Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs
|
|
|
- Georgiana Wilkins
- 5 years ago
- Views:
Transcription
1 Am J Physiol Endocrinol Metab 296: E1239 E1250, First published March 17, 2009; doi: /ajpendo Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs Caroline Bauchart-Thevret, Barbara Stoll, Shaji Chacko, and Douglas G. Burrin US Department of Agriculture/Agricultural Research Service Children s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, Texas Submitted 19 December 2008; accepted in final form 12 March 2009 Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 296: E1239 E1250, First published March 17, 2009; doi: /ajpendo We recently showed that the developing gut is a significant site of methionine transmethylation to homocysteine and transsulfuration to cysteine. We hypothesized that sulfur amino acid (SAA) deficiency would preferentially reduce mucosal growth and antioxidant function in neonatal pigs. Neonatal pigs were enterally fed a control or an SAA-free diet for 7 days, and then whole body methionine and cysteine kinetics were measured using an intravenous infusion of [1-13 C;methyl- 2 H 3 ]methionine and [ 15 N]cysteine. Body weight gain and plasma methionine, cysteine, homocysteine, and taurine and total erythrocyte glutathione concentrations were markedly decreased ( 46% to 85%) in SAA-free compared with control pigs. Whole body methionine and cysteine fluxes were reduced, yet methionine utilization for protein synthesis and methionine remethylation were relatively preserved at the expense of methionine transsulfuration, in response to SAA deficiency. Intestinal tissue concentrations of methionine and cysteine were markedly reduced and hepatic levels were maintained in SAA-free compared with control pigs. SAA deficiency increased the activity of methionine metabolic enzymes, i.e., methionine adenosyltransferase, methionine synthase, and cystathionine -synthase, and S-adenosylmethionine concentration in the jejunum, whereas methionine synthase activity increased and S-adenosylmethionine level decreased in the liver. Small intestine weight and protein and DNA mass were lower, whereas liver weight and DNA mass were unchanged, in SAA-free compared with control pigs. Dietary SAA deficiency induced small intestinal villus atrophy, lower goblet cell numbers, and Ki-67-positive proliferative crypt cells in association with lower tissue glutathione, especially in the jejunum. We conclude that SAA deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. transmethylation; transsulfuration; remethylation; homocysteine INCREASING EVIDENCE INDICATES that sulfur amino acids (SAAs), methionine and cysteine, are implicated in numerous biological functions and diseases, aside from their role in protein synthesis. Methionine is an indispensable amino acid and is transmethylated intracellularly to homocysteine via S-adenosylmethionine (SAM), the principal biological methyl donor in mammalian cells and a precursor for polyamine synthesis (25). Homocysteine is a sulfur-containing amino acid present in blood and tissues but not incorporated into protein (22). Homocysteine can be converted to cysteine via cystathionine through the transsulfuration pathway, an irreversible process (36). Homocysteine can also be methylated back to methionine Address for reprint requests and other correspondence: D. G. Burrin, Children s Nutrition Research Center, 1100 Bates St., Houston, TX ( via the remethylation pathway. The combination of transmethylation and remethylation pathways comprises the methionine cycle, which occurs in most cells. However, the transsulfuration pathway has a limited tissue distribution and is restricted to the liver, kidney, intestine, and pancreas (6). Cysteine is considered a semi-indispensable amino acid, the availability of which is dependent on methionine intake (36). Cysteine is a constituent amino acid of the tripeptide glutathione ( -Glu- Cys-Gly), the major cellular antioxidant in mammals, and serves also as a precursor for the synthesis of taurine, sulfate, and coenzyme A (36). The gastrointestinal tract is a metabolically significant site of SAA metabolism in the body (8). Our recent studies in infant pigs indicate that the gastrointestinal tract metabolizes 20% of dietary methionine intake, which is mainly transmethylated to homocysteine and transsulfurated to cysteine (34). The gastrointestinal tract accounts for 25% of whole body transmethylation and transsulfuration and is a site of net homocysteine release (34). Studies also show that methionine requirements are 30% higher in enterally fed than in parenterally fed piglets, further confirming the important first-pass metabolic demand of the gut (35). SAAs, especially cysteine, play a key role in cellular redox function and susceptibility to oxidant stress in the intestine (13, 18). Glutathione constitutes the most important intracellular antioxidant, has a critical function in cellular detoxification against oxidative and chemical injury, and has a variety of cytoprotective effects (44). Maintaining normal redox status is particularly important in intestinal epithelial cells, which are exposed to high levels of oxidant stress because of the high rate of oxidative metabolism, as well as exposure to luminal toxins and oxidants derived from the diet. Studies in intestinal epithelial cells indicate that increased oxidant stress and redox imbalance suppress cell proliferation and induce apoptosis, in association with a higher oxidized glutathione and cysteine state (17, 30, 32). However, changes in the extracellular cysteine-to-cystine redox status per se have been shown to mediate proliferative signaling that is independent of the intracellular GSH/GSSG status in colon cancer cells (1, 31). Thus it is evident that cysteine availability is important for maintenance of epithelial cell glutathione level and cell survival. In this context, we hypothesized that given the high cellular turnover and metabolic activity of the gut, enteral SAA deficiency would preferentially reduce intestinal epithelial growth and antioxidant function in neonatal pigs. To test this hypothesis, we investigated the effect of dietary SAA deficiency on intestinal epithelial cell proliferation and the redox status in neonatal pigs enterally fed a control or an SAA-free diet for 7 days. Also, using infusion of [1-13 C;methyl- 2 H 3 ]methionine E1239
2 E1240 and [ 15 N]cysteine, we determined the whole body methionine and cysteine kinetics after the 7-day dietary treatment. MATERIALS AND METHODS Animals and Diets The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with National Institutes of Health guidelines. The study was performed on 3-day-old, female cross-bred piglets ( kg body wt) obtained from the Texas Department of Criminal Justice (Huntsville, TX). On arrival at the animal facility at the Children s Nutrition Research Center, the piglets were surgically implanted with Silastic catheters in the external jugular vein, carotid artery, and stomach under isoflurane general anesthesia, as described previously (9). Postoperatively, they were placed on total parenteral nutrition, including elemental nutrients (glucose, amino acids, lipids, electrolytes, trace minerals, and vitamins), as described previously (9), via continuous intravenous infusion at 215 kcal kg 1 day 1. After 24 h of total parenteral nutrition, the animals were randomly assigned to one of two different diets administered enterally via a continuous intragastric infusion (240 ml kg 1 day 1 ) for 7 days. One group (n 9) received a control diet containing free amino acids (in g/l: 3.03 Ala, 2.62 Arg, 4.66 Asp, 3.71 Cys-HCl H 2O, 5.83 Glu, 4.66 Gln, 2.27 Gly, 1.52 His, 3.38 Ile, 6.00 Leu, 5.67 Lys-HCl, 1.40 Met, 3.09 Phe, 4.37 Pro, 3.27 Ser, 3.68 Thr, 0.70 Trp, 0.70 Tyr, and 3.68 Val), lactose (116 g/l), lipids (20% Intralipd, Fresenius Kabi, Bad Homburg, Germany; 21 g/l), electrolytes (in mg kg 1 day 1 : 160 Na, 544 Cl, 312 K, 100 P, 25 Mg, and 129 Ca), and trace minerals and vitamins sufficient to meet the nutrient requirements for young piglets (National Research Council, 1998). The amount of methionine and cysteine in the control diet was 0.25 g kg 1 day 1, such that the total SAA intake was 0.50 g kg 1 day 1. The second group (n 7) received an SAA-free diet with the same composition as the control diet except for the amino acids. Methionine and cysteine from the control diet were replaced by a mixture of glutamine, glycine, and alanine, such that both diets were isonitrogenous; the final concentrations of glutamine, glycine, and alanine in the SAAfree diet were 7.54, 2.94, and 3.92 g/l, respectively. Pigs were weighed daily to adjust the rates of diet infusion. Arterial blood samples (1 ml) were collected in EDTA-containing tubes daily, and the hematocrit was immediately determined using a microcapillary reader after centrifugation. Plasma and erythrocytes were separated by centrifugation (10 min at 3,000 rpm and 4 C) and stored at 70 C until analysis. Infusion and Sampling Protocol The infusion and sampling protocol is depicted in Fig. 1. After 7 days on their respective dietary treatments, all pigs received a primed, Fig. 1. Tracer infusion and blood sampling protocol. continuous intravenous 2-h infusion (5 mol kg 1 h 1 )of[ 13 C]sodium bicarbonate [99% atom percent excess (APE); Cambridge Isotope Laboratories, Andover, MA] for estimation of whole body CO 2 production (V CO2). Immediately thereafter, they received a primed, continuous, intravenous 8-h infusion (20 mol kg 1 h 1 )of[1-13 C- methyl- 2 H 3]methionine (98% APE; Cambridge Isotope Laboratories) with a priming dose of 20 mol/kg. During the last 5hof[1-13 C- methyl- 2 H 3]methionine infusion, pigs also received a primed, continuous intravenous infusion (10 mol kg 1 h 1 ) of [ 15 N]cysteine (98% APE; Cambridge Isotope Laboratories). During the entire 10-h infusion protocol, arterial blood samples (1 ml) were collected at 0, 1.5, 1.75, 2, 8, 9, and 10 h for 13 CO 2 enrichment. At 0, 6, 7, 8, 9, and 10 h, arterial blood samples (1.5 ml) were also collected for analysis of isotopic enrichment of methionine and cysteine tracers. At the end of the 10-h infusion, all pigs