Resistance to Salmon Calcitonin
|
|
|
- Tracey Phelps
- 5 years ago
- Views:
Transcription
1 An Evaluation of Antibodies and Clinical Resistance to Salmon Calcitonin FREDERICK R. SINGER, J. PHILLIP ALDRED, ROBERT M. NEER, STEPHEN M. KRANE, JOHN T. Porrs, JR., and KURT J. BLOCH From the Department of Medicine, Massachusetts General Hospital, Boston 2114, West Roxbury Veterans Administration Hospital, West Roxbury, Massachusetts 2132, and Harvard Medical School, Boston 2115, and the Armour Pharmaceutical Co., Kankakee, Illinois 691 A B S T R A C T 21 patients with Paget's disease of bone and one with osteoporosis were studied to detect development of antibodies to salmon calcitonin during chronic therapy. Antibody titers ranged from 1: 4 to 1: 3, in plasma obtained after treatment of 11 patients. Radioimmunoelectrophoresis revealed that the antibodies were restricted to the YG class. One patient, W. O., with Paget's disease initially responded to treatment with a decrease in bone turnover, but later became resistant to the hormone in association with the appearance of a very high titer (1: 3,) of antibody against salmon calcitonin. A 1:1 dilution of his plasma was shown to completely inactivate 2 mmrc units/ml of salmon calcitonin as detected by bioassay in rats; slight inactivation was detected at a 1: 2 dilution. All other patients continued to respond to salmon calcitonin despite the development of antibody to the hormone in ten cases. No evidence of systemic allergic reactions or other toxicity was found in any patient. The data suggest that although antibody formation may occur in as many as 5% of patients treated with salmon calcitonin, this antibody response is unlikely to be of clinical significance in most patients. However, in an occasional patient, a marked antibody response may occur which interferes with the therapeutic use of the hormone. INTRODUCTION Calcitonin (porcine) was first administered to man in 1966 (1). Since then porcine, salmon, and human cal- This work was presented in part to the American Federation for Clinical Research, Carmel, Calif., 3 February Dr. Singer is a clinical investigator of the Veterans Administration. His present address is Wadsworth Hospital, Los Angeles, Calif. Received for publication, 18 February 1972 auid il reviscd form 18 April citonin have been used to treat patients with a variety of skeletal diseases. It is now well established that the hormone is useful in the suppression of increased skeletal turnover in patients with Paget's disease of bone (2-4). We have treated 22 patients with salmon calcitonin for periods up to 19 months and have tested serial blood samples from these patients to detect antibody to salmon calcitonin. Since salmon calcitonin differs in structure from human calcitonin, treatment with the former might stimulate formation of such antibodies (5). We now report the development of antibodies in 5% of the tested patients. One patient became resistant to salmon calcitonin in association with the development of a high titer of antibodies; the other patients had low titers of antibodv and continued to respond to the hormone. METHODS Chinical. 21 patients with Paget's disease and one with steroid-induced osteoporosis received daily subcutaneous injections of salmon calcitonin from 1.5 to 19 months. The age and sex of the patients, duration of treatment, and dose of hormone administered are shown in Table I. Initially, the patients were treated with natural salmon calcitonin,' and later synthetic salmon calcitonin.2 The hormone was dissolved in 16% gelatin and administered subcutaneously once daily. Gelatin was used in an attempt to prolong the action of the hormone, as had previously been demonstrated in animal studies (6). 5 mg of phenol was present in each milliliter of diluent; inclusion of this preservative permitted the use of a multiple dose vial. Two patients (L. H. and A. R.) developed localied redness and itching at the injection sites; for these patients an acetatebuffered diluent was employed; after 3 months in patient L. H., and after 5 days of treatment in patient A. R. Laboratory. The continued biochemical response to calcitonin after chronic administration was assessed by measuring the plasma calcium (7) serially for 24 hr after the initial and selected subsequent injections; the 24 hr urinary hydroxyproline excretion (8), and the plasma alkaline phos- 1AL125 (Armour Pharmaceutical Co., Kankakee, Ill.). 2 AL977 (Armour Pharmaceutical Co.). The Journal of Clinical Investigation Volume 51 September
2 TABLE I Clinical Data on 22 Patients Treated with Salmon Calcitonin Salmon cal- Duration citonin-125i Patient of Sex Age Dose therapy binding titer yr MIRC U* months Paget's disease 1. W.. M :3, 2. A. M. F :15 3. M.K. F :15 4. J. A. M :5 5. R.WX. F :5 6. H. H. M :5 7. M. D. F :4 8. G.O. F :4 9. A. F. M :2 1. L. H. F : A.R. F :4 12. G. C. F <1:5 13. P. S. M <1:5 14. C.E. F <1:5 15. H.B. F <1:5 16. F.K. F <1:5 17. G.Z. F <1:5 18. B.B. M <1:5 19. F. M. M <1:5 2. I. D. F <1:5 21. L. C. F <1:5 Osteoporosis J.C. M <1:5 * MRC units, daily dose. phatase concentrations (9) were measured 5-7 days before the start of treatment and for similar periods of time at intervals of several months during treatment. Renal, hepatic, and hematologic function was assessed by the usual laboratory tests. Levels of parathyroid hormone in plasma were measured by radioimmunoassay (1) using plasma obtained in the basal state, after the first injection of salmon calcitonin, and during treatment. Plasma binding of salmon calcitonin-l"i. Plasma was obtained before and at intervals during treatment (24 hr after