JRI Thompson Hemiarthroplasty
|
|
|
- Kristian McCormick
- 5 years ago
- Views:
Transcription
1 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6
2 English 3 Page 2 of 6
3 Important Information Please read instructions for use and corresponding operative technique, prior to use in a clinical setting. The Surgeon should be familiar with the appropriate operative technique. Material(s) Material(s): Standard(s): CoCr BS ISO Intended Use The JRI Thompson Hemiarthroplasty Stem is a collared Cobalt Chrome femoral prosthesis, intended for use with cement in hip hemi-arthroplasty for the management of hip fractures. Description JRI Thompson Hemiarthroplasty Stems are a single use, Cobalt chrome, sterile implant intended for use with cement during hemi-hip arthroplasty. The primary design function is the restoration of biomechanics via replacement of the damaged femur and femoral head. JRI Thompson Hemiarthroplasty Stems are supplied sterile via gamma irradiation, in a single pack, double-barrier packaging. Extensive clinical use of the Thompson stem design has proven the biomechanical stability of the device. Various sizes of the implant are available to accommodate anatomical variations of the acetabulum. Indications JRI Thompson Hemiarthroplasty Stem are indicated for the treatment of fractures of the femoral neck. Contra-indications The device should NOT be implanted where there is active infection, insufficient bonestock to support the prosthesis or provide adequate fixation. JRI Thompson Hemiarthroplasty is not intended for use in revision surgery. Further contra-indications may be, but are not limited to the following conditions: Severe muscle, nerve or vascular diseases that endanger the extremity in question Severe deformations, tumours Severe osteoporosis or deficient bone substance that may endanger stable seating of the prosthesis Systematic and metabolic disorders Weakened or compromised immune system (HIV, tumours, infections) Precautions The following conditions require caution and due consideration during pre-operative planning by the surgeon: Page 3 of 6
4 Obese or severely overweight patients Excessive loading through arduous activity Lack of mental faculties to understand post-operative recuperative regime Alcohol dependency or drug abuse History of falls or disabilities. In patients with a high body mass index, where delayed surgery is feasible, it is advisable that a programme of weight reduction is undertaken prior to any joint replacement surgery. Warning This device should only be implanted by operating surgeons who are familiar with the general problems of prosthetic surgery and who are sufficiently trained to the product-specific operative technique. The surgeon is responsible for ensuring the surgery is carried out properly and in line with the instructions provided in the operative technique. As a manufacturer, JRI Orthopaedics Ltd. Is not responsible for any complications arising from incorrect diagnosis, incorrect choice of implant, incorrect operative technique, treatment methods limitations or inadequate asepsis. Pre-operative Pre-operative planning allows the surgeon to assess for implant size and restoration of biomechanics. Failure to carry out proper planning may lead to incorrect choice of implant type/size. Ensure all implant sizes and required instrumentation are available prior to surgery. Consult operative technique and training materials provided by JRI Orthopaedics Ltd. before use. Functionality of surgical instruments should be checked. Use of damaged instruments may lead to early failure of implant. Transient bacteraemia can occur after surgical procedures. To prevent late infection at the implant site, many orthopaedic surgeons advise the use of antibiotic prophylaxis before and after such procedures for their patients. Intra-operative This implant is intended for use with cement. The surgeon must follow the cement manufacturer instructions concerning preparation and technique. Avoid contact with materials that might damage the surface of the implant after removing from packaging. It is important to check the implant before it is inserted to ensure there is no damage. Packaging should only be removed immediately prior to implantation. Prior to wound closure, the surgical site should be cleared from bone cement, particulate matter or other debris. Implants MUST NOT be re-used under any circumstances as the functionality, integrity and/or sterility of that device may have been adversely affected and therefore cannot be guaranteed. Post-Operative Physicians should ensure patients are aware of implant loading limitations and ensure consistent postoperative care, from a suitably qualified professional, is made available. Page 4 of 6
