Gyan Rai, PhD 06/04/2015
|
|
|
- Darrell Wheeler
- 6 years ago
- Views:
Transcription
1 * Gyan Rai, PhD 06/04/2015
2 Objectives: 2 1. Current Definition of Probiotics 2. Regulatory Categorization & Intended Uses 3. Probiotics & Medical Foods- How to Fit? 4. Regulatory Implications & Considerations
3 What are Probiotics? 3 Live microorganisms, which, when administered in adequate amounts, confers a health benefit on the host United Nations FAO/WHO Working Group Report; 2002 Report did not consider the following in the scope: Biotherapeutic agents Beneficial microorganisms not used in food GMOs
4 Only Drugs can make Disease Claims : 4 Diagnosis, Cure, Mitigation, Treatment, or Prevention of disease Claims suggesting the product has an effect on a specific disease or class of disease Protective against XXX Protects the development of XXX Reduces the pain and stiffness associated with diarrhea / arthritis Decreases the effects of XXX Alleviates constipation / diarrhea Signs and symptoms of the disease: Improves memory (Alzeimers ) Improves urine flow in men (prostate) Lowers cholesterol (hypercholesterolemia) Reduces joint pain (arthritis) Relieves headaches (migraine)
5 Probiotics as a Dietary Supplement? 5 Unlike Drugs or Biologics: Do not require pre-market FDA approval Safety, purity, potency, and efficacy need not be established Burden is generally on FDA to prove supplement is unsafe Can make structure/function claims if: Truthful & not misleading Handout articles without referencing product Must be submitted to FDA within 30 days of marketing Must carry FDA disclaimer Containing one or more dietary ingredient : Vitamin Mineral Herb/botanical Amino acid Dietary substance to supplement the diet by increasing total dietary intake Concentrate, metabolite, etc. of any of the above Intended for ingestion; and Not represented as a conventional food or as a sole item of meal/diet (i.e. intended to supplement the diet) (21 U.S.C. 321(ff))
6 What is Medical Food? 6 Food which is formulated to be consumed or administered enterally (through GI tract, whether orally or by tube) under a physician s supervision and which is intended for specific dietary management of a disease or condition for which distinctive nutritional requirements (based on recognized scientific principles) are established by medical evaluation (21 U.S.C. 360ee(b)(3))
7 Types of Medical Food Claims: 7 Examples: Nutrient deficiency: Provides vitamin C and to prevent scurvy (allowed if relevant to the United States population) Nutritionally complete formula to support malnutrition in GI compromised patients A phenylalanine-free food for the nutritional management of hyperphenylalaninemia A nutritionally complete formula providing concentrated source of calories for patients with restricted fluid intake or increased metabolic needs such as oncology patients, hypermetabolic conditions, trauma, sepsis, and post surgery
8 Claim Substantiation of Medical Foods: 8 No specific medical food regulations for clinical substantiation of claims The need for a strong standard of scientific evidence for the composition and effectiveness of medical foods The scientific standard contained in the statutory medical food definition may require some of the same types of data for medical foods as are needed to support drug claims (e.g., data from clinical investigations) FDA proposed to evaluate its medical foods oversight Safety information about the ingredient/product should be widely available (61 Fed. Reg (November 29, 1996))
9 Criteria for Medical Foods: 9 Specially formulated and processed for partial or exclusive feeding of patient orally or by enteral tube; and Intended for use only under medical supervision; and Intended only for patient receiving active/ongoing medical treatment; and Intended for dietary management of a patient which cannot be achieved by the modification of the normal diet (e.g., chronic medical needs; limited/impaired capacity to ingest, digest, etc.; other special medically-determined nutrient needs); and Provides nutritional support to manage the distinct nutritional requirements resulting from a specific disease/condition (21 C.F.R (j)(8)
10 10 The Year 2013:
11 The IRB Guidance: Why is it Important? 11 According to the FDA, Food use is primarily for its taste, aroma, or nutritive value Biological Endpoints require IND: Sections VI.C. & VI.D. extend the existing IND requirements to any clinical research measuring drug/biologic (disease) endpoints that involves any articles of food, dietary supplement, and cosmetics Forces IRB to opt for an IND Essentially bans responsible clinical research in the absence of an IND for articles that are not drugs or new drugs
12 12 Biological end point of a drug v/s food : why claims matter? FDA strictly interprets: intent to study element of clinical study is synonymous to the intended use element of product An Unapproved Drug: A product marketed or promoted as a treatment, prevention or cure for a disease or condition without an approved indication for such, is considered an unapproved and therefore, an illegal drug Biological end points: Blood Pressure Pulse wave velocity Hyperimic blood flow Perfusion RBC deformability HbA1c levels Serum albumin Blood cholesterol Blood lipid
13 IND Guidance: Legal & Regulatory Framework 13 Sec Requirement for an IND: (a) A sponsor shall submit an IND to FDA if the sponsor intends to conduct a clinical investigation with an investigational new drug that is subject to 312.2(a) (b) A sponsor shall not begin a clinical investigation subject to 312.2(a) until the investigation is subject to an IND which is in effect in accordance with
14 OK- I will file an IND.but wait 14 Prohibits the interstate shipment of certain foods to which: An approved drug or a licensed biological product, has been added A drug or a biological product that has been the subject of substantial clinical investigations, the existence of which has been made public, has been added Sec. 301(II) of FDCA
15 FSDU & Medical Foods 15 FSDU is a Conventional Food ; BUT Intended to meet special nutritional needs associated with age, physiological conditions, or disease. Useful in supplying special dietary needs for patient prevented from eating normally, BUT medical supervision not required NOT specifically tailored for distinct nutritional requirements of the patient s disease/condition/treatment
16 Strict FDA Interpretations: 16 FDA interprets the Medical Foods definition more narrowly: To limit foods that make unapproved disease claims Apply to those that appear to provide proven, essential therapies FDA has attempted to further narrow medical food category: Advance Notice of Proposed Rulemaking-1996 (withdrawn 2003) Medical Food FAQs; Draft Guidance IND guidance document- 2013
