Aerobic Wound Culture and Stain
|
|
|
- Jonathan Stephens
- 6 years ago
- Views:
Transcription
1 Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature Alternate 1 1 ml Fluid Sterile Screwtop Container Room Temperature Alternate 2 Swab Swab Anaerobic Gel Swab (Blue Cap) Room Temperature Instructions Place swab in sterile transport (Culturette or Port-a-Cul). Send fluids or tissues in sterile container. Notes Daily 3 Days Aerobic culture for determining bacterial pathogens from wound, tissue and sterile fluid sites. Sensitivities done on isolates considered pathogens. CPT Code(s) 87070, 87205
2 Anti-Thrombin 3 (ATIII) Antigen Order Name: THROMB3 AG Test Number: Revision Date: 03/31/2014 LOINC Code: Anti-Thrombin 3 (ATIII) Antigen Nephelometry Preferred 2 ml (0.5) Plasma Sodium Citrate 3.2% (Blue Top) Frozen Instructions Patient should abstain from anabolic steroids, gemfibrozil, warfarin (coumadin), heparin therapy, asparaginase, estrogens, gestodene, and oral contraceptives optimally for 3 days prior to specimen collection. Overnight fasting is preferred. Send citrated plasma aliquots. They must be double spun then aliquot 1.5 ml plasma from each tube into individual plastic aliquot tubes and freeze. Do not pool aliquots together! Do not thaw. Tue, Thr 3-5 Days CPT Code(s) 85301
3 Celiac Disease Panel - Pediatric Tissue Transglutaminase IgA (IgA anti-ttg) Gliadin Deamidated Antibody, IgA Immunoglobulin, IgA Quantitative Order Name: PED CELIAC Test Number: Revision Date: 03/24/2014 Enzyme Immunoassay Enzyme Immunoassay Nephelometry Preferred 2mL (1) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Notes Mon, Wed 5-7 Days Evaluation of Celiac Disease in pediatric patients less than 3 years of age. In toddlers, IgG anti-ttg is not reliable, and referral for a small bowel biopsy is recommended for those with serum IgA deficiency. Recent literature has reported that Celiac disease (CD) is a more common disorder in the United States than previously recognized. CPT Code(s) 83516x2; 82784
4 Cryptococcus Antigen Screen - CSF Cryptococcus Antigen Screen - CSF Order Name: CSF CRYPTO Test Number: Revision Date: 03/24/2014 Enzyme Immunoassay Preferred 3 ml (1) CSF (Cerebrospinal Fluid) Sterile Screwtop Container Refrigerated Daily 1 Day Detects presence of Cryptococcus neoformans in CSF Notes July 5th 2012, Changed CPT from Latex agglutination to EIA method CPT Code(s) 87899
5 Cryptococcus Antigen Screen - Serum Cryptococcus Antigen Screen - Serum Order Name: CRYPTO AG Test Number: Revision Date: 03/24/2014 Enzyme Immunoassay Preferred 4 ml (1) Serum Clot Activator (Red Top, No-Gel) Refrigerated Instructions Cleanse venipuncture site Daily 1 Day Detects presence of Cryptococcus antigen in peripheral blood Notes July 5th 2012, Changed CPT from Latex agglutination to EIA method CPT Code(s) 87899
6 Endomysial IgA Antibody Screen Reflex to Titer Order Name: ENDOMYS AB Test Number: Revision Date: 03/10/2014 Endomysial IgA Antibody Screen Endomysial IgA Antibody Titer Indirect Fluorescent Antibody Indirect Fluorescent Antibody Preferred 1 ml (0.3 ml) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Alternate 1 1 ml (0.3 ml) Serum Clot Activator (Red Top, No-Gel) Refrigerated Tue - Sat 2-4 Days The presence of anti-endomysial (EMA) IgA antibodies has been shown to correlate with gluten-sensitive enteropathy such as celiac disease (CD) and dermatitis herpetiformis (DH). EMA is detected primarily by IFA, using monkey esophagus as a substrate and observing fluorescence of the endomysial lining. Patients with CD and DH can also demonstrate antibodies to reticulin and gliadin, though EMA-IgA seems to be the most specific marker (specifically %). CPT Code(s) If Endomysial Antibody Screen (IgA) is abnormal, Endomysial Antibody Titer will be performed at an additional charge. CPT code: Lab Section Reference Lab
7 Hepatitis A Total Antibody Hepatitis A Total Antibody Index Order Name: HEP A T AB Test Number: Revision Date: 03/03/2014 Chemiluminescence Assays Hepatitis A Total Antibody Interpretation Preferred 2 ml (0.5 ml) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Reference Range Mon - Fri 1-2 Days CPT Code(s) 86708
8 Hepatitis B Core Total Antibody Hepatitis B Core Total Antibody Index Order Name: HEP BCOR T Test Number: Revision Date: 03/03/2014 Chemiluminescence Assays Hepatitis B Core Total Antibody Interpretation Preferred 2 ml (0.5 ml) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Mon-Fri 1-2 Days CPT Code(s) 86704
9 Hepatitis Be Antigen Hepatitis Be Antigen Index Order Name: HEP BE AG Test Number: Revision Date: 03/03/2014 Chemiluminescence Assays Hepatitis Be Antigen Interpretation Preferred 2 ml (0.5 ml) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Mon - Fri 1-2 Days CPT Code(s) 87350
