A Chinese Patent Medicine for the Secondary Prevention of Myocardial Infarction
|
|
|
- Florence Webb
- 5 years ago
- Views:
Transcription
1 Section 1 01 Clinical Trials A Chinese Patent Medicine for the Secondary Prevention of Myocardial Infarction China Boli Zhang and Junhua Zhang. Tianjin University of Traditional Chinese Medicine, 312 Anshanxi Str., Nankai District, Tianjin, China Tel.: URL: iec.tjutcm.edu.cn/english2015/home.htm zjhtcm@foxmail.com Duration: Total cost: USD 1,000,000 (Ministry of Science and Technology, State Administration of Traditional Chinese Medicine of PR China) 18
2 EXPLORING TRADITIONAL MEDICINE Summary Survivors from acute myocardial infarction (MI) remain at greatly increased risk of serious vascular events. Thus, secondary prevention aimed to decrease mortality and morbidity in survivors after acute MI is of great, and increasing, significance around the world. Several types of chemical drugs have been recommended for the secondary prevention of MI. However, these conventional strategies have several limitations, such as low adherence, high cost, and side effects during long-term use. Novel approaches to this problem are still needed. Our study aimed to evaluate the effectiveness and safety of Qi-Shen-Yi-Qi Dripping Pills (QSYQ), a multiingredient Chinese patent medicine, for the secondary prevention of MI. A total of 3,505 eligible patients were randomly assigned to a QSYQ group (1,746 patients) and an aspirin group (1,759). Patients took their treatments for 12 months. The final follow-up visit took place 6 months later. The 12-month and 18-month estimated incidences of the primary outcome were 2.98% and 3.67%, respectively, in the QSYQ group. The figures were 2.96% and 3.81% in the aspirin group. These data did not show significant difference of primary and secondary outcomes between aspirin and QSYQ in patients who have had an MI. The result suggested that QSYQ has similar effects to aspirin in the secondary prevention of MI. Background and Justification Acute myocardial infarction is a leading cause of death worldwide. More than 7million people a year have a myocardial infarction (MI). Over the past three decades, MI has emerged from an illness seen predominantly in developed countries to becoming more common also in developing countries. Progress in emergency management led to substantial reductions in the mortality rate of acute MI. However, survivors from acute MI remain at greatly increased risk of serious vascular events. Thus, secondary prevention aimed at decreasing mortality and morbidity in survivors of acute MI is of great, and increasing, significance around the world. Platelets play a key role in the development of thrombotic and ischaemic diseases. Antiplatelet therapy is a major strategy for treating and preventing MI. Anti-platelet drugs have been shown to have definite and substantial net benefits for people who have occlusive vascular disease. Aspirin is a safe and cost-effective anti-platelet drug and current guidelines recommend low-dose aspirin ( mg daily) for the secondary prevention of MI in many countries. However, there are several limitations related to this drug. Longterm therapy with aspirin is associated with an increase in the incidence of symptomatic 19
3 peptic ulcer, duodenal ulcers, and gastrointestinal and intracranial haemorrhage, even when used at low doses or in buffered or enteric-coated formulations. In a populationbased cohort with 4.1 million citizens in Italy, for example, aspirin increased the risk of major gastrointestinal or cerebral bleeding episodes, and patients with diabetes had a high rate of bleeding. Furthermore, aspirin resistance has become a notable problem. Several studies have found that about 1 in 4 individuals may express biochemically defined aspirin resistance. Patients who are resistant to aspirin are at greater risk of recurrent serious vascular events than those who are sensitive to aspirin. Current anti-platelet therapies are generally based on a specific signaling pathway in platelet activation, that is a single agent acting on a single target. Hence, the limitations associated with aspirin also exist for other anti-platelet agents, such as clopidogrel. Agents with multiple ingredients acting on multiple targets may be more effective and less harmful. In Traditional Chinese Medicine (TCM), the key pathogenesis of MI is mainly Qixu (vital energy deficiency) and Xueyu (blood stagnation), that is, degradation of body function and thrombosis. Qi-Shen-Yi-Qi Dripping Pills (QSYQ), a Chinese patent medicine for adding qi and resolving stasis, was approved for clinical use for coronary heart disease and MI rehabilitation by the State Food and Drug Administration of China in QSYQ is made of extractions from Danshen (Radix Salviae Miltiorrhizae), Sanqi (Radix Notoginseng), Jiangxiang (Lignum Dalbergiae Odoriferae), and Huangqi (Radix Astragali). The quality control of QSYQ is good. Herbal materials were cultivated according to the good agriculture practices (GAP), and manufacturing processes strictly followed the standard of good manufacturing practices (GMP). Mass spectroscopy was used to analyse seven quality control markers of QSYQ and showed good consistency of the active markers among different batches. Pharmacological studies revealed that constituents of QSYQ could inhibit the platelet aggregation and the over release of β-tg (a platelet protein). Clinical studies have suggested that QSYQ had similar effect to aspirin in inhibiting platelet aggregation. QSYQ is widely used for the secondary prevention of MI in China. However, there is insufficient evidence to know whether QSYQ can be used as an alternative to aspirin. This multi-centre randomized clinical trial aimed to test whether the regular administration of QSYQ would result in a significant reduction in total serious vascular events in patients who had experienced at least one documented MI. 20