5 Post-operative care should incorporate recognised procedures and take into account information from the operative technique and documented according to internal hospital procedures. Failure to do this may result in malalignment, delayed wound/bone healing, implant failure, infection or impaired joint function. Side Effects As with all major surgical procedures, side effects and adverse events can occur. Some of the more common complications include: Problems resulting from anaesthesia and patient positioning (pain, nausea etc.) Infection Aseptic loosening of implant Dislocation, subluxation, insufficient range of movement, leg length discrepancy Damage to soft tissue Fracture of implant, bone or cement Allergy/hypersensitivity reactions Cardiovascular/pulmonary embolism, respiratory infection, venous thrombosis, neuronal dysfunction, haematoma or delayed wound healing Revision In the event the Thompson Hemiarthroplasty requires revising; ensure all fragments or the primary prosthesis are removed. Clean and prepare the area for implantation in line with current Instructions for Use and Operative Technique of selected revision implant, prior to insertion of device. Storage & Handling Store implants in their original protective packaging in a clean and dry atmosphere. Do not use if the packaging is damaged. Do not use this product after the expiry date (year-month) shown on the product packaging. Avoid removing from packaging until immediately prior to use. Inspect device prior to use. Visibly damaged, scratched, improperly handled implants and implants that have already been used must not be implanted under any circumstances as the functionality, integrity and/or sterility of that device may have been adversely affected and therefore cannot be guaranteed. MRI Safety Information Non-clinical testing has demonstrated that JRI Hip Systems are MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions: Static magnetic field of 3.0 T Maximum spatial field gradient of 720 gauss/cm Maximum whole body averaged specific absorption rate (SAR) of 3.0W/kg Under the scan conditions defined above, the JRI Thompson Hemi-Arthroplasty Stem is expected to produce a maximum temperature rise of 1.7ºC after 15 minutes of continuous scanning The image quality may be compromised if the area of interest is in the exact same area, or relatively close to, the position of the medical device. Therefore, optimization of MR imaging parameters to compensate for the presence of this device may be necessary: Pulse Sequence T1-SE T1-SE GRE GRE Signal Void Size 42,763-mm 2 17,875-mm 2 56,249-mm 2 28,917-mm 2 Page 5 of 6
6 Plane Orientation Parallel Perpendicular Parallel Perpendicular Additional information regarding MRI safety is available upon request at JRI Orthopaedics Ltd. Functional Device Lifespan The functional lifespan of an implant may be impacted by surgeon and selected operative technique, patient physiology and activity levels. Whilst it is normally expected that the lifetime of this implant will exceed a minimum of 10 years, it will be subject to wear and tear through normal everyday use. Products intended for single-use must not be re-used. Re-use or reprocessing (e.g. cleaning and re-sterilisation) may compromise the structural integrity of the device and / or lead to device failure which may result in patient injury or death. Reuse or reprocessing of single-use devices may create a risk of contamination (e.g. transmission of infectious material) which may result in injury or death. Further information For product specific operative technique training or further information, please contact your JRI Orthopaedics Ltd Sales Representative or JRI Orthopaedics Ltd directly. JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Tel: +44(0) Fax: +44(0) Page 6 of 6
Furlong H-A.C. THR System
 Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
TriboFit Hip System. Issue 3 - August 2013 English
 Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Ceramic Femoral Heads and Acetabular Cup Liners
 Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
Instructions for use for Adler Cemented Total Hip Replacement Prosthesis
 0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use for ADLER Cemented Bipolar hemi-arthroplasty Prosthesis
 Page 1 of 8 Instructions use Important Inmation on For use by an Accredited Orthopaedic Surgeon only 1. Device Description: ADLER Cemented Bipolar hemi-arthroplasty implants are used during a surgical
Page 1 of 8 Instructions use Important Inmation on For use by an Accredited Orthopaedic Surgeon only 1. Device Description: ADLER Cemented Bipolar hemi-arthroplasty implants are used during a surgical
- October 2013 English
 TOTAL HIP JOINT REPLACEMENT FOR CEMENTED APPLICATIONS FOR THE ATTENTION OF THE OPERATING SURGEON Total hip replacement provides the surgeon with a means of restoring mobility and reducing pain with the
TOTAL HIP JOINT REPLACEMENT FOR CEMENTED APPLICATIONS FOR THE ATTENTION OF THE OPERATING SURGEON Total hip replacement provides the surgeon with a means of restoring mobility and reducing pain with the
Important Notice to Users in the United States: General Safety Instructions
 POLARSTEM Femoral Stems with Ti/HA (Standard, Lateral, Valgus and Collar) Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com 02/2017
POLARSTEM Femoral Stems with Ti/HA (Standard, Lateral, Valgus and Collar) Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com 02/2017
POLARCUP Dual Mobility System. General Safety Instructions. Instructions for Use. Duty to inform the patient. Implant passport
