CIGNA HEALTHCARE COVERAGE POSITION
|
|
|
- Briana Lyons
- 6 years ago
- Views:
Transcription
1 CIGNA HEALTHCARE COVERAGE POSITION Subject Bone Growth Stimulators: Invasive, Noninvasive, Ultrasound Revised Date... 6/15/2005 Original Effective Date... 6/15/2004 Coverage Position Number Table of Contents Coverage Position... 1 General Background... 2 Coding/Billing Information... 6 References... 7 Related Coverage Positions INSTRUCTIONS FOR USE Coverage Positions are intended to supplement certain standard CIGNA HealthCare benefit plans. Please note, the terms of a participant s particular benefit plan document [Group Service Agreement (GSA), Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Positions are based. For example, a participant s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Position. In the event of a conflict, a participant s benefit plan document always supercedes the information in the Coverage Positions. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable group benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Positions and; 4) the specific facts of the particular situation CIGNA Health Corporation Coverage Position Coverage for ultrasound and noninvasive bone growth stimulators is subject to the terms, conditions and limitations of the Durable Medical Equipment (DME) benefit. Please refer to the applicable CIGNA HealthCare benefit plan document and schedules to determine benefit availability and the terms, conditions and limitations of coverage. If coverage is available for this service, the following conditions apply. CIGNA HealthCare covers ultrasound bone growth stimulation as medically necessary when ANY of the following criteria are met: fresh (< 7 days), closed or grade I open, tibial diaphyseal fractures fresh (< 7 days), closed fractures of the distal radius (Colles fracture) nonunion of bones other than the skull or vertebrae in skeletally mature patients, excluding those that are related to malignancy, when the fracture is equal to or greater than three months from the date of injury or initial treatment, and when there is documentation that the healing has ceased or is not progressing as evidenced by two sequential radiographs > 90 days apart CIGNA HealthCare covers noninvasive electrical bone growth stimulators as medically necessary for the treatment of fracture nonunions when ALL of the following criteria are met: The nonunion is located in a long bone (including; clavicle, humerous, radius, ulna, femur, tibia, fibula, metacarpal and metatarsal bones). Serial multiview imaging studies ( two sets, 90 days apart) confirm fracture healing has ceased. The fracture gap is one cm or less. There is demonstrated proof of skeletal maturity or the patient is 20 years of age or older. Page 1 of 9
2 CIGNA HealthCare covers noninvasive or invasive electrical bone growth stimulators as medically necessary as an adjunct to spinal fusion surgery when EITHER of the following criteria is met: The patient has had prior spinal fusion failure or is undergoing a multi-level fusion. The patient has one or more risk factors for nonhealing such as: smoking, obesity, diabetes, renal disease, or other metabolic disease where bone healing is poor. General Background Bones are divided into four major categories. Long bones are found in the extremities and are comprised of a shaft (diaphysis) and two ends (epiphyses). Long bones, which form levers, support weight and provide for motion, include the femur, tibia, fibula, humerus, radius, ulna, phalanges and clavicle. Short bones, which include the tarsal bones in the foot and carpal bones in the hand, are cube-shaped and designed for strength. Flat bones provide protection and areas for muscle attachment and include the cranial bones, sternum, ribs, ilia and scapulae. Irregular bones include the vertebrae, sacrum, coccyx and some facial bones (U.S. National Cancer Institute s Surveillance, Epidemiology and End Results [SEER] Program, 2005) Fractured bones heal in several distinct stages. Initially, bleeding and swelling at the fracture site causes a hematoma to form. As the hematoma necroses, macrophages and osteoclasts arrive at the site to remove the debris and osteoblasts (bone builders) and endothelial cells (blood vessel builders) are stimulated and begin rebuilding the bone and vasculature. Within two weeks, fibroblasts, osteoblasts and chondroblasts combine to form a soft callus that becomes a hard callus of woven, immature bone that bridges the gap in the bone (approximately four weeks post-injury). Two to three months post-fracture, a hard callus develops into a revascularized bony union that will be modified to lamellar bone that may be thicker and stronger than the original bone (SEER, 2005). Several factors play a role in bone healing. At the fracture site, the extent of the bone and soft tissue damage, adequacy of the blood supply, the gap between bone ends and infection may all have an impact on healing. The individual's general health and nutritional status play a significant role in bone healing. Systemic factors such as diabetes, anemia, hormone insufficiencies, osteoporosis and certain medications and behavioral factors such as smoking and activity level can also impact the rate and quality of bone healing (Hayes, 2001). Approximately 5-10% of fractures will not heal properly (Ryaby, 1998). A nonunion occurs when bone healing has stopped prematurely and will not likely continue without medical intervention. A fracture nonunion is considered to exist only when a minimum of two sets of radiographs obtained prior to starting treatment are separated by a minimum of 90 days showing no evidence of fracture healing between the two sets of radiographs (Centers for Medicare and Medicaid Services [CMS], 2000). Currently, a variety of invasive and noninvasive interventions are used to treat nonunions including immobilization/casting, open or closed reduction, pins, screw fixation, intramedullary rods and bone grafting. Bone growth stimulation may be used instead of, or in addition to, other interventions to promote bone healing. Ultrasound Bone Growth Stimulator Ultrasound bone growth stimulation is designed to transmit low-density, pulsed, high-frequency acoustic pressure waves to accelerate healing of fresh fractures and to promote healing of delayed unions and nonunions that are refractory to standard treatment. The Sonic Accelerated Fracture Healing System (SAFHS ) Model 2A was granted approval by the U.S. Food and Drug Administration (FDA) on October 5, 1994, for accelerating the time to a healed fracture for fresh, closed Colles fractures and fresh, closed or open tibial diaphysis fractures in skeletally mature individuals. On July 7, 1995, the FDA granted approval for the SAFHS Model 2A to be used by patients at home. Exogen 2000 (Smith & Nephew, Inc., Memphis, TN) received FDA premarket approval (PMA) in 2000 for treatment of fractures through bone growth stimulation. On February 22, 2000, the FDA granted approval for the SAFHS 2000 and the Exogen 2000 for the noninvasive treatment of established nonunions. The device labeling excludes nonunions of the skull and vertebrae (FDA, 2000). Page 2 of 9
3 Ultrasound has been shown to be effective in promoting healing of fresh fractures of the tibial diaphysis and radial fractures, as illustrated by studies such as those that follow. Heckman et al. (1994) reported a multi-institutional, prospective, randomized, double-blind, placebocontrolled study (n=67) involving closed or grade one fractures of the tibial shaft. Thirty-three participants were treated with an ultrasound stimulating device and thirty-four with a placebo control device. A significant decrease in the time to clinical healing (86 days in the active treated group versus 114 days in the control group [p=0.01]) was noted. Cook et al. (1997) conducted a randomized controlled trial of low-intensity ultrasound in the healing of tibia and distal radius fracture in patients who smoke (tibial fracture n=67, radial fracture n=63). The time needed for healing of a tibial fracture in patients who smoked and were treated with the active ultrasound device was reduced 72 days, or more than 43% less than that of patients who smoked and were treated with a placebo-control device. Kristiansen et al. (1997) conducted a multi-center, randomized, double-blind, placebo-controlled clinical trial to test the efficacy of a low-intensity, non-thermal, pulsed ultrasound device for decreasing the healing time of the radius. Sixty-one patients were enrolled in the study within seven days after experiencing a fracture. One group was treated with the active