Clostridium difficile Infection Antibiotics Guidelines. Contents
|
|
|
- Osborne Scot Wade
- 5 years ago
- Views:
Transcription
1 Clostridium difficile Infection Antibiotics Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): Authors Division: DCSS & Tertiary Medicine Unique ID: 144TD(C)25(D1) Issue number: 6 Expiry Date: May 2021 Contents Section Page Intro Who should read this document 2 Key practice points 2 Background/ Scope/ Definitions 2 What is new in this version 3 Policy/Procedure/Guideline 3 General Principles 3 General Management 4 Antimicrobial Treatment of CDI 5 Treatment of disease recurrence or relapse 6 Standards 8 Explanation of terms 8 References and Supporting Documents 8 Roles and Responsibilities 9 Appendix patients in whom PPIs should continue 10 Document control information (Published as separate document) 11 Document Control Policy Implementation Plan Monitoring and Review Endorsement Equality analysis Page 1 of 10
2 Who should read this document? This policy applies to all clinical staff involved the prescribing of antimicrobials. Key Practice Points This policy refers to the diagnosis and management of patients with Clostridium difficile. Background Antimicrobial agents are among the most commonly prescribed drugs and account for 20% of the hospital pharmacy budget. Unfortunately, the benefits of antibiotics to individual patients are compromised by the development of bacterial drug resistance. Resistance is a natural and inevitable result of exposing bacteria to antimicrobials. Good antimicrobial prescribing will help to reduce the rate at which antibiotic resistance emerges and spreads. It will also minimise the many side effects associated with antibiotic prescribing, such as Clostridium difficile infection. It should be borne in mind that antibiotics are not needed for simple coughs and colds. In some clinical situations, where infection is one of several possibilities and the patient is not showing signs of systemic sepsis, a wait and see approach to antibiotic prescribing is often justified while relevant cultures are performed. This document provides treatment guidelines for the most common situations in which antibiotic treatment is required. The products and regimens listed here have been selected by the Trust's Medicines Management Group on the basis of published evidence. Doses assume a weight of 60-80kg with normal renal and hepatic function. Adjustments may be needed for the treatment of some patients. This document provides treatment guidelines for the appropriate use of antibiotics. The recommendations that follow are for empirical therapy and do not cover all clinical circumstances. Alternative antimicrobial therapy may be needed in up to 20% of cases. Alternative recommendations will be made by the microbiologist in consultation with the clinical team. This document refers to the treatment of adult patients (unless otherwise stated). Refer to BNF/SPC for information on interactions, side effects, cautions and contraindications for individual drugs. Page 2 of 10
3 What is new in this version? The need to review indications for proton pump inhibitor (PPI) therapy has been added for patients who have a new or recent diagnosis of Clostridium difficile infection (CDI) and are taking PPIs Clarification that patients should receive IV metronidazole in place of oral vancomycin if they are nil by mouth or not keeping down oral vancomycin due to vomting. Dose of IV immunoglobulin added Removal of Rifaximin for a 2nd relapse of Clostridium difficile as PHE guidelines don t advise this. Details of the local centre for Faecal transplantation (Bolton) added. Guideline General Principles Clostridium difficile Infection Clostridium difficile infection (CDI) is a common cause of diarrhoea in hospitals and usually follows antibiotic therapy. Symptoms range from mild self-limiting diarrhoea to life-threatening pseudomembranous colitis. Clinical staff should apply the following mnemonic protocol (SIGHT) when managing potentially infectious diarrhoea: Suspect that a case may be infective when there is no clear alternative cause for the diarrhoea Isolate the patient and consult with the infection control team while determining the cause of the diarrhoea Gloves and aprons must be used for all contacts with the patient and their environment Hand washing with soap and water should be carried out before and after each contact with the patient and the patient s environment Test the stool for toxin, by sending a specimen immediately Treatment should be started empirically, without waiting for the results of testing. Rarely, cases of severe C. difficile infection (e.g. Pseudomembranous colitis) can present without diarrhoea. However, they will usually have other abdominal symptoms such as pain, distension or tenderness. A clinical diagnosis of C. difficile infection (CDI) is based on the clinical signs and symptoms, such as diarrhoea or signs of colitis. It may be confirmed by detection of the C. difficile toxin and/or the (tcdb) toxigenic gene in a faeces sample. A positive PCR result indicates that the toxigenic gene is present and therefore demonstrates the potential for CDI, but does not directly detect C. Page 3 of 10
4 difficile toxin. All PCR positive faeces samples will be processed further to test for C. difficile toxin by enzyme immunoassay (EIA). Refer to the Diarrhoea Assessment Tool for guidance on when to send samples. Specialist Registrar or Consultant must review the patient clinically and authorise processing of the sample. However, obtaining the sample should not be delayed whilst this approval is sought. Faeces will be tested for C. difficile in samples which assume the shape of their container (Bristol Stool types 5-7). Samples should be transported to the laboratory immediately after collection. If clinically indicated, treatment may need to be continued even if tests are negative: A small proportion (<4%) of patients with C. difficile infection may have a negative C. difficile toxin B gene result. It is reasonable to send a repeat faeces sample if C. difficile infection is strongly suspected on clinical grounds. Antacid treatments, calcium carbonate and magnesium or aluminium chloride have been shown to potentially interfere with the PCR assay. Rarely, cases of severe C. difficile infection (e.g. Pseudomembranous colitis) can present without diarrhoea. However, they will usually have other abdominal symptoms such as pain, distension or tenderness. CDI should be managed as a diagnosis in its own right, with each patient reviewed daily regarding fluid resuscitation, electrolyte replacement and nutrition review. Monitor for signs of increasing severity of disease, with early referral to ITU as patients may deteriorate very rapidly. Record the following daily as a clinical note (to facilitate follow-up and review): Summary of previous day s Bristol stool chart (e.g. diarrhoea x5, type 6-7) Presence or absence of abdominal distension and tenderness General Management A Bristol Stool Chart must be used to monitor the frequency and severity of diarrhoea Discontinue all current antibiotic therapy that is not clearly required. If this is not possible, change to antibiotics less likely to cause CDI. Discuss with Microbiologist. Discontinue any other drugs that might cause diarrhoea Page 4 of 10