were euthanized with an intravenous injection of pentobarbital sodium (50 mg/kg) and phenytoin sodium (5 mg/kg; Beutanasia-D, Schering-Plough Animal Health, Kenilworth, NJ). The abdomen was opened, and the entire small intestine distal to the ligament of Treitz to the ileocecal junction was immediately flushed with ice-cold saline. Then the small intestine was divided into two equal portions: the proximal half was designated the jejunum and the distal half the ileum. After they were weighed, samples from respective sections were placed in 10% buffered formalin for 24 h and then 70% ethanol for histological analysis, and the remaining tissue sample was snap frozen in liquid nitrogen and stored at 70 C until analysis for tracer enrichments and protein and DNA contents, as described previously (7). A piece of colon was flushed with ice-cold saline and placed in 10% buffered formalin for 24 h and then 70% ethanol for histological analysis. Liver, stomach, and spleen were weighed, and a piece of liver was snap frozen in liquid nitrogen and stored at 70 C until analysis for tracer enrichments and protein and DNA contents, as described previously (7). Amino Acid and Total Glutathione Concentration Plasma and tissue (jejunum, ileum, and liver) free amino acid and taurine concentrations were determined by reverse-phase HPLC of their phenylisothiocyanate derivatives, as described previously (37). Tissue SAM, S-adenosylhomocysteine (SAH), and methylthioadenosine (MTA) concentrations were quantified by reverse-phase HPLC, as described by Farrar and Clarke (14). Plasma, erythrocyte, and tissue homocysteine, cysteine, and glutathione concentrations were quantified by reverse-phase HPLC after derivatization with O-phthalaldehyde using the method described by Wu et al. (43, 45). Briefly, samples were acidified with 1.5 M perchloric acid (PCA) and 12 mm iodoacetic acid (1:1, vol/vol) and then neutralized with 2 M K 2CO 3. The supernatant was reduced with 28 mm -mercapthoethanol and then treated with 25 mm iodoacetic acid to form S-carboxylmethyl derivatives. Redox Potential Calculations Plasma and tissue redox potential values (E h) were calculated from the cysteine-cystine couple using the Nernst equation at ph 7.4: E h (mv) log([cyss]/[cys] 2 ) (1, 19), where [Cys] and [CySS] are the concentrations of cysteine and cystine, respectively, in plasma and tissue (19). Morphometry, Goblet Cell Content, and Mucosal Cell Proliferation Morphometric analysis was performed on formalin-fixed intestinal samples (jejunum, ileum, and colon) that were embedded in paraffin, sectioned ( 5 m), and stained with hematoxylin and eosin, as described previously (10). The mean gut section area, villus height and area, and crypt depth were measured in at least three gut sections per tissue using an Axiophot microscope (Carl Zeiss, Werk Göttingen, Germany) and NIH Image software (version 1.60, National Institutes of Health, Bethesda, MD). The longest well-oriented villi and
3 E1241 crypts were used for the mean villus height and crypt depth. Goblet cell content was determined in villi and crypts of hematoxylin-eosinstained gut tissue by a single, trained and blinded observer counting the number of goblet cells in well-oriented crypts. Mucosal crypt cell proliferation was determined using Ki-67 immunohistochemistry staining on formalin-fixed, paraffin-embedded, and sectioned ( 5 m) gut tissue. Sections were incubated for 1 h at 60 C, deparaffinized, and rehydrated (xylenes, 100% ethanol, and 95% ethanol). Sections were treated with EDTA antigen retrieval for 25 min in a steamer. After incubation with 3% hydrogen peroxide in methanol for 10 min to block endogenous peroxidase, the sections were covered with 2% normal mouse-2% normal goat serum in PBS-Tween for 20 min at room temperature to block nonspecific binding sites. After 30 min of incubation with the primary antibody (1:1,000 dilution; catalog no. VP-k451, Vector Laboratories), the sections were incubated with the goat anti-rabbit secondary antibody for 30 min and Vector Elite ABC kit for 45 min. Detection was carried out using the chromagen 3-amino-9-ethylcarbazole. The sections were counterstained with hematoxylin and dehydrated, and coverslips were applied in an aqueous mounting medium. The proportion of proliferating crypt cells was quantified by a single, trained and blinded observer counting the number of Ki-67-positive cells (Ki-67-stained nuclei) in well-oriented crypts. Enzyme Assays Frozen gut (jejunum and ileum) and liver tissues were homogenized (1:5, wt/vol) in 0.04 M potassium phosphate buffer (ph 7.5) containing 1 mm EDTA and 10 mm -mercaptoethanol, as described by Lambert et al. (20). Methionine synthase (MS) and cystathionine -synthase (CBS) activities were determined as described previously (20) in the presence of 1 mm SAM for CBS activity assay (33). Methionine adenosyltransferase (MAT) activity was assayed by measurement of the formation of SAM by HPLC using a modified method described by Farrar and Clarke (14). Briefly, tissue extract (100 l) was incubated for 60 min at 37 C in the presence of (in final concentrations) 135 mm Tris HCl (ph 8.2), 180 mm KCl, 265 mm MgCl 2, 5 mm DTT, 16 mm ATP, 265 M SAM, and 5 mm methionine at a final volume of 400 l. The reaction was stopped with 100 l of 2 M PCA, neutralized with 4 M KOH, and then analyzed by HPLC, as described previously (14). Each enzyme activity is expressed as nanomoles of product synthesized per hour per milligram of protein in the tissue extract. Mass Spectrometry Plasma and tissue (jejunum, ileum, and liver) isotopic enrichments of [1-13 C;methyl- 2 H 3]methionine, [1-13 C]methionine, [1-13 C]homocysteine, and [ 15 N]cysteine were quantified on the heptafluorobutyric anhydride derivatives by GC-MS. For plasma preparation, we used a modified method from Davis et al. (12). Briefly, plasma samples were acidified with 10% trichloroacetic acid, amino acids were separated by cation exchange (AG 50W-X8, mesh, hydrogen form resin; Bio-Rad, Richmond, CA), treated with DTT (Sigma-Aldrich, St. Louis, MO), and derivatized with heptafluorobutyric anhydride. Tissue samples were homogenized and deproteinized with 2 M PCA, and the PCA-soluble (tissue-free pool) and acid-insoluble (protein-bound pool) fractions were subjected to mass spectrometric analysis similar to that described previously (37). GC-MS analysis was performed by negative chemical ionization on a GC-MS (model HP-6890/5973 MSD, Hewlett Packard, Palo Alto, CA) using a 30-m-long, 0.25-mm-ID column (0.5 m film thickness, model HP-5ms; Agilent Technologies, Palo Alto, CA). Separate runs were performed for methionine, homocysteine, and cysteine. The abundance of specific ions was determined by selected-ion monitoring at the following mass-to-charge ratios: methionine ( ), homocysteine ( ), and cysteine ( ). Isotopic enrichments were expressed as molar percent excess (MPE) of labeled to unlabeled isotopomer ratios [tracer-to-tracee ratios (TTR)] after correction for natural abundance and standard curves, where MPE [TTR/(TTR 1)] 100, as defined by Wolfe and Chinkes (42). Blood 13 CO 2 enrichment was determined by gas isotope ratio mass spectrometry (40). Protein synthesis was calculated as a fractional synthesis rate (FSR; %/day) from [1-13 C;methyl- 2 H 3]methionine or [ 15 N]cysteine FSR E bound /E free 24/t 100 where E bound and E free are the isotopic enrichments (MPE) of [1-13 C; methyl- 2 H 3]methionine or [ 15 N]cysteine of the PCA-insoluble (protein bound) and PCA-soluble (tissue-free) methionine or cysteine pool, t is time of labeling (in hours), and 24 is the number of hours per day. Whole Body Methionine and Cysteine Kinetics Plateau enrichments were calculated as the mean of the plasma isotopic enrichments for the 8- to 10-h time points, where the steady state was achieved. Methionine. Plasma methionine kinetics were calculated on the basis of the model of Storch et al. (38) using methionine enrichment (without correction for intracellular dilution of methionine) or homocysteine isotopic enrichments to correct methionine fluxes, as described by MacCoss et al. (22). Methionine carboxyl flux (Q c) refers to the rate of appearance of the methionine carboxyl group from protein breakdown and the tracer infusion. However, plasma [1-13 C]homocysteine enrichment reflects the intracellular methionine enrichment, because methionine is transmethylated to homocysteine intracellularly. Thus whole body Q c (expressed in mol kg 1 h 1 ) was calculated using the plasma [M 4] and [M 1] [ 13 C]methionine enrichments (Met) or[1-13 C]homocysteine enrichment (Hcy) as the intracellular [1-13 C]methionine enrichment, as follows Q c IR E tracer / E 4 E 1 1 Q c IR E tracer /E Hcy 1 Met Hcy where IR is the [1-13 C;methyl- 2 H 3]methionine tracer infusion rate ( mol kg 1 h 1 ) and E tracer, E 4, E 1, and E Hcy are the isotopic enrichments (expressed in MPE) of infusate, [1-13 C;methyl- 2 H 3]methionine [M 4], [1-13 C]methionine [M 1], and [1-13 C]homocysteine [M 1] in the plasma, respectively. Methionine methyl flux (Q m) includes the same methionine sources described for Q c, as well as the production of methionine via remethylation of homocysteine. With methionine as precursor, the whole body Q m ( mol kg 1 h 1 ) was calculated as follows Q m IR E tracer /E 4 1 Met Q m cannot be calculated using directly [1-13 C]homocysteine enrichment for intracellular enrichment of [1-13 C;methyl- 2 H 3]methionine, since the methyl group is released during the transmethylation pathway and is not present in homocysteine. However, the intracellular enrichment of the methyl group of methionine with [1-13 C]homocysteine enrichment can be estimated to calculate Q m ( mol kg 1 h 1 ) as follows with Q m IR E tracer /E methyl-met 1 E methyl-met E 4 E Hcy / E 1 E 4 Hcy where IR is the [1-13 C;methyl- 2 H 3]methionine tracer infusion rate ( mol kg 1 h 1 ) and E tracer, E methyl-met, E Hcy, E 1, and E 4 represent the isotopic enrichment (expressed in MPE) of the infusate, the methyl group of methionine, [1-13 C]homocysteine [M 1], [1-13 C]methionine [M 1], and [1-13 C;methyl- 2 H 3]methionine [M 4] in the plasma, respectively, as described by Jahoor et al. (15).