17 Let s try to Apply the MF criteria to Probiotics: 17 Specially formulated and processed for partial or exclusive feeding of patient orally or by enteral tube; and Intended for use only under medical supervision; and Intended only for patient receiving active/ongoing medical treatment; and Intended for dietary management of a patient which cannot be achieved by modification of the normal diet (e.g., chronic medical needs; limited/impaired capacity to ingest, digest, etc.; other special medicallydetermined nutrient needs); and Provides nutritional support to manage the unique nutrient needs resulting from a specific disease/condition (per medical evaluation)
18 Evidence Requirements Specific to Probiotics: 18 Safety of the ingredient- Reasonable certainty of no harm: GRAS or Food Additive Notification Usage & Claim: Probiotics as an ingredient/component of a Food, FSDU, or a MF Probiotics as MF- Generate Scientific Knowledge: Demonstrate unique nutrient requirements How is it unique to the product? Can it be achieved by modification of the diet? For the (nutritional) management of a disease/condition? Work with the agency and develop an understanding about: The quality of evidence and the relationship with the nutritional management of the disease/condition Understand the current framework and the existing criteria for MF
19 Probiotics & the Distinct Nutritional Requirement: 19 Specially formulated and processed for partial or exclusive feeding of patient orally or by enteral tube; and Intended for use only under medical supervision; and Intended only for patient receiving active/ongoing medical supervision; and Intended for dietary management of a patient which cannot be achieved by the modification of the normal diet alone (e.g., chronic medical needs; limited/impaired capacity to ingest, digest, etc.; other special medically-determined nutrient needs); and Provides nutritional support to manage the distinct nutritional requirements resulting from a specific disease/condition (21 C.F.R (j)(8)
20 What else can we do? 20 Dialog with the agency for the role of nutrition in health and disease Relying on data to overcome barriers to narrow interpretations of health claims for all food categories, including MFs Encourage & work with the FDA in the development of evidence standard for disease claims for foods and not drugs Encourage & work with the FDA to incorporate & evaluate the developing science Influence product categorization to match the level of evidence for nutrition
The Path to U.S. Market for Functional Food Ingredients
 The Path to U.S. Market for Functional Food Ingredients American College of Nutrition Symposium Morristown, New Jersey November 18, 2011 DIANE B. McCOLL Hyman, Phelps & McNamara, P.C. 700 Thirteenth Street,
The Path to U.S. Market for Functional Food Ingredients American College of Nutrition Symposium Morristown, New Jersey November 18, 2011 DIANE B. McCOLL Hyman, Phelps & McNamara, P.C. 700 Thirteenth Street,
Getting Your Ingredient to Market: Understanding Your Regulatory Options Venable LLP
 Getting Your Ingredient to Market: Understanding Your Regulatory Options 2013 Venable LLP 1 Overview of the Governing Laws 1. The Federal Food, Drug, and Cosmetic Act ("FD&C Act"): Requires foods, cosmetics,
Getting Your Ingredient to Market: Understanding Your Regulatory Options 2013 Venable LLP 1 Overview of the Governing Laws 1. The Federal Food, Drug, and Cosmetic Act ("FD&C Act"): Requires foods, cosmetics,
2014 FDA/JIFSAN Food & Nutrition Webinar
 2014 FDA/JIFSAN Food & Nutrition Webinar Medical Foods Shawne Suggs-Anderson, MMSc, _ RD Infant Formula and Medical Foods Staff ONLDS/CFSAN/FDA September 23, 2014 53 Main Objectives History What is a Medical
2014 FDA/JIFSAN Food & Nutrition Webinar Medical Foods Shawne Suggs-Anderson, MMSc, _ RD Infant Formula and Medical Foods Staff ONLDS/CFSAN/FDA September 23, 2014 53 Main Objectives History What is a Medical
FDA Regulation of Claims on Dietary Supplement and Food Products
 FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
II. History and importance of defining Healthy : Government/regulatory science perspective
 II. History and importance of defining Healthy : Government/regulatory science perspective Barbara Schneeman, Ph.D. Professor Emerita of Nutrition University of California, Davis Main Question What are
II. History and importance of defining Healthy : Government/regulatory science perspective Barbara Schneeman, Ph.D. Professor Emerita of Nutrition University of California, Davis Main Question What are
Determining Whether A Dietary Supplement Study Requires an Investigational New Drug (IND)
 Determining Whether A Dietary Supplement Study Requires an Investigational New Drug (IND) 02/02/16 description Does a study that claims their dietary supplement promotes healthy joints and cartilage or
Determining Whether A Dietary Supplement Study Requires an Investigational New Drug (IND) 02/02/16 description Does a study that claims their dietary supplement promotes healthy joints and cartilage or
October 31, Draft Guidance for Industry, Frequently Asked Questions About Medical Foods; Second Edition
 October 31, 2013 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Rockville, MD 20852 Re: Docket No. FDA-2013-D-0880 Draft Guidance for
October 31, 2013 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Rockville, MD 20852 Re: Docket No. FDA-2013-D-0880 Draft Guidance for
Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018
 Food Ingredients Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018 Disclosures Think Healthy Group, Inc. George Mason University, Department of Nutrition and Food Studies Journal of the American College
Food Ingredients Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018 Disclosures Think Healthy Group, Inc. George Mason University, Department of Nutrition and Food Studies Journal of the American College
Session 2: Direct-to-Consumer Neurotechnology: Dietary Supplements Dietary Ingredients
 18 th Presidential Commission for the Study of Bioethical Issues August 20, 2014 Washington, DC Session 2: Direct-to-Consumer Neurotechnology: Dietary Supplements Dietary Ingredients Freddie Ann Hoffman,
18 th Presidential Commission for the Study of Bioethical Issues August 20, 2014 Washington, DC Session 2: Direct-to-Consumer Neurotechnology: Dietary Supplements Dietary Ingredients Freddie Ann Hoffman,
Silliker Nutrient and Health Claims U.S. and Canadian Regulatory Guide
 Silliker Nutrient and U.S. and Canadian Regulatory Guide Technical Content by Food Consulting Company 2013 Edition With over 40 years of experience and part of the Mérieux Alliance group of companies,
Silliker Nutrient and U.S. and Canadian Regulatory Guide Technical Content by Food Consulting Company 2013 Edition With over 40 years of experience and part of the Mérieux Alliance group of companies,
FDA s Food Additives Program
 FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
Safety Evaluation for Substances Directly Added to Food
 Safety Evaluation for Substances Directly Added to Food Teresa Croce, PhD Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration How Does FDA Regulate
Safety Evaluation for Substances Directly Added to Food Teresa Croce, PhD Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration How Does FDA Regulate
They. amounts. 1 Harvard School of. Every Day, Medical Foods: Pharmaceuticals.