 Important Notice to Users in the United States: This package insert is for product distributed in US only. U.S. Federal law restricts this device to sale by or on the order of a physician. General Safety
Important Notice to Users in the United States: This package insert is for product distributed in US only. U.S. Federal law restricts this device to sale by or on the order of a physician. General Safety
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Important Notice to Users in the United States: General Safety Instructions
 SLR-PLUS Revision Stem, SLR-PLUS Revision Stem Lateral Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com non-sterile cemented non-
SLR-PLUS Revision Stem, SLR-PLUS Revision Stem Lateral Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com non-sterile cemented non-
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
Optimizing function Maximizing survivorship Accelerating recovery
 Surgical Technique Optimizing Function Maximizing Survivorship Accelerating Recovery The company believes in an approach to patient treatment that places equal importance on: Optimizing function Maximizing
Surgical Technique Optimizing Function Maximizing Survivorship Accelerating Recovery The company believes in an approach to patient treatment that places equal importance on: Optimizing function Maximizing
Important notice: the device(s) can be prescribed and implanted only by a doctor legally authorized to perform this type of surgery.
 rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
For use by an Accredited Orthopaedic Surgeon only
 Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Yasargil Permanent Aneurysm Clips
 Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
EasyStep. Operative technique
 Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA
 SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340
SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340
SURGICAL TECHNIQUE. Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM
 SURGICAL TECHNIQUE Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM Important: This essential product information does not include all of the information necessary for selection and use of a device.
SURGICAL TECHNIQUE Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM Important: This essential product information does not include all of the information necessary for selection and use of a device.
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
CAUTION: Ceramic liners are not approved for use in the United States.
 Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS
 SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
BIOLOX delta Option Ceramic Femoral Head System. Product Features and Instructions for Use
 BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia.
BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia.
Scan Bi-Polar 22/28. Operative Technique
 Scan Bi-Polar 22/28 Operative Technique Disclaimer This publication and all content, artwork, photographs, names, logos and marks contained in it are protected by copyright, trademarks and other intellectual
Scan Bi-Polar 22/28 Operative Technique Disclaimer This publication and all content, artwork, photographs, names, logos and marks contained in it are protected by copyright, trademarks and other intellectual
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
SURGICAL TECHNIQUE. Pivot Bipolar Solitude Unipolar
 SURGICAL TECHNIQUE Pivot Bipolar Solitude Unipolar The following technique is a general guide for the instrumentation of the Pivot Bipolar and Solitude Unipolar systems. It is expected that the surgeon
SURGICAL TECHNIQUE Pivot Bipolar Solitude Unipolar The following technique is a general guide for the instrumentation of the Pivot Bipolar and Solitude Unipolar systems. It is expected that the surgeon
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius.
 Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius. Technique Guide Part of the External Fixation System Low-Profile Wrist Fixator Indications Intended for stabilization of
Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius. Technique Guide Part of the External Fixation System Low-Profile Wrist Fixator Indications Intended for stabilization of
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
BIOLOX delta Option Ceramic Femoral Head System
 BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Knees Hips Extremities Cement and Accessories PMI Technology Product Features BIOLOX delta ceramic heads are composed
BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Knees Hips Extremities Cement and Accessories PMI Technology Product Features BIOLOX delta ceramic heads are composed
Bi-Polar 22.2mm & 28mm System - Operative technique
 Disclaimer Biomet UK Ltd, as the manufacturer of this device, does not practice medicine and does not recommend any particular surgical technique for use on a specific patient. The surgeon who performs
Disclaimer Biomet UK Ltd, as the manufacturer of this device, does not practice medicine and does not recommend any particular surgical technique for use on a specific patient. The surgeon who performs
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM
 CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
Greater Component Accuracy...
