FibroTest/FibroMax Scientific Publications
|
|
|
- Rosaline Griselda Townsend
- 6 years ago
- Views:
Transcription
1 /FibroMax Scientific Publications Key Publications for 2018 EASL 2018 Metabolic Liver Diseases (MLD) NASH-Test V2 : a new quantitative test for the diagnosis of nonalcoholic steatohepatitis (NASH) Impact of steatosis and inflammation definitions on the performance of NASH tests. Poynard T, Munteanu M, Charlotte F, et al. FLIP consortium, the FibroFrance-CPAM group; and the FibroFrance-Obese group. Eur J Gastroenterol Hepatol. 2018;30: Authors aimed to construct a new noninvasive quantitative test for the diagnosis of NASH (NIT-NASH) using a simplified histological definition* that permits to identify more cases of NASH than the standard histological NASH-CRN definition (Eur J Gastroenterol Hepatol. 2018). A total of 1,081 metabolic liver disease (MLD) patients were included from the FibroFrance Project (USA-NCT ) and the FLIP European Consortium ( ). NIT-NASH does not include BMI and fasting glucose to avoid variability in obese and type2 diabetic (T2D) patients. The performances [AUROC (95%IC)] were high (0.77 and 0.81), in both training and control groups, respectively. NIT-NASH had higher performances than NAFLD fibrosis score, BARD, FIB-4 and ActiTest. Significant MLD (A2 or F2 as per NIT-NASH combination with ) was strongly associated with diabetes when applied in larger populations (US and French cohorts). In conclusion, the new NIT-NASH enables a quantitative assessment of NASH in subjects with MLD risk. Important fact, this new diagnosis of NASH (NIT-NASH) does not require BMI and glucose any longer. * The new definition of MLD does not require the presence of steatosis and the presence of both lobular inflammation and ballooning and enable the diagnostic of NASH in patients with steatosis less than 5%, and a grade 2 lobular inflammation without ballooning. (Poynard et al. Eur J Gastroenterol Hepatol. 2018) (see next abstract) New quantitative test for the diagnosis of nonalcoholic steatohepatitis (NASH) Diagnostic performance of a new noninvasive test for nonalcoholic steatohepatitis using a simplified histological reference. Poynard T, Munteanu M, Charlotte Fet al; for the FLIP Consortium, the FibroFrance-CPAM Group, the FibroFrance-Obese Group. Eur J Gastroenterol Hepatol doi: /MEG Authors proposed to improve the identification of NASH cases by using a SAF-simplifiedscore, which does not require the presence of steatosis and of both activity features as the standard definition does [NASH-CRN (Kleiner Hepatology 2005)]. The impact of definitions variability on the prevalence of NASH (evaluated by FibroMax) was studied for: 1/ less 5% steatosis for NASH; 2/ no steatosis requirement for NASH; 3/ severe NASH based on the SAF-activity grade at least A2. Patients were from the FibroFrance project (USA-NCT ) and from the FLIP consortium ( The present study confirmed that the variability in the estimated performance of NIT-NASHs is related to the diverse histological definitions of NASH. In conclusion, a simplified definition of NASH, based on activity and not requiring >5% steatosis, had the lowest risk of false-positives/false-negatives. POYNARD 2018 NASHTest v2 NASH-, Metabolic, Steatohepatitis POYNARD 2018 New definition NASHTest v2, NASH-, Metabolic, Steatohepatitis
2 and SteatoTest for the NAFLD screening in Type 2 Diabetes European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver disease: evaluation of their application in people with Type 2 diabetes. Sberna AL, Bouillet B, Rouland A, et al. Diabet Med. 2018;35: The study aimed to evaluate the application of the recently proposed recommendations by the European Associations EASL, EASD and EASO for the diagnosis, treatment and follow-up of non-alcoholic fatty liver disease (NAFLD) in n=179 type 2 diabetes (T2D) patients. According to the guidelines, the following non-invasive tests (NIT) were done for steatosis and fibrosis evaluation: SteatoTest and [FibroMax panel, (BioPredictive, France)], proton magnetic resonance spectroscopy (H-MRS), fatty liver index score (FLI) and nonalcoholic fatty liver disease fibrosis score (NFS). According to H-MRS, 68.7% of participants had steatosis (liver fat content >5.5%) and according to SteatoTest, 78.7% had important steatosis (score >0.57) with 70% concordant results with H-MRS. identified 2.2% T2D having clinically advanced fibrosis ( >0.57), but normal ALT, AST and GGT. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. Vilar-Gomez E, Chalasani N. J Hepatol. 2018;68: Based on the assumption that the diagnostic accuracy can be improved by combining biomarkers, the authors proposed an algorithm using non-invasive tests (NIT) that includes, to assess patients with NAFLD risk. The algorithm combines several prediction rules, free biomarkers (e.g. NAFLD fibrosis score, FIB-4 index and BARD score, Pro-C3 tests) and patented biomarkers (e.g., ELF). The algorithm discriminates between the high risk patients for fibrosis F3 or more ( >0.70) in whom to consider a liver biopsy and the low risk patients ( <0.30) to be monitored with repeated NIT every 2 years. Authors acknowledged that patented markers of fibrosis such as are more specific and have higher positive predictive values for detecting advanced fibrosis and adverse outcomes. Screening for NAFLD with FibroMax in chronic obstructive pulmonary disease (COPD) Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Viglino D, Jullian-Desayes I, Minoves M, Aron-Wisnewsky J, Leroy V, Zarski JP, Tamisier R, Joyeux- Faure M, Pépin JL. Eur Respir J. 2017;49. pii: The authors aimed to investigate the relationship between COPD severity and nonalcoholic fatty liver disease (NAFLD) using validated FibroMax panel. The prevalences of steatosis, NASH and fibrosis as per SteatoTest, NashTest and were 41.4%, 36.9% and 61.3%, respectively. In multivariate analysis, steatosis and fibrosis as per SteatoTest and, respectively, were significantly associated with gender, body mass index (BMI), untreated sleep apnoea and insulin resistance. Only BMI was a risk factor of NASH as per NashTest. The authors concluded that NAFLD is highly prevalent in COPD and biomarkers of liver damage may allow specific lesions to be detected and treated. SBERNA 2018, SteatoTest, NAFLD, T2D VILAR-GOMEZ 2018, NAFLD, NASH VIGLINO 2017 FibroMax SteatoTest NashTest COPD NAFLD
