DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN CLINICAL GUIDELINE V3.0
|
|
|
- Daniel Powers
- 5 years ago
- Views:
Transcription
1 DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN CLINICAL GUIDELINE V3.0
2 1. Aim/Purpose of this Guideline This guideline is relevant to all medical and nursing staff caring for children with Pyloric Stenosis. 2. The Guidance Pyloric stenosis is due to hypertrophy of the pyloric sphincter, leading to narrowing of the gastric outlet. It occurs in 2 in 1000 live births and is the most common cause of gastric outflow obstruction in children under 3 months of age. Risk factors include: Sex (4:1 male-female ratio) 1st born infants Parental history of pyloric stenosis (higher if mother affected) Term infants Caucasian families 2.1. Presentation Pyloric stenosis typically presents between 3 6 weeks of age with forceful nonbilious vomiting which is occasionally blood stained. There may also signs of reduced absorption such as failure to thrive, dry nappies, dry mucous membranes, tachycardia and depressed fontanelle. Jaundice may also be present. It is rare for the condition to present with diarrhoea. (BMJ 2009) Differential diagnoses to consider are GORD, over feeding, infectious diarrhoea and small bowel atresias. (BMJ 2009) Assessment Assess degree of dehydration Observe for visible gastric peristalsis (waves of muscular contraction across abdomen from left upper quadrant to right lower quadrant; requires 2-3 minutes observation) Palpate for pyloric mass (typically olive sized in right upper quadrant) (BMJ 2009) Test feeds can be a useful aid if clinical diagnosis is uncertain, infant if given full strength feed and observed for mechanism of any vomiting e.g. projectile or posset; bilious or non-bilious. Abdominal ultrasound should be arranged in all babies where the diagnosis is suspected. The US is diagnostic if pylorus is greater than 4mm thickness and greater than 17mm in length. Page 2 of 9
3 2.3. Management Plot weight on growth chart and compare to previous measurements Blood gas (venous or capillary): hyperchloraemic hypokalaemic metabolic alkalosis is often present Bloods: FBC, U/E, Glucose, LFT (if jaundiced) NBM Discuss with the surgeons at RCHT initially who may advise further discussion with surgical team at Bristol children s Hospital. Regular BM monitoring 4-6 hourly via heel prick Strict fluid monitoring and correct dehydration NG tube on free drainage with hourly aspirations Replace NG losses ml for ml with 0.9% NaCl + 10mmol KCl per 500mls of intravenous fluid Also give maintenance intravenous fluids 0.45% NaCl + 5% dextrose + 10mmol KCl per 500mls of fluid Serum bicarbonate and potassium are almost always corrected prior to surgery. Families may need to be warned that this correction will usually happen locally before transfer to the surgical centre Surgery Pyloromyotmy is the gold standard surgical procedure. This involves longitudinal splitting of the pylorus muscle by either open or laparoscopic methods Post-operative care Feeding is usually recommenced 4-6 hours after surgery. This will be at the discretion of the surgical team. Vomiting may continue but should resolve within 48 hours. The main complications of the procedure are continued vomiting, infection and bowel perforation. Page 3 of 9
4 3. Monitoring compliance and effectiveness Element to be monitored Lead Tool Frequency Reporting arrangements Acting on recommendations and Lead(s) Compliance with section 2.4 of the guideline Audit lead Patient review, notes audit. At point of patient occurrence as low numbers. Paediatric consultant Child Health Directorate audit and guidelines meeting Paediatric consultants Directorate audit and guidelines meeting Required actions will be identified and completed in 3-6 months. Change in practice and lessons to be shared Required changes to practice will be identified and actioned within 3-6 months. A lead member of the team will be identified to take each change forward where appropriate. Lessons will be shared with all the relevant stakeholders 4. Equality and Diversity 4.1. This document complies with the Royal Cornwall Hospitals NHS Trust service Equality and Diversity statement Equality Impact Assessment The Initial Equality Impact Assessment Screening Form is at Appendix 2. Page 4 of 9
5 Appendix 1. Governance Information Document Title Date Issued/Approved: 10 th Nov 2017 Diagnosis and Management of Pyloric Stenosis in Children Clinical Guideline V3.0 Date Valid From: 10 th Nov 2017 Date Valid To: 10 th Nov 2020 Directorate / Department responsible (author/owner): Chris Williams Paediatric Consultant Contact details: Brief summary of contents Clinical Guideline for the diagnosis and management of pyloric stenosis in children. Suggested Keywords: Target Audience Executive Director responsible for Policy: Child Children Pyloric stenosis RCHT CPFT KCCG Medical Director Date revised: 10 th Nov 2017 This document replaces (exact title of previous version): Approval route (names of committees)/consultation: CLINICAL GUIDELINE FOR THE DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN V2.0 Child health audit and guidelines meeting Divisional Manager confirming approval processes Name and Post Title of additional signatories Name and Signature of Divisional/Directorate Governance Lead confirming approval by specialty and divisional management meetings David Smith Not Required {Original Copy Signed} Name: Caroline Amukusana Signature of Executive Director giving approval Publication Location (refer to Policy on Policies Approvals and Page 5 of 9 {Original Copy Signed} Internet & Intranet Intranet Only
6 Ratification): Document Library Folder/Sub Folder Links to key external standards Related Documents: Training Need Identified? Clinical / Paediatric none Guner YS., Aranda A. and Upperman YS. British medical journal : Best practice Pyloric Stenosis. NO Version Control Table Date Version No Summary of Changes Changes Made by (Name and Job Title) December 2011 January 2014 V1.0 Initial Issue V2.0 Review and reformat Dr Fiona Briant & Dr James Mallen Dr.N.Venkata paediatric consultant and Tabitha Fergus deputy ward manager- (reformat only) Nov 2017 V3.0 Minor Changes to wording in assessment and management sections Chris Williams, Paediatric Consultant All or part of this document can be released under the Freedom of Information Act 2000 This document is to be retained for 10 years from the date of expiry. This document is only valid on the day of printing Controlled Document This document has been created following the Royal Cornwall Hospitals NHS Trust Policy on Document Production. It should not be altered in any way without the express permission of the author or their Line Manager. Page 6 of 9
7 Appendix 2. Initial Equality Impact Assessment Form This assessment will need to be completed in stages to allow for adequate consultation with the relevant groups. Name of Name of the strategy / policy /proposal / service function to be assessed DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN CLINICAL GUIDELINE V3.0 Directorate and service area: Child Health Name of individual completing assessment: Chris Williams Is this a new or existing Policy? Existing Telephone: Policy Aim* Clear guidance for medical staff caring for child with pyloric stenosis. Who is the strategy / policy / proposal / service function aimed at? 2. Policy Objectives* Clear guidance for medical staff caring for child with pyloric stenosis. 3. Policy intended Outcomes* Evidenced based and standardised practice. 4. *How will you measure the outcome? 5. Who is intended to benefit from the policy? 6a Who did you consult with b). Please identify the groups who have been consulted about this procedure. Audit and review Children and families Workforce Patients Local groups x Please record specific names of groups Clinical Guideline Group Child Health Directorate External organisations Other Page 7 of 9
