TitleExtraction Behavior of Metal Acetyl. Author(s) Shigematsu, Tsunenobu; Tabushi, Mas.
|
|
|
- Frank Lang
- 5 years ago
- Views:
Transcription
1 TitleExtraction Behavior of Metal Acetyl Author(s) Shigematsu, Tsunenobu; Tabushi, Mas Citation Bulletin of the Institute for Chemi University (1961), 39(1): Issue Date URL Right Type Departmental Bulletin Paper Textversion publisher Kyoto University
2 Extraction Behavior of Metal Acetylacetonates" Tsunenobu SFIIGEMATSU and Masayuki (Shigematsu Laboratory) Received December 12, 1960 TABusI-n*" The extraction behaviors of several metal acetylacetonates with chloroform were studied, and the extraction curves were presented for aluminium, beryllium, cadmium, cerium, cobalt, copper, ferric iron, gallium, hafnium, indium, lead, magnesium, manganese, nickel, strontium, uranium, yttrium and zinc. Some considerations were made on the relationships between the properties of the central metal ions and the extractability of the chelates. Maximum extraction recovery is correlated with the ionic potential and the ionization potential of the metal, and the pii value for unit distribution ratio decreases linearly with the increase of the ionic potential. These facts may be attributed to that the increase of these potentials rises the bound energy and covalent property of oxygen bond, which is essentially ionic, and so results in the increase of the stability of the chelates and of the solubility into the organic solvent. INTRODUCTION Acetylacetone reacts with many metals to form stable chelates, and many of them can be extracted with various organic solvents. Acetylacetone is not only a chelating reagent but also an extracting solvent. Freiser et al' ' '. studied on the extraction of metals with acetylacetone. The authors also investigated on the solvent extraction of metal acetylacetonates, and proposed the methods for the separation and the spectrophotometric determination of beryllium7'8, uranium''"', and ferric iron10,"'. During the investigations, it became evident that the extractability of metal acetylacetonates may be related to the ionic potentials or the ionization potentials of the central metal ions. Although many reports were already published on the relation between the stability of the metal complexes and the properties of metal ions, such as ionic radii, ionization potentials or electronegativities and the studies were made also on the /3-diketone chelates''=-'", studies on the solvent extractions have not been reported. In the present paper, the relationships between the ionic potentials of metal ions and the maximum per cent extractions of the chelates, the ionization potentials and the maximum per cent extractions, and the ionic potentials and the ph values for the half extractions are discussed respectively. EXPERIMENTAL Spectrophotometric, fluorometric and polarographic measurements for the an- * Presented before the 8 th Annual Meeting of the Japan Society for Analytical Chemistry (Sept. 1958). (35 )
3 Tsunenobu SI-IIGEMATSU and Masayuki TABUSHI alytical determinations were made with a Hitachi's Photoelectric Spectrophotometer, Model EPU-2A, a Shimadzu's Universal Fluorometer, Model OF-1, and a Yanagimoto's Polarograph, Model PA-105 respectively. A Horiba's Glass electrode ph meter, Model M, and a Metro's EIT Scalar, Model 6E with G-M tube (TEN 132), were used for ph measurements and radioactivity countings. In order to obtain the extraction curves for acetylacetonates of metals, such as aluminium, beryllium, cadmium, cerium, cobalt, copper, ferric iron, gallium, hafnium, indium, lead, magnesium, manganese, nickel, strontium, uranium, yttrium, and zinc, the extractions were carried by shaking 50 ml. of aqueous solutions having a constant concentration of acetylacetone, 1 per cent, and various ph values, with 20 ml. of chloroform. Per cent extractions were determined by the measurements of radioactivity, when the radioisotopes were used or by the common chemical analysis, as described in Table 1, after evaporating chloroform solutions and destroying organic materials by fumming with perchloric acid. Table 1. The analytycal method for the determination of the per cent extraction. MetalMethod AlFluorometry (Pontachrome Blue Black Ra)) BeSpectrophotometry (Acetylacetone ; Eriochrome Cyanine Rb') CdPolarography CeRadioactivity (Ce-144) CoRadioactivity (Co-60) Cu! Spectrophotometry (Chloro-Complex) FeSpectrophotometry (Acetylacetone) FeRadioactivity (Fe-55, 59) GaFluorometry (2-Methyloxine"') HfRadioactivity (I-If-181) InFluorometry (2-Methyloxine' ) MgChelatometry MnSpectrophotometry (KMnO4) NiRadioactivity (Ni-63) PbSpectrophotometry (Chloro-Complexe') SrRadioactivity (Sr-89) USpectrophotometry (Acetylacetone ; KSCN) YRadioactivity (Y-91) ZnRadioactivity (Zn-63) Reference a' M. Ishibashi, T. Shigematsu and Y. Nishikawa, Japan Analyst, (1957). b' U. T. Hill, Anal. Chem., 29, 288 (1957). ' M. Ishibashi, T. Shigematsu and Y. Nishikawa, J. Chem. Soc. Japan, Pure Chem. Sec., 78, 1139 (1957). "' M. Ishibashi, T. Shigematsu and Y. Nishikawa, ibid., 77, 1479 (1956). e' Y. Yamamoto, This Bulletin, 36, 139 (1658). RESULTS AND CONCLUSION Extraction curves are shown in Fig. 1-a and Fig. 1-b. Results on the acetylacetonates of alminium, beryllium, gallium, hafnium, indium, iron and uranium show ( 36 )
4 Extraction Behavior of Metal Acetylacetonates. 100 v'~~ 80 ' 60 - F csu e. i. apbpi) a -O a-. Fe " ift. Ga. rill* Cu, O 20 Ga ZnI CuY 0 0 Zn U., ph Fig. 1-a. Extraction curves of metal acetylacetonates. 1ao/Oto.~y N Be In Hf 80 Al,,IIf 60 de U czy B e IIf 1/ Cehr a 40- Al U a On, 20 M n0 Mn 0 In I, t I Ili ( I I ( ph Fig. 1-b. Extraction curves of metal acetylacetonates.. (37)
5 Tsunenobu SHIGEMATSU and Masayuki TABUSHI approximately quantitative extraction, while the chelates of cadmium, cobalt, magnesium, nickel and strontium are not extracted at any ph values, and the extraction of the chelates of cerium, copper, lead, manganese and zinc are incomplete. In Fig. 2, the maximum per cent extractions of metal acetylacetonates are shown as a function of ionic potentials (i onicicradii*ji_e1of the central metal ions. The figure suggests that there may be a positive correlation between the maximum per cent extractions and the ionic potentials : the chelates of metals with higher ionic potentials than 4, are extracted almost completely ; the chelates with lower ionic potential than 2.5 are not extracted ; within the interval from 2.5 to 4, there is a regular increase in the extractability as the ionic potential increases. The chelates of copper and lead show unexpected extractability, and magnesium with larger ionic potential than that of copper, can not be extracted. This reverse trend between copper and magnesium may be caused by the ionization potential (see Fig. 4), and the irregularity of lead chelate will be discussed later O PbCc O Cu Fe Ga Al In0 Ile OIII 0 0 co GO s. v U ,M 20 zn 0 n SrCo OY 0 -O-- Cd II Ni Mg III Ionic potential, Z/r Fig. 2. Relation between the maximum percent extraction and ionic potential. In Fig. 3, the pii 1/2 values (phs corresponding to unit distribution ratio**) of the extractable chelates having the maximum per cent extraction of 30% or more, are plotted as a function of ionic potentials. As shown in the figure, there is a destinct linear relationship between the ph values and the ionic potentials. In this relation, however, copper chelate shows irregularity. Fig. 4 represents the correlation between the maximum per cent extractions for the chelates of bivalent metals and the ionization potentials (E31>31++)050. As seen in the figure, the extractability increases with the ionization potential, except lead chelate which shows unexpected extractability. * E. Kordes : Z. Physik. Chem., B 48, 91 (1940) ** It corresponds to 28.6% extraction yield in the extraction procedure described above. *** "Handbook of Chemistry" (1952) Maruzen Co., Tokyo. ( 38 )