4 EXPLORING TRADITIONAL MEDICINE Description A randomised, double-blind, parallel controlled study was carried out. With such a large scale clinical trial, the quality control of process management was key for the validity and reliability of the results. The study protocol was reviewed and approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine and the trial registered with the World Health Organization Clinical Trial Registering Platform. Patients with a definite diagnosis of STelevation or non-st-elevation MI (ST is a diagnostic section of an ECG readout) were potentially eligible for this study if they met the following criteria: (1) the last documented MI was 4 weeks to 24 months earlier; (2) Traditional Chinese Medicine symptoms were Qixu-Xueyu (vital energy deficiency combining with blood stagnation); (3) aged from 18 to 75 years (the maximum age was adjusted from 65 years to 75 years in July 2006 because of inadequate recruitment). Patients were required to be free of other life-threatening diseases or problems which might have limited the ability to obtain long-term follow up and to be free of any condition which would mean that regular use of the trial drugs was contraindicated. Patients with any of the following conditions were excluded: (1) a history of percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG); (2) pregnant women or those who were breast feeding; (3) contraindication to aspirin (e.g., asthma, active phase peptic ulcer, and haemorrhagic disease); (4) heart function of grade IV(NYHA grade); (5) uncontrolled systemic hypertension (contractive pressure 180 mmhg or diastolic pressure 110 mmhg); (6) uncontrolled serious cardiac arrhythmias (e.g., atrial fibrillation and supraventricular tachycardia); (7) serious primary disease of liver, kidney, and hemopoietic system, or psychosis, or malignant tumor; (8) allergic history to study drugs; (9) participation in other clinical trials during the previous three months. After a comprehensive medical evaluation, patients were given a full explanation of the study by investigators. Each patient was asked for their written informed consent before joining the study. Recruitment took place in 88 hospitals across China. Patients were randomly assigned to two groups (ratio 1:1): QSYQ group and aspirin group. In the QSYQ group, patients took 0.5 g (one package) QSYQ three times per day and 100 mg (in four tablets) simulated enteric-coated aspirin once a day. Patients in the aspirin group took 0.5 g (one package) simulated QSYQ three times per day and 100 mg (in four tablets) enteric-coated aspirin daily. Placebos for QSYQ and aspirin were developed which had the same appearance, colour, and taste as the relevant drug. Patients were prohibited from taking other anti-platelet drugs or Yiqi-Huoxue Chinese medicines during the treatment period. Concomitant medications, such as antihypertensive (e.g., beta-blockers and ACE 21
5 inhibitors), hypoglycaemic agents, and lipid-lowering drugs, could be prescribed at the discretion of the attending physicians and had to be recorded in detail (including drug name, period taken, dosage, and purpose). The treatment period for the trial drugs was 12 months. After this time (or if the trial drugs were stopped for some other reason), patients could be prescribed treatments by their physicians without any limitation. The primary endpoint was a composite of cardiovascular death, non-fatal re-infarction (documented by ECG and enzyme changes), and non-fatal stroke (diagnosed by CT or MRI). After a first visit for collecting baseline data after randomization, enrolled patients, their dependents, or both, were asked to visit clinical centres monthly. If no primary endpoints occurred, there were 12 visits during the treatment period and a final visit (6 months after the termination of the trial drugs). When a patient had one of the primary endpoints, the case was considered completed and there was no further follow-up visit. The number of patients for each group at each stage of the trail are shown in Fig. 1. At the end of the trial period, statistical analyses were carried out on the date from 1,456 patients in the QSYQ cohort and 1,500 in the aspirin group. 22
6 EXPLORING TRADITIONAL MEDICINE Assessed for eligibility (n = 3752) ENROLMENT Excluded (n = 247) Not meeting inclusion criteria (n = 38) Declined to participate (n = 203) Other reasons (n = 6) Randomised (n = 3505) ALLOCATION QSYQ group (n = 1746) Completed followup (n = 1496) Dropped out (n = 250) Aspirin group (n = 1759) Completed followup (n = 1541) Dropped out (n = 218) FOLLOW-UP Lost to followup (n = 73) Discontinued intervention (n = 177) Adverse events (n = 37) Lack of effectiveness (n = 36) Violated protocol (n = 15) Subjects unwillingness (n = 75) Others (n = 14) Lost to followup (n = 57) Discontinued intervention (n = 161) Adverse events (n = 41) Lack of effectiveness (n = 29) Violated protocol (n = 13) Subjects unwillingness (n = 67) Others (n = 11) ANALYSIS Analysed (n = 1746) Intention to treat (n = 1746) Per-protocol (n = 1456) Analysed (n = 1759) Intention to treat (n = 1759) Per-protocol (n = 1500) Figure 1: Flow diagram of participants through each stage of the trial. 23
7 Results In the trial, QSYQ showed similar effects to aspirin for the prevention of recurrent vascular events in patient with a previous MI (Figure 2). The rate of composite endpoints (cardiovascular death, non-fatal MI, and non-fatal stroke) after 12 months of treatment was 2.98% in the QSYQ group and 2.96% in the aspirin group. The incidence of serious vascular events of this trial was lower than previous studies of secondary prevention for MI, which may be due to several factors. Figure 2: Cumulative incidence curves of the primary outcome composed of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke: (left) intention-to-treat analysis; (right) per-protocol analysis). Partnerships Among the main partners of this trial were Peking University First Hospital, the Chinese Academy of Traditional Chinese Medicine and Xiyuan Hospital, Beijing. The protocol of the trial was revised more than ten times after consulting with multidiscipline experts from clinical epidemiology, evidence-based medicine, statistics and cardiology, etc. Before patients were recruited, the protocol of this trial was evaluated by Italian and US experts. The trial was designed, executed and analysed by a steering committee, a clinical monitoring centre, an endpoints committee, a drug management centre, a data management centre, and a biostatistics centre. Investigators in each clinical centre were trained before beginning the study. 24