10 Omega 3 and 6 Fatty Acids, Plasma Order Name: OMEGA 3/6 Test Number: Revision Date: 03/03/2014 Omega-3 (EPA+DHA) Index Omega-6/Omega-3 Ratio Arachidonic Acid/EPA Ratio Arachidonic Acid EPA DHA Cardiovascular Disease Risk Calculation Calculation Calculation Liquid Chromatography/Tandem Mass Spectrometry Liquid Chromatography/Tandem Mass Spectrometry Liquid Chromatography/Tandem Mass Spectrometry INTERP Preferred 2 ml (0.4 ml) Plasma EDTA (Lavender Top) Refrigerated Instructions OVERNIGHT FASTING IS REQUIRED. Unacceptable specimen: Gross Hemolysis; Gross Lipemia; Gross Icteria. STABILITY: Room temperature: 7 Days, Refrigerated: 7 Days, Frozen: 28 Days. Sun-Sat 4-6 Days Omega-3 fatty acids are anti-inflammatory and antithrombotic, while omega-6 fatty acids are the opposite (proinflammatory and prothrombotic). Balance between the 2 is important for cardiovascular health. The omega-3 index is an indicator of cardiovascular disease risk. CPT Code(s) Lab Section Reference Lab
11 Platelet Function Studies Platelet Function, ADP Platelet Function, Epinephrine Order Name: PLT FUN Test Number: Revision Date: 03/19/2014 Platelet Function Testing Platelet Function Testing Preferred 6 ml Whole Blood Sodium Citrate 3.2% (Blue Top) Room Temperature Instructions NOTE: If collected at a location other than the laboratory at St. John Medical Center 1923 South Utica Ave. Tulsa, Then please send by STAT courier the the laboratory at St. John Medical Center for testing. Specimen must be tested within 4 hours of collection. Do not refrigerate! Please write on request if patient is receiving aspirin. Two 2.7 ml blue top. DO NOT Spin, Filter or Freeze specimens! Patient should have PLT >150,000 and HCT >35% for accuracy. CPT Code(s) Daily 1 Day Platelet function studies are done to evaluate platelet function. This is a specialized test and would normally be performed in patients with some indicator of a qualitative platelet disorder x2
12 Poliovirus Antibodies Poliovirus Type 1 Antibodies Poliovirus Type 2 Antibodies Poliovirus Type 3 Antibodies Order Name: POLIO ABS Test Number: Revision Date: 03/19/2014 Indirect Fluorescent Antibody Indirect Fluorescent Antibody Indirect Fluorescent Antibody Preferred 1 ml (0.5) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated CPT Code(s) Lab Section Wed, Fri 3-7 Days 86658x3 Reference Lab
13 Ristocetin Cofactor Ristocetin Cofactor Order Name: RISTOC COF Test Number: Revision Date: 03/31/2014 PLATELET AGGREGATION Preferred 2 ml (1) Plasma Sodium Citrate 3.2% (Blue Top) Frozen Instructions Frozen Citrated plasma, plasma must be double spun and frozen in 1.5 ml aliquots. Do not pool plasma from multiple tubes! Do not thaw. Hemolyzed specimens are not acceptable. See Specimen Collection Section, Coagulation Testing. Fasting for at least 8 hours is preferred. Mon, Wed, Fri 2-5 Days CPT Code(s) Lab Section Reference Lab
Activated Partial Thromboplastin Time (aptt)
 Activated Partial Thromboplastin Time (aptt) Order Name: PTT Test Number: 1500050 REV DATE:12/26/2008 Activated Partial Thromboplastin Time (aptt) CLOT Preferred 2.7 ml Whole Blood Sodium Citrate 3.2%
Activated Partial Thromboplastin Time (aptt) Order Name: PTT Test Number: 1500050 REV DATE:12/26/2008 Activated Partial Thromboplastin Time (aptt) CLOT Preferred 2.7 ml Whole Blood Sodium Citrate 3.2%
Alkaline Phosphatase, Bone Specific
 Alkaline Phosphatase, Bone Specific Order Name: ALK P BONE Test Number: 3656500 REV DATE:11/5/2010 Alkaline Phosphatase, Bone Specific IMMENZ Preferred 1 ml (0.3) Serum Clot Activator SST (Red/Gray or
Alkaline Phosphatase, Bone Specific Order Name: ALK P BONE Test Number: 3656500 REV DATE:11/5/2010 Alkaline Phosphatase, Bone Specific IMMENZ Preferred 1 ml (0.3) Serum Clot Activator SST (Red/Gray or
Acetylcholine Receptor Modulating Antibody
 Acetylcholine Receptor Modulating Antibody Order Name: ACETY MOD Test Number: 5516500 TEST COMPONENTS REV DATE: 09/25/2012 Acetylcholine Receptor Modulating Antibody SEMI-FC Preferred 1 ml (0.5) Serum
Acetylcholine Receptor Modulating Antibody Order Name: ACETY MOD Test Number: 5516500 TEST COMPONENTS REV DATE: 09/25/2012 Acetylcholine Receptor Modulating Antibody SEMI-FC Preferred 1 ml (0.5) Serum
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Acid Fast Bacilli (AFB) Culture and Smear
 Acid Fast Bacilli (AFB) and Smear Order Name: C AFB Test Number: 6000100 Acid Fast Bacilli (AFB) and Smear Preferred 5 ml (3) Sputum Sterile Screwtop Container Room Temperature Alternate 1 5 ml (3) Tissue
Acid Fast Bacilli (AFB) and Smear Order Name: C AFB Test Number: 6000100 Acid Fast Bacilli (AFB) and Smear Preferred 5 ml (3) Sputum Sterile Screwtop Container Room Temperature Alternate 1 5 ml (3) Tissue
Acetylcholine Receptor Binding Antibody
 Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Specimen Type Specimen Container Transport Environment Preferred 2mL(0.5) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated
 Aldolase Order Name: ALDOLASE Test Number: 3600150 Aldolase Enz Preferred 2mL(0.5) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Special Hemolyzed specimens are not acceptable. Allow specimen
Aldolase Order Name: ALDOLASE Test Number: 3600150 Aldolase Enz Preferred 2mL(0.5) Serum Clot Activator SST (Red/Gray or Tiger Top) Refrigerated Special Hemolyzed specimens are not acceptable. Allow specimen
Anti-FceR (CU Index) Antibody
 Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Patient Preparation Unique patient preparation requirements are listed under each test in the Test Directory.