6 Extraction Behavior of Metal Acetylacetonates 8 O I'b 6-O Zo 0 4- OC to O Cc O leiif cl 13e 2 -Fe^ III Ionic potential, Fig. 3. Relation between ph 1,2 and ionic potential. Z/r Be 0 so - u 60 - ro OCu PI) 0 O N Zn G40 MgCi, C(1 0 O O-O o Ni Total ionization potential (ev) Fig. 1. Relation between the maximum percent extraction and ionization potential. CONSIDERATION AND DISCUSSION General Consideration On the solvent extraction of the metal chelates, the extractability is determined by the formation constant and by the distribution coefficient of the chelates. Because acetylacetone is oxygen-oxygen donating ligand, it has a marked tendency to form ionic bond, and it is confirmed by the facts that nickel and cobalt acetylacetonates have not planer configurations, but tetrahedral, as proved by the magnetic properties (3.2 and 4.2 Bohr magnetones, respectively) and the absoption spectra, and that ferric acetylacetonate also is para magnetic (the magnetic moment : 5.95 Bohr magnetone) and therefore 3 d orbital of iron is not occupied by the bond ( 39 )
7 Tsunenobu SI-IIGEMATSU and Masayuki TABUSHI formation'''. In comparision with the covalent bond, the ionic bond may be effected greatly by the ionic potential or the ionization potential of the central metal ion. As these potential values increase, the bond energy as well as covalency in the bond of the chelate becomes greater, that is, both the stability of the chelate and the distribution coefficient to organic solvent increase with these potentials. As mentioned above, the extractability are determined by the formation constant and the bistribution coefficient of the chelate, and therefore the increases in the ionic- or ionization potential results in the increases of the maximum per cent extractions and in the decreases of ph1, value. The regularities represented in Fig. 2, Fig. 3 and Fig. 4, can be explained by these considerations. As previously reported by many workers, there is a positive correlation between the stabilities of the chelates and ionic- or ionization potentials, and so the similar features as shown in figures will be observed also when these are expressed as a function of the stability constant. On Bivalent Cations of the First Transition Series The stabilities of the complexes of bivalent ions of the first transition elements follow Irving and Williams' order of the stability". Zn<Cu>Ni>Co>Mn>Fe regardless of the nature of the coordination ligands. In the solvent extractions of acetylacetonates of these metals, is observed a similar sequence, Cu''>Zn">Ni"--Co"<Mn"? (Fe" unstable). Now, the difference of the position of zinc in the both orders, will present an interesting question, and it is considered as follows. Irving and Williams' order of the stability corresponds essentially to the order of ionic radii of the metal ions, and zinc holds the position between copper and nickel. However, in almost all cases, zinc- complexes are less stable than cobalt complexes, and this disorder may be attributed to the fact that zinc complexes are tetrahedral, and therefore some configurational changes occur by the complex formation from octahedral aquo ion, while the complexes of nickel or of cobalt have planer structure and the replacements of the ligands occur in a usual way. For acetylacetonates, such considerations are not required, because nickel and cobalt form tetrahedral chelate as well as zinc, and on the contrary, the order of the ability to form tetrahedral configuration, Zn">Co">Ni+' >>Cu", must be considered to be what was assumed from the stability of the complexes of triaminoethylamine. Thus, it seems that the sequence of the extractabilities described above is reasonable. This assumption might be confirmed by the fact that zinc chelates with toropolone or its derivatives, which are oxygen-oxygen ligands and have similar structures to acetylacetone, are more stable than nickel and cobalt chelatesl''20', although the stability constant of zinc acetylacetonate measured by the ph titration method is slightly less than those of nickel and cobalt. As shown in Fig. 1, manganese can be extracted at relatively high ph region, (ph 9-10), but it is doubtful whether manganese exists as bivalent ion in this region. Ferrous acetylacetonate is so unstable that it is easily oxidized to ferric chelate and ( 40 )
8 Extraction Behavior of Metal Acetylacetonates the oxidation can not be prevented with ascorbic acid*. Thus, it may be concluded that Irving and Williams' sequence of the stability is valid for the extraction of acetylacetonates. On Anomaly of Copper and Lead In general, the high stability of copper complexes is attributed to its high ionization potential, and the acetylacetonate is not an exception. This can be proved by the result that the unusual features of copper chelate are observed in Fig. 2 and Fig. 3, but not in Fig. 4. The anomaly of lead complexes has been discussed by many workers. Mellor and Maley2" suggested that the stability order, Cu>Ni>Pb>Co>Zn, proposed by themselves for salicylaldehyde complexes, was not justified either by the ionic radii or the electronegativities of the metals. The similar disorder is found also in Monk's sequence of the stability2", Ni>Zn>Pb~Co>Mn, obtained for the chelates with alanine and glycine. The extraction behavior of lead acetylacetonate is the same. It follows Mellor and Maley's or Monk's order of the stability, if one takes account of the configuration of nickel chelate as discussed above. Now, comparing Fig. 2, Fig. 3 and Fig. 4., a question why the irregular behavior of lead acetylacetonate is found in the plots of the maximum percent extractions, but not in those of ph, arises. This observations may suggest the possibility that lead exists as high valent ion in the ph region where the extraction is carried, and this might give a hint to explain the anomaly of lead. In order to answer this question, of course, more detailed investigations must be made, although the valency change of lead might be possible, because of its relatively small third ionization potential. Freiser and coworker2 reported that tervalent cobalt can be extracted with acetylacetone, and the result is also explained in term of the ionic potentials of cobaltous (2.65) and cobaltic (4.78). They proposed also a method for the extraction of chromium", in which chromium was heated with acetylacetone prior to the extraction. Chromium is expected to be extractable from its ionic potential of 4.85, but the formation of inner complex is very slow, because of its inert type electron configuration2", and the latter fact is the reason why the heating in advance is required. In this research, the consideration was made on the results obtained when chloroform was used as an extracting solvent. Acetylacetonates are also extracted with esters, and for some chelates, ester is superior to chloroform, although in general, the latter is the better solvent. And it is expected that there is a correlation between the structure of the chelate and the natrue of the extracting solvent. Further investigation will be carried out on this interesting problem. SUMMARY The extraction curves for metal acetylacetonates were obtained, and the behaviors of the chelates were discussed as a function of the ionic- or ionization poten- * I-Iydroxylarnine can not be used, because it reacts with acetylacetone. ( 41 )
9 Tsunenobu SHIGcMATSU and Masayuki TABUSHI tials of the central metal ions. The increase of ionic- or ionization potential results in the rise of the maximum per cent extraction and in the decrease of ph value, and this may be due to the increase in the stability of the chelate and the covalency of the bond which is related to the solubility in organic solvent. Just after the preparation of this manuscript, W. B. Brown, J. F. Steinbach and W. F. Wanger has published a paper concerning the similar consideration on acetylacetonats of lantanides: J. Inorg. Nuclear Chem., 13, 119 (1960). REFERENCES (1) J. F. Steinbach and H. Freiser, Anal. Chem., 25, 881 (1953). (2) J. F. Steinbach and H. Freiser, ibid., 26, 375 (1954). (3) A. Krishen and H. Freiser, ibid., 29, 288 (1957). (4) J. P. McKaveney and H. Freiser, ibid., 29, 291 (1957). (5) J. P. McKaveney and H. Freiser, ibid., 30, 526 (1958). (6) J. P. McKaveney and H. Freiser, ibid., 30, 1965 (1958). (7) T. Shigematsu and M. Tabushi, J. Chem. Soc. Japan, Pure Chem. Sec., 80, 162 (1958). (8) M. Tabushi, This Bulletin 36, 156 (1958). (9) M. Tabushi, ibid., 37, 226, 237 (1959). (10) M. Tabushi, ibid., 37, 232, 245 (1959). (11) T. Shigematsu and M. Tabushi, J. Chem. Soc. Japan, Pure Chem. Sec., 80, 1018 (1958). (12) R. M. Izatt, W. C. Fernelius and B. P. Blok, J. Phys. Chem., 59, 80 (1955). (13) R. M. Izatt, W. C. Fernelius, C. G. Haas and B. P. Block, ibid., 59, 170 (1955). (14) Le Grand, G van Uitert, W.C. Fernelius and B.E. Douglas, J. Am. Chem. Soc. 75, 2736 (1953). (15) A. E. Martell and M. Calvin, "Chemistry of the Metal Chelate Compounds" pp. 214, 218, 219 (1952) New York. (16) H. Irving and P. J. P. Williams, J. Chem. Soc., 1953, (17) B. E. Bryant, W. C. Fernelius and B.E. Douglas, J. Am. Chem. Soc. 75, 3784 (1953). (18) B. E. Bryant and W. C. Fernelius, ibid., 76, 1696 (1954). (19) B. E. Bryant and W. C. Fernelius, ibid., 76, 3783 (1954). (20) B. E. Bryant and W. C. Fernelius, ibid., 76, 4864 (1954). (21) D.P. Mellor and L. Maley, Nature, 159, 370 (1947). (22) C. B. Monk, Trans. Faraday Soc., 47, 297 (1951). (23) G. H. Morrison and H. Freiser, "Solvent Extraction in Analytical Chemistry" p. 202 (1957) New York. (24) A. E. Martell and M. Calvin, "Chemistry of the Metal Chelate Compounds" p. 233 (1952) New York. ( 42 )
