Adrian C. Peña, M.D., 1 Camilo C. Roa, M.D, 2 Nazario A. Macalintal, M.D. 3 Vincent M. Balanag, Jr. M.D., 4 and Myrna T. Mendoza, M.D.
|
|
|
- Benedict Berry
- 6 years ago
- Views:
Transcription
1 Safety and Efficacy of Intravenous and Oral Azithromycin in Community Acquired Pneumonia: Results of an Open, Multicenter Study Among Filipino Patients Adrian C. Peña, M.D., 1 Camilo C. Roa, M.D, 2 Nazario A. Macalintal, M.D. 3 Vincent M. Balanag, Jr. M.D., 4 and Myrna T. Mendoza, M.D. 5 ( 1 East Avenue Medical Center, 2 Philippine General Hospital, 3 Mandaluyong City Medical Center, 4 Lung Center of the Philippines, 5 National Kidney and Transplant Institute; Correspondence: Dr. Adrian C. Peña, Room 223 University of the East Ramon Magsaysay Memorial Medical Center, Aurora Blvd., Quezon City. First published in the Phil J Internal Medicine 2003; 41: Reprinted with permission with modifications to conform to this journal s format.) ABSTRACT The mortality and morbidity associated with community-acquired pneumonia (CAP) remain significant. Recommendations emphasize the prompt initiation of proper antibiotic treatment, which should include coverage for atypical organisms in hospitalized CAP patients. Newer macrolides, like azithromycin, offer an advantage because of the potential for monotherapy, ease of once-a-day dosing, and convenience for switch therapy. This multicenter, open, non-comparative investigation among adult patients with clinical and radiographic/microbiologic evidence of CAP needing hospitalization was conducted in order to evaluate the efficacy and safety of azithromycin in the treatment of CAP requiring hospitalization. Intravenous azithromycin 500 mg once daily (OD) for at least two days followed by oral azithromycin 500 mg to complete a total of 7-10 days treatment were given to study subjects. Subjects were observed for a total of 35 days. Clinical response, radiographic and microbiological outcome, and adverse events were monitored at regular intervals. A total of fifty patients were enrolled and forty-one completed the study. These were mainly females who were tachypneic and slightly febrile on admission with leukocytosis and neutrophilic predominance. The most frequent microorganism isolated by culture was Streptococcus pneumoniae. Significant improvement in clinical parameters and physical findings were observed from initial to succeeding visits even after intention-to-treat analysis. Clinical improvement was documented in 98% (42/43) after at least 10 days with at least a presumed bacteriological eradication in 74% (32/43). The mean duration of IV therapy was three days with a mean total duration of eight days. Majority was switched to oral formulation by the fourth day. The most common adverse event reported was gastrointestinal disturbance, which occurred in eight subjects. Azithromycin, given as a single agent, is effective in adult CAP patients with no co-morbidities requiring hospitalization. Clinical response was very favorable with no documented relapse. It is generally safe and well tolerated. [Phil J Microbiol Infect Dis 2003; 32(4): ] Key Words: Community acquired pneumonia, CAP, azithromycin, azalide INTRODUCTION Community acquired pneumonia (CAP) is a surging health problem and continues to be a significant cause of mortality and morbidity even in developed countries. In the United States, approximately 1.7 million hospitalizations for CAP are reported each year at an annual cost of about US$ 23 billion. 1 In the Philippines, CAP remains to be among the top five causes of death and the figures have not changed significantly in the past few years. 2 These numbers might be more significant especially among the geriatric age group where death rates approach 40%. 3
2 Various consensus guidelines have been presented with emphasis on the proper diagnostic and therapeutic approach to pneumonia. 1,2,4,5 They consistently stress the critical aspect of initiating the proper antibiotic agents promptly, which is associated with more favorable outcome. 5 The role of empiric therapy is likewise stressed since causative pathogens are identified in fewer than 50% of patients despite exhaustive investigations. The chosen antimicrobial regimen should cover for core organisms such as Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis, as well as atypical organisms. 1,2,4 The role of macrolides as a single agent or in combination with other antimicrobials has been recognized by panel recommendations principally because of its excellent activity against commonly encountered organisms in CAP patients requiring hospitalization. 5 Newer agents of this class, like azithromycin, offer excellent activity against H. influenzae, fewer adverse events compared to erythromycin, convenience of once daily dosing, and high tissue concentrations. The American Thoracic Society (ATS) has recommended monotherapy with intravenous azithromycin for hospitalized CAP patients who do not require ICU admission, without cardiopulmonary disease and modifying factors (group IIIb). 1 The importance of switching therapy from intravenous to oral formulation is likewise an issue because this will potentially increase compliance and was proven to shorten hospital stay and reduce overall cost of care. 6 Antimicrobial preparations with superior bioavailability and tissue concentration after ingestion will definitely offer an advantage in terms of management. This study was primarily undertaken to evaluate the safety of azithromycin and clinical response of patients to azithromycin in the management of CAP requiring hospitalization. This is the first local study to investigate the efficacy of intravenous followed by oral azithromycin as monotherapy among this group of CAP patients. MATERIALS AND METHODS Study Design. This was an open, non-comparative multicenter investigation conducted in in-patient setting of the following centers: East Avenue Medical Center, Philippine General Hospital, National Kidney and Transplant Institute, Lung Center of the Philippines, and Mandaluyong City Medical Center. The study was conducted in accordance with the ethical principles contained in the Declaration of Helsinki and International Conference on Harmonization (ICH) - Good Clinical Practice Guidelines. All patients gave their written informed consent prior to participating in the study. Patient Selection. Patients with clinical and radiological evidence of CAP were included in this study with the following inclusion criteria: A. Men and women who were at least 18 years of age. Women of childbearing potential, including women who are postmenopausal for less than two years, must have a negative urine gonadotropin pregnancy test prior to entry into the study, confirmed by a negative serum gonadotropin pregnancy test. All women with childbearing potential, including women who are postmenopausal for less than 2 years, must use adequate contraception both during and for 3 months after the last dose of study drug has been taken.