8 EXPLORING TRADITIONAL MEDICINE Impact After completing this trial, we established a series of criteria and methods for completing large scale clinical trial. This trial won a National Award of Science and Technology. The methods and experience of this trial have been widely used in post-marketing evaluation of traditional Chinese medicines. After rigorous clinical evaluation, sales of Chinese patent medicines have developed greatly. The trial was successful mainly because it received support from the Ministry of Science and Technology and the State Administration of Traditional Chinese Medicine of China. Replicability The experience, especially the efforts taken to ensure a robust design and analysis of the trial, will be valuable for clinical trials of other traditional medicines. Lessons Learned How to manage the process of randomisation, drug allocation, timely data collection and audit were the main obstacles for the trial. We used a Clinical Research Interactive Voice Respond System (CRIVRS) for dynamic management of this trial. Investigators connected to the CRIVRS by telephone when a patient was ready to be randomised and the CRIVRS then provided a subject number, randomisation code, and drug number to the investigator by voice, , and SMS. Because of the care taken to develop the trial s randomized control design, the results have been widely accepted by clinical practitioners and researchers worldwide. 25
9 Future Plans We intend to evaluate more Chinese patent medicines. For future clinical trials, we will design the protocols with international collaboration. Publications Hongcai Shang, Junhua Zhang, Chen Yao, Baoyan Liu, Xiumei Gao, Ming Ren, Hongbao Cao, Guohua Dai, Weiliang Weng, Sainan Zhu, HuiWang, Hongjuan Xu and Boli Zhang. Qi-Shen-Yi-Qi Dripping Pills for the Secondary Prevention of Myocardial Infarction: A Randomised Clinical Trial. Evidence-Based Complementary and Alternative Medicine Article ID , 26
Stroke secondary prevention. Gill Cluckie Stroke Nurse Consultant St. George s Hospital
 Stroke secondary prevention Gill Cluckie Stroke Nurse Consultant St. George s Hospital Stroke recurrence The risk of recurrent stroke is greatest after first stroke 2 3% of survivors of a first stroke
Stroke secondary prevention Gill Cluckie Stroke Nurse Consultant St. George s Hospital Stroke recurrence The risk of recurrent stroke is greatest after first stroke 2 3% of survivors of a first stroke
Role of Clopidogrel in Acute Coronary Syndromes. Hossam Kandil,, MD. Professor of Cardiology Cairo University
 Role of Clopidogrel in Acute Coronary Syndromes Hossam Kandil,, MD Professor of Cardiology Cairo University ACS Treatment Strategies Reperfusion/Revascularization Therapy Thrombolysis PCI (with/ without
Role of Clopidogrel in Acute Coronary Syndromes Hossam Kandil,, MD Professor of Cardiology Cairo University ACS Treatment Strategies Reperfusion/Revascularization Therapy Thrombolysis PCI (with/ without
ASPIRIN AND VASCULAR DISEASE
 ASPIRIN AND VASCULAR DISEASE SUMMARY Aspirin is an effective antiplatelet agent for patients with cardiovascular and cerebrovascular disease. Incidence of adverse effects and drug interactions increases
ASPIRIN AND VASCULAR DISEASE SUMMARY Aspirin is an effective antiplatelet agent for patients with cardiovascular and cerebrovascular disease. Incidence of adverse effects and drug interactions increases
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS
 CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
Prasugrel vs. Ticagrelor in ACS/PCI Which one to choose? V. Voudris MD FESC FACC 2 nd Cardiology Division Onassis Cardiac Surgery Center
 Prasugrel vs. Ticagrelor in ACS/PCI Which one to choose? V. Voudris MD FESC FACC 2 nd Cardiology Division Onassis Cardiac Surgery Center Hospitalizations in the U.S. Due to ACS Acute Coronary Syndromes
Prasugrel vs. Ticagrelor in ACS/PCI Which one to choose? V. Voudris MD FESC FACC 2 nd Cardiology Division Onassis Cardiac Surgery Center Hospitalizations in the U.S. Due to ACS Acute Coronary Syndromes
Bayer s rivaroxaban submitted to U.S. FDA for approval in patients with coronary and/or peripheral artery disease
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer s rivaroxaban submitted to U.S. FDA for approval in patients with coronary
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer s rivaroxaban submitted to U.S. FDA for approval in patients with coronary
Iroko Pharmaceuticals Receives FDA Approval for VIVLODEX - First Low Dose SoluMatrix Meloxicam for Osteoarthritis Pain
 Iroko Pharmaceuticals Receives FDA Approval for VIVLODEX - First Low Dose SoluMatrix Meloxicam for Osteoarthritis Pain VIVLODEX Developed to Align with FDA NSAID Recommendations Proven Efficacy at Low
Iroko Pharmaceuticals Receives FDA Approval for VIVLODEX - First Low Dose SoluMatrix Meloxicam for Osteoarthritis Pain VIVLODEX Developed to Align with FDA NSAID Recommendations Proven Efficacy at Low
Durlaza. Durlaza (aspirin) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.13 Subject: Durlaza Page: 1 of 4 Last Review Date: September 15, 2016 Durlaza Description Durlaza
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.13 Subject: Durlaza Page: 1 of 4 Last Review Date: September 15, 2016 Durlaza Description Durlaza
ISCHEMIC VASCULAR DISEASE (IVD) MEASURES GROUP OVERVIEW
 ISCHEMIC VASCULAR DISEASE (IVD) MEASURES GROUP OVERVIEW 2014 PQRS OPTIONS F MEASURES GROUPS: 2014 PQRS MEASURES IN ISCHEMIC VASCULAR DISEASE (IVD) MEASURES GROUP: #204. Ischemic Vascular Disease (IVD):
ISCHEMIC VASCULAR DISEASE (IVD) MEASURES GROUP OVERVIEW 2014 PQRS OPTIONS F MEASURES GROUPS: 2014 PQRS MEASURES IN ISCHEMIC VASCULAR DISEASE (IVD) MEASURES GROUP: #204. Ischemic Vascular Disease (IVD):
The University of Mississippi School of Pharmacy
 LONG TERM PERSISTENCE WITH ACEI/ARB THERAPY AFTER ACUTE MYOCARDIAL INFARCTION: AN ANALYSIS OF THE 2006-2007 MEDICARE 5% NATIONAL SAMPLE DATA Lokhandwala T. MS, Yang Y. PhD, Thumula V. MS, Bentley J.P.
LONG TERM PERSISTENCE WITH ACEI/ARB THERAPY AFTER ACUTE MYOCARDIAL INFARCTION: AN ANALYSIS OF THE 2006-2007 MEDICARE 5% NATIONAL SAMPLE DATA Lokhandwala T. MS, Yang Y. PhD, Thumula V. MS, Bentley J.P.
