Toll-Free Customer Service: Instruction Manual. with SAFE-T-Energetics. Toll-Free Customer Service
|
|
|
- Kevin Edwards
- 5 years ago
- Views:
Transcription
1 Toll-Free Customer Service: North Pima Road Scottsdale, AZ K with SAFE-T-Energetics Instruction Manual Toll-Free Customer Service J
2 Table of Contents Introduction 2 Purpose of Provant 2 Safety Information for Patients and Caregivers 3 Product Description 7 General Information about Provant 8 Instructions for Use 9 Setting Up Provant 9 Turning On Provant 9 Placing the Disposable Applicator Cover 9 Positioning the Treatment Applicator Pad 10 Starting a Treatment Session 11 Finishing Treatment 11 Cleaning Provant 12 Servicing Provant 12 Moving and Storing Provant 13 Returning Provant to Regenesis 14 Troubleshooting Provant 15 EMC User information 17 Provant Therapy System Specifications 21 Copyright 2018 Regenesis Biomedical, Inc. All rights reserved. Protected under US patents, 6,334,069; 6,353,763; 6,967,281; 6,974,961; 7,024,239; 7,572,985 and 7,579,555. Additional patents are pending. Provant, Regenesis, Regenesis Biomedical, SAFE-T-ENERGETICS, the Provant yellow, and the Energy Starburst Logo are registered trademarks of Regenesis Biomedical, Inc., Scottsdale, AZ. Save this manual for future reference. 1
3 Introduction This Instruction Manual is intended to assist you in using the Provant Therapy System. Provant is a pulsed electromagnetic field (PEMF) device. Please read this entire instruction manual carefully before using Provant. If you have any questions, please contact Regenesis Biomedical: Phone: Website: info@regenesisbio.com PURPOSE OF PROVANT Provant is indicated for adjunctive use in the palliative treatment of postoperative pain and edema of soft tissue. Pain and edema (i.e., swelling) can be significant obstacles to postoperative recovery. By helping reduce pain and swelling, Provant plays an important role in your postoperative care. 2
4 SAFETY INFORMATION FOR PATIENTS AND CAREGIVERS INDICATIONS FOR USE The Provant Therapy System is indicated for adjunctive use in the palliative treatment of postoperative pain and edema of soft tissue. CONTRAINDICATIONS DO NOT USE Provant Therapy if you have a pacemaker or defibrillator. DO NOT USE Provant Therapy if you are or may be pregnant. DO NOT USE Provant Therapy to treat over bony growth areas in children. DO NOT USE provant Therapy over active or previously treated cancer, either solid or blood-related in origin. WARNINGS AND PRECAUTIONS DO NOT USE Provant Therapy over implanted metal lead wires. Closely follow any directions published by the manufacturer s information for use (IFU). The long-term health effects of Provant Therapy are not known. The long-term biologic effects of exposure to Provant Therapy beyond the manufacturer s recommended dosing are not known. The effect of Provant Therapy on topical medicated dermal applications has not been evaluated. Consult your physician prior to use of Provant Therapy over topical medicated dermal applications. DO NOT USE Provant Therapy if you are using another electronic medical device without first consulting your physician. The effect of Provant Therapy on cancer, either solid or bloodrelated, and precancerous lesions is not known. In some patients, pain (hyperalgesia) with use of Provant Therapy has been reported. 3
5 The Provant Therapy Device may cause radio interference or may disrupt the operation of nearby equipment. It may be necessary to mitigation measures such as: Ensuring that the active side of the Treatment Applicator Pad is not near or pointing toward other electronic devices or cabling while the Provant Therapy Device is in ongoing treatment mode. Reorient or relocate the other electronic device(s). Increase the separation between the Treatment Applicator Pad and other electronic device(s). Reposition cables so that they are not on or near each other. Connect the equipment into an outlet on a circuit different from that to which the other electronic device(s) are connected. DO NOT USE this System if it has a damaged cable or Treatment Applicator. DO NOT USE this System in the presence of flammable anesthetic mixtures. DO NOT allow water or other liquids to be spilled into the System Case. ADVISORIES There are important operating, maintenance and service instructions in the literature accompanying the product. There is a presence of un-insulated dangerous voltage within the product s enclosure that may be of sufficient magnitude to constitute a risk of electric shock. Provant intentionally applies pulsed electromagnetic field (PEMF) energy to cells for medical treatment. Provant is a type BF applied part. This means the Treatment Applicator Pad is safe to place against a living body. Provant is MR unsafe keep away from magnetic resonance imaging (MRI) equipment. 4
6 SUMMARY OF CLINICAL PERFORMANCE The clinical performance of Provant Therapy has been evaluated in a number of post-operative conditions including lumbar surgery, total knee arthroplasty, and bunionectomies. These studies point out the safe use of the Provant Therapy and while the studies were not powered, trends were observed to be consistent with the indication of pain and edema reduction in soft tissue. Clinical Performance in Randomized, Blinded, Sham-Controlled Studies Three studies have been conducted to evaluate the clinical performance of Provant. Study 1 evaluated subjects with chronic post-operative pain 3 to 36 months after a lumbar decompression surgery. Subjects were randomized to receive Provant (N=13) or a sham control (N=14) and treated twice daily for 60 days. Study 2 evaluated subjects with chronic post-operative pain 3 to 36 months after a total knee arthroplasty (TKA). Subjects were randomized to receive Provant (N=24) or a sham control (N=11) and treated twice daily for 60 days. Study 3 evaluated subjects with acute pain directly following bunionectomy surgery. Subjects received treatment with the Provant device (N=71) or a sham control (N=68) for 7 days directly following surgery. An additional open-label study in subjects (N=41) with chronic postoperative pain after low back surgery was also conducted. 5
7 Safety of Provant Therapy The safety of Provant Therapy has been evaluated in 225 subjects in controlled clinical studies. The overall rate of adverse events with Provant Therapy was similar to a matched sham control. Adverse events that occurred more with a higher frequency with Provant Therapy was increased pain (hyperalgesia). Regenesis Biomedical maintains a post-market surveillance program to monitor the safety of the Provant Therapy following prescription of the device. Regenesis has received reports of adverse events of greater than 1 per 1000 patients in pain (hyperalgesia) (2.3% of patients) and skin reaction (0.2% of patients). A subset of hyperalgesia patients was instructed by their prescribers to temporarily discontinue therapy until hyperalgesia improved followed by reintroduction at a lower dose with a gradual dose increase over time were able to tolerate Provant Therapy. Reports received at a rate of 0.1% or less generally related to preexisting pain, the underlying condition being treated, or to the surgical procedures that preceded the use of Provant Therapy. Regenesis does not consider these latter reports as being associated with use of Provant Therapy. Expected Adverse Events Based on a survey where all patients treated with Provant Therapy were asked to report any symptoms and signs, 2.3% reported increased pain and 0.2% reported rash while using Provant Therapy. Infrequent reports of increased pain have also been reported by patients in clinical trials. 6
8 Product Description Provant includes the following: 1 Provant System Case (yellow) 2 Power Switch (lighted rocker switch) 3 Control Panel (with indicator lights) 4 Start/Stop Button 5 Usage Meter (available on certain models) 6 A/C Power Cable (three-prong) 7 Yellow Cable 8 Treatment Applicator Pad 9 Disposable Applicator Covers 10 Guidelines for Use 11 Countdown Timer If any items are missing or appear damaged, contact Regenesis Customer Service at toll free 7
9 General Information About PROVANT Provant must be prescribed by a licensed healthcare provider. This device is intended to be used solely by the patient for whom it is prescribed. Provant Therapy has SAFE-T-Energetics (self-adjusting feedback technology). Provant automatically regulates and controls the delivered dose, adjusting to meet each patient s unique body characteristics. In order to achieve desired results, it is recommended to treat twice a day, about 8 to 12 hours apart, for the full pre-timed 30 minute treatment; however, it is important to treat as your prescribing clinician has directed. Provant treatments are pre-timed and can be administered through clothing, dressings, etc. There is no need to remove these items for treatment. Provant is an electrical device, so access to an electric outlet is required for use. By following the instructions in this manual, you can administer your own treatments. Alternatively, a family member or care provider may administer the treatments. During prolonged use, the Control Panel surface may become slightly warm to the touch. The Disposable Applicator Covers are designed to serve as a barrier, keeping the Treatment Applicator Pad clean, and to provide a comfortable contact surface. Provant will not operate without a new Disposable Applicator Cover in place for each treatment session. The Provant Therapy System contains a radiofrequency identification (RFID) function which transmits and receives at the MHz frequency. This functionality may be interfered with by other equipment, even if that other equipment complies with CISPR emission requirements. 8