3 Screening general population for the screening of unknown liver diseases in the general population Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Harris R, Harman DJ, Card TR et al. Lancet Gastroenterol Hepatol 2017 Published Online S (16) Authors proposed to review 19 studies of fibrosis screening, among which 11 used noninvasive tests. Only TE and were compared with histological endpoints. The prevalence of advanced liver fibrosis and cirrhosis in the general population (GP) were % and %, respectively; even higher prevalences (0 27.9% and %, respectively) than in the GP were reported in targeted populations with risk factors of liver disease, such as non-alcoholic fatty liver disease, hazardous alcohol use, or type 2 diabetes. was considered as the most validated for liver fibrosis screening and to consistently detect otherwise unrecognized liver disease in GP. (Poynard et al. 2010, Zelber-Sagi et al 2012 and Grattagliano et al. 2013). Authors concluded that only the use of validated specific markers like can consistently detect disease that would have otherwise been missed by current referral pathways based on abnormal liver function tests. HARRIS 2017 Elastography Histology Screening Review HIV Liver Fibrosis Regression assessed using during tenofovircontaining ART Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: a prospective cohort study. Boyd A, Bottero J, Miailhes P, Lascoux-Combe C, Rougier H, Girard PM, Serfaty L, Lacombe K. J Int AIDS Soc. 2017;20:1-12. The aim of this study was to evaluate the dynamic of liver fibrosis as per, at baseline and every six to twelve months, in n=167 TDF-treated HIV-HBV co-infected patients followed-up (FU) sixty months (IQR = 36-93). Among patients with baseline F3- F4 fibrosis (28.1%), 14.9% regressed to F0-F2 at last FU-visit. Among patients with F0-F2- baseline fibrosis (71.9%), 16.7% progressed to F3-F4 at last FU-visit. Fibrosis progression was not associated with virus-related factors, but with male gender, older age, lower nadir CD4+ cell count, higher fasting glycaemia and anaemia at TDF-initiation. Authors stressed the importance of continuous fibrosis monitoring as part of routine care in this patient group. BOYD 2017 HBV/HIV HBV Review article on fibrosis assessment for chronic hepatitis B Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Parikh P1, Ryan JD1, Tsochatzis EA1. Ann Transl Med Feb;5(3):40. It was admitted that the detection and quantification of liver fibrosis is a key factor for chronic hepatitis B management and prognostication. Authors stressed that non-invasive algorithms can reliably stage liver disease and are now incorporated into International guidelines for HBV management. Authors reminded that in a meta-analysis of 71 studies, APRI had lower performance than FIB-4, transient elastography (TE) and in both HBV and HCV patients. was identified to rule out cirrhosis as per recent metaanalysis. (Houot et al. Aliment Pharmacol Ther 2016) PARIKH 2017 HBV Review
4 European consensus definition of severe fibrosis using in chronic viral hepatitis Late presentation of chronic viral hepatitis for medical care: a consensus definition. Mauss S, Pol S, Buti M, Duffell E, et al.; European consensus working group on late presentation for Viral Hepatitis Care. BMC Med. 2017;15:92. The European Consensus Working Group* has published the following consensus definitions for the advanced and for the late stage liver disease using non-invasive tools for assessing fibrosis caused by chronic hepatitis B and C in patients who had not received prior treatment. : Advanced liver disease is defined by a score>0.59, APRI score> 1.5, FIB-4> 3.25, transient elastography (TE)> 9.5 kpa or liver biopsy stage METAVIR> = F3. Late-stage liver disease was clinically defined by the presence of decompensated cirrhosis and / or hepatocellular carcinoma. In conclusion, the authors expect the consensus definitions to be an easy-to-use reference for public health authorities and to better assess the clinical situation on a population basis. * The European Consensus Working Group includes experts in viral hepatitis from the European Association for the Study of the Liver (EASL), experts from the HIV Initiative in Europe and other relevant stakeholders, including patient associations, health-policy makers and surveillance and medical experts. MAUSS 2017, Cutoff, Hepatitis B, Hepatitis C Alcoholic Liver Diseases (ALD) New validation of in excessive drinkers from primary and secondary healthcenters Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Thiele M, Stæhr Madsen B, Fuglsang Hansen J, et al. Gastroenterology 2018 Jan 4. doi: /j.gastro Authors proposed to compare prospectively the accuracy of the Enhanced Liver Fibrosis test (ELF),, liver stiffness measurements by TE and 2D-SWE and 6 other biomarkers in detection of advanced liver fibrosis in patients with excessive dinking recruited in primary (n=128) and secondary (n=161) healthcenters. Diagnostic accuracy of was high (AUROC 0.90) for advanced fibrosis (AF) (Kleiner stage >=F3 using biopsy), comparable to ELF (0.92, p=ns). In intention-todiagnose analyses, has comparable performances to TE and 2D-SWE for AF (all p=ns). For the primary care patients, values below 0.58 had 94% negative predictive values for AF. The main strengths of study are the analyses in intention-to-diagnose and the inclusion of patients with metabolic syndrome features and ongoing drinking, which reflect real-life situations. In conclusion, in excessive drinkers from primary and secondary care, can ruled out AF (scores below 0.58) with high diagnostic performances. THIELE 2018, ELF, TE, 2D-SWE, Alcohol
5 HCV Improve of and AFP in HCV-SVR Potent viral suppression and improvements in alpha-fetoprotein and measures of fibrosis in Japanese patients receiving a daclatasvir/asunaprevir/beclabuvir fixed-dose combination for the treatment of HCV genotype-1 infection. Akuta N, Toyota J, Karino Y, et al. J Gastroenterol Mar 2. doi: / s The present study assessed the dynamics of hepatic fibrosis with and alphafetoprotein (AFP) in pre- and post-treatment HCV-genotype 1 patients that achieved SVR in UNITY-3 trial with DAA therapy (daclatasvir/ asunaprevir/ beclabuvir). A total of 217 patients were included, 46% were aged >65 years and 21% had compensated cirrhosis. Both and AFP values improved significantly post-treatment with numerically greater score improvement in cirrhotic patients. stage decreased in 44%, remained stable in 50%, and worsened in 6% of patients at SVR. Improvements in both and AFP scores suggest that HCV-SVR may reduce the risk of future liver disease progression and hepatocellular carcinoma, particularly in those with compensated cirrhosis. used to identify cirrhosis in chronic hepatitis C prognosis study Role of Non-hepatic Medical Comorbidity and Functional Limitations in Predicting Mortality in Patients with HCV. Natarajan Y, White DL, El-Serag HB, Ramsey D, Richardson P, Kuzniarek J, Shukla R, Tansel A, Kanwal F. Dig Dis Sci. 2017;62: Authors proposed to determine the effect of comorbidities and functional status on survival in a cohort of HCV chronic carriers. The functional status was assesd as per Schonberg Index (SI) based on age, gender, and medical comorbidities. Comorbidities and functional limitations as per SI predicted higher mortality in patients with HCV, independently of cirrhosis. Baseline cirrhosis as per had the highest hazard ratio for mortality in HCV patients, higher than congestive heart failure or alcohol ingestion suggesting that is a real marker of severity of the disease. Comorbidities as per SI should be taken also into account for predicting mortality. used to identify cirrhosis in chronic hepatitis C with hepatocellular carcinoma (HCC) Hepatocellular carcinoma (HCC) decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. Prenner SB1, VanWagner LB2, Flamm SL1, Salem R3, Lewandowski RJ3, Kulik L4. J Hepatol. 2017;66: The aim of this study was to assess the efficacy of all-oral-daa regimens in 421 HCV+ cirrhotic patients who have or had HCC compared to those without HCC. Cirrhosis was defined by one of the following: liver biopsy, transient elastography >12.5 kpa, acoustic radiation force impulse >2.0 m/s, MR-elastography >5 kpa, or (FibroSURE) >=0.74. Failure to achieve SVR occurred in 21% of patients with HCC compared to 12% of patients without HCC (p=0.009). Patients with active HCC seemed more likely to fail hepatitis C treatment than patients without HCC. AKUTA 2018, HCV, HCC NATARAJAN 2017 HCV Prognosis PRENNER 2017 HCV HCC
