Cefepime versus Ceftriaxone for Empiric Treatment of Hospitalized Patients with Community-Acquired Pneumonia
|
|
|
- Brian Harrison
- 5 years ago
- Views:
Transcription
1 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1998, p Vol. 42, No /98/$ Copyright 1998, American Society for Microbiology Cefepime versus Ceftriaxone for Empiric Treatment of Hospitalized Patients with Community-Acquired Pneumonia M. ZERVOS, 1 * M. NELSON, 2 AND THE CEFEPIME STUDY GROUP 1,2 Infectious Diseases Division, Wayne State University, William Beaumont Hospital, Royal Oak, Michigan, 1 and Medical Services, Veterans Administration Medical Center, Kansas City, Missouri 2 Received 21 April 1997/Returned for modification 24 June 1997/Accepted 10 January 1998 Effective empiric treatment of pneumonia requires antibiotic coverage against gram-negative and grampositive pathogens, including drug-resistant isolates. We compared the safety and efficacy of intravenous (i.v.) cefepime (2 g administered every 12 h) to those of i.v. ceftriaxone (1 g administered every 12 h) for the empiric treatment of hospitalized patients with community-acquired pneumonia. Of the 115 patients randomized to the study, 86 (cefepime recipients, n 40; ceftriaxone recipients, n 46) were evaluated for clinical efficacy (clinically evaluated patients). Favorable clinical outcomes (cure or improvement) were comparable among clinically evaluated patients in the cefepime and ceftriaxone treatment arms (95.0 versus 97.8%, respectively; 95% confidence interval for treatment difference [data for ceftriaxone group minus data for cefepime group], 5.1 to 10.8%). The most common bacteria isolated from patients in both treatment groups were Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus. In clinically evaluated patients with a microbiologic response, all (100%) of the 32 pathogens from cefepime-treated patients and 97.4% (38 of 39) of the pathogens from ceftriaxone-treated patients were eradicated (documented or presumed eradication). The one persistent infection in the ceftriaxone group was caused by Pseudomonas fluorescens. Both treatments were well tolerated. Our data thus suggest that cefepime and ceftriaxone have comparable safety and efficacy for the treatment of pneumonia in hospitalized patients. Pneumonia is responsible for more than 500,000 hospital admissions annually and continues to be a major cause of morbidity and mortality in the United States (4). This disease is the most frequent infectious cause of death and the sixth most frequent cause of all deaths in the United States (11, 13). The diagnosis of pneumonia can be difficult to establish, particularly in patients with other comorbid medical conditions, such as chronic lung disease, malignancy, congestive heart failure, or adult respiratory distress syndrome (17). Determination of the causative pathogen can also be difficult. Although advances in microbiologic techniques have aided in identifying the pathogens responsible for pneumonia, accurate bacteriologic diagnosis remains elusive for many patients. The treatment of pneumonia is therefore generally empiric and is based on the knowledge of the most prevalent pathogens, the clinical setting, and drug resistance patterns. Pathogens commonly associated with community-acquired pneumonia include Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus, but gram-negative bacteria may also be involved (9, 10). Gram-negative bacteria play a particularly important role in patients who are elderly, who are hospitalized, or who have a serious comorbid illness (5, 6). Cefepime, a fourth-generation cephalosporin recently introduced in the United States, has an extended spectrum of activity that encompasses both gram-positive organisms, such as S. aureus and S. pneumoniae, and gram-negative pathogens, including Pseudomonas aeruginosa, Enterobacter spp., and other members of the family Enterobacteriaceae that are becoming * Corresponding author. Mailing address: William Beaumont Hospital, 3601 West 13 Mile Rd., Royal Oak, MI Phone: (248) Fax: (248) mzervos@beaumont.edu. The Cefepime Study Group includes the following investigators, who enrolled patients in the study: J. Bernstein, D. Kernodle, R. McCabe, N. Memon, R. Player, A. Quartin, C. VanHook, and S. Willsie. increasingly resistant to expanded-spectrum cephalosporins (6, 7, 19, 20). In this study, we therefore compared the empiric use of cefepime to that of ceftriaxone, an expanded-spectrum cephalosporin, for the treatment of hospitalized patients with pneumonia. MATERIALS AND METHODS Study design. This study was an open-label, randomized, comparative, multicenter study involving 115 adult patients with community-acquired pneumonia who were recruited from 10 U.S. centers over a 12-month period. Patients were randomized (1:1) to receive intravenous treatment with either cefepime (2 g every 12 h) or ceftriaxone (1 g every 12 h) for 5 to 10 days (maximum duration, 14 days). The intravenous administration of both drugs occurred over a period of 30 min. The protocol allowed the study drugs to be administered intramuscularly (i.m.) to continue or complete a course of treatment. Concomitant treatment with erythromycin or metronidazole was allowed when infection with Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, or a cephalosporin-resistant anaerobic organism was suspected. Signed informed consent was obtained from all patients or a legally authorized representative. Institutional review board approval was received from all institutions that enrolled patients in the study. Patient eligibility. Hospitalized patients 18 years of age and older with clinical signs and symptoms of pneumonia were eligible for enrollment. Evidence of community-acquired pneumonia was defined by documentation of at least two of three of the following groups of signs and symptoms (i) fever (temperature greater than 38 C) or hypothermia (temperature less than 36 C); (ii) purulent sputum ( 25 polymorphonuclear leukocytes and 10 squamous cells/low-power field), leukocytosis (leukocyte count, 10,000/mm 3 ), worsening arterial oxygen gradients in relation to baseline measurements, or newly developed hypoxia; or (iii) evidence of pulmonary involvement probably due to the presence of bacteria susceptible to cefepime and ceftriaxone (radiologic findings consistent with the diagnosis of aspiration pneumonia, lobar pneumonia, or diffuse unilateral or bilateral pneumonia and/or signs of pulmonary consolidation or pneumonia on physical examination). At least one prestudy sputum or lower respiratory tract specimen was to be obtained for testing of the in vitro susceptibility of the infecting organism(s) to the study drugs. Exclusion criteria for this study included previous treatment with cefepime or ceftriaxone for this episode of pneumonia, a history of hypersensitivity to a cephalosporin or penicillin antibiotic, pregnancy or breast-feeding, creatinine clearance of 11 ml/min, anticipated treatment with other systemic bacterial drugs (with the exception of erythromycin or metronidazole), thrombocytopenia or granulocytopenia expected to last more than 7 days, resistance of the primary 729