Hydroxyquinoline, 8-Mercaptoquinoli. Eiji Suito on the Occasion of his R.
 Comparison of Properties and Struct Title Hydroxyquinoline, 8-Mercaptoquinoli Selenoquinoline (Commemoration Issu Eiji Suito on the Occasion of his R Author(s) Sekido, Eiichi; Fukui, Nobuo Citation Bulletin
Comparison of Properties and Struct Title Hydroxyquinoline, 8-Mercaptoquinoli Selenoquinoline (Commemoration Issu Eiji Suito on the Occasion of his R Author(s) Sekido, Eiichi; Fukui, Nobuo Citation Bulletin
of human hair and nails. Part I. Analytical methodology, Sci. Tot. Environ. 2000, 250/1-3,
 Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Multi Analyte Custom Grade Solution
 1.0 ACCREDITATION / REGISTRATION INORGANIC VENTURES is accredited to ISO Guide 34, "General Requirements for the Competence of Reference Material Producers" and ISO/IEC 17025, "General Requirements for
1.0 ACCREDITATION / REGISTRATION INORGANIC VENTURES is accredited to ISO Guide 34, "General Requirements for the Competence of Reference Material Producers" and ISO/IEC 17025, "General Requirements for
An Investigative Study of Reactions Involving Glucosinolates and Isothiocyanates
 An Investigative Study of Reactions Involving Glucosinolates and Isothiocyanates Alzea Chrisel H. Alea 1, Diane Elaine T. Co 2 and Marissa G Noel 3* 1,2,3Chemistry Department, De La Salle University, 2401
An Investigative Study of Reactions Involving Glucosinolates and Isothiocyanates Alzea Chrisel H. Alea 1, Diane Elaine T. Co 2 and Marissa G Noel 3* 1,2,3Chemistry Department, De La Salle University, 2401
Elemental analysis in clinical practice
 Elemental analysis in clinical practice Nicholas J Miller FRCPath, Laboratory Director, Biolab Medical Unit, ThermoFisher summer symposium 7 th June 2011, QEII Conference Centre Nutritional Elements Macro
Elemental analysis in clinical practice Nicholas J Miller FRCPath, Laboratory Director, Biolab Medical Unit, ThermoFisher summer symposium 7 th June 2011, QEII Conference Centre Nutritional Elements Macro
There are two groups of minerals: Major salt components: K, Na, Ca, Mg, Cl -, sulfate, phosphate, and HCO
 MINERALS INTRODUCTION 90 elements in the earth s s crust, 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
MINERALS INTRODUCTION 90 elements in the earth s s crust, 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
A QUANTITATIVE METHODOLOGY FOR DETERMINATION OF IMIDAZOLIC COMPOUNDS IN PHARMACEUTICAL CREAMS, UTILIZING A COBALT COMPLEX (II) ASSAY
 A QUANTITATIVE METHODOLOGY FOR DETERMINATION OF IMIDAZOLIC COMPOUNDS IN PHARMACEUTICAL CREAMS, UTILIZING A COBALT COMPLEX (II) ASSAY Published en enfarma, accepted by RMCF, Mexico Author: Dr. HEINZ LIECHTI
A QUANTITATIVE METHODOLOGY FOR DETERMINATION OF IMIDAZOLIC COMPOUNDS IN PHARMACEUTICAL CREAMS, UTILIZING A COBALT COMPLEX (II) ASSAY Published en enfarma, accepted by RMCF, Mexico Author: Dr. HEINZ LIECHTI
Bottom Ash Data Week 30
 Bottom Ash Data 2018 Week 30 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on August 14, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 30 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on August 14, 2018. The data represents bottom ash composite results for
Bottom Ash Data Week 38
 Bottom Ash Data 2018 Week 38 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 4, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 38 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 4, 2018. The data represents bottom ash composite results for
Bottom Ash Data Week 37
 Bottom Ash Data 2018 Week 37 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 2, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 37 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 2, 2018. The data represents bottom ash composite results for
Bottom Ash Data Week 12
 Bottom Ash Data 2019 Week 12 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on April 11, 2019. The data represents bottom ash composite results for
Bottom Ash Data 2019 Week 12 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on April 11, 2019. The data represents bottom ash composite results for
Bottom Ash Data Week 49
 Bottom Ash Data 2018 Week 49 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on December 20, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 49 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on December 20, 2018. The data represents bottom ash composite results for
Bottom Ash Data Week 8
 Bottom Ash Data 2019 Week 8 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 6, 2019. The data represents bottom ash composite results for week
Bottom Ash Data 2019 Week 8 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 6, 2019. The data represents bottom ash composite results for week
Bottom Ash Data Week 17
 Bottom Ash Data 2018 Week 17 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on May 16, 2018. The data represents bottom ash composite results for week
Bottom Ash Data 2018 Week 17 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on May 16, 2018. The data represents bottom ash composite results for week
Bottom Ash Data Week 9
 Bottom Ash Data 2018 Week 9 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 26, 2018. The data represents bottom ash composite results for week
Bottom Ash Data 2018 Week 9 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 26, 2018. The data represents bottom ash composite results for week
There are two groups of minerals: Major salt components: K, Na, Ca, Mg, Cl -, sulfate, phosphate, and HCO 3
 MINERALS INTRODUCTION 90 elements in the earth s s crust. 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
MINERALS INTRODUCTION 90 elements in the earth s s crust. 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
Pharmaceutical Analytical Chemistry PHCM223 Lecture 12 Applications on different types of equilibria. Dr. Nesrine El Gohary 12 th lecture
 Pharmaceutical Analytical Chemistry PHCM223 Lecture 12 Applications on different types of equilibria Dr. Nesrine El Gohary 12 th lecture Revision lecture next week Next Saturday (21-5-2016) in the 1 st
Pharmaceutical Analytical Chemistry PHCM223 Lecture 12 Applications on different types of equilibria Dr. Nesrine El Gohary 12 th lecture Revision lecture next week Next Saturday (21-5-2016) in the 1 st
Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A. Ac-ag...Au-Zr. Bearbeitet von B Predel
 Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A Ac-ag...Au-Zr Bearbeitet von B Predel 1. Auflage 2006. Buch. XXX, 334 S. ISBN 978 3 540 43534 1
Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A Ac-ag...Au-Zr Bearbeitet von B Predel 1. Auflage 2006. Buch. XXX, 334 S. ISBN 978 3 540 43534 1
Supplying Nutrients to Crops
 Supplying Nutrients to Crops What is Plant Nutrition? Plants need nutrients for healthy growth and development. Plant nutrition involves the absorption of nutrients for plant growth and is dependent on
Supplying Nutrients to Crops What is Plant Nutrition? Plants need nutrients for healthy growth and development. Plant nutrition involves the absorption of nutrients for plant growth and is dependent on
TREE ESSENTIAL ELEMENT. ZINC (Zn)
 Pub. No. 17 April 2016 TREE ESSENTIAL ELEMENT ZINC (Zn) By Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Zinc (Zn) is a
Pub. No. 17 April 2016 TREE ESSENTIAL ELEMENT ZINC (Zn) By Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Zinc (Zn) is a
Bottom Ash Data Week 40
 Bottom Ash Data 2018 Week 40 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 25, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 40 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 25, 2018. The data represents bottom ash composite results for
People who have a zinc deficiency can take hydrated zinc sulfate (ZnSO 4.xH 2O) as a dietary supplement.