ultrasound device (n=30) and the second group which had thirty-one fractures was treated with placebo. Comparison of the two fracture groups demonstrated significant acceleration of the fracture healing in a mean of 61 days for the patients who were treated with ultrasound stimulation and 98 days for those managed with placebo (p<0.0001). Compared with treatment with placebo, treatment with ultrasound was associated with significantly smaller loss of reduction (20% vs. 43% p<0.01). The authors concluded that the ultrasound signal accelerates the healing of fractures of the distal radial metaphysic and decreases the loss of reduction during fracture healing. Leung et al. (2004) conducted a randomized, prospective, intervention trial to evaluate the potential effect of low-intensity pulsed ultrasound on intact bone for prevention of postmenopausal bone loss in the distal radius. Twenty healthy postmenopausal Chinese women between the ages of met the inclusion criteria. For each woman, the treatment hand was randomly selected, and the contra lateral site served as a control. Integral and trabecular bone mineral density were measured using highly precise multilayer peripheral quantitative computed tomography at the bilateral distal radius at baseline, three months after daily low-intensity pulsed ultrasound treatment, and three months after discontinuing treatment. Results showed that the rate of bone change (trabecular bone mineral density and integral bone mineral density) did not significantly differ between the site treated with low-intensity pulsed ultrasound and the contra lateral control at either follow-up. Also, during the follow-up, bone mineral density did not change significantly in the contra lateral control site. This was the first prospective and randomized study to show that low-intensity pulsed ultrasound at the current regime did not have significant effects on intact bone for prevention of postmenopausal bone loss in the distal radii of older Chinese women. Electrical Bone Growth Stimulators (Noninvasive) Noninvasive bone growth stimulators use inductive and conductive methods to deliver a broad uniform electric field, pulse electromagnetic field (PEMF), or combined electromagnetic (CMF) field to the fracture site via treatment coils or disks placed on the skin and attached to an external power supply. Semiinvasive direct current stimulation uses a cathode implanted in the cortex of one end of the nonunion site and attached to an external power supply. An anode attached to the skin completes the electrical circuit. Invasive direct current stimulation involves threading the cathode through or around the bone with the anode and power supply implanted in the surrounding soft tissue. Noninvasive electrical bone growth stimulators are premarket approved (PMA) FDA-approved as class III devices. The devices include: OL 1000 and SpinaLogic Bone Growth Stimulator (Regentek, a division of dj Orthopedics, LLC (formerly OrthoLogic, Tempe, AZ); Physio-Stim Lite, Spinal-Stim Lite (Orthofix, Inc., Richardson, TX); EBI Bone Healing System, SpinalPak, and OrthoPak (Biolectron, a subsidiary of Electro-Biology, Inc., Parsippany, NJ) (FDA, 2001). All are Class III devices. Studies have supported the use of noninvasive bone growth stimulation for accelerating the healing time to a fresh, closed, posteriorly displaced distal radius fracture and a fresh closed Grade 1 open tibial Page 3 of 9
4 diaphysis fracture in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization. Electrical bone growth stimulation is not indicated for nonunion fractures where the bones are not aligned or a synovial pseudarthrosis exists, when the bone gap is more than one centimeter or greater than one half the diameter of the bone, and for patients who are unable to be compliant with appropriate use of the device or treatment regimens. The safety and effectiveness of electrical bone growth stimulation has not been established in bone pathology such as osteomyelitis, spondylitis, Paget's disease, metastatic cancer, advanced osteoporosis or arthritis, or for avascular or necrotic bone tissues. Patients lacking skeletal maturity, pregnant women and patients with demand pacemakers or implantable defibrillators are not candidates for electrical bone growth stimulator therapy. Fixation devices made from magnetic materials may compromise the effects of electric bone growth stimulators (Orthofix 2005). The bulk of the scientific evidence demonstrating the efficacy of electrical bone growth stimulation addresses its use for nonunion fractures in long bones or as an adjunct to spinal fusion. Electrical stimulation for promoting healing in short bones, particularly the scaphoid, has shown success. In a metaanalysis of available literature, Akai et al. (2002) examined whether electrical stimulation has a specific effect on spinal fusion. Little evidence exists on the efficacy of electrical stimulation for improving the fusion rate of spinal fusion surgery. A total of five randomized controlled trials (RCTs) that assessed healing of spinal fusion were identified and scored on methodological quality. All the identified studies reported positive findings, but the quality score of each trial showed wide flaws. The data was able to be pooled because of the relative homogeneity of the subjects who had spine fusion and radiographic assessment from these studies. Excluding one trial with the lowest score, the combined results of four trials, whose major endpoints were the success rate of the fusion, revealed a statistically significant effect of electrical stimulation with various techniques; however, the selected trials still showed wide variation in view of stimulation modalities and treatment protocol. The pooled result of the studies in this review revealed the efficacy of electrical stimulation based on proven methodological quality. The calculated difference in response rates of treated (stimulated) or not treated (placebo) cases in the five trials shows a pooled rate difference of (95%), strongly supporting the effectiveness of electrical stimulation with various modalities on the healing times of patients with spinal fusion. As problems on therapeutic modality and protocol remain, there is a further need for improvement in design to constitute acceptable proof and to establish treatment programs that better demonstrate electrical stimulation effects on spinal fusion (Akai, et al., 2002). Hotta (1994) completed a health technology review for the Agency for Health Care Policy and Research (AHCPR), currently referred to as the Agency for Healthcare Research and Quality (AHRQ), and determined that direct electrical current stimulates bone formation and that it has been used as a standard of care in the treatment of lone-bone fractures that have failed to fuse. Direct current stimulation may play a similar role in spinal fusion, especially in patients who have had fusion failures or who are at high risk for fusion failures. The available data appear to suggest that an implantable bone-growth stimulator may be a useful adjunct that could enhance the probability of fusion success in patients who have had previous fusion failure or need extensive bone grafting for multiple level fusion. No quantification of results as to efficacy or duration was possible. There is insufficient data to support use of an implantable bone-growth stimulator in high-risk patients such as those who have spondylolisthesis, who are obese, or are smokers. Scott et al. (1994) studied twenty-three patients who had an established non-union of a long bone. They were entered into a prospective, double-blind trial in which electrical capacitive coupling was used for treatment. Twenty-one patients completed the study: ten were actively managed and eleven were managed with a placebo unit. The duration to healing time was thirty-one months for the actively managed group and twenty-six months for the placebo group. The non-union healed in six of the ten patients who had been managed actively but in none of the patients who had been managed with the placebo unit. This difference in the rates of healing between the actively managed and the placebo groups is highly significant (p = 0.004). Page 4 of 9