3 B. Clinical and radiographic findings consistent with CAP: 1. New pulmonary infiltrate on chest x-ray that cannot be attributed to some other etiology 2. Any one of the following: a. cough b. tachypnea (RR> 20/min) c. tachycardia (HR>100/min) d. fever (T>37.8 C) AND 3 at least one abnormal physical chest finding of rhonchi, crackles or wheezes. Pneumonia is of a severity that warrants hospitalization and initial IV therapy. Criteria for exclusion 1. Pregnant or lactating women or women of childbearing potential not practicing adequate methods of contraception 2. Treatment with any other potentially effective antibiotic within 24 hours prior to enrollment or concomitantly with the study drug 3. Known sensitivity or intolerance to macrolide antibiotics 4. Presence of infection at enrollment that may require treatment with an antibiotic other than the study drug 5. Severe pneumonia requiring critical care interventions or additional antimicrobials 6. Patients with underlying lung disease, significant hematological, renal, cardiovascular, or hepatic disease, AIDS, HIV infection, or metastatic tumor 7. Donation of blood or blood products for transfusion during and for 6 weeks after the study Medication. Following a full assessment at the initial screening visit, eligible patients were given azithromycin administered at a dose of 500 mg OD in 250 ml or 500 ml compatible diluent. The infusate concentration and rate of infusion for azithromycin was either 1 mg/ml over 3 hours or 2 mg/ml over 1 hour for 2-5 days, depending upon the clinician s judgment. Azithromycin intravenous administration was followed by the oral capsule preparation at a dose of 500 mg OD to complete a total of 7-10 days of therapy, depending on clinical scenario. Oral azithromycin was given at least one hour before or 2 hours after meals. Monitoring. Each patient was evaluated by the investigator on four subsequent visits: Day 1 or before starting treatment (Visit 1 or baseline visit), after 3 days (Visit 2), after 10 to 14 days (Visit 3), and thereafter on Days 28 to 35 (Visit 4). During the baseline visit, the following were obtained: 1. Baseline physical examination and clinical assessment of signs and symptoms 2. Chest x-ray, postero-anterior view (CXR-PA) 3. CBC with differential count 4. Liver function test, BUN, creatinine, glucose, sodium, potassium
4 5. Prothrombin time, partial thromboplastin time 6. Two sets of blood culture obtained from two different sites. If blood culture turns out to be positive for a pathogen, follow-up blood cultures were obtained during subsequent evaluations until they were negative 7. Urine for pregnancy test on women of childbearing potential and urinalysis 8. Arterial blood gases, if clinically indicated 9. Expectorated sputum or endotracheal aspirate for Gram stain and routine aerobic bacterial culture 10. Any material from the respiratory system like pleural fluid or tissue was sent for culture upon the clinicians discretion Patients with no isolated pathogen from adequate samples were considered bacteriologically non-evaluable but were followed up for clinical response and evaluation Throughout their hospital stay, patients were monitored daily for any adverse event, clinical assessments of signs and symptoms were recorded, changes in concomitant medication were noted, and arterial blood gases were obtained if indicated. On the third day of hospitalization and at the end of treatment, CXR films, urinalysis, baseline blood tests, Gram stain of any respiratory specimen, and two sets of blood culture (if baseline culture was positive) were repeated. Vital signs (temperature, RR, PR, and blood pressure) were monitored and recorded at every visit. The severity of fever, cough, purulent sputum, dyspnea, cyanosis, rales, rhonchi and wheeze were graded from 0 to 3, corresponding to none, mild, moderate, and severe, respectively. After at least one month from entry into the study, individuals were followed up and physical examinations and clinical assessments for signs and symptoms were performed. CXR films were repeated if radiographs taken at initial visits did not show improvement or if the subjects clinical condition deteriorated. Bacteriologic examination of respiratory specimen and a repeat laboratory evaluation was performed if these were indicated or if the subject experienced a clinically significant adverse event. At any point during the study, the investigator could discontinue treatment or withdraw the patient upon his discretion with the surrounding circumstances clearly documented. Assessment of clinical response. The clinical, bacteriological, and radiological responses of the patients were recorded based on a preset criteria (see Appendix I). All adverse events were also recorded. Assessment of safety. This was assessed via: 1) adverse events observed by the investigators or volunteered by patients at each study visit, regardless of suspected causal relationship to the study medication; 2) clinically significant changes in physical examination findings; and 3) changes in standard laboratory test values requiring a change in drug dosage, discontinuation, or other interventions. A serious adverse event is defined as any drug experience occurring at any dose that: 1) results in death; 2) is life-threatening; 3) results in in-patient hospitalization or prolongation of existing hospitalization; 4) results in a persistent or significant disability/incapacity; or 5) results in congenital anomaly/birth defect.
5 Statistical Analysis. Means (for quantitative variables) and percent-ages (for categorical variables) were calculated to describe the characteristics of patients. Temperature, RR, PR and blood pressure were analyzed by repeated measures ANOVA to determine improvement of vital signs over time. The Friedman test was employed to compare each respiratory symptom of fever, cough, purulent sputum, dyspnea, cyanosis, rales, rhonchi and wheeze from baseline up to the last visit. Two sided tests were used and p values of.05 or less were taken to be statistically significant. Intention to treat analysis was carried out for patients who had at least one dose of the test drug. For patients withdrawn before the conclusion of the study, the last available examination was carried forward in the calculations. RESULTS Baseline Characteristics A total of 63 patients were screened of which 50 were subsequently included in the study. The demographics of the 50 subjects are shown in Table 1. Table 1. Demographic and clinical data characteristics of the patients studied (ITT population; n=50) Sex: Male (n, %) 21 (40%) Female (n, %) 29 (60%) Age: mean (range) in years 47.8 (18 89) Temperature at baseline: mean and SD in centigrade / Respiratory rate (bpm): mean and SD 25 +/ White Blood Cell (WBC) count (x 10 9/ L): mean and SD / Organisms isolated (n= 34 culture isolates) Streptococcus pneumoniae 9 Staphylococcus epidermidis 5 Alpha-hemolytic Streptococcus 4 Hemophilus influenzae 4 Klebsiella pneumoniae 2 Hemophilus parainfluenzae 2 Enterobacter aerogenes 2 Others 6 Forty-one subjects completed the study and were available for evaluation. Nine were withdrawn for the following reasons: lost to follow-up, patient went home against medical advice, high suspicion of underlying pulmonary tuberculosis, significant protocol violations in three cases and occurrence of serious adverse events in three cases. The study population was predominantly female with a wide range of ages, the oldest being 89 years old. Majority of the patients were slightly febrile and were tachypneic. All of them demonstrated an abnormal lung finding on physical exam with unilateral crackles being present in 90% of cases. An elevated WBC count was documented, the highest being x 10 9 /L with all cases showing a neutrophilic predominance in the differential count. Clinical Response Evaluation There was a significant (p value <0.001) normalization of temperature, RR and PR from initial visit to succeeding visits (Figure 1). These findings were similar after an
6 intention-to-treat analysis was done (Figure 2). Likewise, there was a significant improvement over succeeding visits for fever, cough, purulent sputum, dyspnea and rales considering the fifty individuals enrolled in the study (p value <0.001) (Figure 3). The finding of crackles was likewise reduced by the third day of therapy. There was no reported relapse in these parameters and improvement was well documented until the end of the study. Decreased breath sounds, chest pain/tightness, back pain, weakness and anorexia were other reported symptoms, which eventually disappeared on succeeding visits. (p value for PR, RR and temperature = <0.001) Figure 1. Mean values of clinical parameters for patients who completed all visits (n= 41) Figure 2. Mean values of clinical parameters after intention-to-treat analysis (n=50)
7 Figure 3. Mean scores of symptoms during visits (n=50) The overall clinical response at visit 3 (day 10-14) can be assessed in 43 patients and 98% (42 out of 43) showed at least an improvement of their clinical condition. At visit 4, overall clinical response of cure is 90% (Table 2). Table 2. Distribution of Patients According to Clinical Response Visit 3 Visit 4 Cure Improvement 21 Failure 1 3 Lost to follow-up 1 (improvement on Visit 3) Withdrawn due to AE 1 (failure on Visit 3) Bacteriological Evaluation In 34 subjects, Streptococcus pneumoniae was the most common causative agent of the CAP based on sputum culture. The other etiologic organisms are shown in Table 1. The remaining organisms included other Staphylococcus species and Enterococcus. Sixteen patients yielded no organism on culture and therefore, their bacteriological response could not be evaluated. In 91% (31 out of 34) of cases, at least a presumed eradication was concluded by the end of a 7-10 day treatment period. All organisms were sensitive to azithromycin except for two cases of Enterobacter aerogenes and one isolate of Pseudomonas aeruginosa. The overall bacteriological response of the 43 patients who had completed visit 3 is shown in table 3. Radiological Evaluation The chest x-rays of the 41 patients who completed the study revealed that only 15 normalized while the rest remained abnormal. Considering those who reached Visit 3 and Visit 4, the radiological response of these 43 patients are summarized in table 4.