2 730 ZERVOS ET AL. ANTIMICROB. AGENTS CHEMOTHER. TABLE 1. Demographic characteristics of randomized patients Characteristic (n 59) (n 56) Sex (no. [%]) a Male 36 (61.0) 43 (76.8) Female 23 (39.0) 13 (23.2) Race (no. [%]) Black 21 (35.6) 21 (37.5) Hispanic 0 2 (3.6) Asian 1 (1.7) 0 White 37 (62.7) 32 (57.1) Other 0 1 (1.8) Mean age (yr) Mean wt (kg) b a P b Data were available for 49 cefepime-treated patients and 47 ceftriaxonetreated patients. infecting organism to the assigned study drug, the presence of pulmonary secretions that were known to be culture negative before therapy was initiated, and the presence of a medically significant condition that could affect study outcome or limit survival during therapy and follow-up (i.e., cystic fibrosis; pneumonia distal to obstructive carcinoma; empyema; positive human immunodeficiency virus status; documented Legionella, Mycoplasma, or Chlamydia pneumonitis; severe cardiac disease characterized by pulmonary edema or unstable congestive heart failure; serious hepatic disease; and concurrent meningitis, osteomyelitis, or infective endocarditis). Study procedures. Upon entering the study, each patient s medical history was recorded and a physical examination, chest radiography, sputum and blood culture analysis, and antibiotic sensitivity testing were performed. Clinical laboratory studies included hematology and serum chemistry analyses and urinalysis. During therapy, clinical evaluations were performed daily during the first week and every 2 to 3 days thereafter to evaluate the efficacy and safety of the study drugs. Blood samples were recultured at least every 3 days until they were negative and at the end of therapy. Clinical laboratory studies were repeated twice weekly during the first week, weekly thereafter during therapy, and within 72 h of the discontinuation of therapy. A posttreatment evaluation, performed within 72 h of the completion of therapy, included a clinical evaluation to determine the presence or absence of signs and symptoms of pneumonia or adverse events, culture analysis of a sputum or lower respiratory tract sample, and chest radiography. Respiratory secretions were cultured for routine respiratory pathogens by standard procedures. Susceptibility to cefepime and ceftriaxone was determined at each study site by either disk diffusion or MIC methods by using National Committee for Clinical Laboratory Standard guidelines. Evaluation of efficacy. Patients were considered clinically evaluable if they met all pretherapy, on-therapy, and posttherapy criteria and received study drug for at least 5 days. The clinical response of each patient was categorized as (i) cure (complete resolution of all signs and symptoms of pneumonia and improvement or lack of progression of all positive findings on chest radiography), (ii) improvement (improvement of one or more signs and symptoms of pneumonia without a complete resolution but a lack of progression of radiographic findings), or (iii) failure (failure to resolve signs and symptoms of pneumonia or progression of infection on chest radiography). The microbiologic response to treatment was defined as follows: (i) eradication (elimination of the original pathogen[s] from subsequent cultures), (ii) presumptive eradication (presumed elimination of the original pathogen[s] as evidenced by the absence of appropriate material for culture at the original site of infection), (iii) persistence (continued presence of the pathogen[s] from the original site of infection during or upon completion of therapy with or without evidence of infection), or (iv) relapse (eradication and then reisolation of the initial pathogen[s]). Pathogens other than the original organism that were isolated from cultures of sputum specimens during therapy or within 3 days of the completion of therapy were judged to be superinfection or colonization. Statistical analysis. Statistical analyses were performed to compare demographic and baseline medical characteristics, efficacy, and safety. In determining the homogeneity of the two treatment groups, including demographic and baseline medical characteristics, categorical variables were analyzed by either the Fisher exact test or the Cochran-Mantel-Haenszel test, and quantitative variables were analyzed by two-way analysis of variance. Clinical and microbiologic response rates were compared by the Cochran-Mantel-Haenszel test, and the rates of incidence of adverse events were compared by the Fisher exact test. RESULTS Patient characteristics. A total of 115 patients with pneumonia were randomized to the study, 59 to the cefepime group and 56 to the ceftriaxone group. The demographic characteristics of these patients are presented in Table 1. There were no significant differences in the races, ages, heights, or weights of the patients entering the study. Both groups had more males than females. Compared to the cefepime group, the ceftriaxone group had significantly more males (36 versus 43; P 0.046). The characteristics of the baseline medical histories that were significantly different between the treatment groups included prevalence of eye, ear, nose, or throat disease (cefepime group, n 16; ceftriaxone group, n 25; P 0.026); the infection under study as the diagnosis for hospitalization (cefepime group, n 50; ceftriaxone group, n 55; P 0.009); and recent trauma (cefepime group, n 4; ceftriaxone group, n 0; P 0.048). Histories of bronchopulmonary disease and tobacco use were each present in approximately 60% of the patients in both groups. The presenting clinical signs and symptoms of pneumonia at study entry were similar for each treatment group (Table 2). Lobar pneumonia was the most common diagnosis. More patients in the cefepime group than in the ceftriaxone group had a right lung infection (47 versus 34; P 0.046); the cefepime group also had more mild infections (11 versus 6; P 0.025). No other significant differences between the study groups were noted. Of the 115 patients who entered the study, 21 (cefepime group, n 12; ceftriaxone group, n 9) discontinued treatment before completing the study. The reasons for discontinuation were protocol violations, including no isolation of a pathogen (cefepime group, n 5; ceftriaxone group, n 2), adverse experience (cefepime group, n 3; ceftriaxone group, n 1); patient s request (cefepime group, n 2; ceftriaxone group, n 2), loss to follow-up or unevaluable (cefepime TABLE 2. Pneumonia characteristics of randomized patients at study entry a Characteristic Cefepime (n 59) Ceftriaxone (n 56) Diagnosis Lobar pneumonia 43 (72.9) 44 (78.6) Bronchopneumonia 15 (25.4) 12 (21.4) Interstitial infiltrate 1 (1.7) 0 Site b Right lung 47 (79.7) 34 (60.7) Left lung 12 (20.3) 22 (39.3) Severity b Mild 11 (18.6) 6 (10.7) Moderate 48 (81.4) 46 (82.1) Severe 0 4 (7.1) Chronicity Acute 58 (98.3) 56 (100) Other 1 (1.7) 0 Classification Single lung lobe 40 (67.8) 38 (67.9) Multiple lung lobe 6 (10.2) 5 (8.9) Both lungs 13 (22.0) 13 (23.2) a The mean number of days of infection before study entry were 4.5 and 5.7 for the cefepime and ceftriaxone groups, respectively. b P 0.05.
3 VOL. 42, 1998 CEFEPIME VERSUS CEFTRIAXONE FOR PNEUMONIA 731 TABLE 3. Clinical response to therapy for clinically evaluated patients Clinical response (n 40) (n 46) Favorable (cured plus improved) 38 (95.0) 45 (97.8) Cured 8 (20.0) 14 (30.4) Improved 30 (75.0) 31 (67.4) Failure 2 (5.0) 1 (2.2) TABLE 4. Bacteriologic response to therapy for patients evaluated for clinical efficacy a Bacteriologic response (n 32) (n 39) Eradicated 32 (100) 38 (97.4) Documented eradication 17 (53.1) 17 (43.6) Presumed eradication 15 (46.9) 21 (53.8) Persistence 0 (0) 1 (2.6) a Multiple pathogens may have been obtained from the same patient. group, n 1; ceftriaxone group, n 3), the patient missed a study drug dose (cefepime group, n 1), and the patient improved on the physician requested discontinuation (ceftriaxone group, n 1). Although the study protocol excluded patients whose pathogens were not susceptible to the study drug in vitro, there were no discontinuations for this reason because all the organisms were susceptible to the treatments that the patients were randomized to receive. A total of 94 patients completed the study (cefepime group, n 47; ceftriaxone group, n 47). Eighty-six patients were evaluable for clinical efficacy (clinically evaluated patients) (cefepime group, n 40; ceftriaxone group, n 46), and 48 (cefepime group, n 23; ceftriaxone group, n 25) of these patients were evaluable for microbiologic efficacy. Under the study protocol, erythromycin and metronidazole were allowed as concomitant antibiotic therapy when infection with L. pneumophila, M. pneumoniae, C. pneumoniae, or a cephalosporin-resistant anaerobic organism was suspected. During the course of the study, 13 patients in the cefepime group and 15 patients in the ceftriaxone group received erythromycin and 2 patients in each group received metronidazole. Seven additional patients who received concomitant antimicrobial agents were approved by the sponsor as evaluable. The additional antimicrobial agents used were augmentin, bacitracin, doxycycline, and rifampin (one patient each in the ceftriaxone group), trimethoprim-sulfamehoxazole (Bactrim DS) (one patient in the cefepime group), and vancomycin (1 patient in the cefepime group and one patient in the ceftriaxone group). Of the patients included in evaluations of clinical response, comparable numbers in each group received concomitant antibiotics (cefepime group, n 12; ceftriaxone group, n 14). Clinical response. The mean duration of treatment for all patients enrolled in the study was approximately 6.5 days (range, 2 to 14 days). A satisfactory clinical response (cure or improvement) was achieved in 38 of 40 (95.0%) patients in the cefepime group and 45 of 46 (97.8%) patients in the ceftriaxone group (P 0.549) (Table 3). The 95% confidence interval (CI) for the difference between cure plus improvement rates in the two treatment groups (data for the ceftriaxone group minus data for the cefepime group) was determined to lie between 5.1 and 10.8%. Similar results were obtained in an intent-to-treat analysis of all randomized patients: a satisfactory clinical response was achieved in 49 of 59 (83.0%) of all randomized patients in the cefepime group and 50 of 56 (89.3%) of all randomized patients in the ceftriaxone group (P 0.307; 95% CI for difference between groups [data for the group cefepime minus data for the ceftriaxone group], 18.8 to 7.9%). The favorable clinical response (cure plus improvement) rate for those who received concomitant antibiotics was 100% (12 of 12) for the cefepime group and 92.9% (13 of 14) for the ceftriaxone group. In comparison, for patients who did not receive concomitant antibiotics, the favorable clinical response rate was 92.9% (26 of 28) for the cefepime group and 100% (32 of 32) for the ceftriaxone group. The difference in response rates between those who did and those did not receive concomitant antibiotics was statistically insignificant (P 0.974, 0.593, and for all patients included in efficacy analyses, the patients in the cefepime group, and the patients in the ceftriaxone group, respectively). Thus, concomitant antibiotics appeared to have little impact on the efficacies of the study drugs. Bacteriology and bacteriologic response. All organisms isolated demonstrated in vitro susceptibility to the assigned study treatments. The bacteria most frequently isolated from the clinically evaluated patients in the cefepime and ceftriaxone treatment groups were S. pneumoniae (seven and eight isolates, respectively), H. influenzae (nine isolates in each group), and S. aureus (six isolates in each group). The 48 clinically evaluated patients with a microbiologic response included 23 patients with 32 pathogens in the cefepime group and 25 patients with 39 pathogens in the ceftriaxone group. Both treatment groups had similar satisfactory bacteriologic response rates by patient and by pathogen (Tables 4 and 5, respectively). For the cefepime group, all 32 (100%) pathogens were eradicated (documented or presumed eradication). Similar results were noted for the ceftriaxone group, TABLE 5. Bacteriologic response by pathogen for patients evaluated for clinical efficacy a Pathogen No. of patients in the following group with the indicated response b : (n 32), eradication (n 39) Eradication Persistence Enterobacter cloacae 1 Escherichia coli 3 2 Haemophilus influenzae 9 9 Haemophilus parahemolyticus 1 3 Haemophilus parainfluenzae 1 3 Haemophilus species 1 1 Klebsiella pneumonia 1 Lactobacillus species 1 Pseudomonas aeruginosa 1 Pseudomonas fluorescens 1 1 Staphylococcus aureus 6 6 Streptococcus ( -hemolytic) 2 Streptococcus faecalis 1 Streptococcus (group C) 1 Streptococcus pneumoniae 7 9 a Multiple pathogens may have been from the same patient. b No organisms persisted in the cefepime group. Eradication was documented or presumed.
4 732 ZERVOS ET AL. ANTIMICROB. AGENTS CHEMOTHER. with 38 of 39 (97.4%) pathogens eradicated (P 0.317; 95% CI for difference in favorable bacteriologic response rates for ceftriaxone minus the rates for cefepime, 7.5 to 2.4%). The one pathogen that did not respond to therapy with ceftriaxone was Pseudomonas fluorescens, which was found to persist at the posttherapy evaluation. Safety evaluation. An analysis of adverse events was performed for all patients who received at least one dose of study medication (all randomized patients). Treatment-emergent adverse events occurred in 40 of 59 (67.8%) patients treated with cefepime and 29 of 56 (51.8%) patients treated with ceftriaxone (P 0.090). There were no statistically significant differences between the treatment groups for any adverse event category. Six adverse events in the cefepime group and four adverse events in the ceftriaxone group were considered to be probably related to study drug. In the cefepime treatment group, these events included enlarged abdomen, fever, arm pain, abdominal pain, dyspepsia, and urinary tract infection. In the ceftriaxone treatment group, adverse events probably related to study drug were two incidents of diarrhea and one incident each of moniliasis (Candida) and oral moniliasis. Seven cefepime-treated and seven ceftriaxone-treated patients received one to six doses of study drug i.m. Poor tolerance to the i.m. injection was reported for one patient who received ceftriaxone; all other patients in both groups tolerated the i.m. injections well. No clinically significant laboratory abnormalities were reported for either treatment group. Three cefepime-treated patients and one ceftriaxone-treated patient died during the study. None of the deaths was considered to be related to the study treatments. DISCUSSION Current therapeutic strategies for the treatment of pneumonia are complicated by the changing etiologies of this disease. In particular, gram-negative pathogens have become increasingly important as the cause of pneumonia, particularly in patients who are elderly, who are hospitalized, or who have coexisting illnesses, and antibiotic resistance appears to be increasing steadily among respiratory tract pathogens (5 7, 17). These factors, combined with the frequent absence of a microbiologic diagnosis, can confound the selection of an antibiotic for empiric use in the treatment of pneumonia. This study compared an extended-spectrum cephalosporin, cefepime, with a widely used expanded-spectrum cephalosporin, ceftriaxone, for the empiric treatment of hospitalized patients with community-acquired pneumonia. The majority of patients in both treatment groups achieved a satisfactory clinical response: 95.0% of the cefepime-treated and 97.8% of the ceftriaxone-treated clinically evaluated patients were cured or improved. These rates are similar to those reported in previous studies for patients with pneumonia treated with cefepime (80 to 100%) (2, 3, 12, 15) or ceftriaxone (70 to 90%) (1, 9). High bacteriologic response rates were also achieved in both the cefepime and the ceftriaxone groups (100 and 97.4%, respectively, for clinically evaluated patients with a microbiologic response). Although certain concomitant antibiotics (primarily erythromycin and metronidazole) were allowed during this trial, concomitant treatment appeared to have little impact on the favorable clinical response rate in the clinically evaluated patients (cefepime group, 100% with and 92.9% without concomitant antibiotics; ceftriaxone group, 92.9% with and 100% without concomitant antibiotics). The only case of documented persistence of a pathogen occurred in a ceftriaxone-treated patient infected with P. fluorescens. Gram-negative pathogens are increasingly playing a role as the cause of pneumonia, particularly in elderly or hospitalized patients (5, 6). Although a larger study is required to assess the comparative efficacies of cefepime and ceftriaxone against pneumonia caused by gram-negative bacteria or pathogens resistant to multiple antimicrobial agents, in vitro studies have demonstrated that cefepime s activity against certain key gram-negative pathogens, particularly Pseudomonas species and the members of the family Enterobacteriaceae, is superior to that of ceftriaxone (6, 18, 19). In one survey of 12,574 clinical isolates, cefepime was active against 92.7% of all gramnegative bacteria whereas ceftriaxone was active against 74.1% of all gram-negative