10 Instructions For Use SETTING UP PROVANT Read Safety Information before using Provant. Place Provant in a nearby convenient, stable location. Open Provant by lifting up the two latches on the outside of the System Case. A hinge will hold the lid open. Make sure you can see and reach the Provant Control Panel before you start treating. Make sure the Yellow Cable and Treatment Applicator Pad can reach to the part of your body being treated. Plug A/C Power Cable into an adjacent three prong power outlet. Make sure no one will trip on the Yellow Cable or A/C Power Cable during your treatment. Please save the original Provant shipping box so you can use it to return the device after your treatment has ended. TURNING ON PROVANT Turn Provant On by pressing the Power Switch on the side of the Control Panel. The green light on the Power Switch will turn on. PLACING THE DISPOSABLE APPLICATOR COVER IMPORTANT: Each Disposable Applicator Cover is to be used only once. Open the silver pack of Disposable Applicator Covers provided with the device and remove one Disposable Applicator Cover. Identify the side of the Disposable Applicator Cover which has a clear window and yellow starburst image. Identify the side of Treatment Applicator Pad which says This side towards patient. Gently insert the Treatment Applicator Pad into the Disposable Applicator Cover so that the words This side towards patient are visible through the clear Disposable Applicator Cover window. Press locking strips at the top of the Cover together to seal the Disposable Applicator Cover on the Treatment Applicator Pad. If the Disposable Applicator Cover has been used previously or is not correctly oriented, Provant will beep three times and treatment will not start when the Start/Stop button is pressed. Additional Disposable Applicator Covers are available from Regenesis Customer Service and may be ordered by calling
11 POSITIONING THE TREATMENT APPLICATOR PAD IMPORTANT: The body part being treated and the Treatment Applicator Pad should rest lightly together. Excess pressure or weight on the Treatment Applicator Pad may trigger the Service Required light and stop treatment. IMPORTANT: Do not push or force the Treatment Applicator Pad underneath you when positioning it, as this may cause friction and injury to your skin. Find a comfortable position sitting or lying down. If you are sitting or lying on a metallic surface, make sure comfortable padding, such as a mattress or cushion, is between the treatment applicator pad and the metal, so as not to interfere with the delivery of the therapeutic PEMF energy. Make sure you are positioned such that the site to be treated is easily reached. It is not necessary to remove any clothing or dressings because Provant PEMF energy penetrates from the Treatment Applicator Pad through clothing and dressings. Position the Treatment Applicator Pad directly against the treatment site. The words This side towards patient should be facing the area you are treating. Center the starburst symbol over the treatment site. You may support the Treatment Applicator Pad next to the treatment site using a pillow or rolled towel to ensure it stays in contact with the treatment site. 10
12 STARTING A TREATMENT SESSION Press the START/STOP button on the Control Panel. A short beep will sound, letting you know that treatment has begun. Three lights will illuminate on the Control Panel during treatment: a green CPI RUNNING light; a stack of green lines that scroll from left to right; and a green 30-minute count-down timer. The count-down timer tells you how much time remains in the treatment session and counts down in one-minute increments. FINISHING TREATMENT At the end of 30 minutes, treatment automatically ends. All indicator lights will turn off and a long beep will sound to let you know the treatment session is finished. Turn Provant Off by pressing the Power Switch on the side of the Control Panel. The green light on the Power Switch will turn off. Remove the Treatment Applicator Pad from the treatment site. Remove the Disposable Applicator Cover from the Treatment Applicator Pad and discard it as normal waste. The Disposable Applicator Covers are not recyclable. You may stop Provant prior to the end of the treatment session by pressing the START/STOP button on the Control Panel, or pressing the Power Switch. 11
13 Cleaning PROVANT IMPORTANT: Do not submerge any part of Provant in any liquid. IMPORTANT: Do not use organic cleaners other than isopropyl alcohol / water solution as this may damage Provant and void the warranty. IMPORTANT: If desired, the Treatment Applicator Pad can be cleaned following the directions below. Before cleaning, first unplug Provant from the electrical outlet. Prepare a dilute solution of rubbing alcohol (70% isopropyl alcohol) and water by mixing equal parts of rubbing alcohol and tap water. Moisten a clean cloth with the rubbing alcohol/water solution and gently wipe the surface of the Treatment Applicator Pad. For further instructions, contact Regenesis Customer Service at Servicing PROVANT All service is provided and conducted by Regenesis. Please call Do not attempt to disassemble or repair Provant. There are no user-serviceable parts in Provant. Any unauthorized attempts to service or repair Provant will void the warranty and may result in additional charges. 12
14 Moving and Storing PROVANT IMPORTANT: To avoid damage, properly pack the A/C Power Cable, Yellow Cable and Treatment Applicator Pad. Unplug the A/C Power Cable from the wall, bundle and place in the space next to the Control Panel. Place the Treatment Applicator Pad on top of the Control Panel. Do NOT wrap cables around the Treatment Applicator Pad. Bundle the Yellow Cable and place in the space next to the Control Panel, on top of the A/C Power Cable. Close and latch the lid, making sure no cables are caught when closing the lid. The Provant Therapy System should not be exposed to environmental conditions outside the following ranges for extended periods of time: Operating Temperature: 54º 90ºF (12º 32ºC) Storage Temperature: -40º 158ºF (-40º 70ºC) Operating/Storage Humidity: 10% 95%, non-condensing Operating Altitude: mbar (-1,000 18,000ft/ m) Handle the Provant Therapy System using the convenient carrying handle. Do not use or leave Provant in direct sunlight, high humidity, or severe heat or cold as this could adversely affect performance. 13
15 Returning PROVANT to Regenesis When your treatment is complete and your prescribing physician has instructed you to discontinue the use of Provant, please call Regenesis Customer Service within 24 hours to arrange the easy return of Provant. Call Locate the original Provant shipping box. Remove the pre-addressed, pre-paid Overnight Shipping return label provided with the device. Apply the shipping label to the Provant shipping box over the original shipping label. Place Provant, handle-side up, into the box by slipping it between the foam packing strips. Close the shipping box and secure the box lid with packing tape. Discard all used Disposable Applicator Covers. Do not return the used Disposable Applicator Covers with Provant. Call Regenesis toll-free at to arrange for door-to-door Overnight Shipping pick-up. If you do not have the original Provant shipping box or return label, call Regenesis for assistance toll-free at
16 Troubleshooting PROVANT During use, problems may arise. This section will help you troubleshoot common problems. Call Regenesis Customer Service for additional assistance by calling toll-free If your problem involves a health issue and you need emergency assistance, please call 911 and/or contact your health care professional. Please report serious side effects to Regenesis Customer Service by calling PROVANT DOES NOT START AND/OR THERE ARE NO INDICATOR LIGHTS: Make sure the A/C Power Cable is firmly plugged into the electrical wall outlet. Make sure that the opposite end of the A/C Power Cable is firmly plugged into the side of the Control Panel. Make sure the Power Switch is On. The Power Switch lights up green when it is On. If problem persists, call Customer Service at WHEN THE START/STOP BUTTON IS PUSHED, PROVANT BEEPS THREE TIMES: Replace the Disposable Applicator Cover with a new, unused cover. Make sure This side toward patient is easily visible through the clear window. Turn the Power Switch Off and then On. Press the Start/Stop button. If the problem persists, repeat with another new cover. If problem persists, call Customer Service at
17 SERVICE REQUIRED LIGHT IS LIT: Make sure only light pressure from the patient s limb or body is on the Treatment Applicator Pad, and turn Provant Off, then On. If problem persists, call Customer Service at PROVANT APPEARS TO INTERFERE WITH OPERATION OF OTHER ELECTRONIC DEVICES: Make sure the Treatment Applicator Pad is not near other electronic devices or their cables. Make sure the Provant A/C Power Cable and Yellow Cable are not near other electronic device or their cables. Plug Provant into a different electrical wall receptacle. Increase the distance between the Treatment Applicator Pad and the other electronic devices. Move other electronic devices to a place where there is no interference. For further troubleshooting assistance, call Regenesis customer service at the toll-free number below. Regenesis Customer Service Toll-Free IMPORTANT: Please have the Serial Number located on the label on the back of Provant ready for Customer Service. 16
