CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS
|
|
|
- Mervin Bridges
- 5 years ago
- Views:
Transcription
1 CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis Directive 75/318/EEC as amended Date of first adoption First adopted February 1987 This version adopted November 1996 Date of entry into May 1997 force Status Last revised 1996 Previous titles/other Anti-anginal drugs (1987) / CPMP/EWP/234/95 references Additional Notes This note for guidance is intended to provide guidance on clinical investigations to determine the value o f medicinal products in preventing attacks of angina pectoris and to increase symptom limited exercise capacity, irrespective of the nature, mode of action, or route of administration of these products. It should be read in conjunction with Part 4 of the Annex of Directive 75/318/EEC, as amended, and other guidelines for conducting clinical trials. This version replaces the previous 1987 guideline: Anti-anginal drugs. CONTENTS 1. INTRODUCTION 2. CRITERIA OF EFFICACY 3. METHODS TO ASSESS EFFICACY 4. SELECTION OF PATIENTS 5. STRATEGY - DESIGN 6. SAFETY ASPECTS 289
2 CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS INTRODUCTION This note is intended to provide guidance on clinical investigations to determine the value of medicinal products in preventing attacks of angina pectoris and to increase symptom limited exercise capacity, irrespective of the nature, mode of action, or route of administration of these products. It is intended for the investigation of medicinal products to be used in patients with stable angina pectoris. This comprises angina induced by exercise or by other stimuli, but not unstable angina pectoris. This note for guidance should be read in conjunction with Part 4 of the Annex to Directive 75/318/EEC as amended, and guidelines for conducting clinical trials, especially those on: Pharmacokinetic Studies in Man Clinical Investigation of Medicinal Products for Long-Term Use Dose Response Information to Support Product Authorisation Biostatistical Methodology in Clinical Trials The Extent of Population Exposure to Assess Clinical Safety for Medicines Intended for Long-Term Treatment of Non-Life-Threatening Conditions The clinical profile of such a medicinal product needs to be studied in an acute stress testing setting, i.e. with provocation of anginal attacks due to cardiac ischaemia, which is assumed to represent the conditions of normal practice. Valid data are only likely to be obtained i f sufficient account is taken of such factors as the pronounced placebo effect in angina pectoris, the substantial variation in the nature and severity of symptoms, and the subjective character of chest pain. 2. CRITERIA OF EFFICACY As the assessment of the effect of antianginal medicinal products based on clinical measurements alone is as yet considered too unreliable because of the possible influence of uncontrolled variables, it has become accepted that measurements of exercise capacity using standardised exercise testing should be, in spite of an intrinsic amount of variability, the major criteria of efficacy. In addition to its objective character, it is assumed that improved exercise capacity may be a surrogate for the patient benefit in terms of better quality of life. Moreover, exercise testing provides evidence that the relief of angina and increased exercise capacity are mediated by an anti-ischaemic effect. In addition, clinical evidence of symptomatic improvement should also be provided in the major therapeutic trials. 291
3 3CC20a 2.1 Exercise based variables Exercise capacity Exercise testing provides a wide array of variables, all of which may contribute to the assessment of the antianginal effect. The assessment of total exercise capacity at trough which is defined as the residual effect at the end of the dose interval, is the major efficacy criterion for antianginal medicinal products. Total exercise capacity (total exercise time or total workload performed) is probably the most meaningful measurement that can be obtained from exercise testing, and thus it should be considered, by itself, as a primary endpoint in therapeutic clinical trials. Total exercise capacity can be defined as the maximal duration or workload of exercise which can be performed by the patient in the setting of a standardised exercise test. The reasons for interrupting exercise should be related to symptoms (e.g. Borg score 3) and signs of myocardial ischaemia. With adequate study conditions (low intra-individual variability of the initial results, adequate patient selection), the variability of consecutive measurements is relatively low, thus ensuring acceptable reproducibility. However, other exercise variables should be used for an adequate characterisation of the effect on antianginal effect in addition to total exercise capacity. A consistent result in these exercise variables should be demonstrated before accepting an effect on exercise capacity Time to the onset of angina Time to the onset of angina has its limitation derived from the subjective nature of pain. Furthermore a significant amount of treated patients might have no limiting angina after treatment or might be limited in their exercise capacity by reasons other than angina Time to ST segment depression Time to 1 mm ST segment depression in the left precordial leads is a more objective variable and it is indicative of an anti-ischaemic effect which is an important fact i n antianginal medicinal product assessment. Nevertheless, at present, its extrapolation i n terms of clinical benefit for the patient is unknown. The above three variables can be considered to be relevant to therapeutic effect, and all of them should be measured in therapeutic trials. They must show consistency or at least no major contradiction between them in order to conclude efficacy of a new medicinal product Other exercise variables Additional exercise variables can also be measured. These include the rate-pressure product, the magnitude of ST depression, gasometric measurements, and radionucleographic evidence of myocardial hypoperfusion or ventricular dysfunction induced by ischaemia. 2.2 Anginal pain / consumption of short acting nitrates Frequency, intensity and duration of anginal pain as well as the concomitant use of short acting nitrates should be documented. Whereas this variable is not considered to be a primary endpoint, it is highly relevant as a secondary endpoint in both short-term and longterm studies. 292