5 Abeed et al. (1998) conducted a prospective descriptive study to determine the extent to which capacitively-coupled electrical stimulation (CCEST) at a long bone fracture site can promote healing of nonunited fractures. Sixteen patients with nonunited fractures of 9-76 months were treated with CCEST. Thirteen patients had previously undergone one or more surgical procedures, and the other three had been given plaster casts. A 63-kilohertz, six-volt peak-to-peak sine wave signal was applied across two 40-millimeter in diameter stainless steel plates placed on the skin at opposite sides of the fracture site. The device was used for up to 30 weeks; if no healing occurred by this time it was removed and considered to have failed. The results indicated that 11 of the nonunions achieved union at an average of 15 weeks of stimulation. The only significant factor determining the success of healing was the distance between the plates; a distance of 80 mm or less resulted in healing in all cases. Healing was not affected significantly by any of the following factors: whether or not the nonunion had been treated surgically prior to stimulation; whether or not it had been infected; whether or not the patient bore weight after treatment; or by the presence or absence of metal from previous surgery at the fracture site. The authors concluded that the findings confirm those of previous studies that CCEST promotes bone healing of fracture nonunions. The dependence of healing on the interplate distance suggests that maintaining sufficient current across the plates is necessary to allow healing, which for larger bones may be achieved by increasing the area of the plates, the applied voltage, or the excitation frequency of the stimulation. Goodwin et al. (1999) conducted a randomized double-blind prospective comparison with a placebo control to evaluate the effect of noninvasive capacitively-coupled electrical stimulation on the success rate of lumbar spine fusion surgery, and to compare active with placebo stimulators as adjuncts to contemporary fusion techniques. Previous studies have established the effectiveness of direct current and electromagnetic field stimulation as adjuncts for some forms of spinal fusion. None of the previous placebo-controlled studies on external bone stimulation included posterolateral fusion techniques, and most were conducted with prior generations of internal fixation hardware. The investigation was conducted by 28 U.S. surgeons. Patients with a primary diagnosis of degenerative disc disease with or without other degenerative changes were selected. The study protocol defined success as a clinical outcome rated as excellent or good and a fusion documented as solid by both the investigator and the blinded independent radiologist. Patients were randomized within three weeks of surgery and were instructed to use the stimulator 24 hours a day until healing occurred, or for nine months if healing was delayed. Disagreements on radiographic success were resolved by a second blinded, independent reviewer. For the 179 patients who completed treatment and evaluation, the overall protocol success rate (both clinical and radiographic results rated as successes) was 84.7% for the active patients and 64.9% for the placebo patients. This difference is highly significant according to the Yates corrected chi-square test (p=0.0043). Best improvements in patient outcomes (20% or greater success rate) occurred when active stimulation was used in conjunction with posterolateral fusion (p=0.006) and when internal fixation also was incorporated (p=0.013). This study was consistent in that active stimulation improved results for each stratification; however, some strata had insufficient numbers of patients for the results to have statistical significance. Improved success rates when capacitively-coupled stimulation is added to internal fixation are hypothesized to result from overcoming the biochemical effects of stress shielding. The authors report that capacitively-coupled stimulation is an effective adjunct to primary spine fusion, especially for patients with posterolateral fusion and those with internal fixation. Electrical Bone Growth Stimulators (Invasive) Invasive bone growth stimulators are implanted devices that deliver electrical energy to a nonhealing fracture or bone fusion site. The goal is to induce osteogenesis, stimulate bone growth and promote fracture healing. The advantage of invasive direct-current electric bone growth systems over noninvasive systems is that a constant current is delivered to the fracture site without the concerns for patient compliance or cooperation. EBI (EBI L.P., Parsippany, NJ) manufactures two FDA-approved implantable bone growth stimulators: the OsteoGen and the SpF Implantable Spine Fusion Stimulator. The OsteoGen and OsteoGen -D are designed for the treatment of fracture nonunion, with the latter model indicated only for use in multiple nonunions or severely comminuted fractures that require more than one electrode to facilitate treatment. Four models of the SpF Implantable Spine Fusion Stimulator are available. The SpF - 2T and SpF -4T are indicated for fusion of one or two levels, while the SpF -XL and SpF - XL IIb are indicated for fusion of three or more levels. In 2003, EBI added the SpF -PLUS to their product range. The FDA has also Page 5 of 9
6 approved the Zimmer Direct Current Bone Growth Stimulator (Zimmer, Inc., Warsaw, IN) for the treatment of fracture nonunions (FDA, 2004). Invasive electrical bone stimulators have also been shown to be effective in promoting bone healing in high-risk individuals undergoing spinal fusion. A high-risk patient is one with a prior fusion failure, who is undergoing a multi-level fusion, or a patient at risk for poor healing such as one who smokes, is obese or has diabetes. Kucharzyk (1999) reported a controlled clinical trial of a series of 65 instrumented patients without stimulation who were compared with a later series of 65 patients with instrumentation and implantable electrical stimulation. The groups were evaluated for risk factors, age, diagnostic groups, levels fused, and radiographic and clinical success to test the efficacy of electrical stimulation in instrumented high-risk lumbar fusions. All patients were instrumented via pedicle screws and autologous bone graft. Diagnostic groups were evaluated, and the risk factors in each group were identified and compared. Postoperation management and follow-up regimen were similar in each group. Radiographs were evaluated and confirmed by an independent radiologist. Clinical success was evaluated and confirmed by a second orthopedic surgeon. Fusion success was 95.6% in the stimulated group compared with 87% in the nonstimulated group (p=0.05). Clinical success was 91% in the stimulated group and 79% in the nonstimulated group (p=0.02). In a workers' compensation subset, fusion success was 93% in the stimulated group and 81% in the nonstimulated group. Clinical success was 57% in the stimulated group and 46% in the nonstimulated group. The authors concluded that the results from using both instrumentation and electrical stimulation in a high-risk pool of patients showed a statistically significant difference, with higher rates of fusion and clinical success than in a similar pool that did not receive stimulation. Rogozinski et al. (1996) reported a randomized controlled trial of 94 patients assigned to groups either with or without implanted bone growth stimulation as an adjunct to instrumented spinal fusion. The trial was conducted between May 1990 and December Consecutive groups with or without stimulation were compared prospectively; a small group was compared with random assignment of surgery with or without stimulation. The study was designed to test the efficacy of implanted bone growth stimulation in instrumented fusion, especially regarding high-risk patient groups including smokers, those with previous back surgery, and those with multiple fusion levels. Fusion surgery was performed by the same two surgeons for all patients, using autologous graft and instrumentation (pedicle screw and rod). Surgical indications and pre- and postoperative regimens were similar for all patients. Average follow-up period was 20.5 months. Ninety-six percent of patients with stimulation had solid fusion versus 85% fusion in patients who did not have stimulation. The authors concluded that implanted bone growth stimulation can improve fusion results in patients with instrumented lumbosacral fusion as has been demonstrated in situ fusions. Patients in high-risk categories (smokers, those with multiple back surgeries, and multilevel fusions) also are demonstrated to have higher fusion rates with implanted bone growth stimulation than those without benefit of stimulation. Summary There is sufficient peer-reviewed scientific literature to support safety and efficacy of ultrasound bone growth therapy in patients with fresh fractures of the distal radius and the tibial diaphysis, when the patients have skeletal maturity, and when the therapy is used as an adjunct to closed reduction and cast immobilization, or for nonunion of bones other than skull or vertebrae. There is sufficient peer-reviewed scientific literature to support the efficacy of noninvasive bone growth stimulation in the healing of an established nonunion acquired secondary to trauma, excluding all vertebrae and flat bones where the width of the nonunion defect is less than one-half the width of the width of the bone to be treated. Literature supports the safety and efficacy of invasive or noninvasive bone growth stimulation devices in the adjunctive treatment of patients with prior spinal fusion failure or who are undergoing a multi-level fusion, or in patients who have one or more risk factors for non-healing such as: smoking, obesity, diabetes, renal disease, or other metabolic disease where bone healing is poor Coding/Billing Information Page 6 of 9