18 Electromagnetic Compatibility (EMC) User Information WARNING: Provant Therapy System needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in this manual. Portable and mobile RF communications equipment can affect the Provant Therapy System. The use of accessories, transducers and cables other than those specified by Regenesis Biomedical may result in increased emissions or decreased immunity of the equipment. This equipment should not be used adjacent to or stacked with other equipment and that if adjacent or stacked use is necessary, the equipment should be observed to verify normal operation in the configuration in which it will be used. NOTE: The EMC tables and other guidelines that are included in the Instruction Manual provide information to the customer or user that is essential in determining the suitability of the equipment or system for the electromagnetic environment of use, and in managing the electromagnetic environment of use to permit the equipment or system to perform its intended use without disturbing other equipment and systems or nonmedical electrical equipment. Guidance and manufacturer s declaration electromagnetic emissions The PROVANT THERAPY SYSTEM is intended for use in the electromagnetic environment specified below. The customer or the user of the PROVANT THERAPY SYSTEM should assure that it is used in such an environment. Emissions Test RF emissions CISPR 11 RF emissions CISPR 11 Harmonic emissions IEC Voltage Fluctuations/ Flicker emissions Compliance Group 2 Class A Class A Complies The PROVANT THERAPY SYSTEM must emit electromagnetic energy in order to perform its intended function. Nearby electronic equipment may be affected. The PROVANT THERAPY SYSTEM is suitable for use in all establishments other than domestic and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. 17
19 Guidance and manufacturer s declaration electromagnetic immunity The PROVANT THERAPY SYSTEM is intended for use in the electromagnetic environment specified below. The customer or the user of the PROVANT THERAPY SYSTEM should assure that it is used in such an environment. Immunity test IEC test level Compliance level Electromagnetic environment guidance Electrostatic discharge (ESD) IEC ±6 kv contact ±8 kv air ±15 kv contact ±30 kv air The relative humidity should be at least 5%. Electrical fast transient/burst IEC ±2 kv for power supply lines ±2 kv for power supply lines Mains power quality should be that of a typical commercial or hospital environment. Surge IEC ±1 kv differential mode ±2 kv common mode ±1 kv differential mode ±2 kv common mode Mains power quality should be that of a typical commercial or hospital environment. Voltage dips, short interruptions and voltage variations on power supply input lines IEC <5 % U T (>95 % dip in U T ) for 0,5 cycle 40 % U T (60 % dip in U T ) for 5 cycles 70 % U T (30 % dip in U T ) for 25 cycles <5 % U T (>95 % dip in U T ) for 5 sec <5 % U T (>95 % dip in U T ) for 0,5 cycle 40 % U T (60 % dip in U T ) for 5 cycles 70 % U T (30 % dip in U T ) for 25 cycles <5 % U T (>95 % dip in U T ) for 5 sec Mains power quality should be that of a typical commercial or hospital environment. If the user of the PROVANT THERAPY SYSTEM requires continued operation during power mains interruptions; it is recommended that the PROVANT THERAPY SYSTEM be powered from an uninterruptible power supply or a battery. (50/60 Hz) magnetic field IEC A/m 30 A/m Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. NOTE U T is the a.c. mains voltage prior to application of the test level. ESSENTIAL PERFORMANCE: The Provant Therapy System shall indicate proper operation or shutdown, alarm or stand-by. 18
20 Guidance and manufacturer s declaration electromagnetic immunity The PROVANT THERAPY SYSTEM is intended for use in the electromagnetic environment specified below. The customer or the user of the PROVANT THERAPY SYSTEM should assure that it is used in such an environment. Immunity test IEC test level Compliance level Electromagnetic environment guidance Portable and mobile RF communications equipment should be used no closer to any part of the PROVANT THERAPY SYSTEM, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Conducted RF IEC Vrms 150 khz to 80 MHz 10 Vrms Recommended separation distance d = 0.35 P Radiated RF IEC V/m 80 MHz to 2,5 GHz 10 V/m d = 0.35 P 80 MHz to 800 MHz d = 0.7 P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey a, should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the PROVANT THERAPY SYSTEM is used exceeds the applicable RF compliance level above, the PROVANT THERAPY SYSTEM should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the PROVANT THERAPY SYSTEM. b Over the frequency range 150 khz to 80 MHz, field strengths should be less than 10 V/m. 19
21 Recommended separation distances between portable and mobile RF communications equipment and the PROVANT THERAPY SYSTEM The PROVANT THERAPY SYSTEM is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the PROVANT THERAPY SYSTEM can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the PROVANT THERAPY SYSTEM as recommended below, according to the maximum output power of the communications equipment. Rated maximum output power of transmitter W Separation distance according to frequency of transmitter m 150 khz to 80 MHz d = 0.35 P 80 MHz to 800 MHz d = 0.35 P 800 MHz to 2.5 GHz d = 0.7 P For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people
22 PROVANT THERAPY SYSTEM SPECIFICATIONS INDICATIONS FOR USE Provant is indicated for adjunctive use in the palliative treatment of postoperative pain and edema of soft tissue. CONTRAINDICATIONS Provant SHOULD NOT BE USED: By patients with cardiac pacemaker or defibrillator By patients during pregnancy Over bony growth areas in children Over active or previously treated cancer, either solid or bloodrelated in origin. WARNINGS AND PRECAUTIONS Provant SHOULD NOT BE USED over ANY implanted metal lead wires. Closely follow any directions published by the manufacturer s information for use (IFU). The long-term health effects of Provant Therapy are not known. The long-term biologic effects of exposure to Provant Therapy beyond the manufacturer s recommended dosing are not known. The effect of Provant Therapy on topical medicated dermal applications has not been evaluated. Consult your physician prior to use of Provant Therapy over topical medicated dermal applications. DO NOT USE Provant Therapy if you are using another electronic medical device without first contacting your physician. The effect of Provant Therapy on cancer, either solid or bloodrelated, and precancerous lesions is not known. In some patients, pain (hyperalgesia) with use of Provant Therapy has been reported. CAUTION Federal law restricts this device to sale by, or on the order of, a licensed healthcare practitioner. OVERVIEW The Provant Therapy System is a solid-state, fixedpower output pulsed electromagnetic field (PEMF) generator and transmitter designed to operate at the Federal Communications Commission authorized medical device frequency of MHz. During therapy, the Provant transmits a fixed dose of non-ionizing, non-thermal shortwave therapy (SWT) energy via an applicator pad that is placed adjacent to the area to be treated. The SWT energy produced by Provant is similar to that of cell phones and AM/FM radio transmissions. The Provant device also contains an RFID function which transmits at the MHz frequency. Provant has been designed with the patient in mind -- it is lightweight, easy to transport and requires little patient or care-provider interface. There are only two switches, a Power On/Off switch and a Treatment START/STOP switch; no adjustments are necessary. Provant automatically delivers and monitors a preset therapeutic energy dose during each 30-minute treatment cycle. The Provant Therapy System complies with the following standards: UL and IEC DETAILED SPECIFICATIONS Size (closed) 13.8 x 10.3 x 6.0 Weight 10 pounds Applicator size 7.5 x 8.5 x 1.0 A/C Power Cable 10 Hospital Grade, 10 AMP, 18 AWG Treatment Applicator 8 RG-316 Coaxial cable, Cable Silver plated copper clad steel Power Requirements Circuit Protection Power Consumption 21 AC V, Hz AC 0.5 Amp Slow Blow 35 Watts Treatment RFID Operating Frequency MHz MHz Transmission Profile Pulsed Sine Wave Square Wave Pulse Duration 42 µsec 100ms Pulse Frequency 1 KHz Single pulse Duty Cycle 4.2% <1ms per 30 min cycle Signal Strength 591 V/m m Effective Radiated Power <3W 200mW
Smart Control remote guide
 Smart Control remote guide Thank you Thank you for choosing Unitron Smart Control remote for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing
Smart Control remote guide Thank you Thank you for choosing Unitron Smart Control remote for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing
Treatment Pad Positioning
 The Provant Therapy System is indicated for adjunctive use in the palliative treatment of postoperative pain and edema of soft tissue. Provant received FDA clearance in 1997, 2010, and 2013. Prior to use,
The Provant Therapy System is indicated for adjunctive use in the palliative treatment of postoperative pain and edema of soft tissue. Provant received FDA clearance in 1997, 2010, and 2013. Prior to use,
This page has been intentionally left blank
 Instruction Manual Table of Contents Introduction... 1 Important Safeguards... 2 Warranty... 5 Classifications and Markings... 6 Aeroneb Go System Parts... 7 Unit Assembly and Usage... 7 Prior to Assembly...