4 E1242 The methionine remethylation rate (RM, mol kg 1 h 1 ) was calculated as follows RM Q m Q c The whole body V CO2 ( mol kg 1 h 1 ) was calculated as follows V CO2 IR E tracer /E13 CO 2 1 where IR and E tracer are the infusion rate ( mol kg 1 h 1 ) and the isotopic enrichment (MPE) of infused [ 13 C]bicarbonate, respectively, and E13 CO 2 is the isotopic enrichment of 13 CO 2 (expressed in APE) in the blood during the [ 13 C]bicarbonate infusion. The whole body 13 CO 2 production (V 13 CO 2, mol kg 1 h 1 ) derived from [1-13 C;methyl- 2 H 3]methionine was calculated as follows V 13 CO 2 V CO2 E13 CO 2 where V CO2 is expressed in mol kg 1 h 1 and E13 CO 2 is the isotopic enrichment of 13 CO 2 (APE) in the blood during the [1-13 C; methyl- 2 H 3]methionine infusion. The whole body transsulfuration rate (TS, mol kg 1 h 1 ), equivalent to the methionine oxidation, was calculated using methionine or homocysteine as precursor of the transsulfuration pathway, as follows TS V 13 CO 2 1/ E 4 E 1 1/E tracer TS V 13 CO 2 1/E Hcy 1/E tracer Met Hcy The rates of methionine transmethylation (TM) and protein synthesis (TM and PS, mol kg 1 h 1 ) were calculated as follows TM RM TS PS Q c TS Cysteine. Cysteine flux rate (Q Cys, mol kg 1 h 1 ) was calculated as for methionine flux Q Cys IR E tracer /E Cys 1 where IR is the [ 15 N]cysteine tracer infusion rate ( mol kg 1 h 1 ), E tracer is the infusate enrichment (MPE), and E Cys is the isotopic enrichment (MPE) of [ 15 N]cysteine [M 1] in the plasma. Statistics Values are means SE. Comparison of control with SAA-free values was performed with the two-tailed Student s test for unpaired data. The whole body methionine kinetic values calculated with methionine or homocysteine isotopic enrichments were compared using the two-tailed Student s test for paired data in each group. Statistical significance was assigned at P RESULTS Blood and Tissue Concentrations of SAAs and Their Metabolites Dietary SAA deficiency caused a marked decrease in plasma methionine, homocysteine, cysteine, cystine, and taurine concentrations ( 55% to 85%), as well as erythrocyte homocysteine (not shown) and total glutathione concentrations ( 46% to 53%) compared with a control diet (Table 1). Although the plasma cystine concentration was lower in the SAA-free than in the control group, the proportion of cystine constituted 45% of the total cyste(i)ne (cysteine cystine) in SAA-free pigs compared with 22% in the control pigs. In the tissue, a lower concentration of homocysteine, taurine (not significant in the jejunum), and total glutathione was found in the jejunum, ileum, and liver of SAA-free than control pigs (Table 1). Methionine ( 52% and 42%) and cysteine ( 84% and 67%) concentrations were significantly lower in the jejunum and ileum, respectively, but were not different in the liver, of SAA-free animals compared with control animals. The cystine concentration was higher ( 12% to 24%) in the three tissues, but only significantly in the ileum of SAA-free than control pigs. Although SAM ( 29%) and SAH ( 49%) concentrations were lower in the liver, SAM concentration was significantly higher ( 140%) in the jejunum than in the ileum in SAA-free than control pigs; no difference in SAH concentration was observed in the small intestine between the two groups. In addition, no difference was noted for MTA concentration between the two groups in the three tissues, but the MTA level was higher in the jejunum than in the ileum and liver. Plasma and Tissue Redox Status During the 7-day period, plasma E h, determined from the cysteine-cystine couple, was significantly increased from the 2nd day of enteral nutrition in SAA-free compared with control pigs (Fig. 2A) because of the significant decrease in plasma cysteine (Fig. 2B) and cystine (data not shown) concentrations. The redox potential was significantly higher in the jejunum and the ileum, but not different in the liver, of SAA-free animals compared with control animals (Fig. 2C). Table 1. Blood and tissue concentrations of SAAs and their metabolites at day 7 Plasma Jejunum Ileum Liver Control SAA-free Control SAA-free Control SAA-free Control SAA-free Methionine SAM NA NA * SAH NA NA MTA NA NA Homocysteine * Cysteine Cystine * Taurine , , , , * 7, Glutathione a 1, , , , Values ( mol/l blood and nmol/g tissue) are means SE of 9 piglets fed the control diet and 7 piglets fed the sulfur amino acid (SAA)-free diet. SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; MTA, methylthioadenosine; NA, not analyzed. a Glutathione concentration was measured in erythrocytes, since glutathione was not detectable in plasma. *P 0.05; P 0.01; P vs. control.
