specificity." Whereas trypsin acts almost exclusively on peptide bonds properties.1 These include molecular weights (approximately 25,000 and 24,000,
|
|
|
- Britney Moody
- 6 years ago
- Views:
Transcription
1 884 BIOCHEMISTRY: WALSH AND NEURATH PROC. N. A. S. 22 Craig, L. G., W. Koenigsberg, and R. J. Hill, Amino Acids and Peptides with Antimetabolic Activity, CIBA Foundation Symposium (1958), p Du Vigneaud, V., personal communication. 24 Mach, B., C. Slayman, and E. L. Tatum, manuscript in preparation. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS BY KENNETH A. WALSH AND HANS NEURATH UNIVERSITY OF WASHINGTON, SEATFLE Communicated August 27, 1964 The striking similarity of many properties of trypsin and chymotrypsin has been well known for many years.1-5 Both enzymes originate in the acinar cells of pancreatic tissue6 at identical rates7 as their inactive precursors trypsinogen and chymotrypsinogen A, and both are activated by the cleavage (by trypsin) of the peptide bond contributed by the basic residue which is closest to the amino terminus of the zymogen.8, 9 Both enzymes catalyze the hydrolysis of peptide, amide, and ester bonds, and both are characterized by a high degree of selectivity toward the amino acids which donate carboxyl groups to these bonds.10-'2 In both cases, the enzymes appear to become acylated through these carboxyl groups as an intermediate step in the catalytic mechanism.."-"5 Both enzymes are inhibited by certain organic fluorophosphates such as DFP, 16 and the site of reaction of these inhibitors has been identified as a unique serine residue in the same tetrapeptide sequence.17' 18 The same serine residue becomes acylated as an intermediate in the catalytic mechanism."9 In addition to serine, a histidine residue has long been implicated as a component of the active site of both chymotrypsin and trypsin.20 Indirect supporting evidence, derived from the ph dependence of the acylation and deacylation steps, and from the effects of photooxidation on enzyme activation of chymotrypsin,2' has been recently strengthened and confirmed by the findings of Shaw and coworkers22-24 that bifunctional reagents, resembling in structure specific substrates, inactivate chymotrypsin and trypsin, respectively, by forming covalent derivatives of a single histidine residue in each enzyme. Chymotrypsinogen and trypsinogen resemble each other in certain chemical properties.1 These include molecular weights (approximately 25,000 and 24,000, respectively) and isoelectric points (approximately 9.3). A comparison of the amino acid compositions of the two zymogens25-27 in Table 1 reveals that the similarity extends to this level also. The compositional similarity is made even more striking if certain residues of similar chemical character are grouped together, such as the total acidic, total basic, total aromatic, total branched hydrophobic, and total hydroxy-amino acid residues. Such comparative data are given at the bottom of Table 1. The only really striking difference between the two enzymes lies in their substrate specificity." Whereas trypsin acts almost exclusively on peptide bonds involving the carboxyl groups of lysine or arginine residues, chymotrypsin has no significant action upon these bonds, but is most active toward linkages involving the
2 VOL. 52, 1964 BIOCHEMISTRY: WALSH AND NEURATH 885 TABLE 1 AMINO ACID COMPOSITIONS OF TRYPSINOGEN26 AND CHYMOTRYPSINOGEN25 Trypsinogen Chymotrypsinogen Asparagine Glutamine Aspartic acid 10 9 Threonine t Serine Glutamic acid 3 5 Proline 9 9 Glycine Alanine Cystine/ Valine Methionine 2 2 Isoleucine Leucine Tyrosine 10 4 Phenylalanine 3 6 Tryptophan 4 8 Lysine Histidine 3 2 Arginine 2 4 Total acidic residues Lysine and arginine Total amides Total aromatic residues Serine + threonine Sum of isoleucine, leucine, and valine carboxyl groups of aromatic amino acids. Although trypsin also catalyzes the cleavage of certain aromatic substrates (characteristic of chymotrypsin), it is less effective than chymotrypsin in this regard, and far less effective than toward