MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS FOR THE APPENDICULAR SKELETON
|
|
|
- Douglas Welch
- 6 years ago
- Views:
Transcription
1 MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS 06/22/16, 06/22/17, 04/26/18 PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an Essential Plan product, covers a specific service, medical policy criteria apply to the benefit. If a Medicare product covers a specific service, and there is no national or local Medicare coverage decision for the service, medical policy criteria apply to the benefit. POLICY STATEMENT: I. ELECTRICAL BONE GROWTH STIMULATOR A. Based upon our criteria and assessment of the peer-reviewed literature, electrical bone growth stimulation has not been demonstrated to improve patient outcomes in the applications of fresh fractures, stress fractures, delayed unions, or fresh bunionectomies and, therefore, is considered investigational. B. Based upon our criteria and review of the peer reviewed literature non-invasive electrical bone growth stimulation for the treatment of non-union secondary to trauma has been medically proven to be effective and therefore medically appropriate for the following indications: 1. The treatment for non-union secondary to trauma of the bones of the appendicular skeleton, including the humerus, ulna, radius, carpals, metacarpals, femur, tibia, fibula, tarsals, metatarsals, phalanges, scapula, clavicle, pelvis and patella. In order for coverage to be available, patients must meet all of the following criteria: a. Greater than or equal to 3 months have elapsed since injury or initial treatment; b. Serial radiographs of the preceding 3 month period have confirmed that no progressive signs of healing have occurred or unless the injury is greater than 6 months, shows no progressive signs of healing, and has not been actively treated; c. The fracture gap is one centimeter or less; and d. The patient can be adequately immobilized and, when appropriate, is likely to comply with non-weight bearing. 2. The treatment of infantile non-union; or 3. The treatment of failed joint fusion secondary to failed arthrodesis of the ankle or knee; or 4. As a non-surgical salvage for pseudoarthrosis (minimum nine months after last lumbar spinal fusion surgery). C. Contraindications to the use of an electrical bone growth stimulation include: 1. Fracture gaps greater than one centimeter; and 2. Patients with a demand-type pacemaker or an implantable cardioverter defibrillator. II. ULTRASONIC BONE GROWTH STIMULATOR A. Based upon our criteria and assessment of the peer reviewed literature, ultrasound accelerated fracture healing systems have been proven to be medically effective when used to treat non-union fractures (excluding fractures of the skull or vertebrae and tumor-related fractures) and are therefore medically appropriate when all of the following criteria are met: 1. At least 3 months have elapsed since injury; 2. Nonunion of the fracture is documented by a minimum of two sets of radiographs obtained prior to starting treatment with the US device, separated by a minimum of 90 days, each including multiple views of the fracture site, and with written interpretation by a physician stating that there has been no clinically significant evidence of fracture healing between the two sets of radiographs; A nonprofit independent licensee of the BlueCross BlueShield Association
2 PAGE: 2 OF: 7 3. The fracture gap is one cm or less; and 4. The patient can be adequately immobilized and is of an age where likely to comply with non-weightbearing. B. Based upon our criteria and review of the peer-reviewed literature ultrasound accelerated fracture healing systems do not significantly improve patient outcomes and are therefore not medically necessary for the following indications: 1. To accelerate healing of fresh, closed, posteriorly displaced distal radius fractures, 2. To accelerate healing of fresh, closed or Grade 1 tibial diaphysis fractures; 3. To accelerate fresh fractures, fusions, or delayed unions of the scaphoid (carpal navicular). 4. To treat delayed union of fractures; or 5. Treatment of congenital pseudoarthrosis; or 6. Treatment of Charcot arthropathy; treatment of fractures related to Charcot arthropathy using ultrasonic bone growth stimulators may be considered medically necessary when all of the criteria listed in IIA are met; or 7. Treatment of Osteogenesis Imperfecta. Refer to Corporate Medical Policy # regarding Bone Growth Stimulators; Invasive and Noninvasive Electrical Stimulation of the Spine. POLICY GUIDELINES: I. Prior authorization is contract dependent. Please refer to your Customer (Member/Provider) Services Department for contract information. II. Durable Medical Equipment rider/coverage is required. III. Ultrasound accelerated healing devices are not to be used in conjunction with any other noninvasive osteogenic stimulation devices. IV. The Federal Employee Health Benefit Program (FEHBP/FEP) requires that procedures, devices or laboratory tests approved by the U.S. Food and Drug Administration (FDA) may not be considered investigational and thus these procedures, devices or laboratory tests may be assessed only on the basis of their medical necessity. DESCRIPTION: I. Electrical bone growth stimulators are used to induce the growth of bones in cases of delayed union or non-union of fractures. Two methods of electrical bone growth stimulation are available: A. Non-invasive stimulators use an external power supply and externally applied coils that produce an electrical current to the fracture site via pulsed electromagnetic fields (PEMFs), combined electromagnetic field (CMF) technology, or capacitive coupling to stimulate bone growth. B. Invasive stimulators use a current generator that is surgically implanted in an intramuscular subcutaneous space and connected to an electrode that is implanted within the bone fragments that are hoped to be fused. The power source is removed in a second surgical procedure once it has discharged. II. Ultrasonic Accelerated Fracture, or Sonic Accelerated Fracture Healing System (SAFHS), is a non-invasive device that uses low intensity, pulsed, ultrasound therapy to stimulate and accelerate fracture healing time. The device consists of two main components: a signal generator about the size of a laptop computer and a small, square transducer connected to the generator by cable. The transducer is applied to the skin over the fracture site using a gel to facilitate transmission of the ultrasound signal. Delayed unions are defined by using clinical and radiographic findings suggesting an un-united fracture where the possibility of healing exists. Healing has not advanced at the "average" rate for the location and type of fracture.