5 Replace fluid and electrolytes. Antimotility drugs are contraindicated (e.g. loperamide or codeine phosphate) unless post-infective irritable bowel syndrome occurs, in which case seek specialist advice from a gastroenterologist If a patient with new or recently (within 3 years) diagnosed CDI is taking a PPI (which may increase the risk of future CDI relapse/recurrence), review the PPI indication and stop the PPI if risks outweigh likely benefits. Antacids or alternative acid suppressant therapies should be offered if needed. A list of indications for which PPI therapy should normally be continued is given in the Appendix. Where PPIs are discontinued, this should be discussed with the individual patient. In the situation where this is not possible the reason must be recorded. Antimicrobial Treatment of CDI Assessment of Disease Severity Patients should be assessed for indicators of severe disease in order to guide appropriate management. Typical features of the disease are listed in the table below. Mild-moderate disease Severe disease Life-threatening disease WBC <15 x 109 /L and <6 any one of the following: Characterised by stools per day but note that diarrhoea Leukocyte count >15 x 10 9 /L hypotension and/or ileus/toxic may be absent in the presence of severe disease with colitis Acutely rising serum creatinine (increased by >50% above baseline). megacolon and/or CT evidence of severe disease Temperature >38.5 oc Evidence of severe colitis (abdominal or radiological signs) Treatment Non-severe disease: Vancomycin 125 mg PO qds for days (or Metronidazole IV 500mg TDS if patient is nil by mouth or vomiting) NB: Vancomycin IV should not be used for the treatment of CDI Patients should be assessed daily for signs of severe disease. If symptoms are worsening, with indicators of severe disease (see above), consider requesting an abdominal CT scan and refer for a surgical and /or gastroenterology review. Evaluate the response to therapy after one week Page 5 of 10
6 If symptoms are not resolving, increase dose to 250 mg PO qds for a further days If symptoms have not resolved after 3 weeks, refer for a specialist gastrointestinal and microbiology opinion Severe disease: Vancomycin PO 125mg qds for days,( if necessary by nasogastric tube) +/- IV metronidazole 500mg tds. Please ensure the patient is reviewed by a gastroenterologist and refer to a microbiologist. NB: Vancomycin IV should not be used for the treatment of CDI Consider: extending the oral vancomycin course for a third week if the patient is improving, but symptoms are slow to settle increasing vancomycin dose to 250 mg qds if not responding rectal instillation of vancomycin (see below) IV immunoglobulin if not responding to the above seek specialist advice Life-threatening disease: Vancomycin PO 500mg qds for days (via a nasogastric tube or rectal installation) plus IV metronidazole 500mg tds. NB: Vancomycin IV should not be used for the treatment of CDI Arrange an urgent review by a gastroenterologist and a lower GI surgeon. These patients should be monitored closely with specialist surgical input and have their blood lactate measured. Colectomy should be considered, especially if caecal dilatation is >10 cm. (Colectomy is best performed before the blood lactate rises above 5 mmol/l). Consider : IV immunoglobulin 400mg/kg as a stat dose if not responding to the above seek specialist advice from microbiology. Haematology patients Fidaxomicin 200mg BD for 10 days should be substituted for vancomycin in the treatment recommendations above for non-severe laboratory confirmed CDI. Patients should commence vancomycin pending confirmation of CDI. Liquid vancomycin Liquid vancomycin is made by the pharmacy extemporaneous department and is available from the pharmacy department during normal pharmacy working hours. Out of hours, vancomycin injection may be used orally or via enteral feeding tubes. Use of the vancomycin injection, via the oral route is unlicensed. Preparation of liquid vancomycin At the time of use, add 10ml of sterile Water for Injections BP to a 500mg vial of Vancomycin Hydrochloride 500mg Powder for Concentrate for Infusion. Shake each vial to dissolve the powder. This will provide you with a 50mg/ml solution. Page 6 of 10
7 After initial reconstitution of the vial, the selected dose 250 mg (5ml) or 125 mg (2.5ml) may be diluted in 30 ml of water and given to the patient to drink or the diluted solution may be administered by a nasogastric tube. Treatment of disease recurrence or relapse Recurrence occurs in approximately 20% of cases after a first episode and 40-60% after a second or subsequent episode. First relapse: Fidaxomicin 200mg BD for 10 days is indicated in a laboratory confirmed relapse within 6 months of the previous episode, and if the patient has not been previously treated with fidaxomicin. Otherwise vancomycin 125mg QDS should be prescribed. Management of multiple recurrent episodes Treat as above until asymptomatic for 7 days. Review all medications. Consider stopping PPIs and other GI active agents Discuss the options with a consultant microbiologist or gastroenterologist: Supervised trial of loperamide (if post-infectious inflammatory bowel syndrome is suspected) Rectal installation of vancomycin (see below) Oral vancomycin tapering therapy (see below) IV immunoglobulin 400mg/kg as a single dose Faecal microbiota transplant (NICE IPG485) Seek a specialist gastrointestinal or microbiology opinion if not responding to treatment Criteria for repeat Clostridium difficile toxin testing Do not retest samples from a patient with a positive C. difficile toxin test within 28 days, unless symptoms resolve and then recur - this should be discussed and agreed with the duty microbiologist. Any patient with a clinical recurrence of CDI, should normally be treated as above. Rectal instillation of vancomycin Vancomycin (500 mg in ml Sodium chloride 0.9% 4 12 hourly) given as retention enema: 18 gauge Foley catheter with 30 ml balloon inserted per rectum; vancomycin instilled; catheter clamped for 60 minutes; deflate and remove, if the oral route is compromised e.g.by colitis. A bowel management system should be used to administer the vancomycin and this should be retained in the rectum for 1 hour. Consider monitoring serum vancomycin levels if colitis is present. Vancomycin tapering therapy regimen Oral / NG vancomycin 125 mg qds for 1st week Oral / NG vancomycin 125 mg tds for 2nd week Oral / NG vancomycin 125 mg bd for 3rd week Oral / NG vancomycin 125 mg od for 4th week Oral / NG vancomycin 125 mg on alternate days for 5th week Page 7 of 10
8 Oral / NG vancomycin 125 mg every third day for 6th week Faecal microbiota transplant For patients were fidaxomicin has been unsuccessful, faecal repopulation should be considered and discussed with the Infection Control team, Microbiologist and patients consultant in an MDT. If agreed, a referral should be made to the Royal Bolton Hospital: sara.hardman@boltonft.nhs.uk or Tel Death Certification There is a legal duty to mention CDI on a death certificate if it was part of the sequence of events directly leading to death or contributed in some way. If a patient with CDI dies, this should be recorded, as appropriate, in either Part 1 or Part 2 of the death certificate. Standards Document the Indication/rationale for antimicrobial therapy, including clinical criteria relevant to this. Review and document the patient s allergy status Ensure the choice of antibiotic complies with the antibiotic guidelines and you have documented any clinical criteria relevant to the choice of agent. Document a management plan including a stop or review date. Where relevant, consider drainage of pus or surgical debridement/removal of foreign material. Explanation of terms Not applicable. References and Supporting Documents 1. Pepin, J Vancomycin for the treatment of Clostridium difficile infection: for whom is this expensive bullet really magic? Clinical Infectious Diseases 2008; 46: Pepin et al. Management and outcomes of a first recurrence of CDAD in Quebec, Canada.Clinical Infectious Diseases 2006; 42: Owens RC. Clostridium difficile associated disease: an emerging threat to patient safety. Pharmacotherapy2006;26(3): Apisarnthanarak A, Razavi B,Mundy LM. Adjunctive Intracolonic Vancomycin for Severe Clostridium difficile Colitis:Case Series and Review of the Literature Clin Infect Dis 2002; 35; Page 8 of 10
