Dissolved and Labile Concentrations of Cd, Cu, Pb, and Zn in Aged Ferrihydrite-Organic Matter Systems
|
|
|
- Annabella Wilson
- 6 years ago
- Views:
Transcription
1 Environ. Sci. Technol. 1999, 33, Dissolved and Labile Concentrations of Cd, Cu, Pb, and Zn in Aged Ferrihydrite-Organic Matter Systems CARMEN ENID MARTIÄ NEZ* AND MURRAY B. MCBRIDE Department of Soil, Crop, and Atmospheric Sciences, Cornell University, Ithaca, New York The relative efficiencies of an organic and a mineral component of soils in controlling Zn, Cd, Cu, and Pb solubility and dissolution initially and during aging at ph 5.5 for up to 200 days is investigated. Metal retention by a natural organic matter (leaf compost) and a mineral model system (ferrihydrite) were tested for the organic and hydrous ferric oxide components separately (ORG and HFO) and in a mixed system (HFO-ORG). Total dissolved (by ICP) and labile (by dpasv) concentrations of metals in solution were measured. Initial Cd and Zn solubility in the systems followed the order: HFO > HFO-ORG > ORG. After aging for about 200 days, however, Cd and Zn solubility was HFO ) HFO-ORG > ORG. Thus, the organic adsorbate proved to be more efficient in Zn and Cd removal from solution under the conditions used in this study. The HFO system resulted in the highest Cu solubility at intermediate aging times. However, during longer aging, total dissolved Cu increased in the ORG system whereas that in the HFO decreased, so that Cu solubility was lower in HFO after about 200 days. Lead solubility generally remained very low (<0.05 µm) except in the ORG system in which the total dissolved Pb reached 0.25 µm. The ORG system shows that about 75% of total dissolved Cu and 80% of total dissolved Pb exist as nonlabile organo-metal complexes, while soluble nonlabile complexes account for about 40% of dissolved Zn. Cadmium complexation (nonlabile) in the ORG system was minimal, thus Cd exists mostly in the free ionic form or as weak (labile) organic complexes. Introduction Iron oxides and organic matter coexist in soils and serve as reactive adsorbents of heavy metals. Both have a high surface area and a high reactivity that is associated with surface functional groups (1). Iron oxides and organic matter are intimately associated in soils, probably as oxide-organic complexes; thus, their individual function is difficult to discern. The tendency of a metal to react with the mineral or the organic component of soils could potentially limit its solubility, which will ultimately determine its bioavailability and toxicity. Due to competition among multiple and mixed solid phases for metal retention, it is difficult to assess what soil component (mineral or organic) is responsible for the long-term solubility control of metals in soils. This results in uncertainty about the importance of reactions with organic * Corresponding author telephone: (607) ; fax: (607) ; cem20@cornell.edu. and iron oxide colloids as mechanisms by which metal ions are retained in soils. Controls on metal solubility by oxides have been attributed to ion exchange reactions, specific adsorption to surface hydroxyl groups, coprecipitation, and at higher loadings and ph, multinuclear complex or mixed-cation hydroxide complex formation at the surface or precipitation as the discrete oxide or hydroxide. Metal retention by hydrous ferric oxide follows the general order: Pb g Cu.. Zn > Cd. Studies on metal adsorption and coprecipitation with iron oxides show a retention time (aging) effect that usually results in decreased metal solubility (2-5). Although iron (hydr)oxides may be an important sink for metal retention in the long term and have been used in attempts to remediate metal-contaminated soils, Martínez and McBride (ref 2 and references therein) showed that, even when metals are coprecipitated with iron (hydr)oxides and aged for long periods of time, residual solubilities of free Cd, Cu, and Zn can be high enough to reach reported phytotoxicity values or allow excessive uptake by crops. Soil organic matter has been recognized as a critical component in the retention of heavy metals in soils (6, 7). Organic matter contains S, O, and N functional groups that bind heavy metals strongly. X-ray absorption spectroscopy (XAS) and electron spin resonance (ESR) studies indicate that Pb, Cu, and Zn form inner-sphere complexes with soil humic substances (8-10). Lead, Cu, and Zn coordinate with oxygen ligands while Zn also coordinates with sulfur (thiol) containing functional groups. Although metal dissolution during long-term decomposition of organic matter has not been investigated in detail, several studies indicate that metals become more available after incubation of soils amended with metal-contaminated organic materials such as sewage sludge (11-13). Increases in metal availability were related to organic matter decomposition. Furthermore, organic matter decomposition produces soluble organic ligands (dissolved organic carbon, DOC) that may change metal speciation in solution, thus influencing metal adsorptivity, mobility, toxicity, and bioavailability. Nevertheless, field studies have shown that chemical extractability (14) and bioavailability (15) remain constant with time after the sludge application is terminated. Few studies have compared the relative importance of an organic and a mineral soil component in their ability to remove metals from solution (16-20). In a study in which individual materials (organic and iron oxide) were equilibrated to simulate a multicomponent system (20), Cu was shown to concentrate in the organic component. Using single systems, organic matter was found a more efficient adsorbent in removing Cu from solution at ph than goethite (16). In the acid ph range (ph < 6.5), mineral-bound organic matter enhances the adsorption of some metals (e.g., Cu and Co), thus reducing their solubility (18, 19). At neutral ph, the mineral component controlled metal solubility while dissolved organic matter increased metal dissolution by forming soluble metal-organic complexes. This study investigates the initial solubility and long-term (up to 200 days) dissolution of important trace elements (Cd, Cu, Pb, and Zn) from separate and mixed iron oxide-organic matter systems. Metal-organic matter (ORG) and ferrihydrite (HFO) and ferrihydrite-organic matter (HFO-ORG) metal coprecipitates are formed, and their efficacy in maintaining low metal concentrations in solution is compared. The mixed system is used in an attempt to represent a model soil. Solution concentration of metals are determined where total dissolved concentrations are measured by inductively coupled /es980576c CCC: $ American Chemical Society VOL. 33, NO. 5, 1999 / ENVIRONMENTAL SCIENCE & TECHNOLOGY Published on Web 01/21/1999
2 plasma emission spectrometry (ICP), and labile concentrations are measured by differential pulse anodic stripping voltammetry (dpasv). Discrimination between total dissolved and labile concentrations of metals provides information on the formation and stability of soluble metal-organic complexes. DPASV-Labile Metals. Labile metal represents the fraction of total dissolved metal determined by stripping analysis. This fraction includes the free hydrated metal ions and the labile metal complexes, which are complexes that dissociate very rapidly to yield the free metal (21). It is often assumed that the labile metal represents the metal fraction that is bioavailable and, therefore, similar to the toxic fraction of the dissolved metal (22). Differential pulse anodic stripping voltammetry (dpasv) involves two steps. In the first, the deposition (preconcentration) step, metal ions in the sample solution are reduced at negative (cathodic) potential and concentrated into a mercury electrode. The amalgamated metals are measured in the second (stripping) step by applying a positive (anodic) potential scan and measuring the peak currents produced as the system reaches the oxidation potential of the metals. Several factors affect what would be measured as dpasvlabile metals. In the theory of labile/inert discrimination, a lability criterion has been developed using the ratio of the kinetic current to the diffusion current (i k/i d) as an index of the lability of complexes. The kinetic current (i k) is the current observed for a metal ion complex and depends on the rate constant for dissociation of the complex. The diffusion current (i d) is the current for the same concentration of the metal ion but in the absence of ligand. It depends on the time necessary to cross the diffusion layer, which in turn depends on the layer thickness and the diffusion coefficient of the metal ion. Using this criterion, together with stability (thermodynamic) equilibrium parameters (e.g., dissociation and formation constants), metal complexes are calculated to be labile or nonlabile by anodic stripping methods [for details on this theory the reader is referred to a review by Florence (23) and the references therein]. The deposition potential, if sufficiently negative, may lead to the direct reduction of some metal complexes. Also, the complex is dpasv-labile if the rate of dissociation is similar (rapid) as compared to the time scale of the stripping measurement. Thus, based on these parameters and on empirical measurements, the free metal ion and weak inorganic and organic complexes are dpasvlabile. Among the characteristics determining the lability of metal-organic complexes are the degree of aromaticity of the organic material and the functional group involved in complexation. Fulvic acids were suggested to form both labile and nonlabile complexes with Cu (24). The fulvic acids varied in degree of aromaticity, with the nonlabile Cu-fulvic acid complexes resulting from the fulvic acid with high aromatic proton signal (by nuclear magnetic resonance, NMR). Also, it has been suggested that Cu binding involves S and N functional groups to form nonlabile organic complexes (23). Experimental Section Batch-type reaction experiments were used to monitor the total dissolved (determined by inductively coupled plasma emission spectrometry, ICP) and labile (determined by differential pulse anodic stripping voltammetry, dpasv) solution concentrations of Cd, Cu, Pb, and Zn after all four metals in combination were sorbed or coprecipitated and aged in single and mixed solid phases. The ph of the suspensions was initially raised to ph 6, because