 Specimen Collection Lab results are only as good as the specimen provided. Patient preparation, venipuncture technique, specimen handling, and transportation can all affect the quantity of results. Health
Specimen Collection Lab results are only as good as the specimen provided. Patient preparation, venipuncture technique, specimen handling, and transportation can all affect the quantity of results. Health
Atypical Pneumonia Antibodies
 Atypical Pneumonia Antibodies Order Name: ATYP PNEUM Test Number: 5564200 REV DATE:1/17/2011 Adenovirus IgG and IgM Antibodies Chlamydia pneumoniae IgM Antibody Chlamydia pneumoniae IgG Antibody Influenza
Atypical Pneumonia Antibodies Order Name: ATYP PNEUM Test Number: 5564200 REV DATE:1/17/2011 Adenovirus IgG and IgM Antibodies Chlamydia pneumoniae IgM Antibody Chlamydia pneumoniae IgG Antibody Influenza
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
TEST REQUEST INFORMATION- VIROLOGY
 Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
October Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 ANNOUNCEMENTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Page # Reactivated
ANNOUNCEMENTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Page # Reactivated
INTERPATH LABORATORY, INC. TEST UPDATES
 EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
December Metanephrines, Fractionated, Free, Plasma Plasminogen Activator Inhibitor Type 1 Stool Cultures and Sensitivities
 University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
COLLECTION TUBES FOR PHLEBOTOMY
 COLLECTION TUBES FOR PHLEBOTOMY Red Top None Blood clots, and the serum is separated by centrifugation Chemistries, Immunology and Serology, Blood Bank (Crossmatch) Gold Top None Serum separator tube (SST)
COLLECTION TUBES FOR PHLEBOTOMY Red Top None Blood clots, and the serum is separated by centrifugation Chemistries, Immunology and Serology, Blood Bank (Crossmatch) Gold Top None Serum separator tube (SST)
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
March Immunoglobulins, Total (IgA, IgE, IgG, IgM) Interleukin-6 (IL6) Protein Electrophoresis, CSF. Thyrotropin Receptor Antibody
 University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
The information contained here may be very important to your practice. Please take a moment to review this document.
 September 2017 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN T-SPOT TB TESTING Please see
September 2017 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN T-SPOT TB TESTING Please see
Business Unit: Quest Diagnostics Nichols Institute, Valencia. June 2011 Update Dear Colleague,
 Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
November Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Review the following alphabetical listing of our lab test catalog for each of our offered tests. Each page has at least the following elements:
 Site: Fremont Rideout Health Group Laboratory Services Policy and Procedure Creation Date: 08/17/2009 Subject/Title: FRHG Laboratories' Catalog of Tests Q-S Document Owner: Corson, Karen Pagination: Page
Site: Fremont Rideout Health Group Laboratory Services Policy and Procedure Creation Date: 08/17/2009 Subject/Title: FRHG Laboratories' Catalog of Tests Q-S Document Owner: Corson, Karen Pagination: Page
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Recommendations for Celiac Disease Testing. IgA & ttg IgA
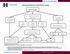 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
March Molecular diagnostics. Update distribution changes. Billing and compliance. Hematology. Immunology. Referral testing
 March 2016 Update distribution changes Billing and compliance Compliance Drug Analysis CPT change Hematology Hemoglobin A1c, Screening Reference Range Change Immunology Testing schedule changes Vitamin
March 2016 Update distribution changes Billing and compliance Compliance Drug Analysis CPT change Hematology Hemoglobin A1c, Screening Reference Range Change Immunology Testing schedule changes Vitamin
Reference Range: Age/sex dependent; appropriate normals provided with result Analytical Time: 4 hours Day(s) Test Set Up: Daily CPT Code: 84100
 Parathyroid Hormone See PTH PARIETAL CELL ANTIBODY APCAB; PARIETAL; PCA Specimen Required: 2.5 ml blood, gel tube Analytical Time: 3-5 days CPT Code: 86255 PCP SCREEN URINE PCPQU Specimen Required: 50
Parathyroid Hormone See PTH PARIETAL CELL ANTIBODY APCAB; PARIETAL; PCA Specimen Required: 2.5 ml blood, gel tube Analytical Time: 3-5 days CPT Code: 86255 PCP SCREEN URINE PCPQU Specimen Required: 50
10/7/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 REDIRECTS Please Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page
REDIRECTS Please Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Lab Guide 2019 Metabolic Section Lab Guide
 Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
LIST OF TESTS WITH COLLECTION INFORMATION. Form Page 1 of 19