3 PAGE: 3 OF: 7 Non-unions are defined as radiographic findings with clinical mobility of the bone fragments and where bone healing has ceased and there has been more than 3 months since the time of the fracture. Delayed union differs from non-union in that in the former, there are no indications that union will fail, while in the latter, there are no longer any visible signs that union will occur. Refer to Corporate Medical Policy # regarding Extracorporeal Shock Wave Therapy Refer to Corporate Medical Policy # regarding Bone Growth Stimulators: Invasive and Noninvasive Electrical Stimulation of the Spine RATIONALE: The FDA has given premarket approval for the EBI Bone Healing System, the Orthologic Bone Growth Stimulator, SpinalPak, Spinal-Stim Lite, Physio-Stim Life, OrthoPak, and SpinaLogic external stimulators and the Orthogen/ Osteogen, Zimmer Direct Current Bone Growth Stimulator, and SpF implanted spinal fusion stimulators. There is sufficient evidence reported in the peer-reviewed literature to conclude that external electrical stimulation improves outcomes for non-union of fractures, for infantile non-union, failed joint fusion, and for non-surgical salvage for pseudoarthrosis. Non-invasive and invasive electrical bone stimulation improves outcomes when used as an adjunct to spinal fusion surgery for patients at high risk for pseudoarthrosis. Improved outcomes have been achieved outside the investigational setting. A randomized controlled trial to determine if interferential current could significantly reduce healing time in new fractures of the tibia or prevent non-union found no significant difference in time to union compared to placebo. A randomized controlled trial to determine if interferential current would accelerate tibial stress fracture healing found no difference in time to healing between treatment and placebo groups. Greater device use and less weightbearing loading enhanced the effectiveness of the active device. A 2002 meta-analysis of trials of the effect of electrical stimulation on musculoskeletal systems included four studies of fresh fractures, all of them failing to provide evidence of efficacy. The FDA approved the BioniCare Stimulator Model BIO-1000 TM in 2003 for use as an adjunctive therapy in reducing the level of pain and symptoms associated with osteoarthritis of the knee. The BioniCare device is purported to stimulate chondrogenesis, however no studies have been performed in humans to evaluate whether chondrogenesis occurs with use of this device. No studies of the use of electrical bone growth stimulators in bunionectomies were identified. FDA premarket approval was granted the Exogen 2000 Sonic Accelerated Fracture Healing System (SAFHS ) in 1994 for treatment of fresh Colles fractures and open tibial diaphysis fractures when managed by closed reduction and casting and expanded to non-unions in Data presented to the FDA as part of the approval process for the SAFHS device demonstrated that 64 of 74 cases of non-union (mean fracture age nearly 3 years) were healed with use of low-intensity ultrasound. Patients receiving drugs which alter bone metabolism were excluded from studies of the device. Two studies of ultrasound after intramedullary nailing and fixation with absorbable screws showed no benefit from ultrasound. Most fresh fractures heal following standard care, such as closed reduction and casting.
4 PAGE: 4 OF: 7 The United Kingdom s National Institute for Health and Clinical Excellence (NICE) updated their guidance on lowintensity pulsed ultrasound for the treatment of nonunion and delayed fracture healing in NICE reached the following conclusions: Clinical evidence shows a high rate of fracture healing which supports the use of the EXOGEN ultrasound bone healing system to treat long-bone fractures with nonunion (failure to heal after 9 months). In addition, the EXOGEN ultrasound bone healing system to treat long-bone fractures with nonunion is associated with an estimated cost saving when compared with current management, through avoiding surgery. There is some radiological evidence of improved healing when the EXOGEN ultrasound bone healing system is used for long-bone fractures with delayed healing (no radiological evidence of healing after approximately 3 months). There are substantial uncertainties about the rate at which bone healing progresses without adjunctive treatment between 3 and 9 months after fracture, and about whether or not surgery would be necessary. These uncertainties result in a range of cost consequences, some cost-saving and others that are more costly than current management. Small randomized controlled trials suggest that ultrasound may accelerate healing of fresh fractures and promote healing in subgroups at risk for non-union. Larger trials are needed to confirm this. No studies were identified that included children less than 17 years old. The mechanism for the effect of ultrasound on bone healing is not fully understood. Foley et al published results of the investigational device exemption study of pulsed electromagnetic field (PEMF) stimulation (Cervical-Stim device from Orthofix) as an adjunct to anterior cervical discectomy and fusion (ACDF) with anterior cervical plates and allograft interbody implants. A total of 323 patients were randomized, 163 to PEMF and 160 to no stimulation. All patients were active smokers (more than one pack of cigarettes per day, 159 patients) or were undergoing multilevel ACDF (192 patients). The patients in the treatment group wore the Cervical-Stim device for 4 hours per day for 3 months starting 1 week after surgery. Efficacy was measured by radiographic analysis at 1, 2, 3, 6, and 12 months. Fusion rates for the 240 evaluable patients at 6 months were 83.6% for the PEMF group and 68.6% for the control group (p=.0065). By intent-to-treat analysis, assuming that nonevaluable patients did not have fusion, PEMF and control groups fusion rates were 65.6% and 56.3%, respectively (p=.0835). Of 245 patients available for follow-up at 12 months, fusion was achieved in116 of 125 PEMF patients and 104 of 120 control patients (p=.1129). Patient compliance, which was automatically monitored by the device, was assessed at each visit; however, compliance data were not included in the paper. The large number of dropouts, non-significant difference in fusion rates by intent-to-treat analysis, and lack of data on functional outcomes (e.g., pain, return to usual activity) limit interpretation of these study results. Thus, this technique is considered investigational for the cervical spine. The American Academy of Orthopedic Surgeons (AAOS) publishes information on Nonunions. Nonunions occur when a broken bone fails to heal and a delayed union is when a fracture takes longer than usual to heal. Some broken bones do not heal even when they get the best surgical or nonsurgical treatment because of inadequate stability, the blood supply is limited or lack of good nutrition to promote healing. Some bones can be expected to heal with minimal treatment due to inherent stability and excellent blood supply (toe bones). Other bones may not heal as quickly due to a limited blood supply (femoral head and neck, small wrist bone (scaphoid). Bones with moderate blood supply (tibia) may not heal quickly because the skin and muscle over the bone was damaged and the external blood supply was impaired. In addition, certain risk factors make it more likely that a bone will fail to heal. These risk factors include tobacco or nicotine use in any form, older age, severe anemia, diabetes, hypothyroidism, infection, certain medications, and low vitamin D level. CODES: Number Description Eligibility for reimbursement is based upon the benefits set forth in the member s subscriber contract. CODES MAY NOT BE COVERED UNDER ALL CIRCUMSTANCES. PLEASE READ THE POLICY AND GUIDELINES STATEMENTS CAREFULLY. Codes may not be all inclusive as the AMA and CMS code updates may occur more frequently than policy updates.