Summary of the risk management plan (RMP) for Kengrexal (cangrelor)
 EMA/78859/2015 Summary of the risk management plan (RMP) for Kengrexal (cangrelor) This is a summary of the risk management plan (RMP) for Kengrexal, which details the measures to be taken in order to
EMA/78859/2015 Summary of the risk management plan (RMP) for Kengrexal (cangrelor) This is a summary of the risk management plan (RMP) for Kengrexal, which details the measures to be taken in order to
Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults
 Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults First Lower Dose NSAID Using SoluMatrix Fine Particle Technology to be Reviewed
Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults First Lower Dose NSAID Using SoluMatrix Fine Particle Technology to be Reviewed
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Bayer s Rivaroxaban Demonstrated Superior Protection Against Recurrent Venous Thromboembolism Compared with Aspirin in EINSTEIN CHOICE Study
 News Release Not intended for U.S. and UK Media Bayer AG Communications and Public Affairs 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com New Late-Breaking Study Data Presented at ACC.17:
News Release Not intended for U.S. and UK Media Bayer AG Communications and Public Affairs 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com New Late-Breaking Study Data Presented at ACC.17:
- May Help Increase Appropriate Early Use in Acute Coronary Syndrome Patients -
 MEDIA INVESTORS Ken Dominski John Elicker Bristol-Myers Squibb Bristol-Myers Squibb 609-252-5251 212-546-3775 ken.dominski@bms.com john.elicker@bms.com Amy Ba Felix Lauscher sanofi-aventis sanofi-aventis
MEDIA INVESTORS Ken Dominski John Elicker Bristol-Myers Squibb Bristol-Myers Squibb 609-252-5251 212-546-3775 ken.dominski@bms.com john.elicker@bms.com Amy Ba Felix Lauscher sanofi-aventis sanofi-aventis
Ticagrelor compared with clopidogrel in patients with acute coronary syndromes the PLATO trial
 compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
Bayer Pharma AG Berlin Germany Tel News Release. Not intended for U.S. and UK Media
 News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the
News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the
Cardiology. Self Learning Package. Module 5: Pharmacology: Treatment of Acute Coronary. Prevention
 Cardiology Self Learning Package Module 5: Pharmacology: Treatment of Acute Coronary Syndromes, Module 5: Pharmacology: Hyperlipidaemia, Treatment of Acute Coronary Hypertension, Symdrome, Hyperlipidaemia,
Cardiology Self Learning Package Module 5: Pharmacology: Treatment of Acute Coronary Syndromes, Module 5: Pharmacology: Hyperlipidaemia, Treatment of Acute Coronary Hypertension, Symdrome, Hyperlipidaemia,
PRASUGREL HYDROCHLORIDE (Effient Eli Lilly Canada Inc.) Indication: Acute Coronary Syndrome
 CEDAC FINAL RECOMMENDATION PRASUGREL HYDROCHLORIDE (Effient Eli Lilly Canada Inc.) Indication: Acute Coronary Syndrome Recommendation: The Canadian Expert Drug Advisory Committee (CEDAC) recommends that
CEDAC FINAL RECOMMENDATION PRASUGREL HYDROCHLORIDE (Effient Eli Lilly Canada Inc.) Indication: Acute Coronary Syndrome Recommendation: The Canadian Expert Drug Advisory Committee (CEDAC) recommends that
NHS Dumfries & Galloway Aspirin Discontinuation Audit May 2011 (updated August 2015)
 Title of Project: NHS Dumfries & Galloway Aspirin Discontinuation Audit May 2011 (updated August 2015) 1 Reason for the review In the UK, low dose aspirin (75mg) is licensed for the prevention of thrombotic
Title of Project: NHS Dumfries & Galloway Aspirin Discontinuation Audit May 2011 (updated August 2015) 1 Reason for the review In the UK, low dose aspirin (75mg) is licensed for the prevention of thrombotic
Addition of clopidogrel to aspirin in patients with acute myocardial infarction: randomised placebo-controlled trial
 Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative
Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative
Blood Pressure Reduction Among Acute Stroke Patients A Randomized Controlled Clinical Trial
 Blood Pressure Reduction Among Acute Stroke Patients A Randomized Controlled Clinical Trial Jiang He, Yonghong Zhang, Tan Xu, Weijun Tong, Shaoyan Zhang, Chung-Shiuan Chen, Qi Zhao, Jing Chen for CATIS
Blood Pressure Reduction Among Acute Stroke Patients A Randomized Controlled Clinical Trial Jiang He, Yonghong Zhang, Tan Xu, Weijun Tong, Shaoyan Zhang, Chung-Shiuan Chen, Qi Zhao, Jing Chen for CATIS
Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the Secondary Prevention of Myo
 Xarelto (Rivaroxaban) Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the Secondary Prevention of Myocardial Infarction and Death in Patients with Coronary or Peripheral Artery
Xarelto (Rivaroxaban) Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the Secondary Prevention of Myocardial Infarction and Death in Patients with Coronary or Peripheral Artery
Manuel Castella MD PhD Hospital Clínic, University of
 Manuel Castella MD PhD Hospital Clínic, University of Barcelona mcaste@clinic.ub.es @mcastellamd www.escardio.org/guidelines European Heart Journal - doi:10.1093/eurheartj/ehw210 Providing integrated care
Manuel Castella MD PhD Hospital Clínic, University of Barcelona mcaste@clinic.ub.es @mcastellamd www.escardio.org/guidelines European Heart Journal - doi:10.1093/eurheartj/ehw210 Providing integrated care
Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre
 Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre Objectives To learn what s new in stroke care 2010-11 1) Acute stroke management Carotid artery stenting versus surgery for symptomatic
Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre Objectives To learn what s new in stroke care 2010-11 1) Acute stroke management Carotid artery stenting versus surgery for symptomatic
Daiichi Sankyo and Lilly Receive U.S. FDA Approval for Effient
 For Immediate Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Takashi Shoda, President and Representative Director (Code no.: 4568, First Section, Tokyo, Osaka and Nagoya Stock Exchanges)
For Immediate Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Takashi Shoda, President and Representative Director (Code no.: 4568, First Section, Tokyo, Osaka and Nagoya Stock Exchanges)
Guideline scope Hypertension in adults (update)
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Hypertension in adults (update) This guideline will update the NICE guideline on hypertension in adults (CG127). The guideline will be
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Hypertension in adults (update) This guideline will update the NICE guideline on hypertension in adults (CG127). The guideline will be
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Measure #204 (NQF 0068): Ischemic Vascular Disease (IVD): Use of Aspirin or Another Antiplatelet National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Measure #204 (NQF 0068): Ischemic Vascular Disease (IVD): Use of Aspirin or Another Antiplatelet National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
ASCEND Randomized placebo-controlled trial of aspirin 100 mg daily in 15,480 patients with diabetes and no baseline cardiovascular disease
 ASCEND Randomized placebo-controlled trial of aspirin 100 mg daily in 15,480 patients with diabetes and no baseline cardiovascular disease Jane Armitage and Louise Bowman on behalf of the ASCEND Study
ASCEND Randomized placebo-controlled trial of aspirin 100 mg daily in 15,480 patients with diabetes and no baseline cardiovascular disease Jane Armitage and Louise Bowman on behalf of the ASCEND Study
Randomized comparison of single versus double mammary coronary artery bypass grafting: 5 year outcomes of the Arterial Revascularization Trial
 Randomized comparison of single versus double mammary coronary artery bypass grafting: 5 year outcomes of the Arterial Revascularization Trial Embargoed until 10:45 a.m. CT, Monday, Nov. 14, 2016 David
Randomized comparison of single versus double mammary coronary artery bypass grafting: 5 year outcomes of the Arterial Revascularization Trial Embargoed until 10:45 a.m. CT, Monday, Nov. 14, 2016 David
Metoprolol Succinate SelokenZOC
 Metoprolol Succinate SelokenZOC Blood Pressure Control and Far Beyond Mohamed Abdel Ghany World Health Organization - Noncommunicable Diseases (NCD) Country Profiles, 2014. 1 Death Rates From Ischemic
Metoprolol Succinate SelokenZOC Blood Pressure Control and Far Beyond Mohamed Abdel Ghany World Health Organization - Noncommunicable Diseases (NCD) Country Profiles, 2014. 1 Death Rates From Ischemic
UPDATES FROM THE 2018 ANTIPLATELET GUIDELINES
 UPDATES FROM THE 2018 ANTIPLATELET GUIDELINES Claudia Bucci BScPhm, PharmD Clinical Coordinator, Cardiovascular Diseases Sunnybrook Health Sciences Centre 21st Annual Contemporary Therapeutic Issues in
UPDATES FROM THE 2018 ANTIPLATELET GUIDELINES Claudia Bucci BScPhm, PharmD Clinical Coordinator, Cardiovascular Diseases Sunnybrook Health Sciences Centre 21st Annual Contemporary Therapeutic Issues in
To provide information on the use of acetyl salicylic acid in the treatment and prevention of vascular events.