this is a ph typical for soils and it prevents the precipitation of metals. The suspension ph was stabilized at ph 5.5 during aging and was kept at that value. All aging experiments used mixed batch reactors that were maintained at room temperature (23 C) in the presence of ambient CO 2. The metal concentrations chosen (in all experiments) were based on approximate levels that may occur in the fine fraction of soils (that fraction that is considered most reactive) subjected to heavy sewage sludge application under the current U.S. EPA regulations and that have been observed in a number of contaminated soils. They are as follows [mg of metal (kg of solid) -1 ]: 100, 1500, 1500, and 3000 mg kg -1 for Cd, Cu, Pb, and Zn, respectively, and they represent the concentrations in the solid phase if complete sorption or coprecipitation occurred. The solid phases studied were hydrous ferric oxide (HFO), organic matter (ORG), and a mixed solid containing hydrous ferric oxide and organic matter (HFO-ORG). The ICP detection limits (3σ criterion) were estimated to be 0.03, 0.003, 0.03, and 0.05 µm for Zn, Cd, Pb, and Cu, respectively, by the method used in this study. We used an axial ICP-OES instrument manufactured by Trulogic Systems, Inc. DPASV detection limits are about one-fifth of those determined by ICP. Analytical methods are within a 5% RSD. Metals-Iron Oxide Coprecipitate (HFO). A coprecipitate was formed by the titration of solutions containing M Fe [as Fe(NO 3) 3], M Cd (as cadmium acetate), M Cu (as copper acetate), M Pb (as lead acetate), and M Zn (as zinc acetate) to ph 6 with 0.1 M KOH in 1 mm KNO 3 background electrolyte and aged for up to 200 days. The Fe concentration used was calculated to yield 0.33 g of iron oxide (as R-FeOOH). The concentrations used represent loadings of mol of Zn 2+ / mol of Fe 3+, mol of Cd 2+ /mol of Fe 3+, mol of Pb 2+ /mol of Fe 3+, and mol of Cu 2+ /mol of Fe 3+ ; all four metals in combination were coprecipitated with Fe 3+ for a total of mol of M 2+ /mol of Fe 3+. At the end of the titration (ph 6), the added OH/Fe ratio was 3.40 in a total volume of 750 ml. This suspension was sampled periodically for metal analysis and ph measurement. At each time interval, a subsample was removed and centrifuged for 10 min at rpm ( rcf). The supernatant was then filtered through a 0.2-µm polycarbonate membrane filter; acidified by the addition of 1 M HCl; and analyzed immediately for labile Cd, Cu, Pb, and Zn by dpasv. Acidification of the supernatants, used to stabilize the solutions during storage, had no effect on dpasv-labile metals in the HFO system because of the absence of soluble metal complexes in this system (2). The filtered supernatants were also analyzed for total dissolved Cd, Cu, Pb, and Zn by ICP. Although the ph of the suspension was initially raised to 6, the HFO coprecipitate was stabilized at a ph of about 5.5 after about 100 days. Metals-Organic System (ORG). A leaf compost (predominantly sugar maple) was used as a natural model system for organic matter in our experiments. It was collected from a rural location near Ithaca, NY, air-dried, ground in a Waring blender, and sieved to <1 mm. The leaf compost had a ph of 7.12 in water and an organic carbon content of 37.9% (by a combustion method). Elemental analysis of the leaf compost, performed by ICP of a HNO 3 digest, was 2.5, 2.6, 56, 4.8, 4.9, 98, 2.5, 19, 18, and 188 mg kg -1 of P, S, Ca, Fe, Al, Si, Cd, Cu, Pb, and Zn, respectively. These values represent the average of duplicate analysis. The ph of the leaf compost was adjusted to about 5.5 by addition of small quantities of 1 M HNO 3, dialyzed to remove excess salts, and freeze-dried prior to use. The ph of a suspension containing Cd ( Mas cadmium acetate), Cu ( M as copper acetate), Pb ( M as lead acetate), Zn ( M as zinc acetate), and leaf compost (0.33 g) was raised to ph 6 by using 0.1 M KOH in 1 mm KNO 3 background electrolyte (total volume of 750 ml) and aged for up to 200 days. Small aliquots of base were needed since the suspension s initial ph was In this system, the ph stabilized at about 5.8 so that addition of acid (1 mm HNO 3) was necessary to keep the ph ENVIRONMENTAL SCIENCE & TECHNOLOGY / VOL. 33, NO. 5, 1999
3 constant at 5.5 using a ph-stat device. The suspension was sampled periodically for metal analysis as described previously. Nonacidified, filtered supernatants were analyzed by dpasv immediately after their collection. Filtered and acidified (for sample preservation) supernatants were analyzed by ICP. The supernatants obtained from this system (ORG) had a yellow color even after filtration. The yellow color was not present in the HFO or HFO-ORG systems, both of which had colorless supernatants. Metals-Iron Oxide-Organic Coprecipitate (HFO-ORG). A coprecipitate was formed by increasing the ph of a suspension containing Cd ( M as cadmium acetate), Cu ( M as copper acetate), Pb ( M as lead acetate), Zn ( M as zinc acetate), Fe ( M as Fe(NO 3) 3), and leaf compost (0.33 g) to ph 6 by using 0.1 M KOH in 1 mm KNO 3 background electrolyte (total volume of 750 ml) and aged for up to 200 days. Thus, a 1:1 iron oxide:leaf compost ratio (by weight) was used in the HFO-ORG system. The suspension had an initial ph of 2.6. At the end of the titration, the OH/Fe ratio was 3.23, and the ph was 6, but decreases in suspension ph with aging of the HFO-ORG coprecipitate were observed. The ph was maintained at 5.5 throughout the experiment using a phstat device. The suspension was sampled periodically in the same manner as described above, and metal analysis was performed as for the ORG system. Base and/or acid additions were insignificant to the total volume of the systems maintained at constant ph over the aging period. Control experiments without trace metals were conducted in the same manner as described above and yielded metal solution concentrations below the detection limits of the methods used in this study. The concentration of dissolved organic carbon (DOC) in the supernatants was estimated by colorimetry. The absorbance of the supernatants was calibrated with the absorbance of soil extracts (at λ ) 254 nm) of known DOC (25). Sulfur (organic plus sulfate) in the supernatants was determined by ICP. The solid phases were characterized by infrared (FTIR) and X-ray diffraction (XRD) as described previously (26). Results and Discussion The solid phases were characterized to test for the presence of iron oxide in the HFO-ORG system since organics may interfere in their formation (1). X-ray diffraction provided evidence for the formation of noncrystalline iron oxide after coprecipitation in the presence of the organic material as a 2-line ferrihydrite was present in both the HFO and HFO- ORG systems. Thus, initial Fe 3+ complexation with organic matter did not prevent oxide formation, although some complexation may have occurred. Furthermore, inspection of the HFO-ORG system by FTIR confirmed the presence of a solid phase with characteristic absorption bands of both the HFO and ORG systems. Metal Dissolution from Single and Mixed Systems. Variable concentrations of Cd, Cu, Pb, and Zn remained in solution after their simultaneous retention by organic matter (ORG) and coprecipitation with iron oxide (HFO) and an iron oxide-organic matter mixed (HFO-ORG) system (Figures 1 and 2, Table 1). Cadmium equilibration with ferrihydrite (HFO) resulted in about 0.3 µm Cd in solution even after 200 days (Figure 1). Initial soluble Cd in the organic (ORG) and mixed (HFO-ORG) systems was lower than in the HFO system; however, solubility in these organiccontaining systems increased with time (Figure 1 and Table 1). Yet, after aging for about 200 days, Cd solubility showed a lower value in the ORG system and reached a similar (higher) concentration in the HFO and HFO-ORG systems. The solubility behavior of Zn was similar to that of Cd in the HFO-ORG and ORG systems, with Zn solubility increasing with time. This behavior of Cd and Zn suggests increases in FIGURE 1. Total dissolved (ICP) and labile (ASV) concentrations of Cd and Zn in ferrihydrite (HFO), organic matter (ORG), and ferrihydrite-organic matter (HFO-ORG) systems as a function of time. Symbols as in Figure 2. dissolved organic matter resulting in the release of metals into solution. Some reduction in dissolved Zn was observed from the HFO system after 200 days of equilibration (Figure 1). After 200 days of aging, labile Zn concentrations were similar in the HFO and HFO-ORG systems and lower in the ORG system. In general, and during reaction for up to 200 days, dissolved (both total and labile) Zn and Cd concentrations decreased in the order: HFO g HFO-ORG > ORG, indicating that the 100% organic system was more effective in Zn and Cd removal from solution (Figure 1). Cadmium and Zn solubilities in the HFO-ORG system, although ferrihydrite was formed, suggest that some of the Fe reacted with organic matter functional groups, forming iron-organic matter complexes. This is suggested in a schematic diagram showing the effect organic matter content has on the formation of various iron oxides (1). As in our experiments (HFO-ORG system), high organic matter content and high Fe supply (1:1 organic matter:iron oxide ratio by weight) can result in the formation of ferrihydrite and iron-organic complexes. Iron complexation with organic matter will block and therefore reduce the quantity of adsorption sites otherwise available for Zn and Cd complexation. Thus, Zn and Cd solubilities encountered in the HFO-ORG system can be explained by the blockage of reaction sites with Fe. This effect is not seen for Pb and Cu (Figure 2), presumably because of their stronger affinity for organic matter, allowing them to compete effectively with Fe and outcompete Zn and Cd for reaction sites (27, 28). Kerndorff and Schnitzer (28) showed that, as the ph increases from 2.4 to 5.8, Pb and Cu VOL. 33, NO. 5, 1999 / ENVIRONMENTAL SCIENCE & TECHNOLOGY 9 747