4 2.3 Quality of life Quality of life measurement can provide valuable information about the effect of therapy on the general health status. 2.4 Morbidity and mortality At present, there is no requirement to prove beneficial effect on these variables in order to obtain a marketing authorisation, although any effects on cardiovascular and total morbidity and mortality should be evaluated. This should be done in particular if there is a reasoned suspicion that a new medicinal product might have detrimental effects on these parameters (See also section on safety). 2.5 Ischaemic episodes Ambulatory recording of ischaemic episodes may give supportive evidence for the efficacy of the medicinal product tested. 3. METHODS TO ASSESS EFFICACY 3.1 Exercise testing Bicycle or treadmill exercise tests should be employed to induce angina. The exercise protocols should be validated and follow the recommendations of international organisations of cardiology. The characterisation of ST segment abnormalities suggesting ischaemia should be based on internationally accepted criteria. At least two standardised exercise tests should be performed at the start of the study. The difference between the tests should not exceed 20%. If patients are included with a larger difference a rationale for this should be given. Other stress tests should be limited to phase II studies. Repeated measurements over time, e.g. every 2 to 4 weeks, should be performed. The physiological testing of the patients must be performed under medical supervision i n facilities equipped to treat any cardiac complication. 3.2 Anginal pain The patient s experience of anginal pain should be recorded in a patient diary as well as the concomitant use of short-acting nitrates. The daily frequency of anginal pain should whenever possible be registered by patients using available log books. 3.3 Quality of life A quality of life assessment may be considered, provided the questionnaire is validated i n the context of the proposed target group. 293
5 3CC20a 3.4 Morbidity and mortality Cardiovascular morbidity and mortality can be measured in specially designed studies, but should always be evaluated and assessed using the pooled data of the (controlled) trials. 3.5 Ischaemic episodes Appropriate information on the number and duration of ischaemic episodes (either symptomatic or silent) can be measured by standardised ambulatory ECG recording (Holter monitoring) over 48 hours. The tapes should be evaluated using acknowledged equipment. 4. SELECTION OF PATIENTS The patients included in the studies must suffer from stable angina pectoris on the basis of coronary heart disease, preferably documented by a history of proven myocardial infarction, previous coronary revascularisation or coronary angiography. With regard to dose-finding studies the documentation of unequivocal coronary heart disease is mandatory. Stable angina is defined as anginal exercise pain of more than 3 months duration in the absence of angina at rest or accelerated chest pain pattern. Patients with a recent (< 3 months) cardiac event such as myocardial infarction, CABG surgery or percutaneous angioplasty should not be included in the studies. The patients to be included in the study must be capable of exercise testing according to standardised protocols. The stability and reproducibility of the patient's symptoms and exercise performance are essential to the studies. The symptoms of angina pectoris and the nitrate consumption must have been stable at least during the 2 weeks preceding the study. Any concomitant medication shall have been unchanged during this period of time. At least in some studies efforts should be made to enrol a study population without concomitant antianginal medicinal product therapy where the investigational product should be given as monotherapy. Some of the trials should also include patients where the investigational product is given as add on therapy and compared to an acceptable active control and/or placebo. Rescue medication with short acting nitrate should always be allowed throughout the whole trial period. 5. STRATEGY DESIGN 5.1 Initial studies Pharmacodynamics These studies should define the pharmacodynamic properties of the active ingredient and of any active metabolites. The studies should include, when appropriate, data on: Haemodynamic effects at rest and during exercise; Myocardial oxygen consumption; Coronary blood flow/diameter of normal and stenosed coronary arteries; Effects on heart rate, rhythm, conduction times and, if necessary, refractory period; 294
6 Effects on renal function and electrolytes; Effects on the pulmonary function; Effects on the metabolism, particularly of glucose and lipids; Neurohormonal effects; Platelet aggregation and other rheological effects; Vital and laboratory parameters; Adverse events; Tolerability Pharmacokinetics The kinetics of any active metabolites should also be studied if they contribute to the effects of the medicinal product. The studies should also provide data in potential at risk patient groups (e.g. the elderly, patients with heart failure, patients with renal or hepatic dysfunction and in slow metabolisers, where applicable) Interactions Pharmacokinetic and pharmacodynamic interactions should be investigated primarily with other frequently coadministered medicinal products in the target population, e.g. other antianginal products, antihypertensive agents, medicinal products to treat heart failure, anticoagulant products. Interactions with other substrates of the metabolising isozymes should also be investigated. 5.2 Therapeutic studies Withdrawal of concomitant antianginal medication prior to randomisation would allow a methodologically proper evaluation of the new antianginal medicinal product as monotherapy. A run-in period with placebo lasting at least 2 weeks should be considered in order to ensure the stability of the disease. Short acting nitrates are allowable for interruption of angina attacks. Efficacy throughout the whole dosing interval should be proven Dose Response studies Dose Response studies should be randomised, placebo-controlled and double-blinded using at least three dosages to establish the clinically useful dose range as well as the optimal dose. These studies should preferably be designed as parallel group studies and should last at least six weeks. The results of the dose response studies of a new antianginal medicinal product should provide robust evidence of its efficacy as compared to placebo, including precise quantitative estimates of its beneficial effects. 295