5 PAGE: 5 OF: 7 CPT: Electrical stimulation to aid bone healing; non-invasive (non-operative) invasive (operative) Low intensity ultrasound stimulation to aid bone healing, noninvasive (nonoperative) Copyright 2018 American Medical Association, Chicago, IL HCPCS: E0747 Osteogenesis stimulator, electrical, non-invasive; other than spinal applications E0748 E0749 E0760 spinal applications surgically implanted Osteogenesis stimulator, low intensity ultrasound, non-invasive ICD10: M80.00xS Age-related osteoporosis with current pathological fracture, unspecified site, sequela M80.021A-M80.879A Osteoporosis with current pathological fracture, initial encounter for fracture (code range) M84.30xS M84.38xS M84.40xS M84.421A-M84.673A M84.68xS Stress fracture, unspecified site, sequela Stress fracture, other site, sequela Pathological fracture, unspecified site, sequela Disorder of continuity of bone, initial encounter for fracture (code range) Pathological fracture in other disease, other site, sequela Q68.8 Other specified congenital musculoskeletal deformities Q71.61 Lobster-claw right hand Q71.63 Lobster-claw hand, bilateral Q74.0-Q74.9 Other congenital malformations of limb(s) (code range) Q87.0 Congenital malformation syndromes predominantly affecting facial appearance S42.201A-S42.92B S49.001A-S49.199A S52.001A-S52.92C S59.101A-S59.199A S72.001A-S72.499C S79.001A-S79.199A Fracture of shoulder and upper arm, initial encounter for closed or open fracture (code range) Other and unspecified injuries of shoulder and upper arm, initial encounter for closed fracture (code range) Fracture of forearm, initial encounter for closed or open fracture, initial encounter for open fracture type IIIA, IIIB, or IIIC (code range) Other and unspecified injuries of elbow and forearm, initial encounter for closed fracture (code range) Fracture of femur, initial encounter for closed fracture, initial encounter for open fracture type I or II, initial encounter for open fracture type IIIA, IIIB, or IIIC (code range) Other and unspecified injuries of hip and thigh, initial encounter for closed fracture, (code range)
6 PAGE: 6 OF: 7 REFERENCES: S82.101A-S82.866C S89.001A-S89.299A S92.301A-S92.356B Fracture of lower leg, including ankle, initial encounter for closed fracture, initial encounter for open fracture type I or II, initial encounter for open fracture type IIIA, IIIB, or IIIC (code range) Other and unspecified injuries of lower leg, initial encounter for closed fracture (code range) Fracture of unspecified metatarsal bone(s) and great toe, initial encounter for closed or open fracture, (code range) *Akai M, et al. Effect of electrical stimulation on musculoskeletal systems; a meta-analysis of controlled clinical trials. Bioelectromagnetics 2002;23: Aleem IS, et al. Efficacy of electrical stimulators for bone healing: a meta-analysis of randomized sham-controlled trials. Sci Rep. Aug ;6: American Academy of Orthopedic Surgeons (AAOS). Nonunions Mar, Updated 2007 Sep, Reviewed 2014 Mar. [ accessed 1/11/18. BlueCross BlueShield Association. Electrical bone growth stimulation of the appendicular skeleton. Medical Policy Reference Manual Policy # Apr 13. BlueCross BlueShield Association. Ultrasound accelerated fracture healing device. Medical Policy Reference Manual Policy # Dec 14. *Busse JW, et al. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ 2002 Feb 19;166(4): *Busse JW, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomized controlled trials. BMJ 2009:338:b351. *Dhawan SK, et al. The effect of pulsed electromagnetic fields on hindfoot arthrodesis: a prospective study. J Foot Ankle Surg 2004 Mar-Apr;43(2):93-6. Ebrahim S, et al. Low-intensity pulsed ultrasonography versus electrical stimulation for fracture healing: a systematic review and network meta-analysis. Can J Surg 2014 Jun;57(3):E *Foley KT, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J 2008;8(3): Griffin XL, et al. Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults. Cochrane Database Syst Rev 2011;(4):CD Griffin XL, et al. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst Rev 2014;6:CD Hannemann PF, et al. CT scan evaluated outcome of pulsed electromagnetic fields in the treatment of acute scaphoid fractures. Bone Joint J Aug 2014;96-B(8): Hannemann PF, et al. The clinical and radiological outcome of pulsed electromagnetic field treatment for acute scaphoid fractures: a randomised double-blind placebo-controlled multicentre trial. J Bone Joint Surg Br 2012;94(10): *Leung KS, et al. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol 2004 Mar;30(3):
7 PAGE: 7 OF: 7 *Livesley PJ, et al. Electrotherapy and the management of minimally displaced fracture of the neck of the humerus. Injury 1992;23(5): National Institute for Health and Care Excellence. NICE medical technology guidance 12: EXOGEN ultrasound bone healing system for long bone fractures with non-union or delayed healing Jan [ accessed 1/11/18. Poolman RW, et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ. Feb ;356:j576. *Rue JPH, et al. The effect of pulsed ultrasound in the treatment of tibial stress fractures. Orthoped 2004 Nov;27(11): *Rutten S, et al. Use of low-intensity pulsed ultrasound for posttraumatic nonunions of the tibia: a review of patients treated in the Netherlands. J Trauma 2007 Apr;62: Schandelmaier S, et al. Low intensity pulsed ultrasound for bone healing: systematic review of randomized controlled trials. BMJ. Feb ;356:j656. Shi H, et al. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of longbone fractures: a prospective randomized controlled study. BMC Musculoskeletal Disorders 2013;14:35. *Simmons JW Jr, et al. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electromagnetic fields. Am J Orthop 2004 Jan;33(1): *Simonis RB, et al. Electrical treatment of tibial non-union: a prospective, randomized, double-blind trial. Injury 2003 May;34(5): *key article KEY WORDS: Bone Growth Stimulator, Osteogenic Stimulator, SAFHS, Ultrasonic Bone Growth Stimulator, US. CMS COVERAGE FOR MEDICARE PRODUCT MEMBERS There is currently a National Coverage Determination (NCD) for Osteogenic Stimulators. Please refer to the following NCD website for Medicare Members:
MEDICAL POLICY SUBJECT: BONE GROWTH STIMULATORS
 MEDICAL POLICY PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Corporate Medical Policy
 Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2017 6/2018 6/2017 Description
Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2017 6/2018 6/2017 Description
Electrical Bone Growth Stimulators (Osteogenic Stimulation)
 Medical Policy Manual Durable Medical Equipment, Policy No. 83.11 Electrical Bone Growth Stimulators (Osteogenic Stimulation) Next Review: July 2018 Last Review: September 2017 Effective: October 1, 2017
Medical Policy Manual Durable Medical Equipment, Policy No. 83.11 Electrical Bone Growth Stimulators (Osteogenic Stimulation) Next Review: July 2018 Last Review: September 2017 Effective: October 1, 2017
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/17 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/17 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
09/12/17. I. Electrical Bone Growth Stimulator (invasive, semi-invasive, or non-invasive) any of the following: A-C
 Reference #: MC/F021 Page: 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/F021 Page: 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/15 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/15 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS)
 09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Low Intensity Ultrasound Accelerated Fracture Healing Device
 Page 1 of 14 Medical Policies - DME Low Intensity Ultrasound Accelerated Fracture Healing Device Print Number: DME101.030 Effective Date: 01-01-2015 Coverage: Low-intensity ultrasound treatment may be
Page 1 of 14 Medical Policies - DME Low Intensity Ultrasound Accelerated Fracture Healing Device Print Number: DME101.030 Effective Date: 01-01-2015 Coverage: Low-intensity ultrasound treatment may be
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 POLICY TITLE ELECTRICAL BONE GROWTH STIMULATION OF THE APPENDICULAR Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 6/1/2018 Effective Date: 10/1/2018 POLICY PRODUCT VARIATIONS
POLICY TITLE ELECTRICAL BONE GROWTH STIMULATION OF THE APPENDICULAR Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 6/1/2018 Effective Date: 10/1/2018 POLICY PRODUCT VARIATIONS
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP029 Section: Medical Benefit Policy Subject: Bone Growth Stimulator I. Policy: Bone Growth Stimulator II. Purpose/Objective: To provide a policy of coverage regarding
POLICIES AND PROCEDURE MANUAL Policy: MP029 Section: Medical Benefit Policy Subject: Bone Growth Stimulator I. Policy: Bone Growth Stimulator II. Purpose/Objective: To provide a policy of coverage regarding
Subject: Ultrasound Osteogenesis Stimulators, Non Invasive
 Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
UTILIZATION MANAGEMENT POLICY
 TITLE: BONE GROWTH STIMULATORS EFFECTIVE DATE: July 1, 2015 UTILIZATION MANAGEMENT POLICY DOCUMENT HISTORY Original Endorsement Date: May, 1991 Subsequent Endorsement Date(s): 02/1992, 03/1994, 03/1995,
TITLE: BONE GROWTH STIMULATORS EFFECTIVE DATE: July 1, 2015 UTILIZATION MANAGEMENT POLICY DOCUMENT HISTORY Original Endorsement Date: May, 1991 Subsequent Endorsement Date(s): 02/1992, 03/1994, 03/1995,
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Electrical Bone Growth Stimulation Page 1 of 42 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Electrical Bone Growth Stimulation of the Appendicular Skeleton
Electrical Bone Growth Stimulation Page 1 of 42 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Electrical Bone Growth Stimulation of the Appendicular Skeleton
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion
FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion
Corporate Medical Policy
 Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation of the Spine as an Adjunct
FEP Medical Policy Manual Effective Date: October 15, 2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation of the Spine as an Adjunct
Original Policy Date 12/1/9512:2013
 MP 1.01.03 Ultrasound Accelerated Fracture Healing Device Medical Policy Section Durable Medical Equipment Issue 12:2013 Original Policy Date 12/1/9512:2013 Last Review Status/Date 12:2013 Return to Medical
MP 1.01.03 Ultrasound Accelerated Fracture Healing Device Medical Policy Section Durable Medical Equipment Issue 12:2013 Original Policy Date 12/1/9512:2013 Last Review Status/Date 12:2013 Return to Medical
Electrical Bone Growth Stimulation of the Appendicular Skeleton
 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date June 11, 2014 Next Review Date December 2015
7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date June 11, 2014 Next Review Date December 2015
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton
FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton
Protocol. Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion (70185) Medical Benefit Effective Date: 07/01/12 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10,
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion (70185) Medical Benefit Effective Date: 07/01/12 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10,
Clinical Policy Title: Bone growth stimulators for non-healing fractures
 Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Geisinger Health Plan, Geisinger Indemnity Insurance Company and Geisinger Quality Options are
 Bone Growth Stimulator Policy # : MP029 Last Review Date : 2015-10-23 Status : Active Cross References : Reviewed Geisinger Health Plan, Geisinger Indemnity Insurance Company and Geisinger Quality Options
Bone Growth Stimulator Policy # : MP029 Last Review Date : 2015-10-23 Status : Active Cross References : Reviewed Geisinger Health Plan, Geisinger Indemnity Insurance Company and Geisinger Quality Options
Bone Growth Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Bone Growth Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Policy #: 331 Latest Review Date: February 2015
 Name of Policy: Bone Growth Stimulators: Ultrasound Policy #: 331 Latest Review Date: February 2015 Category: DME Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue
Name of Policy: Bone Growth Stimulators: Ultrasound Policy #: 331 Latest Review Date: February 2015 Category: DME Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue
Description. Section: Surgery Effective Date: April 15, 2015 Subsection: Surgery Original Policy Date: June 7, 2012 Subject: Page: 1 of 11