bacteria (19). Furthermore, for some pathogens cefepime is associated with a lower level of drug resistance than ceftriaxone and other expanded-spectrum cephalosporins. In the survey discussed above, approximately 35% of the Enterobacter cloacae strains were resistant to ceftriaxone, whereas only 4% were resistant to cefepime (19). These data are supported by the results of clinical studies. Of 16 patients infected with strains of Enterobacter spp. with reduced susceptibility or resistance to the expanded-spectrum cephalosporin ceftazidime, all were successfully treated with cefepime (16). Both cefepime and ceftriaxone eradicated 100% of grampositive pathogens from clinically evaluated patients with a microbiologic response. This finding agrees with the antimicrobial activities of these drugs determined in vitro. In these studies, cefepime and ceftriaxone have demonstrated similar levels of activity against gram-positive bacteria commonly associated with community-acquired pneumonia, including S. pneumoniae, H. influenzae, and S. aureus (8). Of particular note are the activities of these agents against penicillin-resistant strains of S. pneumoniae, an important clinical pathogen. In a study involving 122 penicillin-resistant (MICs, 2.0 mg/ liter) or relatively resistant (MICs, 0.12 to 1.0 mg/liter) S. pneumoniae isolates, 95.1% were susceptible to cefepime and 94.3% were susceptible to ceftriaxone (21). In this trial, both cefepime and ceftriaxone were well tolerated, with a low incidence of drug-related adverse events. The favorable safety profile of cefepime has been extensively documented in clinical trials (14). Although this trial did not include sufficient numbers of patients to detect subtle differences in efficacy and safety between cefepime and ceftriaxone, the data reported here support the conclusion that cefepime and ceftriaxone are therapeutically comparable for the empiric treatment of community-acquired pneumonia in hospitalized patients. These two agents were equally effective against the pathogens in this study, and both were associated with a low rate of adverse effects. ACKNOWLEDGMENT This study was supported by Bristol-Myers Squibb Pharmaceuticals. REFERENCES 1. Bassetti, D., M. Cruciani, M. Solbiati, F. Rubini, L. Gandola, G. Valenti, M. De Palma, R. Corda, and N. Carimeo Comparative efficacy of ceftriaxone versus ceftazidime in the treatment of nosocomial lower respiratory tract infections. Chemotherapy (Basel) 37: Clynes, N., B. E. Scully, and H. C. Neu The use of cefepime (BMY 28142) to treat respiratory infections. Diagn. Microbiol. Infect. Dis. 12: Edelstein, H., V. Chirurgi, S. Oster, R. Karp, K. Cassano, S. Aiken, and R. McCabe A randomized trial of cefepime (BMY 28142) and ceftazidime for the treatment of pneumonia. J. Antimicrob. Chemother. 28: Fang, G. D., M. Fine, J. Orloff, D. Arisumi, V. L. Yu, W. Kapoor, J. T. Grayson, S. P. Wang, R. Kohler, R. R. Muder, et al New and emerging
5 VOL. 42, 1998 CEFEPIME VERSUS CEFTRIAXONE FOR PNEUMONIA 733 etiologies for community-acquired pneumonia with implications for therapy. Medicine (Baltimore) 69: Ginesu, F., P. Pirina, G. Deioloa, S. Ostera, S. Mele, and A. G. Fois Etiology and therapy of community-acquired pneumonia. J. Chemother. 9: Jones, R. N Introduction. Am. J. Med. 100(Suppl. 6A): Jones, R. N Impact of changing pathogens and antimicrobial susceptibility patterns in the treatment of serious infections in hospitalized patients. Am. J. Med. 100(Suppl. 6A): Kessler, R. E., and J. Fung-Tome Susceptibility of bacterial isolates to -lactam antibiotics from US clinical trials over a 5-year period. Am. J. Med. 100(Suppl. 6A): Mangi, R. J., K. M. Peccerillo, J. Ryan, C. Berenson, T. Greco, G. Thornton, and V. T. Andriole Cefoperazone versus ceftriaxone monotherapy of nosocomial pneumonia. Diagn. Microbiol. Infect. Dis. 15: Marrie, T. J., H. Durant, and L. Yates Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev. Infect. Dis. 11: Marrie, T. J Community-acquired pneumonia. Clin. Infect. Dis. 18: McCabe, R., V. Chirurgi, S. A. Farkas, A. Haddow, G. Heinz, and S. Greene A new therapeutic option for the treatment of pneumonia. Am. J. Med. 100(Suppl. 6A): National Center for Health Statistics Advanced Report of Final Mortality Statistics, vol. 43, no. 6 (Suppl). National Center for Health Statistics, Hyattsville, Md. 14. Neu, H. C Safety of cefepime: a new extended-spectrum parenteral cephalosporin. Am. J. Med. 100(Suppl. 6A): Oster, S., H. Edelstein, K. Cassano, and R. McCabe Open trial of cefepime (BMY 28142) for infections in hospitalized patients. Antimicrob. Agents Chemother. 34: Sanders, W. E., Jr., J. H. Tenney, and R. E. Kessler Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin. Infect. Dis. 23: Scheld, W. M., and G. L. Mandell Nosocomial pneumonia: pathogenesis and recent advances in diagnosis and therapy. Rev. Infect. Dis. 13(Suppl. 9): Thornsberry, C., S. D. Brown, Y. C. Yee, S. K. Bouchillon, J. K. Marler, and T. Rich In vitro activity of cefepime and other antimicrobials: survey of European isolates. J. Antimicrob. Chemother. 32(Suppl. B): Thornsberry, C., and Y. C. Yee Comparative activity of eight antimicrobial agents against clinical bacterial isolates from the United States, measured by two methods. Am. J. Med. 100(Suppl. 6A): Washington, J. A., R. N. Jones, E. H. Gerlach, P. R. Murray, S. D. Allen, and C. C. Knapp Multicenter comparison of in vitro activities of FK-037, cefepime, ceftriaxone, ceftazidime, and cefuroxime. Antimicrob. Agents Chemother. 37: Yee, Y. C., C. Thornsberry, S. D. Brown, S. K. Bouchillon, J. K. Marler, and T. Rich A comparative study of the in vitro activity of cefepime and other antimicrobial agents against penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 32(Suppl. B): Downloaded from on October 19, 2018 by guest
Treatment of febrile neutropenia in patients with neoplasia
 Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections
 Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
PNEUMONIA IN CHILDREN. IAP UG Teaching slides
 PNEUMONIA IN CHILDREN 1 INTRODUCTION 156 million new episodes / yr. worldwide 151 million episodes developing world 95% in developing countries 19% of all deaths in children
PNEUMONIA IN CHILDREN 1 INTRODUCTION 156 million new episodes / yr. worldwide 151 million episodes developing world 95% in developing countries 19% of all deaths in children
Hospital-acquired Pneumonia
 Hospital-acquired Pneumonia Hospital-acquired pneumonia (HAP) Pneumonia that occurs at least 2 days after hospital admission. The second most common and the leading cause of death due to hospital-acquired
Hospital-acquired Pneumonia Hospital-acquired pneumonia (HAP) Pneumonia that occurs at least 2 days after hospital admission. The second most common and the leading cause of death due to hospital-acquired
Cefepime/clindamycin vs. ceftriaxone/clindamycin for the empiric treatment of poisoned patients with aspiration pneumonia
 ACTA BIOMED 2008; 79: 117-122 Mattioli 1885 O R I G I N A L A R T I C L E Cefepime/clindamycin vs. ceftriaxone/clindamycin for the empiric treatment of poisoned patients with aspiration pneumonia Haleh
ACTA BIOMED 2008; 79: 117-122 Mattioli 1885 O R I G I N A L A R T I C L E Cefepime/clindamycin vs. ceftriaxone/clindamycin for the empiric treatment of poisoned patients with aspiration pneumonia Haleh
Community Acquired Pneumonia. Abdullah Alharbi, MD, FCCP
 Community Acquired Pneumonia Abdullah Alharbi, MD, FCCP A 68 y/ male presented to the ED with SOB and productive coughing for 2 days. Reports poor oral intake since onset due to nausea and intermittent
Community Acquired Pneumonia Abdullah Alharbi, MD, FCCP A 68 y/ male presented to the ED with SOB and productive coughing for 2 days. Reports poor oral intake since onset due to nausea and intermittent
Mesporin TM. Ceftriaxone sodium. Rapid onset, sustained action, for a broad spectrum of infections
 Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Pathology of Pneumonia
 Pathology of Pneumonia Dr. Atif Ali Bashir Assistant Professor of Pathology College of Medicine Majma ah University Introduction: 5000 sq meters of area.! (olympic track) Filters >10,000 L of air / day!