4 TABLE 1. ICP-Total Dissolved Metal at Initial and Final Equilibration Times Expressed as % of Total Metal Added a metal in solution (% of total added) system equilibration Zn Cd Pb Cu HFO initial 34 (35) 40 (29) 0 (0) 4.8 (1.3) final 26 (21) 33 (32) 0 (0) 2.0 (2.4) HFO-ORG initial 29 (21) 20 (20) 0 (0) 1.1 (1.9) final 34 (25) 30 (28) 0 (0) 0.8 (0.4) ORG initial 6.0 (0.7) 2.6 (1.7) 0.8 (0.1) 2.3 (0.9) final 20 (13) 20 (17) 3.7 (0.4) 8.7 (2.1) a Numbers in parentheses are dpasv-labile metals (as % of total metal added). FIGURE 2. Total dissolved (ICP) and labile (ASV) concentrations of Cu and Pb in ferrihydrite (HFO), organic matter (ORG), and ferrihydrite-organic matter (HFO-ORG) systems as a function of time. become more efficient competitors with Fe for reaction sites on humic acid. As shown in Figure 2, the HFO coprecipitate resulted in the highest concentration of dissolved Cu at intermediate aging times (discussion on possible mechanisms can be found in ref 2). However, after about 200 days, the HFO-ORG system controlled Cu solubility at the lowest level (Figure 2). Copper dissolution from the ORG system was very pronounced, with solubility increasing over the period of the experiment. Reasons for this will be discussed in a later section. Lead was efficiently removed from solution by the HFO and HFO- ORG coprecipitates (Table 1 and Figure 2). Its solubility ranged from below 5 nm to 0.05 µm, suggesting a high efficiency of iron oxide and mixed iron oxide-organic matter in reducing Pb concentrations in solution (Figure 2). As for Cu, total dissolved Pb in the ORG system increased with aging, and possible mechanisms for this will be discussed in a later section. The results obtained for Pb and Cu suggest that these elements compete more effectively than Zn or Cd with Fe for complexation sites in the HFO-ORG system. Comparison of metal dissolution (for up to 200 days) from single and mixed systems indicate that ferrihydrite alone is not as efficient an adsorbent for Zn, Cd, and Cu as organic matter or a mixed ferrihydrite-organic matter system, although it is very effective in removing Pb from solution (Table 1). Concentration in Solution: Total Dissolved (ICP) and Labile (dpasv) Metals. Total dissolved (by ICP) and labile (by dpasv) concentrations of Cd, Cu, Pb, and Zn were similar in the HFO system, indicating that all dissolved metals in this pure mineral system were in the free cation or labile (inorganic) complex form. In contrast, there were differences between total dissolved and labile concentrations of Zn in the HFO-ORG and ORG systems and of Cu and Pb in the ORG system (Figures 1 and 2). During sampling, it was noticed that the supernatants obtained from the HFO and HFO- ORG systems were colorless whereas the supernatants obtained from the ORG system were yellow even after filtration. Thus, we estimated dissolved organic carbon (DOC) in the systems (by colorimetry) and found increased concentrations of DOC in the ORG system as aging time increased (Figure 3). No DOC was detected in the HFO-ORG system by the method used in this study. However, this does not necessarily rule out the presence of soluble low molecular weight organic compounds as aliphatic compounds do not produce color. Increases in DOC with aging of the systems can be accounted for by (i) microbial oxidation of organic matter and (ii) physical disruption and dissolution induced by prolonged stirring. The lack of measurable DOC (by colorimetry) in the HFO-ORG system suggests that either soluble organics are adsorbed by the iron oxide or that the iron oxide acts as a flocculant, preventing oxidation and/or physical disruption of the organic material. In addition, although DOC was not detected colorimetrically in the HFO- ORG system, the concentration of soluble S increased after 175 days ( mg of S L -1 in solution). An increase in soluble S was also observed in the ORG system ( mg of S L -1 in solution). This result may indicate an increase in soluble organics (organic S) or of dissolved sulfate due to organic matter decomposition. Differences in total dissolved (ICP) and labile (dpasv) concentrations of Zn indicate some degree of Zn complexation in the HFO-ORG and ORG systems (Figure 1 and Table 1). Although iron oxide serves as a sink for soluble organics, some may remain in solution and form soluble complexes with Zn. These zinc-organic complexes are evidently not all dpasv-labile (Figure 1). Thus, total dissolved Zn consists of the free metal cation and dpasv-labile and nonlabile complexes. As the dissolved concentration of Zn increases with DOC in the ORG system (Figure 3), labile Zn increases proportionately. That is, at low DOC content (1.7 days) most of the dissolved Zn is complexed (nonlabile), but as DOC (and time) increases, the nonlabile (complexed) Zn is constant ENVIRONMENTAL SCIENCE & TECHNOLOGY / VOL. 33, NO. 5, 1999
5 FIGURE 3. Relationship between dissolved metals (ICP-total dissolved and ASV-labile) and dissolved organic carbon (DOC) in the organic (ORG) system. at about 40% of the total dissolved concentration; thus, labile Zn increases with time. Concentrations of total dissolved and dpasv-labile Cd are similar in the HFO-ORG and ORG systems (Figure 1). But since dissolved Cd (both total and labile) increases in proportion to DOC in the ORG system (Figure 3), it is reasonable to suggest that low molecular weight organics react with Cd to form dpasv-labile complexes that have the effect of increasing Cd solubility over time (21). Then Cd exists in solution as both the free ionic cation and as weak (dpasv-labile) organic complexes. It has been shown that >80% of DOC has a molecular weight of > (29). However, Cd is mostly associated with the DOC fraction with molecular weight between 1000 and (29). Total dissolved and dpasv-labile concentrations of Pb and Cu in the HFO and HFO-ORG coprecipitates are similar (Figure 2). Thus, in the systems containing HFO, dpasv-labile equates to total dissolved metal, and Pb and Cu exist as free ions and labile complexes in solution. Lead and Cu dissolution from the HFO-ORG system is limited to very low concentrations (Figure 2). Although Pb and Cu react strongly with dissolved organic matter that promotes metal dissolution from adsorption sites (1, 30), the HFO-ORG system is evidently very efficient in Cu and Pb removal from solution (Table 1). This is probably the result of the high affinity that iron oxides and organic matter have for Pb and Cu and to the tendency of Pb and Cu to form complexes with high molecular weight soluble organics, which are then preferentially adsorbed by iron oxide surfaces. This leaves little Pb or Cu in solution, mostly in the uncomplexed form. The relationships between Pb and Cu dissolution and dissolved organic carbon (DOC) in the ORG system are presented in Figure 3. DPASV-labile concentrations of Pb ( µm) and Cu ( µm) remain low with aging despite increases in DOC. However, total dissolved concentrations of Pb and Cu increase linearly with DOC, suggesting Pb and Cu complexation with nonlabile soluble organics (Figure 3). After equilibration of the ORG system for 184 days, 80% of the total dissolved Pb and 75% of the total dissolved Cu were complexed. Humic and fulvic acids are known to reduce the dpasv-labile concentrations of Pb and Cu (31). Similar correspondence between DOC concentrations and dissolved Cu and other metals has been observed in soils (32, 33), suggesting that DOC controls solubility of some metals. Differences between total dissolved and labile metal concentrations follow the trend: Pb (ORG) g Cu (ORG) > Zn (ORG and HFO-ORG). Cd (ORG and HFO-ORG). These results could be explained by competition among metals for dissolved organic carbon since Cu and Pb complex more strongly with organic matter than Zn and Cd (34). This competition effect was investigated by Piotrowicz et al. (24) in a study in which fulvic acid was added to a solution containing Pb, Cu, Zn, and Cd. After fulvic acid addition, all the Cd remained labile and only part of the Zn became nonlabile. Environmental Implications. The concentration of dissolved trace metals depends strongly on the particular metal and adsorbent. After equilibration for about 200 days, labile concentrations of Cu and Pb were particularly low in the HFO-ORG and HFO systems, respectively. The organic material limited labile Cd and Zn solubility more effectively than iron oxide. Increases in DOC with aging, however, were accompanied by increases in dissolved concentrations of Zn and Cd (ORG system). In the case of Cd, and to a lesser extent Zn, increases in the labile concentrations of these metals (which is believed to represent the metal fraction that is bioavailable and potentially toxic) also occurred over time. This time-dependent increase in metal lability suggests that decomposition or hydrolysis of organic matter in metalcontaminated soils could increase metal solubility or toxicity. It is recognized, however, that laboratory studies may need corrections for ionic strength, solid concentration effects (solid:solution ratio), and ion competition (e.g., Ca) to make a more representative comparison. Our experiments were run for about 200 days, and although our natural organic system (leaf compost) was relatively stable to decomposition, this equilibration time was sufficient for DOC to appear in VOL. 33, NO. 5, 1999 / ENVIRONMENTAL SCIENCE & TECHNOLOGY 9 749