injection), and stored at - 4C. Salmon calcitonin was iodinated with "I by the method of Hunter and Greenwood (11) and the labeled peptide was purified by absorption and eluation from silica (12). The specific activity of the salmon calcitonin-'i was MsCi/,ug. To detect binding of salmon calcitonin-'5i to a plasma component, multiple dilutions of plasma were incubated at 4C with 3 pg salmon calcitonin-'"i at ph 7 in.2 M phosphate buffer containing 16% pooled human plasma by volume' for hr. Final incubation volume was.5 ml. Control tubes containing the same dilutions of plasma and 3 pg salmon calcitonin-"ji were incubated simultaneously with excess unlabeled salmon calcitonin. Separation of bound from free hormone was accomplished by addition of 1 ml of dioxane, 'The pooled human plasma is used as a part of a standard diluent in our calcitonin radioimmunoassays; this plasma was separately shown not to bind salmon calcitonin Singer, Aldred, Neer, Krane, Potts, and Bloch incubation for 3 min, and centrifugation at 1, rpm at 4 for 1 min. The supernatant was decanted and the radioactivity in the dioxane precipitate (bound fraction) and supernatant (free fraction) was measured in a gamma spectrometer (Packard Instrument Co., Inc., Downers Grove, Ill.). The ratio of bound to free salmon calcitonin- 5I was calculated using a correction for nonspecific binding determined from the control tubes. The formula used is as follows: B Corrected - = F [(B + F)(B+F)] F where B = bound cpm in presence of patient's plasma, F = free cpm in presence of patient's plasma, Be = bound cpm in presence of patient's plasma + excess salmon calcitonin, Fe = free cpm in presence of patient's plasma + excess salmon calcitonin. The plasma binding titer is then defined as the highest dilution of plasma at which specific binding, i.e., a corrected B/F of.5, could be demonstrated. The maximum binding capacity of plasma (13) from patient W.. was determined by incubating a 1: 5 dilution of plasma obtained after 1 yr of treatment with 3 pg salmon calcitonin-'5i plus amounts of unlabeled salmon calcitonin ranging from to 1 ng/ml for 96 hr at 4VC. Separation of bound from free hormone was accomplished as before. The data was analyed by plotting the ratio of bound to free hormone as a function of salmon calcitonin bound and extrapolating to the abscissa. Characteriation of plasma binding. Electrophoresis in agarose gel of control and treatment plasma mixed with salmon calcitonin-'5i was performed according to Laurell (14). Immuno- and radioimmunoelectrophoresis were performed as previously described (15), except that X-ray film was applied to the dry plates for 4-6 days. Monospecific antisera to human G, A, and M immunoglobulins were prepared according to the method of Fahey and McLaughlin (16). Inactivation of salmon calcitonin, by plasma. Plasma samples were also tested to detect inhibition of the hypocalcemic activity of salmon calcitonin by bioassay; control and treatment plasma were examined from six patients. All plasma samples were heated to 56C for 2 min, since it is known this will eliminate the factor in plasma that normally inactivates calcitonin (17). To 5, 25, 1, 5, and 25 ;41 of plasma was added 1 mmrc units (Medical Research Council units) of salmon calcitonin in 1% gelatin solution, and the total volume adjusted to 5. ml with.9% NaCl. These quantities of plasma represent an effective dilution of 1: 1 to 1: 2. After thorough mixing, samples were incubated at room temperature for 3 min, and then analyed by a modification of a standard rat bioassay for calcitonin (18). Each dilution of plasma was injected subcutaneously into six fasted female Sprague- Dawley rats so as to administer a dose of 1 mmrc units/1 grams in.5 ml diluent to each rat. After 3 min, the rats were anesthetied with ether, blood was obtained from the abdominal aorta, and after centrifugation, the concentration of serum calcium was determined (19). The plasma of patient W.. was studied in triplicate. The fall in serum calcium (mean ±SE) at 3 min was plotted for each dilution of plasma studied, including a 1: 1 plasma dilution from a normal subject. In order to estimate the dilution of plasma which would completely inactivate the salmon calcitonin inj ected, the data were ana-
3 lyed by plotting the per cent inhibition of the maximal hypocalcemic response (the response obtained with the 1:1 control plasma) against the plasma dilution on a log scale. A regression line was drawn which was extended through 1%c inhibition to obtain the estimate. RESULTS Plasma binding of salmon calcitonin-125i. The results of tests with plasma from each of the 22 patients are listed in Table I. Nonspecific binding of salmon calcitonin-ai, the fraction of labeled hormone that could not be displaced from plasma by excess unlabeled salmon calcitonin, did not exceed 2% in any of the patients. The control samples of plasma obtained from each patient before treatment in all instances did not bind salmon calcitonin-'i. In samples obtained from 1 patients, the binding after treatment was detected at dilutions of plasma that ranged from 1: 4 to 1: 15. The 11th patient, W. O., with a distinctly higher titer of 1: 3,, followed a unique clinical course that suggested possible inactivation of salmon calcitonin by antibody. Case summiintary. W.. (MGH No ) is a 5 yr old patient with Paget's disease involving the skull and pelvis. He had mild loss of hearing and pelvic pain. He was treated with 1 MRC units of salmon calcitonin daily in.5 ml gelatin diluent for 15 months. Fig. 1 illustrates the result of this treatment on plasma alkaline phosphatase