5 Fig. 2. Redox potential values (E h) from cysteine-cystine (Cys/CySS) couple in plasma (A) and tissue (jejunum, ileum, and liver; C) and plasma cysteine concentration (B) in piglets continuously enterally fed a control or a sulfur amino acid (SAA)-free diet for 7 days. Values are means SE of 9 control and 7 SAA-free animals. **P 0.01; ***P vs. control. Isotopic Enrichments The steady-state plasma isotopic enrichment (MPE) of [1-13 C; methyl- 2 H 3 ]methionine, [1-13 C]methionine, and [1-13 C]homocysteine was achieved between 4 and 6hof[1-13 C;methyl- 2 H 3 ] methionine infusion in both groups. The plasma [ 15 N]cysteine enrichment reached a steady state within 1hof[ 15 N]cysteine infusion (data not shown). Thus all steady-state isotopic enrichments presented in Table 2 were calculated as the mean of plasma isotopic enrichments between 8 and 10 h of infusion for [1-13 C; methyl- 2 H 3 ]methionine and [ 15 N]cysteine (Fig. 1). Overall, isotopic enrichments of methionine, homocysteine, and cysteine in plasma and tissue were higher in SAA-free than control pigs, with higher enrichments in the jejunum than in the ileum (Table 2). Plasma and tissue isotopic enrichments of [1-13 C;methyl- 2 H 3 ]methionine were higher than [1-13 C]methionine enrichments, because [1-13 C]methionine is synthesized from [1-13 C; methyl- 2 H 3 ]methionine after at least one methionine cycle. Methionine and cysteine enrichments were lower in tissue than in plasma in both groups. On the basis of the relative enrichments, plasma [1-13 C]homocysteine was produced mostly from [1-13 C;methyl- 2 H 3 ]methionine by transmethylation, although some can be recycled from [1-13 C]methionine. In control and SAA-free pigs, plasma homocysteine enrichments were lower than plasma [1-13 C;methyl- 2 H 3 ]methionine enrichments. In control pigs, [1-13 C]homocysteine enrichments were higher than [1-13 C;methyl- 2 H 3 ]methionine enrichments in the liver; in SAA-free pigs, all [1-13 C]homocysteine enrichments were higher than [1-13 C;methyl- 2 H 3 ]methionine enrichments in the jejunum, ileum, and liver and were not significantly different from [1-13 C]homocysteine enrichments in the plasma (data not shown). We estimated the tissue methionine cycle activity by calculating the [M 1]-to-[M 4] methionine enrichment ratio, which was significantly higher in the jejunum [ (control) and (SAA-free), P 0.001], ileum [ (control) and (SAA-free), P 0.001], and liver [ (control) and (SAA-free), P 0.01] of SAA-free than control pigs. The protein synthesis rate (FSR, %/day) was calculated in the jejunum, ileum, and liver using [1-13 C;methyl- 2 H 3 ]methionine and [ 15 N]cysteine enrichments. However, the results with [1-13 C;methyl- 2 H 3 ]methionine were not presented, since the tissue free-pool enrichment in the SAA-free group was sufficiently high that the tissue free and protein enrichment were likely equilibrated, thus making calculation invalid. A significant decrease in FSR using [ 15 N]cysteine enrichments Table 2. Steady-state isotopic enrichments in plasma and tissue Control (n 9) SAA-Free (n 4) Plasma 1-13 C;methyl- 2 H 3 methionine C methionine C homocysteine [ 15 N]cysteine Jejunum 1-13 C;methyl- 2 H 3 methionine C methionine * 1-13 C homocysteine [ 15 N]cysteine Ileum 1-13 C;methyl- 2 H 3 methionine C methionine C homocysteine [ 15 N]cysteine * Liver E C;methyl- 2 H 3 methionine C methionine * 1-13 C homocysteine [ 15 N]cysteine Values are means SE of steady-state plasma isotopic enrichments calculated as mean of plasma isotopic enrichments for 8- to 10-h time points and expressed as molar percent excess. Only tissue free-pool enrichments are shown; data for tissue protein-bound pool are not shown. *P 0.05; P 0.01; P vs. control.
6 E1244 was found in the jejunum [ (control) and (SAA-free), P 0.001], ileum [ (control) and (SAA-free), P 0.001], and liver [ (control) and (SAA-free), P 0.01] of SAA-free compared with control pigs. Whole Body Methionine and Cysteine Metabolism Whole body V CO2 was significantly different between the two groups ( and mmol kg 1 h 1 in control and SAA-free, respectively, P 0.01). Whole body methionine and cysteine kinetics are shown in Table 3. Whole body methionine kinetics were calculated based on the model of Storch et al. (38) using methionine isotopic enrichments, without correction for the intracellular isotopic dilution of methionine, or homocysteine isotopic enrichments to correct methionine fluxes, as described by MacCoss et al. (22). In both treatment groups, all estimates of whole body methionine kinetics using homocysteine were significantly higher than those using methionine isotopic enrichments; this includes estimates of methionine Q m and Q c, as well as TM, TS, RM, and PS. However, the mean difference in the calculated methionine fluxes using methionine or homocysteine enrichment was 19% in the control group but was much higher (64%) in the SAA-free group. In general, the rates of whole body methionine kinetics, corrected with homocysteine isotopic enrichments, were markedly lower in SAA-free than control pigs. To estimate the metabolic fate of methionine via TM, TS, and PS, we calculated the fractional rates relative to whole body methionine flux (Q m ; Table 3). The fraction of methionine metabolized via TM (TM/Q m ) and PS (PS/Q m ) was approximately equal in control pigs (0.48 and 0.52, respectively); however, in SAA-free pigs the fraction of methionine used for PS was higher than for TM (0.34 and 0.66). Thus the fractional rate of TM decreased, whereas PS increased, in SAA-free compared with control pigs. Once methionine enters the methionine cycle via conversion to SAM and transmethylation to homocysteine, there are two metabolic fates: remethylation back to methionine or transsulfuration to cysteine (Table 3). Of the whole body Table 3. Whole body methionine and cysteine kinetics Present Study Control (n 9) SAA-free (n 4) methionine that was metabolized via TM, the fraction remethylated back to methionine (RM/TM) was 0.43, whereas the fraction (TS/TM) metabolized via TS was 0.57, in control pigs. However, in SAA-free pigs, the fraction metabolized via RM was higher (0.64) and the fraction metabolized via TS was lower (0.36). Whole body methionine kinetics from our previous study (34) are also depicted in Table 3, where rates were calculated using methionine and homocysteine isotopic enrichments. In our previous study, 3-wk-old pigs received a 6-h intravenous infusion of [1-13 C]methionine and [methyl- 2 H 3 ]methionine at 10 mol kg 1 h 1 for each methionine tracer. Recalculating these results, we also found significantly lower ( 24%) whole body methionine fluxes (Q m and Q c ) when methionine was used as the precursor than when homocysteine was used as the precursor. Interestingly, the methionine fluxes (Q m and Q c ) were significantly higher ( 14% to 36%) in the control neonatal pigs of the present study than in the infant pigs in our previous study (34). In addition, we found higher whole body flux rates of TM ( 151%), TS ( 86%), and RM ( 390%) in the present study than in our previous study (34); yet we found no difference in PS rates. However, the fractional methionine metabolism via TM (TM/Q m ) and that via TS (TS/Q m ) were higher and PS (PS/Q m ) was lower in the present study than in our previous study (34). Moreover, the fractional rate of RM (RM/TM) was higher and the TS (TS/TM) was lower in the present study than in our previous study. In addition to methionine kinetics, we also found a significantly lower ( 82%) whole body [ 15 N]cysteine flux in SAA-free than control pigs. Body and Tissue Weight and Tissue Protein and DNA Mass During the 7-day enteral nutrition period, the body weight gain decreased markedly ( 70%) in SAA-free pigs compared with control pigs (Table 4). After 7 days of enteral nutrition, jejunum and ileum weight, expressed as a proportion (g/kg) of body weight, was significantly lower ( 39%) in SAA-free than control pigs. However, no significant differences in liver, stomach, and spleen weight were observed between the two groups. Protein and DNA masses were lower in the small Riedijk et al. (34) Met Hcy Met Hcy Met Hcy Q m * 91 10* Q c * 71 8* TM * 31 4* TS * 11 2* RM * 20 2* PS * 60 6* TM/Q m * * TS/Q m * * PS/Q m * * RM/TM * * TS/TM * * [ 15 N]cysteine flux * Values ( mol kg 1 h 1 ) are means SE. For methionine kinetics, rates were calculated using methionine (Met) or homocysteine (Hcy) isotopic enrichments. Q m and Q c, methionine methyl and carboxyl flux, respectively; TM, transmethylation; TS, transsulfuration; RM, remethylation; PS, protein synthesis. In the study of Riedijk et al. (34), 3-wk-old piglets received an 6-h intravenous infusion of 1-13 C methionine and methyl- 2 H 3 methionine at 10 mol kg 1 h 1 for each methionine tracer, so total isotopic methionine infusion rate was 20 mol kg 1 h 1.*P 0.05, SAA-free vs. control. P 0.05, Met vs. Hcy. P 0.05, control vs. Riedijk et al. (34).