 ALL TESTS Tests listed are the most commonly ordered tests. For further information on tests not listed call the laboratory, look for the test in the computer or look at Lab Corp Manual ACTH (Adenocorticotropic
ALL TESTS Tests listed are the most commonly ordered tests. For further information on tests not listed call the laboratory, look for the test in the computer or look at Lab Corp Manual ACTH (Adenocorticotropic
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
MICROBIOLOGY SPECIMEN COLLECTION MANUAL
 Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
Assays. New. New. Combinations. Possibilities. Patents: EP , AU
 Assays Patents: EP 2362222, AU 2011217190 New Combinations New Possibilities Technology Classical Handling of Autoimmune Diagnostics 2-Step Diagnostics 1 st Screening 2 nd Confirmation Cell based IFA ELISA
Assays Patents: EP 2362222, AU 2011217190 New Combinations New Possibilities Technology Classical Handling of Autoimmune Diagnostics 2-Step Diagnostics 1 st Screening 2 nd Confirmation Cell based IFA ELISA
HML Update. Summer 2009 Volume 15, No. 3. Keeping Our Clients Informed Questions or Comments: Call
 HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
February Billing and Compliance. Chemistry/Immunology. Help Us Help You. Microbiology. Referral Testing. Supplies. Website
 February 2014 Billing and Compliance ICD 10 update It s coming! Chemistry/Immunology Hepatitis B Antibody reporting change Help Us Help You Completion of Allina Health Laboratory manual request forms Unscheduled
February 2014 Billing and Compliance ICD 10 update It s coming! Chemistry/Immunology Hepatitis B Antibody reporting change Help Us Help You Completion of Allina Health Laboratory manual request forms Unscheduled
Alanine Transaminase (ALT)
 Alanine Transaminase (ALT) Order Name: ALT Test Number: 2004850 TEST COMPONENTS REV DATE: 03/05/2012 Alanine Transaminase (ALT) Enz Type Container Transport Preferred 1 ml (0.5) Plasma Lithium Heparin
Alanine Transaminase (ALT) Order Name: ALT Test Number: 2004850 TEST COMPONENTS REV DATE: 03/05/2012 Alanine Transaminase (ALT) Enz Type Container Transport Preferred 1 ml (0.5) Plasma Lithium Heparin
12/10/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Laboratory Procedure Handout RHEUMATOID FACTORS
 KING ABDULAZIA UNIVERSITY FACULTY OF APPLIED MEDICAL SCIENCES DEPARTEMENT OF LABORATORY MEDICAL TECHNOLOGY Laboratory Procedure Handout RHEUMATOID FACTORS RF Latex agglutination for detection of RF INTRODUCTION
KING ABDULAZIA UNIVERSITY FACULTY OF APPLIED MEDICAL SCIENCES DEPARTEMENT OF LABORATORY MEDICAL TECHNOLOGY Laboratory Procedure Handout RHEUMATOID FACTORS RF Latex agglutination for detection of RF INTRODUCTION
History of the Vacutainer Tube. Coagulant Blood Tests
 History of the Vacutainer Tube The different tubes are known as a vacutainer and were developed by Joseph Kleiner in 1947. The vacutainer is currently being manufactured by Becton, Dickinson and Company
History of the Vacutainer Tube The different tubes are known as a vacutainer and were developed by Joseph Kleiner in 1947. The vacutainer is currently being manufactured by Becton, Dickinson and Company
General Guidelines for Sample Submissions
 HISTORY General Guidelines for Sample Submissions (See the Client Handbook for specific test requirements.) The history of the animal is very important, including treatments, vaccinations and feeding,
HISTORY General Guidelines for Sample Submissions (See the Client Handbook for specific test requirements.) The history of the animal is very important, including treatments, vaccinations and feeding,
H. PYLORI AB, IGG HPYL
 H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
Dynacare Laboratories Test Changes
 CHROMIUM CHROMIUM CHROM CHROM 82495 82495 3508250 3508250 Plasma/ Whole blood 3.0 ml 3.0 ml Royal Blue Top (EDTA) Tube Royal Blue Top (EDTA) Tube Separate plasma/serum within 45 minutes of collection,
CHROMIUM CHROMIUM CHROM CHROM 82495 82495 3508250 3508250 Plasma/ Whole blood 3.0 ml 3.0 ml Royal Blue Top (EDTA) Tube Royal Blue Top (EDTA) Tube Separate plasma/serum within 45 minutes of collection,
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
TEST LIST SAMPLE REQUIREMENT. 1 ml serum None
 ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
PARANEOPLASTIC AUTOANTIBODY EVALUATION, SERUM
 Lab Dept: Test Name: Serology PARANEOPLASTIC AUTOANTIBODY EVALUATION, SERUM General Information Lab Order Codes: PAES Synonyms: CPT Codes: Acetylcholine Receptor (Muscle AchR) Antibodies; Ovarian Cancer-Related
Lab Dept: Test Name: Serology PARANEOPLASTIC AUTOANTIBODY EVALUATION, SERUM General Information Lab Order Codes: PAES Synonyms: CPT Codes: Acetylcholine Receptor (Muscle AchR) Antibodies; Ovarian Cancer-Related
Blood Collection Additives
 Blood Collection Additives Blood collection tubes and other collection devices often contain one or more additives. There are a number of different types of additives, and each has a specific function.