6 HCV Treatment Prioritization in trials using Sofosbuvir/Velpatasvir in Patients With Hepatitis C Virus Genotypes 1-6 and Compensated Cirrhosis or Advanced Fibrosis. Asselah T, Bourgeois S, Pianko S, et al.. Liver Int. 2018;38: Efficacy of 8 Weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in Patients With Chronic HCV Infection: 2 Phase 3 Randomized Trials. Jacobson IM, Lawitz E, Gane EJ,, et al.. Gastroenterology. 2017;153: ASSELAH 2018 SOFO-VELPA JACOBSON 2017 SOFO-VELPA-VOXI Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1 Pol S, Bourliere M, Lucier S, et al. J Hepatol. 2017;66: POL 2017 SOFO-DACLA Real-Life Use of 3 Direct-Acting Antiviral Regimen in a Large Cohort of Patients with Genotype-1b HCV Compensated Cirrhosis. Gheorghe L, Iacob S, Curescu M,et al. J Gastrointestin Liver Dis. 2017;26: Real-world efficacy and safety of ombitasvir, paritaprevir/r+dasabuvir+ribavirin in genotype 1b patients with hepatitis C virus cirrhosis. Preda CM, Popescu CP, Baicus C, et al A. Liver Int doi: /liv Efficacy and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with ribavirin for the treatment of HCV genotype 1b compensated cirrhosis in patients aged 70 years or older. Trifan A, Stanciu C, Gheorghe L, et al. Medicine (Baltimore) 2017; 96: e9271. GHEORGHE 2017 OBV/PTV/r+DSV PREDA 2017 OBV/PTV/r+DSV TRIFAN 2017 OBT/PTV/r+DSV Drug Induced Liver Injury (DILI) DILI-ActiTest a new prognostic biomarker of drug-induced-liver-injury (DILI). Evidence-based Translation consortium. Serum apolipoprotein A1 and haptoglobin, in patients with suspected drug-induced liver injury (DILI) as biomarkers of recovery. Peta V, Tse C, Perazzo H, et al; Drug Induced Liver Injury- Groupe Hospitalier Pitié-Salpêtrière; Drug Induced Liver Group of the Injury Safer and Faster PLoS One. 2017;12:e The primary objective was to analyze in patients with DILI the prognostic performance of ActiTest and proteins apoliprotein-a1 (APOA1), haptoglobin (HAPTO) and alpha-2-macroglobulin (A2M), as predictors of recovery* outcome. N=115 adjucated DILI cases from the European DILI Consortium had at least two samples during 12-weeks follow-up. APOA1 and HAPTO, both acute phase reactants, have had the strongest negative correlation with DILI during the follow-up. A new biomarker, DILI-ActiTest [patent pending] combining ApoA1, Hapto, A2M, GGT, age and gender, resulted in a significant prediction of recovery with 67% accuracy and an significant AUROC of 0.72 (p<0.001 vs hazard). Further validation of the panel DILI ActiTest should be performed in another group of DILI cases. * Recovery outcome was defined as an ALT <2x and BILI <2x the upper limit of normal. PETA 2017 DILI-ActiTest, Haptoglobin, Apolipoproten A1
7
Bio Predictive. FibroTest Scientific Publications. Ratziu 2016 FibroMax NASH. Friedman 2016 FibroTest NASH EASL Key Publications for 2017
 EASL 2017 Scientific Publications Ratziu 2016 NASH Friedman 2016 NASH SteatoTest and sensitive markers of improvement in NASH trial using Elafibranor Elafibranor, an agonist of the peroxisome proliferator
EASL 2017 Scientific Publications Ratziu 2016 NASH Friedman 2016 NASH SteatoTest and sensitive markers of improvement in NASH trial using Elafibranor Elafibranor, an agonist of the peroxisome proliferator
FibroTest/FibroMax Scientific Publications
 /FibroMax Scientific Publications Key Publications for 2019 EASL 2019 Nonalcoholic steatohepatitis (NASH) NASH-Test V2 : a new quantitative test for the diagnosis of nonalcoholic steatohepatitis (NASH)
/FibroMax Scientific Publications Key Publications for 2019 EASL 2019 Nonalcoholic steatohepatitis (NASH) NASH-Test V2 : a new quantitative test for the diagnosis of nonalcoholic steatohepatitis (NASH)
Bio Predictive. FibroTest/FibroSure Scientific Publications. Houot 2015 FibroTest, TE, FIB-4, APRI Meta-analysis
 AASLD 2015 /FibroSure Scientific Publications Section 1 - compared to APRI, FIB-4 and transient elastography Houot 2015, TE, FIB-4, APRI Meta-analysis is superior to TE by Fibroscan, APRI and Fib-4 using
AASLD 2015 /FibroSure Scientific Publications Section 1 - compared to APRI, FIB-4 and transient elastography Houot 2015, TE, FIB-4, APRI Meta-analysis is superior to TE by Fibroscan, APRI and Fib-4 using
Bio Predictive. FibroTest/FibroSure Scientific Publications. Harris 2017 FibroTest Elastography Histology Screening Review
 AASLD 2017 /FibroSure Scientific Publications Screening general population Harris 2017 Histology Screening Review Ginès 2016,, Screening Review for the screening of unknown liver diseases in the general
AASLD 2017 /FibroSure Scientific Publications Screening general population Harris 2017 Histology Screening Review Ginès 2016,, Screening Review for the screening of unknown liver diseases in the general
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
NASH : Diagnosis and investigation. VII Workshop international, Curitiba, Brazil 29/08/2014
 NASH : Diagnosis and investigation VII Workshop international, Curitiba, Brazil 29/08/2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France Usual diagnostic circumstances
NASH : Diagnosis and investigation VII Workshop international, Curitiba, Brazil 29/08/2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France Usual diagnostic circumstances
Worldwide Causes of HCC
 Approach to HCV Treatment in Patients with HCC Mark W. Russo, MD, MPH, FACG Carolinas HealthCare System Charlotte Worldwide Causes of HCC 60% 50% 40% 30% 20% 10% 0% 54% 31% 15% Hepatitis B Hepatitis C
Approach to HCV Treatment in Patients with HCC Mark W. Russo, MD, MPH, FACG Carolinas HealthCare System Charlotte Worldwide Causes of HCC 60% 50% 40% 30% 20% 10% 0% 54% 31% 15% Hepatitis B Hepatitis C
Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease
 Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease Policy Number: 2.04.41 Last Review: 2/2018 Origination: 2/2017 Next Review: 2/2019 Policy Blue Cross and
Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease Policy Number: 2.04.41 Last Review: 2/2018 Origination: 2/2017 Next Review: 2/2019 Policy Blue Cross and
Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease
 Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease Policy Number: 2.04.41 Last Review: 2/2019 Origination: 2/2017 Next Review: 2/2020 Policy Blue Cross and
Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease Policy Number: 2.04.41 Last Review: 2/2019 Origination: 2/2017 Next Review: 2/2020 Policy Blue Cross and
Worldwide Causes of HCC
 Approach to HCV Treatment in Patients with HCC JORGE L. HERRERA, MD, MACG UNIVERSITY OF SOUTH ALABAMA COLLEGE OF MEDICINE Worldwide Causes of HCC 60% 50% 40% 54% 30% 20% 10% 31% 15% 0% Hepatitis B Hepatitis
Approach to HCV Treatment in Patients with HCC JORGE L. HERRERA, MD, MACG UNIVERSITY OF SOUTH ALABAMA COLLEGE OF MEDICINE Worldwide Causes of HCC 60% 50% 40% 54% 30% 20% 10% 31% 15% 0% Hepatitis B Hepatitis
The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis
 The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis Objectives: Liver biopsy is the gold standard for diagnosing the extent of fibrosis in NAFLD/NASH;
The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis Objectives: Liver biopsy is the gold standard for diagnosing the extent of fibrosis in NAFLD/NASH;