7 Table 4. Body and tissue weight and tissue protein and DNA mass intestine and liver (not significant for DNA mass in the liver) of SAA-free than control animals. Morphometry, Goblet Cell Content, and Mucosal Cell Proliferation Histological analysis of the intestine (jejunum, ileum, and colon) showed lower ( 20% to 40%) gut section area, villus height, villus area, and crypt depth (not different in the ileum; Fig. 3, B E), as well as a lower ( 60%) absolute number of goblet cells, in the villi and crypts of the jejunum in SAA-free than control pigs, but the difference was not significant in the ileum (Fig. 3, F and G) in SAA-free compared with control pigs. In addition, the absolute number of Ki-67-positive proliferative crypt cells was lower ( 40%) in the jejunum and colon of SAA-free than control pigs (Fig. 4B). Methionine Metabolic Enzyme Activity in the Tissue MAT activity was increased in the jejunum ( 306%) and ileum ( 288%), but not in the liver, whereas MS activity was higher ( 102% to 325%) in all three tissues of the SAA-free than control pigs (Fig. 5). We also found a higher CBS activity in the jejunum ( 318%) of the SAA-free than control pigs, but not in the ileum or liver. DISCUSSION Control (n 9) SAA-Free (n 7) Body weight gain, g kg 1 day Tissue weight, g/kg body wt Jejunum Ileum Small intestine (jejunum ileum) Liver Stomach Spleen Tissue protein mass, g/kg body wt Jejunum Ileum Liver Tissue DNA mass, mg/kg body wt Jejunum Ileum Liver Values are means SE. P 0.01; P vs. control. The main objective of the present study was to characterize the impact of enteral SAA deficiency on normal intestinal mucosal growth and antioxidant function in neonatal pigs. Our previous work (34) and that of others (35) showed that the gut is an important site of whole body methionine metabolism in the neonatal pig, consuming 20 30% of the dietary intake, and much of the methionine used by the gut is metabolized via transmethylation and transsulfuration. We also were especially interested in the impact of SAA deficiency on the intestinal growth, since previous reports suggest that SAAs are key precursors for maintenance of cell redox status via GSH synthesis, which is a critical determinant of epithelial cell proliferation. Our results showed that SAA deficiency preferentially reduced intestinal growth compared with growth of other tissues such as liver and spleen. Moreover, we showed E1245 that SAA deficiency suppressed intestinal epithelial cell proliferation, especially in the jejunum and, as expected, SAA and GSH concentrations in the small intestine. Our isotope kinetic approach using [1-13 C;methyl- 2 H 3 ]methionine also showed that methionine is preferentially conserved for protein synthesis under SAA-deficient conditions, whereas transmethylation and transsulfuration are suppressed. Whole body methionine kinetics were quantified using the [1-13 C;methyl- 2 H 3 ]methionine tracer approach based on the model of Storch et al. (38). Storch et al. assumed that the intracellular dilution of methionine was the same as that observed for intracellular leucine by Matthews et al. (26), which is 20% lower than plasma leucine enrichment; yet this assumption does not reflect the true physiological effect of methionine metabolism. In the present study, we used plasma homocysteine enrichments to correct the intracellular methionine enrichments, as described by MacCoss et al. (22), since plasma homocysteine can be derived only from intracellular homocysteine formed by transmethylation of methionine. In our calculations, the correction with homocysteine, rather than methionine, enrichments led to higher whole body methionine kinetic rates, but the increase was disproportionally higher in the SAA-free group ( 64%) than in the control group ( 19%). This discrepancy in flux estimates between the groups emphasizes the importance of the kinetic model used to quantify factors that regulate methionine metabolism. All measures of methionine flux and metabolism were markedly lower in SAA-free than control pigs, as we expected, because of the dietary treatment; this occurred whether (or not) the whole body methionine kinetic rates were corrected with homocysteine enrichments. However, the relative metabolic fate of whole body methionine in SAA deficiency conditions was also altered, such that more methionine was directed to protein synthesis, rather than transmethylation and transsulfuration pathways. Although less methionine was metabolized via the transmethylation to homocysteine, the fractional rate of homocysteine remethylation back to methionine was markedly higher in SAA-free than control pigs. This result is consistent with the findings of Storch et al. (38), where 67% of the homocysteine formed was remethylated in adult men given a methionine- and cystine-free diet for 5 days. Under methionine-free conditions, methionine is derived largely from proteolysis and the remethylation of homocysteine. Thus our results suggest that, under SAA-free conditions, whole body methionine metabolism is prioritized so as to preserve methionine for protein synthesis, whereas most of the methionine transmethylated to homocysteine is remethylated to maintain the methionine needs. We also compared results of the present study using 1-wkold neonatal pigs with results of our previous study using 1-mo-old pigs (34). In general, whole body methionine fluxes and metabolic rates were significantly higher in the control group from present study than in our previous study. These findings can be explained in part by the difference in age between the animals. However, despite the higher overall methionine fluxes, the fractional methionine metabolism via transmethylation (TM/Q m ) and that via transsulfuration (TS/ Q m ) were higher, yet the protein synthesis (PS/Q m ) was lower, in the present study of 1-wk-old pigs than in the previous study of 1-mo-old pigs. Moreover, the fractional remethylation rate (RM/TM) was twice as high in neonatal as in infant pigs,
8 E1246 Fig. 3. Intestinal cross sections stained with hematoxylin and eosin (A) and morphological parameters (B G) of jejunum, ileum, and colon of piglets continuously enterally fed a control or an SAA-free diet for 7 days. Values are means SE of 9 control and 7 SAA-free animals. *P 0.05; **P 0.01; ***P vs. control. indicating a more active methionine cycle. Interestingly, the absolute rate of whole body protein synthesis was not significantly different between neonatal and infant pigs. These results suggest higher overall methionine requirements in the neonatal than in the infant pigs, not so much for protein synthesis but, rather, for the synthesis of SAM, the principal methyl donor in mammalian cells and a precursor for polyamine synthesis (25). Another important metabolic function of methionine is transsulfuration in the synthesis of cysteine and, ultimately, glutathione and taurine (16, 21). Cysteine is the limiting amino acid for glutathione synthesis, and it is the only precursor for taurine synthesis (39). Thus the lower transsulfuration rate and cysteine flux in SAA-free than control pigs resulted in lower blood concentrations of glutathione and taurine. Along with glutathione and taurine, cysteine plays an important role as an extracellular reducing agent (2, 5, 27). Cysteine (Cys) and its disulfide cystine (CySS) comprise the predominant low-molecular-weight thiol/disulfide pool found in the plasma and central
9 E1247 Downloaded from Fig. 4. A: intestinal cross sections immunostained for Ki-67. Magnification 100. B: proliferative cell distribution (i.e., Ki-67-positive cells) in crypts of jejunum, ileum, and colon of piglets continuously enterally fed a control or an SAA-free diet for 7 days. Values are means SE of 9 control and 7 SAA-free animals. *P 0.05 vs. control. to the maintenance of the redox state of plasma proteins (19). The present data show that dietary SAA deficiency leads to a more oxidized status of the Cys/CySS E h in plasma, consistent with the previous report (29). The impact of dietary SAA deficiency on organ growth appeared to be specific to the intestine. When expressed relative to body weight, we found a lower small intestine weight in SAA-free than control pigs; yet there were no differences in the liver, stomach, and spleen. Moreover, the DNA mass was also lower in the small intestine but not the liver of SAA-free than control pigs. The lower DNA mass was reflected in intestine villus atrophy and lower crypt depth and crypt cell proliferation, especially in the jejunum. Redox status influences intestinal epithelial cell proliferation and apoptosis, where increased oxidant stress and redox imbalance suppress cell proliferation and induce apoptosis (3). Intestinal redox balance is a complex process involving GSH/GSSG and the Cys-CySS couple (1, 3, 31). Depletion of GSH, induced by buthionine sulfoximine, suppressed growth of jejunal and colonic epithelial cells, and this could be prevented by GSH administration (23). In this study under SAA-free conditions, we found a significant increase in E h, calculated from the Cys-CySS couple. Inasmuch as cysteine, glutathione, and taurine possess an antioxidant activity (2), the oxidant stress generated by the low concentration of these molecules, together with reduced protein synthesis, may be responsible for the intestinal mucosal atrophy observed in the SAA-free animals. As part of the methionine cycle, homocysteine is produced, and our previous study showed that the gut is a net site of homocysteine release into the body (34). In the present study, plasma and tissue homocysteine concentrations were lower in the SAA-free than in the control group. However, in the SAA-free pigs, we found that the isotopic enrichment of homocysteine in the gut and liver tissue was nearly equal to that of the plasma and substantially higher than tissue methionine. Inasmuch as homocysteine is synthesized intracellularly by on June 10, 2017