 ACETYL SALICYLIC ACID TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To provide information on the use of acetyl salicylic acid in the treatment and prevention of vascular events.
ACETYL SALICYLIC ACID TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To provide information on the use of acetyl salicylic acid in the treatment and prevention of vascular events.
Vimovo (delayed-release enteric-coated naproxen with esomeprazole)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.17.01 Subject: Vimovo Page: 1 of 5 Last Review Date: September 18, 2015 Vimovo Description Vimovo (delayed-release
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.17.01 Subject: Vimovo Page: 1 of 5 Last Review Date: September 18, 2015 Vimovo Description Vimovo (delayed-release
Evidence-Based Management of CAD: Last Decade Trials and Updated Guidelines
 Evidence-Based Management of CAD: Last Decade Trials and Updated Guidelines Enrico Ferrari, MD Cardiac Surgery Unit Cardiocentro Ticino Foundation Lugano, Switzerland Conflict of Interests No conflict
Evidence-Based Management of CAD: Last Decade Trials and Updated Guidelines Enrico Ferrari, MD Cardiac Surgery Unit Cardiocentro Ticino Foundation Lugano, Switzerland Conflict of Interests No conflict
» A new drug s trial
 » A new drug s trial A placebo-controlled, double-blind, parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause
» A new drug s trial A placebo-controlled, double-blind, parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Aspirin for the Prevention of Cardiovascular Disease
 Detail-Document #250601 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER June 2009 ~ Volume 25 ~ Number 250601 Aspirin for the Prevention of Cardiovascular
Detail-Document #250601 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER June 2009 ~ Volume 25 ~ Number 250601 Aspirin for the Prevention of Cardiovascular
Program Metrics. New Unique ID. Old Unique ID. Metric Set Metric Name Description. Old Metric Name
 Program Metrics The list below includes the metrics that will be calculated by the PINNACLE Registry for the outpatient office setting. These include metrics for, Atrial Fibrillation, Hypertension and.
Program Metrics The list below includes the metrics that will be calculated by the PINNACLE Registry for the outpatient office setting. These include metrics for, Atrial Fibrillation, Hypertension and.
North Wales Cardiac Network Guidelines on oral antiplatelet therapy in cardiovascular disease
 Guidelines on oral antiplatelet therapy in cardiovascular disease This guidance should be considered as one part of the wider therapeutic management of patients. The indication for antiplatelet therapy
Guidelines on oral antiplatelet therapy in cardiovascular disease This guidance should be considered as one part of the wider therapeutic management of patients. The indication for antiplatelet therapy
Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease. Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.
 Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.Κιλκίς Primary CVD Prevention A co-ordinated set of actions,
Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.Κιλκίς Primary CVD Prevention A co-ordinated set of actions,
LOW DOSE ASPIRIN CARDIOVASCULAR DISEASE FOR PROPHYLAXIS OF FOR BACKGROUND USE ONLY NOT TO BE USED IN DETAILING
 LOW DOSE ASPIRIN FOR PROPHYLAXIS OF CARDIOVASCULAR DISEASE FOR BACKGROUND USE ONLY NOT TO BE USED IN DETAILING Use of Low Dose Aspirin to Treat and Prevent Cardiovascular Disease In recent decades, aspirin
LOW DOSE ASPIRIN FOR PROPHYLAXIS OF CARDIOVASCULAR DISEASE FOR BACKGROUND USE ONLY NOT TO BE USED IN DETAILING Use of Low Dose Aspirin to Treat and Prevent Cardiovascular Disease In recent decades, aspirin
Results from RE-LY and RELY-ABLE
 Results from RE-LY and RELY-ABLE Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in longterm stroke prevention EXECUTIVE SUMMARY Dabigatran etexilate (Pradaxa ) has shown a consistent
Results from RE-LY and RELY-ABLE Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in longterm stroke prevention EXECUTIVE SUMMARY Dabigatran etexilate (Pradaxa ) has shown a consistent
Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost-effectiveness analysis Schleinitz M D, Weiss J P, Owens D K
 Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost-effectiveness analysis Schleinitz M D, Weiss J P, Owens D K Record Status This is a critical abstract of an economic evaluation
Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost-effectiveness analysis Schleinitz M D, Weiss J P, Owens D K Record Status This is a critical abstract of an economic evaluation
Bayer submits application for marketing approval of rivaroxaban for patients with coronary or peripheral artery disease to European Medicines Agency
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer submits application for marketing approval of rivaroxaban for patients
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer submits application for marketing approval of rivaroxaban for patients
Diagnosis and Management of Acute Myocardial Infarction
 Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
A Randomized Trial Evaluating Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 inhibition, in ACS
 A Randomized Trial Evaluating Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 inhibition, in ACS Magnus Ohman MB, on behalf of the GEMINI-ACS-1 Investigators
A Randomized Trial Evaluating Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 inhibition, in ACS Magnus Ohman MB, on behalf of the GEMINI-ACS-1 Investigators
Head-to-Head Study Showed Prasugrel Statistically Superior to Clopidogrel in Reducing Recurrent Cardiovascular Events
 Date: August 21, 2008 Refer to: Carole Copeland (OUS) Eli Lilly and Company 317-277-3661 (office) 317-610-6196 (cell) Tammy Hull (U.S.A.) Eli Lilly and Company 317-651-9116 (office) 317-614-5132 (cell)
Date: August 21, 2008 Refer to: Carole Copeland (OUS) Eli Lilly and Company 317-277-3661 (office) 317-610-6196 (cell) Tammy Hull (U.S.A.) Eli Lilly and Company 317-651-9116 (office) 317-614-5132 (cell)
Should we prescribe aspirin and statins to all subjects over 65? (Or even all over 55?) Terje R.Pedersen Oslo University Hospital Oslo, Norway
 Should we prescribe aspirin and statins to all subjects over 65? (Or even all over 55?) Terje R.Pedersen Oslo University Hospital Oslo, Norway The Polypill A strategy to reduce cardiovascular disease by
Should we prescribe aspirin and statins to all subjects over 65? (Or even all over 55?) Terje R.Pedersen Oslo University Hospital Oslo, Norway The Polypill A strategy to reduce cardiovascular disease by
5/2/2016. Outpatient Stroke Management Sheila Smith MD May 5, 2016
 Outpatient Stroke Management Sheila Smith MD May 5, 2016 1 Management of Outpatient Stroke Objectives Review blood pressure management post stroke Review antithrombotic therapy Review statin therapy Discuss
Outpatient Stroke Management Sheila Smith MD May 5, 2016 1 Management of Outpatient Stroke Objectives Review blood pressure management post stroke Review antithrombotic therapy Review statin therapy Discuss
Myocardial Infarction In Dr.Yahya Kiwan
 Myocardial Infarction In 2007 Dr.Yahya Kiwan New Definition Of Acute Myocardial Infarction The term of myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting
Myocardial Infarction In 2007 Dr.Yahya Kiwan New Definition Of Acute Myocardial Infarction The term of myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting
Review guidance for patients on long-term amiodarone treatment
 Review guidance for patients on long-term amiodarone treatment This review guidance document has been produced in response to: 1. Current supply shortages of branded and generic versions of 100mg and 200mg
Review guidance for patients on long-term amiodarone treatment This review guidance document has been produced in response to: 1. Current supply shortages of branded and generic versions of 100mg and 200mg
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Supplement materials:
 Supplement materials: Table S1: ICD-9 codes used to define prevalent comorbid conditions and incident conditions Comorbid condition ICD-9 code Hypertension 401-405 Diabetes mellitus 250.x Myocardial infarction
Supplement materials: Table S1: ICD-9 codes used to define prevalent comorbid conditions and incident conditions Comorbid condition ICD-9 code Hypertension 401-405 Diabetes mellitus 250.x Myocardial infarction
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Atrial Fibrillaiton and Heart Failure: Anticoagulation therapy in all cases?