and urinary total hydroxyproline excretion over an 11.5 month period. During this time his alkaline phosphatase and hydroxyproline excretion decreased and then rose to pretreatment levels. Concomitant with the reversal of the initial response, was a marked increase in his plasma binding titer of salmon calcitonin-'"j. Fig. 2 indicates his plasma calcium response up to 12 hr after his first injection of salmon calcitonin and intermittently during treatment. Although he initially had a hypocalcemic response of 1 mg/1 ml after a test dose of 2 MRC units (Fig. 2A), he no longer became hypocalcemic after 1 MRC units of salmon calcitonin at 7.5 months (Fig. 2B). After 1 yr of treatment, a rapid i.v. injection of 1 MRC units did lower plasma calcium (Fig. 2C), but 1 MRC units subcutaneously did not (Fig. 2D). W -J Yt._ t C: ac\j E >- a: 2, 15 1 _ 2 8 < Cn o a.i a. 4-) Ma. 5 1X 1 1 CONTROL WEEK WEEK 12 MONTHS 11 t n FIGURE 1 The control and treatment levels of urinary hydroxyproline, plasma alkaline phosphatase, and salmon calcitonin-l'i binding titer during an 11.5 month period when patient WV.. received 1 MRC units of salmon calcitonin daily. The hydroxyproline data points represent the mean ±SE of at least five 24 hr urine collections during a 1 wk period. The upper limit of normal is 4 mg/24 hr. The alkaline phosphatase (Bessey-Lowry-Brock Units) data points represent the mean +SE of at least three blood samples taken during the week. The upper limit of normal is 2.6 U. The binding titer is plotted on a log scale. Evaluation of Antibodies and Clinical Resistance to Salmon Calcitonin 2333
4 E C) D -j C) nl I F A r B SOT 25 I I ' + 1.r c SC T F E -1. OU PCT 1U SUBCUT D SCT 1U SUBCUT F PCT IOOU SUBCUT H O U R S FIGURE 2 The plasma calcium responses to calcitonin during the course of treatment of patient W.. (A) Before salmon calcitonin treatment; (B) 7months; (C) 12months; (D) 15 months; (E) before porcine calcitonin treatment; (F) 3 months. During treatment there was neither clinical evidence of an allergic reaction or any evidence of renal, hepatic, or bone marrow toxicity. Plasma parathyroid hormone levels rose during the initial hypocalcemic period as well as during a similar period induced with 1 MRC units of salmon calcitonin (cited above) after 1 yr of treatment (Fig. 3). The levels of parathyroid hormone attained were similar on both occasions. Basal plasma calcium and phosphorus levels did not change significantly during treatment. There was no detectable radiologic change. The patient's bone pain diminished initially and did not return during the period in which he no longer showed a biochemical response to salmon calcitonin. After 15 months of treatment with salmon calcitonin it was demonstrated that porcine calcitonin' could produce a hypocalcemic response in the patient (Fig. 2E). He was then treated daily with 1 MRC units subcutaneously for 3 months. At the end of this period of treatment, his alkaline phosphatase and hydroxyproline excretion had not changed significantly from control levels, and he had become resistant to the hypocalcemic effect of porcine calcitonin (Fig. 2F). Plasma binding titers of porcine calcitonin-'i were measured using the same method as for salmon calcitonin-'i. At the onset of porcine calcitonin therapy, the porcine calcitonin-'i 'AL831 (Armour Pharmaceutical Co.) Singer, Aldred, Neer, Krane, Potts, and Bloch binding titer was 1: 3, indicating that the antisalmon calcitonin antibody also bound porcine calcitonin. At the end of the 3 months of porcine calcitonin therapy, the titer had not changed significantly. In three patients (P. S., G. Z., and L. H.) plasma alkaline phosphatase and urinary hydroxyproline either fell into the normal range or progressively fell toward normal. Five patients (A. M., J. A., H. H., A. F., and G. C.) showed a 25-75% decrease in skeletal turnover which persisted with continued treatment. These five patients continued to show an acute hypocalcemic response to injected salmon calcitonin throughout the course of treatment. There was no correlation between the development of antibodies and a plateau response. In the remaining patients there was a similar decrease in skeletal turnover but the duration of therapy or frequency of follow-up admissions was insufficient to establish whether these patients attained a plateau response. Characteriation of binding activity: association with innnunoglobuidn. G. Control and treatment plasma from patients W. O., A. M., A. F., H. H., and R. W. was mixed with salmon calcitonin-l2'i and subjected to agarose gel electrophoresis. Radioautography revealed binding to the y-globulin fraction in each of the five treatment plasmas; no binding was detected in any control plasma. The same plasma samples were subjected to immunoelectrophoresis. After yg, -ya, and YM precipitin lines had formed, salmon calcitonin-'=i was added to the troughs for radioautography. In Fig. 4A and B the results obtained with plasma from patient W.. are shown. There was no difference in the precipitin lines ;- 9.5 E J < 8.5 C-) CL HOURS 8 1. E F- c.5 a İ FIGURE 3 Plasma calcium and human parathyroid hormone levels in patient W.. Our normal range for calcium is 8.5 to 1.5 mg/1 ml and for parathyroid hormone.3 to.8 ng/ml. The closed circles indicate the levels after the first injection of salmon calcitonin, (2 MRC units i.v.). The open circles indicate the levels after the 1 MRC unit i.v. salmon calcitonin dose at 12 months. -J