its own basic substrates. Several lines of evidence indicate that this property is intrinsic in trypsin itself, and not the result of a chymotryptic contamination.28 The recent finding of Mares-Guia and Shaw29 that a binding site for aromatic compounds exists at the active center of trypsin indicates that the striking difference in specificity between the two enzymes may turn out to be a quantitative phenomenon, rather than a qualitative one, and draws attention again to the underlying similarity between the two enzymes. The fundamental similarities in biological, mechanistic, and compositional character between the two molecules have led to the logical prediction of an extensive similarity in primary structure,5 including in particular the regions of structure known to have functional importance. However, as the primary structure of the two molecules was evolved,30 no analogous amino acid sequences were found that were larger than the tetrapeptide sequence originally observed to surround the serines of the active centers. More recently,3"-33 a nearly identical nonadecapeptide sequence was observed in chymotrypsin and trypsin containing two histidine residues in sufficiently close proximity to each other to suggest that not one but both of them are functional components of the active center in both enzymes.26 The recent completion of the structure of chymotrypsinogen A25 and a tentative structure for trypsinogen27 31 permit a detailed comparison of the molecular structures of the two enzymes, and a search for evidence of homology. This comparison is made in Table 2 by outlining the linear structures of the two zymogens in such a fashion as to make in each the amino terminal isoleucine residue, formed
3 886 BIOCHEMISTRY: WALSH AND NEURATH PROC. N. A. S. Chymotrypsinogen Trypsinogen TABLE 2 THE STRUCTURAL SIMILARITY OF TRYPSINOGEN AND CHYMOTRYPSINOGEN cys-gly-val-pro-ala-ile-gln-pro-val-leu-ser-gly-leu-ser-arg-ile-val-glyval-asp-asp-asp-asp-lys-ile-val-gly asp-glu-glu-ala-val-pro-gly-ser-trp-pro-trp-gln-val-ser-leu-gln-asp-lys-thr-gly-phe-his-phegly-tyr-thr-cys-gly-ala-asn-thr-val-pro-tyr-gln-val-ser-leu-asn- -ser-gly-tyr-his-phe_ CYS-GLY-GLY-SER-LEU-ILE-ASN-glu-asn-TRP-VAL-VAL-thr-AIA-ALA-HIS-CYS-gly-val-thr-thr-ser-asp- CYS-GLY-GLY-SER-LEU-ILE-ASN-ser-gln-TRP-VAL-VAL-ser-AIA-ALA-HIS-CYS-tyr-lys-ser-gly-ile-gln VAL-val-val-ala-gly-glu-phe-asp-gln-gly-ser-ser-ser-glu-lys-ile-gln-lys-leu-lys-ile-ala-lys- VAL-arg-leu-gly-glu-asp-asn-ile-asn-val-val-glu-gly-asp-glu-gln-phe-ile-ser-ala-ser-lys-ser , val-phe-lys-asn-ser-lys-tyr-asn-ser-leu-thr-ile-asn-asn-asn-ile-thr-leu-leu-lys-leu-ser-thrile-val-his-pro-ser- -TYR-ASN(pro,leuthr,asn)ASN-ASN-asp-ILE-met-LEL-ile-LYS-LEU-lys-ser go AIA-ALA-SER-phe-ser-gln-thr-VAL-ser-ala-val-cys-LEU-PRO-ser-ala-ser-asp-asp-phe-ala-AIA-GLY- AIA-ALA-SER-leu-asn-ser-arg-VAL-ala-ser-ile-ser-LEU-PRO-thr-ser-cys- -41a-ser-AIA-GLY o THR-thr-CYS-val-thr-thr-GLY-TRP-GLY-leu-THR-arg-tyr-thr-asn-ala-asn-thr-PRO-ASP-arg-LEU-gln- THR-gln-CYS-leu-ile-ser-GLY-TRP-GLY-asn-THR-lys-ser-ser-gly-thr-ser-tyr-PRO-ASP-val-LEU-lys gln-ala-ser-leu-pro-leu-leu-ser-asn-thr-asn-cys-lys-lys-tyr-trp-gly-thr-lys-ile-lys-asp-ala- cys-leu-lys-ala-pro-ile-leu-ser-asp-ser-ser-cys-lys-ser-ala-tyr-pro-gly-gln-ile-thr-ser-asn MET-ile-CYS-AIA-GLY-ala-ser-gly-val-ser- -SER-CYS-met-GLY-ASP-SER-GLY-GLY-PRO-leu-VAIL. MET-phe-CYS-ALA-GLY-tyr-leu-glu-gly-gly-lys-asn-SER-CYS-gln-GLY-ASP-SER-GLY-GLY-PRO-val-VAL lj CYS-lys-lys-asn-gly-ala-trp-thr-leu-val-GLY-ILE-VAL-ser-SER-TRP-GLY-SER-ser-thr-cys-ser-thr-,CYS-ser-gly-lys-leu-gln- -GLY-ILE-VAL- -SER-TRP-GLY-SER-gly-cys-ala-gln-lys I ser-thr-pro-gly-val-tyr-ala-arg-val-thr-ala-leu-val-asn-trp-val-gln-gln-thr-leu-aia-ala-asn asn-lys-pro-gly-val-tyr-thr-lys-val-cys-asn-tyr-val-ser-trp-ile-lys-gln-thr-ile-ala-ser-asn The sequence of chymotrypsinogen is taken from Hartley25 and the tentative structure of trypsinogen27 from Walsh, Sampath Kumar, and Kauffman.U The positions of the disulfides are not indicated. The sequence in trypsinogen has not yet been established. Gaps in the sequence