6 the system. Increases in DOC from the ORG system promoted the formation of soluble (nonlabile) organic complexes with Cu and Pb, which could in field situations enhance metal mobility through the soil. However, mixed solid phases (iron oxides and organic matter in soil systems for example) may provide favorable conditions for readsorption of copperand lead-organic complexes, as suggested by the low concentrations of dissolved Cu and Pb found in the HFO- ORG system. Acknowledgments This research was supported by the USDA-NRI Competitive Grants Program, Award Literature Cited (1) Schwertmann, U.; Kodama, H.; Fischer, W. R. In Interactions of soil minerals with natural organics and microbes; Huang, P. M., Schnitzer, M., Eds.; Soil Science Society of America; Madison, WI, 1986; p 223. (2) Martínez, C. E.; McBride, M. B. Environ. Sci. Technol. 1998, 32, 743. (3) Ford, R. G.; Bertsch, P. M.; Farley, K. J. Environ. Sci. Technol. 1997, 31, (4) Ainsworth, C. C.; Pilon, J. L.; Gassman; P. L.; Van Der Sluys, W. G. Soil Sci. Soc. Am. J. 1994, 58, (5) Kolthoff, I. M.; Moskovitz, B. J. Phys. Chem. 1937, 41, 629. (6) Harter, R. D.; Naidu, R. In Advances in Agronomy; Sparks, D. L., Ed.; Academic Press: New York, 1995; Vol.55, p 219. (7) Stevenson, F. J. Soil Biol. Biochem. 1979, 11, 493. (8) Xia, K.; Bleam, W.; Helmke, P. A. Geochim. Cosmochim. Acta 1997, 61, (9) Xia, K.; Bleam, W.; Helmke, P. A. Geochim. Cosmochim. Acta 1997, 61, (10) McBride, M. B. Soil Sci. 1978, 126, 200. (11) Sadovnikova, L.; Otabbong, E.; Iakimenko, O.; Nilsson, I.; Persson, J.; Orlov, D. Agric. Ecosyst. Environ. 1996, 58, 127. (12) Hooda, P. S.; Alloway, B. J. Int. J. Environ. Anal. Chem. 1994, 57, 289. (13) Hooda, P. S.; Alloway, B. J. J. Soil Sci. 1993, 44, 97. (14) McGrath, S. P.; Cegarra, J. J. Soil Sci. 1992, 43, 313. (15) Chang, A. C.; Hyun, H.; Page, A. L. J. Environ. Qual. 1997, 26, 11. (16) McBride, M.; Martínez, C. E.; Sauvé, S.Soil Sci. Soc. Am. J. 1998, 62, (17) Tessier, A.; Fortin, D.; Berzile, N.; DeVitre, R. R.; Leppard, G. G. Geochim. Cosmochim. Acta 1996, 60, 387. (18) Zachara, J. M.; Resch, C. T.; Smith, S. C. Geochim. Cosmochim. Acta 1994, 58, 553. (19) Davis, J. A. Geochim. Cosmochim. Acta 1984, 48, 679. (20) McLaren, R. G.; Williams, J. G.; Swift, R. S. J. Soil Sci. 1983, 34, 325. (21) Wang, J. Stripping analysis: Principles, instrumentation, and applications; VCH Publishers: Deerfield Beach, FL, (22) Tessier, A., Turner, D. R., Eds. Metal speciation and bioavailability in aquatic systems; John Wiley: Chichester, New York, (23) Florence, T. M. Analyst 1986, 111, 489. (24) Piotrowicz, S. R.; Springer-Young, M.; Puig, J. A.; Spencer, M. J. Anal. Chem. 1982, 54, (25) Moore, T. R. Soil Sci. Soc. Am. J. 1985, 49, (26) Martínez, C. E.; McBride, M. B. Clays Clay Miner. 1998, 46, 537. (27) Jin, X.; Bailey, G. W.; Yu, Y. S.; Lynch, A. T. Soil Sci. 1996, 161, 509. (28) Kerndorff, H.; Schnitzer, M. Geochim. Cosmochim. Acta 1980, 44, (29) John, J.; Salbu, B.; Gjessing, E. T.; Bjørnstad, H. E. Water Res. 1988, 22, (30) Saar, R. A.; Weber, J. H. Geochim. Cosmochim. Acta 1980, 44, (31) Aualiitia, T. U.; Pickering, W. F. Water Res. 1986, 20, (32) Romkens, P. F. A. M.; Dolfin, J. Environ. Sci. Technol. 1998, 32, 363. (33) McBride, M. B.; Richards, B. K.; Steenhuis, T.; Russo, J. J.; Sauvé, S. Soil Sci. 1997, 162, 487. (34) Stevenson, F. J. Soil Sci. Soc. Am. J. 1976, 40, 665. Received for review June 5, Revised manuscript received December 7, Accepted December 7, ES980576C ENVIRONMENTAL SCIENCE & TECHNOLOGY / VOL. 33, NO. 5, 1999
UPTAKE OF MAJOR AND TRACE ELEMENTS BY GRASS BIOMASS AFTER AMELIORATION OF DEGRADED SOIL
 General and Applied Plant Physiology 2010, Volume 36 (1 2), pp. 12 16 2010 ISSN 1312-8183 Published by the Institute of Plant Physiology Bulgarian Academy of Sciences Available online at http://www.bio21.bas.bg/ipp/
General and Applied Plant Physiology 2010, Volume 36 (1 2), pp. 12 16 2010 ISSN 1312-8183 Published by the Institute of Plant Physiology Bulgarian Academy of Sciences Available online at http://www.bio21.bas.bg/ipp/
JORIND 9(2) December, ISSN
 SEQUENTIAL EXTRACTION OF Cu, Cd, Pb AND Zn FROM SOIL AROUND INDUSTRIAL WASTE DUMP SITES IN KADUNA ENVIRON USING SIMPLE AND SEQUENTIAL PROCEDURES. H.A Zakari, D.D. Adams, M Shimbayev, P. Nyam Department
SEQUENTIAL EXTRACTION OF Cu, Cd, Pb AND Zn FROM SOIL AROUND INDUSTRIAL WASTE DUMP SITES IN KADUNA ENVIRON USING SIMPLE AND SEQUENTIAL PROCEDURES. H.A Zakari, D.D. Adams, M Shimbayev, P. Nyam Department
The effect of humic acid and fulvic acid on adsorption-desorption behavior of copper and zinc in the yellow soil
 The effect of humic acid and fulvic acid on adsorption-desorption behavior of copper and zinc in the yellow soil Zhantai Wang, Minxia Cao, Wenchang Cai, and Heping Zeng Citation: AIP Conference Proceedings
The effect of humic acid and fulvic acid on adsorption-desorption behavior of copper and zinc in the yellow soil Zhantai Wang, Minxia Cao, Wenchang Cai, and Heping Zeng Citation: AIP Conference Proceedings
Speciation Analysis and Removal of Heavy Metals Zn, Cu, Cd from Sludge by Organic Acid
 5th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2015) Speciation Analysis and Removal of Heavy Metals Zn, Cu, Cd from Sludge by Organic Acid S.W. SONG 1*, L. WANG
5th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2015) Speciation Analysis and Removal of Heavy Metals Zn, Cu, Cd from Sludge by Organic Acid S.W. SONG 1*, L. WANG
Soil Composition. Air
 Soil Composition Air Soil Included Air Approximately 40 to 60% of the volume of a soil is actually empty space between the solid particles (voids). These voids are filled with air and/or water. The air
Soil Composition Air Soil Included Air Approximately 40 to 60% of the volume of a soil is actually empty space between the solid particles (voids). These voids are filled with air and/or water. The air
Plant Nutrients in Mineral Soils
 The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
Students are requested, in their own interests, to write legibly.
 School of Chemistry UNIVERSITY OF KWAZULU-NATAL, WESTVILLE NOVEMBER 2008 EXAMINATIONS CHEM 781: HONOURS ELECTIVES CHEM781: Speciation, Toxicity and Bioavailability DURATION: 1½ Hours TOTAL MARKS: 100 Internal
School of Chemistry UNIVERSITY OF KWAZULU-NATAL, WESTVILLE NOVEMBER 2008 EXAMINATIONS CHEM 781: HONOURS ELECTIVES CHEM781: Speciation, Toxicity and Bioavailability DURATION: 1½ Hours TOTAL MARKS: 100 Internal
Approximating Solubility/Availability & Communicating Information
 Approximating Solubility/Availability & Communicating Information Wayne P. Robarge Professor (Emeritus) Soil Physical Chemistry Department of Crop and Soil Sciences NC State University, Raleigh, NC Presented
Approximating Solubility/Availability & Communicating Information Wayne P. Robarge Professor (Emeritus) Soil Physical Chemistry Department of Crop and Soil Sciences NC State University, Raleigh, NC Presented
ENVE 424 Anaerobic Treatment
 ENVE 424 Anaerobic Treatment Lecture 6 Toxic substances in anaerobic treatment 2012 2013 Fall 01 Nov 2012 Assist. Prof. A. Evren Tugtas Basic Fundamentals Inhibition Competitive Inhibition Uncompetitive
ENVE 424 Anaerobic Treatment Lecture 6 Toxic substances in anaerobic treatment 2012 2013 Fall 01 Nov 2012 Assist. Prof. A. Evren Tugtas Basic Fundamentals Inhibition Competitive Inhibition Uncompetitive
Application note. Determination of metals in soil by microwave plasma - atomic emission spectrometry (MP-AES) using DTPA extraction.
 Determination of metals in soil by microwave plasma - atomic emission spectrometry (MP-AES) using DTPA extraction Application note Agriculture Authors Marília S. Teodoro1, Daniela Schiavo2, Mônica Ferreira
Determination of metals in soil by microwave plasma - atomic emission spectrometry (MP-AES) using DTPA extraction Application note Agriculture Authors Marília S. Teodoro1, Daniela Schiavo2, Mônica Ferreira
Supporting Information
 Supporting Information Extracellular Saccharide-Mediated Reduction of Au 3+ to Gold Nanoparticles: New Insights for Heavy Metals Biomineralization on Microbial Surfaces Fuxing Kang,, Xiaolei Qu, Pedro
Supporting Information Extracellular Saccharide-Mediated Reduction of Au 3+ to Gold Nanoparticles: New Insights for Heavy Metals Biomineralization on Microbial Surfaces Fuxing Kang,, Xiaolei Qu, Pedro
THE USE OF PHOSPHORUS AND OTHER SOIL AMENDMENTS FOR IN SITU STABILIZATION OF SOIL LEAD
 THE USE OF PHOSPHORUS AND OTHER SOIL AMENDMENTS FOR IN SITU STABILIZATION OF SOIL LEAD 1 G.M. Hettiarachchi and 2 G.M. Pierzynski Dept. of Agronomy, Kansas State University, Manhattan, KS 66506, 1 Phone:
THE USE OF PHOSPHORUS AND OTHER SOIL AMENDMENTS FOR IN SITU STABILIZATION OF SOIL LEAD 1 G.M. Hettiarachchi and 2 G.M. Pierzynski Dept. of Agronomy, Kansas State University, Manhattan, KS 66506, 1 Phone:
Thesis Summary THE CHEMICAL AND ANALYTICAL CONTROL OF HUMIC ACIDS IN NATURAL PRODUCTS
 Thesis Summary THE CHEMICAL AND ANALYTICAL CONTROL OF HUMIC ACIDS IN NATURAL PRODUCTS Scientific Coordinator, Prof. PhD Alexandru Popescu PhD student, Irina Daniela Nicolaescu The PhD thesis entitled The
Thesis Summary THE CHEMICAL AND ANALYTICAL CONTROL OF HUMIC ACIDS IN NATURAL PRODUCTS Scientific Coordinator, Prof. PhD Alexandru Popescu PhD student, Irina Daniela Nicolaescu The PhD thesis entitled The
Summary and general discussion
 Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL)
 Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL) Patty Lucas AAPFCO Laboratory Services Committee Meeting Friday, August 7, 2015 Fertilizer Sample
Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL) Patty Lucas AAPFCO Laboratory Services Committee Meeting Friday, August 7, 2015 Fertilizer Sample
New Materials for Removal of Trace Impurities from Environmental Waters
 New Materials for Removal of Trace Impurities from Environmental Waters July, 2010 John Sawyer Life-Changing Research and Development John.Sawyer@matricresearch.com Chemical and Environmental Technologies
New Materials for Removal of Trace Impurities from Environmental Waters July, 2010 John Sawyer Life-Changing Research and Development John.Sawyer@matricresearch.com Chemical and Environmental Technologies
Sequential extraction of Zn in deposol
 ORIGINAL SCIENTIFIC PAPER Sequential extraction of Zn in deposol Ivana Trajkovic 1, Vlado Licina 1, Zoran Atanackovic 1 1 Belgrade University, Faculty of Agriculture, Nemanjina 6, 11080 Belgrade, Serbia,
ORIGINAL SCIENTIFIC PAPER Sequential extraction of Zn in deposol Ivana Trajkovic 1, Vlado Licina 1, Zoran Atanackovic 1 1 Belgrade University, Faculty of Agriculture, Nemanjina 6, 11080 Belgrade, Serbia,
INTERNATIONAL ŒNOLOGICAL CODEX. BENTONITES Bentonita N SIN: 558 (Oeno 11/2003 modified Oeno )
 BENTONITES Bentonita N SIN: 558 (Oeno 11/2003 modified Oeno 441-2011) 1. OBJECT, ORIGIN AND FIELD OF APPLICATION Bentonites are hydrous aluminium silicates belonging to the montmorillonite group. The brute
BENTONITES Bentonita N SIN: 558 (Oeno 11/2003 modified Oeno 441-2011) 1. OBJECT, ORIGIN AND FIELD OF APPLICATION Bentonites are hydrous aluminium silicates belonging to the montmorillonite group. The brute
The Removal of Metal Ions (Cu 2+ and Zn 2+ ) using Waste-reclaimed Adsorbent for Plating Wastewater Treatment Process
 , October 20-22, 2010, San Francisco, USA The Removal of Metal Ions (Cu 2+ and Zn 2+ ) using Waste-reclaimed Adsorbent for Plating Wastewater Treatment Process Young-Hoon Jo, Si-Hyun Do, Yoon-Seok Jang,
, October 20-22, 2010, San Francisco, USA The Removal of Metal Ions (Cu 2+ and Zn 2+ ) using Waste-reclaimed Adsorbent for Plating Wastewater Treatment Process Young-Hoon Jo, Si-Hyun Do, Yoon-Seok Jang,
LONG-TERM GOALS OBJECTIVES
 Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
MESQUITA M. Eulália., VIEIRA e SILVA J.M., DOMINGUES Hermínia Estação Agronómica Nacional, Quinta do Marquês, 2780 Oeiras, Portugal.
