SAMPLE. Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline
|
|
|
- Ambrose Horton
- 5 years ago
- Views:
Transcription
1 Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline This document provides guidelines for patient preparation, specimen collection, transport, and processing for the measurement of trace elements in a variety of biological matrices. A guideline for global application developed through the Clinical and Laboratory Standards Institute consensus process.
2 Clinical and Laboratory Standards Institute Setting the standard for quality in medical laboratory testing around the world. The Clinical and Laboratory Standards Institute (CLSI) is a not-for-profit membership organization that brings together the varied perspectives and expertise of the worldwide laboratory community for the advancement of a common cause: to foster excellence in laboratory medicine by developing and implementing medical laboratory standards and guidelines that help laboratories fulfill their responsibilities with efficiency, effectiveness, and global applicability. Consensus Process Consensus the substantial agreement by materially affected, competent, and interested parties is core to the development of all CLSI documents. It does not always connote unanimous agreement, but does mean that the participants in the development of a consensus document have considered and resolved all relevant objections and accept the resulting agreement. Commenting on Documents CLSI documents undergo periodic evaluation and modification to keep pace with advancements in technologies, procedures, methods, and protocols affecting the laboratory or health care. CLSI s consensus process depends on experts who volunteer to serve as contributing authors and/or as participants in the reviewing and commenting process. At the end of each comment period, the committee that developed the document is obligated to review all comments, respond in writing to all substantive comments, and revise the draft document as appropriate. Comments on published CLSI documents are equally essential, and may be submitted by anyone, at any time, on any document. All comments are managed according to the consensus process by a committee of experts. Appeals Process When it is believed that an objection has not been adequately considered and responded to, the process for appeals, documented in the CLSI Standards Development Policies and Processes, is followed. All comments and responses submitted on draft and published documents are retained on file at CLSI and are available upon request. Get Involved Volunteer! Do you use CLSI documents in your workplace? Do you see room for improvement? Would you like to get involved in the revision process? Or maybe you see a need to develop a new document for an emerging technology? CLSI wants to hear from you. We are always looking for volunteers. By donating your time and talents to improve the standards that affect your own work, you will play an active role in improving public health across the globe. For additional information on committee participation or to submit comments, contact CLSI. Clinical and Laboratory Standards Institute 950 West Valley Road, Suite 2500 Wayne, PA USA P: F: standard@clsi.org
3 September NOTE: This document is no longer being reviewed as part of the CLSI consensus process. However, because of its usefulness to segments of the health care community, it is available for its informational content. Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline Abstract Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline (CLSI document C38- A) is intended for persons responsible for the collection and processing of samples used for trace element determinations. The guideline addresses patient preparation, as well as considerations for collection, transport, and processing of specimens by element. Contamination control and quality assurance programs are also discussed. Clinical and Laboratory Standards Institute (CLSI). Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline. CLSI document (ISBN ). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania USA, The Clinical and Laboratory Standards Institute consensus process, which is the mechanism for moving a document through two or more levels of review by the health care community, is an ongoing process. Users should expect revised editions of any given document. Because rapid changes in technology may affect the procedures, methods, and protocols in a standard or guideline, users should replace outdated editions with the current editions of CLSI documents. Current editions are listed in the CLSI catalog and posted on our website at If your organization is not a member and would like to become one, and to request a copy of the catalog, contact us at: Telephone: ; Fax: ; customerservice@clsi.org; Website: NCCLS VOL.17 NO.13 i
4 ISBN September 1997 ISSN Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline Volume 17 Number 13 Gillian Lockitch, M.D., F.R.C.P.C. Jack D. Fassett, Ph.D. Benjamin Gerson, M.D. David E. Nixon, Ph.D. Patrick J. Parsons, Ph.D. John Savory, Ph.D.
5 Copyright 1997 Clinical and Laboratory Standards Institute. Except as stated below, any reproduction of content from a CLSI copyrighted standard, guideline, companion product, or other material requires express written consent from CLSI. All rights reserved. Interested parties may send permission requests to permissions@clsi.org. CLSI hereby grants permission to each individual member or purchaser to make a single reproduction of this publication for use in its laboratory procedure manual at a single site. To request permission to use this publication in any other manner, permissions@clsi.org. Suggested Citation CLSI. Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline. CLSI document. Wayne, PA: Clinical and Laboratory Standards Institute; Proposed Guideline December 1994 Approved Guideline September 1997 ISBN ISSN NCCLS VOL.17 NO.13 iv
6 Contents Abstract... i Committee Membership... v Active Membership...vii Foreword... 1 Introduction Scope Definitions Reporting Units Universal Precautions Contamination Control Background Contamination from Collection Materials Contamination from the Laboratory Environment Testing Contamination in Phlebotomy Tubes Specimen Selection and Collection Protocols Blood, Plasma, or Serum Urine Hair and Nails Tissues Human Milk Stools Specific Elements Aluminum Arsenic Cadmium Chromium Cobalt Copper Iron Lead Manganese Mercury Molybdenum Nickel Selenium Uranium Vanadium Zinc xiii NCCLS VOL.17 NO.13 xi
7 References Bibliography Summary of Comments and Subcommittee Responses Related NCCLS Publications NCCLS VOL.17 NO.13 xii
8 Foreword Preanalytical factors are probably the most important cause of erroneous trace element reference data in biological matrices today. The development of sensitive, specific, and accurate analytical technology at an acceptable cost has moved determination of trace and ultratrace elements from research facilities into a wide range of clinical laboratories. Expanding knowledge of trace element nutrition and toxicity has increased clinical demand for these assays. However, with increased sensitivity and lower limits of detection, the problem of specimen contamination with the element of interest has been magnified. It is vital that the accurately determined trace element concentration reflects the condition of the patient and not contamination introduced during collection and handling. Elements are classified according to the level at which they occur in the body as "trace" (body 4 content 0.01 to 100 µg/g; 10 to 10 µg/l) or "ultratrace" (body content less than 0.01 µg/g; less than 10 µg/l). Earlier attempts to define reference interval data for many of the trace and ultratrace elements provided ranges that were far wider than are now accepted as "normal." This resulted from a lack of awareness that the ubiquity of many trace elements in the environment required special precautions from preanalytical processes through the actual analysis. In this document, the components of specimen collection and preanalytical processing that can contribute to trace element contamination are addressed and protocols for prevention of contamination are described. The trace elements most commonly tested for clinical purposes are individually listed. For each element, the optimal specimen for assessment, preanalytical factors to consider in patient preparation and reference intervals, or concentrations suggesting toxicity or deficiency, are described. Key Words Trace element, ultratrace element, essential elements, specimen collection, contamination control. NCCLS VOL.17 NO.13 xiii