8 What was the outcome of the consultation? Guideline agreed 7. The Impact Please complete the following table. If you are unsure/don t know if there is a negative impact you need to repeat the consultation step. Are there concerns that the policy could have differential impact on: Equality Strands: Yes No Unsure Rationale for Assessment / Existing Evidence Age Sex (male, female, trans-gender / gender reassignment) Race / Ethnic communities /groups Disability - Learning disability, physical impairment, sensory impairment, mental health conditions and some long term health conditions. Religion / other beliefs Marriage and Civil partnership Pregnancy and maternity Sexual Orientation, Bisexual, Gay, heterosexual, Lesbian You will need to continue to a full Equality Impact Assessment if the following have been highlighted: You have ticked Yes in any column above and No consultation or evidence of there being consultation- this excludes any policies which have been identified as not requiring consultation. or Major this relates to service redesign or development Page 8 of 9
9 8. Please indicate if a full equality analysis is recommended. Yes No 9. If you are not recommending a Full Impact assessment please explain why. No areas indicated Signature of policy developer / lead manager / director Chris Williams Date of completion and submission 10/11/17 Names and signatures of members carrying out the Screening Assessment 1. Chris Williams 2. Human Rights, Equality & Inclusion Lead Keep one copy and send a copy to the Human Rights, Equality and Inclusion Lead c/o Royal Cornwall Hospitals NHS Trust, Human Resources Department, Knowledge Spa, Truro, Cornwall, TR1 3HD This EIA will not be uploaded to the Trust website without the signature of the Human Rights, Equality & Inclusion Lead. A summary of the results will be published on the Trust s web site. Signed Chris Williams Date 10/11/17 Page 9 of 9
Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0. March 2018
 Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0 March 2018 Page 1 of 8 Summary flow chart for monitoring of blood glucose if >11mmol/L For Adults
Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0 March 2018 Page 1 of 8 Summary flow chart for monitoring of blood glucose if >11mmol/L For Adults
METABOLIC BONE DISEASE OF PREMATURITY NEONATAL CLINICAL GUIDELINE V3.0
 METABOLIC BONE DISEASE OF PREMATURITY NEONATAL CLINICAL GUIDELINE V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline To provide guidance on the prevention of metabolic bone disease in the neonate. All
METABOLIC BONE DISEASE OF PREMATURITY NEONATAL CLINICAL GUIDELINE V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline To provide guidance on the prevention of metabolic bone disease in the neonate. All
Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol
 Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol 1. Aim/Purpose of this Guideline 1.1. To Provide safe and efficient administration of Opioids in Recovery.
Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol 1. Aim/Purpose of this Guideline 1.1. To Provide safe and efficient administration of Opioids in Recovery.
CLINICAL GUIDELINE FOR THE MANAGEMENT OF BARRETT S OESOPHAGUS Summary.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF BARRETT S OESOPHAGUS Summary. Page 1 of 12 Recurrent LGD at any point- discuss at MDT And consider RFA Patients with LGD should have a repeat endoscopy in 6 months.
CLINICAL GUIDELINE FOR THE MANAGEMENT OF BARRETT S OESOPHAGUS Summary. Page 1 of 12 Recurrent LGD at any point- discuss at MDT And consider RFA Patients with LGD should have a repeat endoscopy in 6 months.
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF NEBULISED PENTAMIDINE Summary. 1.
 CLINICAL GUIDELINE FOR THE ADMINISTRATION OF NEBULISED PENTAMIDINE Summary. 1. Patient requires nebulised Pentamidine Ensure equipment listed is available Ensure HEPA filtered room in Haematology Clinic
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF NEBULISED PENTAMIDINE Summary. 1. Patient requires nebulised Pentamidine Ensure equipment listed is available Ensure HEPA filtered room in Haematology Clinic
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline
 SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF MESNA WITH IFOSFAMIDE AND CYCLOPHOSPHAMIDE Summary.
 CLINICAL GUIDELINE FOR THE ADMINISTRATION OF MESNA WITH IFOSFAMIDE AND CYCLOPHOSPHAMIDE Summary. Yes Is patient prescribed ifosfamide or cyclophosphamide >1g/m 2? Chemotherapy prescription on Aria should
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF MESNA WITH IFOSFAMIDE AND CYCLOPHOSPHAMIDE Summary. Yes Is patient prescribed ifosfamide or cyclophosphamide >1g/m 2? Chemotherapy prescription on Aria should
PRESEPTAL AND ORBITAL CELLULITIS IN CHILDREN- CLINICAL GUIDELINE V3.0
 PRESEPTAL AND ORBITAL CELLULITIS IN CHILDREN- CLINICAL GUIDELINE V3.0 Page 1 of 8 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff caring for a child with Preseptal
PRESEPTAL AND ORBITAL CELLULITIS IN CHILDREN- CLINICAL GUIDELINE V3.0 Page 1 of 8 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff caring for a child with Preseptal
28 th September Author Jeremy Gilbert Bariatric Nurse Specialist
 POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
HYPOSPADIAS NEONATAL CLINICAL GUIDELINE. 1. Aim/Purpose of this Guideline. 2. The Guidance
 HYPOSPADIAS NEONATAL CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all staff managing the initial care of infants born with hypospadias. It includes assessment and
HYPOSPADIAS NEONATAL CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all staff managing the initial care of infants born with hypospadias. It includes assessment and
Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia or Intravenous Sedation Clinical Guideline V5.0
 Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia or Intravenous Sedation November 2018 Summary. Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia
Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia or Intravenous Sedation November 2018 Summary. Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia
CLINICAL GUIDELINE FOR MANAGEMENT OF NEUTROPENIC SEPSIS IN ADULT CANCER PATIENTS (this guideline excludes haematology patients)
 CLINICAL GUIDELINE FOR MANAGEMENT OF NEUTROPENIC SEPSIS IN ADULT CANCER PATIENTS (this guideline excludes haematology patients) 1. Aim/Purpose of this Guideline 1.1. Systemic cancer treatments and immunological
CLINICAL GUIDELINE FOR MANAGEMENT OF NEUTROPENIC SEPSIS IN ADULT CANCER PATIENTS (this guideline excludes haematology patients) 1. Aim/Purpose of this Guideline 1.1. Systemic cancer treatments and immunological
CLINICAL GUIDELINE FOR THE MANAGEMENT OF ANAPHYLAXIS IN INFANTS AND CHILDREN UNDER SIXTEEN YEARS OF AGE V3.0
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF ANAPHYLAIS IN INFANTS AND CHILDREN UNDER SITEEN YEARS OF AGE V3.0 Page 1 of 16 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide
CLINICAL GUIDELINE FOR THE MANAGEMENT OF ANAPHYLAIS IN INFANTS AND CHILDREN UNDER SITEEN YEARS OF AGE V3.0 Page 1 of 16 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide
School Hearing Screening Policy
 School Hearing Screening Policy V2.1 1st August 2017 Page 1 of 13 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership and Responsibilities...