MP Noninvasive Techniques for the Evaluation and Monitoring of Patients With Chronic Liver Disease
 Medical Policy BCBSA Ref. Policy: 2.04.41 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our medical policies
Medical Policy BCBSA Ref. Policy: 2.04.41 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our medical policies
Follow-up of patients with SVR Lawrence Serfaty Service d Hépatologie, UMR_S 938 Hôpital Saint-Antoine Université Pierre&Marie Curie Paris, France
 9th Paris Hepatitis Conference, January 11-12, 2016 Follow-up of patients with SVR Lawrence Serfaty Service d Hépatologie, UMR_S 938 Hôpital Saint-Antoine Université Pierre&Marie Curie Paris, France Disclosures
9th Paris Hepatitis Conference, January 11-12, 2016 Follow-up of patients with SVR Lawrence Serfaty Service d Hépatologie, UMR_S 938 Hôpital Saint-Antoine Université Pierre&Marie Curie Paris, France Disclosures
The role of ARFI and APRI in diagnosis of liver fibrosis on patients with common chronic liver diseases
 RESEARCH ARTICLE The role of ARFI and APRI in diagnosis of liver fibrosis on patients with common chronic liver diseases Objective: This study aimed to investigate the value of liver fibrosis assessment
RESEARCH ARTICLE The role of ARFI and APRI in diagnosis of liver fibrosis on patients with common chronic liver diseases Objective: This study aimed to investigate the value of liver fibrosis assessment
In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease
 REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
Clinical Criteria for Hepatitis C (HCV) Therapy
 Clinical Criteria for Hepatitis C (HCV) Therapy Pre-Treatment Evaluation o Must have chronic hepatitis C and HCV genotype and sub-genotype documented; o Patients who have prior exposure to DAA therapy
Clinical Criteria for Hepatitis C (HCV) Therapy Pre-Treatment Evaluation o Must have chronic hepatitis C and HCV genotype and sub-genotype documented; o Patients who have prior exposure to DAA therapy
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Noninvasive Techniques for the Evaluation and Monitoring of Page 1 of 38 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Noninvasive Techniques for the Evaluation
Noninvasive Techniques for the Evaluation and Monitoring of Page 1 of 38 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Noninvasive Techniques for the Evaluation
Management of Liver Diseases: A Nonhepatologist s Viewpoint
 Management of Liver Diseases: A Nonhepatologist s Viewpoint Arthur Y. Kim, MD Associate Professor of Medicine Harvard Medical School Director, Viral Hepatitis Clinic Massachusetts General Hospital Boston,
Management of Liver Diseases: A Nonhepatologist s Viewpoint Arthur Y. Kim, MD Associate Professor of Medicine Harvard Medical School Director, Viral Hepatitis Clinic Massachusetts General Hospital Boston,
Transient elastography in chronic liver diseases of other etiologies
 4 Post Meeting A.I.S.F. Unmet Clinical Needs in Hepatology: New and upcoming diagnostic tools" Transient elastography in chronic liver diseases of other etiologies Dr. Vincenza Calvaruso Gastroenterologia
4 Post Meeting A.I.S.F. Unmet Clinical Needs in Hepatology: New and upcoming diagnostic tools" Transient elastography in chronic liver diseases of other etiologies Dr. Vincenza Calvaruso Gastroenterologia
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Noninvasive Techniques for the Evaluation and Page 1 of 34 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Noninvasive Techniques for the Evaluation and Professional
Noninvasive Techniques for the Evaluation and Page 1 of 34 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Noninvasive Techniques for the Evaluation and Professional
Clinical Criteria for Hepatitis C (HCV) Therapy
 Clinical Criteria for Hepatitis C (HCV) Therapy Pre-Treatment Evaluation o Must have chronic hepatitis C and HCV genotype and sub-genotype documented; o Patients who have prior exposure to DAA therapy
Clinical Criteria for Hepatitis C (HCV) Therapy Pre-Treatment Evaluation o Must have chronic hepatitis C and HCV genotype and sub-genotype documented; o Patients who have prior exposure to DAA therapy
Transient elastography the state of the art
 Transient elastography the state of the art Laurent CASTERA, MD PhD Department of Hepatology, Hôpital Beaujon, Clichy Université Paris-7, France White Nights of Hepatology, St Petersburg, Russia, june
Transient elastography the state of the art Laurent CASTERA, MD PhD Department of Hepatology, Hôpital Beaujon, Clichy Université Paris-7, France White Nights of Hepatology, St Petersburg, Russia, june
Invasive. Sampling error. Interobserver variability. Nondynamic evaluation of
 How to assess liver fibrosis Serum markers or FibroScan vs. liver biopsy? Laurent CASTERA & Pierre BEDOSSA Hôpital Beaujon, AP-HP, Clichy Université Paris-VII France 4 th Paris Hepatitis Conference, Paris,
How to assess liver fibrosis Serum markers or FibroScan vs. liver biopsy? Laurent CASTERA & Pierre BEDOSSA Hôpital Beaujon, AP-HP, Clichy Université Paris-VII France 4 th Paris Hepatitis Conference, Paris,
Non-Invasive Testing for Liver Fibrosis
 NORTHWEST AIDS EDUCATION AND TRAINING CENTER Non-Invasive Testing for Liver Fibrosis John Scott, MD, MSc Associate Professor, University of Washington Associate Clinic Director, Hep/Liver Clinic, Harborview
NORTHWEST AIDS EDUCATION AND TRAINING CENTER Non-Invasive Testing for Liver Fibrosis John Scott, MD, MSc Associate Professor, University of Washington Associate Clinic Director, Hep/Liver Clinic, Harborview
Clinical Criteria for Hepatitis C (HCV) Therapy
 Clinical Criteria for Hepatitis C (HCV) Therapy Pre-Treatment Evaluation o Must have chronic hepatitis C and HCV genotype and sub-genotype documented; o Patients who have prior exposure to DAA therapy
Clinical Criteria for Hepatitis C (HCV) Therapy Pre-Treatment Evaluation o Must have chronic hepatitis C and HCV genotype and sub-genotype documented; o Patients who have prior exposure to DAA therapy
5/2/2016. Arthur Y. Kim, MD Assistant Professor of Medicine Harvard Medical School Massachusetts General Hospital Boston, Massachusetts
 Management of Liver Diseases (A Nonhepatologist s Viewpoint) Arthur Y. Kim, MD Assistant Professor of Medicine Harvard Medical School Massachusetts General Hospital Boston, Massachusetts FINAL: 04/11/2016
Management of Liver Diseases (A Nonhepatologist s Viewpoint) Arthur Y. Kim, MD Assistant Professor of Medicine Harvard Medical School Massachusetts General Hospital Boston, Massachusetts FINAL: 04/11/2016
New HCV reimbursement criteria Chronic hepatitis C regardless of fibrosis stage if:
 New HCV reimbursement criteria 01-2017 Chronic hepatitis C with F2 fibrosis stage Chronic hepatitis C regardless of fibrosis stage if: HIV-HCV coinfection HBV-HCV coinfection Listed for or post-solid organ
New HCV reimbursement criteria 01-2017 Chronic hepatitis C with F2 fibrosis stage Chronic hepatitis C regardless of fibrosis stage if: HIV-HCV coinfection HBV-HCV coinfection Listed for or post-solid organ
Meet the Professor: HIV/HCV Coinfection
 Meet the Professor: HIV/HCV Coinfection Vincent Lo Re, MD, MSCE Assistant Professor of Medicine and Epidemiology Division of Infectious Diseases Center for Clinical Epidemiology and Biostatistics University