8 Table 3. Distribution of Patients According to Bacteriological Evaluation Visit 3 Visit 4 Eradication 4 4 Presumed eradication Persistence 3 1 Eradication with reinfection 1 Superinfection Bacteriologically non-evaluable 9 7 Withdrawn 2 Lost to follow-up (1= presumed eradication on Visit 3) Adverse event (1= bacteriologically non-evaluable on Visit 3) Table 4. Distribution of Patients According to Radiologic Evaluation Visit 3 Visit 4 Resolution 6 15 Marked improvement Radiological failure 8 5 Non-evaluable 3 5 No x-ray 2 Withdrawn 2 Lost to follow-up (1= marked improvement on Visit 3) Averse event (1 = radiological failure on Visit 3) Duration of Therapy The mean duration of intravenous treatment was three days with a mean total duration of eight days of therapy. No patient received intravenous azithromycin beyond five days. Oral azithromycin was started by the third day in 6 patients and majority (25 patients) was shifted to oral therapy by the fourth day. Safety Outcome Safety data were available for all 50 patients who took at least one dose of study drug. Table 5 shows a summary of the adverse events. A total of 32 events were reported in 18 patients. Out of these events, seven were considered causally related to the study medication: 2 cases of diarrhea and 5 cases of local pain on intravenous site. All were mild in severity and no serious or severe events classified as treatment-related occurred. The three cases of serious events were withdrawn from the study. One patient had pleural effusion and another had acute respiratory distress syndrome. These medical conditions led to hospitalization and prolongation of existing hospitalization. The patient who had acute myocardial infarction expired on the first day of treatment with azithromycin. According to the investigator s judgment, these serious adverse events were not causally related to the study drug. DISCUSSION The unique structure of azithromycin, being an azalide, makes it ideal for once daily administration. 7 Following an oral dose, azithromycin is rapidly absorbed and distributes rapidly throughout the body. Rapid movement from blood into tissue results in
9 significantly higher drug concentrations in tissue than in plasma. This pharmacokinetic profile and high values for steady-state volumes of distribution suggest that the prolonged half-life is due to extensive uptake and subsequent release of the drug from tissues. Table 5. Adverse Events and Frequency (All Causality) Event Frequency Gastrointestinal disturbance 8 Phlebitis 6 Muscular pain 3 Fever 1 Pruritus 1 Hypertension 3 Cough 1 Asthma exacerbation 1 Chest heaviness 1 Headache 1 Fungal infection 1 Arthritis 1 Angioedema 1 Serious 3 (Myocardial infarction, Pleural effusion Acute Respiratory Distress Syndrome) The causative pathogens identified in this study were similar to other local investigations with S. pneumoniae and H. influenzae accounting for most cases. 8 Although no examination was done to document the possible existence of atypical organisms, the gram stains performed on all sputum samples, which assured a good quality of the respiratory specimen, yielded a variety of organisms. Guidelines on CAP have emphasized adequate antibiotic coverage for patients requiring hospitalization in the treatment of their infection (including atypical organism) who do not have other uncontrolled co-morbidities, a population that was included in this study. 1,4,5 The overall good clinical response to treatment with azithromycin emphasized the excellent activity of this drug in this patient group. The outcomes observed in this study support the in vitro findings demonstrating superior activity against S. pneumoniae, H. influenzae, S. aureus, and atypical organisms such as Mycoplasma pneumoniae and Legionella pneumophila. 7 A number of studies have highlighted the role of atypical organisms as causative agents in hospitalized CAP, 8-12 and antibiotic regimens should have adequate coverage against these pathogens. It is likewise a concern that these groups of bacteria are increasing among the geriatric age group. In our study, the oldest admitted patient was 89 years old. The pharmacokinetics of azithromycin in elderly subjects was shown to be similar to younger patients and no dosage adjustment is necessary. 7 Current investigations have stressed the benefits of adding macrolides into an empiric antibiotic regimen. 13,14 Such practices have led to shorter lengths of hospital stay and lower mortality rates compared to regimens without macrolides. Atypical organisms should always be considered when dealing with hospitalized cases of pneumonia. Majority of the patients (98%) were considered cured or improved at the end of 10 days of treatment. Improvement in clinical parameters from baseline to succeeding visits was significant even after an intention-to-treat analysis was performed.
10 Furthermore, even symptoms were significantly fewer by the third day of treatment in all cases. More than half of these patients were safely switched to oral therapy by the third or fourth day. The favorable clinical response of patients by the second to third day of intravenous treatment made it possible to shift to oral azithromycin rapidly. This implies that the causative organism(s) in these hospitalized pneumonia cases were appropriately covered with azithromycin given as monotherapy. These findings are consistent with a landmark study by Ramirez and colleagues on switch therapy among hospitalized CAP patients. 6 In that study, majority of patients can be safely switched to oral antibiotics by the third day of treatment. In addition, his study demonstrated that such practice leads to a shorter hospital stay and a reduction in the overall cost of care. Overall, this translated to improved patient outcome and satisfaction. A once-a-day drug, which can be given as a single agent, might probably improve patient compliance with treatment. The clinical responses in this study were maintained up to three to four weeks after treatment. There were no reported cases of relapse. Most of the adverse events reported in this study were related to gastrointestinal symptoms, consisting mainly of diarrhea and nausea, and local irritation at the intravenous site. This was similar to a larger study, which showed diarrhea in 5.4% of cases followed by irritation at the insertion site in 3.5%. 15 However these rates are significantly lower compared to a regimen with cefuroxime and erythromycin. 16 Overall, in a larger study among 8,000 subjects, oral azithromycin was well tolerated by the vast majority who received short-duration therapy, regardless of their underlying disease severity and their age. (7) Treatment related side effects occurred in 13.8% and discontinuation due to these reactions occurred in only 0.7% of patients. In our study, the three serious events were attributed mainly to the patients underlying concomitant illness. CONCLUSION Azithromycin is safe, well tolerated and effective among hospitalized CAP patients without co-morbidities. The once daily regimen can be administered as a single agent in this group of patients with the majority exhibiting favorable clinical response by the third day of treatment. Most patients can be switched to oral azithromycin by the 3 rd or 4 th day. Optimal clinical and bacteriological responses were documented and no relapse was evident after approximately a month of follow up. Acknowledgement This study was supported by Pfizer, Inc. REFERENCES 1. American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163: Task Force on CAP, Philippine Clinical Practice Guidelines Group in Infectious Diseases. Community-Acquired Pneumonia: Clinical Practice Guideline. PPG-ID Philippine Society for Microbiology and Infectious Diseases. Volume 1 Nuo.2, Quezon City, Philippines: NCP Publishing Corp., Ruiz M, Ewig S, Marcos MA. Etiology of community-acquired pneumonia: impact of age, co-morbidity, and severity. Am J Respir Crit Care Med 1999; 160:
11 4. Bartlett JG, Donnell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis 2000; 31: ASCAP Panel: Community-Acquired Pneumonia (CAP) Year 2002 Antibiotic Selection and Management Update: evaluation, risk stratification, and current antimicrobial treatment guidelines for hospital-based treatment of CAP. Hospital Medicine Consensus Reports 2002: Ramirez JA, Srinath L, Ahkee S, et al. Early switch from intravenous to oral antibiotics in the treatment of hospitalized patients with community-acquired pneumonia. Arch Intern Med 1995; 155: Pfizer data on file: 19 February Bernas G, Galvez B. Community-acquired pneumonia: etiology, clinical profile, and outcome. Phil J Chest Diseases 1997; 5(1): Bates JH, Campbell GD, Baron AL, et al. Microbial etiology of acute pneumonia in hospitalized patients. Chest 1992; 101: Woodhead MA, MacFarlane JT, McCracken JS, et al. Prospective study of the etiology and outcome of pneumonia in the community. Lancet 1987; 1: Marrie TJ, Durant H, Yates L. Community-acquired pneumonia requiring hospitalization: a 5-year prospective study. Rev Infect Dis 1989; 11: Marston BJ, Plouffe JF, File TM, et al. Incidence of community-acquired pneumonia requiring hospitalization: results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 1997; 157: Stahl J, Barza M, Dejardin J, et al. Effect of macrolides as part of initial empiric therapy on length of stay in patients hospitalized with community-acquired pneumonia. Arch Intern Med 1999; 159: Gleason P, Mechar T, Fine J, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med 1999; 159: Plouffe J, Schwartz D, Kolokathis A, et al. Clinical efficacy of intravenous followed by oral azithromycin monotherapy in hospitalized patients with community-acquired pneumonia. Antimicrob Agents and Chemotherapy 2000; 44(7): Garey KW, Amsden GW. Azithromycin vs. cefuroxime plus erythromycin in CAP. Ann Pharmacother 1999; 33: APPENDIX I CRITERIA FOR EVALUATED RESPONSE Clinical Response The clinical response will be classified by the investigator post-therapy for each patient at the end of treatment visit (10-14 days post therapy) as follows: Cure: Complete resolution of all signs and symptoms of pneumonia, no need to start additional antibiotics for pneumonia after the end of the study treatment. Improvement: Incomplete resolution of all signs and symptoms of pneumonia but no need to start additional antibiotics for pneumonia after the end of study treatment. Failure: Any of the following conditions: persistence or progression of all signs and symptoms after 3 days of therapy; development of new clinical findings consistent with active infection; death from pneumonia; or inability to complete the study because of adverse effects. The clinical response will be evaluated by the investigator post-therapy for ach patient at the 4-6 week posttherapy follow-up visit as follows: Cure: complete resolution of all signs and symptoms of pneumonia. Failure: Any of the following conditions: persistence or progression of all signs and symptoms after 3 days of therapy; development of new clinical findings consistent with active infection; death from pneumonia; or inability to complete the study because of adverse effects. Bacteriological Response The bacteriological response will be classified by the sponsor for each pretreatment causative pathogen for each patient at the end-of-therapy visit(10-14 days post-therapy) and at the 4-6 week post-therapy followup visit as follows:
12 Eradication: Elimination of the original causative organism(s) from the same site during or upon completion of therapy. Presumed eradication: Absence of adequate culture material for evaluation because the patient is clinically improved and there is no more sputum production, or repeat aspiration of pleural fluid is no longer justified. Persistence: Failure to eradicate the original causative organism(s) at all post-baseline time points from site previously listed whether signs of inflammation are present or not. Relapse: Reappearance of original causative organism from the original site of infection within 5 days of discontinuation of treatment or during treatment after a post-baseline culture has been negative. Superinfection: Development of a new lower respiratory tract infection during treatment or within 3 days post-treatment, that is due to a new or resistant pathogen which was not recognized as the original causative organism(s). Colonization: Positive sputum culture yielding a bacterial strain other than the primary causative isolate, appearing greater than 48 hours after starting therapy, persisting in at least two repeated cultures, and not associated with fever, leukocytosis, persistence or progression of pneumonia, or evidence of infection at a distant site. Eradication with reinfection: Elimination of the initial infecting pathogen followed by replacement with a new species, or new serotype or biotype of the same organism, in sputum, pleural fluid or blood, in the presence of signs and symptoms of infection after completion of therapy. Radiological Assessment Consecutive chest x-rays will be evaluated and their evolution graded as: Resolution: Disappearance of all radiological signs of infection. Marked improvement: Significant improvement in the radiological signs of infection compared to the baseline. Radiological failure: No change or worsening in the radiological signs of infection compared to the baseline. APPENDIX II Members of the study group who conducted this trial were: East Avenue Medical Center: Principal Investigator: Adrian C. Peña. Co-investigators: Michelangelo Sabas, Cecil Santos. Study Coordinator: Maria Theresa Gomez. Philippine General Hospital: Principal Investigator: Camilo C. Roa. Co-investigators: Lalaine Mortera, Jennifer Sandoval. Study Coordinator: Rachel Manansala. Mandaluyong City Medical Center: Principal Investigator: Nazario A. Macalintal. Co-investigators: Cesar Matias, Lovey Labayog. Study Coordinator: Czarina Fabiana, Emma Viernes. Lung Center of the Philippines: Principal Investigator: Vincent M. Balanag. Co-investigators: Teresa Barzaga, Romeo Labao Jr. National Kidney and Transplant Institute: Principal Investigator: Myrna T. Mendoza. Co-investigator: Primo Valenzuela
JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections
 Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Community Acquired Pneumonia. Abdullah Alharbi, MD, FCCP
 Community Acquired Pneumonia Abdullah Alharbi, MD, FCCP A 68 y/ male presented to the ED with SOB and productive coughing for 2 days. Reports poor oral intake since onset due to nausea and intermittent
Community Acquired Pneumonia Abdullah Alharbi, MD, FCCP A 68 y/ male presented to the ED with SOB and productive coughing for 2 days. Reports poor oral intake since onset due to nausea and intermittent
The McMaster at night Pediatric Curriculum
 The McMaster at night Pediatric Curriculum Community Acquired Pneumonia Based on CPS Practice Point Pneumonia in healthy Canadian children and youth and the British Thoracic Society Guidelines on CAP Objectives
The McMaster at night Pediatric Curriculum Community Acquired Pneumonia Based on CPS Practice Point Pneumonia in healthy Canadian children and youth and the British Thoracic Society Guidelines on CAP Objectives
Antimicrobial Stewardship in Community Acquired Pneumonia
 Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
Community-Acquired Pneumonia OBSOLETE 2
 Community-Acquired Pneumonia OBSOLETE 2 Clinical practice guidelines serve as an educational reference, and do not supersede the clinical judgment of the treating physician with respect to appropriate
Community-Acquired Pneumonia OBSOLETE 2 Clinical practice guidelines serve as an educational reference, and do not supersede the clinical judgment of the treating physician with respect to appropriate
KAISER PERMANENTE OHIO COMMUNITY ACQUIRED PNEUMONIA
 KAISER PERMANENTE OHIO COMMUNITY ACQUIRED PNEUMONIA Methodology: Expert opinion Issue Date: 8-97 Champion: Pulmonary Medicine Most Recent Update: 6-08, 7-10, 7-12 Key Stakeholders: Pulmonary Medicine,
KAISER PERMANENTE OHIO COMMUNITY ACQUIRED PNEUMONIA Methodology: Expert opinion Issue Date: 8-97 Champion: Pulmonary Medicine Most Recent Update: 6-08, 7-10, 7-12 Key Stakeholders: Pulmonary Medicine,
Community Acquired Pneumonia
 April 2014 References: 1. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL Mace SE, McCracken Jr. GH, Moor MR, St. Peter SD, Stockwell JA, and Swanson JT. The Management of
April 2014 References: 1. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL Mace SE, McCracken Jr. GH, Moor MR, St. Peter SD, Stockwell JA, and Swanson JT. The Management of
WORKSHOP. The Multiple Facets of CAP. Community acquired pneumonia (CAP) continues. Jennifer s Situation
 Practical Pointers pointers For for Your your Practice practice The Multiple Facets of CAP Dr. George Fox, MD, MSc, FRCPC, FCCP Community acquired pneumonia (CAP) continues to be a significant health burden
Practical Pointers pointers For for Your your Practice practice The Multiple Facets of CAP Dr. George Fox, MD, MSc, FRCPC, FCCP Community acquired pneumonia (CAP) continues to be a significant health burden
Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center
 Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Kathy Peters is a 63 y.o. patient that presents to your urgent care office today with a history
Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Kathy Peters is a 63 y.o. patient that presents to your urgent care office today with a history
Setting The setting was secondary care. The economic study was carried out in the USA.
 Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
an inflammation of the bronchial tubes
 BRONCHITIS DEFINITION Bronchitis is an inflammation of the bronchial tubes (or bronchi), which are the air passages that extend from the trachea into the small airways and alveoli. Triggers may be infectious
BRONCHITIS DEFINITION Bronchitis is an inflammation of the bronchial tubes (or bronchi), which are the air passages that extend from the trachea into the small airways and alveoli. Triggers may be infectious
Pneumonia Community-Acquired Healthcare-Associated
 Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Community Acquired Pneumonia. Background & Rationale to North American Guidelines. Lionel Mandell MD FRCPC Brussels Belgium
 Community Acquired Pneumonia Background & Rationale to North American Guidelines Lionel Mandell MD FRCPC Brussels Belgium Consider Impact of the disease Issues to reflect upon Impact of the Disease 3-4
Community Acquired Pneumonia Background & Rationale to North American Guidelines Lionel Mandell MD FRCPC Brussels Belgium Consider Impact of the disease Issues to reflect upon Impact of the Disease 3-4
Chapter 22. Pulmonary Infections
 Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
THE PHARMA INNOVATION - JOURNAL Acute exacerbation of chronic obstructive pulmonary disease, caused by viruses: the need of combined antiinfective
 Received: 19-11-2013 Accepted: 28-12-2013 ISSN: 2277-7695 CODEN Code: PIHNBQ ZDB-Number: 2663038-2 IC Journal No: 7725 Vol. 2 No. 11. 2014 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION
Received: 19-11-2013 Accepted: 28-12-2013 ISSN: 2277-7695 CODEN Code: PIHNBQ ZDB-Number: 2663038-2 IC Journal No: 7725 Vol. 2 No. 11. 2014 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION
Supplementary Online Content
 Supplementary Online Content Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory
Supplementary Online Content Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory
ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: 07/November/2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
PULMONARY EMERGENCIES
 EMERGENCIES I. Pneumonia A. Bacterial Pneumonia (most common cause of a focal infiltrate) 1. Epidemiology a. Accounts for up to 10% of hospital admissions in the U.S. b. Most pneumonias are the result
EMERGENCIES I. Pneumonia A. Bacterial Pneumonia (most common cause of a focal infiltrate) 1. Epidemiology a. Accounts for up to 10% of hospital admissions in the U.S. b. Most pneumonias are the result
Making the Right Call With. Pneumonia. Community-acquired pneumonia (CAP) is a. Community-Acquired. What exactly is CAP?