 Subject: Electrical Bone Growth Stimulation of the Last Review Status/Date: March 2015 Page: 1 of 11 Electrical Bone Growth Stimulation of the Description In the appendicular skeleton, electrical stimulation
Subject: Electrical Bone Growth Stimulation of the Last Review Status/Date: March 2015 Page: 1 of 11 Electrical Bone Growth Stimulation of the Description In the appendicular skeleton, electrical stimulation
Populations Interventions Comparators Outcomes Individuals: With fracture nonunion
 Protocol Electrical Bone Growth Stimulation of the Appendicular Skeleton (70107) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09,
Protocol Electrical Bone Growth Stimulation of the Appendicular Skeleton (70107) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09,
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Last Review Status/Date: December 2013 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Page: 1 of 10 Adjunct to Spinal Fusion Procedures Description Both
Last Review Status/Date: December 2013 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Page: 1 of 10 Adjunct to Spinal Fusion Procedures Description Both
Horizon BCBSNJ Uniform Medical Policy Manual. Treatment. Section:
 Horizon BCBSNJ Uniform Medical Policy Manual Section: Treatment Policy Number: 008 Effective Date: 08/12/2014 Original Policy Date: 10/05/1994 Last Review Date: 03/10/2015 Date Published to Web: 11/23/2011
Horizon BCBSNJ Uniform Medical Policy Manual Section: Treatment Policy Number: 008 Effective Date: 08/12/2014 Original Policy Date: 10/05/1994 Last Review Date: 03/10/2015 Date Published to Web: 11/23/2011
Electrical Bone Growth Stimulation of the Appendicular Skeleton
 Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Medical Policy Bulletin
 Medical Policy Bulletin Title: Policy #: Electrical Bone Growth Stimulation and Low Intensity Ultrasound Accelerated Fracture Healing System 05.00.09h The below medical or claim payment policy is applicable
Medical Policy Bulletin Title: Policy #: Electrical Bone Growth Stimulation and Low Intensity Ultrasound Accelerated Fracture Healing System 05.00.09h The below medical or claim payment policy is applicable
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2014 Origination: 8/2002 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas
Electrical Bone Growth Stimulation of the Appendicular Skeleton
 Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Electrical Bone Growth Stimulation of the Appendicular Skeleton Policy Number: 7.01.07 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 01/01/18 Next Review Date: 09/18 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 01/01/18 Next Review Date: 09/18 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date October 15, 2007 Next Review Date
7.01.85 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Section 7.0 Surgery Subsection Effective Date February 15, 2015 Original Policy Date October 15, 2007 Next Review Date
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14 THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
CIGNA HEALTHCARE COVERAGE POSITION
 CIGNA HEALTHCARE COVERAGE POSITION Subject Bone Growth Stimulators: Invasive, Noninvasive, Ultrasound Revised Date... 6/15/2005 Original Effective Date... 6/15/2004 Coverage Position Number... 0084 Table
CIGNA HEALTHCARE COVERAGE POSITION Subject Bone Growth Stimulators: Invasive, Noninvasive, Ultrasound Revised Date... 6/15/2005 Original Effective Date... 6/15/2004 Coverage Position Number... 0084 Table
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy File name: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures File code:
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy File name: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures File code:
Bone Growth Stimulators AHM
 Bone Growth Stimulators AHM Clinical Indications Ultrasonic osteogenesis stimulator (e.g., the Sonic Accelerated Fracture Healing System (SAFHS)) medically necessary DME to Ultrasonic osteogenesis stimulator
Bone Growth Stimulators AHM Clinical Indications Ultrasonic osteogenesis stimulator (e.g., the Sonic Accelerated Fracture Healing System (SAFHS)) medically necessary DME to Ultrasonic osteogenesis stimulator
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures. Original Policy Date
 MP 7.01.68 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with
MP 7.01.68 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with
Ultrasound Accelerated Fracture Healing Device. Description
 Subsection: Equipment Original Policy March 9, 2012 Subject: Ultrasound Accelerated Fracture Healing Device Date: Page: 1 of 13 Last Review Status/Date: December 2016 Ultrasound Accelerated Fracture Healing
Subsection: Equipment Original Policy March 9, 2012 Subject: Ultrasound Accelerated Fracture Healing Device Date: Page: 1 of 13 Last Review Status/Date: December 2016 Ultrasound Accelerated Fracture Healing
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2017 Origination: 8/2002 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Medical Policy POLICY POLICY GUIDELINES. MP Ultrasound Accelerated Fracture Healing Device
 Medical Policy MP 1.01.05 Last Review: 7/25/2017 Effective Date: 11/01/2017 Issue: 7:2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation
Medical Policy MP 1.01.05 Last Review: 7/25/2017 Effective Date: 11/01/2017 Issue: 7:2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation
Name of Policy: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Name of Policy: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy #: 524 Latest Review Date: May 2017 Category: DME Policy Grade: B Background/Definitions: As a general
Name of Policy: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy #: 524 Latest Review Date: May 2017 Category: DME Policy Grade: B Background/Definitions: As a general
Musculoskeletal System
 Musculoskeletal System CPT CPT copyright 2011 American Medical Association. All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the
Musculoskeletal System CPT CPT copyright 2011 American Medical Association. All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2018 Origination: 8/2002 Next Review: 8/2019 Policy Blue Cross and Blue Shield of Kansas
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Policy Number: 7.01.85 Last Review: 8/2018 Origination: 8/2002 Next Review: 8/2019 Policy Blue Cross and Blue Shield of Kansas