 Medical Foods: Managing Diseas se through Nutrition Medical foods straddle the gap between drugs and conventional foods by targeting the nutritional deficiencies that underlie disease. Given the large
Medical Foods: Managing Diseas se through Nutrition Medical foods straddle the gap between drugs and conventional foods by targeting the nutritional deficiencies that underlie disease. Given the large
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Nutritional Criteria for Labeling Claims
 Nutritional Criteria for Labeling Claims Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements Center for Food Safety and Applied Nutrition/ Food and Drug Administration Department
Nutritional Criteria for Labeling Claims Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements Center for Food Safety and Applied Nutrition/ Food and Drug Administration Department
U.S. FDA Perspective on Food Supplements/TM
 U.S. FDA Perspective on Food Supplements/TM IKHLAS A. KHAN National Center for Natural Products Research, Department of Pharmacognosy and Research Institute of Pharmaceutical Sciences, School of Pharmacy,
U.S. FDA Perspective on Food Supplements/TM IKHLAS A. KHAN National Center for Natural Products Research, Department of Pharmacognosy and Research Institute of Pharmaceutical Sciences, School of Pharmacy,
Translating the science into efficacy claims on probiotic or prebiotic products in the US market
 Translating the science into efficacy claims on probiotic or prebiotic products in the US market American Dairy Science Association Annual Meeting July 13, 2010 Dairy & Food Culture Technologies Consulting
Translating the science into efficacy claims on probiotic or prebiotic products in the US market American Dairy Science Association Annual Meeting July 13, 2010 Dairy & Food Culture Technologies Consulting
memorandum Venable s FDA Practice Group FDA Publishes Draft Guidance on New Dietary Ingredients ( NDIs )
 memorandum TO Valued Dietary Supplement Clients DATE July 5, 2011 FROM Venable s FDA Practice Group RE FDA Publishes Draft Guidance on New Dietary Ingredients ( NDIs ) On July 1, 2011, the U.S. Food and
memorandum TO Valued Dietary Supplement Clients DATE July 5, 2011 FROM Venable s FDA Practice Group RE FDA Publishes Draft Guidance on New Dietary Ingredients ( NDIs ) On July 1, 2011, the U.S. Food and
L A W O F F I C E S HYMAN, PHELPS & MCNAMARA, P.C T H I R T E E N T H S T R E E T, N. W. S U I T E
 A. WES SIEGNER, JR. L A W O F F I C E S 7 0 0 T H I R T E E N T H S T R E E T, N. W. S U I T E 1 2 0 0 W A S H I N G T O N, D. C. 2 0 0 0 5-5929 ( 2 0 2 ) 7 3 7-5600 F A C S I M I L E ( 2 0 2 ) 7 3 7-9329
A. WES SIEGNER, JR. L A W O F F I C E S 7 0 0 T H I R T E E N T H S T R E E T, N. W. S U I T E 1 2 0 0 W A S H I N G T O N, D. C. 2 0 0 0 5-5929 ( 2 0 2 ) 7 3 7-5600 F A C S I M I L E ( 2 0 2 ) 7 3 7-9329
Client Alert. FDA Draft Guidance Broadens New Dietary Ingredient Definition and Extends Notification Requirements 1
 Contact Attorney Regarding This Matter: Emalee G. Murphy 202.677.4052 - direct 202.677.4053 - fax emalee.murphy@agg.com Attorneys at Law 171 17th Street NW Suite 2100 Atlanta, GA 30363-1031 404.873.8500
Contact Attorney Regarding This Matter: Emalee G. Murphy 202.677.4052 - direct 202.677.4053 - fax emalee.murphy@agg.com Attorneys at Law 171 17th Street NW Suite 2100 Atlanta, GA 30363-1031 404.873.8500
Crossing the Line. When does innovative therapy become research?
 Crossing the Line When does innovative therapy become research? Presentation Overview Importance of Distinction Primary Research Regulators HHS FDA Tricky Situations Innovative surgery Quality improvement
Crossing the Line When does innovative therapy become research? Presentation Overview Importance of Distinction Primary Research Regulators HHS FDA Tricky Situations Innovative surgery Quality improvement
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
Food Irradiation: FDA s Perspective Teresa Croce, Ph.D.