Pathology of Pneumonia Dr. Atif Ali Bashir Assistant Professor of Pathology College of Medicine Majma ah University Introduction: 5000 sq meters of area.! (olympic track) Filters >10,000 L of air / day!
Pneumonia Community-Acquired Healthcare-Associated
 Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Chapter 22. Pulmonary Infections
 Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Methicillin-Resistant Staphylococcus aureus (MRSA) S urveillance Report 2008 Background Methods
 Methicillin-Resistant Staphylococcus aureus (MRSA) Surveillance Report 2008 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services
Methicillin-Resistant Staphylococcus aureus (MRSA) Surveillance Report 2008 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services
Received 30 March 2005; returned 16 June 2005; revised 8 September 2005; accepted 12 September 2005
 Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
THE USE OF THE PENICILLINASE-RESISTANT
 Therapeutic problems THE USE OF THE PENICILLINASE-RESISTANT PENICILLIN IN THE PNEUMONIAS OF CHILDREN MARTHA D. Yow, MARY A. SOUTH AND CHARLES G. HESS From the Department of Pediatrics, Baylor University
Therapeutic problems THE USE OF THE PENICILLINASE-RESISTANT PENICILLIN IN THE PNEUMONIAS OF CHILDREN MARTHA D. Yow, MARY A. SOUTH AND CHARLES G. HESS From the Department of Pediatrics, Baylor University
Treatment of serious Pseudomonas infections with azlocillin
 Journal of Antimicrobial Chemotherapy (983), Suppl. B, 53-58 Treatment of serious Pseudomonas infections with azlocillin S. Olive, W. J. Mogabgab, B. Holmes, B. Pollock, B. Pauling and R. Beville Tulane
Journal of Antimicrobial Chemotherapy (983), Suppl. B, 53-58 Treatment of serious Pseudomonas infections with azlocillin S. Olive, W. J. Mogabgab, B. Holmes, B. Pollock, B. Pauling and R. Beville Tulane
Unit II Problem 2 Pathology: Pneumonia
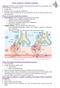 Unit II Problem 2 Pathology: Pneumonia - Definition: pneumonia is the infection of lung parenchyma which occurs especially when normal defenses are impaired such as: Cough reflex. Damage of cilia in respiratory
Unit II Problem 2 Pathology: Pneumonia - Definition: pneumonia is the infection of lung parenchyma which occurs especially when normal defenses are impaired such as: Cough reflex. Damage of cilia in respiratory
Aciphin Ceftriaxone Sodium
 Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Severe β-lactam allergy. Alternative (use for mild-moderate β-lactam allergy) therapy
 Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Ceftizoxime in the treatment of infections in patients with cancer
 Journal of Antimicrobial Chemotherapy (98), Suppl. C, 67-73 Ceftizoxime in the treatment of infections in patients with cancer V. Fainstein, R. Bolivar,. Elting, M. Valdivieso and G. P. Bodey Department
Journal of Antimicrobial Chemotherapy (98), Suppl. C, 67-73 Ceftizoxime in the treatment of infections in patients with cancer V. Fainstein, R. Bolivar,. Elting, M. Valdivieso and G. P. Bodey Department
PULMONARY EMERGENCIES
 EMERGENCIES I. Pneumonia A. Bacterial Pneumonia (most common cause of a focal infiltrate) 1. Epidemiology a. Accounts for up to 10% of hospital admissions in the U.S. b. Most pneumonias are the result
EMERGENCIES I. Pneumonia A. Bacterial Pneumonia (most common cause of a focal infiltrate) 1. Epidemiology a. Accounts for up to 10% of hospital admissions in the U.S. b. Most pneumonias are the result
Efficacy of Ceftriaxone in Serious Bacterial Infections
 ANTIMIROBIAL AGENTS AND HEMOTHERAPY, Mar 1982, p 402-406 0066-4804/82/030402-05$0200/0 Vol 21, No 3 Efficacy of eftriaxone in Serious Bacterial Infections JAY S EPSTEIN, SUSAN M HASSELQUIST, AND GARY L
ANTIMIROBIAL AGENTS AND HEMOTHERAPY, Mar 1982, p 402-406 0066-4804/82/030402-05$0200/0 Vol 21, No 3 Efficacy of eftriaxone in Serious Bacterial Infections JAY S EPSTEIN, SUSAN M HASSELQUIST, AND GARY L
Setting The setting was secondary care. The economic study was carried out in the USA.
 Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia
 High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia Review Andrew F. Shorr, MD, MPH, FCCP Department of Medicine Pulmonary and Critical Care Medicine, Washington Hospital Center, Washington,
High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia Review Andrew F. Shorr, MD, MPH, FCCP Department of Medicine Pulmonary and Critical Care Medicine, Washington Hospital Center, Washington,
Lecture Notes. Chapter 16: Bacterial Pneumonia
 Lecture Notes Chapter 16: Bacterial Pneumonia Objectives Explain the epidemiology Identify the common causes Explain the pathological changes in the lung Identify clinical features Explain the treatment
Lecture Notes Chapter 16: Bacterial Pneumonia Objectives Explain the epidemiology Identify the common causes Explain the pathological changes in the lung Identify clinical features Explain the treatment
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Affinity of Doripenem and Comparators to Penicillin-Binding Proteins in Escherichia coli and ACCEPTED
 AAC Accepts, published online ahead of print on February 00 Antimicrob. Agents Chemother. doi:./aac.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
AAC Accepts, published online ahead of print on February 00 Antimicrob. Agents Chemother. doi:./aac.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Pneumonia. Dr. Rami M Adil Al-Hayali Assistant professor in medicine
 Pneumonia Dr. Rami M Adil Al-Hayali Assistant professor in medicine Definition Pneumonia is an acute respiratory illness caused by an infection of the lung parenchyma, associated with recently developed
Pneumonia Dr. Rami M Adil Al-Hayali Assistant professor in medicine Definition Pneumonia is an acute respiratory illness caused by an infection of the lung parenchyma, associated with recently developed
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES
 DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Chapter 10 Respiratory System J00-J99. Presented by: Jesicca Andrews
 Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Upper...and Lower Respiratory Tract Infections
 Upper...and Lower Respiratory Tract Infections Robin Jump, MD, PhD Cleveland Geriatric Research Education and Clinical Center (GRECC) Louis Stokes Cleveland VA Medical Center Case Western Reserve University
Upper...and Lower Respiratory Tract Infections Robin Jump, MD, PhD Cleveland Geriatric Research Education and Clinical Center (GRECC) Louis Stokes Cleveland VA Medical Center Case Western Reserve University
KAISER PERMANENTE OHIO COMMUNITY ACQUIRED PNEUMONIA
 KAISER PERMANENTE OHIO COMMUNITY ACQUIRED PNEUMONIA Methodology: Expert opinion Issue Date: 8-97 Champion: Pulmonary Medicine Most Recent Update: 6-08, 7-10, 7-12 Key Stakeholders: Pulmonary Medicine,
KAISER PERMANENTE OHIO COMMUNITY ACQUIRED PNEUMONIA Methodology: Expert opinion Issue Date: 8-97 Champion: Pulmonary Medicine Most Recent Update: 6-08, 7-10, 7-12 Key Stakeholders: Pulmonary Medicine,
ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: 07/November/2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Cefotaxime Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES
 DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
Eun-Young Kang, M.D., Jae Wook Lee, M.D., Ji Yung Choo, M.D., Hwan Seok Yong, M.D., Ki Yeol Lee, M.D., Yu-Whan Oh, M.D.