5 Pre Post IA iiiow-... no -mp... X1I'P *. k. AO.P. I.:a t '4 A + + FIGURE 4 Immunoelectrophoresis and radioautography of plasma before and 12 months after the administration of salmon calcitonin to patient W.. Wells contain pre- and post-treatment plasmas as indicated; troughs were filled with monospecific antiserum to human yg (upper two troughs), antiserum to ya (middle two troughs), and antiserum to 'ym (lower two troughs). After development of precipitin lines (IA), salmon calcitonin-'i was added to the troughs; binding of labeled hormone primarily to the "fast" portion G precipitin arc occurred in the post-treatment plasma (IB). observed with plasma obtained before and after therapy; salmon calcitonin-'=i was bound to the YG precipitin arc obtained with treatment plasma, but not with the control plasma. Identical results were obtained with the treatment plasma of the other patients. Maximttium binding capacity of W.. plasma. The maximum binding capacity of the plasma of W.. is illustrated in Fig. 5. The plasma taken after 1 yr of salmon calcitonin therapy bound approximately.6,g/ ml, or 1.5 MRC units/ml of salmon calcitonin. If one assumes a volume of distribution of YG antibody of 85 ml per kilogram of body weight (2), it can be calculated that approximately 1, MRC units of administered salmon could be bound by the total amount of antibody estimated to be present in patient W. O., who weighed 8 kg. Inactivation of hypocalcemic activity of salmon calcitonin by W.. plasma. The results of an additional method of estimating the total amount of antibody present in patient W.. are illustrated in Fig. 6. This method provides an approach that assesses directly inactivation of hormone by antibody rather than binding of hormone to antibody. Increasing quantities of the patient's plasma produced progressively greater inhibition of the hypocalcemic effect of salmon calcitonin in the rat. 5 Al of plasma (the 1: 1 dilution, Fig. 6) Evaluation of Antibodies and Clinical Resistance to Salmon Calcitonin 2*335
6 6 H 5 4 () llj LLJ 3 UL- \ 2 D I m SCT BOUND ng/ml FIGURE 5 The maximum binding capacity of a 1: 5 dilution of plasma from patient W.. after 12 months of salmon calcitonin therapy. The ratio of salmon calcitonin- "2I bound by antibody to free salmon calcitonin-'5i is plotted as a function of bound salmon calcitonin. The maximum binding capacity is obtained by extrapolating the curve to the abscissa. N completely inhibited the hypocalcemic effect of 1 mmrc units of salmon calcitonin in a 1 g rat. When the data are plotted as per cent of hypocalcemic inhibition vs. the plasma dilution on a log scale, a linear regression is found (inset, Fig. 6). By extrapolating the line, it would appear that 1% inhibition could be achieved with plasma diluted 1: 15. This figure is in good agreement with the direct observations that complete inactivation occurs with plasma diluted 1: 1, and incomplete inactivation with plasma diluted 1: 2. From this estimate it was calculated that 1 ml of plasma from W.. inactivates.3 MRC units (15 X 2 mmrc units/ml) of salmon calcitonin. It was further estimated, using the assumptions outlined above, that 2 MRC units of calcitonin could be inactivated by the total quantity of antibody available in patient W.. No inactivation of the biological activity of calcitonin was detected with the control plasma from this patient, or with plasma from five other patients (A. M., R. W., H. H., A. F., and G. C.). DISCUSSION The daily administration of salmon calcitonin to 22 patients for periods up to 19 months was associated with the development of plasma binding of salmon calcitonin- "I in 11 patients. The evidence that antibodies to salmon calcitonin were responsible for the binding included: (a) the binding of salmon calcitonin-lzi was demonstrated only after treatment with this hormone began; (b) the 2336 Singer, Aldred, Neer, Krane, Potts, and Bloch salmon calcitonin-'i could be displaced from its binding sites by excess unlabeled salmon calcitonin; and (c) the binding activity was specifically associated with the IgG fraction of the plasma proteins as determined by radioimmunoelectrophoresis. The clinical significance of the presence of antibodies, other than inhibition of the pharmacologic effect of salmon calcitonin in one patient, appears to be minimal in view of the lack of associated allergic phenomena or any evidence of toxicity. All patients, except W. O., continued to show a response to the hormone during the course of treatment as judged by continued suppression of the indices of skeletal turnover. The clinical course of patient W.. is of great interest since in this patient the development of antibodies appeared responsible for acquired resistance to the hormone. The unique features seen in this patient included: (a) the development of resistance to the biological effects of salmon calcitonin with the loss of the hypocalcemic response to the hormone and a return of indices of skeletal turnover to pretreatment values despite continued treatment; (b) a far greater antibody titer (maximum 1: 3,) than was found in any other patient; and (c) inactivation by his plasma of salmon calcitonin in the rat bioassay. There was no clinical evi- 9.6, E On 9.21 D 9. - < 8.81 : 8.6- :D WLJ 8.4- (I) MINUTES 3 1,1 2 1[5 [io 1:2 l (C C NT) FIGURE 6 The inhibition of the hypocalcemic activity of salmon calcitonin by the plasma of patient W.. The serum calcium (mean ±SE of three separate studies) before and 3 min after injection of 1 mmrc units (2 mmrc units/ ml) salmon calcitonin/1 g rat and varying dilutions of W.. treatment plasma and plasma from a normal control. The inset shows the data plotted as the per cent inhibition of the maximal hypocalcemic response (the dashed line) vs. the plasma dilution on a log scale.