School Hearing Screening Policy V2.1 1st August 2017 Page 1 of 13 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership and Responsibilities...
SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN
 SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the
SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOKALAEMIA
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of acamprosate.
 SHARED CARE GUIDELINE FOR ACAMPROSATE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of
SHARED CARE GUIDELINE FOR ACAMPROSATE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of
Start. What is the serum phosphate concentration? Moderate Hypophosphataemia mmol/l. Replace using oral. phosphate. (See section 3.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOPHOSPHATAEMIA IN ADULTS Summary. Key: General Notes GP/SWASFT ED/MAU/SRU/Acute GP/Amb-Care In-patient wards Start What is the serum concentration? Mild Hypophosphataemia
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOPHOSPHATAEMIA IN ADULTS Summary. Key: General Notes GP/SWASFT ED/MAU/SRU/Acute GP/Amb-Care In-patient wards Start What is the serum concentration? Mild Hypophosphataemia
MANAGEMENT OF THE BLADDER IN THE POSTOPERATIVE PERIOD FOLLOWING UNCOMPLICATED GYNAECOLOGICAL SURGERY CLINICAL GUIDELINES
 MANAGEMENT OF THE BLADDER IN THE POSTOPERATIVE PERIOD FOLLOWING UNCOMPLICATED GYNAECOLOGICAL SURGERY CLINICAL GUIDELINES 1. Aim/Purpose of this Guideline All clinical staff working in the Division of women,
MANAGEMENT OF THE BLADDER IN THE POSTOPERATIVE PERIOD FOLLOWING UNCOMPLICATED GYNAECOLOGICAL SURGERY CLINICAL GUIDELINES 1. Aim/Purpose of this Guideline All clinical staff working in the Division of women,
Captopril and Enalapril (Ace Inhibitor) Therapy Clinical Guideline V1.0
 Captopril and Enalapril (Ace Inhibitor) Therapy Clinical Guideline V1.0 November 2018 Summary Prescribing, monitoring and administration of Captopril and Enalapril FOR STAFF PATIENTS Medical and Nursing
Captopril and Enalapril (Ace Inhibitor) Therapy Clinical Guideline V1.0 November 2018 Summary Prescribing, monitoring and administration of Captopril and Enalapril FOR STAFF PATIENTS Medical and Nursing
CLINICAL GUIDELINE FOR MANAGEMENT OF GALLSTONES PATHOLOGY IN ADULTS
 CLINICAL GUIDELINE FOR MANAGEMENT OF GALLSTONES PATHOLOGY IN ADULTS 1. Aim/Purpose of this Guideline This guideline is for the management of gallstones pathology in adults. It has been benchmarked against
CLINICAL GUIDELINE FOR MANAGEMENT OF GALLSTONES PATHOLOGY IN ADULTS 1. Aim/Purpose of this Guideline This guideline is for the management of gallstones pathology in adults. It has been benchmarked against
CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline 1.1. This guideline contains recommendations about general principles for managing intravenous (IV)
CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline 1.1. This guideline contains recommendations about general principles for managing intravenous (IV)
CLINICAL GUIDELINE FOR NEONATAL BCG VACCINATION V3.0
 CLINICAL GUIDELINE FOR NEONATAL BCG VACCINATION V3.0 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. Tuberculosis is a notifiable disease in the UK and although incidence is low it remains a Public
CLINICAL GUIDELINE FOR NEONATAL BCG VACCINATION V3.0 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. Tuberculosis is a notifiable disease in the UK and although incidence is low it remains a Public
MANAGEMENT OF NEONATAL HYPOTENSION CLINICAL GUIDELINE
 MANAGEMENT OF NEONATAL HYPOTENSION CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. To provide guidance on the assessment and management of infants with hypotension. All involved will benefit from
MANAGEMENT OF NEONATAL HYPOTENSION CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. To provide guidance on the assessment and management of infants with hypotension. All involved will benefit from
GESTATIONAL DIABETES MELLITUS AND SUBSEQUENT MANAGEMENT OF CONFIRMED GESTATIONAL DIABETES MELLITUS (GDM) AND SELECTIVE SCREENING - CLINICAL GUIDELINE
 GESTATIONAL DIABETES MELLITUS AND SUBSEQUENT MANAGEMENT OF CONFIRMED GESTATIONAL DIABETES MELLITUS (GDM) AND SELECTIVE SCREENING - CLINICAL GUIDELINE V 1.5 2017 Screening - Clinical Guideline Page 1 of
GESTATIONAL DIABETES MELLITUS AND SUBSEQUENT MANAGEMENT OF CONFIRMED GESTATIONAL DIABETES MELLITUS (GDM) AND SELECTIVE SCREENING - CLINICAL GUIDELINE V 1.5 2017 Screening - Clinical Guideline Page 1 of
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HIGH BLOOD GLUCOSE LEVELS AND SICK DAYS FOR INSULIN PUMP USERS UNDER THE PAEDIATRIC DIABETES SERVICE. V4.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HIGH BLOOD GLUCOSE LEVELS AND SICK DAYS FOR INSULIN PUMP USERS UNDER THE PAEDIATRIC DIABETES SERVICE. V4.1 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. The
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HIGH BLOOD GLUCOSE LEVELS AND SICK DAYS FOR INSULIN PUMP USERS UNDER THE PAEDIATRIC DIABETES SERVICE. V4.1 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. The
Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018
 Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018 Page 1 of 12 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled
Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018 Page 1 of 12 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled
Clinical guideline for the introduction of Sacubitril Valsartan in primary and secondary care in Cornwall
 Clinical guideline for the introduction of Sacubitril Valsartan in primary and secondary care in Cornwall Page 1 of 15 Summary Patient clinically assessed and reviewed by a Consultant Cardiologist, Cardiology
Clinical guideline for the introduction of Sacubitril Valsartan in primary and secondary care in Cornwall Page 1 of 15 Summary Patient clinically assessed and reviewed by a Consultant Cardiologist, Cardiology
Hypoglycaemia in Adults with Diabetes Clinical Guideline V5.0. March 2018
 March 2018 Page 1 of 11 Adult with Diabetes and blood glucose < 4 mmol/l Treatment as per Guideline: Nursing / Clinical Staff Patient reviewed as per guideline guidance notes: Nursing / Clinical Staff
March 2018 Page 1 of 11 Adult with Diabetes and blood glucose < 4 mmol/l Treatment as per Guideline: Nursing / Clinical Staff Patient reviewed as per guideline guidance notes: Nursing / Clinical Staff
SHARED CARE GUIDELINE FOR RIFAXIMIN FOR PREVENTING EPISODES OF OVERT HEPATIC ENCEPHALOPATHY IN ADULT PATIENTS 1. Aim/Purpose of this Guideline
 SHARED CARE GUIDELINE FOR RIFAXIMIN FOR PREVENTING EPISODES OF OVERT HEPATIC ENCEPHALOPATHY IN ADULT PATIENTS 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy
SHARED CARE GUIDELINE FOR RIFAXIMIN FOR PREVENTING EPISODES OF OVERT HEPATIC ENCEPHALOPATHY IN ADULT PATIENTS 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy
SHARED CARE GUIDELINE FOR MODAFINIL 1. Aim/Purpose of this Guideline. 2. The Guidance
 SHARED CARE GUIDELINE FOR MODAFINIL 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of modafinil.
SHARED CARE GUIDELINE FOR MODAFINIL 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of modafinil.
Prevention and Treatment of Mucositis in Children and Young People with Cancer Clinical Guideline V3.1 December 2018
 Prevention and Treatment of Mucositis in Children and Young People with Cancer Clinical Guideline V3.1 December 2018 1. Aim/Purpose of this Guideline This guideline applies to all medical and nursing staff
Prevention and Treatment of Mucositis in Children and Young People with Cancer Clinical Guideline V3.1 December 2018 1. Aim/Purpose of this Guideline This guideline applies to all medical and nursing staff
CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled Analgesia
CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled Analgesia
CLINICAL GUIDELINE FOR THE MANAGEMENT OF CONVULSIVE STATUS EPILEPTICUS IN CHILDHOOD V3.0
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF CONVULSIVE STATUS EPILEPTICUS IN CHILDHOOD V3.0 Clinical Guideline Template Page 1 of 14 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all nursing
CLINICAL GUIDELINE FOR THE MANAGEMENT OF CONVULSIVE STATUS EPILEPTICUS IN CHILDHOOD V3.0 Clinical Guideline Template Page 1 of 14 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all nursing
CLINICAL GUIDELINE FOR THE EVALUATION OF A CHILD PRESENTING WITH FEVER AND SEIZURE V3.0
 CLINICAL GUIDELINE FOR THE EVALUATION OF A CHILD PRESENTING WITH FEVER AND SEIZURE V3.0 Clinical Guideline Template Page 1 of 18 Page 1 of 13 1. Aim/Purpose of this Guideline 1.1. This guideline applies
CLINICAL GUIDELINE FOR THE EVALUATION OF A CHILD PRESENTING WITH FEVER AND SEIZURE V3.0 Clinical Guideline Template Page 1 of 18 Page 1 of 13 1. Aim/Purpose of this Guideline 1.1. This guideline applies
Suspected Pulmonary embolus Ambulatory Pathway. Document Title. Date Issued/Approved: Date Valid From: 11/11/17. Date Valid To: 11/05/18
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
MUCOSITIS IN CHILDREN AND YOUNG PEOPLE WITH CANCER- CLINICAL GUIDELINE FOR PREVENTION AND TREATMENT V3.0
 MUCOSITIS IN CHILDREN AND YOUNG PEOPLE WITH CANCER- CLINICAL GUIDELINE FOR PREVENTION AND TREATMENT V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all medical and nursing
MUCOSITIS IN CHILDREN AND YOUNG PEOPLE WITH CANCER- CLINICAL GUIDELINE FOR PREVENTION AND TREATMENT V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all medical and nursing
CLINICAL PROCEDURE FOR THE SAFE REMOVAL OF FEMORAL ARTERIAL SHEATHS USING A DIGITAL APPROACH 1. Aim/Purpose of this Guideline
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
CLINICAL GUIDELINE FOR THE MANAGEMENT OF VIRAL LARYNGO-TRACHEOBRONCHITIS (CROUP) V3.0
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF VIRAL LARYNGO-TRACHEOBRONCHITIS (CROUP) V3.0 Clinical Guideline Template Page 1 of 13 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all nursing
CLINICAL GUIDELINE FOR THE MANAGEMENT OF VIRAL LARYNGO-TRACHEOBRONCHITIS (CROUP) V3.0 Clinical Guideline Template Page 1 of 13 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all nursing
CLINICAL GUIDELINE FOR THE USE OF PHENYTOIN IN EPILEPSY
 This applies to adult patients only CLINICAL GUIDELINE FOR THE USE OF PHENYTOIN IN EPILEPSY Key: General Notes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Yes Patient already taking phenytoin?
This applies to adult patients only CLINICAL GUIDELINE FOR THE USE OF PHENYTOIN IN EPILEPSY Key: General Notes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Yes Patient already taking phenytoin?