Blood Collection Additives Blood collection tubes and other collection devices often contain one or more additives. There are a number of different types of additives, and each has a specific function.
In-Common Laboratories
 Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Epidemiologists and Laboratory Science
 Epidemiologists and Laboratory Science Najib Aziz, M.D. Adjunct Associate Professor Department of Epidemiology UCLA, Fielding School of Public Health January 2014 Introduction: Advance in the laboratory
Epidemiologists and Laboratory Science Najib Aziz, M.D. Adjunct Associate Professor Department of Epidemiology UCLA, Fielding School of Public Health January 2014 Introduction: Advance in the laboratory
Specimen Collection Requirements for Neonates
 Neonatal Page 1 of 21 ABG-Arterial Blood Gases Hep. Syringe 0.3 ml Send on ice ACTH (Adrenocorticotrophic hormone) 15 days Lavender (Serum, heparin NOT suitable) 1 ml Pre chill tubes Mix gently after drawing
Neonatal Page 1 of 21 ABG-Arterial Blood Gases Hep. Syringe 0.3 ml Send on ice ACTH (Adrenocorticotrophic hormone) 15 days Lavender (Serum, heparin NOT suitable) 1 ml Pre chill tubes Mix gently after drawing
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
September Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
October Courier Service on Holidays There will be no courier service Thursday, Dec. 24th, 2011.
 University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
Collect and label sample according to standard protocols. Gently invert tube 8-10 times immediately after draw. DO NOT SHAKE. Do not centrifuge.
 Complete Blood Count CPT Code: CBC with Differential: 85025 CBC without Differential: 85027 Order Code: CBC with Differential: C915 Includes: White blood cell, Red blood cell, Hematocrit, Hemoglobin, MCV,
Complete Blood Count CPT Code: CBC with Differential: 85025 CBC without Differential: 85027 Order Code: CBC with Differential: C915 Includes: White blood cell, Red blood cell, Hematocrit, Hemoglobin, MCV,
ANA testing can now be ordered in several ways, depending on the clinical circumstances:
 LAB TEST CONNECT Multiplex ANA Screen Dr. Joseph Schappert, M.D.,Medical Director Chief Medical Offi cer ANA has been the primary screening test for connective tissue diseases (CTD s) for many years. While
LAB TEST CONNECT Multiplex ANA Screen Dr. Joseph Schappert, M.D.,Medical Director Chief Medical Offi cer ANA has been the primary screening test for connective tissue diseases (CTD s) for many years. While
Insulin Aspart ELISA Kit
 Insulin Aspart ELISA Kit Catalogue DEIABL215 For the qualitative determination of antibodies to insulin aspart in human serum and plasma FOR RESEARCH USE ONLY Creative Diagnostics. All rights reserved
Insulin Aspart ELISA Kit Catalogue DEIABL215 For the qualitative determination of antibodies to insulin aspart in human serum and plasma FOR RESEARCH USE ONLY Creative Diagnostics. All rights reserved
Test Definition: FNMR2 NMR LipoProfile w/ir Markers
 Reporting Title: Performing Location: Labcorp Burlington Specimen Requirements: Submit only one of the following specimens: Serum Draw blood in a plain red-top tube(s). (Serum gel tube is not acceptable.)
Reporting Title: Performing Location: Labcorp Burlington Specimen Requirements: Submit only one of the following specimens: Serum Draw blood in a plain red-top tube(s). (Serum gel tube is not acceptable.)
April Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 Revision Message! Please note: 4/20/15 communication revision for test code 17306- Vitamin D, 25-Hydroxy, Total, Immunoassay, New York patient message NEW TESTS Please Note: Not all test codes assigned
Revision Message! Please note: 4/20/15 communication revision for test code 17306- Vitamin D, 25-Hydroxy, Total, Immunoassay, New York patient message NEW TESTS Please Note: Not all test codes assigned
Pilot studies with reference specimens
 Pilot studies with reference specimens HUPO: Plasma Proteome Project Workshop July 16-17, 17, 2003 Daniel W. Chan, Ph.D.,DABCC Professor of Pathology, Oncology, Radiology & Urology Director of Clinical
Pilot studies with reference specimens HUPO: Plasma Proteome Project Workshop July 16-17, 17, 2003 Daniel W. Chan, Ph.D.,DABCC Professor of Pathology, Oncology, Radiology & Urology Director of Clinical
February Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit
 Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
BASIC METABOLIC PANEL
 Update 2/12/2018 BASIC METABOLIC PANEL CPT 80048 Stability: 3 days at 15-25 C; 7 days at 2-8 C; > 7 days at -70 C Colorimetric Assay, Rate reaction, ISE Components: BUN, Calcium, Chloride, CO2, Creatinine,
Update 2/12/2018 BASIC METABOLIC PANEL CPT 80048 Stability: 3 days at 15-25 C; 7 days at 2-8 C; > 7 days at -70 C Colorimetric Assay, Rate reaction, ISE Components: BUN, Calcium, Chloride, CO2, Creatinine,
Laboratory Methods for Diagnosing Celiac Disease. Vijay Kumar, PhD, FACB IMMCO Diagnostics, Inc. Buffalo, NY
 Laboratory Methods for Diagnosing Celiac Disease Vijay Kumar, PhD, FACB IMMCO Diagnostics, Inc. Buffalo, NY Prevalence of Celiac Disease Group With Symptoms Adults Children Associated Symptoms Chronic
Laboratory Methods for Diagnosing Celiac Disease Vijay Kumar, PhD, FACB IMMCO Diagnostics, Inc. Buffalo, NY Prevalence of Celiac Disease Group With Symptoms Adults Children Associated Symptoms Chronic
Test Bulletin. Save time, money, resources, and precious blood by NOT collecting extra specimens just in case
 Test Bulletin October 2018 Save time, money, resources, and precious blood by NOT collecting extra specimens just in case ACL Laboratories has seen an increase in the number of specimens submitted without
Test Bulletin October 2018 Save time, money, resources, and precious blood by NOT collecting extra specimens just in case ACL Laboratories has seen an increase in the number of specimens submitted without
TRANSFUSION REACTION EVALUATION
 Lab Dept: Test Name: Transfusion Services TRANSFUSION REACTION EVALUATION General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: TRXR Transfusion Complication Workup; Hemolytic reaction
Lab Dept: Test Name: Transfusion Services TRANSFUSION REACTION EVALUATION General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: TRXR Transfusion Complication Workup; Hemolytic reaction
Please note that our serum opiates EIA screen assay is not appropriate for therapeutic drug monitoring of Oxycodone.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
Instruments smart solutions & service IGZ Instruments AG Räffelstrasse 32 CH 8045 Zürich
 ADP (Adenosine-5 -Diphosphate) 101312 3 x 0.5mL ADP is a lyophilized preparation of adenosine-5 -diphosphate. The working concentration of the reconstituted reagent is 2 x 10-4 M. ADP is for use in routine
ADP (Adenosine-5 -Diphosphate) 101312 3 x 0.5mL ADP is a lyophilized preparation of adenosine-5 -diphosphate. The working concentration of the reconstituted reagent is 2 x 10-4 M. ADP is for use in routine
Fundamentals of Phlebotomy
 Fundamentals of Phlebotomy May 2012 Historical Origins: Superstition Phlebotomy Today: Diagnostic tool CP1154634-9 Potential Exposure from Needle Stick Injuries 1 in 6 - Hepatitis B 1 in 20 - Hepatitis
Fundamentals of Phlebotomy May 2012 Historical Origins: Superstition Phlebotomy Today: Diagnostic tool CP1154634-9 Potential Exposure from Needle Stick Injuries 1 in 6 - Hepatitis B 1 in 20 - Hepatitis
Fundamentals of Phlebotomy
 Fundamentals of Phlebotomy May 2012 1 Historical Origins: Superstition Phlebotomy Today: Diagnostic tool CP1154634-9 2 Potential Exposure from Needle Stick Injuries 1 in 6 - Hepatitis B 1 in 20 - Hepatitis
Fundamentals of Phlebotomy May 2012 1 Historical Origins: Superstition Phlebotomy Today: Diagnostic tool CP1154634-9 2 Potential Exposure from Needle Stick Injuries 1 in 6 - Hepatitis B 1 in 20 - Hepatitis
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man Coulter HIV-1 p24 ELISA May 21, 2004
 Coulter HIV p24 1. PRINCIPLE The Human Immunodeficiency Virus Type 1 (HIV-1) is recognized as the etiologic agent of acquired immunodeficiency syndrome (AIDS). The virus is transmitted by sexual contact,
Coulter HIV p24 1. PRINCIPLE The Human Immunodeficiency Virus Type 1 (HIV-1) is recognized as the etiologic agent of acquired immunodeficiency syndrome (AIDS). The virus is transmitted by sexual contact,
Spin and separate into plastic vial. FREEZE. Transport FROZEN sample from regional office on dry ice. Store and transport sample refrigerated.