7 dence of hyperparathyroidism and the concentrations of parathyroid hormone induced by hypocalcemia were similar before and during treatment. Hence, resistance to the hormone in this patient can not be attributed to the development of secondary hyperparathyroidism. It was possible to overcome resistance to the hypocalcemic effect of salmon calcitonin by rapid i.v. administration of 1 MRC units of the hormone. One speculation for this difference in response to subcutaneous and i.v. administration is that after i.v. injection enough hormone reached skeletal receptor sites before complete mixing of the hormone and antibody occurred. Antibodies to polypeptide hormones have been demonstrated in the blood of patients treated with bovine and porcine insulin (21), bovine and porcine vasopressin (22), bovine parathyroid hormone (23), porcine and al-24 ACTH (24, 25), and human growth hormone (26). Almost all diabetics treated with bovine and porcine insulin develop antibodies to insulin, but only a few are insulin resistant. Berson and Yalow have pointed out that the responsive diabetics with antibodies have low binding capacities and that the insulin-resistant patients have much greater binding capacities (13). Resistance to vasopressin has not been documented and only one patient with antibodies and resistance to bovine PTH has been described (23). Antibodies to porcine ACTH given in gelatin and as a inc phosphate complex commonly result in antibody formation, but resistance to hormone action has not been proven (24, 25). In these reports the foreign protein nature of the hormones has been used as the explanation for the development of antibodies. Development of antibodies to hormone preparations believed to be identical to endogenous human hormone would appear to violate concepts of immune tolerance. However, synthetic al-24 ACTH did induce antibody formation in man although this polypeptide is identical in structure to the first 24 amino acids of human ACTH (25). Lack of the terminal 15 amino acids may unmask an antigenic site in this peptide. Human growth hormone administration can also result in antibody formation and resistance to its metabolic effects in man (27, 28). Conformational differences resulting from extraction of growth hormone from pituitary glands may render this preparation antigenic in man. Once resistance to salmon calcitonin developed in patient W.. who had initially responded to the hormone, it seemed reasonable to administer to him another form of calcitonin that is structurally distinct, such as porcine calcitonin. However, since porcine calcitonin is quite different in structure from human calcitonin (5), it might be expected that antibodies directed against this hormone would also develop. Patient W. O., who already had a low titer of antibodies which bound porcine calcitonin, received treatment for 3 months with the hormone after it was demonstrated that he was not resistant to its hypocalcemic effect. He became resistant to porcine calcitonin during this period although he did not show a rise in antibody titer. Further studies are required to elucidate the mechanism of clinical resistance developing during treatment with porcine calcitonin in this patient. Human calcitonin has recently become available for therapeutic trials. However, since the structure of synthetic human calcitonin is based on the structure determined from calcitonin extracted from a medullary carcinoma of thyroid (29), it is possible that there is a small difference in structure between the synthetic preparation and endogenous calcitonin in normal subjects. Such a difference could predispose to antibody development to human tumor calcitonin in patients treated with this preparation. In a recent paper the absence of antibody formation was reported in one patient treated with human calcitonin for 1 yr (3). Recently we have started to administer synthetic human calcitonin 6 to patient W.. Preliminary results indicate a definite decrease in skeletal turnover during the 1st wk of treatment. Note added in proof. After 6 months of treatment with human calcitonin the plasma alkaline phosphatase and 24 hr urinary hydroxyproline excretion of patient W.. had plateaued at two to three times normal. These indices had decreased 4%, from control levels. There were no detectable antibodies to human calcitonin in his plasma and there was no evidence for hyperparathyroidism. The factor responsible for the incomplete suppression of his disease remains to be determined. ACKNOWLEDGMENTS We thank Dr. James Lesh, James Bastian, and Karl Hansen of Armour Pharmaceutical for the salmon and porcine calcitonin, and Doctors Alfred Ling and William Darrow of Ciba for the human calcitonin, and Eliabeth Greene, Michael Byrne, Phillip Nagel, and Cyrena Simons for expert technical assistance. This study was supported by grants from the Veterans Administration, John A. Hartford Foundation, Gebbie Foundation, Damon Runyon Memorial Fund for Cancer Research, and U. S. Public Health Service (AM3564, AM11794, AM525, and AM451). 'Calcitonin M (Ciba Pharmaceutical Co., Summit, N. J.). REFERENCES 1. Foster, G. V., G. F. Joplin, I. MacIntyre, K. E. W. Melvin, and E. Slack Effect of thyrocalcitonin in man. Lancet. I: Bijvoet,. L. M., J. van der Sluys Veer, J. Wildiers, and. Smeenk Effects of long-term calcitonin administration to patients. In Calcitonin 1969, Proceedings of the Second International Symposium. S. Taylor and G. Foster, editors. W. Heinemann, London Woodhouse, N. J. Y., M. Reiner, P. Bordier, D. N. Kalu, M. Fisher, G. V. Foster, G. F. Joplin, and I. MacIntyre Human calcitonin in the treatment of Paget's bone disease. Lancet. I: Singer, F. R., H. T. Keutmann, R. M. Neer, J. T. Potts, Jr., and S. M. Krane Pharmacological Evaluation of Antibodies and Clinical Resistance to Salmon Calcitonin 2337