 Greater Component Accuracy... StageOne cement spacer molds are designed to provide the surgeon with choices never before offered in two-stage revision knee surgery for an infected total joint. StageOne
Greater Component Accuracy... StageOne cement spacer molds are designed to provide the surgeon with choices never before offered in two-stage revision knee surgery for an infected total joint. StageOne
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
The short stem hip system Surgical Technique
 The short stem hip system Surgical Technique The short stem hip system cementless The Aida hip system was developed in co-operation with the Aida inventors group, founded by surgeons of 10 surgical department
The short stem hip system Surgical Technique The short stem hip system cementless The Aida hip system was developed in co-operation with the Aida inventors group, founded by surgeons of 10 surgical department
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
FastFrame External Fixation System. Damage Control Surgical Technique
 FastFrame External Fixation System Damage Control Surgical Technique 1 FastFrame External Fixation System Damage Control Surgical Technique Table of Contents Introduction... 2 Indications and Contradictions...
FastFrame External Fixation System Damage Control Surgical Technique 1 FastFrame External Fixation System Damage Control Surgical Technique Table of Contents Introduction... 2 Indications and Contradictions...
Positioning Sleeve Surgical Technique
 Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
SURGICAL TECHNIQUE. Alpine Cementless Hip Stem
 SURGICAL TECHNIQUE Alpine Cementless Hip Stem The following technique is a general guide for the instrumentation of the Alpine Cementless Hip Stem. It is expected that the surgeon is already familiar with
SURGICAL TECHNIQUE Alpine Cementless Hip Stem The following technique is a general guide for the instrumentation of the Alpine Cementless Hip Stem. It is expected that the surgeon is already familiar with
operative technique Kent Hip
 operative technique Kent Hip The Kent Hip Operative Technique The Kent Hip was developed by Mr Cliff Stossel, FRCS in Maidstone, Kent, UK and first implanted in 1986. It was designed to deal with problems
operative technique Kent Hip The Kent Hip Operative Technique The Kent Hip was developed by Mr Cliff Stossel, FRCS in Maidstone, Kent, UK and first implanted in 1986. It was designed to deal with problems
Bone Bangalore
 Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital, Whitefield,
Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital, Whitefield,
KASM Knee Articulating Spacer Mold. Surgical Summary
 KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
Description Materials Indications Patient selection factors to be considered include: Contraindications Absolute contraindications include:
 Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
AVANTEON. Operative Technique & Catalogue Information AVANTEON
 AVANTEON Operative Technique & Catalogue Information AVANTEON H I P S Y S T E M Pre-operative Planning The overall aim of pre-operative planning is to establish anatomical data from the patient to guide
AVANTEON Operative Technique & Catalogue Information AVANTEON H I P S Y S T E M Pre-operative Planning The overall aim of pre-operative planning is to establish anatomical data from the patient to guide
Surgical procedure. METS Coned Hemi-Pelvis
 Surgical procedure METS Coned Hemi-Pelvis Surgical Procedure Contents 1.0 2.0 3.0 4.0 5.0 Product overview 2 1.1 Indications 1.2 Absolute contra-indications 1.3 Relative contra-indications 1.4 Capabilities
Surgical procedure METS Coned Hemi-Pelvis Surgical Procedure Contents 1.0 2.0 3.0 4.0 5.0 Product overview 2 1.1 Indications 1.2 Absolute contra-indications 1.3 Relative contra-indications 1.4 Capabilities
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Metha Short Hip Stem System
 Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Ovation Hip System. Surgical Technique
 Ovation Hip System Surgical Technique Ovation Hip System Surgical Technique Ovation Designing Surgeons: Andrew Petrella, M.D. Lecanto, FL Richard Vlasak, M.D. Gainesville, FL Ovation Tribute Designing
Ovation Hip System Surgical Technique Ovation Hip System Surgical Technique Ovation Designing Surgeons: Andrew Petrella, M.D. Lecanto, FL Richard Vlasak, M.D. Gainesville, FL Ovation Tribute Designing
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
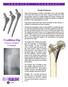
Use Of A Long Femoral Stem In The Treatment Of Proximal Femoral Fractures: A Report Of Four Cases
 ISPUB.COM The Internet Journal of Orthopedic Surgery Volume 5 Number 1 Use Of A Long Femoral Stem In The Treatment Of Proximal Femoral Fractures: A Report Of Four Cases C Yu, V Singh Citation C Yu, V Singh..
ISPUB.COM The Internet Journal of Orthopedic Surgery Volume 5 Number 1 Use Of A Long Femoral Stem In The Treatment Of Proximal Femoral Fractures: A Report Of Four Cases C Yu, V Singh Citation C Yu, V Singh..