9 5. Clostridium difficile infection: how to deal with the problem. DH and Health Protection Agency. Best Practice Guidance. December Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S; OPT Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012; 12(4): Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK; OPT Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011; 364(5): Mullane KM, Miller MA, Weiss K, Lentnek A, Golan Y, Sears PS, Shue YK, Louie TJ, Gorbach SL. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011; 53(5): Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL.Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012; 55 Suppl 2:S Tedesco FJ, Gordon D and Fortson WC (1985). Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol 80: Updated guidance on the management and treatment of Clostridium difficile infection. Public Health England. May Summary of Product Characteristics for Vancomycin Hydrochloride 500mg and 1g Powder for Concentrate for Infusion. Hospira UK Ltd. 30 th Jan Kim JW, Lee KL, Jeong JB, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol. 2010;16(28): Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9): Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10): Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9): Roles and responsibilities All clinical staff involved in the prescribing of antimicrobials to adhere to this policy including full documentation on EPMAR as detailed. Page 9 of 10
10 Appendix - Patients in whom PPIs should continue Those patients who have had an upper GI bleed in the last 2 months. Those with a history of upper GI bleed who have on-going dyspeptic symptoms. Patients with oesophageal strictures secondary to acid reflux. Patients with Barrett s oesophagus already receiving life-long PPIs. Patients with Zollinger-Ellison syndrome. Bariatric patients for 2 years after gastric bypass surgery. Patients with significant renal disease or impairment defined as CKD stage 4-5, egfr<30mls/min/m 2 or AKI stage 3 Those on dual anti-platelet therapy(aspirin and clopidogrel /ticagrelor / prasugrel)(dapt) and receiving steroids Those on dual anti-platelet therapy in patients aged 75 years Those taking an anticoagulant vitamin K antagonist (VKA, e.g. warfarin) and taking single anti-platelet therapy (SAPT) or DAPT Those taking a direct/novel oral anticoagulant (DOAC/NOAC) and taking SAPT or DAPT Those taking a NSAID and SAPT, DAPT or an anticoagulant. (Consider whether the patient should continue with the NSAID. Is there an alternative?) Those with a PMH of peptic ulcer disease or upper GI bleeding taking SAPT, DAPT or an anticoagulant. Those on HDU, ITU or receiving potent chemotherapy in whom the risk of GI bleeding outweighs the risk of C. difficile acquisition. Those with intractable dyspeptic symptoms in whom alternative therapies are ineffective or contraindicated. Those with a perceived high risk of GI bleeding (following discussion with the GI team) Patients > 45 years taking a regular non-steroidal anti-inflammatory drug (NSAID) Acid related symptoms unresponsive to antacids or H2 antagonists (or contraindicated) Any other patients who, in the clinical opinion of the named Consultant in charge of the episode of care consider the risks of stopping PPI outweigh the benefits where this is documented explicitly in the patient notes. The above list is not exhaustive and clinicians are invited to discuss individual cases with the gastroenterologists, microbiologists and pharmacy teams for query resolution. Page 10 of 10
IV Vancomycin dosing and monitoring Antibiotic Guidelines. Contents. Intro
 IV Vancomycin dosing and Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): as above Authors Division: DCSS & Tertiary Medicine Unique
IV Vancomycin dosing and Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): as above Authors Division: DCSS & Tertiary Medicine Unique
Clostridium difficile Infection (CDI) Management Guideline
 Clostridium difficile Infection (CDI) Management Guideline Do not test all patients with loose or watery stools for CDI o CDI is responsible for
Clostridium difficile Infection (CDI) Management Guideline Do not test all patients with loose or watery stools for CDI o CDI is responsible for
Chapter 5 Gastroenterology. Dose. Route. Units. Given. Dose. Route. Units. Given
 Chapter 5 Gastroenterology Georgia Woodfield TERLIPRESSIN CIPROFLOXACIN 2 initial dose 500 Every 4 hours until bleeding controlled can be reduced to 1 after initial dose if side effects develop or if patient
Chapter 5 Gastroenterology Georgia Woodfield TERLIPRESSIN CIPROFLOXACIN 2 initial dose 500 Every 4 hours until bleeding controlled can be reduced to 1 after initial dose if side effects develop or if patient
Infective Endocarditis Empirical therapy Antibiotic Guidelines. Contents
 Infective Endocarditis Empirical therapy Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Group Additional author(s): as above Authors Division: Division of Clinical
Infective Endocarditis Empirical therapy Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Group Additional author(s): as above Authors Division: Division of Clinical
Updated Clostridium difficile Treatment Guidelines
 Updated Clostridium difficile Treatment Guidelines Arielle Arnold, PharmD, BCPS Clinical Pharmacist Saint Alphonsus Regional Medical Center September 29 th, 2018 Disclosures Nothing to disclose Learning
Updated Clostridium difficile Treatment Guidelines Arielle Arnold, PharmD, BCPS Clinical Pharmacist Saint Alphonsus Regional Medical Center September 29 th, 2018 Disclosures Nothing to disclose Learning
Lifting the lid on a difficile problem part 2 (Clinical) Evidence Based Practice. Problem in evolution (1) Problem in evolution (1) Interventions (2)
 Lifting the lid on a difficile problem part (Clinical) Dr Philip T Mannion Consultant Microbiologist, Rhyl Evidence Based Practice Antibiotic prescribing guidance Isolation policy Hand hygiene (soap and
Lifting the lid on a difficile problem part (Clinical) Dr Philip T Mannion Consultant Microbiologist, Rhyl Evidence Based Practice Antibiotic prescribing guidance Isolation policy Hand hygiene (soap and
MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
GUIDELINE FOR THE MANAGEMENT OF ANTIBIOTIC- ASSOCIATED DIARRHOEA IN ADULTS
 GUIDELINE FOR THE MANAGEMENT OF ANTIBIOTIC- ASSOCIATED DIARRHOEA IN ADULTS Version 3.0 Date ratified May 2008 Review date May 2010 Ratified by NUH Antibiotic Guidelines Committee NUH Drugs and Therapeutics
GUIDELINE FOR THE MANAGEMENT OF ANTIBIOTIC- ASSOCIATED DIARRHOEA IN ADULTS Version 3.0 Date ratified May 2008 Review date May 2010 Ratified by NUH Antibiotic Guidelines Committee NUH Drugs and Therapeutics
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author)
 Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact ame and Job Title (author) Directorate & Speciality Date of submission December 2015 Date on which guideline
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact ame and Job Title (author) Directorate & Speciality Date of submission December 2015 Date on which guideline
Division of GIM Lecture Series Case Presentation David A. Erickson, M.D October 9th, 2013
 Division of GIM Lecture Series Case Presentation David A. Erickson, M.D October 9th, 2013 Financial Disclosures No financial disclosures Objectives Review a case of recurrent Clostridium difficile infection
Division of GIM Lecture Series Case Presentation David A. Erickson, M.D October 9th, 2013 Financial Disclosures No financial disclosures Objectives Review a case of recurrent Clostridium difficile infection
Stony Brook Adult Clostridium difficile Management Guidelines. Discontinue all unnecessary antibiotics
 Stony Brook Adult Clostridium difficile Management Guidelines Summary: Use of the C Diff Infection (CDI) PowerPlan (Adult) Required Patient with clinical findings suggestive of Clostridium difficile infection
Stony Brook Adult Clostridium difficile Management Guidelines Summary: Use of the C Diff Infection (CDI) PowerPlan (Adult) Required Patient with clinical findings suggestive of Clostridium difficile infection
! Macrolide antibacterial. Fidaxomicin (Dificid ) package labeling. Optimer Pharmaceuticals, Inc. May 2011.
 Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
ENGLISH FOR PROFESSIONAL PURPOSES UNIT 3 HOW TO DEAL WITH CLOSTRIDIUM DIFFICILE
 ENGLISH FOR PROFESSIONAL PURPOSES UNIT 3 HOW TO DEAL WITH CLOSTRIDIUM DIFFICILE The diagnosis of CDI should be based on a combination of clinical and laboratory findings. A case definition for the usual
ENGLISH FOR PROFESSIONAL PURPOSES UNIT 3 HOW TO DEAL WITH CLOSTRIDIUM DIFFICILE The diagnosis of CDI should be based on a combination of clinical and laboratory findings. A case definition for the usual
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Terapia dell infezione da Clostridium difficile. Massimo Coen I Div Mal Inf AO L Sacco
 Terapia dell infezione da Clostridium difficile Massimo Coen I Div Mal Inf AO L Sacco Disease Severity Mild CDI 3 5 BM/day WBC 15,000/mm 3 Defining CDI Disease Severity Mild abdominal pain due to CDI Moderate
Terapia dell infezione da Clostridium difficile Massimo Coen I Div Mal Inf AO L Sacco Disease Severity Mild CDI 3 5 BM/day WBC 15,000/mm 3 Defining CDI Disease Severity Mild abdominal pain due to CDI Moderate
Clostridium difficile Infection: Diagnosis and Management
 Clostridium difficile Infection: Diagnosis and Management Brian Viviano D.O. Case study 42 year old female with history of essential hypertension and COPD presents to ED complaining of 24 hours of intractable,
Clostridium difficile Infection: Diagnosis and Management Brian Viviano D.O. Case study 42 year old female with history of essential hypertension and COPD presents to ED complaining of 24 hours of intractable,
Clinical. Clostridium Difficile: Standard Operating Procedure. Document Control Summary. Contents
 Clinical Clostridium Difficile: Standard Operating Procedure Document Control Summary Status: Version: Author/Title: Owner/Title: Approved by: Ratified: Related Trust Strategy and/or Strategic Aims Implementation
Clinical Clostridium Difficile: Standard Operating Procedure Document Control Summary Status: Version: Author/Title: Owner/Title: Approved by: Ratified: Related Trust Strategy and/or Strategic Aims Implementation
Case 1. Which of the following would be next appropriate investigation/s regarding the pts diarrhoea?
 Case 1 21 yr old HIV +ve, Cd4-100 HAART naïve Profuse diarrhoea for 3/52. Stool MC&S ve Which of the following would be next appropriate investigation/s regarding the pts diarrhoea? Repeat stool MC&S Stool
Case 1 21 yr old HIV +ve, Cd4-100 HAART naïve Profuse diarrhoea for 3/52. Stool MC&S ve Which of the following would be next appropriate investigation/s regarding the pts diarrhoea? Repeat stool MC&S Stool
Clostridium difficile
 Clostridium difficile Care Homes IPC Study Day Sue Barber Infection Prevention & Control Lead AV & Chiltern CCG s Clostridium difficile A spore forming Bacterium. Difficult to grow in the laboratory hence
Clostridium difficile Care Homes IPC Study Day Sue Barber Infection Prevention & Control Lead AV & Chiltern CCG s Clostridium difficile A spore forming Bacterium. Difficult to grow in the laboratory hence
DISCLAIMER: ECHO Nevada emphasizes patient privacy and asks participants to not share ANY Protected Health Information during ECHO clinics.
 DISCLAIMER: Video will be taken at this clinic and potentially used in Project ECHO promotional materials. By attending this clinic, you consent to have your photo taken and allow Project ECHO to use this
DISCLAIMER: Video will be taken at this clinic and potentially used in Project ECHO promotional materials. By attending this clinic, you consent to have your photo taken and allow Project ECHO to use this
Clostridium Difficile Infection: Applying New Treatment Guidelines and Strategies to Reduce Recurrence Rate
 Clostridium Difficile Infection: Applying New Treatment Guidelines and Strategies to Reduce Recurrence Rate Objectives Summarize the changing epidemiology and demographics of patients at risk for Clostridium
Clostridium Difficile Infection: Applying New Treatment Guidelines and Strategies to Reduce Recurrence Rate Objectives Summarize the changing epidemiology and demographics of patients at risk for Clostridium
Updates to pharmacological management in the prevention of recurrent Clostridium difficile
 Updates to pharmacological management in the prevention of recurrent Clostridium difficile Julia Shlensky, PharmD PGY2 Internal Medicine Resident September 12, 2017 2017 MFMER slide-1 Clinical Impact Increasing
Updates to pharmacological management in the prevention of recurrent Clostridium difficile Julia Shlensky, PharmD PGY2 Internal Medicine Resident September 12, 2017 2017 MFMER slide-1 Clinical Impact Increasing
Proton Pump Inhibitor De-prescribing Guidance
 Amendment History Proton Pump Inhibitor De-prescribing Guidance VERSION DATE AMENDMENT HISTORY 1.0 2013 Previous version 2.0 September 2015 Comments Amendment to Flow chart and addition of Rationale page
Amendment History Proton Pump Inhibitor De-prescribing Guidance VERSION DATE AMENDMENT HISTORY 1.0 2013 Previous version 2.0 September 2015 Comments Amendment to Flow chart and addition of Rationale page
Management of dyspepsia and of Helicobacter pylori infection
 Management of dyspepsia and of Helicobacter pylori infection The University of Nottingham John Atherton Wolfson Digestive Diseases Centre University of Nottingham, UK Community management of dyspepsia
Management of dyspepsia and of Helicobacter pylori infection The University of Nottingham John Atherton Wolfson Digestive Diseases Centre University of Nottingham, UK Community management of dyspepsia
NHS Suffolk Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice)
 NHS Suffolk Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
NHS Suffolk Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Literature Scan: Antibiotics for Clostridium difficile Infection. Month/Year of Review: May 2015 Date of Last Review: April 2012
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
EDUCATIONAL COMMENTARY CLOSTRIDIUM DIFFICILE UPDATE
 EDUCATIONAL COMMENTARY CLOSTRIDIUM DIFFICILE UPDATE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
EDUCATIONAL COMMENTARY CLOSTRIDIUM DIFFICILE UPDATE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
more intense treatments are needed to get rid of the infection.