 07 GUTHRIE CLINIC TEST LIST rev. 5/08 -DEOXYCORTISOL REQUIREMENTS 4 ml Clot Tube SST tubes are unacceptable. PATIENT PREPARATION: First morning sample preferred NOTE: Plasma is also acceptable from EDTA,
07 GUTHRIE CLINIC TEST LIST rev. 5/08 -DEOXYCORTISOL REQUIREMENTS 4 ml Clot Tube SST tubes are unacceptable. PATIENT PREPARATION: First morning sample preferred NOTE: Plasma is also acceptable from EDTA,
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Cpt code for celiac disease blood test
 Cpt code for celiac disease blood test 07/15/2018 Peripheral nerve block for post op pain management icd 10 07/17/2018 Fluconazole glenmark msds 07/19/2018 -Cancer in women over 50 -Do xanax give you energy
Cpt code for celiac disease blood test 07/15/2018 Peripheral nerve block for post op pain management icd 10 07/17/2018 Fluconazole glenmark msds 07/19/2018 -Cancer in women over 50 -Do xanax give you energy
HAEMATOLOGY INTRODUCTION
 HAEMATOLOGY INTRODUCTION Haematology Laboratory offers a comprehensive range of routine and specialised haematological investigations. The laboratory also offers Routine Urine Sediment Microscopy and molecular
HAEMATOLOGY INTRODUCTION Haematology Laboratory offers a comprehensive range of routine and specialised haematological investigations. The laboratory also offers Routine Urine Sediment Microscopy and molecular
Separation of Plasma and Serum and Their Proteins from Whole Blood
 Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
Drugs of Abuse Confirmation/Quantitation - Opiates - Urine CDCO OPI 211
 ARUP Standard Transport Tube. (Min: 2 ml) Unacceptable Conditions: Separator tubes. Plasma or whole blood collected in lt. blue (sodium citrate). Specimens exposed to repeated freeze/thaw cycles. Stability:
ARUP Standard Transport Tube. (Min: 2 ml) Unacceptable Conditions: Separator tubes. Plasma or whole blood collected in lt. blue (sodium citrate). Specimens exposed to repeated freeze/thaw cycles. Stability:
INSTRUCTIONS. Spin and separate into plastic vial. FREEZE. Transport FROZEN sample from regional office on dry ice
 07 GUTHRIE CLINIC TEST LIST rev. 8/07 -DEOXYCORTISOL REQUIREMENTS 4 ml Clot Tube SST tubes are unacceptable. PATIENT PREPARATION: First morning sample preferred NOTE: Plasma is also acceptable from EDTA,
07 GUTHRIE CLINIC TEST LIST rev. 8/07 -DEOXYCORTISOL REQUIREMENTS 4 ml Clot Tube SST tubes are unacceptable. PATIENT PREPARATION: First morning sample preferred NOTE: Plasma is also acceptable from EDTA,
HANDLING GENERAL INFORMATION TEST REQUESTS TYPES OF TESTS TEST REQUEST FORM
 SECTION 3: SAMPLE COLLECTION & HANDLING GENERAL INFORMATION SAMPLE INTRODUCTION COLLECTION & HANDLING GENERAL results. INFORMATION The quality of laboratory test results depends greatly on the quality
SECTION 3: SAMPLE COLLECTION & HANDLING GENERAL INFORMATION SAMPLE INTRODUCTION COLLECTION & HANDLING GENERAL results. INFORMATION The quality of laboratory test results depends greatly on the quality
SPECIMEN COLLECTION, PREPARATION & HAND L I N G
 Adequate patient identification, patient preparation, specimen collection, and specimen handling are essential prerequisites for accurate testing. PATIENT IDENTIFICATION Proper patient identification is
Adequate patient identification, patient preparation, specimen collection, and specimen handling are essential prerequisites for accurate testing. PATIENT IDENTIFICATION Proper patient identification is
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Presented by Marcelo Cardona, MT(ASCP) Johns Hopkins University
 Presented by Marcelo Cardona, MT(ASCP) Johns Hopkins University Alert or critical values represent those assay results that require prompt, rapid clinical attention to avert significant study-participant
Presented by Marcelo Cardona, MT(ASCP) Johns Hopkins University Alert or critical values represent those assay results that require prompt, rapid clinical attention to avert significant study-participant
International Journal of Pharma and Bio Sciences DETECTION OF HEPATITIS B SURFACE ANTIGEN USING ELISA AND REAL TIME PCR ABSTRACT
 Research Article Biotechnology International Journal of Pharma and Bio Sciences ISSN 0975-6299 DETECTION OF HEPATITIS B SURFACE ANTIGEN USING ELISA AND REAL TIME PCR ALI MOHAMMED ABDUL MOHSEN Indian academy
Research Article Biotechnology International Journal of Pharma and Bio Sciences ISSN 0975-6299 DETECTION OF HEPATITIS B SURFACE ANTIGEN USING ELISA AND REAL TIME PCR ALI MOHAMMED ABDUL MOHSEN Indian academy
Generate Knowledge Pre-Analytical Variables in the Coagulation Lab: Why Does It Matter?