 Scientific registration nº 1892 Symposium nº 25 Presentation: poster Competitive sorption of Cu and Zn by sludge amended acid soils Adsorption compétitive de cuivre et zinc par des sols acides amendés
Scientific registration nº 1892 Symposium nº 25 Presentation: poster Competitive sorption of Cu and Zn by sludge amended acid soils Adsorption compétitive de cuivre et zinc par des sols acides amendés
Bio-accessibility of Trace Metals Using Chemical Fractionation and In Vitro Extraction Methods
 Bio-accessibility of Trace Metals Using Chemical Fractionation and In Vitro Extraction Methods Naomi Waissman Assadian 1 and Juan Pedro Flores Margez 2 Abstract Rural and urban border communities at Paso
Bio-accessibility of Trace Metals Using Chemical Fractionation and In Vitro Extraction Methods Naomi Waissman Assadian 1 and Juan Pedro Flores Margez 2 Abstract Rural and urban border communities at Paso
Phosphate removal from secondary effluent of wastewater treatment: characterization and potential re-use as fertilizer of recovered precipitates
 TINOS 2015 Sustainable solid waste management Phosphate removal from secondary effluent of wastewater treatment: characterization and potential re-use as fertilizer of recovered precipitates Raptopoulou
TINOS 2015 Sustainable solid waste management Phosphate removal from secondary effluent of wastewater treatment: characterization and potential re-use as fertilizer of recovered precipitates Raptopoulou
Determination of available nutrients in soil using the Agilent 4200 MP-AES
 Determination of available nutrients in soil using the Agilent 4200 MP-AES Application note Agriculture Author Dharmendra Vummiti Agilent Technologies, India Introduction Multielement testing of soil samples
Determination of available nutrients in soil using the Agilent 4200 MP-AES Application note Agriculture Author Dharmendra Vummiti Agilent Technologies, India Introduction Multielement testing of soil samples
MARGAM SUNITHA, KANWAR L. SAHRAWAT, AND SUHAS P. WANI. Introduction
 Communications in Soil Science and Plant Analysis, 46:627 632, 2015 Copyright Taylor & Francis Group, LLC ISSN: 0010-3624 print / 1532-2416 online DOI: 10.1080/00103624.2015.1005226 Comparative Evaluation
Communications in Soil Science and Plant Analysis, 46:627 632, 2015 Copyright Taylor & Francis Group, LLC ISSN: 0010-3624 print / 1532-2416 online DOI: 10.1080/00103624.2015.1005226 Comparative Evaluation
The implementation of bioavailability in defining PNEC values for trace metals and metalloids in soil
 Department of Earth and Environmental Sciences Division of Soil and Water Management The implementation of bioavailability in defining PNEC values for trace metals and metalloids in soil Erik Smolders
Department of Earth and Environmental Sciences Division of Soil and Water Management The implementation of bioavailability in defining PNEC values for trace metals and metalloids in soil Erik Smolders
AVAILABILITY OF Cd, Ni AND Zn TO RYEGRASS IN SEWAGE SLUDGE-TREATED SOILS AT DIFFERENT TEMPERATURES
 AVAILABILITY OF Cd, Ni AND Zn TO RYEGRASS IN SEWAGE SLUDGE-TREATED SOILS AT DIFFERENT TEMPERATURES V. ANTONIADIS and B. J. ALLOWAY The University of Reading, Department of Soil Science, P.O. Box 233, Reading,
AVAILABILITY OF Cd, Ni AND Zn TO RYEGRASS IN SEWAGE SLUDGE-TREATED SOILS AT DIFFERENT TEMPERATURES V. ANTONIADIS and B. J. ALLOWAY The University of Reading, Department of Soil Science, P.O. Box 233, Reading,
THE REMOBILIZATION OF METALS FROM IRON OXIDES AND SEDIMENTS BY METAL-EDTA COMPLEXES
 THE REMOBILIZATION OF METALS FROM IRON OXIDES AND SEDIMENTS BY METAL-EDTA COMPLEXES B. NOWACK, F. G. KARI and H. G. KRÜGER Swiss Federal Institute for Environmental Science and Technology (EAWAG), Dübendorf,
THE REMOBILIZATION OF METALS FROM IRON OXIDES AND SEDIMENTS BY METAL-EDTA COMPLEXES B. NOWACK, F. G. KARI and H. G. KRÜGER Swiss Federal Institute for Environmental Science and Technology (EAWAG), Dübendorf,
Bioavailability and crop uptake of trace elements in soil columns amended with sewage sludge products
 Plant and Soil 262: 71 84, 2004. 2004 Kluwer Academic Publishers. Printed in the Netherlands. 71 Bioavailability and crop uptake of trace elements in soil columns amended with sewage sludge products M.B.
Plant and Soil 262: 71 84, 2004. 2004 Kluwer Academic Publishers. Printed in the Netherlands. 71 Bioavailability and crop uptake of trace elements in soil columns amended with sewage sludge products M.B.
Effect of salinity on Cd and Zn availability
 Symposium no. 33 Paper no. 08 Presentation: poster Effect of salinity on Cd and Zn availability KHOSHGOFTARMENSH A.H., JAAFARI B. and SHARIATMADARI H. Department of Soil Science, College of Agriculture,
Symposium no. 33 Paper no. 08 Presentation: poster Effect of salinity on Cd and Zn availability KHOSHGOFTARMENSH A.H., JAAFARI B. and SHARIATMADARI H. Department of Soil Science, College of Agriculture,
Junias Adusei-Gyamfi. Supervisors: University of Lille: Justine Criquet, Baghdad Ouddane TU Delft: Bas Heijman, Luuk Rietveld. Second DOC2C's workshop
 Characterization of Natural Organic Matter and processes during drinking water treatments Junias Adusei-Gyamfi 1 Supervisors: University of Lille: Justine Criquet, Baghdad Ouddane TU Delft: Bas Heijman,
Characterization of Natural Organic Matter and processes during drinking water treatments Junias Adusei-Gyamfi 1 Supervisors: University of Lille: Justine Criquet, Baghdad Ouddane TU Delft: Bas Heijman,
 anna.ida3@gmail.com/2013 Have you ever heard HUMUS?? A brown to black complex variable of carbon containing compounds as possessing cellular organization in the form of plant and animal bodies Derived
anna.ida3@gmail.com/2013 Have you ever heard HUMUS?? A brown to black complex variable of carbon containing compounds as possessing cellular organization in the form of plant and animal bodies Derived
The complexing capacities of humic substances isolated from Baltic sediments and their molecular weight fractions towards copper(ii) and iron (III)*
 The complexing capacities of humic substances isolated from Baltic sediments and their molecular weight fractions towards copper(ii) and iron (III)* OCEANOLOGIA, No. 34 pp. 39-47, 1993. PL ISSN 0078-3234
The complexing capacities of humic substances isolated from Baltic sediments and their molecular weight fractions towards copper(ii) and iron (III)* OCEANOLOGIA, No. 34 pp. 39-47, 1993. PL ISSN 0078-3234
CONCENTRATION AND SPECIATION OF Cu, Ni, Pb and Zn IN CULTIVATED AND UNCULTIVATED SOILS
 129 Bulgarian Journal of Agricultural Science, 15 (No 2) 2009, 129-134 Agricultural Academy CONCENTRATION AND SPECIATION OF Cu, Ni, Pb and Zn IN CULTIVATED AND UNCULTIVATED SOILS C. AYDINALP Uludag University,
129 Bulgarian Journal of Agricultural Science, 15 (No 2) 2009, 129-134 Agricultural Academy CONCENTRATION AND SPECIATION OF Cu, Ni, Pb and Zn IN CULTIVATED AND UNCULTIVATED SOILS C. AYDINALP Uludag University,
VOL. 5, NO. 6, June 2015 ISSN ARPN Journal of Science and Technology All rights reserved.
 VOL. 5, NO. 6, June 2015 ISSN 22-7217 Impact of Cumulative Sediment Deposition by Irrigation Water on Soil and Sugarcane in Savannah Sugar Company Limited; Numan, Adamawa State Nigeria 1 R.P. Ali, 2 H.M.
VOL. 5, NO. 6, June 2015 ISSN 22-7217 Impact of Cumulative Sediment Deposition by Irrigation Water on Soil and Sugarcane in Savannah Sugar Company Limited; Numan, Adamawa State Nigeria 1 R.P. Ali, 2 H.M.