 Q1.Zinc forms many different salts including zinc sulfate, zinc chloride and zinc fluoride. (a) People who have a zinc deficiency can take hydrated zinc sulfate (ZnSO 4.xH 2O) as a dietary supplement.
Q1.Zinc forms many different salts including zinc sulfate, zinc chloride and zinc fluoride. (a) People who have a zinc deficiency can take hydrated zinc sulfate (ZnSO 4.xH 2O) as a dietary supplement.
Bottom Ash Data Week 1
 Bottom Ash Data 2019 Week 1 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on January 17, 2019. The data represents bottom ash composite results for
Bottom Ash Data 2019 Week 1 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on January 17, 2019. The data represents bottom ash composite results for
Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka
 , 152-158 Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka W.S. Madurapperuma and D. Kumaragamage 1 Postgraduate Institute of Agriculture University
, 152-158 Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka W.S. Madurapperuma and D. Kumaragamage 1 Postgraduate Institute of Agriculture University
Edward V. Krizhanovsky, Ph.D., Kamila B. Tursunova
 REPORT ON RESEARCH OF BAE SYNERGY LIQUID PREPARATION INFLUENCE ON MINERAL METABOLISM Edward V. Krizhanovsky, Ph.D., Kamila B. Tursunova Bioenergy Technologies Institute, Sveaborgskaia, 12, Saint- Petersburg,
REPORT ON RESEARCH OF BAE SYNERGY LIQUID PREPARATION INFLUENCE ON MINERAL METABOLISM Edward V. Krizhanovsky, Ph.D., Kamila B. Tursunova Bioenergy Technologies Institute, Sveaborgskaia, 12, Saint- Petersburg,
Materials Declaration Form
 Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL)
 Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL) Patty Lucas AAPFCO Laboratory Services Committee Meeting Friday, August 7, 2015 Fertilizer Sample
Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL) Patty Lucas AAPFCO Laboratory Services Committee Meeting Friday, August 7, 2015 Fertilizer Sample
Test Report No.T JP Date: FEB 24, 2017 Page 1 of 6
 Test Report No.T51710190071JP Date: FEB 24, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Test Report No.T51710190071JP Date: FEB 24, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Test Report No.T JP Date: FEB 22, 2017 Page 1 of 6
 Test Report No.T51710190051JP Date: FEB 22, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Test Report No.T51710190051JP Date: FEB 22, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Engineering of Ministry of Education, Institute of Molecular Science, Shanxi University, Taiyuan , China.
 Indicator approach to develop a chemosensor for the colorimetric sensing of thiol-containing in water and its application for the thiol detection in plasma Fang-Jun Huo, a Yu-Tao Yang, b Jing Su, b Yuan-Qiang
Indicator approach to develop a chemosensor for the colorimetric sensing of thiol-containing in water and its application for the thiol detection in plasma Fang-Jun Huo, a Yu-Tao Yang, b Jing Su, b Yuan-Qiang
Materials Declaration Form
 Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
Chinese Zinc Sulfate Monohydrate testing. Dick Camp Kronos Micronutrients
 Chinese Zinc Sulfate Monohydrate testing Dick Camp Kronos Micronutrients THE ISSUE Most if not all Chinese ZSM imports are not all ZSM. They are a combination of ZSM and a Non- Water-Soluble compound of
Chinese Zinc Sulfate Monohydrate testing Dick Camp Kronos Micronutrients THE ISSUE Most if not all Chinese ZSM imports are not all ZSM. They are a combination of ZSM and a Non- Water-Soluble compound of
By Andrew & Erin Oxford, Bethel
 Chemistry in Plant Nutrition & Growth Objectives Review elements of chemistry and apply them to plant nutrition and growth in an agricultural context. Suggested grade levels 9-12 Alaska Content Standards
Chemistry in Plant Nutrition & Growth Objectives Review elements of chemistry and apply them to plant nutrition and growth in an agricultural context. Suggested grade levels 9-12 Alaska Content Standards
Author(s) Ishibashi, Masayoshi; Fujinaga, Tai.
 Title Studies on Boric Acid and Borates. acid and of Borates on the PoIarogr Author(s) Ishibashi, Masayoshi; Fujinaga, Tai Citation Bulletin of the Institute for Chemi University (1958), 36(6): 134-138
Title Studies on Boric Acid and Borates. acid and of Borates on the PoIarogr Author(s) Ishibashi, Masayoshi; Fujinaga, Tai Citation Bulletin of the Institute for Chemi University (1958), 36(6): 134-138
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series DESCRIPTION OF THE MOST IMPORTANT PROPERTIES AND THE POSSIBLE VARIATIONS AND TOLERANCES
 USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
Soil Composition. Air
 Soil Composition Air Soil Included Air Approximately 40 to 60% of the volume of a soil is actually empty space between the solid particles (voids). These voids are filled with air and/or water. The air
Soil Composition Air Soil Included Air Approximately 40 to 60% of the volume of a soil is actually empty space between the solid particles (voids). These voids are filled with air and/or water. The air
A study of redox properties of hydralazine hydrochloride, an antihypertensive drug
 Journal of Saudi Chemical Society () 4, 4 45 King Saud University Journal of Saudi Chemical Society www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE A study of redox properties of hydralazine hydrochloride,
Journal of Saudi Chemical Society () 4, 4 45 King Saud University Journal of Saudi Chemical Society www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE A study of redox properties of hydralazine hydrochloride,
Materials Declaration Form
 Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
CERTIFICATE OF ANALYSIS. tel: fax:
 CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
Matrix Reference Materials - SCP SCIENCE
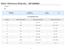 EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
Micronutrient Management. Dorivar Ruiz Diaz Soil Fertility and Nutrient Management
 Micronutrient Management Dorivar Ruiz Diaz Soil Fertility and Nutrient Management Essential Nutrients Thirteen essential nutrients Nitrogen, phosphorus, potassium, calcium, magnesium, sulfur Iron, manganese,
Micronutrient Management Dorivar Ruiz Diaz Soil Fertility and Nutrient Management Essential Nutrients Thirteen essential nutrients Nitrogen, phosphorus, potassium, calcium, magnesium, sulfur Iron, manganese,
There are two groups of minerals: Major salt components: K, Na, Ca, Mg, Cl -, sulfate, phosphate, and HCO 3
 MINERALS INTRODUCTION 90 elements in the earth s s crust. 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
MINERALS INTRODUCTION 90 elements in the earth s s crust. 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
Figure 1. Location of 43 benchmark sites across Alberta.