have been arbitrarily inserted to draw attention to the resulting areas of homology. Amino acid residues that are homologous in the two proteins are shown in capital letters. upon activation, a common starting point. The rationale for this basis of comparison is derived from the analogies in the activation of the two zymogens, which include, among others, the formation of an identical isoleucyl-valyl-glycyl N- terminal sequence in the newly formed enzymes.34' 26 The mutual alignment of the two linear structures shown in Table 2 in addition makes allowance for occasional deletions following a precedent set by the hemoglobin-myoglobin com-
4 VOL. 52, 1964 BIOCHEMISTRY: WALSH AND NEURATH 887 parisons.35 The data of Table 2 indicate that approximately 40 per cent of the amino acid residues occur in identical positions in the two chains. Identical triand even dipeptide sequences which previously have seemed statistically unimportant36 now indicate a striking homology because they are located in analogous positions throughout the two molecules. This type of homology could not be detected until the complete structure of both proteins was known. Allowing for minor amino acid replacements, the longest continuous sequence common to both proteins is the 19-amino-acid peptide containing two histidines, and extending from histidine -29 in trypsinogen (40 in chymotrypsinogen A) to half-cystine 47 in trypsinogen (58 in chymotrypsinogen). The other common area of functional importance extends, with minor modifications, from serine 178 in trypsinogen (190 in chymotrypsinogen A) to half-cystine 189 in trypsinogen (201 in chymotrypsinogen A) and contains in the middle the reactive serine as part of the previously known, common tetrapeptide sequence Gly-Asp-Ser-Gly. The homology in this region is more extensive than previously believed, because of a previous error in the sequence of the active center peptide37 isolated from trypsin. Close examination of the apparent homology of the two proteins indicates some gradation in the frequency of some amino acid replacements, which may well reflect the importance of these residues to the conformation of the proteins. Thus, it may be of structural significance that 6 of the 7 proline residues in the region of chymotrypsin subject to comparison are in identical positions in trypsin, and all 4 tryptophan residues in trypsinogen occur in identical positions in chymotrypsinogen. On the other hand, 50 of the 62 charged residues of both molecules (lysine, arginine, aspartic, and glutamic acid residues) do not occur in homologous positionsan observation which would be consistent with their location on the outer surface of the molecules. Seven or eight of the 10 half-cystines in chymotrypsinogen are found in homologous loci in trypsinogen. The two nonhomologous half-cystines in chymotrypsinogen (nos. 1,122) have been shown to be joined by a disulfide bond.38 Four of the eight homologous half-cystines of chymotrypsinogen have been tentatively shown to be identically paired by homologous disulfide bonds in the two zymogens3l (nos. 42, 58, and nos. 168, 182 in chymotrypsinogen). However, the further extent of this homology cannot be established until the positions of the remaining disulfide bridges in trypsinogen have been completely elucidated. 27 The degree of conservation of character observed with the two enzymes is not as great as that found in either the hemoglobins35 or the cytochromes,39 but the more extensive structural differences between chymotrypsinogen and trypsinogen are accompanied by a clear change in biological specificity, whereas the specific biological function of the hemoproteins is maintained, in spite of structural deviations. While the actual homology between trypsinogen and chymotrypsinogen is approximately 40 per cent, a homology of character can be seen to extend further if one equates certain amino acid residues having analogous chemical properties. For example, if one equates serine to threonine, and leucine to isoleucine to valine, the homology is extended to 50 per cent of the molecule. If one further extends this principle by equating the aromatic amino acids with each other, the lysine and arginine residues with each other, and the asparagine and glutamine residues with each other, the homology extends to 56 per cent of the residues. It would be difficult to ignore the chemical differences between these residues except by in-