 Food Irradiation: FDA s Perspective Teresa Croce, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration March 25, 2015 Federal Food, Drug, &
Food Irradiation: FDA s Perspective Teresa Croce, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration March 25, 2015 Federal Food, Drug, &
NDI: LOOKING BACK & AHEAD
 NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
FDLI ANNUAL CONFERENCE May 4, 2018
 FDLI ANNUAL CONFERENCE May 4, 2018 DIETARY SUPPLEMENTS RETAILER ISSUES AND LIABILITY Jean Frydman, Partner, Chair FDA Practice Fox Rothschild, LLP FDA Concern is Safety Only DHSEA Act of 1994 Know what
FDLI ANNUAL CONFERENCE May 4, 2018 DIETARY SUPPLEMENTS RETAILER ISSUES AND LIABILITY Jean Frydman, Partner, Chair FDA Practice Fox Rothschild, LLP FDA Concern is Safety Only DHSEA Act of 1994 Know what
The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016
 INFORMATION NOTE INTENDED FOR KNOWLEDGE HUB The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016 This note is to alert you to the fact that the English Statutory
INFORMATION NOTE INTENDED FOR KNOWLEDGE HUB The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016 This note is to alert you to the fact that the English Statutory
Understanding Today s Probiotics Regulations in South East Asia. Wai Mun Poon Regulatory Affairs Consultant
 Understanding Today s Probiotics Regulations in South East Asia Wai Mun Poon Regulatory Affairs Consultant waimun@wongsjasia.com Probiotic Foods in South East Asia More probiotic foods are being introduced
Understanding Today s Probiotics Regulations in South East Asia Wai Mun Poon Regulatory Affairs Consultant waimun@wongsjasia.com Probiotic Foods in South East Asia More probiotic foods are being introduced
Original Effective Date: 9/10/09
 Subject: Oral and Tube Fed Enteral Nutrition Policy Number: MCR-070 *(This MCR replaces and combines MCG-070 & 071) Original Effective Date: 9/10/09 Revision Date(s): 6/29/12, 8/7/14 This MCR is no longer
Subject: Oral and Tube Fed Enteral Nutrition Policy Number: MCR-070 *(This MCR replaces and combines MCG-070 & 071) Original Effective Date: 9/10/09 Revision Date(s): 6/29/12, 8/7/14 This MCR is no longer
The opinions expressed are the speaker s and not his company s.
 May 13, 2010 James Brooks, Ph.D. Vice President, Science & Technology Christine Burdick-Bell, J.D. Vice President, Legal & Regulatory Affairs Pharmavite LLC The opinions expressed are the speaker s and
May 13, 2010 James Brooks, Ph.D. Vice President, Science & Technology Christine Burdick-Bell, J.D. Vice President, Legal & Regulatory Affairs Pharmavite LLC The opinions expressed are the speaker s and
Food. [[Page 999]] Part IV. Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 101
![Food. [[Page 999]] Part IV. Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 101 Food. [[Page 999]] Part IV. Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 101](/thumbs/95/126080208.jpg) 1 of 96 12/15/2009 2:32 PM Food Federal Register 65 FR 999 January 6, 1999 -- Regulations on Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of
1 of 96 12/15/2009 2:32 PM Food Federal Register 65 FR 999 January 6, 1999 -- Regulations on Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of
Food & Supplement Labels
 Food & Supplement Labels Food Labels List of Ingredients Nutrition Facts Panel Claims Supplement Regulations & Claims DSHEA Food Certifications Organic & Fair Trade List of Ingredients Listed in descending
Food & Supplement Labels Food Labels List of Ingredients Nutrition Facts Panel Claims Supplement Regulations & Claims DSHEA Food Certifications Organic & Fair Trade List of Ingredients Listed in descending
 1 of 6 8/7/17, 10:45 AM January 15, 2017 By Katie Burns Posted Jan. 4, 2017 A third of all U.S. households with dogs use supplements, as do about a fifth of households with cats, according to a report
1 of 6 8/7/17, 10:45 AM January 15, 2017 By Katie Burns Posted Jan. 4, 2017 A third of all U.S. households with dogs use supplements, as do about a fifth of households with cats, according to a report
The Shifting Federal Regulation of Cannabis Products
 The Durham Bar 2019 CLE Program The Shifting Federal Regulation of Cannabis Products Erica M. Jackson, FDA Partner K&L Gates February 6, 2019 Copyright 2018 by K&L Gates LLP. All rights reserved. OVERVIEW
The Durham Bar 2019 CLE Program The Shifting Federal Regulation of Cannabis Products Erica M. Jackson, FDA Partner K&L Gates February 6, 2019 Copyright 2018 by K&L Gates LLP. All rights reserved. OVERVIEW
IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION
 Case 1:13-cv-03675-WBH Document 14 Filed 01/07/14 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION UNITED STATES OF AMERICA, Plaintiff, v. CIVIL ACTION
Case 1:13-cv-03675-WBH Document 14 Filed 01/07/14 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION UNITED STATES OF AMERICA, Plaintiff, v. CIVIL ACTION
Decoding the Confusing Language of Vitamins. Yuliya Klopouh, M.H., Pharm.D
 Decoding the Confusing Language of Vitamins Yuliya Klopouh, M.H., Pharm.D Nutritional Supplements ( Vitamins, etc) History of Dietary supplements 1990 Nutritional Labeling and Education Act Permitted use
Decoding the Confusing Language of Vitamins Yuliya Klopouh, M.H., Pharm.D Nutritional Supplements ( Vitamins, etc) History of Dietary supplements 1990 Nutritional Labeling and Education Act Permitted use
Public Hearing Before U.S. Food and Drug Administration
 Substances in Food and Labeling; Regulatory Framework for Foods that Companies are Marketing as Functional Foods Public Hearing Before U.S. Food and Drug Administration Ilene Ringel Heller Senior Staff
Substances in Food and Labeling; Regulatory Framework for Foods that Companies are Marketing as Functional Foods Public Hearing Before U.S. Food and Drug Administration Ilene Ringel Heller Senior Staff
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years
 The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
IRB review of device studies
 Washington University School of Medicine Digital Commons@Becker 2011 Device Advice from the FDA 2011 Conferences 2011 IRB review of device studies Jonathan M. Green Washington University School of Medicine
Washington University School of Medicine Digital Commons@Becker 2011 Device Advice from the FDA 2011 Conferences 2011 IRB review of device studies Jonathan M. Green Washington University School of Medicine
GUIDELINES FOR MAKING PRODUCT CLAIMS
 GUIDELINES FOR MAKING PRODUCT CLAIMS This practical guide to making claims provides valuable information for Independent Business Owners (IBOS) to use in their everyday operations. It describes various
GUIDELINES FOR MAKING PRODUCT CLAIMS This practical guide to making claims provides valuable information for Independent Business Owners (IBOS) to use in their everyday operations. It describes various
GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS
 1 CAC/GL 23-1997 GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS CAC/GL 23-1997 Nutrition claims should be consistent with national nutrition policy and support that policy. Only nutrition claims that
1 CAC/GL 23-1997 GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS CAC/GL 23-1997 Nutrition claims should be consistent with national nutrition policy and support that policy. Only nutrition claims that
One generally, applicable definition is "the use of living organisrns to make new products."