 Atrial Fibrillaiton and Heart Failure: Anticoagulation therapy in all cases? Nicolas Lellouche Fédération de Cardiologie Hôpital Henri Mondor Créteil Disclosure Statement of Financial Interest I currently
Atrial Fibrillaiton and Heart Failure: Anticoagulation therapy in all cases? Nicolas Lellouche Fédération de Cardiologie Hôpital Henri Mondor Créteil Disclosure Statement of Financial Interest I currently
TICAGRELOR VERSUS CLOPIDOGREL AFTER THROMBOLYTIC THERAPY IN PATIENTS WITH ST- ELEVATION MYOCARDIAL INFARCTION: A RANDOMIZED CLINICAL TRIAL
 TICAGRELOR VERSUS CLOPIDOGREL AFTER THROMBOLYTIC THERAPY IN PATIENTS WITH ST- ELEVATION MYOCARDIAL INFARCTION: A RANDOMIZED CLINICAL TRIAL Otavio Berwanger, MD, PhD - On behalf of the TREAT Trial Steering
TICAGRELOR VERSUS CLOPIDOGREL AFTER THROMBOLYTIC THERAPY IN PATIENTS WITH ST- ELEVATION MYOCARDIAL INFARCTION: A RANDOMIZED CLINICAL TRIAL Otavio Berwanger, MD, PhD - On behalf of the TREAT Trial Steering
Ticagrelor compared with clopidogrel in patients with acute coronary syndromes the PLATelet Inhibition and patient Outcomes trial
 compared with clopidogrel in patients with acute coronary syndromes the PLATelet Inhibition and patient Outcomes trial Outcomes in patients with and planned PCI Ph.Gabriel Steg*, Stefan James, Robert A
compared with clopidogrel in patients with acute coronary syndromes the PLATelet Inhibition and patient Outcomes trial Outcomes in patients with and planned PCI Ph.Gabriel Steg*, Stefan James, Robert A
Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) Trial of Cardiovascular Events in High-Risk Hypertensive Patients
 1/5 This site became the new ClinicalTrials.gov on June 19th. Learn more. We will be updating this site in phases. This allows us to move faster and to deliver better services. Show less IMPORTANT: Listing
1/5 This site became the new ClinicalTrials.gov on June 19th. Learn more. We will be updating this site in phases. This allows us to move faster and to deliver better services. Show less IMPORTANT: Listing
National Horizon Scanning Centre. Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation
 Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation August 2008 This technology summary is based on information available at the time of
Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation August 2008 This technology summary is based on information available at the time of
Post Operative Troponin Leak: David Smyth Christchurch New Zealand
 Post Operative Troponin Leak: Does It Really Matter? David Smyth Christchurch New Zealand Life Was Simple Once Transmural Infarction Subendocardial Infarction But the Blood Tests Were n t Perfect Creatine
Post Operative Troponin Leak: Does It Really Matter? David Smyth Christchurch New Zealand Life Was Simple Once Transmural Infarction Subendocardial Infarction But the Blood Tests Were n t Perfect Creatine
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #44 (NQF 0236): Coronary Artery Bypass Graft (CABG): Preoperative Beta-Blocker in Patients with Isolated CABG Surgery National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR
Quality ID #44 (NQF 0236): Coronary Artery Bypass Graft (CABG): Preoperative Beta-Blocker in Patients with Isolated CABG Surgery National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR
Antihypertensive Trial Design ALLHAT
 1 U.S. Department of Health and Human Services Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic National Institutes
1 U.S. Department of Health and Human Services Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic National Institutes
Plavix duration of effect
 Plavix duration of effect The Borg System is 100 % Plavix duration of effect Jan 20, 2015. If you have had stent placement after balloon angioplasty for coronary artery disease you will be placed on medications
Plavix duration of effect The Borg System is 100 % Plavix duration of effect Jan 20, 2015. If you have had stent placement after balloon angioplasty for coronary artery disease you will be placed on medications
Mortality. p =
 Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response
National Horizon Scanning Centre. Irbesartan (Aprovel) for heart failure with preserved systolic function. August 2008
 Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
The randomized study of efficiency and safety of antithrombotic therapy in
 .. [ ] 18 150 160 mg/d 2 mg/d INR 2.0 3.0( 75 INR 1.6 2.5) 704 369 335 420 59.7% 63.3 9.9 19 2 24 2.7% 6.0% P =0.03 OR 0.44 95% CI 0.198 0.960 56% 62% 1.8% 4.6% P =0.04 OR 0.38 95% CI 0.147 0.977 52% 10.6%
.. [ ] 18 150 160 mg/d 2 mg/d INR 2.0 3.0( 75 INR 1.6 2.5) 704 369 335 420 59.7% 63.3 9.9 19 2 24 2.7% 6.0% P =0.03 OR 0.44 95% CI 0.198 0.960 56% 62% 1.8% 4.6% P =0.04 OR 0.38 95% CI 0.147 0.977 52% 10.6%
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd.
 rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Prof. Jindřich Špinar, MD
 Prof. Jindřich Špinar, MD Head of the Internal Cardiology dpt., University Hospital Brno Focuses on clinical cardiology, acute and chronic heart failure, ischemic heart gisease, hypertension Vice head
Prof. Jindřich Špinar, MD Head of the Internal Cardiology dpt., University Hospital Brno Focuses on clinical cardiology, acute and chronic heart failure, ischemic heart gisease, hypertension Vice head
Figure 1: Consort diagram; the status of participants in the study
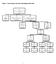 Figure : Consort diagram; the status of participants in the study Figure a. Time to first colorectal cancer in those randomized to aspirin compared with those randomized to aspirin placebo (AP). Kaplan-Meier
Figure : Consort diagram; the status of participants in the study Figure a. Time to first colorectal cancer in those randomized to aspirin compared with those randomized to aspirin placebo (AP). Kaplan-Meier
Cardiovascular Concerns in Intermediate Care
 Cardiovascular Concerns in Intermediate Care GINA ST. JEAN RN, MSN, CCRN-CSC CLINICAL NURSE EDUCATOR HEART AND & CRITICAL AND INTERMEDIATE CARE Objectives: Identify how to do a thorough assessment of the
Cardiovascular Concerns in Intermediate Care GINA ST. JEAN RN, MSN, CCRN-CSC CLINICAL NURSE EDUCATOR HEART AND & CRITICAL AND INTERMEDIATE CARE Objectives: Identify how to do a thorough assessment of the
Advances in Cardiovascular Diagnosis and Therapy. No disclosure or conflicts. Outline
 Advances in Cardiovascular Diagnosis and Therapy Firas Zahr, MD Assistant Professor of Medicine Interventional Cardiology University Of Iowa No disclosure or conflicts Outline What is new with revascularization?
Advances in Cardiovascular Diagnosis and Therapy Firas Zahr, MD Assistant Professor of Medicine Interventional Cardiology University Of Iowa No disclosure or conflicts Outline What is new with revascularization?
6/1/18 LEARNING OBJECTIVES PATIENT POPULATION PRESENTATIONS
 PREVENTING HOSPITAL READMISSIONS IN CARDIOVASCULAR PATIENTS Christina Cortez Perry, MSN, FNP-C, CCCC Cardiology Coordinator- Corpus Christi Medical Center 1 2 LEARNING OBJECTIVES Identify the target patient
PREVENTING HOSPITAL READMISSIONS IN CARDIOVASCULAR PATIENTS Christina Cortez Perry, MSN, FNP-C, CCCC Cardiology Coordinator- Corpus Christi Medical Center 1 2 LEARNING OBJECTIVES Identify the target patient
Setting The setting was secondary care. The economic study was carried out in the UK.
 A cost-utility analysis of clopidogrel in patients with non-st-segment-elevation acute coronary syndromes in the UK Karnon J, Bakhai A, Brennan A, Pandor A, Flather M, Warren E, Gray D, Akehurst R Record
A cost-utility analysis of clopidogrel in patients with non-st-segment-elevation acute coronary syndromes in the UK Karnon J, Bakhai A, Brennan A, Pandor A, Flather M, Warren E, Gray D, Akehurst R Record
2 Summary of NICE TA 249: Atrial fibrillation - Dabigatran Etexilate
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Clinical Trial Synopsis TL-OPI-516, NCT#
 Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
FastTest. You ve read the book now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Quality Payment Program: Cardiology Specialty Measure Set
 Measure Title * Reportable via PINNACLE α Reportable via Diabetes Collaborative CQMC v1.0 Measure High Priority Measure Cross Cutting Measure Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor
Measure Title * Reportable via PINNACLE α Reportable via Diabetes Collaborative CQMC v1.0 Measure High Priority Measure Cross Cutting Measure Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor
HYPERTENSION: UPDATE 2018
 HYPERTENSION: UPDATE 2018 From the Cardiologist point of view Richard C Padgett, MD I have no disclosures HYPERTENSION ALWAYS THE ELEPHANT IN THE EXAM ROOM BUT SOMETIMES IT CHARGES HTN IN US ~78 million
HYPERTENSION: UPDATE 2018 From the Cardiologist point of view Richard C Padgett, MD I have no disclosures HYPERTENSION ALWAYS THE ELEPHANT IN THE EXAM ROOM BUT SOMETIMES IT CHARGES HTN IN US ~78 million
Summary Protocol ISRCTN / NCT REVIVED-BCIS2 Summary protocol version 4, May 2015 Page 1 of 6
 Summary Protocol REVIVED-BCIS2 Summary protocol version 4, May 2015 Page 1 of 6 Background: Epidemiology In 2002, it was estimated that approximately 900,000 individuals in the United Kingdom had a diagnosis
Summary Protocol REVIVED-BCIS2 Summary protocol version 4, May 2015 Page 1 of 6 Background: Epidemiology In 2002, it was estimated that approximately 900,000 individuals in the United Kingdom had a diagnosis
ASCEND A randomized trial of omega-3 fatty acids (fish oil) versus placebo for primary cardiovascular prevention in 15,480 patients with diabetes
 ASCEND A randomized trial of omega-3 fatty acids (fish oil) versus placebo for primary cardiovascular prevention in 15,480 patients with diabetes Jane Armitage and Louise Bowman on behalf of the ASCEND
ASCEND A randomized trial of omega-3 fatty acids (fish oil) versus placebo for primary cardiovascular prevention in 15,480 patients with diabetes Jane Armitage and Louise Bowman on behalf of the ASCEND
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST Is there a mortality risk associated with aspirin use in heart failure? Results from a large community based cohort Margaret Bermingham, Mary-Kate Shanahan, Saki Miwa,
DECLARATION OF CONFLICT OF INTEREST Is there a mortality risk associated with aspirin use in heart failure? Results from a large community based cohort Margaret Bermingham, Mary-Kate Shanahan, Saki Miwa,
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Belinda Green, Cardiologist, SDHB, 2016
 Acute Coronary syndromes All STEMI ALL Non STEMI Unstable angina Belinda Green, Cardiologist, SDHB, 2016 Thrombus in proximal LAD Underlying pathophysiology Be very afraid for your patient Wellens
Acute Coronary syndromes All STEMI ALL Non STEMI Unstable angina Belinda Green, Cardiologist, SDHB, 2016 Thrombus in proximal LAD Underlying pathophysiology Be very afraid for your patient Wellens