Long-term acid generation containing heavy metals from the tailings of a closed mine and its countermeasures
 Long-term acid generation containing heavy metals from the tailings of a closed mine and its countermeasures Kimleang KHOEURN Candidate for the Degree of Doctorate in Engineering Supervisor : Professor
Long-term acid generation containing heavy metals from the tailings of a closed mine and its countermeasures Kimleang KHOEURN Candidate for the Degree of Doctorate in Engineering Supervisor : Professor
DOMOGRAN 45 ACTIVATING YOUR NUTRIENT POTENTIAL THE NITROGEN-SULFUR FERTILIZER FROM LEUNA
 DOMOGRAN 45 ACTIVATING YOUR NUTRIENT POTENTIAL THE NITROGEN-SULFUR FERTILIZER FROM LEUNA www.domogran.de DOMOGRAN 45 nitrogen and sulfur fertilizer for positive nutrient dynamics Because of its attraction
DOMOGRAN 45 ACTIVATING YOUR NUTRIENT POTENTIAL THE NITROGEN-SULFUR FERTILIZER FROM LEUNA www.domogran.de DOMOGRAN 45 nitrogen and sulfur fertilizer for positive nutrient dynamics Because of its attraction
Trace metals in the ocean Lecture January 23, 2006
 Trace metals in the ocean 12.097 Lecture January 23, 2006 Metals of interest Required for metabolic functions Mn, Fe, Co, Ni, Cu, Zn Deficiency limits production (photosynthetic ability) Excess limits
Trace metals in the ocean 12.097 Lecture January 23, 2006 Metals of interest Required for metabolic functions Mn, Fe, Co, Ni, Cu, Zn Deficiency limits production (photosynthetic ability) Excess limits
AD-Net Research Colloquium Sept 2017 Choosing Trace Elements to Maximise Benefits (to AD)
 AD-Net Research Colloquium 11-12 Sept 2017 Choosing Trace Elements to Maximise Benefits (to AD) Cynthia Carliell-Marquet Honorary Senior Research Fellow, University of Birmingham Senior Process Designer,
AD-Net Research Colloquium 11-12 Sept 2017 Choosing Trace Elements to Maximise Benefits (to AD) Cynthia Carliell-Marquet Honorary Senior Research Fellow, University of Birmingham Senior Process Designer,
Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka
 , 152-158 Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka W.S. Madurapperuma and D. Kumaragamage 1 Postgraduate Institute of Agriculture University
, 152-158 Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka W.S. Madurapperuma and D. Kumaragamage 1 Postgraduate Institute of Agriculture University
MULTI-COMPONENT ANALYSIS OF HEAVY METALS
 MULTI-COMPONENT ANALYSIS OF HEAVY METALS in Various Foods Using Shimadzu s AA, ICP, ICPMS & EDX HEAVY METALS TESTING HEAVY METALS POSE A SIGNIFICANT THREAT TO HUMAN HEALTH The U.S. Food and Drug Administration
MULTI-COMPONENT ANALYSIS OF HEAVY METALS in Various Foods Using Shimadzu s AA, ICP, ICPMS & EDX HEAVY METALS TESTING HEAVY METALS POSE A SIGNIFICANT THREAT TO HUMAN HEALTH The U.S. Food and Drug Administration
BOTANY AND PLANT GROWTH Lesson 9: PLANT NUTRITION. MACRONUTRIENTS Found in air and water carbon C oxygen hydrogen
 BOTANY AND PLANT GROWTH Lesson 9: PLANT NUTRITION Segment One Nutrient Listing Plants need 17 elements for normal growth. Carbon, oxygen, and hydrogen are found in air and water. Nitrogen, phosphorus,
BOTANY AND PLANT GROWTH Lesson 9: PLANT NUTRITION Segment One Nutrient Listing Plants need 17 elements for normal growth. Carbon, oxygen, and hydrogen are found in air and water. Nitrogen, phosphorus,
Total zinc was determined by digesting the soil ZnSO 4
 ADVANCE RESEARCH JOURNAL OF CROP IMPROVEMENT Volume 2 Issue 2 (December, 2011) Page : 203-207 Received : September, 2011; Revised : October, 2011; Accepted : November, 2011 Research Paper See end of the
ADVANCE RESEARCH JOURNAL OF CROP IMPROVEMENT Volume 2 Issue 2 (December, 2011) Page : 203-207 Received : September, 2011; Revised : October, 2011; Accepted : November, 2011 Research Paper See end of the
Nutrients & Diagnosing Nutrient Needs. Carrie Laboski Dept. of Soil Science UW-Madison
 Nutrients & Diagnosing Nutrient Needs Carrie Laboski Dept. of Soil Science UW-Madison Sources of nutrients available for plant uptake Nutrients in the soil solution are: In ionic form At low concentration
Nutrients & Diagnosing Nutrient Needs Carrie Laboski Dept. of Soil Science UW-Madison Sources of nutrients available for plant uptake Nutrients in the soil solution are: In ionic form At low concentration
SUPPORTING INFORMATION. Zinc isotope fractionation as an indicator of geochemical attenuation processes
 Zinc isotope fractionation as an indicator of geochemical attenuation processes Harish Veeramani 1 *, Jane Eagling 1, Julia H. Jamieson-Hanes 1, Lingyi Kong 1, Carol J. Ptacek 1 and David W Blowes 1 1
Zinc isotope fractionation as an indicator of geochemical attenuation processes Harish Veeramani 1 *, Jane Eagling 1, Julia H. Jamieson-Hanes 1, Lingyi Kong 1, Carol J. Ptacek 1 and David W Blowes 1 1
Biochar: Contaminant source or sink?
 Federal Department of Economic Affairs FDEA Agroscope ReckenholzTänikon Research Station ART Biochar: Contaminant source or sink? Isabel Hilber, Franziska Blum, Hans Peter Schmidt, Sarah Hale, Gerard Cornelissen,
Federal Department of Economic Affairs FDEA Agroscope ReckenholzTänikon Research Station ART Biochar: Contaminant source or sink? Isabel Hilber, Franziska Blum, Hans Peter Schmidt, Sarah Hale, Gerard Cornelissen,
Welcome. Greg Patterson C.C.A. President A&L Canada Laboratories
 Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Discussion Soil test levels Dropping P,K Organic matter levels dropping Cost of Fertilizer Increasing due to Global Demand Environmental
Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Discussion Soil test levels Dropping P,K Organic matter levels dropping Cost of Fertilizer Increasing due to Global Demand Environmental
Scientific registration number: 2271 Symposium n o : 25 Presentation : poster. OLIVEIRA Fernando Carvalho (2) ; MATTIAZZO Maria Emilia (2)
 Scientific registration number: 2271 Symposium n o : 25 Presentation : poster Copper, Nickel and Zinc availability to corn plants in acid soils amended with sewage sludge (1) Disponibilité en cuivre, en
Scientific registration number: 2271 Symposium n o : 25 Presentation : poster Copper, Nickel and Zinc availability to corn plants in acid soils amended with sewage sludge (1) Disponibilité en cuivre, en
Ion-exchange technique (IET) for measuring Cu 2+, Ni 2+ and Zn 2+ activities in soils contaminated with metal mixtures
 , 14, 55 63 Supplementary material Ion-exchange technique (IET) for measuring Cu 2+, Ni 2+ and Zn 2+ activities in soils contaminated with metal mixtures D. M. Schwertfeger A,B and W. H. Hendershot A,C
, 14, 55 63 Supplementary material Ion-exchange technique (IET) for measuring Cu 2+, Ni 2+ and Zn 2+ activities in soils contaminated with metal mixtures D. M. Schwertfeger A,B and W. H. Hendershot A,C
TRACE ELEMENTS IN URINE. Event #1, 2010
 TRACE ELEMENTS IN URINE Event #, 00 March 6, 00 Event #, 00 Urine Arsenic The source of the test materials is human urine obtained from donor volunteers with informed consent. Urine was collected into
TRACE ELEMENTS IN URINE Event #, 00 March 6, 00 Event #, 00 Urine Arsenic The source of the test materials is human urine obtained from donor volunteers with informed consent. Urine was collected into
Understanding a Soil Report
 Understanding a Soil Report AGRONOMY SOIL ANALYSIS 1. Soil ph Soil ph is a measure of the acidity in the soil. An acidic soil has a greater amount of hydrogen (H+) ions and a ph below 7.0. Values above
Understanding a Soil Report AGRONOMY SOIL ANALYSIS 1. Soil ph Soil ph is a measure of the acidity in the soil. An acidic soil has a greater amount of hydrogen (H+) ions and a ph below 7.0. Values above
FACT SHEET. Understanding Cation Exchange Capacity and % Base Saturation
 Understanding Cation Exchange Capacity and % Base Saturation FACT SHEET A & L CANADA LABORATORIES, INC. 2136 Jetstream Rd. London, ON N5V 3P5 Phone: 519-457-2575 Fax: 519-457-2664 Aginfo@alcanada.com www.alcanada.com
Understanding Cation Exchange Capacity and % Base Saturation FACT SHEET A & L CANADA LABORATORIES, INC. 2136 Jetstream Rd. London, ON N5V 3P5 Phone: 519-457-2575 Fax: 519-457-2664 Aginfo@alcanada.com www.alcanada.com
Effects of Aluminum and Silicon as Additive Materials for the Zinc Anode in Zn-Air Batteries
 Journal of the Korean Electrochemical Society Vol. 21, No. 1, 2018, 12-20 https://doi.org/10.5229/jkes.2018.21.1.12 pissn 1229-1935 eissn 2288-9000 Effects of Aluminum and Silicon as Additive Materials
Journal of the Korean Electrochemical Society Vol. 21, No. 1, 2018, 12-20 https://doi.org/10.5229/jkes.2018.21.1.12 pissn 1229-1935 eissn 2288-9000 Effects of Aluminum and Silicon as Additive Materials
AVAILABLE Cd CONTENT OF SALT AFFECTED AND NORMAL SOILS OF HALASTRA KALOHORI AREA
 Global NEST Journal, Vol 9, No 3, pp 195-200, 2007 Copyright 2007 Global NEST Printed in Greece. All rights reserved AVAILABLE Cd CONTENT OF SALT AFFECTED AND NORMAL SOILS OF HALASTRA KALOHORI AREA TH.
Global NEST Journal, Vol 9, No 3, pp 195-200, 2007 Copyright 2007 Global NEST Printed in Greece. All rights reserved AVAILABLE Cd CONTENT OF SALT AFFECTED AND NORMAL SOILS OF HALASTRA KALOHORI AREA TH.
