Mandatory Reflex Testing Approved by Medical Executive Committee (MEC) October 2018
|
|
|
- Bernadette Tucker
- 5 years ago
- Views:
Transcription
1 Mandatory Reflex Testing Approved by Medical Executive Committee (MEC) October 2018 INITIAL TEST and RESULT All newly diagnosed patients with Acute Myeloid Leukemia under age 80, unless otherwise specified by physician. (Bone Marrow or Whole Blood) aptt >150 without clot detection aptt Mixing Study aptt Mixing Studies (Test Code 4120) greater than or equal to 6 seconds above the normal range. Anaerobic Culture Antibody Screens AntiNuclear Antibody (ANA) EIA Screen AntiNuclear Antibody (ANA) Hep-2 Substrate with Reflex positive Blood Culture (Test Codes 8894 & 8896); if positive for growth of bacteria All newly diagnosed patients with B-Cell Lymphomas under age 80, unless otherwise specified by physician All patients with Invasive ductal, lobular, mixed ductal/lobular, mammary NOS, or micrometastatic lymph node breast carcinoma meeting the following criteria: Age less than 70 years Not pure tubular, mucinous, or colloid carcinoma (grade 1 special subtypes with good prognosis) Tumor is pathologic stage pt1b, T1c, T2, or T3 Tumor is not stage T4 Tumor is pathologic stage N0 or N1mi Tumor is ER positive and Her2/neu negative Not post-treatment (y), recurrent tumor (r) or with known distant metastatic tumor (M1) All newly diagnosed or previously untested Adult Granulosa Cell Tumors, Serous Boderline Tumors, and Low Grade Serous Carcinomas Bone Marrow samples with clinical indications for possible need Heme Molecular Sequence Analysis Unfractionated Heparin APTT and, if the APTT Mixing Study does not correct, order a Lupus Anticoagulant Screen (LA1) Heparin Neutralization (Policy 6679) to rule out anticoagulant: If aptt corrects to normal, then a Mixing Study is not indicated. If aptt remains elevated, then Unfractionated Heparin Level (Test Code 8101) assay is ordered. If Unfractionated Heparin Level is greater than 1.0 U/mL, then a Mixing Study is not indicated. Aerobic culture on all orders that do not already have an aerobic culture ordered on the same specimen. ABO/RH AntiNuclear Antibody (ANA) Hep-2 Substrate with Reflex positive AntiNuclear Antibody (ANA) Titer and Pattern Bacterial identification will be performed if growth occurs any bottle. An antibiotic susceptibility will be performed on all pathogenic isolates. MyD testing on bone marrow aspirate Send for Oncotype DX testing: 1) If multifocal,and meet above criteria 2). Perform Oncotype on largest primary tumor (if same histology) 3) Perform Oncotype on up to three primary tumors (if different histology), indicate order of testing to Oncotype (as they will stop further testing if high recurrence score is resulted 4) If bilateral, perform Oncotype on both sides. 5) If high risk tumor: stage (T4, N1b+), ER-, or Her2 positive concurrent cancer that would not reflex, do not reflect test lower risk tumor(s) IHC for Estrogen and Progesterone receptors Sort CD138 to purify (concentrate) sample and hold
2 INITIAL TEST and RESULT to perform FISH Myltiple Myeloma Panel for possible FISH Multiple Myeloma Panel Diagnostic sample of B-lymphoblastic leukemia (B-ALL) and B-cell non-hodgkin lymphoma with anti CD-19 therapy Flow cytometry Blinatumomab tube (anti-cd19 therapy tubes) CBC w/man diff - will reflex to CBC w/out diff "if" the WBC is less CBC w/out differential than 0.4. CBC w/man diff - will reflex to CBC w/out diff "if" requested within CBC w/out differential in 24 hours. Flow cytometry testing 1. CBC w diff on all orders that do not already have a CBC w diff ordered on patient on the same date and specimen is less than 10 hours old. 2. And Flow cytometry is unable to get WBC and automated differential. Celiac Disease Cascade Normal IgA, negative TTG (IgA at or above Normal Low for patient's age, TTG IgA <=6.9) Celiac TTG Ab IgA and Celiac Total IgA performed initially (Celiac disease unlikely. Further reflex testing not indicated.) Normal IgA, positive TTG (IgA at or above Normal Low for patient's age, TTG IgA >=10.1) (Celiac disease likely. Further reflex testing not indicated.) Normal IgA, equivocal TTG (IgA at or above Normal Low for patient's age, TTG IgA ) (Endomysial Ab IgA ordered by reflex rule for further evaluation) Decreased IgA, negative TTG (IgA below Normal Low for patient's age, TTG IgA <=6.9), (Transglutaminase Ab IgG, Gliadin Ab IgG, and Gliadin Ab IgA ordered by reflex rule for further evaluation.) Decreased IgA, positive TTG IgA (IgA below Normal Low for patient's age, TTG IgA>=10.1) (Transglutaminase Ab IgG, Gliadin Ab IgG, and Gliadin Ab IgA ordered by reflex rule for further evaluation.) Decreased IgA, equivocal TTG IgA (IgA below Normal Low for patient's age, TTG IgA ): (Endomysial Ab IgA, Transglutaminase Ab IgG, Gliadin Ab IgG, and Gliadin Ab IgA ordered by reflex rules for further evaluation. Cerebral Spinal Fluid (CSF) If Protein Electrophoresis [Test Code 8651] is requested IgG Index Synthesis CSF Careset [Test Code 11593], IgG Index Serum [Test Code 11592] Cerebral Spinal Fluid (CSF) RBC Cell Count greater than or equal to Additional count of tube 1 25 cells in tube 3 Cerebral Spinal Fluid (CSF) WBC Cell Count greater than 0, in tube Manual differential 3 Cryptococcus Antigen ordered on CSF Cryptococcus CSF Panel (Cryptococcus Ag and
3 INITIAL TEST and RESULT Clostridium difficile by EIA Toxin Positive Antigen Negative Toxin Negative Antigen Positive Culture from catheter tip or foreign bodies Direct Antiglobulin Mononucleosis Screen, Epstein Barr (EBV) IgM if Negative (Test Code 236): If Mononucleosis Screen is Negative All Hematopoietic neoplasms Hemoglobin (Hgb) A2 result is greater than 10% Hemoglobin Fractionation that identifies new Hemoglobin S Hepatitis C Genotype w/amplification: Positive Hepatitis C Virus amplification result Her2/neu immunohistochemistry 2+ positive Herpes Simplex, IgM, Antibody Screen result is positive or equivocal HIV 1/2 Antibody EIA and Quick Test If HIV 1/2 Antibody is positive HIV Quick (Spectrum Health Regional Lab only) Immunohistochemical (IHC) Stain [Test Code 1098] All newly diagnosed patients with endometrial cancer, including cases of recurrence when no IHC was previously performed. a) If MLH1 is absent: b) If PMS2, MSH2, and/or MSH6 is/are absent: Fungal Culture) Clostridium difficile by PCR Culture catheter/device ABO/RH Epstein Barr (EBV) VCA IgM Acute Antibody (Test Code 4268) DNA extraction and hold, cytogenetics, and flow cytometry Capillarys Hemoglobin Electrophoresis (to confirm Hgb E) Sickle Cell Screen Hepatitis C Genotype FISH for Her2/neu Herpes Ab IgM IFA a) HIV Quick test. If Positive: reflex to Roche HIV. b) Roche HIV: If positive for Antibody then reflex to Geenius for confirmation. (Positive Antigens do not reflex; HIV RNA testing requires a new order and a new specimen). Cancel and reorder as HIV ½ Antibody EIA MLH1 Promoter Hypermethylation Analysis [Test Code 7091] If hypermethylation is absent: refer to genetics consult. Refer to genetics consult.