 Making the Right Call With Community-Acquired Pneumonia In this article: By Thomas J. Marrie, MD The case of Allyson Allyson, 32, presented to the emergency department with a 48-hour history of anorexia,
Making the Right Call With Community-Acquired Pneumonia In this article: By Thomas J. Marrie, MD The case of Allyson Allyson, 32, presented to the emergency department with a 48-hour history of anorexia,
PNEUMONIA. I. Background 6 th most common cause of death in U.S. Most common cause of infection related mortality
 Page 1 of 8 September 4, 2001 Donald P. Levine, M.D. University Health Center Suite 5C Office: 577-0348 dlevine@intmed.wayne.edu Assigned reading: pages 153-160; 553-563 PNEUMONIA the most widespread and
Page 1 of 8 September 4, 2001 Donald P. Levine, M.D. University Health Center Suite 5C Office: 577-0348 dlevine@intmed.wayne.edu Assigned reading: pages 153-160; 553-563 PNEUMONIA the most widespread and
Chapter 10 Respiratory System J00-J99. Presented by: Jesicca Andrews
 Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Epidemiology and Etiology of Community-Acquired Pneumonia 761 Lionel A. Mandell
 LOWER RESPIRATORY TRACT INFECTIONS Preface Thomas M. File, Jr xiii Community-Acquired Pneumonia: Pathophysiology and Host Factors with Focus on Possible New Approaches to Management of Lower Respiratory
LOWER RESPIRATORY TRACT INFECTIONS Preface Thomas M. File, Jr xiii Community-Acquired Pneumonia: Pathophysiology and Host Factors with Focus on Possible New Approaches to Management of Lower Respiratory
PNEUMONIA IN CHILDREN. IAP UG Teaching slides
 PNEUMONIA IN CHILDREN 1 INTRODUCTION 156 million new episodes / yr. worldwide 151 million episodes developing world 95% in developing countries 19% of all deaths in children
PNEUMONIA IN CHILDREN 1 INTRODUCTION 156 million new episodes / yr. worldwide 151 million episodes developing world 95% in developing countries 19% of all deaths in children
Management of Acute Exacerbations
 15 Management of Acute Exacerbations Cenk Kirakli Izmir Dr. Suat Seren Chest Diseases and Surgery Training Hospital Turkey 1. Introduction American Thoracic Society (ATS) and European Respiratory Society
15 Management of Acute Exacerbations Cenk Kirakli Izmir Dr. Suat Seren Chest Diseases and Surgery Training Hospital Turkey 1. Introduction American Thoracic Society (ATS) and European Respiratory Society
Community-acquired pneumonia in adults
 Prim Care Clin Office Pract 30 (2003) 155 171 Community-acquired pneumonia in adults Julio A. Ramirez, MD a,b, * a Department of Medicine, University of Louisville School of Medicine, 512 S. Hancock Street,
Prim Care Clin Office Pract 30 (2003) 155 171 Community-acquired pneumonia in adults Julio A. Ramirez, MD a,b, * a Department of Medicine, University of Louisville School of Medicine, 512 S. Hancock Street,
Lecture Notes. Chapter 16: Bacterial Pneumonia
 Lecture Notes Chapter 16: Bacterial Pneumonia Objectives Explain the epidemiology Identify the common causes Explain the pathological changes in the lung Identify clinical features Explain the treatment
Lecture Notes Chapter 16: Bacterial Pneumonia Objectives Explain the epidemiology Identify the common causes Explain the pathological changes in the lung Identify clinical features Explain the treatment
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for
Treatment of febrile neutropenia in patients with neoplasia
 Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
To Study The Cinico-Radiological Features And Associated Co-Morbid Conditions
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 17, Issue 7 Ver. 16 (July. 2018), PP 58-62 www.iosrjournals.org To study the clinico-radiological features
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 17, Issue 7 Ver. 16 (July. 2018), PP 58-62 www.iosrjournals.org To study the clinico-radiological features
Upper...and Lower Respiratory Tract Infections
 Upper...and Lower Respiratory Tract Infections Robin Jump, MD, PhD Cleveland Geriatric Research Education and Clinical Center (GRECC) Louis Stokes Cleveland VA Medical Center Case Western Reserve University
Upper...and Lower Respiratory Tract Infections Robin Jump, MD, PhD Cleveland Geriatric Research Education and Clinical Center (GRECC) Louis Stokes Cleveland VA Medical Center Case Western Reserve University
Pneumonia. Dr. Rami M Adil Al-Hayali Assistant professor in medicine
 Pneumonia Dr. Rami M Adil Al-Hayali Assistant professor in medicine Definition Pneumonia is an acute respiratory illness caused by an infection of the lung parenchyma, associated with recently developed
Pneumonia Dr. Rami M Adil Al-Hayali Assistant professor in medicine Definition Pneumonia is an acute respiratory illness caused by an infection of the lung parenchyma, associated with recently developed
UPDATE IN HOSPITAL MEDICINE
 UPDATE IN HOSPITAL MEDICINE FLORIDA CHAPTER ACP MEETING 2016 Himangi Kaushal, M.D., F.A.C.P. Program Director Memorial Healthcare System Internal Medicine Residency DISCLOSURES None OBJECTIVES Review some
UPDATE IN HOSPITAL MEDICINE FLORIDA CHAPTER ACP MEETING 2016 Himangi Kaushal, M.D., F.A.C.P. Program Director Memorial Healthcare System Internal Medicine Residency DISCLOSURES None OBJECTIVES Review some
Bacterial pneumonia with associated pleural empyema pleural effusion
 EMPYEMA Synonyms : - Parapneumonic effusion - Empyema thoracis - Bacterial pneumonia - Pleural empyema, pleural effusion - Lung abscess - Complicated parapneumonic effusions (CPE) 1 Bacterial pneumonia
EMPYEMA Synonyms : - Parapneumonic effusion - Empyema thoracis - Bacterial pneumonia - Pleural empyema, pleural effusion - Lung abscess - Complicated parapneumonic effusions (CPE) 1 Bacterial pneumonia
Respiratory Diseases and Disorders
 Chapter 9 Respiratory Diseases and Disorders Anatomy and Physiology Chest, lungs, and conducting airways Two parts: Upper respiratory system consists of nose, mouth, sinuses, pharynx, and larynx Lower
Chapter 9 Respiratory Diseases and Disorders Anatomy and Physiology Chest, lungs, and conducting airways Two parts: Upper respiratory system consists of nose, mouth, sinuses, pharynx, and larynx Lower
ETIOLOGIES AND TREATMENT OUTCOMES IN PATIENTS HOSPITALIZED WITH COMMUNITY-ACQUIRED PNEUMONIA (CAP) AT SRINAGARIND HOSPITAL, KHON KAEN, THAILAND
 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH ETIOLOGIES AND TREATMENT OUTCOMES IN PATIENTS HOSPITALIZED WITH COMMUNITY-ACQUIRED PNEUMONIA (CAP) AT SRINAGARIND HOSPITAL, KHON KAEN, THAILAND Wipa Reechaipichitkul
SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH ETIOLOGIES AND TREATMENT OUTCOMES IN PATIENTS HOSPITALIZED WITH COMMUNITY-ACQUIRED PNEUMONIA (CAP) AT SRINAGARIND HOSPITAL, KHON KAEN, THAILAND Wipa Reechaipichitkul
Beta-lactamase production should have no effect on Azithromycin activity.
 AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
How do we define pneumonia?
 Robert L. Keith MD FCCP Associate Professor of Medicine Division of Pulmonary Sciences & Critical Care Medicine Denver VA Medical Center University of Colorado Denver How do we define pneumonia? Fever
Robert L. Keith MD FCCP Associate Professor of Medicine Division of Pulmonary Sciences & Critical Care Medicine Denver VA Medical Center University of Colorado Denver How do we define pneumonia? Fever
Cefepime versus Ceftriaxone for Empiric Treatment of Hospitalized Patients with Community-Acquired Pneumonia
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1998, p. 729 733 Vol. 42, No. 4 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology Cefepime versus Ceftriaxone for Empiric Treatment of
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1998, p. 729 733 Vol. 42, No. 4 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology Cefepime versus Ceftriaxone for Empiric Treatment of
Diagnosis of Ventilator- Associated Pneumonia: Where are we now?
 Diagnosis of Ventilator- Associated Pneumonia: Where are we now? Gary French Guy s & St. Thomas Hospital & King s College, London BSAC Guideline 2008 Masterton R, Galloway A, French G, Street M, Armstrong
Diagnosis of Ventilator- Associated Pneumonia: Where are we now? Gary French Guy s & St. Thomas Hospital & King s College, London BSAC Guideline 2008 Masterton R, Galloway A, French G, Street M, Armstrong
PEDIATRIC INFLUENZA CLINICAL PRACTICE GUIDELINES
 PEDIATRIC INFLUENZA CLINICAL PRACTICE GUIDELINES DEFINITIONS AND BACKGROUND Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms. Signs
PEDIATRIC INFLUENZA CLINICAL PRACTICE GUIDELINES DEFINITIONS AND BACKGROUND Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms. Signs
Patient information: Pneumonia in adults (Beyond the Basics)
 Page 1 of 8 Official reprint from UpToDate www.uptodate.com 2014 UpToDate Patient information: Pneumonia in adults (Beyond the Basics) Authors Thomas J Marrie, MD Thomas M File, Jr, MD Section Editor John
Page 1 of 8 Official reprint from UpToDate www.uptodate.com 2014 UpToDate Patient information: Pneumonia in adults (Beyond the Basics) Authors Thomas J Marrie, MD Thomas M File, Jr, MD Section Editor John
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Pneumonia and influenza combined are the fifth leading
 Community-Acquired Pneumonia in Older Veterans: Does the Pneumonia Prognosis Index Help? Lona Mody, MD,* Rongjun Sun, PhD, and Suzanne Bradley, MD* OBJECTIVES: Mortality rates from pneumonia increase steadily
Community-Acquired Pneumonia in Older Veterans: Does the Pneumonia Prognosis Index Help? Lona Mody, MD,* Rongjun Sun, PhD, and Suzanne Bradley, MD* OBJECTIVES: Mortality rates from pneumonia increase steadily
Exam 1 Review. Cardiopulmonary Symptoms Physical Examination Clinical Laboratory Studies
 Exam 1 Review Cardiopulmonary Symptoms Physical Examination Clinical Laboratory Studies WBC Count Differential A patient had been admitted to the hospital for acute shortness of breath. A CXR examination
Exam 1 Review Cardiopulmonary Symptoms Physical Examination Clinical Laboratory Studies WBC Count Differential A patient had been admitted to the hospital for acute shortness of breath. A CXR examination
POLICY FOR TREATMENT OF LOWER RESPIRATORY TRACT INFECTIONS
 POLICY F TREATMENT OF LOWER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: June 2018 Approved by: The Drugs & Therapeutics Committee Date: July 2018 Implementation
POLICY F TREATMENT OF LOWER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: June 2018 Approved by: The Drugs & Therapeutics Committee Date: July 2018 Implementation
PANEL KEGAWAT DARURATAN SISTEM PERNAPASAN (SERANGAN ASMA AKUT, PNEUMONIA DAN COPD) dan EDEMA PARU
 1 PANEL KEGAWAT DARURATAN SISTEM PERNAPASAN (SERANGAN ASMA AKUT, PNEUMONIA DAN COPD) dan EDEMA PARU ASTHMA 2 2 Agonist Bronchodilator Response Anticholinergic Asthma Response Panel A COPD Response Panel
1 PANEL KEGAWAT DARURATAN SISTEM PERNAPASAN (SERANGAN ASMA AKUT, PNEUMONIA DAN COPD) dan EDEMA PARU ASTHMA 2 2 Agonist Bronchodilator Response Anticholinergic Asthma Response Panel A COPD Response Panel
Pneumonia Severity Scores:
 Pneumonia Severity Scores: Are they Accurate Predictors of Mortality? JILL McEWEN, MD FRCPC Clinical Professor Department of Emergency Medicine University of British Columbia Vancouver, BC Canada President,
Pneumonia Severity Scores: Are they Accurate Predictors of Mortality? JILL McEWEN, MD FRCPC Clinical Professor Department of Emergency Medicine University of British Columbia Vancouver, BC Canada President,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
Clinical, radiological and bacteriological profile of patients with community acquired pneumonia (CAP)
 Original Research Article Clinical, radiological and bacteriological profile of patients with community acquired pneumonia (CAP) R. S. Pushpa Kumari 1*, V. A. Vipula 2, Sonal Jain 3 1 Professor, Department
Original Research Article Clinical, radiological and bacteriological profile of patients with community acquired pneumonia (CAP) R. S. Pushpa Kumari 1*, V. A. Vipula 2, Sonal Jain 3 1 Professor, Department
Ceftizoxime in the treatment of infections in patients with cancer
 Journal of Antimicrobial Chemotherapy (98), Suppl. C, 67-73 Ceftizoxime in the treatment of infections in patients with cancer V. Fainstein, R. Bolivar,. Elting, M. Valdivieso and G. P. Bodey Department
Journal of Antimicrobial Chemotherapy (98), Suppl. C, 67-73 Ceftizoxime in the treatment of infections in patients with cancer V. Fainstein, R. Bolivar,. Elting, M. Valdivieso and G. P. Bodey Department
Pharmacokinetics of intravenous azithromycin and ceftriaxone when administered alone and concurrently to healthy volunteers
 Journal of Antimicrobial Chemotherapy (2002) 50, 1075 1079 DOI: 10.1093/jac/dkg003 Pharmacokinetics of intravenous azithromycin and ceftriaxone when administered alone and concurrently to healthy volunteers
Journal of Antimicrobial Chemotherapy (2002) 50, 1075 1079 DOI: 10.1093/jac/dkg003 Pharmacokinetics of intravenous azithromycin and ceftriaxone when administered alone and concurrently to healthy volunteers
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Hospital-acquired Pneumonia
 Hospital-acquired Pneumonia Hospital-acquired pneumonia (HAP) Pneumonia that occurs at least 2 days after hospital admission. The second most common and the leading cause of death due to hospital-acquired
Hospital-acquired Pneumonia Hospital-acquired pneumonia (HAP) Pneumonia that occurs at least 2 days after hospital admission. The second most common and the leading cause of death due to hospital-acquired
The Importance of Appropriate Treatment of Chronic Bronchitis
 ...CLINICIAN INTERVIEW... The Importance of Appropriate Treatment of Chronic Bronchitis An interview with Antonio Anzueto, MD, Associate Professor of Medicine, University of Texas Health Science Center,
...CLINICIAN INTERVIEW... The Importance of Appropriate Treatment of Chronic Bronchitis An interview with Antonio Anzueto, MD, Associate Professor of Medicine, University of Texas Health Science Center,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement
 CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
Unit II Problem 2 Pathology: Pneumonia
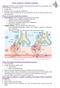 Unit II Problem 2 Pathology: Pneumonia - Definition: pneumonia is the infection of lung parenchyma which occurs especially when normal defenses are impaired such as: Cough reflex. Damage of cilia in respiratory
Unit II Problem 2 Pathology: Pneumonia - Definition: pneumonia is the infection of lung parenchyma which occurs especially when normal defenses are impaired such as: Cough reflex. Damage of cilia in respiratory
Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis
 Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Respiratory tract infections. Krzysztof Buczkowski
 Respiratory tract infections Krzysztof Buczkowski Etiology Viruses Rhinoviruses Adenoviruses Coronaviruses Influenza and Parainfluenza Viruses Respiratory Syncitial Viruses Enteroviruses Etiology Bacteria
Respiratory tract infections Krzysztof Buczkowski Etiology Viruses Rhinoviruses Adenoviruses Coronaviruses Influenza and Parainfluenza Viruses Respiratory Syncitial Viruses Enteroviruses Etiology Bacteria
To develop guidelines for the use of appropriate antibiotics for adult patients with CAP and guidance on IV to PO conversion.