10 E1248 Fig. 5. Enzyme activity of methionine adenosyltransferase (MAT; A), methionine synthase (MS; B), and cystathionine -synthase (CBS; C) in jejunum, ileum, and liver of piglets continuously enterally fed a control or an SAA-free diet for 7 days. Values are means SE of 9 control and 7 SAA-free animals. *P 0.05; **P 0.01; ***P vs. control. from methionine via the transmethylation pathway, these results indicated that the tissue homocysteine pool was derived from plasma uptake, rather than intracellular synthesis, or that subcellular compartmentation of these pools exists. We also found a higher (2-fold) fractional rate of remethylation estimated with [M 1]/[M 4] methionine enrichment in the jejunum and the ileum of the SAA-free than control pigs. This was consistent with higher MS activity in the jejunum and ileum of SAA-free than control pigs. MS is ubiquitously distributed and is a dominant enzyme that catalyzes the synthesis of methionine from homocysteine in the intestine (6). The transsulfuration pathway is an irreversible process that converts homocysteine to cysteine. In contrast to the methionine cycle, the transsulfuration pathway is restricted to the liver, kidney, intestine, and pancreas (6), and we previously showed that CBS, the rate-limiting enzyme in the transsulfuration pathway, was expressed in intestinal epithelial cells of piglets (34). Although we cannot estimate the fractional rate of transsulfuration, the low amount of cysteine and glutathione found in the jejunum and ileum of SAA-free pigs suggests a low transsulfuration rate. Thus, as with the whole body metabolism, methionine catabolism via transsulfuration is limited to preserve methionine for protein synthesis. These factors contribute to the low intestinal cysteine concentration in the SAA-free pigs, which may explain the lower goblet cell numbers, especially in the jejunum, since goblet cells secrete cysteine-rich mucins involved in the innate immune function of the intestine (41). On the basis of these results, we speculate that the high fractional rate of transmethylation and remethylation in all tissue from SAA-free pigs could provide methionine for SAM synthesis. SAM is synthesized from methionine by MAT, which exists through three distinct isoforms: MAT I and MAT III, which are expressed in liver, and MAT II, which is widely expressed in extrahepatic tissue, including the gut (25). Interestingly, in response to SAA deficiency, MAT activity markedly increased in the small intestine, where it was higher in the jejunum than in the ileum, whereas it was unchanged in the liver. Similarly, SAM levels were much higher in the jejunum than in the ileum after an SAA-free diet. SAM has two main metabolic fates: as the major methyl donor in cells and in the synthesis of polyamines, which play a key role in cell proliferation and expression of growth-related genes (4, 24, 28). A recent report (11) showed that the MAT2A gene, which encodes for MAT II, was upregulated in human colon cancer cells, and its inhibition led to cell death. These findings indicate that MAT2A gene expression, and hence cellular SAM synthesis, is critical for survival and proliferation in intestinal epithelial cells. Our results suggest that, during SAA deficiency, the upregulation of MAT activity and the increase of SAM concentration in the intestine, especially in the jejunum, may be to maintain epithelial polyamine synthesis for cell proliferation. SAM is also involved in the transsulfuration pathway by activating CBS (33). Thus the higher SAM concentration in the jejunum than in the ileum in SAA-free conditions may explain the increase in CBS activity observed in the jejunum but not in the ileum. In conclusion, we show here that dietary SAA deficiency preferentially reduced intestinal growth in neonatal pigs. The suppression of intestinal growth was associated with villus atrophy, reduced epithelial cell proliferation and lower goblet cell numbers, and diminished redox capacity. Isotopic kinetic analyses at the whole body and tissue level show that, under SAA-deficient conditions, methionine metabolism is prioritized in a coordinate manner, such that protein synthesis is preserved over methionine transmethylation and the methionine pool is preserved by upregulation of homocysteine remethylation and suppression of transsulfuration. The suppression of transsulfuration contributed to the diminished cellular cysteine and GSH concentrations and increased oxidant stress, which seemed to preferentially impact intestinal growth, especially in the jejunum. We also show that the methionine flux, and hence metabolic requirement, appeared to be higher in neonatal than infant pigs, but this difference in methionine use was solely attributed to transmethylation and transsulfuration. These results highlight the methionine requirements for metabolic functions, such as methylation and polyamine synthesis during early development, and warrant further study as to the
Cysteine Supplementation in Parenteral-fed Critically ill Neonates:
 Cysteine Supplementation in Parenteral-fed Critically ill Neonates: A Randomized Trial Investigating Glutathione Synthesis Stephen B. Shew, M.D. UCLA Division of Pediatric Surgery Nov 29, 2006 Background
Cysteine Supplementation in Parenteral-fed Critically ill Neonates: A Randomized Trial Investigating Glutathione Synthesis Stephen B. Shew, M.D. UCLA Division of Pediatric Surgery Nov 29, 2006 Background
5th Amino Acid Assessment Workshop
 5th Amino Acid Assessment Workshop The In Vivo Sparing of Methionine by Cysteine in Sulfur Amino Acid Requirements in Animal Models and Adult Humans 1,2 Ronald O. Ball,* y3 Glenda Courtney-Martin, y and
5th Amino Acid Assessment Workshop The In Vivo Sparing of Methionine by Cysteine in Sulfur Amino Acid Requirements in Animal Models and Adult Humans 1,2 Ronald O. Ball,* y3 Glenda Courtney-Martin, y and
Amino acid metabolism
 Amino acid metabolism The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. The product ammonia is excreted after conversion to urea or other
Amino acid metabolism The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. The product ammonia is excreted after conversion to urea or other
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Midterm 2. Low: 14 Mean: 61.3 High: 98. Standard Deviation: 17.7
 Midterm 2 Low: 14 Mean: 61.3 High: 98 Standard Deviation: 17.7 Lecture 17 Amino Acid Metabolism Review of Urea Cycle N and S assimilation Last cofactors: THF and SAM Synthesis of few amino acids Dietary
Midterm 2 Low: 14 Mean: 61.3 High: 98 Standard Deviation: 17.7 Lecture 17 Amino Acid Metabolism Review of Urea Cycle N and S assimilation Last cofactors: THF and SAM Synthesis of few amino acids Dietary
9 Metabolic trigger: control of methionine metabolism
 9 Metabolic trigger: control of methionine metabolism M.V. Martinov 1,V.M.Vitvitsky 1,E.V.Mosharov 2,R.Banerjee 2,F.I.Ataullakhanov 1 1 National Research Center for Hematology, Moscow, Russia 125167 2
9 Metabolic trigger: control of methionine metabolism M.V. Martinov 1,V.M.Vitvitsky 1,E.V.Mosharov 2,R.Banerjee 2,F.I.Ataullakhanov 1 1 National Research Center for Hematology, Moscow, Russia 125167 2
Skeletal muscle metabolism was studied by measuring arterio-venous concentration differences
 Supplemental Data Dual stable-isotope experiment Skeletal muscle metabolism was studied by measuring arterio-venous concentration differences across the forearm, adjusted for forearm blood flow (FBF) (1).
Supplemental Data Dual stable-isotope experiment Skeletal muscle metabolism was studied by measuring arterio-venous concentration differences across the forearm, adjusted for forearm blood flow (FBF) (1).
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Metabolism of amino acids. Vladimíra Kvasnicová
 Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
AMINO ACID METABOLISM
 AMINO ACID METABOLISM Synthesis of Urea in Liver The series of reactions that form urea is known as the Urea Cycle or the Krebs-Henseleit Cycle. The urea cycle operates only to eliminate excess nitrogen.
AMINO ACID METABOLISM Synthesis of Urea in Liver The series of reactions that form urea is known as the Urea Cycle or the Krebs-Henseleit Cycle. The urea cycle operates only to eliminate excess nitrogen.
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo
 Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo BERNHARD H. LAUTERBURG, GEORGE B. CORCORAN, and JERRY R. MITCHELL, Baylor College of
Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo BERNHARD H. LAUTERBURG, GEORGE B. CORCORAN, and JERRY R. MITCHELL, Baylor College of
Classification of amino acids: -
 Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
number Done by Corrected by Doctor Dr.Diala
 number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
Catabolism of Carbon skeletons of Amino acids. Amino acid metabolism
 Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
III. TOXICOKINETICS. Studies relevant to the toxicokinetics of inorganic chloramines are severely
 III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Biochemistry 2 Recita0on Amino Acid Metabolism
 Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Midterm 2 Results. Standard Deviation:
 Midterm 2 Results High: Low: Mean: Standard Deviation: 97.5% 16% 58% 16.3 Lecture 17 Amino Acid Metabolism Urea Cycle N and S assimilation Last cofactors: THF and SAM Dietary (Exogenous) Proteins Hydrolyzed
Midterm 2 Results High: Low: Mean: Standard Deviation: 97.5% 16% 58% 16.3 Lecture 17 Amino Acid Metabolism Urea Cycle N and S assimilation Last cofactors: THF and SAM Dietary (Exogenous) Proteins Hydrolyzed
Evaluation of Stable Isotope Labeling Technique in Measuring the Tissues Protein Fractional Synthesis Rates in Rats
 Available online at www.annclinlabsci.org Annals of Clinical & Laboratory Science, vol. 45, no. 2, 2015 187 Evaluation of Stable Isotope Labeling Technique in Measuring the Tissues Protein Fractional Synthesis
Available online at www.annclinlabsci.org Annals of Clinical & Laboratory Science, vol. 45, no. 2, 2015 187 Evaluation of Stable Isotope Labeling Technique in Measuring the Tissues Protein Fractional Synthesis
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Amino acids-incorporated nanoflowers with an
 Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity Zhuo-Fu Wu 1,2,+, Zhi Wang 1,+, Ye Zhang 3, Ya-Li Ma 3, Cheng-Yan He 4, Heng Li 1, Lei Chen 1, Qi-Sheng Huo 3, Lei Wang 1,*
Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity Zhuo-Fu Wu 1,2,+, Zhi Wang 1,+, Ye Zhang 3, Ya-Li Ma 3, Cheng-Yan He 4, Heng Li 1, Lei Chen 1, Qi-Sheng Huo 3, Lei Wang 1,*
Oxidation and Methylation in Human Brain: Implications for vaccines
 Oxidation and Methylation in Human Brain: Implications for vaccines 1 Life can be viewed through the perspective of oxidation and reduction, which involves the loss and gain of electrons, respectively.