7 Note: This list of codes may not be all-inclusive. Covered when medically necessary: CPT * Description Codes Low intensity ultrasound stimulation to aid bone healing, noninvasive (nonoperative) Electrical stimulation to aid bone healing; invasive (operative) Electrical stimulation to aid bone healing; non invasive (nonoperative) HCPCS Codes E0760 E0749 E0748 E0747 Description Osteogenesis stimulator, low intensity ultrasound, noninvasive Osteogenesis stimulator; electrical, surgically implanted Osteogenesis stimulator; electrical, noninvasive, spinal application Osteogenesis stimulator; electrical, surgically noninvasive, other than spinal applications ICD-9-CM Description Diagnosis Codes nonunion of fracture; nonunion of fracture pseudarthrosis (bone) *Current Procedural Terminology (CPT ) 2004 American Medical Association: Chicago, IL. References 1. Abeed R, Naseer M, Abel E. Capacitively coupled electrical stimulation treatment: results from patients with failed long bone fracture unions. J Orth Trauma. 1998;12(7): Akai M, Kawashima N, Kimura T, Hayashi K. Electrical stimulation as an adjunct to spinal fusion: a meta-analysis of controlled clinical trials. Bioelectromagnetics Oct;23(7): Bora F, Osterman A, Woodbury D, Brighton C. Treatment of nonunion of the scaphoid by direct current. Orthop Clinics of N Am. 2984;15(1): Bhandari M, Schemitsch E. Clinical advances in the treatment of fracture nonunion: the response to mechanical stimulation. Curr Op Orthop. 2000;11(5): Brighton C, Shaman P, Heppenstall R, Esterhai J, Pollack S, Friedenberg Z. Tibial nonunion treated with direct current, capacitive coupling or bone graft. Clin Orthop. 1995;321: Brighton C, Black J. Friedenberg Z, Esterhai J, Day L, Connolly J. A multicenter study of the treatment of nonunion with constant direct current. J Bone Joint Surg Am. 1981;63(1): Brighton C. The semi-invasive method of treating nonunion with direct current. Orthop Clin North Am 1984;15(1): Busse J, Bhandari M, Kulkarni A, Tunks E. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ 2002;166(4): Cook S, Ryaby J, McCabe J, Frey J, Heckman J, Kristiansen T. Acceleration of time and distal radius fracture healing in patients who smoke. Clin Orthop 1997;337: Page 7 of 9
8 10. CIGNA Healthcare Medicare Administration DMERC Region D. Part 35 Medical Procedures Coverage Issue Osteogenic Stimulation. Accessed May 3, 2004, May 5, Available at URL address: Centers for Medicare & Medicaid (CMS).Services. National Coverage Determinations. Osteogenic Stimulation. Publication Number 6, Transmittal Number 131. January Centers for Medicare and Medicaid Services. National Coverage Policy Revision. Electrical stimulation for fracture healing (CAG-00022). Decision Memorandum. July XEBI Medical. Accessed May 3, 2004, May 5, Available at URL address: Exogen, Inc. Accessed May 3, 2004, May 5, Available at URL address: Food and Drug Administration. Center for Devices and Radiological Health (CDRH). Premarket approvals page. Summary of safety and effectiveness. Updated November 5, Accessed May 3, 2004, May 5, Available at URL address: Gebauer D, Mayr E, Orthner E. Nonunions treated by pulsed low intensity ultrasound [Abstract from the 15th annual meeting of the Orthopedic Trauma Association]. J Orth Trauma 2000;14(2) Goodwin C, Brighton C, Guyer R, Johnson J, Light K, Yuan H. A double blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine 1999;24(13): HAYES Medical Technology Directory. Electrical Bone Growth Stimulation, Invasive. Lansdale PA: HAYES, Inc Winifred S. Hayes, INC February. 19. HAYES Medical Technology Directory. Electrical Bone Growth Stimulation, Noninvasive. Lansdale PA: HAYES, Inc Winifred S. Hayes, INC August. Update October 14, HAYES Medical Technology Directory. Ultrasound bone growth stimulators. Lansdale PA: HAYES, Inc Winifred S. Hayes, INC October. 21. Hadjiargyrou M, McLeod K, Ryaby J, Clinton R. Enhancement of fracture healing by low intensity ultrasound. Clin Orthop 1998;355S:S216-S Hannaouche D, Petite H, Sedel L. Current trends in the enhancement of fracture healing. JBJS B(2): Heckman J, Ryaby J, McCabe J, Frey J,Kilcoyne R. Acceleration of tibial fracture-healing by noninvasive, low-intensity pulsed ultrasound. JBJS 1994;76-A(1): Hotta S. Electrical bone-growth stimulation and spinal fusion. Health technology assessment review No Agency for Health Care Policy and Research (AHCPR) Pub No Accessed May 3, 2004, May 5, Available at URL address: Kristiansen T, Ryaby J, McCabe J, Frey J, Roe L. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, doubleblind, placebo-controlled study. JBJS 1997;79-A(7): Page 8 of 9
9 26. Kucharzyk D. A controlled prospective outcome study of implantable electrical stimulation with spinal instrumentation in a high-risk spinal fusion population. Spin 1999;24(5): Leung KS, Lee WS, Tsui HF, Liu PP, Cheung WH. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol Mar;30(3): Mayr E, Frankel V, Rüter A. Ultrasound: an alternative healing method for nonunions? Arch Orthop Trauma Surg 2000;120: Nolte P, van der Krans A, Patka P, Janssen I, Ryaby J, Albers G. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma 2001;51(4): Orthofix. Bone growth stimulators. Accessed May 3, 2004, May 5, Available at URL address: OrthoLogic Products. Accessed May 3, 2004, May 5, Available at URL address: Otter MW, McLeod KJ, Rubin CT. Effects of electromagnetic fields in experimental fracture repair. Clin Orthop 1998;355S:S Paterson D. Treatment of nonunion with a constant direct current: a totally implantable system. Orthop Clin North Am 1984;15(1): Rogozinski A, Rogozinski C. Efficacy of implanted bone growth stimulation in instrumented lumbosacral spinal fusion. Spine 1996;21(21): Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. JBJS 2001;83-A(2): Ryaby JT. Clinical effects of electromagnetic and electric fields on fracture healing. Clin Orthop S:S Scott G, King J. A prospective, double blind trial of electrical capacitive coupling in the treatment of nonunion long bones. J Bone Joint Surg 1994;76-A(6): U.S. National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program, with Emory University, Atlanta, Georgia, U.S.A. Anatomy and physiology: classification of bones. Accessed May 6,2004. Available at URL address: Page 9 of 9
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton
FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP029 Section: Medical Benefit Policy Subject: Bone Growth Stimulator I. Policy: Bone Growth Stimulator II. Purpose/Objective: To provide a policy of coverage regarding
POLICIES AND PROCEDURE MANUAL Policy: MP029 Section: Medical Benefit Policy Subject: Bone Growth Stimulator I. Policy: Bone Growth Stimulator II. Purpose/Objective: To provide a policy of coverage regarding
MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS
 MEDICAL POLICY PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
UTILIZATION MANAGEMENT POLICY
 TITLE: BONE GROWTH STIMULATORS EFFECTIVE DATE: July 1, 2015 UTILIZATION MANAGEMENT POLICY DOCUMENT HISTORY Original Endorsement Date: May, 1991 Subsequent Endorsement Date(s): 02/1992, 03/1994, 03/1995,
TITLE: BONE GROWTH STIMULATORS EFFECTIVE DATE: July 1, 2015 UTILIZATION MANAGEMENT POLICY DOCUMENT HISTORY Original Endorsement Date: May, 1991 Subsequent Endorsement Date(s): 02/1992, 03/1994, 03/1995,
Protocol. Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion (70185) Medical Benefit Effective Date: 07/01/12 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10,
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion (70185) Medical Benefit Effective Date: 07/01/12 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10,