 1. What is Biotechnology'? A B One generally, applicable definition is "the use of living organisrns to make new products." Biotechnology is not new. For thousands of years, people have been using, living
1. What is Biotechnology'? A B One generally, applicable definition is "the use of living organisrns to make new products." Biotechnology is not new. For thousands of years, people have been using, living
The United Natural Products Alliance (UNPA) submits these comments in. response to the Food and Drug Administration s Draft Guidance on New Dietary
 December 2, 2011 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Draft Guidance for Industry Dietary
December 2, 2011 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Draft Guidance for Industry Dietary
Re: National Bioengineered Food Disclosure Standard; Proposed Rule; Request for Comments, 83 Fed. Reg (May 4, 2018), Docket No.
 VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
Policy Statement Food for Special Medical Purposes Policy Statement
 Policy Statement Food for Special Medical Purposes Policy Statement Summary The PARNUTS directive has been replaced with the Foods for Specific Groups Regulation. This seeks to ensure a consistent approach
Policy Statement Food for Special Medical Purposes Policy Statement Summary The PARNUTS directive has been replaced with the Foods for Specific Groups Regulation. This seeks to ensure a consistent approach
Ornsurang Teerawat
 ASEAN TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS REGULATORY AND HARMONIZATION UPDATE Ornsurang Teerawat 21.10.2014 \ http://trainstudenytp.wordpress.com ACCSQ (ASEAN Consultative Committee for Standard
ASEAN TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS REGULATORY AND HARMONIZATION UPDATE Ornsurang Teerawat 21.10.2014 \ http://trainstudenytp.wordpress.com ACCSQ (ASEAN Consultative Committee for Standard
FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS
 FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS Presented by W. Patrick Noonan And Chris Noonan For NUTRITION INDUSTRY ASSOCIATION W. Patrick Noonan, P.C. 21800 Oxnard Street, Suite 840 Woodland Hills,
FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS Presented by W. Patrick Noonan And Chris Noonan For NUTRITION INDUSTRY ASSOCIATION W. Patrick Noonan, P.C. 21800 Oxnard Street, Suite 840 Woodland Hills,
Revised Version Text of final Codex Vitamin and Mineral Guideline. Marked for Amendment. Deletions struck through; additions underlined.
 Revised Version Text of final Codex Vitamin and Mineral Guideline. Marked for Amendment Deletions struck through; additions underlined. Finalized by the Codex Alimentarius Commission July 4, 2005 Guidelines
Revised Version Text of final Codex Vitamin and Mineral Guideline. Marked for Amendment Deletions struck through; additions underlined. Finalized by the Codex Alimentarius Commission July 4, 2005 Guidelines
Feed Ingredient Utility. Mika Alewynse Division of Animal Feeds Center for Veterinary Medicine FDA
 Feed Ingredient Utility Mika Alewynse Division of Animal Feeds Center for Veterinary Medicine FDA AAFCO Definition Process Proposed definition Description/ purpose of the ingredient Rationale for request
Feed Ingredient Utility Mika Alewynse Division of Animal Feeds Center for Veterinary Medicine FDA AAFCO Definition Process Proposed definition Description/ purpose of the ingredient Rationale for request
Chemical food safety in the U.S. analysis of FDA s scientific basis for assessing chemical risk. Tom Neltner October 9, 2014
 Chemical food safety in the U.S. analysis of FDA s scientific basis for assessing chemical risk Tom Neltner October 9, 2014 Topics 1. Current focus of U.S. public interest community 2. Comparison of U.S.
Chemical food safety in the U.S. analysis of FDA s scientific basis for assessing chemical risk Tom Neltner October 9, 2014 Topics 1. Current focus of U.S. public interest community 2. Comparison of U.S.
GSCI 2202 FOOD FOR HEALTH. Eat to compete: Dietary Supplements
 GSCI 2202 FOOD FOR HEALTH Eat to compete: Dietary Supplements Dietary supplements on the market are: NOT regulated Could contain illegal substances Could be costly Could be harmful Current laws on dietary
GSCI 2202 FOOD FOR HEALTH Eat to compete: Dietary Supplements Dietary supplements on the market are: NOT regulated Could contain illegal substances Could be costly Could be harmful Current laws on dietary
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17
 TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
Paul M. Coates, Ph.D.
 Dietary Supplements in the United States: Claims and Evidence The National Institute of Health and Nutrition December 2008 Paul M. Coates, Ph.D. Office of Dietary Supplements National Institutes of Health
Dietary Supplements in the United States: Claims and Evidence The National Institute of Health and Nutrition December 2008 Paul M. Coates, Ph.D. Office of Dietary Supplements National Institutes of Health
General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff
 General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff Document issued on: July 29, 2016. The draft of this document was issued on January 20, 2015.
General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff Document issued on: July 29, 2016. The draft of this document was issued on January 20, 2015.