Meet the Professor: HIV/HCV Coinfection Vincent Lo Re, MD, MSCE Assistant Professor of Medicine and Epidemiology Division of Infectious Diseases Center for Clinical Epidemiology and Biostatistics University
The impact of the treatment of HCV in developing Hepatocellular Carcinoma
 The impact of the treatment of HCV in developing Hepatocellular Carcinoma Paul Y Kwo, MD Professor of Medicine Medical Director, Liver Transplantation Gastroenterology/Hepatology Division Indiana University
The impact of the treatment of HCV in developing Hepatocellular Carcinoma Paul Y Kwo, MD Professor of Medicine Medical Director, Liver Transplantation Gastroenterology/Hepatology Division Indiana University
Hepatitis C in Disclosures
 Hepatitis C in 2018 Sandeep Mukherjee, MD CHI Health and Creighton University Medical Center Division of Gastroenterology Grant support: Abbvie Disclosures Speaker: Abbvie, Gilead, Merck Section editor
Hepatitis C in 2018 Sandeep Mukherjee, MD CHI Health and Creighton University Medical Center Division of Gastroenterology Grant support: Abbvie Disclosures Speaker: Abbvie, Gilead, Merck Section editor
HCV care after cure. This program is supported by educational grants from
 HCV care after cure This program is supported by educational grants from Raffaele Bruno,MD Department of Infectious Diseases, Hepatology Outpatients Unit University of Pavia Fondazione IRCCS Policlinico
HCV care after cure This program is supported by educational grants from Raffaele Bruno,MD Department of Infectious Diseases, Hepatology Outpatients Unit University of Pavia Fondazione IRCCS Policlinico
National Horizon Scanning Centre. Enhanced Liver Fibrosis Test (ELF) for evaluating liver fibrosis. June 2008
 Enhanced Liver Fibrosis Test (ELF) for evaluating liver fibrosis June 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Enhanced Liver Fibrosis Test (ELF) for evaluating liver fibrosis June 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Transient elastography in chronic viral liver diseases
 4 th AISF POST-MEETING COURSE Roma, 26 Febbraio 2011 Transient elastography in chronic viral liver diseases CRISTINA RIGAMONTI, M.D., Ph.D. Transient elastography (TE): a rapid, non-invasive technique
4 th AISF POST-MEETING COURSE Roma, 26 Febbraio 2011 Transient elastography in chronic viral liver diseases CRISTINA RIGAMONTI, M.D., Ph.D. Transient elastography (TE): a rapid, non-invasive technique
Screening for HCCwho,
 Screening for HCCwho, how and how often? Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital HCC Global Epidemiology
Screening for HCCwho, how and how often? Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital HCC Global Epidemiology
New HCV reimbursement criteria Chronic hepatitis C regardless of fibrosis stage if:
 New HCV reimbursement criteria 01-2018 Chronic hepatitis C with F2 fibrosis stage Chronic hepatitis C regardless of fibrosis stage if: HIV-HCV coinfection HBV-HCV coinfection Listed for or post-solid organ
New HCV reimbursement criteria 01-2018 Chronic hepatitis C with F2 fibrosis stage Chronic hepatitis C regardless of fibrosis stage if: HIV-HCV coinfection HBV-HCV coinfection Listed for or post-solid organ
Hepatology For The Nonhepatologist
 Hepatology For The Nonhepatologist Andrew Aronsohn, MD Associate Professor of Medicine University of Chicago Chicago, Illinois Learning Objectives After attending this presentation, learners will be able
Hepatology For The Nonhepatologist Andrew Aronsohn, MD Associate Professor of Medicine University of Chicago Chicago, Illinois Learning Objectives After attending this presentation, learners will be able
New Tools for Assessing Disease Sererity, progression and regression in HBV and HCV
 New Tools for Assessing Disease Sererity, progression and regression in HBV and HCV Jidong Jia, MD, PhD Beijing Friendship Hospital, Capital Medical University 2016-3-14 Disclosure Consultation for Abbvie
New Tools for Assessing Disease Sererity, progression and regression in HBV and HCV Jidong Jia, MD, PhD Beijing Friendship Hospital, Capital Medical University 2016-3-14 Disclosure Consultation for Abbvie
When to Treat: Staging Liver Disease David L. Thomas, MD, MPH
 When to Treat: Staging Liver Disease David L. Thomas, MD, MPH Professor of Medicine Johns Hopkins School of Medicine Disclosures Received royalties from UpToDate, Inc. Staging refers to the assessment
When to Treat: Staging Liver Disease David L. Thomas, MD, MPH Professor of Medicine Johns Hopkins School of Medicine Disclosures Received royalties from UpToDate, Inc. Staging refers to the assessment
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
FibroTest/FibroSure Scientific Publications Posters for AASLD 2012
 AASLD Survival Tuesday, Nov. 13 Poster 1733 /FibroSure Scientific Publications Posters for AASLD Long term survival of liver fibrosis after virological cure (SVR) in patients with chronic hepatitis C (CHC):
AASLD Survival Tuesday, Nov. 13 Poster 1733 /FibroSure Scientific Publications Posters for AASLD Long term survival of liver fibrosis after virological cure (SVR) in patients with chronic hepatitis C (CHC):
WHEN HCV TREATMENT IS DEFERRED WV HEPC ECHO PROJECT
 WHEN HCV TREATMENT IS DEFERRED WV HEPC ECHO PROJECT October 13, 2016 Reminder - treatment is recommended for all patients with chronic HCV infection Except short life expectancies that cannot be remediated
WHEN HCV TREATMENT IS DEFERRED WV HEPC ECHO PROJECT October 13, 2016 Reminder - treatment is recommended for all patients with chronic HCV infection Except short life expectancies that cannot be remediated
Special developments in the management of Hepatitis C. Disclosures
 Special developments in the management of Hepatitis C Sandeep Mukherjee,MD Division of Gastroenterology CHI Health and Creighton University Medical Center Omaha, NE 68154 Sandeep.Mukherjee@alegent.org
Special developments in the management of Hepatitis C Sandeep Mukherjee,MD Division of Gastroenterology CHI Health and Creighton University Medical Center Omaha, NE 68154 Sandeep.Mukherjee@alegent.org
Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR
 Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR This program is supported by educational grants from AbbVie, Gilead Sciences, and Merck About These Slides Please feel free to use,
Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR This program is supported by educational grants from AbbVie, Gilead Sciences, and Merck About These Slides Please feel free to use,
INHSU th International Symposium on Hepatitis Care In Substance Users
 INHSU 2015 4 th International Symposium on Hepatitis Care In Substance Users Sydney, 7 October 2015 Non-invasive liver fibrosis assessment: Opportunities for enhanced liver disease assessment and treatment
INHSU 2015 4 th International Symposium on Hepatitis Care In Substance Users Sydney, 7 October 2015 Non-invasive liver fibrosis assessment: Opportunities for enhanced liver disease assessment and treatment
New HCV reimbursement criteria Chronic hepatitis C regardless of fibrosis stage if:
 New HCV reimbursement criteria 01-2018 Chronic hepatitis C with F2 fibrosis stage Chronic hepatitis C regardless of fibrosis stage if: HIV-HCV coinfection HBV-HCV coinfection Listed for or post-solid organ
New HCV reimbursement criteria 01-2018 Chronic hepatitis C with F2 fibrosis stage Chronic hepatitis C regardless of fibrosis stage if: HIV-HCV coinfection HBV-HCV coinfection Listed for or post-solid organ