9 1 Introduction Control of Preanalytical Variation in Trace Element Determinations; Approved Guideline It is recognized that much of the pioneering research published in trace element literature is based on erroneously derived reference 1 interval data. The source of the problem was in the lack of recognition of exogenous specimen contamination, which could have occurred at the collection, transport, processing, or analytical stages. Thus, reference intervals for ultratrace elements, such as chromium, or acceptable blood concentrations for toxic elements, such as aluminum, have decreased several fold over the past two decades. The use of increasingly sensitive methods, such as electrothermal atomic absorption spectrometry (ETAAS) or inductively coupled plasma mass spectrometry (ICPMS); increasing interest in ultratrace elements; and the need for precise and accurate analyses for elements such as lead, at extremely low levels, have accentuated the problems of analytical and preanalytical contamination. 2 The intent of this guideline is to (1) develop an awareness of the factors that affect the determination of trace elements in a variety of specimen types, (2) foster communication between the laboratorian performing the test and those responsible for collecting the specimen, and (3) provide definitive protocols for eliminating preanalytical variability. If a specimen is to be sent to a reference trace element laboratory for analysis, it is suggested that the laboratory be consulted in advance for special collection and handling instructions. 2 Scope This guideline provides directions for patient preparation, specimen collection, transport, and processing for analysis of trace elements in biological matrices (i.e., body fluids, such as blood, urine, breast milk, and tissues). Specific reference is made to those elements that are known to be essential or toxic for humans and are, therefore, most likely to be measured for clinical reasons. 2.1 Definitions For the purposes of this document, the following definitions apply: Trace element, n - An element that occurs at a level of 0.01 to 100 µg/g (10 µg/l to µg/l). Ultratrace element, n - Arbitrarily defined as one that occurs at a level of less than 0.01 µg/g (less than 10 µg/l). 1 From the perspective of preventing preanalytical or analytical contamination, classification of an element as trace or ultratrace depends on (1) the expected concentration in the sample matrix and (2) the sensitivity of the analytical method used for that element in a specific matrix. Thus, for example, while aluminum occurs in the serum of healthy persons as an ultratrace element, in a patient on dialysis who has aluminum toxicity, aluminum may be considered a trace element. Tables 1 and 2 categorize clinically important elements found in blood and urine. Essential element, n - That a specific trace element is consistently detectable in human tissues or fluids does not imply that it is essential. Many trace elements are so ubiquitous in the environment (e.g., Al, Pb) that it is hardly surprising that they are "normally" found in human tissues and fluids. As analytical detection limits are improved further, other rare elements could also be detected at ultratrace levels. The criteria used to establish essentiality in other areas of life 3,4 science, e.g., plant growth can be adapted, with some qualification, to the animal kingdom. An element is considered essential (a) if without it, the species cannot achieve normal, healthy growth or complete its normal life cycle and (b) if it is part of a molecule of an essential constituent or metabolite. In addition, the element must be specific and not be replaceable by another, and it must exert its effect, directly on growth or metabolism NCCLS VOL.17 NO.13 1
10 and not by some indirect effect, such as International d'unités (SI), these do not antagonism of another element present at always coincide with toxic levels. the units recommended by the International Union of Pure and Applied Chemistry (IUPAC) Based on these criteria, a number of trace and by the International Federation of Clinical elements have been clearly identified as Chemistry (IFCC) for reporting results of essential for normal, healthy growth in clinical laboratory measurements. Because SI humans. While there may be some elements units are used worldwide but there is not yet that are not universally accepted, due to the a consensus in the United States, NCCLS paucity of data supporting claims for documents include the IUPAC/IFCC essentiality, they may be considered recommended units of volume (L) and borderline candidates. The concept of substance (molecular) concentration (mol/l) in essentiality, and arguments over accepted parentheses, where appropriate. In this 5 criteria, are discussed in detail by Davies. document, wherever possible, we use conventional mass/volume (e.g., µg/dl) units, Tables 1 and 2 list those trace and ultra trace or mass/mass (µg/g) units, to describe normal elements, which are the focus of this and abnormal concentration ranges, followed document, in alphabetical order. Some are by IUPAC/IFCC-recommended equivalents in considered essential for normal, healthy parentheses. growth in humans, others are borderline. Several are nonessential toxic elements. Results for trace elements in urine can be Elements in Groups I, II, and VII (i.e. the alkali calculated as an excretion rate if a timed and alkaline earth metals, and the halogens) specimen is obtained. Usually, such results are not included, although some of these are are reported as µg (or mg) element per 24 essential. hours, or as Fg element per g (urinary) 2.2 Reporting Units creatinine (see Section 5.2.2). In the analytical laboratory, it is commonplace to use "bench" units, such as parts-per-million (ppm) A variety of units are currently used throughout the United States for reporting trace element concentrations in human body fluids and tissues (see Table 3). Although NCCLS documents generally use units that are fully acceptable within the Système or parts-per-billion (ppb) for concentration. These units should not be used to report trace element concentrations in clinical specimens. They are confusing and ambiguous to nonanalytical personnel, since they do not indicate if the concentration is based on a mass/volume or a mass/mass ratio. NCCLS VOL.17 NO.13 2
11 Related NCCLS Publications 2 C3-A3 GP16-A H3-A3 H4-A3 H14-A2 H18-A H24-T H31-P H35-T Preparation and Testing of Reagent Water in the Clinical Laboratory Third Edition; Approved Guideline (1997). C3-A3 addresses the requirements for purified water, methods for monitoring quality and testing for specific contaminants, and systemdesign considerations. Routine Urinalysis and Collection, Transportation, and Preservation of Urine Specimens; Approved Guideline (1995). GP16-A discusses procedures that address materials and equipment, macroscopic examinations, clinical analyses, and microscopic evaluations. Also, the document offers information on collection, specimen criteria, and storage. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture Third Edition; Approved Standard (1991). H3-A3 discusses methods of collection, as well as a training program for increasing integrity and for minimizing error. Procedures for the Collection of Diagnostic Blood Specimens by Skin Puncture Third Edition; Approved Standard (1991). H4-A3 describes proper collection techniques and discusses hazards to patients. Devices for Collection of Skin Puncture Blood Specimens Second Edition; Approved Guideline (1990). H14-A2 gives specifications of disposable devices for collecting, processing, and transferring diagnostic blood specimens obtained by skin puncture. Procedures for the Handling and Processing of Blood Specimens; Approved Guideline (1990). H18-A addresses the multiple factors involved in the handling and processing of specimens that can introduce imprecision or systematic bias into results. Additives to Blood Collection Devices: Heparin; Tentative Standard (1988). H24-T contains a technical description of heparin compounds used in devices. The document also addresses evaluation of the suitability of heparin-containing devices and the quantitation of heparin. Collection Containers for Specimens for Toxicological Analysis; Proposed Guideline (1986). H31-P discusses the recommended toxicology/drug monitoring requirements. Additives to Blood Collection Devices: Edta; Tentative Standard (1992). H35-T offers a technical description of ethylenediaminetetra-acetic acid (EDTA) and its use in blood collection products. 2 Proposed- and tentative-level documents are being advanced through the NCCLS consensus process; therefore, readers should refer to the most recent editions. NCCLS VOL.17 NO.13 30
12 E PL Explore the Latest Offerings From CLSI! As we continue to set the global standard for quality in laboratory testing, we are adding products and programs to bring even more value to our members and customers. M By becoming a CLSI member, your laboratory will join 1,600+ other influential organizations all working together to further CLSI s efforts to improve health care outcomes. You can play an active role in raising global laboratory testing standards in your laboratory, and around the world. SA Find out which membership option is best for you at Find what your laboratory needs to succeed! CLSI U provides convenient, cost-effective continuing education and training resources to help you advance your professional development. We have a variety of easy-to-use, online educational resources that make elearning stress-free and convenient for you and your staff. See our current educational offerings at When laboratory testing quality is critical, standards are needed and there is no time to waste. eclipse Ultimate Access, our cloud-based online portal of the complete library of CLSI standards, makes it easy to quickly find the CLSI resources you need. Learn more and purchase eclipse at clsi.org/eclipse. For more information, visit today.