Oxidation and Methylation in Human Brain: Implications for vaccines 1 Life can be viewed through the perspective of oxidation and reduction, which involves the loss and gain of electrons, respectively.
What location in the gastrointestinal (GI) tract has tight, or impermeable, junctions between the epithelial cells?
 CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
HRP cytochemistry. Division of Radiooncology, Deutsches Krebsforschungszentrum, Heidelberg, Germany
 HRP cytochemistry WOLF D. KUHLMANN, M.D. Division of Radiooncology, Deutsches Krebsforschungszentrum, 69120 Heidelberg, Germany A range of substrates is available for the cytochemical staining of peroxidase
HRP cytochemistry WOLF D. KUHLMANN, M.D. Division of Radiooncology, Deutsches Krebsforschungszentrum, 69120 Heidelberg, Germany A range of substrates is available for the cytochemical staining of peroxidase
AMINO ACID METABOLISM. Sri Widia A Jusman Dept. of Biochemistry & Molecular Biology FMUI
 AMINO ACID METABOLISM Sri Widia A Jusman Dept. of Biochemistry & Molecular Biology FMUI Amino acids derived from dietary protein absorbed from intestine through blood taken up by tissues used for biosynthesis
AMINO ACID METABOLISM Sri Widia A Jusman Dept. of Biochemistry & Molecular Biology FMUI Amino acids derived from dietary protein absorbed from intestine through blood taken up by tissues used for biosynthesis
Mechanism of Detoxification
 Mechanism of Detoxification Prof.Dr. Hedef Dhafir El-Yassin 1 Objectives: 1. To list the detoxification pathways 2. To describe detoxification pathways and phases in the liver, 2 3 4 o Xenobiotics are
Mechanism of Detoxification Prof.Dr. Hedef Dhafir El-Yassin 1 Objectives: 1. To list the detoxification pathways 2. To describe detoxification pathways and phases in the liver, 2 3 4 o Xenobiotics are
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Whole body and skeletal muscle glutamine metabolism in healthy subjects
 Am J Physiol Endocrinol Metab 280: E323 E333, 2001. Whole body and skeletal muscle glutamine metabolism in healthy subjects B. MITTENDORFER, 1 E. VOLPI, 2,4 AND R. R. WOLFE 1,3,4 Departments of 1 Surgery,
Am J Physiol Endocrinol Metab 280: E323 E333, 2001. Whole body and skeletal muscle glutamine metabolism in healthy subjects B. MITTENDORFER, 1 E. VOLPI, 2,4 AND R. R. WOLFE 1,3,4 Departments of 1 Surgery,
Nitrogen Metabolism. Overview
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
~PENTOSE PHOSPHATE PATHWAY~ DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU
 ~PENTOSE PHOSPHATE PATHWAY~ DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU OVERVIEW The pentose phosphate pathway (also called the hexose monophosphate shunt, or 6- phosphogluconate pathway) occurs in the cytosol
~PENTOSE PHOSPHATE PATHWAY~ DR. A. TARAB DEPT. OF BIOCHEMISTRY HKMU OVERVIEW The pentose phosphate pathway (also called the hexose monophosphate shunt, or 6- phosphogluconate pathway) occurs in the cytosol
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Nitrogen Metabolism. Overview
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
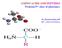 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
9/16/15. Properties of Water. Benefits of Water. More properties of water
 Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
SIMPLE BASIC METABOLISM
 SIMPLE BASIC METABOLISM When we eat food such as a tuna fish sandwich, the polysaccharides, lipids, and proteins are digested to smaller molecules that are absorbed into the cells of our body. As these
SIMPLE BASIC METABOLISM When we eat food such as a tuna fish sandwich, the polysaccharides, lipids, and proteins are digested to smaller molecules that are absorbed into the cells of our body. As these
Human Nutrition and Metabolism
 Human Nutrition and Metabolism Regulation of Sulfur Amino Acid Metabolism in Men in Response to Changes in Sulfur Amino Acid Intakes 1,2 Marco Di Buono,* Linda J. Wykes,** David E.C. Cole, Ronald O. Ball*
Human Nutrition and Metabolism Regulation of Sulfur Amino Acid Metabolism in Men in Response to Changes in Sulfur Amino Acid Intakes 1,2 Marco Di Buono,* Linda J. Wykes,** David E.C. Cole, Ronald O. Ball*
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
!!"#$%&'#()*+,-).(&"/+0&'12'
 LAB #: Sample Report PATIENT: Sample Patient ID: SEX: Female DOB: 01/01/1985 AGE: 33 CLIENT #: 12345 DOCTOR: Sample Doctor Doctors Data Inc 3755 Illinois Ave St. Charles, IL 60174 U.S.A.!!"#$%&'#()*+,-).(&"/+0&'12'
LAB #: Sample Report PATIENT: Sample Patient ID: SEX: Female DOB: 01/01/1985 AGE: 33 CLIENT #: 12345 DOCTOR: Sample Doctor Doctors Data Inc 3755 Illinois Ave St. Charles, IL 60174 U.S.A.!!"#$%&'#()*+,-).(&"/+0&'12'
Speaker: Paul De Smet. Thursday June 4th 2015
 Speaker: Paul De Smet Thursday June 4th 2015 Your logo Stress Support: the nutritional answer to stress, infection and disease Thursday June 4th 2015 Table of content Stress Support: the nutritional answer
Speaker: Paul De Smet Thursday June 4th 2015 Your logo Stress Support: the nutritional answer to stress, infection and disease Thursday June 4th 2015 Table of content Stress Support: the nutritional answer
Integrative Metabolism: Significance
 Integrative Metabolism: Significance Energy Containing Nutrients Carbohydrates Fats Proteins Catabolism Energy Depleted End Products H 2 O NH 3 ADP + Pi NAD + NADP + FAD + Pi NADH+H + NADPH+H + FADH2 Cell
Integrative Metabolism: Significance Energy Containing Nutrients Carbohydrates Fats Proteins Catabolism Energy Depleted End Products H 2 O NH 3 ADP + Pi NAD + NADP + FAD + Pi NADH+H + NADPH+H + FADH2 Cell
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion
 Am J Physiol Endocrinol Metab 284: E76 E89, 2003. First published September 11, 2002; 10.1152/ajpendo.00234.2002. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino
Am J Physiol Endocrinol Metab 284: E76 E89, 2003. First published September 11, 2002; 10.1152/ajpendo.00234.2002. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
1. Describe the relationship of dietary protein and the health of major body systems.
 Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2*
 Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
EFFECTS OF AMINO ACID SUBSTITUTIONS FOR WHEY PROTEIN CONCENTRATE ON WEANLING PIG PERFORMANCE. Authors: J. Chung, S.D. Carter and J.C.
 EFFECTS OF AMINO ACID SUBSTITUTIONS FOR WHEY PROTEIN CONCENTRATE ON WEANLING PIG PERFORMANCE 1999 Animal Science Research Report Authors: Story in Brief Pages 266-272 J. Chung, S.D. Carter and J.C. Whisenhunt
EFFECTS OF AMINO ACID SUBSTITUTIONS FOR WHEY PROTEIN CONCENTRATE ON WEANLING PIG PERFORMANCE 1999 Animal Science Research Report Authors: Story in Brief Pages 266-272 J. Chung, S.D. Carter and J.C. Whisenhunt
Organic Acids Part 10 Dr. Jeff Moss
 Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part X Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 2 Sulfur
Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part X Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 2 Sulfur
Electrolytes Solution
 Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77
 PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77 DIGESTION & ABSORPTION DIETARY PROTEINS SERVE 3 FUNCTIONS 1. THEIR CONSTITUTENT AMINOACIDS ARE USED FOR SYNTHESIS OF BODY PROTEINS
PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77 DIGESTION & ABSORPTION DIETARY PROTEINS SERVE 3 FUNCTIONS 1. THEIR CONSTITUTENT AMINOACIDS ARE USED FOR SYNTHESIS OF BODY PROTEINS
The Basics: A general review of molecular biology:
 The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
paper and beads don t fall off. Then, place the beads in the following order on the pipe cleaner:
 Beady Pipe Cleaner Proteins Background: Proteins are the molecules that carry out most of the cell s dayto-day functions. While the DNA in the nucleus is "the boss" and controls the activities of the cell,
Beady Pipe Cleaner Proteins Background: Proteins are the molecules that carry out most of the cell s dayto-day functions. While the DNA in the nucleus is "the boss" and controls the activities of the cell,
MPRP Annual Report (January 2012)
 MPRP Annual Report (January 2012) Further Evaluation of a New Precision-Fed Chick Assay for Determining Amino Acid Digestibility and Metabolizable Energy of Feed Ingredients C.M. Parsons University of
MPRP Annual Report (January 2012) Further Evaluation of a New Precision-Fed Chick Assay for Determining Amino Acid Digestibility and Metabolizable Energy of Feed Ingredients C.M. Parsons University of
This student paper was written as an assignment in the graduate course
 77:222 Spring 2003 Free Radicals in Biology and Medicine Page 0 This student paper was written as an assignment in the graduate course Free Radicals in Biology and Medicine (77:222, Spring 2003) offered
77:222 Spring 2003 Free Radicals in Biology and Medicine Page 0 This student paper was written as an assignment in the graduate course Free Radicals in Biology and Medicine (77:222, Spring 2003) offered
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
EFFECTS OF REPLACING WHEY PROTEIN CONCENTRATE WITH CRYSTALLINE AMINO ACIDS ON WEANLING PIG PERFORMANCE
 EFFECTS OF REPLACING WHEY PROTEIN CONCENTRATE WITH CRYSTALLINE AMINO ACIDS ON WEANLING PIG PERFORMANCE 1999 Animal Science Research Report Authors: Story in Brief Pages 258-265 J. Chung, S.D. Carter,C.V.