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Ultrasound Accelerated Fracture Healing Device Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ultrasound Accelerated Fracture Healing Device Professional
Ultrasound Accelerated Fracture Healing Device Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ultrasound Accelerated Fracture Healing Device Professional
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14, 09/15/15,09/21/17. THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION DEVICES FOR VENOUS THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION REVISED DATE: 06/26/14, 10/15/15, 06/16/16, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION REVISED DATE: 06/26/14, 10/15/15, 06/16/16, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria
61 (17 direct current, 22 pulsed electromagnetic field, 22 control) 116 Osteonecrosis radiographic progression and Harris hip score
 Page 1 Appendix TABLE E-1 Summary of Included Clinical Studies* Type of Stimulation and Study Level of Evidence Indication Tested No. of Patients Outcome Measure Result Direct current Andersen 44 I Spinal
Page 1 Appendix TABLE E-1 Summary of Included Clinical Studies* Type of Stimulation and Study Level of Evidence Indication Tested No. of Patients Outcome Measure Result Direct current Andersen 44 I Spinal
WHEN TO ADD BONE STIMULATION
 WHEN TO ADD BONE STIMULATION John Ketz, MD CSFS 2017 Bone Healing Fracture Hematoma (Immediate) Inflammatory Phase (Days) Reparative Phase (Weeks) Remodeling Phase (Months) 1/30/2017 2 Bone Healing Multiple
WHEN TO ADD BONE STIMULATION John Ketz, MD CSFS 2017 Bone Healing Fracture Hematoma (Immediate) Inflammatory Phase (Days) Reparative Phase (Weeks) Remodeling Phase (Months) 1/30/2017 2 Bone Healing Multiple
ICD-10 Service Line Overview Musculoskeletal
 ICD-10 Service Line Overview Musculoskeletal ICD-10 incorporates much greater clinical detail and specificity as well as updated terminology to be consistent with current clinical practices. ICD-10-CM
ICD-10 Service Line Overview Musculoskeletal ICD-10 incorporates much greater clinical detail and specificity as well as updated terminology to be consistent with current clinical practices. ICD-10-CM
Bone Composition. Bone is very strong for its relatively light weight The major components of bone are:
 Human Bones Bone Composition Bone is very strong for its relatively light weight The major components of bone are: Calcium carbonate Calcium phosphate Collagen Water Cortical Bone Spongy Bone Medullary
Human Bones Bone Composition Bone is very strong for its relatively light weight The major components of bone are: Calcium carbonate Calcium phosphate Collagen Water Cortical Bone Spongy Bone Medullary
HOW PEMF WORKS. Orthofix Pulsed Electromagnetic Field Technology
 HOW PEMF WORKS Orthofix Pulsed Electromagnetic Field Technology HOW PEMF STIMULATES SPINE FUSION How does PEMF affect spinal fusion? A successful spinal fusion depends on many complex healing processes.
HOW PEMF WORKS Orthofix Pulsed Electromagnetic Field Technology HOW PEMF STIMULATES SPINE FUSION How does PEMF affect spinal fusion? A successful spinal fusion depends on many complex healing processes.
Fractures cause considerable
 Clin Orthop Relat Res (2016) 474:1553 1559 / DOI 10.1007/s11999-016-4816-1 Clinical Orthopaedics and Related Research A Publication of The Association of Bone and Joint Surgeons Published online: 5 April
Clin Orthop Relat Res (2016) 474:1553 1559 / DOI 10.1007/s11999-016-4816-1 Clinical Orthopaedics and Related Research A Publication of The Association of Bone and Joint Surgeons Published online: 5 April
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS
 Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title COMPUTERIZED MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS Policy Number 2.01.13 Category Technology Assessment Effective Date
Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title COMPUTERIZED MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS Policy Number 2.01.13 Category Technology Assessment Effective Date
The Human Body. Lesson Goal. Lesson Objectives 9/10/2012. Provide a brief overview of body systems, anatomy, physiology, and topographic anatomy
 The Human Body Lesson Goal Provide a brief overview of body systems, anatomy, physiology, and topographic anatomy Medial Lateral Proximal Distal Superior Inferior Anterior Lesson Objectives Explain the
The Human Body Lesson Goal Provide a brief overview of body systems, anatomy, physiology, and topographic anatomy Medial Lateral Proximal Distal Superior Inferior Anterior Lesson Objectives Explain the
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- AIDED DETECTION (CAD) POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Human Skeletal System Glossary
 Acromegaly Apatite Acromegaly - is a condition which involves excessive growth of the jaw, hands, and feet. It results from overproduction of somatotropin in adults (after fusion of the ossification centres
Acromegaly Apatite Acromegaly - is a condition which involves excessive growth of the jaw, hands, and feet. It results from overproduction of somatotropin in adults (after fusion of the ossification centres
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND (TRUS)
 MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
Bone Growth Stimulators: Electrical (Invasive, Noninvasive), Ultrasound
 Medical Coverage Policy Effective Date...05/15/2018 Next Review Date...04/15/2019 Coverage Policy Number... 0084 Bone Growth Stimulators: Electrical (Invasive, Noninvasive), Ultrasound Table of Contents
Medical Coverage Policy Effective Date...05/15/2018 Next Review Date...04/15/2019 Coverage Policy Number... 0084 Bone Growth Stimulators: Electrical (Invasive, Noninvasive), Ultrasound Table of Contents
A. Incorrect! The appendicular skeleton includes bones of the shoulder, arm, hand, pelvis, leg and foot.
 Anatomy and Physiology - Problem Drill 08: The Skeletal System III No. 1 of 10 1. Which of the following statements about the appendicular skeleton is correct? A. The appendicular skeleton includes bones
Anatomy and Physiology - Problem Drill 08: The Skeletal System III No. 1 of 10 1. Which of the following statements about the appendicular skeleton is correct? A. The appendicular skeleton includes bones
The Skeletal System in Action!! The Skeletal System in Action!