5 888 BIOCHEMISTRY: WALSH AND NEURATH Pioc. N. A. S. dicating that a gross change in chemical nature (and conformational consequences) would not be involved in any of these hypothetical transformations. The high degree of conservation inherent in this homology must reflect either areas of the molecule which have evolved spontaneously and independently by virtue of their necessity for the induction of proteolytic activity by whatever mechanism, or areas which have persisted in the course of evolution of the two proteins from a common ancestral precursor because they are necessary for function. It is difficult to accept the former explanation, because it would necessitate that 40 per cent of each molecule be directly or indirectly involved in the actual function of the enzyme. On the other hand, the latter explanation requires only that certain areas which are of functional importance should not change, but dictates nothing about the degree of change or lack thereof in the remainder of the molecule. If the theory is valid that these two enzymes have evolved from a common precursor, there has been in the course of evolution not only a change of 60 per cent of the molecule, and a consequential marked change in specificity, but also at the same time an equally marked preservation of both the basic physicochemical character of the molecule, and the fundamental catalytic mechanism. From the viewpoint of enzymatic specificity, the differences in covalent structure are more important than their similarities. Somewhere along the sequence of the two enzymes there must be an array of amino acid residues specific for each enzyme, and distributed in such a manner as to give rise in the tertiary structure to the configuration characteristic for tryptic and chymotryptic specificity, respectively. Unfortunately, no method of labeling has yet been found which would identify in the degraded enzyme the residues responsible for the specific substrate binding, and hence lend itself to direct solution in the manner of the present analysis. It can be approached, however, by comparison of the covalent structure of enzymes having similar specificity and occurring in various species of animals.32 Some homology is clearly indicated between bovine and porcine trypsinogen,40 and between bovine chymotrypsinogens A and B.34 It seems a reasonable prediction that the similarities of composition, end-groups, and activation between bovine and porcine trypsin would extend to the level of the structural homology. The recent findings of proteolytic activity with parallel specificities in the pancreas of lower animals (dogfish)4' suggest the feasibility of exploring the extent of homology in a particular enzyme as it evolved, concurrent with the appearance of individual species of animals. At this stage, it seems probable that one can anticipate a parallel between paleontological evidence for the order of evolution of biological structure, that is, of species, and concurrent evolution of chemical structure, that is, the specificity and mechanism of enzymes. Perhaps, in fact, one must anticipate a closer relationship between many proteins now thought to be completely unrelated. ' Green, N. M., and H. Neurath, in The Proteins, ed. H. Neurath and K. Bailey (New York: Academic Press, 1954), vol. 2B, p Desnuelle, P., in The Enzymes, ed. P. D. Boyer, H. Lardy, and K. Myrback (New York: Academic Press, 1960), vol Neurath, H., G. H. Dixon, and J.-F. Pechere, Proc. Intern. Congr. Biochem. 4th, 1960, 63 (vol. 8). 4 Hartley, B. S., Ann. Rev. Biochem., 29, 45 (1960). 6 Sorm, F., and B. Keil, Advan. Protein Chem., 17, 167 (1962). 6 Siekevitz, P., and G. Palade, J. Biophys. Biochem. Cytol., 7, 619, 631 (1960).