Geisinger Health Plan, Geisinger Indemnity Insurance Company and Geisinger Quality Options are
 Bone Growth Stimulator Policy # : MP029 Last Review Date : 2015-10-23 Status : Active Cross References : Reviewed Geisinger Health Plan, Geisinger Indemnity Insurance Company and Geisinger Quality Options
Bone Growth Stimulator Policy # : MP029 Last Review Date : 2015-10-23 Status : Active Cross References : Reviewed Geisinger Health Plan, Geisinger Indemnity Insurance Company and Geisinger Quality Options
Subject: Ultrasound Osteogenesis Stimulators, Non Invasive
 Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Last Review Status/Date: December 2013 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Page: 1 of 10 Adjunct to Spinal Fusion Procedures Description Both
Last Review Status/Date: December 2013 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Page: 1 of 10 Adjunct to Spinal Fusion Procedures Description Both
MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS FOR THE APPENDICULAR SKELETON
 MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS 06/22/16, 06/22/17, 04/26/18 PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If
MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS 06/22/16, 06/22/17, 04/26/18 PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If
Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS)
 09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy File name: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures File code:
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy File name: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures File code:
09/12/17. I. Electrical Bone Growth Stimulator (invasive, semi-invasive, or non-invasive) any of the following: A-C
 Reference #: MC/F021 Page: 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/F021 Page: 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/15 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/15 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Corporate Medical Policy
 Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2017 6/2018 6/2017 Description
Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2017 6/2018 6/2017 Description
Low Intensity Ultrasound Accelerated Fracture Healing Device
 Page 1 of 14 Medical Policies - DME Low Intensity Ultrasound Accelerated Fracture Healing Device Print Number: DME101.030 Effective Date: 01-01-2015 Coverage: Low-intensity ultrasound treatment may be
Page 1 of 14 Medical Policies - DME Low Intensity Ultrasound Accelerated Fracture Healing Device Print Number: DME101.030 Effective Date: 01-01-2015 Coverage: Low-intensity ultrasound treatment may be
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures. Original Policy Date
 MP 7.01.68 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with
MP 7.01.68 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/17 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/17 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Original Policy Date 12/1/9512:2013
 MP 1.01.03 Ultrasound Accelerated Fracture Healing Device Medical Policy Section Durable Medical Equipment Issue 12:2013 Original Policy Date 12/1/9512:2013 Last Review Status/Date 12:2013 Return to Medical
MP 1.01.03 Ultrasound Accelerated Fracture Healing Device Medical Policy Section Durable Medical Equipment Issue 12:2013 Original Policy Date 12/1/9512:2013 Last Review Status/Date 12:2013 Return to Medical
Bone Growth Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Bone Growth Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Electrical Bone Growth Stimulation of the Appendicular Skeleton
 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date June 11, 2014 Next Review Date December 2015
7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date June 11, 2014 Next Review Date December 2015
Corporate Medical Policy
 Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Electrical Bone Growth Stimulation Page 1 of 42 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Electrical Bone Growth Stimulation of the Appendicular Skeleton
Electrical Bone Growth Stimulation Page 1 of 42 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Electrical Bone Growth Stimulation of the Appendicular Skeleton
Bone Growth Stimulators: Electrical (Invasive, Noninvasive), Ultrasound
 Medical Coverage Policy Effective Date...05/15/2018 Next Review Date...04/15/2019 Coverage Policy Number... 0084 Bone Growth Stimulators: Electrical (Invasive, Noninvasive), Ultrasound Table of Contents
Medical Coverage Policy Effective Date...05/15/2018 Next Review Date...04/15/2019 Coverage Policy Number... 0084 Bone Growth Stimulators: Electrical (Invasive, Noninvasive), Ultrasound Table of Contents
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date October 15, 2007 Next Review Date
7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date October 15, 2007 Next Review Date
Name of Policy: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Name of Policy: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy #: 524 Latest Review Date: May 2017 Category: DME Policy Grade: B Background/Definitions: As a general
Name of Policy: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy #: 524 Latest Review Date: May 2017 Category: DME Policy Grade: B Background/Definitions: As a general
Electrical Bone Growth Stimulators (Osteogenic Stimulation)
 Medical Policy Manual Durable Medical Equipment, Policy No. 83.11 Electrical Bone Growth Stimulators (Osteogenic Stimulation) Next Review: July 2018 Last Review: September 2017 Effective: October 1, 2017
Medical Policy Manual Durable Medical Equipment, Policy No. 83.11 Electrical Bone Growth Stimulators (Osteogenic Stimulation) Next Review: July 2018 Last Review: September 2017 Effective: October 1, 2017
Policy #: 331 Latest Review Date: February 2015
 Name of Policy: Bone Growth Stimulators: Ultrasound Policy #: 331 Latest Review Date: February 2015 Category: DME Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue
Name of Policy: Bone Growth Stimulators: Ultrasound Policy #: 331 Latest Review Date: February 2015 Category: DME Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Description. Section: Surgery Effective Date: April 15, 2015 Subsection: Surgery Original Policy Date: June 7, 2012 Subject: Page: 1 of 11
 Subject: Electrical Bone Growth Stimulation of the Last Review Status/Date: March 2015 Page: 1 of 11 Electrical Bone Growth Stimulation of the Description In the appendicular skeleton, electrical stimulation
Subject: Electrical Bone Growth Stimulation of the Last Review Status/Date: March 2015 Page: 1 of 11 Electrical Bone Growth Stimulation of the Description In the appendicular skeleton, electrical stimulation
Electrical Bone Growth Stimulation of the Appendicular Skeleton
 Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Medical Policy Bulletin
 Medical Policy Bulletin Title: Policy #: Electrical Bone Growth Stimulation and Low Intensity Ultrasound Accelerated Fracture Healing System 05.00.09h The below medical or claim payment policy is applicable
Medical Policy Bulletin Title: Policy #: Electrical Bone Growth Stimulation and Low Intensity Ultrasound Accelerated Fracture Healing System 05.00.09h The below medical or claim payment policy is applicable
Bone Growth Stimulators AHM
 Bone Growth Stimulators AHM Clinical Indications Ultrasonic osteogenesis stimulator (e.g., the Sonic Accelerated Fracture Healing System (SAFHS)) medically necessary DME to Ultrasonic osteogenesis stimulator
Bone Growth Stimulators AHM Clinical Indications Ultrasonic osteogenesis stimulator (e.g., the Sonic Accelerated Fracture Healing System (SAFHS)) medically necessary DME to Ultrasonic osteogenesis stimulator
Clinical Policy Title: Bone growth stimulators for non-healing fractures
 Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 POLICY TITLE ELECTRICAL BONE GROWTH STIMULATION OF THE APPENDICULAR Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 6/1/2018 Effective Date: 10/1/2018 POLICY PRODUCT VARIATIONS
POLICY TITLE ELECTRICAL BONE GROWTH STIMULATION OF THE APPENDICULAR Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 6/1/2018 Effective Date: 10/1/2018 POLICY PRODUCT VARIATIONS
HOW PEMF WORKS. Orthofix Pulsed Electromagnetic Field Technology
 HOW PEMF WORKS Orthofix Pulsed Electromagnetic Field Technology HOW PEMF STIMULATES SPINE FUSION How does PEMF affect spinal fusion? A successful spinal fusion depends on many complex healing processes.
HOW PEMF WORKS Orthofix Pulsed Electromagnetic Field Technology HOW PEMF STIMULATES SPINE FUSION How does PEMF affect spinal fusion? A successful spinal fusion depends on many complex healing processes.