Instruction Manual Table of Contents Introduction... 1 Important Safeguards... 2 Warranty... 5 Classifications and Markings... 6 Aeroneb Go System Parts... 7 Unit Assembly and Usage... 7 Prior to Assembly...
Provider s Guide. Table of Contents
 Table of Contents 2 3 5 6 7 8 9 10 10 11 13 14 15 17 21 21 21 22 23 How to Use This Guide Warnings, Cautions, and Contraindications Intended Use What is? System Contents Symbols RUSleeping Display Instructions
Table of Contents 2 3 5 6 7 8 9 10 10 11 13 14 15 17 21 21 21 22 23 How to Use This Guide Warnings, Cautions, and Contraindications Intended Use What is? System Contents Symbols RUSleeping Display Instructions
BiPAP Pro Bi-Flex. Accessing the Provider Mode Screens PROVIDER GUIDE
 BiPAP Pro Bi-Flex PROVIDER GUIDE IMPORTANT! Remove this guide before giving the device to the patient. Only medical professionals should adjust pressure settings. This guide provides you with instructions
BiPAP Pro Bi-Flex PROVIDER GUIDE IMPORTANT! Remove this guide before giving the device to the patient. Only medical professionals should adjust pressure settings. This guide provides you with instructions
Video Otoscope MS101. User s Manual UM-AP A
 Video Otoscope MS0 User s Manual UM-AP005-08-A Thank you for choosing the MDSCOPE Video Otoscope, the Video Otoscope choice for health care professionals. The operating and maintenance instructions found
Video Otoscope MS0 User s Manual UM-AP005-08-A Thank you for choosing the MDSCOPE Video Otoscope, the Video Otoscope choice for health care professionals. The operating and maintenance instructions found
INSTALLATION MANUAL. MedRx TINNOMETER. Revolutionary Tinnitus Assessment.
 INSTALLATION MANUAL Revolutionary Tinnitus Assessment MedRx TINNOMETER www.medrx-usa.com Contents Getting to Know Your Tinnometer 3 Computer Requirements 4 Tinnometer 5 Transducers and Accessories 5 Software
INSTALLATION MANUAL Revolutionary Tinnitus Assessment MedRx TINNOMETER www.medrx-usa.com Contents Getting to Know Your Tinnometer 3 Computer Requirements 4 Tinnometer 5 Transducers and Accessories 5 Software
Jazz Ultra Instruments
 Jazz Ultra Instruments Instructions for use Next Contents 1. Introduction...p3 2. Symbols...p4 3. Safety 3.1 Device Classification 3.2 Warnings and cautions...p5 4. Cleaning instructions 4.1 Sterilisation...p6
Jazz Ultra Instruments Instructions for use Next Contents 1. Introduction...p3 2. Symbols...p4 3. Safety 3.1 Device Classification 3.2 Warnings and cautions...p5 4. Cleaning instructions 4.1 Sterilisation...p6
The Biomet EBI Bone Healing System. Patient Manual
 The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
SIX PACK ABS Item No INSTRUCTION MANUAL. Read entire manual before operating this product. Use only as directed.
 SIX PACK ABS Item No. 206098 INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. WARNINGS If you are in the care of a physician, consult your physician before using
SIX PACK ABS Item No. 206098 INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. WARNINGS If you are in the care of a physician, consult your physician before using
SLEEP IMPROVING WRISTBAND. Item No Owner s Guide
 SLEEP IMPROVING WRISTBAND Item No. 205350 Owner s Guide Thank you for purchasing the Sharper Image Sleep Improving Wristband. Based on ancient Chinese acupuncture principles, this biofeedback device uses
SLEEP IMPROVING WRISTBAND Item No. 205350 Owner s Guide Thank you for purchasing the Sharper Image Sleep Improving Wristband. Based on ancient Chinese acupuncture principles, this biofeedback device uses
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T
 Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
ER75 Electro-Acoustic Ear Simulator. Operating Manual
 ER75 Electro-Acoustic Ear Simulator Operating Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. Amplivox Ltd. 6 Oasis Park, Eynsham Oxfordshire, OX29 4TP United
ER75 Electro-Acoustic Ear Simulator Operating Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. Amplivox Ltd. 6 Oasis Park, Eynsham Oxfordshire, OX29 4TP United
PainShield MD User Manual
 PainShield MD User Manual Cat. # PSUM003 Ver. 07 (KEV) Table of Contents GENERAL INFORMATION 4 IMPORTANT GENERAL SAFETY CAUTIONS AND NOTICES 7 CLINICAL INFORMATION 8 GENERAL DESCRIPTION 10 PAINSHIELD
PainShield MD User Manual Cat. # PSUM003 Ver. 07 (KEV) Table of Contents GENERAL INFORMATION 4 IMPORTANT GENERAL SAFETY CAUTIONS AND NOTICES 7 CLINICAL INFORMATION 8 GENERAL DESCRIPTION 10 PAINSHIELD
INSTALLATION MANUAL. AVANT Air, Bone, Speech and Masking Audiometry AUDIOMETERS.
 INSTALLATION MANUAL AVANT Air, Bone, Speech and Masking Audiometry AUDIOMETERS www.medrx-int.com Contents Getting to Know Your Audiometer.. 3 Computer Requirements.. 4 Avant A2D+ 5 Avant Stealth... 6 Transducers
INSTALLATION MANUAL AVANT Air, Bone, Speech and Masking Audiometry AUDIOMETERS www.medrx-int.com Contents Getting to Know Your Audiometer.. 3 Computer Requirements.. 4 Avant A2D+ 5 Avant Stealth... 6 Transducers
Mini Remote Microphone OPERATIONS MANUAL
 Mini Remote Microphone OPERATIONS MANUAL Table of Contents Overview..................................... 4 Basic Use..................................... 7 Daily Use.....................................