13 950 West Valley Road, Suite 2500, Wayne, PA USA P: Toll Free (US): F: ISBN E:
SAMPLE. Clinical and Laboratory Standards Institute Advancing Quality in Health Care Testing
 Vol. 32 No. 12 Replaces EP27-P Vol. 29 No. 16 How to Construct and Interpret an Error Grid for Quantitative Diagnostic Assays; Approved Guideline This guideline describes what an error grid is, why it
Vol. 32 No. 12 Replaces EP27-P Vol. 29 No. 16 How to Construct and Interpret an Error Grid for Quantitative Diagnostic Assays; Approved Guideline This guideline describes what an error grid is, why it
SAMPLE I/LA20. 3rd Edition
 3rd Edition I/LA20 Analytical Performance Characteristics, Quality Assurance, and Clinical Utility of Immunological Assays for Human Immunoglobulin E Antibodies of Defined Allergen Specificities This report
3rd Edition I/LA20 Analytical Performance Characteristics, Quality Assurance, and Clinical Utility of Immunological Assays for Human Immunoglobulin E Antibodies of Defined Allergen Specificities This report
SAMPLE. Reference and Selected Procedures for the Quantitative Determination of Hemoglobin in Blood; Approved Standard Third Edition
 December 2000 Reference and Selected Procedures for the Quantitative Determination of Hemoglobin in Blood; Approved Standard Third Edition This document describes the principle, materials, and procedure
December 2000 Reference and Selected Procedures for the Quantitative Determination of Hemoglobin in Blood; Approved Standard Third Edition This document describes the principle, materials, and procedure
C30-A2 ISBN ISSN Point-of-Care Blood Glucose Testing in Acute and Chronic Care Facilities; Approved Guideline Second Edition
 ISBN 1-56238-471-6 ISSN 0273-3099 Point-of-Care Blood Glucose Testing in Acute and Chronic Care Facilities; Approved Guideline Second Edition Volume 22 Number 17 David B. Sacks, M.D., Chairholder Patricia
ISBN 1-56238-471-6 ISSN 0273-3099 Point-of-Care Blood Glucose Testing in Acute and Chronic Care Facilities; Approved Guideline Second Edition Volume 22 Number 17 David B. Sacks, M.D., Chairholder Patricia
SAMPLE. Laboratory Detection and Identification of Mycobacteria; Approved Guideline
 May 2008 Laboratory Detection and Identification of Mycobacteria; Approved Guideline This document provides guidance to clinical mycobacteriology laboratories on the most optimum approach for the diagnosis
May 2008 Laboratory Detection and Identification of Mycobacteria; Approved Guideline This document provides guidance to clinical mycobacteriology laboratories on the most optimum approach for the diagnosis
SAMPLE. Design and Validation of Immunoassays for Assessment of Human Allergenicity of New Biotherapeutic Drugs; Approved Guideline
 June 2011 Design and Validation of Immunoassays for Assessment of Human Allergenicity of New Biotherapeutic Drugs; Approved Guideline This document provides guidance for the design, validation, analytical
June 2011 Design and Validation of Immunoassays for Assessment of Human Allergenicity of New Biotherapeutic Drugs; Approved Guideline This document provides guidance for the design, validation, analytical
SAMPLE. Glucose Monitoring in Settings Without Laboratory Support
 POCT13c Glucose Monitoring in Settings Without Laboratory Support This guideline focuses on performance of point-of-care glucose monitoring systems, with an emphasis on safety practices, quality control,
POCT13c Glucose Monitoring in Settings Without Laboratory Support This guideline focuses on performance of point-of-care glucose monitoring systems, with an emphasis on safety practices, quality control,
TOXIC AND ESSENTIAL ELEMENTS
 TOXIC AND ESSENTIAL ELEMENTS Assessment of Key Minerals and Harmful Metals Whole Blood, Red Blood Cell and Serum Elements Urine Toxic and Essential Elements Creatinine Clearance Hair Elements Fecal Metals
TOXIC AND ESSENTIAL ELEMENTS Assessment of Key Minerals and Harmful Metals Whole Blood, Red Blood Cell and Serum Elements Urine Toxic and Essential Elements Creatinine Clearance Hair Elements Fecal Metals
SAMPLE. Protection of Laboratory Workers From Occupationally Acquired Infections; Approved Guideline Fourth Edition
 May 2014 Protection of Laboratory Workers From Occupationally Acquired Infections; Approved Guideline Fourth Edition Based on US regulations, this document provides guidance on the risk of transmission
May 2014 Protection of Laboratory Workers From Occupationally Acquired Infections; Approved Guideline Fourth Edition Based on US regulations, this document provides guidance on the risk of transmission
TRACE ELEMENTS IN SERUM
 TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 05 November th, 05 Event #, 05 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services,
TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 05 November th, 05 Event #, 05 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services,
TRACE ELEMENTS IN SERUM
 TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 0 March th, 0 Event #, 0 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services,
TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 0 March th, 0 Event #, 0 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services,
Bottom Ash Data Week 8
 Bottom Ash Data 2019 Week 8 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 6, 2019. The data represents bottom ash composite results for week
Bottom Ash Data 2019 Week 8 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 6, 2019. The data represents bottom ash composite results for week
Multi Analyte Custom Grade Solution
 1.0 ACCREDITATION / REGISTRATION INORGANIC VENTURES is accredited to ISO Guide 34, "General Requirements for the Competence of Reference Material Producers" and ISO/IEC 17025, "General Requirements for
1.0 ACCREDITATION / REGISTRATION INORGANIC VENTURES is accredited to ISO Guide 34, "General Requirements for the Competence of Reference Material Producers" and ISO/IEC 17025, "General Requirements for
TRACE ELEMENTS IN URINE. Event #1, 2010
 TRACE ELEMENTS IN URINE Event #, 00 March 6, 00 Event #, 00 Urine Arsenic The source of the test materials is human urine obtained from donor volunteers with informed consent. Urine was collected into
TRACE ELEMENTS IN URINE Event #, 00 March 6, 00 Event #, 00 Urine Arsenic The source of the test materials is human urine obtained from donor volunteers with informed consent. Urine was collected into
Bottom Ash Data Week 38