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Briefing paper QOF indicator area: Peripheral arterial disease Potential output:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Briefing paper QOF indicator area: Peripheral arterial disease Potential output:
ST Elevated Myocardial Infarction- Latest AHA recommendations
 ST Elevated Myocardial Infarction- Latest AHA recommendations Sherry Turner, DO, MPH, FACOEP Medical Director Emergency Services Wesley Medical Center The Problem 250,000 Americans each year 30% fail to
ST Elevated Myocardial Infarction- Latest AHA recommendations Sherry Turner, DO, MPH, FACOEP Medical Director Emergency Services Wesley Medical Center The Problem 250,000 Americans each year 30% fail to
325 mg aspirin and plavix
 325 mg aspirin and plavix 12-3-2006 Background Dual antiplatelet therapy with clopidogrel plus low-dose aspirin has not been studied in a broad population of patients at high risk for. Compare Aspirin
325 mg aspirin and plavix 12-3-2006 Background Dual antiplatelet therapy with clopidogrel plus low-dose aspirin has not been studied in a broad population of patients at high risk for. Compare Aspirin
CLOSE. Closure of Patent Foramen Ovale, Oral anticoagulants or Antiplatelet Therapy to Prevent Stroke Recurrence
 CLOSE Closure of Patent Foramen Ovale, Oral anticoagulants or Antiplatelet Therapy to Prevent Stroke Recurrence Guillaume TURC, MD, PhD Paris Descartes University Sainte-Anne hospital Paris, France On
CLOSE Closure of Patent Foramen Ovale, Oral anticoagulants or Antiplatelet Therapy to Prevent Stroke Recurrence Guillaume TURC, MD, PhD Paris Descartes University Sainte-Anne hospital Paris, France On
DR ALEXIA STAVRATI CARDIOLOGIST, DIRECTOR OF CARDIOLOGY DEPT, "G. PAPANIKOLAOU" GH, THESSALONIKI
 The Impact of AF on Natural History of CAD DR ALEXIA STAVRATI CARDIOLOGIST, DIRECTOR OF CARDIOLOGY DEPT, "G. PAPANIKOLAOU" GH, THESSALONIKI CAD MOST COMMON CARDIOVASCULAR DISEASE MOST COMMON CAUSE OF DEATH
The Impact of AF on Natural History of CAD DR ALEXIA STAVRATI CARDIOLOGIST, DIRECTOR OF CARDIOLOGY DEPT, "G. PAPANIKOLAOU" GH, THESSALONIKI CAD MOST COMMON CARDIOVASCULAR DISEASE MOST COMMON CAUSE OF DEATH
Aspirin to Prevent Heart Attack and Stroke: What s the Right Dose?
 The American Journal of Medicine (2006) 119, 198-202 REVIEW Aspirin to Prevent Heart Attack and Stroke: What s the Right Dose? James E. Dalen, MD, MPH Professor Emeritus, University of Arizona, Tucson
The American Journal of Medicine (2006) 119, 198-202 REVIEW Aspirin to Prevent Heart Attack and Stroke: What s the Right Dose? James E. Dalen, MD, MPH Professor Emeritus, University of Arizona, Tucson
Antiplatelet and Anti-Thrombotic Therapy. Ivan Anderson, MD RIHVH Cardiology
 Antiplatelet and Anti-Thrombotic Therapy Ivan Anderson, MD RIHVH Cardiology Outline Anti-thrombotic therapy Risk stratification of stroke with atrial fibrillation DVT and PE treatment Pharmacology Anti-platelet
Antiplatelet and Anti-Thrombotic Therapy Ivan Anderson, MD RIHVH Cardiology Outline Anti-thrombotic therapy Risk stratification of stroke with atrial fibrillation DVT and PE treatment Pharmacology Anti-platelet
Is There A LIfe for DES after discontinuation of Clopidogrel
 EMBARGOED UNTIL 3:45pm CT, Sunday, 11/16/14 - FOR MEDIA BACKGROUND ONLY - Do Not Publish Chicago 2014 Is There A LIfe for DES after discontinuation of Clopidogrel Six-month versus 24-month dual antiplatelet
EMBARGOED UNTIL 3:45pm CT, Sunday, 11/16/14 - FOR MEDIA BACKGROUND ONLY - Do Not Publish Chicago 2014 Is There A LIfe for DES after discontinuation of Clopidogrel Six-month versus 24-month dual antiplatelet
Dr Kerry Gunn. Dr Nicola Broadbent. Anaesthesiologist Auckland City Hospital Auckland. Specialist Anaesthetist Auckland City Hospital Auckland
 Dr Kerry Gunn Anaesthesiologist Auckland City Hospital Auckland Dr Nicola Broadbent Specialist Anaesthetist Auckland City Hospital Auckland 8:30-9:25 WS #96: Optimising Patients for Surgery - Defining
Dr Kerry Gunn Anaesthesiologist Auckland City Hospital Auckland Dr Nicola Broadbent Specialist Anaesthetist Auckland City Hospital Auckland 8:30-9:25 WS #96: Optimising Patients for Surgery - Defining
Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care AF symptoms Tachycardia
 Supplementary Table S1 International Classification of Disease 10 (ICD-10) codes Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care I48 AF
Supplementary Table S1 International Classification of Disease 10 (ICD-10) codes Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care I48 AF
Trends and Variations in General Medical Services Indicators for Coronary Heart Disease: Analysis of QRESEARCH Data
 Trends and Variations in General Medical Services Indicators for Coronary Heart Disease: Analysis of QRESEARCH Data Authors: Professor Julia Hippisley-Cox Professor Mike Pringle Professor of Clinical Epidemiology
Trends and Variations in General Medical Services Indicators for Coronary Heart Disease: Analysis of QRESEARCH Data Authors: Professor Julia Hippisley-Cox Professor Mike Pringle Professor of Clinical Epidemiology
Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation
 Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation Roberto Marchioli, MD, on behalf of the OPERA Investigators American Heart Association, Los Angeles November 5, 2012 Published online
Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation Roberto Marchioli, MD, on behalf of the OPERA Investigators American Heart Association, Los Angeles November 5, 2012 Published online
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
15.0 CONTROL OF STUDY DRUG
 15.0 CONTROL OF STUDY DRUG In compliance with 21 CFR 312.60, investigators in the POINT Trial are responsible for: ensuring that the investigation is conducted according to the signed statement, the investigational
15.0 CONTROL OF STUDY DRUG In compliance with 21 CFR 312.60, investigators in the POINT Trial are responsible for: ensuring that the investigation is conducted according to the signed statement, the investigational