4 INITIAL TEST and RESULT Leukemia/Lymphoma/Myeloma and/or Non-Hodgkin lymphoma panels by flow cytometry: if indicated, reflex testing may be added to further characterize possible abnormal cell populations identified by the screening panel. These panels are reviewed continuously in multidisciplinary conferences and by the flow cytometry laboratory and hematopathologists. NICU patients with a cord blood workup Non SHGR 1) Patients with a diagnosis of Influenza during Influenza season 2) Patients with a diagnosis of Influenza during Non-influenza season The following add on panels may be employed after initial testing, as needed and appropriate, to further evaluate any possible abnormal population of cells. B lymphoblastic leukemia (B-ALL) panel T lymphoblastic leukemia (T-ALL) panel Chronic lymphocytic leukemia (CLL) panel Hairy cell leukemia (HCL) panel Extended B-cell tube panel Extended T-cell tube panel NK cell or LGL panel CD10 positive B-cell panel CD5 positive B-cell panel Acute myeloid leukemia (AML) panel Extended myeloid or monocytic panel Plasma cell panel Mast cell panel Solid tumor panel Neuroblastoma panel Antibody Screen Influenza (Flu) A/B Rapid [test code 4626]; If Influenza (Flu) A/B Rapid is Negative, then Influenza (Flu) A/B PCR [test code 156] Influenza (Flu) A/B PCR [test code 156] SHGR: Influenza A/B Rapid (Test Code 155); if negative Influenza A/B PCR (Test Code 156) Adults (pts >17) Inpatient or Observational at Butterworth or Blodgett with active warfarin order and no active INR order Malaria Rapid Screen (Test Code: 7087) 1) If presumptive positive for malaria antigens 2) If presumptive negative for malaria antigens Cerner will automatically order a PT/INR when: The rule is evoked upon administration of a warfarin dose being signed in the emar. Once evoked the rule looks for: 1. Pt age > 17 years, 2. Inpatient, Observation enounters at BW, BL if 1 and 2 are true then rule keeps evaluating for, 3. an active PT(with INR) order with a requested date and time within a future 24 hours of the administration of the warfarin dose. or 4. an active PT (with INR) with a frequency of; q 24hr, q am, q daily. (child order to be placed approx 00:30 with an ops job) If neither 3 or 4 of these conditions are met the rule will order orderable Protime (with INR) w/ order entry fields. 1) Parasitemia Level (Test Code: 7089); Malaria speciation confirmation by thin/thick smear microscopy evaluation 2) Negative result confirmed by thin/thick smear microscopy evaluation. If smear is positive,
5 INITIAL TEST and RESULT then reflex to Parasitemia Level (Test Code: 7089) Myeloproliferative Neoplasms (MPN) If negative for JAK2 V617F mutation (Test Code 7093) Then MPN Expanded Panel (Test Code 7090) All newly diagnosed of MDS and MDS/MPN Heme molecular Sequence Analysis on all adult Under age 80 patients Pathologist Review (Test Code: 8367) If review of peripheral blood smear is ordered without required Complete Blood Count (CBC) with Differential accompanying CBC with differential, and if the specimen is within (Test Code: 8411) 10 hours of collection. Platelet Count less than 75,000/µL Immature platelet fraction (IPF) Patient is less than 6 months of age and has suspected Hgb S by Capillarys Hemoglobin Electrophoresis the Hgb fractionation test Patient is suspected of having Hgb C by the Hgb fractionation test Capillarys Hemoglobin Electrophoresis Platelet Aggregation Studies Platelet Function Assay (PFA 100) with Collagen/Epinephrine results greater than 180 seconds. Platelet Function Assay (PFA) Positive Antibody Screen, or a positive Direct Antiglobulin Test (DAT) on inpatients and surgical patients Positive culture for pathogen or organism with clinically significant concentration (bacteria or mycobacteria) Positive Cryoglobulin test Positive Group B Strep, Penicillin allergy, PCR on OB patients Positive Hepatitis B surface antigen Positive Lyme Disease Screen Positive Amphetamine, Cannabinoids, Ethanol, Methadone, opiates, Oxycodone or cocaine on a Drug of Abuse screen for Inpatient Obstetric Patients (Mothers and their babies). Positive opiates on Comprehensice Drug Screens Positive Gamma HydroxyButyrate (GHB) Positive prenatal Profile Type &Antibody Screen Prostate Specific Antigen (PSA) Free Level Positive Syphilis IgG Antibody Rapid Plasma Reagin (RPR) test for syphilis Prothrombin Time (PT) Mixing Study Random urine microalbumin Sputum Culture without gram stain order Sputum Gram Stain without culture Thyroglobulin Type & Screen (T&S) on a patient with autologous or directed Platelet Count Collagen/ADP test Platelet Count and hematocrit Relevant studies as needed including antibody identification, antigen typing, elution and absorption. In addition, packed blood cells will be antigen typed and crossmatched. Susceptibility and typing as necessary. Positive Cryoglobulins which have not had an identification in the past 2 months will have the Reflex Cryoglobulin Interpretation ordered. Susceptibility testing. HbsAg Confirmation test Western Blot GC/MS Confirmation GC/MS Confirmation GC/MS Confirmation Antibody identification with titer if identified antibody is clinically significant PSA total on all orders that do not already have a PSA ordered on the same specimen. RPR Titer at Spectrum Health, also confirmation by MHA-TPA at State Health Department SYPHILIS IgG SCREEN will be performed instead of the RPR. PT and, if the PT Mixing Study does not correct, order a Lupus Anticoagulant Screen (LA1) Urine creatinine Gram stain for specimen suitability. If unsuitable, culture is cancelled. Sputum Culture ordered. If Gram Stain is unsuitable, culture is cancelled. Automatically cancel AntiTgAB request when ordered with srtg Crossmatch of the units
6 INITIAL TEST and RESULT units Type & Screen (T&S) on a pre-op patient with an antibody Metastatic Gastric and Esophageal Adenocarcinomas Crossmatch of two antigen negative units Perform Her2/neu immunohistochemistry. When metastasis is pathologically confirmed (can use either metastatic site or primary tumor). Equivocal immuno results will reflex to FISH testing. All colorectal carcinoma resections at Butterworth and Blodgett Immunohistochemical (IHC) protein studies for Hospitals MSH2, MSH6, MLH1, PMS2 [Test Code 1098] PCR for Microsatellite Instability (MSI)[Test Code 904] Additional reflex to BRAF [Test Code 709], if MSI-H with loss of MLH1 on IHC Metastatic Colorectal Carcinoma Colon Mutation Analysis Panel (Test Code 7094) -includes genotyping for KRAS, NRAS, and BRAF mutations All Diffuse Large B-cell Lymphoma/other B-cell Lymphoma 1. Immunohistochemical/In situ hybridization staining to include: CD3, CD20, CD5, CD10, BCL-1, Ki-67, EBERish, BCL-2, BCL-6, MUM1, MYC, CD30, and CD45 2. Cytogenetic FISH testing to include: BCL-2, BCL-6, and MYC rearrangement testing. All newly diagnosed lung (non-small cell) adenocarcinomas Molecular Lung Cancer Panel (Test Code 7030) Metastatic melanoma Including Cases with Positive lymph nodes BRAF (Test Code 709) Glioblastoma (brain) Grade 4 Anaplastic Astrocytoma IDH-wildtype MGMT Methylation Analysis [Test Code706] p53 [Test Code1098] IDH1 [Test Code 1098] Cancer Hotspot analysis Glioblastoma (brain) Grade 4 Recurrent Glioblastoma initially MGMT positive (Methylated) MGMT Methylation Analysis [Test Code706] Glioma Grade 2 and 3 p53 [Test Code 1098] IDH1 [Test Code 1098] Del 1p/19q by FISH [Test Code 955] Ki-67 [Test Code 1098] ATRX by Immunohistochemistry All newly diagnosed or previously untested oropharyngeal squamous cell carcinomas p16 immunohistochemistry (surrogate marker for HPV) Pain Management Panel If positive screen result: o Amphetamines-reflex to methamphetamine and amphetamine if screen positive Barbituarates-reflex to Butalbital, Secobarbital, and Pentobarbital if screen positive o Benzodiazapines-reflex to Benzodiazapines Urine Level Panel if screen positive o Cannabinoids-reflex to confirmation if positive o Cocaine Metabolite-reflex to confirmation if screen positive o Ethanol-reflex to confirmation if screen positive o Methadone Metabolite-reflex to Methadone and EDDP if screen positive UA or UA culture if (where volume is inadequate for microscopic exam Antibody Titer Urine Dipstick (U dip) ABO/Rh and Antibody Screen