 Bottom Ash Data 2018 Week 38 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 4, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 38 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 4, 2018. The data represents bottom ash composite results for
TRACE ELEMENTS IN SERUM. Event #3, 2011
 TRACE ELEMENTS IN SERUM Event #, 0 November 5, 0 Event #, 0 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services, Inc. The units were tested
TRACE ELEMENTS IN SERUM Event #, 0 November 5, 0 Event #, 0 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services, Inc. The units were tested
CERTIFICATE OF ANALYSIS. tel: fax:
 CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
Bottom Ash Data Week 49
 Bottom Ash Data 2018 Week 49 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on December 20, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 49 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on December 20, 2018. The data represents bottom ash composite results for
TRACE ELEMENTS IN SERUM
 TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 0 July 5 th, 0 Event #, 0 Serum Aluminum Serum materials were obtained from the Dutch EQA (External Quality Assessment) scheme SKML (Stichting
TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 0 July 5 th, 0 Event #, 0 Serum Aluminum Serum materials were obtained from the Dutch EQA (External Quality Assessment) scheme SKML (Stichting
TRACE ELEMENTS IN SERUM
 TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 0 November 6 th, 0 Event #, 0 Serum Aluminum Serum materials were obtained from the Dutch EQA (External Quality Assessment) scheme SKML (Stichting
TRACE ELEMENTS IN SERUM Proficiency Test Report Event #, 0 November 6 th, 0 Event #, 0 Serum Aluminum Serum materials were obtained from the Dutch EQA (External Quality Assessment) scheme SKML (Stichting
Bottom Ash Data Week 12
 Bottom Ash Data 2019 Week 12 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on April 11, 2019. The data represents bottom ash composite results for
Bottom Ash Data 2019 Week 12 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on April 11, 2019. The data represents bottom ash composite results for
MULTI-COMPONENT ANALYSIS OF HEAVY METALS
 MULTI-COMPONENT ANALYSIS OF HEAVY METALS in Various Foods Using Shimadzu s AA, ICP, ICPMS & EDX HEAVY METALS TESTING HEAVY METALS POSE A SIGNIFICANT THREAT TO HUMAN HEALTH The U.S. Food and Drug Administration
MULTI-COMPONENT ANALYSIS OF HEAVY METALS in Various Foods Using Shimadzu s AA, ICP, ICPMS & EDX HEAVY METALS TESTING HEAVY METALS POSE A SIGNIFICANT THREAT TO HUMAN HEALTH The U.S. Food and Drug Administration
TRACE ELEMENTS IN SERUM. Event #2, 2010
 TRACE ELEMENTS IN SERUM Event #, 00 June 8, 00 Event #, 00 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services, Inc. The units were tested
TRACE ELEMENTS IN SERUM Event #, 00 June 8, 00 Event #, 00 Serum Aluminum The test materials for serum Al were prepared from human serum obtained from Tennessee Blood Services, Inc. The units were tested
Bottom Ash Data Week 30
 Bottom Ash Data 2018 Week 30 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on August 14, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 30 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on August 14, 2018. The data represents bottom ash composite results for
DETERMINATION OF TRACE ELEMENTS IN WATERS AND WASTES BY INDUCTIVELY COUPLED PLASMA - MASS SPECTROMETRY EPA Method (Revision 5.
 DETERMINATION OF TRACE ELEMENTS IN WATERS AND WASTES BY INDUCTIVELY COUPLED PLASMA - MASS SPECTROMETRY EPA Method 200.8 (Revision 5.4, 1994) Table 1A. Summary of Holding Times and Preservation for Trace
DETERMINATION OF TRACE ELEMENTS IN WATERS AND WASTES BY INDUCTIVELY COUPLED PLASMA - MASS SPECTROMETRY EPA Method 200.8 (Revision 5.4, 1994) Table 1A. Summary of Holding Times and Preservation for Trace
water & dialysate testing services
 water & dialysate testing services Spectra Laboratories, the leading provider of renal-specific testing services, offers clinicians comprehensive water and dialysate testing to help monitor the safety
water & dialysate testing services Spectra Laboratories, the leading provider of renal-specific testing services, offers clinicians comprehensive water and dialysate testing to help monitor the safety
Greg Patterson C.C.A. President A&L Canada Laboratories
 Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Compost Quality Consumer of the Future Population dynamics - more and more people involved in use of compost Better educated on what is expected
Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Compost Quality Consumer of the Future Population dynamics - more and more people involved in use of compost Better educated on what is expected
Bottom Ash Data Week 37
 Bottom Ash Data 2018 Week 37 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 2, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 37 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 2, 2018. The data represents bottom ash composite results for
Bottom Ash Data Week 9
 Bottom Ash Data 2018 Week 9 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 26, 2018. The data represents bottom ash composite results for week
Bottom Ash Data 2018 Week 9 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on March 26, 2018. The data represents bottom ash composite results for week
Bottom Ash Data Week 17
 Bottom Ash Data 2018 Week 17 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on May 16, 2018. The data represents bottom ash composite results for week
Bottom Ash Data 2018 Week 17 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on May 16, 2018. The data represents bottom ash composite results for week
Elemental analysis in clinical practice
 Elemental analysis in clinical practice Nicholas J Miller FRCPath, Laboratory Director, Biolab Medical Unit, ThermoFisher summer symposium 7 th June 2011, QEII Conference Centre Nutritional Elements Macro
Elemental analysis in clinical practice Nicholas J Miller FRCPath, Laboratory Director, Biolab Medical Unit, ThermoFisher summer symposium 7 th June 2011, QEII Conference Centre Nutritional Elements Macro
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 15197 First edition 2003-05-01 In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus Systèmes d'essais
INTERNATIONAL STANDARD ISO 15197 First edition 2003-05-01 In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus Systèmes d'essais
Grinder-Pedersen L, Rasmussen SE, Bugel S, Jorgensen LV, Dragsted LO, Gundersen V, Sandstrom B.