7 3CC20a Main therapeutic studies The objectives of the main therapeutic studies should be to confirm the efficacy and safety of the product. These studies should be performed in a randomised, double-blind, controlled, parallel group design. Active controlled studies, of at least 12 weeks duration, should demonstrate comparable efficacy and safety to an appropriate standard therapy. These studies may last even longer in order to allow a comparison with respect to adverse drug reactions as well. As stated in section 4, some of the trials should also include patients where the investigational product should be given as add-on treatment and compared to an acceptable active control and /or placebo. 6. SAFETY ASPECTS Results of pharmacoepidemiological studies suggest that the influence of different antianginal medicinal products classes on (cardiovascular) morbidity and mortality may not be alike even though the antianginal effect is comparable. Effects on cardiovascular and total morbidity and mortality should be evaluated on the basis of phase II/III trials. Long-term data on adverse events must be presented as the medicinal product may be administered for long periods of time. Thus at least 300 patients should be treated for a minimum of 6 months or 100 patients for one year, preferably in controlled trials. All adverse events occurring during the clinical trials as well as morbidity and mortality data must be fully described, assessed and discussed in the context of current knowledge about antianginal medicinal product therapy. Special attention should also be paid to serious adverse events and reasons for treatment withdrawals. The safety aspects should usually address the following points: Specific effects such as pro-anginal and pro-arrhythmic effects as well as other cardiovascular effects, withdrawal phenomena and any effects related to rebound phenomena or general effects influencing the function of other organ systems, e.g. through alterations of regional blood flow. Both the time of occurrence and the frequency, severity, relevance and outcome of any adverse events must be documented and discussed. Special attention should be paid to high risk groups (e.g. heart failure, renal and hepatic failure, etc.) and to possible interactions with other medicinal products. 296
MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS
 MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS Guideline Title Medicinal Products for the Treatment of Arrhythmias Legislative basis Directive 75/318/EEC as amended Date of first adoption November
MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS Guideline Title Medicinal Products for the Treatment of Arrhythmias Legislative basis Directive 75/318/EEC as amended Date of first adoption November
MEDICINAL PRODUCTS IN THE TREATMENT OF CARDIAC FAILURE
 MEDICINAL PRODUCTS IN THE TREATMENT OF CARDIAC FAILURE Guideline Title Medicinal Products in the Treatment of Cardiac Failure Legislative basis Directive 75/318/EEC as amended Date of first adoption November
MEDICINAL PRODUCTS IN THE TREATMENT OF CARDIAC FAILURE Guideline Title Medicinal Products in the Treatment of Cardiac Failure Legislative basis Directive 75/318/EEC as amended Date of first adoption November
CLINICAL INVESTIGATION OF MEDICAL PRODUCTS IN THE TREATMENT OF GENERALISED ANXIETY DISORDER, PANIC DISORDER AND OBSESSIVE- COMPULSIVE DISORDER
 CLINICAL INVESTIGATION OF MEDICAL PRODUCTS IN THE TREATMENT OF GENERALISED ANXIETY DISORDER, PANIC DISORDER AND OBSESSIVE- COMPULSIVE DISORDER Guideline Title Clinical Investigation of Medical Products
CLINICAL INVESTIGATION OF MEDICAL PRODUCTS IN THE TREATMENT OF GENERALISED ANXIETY DISORDER, PANIC DISORDER AND OBSESSIVE- COMPULSIVE DISORDER Guideline Title Clinical Investigation of Medical Products
CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN GERIATRICS *)
 3CC9a CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN GERIATRICS *) Guideline Title Clinical Investigation of Medicinal Products in Geriatrics*) Legislative basis Directive 75/318/EEC, as amended Date
3CC9a CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN GERIATRICS *) Guideline Title Clinical Investigation of Medicinal Products in Geriatrics*) Legislative basis Directive 75/318/EEC, as amended Date
TRANSPARENCY COMMITTEE OPINION. 4 November 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
Reflection paper on assessment of cardiovascular safety profile of medicinal products
 25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 2004 CPMP/EWP/3020/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 2004 CPMP/EWP/3020/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN THE TREATMENT OF HYPERTENSION
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 19 November 1998 CPMP/EWP/238/95 Rev. 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 19 November 1998 CPMP/EWP/238/95 Rev. 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR
CLINICAL INVESTIGATION OF ORAL CONTRACEPTIVES
 CLINICAL INVESTIGATION OF ORAL CONTRACEPTIVES Guideline Title Clinical Investigation of Oral Contraceptives Legislative basis Directive 75/318/EEC as amended Date of first adoption February 1987 Date of
CLINICAL INVESTIGATION OF ORAL CONTRACEPTIVES Guideline Title Clinical Investigation of Oral Contraceptives Legislative basis Directive 75/318/EEC as amended Date of first adoption February 1987 Date of
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice)
 Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Regulatory Experience in Reviewing CV Safety for Diabetes
 Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 15 November 2007 Doc. Ref. EMEA/CHMP/EWP/517497/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED
European Medicines Agency London, 15 November 2007 Doc. Ref. EMEA/CHMP/EWP/517497/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED
Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular and metabolic diseases Draft
 1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
SCS in angina pectoris
 SCS in angina pectoris STOCKHOLM 100829 Mats Borjesson, FESC MD, PhD, assoc prof Goteborg, Sweden Paincenter, Dept of Medicine Sahlgrenska University Hospital/Östra, Göteborg, Sweden Refractory Angina