DIABETES IN PREGNANCY, TYPE 1 AND TYPE 2 DIABETES MELLITUS (DM), CLINICAL GUIDELINE FOR MIDWIVES V1.4
 DIABETES IN PREGNANCY, TYPE 1 AND TYPE 2 DIABETES MELLITUS (DM), CLINICAL GUIDELINE FOR MIDWIVES V1.4 1 1. Aim/Purpose of this Guideline To provide guidance to Midwives, Obstetricians and the Joint Obstetric/Diabetes
DIABETES IN PREGNANCY, TYPE 1 AND TYPE 2 DIABETES MELLITUS (DM), CLINICAL GUIDELINE FOR MIDWIVES V1.4 1 1. Aim/Purpose of this Guideline To provide guidance to Midwives, Obstetricians and the Joint Obstetric/Diabetes
CLINICAL GUIDELINE FOR AN EPIDURAL INFUSION IN CHILD HEALTH 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR AN EPIDURAL INFUSION IN CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide guidance on caring for children who are receiving epidural
CLINICAL GUIDELINE FOR AN EPIDURAL INFUSION IN CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide guidance on caring for children who are receiving epidural
CLINICAL GUIDELINE FOR MANAGEMENT OF ACUTE CHOLECYSTITIS IN ADULTS
 CLINICAL GUIDELINE FOR MANAGEMENT OF ACUTE CHOLECYSTITIS IN ADULTS 1. Aim/Purpose of this Guideline This guideline is for the management of acute cholecystitis in adults. It has been benchmarked against
CLINICAL GUIDELINE FOR MANAGEMENT OF ACUTE CHOLECYSTITIS IN ADULTS 1. Aim/Purpose of this Guideline This guideline is for the management of acute cholecystitis in adults. It has been benchmarked against
The Management of Children and Young People with Newly Presenting Diabetes Clinical Guideline V5.0 December 2018
 The Management of Children and Young People with Newly Presenting Diabetes Clinical Guideline December 2018 Summary GP suspects diabetes Under 16 years or still in School Year 11 NO Refer Adults YES Section
The Management of Children and Young People with Newly Presenting Diabetes Clinical Guideline December 2018 Summary GP suspects diabetes Under 16 years or still in School Year 11 NO Refer Adults YES Section
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPONATRAEMIA Summary. Start. End. Key: Na + below normal range ( mmol/L) Symptomatic?
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPONATRAEMIA Summary Key: General tes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Na + below normal range (135 145mmol/L) Refer to endocrinology
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPONATRAEMIA Summary Key: General tes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Na + below normal range (135 145mmol/L) Refer to endocrinology
Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0
 Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0 11 October 2017 Table of Contents 1. Introduction.. 3 2. Purpose of this Policy/Procedure. 3 3. Scope
Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0 11 October 2017 Table of Contents 1. Introduction.. 3 2. Purpose of this Policy/Procedure. 3 3. Scope
Pelvic Inflammatory Disease Clinical Guideline V1.0 February 2019
 Pelvic Inflammatory Disease Clinical Guideline V1.0 February 2019 Summary This guideline will provide evidence based guidance on the management of Pelvic Inflammatory Disease A low threshold for empirical
Pelvic Inflammatory Disease Clinical Guideline V1.0 February 2019 Summary This guideline will provide evidence based guidance on the management of Pelvic Inflammatory Disease A low threshold for empirical
Admission of a Child/Young Person with Cystic Fibrosis Clinical Guideline V3.0 May 2018
 Admission of a Child/Young Person with Cystic Fibrosis Clinical Guideline V3.0 May 2018 Page 1 of 16 1. Aim/Purpose of this Guideline This guideline applies to all staff caring for children/young people
Admission of a Child/Young Person with Cystic Fibrosis Clinical Guideline V3.0 May 2018 Page 1 of 16 1. Aim/Purpose of this Guideline This guideline applies to all staff caring for children/young people
Osteoblasts (cells which form new bone) Osteoclasts (cells which break down old bone)
 Clinical guideline for the administration of Bisphosphonates and other drugs affecting bone metabolism in Haematology and Oncology patients 1. Aim/Purpose of this Guideline 1.1. To provide education to
Clinical guideline for the administration of Bisphosphonates and other drugs affecting bone metabolism in Haematology and Oncology patients 1. Aim/Purpose of this Guideline 1.1. To provide education to
SHARED CARE GUIDELINE FOR TREATMENT OF DEMENTIA 1. Aim/Purpose of this Guideline
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
Blood Transfusion Policy for Children and Neonates. November 2017
 Blood Transfusion Policy for Children and Neonates V5 November 2017 1. Introduction 3 2. Purpose of this Policy/Procedure 3 3. Scope 3 4. Ownership and Responsibilities 3 4.1. Role of the Managers 3 4.2.
Blood Transfusion Policy for Children and Neonates V5 November 2017 1. Introduction 3 2. Purpose of this Policy/Procedure 3 3. Scope 3 4. Ownership and Responsibilities 3 4.1. Role of the Managers 3 4.2.