 Page 1 of 5 TITLE: COMMUNITY-ACQUIRED PNEUMONIA (CAP) EMPIRIC MANAGEMENT OF ADULT PATIENTS AND IV TO PO CONVERSION GUIDELINES: These guidelines serve to aid clinicians in the diagnostic work-up, assessment
Page 1 of 5 TITLE: COMMUNITY-ACQUIRED PNEUMONIA (CAP) EMPIRIC MANAGEMENT OF ADULT PATIENTS AND IV TO PO CONVERSION GUIDELINES: These guidelines serve to aid clinicians in the diagnostic work-up, assessment
High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia
 High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia Review Andrew F. Shorr, MD, MPH, FCCP Department of Medicine Pulmonary and Critical Care Medicine, Washington Hospital Center, Washington,
High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia Review Andrew F. Shorr, MD, MPH, FCCP Department of Medicine Pulmonary and Critical Care Medicine, Washington Hospital Center, Washington,
Brice Taylor Assistant Professor Division of Pulmonary and Critical Care Medicine
 Brice Taylor Assistant Professor Division of Pulmonary and Critical Care Medicine Discuss advances in predicting prognosis Understand dwhat we know (and don t know) about the Microbiology Recognize important
Brice Taylor Assistant Professor Division of Pulmonary and Critical Care Medicine Discuss advances in predicting prognosis Understand dwhat we know (and don t know) about the Microbiology Recognize important
CAP, HCAP, HAP, VAP. 1. In 1898, William Osler described community-acquired pneumonia as:
 1. In 1898, William Osler described community-acquired pneumonia as: Brad Sharpe, M.D. Professor of Clinical Medicine Department of Medicine UCSF sharpeb@medicine.ucsf.edu I have no relevant financial
1. In 1898, William Osler described community-acquired pneumonia as: Brad Sharpe, M.D. Professor of Clinical Medicine Department of Medicine UCSF sharpeb@medicine.ucsf.edu I have no relevant financial
Repeated Pneumonia Severity Index Measurement After Admission Increases its Predictive Value for Mortality in Severe Community-acquired Pneumonia
 ORIGINAL ARTICLE Repeated Pneumonia Severity Index Measurement After Admission Increases its Predictive Value for Mortality in Severe Community-acquired Pneumonia Chiung-Zuei Chen, 1 Po-Sheng Fan, 2 Chien-Chung
ORIGINAL ARTICLE Repeated Pneumonia Severity Index Measurement After Admission Increases its Predictive Value for Mortality in Severe Community-acquired Pneumonia Chiung-Zuei Chen, 1 Po-Sheng Fan, 2 Chien-Chung
Respiratory Infections
 Respiratory Infections NISHANT PRASAD, MD THE DR. JAMES J. RAHAL, JR. DIVISION OF INFECTIOUS DISEASES NEWYORK-PRESBYTERIAN QUEENS Disclosures Stockholder: Contrafect Corp., Bristol-Myers Squibb Co Research
Respiratory Infections NISHANT PRASAD, MD THE DR. JAMES J. RAHAL, JR. DIVISION OF INFECTIOUS DISEASES NEWYORK-PRESBYTERIAN QUEENS Disclosures Stockholder: Contrafect Corp., Bristol-Myers Squibb Co Research
A Study of Prognosis and Outcome of Community Acquired Pneumonia in a Tertiary Care Centre
 ISSN: 2319-7706 Volume 4 Number 8 (2015) pp. 763-769 http://www.ijcmas.com Original Research Article A Study of Prognosis and Outcome of Community Acquired Pneumonia in a Tertiary Care Centre Bhadra Reddy
ISSN: 2319-7706 Volume 4 Number 8 (2015) pp. 763-769 http://www.ijcmas.com Original Research Article A Study of Prognosis and Outcome of Community Acquired Pneumonia in a Tertiary Care Centre Bhadra Reddy
Accurate Diagnosis Of Postoperative Pneumonia Requires Objective Data
 Accurate Diagnosis Of Postoperative Pneumonia Requires Objective Data David Ebler, MD David Skarupa, MD Andrew J. Kerwin, MD, FACS Jhun de Villa, MD Michael S. Nussbaum, MD, FACS J.J. Tepas III, MD, FACS
Accurate Diagnosis Of Postoperative Pneumonia Requires Objective Data David Ebler, MD David Skarupa, MD Andrew J. Kerwin, MD, FACS Jhun de Villa, MD Michael S. Nussbaum, MD, FACS J.J. Tepas III, MD, FACS
AWMSG SECRETARIAT ASSESSMENT REPORT. Azithromycin (Zedbac ) 500 mg powder for solution for infusion. Reference number: 2476 LIMITED SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Azithromycin (Zedbac ) 500 mg powder for solution for infusion Reference number: 2476 LIMITED SUBMISSION This report has been prepared by the All Wales Therapeutics
AWMSG SECRETARIAT ASSESSMENT REPORT Azithromycin (Zedbac ) 500 mg powder for solution for infusion Reference number: 2476 LIMITED SUBMISSION This report has been prepared by the All Wales Therapeutics
Acute pneumonia Simple complement
 Acute pneumonia Simple complement 1. Clinical variants of acute pneumonia in children are, except: A. Bronchopneumonia B. Lobar confluent pneumonia C. Viral pneumonia D. Interstitial pneumonia E. Chronic
Acute pneumonia Simple complement 1. Clinical variants of acute pneumonia in children are, except: A. Bronchopneumonia B. Lobar confluent pneumonia C. Viral pneumonia D. Interstitial pneumonia E. Chronic
11/19/2012. The spectrum of pulmonary diseases in HIV-infected persons is broad.
 The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Drug Class Review on Macrolides
 Drug Class Review on Macrolides Preliminary Scan Report 5 July 2014 Last Report: Original August 2006 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review on Macrolides Preliminary Scan Report 5 July 2014 Last Report: Original August 2006 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Polmoniti: Steroidi sì, no, quando. Alfredo Chetta Clinica Pneumologica Università degli Studi di Parma
 Polmoniti: Steroidi sì, no, quando Alfredo Chetta Clinica Pneumologica Università degli Studi di Parma Number of patients Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive
Polmoniti: Steroidi sì, no, quando Alfredo Chetta Clinica Pneumologica Università degli Studi di Parma Number of patients Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive
Guideline for the Management of Fever and Neutropenia in Children with Cancer and/or Undergoing Hematopoietic Stem-Cell Transplantation
 Guideline for the Management of Fever Neutropenia in Children with Cancer /or Undergoing Hematopoietic Stem-Cell Transplantation COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive
Guideline for the Management of Fever Neutropenia in Children with Cancer /or Undergoing Hematopoietic Stem-Cell Transplantation COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive
ISF criteria (International sepsis forum consensus conference of infection in the ICU) Secondary peritonitis
 Appendix with supplementary material. This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Supplementary Tables Table S1. Definitions
Appendix with supplementary material. This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Supplementary Tables Table S1. Definitions
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Critical Care Nursing Theory. Pneumonia. - Pneumonia is an acute infection of the pulmonary parenchyma
 - is an acute infection of the pulmonary parenchyma - is a common infection encountered by critical care nurses when it complicates the course of a serious illness or leads to acute respiratory distress.