 1. Organic vs. Commercially Grown Fruits and Vegetables. 2. Effect of diets based on foods from conventional versus organic production on intake and excretion of flavonoids and markers of antioxidative
1. Organic vs. Commercially Grown Fruits and Vegetables. 2. Effect of diets based on foods from conventional versus organic production on intake and excretion of flavonoids and markers of antioxidative
THE NATIONAL ACADEMIES
 THE NATIONAL ACADEMIES NATIONAL ACADEMY OF SCIENCES INSTITUTE OF MEDICINE NATIONAL ACADEMY OF ENGINEERING NATIONAL RESEARCH COUNCIL DIVISION ON EARTH AND LIFE STUDIES BOARD ON AGRICULTURE AND NATURAL RESOURCES
THE NATIONAL ACADEMIES NATIONAL ACADEMY OF SCIENCES INSTITUTE OF MEDICINE NATIONAL ACADEMY OF ENGINEERING NATIONAL RESEARCH COUNCIL DIVISION ON EARTH AND LIFE STUDIES BOARD ON AGRICULTURE AND NATURAL RESOURCES
TRACE ELEMENTS ANO IRON IN HUMAN METABOLISM
 TRACE ELEMENTS ANO IRON IN HUMAN METABOLISM TOPICS IN HEMATOLOGV Series Editor: Maxwell M. Wintrobe, M.D. University of Utah, Salt Lake City THE RESPIRATORY FUNCTIONS OF BLOOD Lars Garby, M.D. and Jerry
TRACE ELEMENTS ANO IRON IN HUMAN METABOLISM TOPICS IN HEMATOLOGV Series Editor: Maxwell M. Wintrobe, M.D. University of Utah, Salt Lake City THE RESPIRATORY FUNCTIONS OF BLOOD Lars Garby, M.D. and Jerry
Test Report No.T JP Date: FEB 24, 2017 Page 1 of 6
 Test Report No.T51710190071JP Date: FEB 24, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Test Report No.T51710190071JP Date: FEB 24, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
of human hair and nails. Part I. Analytical methodology, Sci. Tot. Environ. 2000, 250/1-3,
 Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
AAFCO Check Sample 2015 Minerals Program. A Targeted and Engineered Concentration Program
 AAFC Check Sample 215 Minerals Program A Targeted and Engineered Concentration Program Approach: 16 Target Elements This quarterly program focuses on minerals of health and toxicological importance in
AAFC Check Sample 215 Minerals Program A Targeted and Engineered Concentration Program Approach: 16 Target Elements This quarterly program focuses on minerals of health and toxicological importance in
Blood collection: peripheral venipuncture v1.0
 Resistance Network (WWARN). The original template and several other procedures are available on our website www.wwarn.org. If you download and adjust this document to suit your study design, please retain
Resistance Network (WWARN). The original template and several other procedures are available on our website www.wwarn.org. If you download and adjust this document to suit your study design, please retain
ESSENTIAL TRACE ELEMENTS PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY
 ESSENTIAL TRACE ELEMENTS PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Back to Basics: Elements Remember that all matter is made of elements An element is a substance in its simplest
ESSENTIAL TRACE ELEMENTS PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Back to Basics: Elements Remember that all matter is made of elements An element is a substance in its simplest
GB Translated English of Chinese Standard: GB NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: GB5009.268-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.268-2016
Translated English of Chinese Standard: GB5009.268-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.268-2016
Bottom Ash Data Week 40
 Bottom Ash Data 2018 Week 40 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 25, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 40 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 25, 2018. The data represents bottom ash composite results for
GB/T Translated English of Chinese Standard: GB/T
 Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
Product Stewardship Information Sheet CH350LN
 Product Stewardship Information Sheet CH350LN Contents Product Manufacturer... 2 Food Contact... 2 European Union (EU) Food Contact... 2 US Food and Drug Administration (FDA)... 2 Canada Health Protection
Product Stewardship Information Sheet CH350LN Contents Product Manufacturer... 2 Food Contact... 2 European Union (EU) Food Contact... 2 US Food and Drug Administration (FDA)... 2 Canada Health Protection
Analyte Proficiency From All Labs # Analytes: 26 Sample # Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016
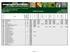 Analyte Proficiency From All Labs # Analytes: 26 Sample # 160511 Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016 Analyte Code Analyte # Tests Submitted # Tests in Robust Calculations
Analyte Proficiency From All Labs # Analytes: 26 Sample # 160511 Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016 Analyte Code Analyte # Tests Submitted # Tests in Robust Calculations
Bottom Ash Data Week 1
 Bottom Ash Data 2019 Week 1 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on January 17, 2019. The data represents bottom ash composite results for
Bottom Ash Data 2019 Week 1 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on January 17, 2019. The data represents bottom ash composite results for
Listing of Color Additives Exempt from Certification; Synthetic Iron Oxide
 This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
Product Stewardship Information Sheet CH200LN-02
 Product Stewardship Information Sheet CH200LN-02 Contents Product Manufacturer... 2 Food Contact... 2 European Union (EU) Food Contact... 2 US Food and Drug Administration (FDA)... 2 Canada Health Protection
Product Stewardship Information Sheet CH200LN-02 Contents Product Manufacturer... 2 Food Contact... 2 European Union (EU) Food Contact... 2 US Food and Drug Administration (FDA)... 2 Canada Health Protection
Test Report No.T JP Date: FEB 22, 2017 Page 1 of 6
 Test Report No.T51710190051JP Date: FEB 22, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Test Report No.T51710190051JP Date: FEB 22, 2017 Page 1 of 6 JIAN PLASTIC & METAL PRODUCTS LTD NO.3-1, SOUTH GAOBU BLVD, GAOBU TOWN, DONGGUAN CITY, GUANGDONG PROVINCE, CHINA,ZIP CODE:523278 The following
Specimen Collection Policies
 Specimen Collection Policies Purpose Great River Medical Center Laboratory is a hospital-based and outreach laboratory with specific standards of excellence. To best serve our patients, all specimens will
Specimen Collection Policies Purpose Great River Medical Center Laboratory is a hospital-based and outreach laboratory with specific standards of excellence. To best serve our patients, all specimens will