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2018 Origination: 8/2002 Next Review: 8/2019 Policy Blue Cross and Blue Shield of Kansas
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2018 Origination: 8/2002 Next Review: 8/2019 Policy Blue Cross and Blue Shield of Kansas
Horizon BCBSNJ Uniform Medical Policy Manual. Treatment. Section:
 Horizon BCBSNJ Uniform Medical Policy Manual Section: Treatment Policy Number: 008 Effective Date: 08/12/2014 Original Policy Date: 10/05/1994 Last Review Date: 03/10/2015 Date Published to Web: 11/23/2011
Horizon BCBSNJ Uniform Medical Policy Manual Section: Treatment Policy Number: 008 Effective Date: 08/12/2014 Original Policy Date: 10/05/1994 Last Review Date: 03/10/2015 Date Published to Web: 11/23/2011
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion
FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion
Ultrasound Accelerated Fracture Healing Device. Description
 Subsection: Equipment Original Policy March 9, 2012 Subject: Ultrasound Accelerated Fracture Healing Device Date: Page: 1 of 13 Last Review Status/Date: December 2016 Ultrasound Accelerated Fracture Healing
Subsection: Equipment Original Policy March 9, 2012 Subject: Ultrasound Accelerated Fracture Healing Device Date: Page: 1 of 13 Last Review Status/Date: December 2016 Ultrasound Accelerated Fracture Healing
61 (17 direct current, 22 pulsed electromagnetic field, 22 control) 116 Osteonecrosis radiographic progression and Harris hip score
 Page 1 Appendix TABLE E-1 Summary of Included Clinical Studies* Type of Stimulation and Study Level of Evidence Indication Tested No. of Patients Outcome Measure Result Direct current Andersen 44 I Spinal
Page 1 Appendix TABLE E-1 Summary of Included Clinical Studies* Type of Stimulation and Study Level of Evidence Indication Tested No. of Patients Outcome Measure Result Direct current Andersen 44 I Spinal
Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature
 Assiotis et al. Journal of Orthopaedic Surgery and Research 2012, 7:24 RESEARCH ARTICLE Open Access Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective
Assiotis et al. Journal of Orthopaedic Surgery and Research 2012, 7:24 RESEARCH ARTICLE Open Access Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective
Populations Interventions Comparators Outcomes Individuals: With fracture nonunion
 Protocol Electrical Bone Growth Stimulation of the Appendicular Skeleton (70107) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09,
Protocol Electrical Bone Growth Stimulation of the Appendicular Skeleton (70107) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09,
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation of the Spine as an Adjunct
FEP Medical Policy Manual Effective Date: October 15, 2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation of the Spine as an Adjunct
The Skeletal System. Chapter 7a. Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life
 The Skeletal System Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life Chapter 7a Support Protection Movement Storage areas Minerals Lipids Hemopoiesis
The Skeletal System Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life Chapter 7a Support Protection Movement Storage areas Minerals Lipids Hemopoiesis
Chapter 5 The Skeletal System
 Chapter 5 The Skeletal System The Skeletal System Parts of the skeletal system Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle) Divided into two divisions Axial skeleton:
Chapter 5 The Skeletal System The Skeletal System Parts of the skeletal system Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle) Divided into two divisions Axial skeleton:
Mechanism of Action. Activating bone healing at every stage
 Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Ultrasound Bone Healing System is an FDA-approved bone healing device that uses low intensity
Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Ultrasound Bone Healing System is an FDA-approved bone healing device that uses low intensity
The Impact of Low-Intensity Pulsed Ultrasound (LIPUS) on Risk Factors for Fracture Nonunion
 ORS would like to highlight the following article supported by funding from Bioventus LLC, Durham, NC as our thank you for being a Champion Corporate Supporter. To learn more about opportunities to support
ORS would like to highlight the following article supported by funding from Bioventus LLC, Durham, NC as our thank you for being a Champion Corporate Supporter. To learn more about opportunities to support
5.1 BONES: AN OVERVIEW
 Unit 5 Skeletal System 5.1 BONES: AN OVERVIEW Section Objectives Identify the major structures and functions of the skeletal system. Differentiate between the two divisions (axial and appendicular) of
Unit 5 Skeletal System 5.1 BONES: AN OVERVIEW Section Objectives Identify the major structures and functions of the skeletal system. Differentiate between the two divisions (axial and appendicular) of
Electrical Bone Growth Stimulation of the Appendicular Skeleton
 Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 01/01/18 Next Review Date: 09/18 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 01/01/18 Next Review Date: 09/18 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Mechanism of Action. Activating bone healing at every stage
 Mechanism of Action Activating bone healing at every stage Ultrasound Bone Healing System Mechanism of Action The Bone Healing System is a unique fracture healing device that uses low intensity pulsed
Mechanism of Action Activating bone healing at every stage Ultrasound Bone Healing System Mechanism of Action The Bone Healing System is a unique fracture healing device that uses low intensity pulsed
Human Skeletal System Glossary
 Acromegaly Apatite Acromegaly - is a condition which involves excessive growth of the jaw, hands, and feet. It results from overproduction of somatotropin in adults (after fusion of the ossification centres
Acromegaly Apatite Acromegaly - is a condition which involves excessive growth of the jaw, hands, and feet. It results from overproduction of somatotropin in adults (after fusion of the ossification centres
Tobacco and Bone Health
 Tobacco and Bone Health Prof. Dr. Alok Chandra Agrawal MS Orthopaedics, DNB Orthopaedics, PhD Orthopaedics MAMS All India Institute of Medical Sciences Raipur CG Cigarette smoking is commonly identified
Tobacco and Bone Health Prof. Dr. Alok Chandra Agrawal MS Orthopaedics, DNB Orthopaedics, PhD Orthopaedics MAMS All India Institute of Medical Sciences Raipur CG Cigarette smoking is commonly identified
Mechanism of Action. Activating bone healing at every stage
 Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Bone Healing System is a unique fracture healing device that uses low intensity pulsed ultrasound
Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Bone Healing System is a unique fracture healing device that uses low intensity pulsed ultrasound
.org. Tibia (Shinbone) Shaft Fractures. Anatomy. Types of Tibial Shaft Fractures
 Tibia (Shinbone) Shaft Fractures Page ( 1 ) The tibia, or shinbone, is the most common fractured long bone in your body. The long bones include the femur, humerus, tibia, and fibula. A tibial shaft fracture
Tibia (Shinbone) Shaft Fractures Page ( 1 ) The tibia, or shinbone, is the most common fractured long bone in your body. The long bones include the femur, humerus, tibia, and fibula. A tibial shaft fracture
The formation of blood cells is called. hemopoiesis. What does our bone store? Where do our bones store fat? yellow marrow.
 What are the 5/6 functions of the skeletal system? support, protection, movement, blood cell formation, storage, homeostasis The formation of blood cells is called hemopoiesis What does our bone store?
What are the 5/6 functions of the skeletal system? support, protection, movement, blood cell formation, storage, homeostasis The formation of blood cells is called hemopoiesis What does our bone store?