Determination of metals in industrial wastewaters by microwave plasmaatomic emission spectrometry
 Determination of metals in industrial wastewaters by microwave plasmaatomic emission spectrometry Application note Environmental Author Terrance Hettipathirana Agilent Technologies Melbourne, Australia
Determination of metals in industrial wastewaters by microwave plasmaatomic emission spectrometry Application note Environmental Author Terrance Hettipathirana Agilent Technologies Melbourne, Australia
Removal of Cu 2+ and Zn 2+ in Aqueous Solutions by Sorption onto Fly Ash and Fly Ash Mixtures
 Removal of Cu 2+ and Zn 2+ in Aqueous Solutions by Sorption onto Fly Ash and Fly Ash Mixtures V. Héquet 1, P. Ricou 1, I. Lecuyer 2 and P. Le Cloirec 1 1 Ecole des Mines de Nantes, Dept. Systèmes Energétiques
Removal of Cu 2+ and Zn 2+ in Aqueous Solutions by Sorption onto Fly Ash and Fly Ash Mixtures V. Héquet 1, P. Ricou 1, I. Lecuyer 2 and P. Le Cloirec 1 1 Ecole des Mines de Nantes, Dept. Systèmes Energétiques
Essential Elements. Original research don by Julius von Sachs 1860 using hydroponics
 Essential Elements Original research don by Julius von Sachs 1860 using hydroponics Using various solutions found ones that supported plant life Sachs found several elements that were needed in relatively
Essential Elements Original research don by Julius von Sachs 1860 using hydroponics Using various solutions found ones that supported plant life Sachs found several elements that were needed in relatively
Leaching Behavior of Coal Combustion Products and the Environmental Implication in Road Construction. Project Progress Report. Jianmin Wang NUTC R201
 Leaching Behavior of Coal Combustion Products and the Environmental Implication in Road Construction Project Progress Report by Jianmin Wang NUTC R21 Disclaimer The contents of this report reflect the
Leaching Behavior of Coal Combustion Products and the Environmental Implication in Road Construction Project Progress Report by Jianmin Wang NUTC R21 Disclaimer The contents of this report reflect the
Zinc isotopes in the Seine River water, France: a probe of. anthropogenic contamination
 Zinc isotopes in the Seine River water, France: a probe of anthropogenic contamination Jiubin Chen*, Jérôme Gaillardet and Pascale Louvat Equipe Géochimie et Cosmochimie, Institut de Physique du Globe
Zinc isotopes in the Seine River water, France: a probe of anthropogenic contamination Jiubin Chen*, Jérôme Gaillardet and Pascale Louvat Equipe Géochimie et Cosmochimie, Institut de Physique du Globe
Basic Consideration on EAF Dust Treatment Using Hydrometallurgical Processes
 Resources Processing 55 : 144 148 (2008) Original Paper RESOURCES PROCESSING Basic Consideration on EAF Dust Treatment Using Hydrometallurgical Processes T. NAKAMURA 1, E. SHIBATA 1, T. TAKASU 2 and H.
Resources Processing 55 : 144 148 (2008) Original Paper RESOURCES PROCESSING Basic Consideration on EAF Dust Treatment Using Hydrometallurgical Processes T. NAKAMURA 1, E. SHIBATA 1, T. TAKASU 2 and H.
Supplying Nutrients to Crops
 Supplying Nutrients to Crops What is Plant Nutrition? Plants need nutrients for healthy growth and development. Plant nutrition involves the absorption of nutrients for plant growth and is dependent on
Supplying Nutrients to Crops What is Plant Nutrition? Plants need nutrients for healthy growth and development. Plant nutrition involves the absorption of nutrients for plant growth and is dependent on
Using DGT to Measure Bioavailable Metals in a Constructed Wetland Treatment System
 Using DGT to Measure Bioavailable Metals in a Constructed Wetland Treatment System Michael H. Paller (Savannah River National Laboratory) Anna Sophia Knox (Savannah River National Laboratory) Coral Springs,
Using DGT to Measure Bioavailable Metals in a Constructed Wetland Treatment System Michael H. Paller (Savannah River National Laboratory) Anna Sophia Knox (Savannah River National Laboratory) Coral Springs,
EconovaPlus Fertiliser
 EconovaPlus Fertiliser The complete plant growth fertiliser, bio-stimulater & carbon control solution. A bio-fertiliser based on the need for organic mineral complexes in the soil. Manufactured by building
EconovaPlus Fertiliser The complete plant growth fertiliser, bio-stimulater & carbon control solution. A bio-fertiliser based on the need for organic mineral complexes in the soil. Manufactured by building
Sulfate Radical-Mediated Degradation of Sulfadiazine by CuFeO 2 Rhombohedral Crystal-Catalyzed Peroxymonosulfate: Synergistic Effects and Mechanisms
 Supporting Information for Sulfate Radical-Mediated Degradation of Sulfadiazine by CuFeO 2 Rhombohedral Crystal-Catalyzed Peroxymonosulfate: Synergistic Effects and Mechanisms Submitted by Yong Feng, Deli
Supporting Information for Sulfate Radical-Mediated Degradation of Sulfadiazine by CuFeO 2 Rhombohedral Crystal-Catalyzed Peroxymonosulfate: Synergistic Effects and Mechanisms Submitted by Yong Feng, Deli
There are two groups of minerals: Major salt components: K, Na, Ca, Mg, Cl -, sulfate, phosphate, and HCO
 MINERALS INTRODUCTION 90 elements in the earth s s crust, 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
MINERALS INTRODUCTION 90 elements in the earth s s crust, 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
Supporting information for the manuscript
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supporting information for the manuscript Toward enhanced photoactivity
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supporting information for the manuscript Toward enhanced photoactivity
Report for using aquatic plant as phytoremediation for removing heavy metals
 Report for using aquatic plant as phytoremediation for removing heavy metals Vu Thi Dieu Huong (M2) 1. INTRODUCTION Charophytes are submerged macrophytes grown in wide range of water bodies and its existence
Report for using aquatic plant as phytoremediation for removing heavy metals Vu Thi Dieu Huong (M2) 1. INTRODUCTION Charophytes are submerged macrophytes grown in wide range of water bodies and its existence
Bioavailability of Cd to Food Crops in
 Vol. 28, pp. 39-43, /979 Bioavailability of Cd to Food Crops in Relation to Heavy Metal Content of Sludge-Amended Soil by Frank T. Bingham* Results of greenhouse and laboratory experiments on factors influencing
Vol. 28, pp. 39-43, /979 Bioavailability of Cd to Food Crops in Relation to Heavy Metal Content of Sludge-Amended Soil by Frank T. Bingham* Results of greenhouse and laboratory experiments on factors influencing
TREE ESSENTIAL ELEMENT. ZINC (Zn)
 Pub. No. 17 April 2016 TREE ESSENTIAL ELEMENT ZINC (Zn) By Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Zinc (Zn) is a
Pub. No. 17 April 2016 TREE ESSENTIAL ELEMENT ZINC (Zn) By Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Zinc (Zn) is a
Detect, remove and re-use: a new paradigm in sensing and removal of Hg (II) from wastewater via SERS-active ZnO/Ag nano-arrays
 Supporting Information 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Detect, remove and re-use: a new paradigm in sensing and removal of Hg (II) from wastewater via SERS-active ZnO/Ag nano-arrays
Supporting Information 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Detect, remove and re-use: a new paradigm in sensing and removal of Hg (II) from wastewater via SERS-active ZnO/Ag nano-arrays
Determination of Copper in Green Olives using ICP-OES
 Application Note Food and Agriculture Determination of Copper in Green Olives using ICP-OES Intelligent Rinse function reduced analysis time by 60%, saving 191.4 L of argon Authors Ryley Burgess, Agilent
Application Note Food and Agriculture Determination of Copper in Green Olives using ICP-OES Intelligent Rinse function reduced analysis time by 60%, saving 191.4 L of argon Authors Ryley Burgess, Agilent
Qualitative chemical reaction of functional group in protein
 Qualitative chemical reaction of functional group in protein Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product formed by
Qualitative chemical reaction of functional group in protein Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product formed by
EUROPEAN QUALIFYING EXAMINATION 2006
 EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
Highly selective colorimetric schiff base chemosensor for detection of Cu 2+
 Indian Journal of Chemistry Vol. 57A, April 2018, pp. 490-494 Highly selective colorimetric schiff base chemosensor for detection of Cu 2+ Dan Liu, Hua Zhu* & Li Yang School of Chemistry and Chemical Engineering,
Indian Journal of Chemistry Vol. 57A, April 2018, pp. 490-494 Highly selective colorimetric schiff base chemosensor for detection of Cu 2+ Dan Liu, Hua Zhu* & Li Yang School of Chemistry and Chemical Engineering,
HEPARIN SODIUM. Heparinum natricum
 Pharmeuropa 25.1 1 Reference: PA/PH/Exp. 6/T (12) 37 ANP NOTE ON THE MONOGRAPH Definition. It is proposed to restrict the scope to heparin material of porcine origin since some of the latest requirements
Pharmeuropa 25.1 1 Reference: PA/PH/Exp. 6/T (12) 37 ANP NOTE ON THE MONOGRAPH Definition. It is proposed to restrict the scope to heparin material of porcine origin since some of the latest requirements
D. E. Crowley and W. Smith Department of Soil and Environmental Sciences, University of California, Riverside, California.