Traditional Chinese Medicine Determination of heavy metals in herbal medicines used in Traditional Chinese Medicine
 INTERNATIONAL STANDARD ISO 18664 First edition 2015-08-01 Traditional Chinese Medicine Determination of heavy metals in herbal medicines used in Traditional Chinese Medicine Médecine traditionnelle chinoise
INTERNATIONAL STANDARD ISO 18664 First edition 2015-08-01 Traditional Chinese Medicine Determination of heavy metals in herbal medicines used in Traditional Chinese Medicine Médecine traditionnelle chinoise
Scientific Working Group on Digital Evidence
 Disclaimer: As a condition to the use of this document and the information contained therein, the SWGDE requests notification by e-mail before or contemporaneous to the introduction of this document, or
Disclaimer: As a condition to the use of this document and the information contained therein, the SWGDE requests notification by e-mail before or contemporaneous to the introduction of this document, or
2 Codex Standard
 1 Codex Standard 108-1981 STANDARD FOR NATURAL MINERAL WATERS 1. SCOPE Adopted 1981. Amendment 2001, 2011. Revisions 1997, 2008. CODEX STAN 108-1981 This standard applies to all packaged natural mineral
1 Codex Standard 108-1981 STANDARD FOR NATURAL MINERAL WATERS 1. SCOPE Adopted 1981. Amendment 2001, 2011. Revisions 1997, 2008. CODEX STAN 108-1981 This standard applies to all packaged natural mineral
Committed to Environment, Health, & Safety
 Committed to Environment, Health, & Safety Environment, Health, and Safety Management System and Policy of W. R. Grace & Co. November 8, 2018 The Grace Environment, Health, and Safety Management System,
Committed to Environment, Health, & Safety Environment, Health, and Safety Management System and Policy of W. R. Grace & Co. November 8, 2018 The Grace Environment, Health, and Safety Management System,
Model 380B DNA Synthesizer Version 1.1
 Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
Safety Data Sheet Product Name: Heparin / PF4 Serum Panel - For In Vitro Diagnostic Use Only
 Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
SAFETY DATA SHEET. Phone: Phone: Emergency Phone:
 SAFETY DATA SHEET SECTION 1. IDENTIFICATION Catalog #: 60-9549-0 Product Name: HIV Rapid Test Control Pack Synonyms: The Chembio HIV Reactive/Nonreactive Controls are quality control reagents for use with
SAFETY DATA SHEET SECTION 1. IDENTIFICATION Catalog #: 60-9549-0 Product Name: HIV Rapid Test Control Pack Synonyms: The Chembio HIV Reactive/Nonreactive Controls are quality control reagents for use with
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 12891-2 Second edition 2014-09-15 Retrieval and analysis of surgical implants Part 2: Analysis of retrieved surgical implants Retrait et analyse des implants chirurgicaux Partie
INTERNATIONAL STANDARD ISO 12891-2 Second edition 2014-09-15 Retrieval and analysis of surgical implants Part 2: Analysis of retrieved surgical implants Retrait et analyse des implants chirurgicaux Partie
FDM Training Program; Mod 7 * The Biochemical Effects of Iodine Wayne L. Sodano, D.C., D.A.B.C.I. & Ron Grisanti, D.C., D.A.B.C.O., M.S.
 Functional Diagnostic Medicine Training Program Module 7 * FMDT 561D The Biochemical Effects of Iodine (Review of Nutrient Element Status Testing) By Wayne L. Sodano, D.C., D.A.B.C.I. & Ron Grisanti, D.C.,
Functional Diagnostic Medicine Training Program Module 7 * FMDT 561D The Biochemical Effects of Iodine (Review of Nutrient Element Status Testing) By Wayne L. Sodano, D.C., D.A.B.C.I. & Ron Grisanti, D.C.,
OFFICIAL METHODS FOR CHEMICAL ELEMENTS IN FOOD OF ANIMAL ORIGIN
 EN 13804 Performance criteria, general considerations and sample preparation 2002 EN 13805 Pressure digestion 2002 EN 13806 Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS)
EN 13804 Performance criteria, general considerations and sample preparation 2002 EN 13805 Pressure digestion 2002 EN 13806 Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS)
Analyses no.: Telephone: Fax: Telephone: Fax:
 Analysis order for biogas Analyses no.: Date of receipt: Sampling vessel: Contractor = Invoice recipient Copy of the test report for: LUFA Customer no. (if available) LUFA Customer no. (if available) Name,
Analysis order for biogas Analyses no.: Date of receipt: Sampling vessel: Contractor = Invoice recipient Copy of the test report for: LUFA Customer no. (if available) LUFA Customer no. (if available) Name,
TERMS AND CONDITIONS NATIONAL GOOD LABORATORY PRACTICE (GLP) COMPLIANCE MONITORING AUTHORITY
 TERMS AND CONDITIONS OF NATIONAL GOOD LABORATORY PRACTICE (GLP) COMPLIANCE MONITORING AUTHORITY FOR OBTAINING AND MAINTAINING ITS GLP CERTIFICATION BY A TEST FACILITY Document No.GLP-101 Version/Issue
TERMS AND CONDITIONS OF NATIONAL GOOD LABORATORY PRACTICE (GLP) COMPLIANCE MONITORING AUTHORITY FOR OBTAINING AND MAINTAINING ITS GLP CERTIFICATION BY A TEST FACILITY Document No.GLP-101 Version/Issue
DRYDEN AQUA LTD Safety Data Sheet 18
 DRYDEN AQUA LTD Safety Data Sheet 18 According to Regulation (EC) No. 1907/2006 Date of compilation: 04/12/12 Print date: 03/02/13 Section 1 Identification of the substance/mixture and of the company/undertaking
DRYDEN AQUA LTD Safety Data Sheet 18 According to Regulation (EC) No. 1907/2006 Date of compilation: 04/12/12 Print date: 03/02/13 Section 1 Identification of the substance/mixture and of the company/undertaking
GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON THE CONDUCT OF GCP INSPECTIONS BY COMPETENT AUTHORITIES OF THE DIFFERENT MEMBER STATES
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 5 November 2008 ENTR/F/2/SF D(2008) 34957 GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 5 November 2008 ENTR/F/2/SF D(2008) 34957 GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON
Candidates sitting for the examination for licensure purposes in California should study and be familiar with the following test plan:
 Candidates sitting for the examination for licensure purposes in California should study and be familiar with the following test plan: NHA Certified Phlebotomy Technician (CPT) CA-Specific Detailed Test
Candidates sitting for the examination for licensure purposes in California should study and be familiar with the following test plan: NHA Certified Phlebotomy Technician (CPT) CA-Specific Detailed Test
EDXRF APPLICATION NOTE
 EDXRF APPLICATION NOTE ANALYSIS OF ANIMAL FEEDS # 1279 SCOPE The analysis of finished animal feeds and premixes is demonstrated using EDXRF with indirect excitation and Fundamental Parameters software,