 Skeletal System The Skeletal System in Action!! The Skeletal System in Action! 5 Functions of the Skeletal System 1. Movement: Skeletal system provides points of attachment for muscles. Your legs and arms
Skeletal System The Skeletal System in Action!! The Skeletal System in Action! 5 Functions of the Skeletal System 1. Movement: Skeletal system provides points of attachment for muscles. Your legs and arms
Surgical Care at the District Hospital. EMERGENCY & ESSENTIAL SURGICAL CARE
 Surgical Care at the District Hospital 1 18 Orthopedic Trauma Key Points 2 18.1 Upper Extremity Injuries Clavicle Fractures Diagnose fractures from the history and by physical examination Treat with a
Surgical Care at the District Hospital 1 18 Orthopedic Trauma Key Points 2 18.1 Upper Extremity Injuries Clavicle Fractures Diagnose fractures from the history and by physical examination Treat with a
Dr.Israa H. Mohsen. Lecture 5. The vertebral column
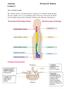 Anatomy Lecture 5 Dr.Israa H. Mohsen The vertebral column The vertebral column a flexible structure consisting of 33 vertebrae holds the head and torso upright, serves as an attachment point for the legs,
Anatomy Lecture 5 Dr.Israa H. Mohsen The vertebral column The vertebral column a flexible structure consisting of 33 vertebrae holds the head and torso upright, serves as an attachment point for the legs,
.org. Tibia (Shinbone) Shaft Fractures. Anatomy. Types of Tibial Shaft Fractures
 Tibia (Shinbone) Shaft Fractures Page ( 1 ) The tibia, or shinbone, is the most common fractured long bone in your body. The long bones include the femur, humerus, tibia, and fibula. A tibial shaft fracture
Tibia (Shinbone) Shaft Fractures Page ( 1 ) The tibia, or shinbone, is the most common fractured long bone in your body. The long bones include the femur, humerus, tibia, and fibula. A tibial shaft fracture
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
ICD-10 CM Training. Orthopaedic
 ICD-10 CM Training Orthopaedic ICD-10-CM Compliance Dates ICD-10-CM will be valid for dates of service on or after October 1, 2015 Outpatient dates of service of October 1, 2015 and beyond. Inpatient hospital
ICD-10 CM Training Orthopaedic ICD-10-CM Compliance Dates ICD-10-CM will be valid for dates of service on or after October 1, 2015 Outpatient dates of service of October 1, 2015 and beyond. Inpatient hospital
CHAPTER 8 LECTURE OUTLINE
 CHAPTER 8 LECTURE OUTLINE I. INTRODUCTION A. The appendicular skeleton includes the bones of the upper and lower extremities and the shoulder and hip girdles. B. The appendicular skeleton functions primarily
CHAPTER 8 LECTURE OUTLINE I. INTRODUCTION A. The appendicular skeleton includes the bones of the upper and lower extremities and the shoulder and hip girdles. B. The appendicular skeleton functions primarily
Part I : Study of Osteoporotic Fractures (SOF) Fractures and Falls History: History of Fractures Questionnaire
 PhenX Measure: Fracture History (#170900) PhenX Protocol: Fracture History (#170901) Date of Interview/Examination (MM/DD/YYYY): Part I : Study of Osteoporotic Fractures (SOF) Fractures and Falls History:
PhenX Measure: Fracture History (#170900) PhenX Protocol: Fracture History (#170901) Date of Interview/Examination (MM/DD/YYYY): Part I : Study of Osteoporotic Fractures (SOF) Fractures and Falls History:
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
MEDICAL POLICY SUBJECT: SACROILIAC JOINT FUSION/STABILIZATION: OPEN AND PERCUTANEOUS METHODS EFFECTIVE DATE: 12/15/16 REVISED DATE: 02/15/18
 MEDICAL POLICY SUBJECT: SACROILIAC JOINT FUSION/STABILIZATION: PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: SACROILIAC JOINT FUSION/STABILIZATION: PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL REVASCULARIZATION
 MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT POLICY GUIDELINES. Page: 1 of 5. Medical Policy Title CRANIAL ORTHOTICS Policy Number 1.01.
 Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title CRANIAL ORTHOTICS Policy Number 1.01.32 Category Equipment/Supplies Effective Date 10/18/01 Revised Date 06/27/02, 07/24/03, 06/24/04,
Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title CRANIAL ORTHOTICS Policy Number 1.01.32 Category Equipment/Supplies Effective Date 10/18/01 Revised Date 06/27/02, 07/24/03, 06/24/04,
Foot and Ankle Systems Coding Reference Guide
 Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
Chapter 8 The Skeletal System: The Appendicular Skeleton. Copyright 2009 John Wiley & Sons, Inc.
 Chapter 8 The Skeletal System: The Appendicular Skeleton Appendicular Skeleton It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton The pectoral girdle consists
Chapter 8 The Skeletal System: The Appendicular Skeleton Appendicular Skeleton It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton The pectoral girdle consists
Corporate Medical Policy
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
AMERICAN RED CROSS FIRST AID RESPONDING TO EMERGENCIES FOURTH EDITION Copyright 2006 by The American National Red Cross All rights reserved.
 Musculoskeletal injuries are most commonly caused by Mechanical forms of energy. Chemicals. Electrical energy. Heat Mechanical energy produces direct, indirect, twisting and contracting forces. Can be
Musculoskeletal injuries are most commonly caused by Mechanical forms of energy. Chemicals. Electrical energy. Heat Mechanical energy produces direct, indirect, twisting and contracting forces. Can be
Anatomy. Anatomy deals with the structure of the human body, and includes a precise language on body positions and relationships between body parts.
 Anatomy deals with the structure of the human body, and includes a precise language on body positions and relationships between body parts. Proper instruction on safe and efficient exercise technique requires
Anatomy deals with the structure of the human body, and includes a precise language on body positions and relationships between body parts. Proper instruction on safe and efficient exercise technique requires
The Musculoskeletal System
 The Musculoskeletal System Introduction The skeletal system and muscular system are often considered together because they are close in terms of structure and function. The two systems are referred to
The Musculoskeletal System Introduction The skeletal system and muscular system are often considered together because they are close in terms of structure and function. The two systems are referred to
Payment Policy. Chiropractic Care. Policy Specific Section: September 10, 2012 November 10, 2012
 Payment Policy Chiropractic Care Type: Payment Policy Policy Specific Section: Payment Original Policy Date: Effective Date: September 10, 2012 November 10, 2012 Description Chiropractic is a branch of
Payment Policy Chiropractic Care Type: Payment Policy Policy Specific Section: Payment Original Policy Date: Effective Date: September 10, 2012 November 10, 2012 Description Chiropractic is a branch of
Exercise 11. The Appendicular Skeleton
 Exercise 11 The Appendicular Skeleton The Appendicular Skeleton The appendicular skeleton contains 126 bones. Consists of the upper and lower limbs, the pectoral girdles, and the pelvic girdles. The pectoral
Exercise 11 The Appendicular Skeleton The Appendicular Skeleton The appendicular skeleton contains 126 bones. Consists of the upper and lower limbs, the pectoral girdles, and the pelvic girdles. The pectoral
Bones of Thorax (Rib Cage)
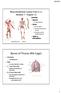 Musculoskeletal System (Part A-2) Module 7 -Chapter 10 Overview Muscles Attachments Bones Bone types Surface features of bones Divisions of the skeletal system Joints or Articulations Susie Turner, M.D.