SUMMARY INTRODUCTION. The Food, Drug, and established provided that duce cancer when ingested
 SUMMARY 1 SUMMARY INTRODUCTION The Food, Drug, and established provided that duce cancer when ingested Cosmetic Act requires that safety of food additives be no additive shall be deemed to be safe if it
SUMMARY 1 SUMMARY INTRODUCTION The Food, Drug, and established provided that duce cancer when ingested Cosmetic Act requires that safety of food additives be no additive shall be deemed to be safe if it
New England Compounding Center 04-Dec-06
 New England Compounding Center 04-Dec-06 Department of Health and Human Services Public Health Service Food and Drug Administration New England District One Montvale Avenue Stoneham, Massachusetts 02180
New England Compounding Center 04-Dec-06 Department of Health and Human Services Public Health Service Food and Drug Administration New England District One Montvale Avenue Stoneham, Massachusetts 02180
SAFETY ASSESSMENT OF FOOD ADDITIVES
 SAFETY ASSESSMENT OF FOOD ADDITIVES Department of Health and Human Services U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Acronyms to Know Center for Food Safety and Applied
SAFETY ASSESSMENT OF FOOD ADDITIVES Department of Health and Human Services U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Acronyms to Know Center for Food Safety and Applied
Module 34: Legal aspects, ADI and GRAS status of food additives
 Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
The father of Western medicine, Hippocrates (460 BC BC) is known to have used many plants and herbs for medicinal purposes. Hippocrates' use of
 UNIT 11 INVESTIGATE WESTERN HERBAL PRODUCTS HISTORY OF TRADITIONAL WESTERN HERBAL MEDICINE The father of Western medicine, Hippocrates (460 BC - 377 BC) is known to have used many plants and herbs for
UNIT 11 INVESTIGATE WESTERN HERBAL PRODUCTS HISTORY OF TRADITIONAL WESTERN HERBAL MEDICINE The father of Western medicine, Hippocrates (460 BC - 377 BC) is known to have used many plants and herbs for
Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of
 This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
UNICEF Nutrition Supplier Meeting
 UNICEF Nutrition Supplier Meeting Copenhagen, 5-6 October 2009 Developing Standards for Foods for Malnourished Children Selma H. Doyran Joint FAO/WHO Food Standards Programme FAO Nutrition and Consumer
UNICEF Nutrition Supplier Meeting Copenhagen, 5-6 October 2009 Developing Standards for Foods for Malnourished Children Selma H. Doyran Joint FAO/WHO Food Standards Programme FAO Nutrition and Consumer
The first women s whole food multivitamin with targeted stress support
 NatureWise Women s Stress Support Multivitamin & Minerals with Sensoril Ashwagandha, Whole Food Complete Multivitamin For Women, Reduce Stress, Boost Energy, Allergen Free, 60 count About the product EFFECTIVE
NatureWise Women s Stress Support Multivitamin & Minerals with Sensoril Ashwagandha, Whole Food Complete Multivitamin For Women, Reduce Stress, Boost Energy, Allergen Free, 60 count About the product EFFECTIVE
Guidance Document The Use of Probiotic Microorganisms in Food
 Guidance Document The Use of Probiotic Microorganisms in Food Food Directorate Health Products and Food Branch Health Canada April 2009 Page 1 of 8 I. Introduction Purpose 1. The purpose of this Guidance
Guidance Document The Use of Probiotic Microorganisms in Food Food Directorate Health Products and Food Branch Health Canada April 2009 Page 1 of 8 I. Introduction Purpose 1. The purpose of this Guidance
April 30, By Electronic Mail
 April 30, 2018 By Electronic Mail Dr. Scott Gottlieb, Commissioner Office of the Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 CommissionerFDA@fda.hhs.gov
April 30, 2018 By Electronic Mail Dr. Scott Gottlieb, Commissioner Office of the Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 CommissionerFDA@fda.hhs.gov
Overview of Regulatory Science of Food Contact Substances
 Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Personal Importation Policy (PIP)
 Personal Importation Policy (PIP) CDR Paras Patel, R.Ph., MBA US Food & Drug Administration Center for Drug Evaluation and Research Drug Shortage Staff Objectives Describe the purpose of the Personal Importation
Personal Importation Policy (PIP) CDR Paras Patel, R.Ph., MBA US Food & Drug Administration Center for Drug Evaluation and Research Drug Shortage Staff Objectives Describe the purpose of the Personal Importation
Consistent with Labeling Final Guidance: Implications for Devices
 Consistent with Labeling Final Guidance: Implications for Devices Vernessa Pollard, Partner, McDermott Will & Emery Cassie Scherer, Principal Legal Counsel, Medtronic Jeffrey Shapiro, Director, Hyman,
Consistent with Labeling Final Guidance: Implications for Devices Vernessa Pollard, Partner, McDermott Will & Emery Cassie Scherer, Principal Legal Counsel, Medtronic Jeffrey Shapiro, Director, Hyman,
FDA Oversight of Nanotechnology Applications in Foods, Food Packaging, and Nutrient Delivery
 FDA Oversight of Nanotechnology Applications in Foods, Food Packaging, and Nutrient Delivery Laura M. Tarantino, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition (CFSAN)
FDA Oversight of Nanotechnology Applications in Foods, Food Packaging, and Nutrient Delivery Laura M. Tarantino, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition (CFSAN)
Final Report. NIH Grant Number: 5R01HG November 15, 2012 (rev d September 2013, rev d January 2016) Submitted by:
 Final Report Federal Regulation of Probiotics: An Analysis of the Existing Regulatory Framework and Recommendations for Alternative Frameworks NIH Grant Number: 5R01HG005171-02 November 15, 2012 (rev d
Final Report Federal Regulation of Probiotics: An Analysis of the Existing Regulatory Framework and Recommendations for Alternative Frameworks NIH Grant Number: 5R01HG005171-02 November 15, 2012 (rev d
Health Claims and FTC Advertising Law
 Health Claims and FTC Advertising Law Institute of Medicine Food Forum Workshop The Human Microbiome, Diet, and Health Michelle Rusk, Attorney* Division of Advertising Practices *Staff presentation does
Health Claims and FTC Advertising Law Institute of Medicine Food Forum Workshop The Human Microbiome, Diet, and Health Michelle Rusk, Attorney* Division of Advertising Practices *Staff presentation does
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS
 WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
Update FDA/Center for Food Safety and Applied Nutrition
 Update FDA/Center for Food Safety and Applied Nutrition Comments by Susan Mayne, Ph.D. Director Center for Food Safety and Applied Nutrition FDLI Annual Conference May 3, 2018 Food Safety, Nutrition and
Update FDA/Center for Food Safety and Applied Nutrition Comments by Susan Mayne, Ph.D. Director Center for Food Safety and Applied Nutrition FDLI Annual Conference May 3, 2018 Food Safety, Nutrition and
هيئة التقييس لدول مجلس التعاون لدول الخليج العربية
 هيئة التقييس لدول مجلس التعاون لدول الخليج العربية GCC STANDARDIZATION ORGANIZATION (GSO) Final Draft GSO 05 FDS 1366 / 2016 اشت ارطات عامة لتداول األغذية المستعملة ألغ ارض طبية خاصة General Requirements
هيئة التقييس لدول مجلس التعاون لدول الخليج العربية GCC STANDARDIZATION ORGANIZATION (GSO) Final Draft GSO 05 FDS 1366 / 2016 اشت ارطات عامة لتداول األغذية المستعملة ألغ ارض طبية خاصة General Requirements
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Petition for Rulemaking on ) Functional Foods and ) Docket No. Request to Establish an ) Advisory Committee ) ) Submitted by the
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Petition for Rulemaking on ) Functional Foods and ) Docket No. Request to Establish an ) Advisory Committee ) ) Submitted by the
Which was the greatest problem with patent medicines in early America that lead to drug legislation?