EDXRF APPLICATION NOTE ANALYSIS OF ANIMAL FEEDS # 1279 SCOPE The analysis of finished animal feeds and premixes is demonstrated using EDXRF with indirect excitation and Fundamental Parameters software,
Toxicology Blood Lead Comprehensive Testing
 Clinical Laboratory s of Practice Blood Lead Comprehensive Testing The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where
Clinical Laboratory s of Practice Blood Lead Comprehensive Testing The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where
ISLAND GOLD TOXIC SUBSTANCE REDUCTION PLAN SUMMARY SUPPORTING VARIOUS TOXIC SUBSTANCE REDUCTION PLANS
 ISLAND GOLD TOXIC SUBSTANCE REDUCTION PLAN SUMMARY SUPPORTING VARIOUS TOXIC SUBSTANCE REDUCTION PLANS TOXIC SUBSTANCE REDUCTION PLAN SUMMARY This Toxic Substance Reduction Plan Summary has been prepared
ISLAND GOLD TOXIC SUBSTANCE REDUCTION PLAN SUMMARY SUPPORTING VARIOUS TOXIC SUBSTANCE REDUCTION PLANS TOXIC SUBSTANCE REDUCTION PLAN SUMMARY This Toxic Substance Reduction Plan Summary has been prepared
DRAFT UGANDA STANDARD
 DRAFT UGANDA STANDARD DUS 2037 First Edition 2018-mm-dd Kombucha Specification Reference number DUS 2037: 2018 UNBS 2018 DUS 2030: 2018 Compliance with this standard does not, of itself confer immunity
DRAFT UGANDA STANDARD DUS 2037 First Edition 2018-mm-dd Kombucha Specification Reference number DUS 2037: 2018 UNBS 2018 DUS 2030: 2018 Compliance with this standard does not, of itself confer immunity
2.T7. Explain the phlebotomy procedure to be performed to the patient. 1.C. Review the requisition for testing
 2011 to 2016 CPT Test Plan Crosswalk Crosswalk Section: The following bridges tasks on the 2011 CPT test plan with task statements on the 2016 CPT test plan. 2011 NHA Test TASK DESCRIPTION 2016 NHA TEST
2011 to 2016 CPT Test Plan Crosswalk Crosswalk Section: The following bridges tasks on the 2011 CPT test plan with task statements on the 2016 CPT test plan. 2011 NHA Test TASK DESCRIPTION 2016 NHA TEST
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series DESCRIPTION OF THE MOST IMPORTANT PROPERTIES AND THE POSSIBLE VARIATIONS AND TOLERANCES
 USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210
 a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 for SMEs a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 Copyright protected document All rights reserved.
a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 for SMEs a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 Copyright protected document All rights reserved.
N.O.S. (Corrosive) Group Standard HSR002618
 N.O.S. (Corrosive) Group Standard 2017 - HSR002618 GROUP STANDARD UNDER THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 N.O.S. (Corrosive) Group Standard 2017 N.O.S. (Corrosive) Group Standard 2017
N.O.S. (Corrosive) Group Standard 2017 - HSR002618 GROUP STANDARD UNDER THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 N.O.S. (Corrosive) Group Standard 2017 N.O.S. (Corrosive) Group Standard 2017
General Chapter/Section: <232> Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No.
 General Chapter/Section: Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No. of Commenters: 18 Editorial changes suggested by commenters have been reviewed by
General Chapter/Section: Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No. of Commenters: 18 Editorial changes suggested by commenters have been reviewed by
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 18645 First edition 2016-03-15 Fertilizers and soil conditioners Water soluble fertilizer General requirements Matières fertilisantes Engrais soluble dans l eau Exigences générales
INTERNATIONAL STANDARD ISO 18645 First edition 2016-03-15 Fertilizers and soil conditioners Water soluble fertilizer General requirements Matières fertilisantes Engrais soluble dans l eau Exigences générales
232 ELEMENTAL IMPURITIES LIMITS
 BRIEFING 232 Elemental Impurities Limits, USP 39 page 268. This chapter is being revised to address comments received and to further align this chapter with ICH Q3D. USP s Elemental Impurities Expert Panel
BRIEFING 232 Elemental Impurities Limits, USP 39 page 268. This chapter is being revised to address comments received and to further align this chapter with ICH Q3D. USP s Elemental Impurities Expert Panel
Version No.: 5.0 TITLE: SDS FOR FECAL OCCULT BLOOD TEST KIT T1-CK SERIES Page 1 of 5
 TITLE: SDS FOR FECAL OCCULT BLOOD TEST KIT T1-CK SERIES Page 1 of 5 Section 1: Product and Company Identification Product Name: Catalog Number: Hemosure Immunological Fecal Occult Blood Test Cassette Kit
TITLE: SDS FOR FECAL OCCULT BLOOD TEST KIT T1-CK SERIES Page 1 of 5 Section 1: Product and Company Identification Product Name: Catalog Number: Hemosure Immunological Fecal Occult Blood Test Cassette Kit
Toxicology Blood Lead Comprehensive Testing
 Clinical Laboratory s of Practice Blood Lead Comprehensive Testing The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where
Clinical Laboratory s of Practice Blood Lead Comprehensive Testing The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where
TANZANIA BUREAU OF STANDARDS
 TBS / AFDC 09 (4842) P3 Pig premixes Specification DRAFT TANZANIA STANDARD TANZANIA BUREAU OF STANDARDS Pig premixes Specification TBS/AFDC 09 (4842) P3 0 FOREWORD This Tanzania standard aimed to control
TBS / AFDC 09 (4842) P3 Pig premixes Specification DRAFT TANZANIA STANDARD TANZANIA BUREAU OF STANDARDS Pig premixes Specification TBS/AFDC 09 (4842) P3 0 FOREWORD This Tanzania standard aimed to control
Quality Management in PCR. Sioban SenGupta UCL Centre for PGD UK
 Quality Management in PCR Sioban SenGupta UCL Centre for PGD UK Outline 5.2 Accommodation & environmental conditions 5.3 Laboratory equipment 5.4 Pre-examination procedures 5.5 Examination procedures 5.6
Quality Management in PCR Sioban SenGupta UCL Centre for PGD UK Outline 5.2 Accommodation & environmental conditions 5.3 Laboratory equipment 5.4 Pre-examination procedures 5.5 Examination procedures 5.6
Agenda. 15 Dec
 Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Analytical methods and laboratory choice for food composition, an introduction
 Analytical methods and laboratory choice for food composition, an introduction Paul Hulshof, Global challenges Research Fund Foodomp workshop, Pretoria, 5-9 Feb, 2018 General Reasons for analyzing food
Analytical methods and laboratory choice for food composition, an introduction Paul Hulshof, Global challenges Research Fund Foodomp workshop, Pretoria, 5-9 Feb, 2018 General Reasons for analyzing food
GLUH PRINCIPLE REF B ANNUAL REVIEW Reviewed by. Date. Date INTENDED USE
 SYNCHRON System(s) Chemistry Information Sheet Copyright 2014 Beckman Coulter, Inc. GLUH Glucose REF B24985 For In Vitro Diagnostic Use Rx Only ANNUAL REVIEW Reviewed by Date Reviewed by Date PRINCIPLE
SYNCHRON System(s) Chemistry Information Sheet Copyright 2014 Beckman Coulter, Inc. GLUH Glucose REF B24985 For In Vitro Diagnostic Use Rx Only ANNUAL REVIEW Reviewed by Date Reviewed by Date PRINCIPLE
STANDARD METHODS FOR CHEMICAL ELEMENTS IN FOOD OF ANIMAL ORIGIN
 EN 13804 Performance criteria, general considerations and sample preparation 2013 EN 13805 Pressure digestion 2014 EN 13806 Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS)
EN 13804 Performance criteria, general considerations and sample preparation 2013 EN 13805 Pressure digestion 2014 EN 13806 Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS)
Toxicology Blood Lead Comprehensive Testing
 Blood Lead Comprehensive Testing The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where applicable to the scope of services
Blood Lead Comprehensive Testing The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where applicable to the scope of services
DRAFT UGANDA STANDARD
 DRAFT UGANDA STANDARD DUS 1635 First Edition 2016-mm-dd Shea butter Specification Reference number DUS 1635: 2016 UNBS 2016 DUS 1635: 2016 Compliance with this standard does not, of itself confer immunity
DRAFT UGANDA STANDARD DUS 1635 First Edition 2016-mm-dd Shea butter Specification Reference number DUS 1635: 2016 UNBS 2016 DUS 1635: 2016 Compliance with this standard does not, of itself confer immunity
Non-destructive testing Penetrant testing. Part 2: Testing of penetrant materials
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 3452-2 Third edition 2013-11-15 Non-destructive testing Penetrant testing Part 2: Testing of penetrant materials Essais non destructifs Examen
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 3452-2 Third edition 2013-11-15 Non-destructive testing Penetrant testing Part 2: Testing of penetrant materials Essais non destructifs Examen
KENYA STANDARD DKS Moringa leaf products Specification
 KENYA STANDARD DKS 2848 Moringa leaf products Specification KEBS 2018 First Edition 2018 TECHNICAL COMMITTEE REPRESENTATION The following organizations were represented on the Technical Committee: Ministry
KENYA STANDARD DKS 2848 Moringa leaf products Specification KEBS 2018 First Edition 2018 TECHNICAL COMMITTEE REPRESENTATION The following organizations were represented on the Technical Committee: Ministry
Summary p. 1 What Are Dietary Reference Intakes? p. 2 Approach for Setting Dietary Reference Intakes p. 7 Nutrient Functions and the Indicators Used
 Summary p. 1 What Are Dietary Reference Intakes? p. 2 Approach for Setting Dietary Reference Intakes p. 7 Nutrient Functions and the Indicators Used to Estimate Requirements p. 10 Criteria and Proposed
Summary p. 1 What Are Dietary Reference Intakes? p. 2 Approach for Setting Dietary Reference Intakes p. 7 Nutrient Functions and the Indicators Used to Estimate Requirements p. 10 Criteria and Proposed
Order of Draw of Blood Collection Tubes. The procedure follows guidelines set forth by the Clinical and Laboratory Standards Institute.
 Order of Draw of Blood Collection Tubes. The procedure follows guidelines set forth by the Clinical and Laboratory Standards Institute. (formerly known as NCCLS) in order to insure that quality specimens
Order of Draw of Blood Collection Tubes. The procedure follows guidelines set forth by the Clinical and Laboratory Standards Institute. (formerly known as NCCLS) in order to insure that quality specimens
Hardness, Total, Sequential
 Hardness, Total, Sequential DOC316.53.01159 Titration Method with EDTA 1,2 Method 8338 0 25,000 mg/l as CaCO 3 Buret Titration Scope and application: For water, wastewater and seawater. 1 USEPA accepted
Hardness, Total, Sequential DOC316.53.01159 Titration Method with EDTA 1,2 Method 8338 0 25,000 mg/l as CaCO 3 Buret Titration Scope and application: For water, wastewater and seawater. 1 USEPA accepted
Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry.
 2013/07/30 A93A01315AUS A11A01641 60 ml 15 ml Pentra C400 Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry. QUAL-QA-TEMP-0846
2013/07/30 A93A01315AUS A11A01641 60 ml 15 ml Pentra C400 Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry. QUAL-QA-TEMP-0846
Understanding and Utilizing Feed Tags for Effective. Mineral Supplementation
 Understanding and Utilizing Feed Tags for Effective Mineral Supplementation Feed tags on mineral supplements, unlike other types of commercial feeds, can be very useful in evaluating the relative value
Understanding and Utilizing Feed Tags for Effective Mineral Supplementation Feed tags on mineral supplements, unlike other types of commercial feeds, can be very useful in evaluating the relative value
N.O.S. (Subsidiary Hazard) Group Standard HSR002624
 N.O.S. (Subsidiary Hazard) Group Standard 2017 - HSR002624 GROUP STANDARD UNDER THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 N.O.S. (Subsidiary Hazard) Group Standard 2017 Pursuant to clause 5 of
N.O.S. (Subsidiary Hazard) Group Standard 2017 - HSR002624 GROUP STANDARD UNDER THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 N.O.S. (Subsidiary Hazard) Group Standard 2017 Pursuant to clause 5 of
Main Challenges Related to Measuring Biomarkers of Exposure of Bisphenol A and Triclosan
 Main Challenges Related to Measuring Biomarkers of Exposure of Bisphenol A and Triclosan Antonia M. Calafat, PhD Organic Analytical Toxicology Branch Division of Laboratory Sciences National Center for
Main Challenges Related to Measuring Biomarkers of Exposure of Bisphenol A and Triclosan Antonia M. Calafat, PhD Organic Analytical Toxicology Branch Division of Laboratory Sciences National Center for
1 OJ L 354, , p OJ L 80, , p. 19.
 Call for scientific and technical data on the permitted food additives E 140(i) chlorophylls, E 140(ii) chlorophyllins, E 141(i) copper complexes of chlorophylls and E 141(ii) copper complexes of chlorophyllins
Call for scientific and technical data on the permitted food additives E 140(i) chlorophylls, E 140(ii) chlorophyllins, E 141(i) copper complexes of chlorophylls and E 141(ii) copper complexes of chlorophyllins