SCS in angina pectoris STOCKHOLM 100829 Mats Borjesson, FESC MD, PhD, assoc prof Goteborg, Sweden Paincenter, Dept of Medicine Sahlgrenska University Hospital/Östra, Göteborg, Sweden Refractory Angina
Migraine: Developing Drugs for Acute Treatment Guidance for Industry
 Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
SYNOPSIS. International co-ordinating investigator Redacted
 Drug product: Drug substance(s): AZD6140 Document No.: D5130C00002 Edition No.: 01 Study code: D5130C00002 Date: 12 October 2005 SYNOPSIS A Double-blind, Double-dummy, Parallel Group Randomised Dose Confirmation
Drug product: Drug substance(s): AZD6140 Document No.: D5130C00002 Edition No.: 01 Study code: D5130C00002 Date: 12 October 2005 SYNOPSIS A Double-blind, Double-dummy, Parallel Group Randomised Dose Confirmation
PROCORALAN MAKING A STRONG ENTRY TO THE NEW ESC GUIDELINES FOR THE MANAGEMENT OF HEART FAILURE
 Press Release Issued on behalf of Servier Date: June 6, 2012 PROCORALAN MAKING A STRONG ENTRY TO THE NEW ESC GUIDELINES FOR THE MANAGEMENT OF HEART FAILURE The new ESC guidelines for the diagnosis and
Press Release Issued on behalf of Servier Date: June 6, 2012 PROCORALAN MAKING A STRONG ENTRY TO THE NEW ESC GUIDELINES FOR THE MANAGEMENT OF HEART FAILURE The new ESC guidelines for the diagnosis and
Month/Year of Review: November 2014 Date of Last Review: June 2012 PDL Classes: Anti-anginals, Cardiovascular
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Copyright 2012 Oregon State University. All Rights
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Copyright 2012 Oregon State University. All Rights
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *)
 DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine)
 EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
Dronedarone for the treatment of non-permanent atrial fibrillation
 Dronedarone for the treatment of non-permanent atrial Issued: August 2010 last modified: December 2012 guidance.nice.org.uk/ta197 NICE has accredited the process used by the Centre for Health Technology
Dronedarone for the treatment of non-permanent atrial Issued: August 2010 last modified: December 2012 guidance.nice.org.uk/ta197 NICE has accredited the process used by the Centre for Health Technology
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 17 February 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON NON-CLINICAL AND CLINICAL DEVELOPMENT OF
European Medicines Agency Evaluation of Medicines for Human Use London, 17 February 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON NON-CLINICAL AND CLINICAL DEVELOPMENT OF
Effects of felodipine on haemodynamics and exercise capacity in patients with angina pectoris
 Br. J. clin. Pharmac. (1987), 23, 391-396 Effects of felodipine on haemodynamics and exercise capacity in patients with angina pectoris J. V. SHERIDAN, P. THOMAS, P. A. ROUTLEDGE & D. J. SHERIDAN Departments
Br. J. clin. Pharmac. (1987), 23, 391-396 Effects of felodipine on haemodynamics and exercise capacity in patients with angina pectoris J. V. SHERIDAN, P. THOMAS, P. A. ROUTLEDGE & D. J. SHERIDAN Departments
Medicine Dr. Omed Lecture 2 Stable and Unstable Angina
 Medicine Dr. Omed Lecture 2 Stable and Unstable Angina Risk stratification in stable angina. High Risk; *post infarct angina, *poor effort tolerance, *ischemia at low workload, *left main or three vessel
Medicine Dr. Omed Lecture 2 Stable and Unstable Angina Risk stratification in stable angina. High Risk; *post infarct angina, *poor effort tolerance, *ischemia at low workload, *left main or three vessel
Role of Clopidogrel in Acute Coronary Syndromes. Hossam Kandil,, MD. Professor of Cardiology Cairo University
 Role of Clopidogrel in Acute Coronary Syndromes Hossam Kandil,, MD Professor of Cardiology Cairo University ACS Treatment Strategies Reperfusion/Revascularization Therapy Thrombolysis PCI (with/ without
Role of Clopidogrel in Acute Coronary Syndromes Hossam Kandil,, MD Professor of Cardiology Cairo University ACS Treatment Strategies Reperfusion/Revascularization Therapy Thrombolysis PCI (with/ without
Guideline on clinical investigation of medicinal products in the treatment of lipid disorders
 19 December 2013 Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical investigation of medicinal products in the treatment of lipid disorders Adopted by CHMP July 2004 Date for coming
19 December 2013 Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical investigation of medicinal products in the treatment of lipid disorders Adopted by CHMP July 2004 Date for coming
Clinical Trial Synopsis TL-OPI-516, NCT#
 Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Ischaemic heart disease. IInd Chair and Clinic of Cardiology
 Ischaemic heart disease IInd Chair and Clinic of Cardiology Definition Syndrome due to chronic insufficient oxygen supply to myocardial cells Nomenclature: ischaemic heart disease (IHD), coronary artery
Ischaemic heart disease IInd Chair and Clinic of Cardiology Definition Syndrome due to chronic insufficient oxygen supply to myocardial cells Nomenclature: ischaemic heart disease (IHD), coronary artery
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 04 CPMP/EWP/2986/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 04 CPMP/EWP/2986/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
The Canadian Cardiovascular Society Functional Classification of Angina
 Applications of EECP Studies have shown EECP is a means of improving perfusion (blood supply) to other organs of the body than just the heart. Studies also support a positive clinical benefit for: Erectile
Applications of EECP Studies have shown EECP is a means of improving perfusion (blood supply) to other organs of the body than just the heart. Studies also support a positive clinical benefit for: Erectile
RANOLAZINE AND IVABRADINE - THEIR CURRENT USE
 3 : 35 RANOLAZINE AND IVABRADINE - THEIR CURRENT USE Abstract Chronic stable angina is a debilitating illness affecting millions worldwide. Considerable progress has been made over the last 30 years in
3 : 35 RANOLAZINE AND IVABRADINE - THEIR CURRENT USE Abstract Chronic stable angina is a debilitating illness affecting millions worldwide. Considerable progress has been made over the last 30 years in
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 26 June 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON THE CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 26 June 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON THE CLINICAL
Zachary I. Hodes, M.D., Ph.D., F.A.C.C.