EFFECTS OF REPLACING WHEY PROTEIN CONCENTRATE WITH CRYSTALLINE AMINO ACIDS ON WEANLING PIG PERFORMANCE 1999 Animal Science Research Report Authors: Story in Brief Pages 258-265 J. Chung, S.D. Carter,C.V.
LAB#23: Biochemical Evidence of Evolution Name: Period Date :
 LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
Cells N5 Homework book
 1 Cells N5 Homework book 2 Homework 1 3 4 5 Homework2 Cell Ultrastructure and Membrane 1. Name and give the function of the numbered organelles in the cell below: A E B D C 2. Name 3 structures you might
1 Cells N5 Homework book 2 Homework 1 3 4 5 Homework2 Cell Ultrastructure and Membrane 1. Name and give the function of the numbered organelles in the cell below: A E B D C 2. Name 3 structures you might
Nitrogen Metabolism. Pratt and Cornely Chapter 18
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
1. (38 pts.) 2. (25 pts.) 3. (15 pts.) 4. (12 pts.) 5. (10 pts.) Bonus (12 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
Pro-Oxidant Environmental Exposures: Implications of Redox Imbalance in Autism S. Jill James, Ph.D.
 Pro-Oxidant Environmental Exposures: Implications of Redox Imbalance in Autism S. Jill James, Ph.D. Professor, Department of Pediatrics Director, Autism Metabolic Genomics Laboratory Arkansas Children
Pro-Oxidant Environmental Exposures: Implications of Redox Imbalance in Autism S. Jill James, Ph.D. Professor, Department of Pediatrics Director, Autism Metabolic Genomics Laboratory Arkansas Children
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Development and Evaluation of a New Precision-Fed Chick Assay for Determining Amino Acid Digestibility and Metabolizable Energy of Feed Ingredients
 Development and Evaluation of a New Precision-Fed Chick Assay for Determining Amino Acid Digestibility and Metabolizable Energy of Feed Ingredients C.M. Parsons University of Illinois 1207 W. Gregory Drive
Development and Evaluation of a New Precision-Fed Chick Assay for Determining Amino Acid Digestibility and Metabolizable Energy of Feed Ingredients C.M. Parsons University of Illinois 1207 W. Gregory Drive
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Amino Acids. Amino Acids. Fundamentals. While their name implies that amino acids are compounds that contain an NH. 3 and CO NH 3
 Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
BASIC SCIENCES & BIOCHEMISTRY FOR BETZPAENIC BRIMBLERS
 BASIC SCIENCES & BIOCHEMISTRY FOR BETZPAENIC BRIMBLERS Lymphatic Vessels One main lymph vessel receives lymph from the right upper arm and the right side of the head and the thorax and empties into the
BASIC SCIENCES & BIOCHEMISTRY FOR BETZPAENIC BRIMBLERS Lymphatic Vessels One main lymph vessel receives lymph from the right upper arm and the right side of the head and the thorax and empties into the
Electronic Supplementary Information. Table of Contents
 Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Series Editors: Daniel Kamin, MD and Christine Waasdorp Hurtado, MD
 NASPGHAN Physiology Lecture Series GI Physiology Module: Absorption of Water and Ions Jason Soden, MD Reviewers: George Fuchs MD: UAMS College of Medicine / Arkansas Children s Hospital Wayne Lencer MD:
NASPGHAN Physiology Lecture Series GI Physiology Module: Absorption of Water and Ions Jason Soden, MD Reviewers: George Fuchs MD: UAMS College of Medicine / Arkansas Children s Hospital Wayne Lencer MD:
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
Introduction to Peptide Sequencing
 Introduction to Peptide equencing Quadrupole Ion Traps tructural Biophysics Course December 3, 2014 12/8/14 Introduction to Peptide equencing - athan Yates 1 Why are ion traps used to sequence peptides?
Introduction to Peptide equencing Quadrupole Ion Traps tructural Biophysics Course December 3, 2014 12/8/14 Introduction to Peptide equencing - athan Yates 1 Why are ion traps used to sequence peptides?
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:!
 LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Amino acids. Ing. Petrová Jaroslava. Workshop on Official Controls of Feed AGR 46230, , Ankara. Turkey ÚKZÚZ - NRL RO Praha 1
 Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Short Bowel Syndrome: Medical management
 Short Bowel Syndrome: Medical management La Sindrome dell'intestino Corto in età pediatrica Brescia 18 marzo 2011 Jon A.Vanderhoof, M.D. Division of Pediatric GI Harvard Medical School Children s Hospital,
Short Bowel Syndrome: Medical management La Sindrome dell'intestino Corto in età pediatrica Brescia 18 marzo 2011 Jon A.Vanderhoof, M.D. Division of Pediatric GI Harvard Medical School Children s Hospital,
Improvement of Intracellular Glutathione Content. in Baker s Yeast. for Nutraceutical Application
 Improvement of Intracellular Glutathione Content in Baker s Yeast for Nutraceutical Application Manuela Rollini, Alida Musatti DeFENS, Section of Food Microbiology and Bioprocessing Vienna, 28 th June
Improvement of Intracellular Glutathione Content in Baker s Yeast for Nutraceutical Application Manuela Rollini, Alida Musatti DeFENS, Section of Food Microbiology and Bioprocessing Vienna, 28 th June
Glutathione Assay Kit
 Glutathione Assay Kit Catalog Number KA1649 250 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Glutathione Assay Kit Catalog Number KA1649 250 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Organic Acids Part 11 Dr. Jeff Moss
 Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part XI Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 USA Today,
Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part XI Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 USA Today,
Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract
 Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract Maaike A. Riedijk*, Barbara Stoll, Shaji Chacko, Henk Schierbeek*, Agneta L. Sunehag, Johannes B. van Goudoever*, and
Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract Maaike A. Riedijk*, Barbara Stoll, Shaji Chacko, Henk Schierbeek*, Agneta L. Sunehag, Johannes B. van Goudoever*, and
Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
 Acta Univ. Sapientiae, Alimentaria, 1 (2008) 49 60 Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
Acta Univ. Sapientiae, Alimentaria, 1 (2008) 49 60 Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Parenteral Nutrition. Outline. Potential Biomarkers for Use in Intestinal Adaptation. Jejunum is primary site of digestion and absorption
 Outline Potential Biomarkers for Use in Intestinal Adaptation Kelly A. Tappenden, Ph.D., R.D. Professor of Nutrition and GI Physiology 1. Intestinal Adaptation potential regulators 2. Intestinal mucosal
Outline Potential Biomarkers for Use in Intestinal Adaptation Kelly A. Tappenden, Ph.D., R.D. Professor of Nutrition and GI Physiology 1. Intestinal Adaptation potential regulators 2. Intestinal mucosal
Amino acid Catabolism
 Enzymatic digestion of dietary proteins in gastrointestinal-tract. Amino acid Catabolism Amino acids: 1. There are 20 different amino acid, they are monomeric constituents of proteins 2. They act as precursors
Enzymatic digestion of dietary proteins in gastrointestinal-tract. Amino acid Catabolism Amino acids: 1. There are 20 different amino acid, they are monomeric constituents of proteins 2. They act as precursors
endopeptidases aminopeptidases carboxypeptidases hydrolyzes a peptide bond somewhere in the middle of the polypeptide
 1 Amino Acid Metabolism: The primary purpose for s in the body is to provide the building blocks for proteins R other s. owever, if there is no protein synthesis occurring, the s can be broken down (i.e.
1 Amino Acid Metabolism: The primary purpose for s in the body is to provide the building blocks for proteins R other s. owever, if there is no protein synthesis occurring, the s can be broken down (i.e.