 California Avocado Society 1995 Yearbook 79: 171-183 SOIL FACTORS ASSOCIATED WITH ZINC DEFICIENCY IN AVOCADO D. E. Crowley and W. Smith Department of Soil and Environmental Sciences, University of California,
California Avocado Society 1995 Yearbook 79: 171-183 SOIL FACTORS ASSOCIATED WITH ZINC DEFICIENCY IN AVOCADO D. E. Crowley and W. Smith Department of Soil and Environmental Sciences, University of California,
THE EFFECT OF SELENIUM ON THE ACCUMULATION OF SOME METALS IN Zea mays L. PLANTS TREATED WITH INDOLE-3-ACETIC ACID
 CELLULAR & MOLECULAR BIOLOGY LETTERS Volume 8, (2003) pp 97 103 http://www.cmbl.org.pl Received 14 October 2002 Accepted 24 January 2003 Short Communication THE EFFECT OF SELENIUM ON THE ACCUMULATION OF
CELLULAR & MOLECULAR BIOLOGY LETTERS Volume 8, (2003) pp 97 103 http://www.cmbl.org.pl Received 14 October 2002 Accepted 24 January 2003 Short Communication THE EFFECT OF SELENIUM ON THE ACCUMULATION OF
CATION-EXCHANGE CHARACTERISTICS OF WHEAT, BARLEY AND PEA DEPENDING ON THE OSMOTIC PRESSURE IN NUTRIENT SOLUTIONS OF LOW ph
 BULG. J. PLANT PHYSIOL., 2002, 28(3 4), 35 45 35 CATION-EXCHANGE CHARACTERISTICS OF WHEAT, BARLEY AND PEA DEPENDING ON THE OSMOTIC PRESSURE IN NUTRIENT SOLUTIONS OF LOW ph Antoaneta Arsova * N. Pushkarov
BULG. J. PLANT PHYSIOL., 2002, 28(3 4), 35 45 35 CATION-EXCHANGE CHARACTERISTICS OF WHEAT, BARLEY AND PEA DEPENDING ON THE OSMOTIC PRESSURE IN NUTRIENT SOLUTIONS OF LOW ph Antoaneta Arsova * N. Pushkarov
Structural and Zn-binding features of ortho- and pyrophosphate modified soil metal-humus sorbents (Features of P-modified humus sorbents)
 Int. Agrophysics, 22, 16, 197 22 INTERNATIONAL Agrophysics www.ipan.lublin.pl/int-agrophysics Structural and Zn-binding features of ortho- and pyrophosphate modified soil metal-humus sorbents (Features
Int. Agrophysics, 22, 16, 197 22 INTERNATIONAL Agrophysics www.ipan.lublin.pl/int-agrophysics Structural and Zn-binding features of ortho- and pyrophosphate modified soil metal-humus sorbents (Features
Improving Micronutrient Fluid Fertilizers using Novel Chelating Agents
 Improving Micronutrient Fluid Fertilizers using Novel Chelating Agents Mike McLaughlin 12 1,2 and dsamuel lstacey 1 1 Earth and Environmental Sciences, The University of Adelaide, AUSTRALIA 2 CSIRO Land
Improving Micronutrient Fluid Fertilizers using Novel Chelating Agents Mike McLaughlin 12 1,2 and dsamuel lstacey 1 1 Earth and Environmental Sciences, The University of Adelaide, AUSTRALIA 2 CSIRO Land
Appendix C Metal Speciation
 Appendix C Metal Speciation GHD Report for Vista Gold Australia - Mt Todd Gold Mine, 43/22187 SPECIATION MODELLING OF METALS IN SURFACE WATERS OF THE EDITH RIVER DURING WET SEASON DISCHARGE OF WASTEWATER
Appendix C Metal Speciation GHD Report for Vista Gold Australia - Mt Todd Gold Mine, 43/22187 SPECIATION MODELLING OF METALS IN SURFACE WATERS OF THE EDITH RIVER DURING WET SEASON DISCHARGE OF WASTEWATER
INTERNATIONAL SIMPOSIUM 1996
 INTERNATIONAL SIMPOSIUM 1996 MICROFERTIGATION Dr. Andres Rrevalo Fuentes EURONOVEOHDES RCRICOLBS SPfllK simposiuin internacional 199G HON. PRINCIPAL HDVflNTEGES RND DISRDVRNTRGES OF FER- ADVANTAGES: *
INTERNATIONAL SIMPOSIUM 1996 MICROFERTIGATION Dr. Andres Rrevalo Fuentes EURONOVEOHDES RCRICOLBS SPfllK simposiuin internacional 199G HON. PRINCIPAL HDVflNTEGES RND DISRDVRNTRGES OF FER- ADVANTAGES: *
Award Number: N
 Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Immobilizing Arsenic in Contaminated Soil Using Humic Mineral Concentrates
 Immobilizing in Contaminated Soil Using Humic Mineral Concentrates Alexander I.Shulgin 1 and D. Joseph Hagerty 2, F. ASCE, PE 1. Research Professor, Chemical Engineering Dept., U. Louisville, Louisville,
Immobilizing in Contaminated Soil Using Humic Mineral Concentrates Alexander I.Shulgin 1 and D. Joseph Hagerty 2, F. ASCE, PE 1. Research Professor, Chemical Engineering Dept., U. Louisville, Louisville,
Topics. Phosphorus cycle. Soil Fertility Management 5 Phosphorus. ZEF IPADS Joint Lecture (18 22 Jan. 2016)
 International Program in Agricultural Development Studies (IPADS) 20 January 2016 IPADS Soil Fertility Management 6 Phosphorus Department of Global Agricultural Sciences IPADS Kensuke OKADA (akokada@mail.ecc.u
International Program in Agricultural Development Studies (IPADS) 20 January 2016 IPADS Soil Fertility Management 6 Phosphorus Department of Global Agricultural Sciences IPADS Kensuke OKADA (akokada@mail.ecc.u
Biochemical Techniques 06 Salt Fractionation of Proteins. Biochemistry
 . 1 Description of Module Subject Name Paper Name 12 Module Name/Title 2 1. Objectives Understanding the concept of protein fractionation Understanding protein fractionation with salt 2. Concept Map 3.
. 1 Description of Module Subject Name Paper Name 12 Module Name/Title 2 1. Objectives Understanding the concept of protein fractionation Understanding protein fractionation with salt 2. Concept Map 3.
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
FACTORS AFFECTING WATER QUALITY
 TECHNICAL PAPER WATER QUALITY PLANT HEALTH FACTORS Water quality is one of the most important factors affecting plant growth, as unwanted components in water can interfere with nutrient availability and
TECHNICAL PAPER WATER QUALITY PLANT HEALTH FACTORS Water quality is one of the most important factors affecting plant growth, as unwanted components in water can interfere with nutrient availability and
THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS
 THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS Richard Murphy Ph.D. Alltech European Bioscience Centre Ireland THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS
THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS Richard Murphy Ph.D. Alltech European Bioscience Centre Ireland THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS
Matrix Reference Materials - SCP SCIENCE
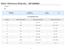 EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
GEOCHEMICAL MODELING TO IMPROVE METALS REMEDIATION
 GEORGIA ENVIRONMENTAL CONFERENCE JEKYLL ISLAND GEOCHEMICAL MODELING TO IMPROVE METALS REMEDIATION 1 GEOCHEMICAL MODELING BASICS Data Required Field Parameters (ph, ORP) Water quality parameters Major cations/anions
GEORGIA ENVIRONMENTAL CONFERENCE JEKYLL ISLAND GEOCHEMICAL MODELING TO IMPROVE METALS REMEDIATION 1 GEOCHEMICAL MODELING BASICS Data Required Field Parameters (ph, ORP) Water quality parameters Major cations/anions
Salts and Chlorides Remediation
 Salts and Chlorides Remediation The remediation of salts and chlorides is not a process of consumption but rather a process of binding, buffering, immobilization, detoxification, filtering, or conversion
Salts and Chlorides Remediation The remediation of salts and chlorides is not a process of consumption but rather a process of binding, buffering, immobilization, detoxification, filtering, or conversion
Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, , P. R.
 Electrochemical synthesis of p-type Zn-Doped α-fe 2 O 3 nanotube arrays for photoelectrochemical water splitting Xiaopeng Qi, a,b Guangwei She,* a Meng Wang, a,b Lixuan Mu, a and Wensheng Shi* a a Key
Electrochemical synthesis of p-type Zn-Doped α-fe 2 O 3 nanotube arrays for photoelectrochemical water splitting Xiaopeng Qi, a,b Guangwei She,* a Meng Wang, a,b Lixuan Mu, a and Wensheng Shi* a a Key
US EPA SW-846 Method 6010D using the Thermo Scientific icap 7400 ICP-OES Duo
 APPLICATION NOTE 43152 US EPA SW-846 Method 6010D using the Thermo Scientific icap 7400 ICP-OES Duo Authors James Hannan, Application Specialist, Thermo Fisher Scientific, Hemel Hempstead, UK Keywords
APPLICATION NOTE 43152 US EPA SW-846 Method 6010D using the Thermo Scientific icap 7400 ICP-OES Duo Authors James Hannan, Application Specialist, Thermo Fisher Scientific, Hemel Hempstead, UK Keywords
Morphology Control of ZnO with Citrate: A Time and Concentration Dependent Mechanistic Insight. Somnath Das, Kingshuk Dutta and Amitava Pramanik*
 Morphology Control of ZnO with Citrate: A Time and Concentration Dependent Mechanistic Insight Somnath Das, Kingshuk Dutta and Amitava Pramanik* Unilever R and D Bangalore, 64 Main Road, Whitefield, Bangalore
Morphology Control of ZnO with Citrate: A Time and Concentration Dependent Mechanistic Insight Somnath Das, Kingshuk Dutta and Amitava Pramanik* Unilever R and D Bangalore, 64 Main Road, Whitefield, Bangalore
25 FOLIAR SPRAYS. average 4750g dried leaf material (Embelton in The Citrus Industry Vol 2).
 25 Plants can obtain all their non-gaseous requirements via the root system. However, most plant organs including woody parts can also absorbed nutrients from solutions (Wittwer, 1963). Although the leaves
25 Plants can obtain all their non-gaseous requirements via the root system. However, most plant organs including woody parts can also absorbed nutrients from solutions (Wittwer, 1963). Although the leaves
For nmental. Written By: Agustin o, Professor. Developed in. and justice for all. Department of. funded by activities. )
 Site-Specificc Nutrient Management For Nutrient Management Planning To Improve Crop Production, Environ nmental Quality, and Economic Return Calcium and Magnesium: Chapter 6 of 10 Written By: Agustin Pagani,,
Site-Specificc Nutrient Management For Nutrient Management Planning To Improve Crop Production, Environ nmental Quality, and Economic Return Calcium and Magnesium: Chapter 6 of 10 Written By: Agustin Pagani,,
TITLE: Phosphorus availability to beans via interactions between mycorrhizae and biochar
 TITLE: Phosphorus availability to beans via interactions between mycorrhizae and biochar SUPPLEMENTARY ONLINE INFORMATION Authors: Steven J. Vanek 1* and Johannes Lehmann 1 1 Department of Geography, Penn
TITLE: Phosphorus availability to beans via interactions between mycorrhizae and biochar SUPPLEMENTARY ONLINE INFORMATION Authors: Steven J. Vanek 1* and Johannes Lehmann 1 1 Department of Geography, Penn
Evaluation of AB - DTPA Extractant for Multinutrients Extraction in Soils
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-776 Volume 7 Number 3 (218) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/1.2546/ijcmas.218.73.141
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-776 Volume 7 Number 3 (218) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/1.2546/ijcmas.218.73.141
BRIEFING Assay + + +
 BRIEFING Sodium Starch Glycolate, NF 22 page 2933 and page 3202 of PF 22(6) [Nov. Dec. 1996]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the
BRIEFING Sodium Starch Glycolate, NF 22 page 2933 and page 3202 of PF 22(6) [Nov. Dec. 1996]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the