 Zachary I. Hodes, M.D., Ph.D., F.A.C.C. Disclamer: I personally have no financial relationship with any company mentioned today. The Care Group, LLC does have a contract with Cardium to participate in
Zachary I. Hodes, M.D., Ph.D., F.A.C.C. Disclamer: I personally have no financial relationship with any company mentioned today. The Care Group, LLC does have a contract with Cardium to participate in
Annex II Scientific conclusions and grounds for variation to the terms of the Marketing Authorisations
 Annex II Scientific conclusions and grounds for variation to the terms of the Marketing Authorisations 25/41 Scientific conclusions Overall summary of the scientific evaluation of trimetazidine containing
Annex II Scientific conclusions and grounds for variation to the terms of the Marketing Authorisations 25/41 Scientific conclusions Overall summary of the scientific evaluation of trimetazidine containing
Setting The setting was a hospital. The economic study was carried out in Australia.
 Coronary artery bypass grafting (CABG) after initially successful percutaneous transluminal coronary angioplasty (PTCA): a review of 17 years experience Barakate M S, Hemli J M, Hughes C F, Bannon P G,
Coronary artery bypass grafting (CABG) after initially successful percutaneous transluminal coronary angioplasty (PTCA): a review of 17 years experience Barakate M S, Hemli J M, Hughes C F, Bannon P G,
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use plondon, 20 February 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) APPENDIX TO THE NOTE FOR
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use plondon, 20 February 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) APPENDIX TO THE NOTE FOR
Going to high altitude with heart disease. Prof. Dr. med. Stefano Rimoldi High Altitude Medicine Inselspital Bern
 Going to high altitude with heart disease Prof. Dr. med. Stefano Rimoldi High Altitude Medicine Inselspital Bern stefano.rimoldi@insel.ch There are very few studies on patients with heart disease going
Going to high altitude with heart disease Prof. Dr. med. Stefano Rimoldi High Altitude Medicine Inselspital Bern stefano.rimoldi@insel.ch There are very few studies on patients with heart disease going
Clinical Policy: Holter Monitors Reference Number: CP.MP.113
 Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
Emerging Drug List RANOLAZINE
 Generic (Trade Name): Manufacturer: Indication: Ranolazine (Ranexa TM ) CV Therapeutics, Inc. For the treatment of stable angina. 1 NO. 58 MAY 2004 Current Regulatory Status: Description: The US Food and
Generic (Trade Name): Manufacturer: Indication: Ranolazine (Ranexa TM ) CV Therapeutics, Inc. For the treatment of stable angina. 1 NO. 58 MAY 2004 Current Regulatory Status: Description: The US Food and
Clinical guideline Published: 23 July 2011 nice.org.uk/guidance/cg126
 Stable angina: management Clinical guideline Published: 23 July 2011 nice.org.uk/guidance/cg126 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Stable angina: management Clinical guideline Published: 23 July 2011 nice.org.uk/guidance/cg126 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Long-term outcome after normal myocardial perfusion imaging in suspected ischaemic heart disease
 Dan Med J 65/2 February 2018 DANISH MEDICAL JOURNAL 1 Long-term outcome after normal myocardial perfusion imaging in suspected ischaemic heart disease Pia Hedegaard Johnsen 1, Martin Berg Johansen 1, 2
Dan Med J 65/2 February 2018 DANISH MEDICAL JOURNAL 1 Long-term outcome after normal myocardial perfusion imaging in suspected ischaemic heart disease Pia Hedegaard Johnsen 1, Martin Berg Johansen 1, 2
Ticagrelor compared with clopidogrel in patients with acute coronary syndromes the PLATO trial
 compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
New Zealand Data Sheet. LYCINATE Sublingual Tablets contain 0.6mg (600mcg) glyceryl trinitrate.
 LYCINATE New Zealand Data Sheet Glyceryl trinitrate 600mcg Tablets Presentation LYCINATE Sublingual Tablets contain 0.6mg (600mcg) glyceryl trinitrate. Uses Actions Glyceryl trinitrate causes smooth muscle
LYCINATE New Zealand Data Sheet Glyceryl trinitrate 600mcg Tablets Presentation LYCINATE Sublingual Tablets contain 0.6mg (600mcg) glyceryl trinitrate. Uses Actions Glyceryl trinitrate causes smooth muscle
Summary of safety concerns Important identified risks
 Valley VI.2 Elements for a public summary NL/H/3661/001-002/DC - Ivabradin Medical VI.2.1 Overview of disease epidemiology Ivabradine is a medicine used for two long-term (chronic) heart conditions: to
Valley VI.2 Elements for a public summary NL/H/3661/001-002/DC - Ivabradin Medical VI.2.1 Overview of disease epidemiology Ivabradine is a medicine used for two long-term (chronic) heart conditions: to
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Unstable angina and NSTEMI
 Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
Severe Hypertension. Pre-referral considerations: 1. BP of arm and Leg 2. Ambulatory BP 3. Renal causes
 Severe Hypertension *Prior to making a referral, call office or Doc Halo, to speak with a Cardiologist or APP to discuss patient and possible treatment options. Please only contact the patient's cardiologist.
Severe Hypertension *Prior to making a referral, call office or Doc Halo, to speak with a Cardiologist or APP to discuss patient and possible treatment options. Please only contact the patient's cardiologist.
PRODUCT INFORMATION ISORDIL ORAL AND SUBLINGUAL TABLETS
 PRODUCT INFORMATION ISORDIL ORAL AND SUBLINGUAL TABLETS NAME OF THE MEDICINE Isosorbide dinitrate; also known as sorbide nitrate. The chemical name for isosorbide dinitrate is 1,4:3,6- dianhydro-2,5-di-o-nitro-d-glucitol.