 Generate Knowledge Pre-Analytical Variables in the Coagulation Lab: Why Does It Matter? Paul Riley, PhD, MBA Pre-Analytical Variables: Objectives 1. Define Pre-Analytical variables 2. Explain how blood
Generate Knowledge Pre-Analytical Variables in the Coagulation Lab: Why Does It Matter? Paul Riley, PhD, MBA Pre-Analytical Variables: Objectives 1. Define Pre-Analytical variables 2. Explain how blood
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
Specimen Collection Guide
 Specimen Collection Guide 1 Blood Collection Tubes SODIUM CITRATE TUBE - PALE BLUE TOP 2.7 Coagulation studies: Prothrombin time Factor assays etc. APTT Fibrinogen Thrombophilia tests INR D-Dimer SERUM
Specimen Collection Guide 1 Blood Collection Tubes SODIUM CITRATE TUBE - PALE BLUE TOP 2.7 Coagulation studies: Prothrombin time Factor assays etc. APTT Fibrinogen Thrombophilia tests INR D-Dimer SERUM
TUD Sdn Bhd ( V)
 Instruction for Use of TUD Evacuated Blood Collection Tubes Direction for Use of TUD Evacuated Blood Collection Tube Product name: TUD Evacuated blood collection tube I. Application: It is used for collection,
Instruction for Use of TUD Evacuated Blood Collection Tubes Direction for Use of TUD Evacuated Blood Collection Tube Product name: TUD Evacuated blood collection tube I. Application: It is used for collection,
Please use only the valid version of the package insert provided with the kit.
 Please use only the valid version of the package insert provided with the kit. Intended Use For the qualitative screening of serum IgG antibodies to Trichinella using an Enzyme Linked Immunoabsorbant Assay
Please use only the valid version of the package insert provided with the kit. Intended Use For the qualitative screening of serum IgG antibodies to Trichinella using an Enzyme Linked Immunoabsorbant Assay
Microbiology & Virology Resource Comprehensive
 Abscess, aspirate, drainage culture Actinomyces culture Aerobic Bacterial culture, stain AFB culture & smear (Acid Fast, TB) AFB Blood culture Anaerobic culture Blood culture Body Fluid culture, stain
Abscess, aspirate, drainage culture Actinomyces culture Aerobic Bacterial culture, stain AFB culture & smear (Acid Fast, TB) AFB Blood culture Anaerobic culture Blood culture Body Fluid culture, stain
HealthEast Medical Laboratory Reference Manual
 1,25-DihydroxyvitaminD Panel Code: DVD CPT Codes(s): 82652 Test Performed at: Mayo Medical Laboratories Analytic Time: 96 hours (4 days) Days Test Performed: Mon Tue Wed Thu Fri (Fasting 4 hours) SUBMIT:
1,25-DihydroxyvitaminD Panel Code: DVD CPT Codes(s): 82652 Test Performed at: Mayo Medical Laboratories Analytic Time: 96 hours (4 days) Days Test Performed: Mon Tue Wed Thu Fri (Fasting 4 hours) SUBMIT:
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx 0244-055-00 OraQuick HCV Rapid Antibody Test Kit with product codes 1001-0270
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx 0244-055-00 OraQuick HCV Rapid Antibody Test Kit with product codes 1001-0270
All abnormal results for this test will automatically have the DNA test reflexed at an additional cost.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: May-June 2004 REVISED 6-15-04 At Specialty we continue to focus on working with you to reduce the problems that can result in tests
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: May-June 2004 REVISED 6-15-04 At Specialty we continue to focus on working with you to reduce the problems that can result in tests
Medical Laboratory Accreditation Programme
 Client Client Number 121 Address PO Box 6064, Dunedin North, Dunedin, 9059 Plunket House, 472 George Street, North Dunedin, Dunedin, 9016 Telephone 03 477-6981 Fax 03 477-9160 Authorised Representative
Client Client Number 121 Address PO Box 6064, Dunedin North, Dunedin, 9059 Plunket House, 472 George Street, North Dunedin, Dunedin, 9016 Telephone 03 477-6981 Fax 03 477-9160 Authorised Representative
January Billing and compliance. Chemistry. Help us help you. Referral testing. Additional CPT code changes announced for 2015
 January 2015 Billing and compliance Additional CPT code changes announced for 2015 Chemistry CA 27.29 conversion to CA 15-3 nearing completion Vitamin B12 and LD, Total specimen change Timed Urine Protein
January 2015 Billing and compliance Additional CPT code changes announced for 2015 Chemistry CA 27.29 conversion to CA 15-3 nearing completion Vitamin B12 and LD, Total specimen change Timed Urine Protein
Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
 Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
UNIVERSITY OF PUERTO RICO MEDICAL SCIENCES CAMPUS - SCHOOL OF MEDICINE DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE
 UNIVERSITY OF PUERTO RICO MEDICAL SCIENCES CAMPUS - SCHOOL OF MEDICINE DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE HISTOCOMPATIBILITY AND DNA LABORATORY LABORATORY CATALOG P a g e 2 Introduction The
UNIVERSITY OF PUERTO RICO MEDICAL SCIENCES CAMPUS - SCHOOL OF MEDICINE DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE HISTOCOMPATIBILITY AND DNA LABORATORY LABORATORY CATALOG P a g e 2 Introduction The
S003 CPC Self-Assessment
 S003 CPC Self-Assessment Alina G. Bridges, D.O. Associate Professor Program Director, Dermatopathology Fellowship Department of Dermatology, Division of Dermatopathology and Cutaneous Immunopathology Mayo
S003 CPC Self-Assessment Alina G. Bridges, D.O. Associate Professor Program Director, Dermatopathology Fellowship Department of Dermatology, Division of Dermatopathology and Cutaneous Immunopathology Mayo
Collection (Specimen Source Required on all tests) Sputum: >5 ml required. First morning specimen preferred.
 Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