 1.0 INTRODUCTION This report describes the micronutrient and trace element status of the AESA (Alberta Environmentally Sustainable Agriculture) Soil Quality Benchmark Sites. Previous reports completed
1.0 INTRODUCTION This report describes the micronutrient and trace element status of the AESA (Alberta Environmentally Sustainable Agriculture) Soil Quality Benchmark Sites. Previous reports completed
CHAPTER 8 SYNTHESIS, STRUCTURAL, OPTICAL AND ELECTRICAL PROPERTIES OF. TRANSITION METAL (TM) DOPED ZnO NANORODS. (TM=Mn, Co, Ni AND Fe).
 190 CHAPTER 8 SYNTHESIS, STRUCTURAL, OPTICAL AND ELECTRICAL PROPERTIES OF TRANSITION METAL (TM) DOPED ZnO NANORODS (TM=Mn, Co, Ni AND Fe). 8.1 Introduction The important and fundamental work for developing
190 CHAPTER 8 SYNTHESIS, STRUCTURAL, OPTICAL AND ELECTRICAL PROPERTIES OF TRANSITION METAL (TM) DOPED ZnO NANORODS (TM=Mn, Co, Ni AND Fe). 8.1 Introduction The important and fundamental work for developing
Soil Conditions Favoring Micronutrient Deficiencies and Responses in 2001
 Soil Conditions Favoring Micronutrient Deficiencies and Responses in 2001 K.A. Kelling and P.E. Speth Department of Soil Science University of Wisconsin-Madison Why micronutrients now: Higher yield, therefore
Soil Conditions Favoring Micronutrient Deficiencies and Responses in 2001 K.A. Kelling and P.E. Speth Department of Soil Science University of Wisconsin-Madison Why micronutrients now: Higher yield, therefore
Plant Nutrients in Mineral Soils
 The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
Fertilizer Compatibility. Raun Lohry Terry Robinson Doyle Meeker
 Fertilizer Compatibility Raun Lohry Terry Robinson Doyle Meeker NPK s & Micros Concentration Chemistry Constraints Can t put 6 gallons in a 5 gallon bucket! Certain elements need help to stay in solution
Fertilizer Compatibility Raun Lohry Terry Robinson Doyle Meeker NPK s & Micros Concentration Chemistry Constraints Can t put 6 gallons in a 5 gallon bucket! Certain elements need help to stay in solution
C 6 H 12 O 6 + 6O 2 6CO 2 + 6H 2 O
 Sample Questions for the Individual Round Exam 1. Glucose is the most basic sugar involved in human metabolism. Its structure is provided below: a. The overall reaction of glucose metabolism is given below.
Sample Questions for the Individual Round Exam 1. Glucose is the most basic sugar involved in human metabolism. Its structure is provided below: a. The overall reaction of glucose metabolism is given below.
ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES"
 (41) Vol. 41, No.6 (1985) T-235 (Received May 24, 1984) ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES" By Toshihiko Sato, Shigenori
(41) Vol. 41, No.6 (1985) T-235 (Received May 24, 1984) ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES" By Toshihiko Sato, Shigenori
Test Report No.T TC Date: JUN 29, 2018 Page 1 of 5
 Test Report No.T31820241114TC Date: JUN 29, 2018 Page 1 of 5 POLYKOTE INK & PAINT (SHENZHEN) LTD. NO. 118, HUANG PU ROAD, SHAJING SUBDISTRICT, BAOAN, SHENZHEN, 518125, CHINA The following samples were
Test Report No.T31820241114TC Date: JUN 29, 2018 Page 1 of 5 POLYKOTE INK & PAINT (SHENZHEN) LTD. NO. 118, HUANG PU ROAD, SHAJING SUBDISTRICT, BAOAN, SHENZHEN, 518125, CHINA The following samples were
Analyte Proficiency From All Labs # Analytes: 26 Sample # Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016
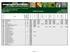 Analyte Proficiency From All Labs # Analytes: 26 Sample # 160511 Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016 Analyte Code Analyte # Tests Submitted # Tests in Robust Calculations
Analyte Proficiency From All Labs # Analytes: 26 Sample # 160511 Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016 Analyte Code Analyte # Tests Submitted # Tests in Robust Calculations
Analysis of e-liquid and e-cigarettes Vapoting test results
 Report n 14/282 Analysis of e-liquid and e-cigarettes Vapoting test results Study for : INSMOKE Attention of : Mr MEILE Customer request nr : Agreement dated 29.04.14 Certech request nr: T277 Certech quotation
Report n 14/282 Analysis of e-liquid and e-cigarettes Vapoting test results Study for : INSMOKE Attention of : Mr MEILE Customer request nr : Agreement dated 29.04.14 Certech request nr: T277 Certech quotation
Materials Declaration Form
 Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
Materials Declaration Form IPC 1752 Version 2 Form Type * Distribute Sectionals * Material Info Subsectionals * A-D Manufacturing Info * : Required Field Supplier Information Company Name * STMicroelectronics
 The Presence of Pyruvate Residues i TitleSimilar Polysaccharide (Commemorati Professor Sango Kunichika On the Oc Author(s) Hirase, Susumu; Watanabe, Kyoko Citation Bulletin of the Institute for Chemi University
The Presence of Pyruvate Residues i TitleSimilar Polysaccharide (Commemorati Professor Sango Kunichika On the Oc Author(s) Hirase, Susumu; Watanabe, Kyoko Citation Bulletin of the Institute for Chemi University
THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS
 THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS Richard Murphy Ph.D. Alltech European Bioscience Centre Ireland THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS
THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS Richard Murphy Ph.D. Alltech European Bioscience Centre Ireland THE INFLUENCE OF MINERALS ON THE STABILITY OF PREMIX AND FEED COMPONENTS
University of Groningen. Magnesium and zinc hydride complexes Intemann, Julia
 University of Groningen Magnesium and zinc hydride complexes Intemann, Julia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Magnesium and zinc hydride complexes Intemann, Julia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
Test Report No.: GZHL IP Date: Sep 01, 2016 Page 1 of 12
 Test Report No.: GZHL1604014433IP Date: Sep 01, 2016 Page 1 of 12 DAILI DND GLOBAL CO., LTD. NO.5 ALY.3, IN.79, XINXING ST., YONGKANG DIST., TAINAN CITY 71C, TAIWAN (R.O.C) The following sample(s) was/were
Test Report No.: GZHL1604014433IP Date: Sep 01, 2016 Page 1 of 12 DAILI DND GLOBAL CO., LTD. NO.5 ALY.3, IN.79, XINXING ST., YONGKANG DIST., TAINAN CITY 71C, TAIWAN (R.O.C) The following sample(s) was/were
Author(s) Hatano, Hiroyuki; Ganno, Shigetake;
 Electron Spin Resonance Studies on Title Inactivation by Direct Action of Ga Issue on Physical, Chemical and Bio Radiation, IV) Author(s) Hatano, Hiroyuki; Ganno, Shigetake; Citation Bulletin of the Institute
Electron Spin Resonance Studies on Title Inactivation by Direct Action of Ga Issue on Physical, Chemical and Bio Radiation, IV) Author(s) Hatano, Hiroyuki; Ganno, Shigetake; Citation Bulletin of the Institute
Chemistry 201 Laboratory Fall 2006 page 1 of 4
 Chemistry 201 Laboratory Fall 2006 page 1 of 4 Experiment: Determination of Iron in a Ferrous Ammonium Sulfate Sample (Fe) This experiment involves the determination of the percentage of ferrous iron in
Chemistry 201 Laboratory Fall 2006 page 1 of 4 Experiment: Determination of Iron in a Ferrous Ammonium Sulfate Sample (Fe) This experiment involves the determination of the percentage of ferrous iron in
Application note. Determination of metals in soil by microwave plasma - atomic emission spectrometry (MP-AES) using DTPA extraction.