SHARED CARE GUIDELINE FOR MYCOPHENOLATE MOFETIL FOR RHEUMATOLOGY INDICATIONS 1. Aim/Purpose of this Guideline
 SHARED CARE GUIDELINE FOR MYCOPHENOLATE MOFETIL FOR RHEUMATOLOGY INDICATIONS 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate
SHARED CARE GUIDELINE FOR MYCOPHENOLATE MOFETIL FOR RHEUMATOLOGY INDICATIONS 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate
Spirometry Clinical Guideline V2.0. May 2018
 Spirometry Clinical Guideline V2.0 May 2018 Spirometry Clinical Guideline V2.0 1 Summary REFERRAL FOR SPIROMETRY QUALITY ASSURED SPIROMETRY PERFORMED BY TECHNICIAN TRAINED AND ASSESSED AS COMPETENT SPIROMETRY
Spirometry Clinical Guideline V2.0 May 2018 Spirometry Clinical Guideline V2.0 1 Summary REFERRAL FOR SPIROMETRY QUALITY ASSURED SPIROMETRY PERFORMED BY TECHNICIAN TRAINED AND ASSESSED AS COMPETENT SPIROMETRY
Clinical Guideline for the Management of Pot Operative Atrial Fibrillation
 Clinical Guideline for the Management of Pot Operative Atrial Fibrillation 1. Aim/Purpose of this Guideline 1.1. Atrial Fibrillation is the most common cardiac arrhythmia with a prevalence of around 0.5%
Clinical Guideline for the Management of Pot Operative Atrial Fibrillation 1. Aim/Purpose of this Guideline 1.1. Atrial Fibrillation is the most common cardiac arrhythmia with a prevalence of around 0.5%
MANAGEMENT OF DIABETES MELLITUS (DM) IN PREGNANCY TYPE 1, TYPE 2 AND GESTATIONAL DIABETES (GDM),CLINICAL GUIDELINE. V1.0
 MANAGEMENT OF DIABETES MELLITUS (DM) IN PREGNANCY TYPE 1, TYPE 2 AND GESTATIONAL DIABETES (GDM),CLINICAL GUIDELINE. V1.0 1 1. Aim/Purpose of this Guideline To provide guidance to Midwives, Obstetricians
MANAGEMENT OF DIABETES MELLITUS (DM) IN PREGNANCY TYPE 1, TYPE 2 AND GESTATIONAL DIABETES (GDM),CLINICAL GUIDELINE. V1.0 1 1. Aim/Purpose of this Guideline To provide guidance to Midwives, Obstetricians
CLINICAL GUIDELINE FOR THE MANAGEMENT OF IMMUNE THROMBOCYTOPENIA (ITP) IN CHILDREN V3.0
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF IMMUNE THROMBOCYTOPENIA (ITP) IN CHILDREN V3.0 Clinical Guideline Template Page 1 of 16 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical
CLINICAL GUIDELINE FOR THE MANAGEMENT OF IMMUNE THROMBOCYTOPENIA (ITP) IN CHILDREN V3.0 Clinical Guideline Template Page 1 of 16 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical
Melatonin shared care guideline. Document Title. Corporate: Clinical. Type of document. Brief summary of contents
 Document Title Melatonin shared care guideline Type of document Corporate: Clinical Brief summary of contents Executive Director responsible for Policy: Directorate / Department responsible (author/owner):
Document Title Melatonin shared care guideline Type of document Corporate: Clinical Brief summary of contents Executive Director responsible for Policy: Directorate / Department responsible (author/owner):
CLINICAL GUIDELINE FOR THE MANAGEMENT OF INPATIENTS WITH PARKINSON S DISEASE
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF INPATIENTS WITH PARKINSON S DISEASE 1. Aim/Purpose of this Guideline To assist all doctors and nurses in the care of inpatients with Parkinson s disease. This guideline
CLINICAL GUIDELINE FOR THE MANAGEMENT OF INPATIENTS WITH PARKINSON S DISEASE 1. Aim/Purpose of this Guideline To assist all doctors and nurses in the care of inpatients with Parkinson s disease. This guideline
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Pre-filled Patient Controlled Analgesia (PCA) syringes
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
SHARED CARE GUIDELINE FOR LITHIUM. 1. Aim/Purpose of this Guideline. 2. The Guidance
 SHARED CARE GUIDELINE FOR LITHIUM 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of lithium.
SHARED CARE GUIDELINE FOR LITHIUM 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of lithium.
AROMATHERAPY - CLINICAL GUIDELINE FOR MIDWIVES. January 2018
 AROMATHERAPY - CLINICAL GUIDELINE FOR MIDWIVES V2 January 2018 1. Aim/Purpose of this Guideline 1.1. Aromatherapy is the use of essential oils, which can be administered by topical application via massage,
AROMATHERAPY - CLINICAL GUIDELINE FOR MIDWIVES V2 January 2018 1. Aim/Purpose of this Guideline 1.1. Aromatherapy is the use of essential oils, which can be administered by topical application via massage,
CLINICAL GUIDELINE FOR ANTIEMETIC USE IN PAEDIATRIC ONCOLOGY 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR ANTIEMETIC USE IN PAEDIATRIC ONCOLOGY 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff working with paediatric oncology patients. 2. The
CLINICAL GUIDELINE FOR ANTIEMETIC USE IN PAEDIATRIC ONCOLOGY 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff working with paediatric oncology patients. 2. The
Clinical Guideline for the management of paediatric patients with Diabetes Type 1 & 2 requiring Surgery or General Anaesthetic. V4
 Clinical Guideline for the management of paediatric patients with Diabetes Type 1 & 2 requiring Surgery or General Anaesthetic. V4 Page 1 of 27 Abbreviation Meaning PDSN DKA BG SC IV RCHT HDU PEWS Paediatric
Clinical Guideline for the management of paediatric patients with Diabetes Type 1 & 2 requiring Surgery or General Anaesthetic. V4 Page 1 of 27 Abbreviation Meaning PDSN DKA BG SC IV RCHT HDU PEWS Paediatric
Acutely Painful testes
 2.0 FINAL Guideline adopted from the Bedside Clinical Guideline Partnership EQUALITY IMPACT The Trust strives to ensure equality of opportunity for all both as a major employer and as a provider of health
2.0 FINAL Guideline adopted from the Bedside Clinical Guideline Partnership EQUALITY IMPACT The Trust strives to ensure equality of opportunity for all both as a major employer and as a provider of health
SHARED CARE GUIDELINE FOR CICLOSPORIN IN DERMATOLOGY. 1. Aim/Purpose of this Guideline. 2. The Guidance
 SHARED CARE GUIDELINE FOR CICLOSPORIN IN DERMATOLOGY 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration
SHARED CARE GUIDELINE FOR CICLOSPORIN IN DERMATOLOGY 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration
Small bowel atresia. Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families
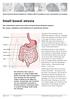 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Small bowel atresia This information sheet from Great Ormond Street Hospital explains the causes, symptoms and treatment
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Small bowel atresia This information sheet from Great Ormond Street Hospital explains the causes, symptoms and treatment
Title Use of Pre-Operative Tests for Elective Surgery Guideline. Department. Anaesthetics. Comment / Changes / Approval
 Document Control Title Use of Pre-Operative Tests for Elective Surgery Guideline Author Consultant Anaesthetist Author s job title Consultant Anaesthetist Directorate Clinical Support Services Department
Document Control Title Use of Pre-Operative Tests for Elective Surgery Guideline Author Consultant Anaesthetist Author s job title Consultant Anaesthetist Directorate Clinical Support Services Department
PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE. Chief Pharmacist. Chief Pharmacist
 REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
ASSESSMENT OF NEONATAL HYPOTONIA CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline
 ASSESSMENT OF NEONATAL HYPOTONIA CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. To provide guidance on the management of hypotonic infants. All involved will benefit from the improvement in service
ASSESSMENT OF NEONATAL HYPOTONIA CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. To provide guidance on the management of hypotonic infants. All involved will benefit from the improvement in service