7 INITIAL TEST and RESULT Tissue Specimens ordered as a Body Fluid Culture Body Fluid Specimens ordered as a Tissue Culture Specimens in Abbott Multi Collect tube ordered as Aptima specimens Specimens in Aptima collection tube ordered as Abbott Multi Collect tube specimens Lupus Screen LA1 elevated Heparin Dependent Antibody (HIT) Positive or Borderline Platelet Aggregation Cancel and Order as a Tissue Culture Cancel and Order as a Body Fluid Culture Cancel Aptima test and reorder corresponding Abbott test for Chlamydia PCR, Gonococcus PCR Cancel Abbott test and reorder corresponding Aptima test for Chlamydia NAAT, Gonococcus NAAT, or Trichomonas Only LA2 if elevated perform Protime and aptt if these are elevated perform mixing study for Protime/aPTT Pathologist interpretation reported with elevated results and/or mixing study Serotonin Release Assay (SRA) Hematocrit
8 Optional Reflex Testing ORDER INITIAL TEST and RESULT OPTIONAL FOLLOW UP TESTING ANA Screen ANA EIA if positive ANA Hep2 (IFA) if positive reflex to titer. If titer is greater than 1:160 reflex to AntidsDNA, anti-sm, anti-rnp, anti-ssa, anti-ssb, anti Scl70, anti-centromere and anti-jo1 Breast Biopsy Primary and recurrent/metastatic Invasive Mammary Carcinoma (excisional biopsy / lumpectomy / mastectomy) Immunohistochemistry for estrogen receptor, progesterone receptor, Her2/neu protein Breast Biopsy Ductal Carcinoma In Situ Immunohistochemistry stains for estrogen receptor, progesterone CBC w/diff WBC less than 3.0 or greater than 18.0 Manual WBC differential HGB less than 8.0 or greater than 18.0 MCV less than 75.0 or greater than Absolute neut count less than 1.50 or greater than 9.00 Absolute lymph count less than 0.39 or greater than 4.50 Absolute mono count greater than 1.50 Absolute eos count greater than 1.00 Absolute bas count greater than 0.20 Abnormal instrument flags suggesting abnormality CBC CBC specimens that fulfill criteria listed in Pathologist review Pathologist Review (Hematology #6783) CBC w/ Diff CBC with any of the following criteria: -WBC less 1.5 x10 3 /µl and not due to known chemotherapy -WBC greater than 30.0 x10 3 /µl and not due to known acute infection, acute trama or steroids -HGB less than 7.0 and not due to know acute blood loss -HGB greater than 19.0 (adult only) -MCV less than 60 fl.0 or greater than fl -NRBC greater than 10 in 100 manual cell differential (not newborn) -Moderate or many:schistocytes, spherocytes, teardrops on erythrocyte morphology -Absolute neut count less than 0.50 x10 3 /µl (adult only) -Absolute lymph count greater than 5.0 x10 3 /µl(adult only) -Absolute mono count greater than 2.00 x10 3 /µl and greater than 30% on differential (adult only) -Absolute eos count greater than 2.00 x10 3 /µl -Absolute bas count greater than 0.50 x10 3 /µl - Blasts or immature cells on manual differential -Abnormal or atyptical lymphocytes -Platelets less than 50 x10 3 /µl or greater Pathologist Review
9 ORDER INITIAL TEST and RESULT OPTIONAL FOLLOW UP TESTING than 600 x10 3 /µl -Circulating megakaryocytes Cell Ct only BFL Body fluid specimens that fulfill criteria listed in Pathologist Review (Hematology #6783) Pathologist review Fetal Cells by Flow Cytometry Triglyceride do LDL Direct if >400 Lipid Panel do LDL Direct if Triglycerides >400 Pap do HPV if PAP Test - HPV if ASCUS or AGUS Ordered STAT and received in lab outside of flow cytometry testing hours (after 3:30 Mon-Fri or after 10:00am Sat or anytime Sunday) LDL Direct if the triglycerides are greater than 400. LDL Direct if the triglycerides are greater than 400. Cervical Cytology with ASCUS, ASC-H OR LSIL Cervical Cytology with ASCUS or AGUS Fetal Hemoglobin by Kleihauer Betke performed in place of flow cytometry test LDL Direct LDL Direct HPV-high risk PAP Test - HPV if NIL, Cervical Cytology with NIL, ASCUS or HPV ASCUS or AGUS AGUS Preg Serum Quant HCG greater than 5 miu/ml Progesterone level Progesterone if PSA Sym FPSA if PSA between 2.5 and 10 ng/ml Free PSA Serum Protein Electrophoresis Do IFE if indicated Serum Protein Electrophoresis with paraprotein found or elevation of protein fraction in electrophoresis pattern. Immunofixation for identification. 24 hour Urine Protein Electrophoresis Do IFE if indicated Random Urine Protein Electrophoresis Do IFE if indicated Urine Protein Electrophoresis with paraprotein found or elevation of protein fraction in electrophoresis pattern. Urine Protein Electrophoresis with paraprotein found or elevation of protein fraction in electrophoresis pattern. Strep A, PCR if Negative Negative rapid Strep A Throat Culture TSH FT4 if indicated TSH less than 0.3 OR greater than 5.0 mcu/ml Biopsy of Thyroid Aspirate Diagnosis of atypical follicular neoplasm of uncertain significance and suspicious for follicular/hurthle cell neoplasm (Bethesda Classification-Categories 3 and 4) HPV Immunofixation for identification. Immunofixation for identification. Free T4 Afirma GEC (Veracyte) Thyroid Function Cascade 1. TSH above TSH below 0.3 a. TSH below 0.1 and FT4 below 1.6 UA culture if Urinalysis (UA) with two or more of the following abnormal findings, provided there are less than 10 squamous epithelial cells observed per high power field: -Greater than or equal to 10 WBC -Positive leukocyte esterase -Positive nitrite 1. Reflex to FT4 and TPO if TSH is high (above 5.0). 2. Reflex to FT4 if TSH is low (below 0.3). a. Reflex to Free T3 if TSH is very low (below 0.1) and FT4 is normal (below 1.6) Culture and sensitivity
10 ORDER INITIAL TEST and RESULT OPTIONAL FOLLOW UP TESTING OR if specimen is -Grossly bloody UA culture if (Where volume is inadequate for microscopic exam ) Hepatitis C Virus Antibody do HCV RNA if indicated Leukemia or Non-Hodgkins Lymphoma Panel by Flow Cytometry Volume is inadequate for microscopic exam and Urine Dipstick (U dip) with one or more of the following abnormal findings: -Positive leukocyte esterase -Positive nitrite Positive or Indeterminate Hepatitis C Virus Antibody result Cell population is diagnostic of circulating leukemia/lymphoma/myeloma Patients under age of 80 with new diagnosis. Culture and sensitivity HCV RNA FISH testing Peanut IgE Reflex Peanut IgE => 0.10 ku/l Peanut component allergen panel (Peanut Ara h 1 IgE, Peanut Ara h 2 IgE, Peanut Ara h 3 IgE, Peanut Ara h 8 IgE, and Peanut Ara h 9 IgE) Egg IgE Reflex Egg white IgE =>0.10 ku/l Egg component allergen Panel (Ovomucoid IgE and Ovalbumin IgE) Milk IgE Reflex Milk (cow) =>0.10 ku/l Milk component allergen Panel (Casein IgE, Alpha-Lactalbumin IgE, and Beta-lactoglobulin IgE) Thyroglobulin Antibody Screen with Reflex If the Thyroglobulin Ab screen result is 22 or greater (Positive), then reflex to Thyroglobulin Mass Spectrometry send out to Mayo Medical Laboratories. If the Thyroglobulin Ab Screen result is <21 (<20, 20, or 21) (Negative), then reflex not indicated. Thyroglobulin Mass Spectrometry (send out to MML #TGMS)
11 Reference Laboratory Required Reflex Algorithms INITIAL TEST and RESULT Paraneoplastic Autoantibody Evaluation, Serum If IFA (83381, 83382, 83137, 83383, 83138, 83076, 83386, 83077) patterns are indeterminate, paraneoplastic autoantibody Western blot is performed at an additional charge. If client requests or if IFA patterns suggests CRMP-5-IgG, CRMP-5-IgG Western blot is performed at an additional charge. If calcium channel P/Q-Type or N-Type is greater than 20, CRMP-5-IgG Western blot is performed at an additional charge. If IFA patterns suggests GAD65 antibody, GAD65 antibody radioimmunoassay is performed at an additional charge. If Ach receptor binding antibody is greater than 0.02 or if striational antibodies are > or = 1:60, Ach receptor modulating antibodies and CRMP-5-IgG Western blot are performed at an additional charge. If Ach receptor modulating antibodies are 40% or greater loss, radioimmunoassay for Ach receptor blocking antibodies is performed at an additional charge. If AchR ganglionic neuronal antibodies are 0.03 or greater CRMP-5-IgG Western blot is performed at an additional charge. Myasthenia Gravis (MG) Evaluation, Adult If AchR modulating antibodies are % loss or indeterminate, AchR blocking antibodies will be performed at an additional charge. If AchR modulating antibody is >= 90% and striational antibodies are 1:60 or greater, AchR ganglionic neuronal antibody and CRMP-5-IgG Western blot will be performed at an additional charge. C2 Complement, Functional, Serum If the C2 result is less than 15 U/mL, thenc3, C4, and C2AG will be performed at an additional charge. Human T-Cell Lymphotropic Virus-I/II (HTLV-I/II) Antibody, Serum