Mini Remote Microphone OPERATIONS MANUAL Table of Contents Overview..................................... 4 Basic Use..................................... 7 Daily Use.....................................
Amplivox Ltd Model 116 Screening Audiometer Operating Manual
 Amplivox Ltd Model 116 Screening Audiometer Operating Manual (Applies from serial number 23310 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
Amplivox Ltd Model 116 Screening Audiometer Operating Manual (Applies from serial number 23310 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
Aeroneb Solo System Instruction Manual
 www.aerogen.com Aeroneb Solo System Instruction Manual Aeroneb Solo System Instruction Manual Introduction 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 10 Installation
www.aerogen.com Aeroneb Solo System Instruction Manual Aeroneb Solo System Instruction Manual Introduction 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 10 Installation
Mini Pulse Electronic Stimulator
 Mini Pulse Electronic Stimulator Model: PM-180 Operating Manual IMPORTANT: Please read all instructions before using this product. Retain this manual for future reference. www.santamedical.com IMPORTANT
Mini Pulse Electronic Stimulator Model: PM-180 Operating Manual IMPORTANT: Please read all instructions before using this product. Retain this manual for future reference. www.santamedical.com IMPORTANT
Remote control 2 guide
 Remote control 2 guide Thank you Thank you for choosing remote control 2 for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare
Remote control 2 guide Thank you Thank you for choosing remote control 2 for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare
I N S T R U C T I O N
 I N S T R U C T I O N M ANUAL model V2x Acoustical airway clearance device. 5.04.1 SAFETY SYMBOLS This manual uses the following safety symbols. They denote critical information. Please read them carefully.
I N S T R U C T I O N M ANUAL model V2x Acoustical airway clearance device. 5.04.1 SAFETY SYMBOLS This manual uses the following safety symbols. They denote critical information. Please read them carefully.
System Instruction Manual
 System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 5 Assembly & Installation 9 Installation For Use With A Ventilator 12 Nebulization 16 Installation
System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 5 Assembly & Installation 9 Installation For Use With A Ventilator 12 Nebulization 16 Installation
Instruction Manual Aerogen, Inc. Part No. AG-AL1010 Rev. C
 Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
System Instruction Manual
 System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 14 Installation
System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 14 Installation
Instruction Manual. Copyright 2016 by Mettler Electronics Corp. Anaheim, CA
 Instruction Manual Distributed by: Mettler Electronics Corp. 1333 South Claudina Street Anaheim, CA 92805 U.S.A. 800.854.9305 1.714.533.2221 Fax: 1.714.635.7539 www.mettlerelectronics.com mail@mettlerelectronics.com
Instruction Manual Distributed by: Mettler Electronics Corp. 1333 South Claudina Street Anaheim, CA 92805 U.S.A. 800.854.9305 1.714.533.2221 Fax: 1.714.635.7539 www.mettlerelectronics.com mail@mettlerelectronics.com
Operator s Manual FLASH GLUCOSE MONITORING SYSTEM. CAUTION: Federal law restricts this device to sale by or on the order of a physician.
 Operator s Manual FLASH GLUCOSE MONITORING SYSTEM CAUTION: Federal law restricts this device to sale by or on the order of a physician. Reader Symbols... 1 Contents Important Safety Information... 2 Indications
Operator s Manual FLASH GLUCOSE MONITORING SYSTEM CAUTION: Federal law restricts this device to sale by or on the order of a physician. Reader Symbols... 1 Contents Important Safety Information... 2 Indications
CP Relief Wand. Model CP 1000 (Rx Only) Operation Manual. Mid-America Medical Innovations, LLC Jefferson City, MO
 CP Relief Wand Model CP 1000 (Rx Only) Operation Manual Mid-America Medical Innovations, LLC Jefferson City, MO RoHS Compliant Attachment 13.6 Page 13.6-1 INDEX I. Introduction General What is pain? How
CP Relief Wand Model CP 1000 (Rx Only) Operation Manual Mid-America Medical Innovations, LLC Jefferson City, MO RoHS Compliant Attachment 13.6 Page 13.6-1 INDEX I. Introduction General What is pain? How
System Instruction Manual
 System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 13 Installation
System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 13 Installation
Phonak PilotOne II. User Guide
 Phonak PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On/Off 7 3.3 Holding PilotOne II 7 3.4 Changing hearing aid
Phonak PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On/Off 7 3.3 Holding PilotOne II 7 3.4 Changing hearing aid
Anodyne Therapy. Model 120 Professional. Important Safety Information and Instructions. Read Entire Booklet Before Operating
 Anodyne Therapy Model 120 Professional Manufacturer: Anodyne Therapy, LLC Address: 14105 McCormick Drive Tampa, FL 33626 Tel: 1-800-521-6664 Fax: 1-800-835-4581 Website: www.anodynetherapy.com Anodyne
Anodyne Therapy Model 120 Professional Manufacturer: Anodyne Therapy, LLC Address: 14105 McCormick Drive Tampa, FL 33626 Tel: 1-800-521-6664 Fax: 1-800-835-4581 Website: www.anodynetherapy.com Anodyne
ihealth PO3 Fingertip Pulse Oximeter OPERATION GUIDE INDEX
 ihealth PO3 Fingertip Pulse Oximeter OPERATION GUIDE INDEX INTRODUCTION AND INTENDED USE...2 CONTENTS AND DISPLAY INDICATORS...2 PRODUCT DESCRIPTION...3 SPECIFICATIONS...3 CAUTIONS...3 Cautions...3 USING
ihealth PO3 Fingertip Pulse Oximeter OPERATION GUIDE INDEX INTRODUCTION AND INTENDED USE...2 CONTENTS AND DISPLAY INDICATORS...2 PRODUCT DESCRIPTION...3 SPECIFICATIONS...3 CAUTIONS...3 Cautions...3 USING
THE REBUILDER SYSTEM. ReBuilder Model 300
 All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
THE REBUILDER SYSTEM. ReBuilder Model 300. ReBuilder Medical Inc. 636 Treeline Drive, Suite A Charles Town, WV
 THE REBUILDER SYSTEM ReBuilder Model 300 ReBuilder Medical Inc. 636 Treeline Drive, Suite A Charles Town, WV 25414 Phone: 304-725-2202 Fax: 304-725-4915 GET TO KNOW YOUR REBUILDER Confirm all the following
THE REBUILDER SYSTEM ReBuilder Model 300 ReBuilder Medical Inc. 636 Treeline Drive, Suite A Charles Town, WV 25414 Phone: 304-725-2202 Fax: 304-725-4915 GET TO KNOW YOUR REBUILDER Confirm all the following
InsuPad User Manual. Charger Base The charger base can be connected by the attached USB cable to the power adapter supplied for charging.
 InsuPad User Manual System overview The InsuPad is designed to improve the delivery of injected insulin into the blood by controlled warming of the area which surrounds the point of injection. The device
InsuPad User Manual System overview The InsuPad is designed to improve the delivery of injected insulin into the blood by controlled warming of the area which surrounds the point of injection. The device
Joint Relaxation Heated Vibration Massager. Knee and Joint Physiotherapy Unit
 Joint Relaxation Heated Vibration Massager Knee and Joint Physiotherapy Unit Instruction Manual Model: KH317 This user manual provides both operation information of this product and its detailed specifications.
Joint Relaxation Heated Vibration Massager Knee and Joint Physiotherapy Unit Instruction Manual Model: KH317 This user manual provides both operation information of this product and its detailed specifications.