Musculoskeletal System (Part A-2) Module 7 -Chapter 10 Overview Muscles Attachments Bones Bone types Surface features of bones Divisions of the skeletal system Joints or Articulations Susie Turner, M.D.
The Skeletal System THE APPENDICULAR SKELETON
 The Skeletal System THE APPENDICULAR SKELETON The appendicular skeleton consists of the girdles and the skeleton of the limbs. The upper (anterior) limbs are attached to the pectoral (shoulder) girdle
The Skeletal System THE APPENDICULAR SKELETON The appendicular skeleton consists of the girdles and the skeleton of the limbs. The upper (anterior) limbs are attached to the pectoral (shoulder) girdle
Lab-1. Miss. Lina Al-Onazy & samar Al-Wgeet =)
 Lab-1 Introduction The human skeleton is composed of 300 bones at birth and by the time adulthood is reached, some bones have fused together to give a total of 206 bones in the body. The human skeleton
Lab-1 Introduction The human skeleton is composed of 300 bones at birth and by the time adulthood is reached, some bones have fused together to give a total of 206 bones in the body. The human skeleton
Osteoarthrosis, unspecified whether generalized or localized, lower leg. Osteoarthrosis, localized, not specified whether primary or secondary, pelvic
 Page 1 Appendix TABLE E-1 Codes (and Definitions) in Humana Database Used for Study Inclusion and Exclusion of Patients Who Underwent,, or 1 to 2-Level Inclusion ICD-9-P-8154 Total knee replacement ICD-9-D-71596
Page 1 Appendix TABLE E-1 Codes (and Definitions) in Humana Database Used for Study Inclusion and Exclusion of Patients Who Underwent,, or 1 to 2-Level Inclusion ICD-9-P-8154 Total knee replacement ICD-9-D-71596
Successful Bone Healing of Nonunion After Ulnar Shortening Osteotomy for Smokers Treated With Teriparatide
 Successful Bone Healing of Nonunion After Ulnar Shortening Osteotomy for Smokers Treated With Teriparatide Takuya Uemura, MD, PhD; Mitsuhiro Okada, MD, PhD; Takuya Yokoi, MD; Kosuke Shintani, MD; Hiroaki
Successful Bone Healing of Nonunion After Ulnar Shortening Osteotomy for Smokers Treated With Teriparatide Takuya Uemura, MD, PhD; Mitsuhiro Okada, MD, PhD; Takuya Yokoi, MD; Kosuke Shintani, MD; Hiroaki
Injuries to the Extremities
 Injuries to the Extremities KNOWLEDGE OBJECTIVES 1. List seven signs and symptoms that suggest a serious extremity injury. 2. Describe how to care for injuries to the shoulder, upper arm, and elbow. 3.
Injuries to the Extremities KNOWLEDGE OBJECTIVES 1. List seven signs and symptoms that suggest a serious extremity injury. 2. Describe how to care for injuries to the shoulder, upper arm, and elbow. 3.
The skeletal system is the framework for the muscular system to attach to so we can move.
 Skeletal System The skeletal system is the framework for the muscular system to attach to so we can move. BONE: A rigid connective tissue Helps to move & support the body Protect the organs (skull, ribs)
Skeletal System The skeletal system is the framework for the muscular system to attach to so we can move. BONE: A rigid connective tissue Helps to move & support the body Protect the organs (skull, ribs)
FLORIDA MEDICARE PART B LOCAL MEDICAL REVIEW POLICY
 FLORIDA MEDICARE PART B LOCAL MEDICAL REVIEW POLICY CPT/HCPCS Codes 93925 Duplex scan of lower extremity arteries or arterial bypass grafts; complete bilateral study 93926 unilateral or limited study Policy
FLORIDA MEDICARE PART B LOCAL MEDICAL REVIEW POLICY CPT/HCPCS Codes 93925 Duplex scan of lower extremity arteries or arterial bypass grafts; complete bilateral study 93926 unilateral or limited study Policy
ICD-10 Service Line Overview Surgical
 ICD-10 Service Line Overview Surgical ICD-10 incorporates much greater clinical detail and specificity as well as updated terminology to be consistent with current clinical practices. ICD-10-CM and ICD-10-PCS
ICD-10 Service Line Overview Surgical ICD-10 incorporates much greater clinical detail and specificity as well as updated terminology to be consistent with current clinical practices. ICD-10-CM and ICD-10-PCS
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
11/25/2012. Chapter 7 Part 2: Bones! Skeletal Organization. The Skull. Skull Bones to Know Cranium
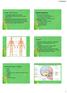 Chapter 7 Part 2: Bones! 5) Distinguish between the axial and appendicular skeletons and name the major parts of each 6) Locate and identify the bones and the major features of the bones that compose the
Chapter 7 Part 2: Bones! 5) Distinguish between the axial and appendicular skeletons and name the major parts of each 6) Locate and identify the bones and the major features of the bones that compose the
MEDICAL POLICY SUBJECT: BULKING AGENTS FOR TREATMENT OF URINARY OR FECAL INCONTINENCE. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific
MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific
10/12/2010. Upper Extremity. Pectoral (Shoulder) Girdle. Clavicle (collarbone) Skeletal System: Appendicular Skeleton
 Skeletal System: Appendicular Skeleton Pectoral girdle Pelvic girdle Upper limbs Lower limbs 8-1 Pectoral (Shoulder) Girdle Consists of scapula and clavicle Clavicle articulates with sternum (Sternoclavicular
Skeletal System: Appendicular Skeleton Pectoral girdle Pelvic girdle Upper limbs Lower limbs 8-1 Pectoral (Shoulder) Girdle Consists of scapula and clavicle Clavicle articulates with sternum (Sternoclavicular
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER AUGMENTATION FOR THE TREATMENT OF GASTROESOPHAGEAL REFLUX DISEASE (GERD)
 MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY. 1 Proprietary Information of YourCare Health Plan
 MEDICAL POLICY INTERFERENTIAL STIMULATORS Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized
MEDICAL POLICY INTERFERENTIAL STIMULATORS Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized