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Religion, Belief and Cultural Practice Policy Meeting the needs of Patients and Carers
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Religion, Belief and Cultural Practice Policy Meeting the needs of Patients and Carers Version No.: 3.2 Effective From: 13 March 2018 Expiry Date:
The Newcastle upon Tyne Hospitals NHS Foundation Trust Religion, Belief and Cultural Practice Policy Meeting the needs of Patients and Carers Version No.: 3.2 Effective From: 13 March 2018 Expiry Date:
Index No: MMG11/1. Version: 1. Date ratified: 12 th November 2013
 Index No: Intravenous fluid prescription in children For previously well children aged one month to 16 years (excluding renal, cardiac, diabetic ketoacidosis and acute burns patients) Version: 1 Date ratified:
Index No: Intravenous fluid prescription in children For previously well children aged one month to 16 years (excluding renal, cardiac, diabetic ketoacidosis and acute burns patients) Version: 1 Date ratified:
SWISS SOCIETY OF NEONATOLOGY. Hypertrophic pyloric stenosis in a preterm infant
 SWISS SOCIETY OF NEONATOLOGY Hypertrophic pyloric stenosis in a preterm infant February 2014 2 Georgi R, Berger M, Schnyder I, Berger S, McDougall J, Division of Neonatology (GR, BM, McDJ), Department
SWISS SOCIETY OF NEONATOLOGY Hypertrophic pyloric stenosis in a preterm infant February 2014 2 Georgi R, Berger M, Schnyder I, Berger S, McDougall J, Division of Neonatology (GR, BM, McDJ), Department
FOR CHILDREN ATTENDING FOR EXODONTIA UNDER GENERAL ANAESTHETIC
 CLINICAL PROTOCOL FOR CHILDREN ATTENDING FOR EXODONTIA UNDER GENERAL ANAESTHETIC CLINICAL RECORDS AND PATIENT INFORMATION RATIONALE The purpose of this clinical protocol is to ensure that the clinical
CLINICAL PROTOCOL FOR CHILDREN ATTENDING FOR EXODONTIA UNDER GENERAL ANAESTHETIC CLINICAL RECORDS AND PATIENT INFORMATION RATIONALE The purpose of this clinical protocol is to ensure that the clinical
Trust Policy 218 Ionising Radiation Safety Policy
 Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
NO SMOKING POLICY. Organisational
 NO SMOKING POLICY Policy Title State previous title where relevant. State if Policy New or Revised Policy Strand Org, HR, Clinical, H&S, Infection Control, Finance For clinical policies only - state index
NO SMOKING POLICY Policy Title State previous title where relevant. State if Policy New or Revised Policy Strand Org, HR, Clinical, H&S, Infection Control, Finance For clinical policies only - state index
Patient Group Directions Policy
 Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Specialised Services Commissioning Policy. CP29: Bariatric Surgery
 Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN)
 Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN) Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of
Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN) Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of
Patients undergoing Bariatric surgery with type 2 diabetes on antidiabetic drugs WITHOUT insulin, when starting liver shrinking diet: Guidance for GPs
 Patients undergoing Bariatric surgery with type 2 diabetes on antidiabetic drugs WITHOUT insulin, when starting liver shrinking diet: Guidance for GPs Subject: Policy Number Ratified By: Patients with
Patients undergoing Bariatric surgery with type 2 diabetes on antidiabetic drugs WITHOUT insulin, when starting liver shrinking diet: Guidance for GPs Subject: Policy Number Ratified By: Patients with
Right Iliac Fossa Pain
 Princess Margaret Hospital for Children PAEDIATRIC ACUTE CARE GUIDELINE Right Iliac Fossa Pain Scope (Staff): Scope (Area): All Emergency Department Clinicians Emergency Department This document should
Princess Margaret Hospital for Children PAEDIATRIC ACUTE CARE GUIDELINE Right Iliac Fossa Pain Scope (Staff): Scope (Area): All Emergency Department Clinicians Emergency Department This document should
Acute Alcohol Withdrawal Protocol
 Acute Alcohol Withdrawal Protocol Controlled document This document is uncontrolled when downloaded or printed Reference number Version 1 Author WHHT: C268 Dr Mohamed Shariff Date ratified August 2014
Acute Alcohol Withdrawal Protocol Controlled document This document is uncontrolled when downloaded or printed Reference number Version 1 Author WHHT: C268 Dr Mohamed Shariff Date ratified August 2014
Specialised Services Policy:
 Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
31 December a programme of dates of meetings for Full Council, Policy Page: 241 Committees, and Area Committees for the twelve months commencing 1 May
 Page: 240 Business Services REPORT TO ABERDEENSHIRE COUNCIL 28 SEPTEMBER, 2017 TIMETABLE OF MEETINGS 2018/19 1 Recommendation 1.1 The Council is recommended to approve the timetable of Council, Policy
Page: 240 Business Services REPORT TO ABERDEENSHIRE COUNCIL 28 SEPTEMBER, 2017 TIMETABLE OF MEETINGS 2018/19 1 Recommendation 1.1 The Council is recommended to approve the timetable of Council, Policy
Clinical Protocol Documentation for Patients Attending for Exodontia under General Anaesthetic Clinical Records and Patient Information
 Clinical Protocol Documentation for Patients Attending for Exodontia under General Anaesthetic Clinical Records and Patient Information CP:92 [Version:1] Applies to:- Specialist Dental Services Sub Committee
Clinical Protocol Documentation for Patients Attending for Exodontia under General Anaesthetic Clinical Records and Patient Information CP:92 [Version:1] Applies to:- Specialist Dental Services Sub Committee
Smoke Free Policy. Version 2.0
 Smoke Free Policy Version 2.0 Important: This document can only be considered valid when viewed on the CCG s internet site. If this document has been printed or saved to another location, you must check
Smoke Free Policy Version 2.0 Important: This document can only be considered valid when viewed on the CCG s internet site. If this document has been printed or saved to another location, you must check
Insert heading depending line on length; please delete delete. length; please delete other cover options once
 Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Specialised Services Commissioning Policy: CP34 Circumcision for children
 Specialised Services Commissioning Policy: CP34 Circumcision for children March 2019 Version 3.0 Document information Document purpose Document name Author Policy Circumcision for Children Welsh Health
Specialised Services Commissioning Policy: CP34 Circumcision for children March 2019 Version 3.0 Document information Document purpose Document name Author Policy Circumcision for Children Welsh Health
Specialised Services Policy Position PP104
 Specialised Services Policy Position PP104 Personalised External Aortic Root Support (PEARS) for surgical management of enlarged aortic root (adults) March 2019 Version 1.0 Document information Document
Specialised Services Policy Position PP104 Personalised External Aortic Root Support (PEARS) for surgical management of enlarged aortic root (adults) March 2019 Version 1.0 Document information Document
Commissioning Policy. Treatment of Snoring. April 2010
 Commissioning Policy Treatment of Snoring April 2010 This commissioning policy applies to patients within: South Worcestershire Clinical Commissioning Group (CCG) Redditch & Bromsgrove Clinical Commissioning
Commissioning Policy Treatment of Snoring April 2010 This commissioning policy applies to patients within: South Worcestershire Clinical Commissioning Group (CCG) Redditch & Bromsgrove Clinical Commissioning
Adult Chest Wall Injury Pathway V2.0 May 2018
 ` Adult Chest Wall Injury Pathway V2.0 May 2018 Page 1 of 22 Summary Adult Chest Wall Injury Pathway Blunt chest wall injury requiring admission. Trauma Call if appropriate. Strongly consider Trauma CT.