Model 170. Operation Manual
 Model 170 Operation Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. This manual is valid for the Model 170 (applies from firmware version 2N22 onwards please
Model 170 Operation Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. This manual is valid for the Model 170 (applies from firmware version 2N22 onwards please
Dear HighQ Check System Owner :
 Dear HighQ Check System Owner : Thank you for purchasing the HighQ Check Blood Glucose Monitoring System. This manual provides important information to help you to use the system properly. Before using
Dear HighQ Check System Owner : Thank you for purchasing the HighQ Check Blood Glucose Monitoring System. This manual provides important information to help you to use the system properly. Before using
Portable Equine Nebuliser System. User Manual
 Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
Unitron remote control guide
 Unitron remote control guide Thank you Thank you for choosing a Unitron remote control for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing
Unitron remote control guide Thank you Thank you for choosing a Unitron remote control for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing
Patient Therapy Controller Guide For use with Prometra Programmable Infusion Systems
 Patient Therapy Controller Guide For use with Prometra Programmable Infusion Systems PTC MR MR Unsafe Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Explanation
Patient Therapy Controller Guide For use with Prometra Programmable Infusion Systems PTC MR MR Unsafe Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Explanation
PilotOne II. User Guide
 PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On / Off 7 3.3 Holding correctly 7 3.4 Changing hearing aid volume
PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On / Off 7 3.3 Holding correctly 7 3.4 Changing hearing aid volume
Instructions for Use. Oscilla TSM400 Screening Tympanometer. Specifications are subject to change without notice ID: 6795 / ver.
 Instructions for Use Oscilla TSM400 Screening Tympanometer Specifications are subject to change without notice 2017-02-09 1 Contents General description... 3 Installation... 4 Device overview... 5 Rear
Instructions for Use Oscilla TSM400 Screening Tympanometer Specifications are subject to change without notice 2017-02-09 1 Contents General description... 3 Installation... 4 Device overview... 5 Rear
For more information visit or contact hearx:
 USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
Your umic. Thank you. Hearing healthcare professional: Telephone: Serial number of umic: Warranty: Date of purchase:
 umic guide Thank you Thank you for choosing the Unitron umic. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare professionals to make advanced, purpose-driven
umic guide Thank you Thank you for choosing the Unitron umic. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare professionals to make advanced, purpose-driven
THE REBUILDER SYSTEM. ReBuilder Model 2407
 All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
Biomet SpinalPak Non-Invasive Spine Fusion Stimulator System
 Biomet SpinalPak Non-Invasive Spine Fusion Stimulator System A Patient s Guide 100 Interpace Parkway Parsippany, NJ 07054 800-526-2579 www.biomet.com BNS231003 2009 EBI, LLC. All trademarks are the property
Biomet SpinalPak Non-Invasive Spine Fusion Stimulator System A Patient s Guide 100 Interpace Parkway Parsippany, NJ 07054 800-526-2579 www.biomet.com BNS231003 2009 EBI, LLC. All trademarks are the property
Compressor Nebulizer Instruction Manual Part No.: 5055
 Compressor Nebulizer Instruction Manual Part No.: 5055 DISTRIBUTED BY: SAVE THESE INSTRUCTIONS. CAUTION - U.S. Federal Law restricts this device to sale by or on the order of a physician. 666002-6310 V1.3
Compressor Nebulizer Instruction Manual Part No.: 5055 DISTRIBUTED BY: SAVE THESE INSTRUCTIONS. CAUTION - U.S. Federal Law restricts this device to sale by or on the order of a physician. 666002-6310 V1.3
Operation Manual MA 27 / MA 27e
 Operation Manual MA 27 / MA 27e Table of Contents Page 1 Introduction 2 2 Intended Use 3 2.1 Description 3 2.2 Extended Function 3 3 Important Safety Note 5 4 Getting started 8 4.1 Unpacking and Checking
Operation Manual MA 27 / MA 27e Table of Contents Page 1 Introduction 2 2 Intended Use 3 2.1 Description 3 2.2 Extended Function 3 3 Important Safety Note 5 4 Getting started 8 4.1 Unpacking and Checking
InWave. User s Manual
 InWave User s Manual This page intentionally left blank. InWave Table of Contents Contact Information / Customer Service / Supplies / Support... 4 Description... 5 Safety Information... 6 Probe Setup...
InWave User s Manual This page intentionally left blank. InWave Table of Contents Contact Information / Customer Service / Supplies / Support... 4 Description... 5 Safety Information... 6 Probe Setup...
Charging base guide. A Sonova brand
 Charging base guide A Sonova brand Thank you Thank you for choosing this rechargeable solution. We care deeply about people with hearing loss. We work closely with hearing healthcare professionals to make
Charging base guide A Sonova brand Thank you Thank you for choosing this rechargeable solution. We care deeply about people with hearing loss. We work closely with hearing healthcare professionals to make
User Training Manual
 Select Language AIM (Aerosol Inhalation Monitor) Model 4500 User Training Manual https://vitalograph.com/downloads/display/41 1/17 UK Sales Vitalograph Ltd. Maids Moreton, Buckingham, MK18 1SW, England
Select Language AIM (Aerosol Inhalation Monitor) Model 4500 User Training Manual https://vitalograph.com/downloads/display/41 1/17 UK Sales Vitalograph Ltd. Maids Moreton, Buckingham, MK18 1SW, England
Table of Contents. Introduction Indications For Use Contraindications Warnings Precautions...5
 User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
Medescan Nebuliser Med - S600A Instructions Manual
 Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Dual Channel TENS. Natural, drug-free pain relief. Target-specific pain relief. Clinically Proven. User Manual. Model ACRL-3000
 Dual Channel TENS Natural, drug-free pain relief Target-specific pain relief. Clinically Proven User Manual Model ACRL-3000 Click here to view detailed product descriptions and prices. Call 800-397-5899
Dual Channel TENS Natural, drug-free pain relief Target-specific pain relief. Clinically Proven User Manual Model ACRL-3000 Click here to view detailed product descriptions and prices. Call 800-397-5899
OsseoCare Pro. Instructions for use - drilling unit. Manufactured by
 OsseoCare Pro Instructions for use - drilling unit Manufactured by Disclaimer English Limitations of liability This product is part of an overall concept and may only be used in conjunction with the associated
OsseoCare Pro Instructions for use - drilling unit Manufactured by Disclaimer English Limitations of liability This product is part of an overall concept and may only be used in conjunction with the associated
Electrodes (for TransAeris System)
 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Connevans.info. DeafEquipment.co.uk. This product may be purchased from Connevans Limited secure online store at
 Connevans.info Solutions to improve the quality of life Offering you choice Helping you choose This product may be purchased from Connevans Limited secure online store at www.deafequipment.co.uk DeafEquipment.co.uk
Connevans.info Solutions to improve the quality of life Offering you choice Helping you choose This product may be purchased from Connevans Limited secure online store at www.deafequipment.co.uk DeafEquipment.co.uk
Blood Glucose Monitoring System. User Guide
 Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
BODY ANALYZER SCALE PS 8710
 02PS8710_en 20.10.2008 13:30 Uhr Seite 14 Safety Please note the following instructions when using the appliance. 7 The appliance is designed for domestic use only. 7 Do not use the appliance on a carpet
02PS8710_en 20.10.2008 13:30 Uhr Seite 14 Safety Please note the following instructions when using the appliance. 7 The appliance is designed for domestic use only. 7 Do not use the appliance on a carpet
User Manual.
 User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
AWAY Model # RTLAGF-900 REV
 AWAY Model # RTLAGF-900 REV2.4.9.15 Contents User manual English 2 General Description 2 Intended Use 3 Included in this Package 4 Instructions for Use 7 Application Duration 7 Electrode Placement 8 Tips
AWAY Model # RTLAGF-900 REV2.4.9.15 Contents User manual English 2 General Description 2 Intended Use 3 Included in this Package 4 Instructions for Use 7 Application Duration 7 Electrode Placement 8 Tips
INSTRUCTION MANUAL. Compressor Nebulizer NE-C801
 active forever.com INSTRUCTION MANUAL TM Compressor Nebulizer NE-C801 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician or licensed healthcare practitioner ENGLISH
active forever.com INSTRUCTION MANUAL TM Compressor Nebulizer NE-C801 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician or licensed healthcare practitioner ENGLISH
ProPocket TM. User Guide
 ProPocket TM User Guide A1 A Introduction Dear customer, Your hearing instruments are equipped with wireless technology and can therefore be controlled by your ProPocket. These instruction describes how
ProPocket TM User Guide A1 A Introduction Dear customer, Your hearing instruments are equipped with wireless technology and can therefore be controlled by your ProPocket. These instruction describes how
Muscle Stimulation. Page 1
 Muscle Stimulation Page 1 Page 2 Page 3 Table of Contents: Get Started with the E-Wave Pg 4 Controls and Features Pg 4 Factory Settings Pg 6 Electrodes and Skin Care Pg 7 Batteries Pg 7 Indications Pg
Muscle Stimulation Page 1 Page 2 Page 3 Table of Contents: Get Started with the E-Wave Pg 4 Controls and Features Pg 4 Factory Settings Pg 6 Electrodes and Skin Care Pg 7 Batteries Pg 7 Indications Pg
Glucose Meter. User Guide. Veterinary Monitoring System. For dog and cat use only
 Glucose Meter User Guide Veterinary Monitoring System For dog and cat use only Gpet instruction Manual 31/5/09 18:06 Page 2 Gpet instruction Manual 31/5/09 18:06 Page 3 TABLE OF CONTENTS Your g-pet system
Glucose Meter User Guide Veterinary Monitoring System For dog and cat use only Gpet instruction Manual 31/5/09 18:06 Page 2 Gpet instruction Manual 31/5/09 18:06 Page 3 TABLE OF CONTENTS Your g-pet system
BRS GM100 Glucose Monitoring System. User Instruction Manual Ver. 3.5
 BRS GM100 Glucose Monitoring System User Instruction Manual Ver. 3.5 TABLE OF CONTENTS Introduction... 3 Intended Use... 3 Understanding the GM100 Glucose Monitoring System... 4 Explanation of the Full
BRS GM100 Glucose Monitoring System User Instruction Manual Ver. 3.5 TABLE OF CONTENTS Introduction... 3 Intended Use... 3 Understanding the GM100 Glucose Monitoring System... 4 Explanation of the Full
Recharge. Relax.Repeat.
 Recharge. Relax.Repeat. Recharge. Relax.Repeat. What s Inside Your rechargeable hearing aids will be delivered to you in two boxes. Please note that both ZPower rechargeable batteries and disposable batteries
Recharge. Relax.Repeat. Recharge. Relax.Repeat. What s Inside Your rechargeable hearing aids will be delivered to you in two boxes. Please note that both ZPower rechargeable batteries and disposable batteries
Compressor Nebulizer. Guidebook
 NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT. Instruction Manual
 HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
CentriVet GK Blood Glucose & Ketone Monitoring System
 CentriVet GK Blood Glucose & Ketone Monitoring System FOR ANIMAL USE. NOT FOR HUMAN USE. Welcome and thank you for choosing the CentriVet GK Blood Glucose & Ketone Monitoring System. The CentriVet GK Blood
CentriVet GK Blood Glucose & Ketone Monitoring System FOR ANIMAL USE. NOT FOR HUMAN USE. Welcome and thank you for choosing the CentriVet GK Blood Glucose & Ketone Monitoring System. The CentriVet GK Blood
UPC 200-A. Stimulator Instructions for Use
 For the treatment of Overactive Bladder (OAB) and associated symptoms of urinary urgency, urinary frequency, and urge incontinence. UPC 200-A Stimulator Instructions for Use CONTENTS Description of Symbols...
For the treatment of Overactive Bladder (OAB) and associated symptoms of urinary urgency, urinary frequency, and urge incontinence. UPC 200-A Stimulator Instructions for Use CONTENTS Description of Symbols...
Operating Instructions. ULTRATHERM 1008 short-wave therapy unit
 Operating Instructions ULTRATHERM 1008 short-wave therapy unit gbo Medizintechnik AG The gbo Medizintechnik AG has taken care in preparation of this manual, but makes no expressed or implied warranty of
Operating Instructions ULTRATHERM 1008 short-wave therapy unit gbo Medizintechnik AG The gbo Medizintechnik AG has taken care in preparation of this manual, but makes no expressed or implied warranty of
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606
 PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
LEARNING TO USE YOUR FIRST HEARING AID
 Hearing Aid User Manual PHLHA46 LEARNING TO USE YOUR FIRST HEARING AID Your hearing aid cannot return your level of hearing to normal or halt further hearing deterioration, but proper use of your hearing
Hearing Aid User Manual PHLHA46 LEARNING TO USE YOUR FIRST HEARING AID Your hearing aid cannot return your level of hearing to normal or halt further hearing deterioration, but proper use of your hearing
Identification. Eartips. Battery Door. 2-Position Switch. Eartip. Removal Cord. Flexible Neck Cord Protective Case. Filter Tool and Extra ACCU Filters
 English User Manual Warnings Failure to follow these recommendations may severely reduce the amount of hearing protection provided by the earplugs. Earplugs comply with EN-352 standard of measurement.
English User Manual Warnings Failure to follow these recommendations may severely reduce the amount of hearing protection provided by the earplugs. Earplugs comply with EN-352 standard of measurement.
Salus-Talent User Guide
 1/40 The user of this device must sufficiently understand its functions and the precautions that must be observed for its safe use and stable performance. For the safe use and post management of the device,
1/40 The user of this device must sufficiently understand its functions and the precautions that must be observed for its safe use and stable performance. For the safe use and post management of the device,
Comfortmax Thermax Hot/Cold Water Circulation System. Instructions for Use
 Comfortmax Thermax Hot/Cold Water Circulation System Instructions for Use THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS
Comfortmax Thermax Hot/Cold Water Circulation System Instructions for Use THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS
SureTemp Plus. REF 690 and 692. Directions for use
 SureTemp Plus REF 690 and 692 Directions for use ii Welch Allyn SureTemp 2016 Welch Allyn, Inc. To support the intended use of the product described in this publication, the purchaser of the product is
SureTemp Plus REF 690 and 692 Directions for use ii Welch Allyn SureTemp 2016 Welch Allyn, Inc. To support the intended use of the product described in this publication, the purchaser of the product is
Amplivox Ltd Otosure Automatic Audiometer Operating Manual (Desktop Version) (Audibase Software Version 5.5)
 Amplivox Ltd Otosure Automatic Audiometer Operating Manual (Desktop Version) (Audibase Software Version 5.5) (Applies from serial number 58000 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29
Amplivox Ltd Otosure Automatic Audiometer Operating Manual (Desktop Version) (Audibase Software Version 5.5) (Applies from serial number 58000 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29
User Guide and Operating Manual
 Deep Penetrating Infrared Light Therapy Panel System User Guide and Operating Manual Roscoe Medical, Inc. 21973 Commerce Parkway Strongsville, Ohio 44149 1.16.13 INCLUDES IMPORTANT SAFETY INFORMATION READ
Deep Penetrating Infrared Light Therapy Panel System User Guide and Operating Manual Roscoe Medical, Inc. 21973 Commerce Parkway Strongsville, Ohio 44149 1.16.13 INCLUDES IMPORTANT SAFETY INFORMATION READ
CAUTION: FEDERAL (U.S.) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN
 IPC USER S GUIDE CAUTION: FEDERAL (U.S.) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN READ ALL DIRECTIONS IN THIS GUIDE BEFORE USING THE MAXUM SYSTEM. CONTACT YOUR HEARING CARE PROFESSIONAL
IPC USER S GUIDE CAUTION: FEDERAL (U.S.) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN READ ALL DIRECTIONS IN THIS GUIDE BEFORE USING THE MAXUM SYSTEM. CONTACT YOUR HEARING CARE PROFESSIONAL
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Dr FuelCell Load Measurement Box
 1 (855) 251-0016 sales@fuelcellstore.com Dr FuelCell Load Measurement Box Instruction Manual www.fuelcellstore.com Instruction Manual for Dr FuelCell TM Load Measurement Box Version 1.0.1 November 2008
1 (855) 251-0016 sales@fuelcellstore.com Dr FuelCell Load Measurement Box Instruction Manual www.fuelcellstore.com Instruction Manual for Dr FuelCell TM Load Measurement Box Version 1.0.1 November 2008
IMPACT Pro R Instructions and guidance
 IMPACT Pro R Instructions and guidance Your IMPACT Pro R hearing instrument Features 1 Receiver Unit 2 Click Dome 3 Battery compartment 4 Push Button 5 Microphone Cover 6 Click Mould 7 Grid 8 HF-4 Wax
IMPACT Pro R Instructions and guidance Your IMPACT Pro R hearing instrument Features 1 Receiver Unit 2 Click Dome 3 Battery compartment 4 Push Button 5 Microphone Cover 6 Click Mould 7 Grid 8 HF-4 Wax
FREQUENTLY ASKED QUESTIONS. Frequently Asked Questions
 Frequently Asked Questions THE DEVICE Do I need a prescription to use the device? Yes. You need a prescription from your eye care professional to use the TrueTear device. How and where can I purchase the
Frequently Asked Questions THE DEVICE Do I need a prescription to use the device? Yes. You need a prescription from your eye care professional to use the TrueTear device. How and where can I purchase the
User Manual Table of Contents. Instructions for Using the LaserTouchOne Charging the Unit... 5 Holding the Device... 5 Treatment Steps...
 User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
Amplivox Ltd Model 240 Diagnostic Audiometer Operating Manual
 Amplivox Ltd Model 240 Diagnostic Audiometer Operating Manual (Applies from serial number 31657 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
Amplivox Ltd Model 240 Diagnostic Audiometer Operating Manual (Applies from serial number 31657 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
Pain Relief Patch. Operation Manual Read Before Using MODEL # ET
 Pain Relief Patch Operation Manual Read Before Using Intended Use The ireliev Pain Relief Patch is intended for temporary relief of pain associated with sore and aching muscles in the upper and lower extremities
Pain Relief Patch Operation Manual Read Before Using Intended Use The ireliev Pain Relief Patch is intended for temporary relief of pain associated with sore and aching muscles in the upper and lower extremities
Compatible with the following products TENS. Replacement Pads. User Manual. Last revised: V Last revised: V
 Compatible with the following products TENS Replacement Pads User Manual Last revised: V11-160923 Last revised: V4-170619 Contents Introduction 2-3 Parts 3 General Warnings and Safety 4-10 Using your TENS
Compatible with the following products TENS Replacement Pads User Manual Last revised: V11-160923 Last revised: V4-170619 Contents Introduction 2-3 Parts 3 General Warnings and Safety 4-10 Using your TENS
ENGLISH. USER MANUAL Register your product at: multi-action toothbrush
 USER MANUAL Register your product at: www.acteh.com/register ENGLISH multi-action toothbrush To reorder toothbrush heads, please visit: www.acteh.com/heads 15% of your next order at www.acteh.com/store
USER MANUAL Register your product at: www.acteh.com/register ENGLISH multi-action toothbrush To reorder toothbrush heads, please visit: www.acteh.com/heads 15% of your next order at www.acteh.com/store
OWNER S MANUAL. BODYCRAFT 7699 Green Meadows Dr., Lewis Center, Ohio Tel:
 OWNER S MANUAL BODYCRAFT 7699 Green Meadows Dr., Lewis Center, Ohio 43035 Tel: 800.990.5556 1 www.bodycraft.com SAFETY INSTRUCTION WARNING: To reduce the risk of serious injury, read the following safety
OWNER S MANUAL BODYCRAFT 7699 Green Meadows Dr., Lewis Center, Ohio 43035 Tel: 800.990.5556 1 www.bodycraft.com SAFETY INSTRUCTION WARNING: To reduce the risk of serious injury, read the following safety
FlexAbs EMS ABDOMINAL STIMULATOR. FlexBody. EMS Body Stimulator INSTRUCTION MANUAL
 FlexAbs TM EMS ABDOMINAL STIMULATOR FlexBody TM EMS Body Stimulator INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. INTRODUCTION The FlexAbs TM and FlexBody TM
FlexAbs TM EMS ABDOMINAL STIMULATOR FlexBody TM EMS Body Stimulator INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. INTRODUCTION The FlexAbs TM and FlexBody TM
Elise_Instruction_Manual_amend_Layout 1 27/06/ :40 Page 1. Instructions for use
 Elise_Instruction_Manual_amend_Layout 1 27/06/2011 13:40 Page 1 Instructions for use Elise_Instruction_Manual_amend_Layout 1 27/06/2011 13:40 Page 2 INTELLIGENT CONTINENCE STIMULATOR CONTENTS Section:
Elise_Instruction_Manual_amend_Layout 1 27/06/2011 13:40 Page 1 Instructions for use Elise_Instruction_Manual_amend_Layout 1 27/06/2011 13:40 Page 2 INTELLIGENT CONTINENCE STIMULATOR CONTENTS Section:
Blood Glucose & Ketone Monitoring System
 Blood Glucose & Ketone Monitoring System Self monitoring of blood glucose is an integral part of diabetes care, but the high cost of testing can make this impossible. At ACON, our goal is to provide high
Blood Glucose & Ketone Monitoring System Self monitoring of blood glucose is an integral part of diabetes care, but the high cost of testing can make this impossible. At ACON, our goal is to provide high
Salus-Talent Pro User Guide
 1/45 2017.08.16 Ver M-1.0.0 The user of this device must sufficiently understand its functions and the precautions that must be observed for its safe use and stable performance. For the safe use and post
1/45 2017.08.16 Ver M-1.0.0 The user of this device must sufficiently understand its functions and the precautions that must be observed for its safe use and stable performance. For the safe use and post
2. Before Testing Monitor Checker Test...10 Inserting Lancets into Lancing Device...11 Quality Control Testing...13
 Table of Contents 1. About your HemoSmart Haemoglobin Screening System Contents of Kit...4 HemoSmart Haemoglobin Meter...5 HemoSmart Haemoglobin Test Strip...7 Adjustable Lancing Device and Lancets...8
Table of Contents 1. About your HemoSmart Haemoglobin Screening System Contents of Kit...4 HemoSmart Haemoglobin Meter...5 HemoSmart Haemoglobin Test Strip...7 Adjustable Lancing Device and Lancets...8
Hearit M. user guide. To find your local supplier visit
 Hearit M UK user guide To find your local supplier visit www.phonicear.com table of contents Hearit.............................................................. 4 Hearit features.........................................................5
Hearit M UK user guide To find your local supplier visit www.phonicear.com table of contents Hearit.............................................................. 4 Hearit features.........................................................5
Compressor Nebulizer System
 MedPro The Professional Choice Le choix des professionnels MedProTM MC Compressor Nebulizer System 705-445 Instruction Manual Index 1. Introduction...2 2. Product Identification...2 3. Important Safeguards...3
MedPro The Professional Choice Le choix des professionnels MedProTM MC Compressor Nebulizer System 705-445 Instruction Manual Index 1. Introduction...2 2. Product Identification...2 3. Important Safeguards...3
System Instruction Manual
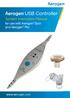 System Instruction Manual for use with Aerogen Solo and Aerogen Pro www.aerogen.com Contents Introduction 2 Intended Use 2 Set Up 5 System Warnings 8 Controls & Indicators 12 Accessories 13 Functional
System Instruction Manual for use with Aerogen Solo and Aerogen Pro www.aerogen.com Contents Introduction 2 Intended Use 2 Set Up 5 System Warnings 8 Controls & Indicators 12 Accessories 13 Functional