PRODUCT INFORMATION ISORDIL ORAL AND SUBLINGUAL TABLETS NAME OF THE MEDICINE Isosorbide dinitrate; also known as sorbide nitrate. The chemical name for isosorbide dinitrate is 1,4:3,6- dianhydro-2,5-di-o-nitro-d-glucitol.
Exercise Test: Practice and Interpretation. Jidong Sung Division of Cardiology Samsung Medical Center Sungkyunkwan University School of Medicine
 Exercise Test: Practice and Interpretation Jidong Sung Division of Cardiology Samsung Medical Center Sungkyunkwan University School of Medicine 2 Aerobic capacity and survival Circulation 117:614, 2008
Exercise Test: Practice and Interpretation Jidong Sung Division of Cardiology Samsung Medical Center Sungkyunkwan University School of Medicine 2 Aerobic capacity and survival Circulation 117:614, 2008
Angina Pectoris Dr. Shariq Syed
 Angina Pectoris Dr. Syed 1 What is Angina Pectoris (AP)? Commonly known as angina is chest pain often due to ischemia of the heart muscle, Because of obstruction or spasm of the coronary arteries 2 What
Angina Pectoris Dr. Syed 1 What is Angina Pectoris (AP)? Commonly known as angina is chest pain often due to ischemia of the heart muscle, Because of obstruction or spasm of the coronary arteries 2 What
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Mortality. p =
 Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Rehabilitation in ischaemic heart disease
 Rehabilitation in ischaemic heart disease Folia Cardiologica 2004 suppl. A Cybulska K., Mamcarz A. Rehabilitacja kardiologiczna w chorobie niedokrwiennej serca Piotrowicz R., Wolszczakiewicz J. Rehabilitacja
Rehabilitation in ischaemic heart disease Folia Cardiologica 2004 suppl. A Cybulska K., Mamcarz A. Rehabilitacja kardiologiczna w chorobie niedokrwiennej serca Piotrowicz R., Wolszczakiewicz J. Rehabilitacja
Opinion 15 May ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: )
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: 34009 563 619 7 7) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: 34009 563 619 7 7) Applicant:
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Outpatient Cardiac Rehabilitation
 Last Review Date: May 12, 2017 Number: MG.MM.ME.26bC3v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: May 12, 2017 Number: MG.MM.ME.26bC3v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Trials Enrolled subjects Findings Fox et al. 2014, SIGNIFY 1
 Appendix 5 (as supplied by the authors): Published trials on the effect of ivabradine on outcomes including mortality in patients with different cardiovascular diseases Trials Enrolled subjects Findings
Appendix 5 (as supplied by the authors): Published trials on the effect of ivabradine on outcomes including mortality in patients with different cardiovascular diseases Trials Enrolled subjects Findings
Management of Coronary Artery Spasm
 Management of Coronary Artery Spasm J. C. Kaski and R. Arroyo-Espliguero Cardiovascular Biology Research Centre Division of Cardiac and Vascular Sciences St George s, University of London Prinzmetal s
Management of Coronary Artery Spasm J. C. Kaski and R. Arroyo-Espliguero Cardiovascular Biology Research Centre Division of Cardiac and Vascular Sciences St George s, University of London Prinzmetal s
ranolazine, 375mg, 500mg and 750mg prolonged-release tablets (Ranexa ) SMC No. (565/09) A. Menarini Pharma UK SRL
 2 nd Re-Submission ranolazine, 375mg, 500mg and 750mg prolonged-release tablets (Ranexa ) SMC No. (565/09) A. Menarini Pharma UK SRL 09 December 2011 The Scottish Medicines Consortium (SMC) has completed
2 nd Re-Submission ranolazine, 375mg, 500mg and 750mg prolonged-release tablets (Ranexa ) SMC No. (565/09) A. Menarini Pharma UK SRL 09 December 2011 The Scottish Medicines Consortium (SMC) has completed
ClinicalTrials.gov Identifier: NCT Sponsor/company: Sanofi-Aventis. Date: 08/02/ 2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
Independent Review Panel (IRP)
 Independent Review Panel (IRP) ranolazine, 375mg, 500mg and 750mg prolonged-release tablets (Ranexa ) SMC No. (565/09) A. Menarini Pharma UK SRL 05 October 2012 The Scottish Medicines Consortium (SMC)
Independent Review Panel (IRP) ranolazine, 375mg, 500mg and 750mg prolonged-release tablets (Ranexa ) SMC No. (565/09) A. Menarini Pharma UK SRL 05 October 2012 The Scottish Medicines Consortium (SMC)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 May 2007
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2007 RECTOGESIC 4 mg/g, rectal ointment B/1 (CIP 376 537-0) Applicant : PROSTRAKAN PHARMA SAS Glyceryl trinitrate
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2007 RECTOGESIC 4 mg/g, rectal ointment B/1 (CIP 376 537-0) Applicant : PROSTRAKAN PHARMA SAS Glyceryl trinitrate
Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre
 Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre Objectives To learn what s new in stroke care 2010-11 1) Acute stroke management Carotid artery stenting versus surgery for symptomatic
Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre Objectives To learn what s new in stroke care 2010-11 1) Acute stroke management Carotid artery stenting versus surgery for symptomatic
Sponsor Novartis. Generic Drug Name. Valsartan and amlodipine Trial Indication(s) Hypertension Protocol Number CVAA489A2306 Protocol Title
 Sponsor Novartis Generic Drug Name Valsartan and amlodipine Trial Indication(s) Hypertension Protocol Number CVAA489A2306 Protocol Title A randomized, double-blind, multi-center, active-controlled, parallel
Sponsor Novartis Generic Drug Name Valsartan and amlodipine Trial Indication(s) Hypertension Protocol Number CVAA489A2306 Protocol Title A randomized, double-blind, multi-center, active-controlled, parallel
Instruct patient and caregivers: Need for constant monitoring Potential complications of drug therapy
 Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
Investigator Responsibilities in Protecting Participants in the Conduct of Bioequivalence Studies Cecilia C. Maramba, MD, MScID
 Investigator Responsibilities in Protecting Participants in the Conduct of Bioequivalence Studies Cecilia C. Maramba, MD, MScID Bioavailability Unit Department of Pharmacology and Toxicology University
Investigator Responsibilities in Protecting Participants in the Conduct of Bioequivalence Studies Cecilia C. Maramba, MD, MScID Bioavailability Unit Department of Pharmacology and Toxicology University
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Otamixaban for non-st-segment elevation acute coronary syndrome
 Otamixaban for non-st-segment elevation acute coronary syndrome September 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not
Otamixaban for non-st-segment elevation acute coronary syndrome September 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not
Clinical Policy Title: Cardiac rehabilitation
 Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Chest pain and troponins on the acute take. J N Townend Queen Elizabeth Hospital Birmingham
 Chest pain and troponins on the acute take J N Townend Queen Elizabeth Hospital Birmingham 3 rd Universal Definition of Myocardial Infarction Type 1: Spontaneous MI related to atherosclerotic plaque rupture
Chest pain and troponins on the acute take J N Townend Queen Elizabeth Hospital Birmingham 3 rd Universal Definition of Myocardial Infarction Type 1: Spontaneous MI related to atherosclerotic plaque rupture
Balloon angioplasty versus bypass grafting in the era of coronary stenting Ekstein S, Elami A, Merin G, Gotsman M S, Lotan C
 Balloon angioplasty versus bypass grafting in the era of coronary stenting Ekstein S, Elami A, Merin G, Gotsman M S, Lotan C Record Status This is a critical abstract of an economic evaluation that meets
Balloon angioplasty versus bypass grafting in the era of coronary stenting Ekstein S, Elami A, Merin G, Gotsman M S, Lotan C Record Status This is a critical abstract of an economic evaluation that meets
Chapter 21: Clinical Exercise Testing Procedures
 Publisher link: thepoint http://thepoint.lww.com/book/show/2930 Chapter 21: Clinical Exercise Testing Procedures American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise
Publisher link: thepoint http://thepoint.lww.com/book/show/2930 Chapter 21: Clinical Exercise Testing Procedures American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise
Supplementary material 1. Definitions of study endpoints (extracted from the Endpoint Validation Committee Charter) 1.
 Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Abbreviated New Drug Evaluation: Ranolazine
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 DUOPLAVIN 75 mg/75 mg, film-coated tablet B/30 (CIP code: 359 022-6) B/30 (CIP code: 382 063-7) DUOPLAVIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 DUOPLAVIN 75 mg/75 mg, film-coated tablet B/30 (CIP code: 359 022-6) B/30 (CIP code: 382 063-7) DUOPLAVIN
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
Final Appraisal Report. Ranolazine (Ranexa ) as add-on therapy for symptomatic treatment of stable angina pectoris. A. Menarini Pharma UK SRL
 Final Appraisal Report Ranolazine (Ranexa ) as add-on therapy for symptomatic treatment of stable angina pectoris A. Menarini Pharma UK SRL Advice No: 1909 December 2009 Recommendation of AWMSG Ranolazine
Final Appraisal Report Ranolazine (Ranexa ) as add-on therapy for symptomatic treatment of stable angina pectoris A. Menarini Pharma UK SRL Advice No: 1909 December 2009 Recommendation of AWMSG Ranolazine
Screening for Asymptomatic Coronary Artery Disease: When, How, and Why?
 Screening for Asymptomatic Coronary Artery Disease: When, How, and Why? Joseph S. Terlato, MD FACC Clinical Assistant Professor, Brown Medical School Coastal Medical Definition The presence of objective
Screening for Asymptomatic Coronary Artery Disease: When, How, and Why? Joseph S. Terlato, MD FACC Clinical Assistant Professor, Brown Medical School Coastal Medical Definition The presence of objective
2.0 Synopsis. Choline fenofibrate capsules (ABT-335) M Clinical Study Report R&D/06/772. (For National Authority Use Only) Name of Study Drug:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
Adults With Diagnosed Diabetes
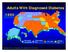 Adults With Diagnosed Diabetes 1990 No data available Less than 4% 4%-6% Above 6% Mokdad AH, et al. Diabetes Care. 2000;23(9):1278-1283. Adults With Diagnosed Diabetes 2000 4%-6% Above 6% Mokdad AH, et
Adults With Diagnosed Diabetes 1990 No data available Less than 4% 4%-6% Above 6% Mokdad AH, et al. Diabetes Care. 2000;23(9):1278-1283. Adults With Diagnosed Diabetes 2000 4%-6% Above 6% Mokdad AH, et
function in patients with ischaemic heart disease
 Br. J. clin. Pharmac. (1986), 22, 319S-324S Calcium antagonist treatment and its effects on left ventricular function in patients with ischaemic heart disease E. A. RODRIGUES, I. M. AL-KHAWAJA, A. LAHIRI
Br. J. clin. Pharmac. (1986), 22, 319S-324S Calcium antagonist treatment and its effects on left ventricular function in patients with ischaemic heart disease E. A. RODRIGUES, I. M. AL-KHAWAJA, A. LAHIRI
TRANSPARENCY COMMITTEE OPINION. 2 April 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 2 April 2008 LOVENOX 6,000 IU anti-xa/0.6 ml, injectable solution (S.C.) in prefilled syringe Box of 2 (CIP: 364 690-3)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 2 April 2008 LOVENOX 6,000 IU anti-xa/0.6 ml, injectable solution (S.C.) in prefilled syringe Box of 2 (CIP: 364 690-3)
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Type of intervention Diagnosis. Economic study type Cost-effectiveness analysis.