 Pharmacology Connections to Nursing Practice 3rd Edition Adams Test Bank Full Download: http://testbanklive.com/download/pharmacology-connections-to-nursing-practice-3rd-edition-adams-test-bank/ Adams
Pharmacology Connections to Nursing Practice 3rd Edition Adams Test Bank Full Download: http://testbanklive.com/download/pharmacology-connections-to-nursing-practice-3rd-edition-adams-test-bank/ Adams
The Tobacco Control Act s Premarket Review Authorities: Reports on Substantial Equivalence and Exemption Requests (905(j))
 The Tobacco Control Act s Premarket Review Authorities: Reports on Substantial Equivalence and Exemption Requests (905(j)) January 12, 2011 Cristi Stark, MS Senior Regulatory Health Project Manager Office
The Tobacco Control Act s Premarket Review Authorities: Reports on Substantial Equivalence and Exemption Requests (905(j)) January 12, 2011 Cristi Stark, MS Senior Regulatory Health Project Manager Office
Medical Weight loss - Chiropractic Articles - The Village Chiropractic Center of Florida
 What is ChiroThin and how does it work? The ChiroThin formula by ChiroNutraceutical is a natural dietary supplement. It contains a host of nutritional ingredients that are well known to aid in fatty acid
What is ChiroThin and how does it work? The ChiroThin formula by ChiroNutraceutical is a natural dietary supplement. It contains a host of nutritional ingredients that are well known to aid in fatty acid
RE: Permits under the Arizona Pharmacy Act should not be required for dietary supplements
 Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Health Canada s Safety Assessment Process for Sugar Substitutes
 Health Canada s Safety Assessment Process for Sugar Substitutes Janice Weightman Bureau of Chemical Safety Food Directorate, Health Canada April 24, 2014 Overview Canadians generally have a taste for sweet
Health Canada s Safety Assessment Process for Sugar Substitutes Janice Weightman Bureau of Chemical Safety Food Directorate, Health Canada April 24, 2014 Overview Canadians generally have a taste for sweet
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Re: Bayer s false and deceptive marketing for its Men s Multis for prevention of cancer
 June 18, 2009 VIA REGULAR MAIL AND FAX TO 973-254-4853 Gary S. Balkema, President Consumer Care Division Bayer HealthCare LLC 36 Columbia Rd Morristown, NJ 07962-1910 Re: Bayer s false and deceptive marketing
June 18, 2009 VIA REGULAR MAIL AND FAX TO 973-254-4853 Gary S. Balkema, President Consumer Care Division Bayer HealthCare LLC 36 Columbia Rd Morristown, NJ 07962-1910 Re: Bayer s false and deceptive marketing
E-Siong Tee, PhD Scientific Director, ILSI SEA Region
 E-Siong Tee, PhD Scientific Director, ILSI SEA Region (nutrihealth.tes@myjaring.net) 1 Background. Health claims permitted in some countries in SEA region mostly arising from applications from food industry
E-Siong Tee, PhD Scientific Director, ILSI SEA Region (nutrihealth.tes@myjaring.net) 1 Background. Health claims permitted in some countries in SEA region mostly arising from applications from food industry
STANDARDIZATION ORGANIZATION FOR G.C.C (GSO)
 ھيئة التقييس لدول مجلس التعاون لدول الخليج العربية STANDARDIZATION ORGANIZATION FOR G.C.C (GSO) Final Draft GSO5/FDS/CAC./2013 اشتراطات األغذية ذات اإلدعاءات التغذوية والصحية Nutritional and Health Claims
ھيئة التقييس لدول مجلس التعاون لدول الخليج العربية STANDARDIZATION ORGANIZATION FOR G.C.C (GSO) Final Draft GSO5/FDS/CAC./2013 اشتراطات األغذية ذات اإلدعاءات التغذوية والصحية Nutritional and Health Claims
Using new scientific knowledge to update regulations in the U.S.
 Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
The Tool-Kit includes six core vitamin D intake variables and five ancillary vitamin D intake variables:
 Page 1 of 7 Pediatric MS Tool-Kit Variables The Tool-Kit vitamin D intake variables were selected and defined to address the following research question: Everything else being equal, are children with
Page 1 of 7 Pediatric MS Tool-Kit Variables The Tool-Kit vitamin D intake variables were selected and defined to address the following research question: Everything else being equal, are children with
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Panel on Defining Healthy and Natural
 Panel on Defining Healthy and Natural Panelists: Shirley Blakely, Janet Collins, and Tony Pavel Moderator: Mary Christ-Erwin This image cannot currently be displayed. Natural What is Natural? From a food
Panel on Defining Healthy and Natural Panelists: Shirley Blakely, Janet Collins, and Tony Pavel Moderator: Mary Christ-Erwin This image cannot currently be displayed. Natural What is Natural? From a food
Give Me a Break, Is That Really a Disease Claim?
 Give Me a Break, Is That Really a Disease Claim? Najla Guthrie, President/CEO KGK Synergize Todd A. Harrison Partner Venable LLP Claudia A. Lewis, Partner Venable LLP ENGREDEA 2014 March 7, 2014 2012 Venable
Give Me a Break, Is That Really a Disease Claim? Najla Guthrie, President/CEO KGK Synergize Todd A. Harrison Partner Venable LLP Claudia A. Lewis, Partner Venable LLP ENGREDEA 2014 March 7, 2014 2012 Venable
OFF All Dr. Clark Cleanses
 Dr. Clark Quick Digestive Optional directions based on the Protocols of Dr. Hulda Clark Since I want you to have the greatest possible benefits from our cleanses please consider the following carefully:
Dr. Clark Quick Digestive Optional directions based on the Protocols of Dr. Hulda Clark Since I want you to have the greatest possible benefits from our cleanses please consider the following carefully:
BEFORE THE FEDERAL COMMUNICATIONS COMMISSION WASHINGTON, DC 20554
 BEFORE THE FEDERAL COMMUNICATIONS COMMISSION WASHINGTON, DC 20554 In the Matter of ) ) Telecommunications Relay Services and ) CG Docket No. 03-123 Speech-to-Speech Services for Individuals ) With Hearing
BEFORE THE FEDERAL COMMUNICATIONS COMMISSION WASHINGTON, DC 20554 In the Matter of ) ) Telecommunications Relay Services and ) CG Docket No. 03-123 Speech-to-Speech Services for Individuals ) With Hearing
The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications
 The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications RadTech UV & EB Technology Expo & Conference 2012 Presented by David J. Ettinger Partner Keller and Heckman LLP
The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications RadTech UV & EB Technology Expo & Conference 2012 Presented by David J. Ettinger Partner Keller and Heckman LLP
Nestle Infant Nutrition 10/31/14
 U.S. Food and Drug Administration Protecting and Promoting Your Health Nestle Infant Nutrition 10/31/14 OCT 31, 2014 Department of Health and Human Services Public Health Service Food and Drug Administration
U.S. Food and Drug Administration Protecting and Promoting Your Health Nestle Infant Nutrition 10/31/14 OCT 31, 2014 Department of Health and Human Services Public Health Service Food and Drug Administration
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
The Safety and Effectiveness of Dietary Supplements
 Assignment 4 The Safety and Effectiveness of Dietary Supplements In Depth: Supplements Supplements, according to the FDA, are a product containing ingredients like vitamins, minerals, herms, amino acids,
Assignment 4 The Safety and Effectiveness of Dietary Supplements In Depth: Supplements Supplements, according to the FDA, are a product containing ingredients like vitamins, minerals, herms, amino acids,
First Amendment Issues in Advertising and Product Packaging
 First Amendment Issues in Advertising and Product Packaging Jonathan H. Adler Johan Verheij Memorial Professor of Law Director, Center for Business Law & Regulation Case Western Reserve University School
First Amendment Issues in Advertising and Product Packaging Jonathan H. Adler Johan Verheij Memorial Professor of Law Director, Center for Business Law & Regulation Case Western Reserve University School
4 SACCHARIN BENEFITS
 4 SACCHARIN BENEFITS SACCHARIN BENEFITS POTENTIAL BENEFITS As part of this study, the OTA was asked to evaluate the potential health benefits, - including psychological benefits, of saccharin availability
4 SACCHARIN BENEFITS SACCHARIN BENEFITS POTENTIAL BENEFITS As part of this study, the OTA was asked to evaluate the potential health benefits, - including psychological benefits, of saccharin availability
WHY NEO LIFE FOR MY LIFE
 WHY NEO LIFE FOR MY LIFE (1) Provitality pack contains (52) of the (59) nutrient families. (2) Tre en en is the most (IMPORTANT) nutrient for cellular health. (3) The (ONE) (PHYTOSTEROL) and (TWO) (PHYTOLIPIDS)
WHY NEO LIFE FOR MY LIFE (1) Provitality pack contains (52) of the (59) nutrient families. (2) Tre en en is the most (IMPORTANT) nutrient for cellular health. (3) The (ONE) (PHYTOSTEROL) and (TWO) (PHYTOLIPIDS)
Codex Alimentarius and the US Dietary Supplement Industry. Mark A. Le Doux, Chairman and CEO Natural Alternatives International, Inc.
 Codex Alimentarius and the US Dietary Supplement Industry Mark A. Le Doux, Chairman and CEO Natural Alternatives International, Inc. UNDERSTANDING THE CODEX ALIMENTARIUS Since the first steps were taken
Codex Alimentarius and the US Dietary Supplement Industry Mark A. Le Doux, Chairman and CEO Natural Alternatives International, Inc. UNDERSTANDING THE CODEX ALIMENTARIUS Since the first steps were taken
Distillers Grains Opportunities and Challenges
 Distillers Grains Opportunities and Challenges RFA National Ethanol Conference February 26, 2008 Linda A. Benjamin, PhD FDA/Center for Veterinary Medicine Center for Veterinary Medicine Approves safe food
Distillers Grains Opportunities and Challenges RFA National Ethanol Conference February 26, 2008 Linda A. Benjamin, PhD FDA/Center for Veterinary Medicine Center for Veterinary Medicine Approves safe food
Nutrition Labeling Laws for Food and Supplements Timeline
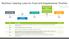 Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