6 VOL. 52, 1964 BIOCHEMISTRY: WALSH AND NEURATH 889 7Keller, P. J., E. Cohen, and H. Neurath, J. Biol. Chem., 234, 311 (1959). 8 Neurath, H., Advan. Protein Chem., 12, 320 (1957). 9 Neurath, H., and G. H. Dixon, Federation Proc., 16, 791 (1957). 10 Bergmann, M., and J. S. Fruton, Advan. Enzymol., 1, 63 (1941). 11 Neurath, H., and G. W. Schwert, Chem. Rev., 46, 70 (1950). 12 Hein, G., and C. Niemann, these PROCEEDINGS, 47, 1341 (1961). 13 Hartley, B. S., and B. A. Kilby, Biochem. J., 56, 288 (1954). 14 Gutfreund, H., and J. M. Sturtevant, these PROCEEDINGS, 42, 719 (1956). 16 Bender, M. L., and E. T. Kaiser, J. Am. Chem. Soc., 84, 2557 (1962). 16 Jansen, E. F., and A. K. Balls, J. Biol. Chem., 194, 721 (1952). 17 Schaffer, N. K., C. S. May, Jr., and W. H. Summerson, J. Biol. Chem., 202, 67 (1953). 1 Dixon, G. H., D. L. Kauffman, and H. Neurath, J. Biol. Chem., 233, 1373 (1958). 19 Oosterbaan, R. A., and M. E. Van Adrichem, Biochim. Biophys. Acta, 27, 423 (1958). 20 Dixon, G. H., H. Neurath, and J.-F. Pech~re, Ann. Rev. Biochem., 27, 489 (1958). 21 Koshland, D. E., Jr., D. H. Strumeyer, and W. J. Ray, Jr., in Enzyme Models and Enzyme Structure, Brookhaven Symposia in Biology, No. 15 (1962), p Schoellmann, G., and E. Shaw, Biochemistry, 2, 252 (1963). 23 Mares-Guia, M., and E. Shaw, Federation Proc., 22, 528 (1963). 24 Ong, E. B., E. Shaw, and G. Schoellmann, J. Am. Chem. Soc., 86, 1271 (1964). 25 Hartley, B. S., Nature, 201, 1284 (1964). 26 Walsh, K. A., D. L. Kauffman, K. S. V. Sampath Kumar, and H. Neurath, these PROCEED- INGS, 51, 301 (1964). A series of papers giving the complete proof of structure of bovine trypsinogen is in preparation, to be published in Biochemistry. 28 Inagami, T., and J. M. Sturtevant, J. Biol. Chem., 235, 1019 (1960). 29 Mares-Guia, M., and E. Shaw, Abstracts, 6th International Congress of Biochemistry, 1964, vol. 4, p Walsh, K. A., D. L. Kauffman, and H. Neurath, Federation Proc., 20, 385 (1961). '7 Walsh, K. A., K. S. V. Sampath Kumar, and D. L. Kauffman, Abstracts, 6th International Congress of Biochemistry, 1964, vol. 2, p Neurath, H., Abstracts, 6th International Congress of Biochemistry, 1964, vol. 4, p Hartley, B. S., Abstracts, 6th International Congress of Biochemistry, 1964, vol. 4, p Desnuelle, P., and M. Rovery, Advan. Protein Chem., 16, 139 (1961). 3 Braunitzer, G., R. Gehring-Muller, N. Hilschmann, K. Hilse, G. Hobom, V. Rudloff, and B. Wittmann-Liebold, Z. Physiol. Chemie, 325, 283 (1961). 36 Williams, J., J. B. Clegg, and M. 0. Mutch, J. Mol. Biol., 3, 532 (1963). 37 Dixon, G. H., D. L. Kauffman, and H. Neurath, J. Biol. Chem., 233, 1373 (1958). 38 Brown, J. R., and B. S. Hartley, Biochem. J., 89, 59P (1963). 39 Margoliash, E., these PROCEEDINGS, 50, 672 (1963). 40 Charles, M., M. Rovery, A. Guidoni, and P. Desnuelle, Biochim. Biophys. Acta, 69, 115 (1963). 41 Prahl, J. W., and H. Neurath, Abstracts, 142nd National Meeting of the American Chemical Society, 1962.
LAB#23: Biochemical Evidence of Evolution Name: Period Date :
 LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
1. Describe the relationship of dietary protein and the health of major body systems.
 Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Amino Acids. Amino Acids. Fundamentals. While their name implies that amino acids are compounds that contain an NH. 3 and CO NH 3
 Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Methionine (Met or M)
 Fig. 5-17 Nonpolar Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine (Leu or L) Isoleucine (Ile or I) Methionine (Met or M) Phenylalanine (Phe or F) Polar Trypotphan (Trp
Fig. 5-17 Nonpolar Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine (Leu or L) Isoleucine (Ile or I) Methionine (Met or M) Phenylalanine (Phe or F) Polar Trypotphan (Trp
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
AP Bio. Protiens Chapter 5 1
 Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
BIOCHEMISTRY REVIEW. Overview of Biomolecules. Chapter 4 Protein Sequence
 BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 4 Protein Sequence 2 3 4 Are You Getting It?? A molecule of hemoglobin is compared with a molecule of lysozyme. Which characteristics do they share?
BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 4 Protein Sequence 2 3 4 Are You Getting It?? A molecule of hemoglobin is compared with a molecule of lysozyme. Which characteristics do they share?
Amino Acids. Review I: Protein Structure. Amino Acids: Structures. Amino Acids (contd.) Rajan Munshi
 Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Amino acids-incorporated nanoflowers with an
 Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity Zhuo-Fu Wu 1,2,+, Zhi Wang 1,+, Ye Zhang 3, Ya-Li Ma 3, Cheng-Yan He 4, Heng Li 1, Lei Chen 1, Qi-Sheng Huo 3, Lei Wang 1,*
Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity Zhuo-Fu Wu 1,2,+, Zhi Wang 1,+, Ye Zhang 3, Ya-Li Ma 3, Cheng-Yan He 4, Heng Li 1, Lei Chen 1, Qi-Sheng Huo 3, Lei Wang 1,*
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Introduction to Peptide Sequencing
 Introduction to Peptide equencing Quadrupole Ion Traps tructural Biophysics Course December 3, 2014 12/8/14 Introduction to Peptide equencing - athan Yates 1 Why are ion traps used to sequence peptides?
Introduction to Peptide equencing Quadrupole Ion Traps tructural Biophysics Course December 3, 2014 12/8/14 Introduction to Peptide equencing - athan Yates 1 Why are ion traps used to sequence peptides?
Protein Investigator. Protein Investigator - 3
 Protein Investigator Objectives To learn more about the interactions that govern protein structure. To test hypotheses regarding protein structure and function. To design proteins with specific shapes.
Protein Investigator Objectives To learn more about the interactions that govern protein structure. To test hypotheses regarding protein structure and function. To design proteins with specific shapes.
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Chemical Mechanism of Enzymes
 Chemical Mechanism of Enzymes Enzyme Engineering 5.2 Definition of the mechanism 1. The sequence from substrate(s) to product(s) : Reaction steps 2. The rates at which the complex are interconverted 3.
Chemical Mechanism of Enzymes Enzyme Engineering 5.2 Definition of the mechanism 1. The sequence from substrate(s) to product(s) : Reaction steps 2. The rates at which the complex are interconverted 3.
1. (38 pts.) 2. (25 pts.) 3. (15 pts.) 4. (12 pts.) 5. (10 pts.) Bonus (12 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version]
![Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version] Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version]](/thumbs/84/90818314.jpg) Earth/matriX: SCIENCE TODAY Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version] By Charles William Johnson Earth/matriX Editions P.O.
Earth/matriX: SCIENCE TODAY Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version] By Charles William Johnson Earth/matriX Editions P.O.
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
PROTEINS. Amino acids are the building blocks of proteins. Acid L-form * * Lecture 6 Macromolecules #2 O = N -C -C-O.
 Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Chapter 21 Lecture Outline
 Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
BIO th September, 1997
 BIO 451 26th September, 1997 EXAM I This exam will be taken apart for grading. Please PRINT your name on each page. If you do not have sufficient room for your answer in the space provided, please continue
BIO 451 26th September, 1997 EXAM I This exam will be taken apart for grading. Please PRINT your name on each page. If you do not have sufficient room for your answer in the space provided, please continue
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Four Classes of Biological Macromolecules. Biological Macromolecules. Lipids
 Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Anfinsen,'0 and the cystines were then reduced and converted to analogues of
 ON THE STRUCTURE AND FUNCTION OF BOVINE TRYPSINOGEN AND TRYPSIN* BY KENNETH A. WALSH, DOROTHY L. KAUFFMAN, K. S. V. SAMPATH KUMAR, AND HANS NEURATH UNIVERSITY OF WASHINGTON, SEATTLE Communicated December
ON THE STRUCTURE AND FUNCTION OF BOVINE TRYPSINOGEN AND TRYPSIN* BY KENNETH A. WALSH, DOROTHY L. KAUFFMAN, K. S. V. SAMPATH KUMAR, AND HANS NEURATH UNIVERSITY OF WASHINGTON, SEATTLE Communicated December
Cells N5 Homework book
 1 Cells N5 Homework book 2 Homework 1 3 4 5 Homework2 Cell Ultrastructure and Membrane 1. Name and give the function of the numbered organelles in the cell below: A E B D C 2. Name 3 structures you might
1 Cells N5 Homework book 2 Homework 1 3 4 5 Homework2 Cell Ultrastructure and Membrane 1. Name and give the function of the numbered organelles in the cell below: A E B D C 2. Name 3 structures you might
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Practice Problems 3. a. What is the name of the bond formed between two amino acids? Are these bonds free to rotate?
 Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
A Chemical Look at Proteins: Workhorses of the Cell
 A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
Chapter 4: Information and Knowledge in the Protein Insulin
 Chapter 4: Information and Knowledge in the Protein Insulin This chapter will calculate the information and molecular knowledge in a real protein. The techniques discussed in this chapter to calculate
Chapter 4: Information and Knowledge in the Protein Insulin This chapter will calculate the information and molecular knowledge in a real protein. The techniques discussed in this chapter to calculate
PROTEINS. Building blocks, structure and function. Aim: You will have a clear picture of protein construction and their general properties
 PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
Introduction. Basic Structural Principles PDB
 BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
Classification of amino acids: -
 Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Biology. Lectures winter term st year of Pharmacy study
 Biology Lectures winter term 2008 1 st year of Pharmacy study 3 rd Lecture Chemical composition of living matter chemical basis of life. Atoms, molecules, organic compounds carbohydrates, lipids, proteins,
Biology Lectures winter term 2008 1 st year of Pharmacy study 3 rd Lecture Chemical composition of living matter chemical basis of life. Atoms, molecules, organic compounds carbohydrates, lipids, proteins,
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 2 Biosynthesis of Enzymes
 Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Chymotrypsin Lecture. Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin
 Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Macromolecules Structure and Function
 Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Review II: The Molecules of Life
 Review II: The Molecules of Life Judy Wieber BBSI @ Pitt 2007 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2007 Outline Introduction Proteins Carbohydrates Lipids
Review II: The Molecules of Life Judy Wieber BBSI @ Pitt 2007 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2007 Outline Introduction Proteins Carbohydrates Lipids
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination #5: Section Five December 7, Name: (print) Section:
 Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
Introduction to Protein Structure Collection
 Introduction to Protein Structure Collection Teaching Points This collection is designed to introduce students to the concepts of protein structure and biochemistry. Different activities guide students
Introduction to Protein Structure Collection Teaching Points This collection is designed to introduce students to the concepts of protein structure and biochemistry. Different activities guide students
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
Catabolism of Carbon skeletons of Amino acids. Amino acid metabolism
 Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth 2 points.
 MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Cells. Variation and Function of Cells
 Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Bio 100 Serine Proteases 9/26/11
 Assigned Reading: 4th ed. 6.4.1 The Chymotrypsin Mechanism Involves Acylation And Deacylation Of A Ser Residue p. 213 BOX 20-1 Penicillin and β-lactamase p. 779 6.5.7 Some Enzymes Are Regulated By Proteolytic
Assigned Reading: 4th ed. 6.4.1 The Chymotrypsin Mechanism Involves Acylation And Deacylation Of A Ser Residue p. 213 BOX 20-1 Penicillin and β-lactamase p. 779 6.5.7 Some Enzymes Are Regulated By Proteolytic
2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry
 Dr. Sanjeeva Srivastava 1. Fundamental of Mass Spectrometry Role of MS and basic concepts 2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry 2 1 MS basic concepts Mass spectrometry - technique
Dr. Sanjeeva Srivastava 1. Fundamental of Mass Spectrometry Role of MS and basic concepts 2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry 2 1 MS basic concepts Mass spectrometry - technique
Arginine side chain interactions and the role of arginine as a mobile charge carrier in voltage sensitive ion channels. Supplementary Information
 Arginine side chain interactions and the role of arginine as a mobile charge carrier in voltage sensitive ion channels Craig T. Armstrong, Philip E. Mason, J. L. Ross Anderson and Christopher E. Dempsey
Arginine side chain interactions and the role of arginine as a mobile charge carrier in voltage sensitive ion channels Craig T. Armstrong, Philip E. Mason, J. L. Ross Anderson and Christopher E. Dempsey
Metabolism of amino acids. Vladimíra Kvasnicová
 Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
number Done by Corrected by Doctor Dr.Diala
 number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
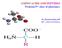 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Date: EXERCISE 4. Figure 1. Amino acid structure.
 Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
Bio Factsheet. Proteins and Proteomics. Number 340
 Number 340 Proteins and Proteomics Every living thing on the planet is composed of cells, and cells in turn are made of many types of molecules, including the biological molecules carbohydrates, lipids,
Number 340 Proteins and Proteomics Every living thing on the planet is composed of cells, and cells in turn are made of many types of molecules, including the biological molecules carbohydrates, lipids,
Maha AbuAjamieh. Tamara Wahbeh. Mamoon Ahram
 12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
Protein and Amino Acid Analysis. Chemistry M3LC
 Protein and Amino Acid Analysis Chemistry M3LC Proteins Proteins are made up of amino acids: H2N-CHR-COOH + H3N-CHR-COO - neutral form zwitterionic form There are twenty standard amino acids: A ala alanine
Protein and Amino Acid Analysis Chemistry M3LC Proteins Proteins are made up of amino acids: H2N-CHR-COOH + H3N-CHR-COO - neutral form zwitterionic form There are twenty standard amino acids: A ala alanine
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids. Side chain. -Carbon atom. Carboxyl group. Amino group
 PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
Judy Wieber. Department of Computational Biology. May 27, 2008
 Review II: The Molecules of Life Judy Wieber BBSI @ Pitt 2008 Department of Computational Biology University it of Pittsburgh School of Medicine i May 27, 2008 Outline Introduction Proteins Carbohydrates
Review II: The Molecules of Life Judy Wieber BBSI @ Pitt 2008 Department of Computational Biology University it of Pittsburgh School of Medicine i May 27, 2008 Outline Introduction Proteins Carbohydrates
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Phenylketonuria (PKU) Structure of Phenylalanine Hydroxylase. Biol 405 Molecular Medicine
 Phenylketonuria (PKU) Structure of Phenylalanine Hydroxylase Biol 405 Molecular Medicine 1998 Crystal structure of phenylalanine hydroxylase solved. The polypeptide consists of three regions: Regulatory
Phenylketonuria (PKU) Structure of Phenylalanine Hydroxylase Biol 405 Molecular Medicine 1998 Crystal structure of phenylalanine hydroxylase solved. The polypeptide consists of three regions: Regulatory
Enzyme Catalytic Mechanisms. Dr. Kevin Ahern
 Enzyme Catalytic Mechanisms Dr. Kevin Ahern Cleave Peptide Bonds Specificity of Cutting Common Active Site Composition/Structure Mechanistically Well Studied Chymotrypsin Chymotrypsin Catalysis H2O Chymotrypsin
Enzyme Catalytic Mechanisms Dr. Kevin Ahern Cleave Peptide Bonds Specificity of Cutting Common Active Site Composition/Structure Mechanistically Well Studied Chymotrypsin Chymotrypsin Catalysis H2O Chymotrypsin
SEQUENCE ON PROTEIN STRUCTURE
 THE INFLUENCE OF AMINO-ACID SEQUENCE ON PROTEIN STRUCTURE ANTHONY V. GUZZO From the Department of Biophysics, University of Chicago, Chicago. Dr. Guzzo's present address is the Department of Chemistry,
THE INFLUENCE OF AMINO-ACID SEQUENCE ON PROTEIN STRUCTURE ANTHONY V. GUZZO From the Department of Biophysics, University of Chicago, Chicago. Dr. Guzzo's present address is the Department of Chemistry,
Chapter 5: Structure and Function of Macromolecules AP Biology 2011
 Chapter 5: Structure and Function of Macromolecules AP Biology 2011 1 Macromolecules Fig. 5.1 Carbohydrates Lipids Proteins Nucleic Acids Polymer - large molecule consisting of many similar building blocks
Chapter 5: Structure and Function of Macromolecules AP Biology 2011 1 Macromolecules Fig. 5.1 Carbohydrates Lipids Proteins Nucleic Acids Polymer - large molecule consisting of many similar building blocks
