To turn on
BOOKING RULES REVISIONS & SUPPORT FOR MULTIPLE CRS
 CloudPM v5.4 BOOKING RULES REVISIONS & SUPPORT FOR MULTIPLE CRS INTERFACES QUICK REFERENCE GUIDE FOR MSI CLOUDPM USERS ED TRUXAL MSI SOLUTIONS 7600 N. 15th Street Suite #250 Phoenix, AZ 85020 Table of
CloudPM v5.4 BOOKING RULES REVISIONS & SUPPORT FOR MULTIPLE CRS INTERFACES QUICK REFERENCE GUIDE FOR MSI CLOUDPM USERS ED TRUXAL MSI SOLUTIONS 7600 N. 15th Street Suite #250 Phoenix, AZ 85020 Table of
DuraMatrix portfolio. Restore with confidence
 DuraMatrix portfolio Restore with confidence History Since 2003, Stryker has partnered with Collagen Matrix, Inc. to provide our customers with quality products that are safe and effective in the restoration
DuraMatrix portfolio Restore with confidence History Since 2003, Stryker has partnered with Collagen Matrix, Inc. to provide our customers with quality products that are safe and effective in the restoration
Wimba Classroom Captioner Guide
 Wimba Classroom Captioner Guide Wimba Classroom Captioner Guide 1 Background 1 Best Practices 1 Enabling Closed Captioning 2 Captioning a Live Presentation 2 Using the Wimba Classroom Interface 2 Using
Wimba Classroom Captioner Guide Wimba Classroom Captioner Guide 1 Background 1 Best Practices 1 Enabling Closed Captioning 2 Captioning a Live Presentation 2 Using the Wimba Classroom Interface 2 Using
Fitting System Instructions for Use
 Including 2017 2018.2 Fitting System Instructions for Use Version 1.0 www.sonici.com Table of contents 1. Introduction 4 2. Installation 5 3. System requirements 6 4. Getting started with Expressfit Pro
Including 2017 2018.2 Fitting System Instructions for Use Version 1.0 www.sonici.com Table of contents 1. Introduction 4 2. Installation 5 3. System requirements 6 4. Getting started with Expressfit Pro
Clay Tablet Connector for hybris. User Guide. Version 1.5.0
 Clay Tablet Connector for hybris User Guide Version 1.5.0 August 4, 2016 Copyright Copyright 2005-2016 Clay Tablet Technologies Inc. All rights reserved. All rights reserved. This document and its content
Clay Tablet Connector for hybris User Guide Version 1.5.0 August 4, 2016 Copyright Copyright 2005-2016 Clay Tablet Technologies Inc. All rights reserved. All rights reserved. This document and its content
Points to Consider CPhI Japan Briefing Session on Pharmaceutical Regulations in Japan
 2013 CPhI Japan Briefing Session on Pharmaceutical Regulations in Japan The Master File System and Points to Consider Kentaro Hashimoto, Master File Management Group, Division of Pharmacopoeia and Standards
2013 CPhI Japan Briefing Session on Pharmaceutical Regulations in Japan The Master File System and Points to Consider Kentaro Hashimoto, Master File Management Group, Division of Pharmacopoeia and Standards
Public Assessment Report Scientific discussion. Trelema (lacosamide) SE/H/1648/01-07/DC
 Public Assessment Report Scientific discussion Trelema (lacosamide) SE/H/1648/01-07/DC This module reflects the scientific discussion for the approval of Trelema. The procedure was finalised on 2018-03-08.
Public Assessment Report Scientific discussion Trelema (lacosamide) SE/H/1648/01-07/DC This module reflects the scientific discussion for the approval of Trelema. The procedure was finalised on 2018-03-08.
Caprelsa. Caprelsa (vandetanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.16 Subject: Caprelsa Page: 1 of 5 Last Review Date: June 22, 2018 Caprelsa Description Caprelsa (vandetanib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.16 Subject: Caprelsa Page: 1 of 5 Last Review Date: June 22, 2018 Caprelsa Description Caprelsa (vandetanib)
Multi-modal Patient Cohort Identification from EEG Report and Signal Data
 Multi-modal Patient Cohort Identification from EEG Report and Signal Data Travis R. Goodwin and Sanda M. Harabagiu The University of Texas at Dallas Human Language Technology Research Institute http://www.hlt.utdallas.edu
Multi-modal Patient Cohort Identification from EEG Report and Signal Data Travis R. Goodwin and Sanda M. Harabagiu The University of Texas at Dallas Human Language Technology Research Institute http://www.hlt.utdallas.edu
PUBLIC ASSESSMENT REPORT Scientific Discussion
 Direction de l Evaluation des Médicaments et des Produits Biologiques PUBLIC ASSESSMENT REPORT Scientific Discussion STAMICIS Kit for preparation of Technetium ( 99m Tc), Sestamibi injection [Tetrakis(2-methoxy-2-methylpropyl-1
Direction de l Evaluation des Médicaments et des Produits Biologiques PUBLIC ASSESSMENT REPORT Scientific Discussion STAMICIS Kit for preparation of Technetium ( 99m Tc), Sestamibi injection [Tetrakis(2-methoxy-2-methylpropyl-1
SAGE. Nick Beard Vice President, IDX Systems Corp.