- is an acute infection of the pulmonary parenchyma - is a common infection encountered by critical care nurses when it complicates the course of a serious illness or leads to acute respiratory distress.
Community Acquired Pneumonia Pediatric Ages 3 month to 18 years Clinical Practice Guideline MedStar Health Antibiotic Stewardship
 Community Acquired Pneumonia Pediatric Ages 3 month to 18 years Clinical Practice Guideline MedStar Health Antibiotic Stewardship These guidelines are provided to assist physicians and other clinicians
Community Acquired Pneumonia Pediatric Ages 3 month to 18 years Clinical Practice Guideline MedStar Health Antibiotic Stewardship These guidelines are provided to assist physicians and other clinicians
Guidelines/Guidance/CAP/ Hospitalized Child. PHM Boot Camp 2014 Jay Tureen, MD June 19, 2014
 Guidelines/Guidance/CAP/ Hospitalized Child PHM Boot Camp 2014 Jay Tureen, MD June 19, 2014 CAP in Children: Epi Greatest cause of death in children worldwide Estimated > 2 M deaths in children In developed
Guidelines/Guidance/CAP/ Hospitalized Child PHM Boot Camp 2014 Jay Tureen, MD June 19, 2014 CAP in Children: Epi Greatest cause of death in children worldwide Estimated > 2 M deaths in children In developed
Pneumonia. Definition of pneumonia Infection of the lung parenchyma Usually bacterial
 Pneumonia Definition of pneumonia Infection of the lung parenchyma Usually bacterial Epidemiology of pneumonia Commonest infectious cause of death in the UK and USA Incidence - 5-11 per 1000 per year Worse
Pneumonia Definition of pneumonia Infection of the lung parenchyma Usually bacterial Epidemiology of pneumonia Commonest infectious cause of death in the UK and USA Incidence - 5-11 per 1000 per year Worse
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective:
 GSK Medicine: abacavir (ABC)/dolutegravir (DTG)/lamivudine (3TC) Study Number: 201147 Title: A IIIb, randomized, open-label study of the safety, efficacy, and tolerability of switching to a fixed-dose
GSK Medicine: abacavir (ABC)/dolutegravir (DTG)/lamivudine (3TC) Study Number: 201147 Title: A IIIb, randomized, open-label study of the safety, efficacy, and tolerability of switching to a fixed-dose
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
GOALS AND INSTRUCTIONAL OBJECTIVES
 October 4-7, 2004 Respiratory GOALS: GOALS AND INSTRUCTIONAL OBJECTIVES By the end of the week, the first quarter student will have an in-depth understanding of the diagnoses listed under Primary Diagnoses
October 4-7, 2004 Respiratory GOALS: GOALS AND INSTRUCTIONAL OBJECTIVES By the end of the week, the first quarter student will have an in-depth understanding of the diagnoses listed under Primary Diagnoses
Recognizing MDR-TB in Children. Ma. Cecilia G. Ama, MD 23 rd PIDSP Annual Convention February 2016
 Recognizing MDR-TB in Children Ma. Cecilia G. Ama, MD 23 rd PIDSP Annual Convention 17-18 February 2016 Objectives Review the definitions and categorization of drugresistant tuberculosis Understand the
Recognizing MDR-TB in Children Ma. Cecilia G. Ama, MD 23 rd PIDSP Annual Convention 17-18 February 2016 Objectives Review the definitions and categorization of drugresistant tuberculosis Understand the
Severe β-lactam allergy. Alternative (use for mild-moderate β-lactam allergy) therapy
 Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Integrated Cardiopulmonary Pharmacology Third Edition
 Integrated Cardiopulmonary Pharmacology Third Edition Chapter 13 Pharmacologic Management of Asthma, Chronic Bronchitis, and Emphysema Multimedia Directory Slide 7 Slide 12 Slide 60 COPD Video Passive
Integrated Cardiopulmonary Pharmacology Third Edition Chapter 13 Pharmacologic Management of Asthma, Chronic Bronchitis, and Emphysema Multimedia Directory Slide 7 Slide 12 Slide 60 COPD Video Passive
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Webposting Clinical Trial Results Synopsis
 Study Summary This summary information is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This summary information is not intended to replace
Study Summary This summary information is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This summary information is not intended to replace
Beta-lactamase production should have no effect on Azithromycin activity.
 AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules & Powder Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Azimex
AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules & Powder Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Azimex
Turkish Thoracic Society
 Türk Toraks Derneği Turkish Thoracic Society Pocket Books Series Diagnosis and Treatment of Community Acquired Pneumonia in Children Short Version (Handbook) in English www.toraks.org.tr This report was
Türk Toraks Derneği Turkish Thoracic Society Pocket Books Series Diagnosis and Treatment of Community Acquired Pneumonia in Children Short Version (Handbook) in English www.toraks.org.tr This report was
(cilia) that help sweep away fluids and/or particles International Journal of Pharmaceutical Sciences and Research 2055
 IJPSR (2014), Vol. 5, Issue 5 (Research Article) Received on 29 November, 2013; received in revised form, 21 February, 2014; accepted, 16 April, 2014; published 01 May, 2014 EVALUATION OF EFFICACY AND
IJPSR (2014), Vol. 5, Issue 5 (Research Article) Received on 29 November, 2013; received in revised form, 21 February, 2014; accepted, 16 April, 2014; published 01 May, 2014 EVALUATION OF EFFICACY AND
Community Acquired & Nosocomial Pneumonias
 Community Acquired & Nosocomial Pneumonias IDSA/ATS 2007 & 2016 Guidelines José Luis González, MD Clinical Assistant Professor of Medicine Outline Intro - Definitions & Diagnosing CAP treatment VAP & HAP
Community Acquired & Nosocomial Pneumonias IDSA/ATS 2007 & 2016 Guidelines José Luis González, MD Clinical Assistant Professor of Medicine Outline Intro - Definitions & Diagnosing CAP treatment VAP & HAP
Investigation and Management of Community-Acquired Pneumonia (CAP) Frequently Asked Questions
 Investigation and Management of Community-Acquired Pneumonia (CAP) Frequently Asked Questions 1. Why was this algorithm developed? Emergency department physicians were seeking guidance about best antimicrobial
Investigation and Management of Community-Acquired Pneumonia (CAP) Frequently Asked Questions 1. Why was this algorithm developed? Emergency department physicians were seeking guidance about best antimicrobial
NCT ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: HMR3647A_4019. Study Code: Telithromycin. Generic drug name:
 Sponsor/company: Generic drug name: These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription
Sponsor/company: Generic drug name: These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription
High-Dose, Short-Course Levofloxacin for Community-Acquired Pneumonia: A New Treatment Paradigm
 MAJOR ARTICLE High-Dose, Short-Course Levofloxacin for Community-Acquired Pneumonia: A New Treatment Paradigm Lala M. Dunbar, 1 Richard G. Wunderink, 2 Michael P. Habib, 3 Leon G. Smith, 4 Alan M. Tennenberg,
MAJOR ARTICLE High-Dose, Short-Course Levofloxacin for Community-Acquired Pneumonia: A New Treatment Paradigm Lala M. Dunbar, 1 Richard G. Wunderink, 2 Michael P. Habib, 3 Leon G. Smith, 4 Alan M. Tennenberg,
Bronchitis/Pneumonia Core Content Keith Conover, M.D., FACEP /15/02 Clinical Spectrum Chest pain, shoulder pain, neck pain, abdominal pain,
 Bronchitis/Pneumonia Core Content Keith Conover, M.D., FACEP 1.0 10/15/02 Clinical Spectrum Chest pain, shoulder pain, neck pain, abdominal pain, headache Links with smoking, pollen count, FH of asthma
Bronchitis/Pneumonia Core Content Keith Conover, M.D., FACEP 1.0 10/15/02 Clinical Spectrum Chest pain, shoulder pain, neck pain, abdominal pain, headache Links with smoking, pollen count, FH of asthma