 Determination of metals in soil by microwave plasma - atomic emission spectrometry (MP-AES) using DTPA extraction Application note Agriculture Authors Marília S. Teodoro1, Daniela Schiavo2, Mônica Ferreira
Determination of metals in soil by microwave plasma - atomic emission spectrometry (MP-AES) using DTPA extraction Application note Agriculture Authors Marília S. Teodoro1, Daniela Schiavo2, Mônica Ferreira
Product List Dated: March 1 st, 2015
 Product List Dated: March 1 st, 2015 Omega-3 Powder (Patented) NovoOmega Omega-3 Powder (No. 6000-6400) 6030 (HALAL) Omega-3 F-30 Powder (Fish Origin) 30% (EPA+DHA) : 30%+2% 12.5 25 6032 (Kosher & HALAL)
Product List Dated: March 1 st, 2015 Omega-3 Powder (Patented) NovoOmega Omega-3 Powder (No. 6000-6400) 6030 (HALAL) Omega-3 F-30 Powder (Fish Origin) 30% (EPA+DHA) : 30%+2% 12.5 25 6032 (Kosher & HALAL)
Mac-life-Plus. A Natural and Unique approach to support and boost your Immune System
 Mac-life-Plus A Natural and Unique approach to support and boost your Immune System 101.11101.0.09039(2017) o Thailand: License N 10-604988 o Germany: registration N o EU other: pending o International:
Mac-life-Plus A Natural and Unique approach to support and boost your Immune System 101.11101.0.09039(2017) o Thailand: License N 10-604988 o Germany: registration N o EU other: pending o International:
0620 CHEMISTRY. Mark schemes should be read in conjunction with the question paper and the Principal Examiner Report for Teachers.
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/31 Paper 3 (Extended Theory), maximum raw mark
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/31 Paper 3 (Extended Theory), maximum raw mark
Nutra-C Non-Acidic and Highly Effective Vitamin-C Ingredient Product No. Product Name Description Available Pack Size
 Product List Omega-3 Powders (Patented) NovoOmega Omega-3 Powders & DHA oil 6030F (HALAL) Omega-3 F-30 Powder (Fish 30% (EPA+DHA) : 30%+2% 12.5 25 Origin) 6010A (Kosher & HALAL) Omega-3 A-10 DHA 10% DHA
Product List Omega-3 Powders (Patented) NovoOmega Omega-3 Powders & DHA oil 6030F (HALAL) Omega-3 F-30 Powder (Fish 30% (EPA+DHA) : 30%+2% 12.5 25 Origin) 6010A (Kosher & HALAL) Omega-3 A-10 DHA 10% DHA
Micro-review. Element reducing oxidizing Fe Fe 2+ (high) Fe 3+ (low) Cu Cu sulfides Cu 2+ (moderate) S HS - (high) SO4 2- (high) Mo
 Micro-review Element reducing oxidizing environment environment Fe Fe 2+ (high) Fe 3+ (low) Cu Cu sulfides Cu 2+ (moderate) (low) S HS - (high) S4 2- (high) Mo [MonS4-n] 2- MoS2 (low) V V 3+, V 4+ sulfides
Micro-review Element reducing oxidizing environment environment Fe Fe 2+ (high) Fe 3+ (low) Cu Cu sulfides Cu 2+ (moderate) (low) S HS - (high) S4 2- (high) Mo [MonS4-n] 2- MoS2 (low) V V 3+, V 4+ sulfides
Supporting information for the article
 Supporting information for the article S1 Exceptional behavior of Ni 2 O 2 species revealed by ESI-MS and MS/MS studies in solution. Application of superatomic core to facilitate new chemical transformations
Supporting information for the article S1 Exceptional behavior of Ni 2 O 2 species revealed by ESI-MS and MS/MS studies in solution. Application of superatomic core to facilitate new chemical transformations
Lesson 3 Understanding Nutrients and Their Importance
 Unit B Understanding Animal Body Systems Lesson 3 Understanding Nutrients and Their Importance 1 Terms Balanced ration Carbohydrates Complex carbohydrates Disaccharides Essential nutrients Ether Fat Fat-soluble
Unit B Understanding Animal Body Systems Lesson 3 Understanding Nutrients and Their Importance 1 Terms Balanced ration Carbohydrates Complex carbohydrates Disaccharides Essential nutrients Ether Fat Fat-soluble
Chromium and Manganese in Japanese Diet. MURAKAMI Yukio *SUZUKI Yoshie **YAMAGATA * Toshiko
 Chromium and Manganese in Japanese Diet MURAKAMI Yukio *SUZUKI Yoshie **YAMAGATA * Toshiko (Received, Jan. 11, 1966) ABSTRACT Measurements of chromium and manganese in the complete diet samples collected
Chromium and Manganese in Japanese Diet MURAKAMI Yukio *SUZUKI Yoshie **YAMAGATA * Toshiko (Received, Jan. 11, 1966) ABSTRACT Measurements of chromium and manganese in the complete diet samples collected
A & L GREAT LAKES LABORATORIES, INC.
 Report No. F10035-0027 59018 3505 Conestoga Drive Fort Wayne, Indiana 46808 260-483-4759 Fax 260-483-5274 Account No. Date Reported: 02/08/2010 SOIL TEST REPORT Page: 1 of 2 Cation Lab Organic Phosphorus
Report No. F10035-0027 59018 3505 Conestoga Drive Fort Wayne, Indiana 46808 260-483-4759 Fax 260-483-5274 Account No. Date Reported: 02/08/2010 SOIL TEST REPORT Page: 1 of 2 Cation Lab Organic Phosphorus
Welcome. Greg Patterson C.C.A. President A&L Canada Laboratories
 Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Discussion Soil test levels Dropping P,K Organic matter levels dropping Cost of Fertilizer Increasing due to Global Demand Environmental
Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Discussion Soil test levels Dropping P,K Organic matter levels dropping Cost of Fertilizer Increasing due to Global Demand Environmental
ON TEA TANNIN ISOLATED FROM GREEN TEA.