8 effects of salman calcitonin in man. Calcium, Parathyroid Hormone and the Calcitonins. R. V. Talmage and P. L. Munson, editors. Excerpta Medica Foundation, Amsterdam Potts, J. T., Jr., H. D. Niall, H. T. Keutmann, L. J. Deftos, and J. A. Parsons Calcitonin: Recent chemical and immunological studies. In Calcitonin 1969, Proceedings of the Second International Symposium, S. Taylor and G. Foster, editors. W. Heinemann, London, Aldred, J. P., G. R. Clements, R. J. Schlueter, J. W. Bastian, J. P. Dailey and F. X. Waeter Pharmacologic and toxicologic effects of porcine thyrocalcitonin in animals. In Calcitonin, Proceedings of the Symposium on Thyrocalcitonin and the C Cells. S. Taylor, editor. W. Heinemann, London MacIntyre, I Flame photometry. Adv. Clin. Chem. 4: Prockop, D. J., and S. Udenfriend A specific method for the analysis of hydroxyproline in tissues and urine. Anal. Biochem. 1: Bessey,. A.,. H. Lowry, and M. J. Brock A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 164: Potts, J. T., Jr., and L. J. Deftos Parathyroid hormone, thyrocalcitonin, vitamin D, bone and bone mineral metabolism. In Duncan's Diseases of Metabolism. P. K. Bondy, editor. W. B. Saunders Co., Philadelphia. 6th edition. 11. Hunter, W. M., and F. C. Greenwood Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature (Lond.). 194: Rosselin, G., R. Assan, R. S. Yalow, and S. A. Berson, Separation of antibody-bound and unbound peptide hormones labelled with 131I by talcum powder and precipitated silica. Nature (Lond.). 212: Berson, S. A., and R. S. Yalow Quantitative aspects of the reaction between insulin and insulin-binding antibody. J. Clin. Invest. 38: Laurell, C. B Electrophoretic microheterogeneity of serum al-antitrypsin. Scand. J. Clin. Lab. Invest. 17: Bloch, K. J., H. C. Morse III, and K. F. Austen Biological properties of rat antibodies. I. Antigen binding by four classes of anti-dnp antibodies. J. Immunol. 11: Fahey, J. L., and C. McLaughlin Preparation of antisera specific for 6.6S y-globulin,,2a-globulins, ylmicroglobulins, and for type I and II common y- globulin determinants. J. ImniUnol. 91: Tashjian, A. H., Jr., and E. F. Voelkel Studies of the inactivation of thyrocalcitonin by serum. In Parathyroid Hormone and Thyrocalcitonin (Calcitonin), Proceedings of the Third Parathyroid Conference. R. V. Talmage and L. F. Belanger, editors. Excerpta Medica Foundation, Amsterdam Schlueter, R. J., J. P. Aldred, and J. D. Fisher Thyrocalcitonin-bioassay characteristics of purified preparations. Acta Endocrinol. 58: Gitelman, H. J An improved automated procedure for the determination of calcium in biological specimens. Anal. Biochem. 18: Waldmann, T. A., and W. Strober Metabolism of immunoglobulins. Prog. Allergy. 13: Berson, S. A., R. S. Yalow, A. Bauman, M. A. Rothschild, and K. Newerly Insulin-"1'I metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J. Clin. Invest. 35: Roth, J., S. M. Glick, L. A. Klein, and M. J. Peterson Specific antibody to vasopressin in man. J. Clin. Endocrinol. Metab. 26: Melick, R. A., J. R. Gill, Jr., S. A. Berson, R. S. Yalow, F. C. Bartter, J. T. Potts, Jr., and G. D. Aurbach Antibodies and clinical resistance to parathyroid hormone. N. Engi. J. Med. 276: Fleischer, N., K. Abe, G. W. Liddle, D. N. Orth, and W. E. Nicholson ACTH antibodies in patients receiving depot porcine ACTH to hasten recovery from pituitary-adrenal suppression. J. Clin. Invest. 46: Ratcliffe, J. G., M. Pritchard, and A. H. El-Shaboury The production of antibodies to porcine corticotrophin and to synacthen. J. Endocrinol. 43: Roth, J., S. M. Glick, R. S. Yalow, and S. A. Berson Antibodies to human growth hormone in human subjects treated with HGH. J. Clin. Invest. 43: Parker, M. L., I. K. Mari, and W. H. Daughaday Resistance to human growth hormone in pituitary dwarfs: Clinical and immunologic studies. J. Clin. Endocrinol. Metab. 24: Prader, A., H. Wagner, J. Seky, R. Illey, J. L. Tauber, and D. Maingay Acquired resistance to human growth hormone caused by specific antibodies. Lancet. I: Neher, R., B. Riniker, R. Maier, P. G. H. Byfield, T. V. Gudmundsson, and I. MacIntyre Human calcitonin. Nature (Lond.). 22: Singer, Aldred, Neer, Krane, Potts, and Bloch
Secretion of Calcitonin in Hypocalcemic States in Man
 Secretion of Calcitonin in Hypocalcemic States in Man LEONARD J. DEFos, DAVI JoHN T. Porrs, JR. POWELL, JACQUELINE G. PARTEMORE, and From the Department of Medicine (Endocrinology), University of California,
Secretion of Calcitonin in Hypocalcemic States in Man LEONARD J. DEFos, DAVI JoHN T. Porrs, JR. POWELL, JACQUELINE G. PARTEMORE, and From the Department of Medicine (Endocrinology), University of California,
Radioimmunoassay Specific for Amino (N) and Carboxyl (C) Terminal Portion of Parathyroid Hormone
 Endocrinol. Japon. 1975, 22 (6), 471 `477 Radioimmunoassay Specific for Amino (N) and Carboxyl (C) Terminal Portion of Parathyroid Hormone MASAHIRO TANAKA, KAORU ABE, IsAMu ADACHI KEN YAMAGUCHI, SUMIKO
Endocrinol. Japon. 1975, 22 (6), 471 `477 Radioimmunoassay Specific for Amino (N) and Carboxyl (C) Terminal Portion of Parathyroid Hormone MASAHIRO TANAKA, KAORU ABE, IsAMu ADACHI KEN YAMAGUCHI, SUMIKO
Calcitonin Resistance: Clinical and Immunologic Studies
 Calcitonin Resistance: Clinical and Immunologic Studies in Subjects with Paget's Disease of Bone lfreated with Porcine and Salmon Calcitonins JoHN G. HADDAD, JR. and JOSEPH G. CALDWELL From the Department
Calcitonin Resistance: Clinical and Immunologic Studies in Subjects with Paget's Disease of Bone lfreated with Porcine and Salmon Calcitonins JoHN G. HADDAD, JR. and JOSEPH G. CALDWELL From the Department