ZUSAMMENSTELLUNG VON WISSENSCHAFTLICHEN PUBLIKATIONEN ZUR WIRKUNG DER PULSIERENDER MAGNETFELDTHERAPIE AUF KNOCHEN- UND KNORPELZELLEN
 ZUSAMMENSTELLUNG VON WISSENSCHAFTLICHEN PUBLIKATIONEN ZUR WIRKUNG DER PULSIERENDER MAGNETFELDTHERAPIE AUF KNOCHEN- UND KNORPELZELLEN 1982 Effects of a pulsed electromagnetic field on a mixed chondroplastic
ZUSAMMENSTELLUNG VON WISSENSCHAFTLICHEN PUBLIKATIONEN ZUR WIRKUNG DER PULSIERENDER MAGNETFELDTHERAPIE AUF KNOCHEN- UND KNORPELZELLEN 1982 Effects of a pulsed electromagnetic field on a mixed chondroplastic
Fractures cause considerable
 Clin Orthop Relat Res (2016) 474:1553 1559 / DOI 10.1007/s11999-016-4816-1 Clinical Orthopaedics and Related Research A Publication of The Association of Bone and Joint Surgeons Published online: 5 April
Clin Orthop Relat Res (2016) 474:1553 1559 / DOI 10.1007/s11999-016-4816-1 Clinical Orthopaedics and Related Research A Publication of The Association of Bone and Joint Surgeons Published online: 5 April
ELECTRICITY: THE HISTORY AND SCIENCE OF BONE GROWTH STIMULATION FOR SPINAL FUSION
 ELECTRICITY: THE HISTORY AND SCIENCE OF BONE GROWTH STIMULATION FOR SPINAL FUSION PAUL ANDREW GLAZER, M.D., LIANE CLAMEN GLAZER, M.D. BETH ISRAEL-DEACONESS MEDICAL CENTER INTRODUCTION Despite advancements
ELECTRICITY: THE HISTORY AND SCIENCE OF BONE GROWTH STIMULATION FOR SPINAL FUSION PAUL ANDREW GLAZER, M.D., LIANE CLAMEN GLAZER, M.D. BETH ISRAEL-DEACONESS MEDICAL CENTER INTRODUCTION Despite advancements
Due in Lab. Due next week in lab - Scientific America Article Select one article to read and complete article summary
 Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Ethan M. Braunstein, M.D. 1, Steven A. Goldstein, Ph.D. 2, Janet Ku, M.S. 2, Patrick Smith, M.D. 2, and Larry S. Matthews, M.D. 2
 Skeletal Radiol (1986) 15:27-31 Skeletal Radiology Computed tomography and plain radiography in experimental fracture healing Ethan M. Braunstein, M.D. 1, Steven A. Goldstein, Ph.D. 2, Janet Ku, M.S. 2,
Skeletal Radiol (1986) 15:27-31 Skeletal Radiology Computed tomography and plain radiography in experimental fracture healing Ethan M. Braunstein, M.D. 1, Steven A. Goldstein, Ph.D. 2, Janet Ku, M.S. 2,
Chapter 6 & 7 The Skeleton
 Chapter 6 & 7 The Skeleton Try this Make clockwise circles with your RIGHT foot, while doing this, draw the number 6 in the air with you RIGHT hand what happens to your foot???? Bony Background Adult body
Chapter 6 & 7 The Skeleton Try this Make clockwise circles with your RIGHT foot, while doing this, draw the number 6 in the air with you RIGHT hand what happens to your foot???? Bony Background Adult body
Parts of the skeletal system. Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle)
 The Skeletal System The Skeletal System Parts of the skeletal system Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle) Divided into two divisions Axial skeleton Appendicular
The Skeletal System The Skeletal System Parts of the skeletal system Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle) Divided into two divisions Axial skeleton Appendicular
PowerPoint Lecture Slides. Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College. The Skeletal System Pearson Education, Inc.
 PowerPoint Lecture Slides Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College CHAPTER 5 The Skeletal System 2012 Pearson Education, Inc. Title Classification of Bones and Gross Anatomy
PowerPoint Lecture Slides Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College CHAPTER 5 The Skeletal System 2012 Pearson Education, Inc. Title Classification of Bones and Gross Anatomy
The Skeletal System ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY PART A ELAINE N. MARIEB EIGHTH EDITION
 5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Ultrasound Accelerated Fracture Healing Device Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ultrasound Accelerated Fracture Healing Device Professional
Ultrasound Accelerated Fracture Healing Device Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ultrasound Accelerated Fracture Healing Device Professional
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
The Use of Bone Stimulators With Athletes. James Sullivan DPM, ATC EATA Symposium 2006 Philadelphia, Pennsylvania
 The Use of Bone Stimulators With Athletes James Sullivan DPM, ATC EATA Symposium 2006 Philadelphia, Pennsylvania Bone Anatomical Structure Physiological Organ Bone Anatomical Structure Provides rigid framework
The Use of Bone Stimulators With Athletes James Sullivan DPM, ATC EATA Symposium 2006 Philadelphia, Pennsylvania Bone Anatomical Structure Physiological Organ Bone Anatomical Structure Provides rigid framework
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
Original Contribution
 doi:10.1016/j.ultrasmedbio.2003.11.008 Ultrasound in Med. & Biol., Vol. 30, No. 3, pp. 389 395, 2004 Copyright 2004 World Federation for Ultrasound in Medicine & Biology Printed in the USA. All rights
doi:10.1016/j.ultrasmedbio.2003.11.008 Ultrasound in Med. & Biol., Vol. 30, No. 3, pp. 389 395, 2004 Copyright 2004 World Federation for Ultrasound in Medicine & Biology Printed in the USA. All rights
The Skeletal System. Chapter 4
 The Skeletal System Chapter 4 FUNCTIONS OF THE SKELETAL SYSTEM Support o Provides shape Protection o Internal organs Movement o Provides structure for muscle to act upon Storage o Minerals & fat Blood
The Skeletal System Chapter 4 FUNCTIONS OF THE SKELETAL SYSTEM Support o Provides shape Protection o Internal organs Movement o Provides structure for muscle to act upon Storage o Minerals & fat Blood
WHEN TO ADD BONE STIMULATION
 WHEN TO ADD BONE STIMULATION John Ketz, MD CSFS 2017 Bone Healing Fracture Hematoma (Immediate) Inflammatory Phase (Days) Reparative Phase (Weeks) Remodeling Phase (Months) 1/30/2017 2 Bone Healing Multiple
WHEN TO ADD BONE STIMULATION John Ketz, MD CSFS 2017 Bone Healing Fracture Hematoma (Immediate) Inflammatory Phase (Days) Reparative Phase (Weeks) Remodeling Phase (Months) 1/30/2017 2 Bone Healing Multiple
Unit 5 Skeletal System
 Unit 5 Skeletal System Nov 21 10:24 PM I. Functions A. Support: > internal framework, structure, anchors & supports soft tissue organs B. Protection: > protects vital organs C. Movement: > provides attach
Unit 5 Skeletal System Nov 21 10:24 PM I. Functions A. Support: > internal framework, structure, anchors & supports soft tissue organs B. Protection: > protects vital organs C. Movement: > provides attach
Skeletal Tissues Dr. Ali Ebneshahidi
 Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
AUGMENT. Bone Graft. Tibiotalar Fusion. rhpdgf-bb/β-tcp THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS
 rhpdgf-bb/β-tcp AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS CASE REPORT Tibiotalar Fusion as presented by Mark Glazebrook, MD Assoc Professor
rhpdgf-bb/β-tcp AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS CASE REPORT Tibiotalar Fusion as presented by Mark Glazebrook, MD Assoc Professor
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Skeletal Tissues. Dr. Ali Ebneshahidi
 Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection : Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection : Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Figure ) The area that causes the lengthwise growth of a long bone is indicated by letter. Diff: 2 Page Ref:
 Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 5 The Skeletal System Short Answer Figure 5.1 Using Figure 5.1, identify the following: 1) Spongy bone is indicated by letter. Diff: 1 Page Ref:
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 5 The Skeletal System Short Answer Figure 5.1 Using Figure 5.1, identify the following: 1) Spongy bone is indicated by letter. Diff: 1 Page Ref:
ProDisc-L Total Disc Replacement. IDE Clinical Study.
 ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
Understanding Osteoporosis
 Understanding Osteoporosis Professor Juliet E. Compston Published by Family Doctor Publications Limited in association with the British Medical Association IMPORTANT NOTICE This book is intended not as
Understanding Osteoporosis Professor Juliet E. Compston Published by Family Doctor Publications Limited in association with the British Medical Association IMPORTANT NOTICE This book is intended not as
The Skeletal System Vertebral column Sacrum. Osseous tissue For the body and soft organs. Magnesium, sodium, fluoride Levers for muscle action
 10/1/2016 Cranium Facial s Skull Clavicle Scapula Sternum Rib Humerus Vertebra Radius Ulna Carpals Thoracic cage (ribs and sternum) The Skeletal System Vertebral column Sacrum Phalanges Metacarpals Femur
10/1/2016 Cranium Facial s Skull Clavicle Scapula Sternum Rib Humerus Vertebra Radius Ulna Carpals Thoracic cage (ribs and sternum) The Skeletal System Vertebral column Sacrum Phalanges Metacarpals Femur
UNIT 5 THE SKELETAL SYSTEM
 UNIT 5 THE SKELETAL SYSTEM Nov 20 12:02 PM I. Functions A. Support: Internal framework, Structure, Anchors & Supports soft tissue/organs B. Protection: Protects vital organs C. Movement: Provide attach
UNIT 5 THE SKELETAL SYSTEM Nov 20 12:02 PM I. Functions A. Support: Internal framework, Structure, Anchors & Supports soft tissue/organs B. Protection: Protects vital organs C. Movement: Provide attach
Medical Policy POLICY POLICY GUIDELINES. MP Ultrasound Accelerated Fracture Healing Device
 Medical Policy MP 1.01.05 Last Review: 7/25/2017 Effective Date: 11/01/2017 Issue: 7:2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation
Medical Policy MP 1.01.05 Last Review: 7/25/2017 Effective Date: 11/01/2017 Issue: 7:2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation
The Skeletal System. Mosby items and derived items 2010, 2006, 2002, 1997, 1992 by Mosby, Inc., an affiliate of Elsevier Inc.
 The Skeletal System Functions of Skeletal System Provides internal framework that supports the body Protects internal organs Helps fight disease by producing white blood cells 2 Functions of Skeletal System
The Skeletal System Functions of Skeletal System Provides internal framework that supports the body Protects internal organs Helps fight disease by producing white blood cells 2 Functions of Skeletal System
The scapula is located on the back side of the ribcage and helps provide part of the shoulder joint and movement for the arms.
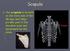 The scapula is located on the back side of the ribcage and helps provide part of the shoulder joint and movement for the arms. Scapula Humerus (Upper Arm Bone) Radius and Ulna Radius on Top Ulna on Bottom
The scapula is located on the back side of the ribcage and helps provide part of the shoulder joint and movement for the arms. Scapula Humerus (Upper Arm Bone) Radius and Ulna Radius on Top Ulna on Bottom
Functions of Skeletal System
 Skeletal System Skeletal System Adult Human has 206 Bones This slideshow will offer fun ways to remember the names of some of the bones, and you can come up with your own tricks! Functions of Skeletal
Skeletal System Skeletal System Adult Human has 206 Bones This slideshow will offer fun ways to remember the names of some of the bones, and you can come up with your own tricks! Functions of Skeletal
Chapter 7 /8 pgs SKELETAL TISSUES AND THE SKELETAL SYSTEM
 Chapter 7 /8 pgs. 189-250 SKELETAL TISSUES AND THE SKELETAL SYSTEM Skeletal Tissue Introduction Bone and cartilage are a specialized types of connective tissue Individual Bones are considered separate
Chapter 7 /8 pgs. 189-250 SKELETAL TISSUES AND THE SKELETAL SYSTEM Skeletal Tissue Introduction Bone and cartilage are a specialized types of connective tissue Individual Bones are considered separate
Skeletal System worksheet
 Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Enhancement of fracture healing-current trends
 Enhancement of fracture healing-current trends DR. CHETAN PRADHAN MS, F.ASIF (Swiss) CONSULTANT ORTHOPAEDIC SURGEON SANCHETI HOSPITAL, PUNE. Iliac crest : the gold standard Osteogenicity : ability to form
Enhancement of fracture healing-current trends DR. CHETAN PRADHAN MS, F.ASIF (Swiss) CONSULTANT ORTHOPAEDIC SURGEON SANCHETI HOSPITAL, PUNE. Iliac crest : the gold standard Osteogenicity : ability to form
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
Effect of Low-Intensity Pulsed Ultrasound Treatment for Delayed and Non-union Stress Fractures of the Anterior Mid-Tibia in Five Athletes
 Tokai J Exp Clin Med., Vol. 32, No. 4, pp. 121-125, 2007 Effect of Low-Intensity Pulsed Ultrasound Treatment for Delayed and Non-union Stress Fractures of the Anterior Mid-Tibia in Five Athletes Yoshiyasu
Tokai J Exp Clin Med., Vol. 32, No. 4, pp. 121-125, 2007 Effect of Low-Intensity Pulsed Ultrasound Treatment for Delayed and Non-union Stress Fractures of the Anterior Mid-Tibia in Five Athletes Yoshiyasu
Electrical Stimulation to Enhance Spinal Fusion: A Systematic Review
 Systematic Review 87 Electrical Stimulation to Enhance Spinal Fusion: A Systematic Review Paul Park 1 Darryl Lau 2 Erika D. Brodt 3 Joseph R. Dettori 3 1 Department of Neurological Surgery, University
Systematic Review 87 Electrical Stimulation to Enhance Spinal Fusion: A Systematic Review Paul Park 1 Darryl Lau 2 Erika D. Brodt 3 Joseph R. Dettori 3 1 Department of Neurological Surgery, University
7/23/2018 DESCRIBING THE FRACTURE. Pattern Open vs closed Location BASIC PRINCIPLES OF FRACTURE MANAGEMENT. Anjan R. Shah MD July 21, 2018.
 BASIC PRINCIPLES OF FRACTURE MANAGEMENT Anjan R. Shah MD July 21, 2018 DESCRIBING THE FRACTURE Pattern Open vs closed Location POLL OPEN HOW WOULD YOU DESCRIBE THIS FRACTURE PATTERN? 1 Spiral 2 Transverse
BASIC PRINCIPLES OF FRACTURE MANAGEMENT Anjan R. Shah MD July 21, 2018 DESCRIBING THE FRACTURE Pattern Open vs closed Location POLL OPEN HOW WOULD YOU DESCRIBE THIS FRACTURE PATTERN? 1 Spiral 2 Transverse
The Skeletal System in Action!! The Skeletal System in Action!
 Skeletal System The Skeletal System in Action!! The Skeletal System in Action! 5 Functions of the Skeletal System 1. Movement: Skeletal system provides points of attachment for muscles. Your legs and arms
Skeletal System The Skeletal System in Action!! The Skeletal System in Action! 5 Functions of the Skeletal System 1. Movement: Skeletal system provides points of attachment for muscles. Your legs and arms
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