 The utility and potential cost-effectiveness of stress myocardial perfusion thallium SPECT imaging in hospitalized patients with chest pain and normal or non-diagnostic electrocardiogram Ben-Gal T, Zafrir
The utility and potential cost-effectiveness of stress myocardial perfusion thallium SPECT imaging in hospitalized patients with chest pain and normal or non-diagnostic electrocardiogram Ben-Gal T, Zafrir
ANGINA PECTORIS. angina pectoris is a symptom of myocardial ischemia in the absence of infarction
 Pharmacology Ezra Levy, Pharm.D. ANGINA PECTORIS A. Definition angina pectoris is a symptom of myocardial ischemia in the absence of infarction angina usually implies severe chest pain or discomfort during
Pharmacology Ezra Levy, Pharm.D. ANGINA PECTORIS A. Definition angina pectoris is a symptom of myocardial ischemia in the absence of infarction angina usually implies severe chest pain or discomfort during
Synopsis. Study title. Investigational Product Indication Design of clinical trial. Number of trial sites Duration of clinical trial / Timetable
 Synopsis Study title Investigational Product Indication Design of clinical trial Number of trial sites Duration of clinical trial / Timetable Repetitive levosimendan infusions for patients with advanced
Synopsis Study title Investigational Product Indication Design of clinical trial Number of trial sites Duration of clinical trial / Timetable Repetitive levosimendan infusions for patients with advanced
Cardiac Rehabilitation
 Easy Choice Health Plan Harmony Health Plan of Illinois Missouri Care Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona OneCare (Care1st Health Plan Arizona, Inc.) Staywell of Florida
Easy Choice Health Plan Harmony Health Plan of Illinois Missouri Care Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona OneCare (Care1st Health Plan Arizona, Inc.) Staywell of Florida
Off-white, round, with faceted edges, scored on one side and bearing the inscription IK10 (10mg) or IK20 (20mg).
 1 TRADE NAME OF THE MEDICINAL PRODUCT Ikorel 10mg and 20mg Tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Nicorandil 10mg or 20mg For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM
1 TRADE NAME OF THE MEDICINAL PRODUCT Ikorel 10mg and 20mg Tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Nicorandil 10mg or 20mg For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM
Ischemic Heart Disease Interventional Treatment
 Ischemic Heart Disease Interventional Treatment Cardiac Catheterization Laboratory Procedures (N = 11,61) is a regional and national referral center for percutaneous coronary intervention (PCI). A total
Ischemic Heart Disease Interventional Treatment Cardiac Catheterization Laboratory Procedures (N = 11,61) is a regional and national referral center for percutaneous coronary intervention (PCI). A total
Cardiovascular Disorders Lecture 3 Coronar Artery Diseases
 Cardiovascular Disorders Lecture 3 Coronar Artery Diseases By Prof. El Sayed Abdel Fattah Eid Lecturer of Internal Medicine Delta University Coronary Heart Diseases It is the leading cause of death in
Cardiovascular Disorders Lecture 3 Coronar Artery Diseases By Prof. El Sayed Abdel Fattah Eid Lecturer of Internal Medicine Delta University Coronary Heart Diseases It is the leading cause of death in
Individual Study Table Referring to Item of the Submission: Volume: Page:
 2.0 Synopsis Name of Company: Abbott Laboratories Name of Study Drug: Meridia Name of Active Ingredient: Sibutramine hydrochloride monohydrate Individual Study Table Referring to Item of the Submission:
2.0 Synopsis Name of Company: Abbott Laboratories Name of Study Drug: Meridia Name of Active Ingredient: Sibutramine hydrochloride monohydrate Individual Study Table Referring to Item of the Submission:
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
GUIDELINE 14 ACUTE CORONARY SYNDROMES
 AUSTRALIAN RESUSCITATION COUNCIL GUIDELINE 14 ACUTE CORONARY SYNDROMES OVERVIEW AND SUMMARY As a part of the International Liaison Committee on Resuscitation (ILCOR) process that led to the International
AUSTRALIAN RESUSCITATION COUNCIL GUIDELINE 14 ACUTE CORONARY SYNDROMES OVERVIEW AND SUMMARY As a part of the International Liaison Committee on Resuscitation (ILCOR) process that led to the International
Inter-regional differences and outcome in unstable angina
 European Heart Journal (2000) 21, 1433 1439 doi:10.1053/euhj.1999.1983, available online at http://www.idealibrary.com on Inter-regional differences and outcome in unstable angina Analysis of the International
European Heart Journal (2000) 21, 1433 1439 doi:10.1053/euhj.1999.1983, available online at http://www.idealibrary.com on Inter-regional differences and outcome in unstable angina Analysis of the International
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 30 May 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 30 May 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Assessment and immediate management of suspected acute coronary syndrome bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Assessment and immediate management of suspected acute coronary syndrome bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
How to approach non-infarct related artery disease in patients with STEMI in a limited resource setting
 How to approach non-infarct related artery disease in patients with STEMI in a limited resource setting Ahmed A A Suliman, MBBS, FACP, FESC Associate Professor, University of Khartoum Interventional Cardiologist,
How to approach non-infarct related artery disease in patients with STEMI in a limited resource setting Ahmed A A Suliman, MBBS, FACP, FESC Associate Professor, University of Khartoum Interventional Cardiologist,