If HTLV I/II Ab is reactive, HTLV I/II Ab confirmation by line immunoassay is performed at an additional charge. Hemolytic Anemia Evaluation This is a consultative evaluation in which the case will be evaluated at Mayo Medical Laboratories, the appropriate tests performed at an additional charge, and the results interpreted.note: #23544 "Reflexed RBC Enzymes, Blood" includes: adenosine deaminase, adenylate kinase, phosphofructokinase, phosphoglycerate kinase, triosephosphate isomerase, and pyrimidine 5'nucleotidase. Glutathione, Blood Hexosaminidase A and Total, Leukocytes/Molecular Reflex If hexosaminidase A is less than 63%, then #82588 "Tay-Sachs Disease, Mutation Analysis, HEXA" will be added and performed at an additional charge. Hemoglobin Electrophoresis Cascade, Blood Level 1 Testing - Hgb electrophoresis cascade will always include: hgb A(2) and F and hgb electrophoresis. If results so warrant, it may also include: agar elec. confirms and hgb S. Level 2 Testing -Hgb electrophoresis cascade performed at additional charge, may include any or all of the following as indicated to identify a rare hemoglobin variant: unstable hgb, IEF confirms, globin elec. confirms, and hgb F red cell distribution. HIV Type-1 RNA Standard Quantification with Reflex to HIV-1 Genotypic Drug Resistance Mutation Analysis, Plasma If HIV-1 RNA titer is 1,000 copies/ml or greater, then HIV-1 genotypic drug resistance mutations will be determined at an additional charge. ADDITIONAL Glutamic Acid Decarboxylase (GAD65) Ab Assay, Serum Ach Receptor (Muscle) Blocking Ab C4 (Fourth Component of Complement), Serum HTLV I/II Ab confirmation by line immunoassay is. Glutathione, Blood Tay-Sachs Diagnosis and Carrier Detection Hemoglobin Electrophoresis Cascade, Level 2 Human Immunodeficiency Virus Type 1 (HIV-1) Genotyping
12 Paraneoplastic Autoantibody Evaluation, Spinal Fluid If IFA (3852, 7472, 21633, 3988, 21632, 21631, 5906, 21650) is indeterminate, paraneoplastic autoantibody Western blot is performed at an additional charge If client requests or if IFA suggests CRMP-5-IgG, CRMP-5-IgG Western blot is performed at an additional charge If IFA suggests GAD65 antibody, GAD65 antibody radioimmuno- preciptation assay is performed at an additional charge Galactosemia Confirmation Test, Blood Testing begins with #8333 "Galactose-1-Phosphate Uridyltransferase (GALT), Blood." If galactose-1-phosphate uridyltransferase is 18.5 U/g or greater of hemoglobin, testing is complete. No molecular test is ordered. If galactose-1-phosphate uridyltransferase is less than 18.5 U/g of hemoglobin, then the galactosemia gene analysis (6 mutation molecular test) will be performed at an additional charge. If quantitative enzyme analysis (#8333 Galactose-1- Phosphate Uridyltransferase [GALT], Blood") and galactosemia gene analysis results are inconsistent (example: no "G" mutation is found in a patient who appears "DG" by quantitative enzyme value), #80341 "Galactose-1-Phosphate Uridyltransferase Biochemical Phenotyping, Erythrocytes" will be performed at an additional charge. A combined interpretive report is issued consistent with tests performed. Alpha-1-Antitrypsin Proteotype S/Z by LC-MS, Serum [Test Code 7049] This test includes Alpha-1-Antitrypsin (82103) and A1AT proteotype for S/Z mutations by LC-MS (83788). When appropriate, an A1AT Phenotype will be performed. Paraneoplastic Autoantibody Western Blot, CSF "Galactose-1- Phosphate Uridyltransferase Biochemical Phenotyping, Erythrocytes" A1AT Phenotype (82104) ADAMTS13 Evaluation ADAMTS13 Evaluation is a reflexive testing algorithm. Activity is always performed. If activity result is <= 30%, the inhibitor assay (1297) will be performed. If inhibitor result is <= 0.7 Inhibitor Units, the antibody assay (1299) will be performed. Blood Center of Wisconsin Alpha-Fetoprotein, Amniotic Fluid [Test Code 1066] If alpha-fetoprotein (AFP) is positive, >2.0 MoM, then acetylcholinesterase (AChE) will be performed at an additional charge. Because false-positive AChE may occur from a bloody tap, specimens with positive AChE results will also be tested for the presence of fetal hemoglobin at no additional charge. Histoplasma Antigen, Urine [8790] If antigen test is indeterminate, the specimen will be sent to Mira Vista Laboratories and Mvista Histoplasma antigen, Urine will be performed at an additional charge. ADAMTS13 Inhibitor assay OR ADAMTS13 Antibody Assay Acetylcholinesterase (AChE) Mayo (8115) Histoplasma Antigen, Urine to Mvista Coccidioides Antibody w/reflex, Serum [8285] If result is positive by EIA screening method, then Coccidioides by complement fixation and immunodiffusion will be performed at an additional charge Coccidioides Ab, CF/ID (RSCOC) Brucella Ab Screen, IgG and IgM, Serum [306] If Brucella Ab Screen, IgG and IgM is positive or equivocal, then Brucella Total Antibody Confirmation, Agglutination will be performed at an additional charge. Brucella Total Antibody Confirmation, Agglutination (BRUTA)
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
REFLEX TESTING LIST March 2012
 Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Reflex Test Protocols
 University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
A written order to request the performance of a laboratory test can be initiated by the following parties:
 Requests/Reporting Requests for Laboratory Services A written order to request the performance of a laboratory test can be initiated by the following parties: Staff physicians and dentists who have been
Requests/Reporting Requests for Laboratory Services A written order to request the performance of a laboratory test can be initiated by the following parties: Staff physicians and dentists who have been
Current September 2017
 1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
Tumor Markers Yesterday, Today & Tomorrow. Steven E. Zimmerman M.D. Vice President & Chief Medical Director
 Tumor Markers Yesterday, Today & Tomorrow Steven E. Zimmerman M.D. Vice President & Chief Medical Director Tumor Marker - Definition Substances produced by cancer cells or other cells in response to cancer
Tumor Markers Yesterday, Today & Tomorrow Steven E. Zimmerman M.D. Vice President & Chief Medical Director Tumor Marker - Definition Substances produced by cancer cells or other cells in response to cancer
Case Workshop of Society for Hematopathology and European Association for Haematopathology
 Case 148 2007 Workshop of Society for Hematopathology and European Association for Haematopathology Robert P Hasserjian Department of Pathology Massachusetts General Hospital Boston, MA Clinical history
Case 148 2007 Workshop of Society for Hematopathology and European Association for Haematopathology Robert P Hasserjian Department of Pathology Massachusetts General Hospital Boston, MA Clinical history
TEST LIST SAMPLE REQUIREMENT. 1 ml serum None
 ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
Rapid Laboratories In House Tests
 Electrolytes CL CL (CHLORIDE) Electrolytes CO2 CO2 (BICARBONATE) Electrolytes K K (POTASSIUM) Electrolytes NA NA (SODIUM) Basic Metabolic Panel (BMP) GLU GLU (GLUCOSE) Basic Metabolic Panel (BMP) CA CA
Electrolytes CL CL (CHLORIDE) Electrolytes CO2 CO2 (BICARBONATE) Electrolytes K K (POTASSIUM) Electrolytes NA NA (SODIUM) Basic Metabolic Panel (BMP) GLU GLU (GLUCOSE) Basic Metabolic Panel (BMP) CA CA
1. Purpose 1.1. To define testing locations, schedule and order priority for each test performed in the core laboratory.
 Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
In-OfficeLabTesting. Effective date: August 1, 2017
 Effective date: August 1, 2017 In-OfficeLabTesting The lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted
Effective date: August 1, 2017 In-OfficeLabTesting The lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted
Sutter Health Plus Effective for Calendar Year 2015
 Sutter Health Plus Effective for Calendar Year 2015 CPTs CPT Descriptions 2015 Cost Under Deducible (Single Unit) Doctor's Office Visit for a New Patient (Also Urgent Care) 99201 Low Level Visit $99.00
Sutter Health Plus Effective for Calendar Year 2015 CPTs CPT Descriptions 2015 Cost Under Deducible (Single Unit) Doctor's Office Visit for a New Patient (Also Urgent Care) 99201 Low Level Visit $99.00
Collect and label sample according to standard protocols. Gently invert tube 8-10 times immediately after draw. DO NOT SHAKE. Do not centrifuge.