J. Biosci., Vol. 7, Number 2, March 1985, pp Printed in India.
 J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
OGY. IV. THE METABOLISM OF IODINE IN
 RADIOACTIVE IODINE AS AN INDICATOR IN THYROID PHYSIOL- OGY IV THE METABOLISM OF IODINE IN GRAVES' 1 By S HERTZ, A ROBERTS, AND W T SALTER (From the Thyroid Clinic of the Massachusetts General Hospital,
RADIOACTIVE IODINE AS AN INDICATOR IN THYROID PHYSIOL- OGY IV THE METABOLISM OF IODINE IN GRAVES' 1 By S HERTZ, A ROBERTS, AND W T SALTER (From the Thyroid Clinic of the Massachusetts General Hospital,
Immunologic Cross-Reaction Between Luteinizing Hormone and Human Chorionic Gonadotropin
 Immunologic Cross-Reaction Between Luteinizing Hormone and Human Chorionic Gonadotropin MELVIN L. TAYMOR, M.D., DONALD A. GOSS, M.D., and ALBERT BUYTENDORP, M.D. RECENTLY a number of reports 2 4 have indicated
Immunologic Cross-Reaction Between Luteinizing Hormone and Human Chorionic Gonadotropin MELVIN L. TAYMOR, M.D., DONALD A. GOSS, M.D., and ALBERT BUYTENDORP, M.D. RECENTLY a number of reports 2 4 have indicated
Parathyroid Hormone: Secretion and Metabolism In Vivo
 Proc. Nat. Acad. Sci. USA Vol. 68, No. 12, pp. 2986-2991, December 1971 Parathyroid Hormone: Secretion and Metabolism In Vivo (human/bovine/gel filtration/radioii catheterization) JOEL F. HABENER, DAVID
Proc. Nat. Acad. Sci. USA Vol. 68, No. 12, pp. 2986-2991, December 1971 Parathyroid Hormone: Secretion and Metabolism In Vivo (human/bovine/gel filtration/radioii catheterization) JOEL F. HABENER, DAVID
Ca, Mg metabolism, bone diseases. Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary
 Ca, Mg metabolism, bone diseases Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary Calcium homeostasis Ca 1000g in adults 99% in bones (extracellular with Mg, P) Plasma/intracellular
Ca, Mg metabolism, bone diseases Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary Calcium homeostasis Ca 1000g in adults 99% in bones (extracellular with Mg, P) Plasma/intracellular
norepinephrinee." 2 PNMT activity is stimulated by certain adrenocortical markedly,3' 4 but can be restored to normal by the administration of
 IMPAIRED SECRETION OF EPINEPHRINE IN RESPONSE TO INSULIN AMONG HYPOPHYSECTOMIZED DOGS* BY RICHARD J. WURTMAN, ALFRED CASPER, LARISSA A. POHORECKY, AND FREDERIC C. BARTTER DEPARTMENT OF NUTRITION AND FOOD
IMPAIRED SECRETION OF EPINEPHRINE IN RESPONSE TO INSULIN AMONG HYPOPHYSECTOMIZED DOGS* BY RICHARD J. WURTMAN, ALFRED CASPER, LARISSA A. POHORECKY, AND FREDERIC C. BARTTER DEPARTMENT OF NUTRITION AND FOOD
Key words: Collagen synthesis - N-Terminal peptide of type III procollagen - Tumor marker - Liver cancer - Liver cirrhosis
 [Gann, 75, 130-135; February, 1984] HIGH CONCENTRATIONS OF N-TERMINAL PEPTIDE OF TYPE III PROCOLLAGEN IN THE SERA OF PATIENTS WITH VARIOUS CANCERS, WITH SPECIAL REFERENCE TO LIVER CANCER Terumasa HATAHARA,
[Gann, 75, 130-135; February, 1984] HIGH CONCENTRATIONS OF N-TERMINAL PEPTIDE OF TYPE III PROCOLLAGEN IN THE SERA OF PATIENTS WITH VARIOUS CANCERS, WITH SPECIAL REFERENCE TO LIVER CANCER Terumasa HATAHARA,
Actions of Parathyroid Hormone in the Vitamin D-deficient
 Journal of Clinical Investigation Vol. 44, No. 12, 1965 Actions of Parathyroid Hormone in the Vitamin D-deficient Dog * ROBERT L. NEY,t WILLIAM Y. W. Au,t GERALD KELLY, I. RADDE, AND FREDERIC C. BARTTER
Journal of Clinical Investigation Vol. 44, No. 12, 1965 Actions of Parathyroid Hormone in the Vitamin D-deficient Dog * ROBERT L. NEY,t WILLIAM Y. W. Au,t GERALD KELLY, I. RADDE, AND FREDERIC C. BARTTER
Thyrocalcitonin and the Jejunal Absorption of Calcium, Water, and Electrolytes in Normal Subjects