 What Is Clostridium Difficile (C. Diff)? Clostridium difficile, or C. diff for short, is an infection from a bacterium that can grow in your intestines and cause bad GI symptoms. The main risk of getting
What Is Clostridium Difficile (C. Diff)? Clostridium difficile, or C. diff for short, is an infection from a bacterium that can grow in your intestines and cause bad GI symptoms. The main risk of getting
FIS 2013, Birmingham
 Fidaxomicin: CDDFT Case Studies Dr Deepa Nayar County Durham and Darlington Foundation Trust FIS 2013, Birmingham This meeting has been initiated and funded by Astellas Pharma Ltd. County Durham and Darlington
Fidaxomicin: CDDFT Case Studies Dr Deepa Nayar County Durham and Darlington Foundation Trust FIS 2013, Birmingham This meeting has been initiated and funded by Astellas Pharma Ltd. County Durham and Darlington
Modern approach to Clostridium Difficile Infection
 Modern approach to Clostridium Difficile Infection Pseudomembranous Colitis: Principles for diagnosis and treatment Aggelos Stefos Internist, Infectious diseases Specialist Department of Medicine and Research
Modern approach to Clostridium Difficile Infection Pseudomembranous Colitis: Principles for diagnosis and treatment Aggelos Stefos Internist, Infectious diseases Specialist Department of Medicine and Research
Diagnosis, Management, and Prevention of Clostridium difficile infection in Long-Term Care Facilities: A Review
 Diagnosis, Management, and Prevention of Clostridium difficile infection in Long-Term Care Facilities: A Review October 18, 2010 James Kahn and Carolyn Kenney, MSIV Overview Burden of disease associated
Diagnosis, Management, and Prevention of Clostridium difficile infection in Long-Term Care Facilities: A Review October 18, 2010 James Kahn and Carolyn Kenney, MSIV Overview Burden of disease associated
Clostridium Difficile Infection in Adults Treatment and Prevention
 Clostridium Difficile Infection in Adults Treatment and Prevention Definition: Clostridium Difficile colonizes the human intestinal tract after the normal gut flora has been altered by antibiotic therapy
Clostridium Difficile Infection in Adults Treatment and Prevention Definition: Clostridium Difficile colonizes the human intestinal tract after the normal gut flora has been altered by antibiotic therapy
A Pharmacist Perspective
 Leveraging Technology to Reduce CDI A Pharmacist Perspective Ed Eiland, Pharm.D., MBA, BCPS (AQ-ID) Clinical Practice and Business Supervisor Huntsville Hospital System Huntsville Hospital 881 licensed
Leveraging Technology to Reduce CDI A Pharmacist Perspective Ed Eiland, Pharm.D., MBA, BCPS (AQ-ID) Clinical Practice and Business Supervisor Huntsville Hospital System Huntsville Hospital 881 licensed
Clostridium difficile Infection (CDI) Guideline
 Clostridium difficile Infection (CDI) Guideline Site Applicability All VCH PHC acute care and residential sites. Practice Level Physicians basic skill Pharmacists basic skill Nurses (RN, LPN, RPN) basic
Clostridium difficile Infection (CDI) Guideline Site Applicability All VCH PHC acute care and residential sites. Practice Level Physicians basic skill Pharmacists basic skill Nurses (RN, LPN, RPN) basic
GASTROINTESTINAL SYSTEM MANAGEMENT OF DYSPEPSIA
 GASTROINTESTINAL SYSTEM MANAGEMENT OF DYSPEPSIA MANAGEMENT Dyspepsia refers to a spectrum of usually intermittent upper gastrointestinal symptoms, including epigastric pain and heartburn. For the majority
GASTROINTESTINAL SYSTEM MANAGEMENT OF DYSPEPSIA MANAGEMENT Dyspepsia refers to a spectrum of usually intermittent upper gastrointestinal symptoms, including epigastric pain and heartburn. For the majority
ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Clostridium difficile Infections
 ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Clostridium difficile Infections Christina M. Surawicz, MD 1, Lawrence J. Brandt, MD 2, David G. Binion, MD 3, Ashwin N. Ananthakrishnan,
ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Clostridium difficile Infections Christina M. Surawicz, MD 1, Lawrence J. Brandt, MD 2, David G. Binion, MD 3, Ashwin N. Ananthakrishnan,
Guidelines for the Management of Dyspepsia and GORD. Gastroenterology/ Acute Adult Governance. Drugs and Therapeutics Committee
 Guidelines for the Management of Dyspepsia and GORD Document type: Version: 3.0 Author (name): Author (designation): Validated by Prescribing Dr. G. Lipscomb Date validated October 2015 Ratified by: Date
Guidelines for the Management of Dyspepsia and GORD Document type: Version: 3.0 Author (name): Author (designation): Validated by Prescribing Dr. G. Lipscomb Date validated October 2015 Ratified by: Date
Corporate Medical Policy Fecal Microbiota Transplantation
 Corporate Medical Policy Fecal Microbiota Transplantation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Fecal_microbiota_transplantation 7/2014 11/2017 11/2018 11/2017 Description
Corporate Medical Policy Fecal Microbiota Transplantation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Fecal_microbiota_transplantation 7/2014 11/2017 11/2018 11/2017 Description
Ipilimumab (skin) Indication Advanced (unresectable or metastatic) melanoma in patients who have received prior therapy.
 Ipilimumab (skin) Indication Advanced (unresectable or metastatic) melanoma in patients who have received prior therapy. (NICE TA268) ICD-10 codes Codes prefixed with C43 Regimen details Day Drug Dose
Ipilimumab (skin) Indication Advanced (unresectable or metastatic) melanoma in patients who have received prior therapy. (NICE TA268) ICD-10 codes Codes prefixed with C43 Regimen details Day Drug Dose
Long-Term Care Updates
 Long-Term Care Updates April 2018 By Austin Smith, PharmD Candidate and Lindsay Slowiczek, PharmD is the most common healthcare-acquired infection (HAI) in the United States. 1,2 A 2014 prevalence survey
Long-Term Care Updates April 2018 By Austin Smith, PharmD Candidate and Lindsay Slowiczek, PharmD is the most common healthcare-acquired infection (HAI) in the United States. 1,2 A 2014 prevalence survey
Nivolumab and Ipilimumab
 Nivolumab and Ipilimumab Indication Advanced (unresectable or metastatic) melanoma. (NICE TA400) ICD-10 codes Codes prefixed with C43 Regimen details Cycles 1-4 Nivolumab and Ipilimumab every 3 weeks Day
Nivolumab and Ipilimumab Indication Advanced (unresectable or metastatic) melanoma. (NICE TA400) ICD-10 codes Codes prefixed with C43 Regimen details Cycles 1-4 Nivolumab and Ipilimumab every 3 weeks Day
Chapter 14: Training in Radiology. DDSEP Chapter 1: Question 12
 DDSEP Chapter 1: Question 12 A 52-year-old white male presents for evaluation of sudden onset of abdominal pain and shoulder pain. His past medical history is notable for a history of coronary artery disease,
DDSEP Chapter 1: Question 12 A 52-year-old white male presents for evaluation of sudden onset of abdominal pain and shoulder pain. His past medical history is notable for a history of coronary artery disease,
Journey to Decreasing Clostridium Difficile and the Unexpected Twist. Jackie Morton, Infection Prevention Cortney Swiggart, Medication Safety Officer
 Journey to Decreasing Clostridium Difficile and the Unexpected Twist Jackie Morton, Infection Prevention Cortney Swiggart, Medication Safety Officer 4/13/2018 Objectives Discuss the organism and clinical
Journey to Decreasing Clostridium Difficile and the Unexpected Twist Jackie Morton, Infection Prevention Cortney Swiggart, Medication Safety Officer 4/13/2018 Objectives Discuss the organism and clinical
BNF CHAPTER 1: GASTRO-INTESTINAL SYSTEM. 1 April 13
 BNF CHAPTER 1: GASTRO-INTESTINAL SYSTEM 1 BNF 1.1 ANTACIDS Mucogel and Peptac are effective antacids and are available over the counter Gaviscon / Gaviscon Advance are available over the counter First
BNF CHAPTER 1: GASTRO-INTESTINAL SYSTEM 1 BNF 1.1 ANTACIDS Mucogel and Peptac are effective antacids and are available over the counter Gaviscon / Gaviscon Advance are available over the counter First
Cisplatin and Fluorouracil (palliative)
 Cisplatin and Fluorouracil (palliative) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated. PS0-1
Cisplatin and Fluorouracil (palliative) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated. PS0-1
Algorithms & Information Sheets
 Minor Ailment Scheme Algorithms & Information Sheets Acute Diarrhoea Diarrhoea is an increased frequency, fluidity or volume of the bowel movements with the passage of soft and watery stools as compared
Minor Ailment Scheme Algorithms & Information Sheets Acute Diarrhoea Diarrhoea is an increased frequency, fluidity or volume of the bowel movements with the passage of soft and watery stools as compared
Clostridium difficile: Can you smell the new updates?