 Eun-Young Kang, M.D., Jae Wook Lee, M.D., Ji Yung Choo, M.D., Hwan Seok Yong, M.D., Ki Yeol Lee, M.D., Yu-Whan Oh, M.D. Department of Radiology, Korea University Guro Hospital, College of Medicine, Korea
Eun-Young Kang, M.D., Jae Wook Lee, M.D., Ji Yung Choo, M.D., Hwan Seok Yong, M.D., Ki Yeol Lee, M.D., Yu-Whan Oh, M.D. Department of Radiology, Korea University Guro Hospital, College of Medicine, Korea
Discrepancies in the recovery of bacteria from multiple sinuses in acute and chronic sinusitis
 Journal of Medical Microbiology (2004), 53, 879 885 DOI 10.1099/jmm.0.45655-0 Short Communication Correspondence Itzhak Brook ib6@georgetown.edu Received 1 March 2004 Accepted 18 May 2004 Discrepancies
Journal of Medical Microbiology (2004), 53, 879 885 DOI 10.1099/jmm.0.45655-0 Short Communication Correspondence Itzhak Brook ib6@georgetown.edu Received 1 March 2004 Accepted 18 May 2004 Discrepancies
Community-acquired pneumonia in adults
 Prim Care Clin Office Pract 30 (2003) 155 171 Community-acquired pneumonia in adults Julio A. Ramirez, MD a,b, * a Department of Medicine, University of Louisville School of Medicine, 512 S. Hancock Street,
Prim Care Clin Office Pract 30 (2003) 155 171 Community-acquired pneumonia in adults Julio A. Ramirez, MD a,b, * a Department of Medicine, University of Louisville School of Medicine, 512 S. Hancock Street,
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Online Supplement for:
 Online Supplement for: INFLUENCE OF COMBINED INTRAVENOUS AND TOPICAL ANTIBIOTIC PROPHYLAXIS ON THE INCIDENCE OF INFECTIONS, ORGAN DYSFUNCTIONS, AND MORTALITY IN CRITICALLY ILL SURGICAL PATIENTS A PROSPECTIVE,
Online Supplement for: INFLUENCE OF COMBINED INTRAVENOUS AND TOPICAL ANTIBIOTIC PROPHYLAXIS ON THE INCIDENCE OF INFECTIONS, ORGAN DYSFUNCTIONS, AND MORTALITY IN CRITICALLY ILL SURGICAL PATIENTS A PROSPECTIVE,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement
 CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
Healthcare-associated infections acquired in intensive care units
 SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Healthcare-associated infections acquired in intensive care units Key facts In 2015, 11 788 (8.3%) of patients staying in an intensive care unit
SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Healthcare-associated infections acquired in intensive care units Key facts In 2015, 11 788 (8.3%) of patients staying in an intensive care unit
Respiratory tract infections. Krzysztof Buczkowski
 Respiratory tract infections Krzysztof Buczkowski Etiology Viruses Rhinoviruses Adenoviruses Coronaviruses Influenza and Parainfluenza Viruses Respiratory Syncitial Viruses Enteroviruses Etiology Bacteria
Respiratory tract infections Krzysztof Buczkowski Etiology Viruses Rhinoviruses Adenoviruses Coronaviruses Influenza and Parainfluenza Viruses Respiratory Syncitial Viruses Enteroviruses Etiology Bacteria
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Cefuroxime iv Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefuroxime iv Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the
Cefuroxime iv Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the
Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center
 Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Kathy Peters is a 63 y.o. patient that presents to your urgent care office today with a history
Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Kathy Peters is a 63 y.o. patient that presents to your urgent care office today with a history
PNEUMONIA. I. Background 6 th most common cause of death in U.S. Most common cause of infection related mortality
 Page 1 of 8 September 4, 2001 Donald P. Levine, M.D. University Health Center Suite 5C Office: 577-0348 dlevine@intmed.wayne.edu Assigned reading: pages 153-160; 553-563 PNEUMONIA the most widespread and
Page 1 of 8 September 4, 2001 Donald P. Levine, M.D. University Health Center Suite 5C Office: 577-0348 dlevine@intmed.wayne.edu Assigned reading: pages 153-160; 553-563 PNEUMONIA the most widespread and
Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis
 Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH)
 Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Ailyn T. Isais-Agdeppa, MD*, Lulu Bravo, MD*
 A FIVE-YEAR RETROSPECTIVE STUDY ON THE COMMON MICROBIAL ISOLATES AND SENSITIVITY PATTERN ON BLOOD CULTURE OF PEDIATRIC CANCER PATIENTS ADMITTED AT THE PHILIPPINE GENERAL HOSPITAL FOR FEBRILE NEUTROPENIA
A FIVE-YEAR RETROSPECTIVE STUDY ON THE COMMON MICROBIAL ISOLATES AND SENSITIVITY PATTERN ON BLOOD CULTURE OF PEDIATRIC CANCER PATIENTS ADMITTED AT THE PHILIPPINE GENERAL HOSPITAL FOR FEBRILE NEUTROPENIA
Pharmacologyonline 1: (2010) ewsletter Singh and Kochbar. Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of
 Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
ZINEX. Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg
 ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
Respiratory Infections
 Respiratory Infections NISHANT PRASAD, MD THE DR. JAMES J. RAHAL, JR. DIVISION OF INFECTIOUS DISEASES NEWYORK-PRESBYTERIAN QUEENS Disclosures Stockholder: Contrafect Corp., Bristol-Myers Squibb Co Research
Respiratory Infections NISHANT PRASAD, MD THE DR. JAMES J. RAHAL, JR. DIVISION OF INFECTIOUS DISEASES NEWYORK-PRESBYTERIAN QUEENS Disclosures Stockholder: Contrafect Corp., Bristol-Myers Squibb Co Research
Community Acquired Pneumonia
 April 2014 References: 1. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL Mace SE, McCracken Jr. GH, Moor MR, St. Peter SD, Stockwell JA, and Swanson JT. The Management of
April 2014 References: 1. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL Mace SE, McCracken Jr. GH, Moor MR, St. Peter SD, Stockwell JA, and Swanson JT. The Management of
The McMaster at night Pediatric Curriculum
 The McMaster at night Pediatric Curriculum Community Acquired Pneumonia Based on CPS Practice Point Pneumonia in healthy Canadian children and youth and the British Thoracic Society Guidelines on CAP Objectives
The McMaster at night Pediatric Curriculum Community Acquired Pneumonia Based on CPS Practice Point Pneumonia in healthy Canadian children and youth and the British Thoracic Society Guidelines on CAP Objectives
ISF criteria (International sepsis forum consensus conference of infection in the ICU) Secondary peritonitis
 Appendix with supplementary material. This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Supplementary Tables Table S1. Definitions
Appendix with supplementary material. This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Supplementary Tables Table S1. Definitions
Acute pneumonia Simple complement
 Acute pneumonia Simple complement 1. Clinical variants of acute pneumonia in children are, except: A. Bronchopneumonia B. Lobar confluent pneumonia C. Viral pneumonia D. Interstitial pneumonia E. Chronic
Acute pneumonia Simple complement 1. Clinical variants of acute pneumonia in children are, except: A. Bronchopneumonia B. Lobar confluent pneumonia C. Viral pneumonia D. Interstitial pneumonia E. Chronic
The clinical implication and prognostic predictors of Tigecycline treatment for pneumonia involving multidrug-resistant Acinetobacter baumannii
 Journal of Infection (2011) 63, 351e361 The clinical implication and prognostic predictors of Tigecycline treatment for pneumonia involving multidrug-resistant Acinetobacter baumannii R 陳南丞 VS 余文良醫師 本檔僅供內部教學使用檔案內所使用之照片之版權仍屬於原期刊公開使用時,
Journal of Infection (2011) 63, 351e361 The clinical implication and prognostic predictors of Tigecycline treatment for pneumonia involving multidrug-resistant Acinetobacter baumannii R 陳南丞 VS 余文良醫師 本檔僅供內部教學使用檔案內所使用之照片之版權仍屬於原期刊公開使用時,
Clinical, radiological and bacteriological profile of patients with community acquired pneumonia (CAP)
 Original Research Article Clinical, radiological and bacteriological profile of patients with community acquired pneumonia (CAP) R. S. Pushpa Kumari 1*, V. A. Vipula 2, Sonal Jain 3 1 Professor, Department