` Adult Chest Wall Injury Pathway V2.0 May 2018 Page 1 of 22 Summary Adult Chest Wall Injury Pathway Blunt chest wall injury requiring admission. Trauma Call if appropriate. Strongly consider Trauma CT.
Tony Gray Head of Safety and Security. Tony Gray Head of Safety and Security. Contents Section Description Page No.
 Health and Safety Policy Practice Guidance Note Latex Sensitivity V04 Date Issued Planned Review Issue 1 Oct 17 Oct 2020 HS-PGN-10 Part of NTW(O)20 Health and Safety Policy Author/Designation Tony Gray
Health and Safety Policy Practice Guidance Note Latex Sensitivity V04 Date Issued Planned Review Issue 1 Oct 17 Oct 2020 HS-PGN-10 Part of NTW(O)20 Health and Safety Policy Author/Designation Tony Gray
Wessex Care Pathway for Term Infants Referred with Bilious Vomiting for Exclusion of Malrotation
 Wessex Care Pathway for Term Infants Referred with Bilious Vomiting for Exclusion of Malrotation Version: 1.3 Issued: Review date: Author: Melanie Drewett The procedural aspects of this guideline can be
Wessex Care Pathway for Term Infants Referred with Bilious Vomiting for Exclusion of Malrotation Version: 1.3 Issued: Review date: Author: Melanie Drewett The procedural aspects of this guideline can be
LOCAL EQUALITY ADVISORY FORUM (LEAF) A Staffordshire CCGs Equality & Inclusion Group. Terms of Reference
 LOCAL EQUALITY ADVISORY FORUM (LEAF) A Staffordshire CCGs Equality & Inclusion Group Terms of Reference (1) Introduction and Purpose Promoting equality and inclusion is at the heart of our values. We will
LOCAL EQUALITY ADVISORY FORUM (LEAF) A Staffordshire CCGs Equality & Inclusion Group Terms of Reference (1) Introduction and Purpose Promoting equality and inclusion is at the heart of our values. We will
Pyloric Stenosis Advice for Parents & Carers
 Pyloric Stenosis Advice for Parents & Carers Children s Services The aim of this leaflet is to provide you as carers or parents all the relevant information regarding Pyloric Stenosis and answer some common
Pyloric Stenosis Advice for Parents & Carers Children s Services The aim of this leaflet is to provide you as carers or parents all the relevant information regarding Pyloric Stenosis and answer some common
GUIDELINE FOR ENTERAL TUBE FEEDING (NASOGASTRIC OR PEG) IN PATIENTS WITH DIABETES MELLITUS TREATED WITH INSULIN
 GUIDELINE FOR ENTERAL TUBE FEEDING (NASOGASTRIC OR PEG) IN PATIENTS WITH DIABETES MELLITUS TREATED WITH INSULIN This guidance does not override the individual responsibility of health professionals to
GUIDELINE FOR ENTERAL TUBE FEEDING (NASOGASTRIC OR PEG) IN PATIENTS WITH DIABETES MELLITUS TREATED WITH INSULIN This guidance does not override the individual responsibility of health professionals to
Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital
 Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Medical management of Hypercyanotic spells in neonates, infants with Tetralogy of Fallot
 Medical management of Hypercyanotic spells in neonates, infants with Tetralogy of Fallot Version: 3 Approval Committee (e.g. Clinical network): Date of Approval: 13 June 2018 Signature of approving Group
Medical management of Hypercyanotic spells in neonates, infants with Tetralogy of Fallot Version: 3 Approval Committee (e.g. Clinical network): Date of Approval: 13 June 2018 Signature of approving Group
IT and Information Acceptable Use Policy
 BMI IMpol04 Information Management IT and Information Acceptable Use Policy This is a controlled document and whilst this document may be printed, the electronic version posted on the intranet/shared drive
BMI IMpol04 Information Management IT and Information Acceptable Use Policy This is a controlled document and whilst this document may be printed, the electronic version posted on the intranet/shared drive
Falls The Assessment, Prevention and Management of Patient Falls (Adult Services) 1.34
 SECTION: 1 PATIENT CARE Including Physical Healthcare POLICY /PROCEDURE: 1.34 NATURE AND SCOPE: SUBJECT (Title): POLICY AND PROCEDURE - TRUST WIDE FALLS: THE ASSESSMENT, PREVENTION AND MANAGEMENT OF PATIENT
SECTION: 1 PATIENT CARE Including Physical Healthcare POLICY /PROCEDURE: 1.34 NATURE AND SCOPE: SUBJECT (Title): POLICY AND PROCEDURE - TRUST WIDE FALLS: THE ASSESSMENT, PREVENTION AND MANAGEMENT OF PATIENT