 Complete Blood Count CPT Code: CBC with Differential: 85025 CBC without Differential: 85027 Order Code: CBC with Differential: C915 Includes: White blood cell, Red blood cell, Hematocrit, Hemoglobin, MCV,
Complete Blood Count CPT Code: CBC with Differential: 85025 CBC without Differential: 85027 Order Code: CBC with Differential: C915 Includes: White blood cell, Red blood cell, Hematocrit, Hemoglobin, MCV,
In Office Lab Testing
 Effective January 1, 201, the lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted laboratory providers.
Effective January 1, 201, the lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted laboratory providers.
Chapter 17 Worksheet Code It
 Class: Date: Chapter 17 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. CPT laboratory codes include collection of the specimen. 2. A dipstick is a small piece of
Class: Date: Chapter 17 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. CPT laboratory codes include collection of the specimen. 2. A dipstick is a small piece of
Out-Patient Billing CPT Codes
 Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
ADx Bone Marrow Report. Patient Information Referring Physician Specimen Information
 ADx Bone Marrow Report Patient Information Referring Physician Specimen Information Patient Name: Specimen: Bone Marrow Site: Left iliac Physician: Accession #: ID#: Reported: 08/19/2014 - CHRONIC MYELOGENOUS
ADx Bone Marrow Report Patient Information Referring Physician Specimen Information Patient Name: Specimen: Bone Marrow Site: Left iliac Physician: Accession #: ID#: Reported: 08/19/2014 - CHRONIC MYELOGENOUS
Molecular. Oncology & Pathology. Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine. Liquid Biopsy.
 Molecular Oncology & Pathology Hereditary Cancer Somatic Cancer Liquid Biopsy Next-Gen Sequencing qpcr Sanger Sequencing Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine
Molecular Oncology & Pathology Hereditary Cancer Somatic Cancer Liquid Biopsy Next-Gen Sequencing qpcr Sanger Sequencing Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine
Results that require action
 Following is a list of Allina Health Laboratory Results That Require Action (formerly Critical Values). Results That Require Action describes a lab test result that may indicate a lifethreatening situation.
Following is a list of Allina Health Laboratory Results That Require Action (formerly Critical Values). Results That Require Action describes a lab test result that may indicate a lifethreatening situation.
Beyond the CBC Report: Extended Laboratory Testing in the Evaluation for Hematologic Neoplasia Disclosure
 Beyond the CBC Report: Extended Laboratory Testing in the Evaluation for Hematologic Neoplasia Disclosure I am receiving an honorarium from Sysmex for today s presentation. 1 Determining the Etiology for
Beyond the CBC Report: Extended Laboratory Testing in the Evaluation for Hematologic Neoplasia Disclosure I am receiving an honorarium from Sysmex for today s presentation. 1 Determining the Etiology for
Recommendations for Celiac Disease Testing. IgA & ttg IgA
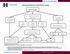 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
ALPS Screen Order Set B-Cell Panel Order Set (% and absolute #) (% and Absolute #) ALPS (CD3+/CD4-/CD8-/TCR αβ+)
 All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
Specific Panels. Celiac disease panel. Pancreas Panel:
 Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Laboratory Values, Critical. ThedaCare. Date Last Reviewed: 4/5/2018 Reviewing Body(s): Quality; Laboratory Leadership
 Laboratory Values, Critical ThedaCare Policy & Procedure Policy Title: Laboratory Values, Critical Policy Number: 694 Location(s): All ThedaCare Department(s): Laboratory Date Last Reviewed: 4/5/2018 Reviewing
Laboratory Values, Critical ThedaCare Policy & Procedure Policy Title: Laboratory Values, Critical Policy Number: 694 Location(s): All ThedaCare Department(s): Laboratory Date Last Reviewed: 4/5/2018 Reviewing
2007 Workshop of Society for Hematopathology & European Association for Hematopathology Indianapolis, IN, USA Case # 228
 2007 Workshop of Society for Hematopathology & European Association for Hematopathology Indianapolis, IN, USA Case # 228 Vishnu V. B Reddy, MD University of Alabama at Birmingham Birmingham, AL USA 11/03/07
2007 Workshop of Society for Hematopathology & European Association for Hematopathology Indianapolis, IN, USA Case # 228 Vishnu V. B Reddy, MD University of Alabama at Birmingham Birmingham, AL USA 11/03/07
2013 AAIM Pathology Workshop
 2013 AAIM Pathology Workshop John Schmieg, M.D., Ph.D. None Disclosures 1 Pathology Workshop Objectives Define the general philosophy of reviewing pathology reports Review the various components of Bone
2013 AAIM Pathology Workshop John Schmieg, M.D., Ph.D. None Disclosures 1 Pathology Workshop Objectives Define the general philosophy of reviewing pathology reports Review the various components of Bone
A Look Into the Determination of Cell Morphology in Hematology in the 21 st Century. Ramon Simon-Lopez, MD Global Scientific Director Beckman Coulter
 A Look Into the Determination of Cell Morphology in Hematology in the 21 st Century Ramon Simon-Lopez, MD Global Scientific Director Beckman Coulter Is cell morphology important? AML M7 CLL CD5 CD19 NHL
A Look Into the Determination of Cell Morphology in Hematology in the 21 st Century Ramon Simon-Lopez, MD Global Scientific Director Beckman Coulter Is cell morphology important? AML M7 CLL CD5 CD19 NHL
2014 Notice to Physicians
 2014 Notice to Physicians August 20, 2014 Dear Physician, will only pay for tests that are deemed medically necessary. These regulations impact physicians who order the tests as well as the laboratories
2014 Notice to Physicians August 20, 2014 Dear Physician, will only pay for tests that are deemed medically necessary. These regulations impact physicians who order the tests as well as the laboratories
Alaska Native Medical Center Anchorage, AK
 ANMC Lab Test Requirements Key: Room Temp (20-25C), Refrigerated (2-8C), (-15 to -25C), Hr (Hours), D (Days), W (Weeks), Mo (Months), Yr (Years). Basic Processing Instructions: Centrifuge all blood specimens
ANMC Lab Test Requirements Key: Room Temp (20-25C), Refrigerated (2-8C), (-15 to -25C), Hr (Hours), D (Days), W (Weeks), Mo (Months), Yr (Years). Basic Processing Instructions: Centrifuge all blood specimens
Hematology & Coagulation Practicum Objectives CLS - 647
 Hematology & Coagulation Practicum Objectives CLS - 647 The following objectives are to be completed by the student for successful completion of this clinical rotation. The objectives within the psychomotor
Hematology & Coagulation Practicum Objectives CLS - 647 The following objectives are to be completed by the student for successful completion of this clinical rotation. The objectives within the psychomotor
Molecular Testing Updates. Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine
 Molecular Testing Updates Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine Keeping Up with Predictive Molecular Testing in Oncology: Technical
Molecular Testing Updates Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine Keeping Up with Predictive Molecular Testing in Oncology: Technical
BID ANNUAL CONTRACT FOR LAB TESTING
 1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
Test Result Reference Range Flag
 Date of Last Result Test Result Reference Range Flag Dec 07, 2016 25-Hydroxy Vitamin D Total 53 ng/ml 30-100 ng/ml Activated Partial Thromboplast Time Alanine Aminotransferase (ALT/SGPT) 25 sec 24-35 sec
Date of Last Result Test Result Reference Range Flag Dec 07, 2016 25-Hydroxy Vitamin D Total 53 ng/ml 30-100 ng/ml Activated Partial Thromboplast Time Alanine Aminotransferase (ALT/SGPT) 25 sec 24-35 sec
Change Log V1.3- v1.4
 Change Log V1.3- v1.4 This document shows the changes that were made to the SSDI manual and the Grade manual for the SEER*RSA version 1.4 release on (Date TBD). SSDI Manual Section: General Instructions
Change Log V1.3- v1.4 This document shows the changes that were made to the SSDI manual and the Grade manual for the SEER*RSA version 1.4 release on (Date TBD). SSDI Manual Section: General Instructions
Aerobic Wound Culture and Stain
 Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: 6000153 Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature
Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: 6000153 Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Lymphoma Case Scenario 1
 Lymphoma Case Scenario 1 HISTORY: A 23-year-old healthy female presented with a month-long history of persistent headache of increasing severity. She noted episodic nausea and vomiting in association with
Lymphoma Case Scenario 1 HISTORY: A 23-year-old healthy female presented with a month-long history of persistent headache of increasing severity. She noted episodic nausea and vomiting in association with
Overseas Referred Tests 2014
 OVERSEAS REFERRALS Listed below are some of the tests currently being offered by CML via Quest Diagnostics and Quest Nichols Institute The fees are quoted in US$ and a JA$ handling charge is also applicable.
OVERSEAS REFERRALS Listed below are some of the tests currently being offered by CML via Quest Diagnostics and Quest Nichols Institute The fees are quoted in US$ and a JA$ handling charge is also applicable.
OHIOHEALTH Laboratory Services INPATIENT, ED AND OTHER HOSPITAL BASED PATIENT CRITICAL VALUE NOTIFICATION LIST
 11/5/2018 OHIOHEALTH Laboratory Services INPATIENT, ED AND OTHER HOSPITAL BASED PATIENT CRITICAL VALUE NOTIFICATION LIST Test Critical Low Critical High Alerting CHEMISTRY Amylase > 400 U/L Emergency Dept
11/5/2018 OHIOHEALTH Laboratory Services INPATIENT, ED AND OTHER HOSPITAL BASED PATIENT CRITICAL VALUE NOTIFICATION LIST Test Critical Low Critical High Alerting CHEMISTRY Amylase > 400 U/L Emergency Dept
Kimberly Rohan ANP-BC, AOCN Nurse Practitioner Edward Cancer Center
 Kimberly Rohan ANP-BC, AOCN Nurse Practitioner Edward Cancer Center Objective The nurse will be able to explain biomarkers and their implications to patient s prescribed treatment plan Biomarkers Use in
Kimberly Rohan ANP-BC, AOCN Nurse Practitioner Edward Cancer Center Objective The nurse will be able to explain biomarkers and their implications to patient s prescribed treatment plan Biomarkers Use in
Diagnostic Approach for Eosinophilia and Mastocytosis. Curtis A. Hanson, M.D.
 Diagnostic Approach for Eosinophilia and Mastocytosis Curtis A. Hanson, M.D. 2014 MFMER slide-1 DISCLOSURES: Relevant Financial Relationship(s) None Off Label Usage None 2014 MFMER slide-2 Molecular Classification
Diagnostic Approach for Eosinophilia and Mastocytosis Curtis A. Hanson, M.D. 2014 MFMER slide-1 DISCLOSURES: Relevant Financial Relationship(s) None Off Label Usage None 2014 MFMER slide-2 Molecular Classification
Case Log Number(s) Veterinarian or VTS Accurately report test results, using appropriate units of measurement Quality Control/Assurance Date Mastered
 AVCPT Skills List Candidate: Understanding of test methodology, techniques and ability to perform testing must be applied to each skill. The overall goal is to provide accurate and valid results to assist
AVCPT Skills List Candidate: Understanding of test methodology, techniques and ability to perform testing must be applied to each skill. The overall goal is to provide accurate and valid results to assist
Applications of IHC. Determination of the primary site in metastatic tumors of unknown origin
 Applications of IHC Determination of the primary site in metastatic tumors of unknown origin Classification of tumors that appear 'undifferentiated' by standard light microscopy Precise classification
Applications of IHC Determination of the primary site in metastatic tumors of unknown origin Classification of tumors that appear 'undifferentiated' by standard light microscopy Precise classification
Whole Blood. Lab 29A. Blood. Plasma. Whole Blood. Formed Elements. Plasma: Fluid component. Formed elements: Cells and fragments
 Whole Blood Lab 29A. Blood Plasma: Fluid component Water (90%) Dissolved plasma proteins Other solutes Formed elements: Cells and fragments RBCs (carry Oxygen) WBCs (immunity) Platelets (cell fragments
Whole Blood Lab 29A. Blood Plasma: Fluid component Water (90%) Dissolved plasma proteins Other solutes Formed elements: Cells and fragments RBCs (carry Oxygen) WBCs (immunity) Platelets (cell fragments
Ordering Physician CLIENT,CLIENT. Collected REVISED REPORT
 HPWET Hematopathology Consultation, MML Embed Client Hematopathology Consult REVISED INAL DIAGNOSIS Interpretation Peripheral blood, bone marrow aspirate and biopsies, bilateral iliac crests: 1. Normocellular
HPWET Hematopathology Consultation, MML Embed Client Hematopathology Consult REVISED INAL DIAGNOSIS Interpretation Peripheral blood, bone marrow aspirate and biopsies, bilateral iliac crests: 1. Normocellular
Neoplasia 2018 lecture 11. Dr H Awad FRCPath
 Neoplasia 2018 lecture 11 Dr H Awad FRCPath Clinical aspects of neoplasia Tumors affect patients by: 1. their location 2. hormonal secretions 3. paraneoplastic syndromes 4. cachexia Tumor location Even
Neoplasia 2018 lecture 11 Dr H Awad FRCPath Clinical aspects of neoplasia Tumors affect patients by: 1. their location 2. hormonal secretions 3. paraneoplastic syndromes 4. cachexia Tumor location Even
HIV Basics: Clinical Tests and Guidelines
 HIV Basics: Clinical Tests and Guidelines ACTHIV 2010 Zelalem Temesgen MD Mayo Clinic Topics Baseline laboratory evaluation Laboratory monitoring through the continuum of care Patients not on antiretroviral
HIV Basics: Clinical Tests and Guidelines ACTHIV 2010 Zelalem Temesgen MD Mayo Clinic Topics Baseline laboratory evaluation Laboratory monitoring through the continuum of care Patients not on antiretroviral
Test Critical Low Critical High Alerting Category
 Test Critical Low Critical High Alerting CHEMISTRY Amylase > 400 U/L Emergency Dept only Amylase, Pancreatic >300 U/L Emergency Dept only Bicarb (Carbon Dioxide) 40 mmol/l Bilirubin, Total
Test Critical Low Critical High Alerting CHEMISTRY Amylase > 400 U/L Emergency Dept only Amylase, Pancreatic >300 U/L Emergency Dept only Bicarb (Carbon Dioxide) 40 mmol/l Bilirubin, Total
October Billing and compliance. Coagulation. Immunohistochemistry. Immunology. Referral testing ICD 10
 October 2015 Billing and compliance ICD 10 Coagulation Processing, transport and stability changes for multiple coagulation tests Immunohistochemistry IHC stain updates Immunology Lyme Western Blot testing
October 2015 Billing and compliance ICD 10 Coagulation Processing, transport and stability changes for multiple coagulation tests Immunohistochemistry IHC stain updates Immunology Lyme Western Blot testing
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN
 TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility CA19-9 (CA-GI) 8 hours 48 hours UNDETERMINED CA 19-9 may be ordered along with other tests,
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility CA19-9 (CA-GI) 8 hours 48 hours UNDETERMINED CA 19-9 may be ordered along with other tests,
Hematology 101. Blanche P Alter, MD, MPH, FAAP Clinical Genetics Branch Division of Cancer Epidemiology and Genetics Bethesda, MD
 Hematology 101 Blanche P Alter, MD, MPH, FAAP Clinical Genetics Branch Division of Cancer Epidemiology and Genetics Bethesda, MD Hematocrits Plasma White cells Red cells Normal, Hemorrhage, IDA, Leukemia,
Hematology 101 Blanche P Alter, MD, MPH, FAAP Clinical Genetics Branch Division of Cancer Epidemiology and Genetics Bethesda, MD Hematocrits Plasma White cells Red cells Normal, Hemorrhage, IDA, Leukemia,
Inspector's Accreditation Unit Activity Menu
 01/12/20XX 15:58:57 Laboratory Accreditation Program Page 1 of 9 CHEMISTRY 1501 ALT, serum/plasma 1502 Albumin, serum/plasma 1504 Alkaline phosphatase, serum/plasma 1506 Amylase, serum/plasma 1508 Bilirubin,
01/12/20XX 15:58:57 Laboratory Accreditation Program Page 1 of 9 CHEMISTRY 1501 ALT, serum/plasma 1502 Albumin, serum/plasma 1504 Alkaline phosphatase, serum/plasma 1506 Amylase, serum/plasma 1508 Bilirubin,
SydPath Reference Intervals for Clinical Trials (Contract Pathology Unit) Unauthorised Copy
 HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
Chronic Lymphocytic Leukemia (CLL)
 Page 1 of 10 PATIENT EDUCATION Chronic Lymphocytic Leukemia (CLL) Introduction Chronic lymphocytic leukemia (CLL) is a type of cancer of the lymphocytes (a kind of white blood cell). It is also referred
Page 1 of 10 PATIENT EDUCATION Chronic Lymphocytic Leukemia (CLL) Introduction Chronic lymphocytic leukemia (CLL) is a type of cancer of the lymphocytes (a kind of white blood cell). It is also referred
EBV and Infectious Mononucleosis. Infectious Disease Definitions. Infectious Diseases
 Infectious Disease Definitions Infection when a microorganism invades a host and multiplies enough to disrupt normal function by causing signs and symptoms Pathogencity ability of an organism to cause
Infectious Disease Definitions Infection when a microorganism invades a host and multiplies enough to disrupt normal function by causing signs and symptoms Pathogencity ability of an organism to cause
Hematology 101. Rachid Baz, M.D. 5/16/2014
 Hematology 101 Rachid Baz, M.D. 5/16/2014 Florida 101 Epidemiology Estimated prevalence 8,000 individuals in U.S (compare with 80,000 MM patients) Annual age adjusted incidence 3-8/million-year 1 More
Hematology 101 Rachid Baz, M.D. 5/16/2014 Florida 101 Epidemiology Estimated prevalence 8,000 individuals in U.S (compare with 80,000 MM patients) Annual age adjusted incidence 3-8/million-year 1 More
Code TEST SPECIMEN REQUIREMENT TAT Referral centre. 1 week BP GM (Specimen stability: 2-8 C for 48 hours) 3240 Anti-ds DNA 3 ml Plain Blood/Serum
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
A007 Allergy Basic Profile (28 parameters) 5 ml Plain Blood/Serum week BPGM
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
GRADING CRITERIA for CMS Regulated Analytes
 CLIA '88 AND GRADING The Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) were established by the federal government (CMS) to regulate clinical laboratories and proficiency test providers
CLIA '88 AND GRADING The Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) were established by the federal government (CMS) to regulate clinical laboratories and proficiency test providers
Hematology Case Conference 8/5/03
 Hematology Case Conference 8/5/03 Bone Marrow Case Patient: Emmxxx Lylexxx 74 year old AA female S/P craniotomy for SDH. Pt has Hx of HTN, DM, Crohn s disease, (R) nephrectomy. Bone marrow for abnormal
Hematology Case Conference 8/5/03 Bone Marrow Case Patient: Emmxxx Lylexxx 74 year old AA female S/P craniotomy for SDH. Pt has Hx of HTN, DM, Crohn s disease, (R) nephrectomy. Bone marrow for abnormal
Differential diagnosis of hematolymphoid tumors composed of medium-sized cells. Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital
 Differential diagnosis of hematolymphoid tumors composed of medium-sized cells Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital Lymphoma classification Lymphoma diagnosis starts with morphologic
Differential diagnosis of hematolymphoid tumors composed of medium-sized cells Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital Lymphoma classification Lymphoma diagnosis starts with morphologic
H. PYLORI AB, IGG HPYL
 H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
Transfusion Medicine (Comprehensive, Limited) J, J1
 www.cap.org Transfusion Medicine Analytes/procedures in bold type are regulated for proficiency testing by the Centers for Medicare & Medicaid Services (CMS). Transfusion Medicine (Comprehensive, Limited)
www.cap.org Transfusion Medicine Analytes/procedures in bold type are regulated for proficiency testing by the Centers for Medicare & Medicaid Services (CMS). Transfusion Medicine (Comprehensive, Limited)
Tumor Markers & Cytopathology
 Tumor Markers & Cytopathology Objectives: After learning, student should be able to 1. Describe the basic concepts of tumor markers and Asst. Prof. Prasit Suwannalert, Ph.D. (Email: prasit.suw@mahidol.ac.th)
Tumor Markers & Cytopathology Objectives: After learning, student should be able to 1. Describe the basic concepts of tumor markers and Asst. Prof. Prasit Suwannalert, Ph.D. (Email: prasit.suw@mahidol.ac.th)
Hematology Revision. By Dr.AboRashad . Mob
 1 1- Hb A2 is consisting of: a) 3 ά chains and 2 γ chains b) 2 ά chains and 2 β chains c) 2 ά chains and 2 δ chains** d) 2 ά chains and 3 δ chains e) 3 ά chains and 2 δ chains 2- The main (most) Hb found
1 1- Hb A2 is consisting of: a) 3 ά chains and 2 γ chains b) 2 ά chains and 2 β chains c) 2 ά chains and 2 δ chains** d) 2 ά chains and 3 δ chains e) 3 ά chains and 2 δ chains 2- The main (most) Hb found
FINALIZED SEER SINQ S NOVEMBER 2011
 : 20110133 Multiple primaries/heme & Lymphoid Neoplasms: A patient was diagnosed 7/31/08 with DLBCL (9680/3) (biopsy left supraclav. node), stage IIIB. Treated with chemo. 10/14/10 biopsy right supraclav.
: 20110133 Multiple primaries/heme & Lymphoid Neoplasms: A patient was diagnosed 7/31/08 with DLBCL (9680/3) (biopsy left supraclav. node), stage IIIB. Treated with chemo. 10/14/10 biopsy right supraclav.
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Barriers to Understanding
 Behind the Scenes: The Critical Importance of Cancer Cell Pathology and the Pathologist Sherry T. Emery, M.D., Chief of Pathology Northeast Health System Barriers to Understanding Questions for 2010 What
Behind the Scenes: The Critical Importance of Cancer Cell Pathology and the Pathologist Sherry T. Emery, M.D., Chief of Pathology Northeast Health System Barriers to Understanding Questions for 2010 What
From Morphology to Molecular Pathology: A Practical Approach for Cytopathologists Part 1-Cytomorphology. Songlin Zhang, MD, PhD LSUHSC-Shreveport
 From Morphology to Molecular Pathology: A Practical Approach for Cytopathologists Part 1-Cytomorphology Songlin Zhang, MD, PhD LSUHSC-Shreveport I have no Conflict of Interest. FNA on Lymphoproliferative
From Morphology to Molecular Pathology: A Practical Approach for Cytopathologists Part 1-Cytomorphology Songlin Zhang, MD, PhD LSUHSC-Shreveport I have no Conflict of Interest. FNA on Lymphoproliferative
Collection (Specimen Source Required on all tests) Sputum: >5 ml required. First morning specimen preferred.
 Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
Interpreting Hematology Scatter-Plots; One Cancer Center s Keys to Seeing the BIG Picture
 Interpreting Hematology Scatter-Plots; One Cancer Center s Keys to Seeing the BIG Picture Barbara L. Burch, MHA MT (ASCP) Laboratory Manager New York University Clinical Cancer Center Disclosure Ms Burch
Interpreting Hematology Scatter-Plots; One Cancer Center s Keys to Seeing the BIG Picture Barbara L. Burch, MHA MT (ASCP) Laboratory Manager New York University Clinical Cancer Center Disclosure Ms Burch
Annual Notice to Providers (2014)
 8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
Serrated Polyps and a Classification of Colorectal Cancer
 Serrated Polyps and a Classification of Colorectal Cancer Ian Chandler June 2011 Structure Serrated polyps and cancer Molecular biology The Jass classification The familiar but oversimplified Vogelsteingram
Serrated Polyps and a Classification of Colorectal Cancer Ian Chandler June 2011 Structure Serrated polyps and cancer Molecular biology The Jass classification The familiar but oversimplified Vogelsteingram
Current Status of Biomarkers (including DNA Tumor Markers and Immunohistochemistry in the Laboratory Diagnosis of Tumors)
 Current Status of Biomarkers (including DNA Tumor Markers and Immunohistochemistry in the Laboratory Diagnosis of Tumors) Kael Mikesell, DO McKay-Dee Hospital May 14, 2015 Outline Update to DNA Testing
Current Status of Biomarkers (including DNA Tumor Markers and Immunohistochemistry in the Laboratory Diagnosis of Tumors) Kael Mikesell, DO McKay-Dee Hospital May 14, 2015 Outline Update to DNA Testing
Medicaid Family Planning Waiver Services CPT Codes and ICD-10 Diagnosis Codes
 CPT Code Description of Covered Codes Evaluation and Management 99384FP 99385FP Family planning new visit 99386FP 99394FP 99395FP Family planning established visit 99396FP 99401FP HIV counseling (pre-test)
CPT Code Description of Covered Codes Evaluation and Management 99384FP 99385FP Family planning new visit 99386FP 99394FP 99395FP Family planning established visit 99396FP 99401FP HIV counseling (pre-test)
Limitations - CEA Limitations Beta HCG
 Frequency Limitations often determine the coverage a patient receives for certain tests. The following information is obtained directly from the NCD/LCD policies for your convenience. Limitations : Glycated
Frequency Limitations often determine the coverage a patient receives for certain tests. The following information is obtained directly from the NCD/LCD policies for your convenience. Limitations : Glycated
2007 Workshop of SH/EAHP. Session 5 Therapy-related myeloid neoplasms
 2007 Workshop of SH/EAHP Session 5 Therapy-related myeloid neoplasms Classification: Key issues MDS vs. AML-M6 MDS vs. MDS/MPD Genetically defined entities Relevance of morphologic classification Clinical
2007 Workshop of SH/EAHP Session 5 Therapy-related myeloid neoplasms Classification: Key issues MDS vs. AML-M6 MDS vs. MDS/MPD Genetically defined entities Relevance of morphologic classification Clinical
Cost-Effective Strategies in the Workup of Hematologic Neoplasm. Karl S. Theil, Claudiu V. Cotta Cleveland Clinic
 Cost-Effective Strategies in the Workup of Hematologic Neoplasm Karl S. Theil, Claudiu V. Cotta Cleveland Clinic In the past 12 months, we have not had a significant financial interest or other relationship
Cost-Effective Strategies in the Workup of Hematologic Neoplasm Karl S. Theil, Claudiu V. Cotta Cleveland Clinic In the past 12 months, we have not had a significant financial interest or other relationship
Epic Labs Orderable As STAT PRIORITY As of 06/22/2016
 ABG+HB(CORDARTERIAL) - BABY A ABG+HB(CORD ARTERIAL)- BABY B ABG+HB(CORD ARTERIAL)- BABY C ACETAMINOPHEN LEVEL ALANINE AMINOTRANSFERASE (ALT) ALBUMIN, FLUID ALBUMIN, PLEURAL FLUID ALBUMIN, SYNOVIAL FLUID
ABG+HB(CORDARTERIAL) - BABY A ABG+HB(CORD ARTERIAL)- BABY B ABG+HB(CORD ARTERIAL)- BABY C ACETAMINOPHEN LEVEL ALANINE AMINOTRANSFERASE (ALT) ALBUMIN, FLUID ALBUMIN, PLEURAL FLUID ALBUMIN, SYNOVIAL FLUID
Medical Laboratory Accreditation Programme
 Client Client Number 121 Address PO Box 6064, Dunedin North, Dunedin, 9059 Plunket House, 472 George Street, North Dunedin, Dunedin, 9016 Telephone 03 477-6981 Fax 03 477-9160 Authorised Representative
Client Client Number 121 Address PO Box 6064, Dunedin North, Dunedin, 9059 Plunket House, 472 George Street, North Dunedin, Dunedin, 9016 Telephone 03 477-6981 Fax 03 477-9160 Authorised Representative
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
August 17, Dear Valued Client:
 August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
Physician Office Laboratory Tests
 Important Change Effective March 1, 2018 Physician Office Laboratory Tests Molina Healthcare of Michigan has updated its list of payable laboratory tests that may be performed in a physician s office.
Important Change Effective March 1, 2018 Physician Office Laboratory Tests Molina Healthcare of Michigan has updated its list of payable laboratory tests that may be performed in a physician s office.
ELISA. AccuDiag Autoimmune Diseases and Others. ANA Screen Qualitative ~135 min 90.4% 100%
 / AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
/ AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
2015 American Thyroid Association Thyroid Nodule and Cancer Guidelines
 2015 American Thyroid Association Thyroid Nodule and Cancer Guidelines Angela M. Leung, MD, MSc, ECNU November 5, 2016 Outline Workup of nontoxic thyroid nodule(s) Ultrasound FNAB Management of FNAB results
2015 American Thyroid Association Thyroid Nodule and Cancer Guidelines Angela M. Leung, MD, MSc, ECNU November 5, 2016 Outline Workup of nontoxic thyroid nodule(s) Ultrasound FNAB Management of FNAB results
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
MICROBIOLOGY SPECIMEN COLLECTION MANUAL
 Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
PEACEHEALTH LABORATORIES
 360-414-2306 www.peacehealthlabs.org Critical Values Call List - Longview Critical values are reported per the criteria published below. Laboratory results meeting these criteria indicate potential life-threatening
360-414-2306 www.peacehealthlabs.org Critical Values Call List - Longview Critical values are reported per the criteria published below. Laboratory results meeting these criteria indicate potential life-threatening
Form 2033 R3.0: Wiskott-Aldrich Syndrome Pre-HSCT Data
 Key Fields Sequence Number: Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Has this patient's data been previously reported to USIDNET? USIDNET ID: Today's Date: - - Date of HSCT for which
Key Fields Sequence Number: Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Has this patient's data been previously reported to USIDNET? USIDNET ID: Today's Date: - - Date of HSCT for which
Please contact Customer Service with any questions. Ph: option 5
 Sarasota Memorial Laboratory Services is pleased to announce that we are now performing the following testing in house: Immature Platelet Fraction (IPF) The Immature Platelet Fraction (IPF) is used to
Sarasota Memorial Laboratory Services is pleased to announce that we are now performing the following testing in house: Immature Platelet Fraction (IPF) The Immature Platelet Fraction (IPF) is used to
Cancers of unknown primary : Knowing the unknown. Prof. Ahmed Hossain Professor of Medicine SSMC
 Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Anemia. A case-based approach. David B. Sykes, MD, PhD Hematology, MGH Cancer Center June 8, 2017
 Anemia A case-based approach David B. Sykes, MD, PhD Hematology, MGH Cancer Center June 8, 2017 Recognizing trends Learning Objectives MCV, RDW, Ferritin, LDH, Reticulocytes Managing complex patients 1.
Anemia A case-based approach David B. Sykes, MD, PhD Hematology, MGH Cancer Center June 8, 2017 Recognizing trends Learning Objectives MCV, RDW, Ferritin, LDH, Reticulocytes Managing complex patients 1.
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017
 M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
Abstracting Hematopoietic Neoplasms
 CASE 1: LYMPHOMA PHYSICAL EXAMINATION 43yo male with a history of lower gastrointestinal bleeding and melena undergoing colonoscopy and biopsy to rule out neoplasm versus inflammation. Patient had no other
CASE 1: LYMPHOMA PHYSICAL EXAMINATION 43yo male with a history of lower gastrointestinal bleeding and melena undergoing colonoscopy and biopsy to rule out neoplasm versus inflammation. Patient had no other
AllinaHealthSystems 1
 Overview Biology and Introduction to the Genetics of Cancer Denise Jones, MS, CGC Certified Genetic Counselor Virginia Piper Cancer Service Line I. Our understanding of cancer the historical perspective
Overview Biology and Introduction to the Genetics of Cancer Denise Jones, MS, CGC Certified Genetic Counselor Virginia Piper Cancer Service Line I. Our understanding of cancer the historical perspective
Disclosures/COI. Cases in Hematopathology. Outline. Heme Path Findings Not to Miss. Normal Peripheral Smear 6/30/2016
 Disclosures/COI Cases in Hematopathology Vamsi Kota Assistant Professor Department of Hematology & Medical Oncology Leukemia/BMT I have no disclosures or conflicts of interest regarding this presentation.
Disclosures/COI Cases in Hematopathology Vamsi Kota Assistant Professor Department of Hematology & Medical Oncology Leukemia/BMT I have no disclosures or conflicts of interest regarding this presentation.
TUMOR M ARKERS MARKERS
 TUMOR MARKERS M.Shekarabi IUMS Definition Many cancers are associated with the abnormal production of some molecules l which h can be measured in plasma. These molecules are known as tumor markers. A good
TUMOR MARKERS M.Shekarabi IUMS Definition Many cancers are associated with the abnormal production of some molecules l which h can be measured in plasma. These molecules are known as tumor markers. A good