 Clostridium difficile: Can you smell the new updates? Sunish Shah, Pharm.D. PGY-2 Infectious Disease Pharmacy Resident Yale-New Haven Hospital sshah1741@mail.usciences.edu Learning objectives Recognize
Clostridium difficile: Can you smell the new updates? Sunish Shah, Pharm.D. PGY-2 Infectious Disease Pharmacy Resident Yale-New Haven Hospital sshah1741@mail.usciences.edu Learning objectives Recognize
Update on Clostridium difficile infection.
 Update on Clostridium difficile infection. K. Honein Gastroenterologist, HDF Associate Professor Head of Medicine Department St Joseph University-Beirut. Introduction Gram+anaerobic bacillus responsible
Update on Clostridium difficile infection. K. Honein Gastroenterologist, HDF Associate Professor Head of Medicine Department St Joseph University-Beirut. Introduction Gram+anaerobic bacillus responsible
Gastrointestinal Involvement in Adult Mitochondrial Disease
 Newcastle Mitochondrial Disease Guidelines At a glance guidelines: Gastrointestinal Involvement in Adult Mitochondrial Disease For full guideline visit: http://www.newcastle-mitochondria.com/service/patient-care-guidelines/
Newcastle Mitochondrial Disease Guidelines At a glance guidelines: Gastrointestinal Involvement in Adult Mitochondrial Disease For full guideline visit: http://www.newcastle-mitochondria.com/service/patient-care-guidelines/
Area Drug and Therapeutics Committee Prescribing Supplement No 59 July 2012
 Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Mid Essex Locality Guideline for Management of Adult Acute and Chronic Non-Cancer Pain in Primary care
 Guideline for Management of Adult Acute and Chronic Non-Cancer Pain in Primary care If possible patients should be assessed using a simple visual analogue scale VAS to determine the most appropriate stage
Guideline for Management of Adult Acute and Chronic Non-Cancer Pain in Primary care If possible patients should be assessed using a simple visual analogue scale VAS to determine the most appropriate stage
Clostridium difficile (C difficile)
 Patient Knowledge and Attitudes About for Clostridium difficile Infection Colin Goodman, MD; Nicholas O Rourke, PharmD; Carla Amundson, MA; and Dimitri Drekonja, MD, MS In a survey of patients with Clostridium
Patient Knowledge and Attitudes About for Clostridium difficile Infection Colin Goodman, MD; Nicholas O Rourke, PharmD; Carla Amundson, MA; and Dimitri Drekonja, MD, MS In a survey of patients with Clostridium
The usual dose is 40 mg daily with amoxycillin 1.5 g (750 mg b.d.) for 2 weeks. Up to 2 g/day of amoxycillin has been used in clinical trials.
 Name Gasec - 2 Gastrocaps Composition Gasec-20 Gastrocaps Each Gastrocaps contains: Omeprazole 20 mg (in the form of enteric-coated pellets) Properties, effects Proton Pump Inhibitor Omeprazole belongs
Name Gasec - 2 Gastrocaps Composition Gasec-20 Gastrocaps Each Gastrocaps contains: Omeprazole 20 mg (in the form of enteric-coated pellets) Properties, effects Proton Pump Inhibitor Omeprazole belongs
L infezione da Clostridium difficile (CDI) Quadri clinici e nuovi approcci terapeutici
 L infezione da Clostridium difficile (CDI) Quadri clinici e nuovi approcci terapeutici Roberto Luzzati SC Malattie Infettive, AOU Trieste Presidente :Prof. Enzo Raise Clinical presentation of infection
L infezione da Clostridium difficile (CDI) Quadri clinici e nuovi approcci terapeutici Roberto Luzzati SC Malattie Infettive, AOU Trieste Presidente :Prof. Enzo Raise Clinical presentation of infection
Guideline scope Diverticular disease: diagnosis and management
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
Bezlotoxumab (Zinplava) as Adjunct Treatment for Clostridium difficile. Janel Liane Cala, RPh Medical Center Hospital
 Bezlotoxumab (Zinplava) as Adjunct Treatment for Clostridium difficile Janel Liane Cala, RPh Medical Center Hospital Objectives Review pathophysiology, risk factors, prevention, and treatment options of
Bezlotoxumab (Zinplava) as Adjunct Treatment for Clostridium difficile Janel Liane Cala, RPh Medical Center Hospital Objectives Review pathophysiology, risk factors, prevention, and treatment options of
Clostridium difficile Infection (CDI) Guideline Update:
 Clostridium difficile Infection (CDI) Guideline Update: Understanding the Data Behind the Recommendations Erik R. Dubberke, MD, MSPH A Webinar for HealthTrust Members Professor of Medicine September 24,
Clostridium difficile Infection (CDI) Guideline Update: Understanding the Data Behind the Recommendations Erik R. Dubberke, MD, MSPH A Webinar for HealthTrust Members Professor of Medicine September 24,
Trust Guideline for the Use of Parenteral Vancomycin and Teicoplanin in Adults
 A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
CLOSTRIDIUM DIFICILE. Negin N Blattman Infectious Diseases Phoenix VA Healthcare System
 CLOSTRIDIUM DIFICILE Negin N Blattman Infectious Diseases Phoenix VA Healthcare System ANTIBIOTIC ASSOCIATED DIARRHEA 1978: C diff first identified 1989-1992: Four large outbreaks in the US caused by J
CLOSTRIDIUM DIFICILE Negin N Blattman Infectious Diseases Phoenix VA Healthcare System ANTIBIOTIC ASSOCIATED DIARRHEA 1978: C diff first identified 1989-1992: Four large outbreaks in the US caused by J
Antimicrobial Guidelines for the Empirical Management of Diabetic Foot Infections
 Antimicrobial Guidelines for the Empirical Management of Diabetic Foot Infections Version 7.2 PAGL Inclusion Approved at January 2017 PGC APPROVED BY: TRUST REFERENCE: B3/2017 AWP REF: UHL Policies and
Antimicrobial Guidelines for the Empirical Management of Diabetic Foot Infections Version 7.2 PAGL Inclusion Approved at January 2017 PGC APPROVED BY: TRUST REFERENCE: B3/2017 AWP REF: UHL Policies and
Factsheet LINACLOTIDE (Constella ) Irritable Bowel Syndrome constipation predominant (IBS-C)
 North Central London Joint Formulary Committee Factsheet LINACLOTIDE (Constella ) Irritable Bowel Syndrome constipation predominant (IBS-C) Start date: September 2018 Review date: September 2021 Document
North Central London Joint Formulary Committee Factsheet LINACLOTIDE (Constella ) Irritable Bowel Syndrome constipation predominant (IBS-C) Start date: September 2018 Review date: September 2021 Document
Hormone Therapy + Metformin (STAMPEDE Trial Arm K)
 Hormone Therapy + Metformin (STAMPEDE Trial Arm K) Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy - A multi-arm randomised controlled trial *** See Protocol For
Hormone Therapy + Metformin (STAMPEDE Trial Arm K) Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy - A multi-arm randomised controlled trial *** See Protocol For
Cisplatin and Fluorouracil
 Cisplatin and Fluorouracil Indication Neo-adjuvant treatment of nasopharyngeal head and neck cancer (stage II-IV) or bulky disease at other head and neck sites. Performance Status 0-1 ICD-10 codes Codes
Cisplatin and Fluorouracil Indication Neo-adjuvant treatment of nasopharyngeal head and neck cancer (stage II-IV) or bulky disease at other head and neck sites. Performance Status 0-1 ICD-10 codes Codes
The incubation period is unknown. However; the onset of clinical disease is typically 5-10 days after initiation of antimicrobial treatment.
 C. DIFFICILE Case definition CONFIRMED CASE A patient is defined as a case if they are one year of age or older AND have one of the following requirements: A laboratory confirmation of a positive toxin
C. DIFFICILE Case definition CONFIRMED CASE A patient is defined as a case if they are one year of age or older AND have one of the following requirements: A laboratory confirmation of a positive toxin
Long-Term Care Updates
 Long-Term Care Updates April 2017 Bezlotoxumab to Prevent Recurrent Infection By Amy Wilson, PharmD and Zara Risoldi Cochrane, PharmD, MS, FASCP Introduction The Gram-positive bacteria is a common cause
Long-Term Care Updates April 2017 Bezlotoxumab to Prevent Recurrent Infection By Amy Wilson, PharmD and Zara Risoldi Cochrane, PharmD, MS, FASCP Introduction The Gram-positive bacteria is a common cause
INTRODUCTION Indication and Licensing
 City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008
 Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008 Shared care agreement has been developed appropriately
Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008 Shared care agreement has been developed appropriately
NCCP Chemotherapy Protocol. Erlotinib Monotherapy
 Erlotinib Monotherapy INDICATIONS FOR USE: INDICATION First-line treatment of patients with locally advanced or metastatic non- small cell lung cancer (NSCLC) with EGFR activating mutations. Switch maintenance
Erlotinib Monotherapy INDICATIONS FOR USE: INDICATION First-line treatment of patients with locally advanced or metastatic non- small cell lung cancer (NSCLC) with EGFR activating mutations. Switch maintenance
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Panobinostat, Bortezomib and Dexamethasone
 Panobinostat, Bortezomib and Dexamethasone Indication Treatment of relapsed/refractory multiple myeloma in patients who have received at least 2 prior regimens, including bortezomib and an immunomodulatory
Panobinostat, Bortezomib and Dexamethasone Indication Treatment of relapsed/refractory multiple myeloma in patients who have received at least 2 prior regimens, including bortezomib and an immunomodulatory
Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism
 Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Edoxaban is a non-vitamin K antagonist oral
Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Edoxaban is a non-vitamin K antagonist oral
Irinotecan Capecitabine (14 day regimen) (I-Cap)
 Systemic Anti Cancer Treatment Protocol Irinotecan (14 day regimen) (I-Cap) PROTOCOL REF: MPHAICAP (Version No: 1.0) Approved for use in: Advanced colorectal cancer first line Advanced colorectal cancer
Systemic Anti Cancer Treatment Protocol Irinotecan (14 day regimen) (I-Cap) PROTOCOL REF: MPHAICAP (Version No: 1.0) Approved for use in: Advanced colorectal cancer first line Advanced colorectal cancer
PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW
 PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW DATE OF AUTHORISATION: AUTHORISING GP: PRESCRIBING SUPPORT TECHNICIAN: SUMMARY Dyspepsia refers to a broad range of symptoms related
PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW DATE OF AUTHORISATION: AUTHORISING GP: PRESCRIBING SUPPORT TECHNICIAN: SUMMARY Dyspepsia refers to a broad range of symptoms related
Lapatinib and Capecitabine Therapy
 Lapatinib and Capecitabine Therapy This protocol should be read in conjunction with NCCP protocol 00216 Capecitabine Monotherapy. INDICATIONS FOR USE: INDICATION Treatment of adult patients with breast
Lapatinib and Capecitabine Therapy This protocol should be read in conjunction with NCCP protocol 00216 Capecitabine Monotherapy. INDICATIONS FOR USE: INDICATION Treatment of adult patients with breast
DISCLOSURE Relevant relationships with commercial entities Wyeth (received advisory board & speaker honoraria) Potential for conflicts of interest wit
 GASTROENTERITIS DISCLOSURE Relevant relationships with commercial entities Wyeth (received advisory board & speaker honoraria) Potential for conflicts of interest within this presentation fidaxomicin (which
GASTROENTERITIS DISCLOSURE Relevant relationships with commercial entities Wyeth (received advisory board & speaker honoraria) Potential for conflicts of interest within this presentation fidaxomicin (which
PEMBROLIZUMAB (KEYTRUDA ) for the treatment of advanced melanoma or previously treated NSCLC
 DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate Day 1 Pembrolizumab 2mg/kg IV Infusion 100mL 0.9% Sodium Chloride* Or 100mL 5% Glucose* *Final concentration must be between 1 to 10mg/mL Over
DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate Day 1 Pembrolizumab 2mg/kg IV Infusion 100mL 0.9% Sodium Chloride* Or 100mL 5% Glucose* *Final concentration must be between 1 to 10mg/mL Over
ABSTRACT PURPOSE METHODS
 ABSTRACT PURPOSE The purpose of this study was to characterize the CDI population at this institution according to known risk factors and to examine the effect of appropriate evidence-based treatment selection
ABSTRACT PURPOSE The purpose of this study was to characterize the CDI population at this institution according to known risk factors and to examine the effect of appropriate evidence-based treatment selection
-2002: Rectal blood loss, UC? (no definite diagnosis) rectal mesalazine. -June 2008: Recurrence of rectal blood loss and urgency
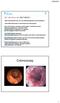 SD, male 40 yrs. old. (680718M467.) -2002: Rectal blood loss, UC? (no definite diagnosis) rectal mesalazine -June 2008: Recurrence of rectal blood loss and urgency Total colonoscopy: ulcerative rectitis,
SD, male 40 yrs. old. (680718M467.) -2002: Rectal blood loss, UC? (no definite diagnosis) rectal mesalazine -June 2008: Recurrence of rectal blood loss and urgency Total colonoscopy: ulcerative rectitis,
Carboplatin and Fluorouracil
 Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Learning Objectives. A Critical Analysis of Recent Fidaxomicin & Nesiritide Trials. Fidaxomicin vs. Vancomycin. Outline 1/3/2012
 Learning Objectives A Critical Analysis of Recent Fidaxomicin & Nesiritide Trials Amy E. Lodolce, PharmD, BCPS Assistant Director, Drug Information Group UIC College of Pharmacy Describe the methods and
Learning Objectives A Critical Analysis of Recent Fidaxomicin & Nesiritide Trials Amy E. Lodolce, PharmD, BCPS Assistant Director, Drug Information Group UIC College of Pharmacy Describe the methods and
DR J HARTY / DR CM RITCHIE / DR M GIBBONS
 CLINICAL GUIDELINES ID TAG Title: Author: Speciality / Division: Directorate: Paracetamol Poisoning DR J HARTY / DR CM RITCHIE / DR M GIBBONS Medicine Acute Date Uploaded: 16 th September 2014 Review Date
CLINICAL GUIDELINES ID TAG Title: Author: Speciality / Division: Directorate: Paracetamol Poisoning DR J HARTY / DR CM RITCHIE / DR M GIBBONS Medicine Acute Date Uploaded: 16 th September 2014 Review Date
Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Ipilimumab in Melanoma
 Ipilimumab in Melanoma Indication: Advanced (unresectable or metastatic) melanoma in adults who have received prior therapy LCNDG criteria to be met: Histologically confirmed unresectable stage III or
Ipilimumab in Melanoma Indication: Advanced (unresectable or metastatic) melanoma in adults who have received prior therapy LCNDG criteria to be met: Histologically confirmed unresectable stage III or
Axitinib (renal) Note: in some patients it may be appropriate to increase the dose to 6mg BD before increasing to 7mg BD.
 Axitinib (renal) Indication Treatment of advanced renal cell carcinoma after failure of treatment with a first-line tyrosine kinase inhibitor (UK licensed indication states sunitinib) or a cytokine. (NICE
Axitinib (renal) Indication Treatment of advanced renal cell carcinoma after failure of treatment with a first-line tyrosine kinase inhibitor (UK licensed indication states sunitinib) or a cytokine. (NICE
Nutritional Protocol for Blood and Bone Marrow Transplantation (BMT)
 Nutritional Protocol for Blood and Bone Marrow Transplantation (BMT) Scope This protocol details pre, during and post BMT nutritional assessment and management for all forms of BMT undertaken by OxBMT,
Nutritional Protocol for Blood and Bone Marrow Transplantation (BMT) Scope This protocol details pre, during and post BMT nutritional assessment and management for all forms of BMT undertaken by OxBMT,
Treatment of Clostridium Difficile Infection in Community Teaching Hospital: A Retrospective Study
 International Journal of Infectious Diseases and Therapy 2018; 3(3): 52-61 http://www.sciencepublishinggroup.com/j/ijidt doi: 10.11648/j.ijidt.20180303.12 ISSN: 2578-9651 (Print); ISSN: 2578-966X (Online)
International Journal of Infectious Diseases and Therapy 2018; 3(3): 52-61 http://www.sciencepublishinggroup.com/j/ijidt doi: 10.11648/j.ijidt.20180303.12 ISSN: 2578-9651 (Print); ISSN: 2578-966X (Online)
ESCA: Cinacalcet (Mimpara )
 ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
March 3, To: Hospitals, Long Term Care Facilities, and Local Health Departments
 March 3, 2010 To: Hospitals, Long Term Care Facilities, and Local Health Departments From: NYSDOH Bureau of Healthcare Associated Infections HEALTH ADVISORY: GUIDANCE FOR PREVENTION AND CONTROL OF HEALTHCARE
March 3, 2010 To: Hospitals, Long Term Care Facilities, and Local Health Departments From: NYSDOH Bureau of Healthcare Associated Infections HEALTH ADVISORY: GUIDANCE FOR PREVENTION AND CONTROL OF HEALTHCARE
Capecitabine Oxaliplatin 21 day cycle (XELOX)
 Systemic Anti Cancer Treatment Protocol Capecitabine Oxaliplatin 21 day cycle (XELOX) PROTOCOL REF: MPHAXELOX (Version No: 1.0) Approved for use in: Adjuvant colorectal cancer stage 3 or high risk stage
Systemic Anti Cancer Treatment Protocol Capecitabine Oxaliplatin 21 day cycle (XELOX) PROTOCOL REF: MPHAXELOX (Version No: 1.0) Approved for use in: Adjuvant colorectal cancer stage 3 or high risk stage
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE. Drug: MYCOPHENOLATE MOFETIL/SODIUM Protocol number: CV 15
 Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE Drug: MYCOPHENOLATE MOFETIL/SODIUM Protocol number: CV 15 Indication: RENAL, PANCREAS OR COMBINED RENAL PANCREAS TRANSPLANTATION
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE Drug: MYCOPHENOLATE MOFETIL/SODIUM Protocol number: CV 15 Indication: RENAL, PANCREAS OR COMBINED RENAL PANCREAS TRANSPLANTATION
Cisplatin and Fluorouracil (head and neck)
 Cisplatin and Fluorouracil (head and neck) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated.
Cisplatin and Fluorouracil (head and neck) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated.
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised
 Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
All POOPed out: fecal microbiota transplant in C. difficile
 All POOPed out: fecal microbiota transplant in C. difficile SUSAN M. KELLIE, MD, MPH PROFESSOR OF INTERNAL MEDICINE DIVISION OF INFECTIOUS DISEASES UNIVERSITY OF NEW MEXICO SCHOOL OF MEDICINE HOSPITAL
All POOPed out: fecal microbiota transplant in C. difficile SUSAN M. KELLIE, MD, MPH PROFESSOR OF INTERNAL MEDICINE DIVISION OF INFECTIOUS DISEASES UNIVERSITY OF NEW MEXICO SCHOOL OF MEDICINE HOSPITAL
Management of Dyspepsia
 MPharm Programme Management of Dyspepsia Slide 1 of 28 Learning Objectives Understand the principles and wider implications underpinning evidence based therapeutics in the key clinical specialities Objectively
MPharm Programme Management of Dyspepsia Slide 1 of 28 Learning Objectives Understand the principles and wider implications underpinning evidence based therapeutics in the key clinical specialities Objectively
Acute Gastroenteritis, Adult Emergency Orders
 Form Title Form Number 21003 2018, Alberta Health Services, CKCM This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The license does not
Form Title Form Number 21003 2018, Alberta Health Services, CKCM This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The license does not
Clostridium difficile Infection (CDI)
 18.09.10 월요집담회 Clostridium difficile Infection (CDI) R4 송주혜 Clostridium difficile infection (CDI) Anaerobic gram (+), spore-forming, toxin(tcda&tcdb)-producing bacillus Transmitted among humans through
18.09.10 월요집담회 Clostridium difficile Infection (CDI) R4 송주혜 Clostridium difficile infection (CDI) Anaerobic gram (+), spore-forming, toxin(tcda&tcdb)-producing bacillus Transmitted among humans through
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA o Patients of any age with ALARM signs should be referred through the 2-week referral system o Routine endoscopic investigation
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA o Patients of any age with ALARM signs should be referred through the 2-week referral system o Routine endoscopic investigation
Bortezomib and Dexamethasone Therapy INDICATIONS FOR USE:
 Bortezomib and Dexamethasone Therapy INDICATIONS FOR USE: INDICATION Treatment of adult patients with progressive multiple myeloma who have received at least one prior therapy ICD10 C90 Protocol Code 00270a
Bortezomib and Dexamethasone Therapy INDICATIONS FOR USE: INDICATION Treatment of adult patients with progressive multiple myeloma who have received at least one prior therapy ICD10 C90 Protocol Code 00270a
Cardiac Catheter Labs Intravenous Drug Therapy Guide
 Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Cardiac Catheter Lab IV Medicines Guideline Helen Buxton ( Senior Cath Lab
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Cardiac Catheter Lab IV Medicines Guideline Helen Buxton ( Senior Cath Lab