Original Research Article Clinical, radiological and bacteriological profile of patients with community acquired pneumonia (CAP) R. S. Pushpa Kumari 1*, V. A. Vipula 2, Sonal Jain 3 1 Professor, Department
POLICY FOR TREATMENT OF LOWER RESPIRATORY TRACT INFECTIONS
 POLICY F TREATMENT OF LOWER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: June 2018 Approved by: The Drugs & Therapeutics Committee Date: July 2018 Implementation
POLICY F TREATMENT OF LOWER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: June 2018 Approved by: The Drugs & Therapeutics Committee Date: July 2018 Implementation
Pseudomonas aeruginosa
 JOURNAL OF CLINICAL MICROBIOLOGY, July 1983, p. 16-164 95-1137/83/716-5$2./ Copyright C) 1983, American Society for Microbiology Vol. 18, No. 1 A Three-Year Study of Nosocomial Infections Associated with
JOURNAL OF CLINICAL MICROBIOLOGY, July 1983, p. 16-164 95-1137/83/716-5$2./ Copyright C) 1983, American Society for Microbiology Vol. 18, No. 1 A Three-Year Study of Nosocomial Infections Associated with
Antimicrobial Stewardship in Community Acquired Pneumonia
 Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
APPENDIX EXHIBITS. Appendix Exhibit A2: Patient Comorbidity Codes Used To Risk- Standardize Hospital Mortality and Readmission Rates page 10
 Ross JS, Bernheim SM, Lin Z, Drye EE, Chen J, Normand ST, et al. Based on key measures, care quality for Medicare enrollees at safety-net and non-safety-net hospitals was almost equal. Health Aff (Millwood).
Ross JS, Bernheim SM, Lin Z, Drye EE, Chen J, Normand ST, et al. Based on key measures, care quality for Medicare enrollees at safety-net and non-safety-net hospitals was almost equal. Health Aff (Millwood).
TRANSPARENCY COMMITTEE OPINION. 15 October Date of Marketing Authorisation (national procedure): 16 April 1997, variation of 18 February 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
December 3, 2015 Severe Sepsis and Septic Shock Antibiotic Guide
 Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
WORKSHOP. The Multiple Facets of CAP. Community acquired pneumonia (CAP) continues. Jennifer s Situation
 Practical Pointers pointers For for Your your Practice practice The Multiple Facets of CAP Dr. George Fox, MD, MSc, FRCPC, FCCP Community acquired pneumonia (CAP) continues to be a significant health burden
Practical Pointers pointers For for Your your Practice practice The Multiple Facets of CAP Dr. George Fox, MD, MSc, FRCPC, FCCP Community acquired pneumonia (CAP) continues to be a significant health burden
Choosing an appropriate antimicrobial agent. 3) the spectrum of potential pathogens
 Choosing an appropriate antimicrobial agent Consider: 1) the host 2) the site of infection 3) the spectrum of potential pathogens 4) the likelihood that these pathogens are resistant to antimicrobial agents
Choosing an appropriate antimicrobial agent Consider: 1) the host 2) the site of infection 3) the spectrum of potential pathogens 4) the likelihood that these pathogens are resistant to antimicrobial agents
Streptococci facultative anaerobe
 THE GENUS STREPTOCOCCUS The genus Streptococcus obtains Gram-positive cocci, nonmotile, nonsporeforming, arranged mostly in chains or in pairs. Most species are facultative anaerobes. Some of streptococci
THE GENUS STREPTOCOCCUS The genus Streptococcus obtains Gram-positive cocci, nonmotile, nonsporeforming, arranged mostly in chains or in pairs. Most species are facultative anaerobes. Some of streptococci
Antibiotic Resistance Pattern of Blood and CSF Culture Isolates At NHLS Academic Laboratories (2005)
 Antibiotic Resistance Pattern of Blood and CSF Culture Isolates At NHLS Academic Laboratories (2005) Streptococcus pneumoniae (SP) Blood Culture Isolates Penicillin intermediate Penicillin Cefotaxime 336
Antibiotic Resistance Pattern of Blood and CSF Culture Isolates At NHLS Academic Laboratories (2005) Streptococcus pneumoniae (SP) Blood Culture Isolates Penicillin intermediate Penicillin Cefotaxime 336
Influenza A (H1N1)pdm09 in Minnesota Epidemiology
 Influenza A (H1N1)pdm09 in Minnesota Epidemiology Infectious Disease Epidemiology, Prevention and Control Division PO Box 64975 St. Paul, MN 55164-0975 Number of Influenza Hospitalizations by Influenza
Influenza A (H1N1)pdm09 in Minnesota Epidemiology Infectious Disease Epidemiology, Prevention and Control Division PO Box 64975 St. Paul, MN 55164-0975 Number of Influenza Hospitalizations by Influenza
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study
 Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
Twice daily ceftriaxone therapy for serious bacterial infections in children
 Journal of Antimicrobial Chemotherapy (1984) 13, 511-516 Twice daily ceftriaxone therapy for serious bacterial infections in children Tasnee Chonmaitree*, Blaise L. Congenif, Jose Munoz+, Tamara A. Rakusan*,
Journal of Antimicrobial Chemotherapy (1984) 13, 511-516 Twice daily ceftriaxone therapy for serious bacterial infections in children Tasnee Chonmaitree*, Blaise L. Congenif, Jose Munoz+, Tamara A. Rakusan*,
Laboratory CLSI M100-S18 update. Paul D. Fey, Ph.D. Associate Professor/Associate Director Josh Rowland, M.T. (ASCP) State Training Coordinator
 Nebraska Public Health Laboratory 2008 CLSI M100-S18 update Paul D. Fey, Ph.D. Associate Professor/Associate Director Josh Rowland, M.T. (ASCP) State Training Coordinator Agenda Discuss 2008 M100- S18
Nebraska Public Health Laboratory 2008 CLSI M100-S18 update Paul D. Fey, Ph.D. Associate Professor/Associate Director Josh Rowland, M.T. (ASCP) State Training Coordinator Agenda Discuss 2008 M100- S18
Outpatient treatment in women with acute pyelonephritis after visiting emergency department
 LETTER TO THE EDITOR Korean J Intern Med 2017;32:369-373 Outpatient treatment in women with acute pyelonephritis after visiting emergency department Hee Kyoung Choi 1,*, Jin-Won Chung 2, Won Sup Oh 3,
LETTER TO THE EDITOR Korean J Intern Med 2017;32:369-373 Outpatient treatment in women with acute pyelonephritis after visiting emergency department Hee Kyoung Choi 1,*, Jin-Won Chung 2, Won Sup Oh 3,
Fever Without a Source Age: 0-28 Day Pathway - Emergency Department Evidence Based Outcome Center
 Age: 0-28 Day Pathway - Emergency Department EXCLUSION CRITERIA Toxic appearing No fever Born < 37 weeks gestational age INCLUSION CRITERIA Non-toxic with temperature > 38 C (100.4 F) < 36 C (96.5 F) measured
Age: 0-28 Day Pathway - Emergency Department EXCLUSION CRITERIA Toxic appearing No fever Born < 37 weeks gestational age INCLUSION CRITERIA Non-toxic with temperature > 38 C (100.4 F) < 36 C (96.5 F) measured
Community acquired pneumonia
 Community acquired pneumonia definition Symptoms of an acute LRTI New focal signs on chest examination At least one systemic feature New radiographic shadow Defination{Crofton} IT IS A SYNDROME CAUSED
Community acquired pneumonia definition Symptoms of an acute LRTI New focal signs on chest examination At least one systemic feature New radiographic shadow Defination{Crofton} IT IS A SYNDROME CAUSED
an inflammation of the bronchial tubes
 BRONCHITIS DEFINITION Bronchitis is an inflammation of the bronchial tubes (or bronchi), which are the air passages that extend from the trachea into the small airways and alveoli. Triggers may be infectious
BRONCHITIS DEFINITION Bronchitis is an inflammation of the bronchial tubes (or bronchi), which are the air passages that extend from the trachea into the small airways and alveoli. Triggers may be infectious
Pneumococcal Pneumonia: Update on Therapy in the Era of Antibiotic Resistance
 a of Antibiotic Resistance March 01, 2003 By Bernard Karnath, MD [1], Akua Agyeman, MD [2], and Albert Lai, MD [3] Sir William Osler once called pneumococcal pneumonia the captain of the men of death.