Hepatocellular Carcinoma Surveillance
 Amit G. Singal, MD, MS Hepatocellular Carcinoma Surveillance Postgraduate Course: Challenges in Management of Common Liver Diseases 308 1 Patient Case 69 year-old otherwise healthy male with compensated
Amit G. Singal, MD, MS Hepatocellular Carcinoma Surveillance Postgraduate Course: Challenges in Management of Common Liver Diseases 308 1 Patient Case 69 year-old otherwise healthy male with compensated
Length of Authorization: 8-16 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis
 Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
REVIEWS. Introduction. FibroMax complete diagnosis of liver injury. Abbreviations
 REVIEWS Noninvasive Biomarkers for the Screening of Fibrosis, Steatosis and Steatohepatitis in Patients with Metabolic Risk Factors: FibroTest-FibroMa Eperience Mona Munteanu, Vlad Ratziu 2, Rachel Morra,
REVIEWS Noninvasive Biomarkers for the Screening of Fibrosis, Steatosis and Steatohepatitis in Patients with Metabolic Risk Factors: FibroTest-FibroMa Eperience Mona Munteanu, Vlad Ratziu 2, Rachel Morra,
/ FIB4 Index , simple steatosis. FIB4 Index. FIB4 Index. FIB4 Index FIB4 Index. Sterling FIB4 Index. FIB4 Index AST AST ALT
 原 著 29 34-41, 2014 FIB4 Index 1 1 1 1 2 1 1 FIB4 Index FIB4 Index cut off 2.67 2.67 12,059 FIB4 IndexFIB4 Index 2.67 / FIB4 Index AST ALT FIB4 Index 2.67 161 1.3% FIB4 Index 5 FIB4 Index 1.1 5 1.6 FIB4
原 著 29 34-41, 2014 FIB4 Index 1 1 1 1 2 1 1 FIB4 Index FIB4 Index cut off 2.67 2.67 12,059 FIB4 IndexFIB4 Index 2.67 / FIB4 Index AST ALT FIB4 Index 2.67 161 1.3% FIB4 Index 5 FIB4 Index 1.1 5 1.6 FIB4
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Harris R, Harman DJ, Card TR, Aithal GP, Guha
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Harris R, Harman DJ, Card TR, Aithal GP, Guha
Learning Objectives. After attending this presentation, participants will be able to:
 Learning Objectives After attending this presentation, participants will be able to: Describe HCV in 2015 Describe how to diagnose advanced liver disease and cirrhosis Identify the clinical presentation
Learning Objectives After attending this presentation, participants will be able to: Describe HCV in 2015 Describe how to diagnose advanced liver disease and cirrhosis Identify the clinical presentation
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
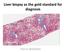 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
How to optimize treatment in G3 patients? Jérôme GOURNAY, MD Hépatologie Centre Hospitalier Universitaire de Nantes France
 How to optimize treatment in G3 patients? Jérôme GOURNAY, MD Hépatologie Centre Hospitalier Universitaire de Nantes France Paris Hepatitis Conference, January 12, 2016 Disclosures I have received funding
How to optimize treatment in G3 patients? Jérôme GOURNAY, MD Hépatologie Centre Hospitalier Universitaire de Nantes France Paris Hepatitis Conference, January 12, 2016 Disclosures I have received funding
Liver Pathology in the 0bese
 Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Length of Authorization: 8-16 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Practical Diagnosis and Staging of Nonalcoholic Fatty Liver Disease: A Narrative Review
 Practical Diagnosis and Staging of Nonalcoholic Fatty Liver Disease: A Narrative Review Authors: Jennifer Gallacher, 1 *Stuart McPherson 1,2 1. Liver Unit, Newcastle Upon Tyne Hospitals NHS Trust, Freeman
Practical Diagnosis and Staging of Nonalcoholic Fatty Liver Disease: A Narrative Review Authors: Jennifer Gallacher, 1 *Stuart McPherson 1,2 1. Liver Unit, Newcastle Upon Tyne Hospitals NHS Trust, Freeman
Hepatitis C Policy Discussion
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Patients with compensated cirrhosis: how to treat and follow-up
 Patients with compensated cirrhosis: how to treat and follow-up Thomas Berg Sektion Hepatologie Klinik und Poliklinik für Gastroenterologie und Rheumatologie Universitätsklinikum Leipzig Leber- und Studienzentrum
Patients with compensated cirrhosis: how to treat and follow-up Thomas Berg Sektion Hepatologie Klinik und Poliklinik für Gastroenterologie und Rheumatologie Universitätsklinikum Leipzig Leber- und Studienzentrum
Length of Authorization: 8-12 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Liver 102: Injury and Healing
 Liver 102: Injury and Healing Dawn Pease, MSN, RN, ANP-BC Brackenridge Specialty Clinics University Medical Center Brackenridge Austin, TX Seton Healthcare Family Liver 102 Outline Biochemical patterns
Liver 102: Injury and Healing Dawn Pease, MSN, RN, ANP-BC Brackenridge Specialty Clinics University Medical Center Brackenridge Austin, TX Seton Healthcare Family Liver 102 Outline Biochemical patterns
Pretreatment Evaluation
 Pretreatment Evaluation Disclosures Research supported by Gilead Sciences Inc.: Site investigator for HIV/HCV SWITCH Registry Study Key personnel for FOCUS HCV Screening Program through Vanderbilt University
Pretreatment Evaluation Disclosures Research supported by Gilead Sciences Inc.: Site investigator for HIV/HCV SWITCH Registry Study Key personnel for FOCUS HCV Screening Program through Vanderbilt University
Alternatives to Liver Biopsy for Assessing Liver Disease
 Alternatives to Liver Biopsy for Assessing Liver Disease a report by Thierry Poynard Professor of Medicine, University of Paris The consensus conference statements recommend liver biopsy in the management
Alternatives to Liver Biopsy for Assessing Liver Disease a report by Thierry Poynard Professor of Medicine, University of Paris The consensus conference statements recommend liver biopsy in the management
Non-Invasive Assessment of Liver Fibrosis. Patricia Slev, PhD University of Utah Department of Pathology
 Non-Invasive Assessment of Liver Fibrosis Patricia Slev, PhD University of Utah Department of Pathology Disclosure Patricia Slev has no relevant financial relationships to disclose. 2 Chronic Liver Disease
Non-Invasive Assessment of Liver Fibrosis Patricia Slev, PhD University of Utah Department of Pathology Disclosure Patricia Slev has no relevant financial relationships to disclose. 2 Chronic Liver Disease
Bio Predictive. FibroTest/FibroSure Scientific Publications. Perazzo 2014 FibroMax Prognosis Metabolic. Park 2014 FibroTest HBV Prognostic
 AASLD 2014 /FibroSure Scientific Publications Perazzo 2014 FibroMax Prognosis Metabolic Park 2014 Prognostic Bonnard 2014 HCV Genotype 4 Schistosomiasis Prognostic value of liver fibrosis and steatosis
AASLD 2014 /FibroSure Scientific Publications Perazzo 2014 FibroMax Prognosis Metabolic Park 2014 Prognostic Bonnard 2014 HCV Genotype 4 Schistosomiasis Prognostic value of liver fibrosis and steatosis
Length of Authorization: 8-16 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Clinical Policy Title: Noninvasive assessment of hepatic fibrosis
 Clinical Policy Title: Noninvasive assessment of hepatic fibrosis Clinical Policy Number: 08.01.03 Effective Date: Oct. 1, 2014 Initial Review Date: June 18, 2014 Most Recent Review Date: August 17, 2017
Clinical Policy Title: Noninvasive assessment of hepatic fibrosis Clinical Policy Number: 08.01.03 Effective Date: Oct. 1, 2014 Initial Review Date: June 18, 2014 Most Recent Review Date: August 17, 2017
Abstract and Introduction. Patients and Methods. M. Hedenstierna; A. Nangarhari; A. El-Sabini; O. Weiland; S.
 www.medscape.com Cirrhosis, High Age and High Body Mass Index Are Risk Factors for Persisting Advanced Fibrosis After Sustained Virological Response in Chronic Hepatitis C M. Hedenstierna; A. Nangarhari;
www.medscape.com Cirrhosis, High Age and High Body Mass Index Are Risk Factors for Persisting Advanced Fibrosis After Sustained Virological Response in Chronic Hepatitis C M. Hedenstierna; A. Nangarhari;
Pretreatment Evaluation
 Pretreatment Evaluation Objective At the end of this lecture, the learner will be able to: Outline the appropriate evaluation of a person infected with HCV in order to assess the benefits and risks of
Pretreatment Evaluation Objective At the end of this lecture, the learner will be able to: Outline the appropriate evaluation of a person infected with HCV in order to assess the benefits and risks of
Screening cardiac patients for advanced liver disease
 HKASLD 30 th ASM and International Symposium on Hepatology 2017 Screening cardiac patients for advanced liver disease 5 Nov 2017 Dr. Lau Yue Leung Joulen Pamela Youde Nethersole Eastern Hospital NAFLD
HKASLD 30 th ASM and International Symposium on Hepatology 2017 Screening cardiac patients for advanced liver disease 5 Nov 2017 Dr. Lau Yue Leung Joulen Pamela Youde Nethersole Eastern Hospital NAFLD
Page: 1 of 19. Noninvasive Techniques for the Evaluation and Monitoring of Patients with Chronic Liver Disease
 and Monitoring of Patients with Chronic Liver Page: 1 of 19 Last Review Status/Date: March 2015 and Monitoring of Patients with Chronic Liver Description Options for noninvasive monitoring of liver fibrosis
and Monitoring of Patients with Chronic Liver Page: 1 of 19 Last Review Status/Date: March 2015 and Monitoring of Patients with Chronic Liver Description Options for noninvasive monitoring of liver fibrosis
NAFLD: evidence-based management. Curso de residentes AEEH Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain
 NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
HEPATOCELLULAR CARCINOMA: SCREENING, DIAGNOSIS, AND TREATMENT
 HEPATOCELLULAR CARCINOMA: SCREENING, DIAGNOSIS, AND TREATMENT INTRODUCTION: Hepatocellular carcinoma (HCC): Fifth most common cancer worldwide Third most common cause of cancer mortality In Egypt: 2.3%
HEPATOCELLULAR CARCINOMA: SCREENING, DIAGNOSIS, AND TREATMENT INTRODUCTION: Hepatocellular carcinoma (HCC): Fifth most common cancer worldwide Third most common cause of cancer mortality In Egypt: 2.3%
New York State HCV Provider Webinar Series
 New York State HCV Provider Webinar Series Overview of Fibrosis-Staging, Child s Pugh, MELD Scores Paul J Gaglio, MD, FACP, AGAF, FAASLD Director: Hepatology Outreach Professor of Medicine (in Surgery)
New York State HCV Provider Webinar Series Overview of Fibrosis-Staging, Child s Pugh, MELD Scores Paul J Gaglio, MD, FACP, AGAF, FAASLD Director: Hepatology Outreach Professor of Medicine (in Surgery)
Hepatitis C Infection: Updated Information for Front Line Workers in Primary Care Settings MAMTA K. JAIN, MD, MPH 2/14/18
 Hepatitis C Infection: Updated Information for Front Line Workers in Primary Care Settings MAMTA K. JAIN, MD, MPH 2/14/18 Overview Hepatitis C Virus Prevalence Effects of Hepatitis C Prevention Diagnosis
Hepatitis C Infection: Updated Information for Front Line Workers in Primary Care Settings MAMTA K. JAIN, MD, MPH 2/14/18 Overview Hepatitis C Virus Prevalence Effects of Hepatitis C Prevention Diagnosis
Liver stiffness predicts liver related events and mortality in HIV/HCV coinfected patients
 Liver stiffness predicts liver related events and mortality in HIV/HCV coinfected patients José Vicente Fernández-Montero, Pablo Barreiro, Eugenia Vispo, Pablo Labarga, Francisco Blanco, Fernanda Rick,
Liver stiffness predicts liver related events and mortality in HIV/HCV coinfected patients José Vicente Fernández-Montero, Pablo Barreiro, Eugenia Vispo, Pablo Labarga, Francisco Blanco, Fernanda Rick,
The Effect of Antiviral Therapy on Liver Fibrosis in CHC. Jidong Jia Beijing Friendship Hospital, Capital Medical University
 The Effect of Antiviral Therapy on Liver Fibrosis in CHC Jidong Jia Beijing Friendship Hospital, Capital Medical University 2016-5-29 1 Disclosure Consultation for Abbvie, BMS, Gilead, MSD, Novartis and
The Effect of Antiviral Therapy on Liver Fibrosis in CHC Jidong Jia Beijing Friendship Hospital, Capital Medical University 2016-5-29 1 Disclosure Consultation for Abbvie, BMS, Gilead, MSD, Novartis and
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Vosevi) Reference Number: CP.HNMC.41 Effective Date: 07.26.17 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Vosevi) Reference Number: CP.HNMC.41 Effective Date: 07.26.17 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
HCV Viremia Was Associated With Increased Mortality in a Prospective Taiwanese Cohort Study
 Tram T. Tran, MD, FACG Approach to HCV Treatment in Patients with HCC Tram T. Tran, MD, FACG Professor of Medicine Medical Director, Liver Transplant Cedars Sinai Medical Center Natural History of HCV
Tram T. Tran, MD, FACG Approach to HCV Treatment in Patients with HCC Tram T. Tran, MD, FACG Professor of Medicine Medical Director, Liver Transplant Cedars Sinai Medical Center Natural History of HCV
Clinical Policy: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) Reference Number: GA.PMN.25 Product: Medicaid Effective Date: 9/17
 Clinical Policy: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) Reference Number: GA.PMN.25 Product: Medicaid Effective Date: 9/17 Last Review Date: 9/17 Revision Log See Important Reminder at the end of
Clinical Policy: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) Reference Number: GA.PMN.25 Product: Medicaid Effective Date: 9/17 Last Review Date: 9/17 Revision Log See Important Reminder at the end of
Module 1 Introduction of hepatitis
 Module 1 Introduction of hepatitis 1 Training Objectives At the end of the module, trainees will be able to ; Demonstrate improved knowledge of the global epidemiology of the viral hepatitis Understand
Module 1 Introduction of hepatitis 1 Training Objectives At the end of the module, trainees will be able to ; Demonstrate improved knowledge of the global epidemiology of the viral hepatitis Understand
Why to biopsy? Indications for liver biopsy in common medical liver diseases- how are they changing?
 Why to biopsy? Indications for liver biopsy in common medical liver diseases- how are they changing? Stephen D Ryder Nottingham University Hospitals NHS Trust and Biomedical research Unit What are we currently
Why to biopsy? Indications for liver biopsy in common medical liver diseases- how are they changing? Stephen D Ryder Nottingham University Hospitals NHS Trust and Biomedical research Unit What are we currently
Medical Policy Non-invasive Tests for Hepatic Fibrosis
 Medical Policy Non-invasive Tests for Hepatic Fibrosis Subject: Non-invasive Tests for Hepatic Fibrosis Background: Accurately diagnosing liver fibrosis and inflammatory activity are the most important
Medical Policy Non-invasive Tests for Hepatic Fibrosis Subject: Non-invasive Tests for Hepatic Fibrosis Background: Accurately diagnosing liver fibrosis and inflammatory activity are the most important
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
The New World of HCV Therapy
 HCV: Assessing the Patient Prior to Treatment: Diagnostic Testing and Strategy JORGE L. HERRERA M.D., MACG UNIVERSITY OF SOUTH ALABAMA COLLEGE OF MEDICINE, MOBILE, AL The New World of HCV Therapy Interferon-free
HCV: Assessing the Patient Prior to Treatment: Diagnostic Testing and Strategy JORGE L. HERRERA M.D., MACG UNIVERSITY OF SOUTH ALABAMA COLLEGE OF MEDICINE, MOBILE, AL The New World of HCV Therapy Interferon-free
We don t need a liver biopsy. We have non-invasive tests
 We don t need a liver biopsy We have non-invasive tests Laurent CASTERA, MD PhD Department of Hepatology, Hôpital Beaujon, Clichy Université Paris Diderot, France PHC 2018 www.aphc.info Liver biopsy: an
We don t need a liver biopsy We have non-invasive tests Laurent CASTERA, MD PhD Department of Hepatology, Hôpital Beaujon, Clichy Université Paris Diderot, France PHC 2018 www.aphc.info Liver biopsy: an
Improving Access to Quality Medical Care Webinar Series
 Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
5/12/2016. Learning Objectives. Management of Hepatitis C Virus Genotype 2 or 3 Infected Treatment-Naive or Experienced Patients
 5/12/216 Management of Hepatitis C Virus Genotype 2 or 3 Infected Treatment-Naive or Experienced Patients Alexander Monto, MD Professor of Clinical Medicine University of California San Francisco San Francisco,
5/12/216 Management of Hepatitis C Virus Genotype 2 or 3 Infected Treatment-Naive or Experienced Patients Alexander Monto, MD Professor of Clinical Medicine University of California San Francisco San Francisco,
Management of HCV in Decompensated Liver Disease
 Management of HCV in Decompensated Liver Disease Michael P. Manns Hannover Medical School (MHH) Department of Gastroenterology, Hepatology and Endocrinology Helmholtz Center for Infection Research (HZI),
Management of HCV in Decompensated Liver Disease Michael P. Manns Hannover Medical School (MHH) Department of Gastroenterology, Hepatology and Endocrinology Helmholtz Center for Infection Research (HZI),
The Impact of HBV Therapy on Fibrosis and Cirrhosis
 The Impact of HBV Therapy on Fibrosis and Cirrhosis Jordan J. Feld, MD, MPH Associate Professor of Medicine University of Toronto Hepatologist Toronto Centre for Liver Disease Sandra Rotman Centre for
The Impact of HBV Therapy on Fibrosis and Cirrhosis Jordan J. Feld, MD, MPH Associate Professor of Medicine University of Toronto Hepatologist Toronto Centre for Liver Disease Sandra Rotman Centre for
Section: Laboratory Last Reviewed Date: June Policy No: 47 Effective Date: August 1, 2013
 Medical Policy Manual Topic: Multianalyte Assays with Algorithmic Analysis for the Evaluation and Monitoring of Patients with Chronic Liver Disease Date of Origin: June 2013 Section: Laboratory Last Reviewed
Medical Policy Manual Topic: Multianalyte Assays with Algorithmic Analysis for the Evaluation and Monitoring of Patients with Chronic Liver Disease Date of Origin: June 2013 Section: Laboratory Last Reviewed
The Regression of Liver Fibrosis in HCV Cirrhotic Patients Who Registered SVR after DAA Therapy
 The Regression of Liver Fibrosis in HCV Cirrhotic Patients Who Registered SVR after DAA Therapy Leustean Anca 1,2, Nastase Raluca 1, Molagic Violeta 1, Radulescu Mihaela 1,2, Munteanu Daniela 1,2, Tiliscan
The Regression of Liver Fibrosis in HCV Cirrhotic Patients Who Registered SVR after DAA Therapy Leustean Anca 1,2, Nastase Raluca 1, Molagic Violeta 1, Radulescu Mihaela 1,2, Munteanu Daniela 1,2, Tiliscan
National Horizon Scanning Centre. Transient elastography (FibroScan) for evaluating liver fibrosis. April 2008
 Transient elastography (FibroScan) for evaluating liver fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Transient elastography (FibroScan) for evaluating liver fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
NONINVASIVE IMAGING METHODS FOR ASSESSMENT OF LIVER DAMAGE IN NASH
 NONINVASIVE IMAGING METHODS FOR ASSESSMENT OF LIVER DAMAGE IN NASH Bachir Taouli, MD Director of Body MRI and Cancer Imaging Program Department of Radiology / Translational and Molecular Imaging Institute
NONINVASIVE IMAGING METHODS FOR ASSESSMENT OF LIVER DAMAGE IN NASH Bachir Taouli, MD Director of Body MRI and Cancer Imaging Program Department of Radiology / Translational and Molecular Imaging Institute
Pretreatment Evaluation
 Pretreatment Evaluation Disclosures Research supported by Gilead Sciences Inc.: Site investigator for HIV/HCV SWITCH Registry Study Key personnel for FOCUS HCV Screening Program through Vanderbilt University
Pretreatment Evaluation Disclosures Research supported by Gilead Sciences Inc.: Site investigator for HIV/HCV SWITCH Registry Study Key personnel for FOCUS HCV Screening Program through Vanderbilt University
Multianalyte assays with algorithmic analyses are considered investigational for the diagnosis or monitoring of patients with chronic liver disease.
 2.04.41 Multianalyte Assays with Algorithmic Analysis for the Evaluation and Monitoring of Patients with Chronic Liver Disease Section 2.0 Medicine Subsection 2.04 Pathology/Laboratory Effective Date September
2.04.41 Multianalyte Assays with Algorithmic Analysis for the Evaluation and Monitoring of Patients with Chronic Liver Disease Section 2.0 Medicine Subsection 2.04 Pathology/Laboratory Effective Date September
Hepatology for the Nonhepatologist
 Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