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand Institute Miami, Florida. System Overview The ALIGN Radial Head System is designed to restore the kinematics of the native radial
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand Institute Miami, Florida. System Overview The ALIGN Radial Head System is designed to restore the kinematics of the native radial
INTRAMEDULLARY NAILING TIBIA AND FEMUR 1.3 TIBIAL AND FEMORAL NAILING FEMORAL NAILING WITH RETROGRADE INSERTION IMPLANTS AND OPERATING MANUAL
 1.3 TIBIA AND FEMORA NAIING FEMORA NAIING WITH RETROGRADE INSERTION IMPANTS AND OPERATING MANUA INTRAMEDUARY NAIING TIBIA AND FEMUR Medical Products Manufacturing and Trading td. General informations
1.3 TIBIA AND FEMORA NAIING FEMORA NAIING WITH RETROGRADE INSERTION IMPANTS AND OPERATING MANUA INTRAMEDUARY NAIING TIBIA AND FEMUR Medical Products Manufacturing and Trading td. General informations
Tradition Hip Primary Surgical Technique
 Design Rationale Many total hip designs in today s marketplace do not take advantage of the known forces present in the femur. Long term stability of a total hip prosthesis requires an implant design and
Design Rationale Many total hip designs in today s marketplace do not take advantage of the known forces present in the femur. Long term stability of a total hip prosthesis requires an implant design and
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps.
 Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
HELIOS h i p s y s t e m
 HELIOS h i p s y s t e m Design The Helios stem is a highly polished, High Nitrogen Stainless Steel (ISO5832-9) dual tapered cemented stem. The design of the stem is based on the clinically lly successful
HELIOS h i p s y s t e m Design The Helios stem is a highly polished, High Nitrogen Stainless Steel (ISO5832-9) dual tapered cemented stem. The design of the stem is based on the clinically lly successful
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Telya. Varisation staple. Surgical Technique. Irish Distributer: O'Mara Medical Tel
 Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Surgical technique. Dynamic Simulation Personalised Solution
 Surgical technique Dynamic Simulation Personalised Solution 2 Contents Operative technique 4 1. Femoral guide placement 4 2. Neck osteotomy 4 3. Laser canister assembly 5 4. Pelvic screw attachment 6 5.
Surgical technique Dynamic Simulation Personalised Solution 2 Contents Operative technique 4 1. Femoral guide placement 4 2. Neck osteotomy 4 3. Laser canister assembly 5 4. Pelvic screw attachment 6 5.
TaperFill. Surgical Technique
 TaperFill Surgical Technique Table of Contents Indications and Contraindications 3 TaperFill Hip Size Charts 4-5 DJO Surgical 9800 Metric Boulevard Austin, TX (800) 456-8696 www.djosurgical.com Preoperative
TaperFill Surgical Technique Table of Contents Indications and Contraindications 3 TaperFill Hip Size Charts 4-5 DJO Surgical 9800 Metric Boulevard Austin, TX (800) 456-8696 www.djosurgical.com Preoperative
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
North of England Bone and Soft Tissue Tumour Service
 North of England Bone and Soft Tissue Tumour Service Guidelines for rehabilitation after replacement of the proximal femur Proximal femoral replacement surgery is usually carried out as part of treatment
North of England Bone and Soft Tissue Tumour Service Guidelines for rehabilitation after replacement of the proximal femur Proximal femoral replacement surgery is usually carried out as part of treatment
SURGICAL TECHNIQUE. Alpine Cemented Hip Stem
 SURGICAL TECHNIQUE Alpine Cemented Hip Stem The following technique is a general guide for the instrumentation of the Alpine Cemented Hip Stem. It is expected that the surgeon is already familiar with
SURGICAL TECHNIQUE Alpine Cemented Hip Stem The following technique is a general guide for the instrumentation of the Alpine Cemented Hip Stem. It is expected that the surgeon is already familiar with
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
External Distal Radius Fixator. Supplement to the 8 mm rod fixator system
 External Distal Radius Fixator. Supplement to the 8 mm rod fixator system Surgical technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation
External Distal Radius Fixator. Supplement to the 8 mm rod fixator system Surgical technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use.
 Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