a of Antibiotic Resistance March 01, 2003 By Bernard Karnath, MD [1], Akua Agyeman, MD [2], and Albert Lai, MD [3] Sir William Osler once called pneumococcal pneumonia the captain of the men of death.
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Nosocomial Pneumonia. <5 Days: Non-Multidrug-Resistant Bacteria
 Nosocomial Pneumonia Meredith Deutscher, MD Troy Schaffernocker, MD Ohio State University Burden of Hospital-Acquired Pneumonia Second most common nosocomial infection in the U.S. 5-10 episodes per 1000
Nosocomial Pneumonia Meredith Deutscher, MD Troy Schaffernocker, MD Ohio State University Burden of Hospital-Acquired Pneumonia Second most common nosocomial infection in the U.S. 5-10 episodes per 1000
Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis
 Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis Vallières, E., Tumelty, K., Tunney, M. M., Hannah, R., Hewitt, O., Elborn, J. S., & Downey, D. G. (2017). Efficacy of Pseudomonas
Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis Vallières, E., Tumelty, K., Tunney, M. M., Hannah, R., Hewitt, O., Elborn, J. S., & Downey, D. G. (2017). Efficacy of Pseudomonas
without the permission of the author Not to be copied and distributed to others
 Emperor s Castle interior-prato What is the Role of Inhaled Polymyxins for Treatment of Respiratory Tract Infections? Helen Giamarellou CONCLUSIONS: Patients with Pseudomonas and Acinetobacter VAP may
Emperor s Castle interior-prato What is the Role of Inhaled Polymyxins for Treatment of Respiratory Tract Infections? Helen Giamarellou CONCLUSIONS: Patients with Pseudomonas and Acinetobacter VAP may
Clinical Comparison of Cefotaxime with Gentamicin plus Clindamycin in the Treatment of Peritonitis and Other Soft-Tissue Infections
 REVIEWS OF INFECTIOUS DISEASES. VOL. 4, SUPPLEMENT. SEPTEMBER-OCTOBER 982 982 by The University of Chicago. All rights reserved. 062-0886/82/0405-022$02.00 Clinical Comparison of with Gentamicin plus Clindamycin
REVIEWS OF INFECTIOUS DISEASES. VOL. 4, SUPPLEMENT. SEPTEMBER-OCTOBER 982 982 by The University of Chicago. All rights reserved. 062-0886/82/0405-022$02.00 Clinical Comparison of with Gentamicin plus Clindamycin
Adrian C. Peña, M.D., 1 Camilo C. Roa, M.D, 2 Nazario A. Macalintal, M.D. 3 Vincent M. Balanag, Jr. M.D., 4 and Myrna T. Mendoza, M.D.
 Safety and Efficacy of Intravenous and Oral Azithromycin in Community Acquired Pneumonia: Results of an Open, Multicenter Study Among Filipino Patients Adrian C. Peña, M.D., 1 Camilo C. Roa, M.D, 2 Nazario
Safety and Efficacy of Intravenous and Oral Azithromycin in Community Acquired Pneumonia: Results of an Open, Multicenter Study Among Filipino Patients Adrian C. Peña, M.D., 1 Camilo C. Roa, M.D, 2 Nazario
Correlation of Sputum Gram Stain and Sputum Culture for Respiratory Tract Infections in a Tertiary Care Hospital, Ballari, India
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 6 (2017) pp. 3008-3012 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.606.357
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 6 (2017) pp. 3008-3012 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.606.357
Session Guidelines. This is a 15 minute webinar session for CNC physicians and staff
 Respiratory Disease Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars monthly to address topics related to risk adjustment documentation and coding
Respiratory Disease Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars monthly to address topics related to risk adjustment documentation and coding
Creating a User Defined Pneumonia-Specific Syndrome in ESSENCE. Preventive Medicine Directorate September 2016
 Creating a User Defined Pneumonia-Specific Syndrome in ESSENCE Preventive Medicine Directorate September 2016 0 Pneumonia-Specific Syndrome NMCPHC retrospective analyses suggest that surveillance using
Creating a User Defined Pneumonia-Specific Syndrome in ESSENCE Preventive Medicine Directorate September 2016 0 Pneumonia-Specific Syndrome NMCPHC retrospective analyses suggest that surveillance using
ORIGINAL ARTICLE /j x
 ORIGINAL ARTICLE 10.1111/j.1469-0691.2005.01297.x Piperacillin tazobactam monotherapy in high-risk febrile and neutropenic cancer patients C. Viscoli 1, A. Cometta 2, W. V. Kern 3, R. De Bock 4, M. Paesmans
ORIGINAL ARTICLE 10.1111/j.1469-0691.2005.01297.x Piperacillin tazobactam monotherapy in high-risk febrile and neutropenic cancer patients C. Viscoli 1, A. Cometta 2, W. V. Kern 3, R. De Bock 4, M. Paesmans
Activity of Ceftolozane/Tazobactam Against a Broad Spectrum of Recent Clinical Anaerobic Isolates
 AAC Accepts, published online ahead of print on 25 November 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.02253-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. Activity
AAC Accepts, published online ahead of print on 25 November 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.02253-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. Activity
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
Objectives. Pneumonia. Pneumonia. Epidemiology. Prevalence 1/7/2012. Community-Acquired Pneumonia in infants and children
 Objectives Community-Acquired in infants and children Review of Clinical Practice Guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America - 2011 Sabah Charania,
Objectives Community-Acquired in infants and children Review of Clinical Practice Guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America - 2011 Sabah Charania,
Upper Respiratory Infections. Mehreen Arshad, MD Assistant Professor Pediatric Infectious Diseases Duke University
 Upper Respiratory Infections Mehreen Arshad, MD Assistant Professor Pediatric Infectious Diseases Duke University Disclosures None Objectives Know the common age- and season-specific causes of pharyngitis
Upper Respiratory Infections Mehreen Arshad, MD Assistant Professor Pediatric Infectious Diseases Duke University Disclosures None Objectives Know the common age- and season-specific causes of pharyngitis
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August Pneumococcal Disease, Invasive (IPD)
 August 2011 Pneumococcal Disease, Invasive (IPD) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August
August 2011 Pneumococcal Disease, Invasive (IPD) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August
11/19/2012. The spectrum of pulmonary diseases in HIV-infected persons is broad.
 The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