 SAGE Nick Beard Vice President, IDX Systems Corp. Sharable Active Guideline Environment An R&D consortium to develop the technology infrastructure to enable computable clinical guidelines, that will be
SAGE Nick Beard Vice President, IDX Systems Corp. Sharable Active Guideline Environment An R&D consortium to develop the technology infrastructure to enable computable clinical guidelines, that will be
Drug prescriptions (Pharm) Exposure (36/48 months)
 ANNEX SECTION PART A - Study design: Figure 1 overview of the study design Drug prescriptions (Pharm) 2006-2010 Exposure (36/48 months) flexible time windows (e.g. 90 days) time index date (hospital discharge)
ANNEX SECTION PART A - Study design: Figure 1 overview of the study design Drug prescriptions (Pharm) 2006-2010 Exposure (36/48 months) flexible time windows (e.g. 90 days) time index date (hospital discharge)
Anti-epilepsy Drug VIMPAT for I.V. infusion 200mg Launched in Japan
 Press Release March 25, 2019 UCB Japan Co., Ltd. Daiichi Sankyo Company, Limited Anti-epilepsy Drug VIMPAT for I.V. infusion 200mg Launched in Japan UCB Japan Co., Ltd. (headquarters: Shinjuku-ku, Tokyo;
Press Release March 25, 2019 UCB Japan Co., Ltd. Daiichi Sankyo Company, Limited Anti-epilepsy Drug VIMPAT for I.V. infusion 200mg Launched in Japan UCB Japan Co., Ltd. (headquarters: Shinjuku-ku, Tokyo;
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL VOLUME 2C. Guidelines. Medicinal products for human use
 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Brussels, March 2018 SANTE-2017-11668 Revision 2 NOTICE TO APPLICANTS VOLUME 2C Guidelines Medicinal products for human use Safety, environment
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Brussels, March 2018 SANTE-2017-11668 Revision 2 NOTICE TO APPLICANTS VOLUME 2C Guidelines Medicinal products for human use Safety, environment
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
Public Assessment Report. Scientific discussion. Kaliumklorid "EQL Pharma" (Potassium chloride) DK/H/2662/001/DC. Date:
 CMDh/223/2005 February 2014 Public Assessment Report Scientific discussion Kaliumklorid "EQL Pharma" (Potassium chloride) DK/H/2662/001/DC Date: 16-06-2017 This module reflects the scientific discussion
CMDh/223/2005 February 2014 Public Assessment Report Scientific discussion Kaliumklorid "EQL Pharma" (Potassium chloride) DK/H/2662/001/DC Date: 16-06-2017 This module reflects the scientific discussion
Modular Program Report
 Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Cerner COMPASS ICD-10 Transition Guide
 Cerner COMPASS ICD-10 Transition Guide Dx Assistant Purpose: To educate Seton clinicians regarding workflow changes within Cerner COMPASS subsequent to ICD-10 transition. Scope: Basic modules and functionality
Cerner COMPASS ICD-10 Transition Guide Dx Assistant Purpose: To educate Seton clinicians regarding workflow changes within Cerner COMPASS subsequent to ICD-10 transition. Scope: Basic modules and functionality
ReSound Assist quick guide. A guide for professionals
 ReSound Assist quick guide A guide for professionals How to complete a ReSound Assist fine-tuning Activate remote fine-tuning. From the Patient screen, select ReSound Assist from the lower navigation row..
ReSound Assist quick guide A guide for professionals How to complete a ReSound Assist fine-tuning Activate remote fine-tuning. From the Patient screen, select ReSound Assist from the lower navigation row..
MOVICOL Launched in Japan -The First Polyethylene Glycol Preparation for Chronic Constipation in Japan-
 November 29, 2018 EA Pharma Co., Ltd. Eisai Co., Ltd. Mochida Pharmaceutical Co., Ltd. MOVICOL Launched in Japan -The First Polyethylene Glycol Preparation for Chronic Constipation in Japan- EA Pharma
November 29, 2018 EA Pharma Co., Ltd. Eisai Co., Ltd. Mochida Pharmaceutical Co., Ltd. MOVICOL Launched in Japan -The First Polyethylene Glycol Preparation for Chronic Constipation in Japan- EA Pharma
Using new scientific knowledge to update regulations in the U.S.
 Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