 70 [Vol. 6 ON TEA TANNIN ISOLATED FROM GREEN TEA. By MICHIYO TSUJIMIIRA. (Received September 8th., 1930). The author(1) has recently isolated Tea catechin from green tea and pro posed the following formula
70 [Vol. 6 ON TEA TANNIN ISOLATED FROM GREEN TEA. By MICHIYO TSUJIMIIRA. (Received September 8th., 1930). The author(1) has recently isolated Tea catechin from green tea and pro posed the following formula
Raymond C. Ward Ward Laboratories, Inc Kearney, NE
 Raymond C. Ward Ward Laboratories, Inc Kearney, NE www.wardlab.com There is More Than N P K Major Nutrients N, P, and K Secondary Nutrients Calcium, Magnesium, and Sulfur Micro-Nutrients Zinc, Iron, Manganese,
Raymond C. Ward Ward Laboratories, Inc Kearney, NE www.wardlab.com There is More Than N P K Major Nutrients N, P, and K Secondary Nutrients Calcium, Magnesium, and Sulfur Micro-Nutrients Zinc, Iron, Manganese,
Chemicals (E) With the partnership of LACH:NER a Czech chemical company
 Chemicals (E) With the partnership of LACH:NER a Czech chemical company INDEX EDETIC ACID 2 ERIOCHROME BLACK T 2 ETHYL ACETATE 2 ETHYL ALCOHOL 3 ETHYL METHYL KETONE 4 ETHYLENE GLYCOL MONOETHYL ETHER 4
Chemicals (E) With the partnership of LACH:NER a Czech chemical company INDEX EDETIC ACID 2 ERIOCHROME BLACK T 2 ETHYL ACETATE 2 ETHYL ALCOHOL 3 ETHYL METHYL KETONE 4 ETHYLENE GLYCOL MONOETHYL ETHER 4
Cranberry Nutrition: An A Z Guide. Joan R. Davenport Soil Scientist Washington State University
 Cranberry Nutrition: An A Z Guide Joan R. Davenport Soil Scientist Washington State University Soil Derived Plant Essential Elements Macro Micro Nitrogen (N) Phosphorus (P) Sulfur (S) Potassium (K) Calcium
Cranberry Nutrition: An A Z Guide Joan R. Davenport Soil Scientist Washington State University Soil Derived Plant Essential Elements Macro Micro Nitrogen (N) Phosphorus (P) Sulfur (S) Potassium (K) Calcium
Official Journal of the European Union. (Non-legislative acts) REGULATIONS
 22.8.2017 EN L 216/1 II (Non-legislative acts) REGULATIONS COMMISSION IMPLEMENTING REGULATION (EU) 2017/1490 of 21 August 2017 concerning the of manganous chloride tetrahydrate, manganese (II) oxide, manganous
22.8.2017 EN L 216/1 II (Non-legislative acts) REGULATIONS COMMISSION IMPLEMENTING REGULATION (EU) 2017/1490 of 21 August 2017 concerning the of manganous chloride tetrahydrate, manganese (II) oxide, manganous
EDXRF APPLICATION NOTE
 EDXRF APPLICATION NOTE ANALYSIS OF ANIMAL FEEDS # 1279 SCOPE The analysis of finished animal feeds and premixes is demonstrated using EDXRF with indirect excitation and Fundamental Parameters software,
EDXRF APPLICATION NOTE ANALYSIS OF ANIMAL FEEDS # 1279 SCOPE The analysis of finished animal feeds and premixes is demonstrated using EDXRF with indirect excitation and Fundamental Parameters software,
Product List (HALAL) Borage Powder GLA 17 17% GLA+ 2% (HALAL) Evening Primrose Powder GLA 9 9% GLA + 2%
 Product List Dated: August 1, 2015 Omega-3 Powders (Patented) NovoOmega Omega-3 Powders & DHA oil 6030F (HALAL) Omega-3 F-30 Powder (Fish Origin) 30% (EPA+DHA) : 30%+2% 12.5 25 6032A-10.0 (Kosher & HALAL)
Product List Dated: August 1, 2015 Omega-3 Powders (Patented) NovoOmega Omega-3 Powders & DHA oil 6030F (HALAL) Omega-3 F-30 Powder (Fish Origin) 30% (EPA+DHA) : 30%+2% 12.5 25 6032A-10.0 (Kosher & HALAL)
VOL. 5, NO. 6, June 2015 ISSN ARPN Journal of Science and Technology All rights reserved.
 VOL. 5, NO. 6, June 2015 ISSN 22-7217 Impact of Cumulative Sediment Deposition by Irrigation Water on Soil and Sugarcane in Savannah Sugar Company Limited; Numan, Adamawa State Nigeria 1 R.P. Ali, 2 H.M.
VOL. 5, NO. 6, June 2015 ISSN 22-7217 Impact of Cumulative Sediment Deposition by Irrigation Water on Soil and Sugarcane in Savannah Sugar Company Limited; Numan, Adamawa State Nigeria 1 R.P. Ali, 2 H.M.
Enzyme Mimics. Principles Cyclodextrins as Mimics Corands as Mimics Metallobiosites
 Enzyme Mimics Principles Cyclodextrins as Mimics Corands as Mimics Metallobiosites 1 Enzyme Mimics Biochemical systems: Binding is a trigger to events: Binding induces a conformational change in the receptor
Enzyme Mimics Principles Cyclodextrins as Mimics Corands as Mimics Metallobiosites 1 Enzyme Mimics Biochemical systems: Binding is a trigger to events: Binding induces a conformational change in the receptor
Experiences with Zinc Injection at Obrigheim Nuclear Power Plant. Ingolf BRIESEN, Obrigheim NPP, Germany
 Experiences with Zinc Injection at Obrigheim Nuclear Power Plant Ingolf BRIESEN, Obrigheim NPP, Germany 1. Introduction/Background Obrigheim Nuclear Power Plant (KWO) went on-line in October 1968. The
Experiences with Zinc Injection at Obrigheim Nuclear Power Plant Ingolf BRIESEN, Obrigheim NPP, Germany 1. Introduction/Background Obrigheim Nuclear Power Plant (KWO) went on-line in October 1968. The
THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE*
 THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE* BY W. R. BROWN (From Ihe Laboratory of Biochemistry, University of Cincinnati, Cincinnati) (Received for publication, November 21, 1935)
THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE* BY W. R. BROWN (From Ihe Laboratory of Biochemistry, University of Cincinnati, Cincinnati) (Received for publication, November 21, 1935)
Polychalcone derived from 8-acetoxyquinoline-5-aidehyde
 Prec. Indian Acad. Sci. (Chem. Sci.), Vol. 96, No. 5, March 1986, pp. 321-326. 9 Printed in India. Polychalcone derived from 8-acetoxyquinoline-5-aidehyde H S PATEL Department of Chemistry, Sardar Patel
Prec. Indian Acad. Sci. (Chem. Sci.), Vol. 96, No. 5, March 1986, pp. 321-326. 9 Printed in India. Polychalcone derived from 8-acetoxyquinoline-5-aidehyde H S PATEL Department of Chemistry, Sardar Patel
Manufacturer of High Purity Chemicals PRODUCT LIST
 Manufacturer of High Purity Chemicals PRODUCT LIST Ammonium NH4 Ammonium Jost Chemical Co. manufactures a range of high purity ammonium phosphates, sulfates and citrates. Ammonium phosphates can be used
Manufacturer of High Purity Chemicals PRODUCT LIST Ammonium NH4 Ammonium Jost Chemical Co. manufactures a range of high purity ammonium phosphates, sulfates and citrates. Ammonium phosphates can be used
Correction of Zinc Deficiency in Avocado
 1997 California Avocado Research Symposium pages 9-12 California Avocado Society and University of California, Riverside Correction of Zinc Deficiency in Avocado Final Report for Project Year 4 of 4 Cooperating
1997 California Avocado Research Symposium pages 9-12 California Avocado Society and University of California, Riverside Correction of Zinc Deficiency in Avocado Final Report for Project Year 4 of 4 Cooperating
EconovaPlus Fertiliser
 EconovaPlus Fertiliser The complete plant growth fertiliser, bio-stimulater & carbon control solution. A bio-fertiliser based on the need for organic mineral complexes in the soil. Manufactured by building
EconovaPlus Fertiliser The complete plant growth fertiliser, bio-stimulater & carbon control solution. A bio-fertiliser based on the need for organic mineral complexes in the soil. Manufactured by building
Water Soluble Fertilizer for Foliar Application
 COMPO EXPERT EXPERTS FOR GROWTH Water Soluble Fertilizer for Foliar Application Highly efficient combination of macro and micro elements Fully chelated trace elements Fast and completely water soluble
COMPO EXPERT EXPERTS FOR GROWTH Water Soluble Fertilizer for Foliar Application Highly efficient combination of macro and micro elements Fully chelated trace elements Fast and completely water soluble
Classification of feed grade sources of trace minerals
 Classification of feed grade sources of trace minerals Stéphane Durosoy Animine Frankfurt 29 Sept. 2017 Classification of goods Product identity and nomenclature Trade facilitation Market segmentation
Classification of feed grade sources of trace minerals Stéphane Durosoy Animine Frankfurt 29 Sept. 2017 Classification of goods Product identity and nomenclature Trade facilitation Market segmentation
TOXIC AND ESSENTIAL ELEMENTS
 TOXIC AND ESSENTIAL ELEMENTS Assessment of Key Minerals and Harmful Metals Whole Blood, Red Blood Cell and Serum Elements Urine Toxic and Essential Elements Creatinine Clearance Hair Elements Fecal Metals
TOXIC AND ESSENTIAL ELEMENTS Assessment of Key Minerals and Harmful Metals Whole Blood, Red Blood Cell and Serum Elements Urine Toxic and Essential Elements Creatinine Clearance Hair Elements Fecal Metals
Greg Patterson C.C.A. President A&L Canada Laboratories
 Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Compost Quality Consumer of the Future Population dynamics - more and more people involved in use of compost Better educated on what is expected
Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Compost Quality Consumer of the Future Population dynamics - more and more people involved in use of compost Better educated on what is expected
TEST REPORT. LAB LOCATION:TURKEY LAB NO. : (7216) SERVICE TYPE: Regular DATE IN: November 29 th, 2016 DATE OUT: December 01 th, 2016
 TEST REPORT LAB LOCATIONTURKEY LAB NO. (7216)333-0351 SERVICE TYPE Regular DATE IN November 29 th, 2016 DATE OUT December 01 th, 2016 MATERIAL SUBMITTED LISANS KIRTASIYE OFIS GERECLERI SAN. TIC. A.S. (Şerifali
TEST REPORT LAB LOCATIONTURKEY LAB NO. (7216)333-0351 SERVICE TYPE Regular DATE IN November 29 th, 2016 DATE OUT December 01 th, 2016 MATERIAL SUBMITTED LISANS KIRTASIYE OFIS GERECLERI SAN. TIC. A.S. (Şerifali
Simultaneous Single Particle Mass Spectrometry Measurements at Two Different Urban Sites and Comparison with Quantitative Techniques
 Simultaneous Single Particle Mass Spectrometry Measurements at Two Different Urban Sites and Comparison with Quantitative Techniques Eoin McGillicuddy 1, Manuel Dall Osto 2, Franco Lucarelli 3, Silvia
Simultaneous Single Particle Mass Spectrometry Measurements at Two Different Urban Sites and Comparison with Quantitative Techniques Eoin McGillicuddy 1, Manuel Dall Osto 2, Franco Lucarelli 3, Silvia
EUROPEAN QUALIFYING EXAMINATION 2006
 EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
Structural and Zn-binding features of ortho- and pyrophosphate modified soil metal-humus sorbents (Features of P-modified humus sorbents)
 Int. Agrophysics, 22, 16, 197 22 INTERNATIONAL Agrophysics www.ipan.lublin.pl/int-agrophysics Structural and Zn-binding features of ortho- and pyrophosphate modified soil metal-humus sorbents (Features
Int. Agrophysics, 22, 16, 197 22 INTERNATIONAL Agrophysics www.ipan.lublin.pl/int-agrophysics Structural and Zn-binding features of ortho- and pyrophosphate modified soil metal-humus sorbents (Features
Immobilizing Arsenic in Contaminated Soil Using Humic Mineral Concentrates
 Immobilizing in Contaminated Soil Using Humic Mineral Concentrates Alexander I.Shulgin 1 and D. Joseph Hagerty 2, F. ASCE, PE 1. Research Professor, Chemical Engineering Dept., U. Louisville, Louisville,
Immobilizing in Contaminated Soil Using Humic Mineral Concentrates Alexander I.Shulgin 1 and D. Joseph Hagerty 2, F. ASCE, PE 1. Research Professor, Chemical Engineering Dept., U. Louisville, Louisville,
Kinetic testing program
 APPENDIX F6 Kinetic testing program 1 Olympic Dam Expansion Supplementary Environmental Impact Statement 2010 Olympic Dam Expansion BHP Billiton 13 th May 2010 Document No: B6045101_FinalRPT_11May10 Olympic
APPENDIX F6 Kinetic testing program 1 Olympic Dam Expansion Supplementary Environmental Impact Statement 2010 Olympic Dam Expansion BHP Billiton 13 th May 2010 Document No: B6045101_FinalRPT_11May10 Olympic
Trace Elements in Manure
 Trace Elements in Manure Purpose: Whenever soil profiles are examined for nutrient trace elements, it has been demonstrated that the metals tend to accumulate in the topsoil. Soil properties affect plant
Trace Elements in Manure Purpose: Whenever soil profiles are examined for nutrient trace elements, it has been demonstrated that the metals tend to accumulate in the topsoil. Soil properties affect plant
E55A GELATIN, GELLING GRADE Gelatina
 00-0PDG.pdf 0 0 0 0 EA GELATIN, GELLING GRADE Gelatina DEFINITION Purified protein obtained from collagen of animals (including fish and poultry) by partial alkaline and/or acid hydrolysis, by enzymatic
00-0PDG.pdf 0 0 0 0 EA GELATIN, GELLING GRADE Gelatina DEFINITION Purified protein obtained from collagen of animals (including fish and poultry) by partial alkaline and/or acid hydrolysis, by enzymatic
Test Report No.: T TY Date: DEC 19, 2018 Page 1 of 10
 Test Report No.: T51810301178TY Date: DEC 19, 2018 Page 1 of 10 GUANGDONG XINYU TECHNOLOGY INDUSTRIAL CO., LTD. LAIMEI INDUSTRIAL ZONE, CHENGHAI DISTRICT, SHANTOU, GUANGDONG The following samples were
Test Report No.: T51810301178TY Date: DEC 19, 2018 Page 1 of 10 GUANGDONG XINYU TECHNOLOGY INDUSTRIAL CO., LTD. LAIMEI INDUSTRIAL ZONE, CHENGHAI DISTRICT, SHANTOU, GUANGDONG The following samples were
Simultaneous Determination of Copper, Zinc and Selenium in Chicken Liver by Differential Pulse Polarography
 Turk J Chem 27 (2003), 347 355. c TÜBİTAK Simultaneous Determination of Copper, Zinc and Selenium in Chicken Liver by Differential Pulse Polarography Güler EKMEKCİ Gazi University, Gazi Education Faculty,
Turk J Chem 27 (2003), 347 355. c TÜBİTAK Simultaneous Determination of Copper, Zinc and Selenium in Chicken Liver by Differential Pulse Polarography Güler EKMEKCİ Gazi University, Gazi Education Faculty,
0620 CHEMISTRY. 0620/23 Paper 2 (Core Theory), maximum raw mark 80
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/23 Paper 2 (Core Theory), maximum raw mark 80
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/23 Paper 2 (Core Theory), maximum raw mark 80