 Thyrocalcitonin and the Jejunal Absorption of Calcium, Water, and Electrolytes in Normal Subjects T. KENNEY GRAY, FREDERICK A. BIEBERDORF, and JOHN S. FORDTRAN From the Department of Internal Medicine
Thyrocalcitonin and the Jejunal Absorption of Calcium, Water, and Electrolytes in Normal Subjects T. KENNEY GRAY, FREDERICK A. BIEBERDORF, and JOHN S. FORDTRAN From the Department of Internal Medicine
Estimation of Hydrocortisone Secretion
 Estimation of Hydrocortisone Secretion Method of Calculation from Urinary-Excretion Data Robert H. Silber IN1938, Anderson, Haymaker, and Joseph (1) reported the finding of increased concentrations of
Estimation of Hydrocortisone Secretion Method of Calculation from Urinary-Excretion Data Robert H. Silber IN1938, Anderson, Haymaker, and Joseph (1) reported the finding of increased concentrations of
The Third Department of Internal Medicine, University of Tokyo Faculty of Medicine, Hongo, Tokyo 113
 Endocrinol. Japon. 1974, 21 (2), 115 ` 119 A Radioimmunoassay for Serum Dehydroepiandrosterone HISAHIKO SEKIHARA, TOHRU YAMAJI, NAKAAKI OHSAWA AND HIROSHI IBAYASHI * The Third Department of Internal Medicine,
Endocrinol. Japon. 1974, 21 (2), 115 ` 119 A Radioimmunoassay for Serum Dehydroepiandrosterone HISAHIKO SEKIHARA, TOHRU YAMAJI, NAKAAKI OHSAWA AND HIROSHI IBAYASHI * The Third Department of Internal Medicine,
A Study of Urinary Excretion
 A Study of Urinary Excretion of Parathyroid Hormone in Man JOHN E. BETHUNE and RANDOLPH A. TURPIN From the Department of Medicine, Los Angeles County-University of Southern California Medical Center, Los
A Study of Urinary Excretion of Parathyroid Hormone in Man JOHN E. BETHUNE and RANDOLPH A. TURPIN From the Department of Medicine, Los Angeles County-University of Southern California Medical Center, Los
Department of Obstetrics and Gynecology, School of Medicine, Chiba University, Chiba
 Endocrinol. Japon. 1971, 18 (6), 477 `485 Radioimmunoassays for Rat Follicle Stimulating and Luteinizing Hormones KATSUYOSHI SEKI, MITSUNORI SEKI, TERUFUMI YOSHIHARA AND HIDEYASU MAEDA Department of Obstetrics
Endocrinol. Japon. 1971, 18 (6), 477 `485 Radioimmunoassays for Rat Follicle Stimulating and Luteinizing Hormones KATSUYOSHI SEKI, MITSUNORI SEKI, TERUFUMI YOSHIHARA AND HIDEYASU MAEDA Department of Obstetrics
INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT
 Brit. J. Phawmacol. (1951), 6, 289. INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT BY From the Pharmacological Laboratory, University of St. Andrews, Medical School, Dundee (Received February 2, 1951)
Brit. J. Phawmacol. (1951), 6, 289. INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT BY From the Pharmacological Laboratory, University of St. Andrews, Medical School, Dundee (Received February 2, 1951)
A DECREASE IN SERUM PROTEIN concentration during pregnancy in humans
 Electrophoretic Studies of Serum Proteins of Nonpregnant and Pregnant Hamsters ROY P. PETERSON, M.A.," CHARLES L. TURBYFILL, M.A.,f and A. L. SODERWALL, Ph.D. A DECREASE IN SERUM PROTEIN concentration
Electrophoretic Studies of Serum Proteins of Nonpregnant and Pregnant Hamsters ROY P. PETERSON, M.A.," CHARLES L. TURBYFILL, M.A.,f and A. L. SODERWALL, Ph.D. A DECREASE IN SERUM PROTEIN concentration
PRODUCT MONOGRAPH. CALCIMAR SOLUTION (Synthetic Calcitonin-Salmon) Calcium Regulator
 PRODUCT MONOGRAPH CALCIMAR SOLUTION (Synthetic Calcitonin-Salmon) Calcium Regulator sanofi-aventis Canada Inc. 2905 Place Louis-R.-Renaud Laval, Quebec H7V 0A3 Date of Revision: April 15, 2014 Submission
PRODUCT MONOGRAPH CALCIMAR SOLUTION (Synthetic Calcitonin-Salmon) Calcium Regulator sanofi-aventis Canada Inc. 2905 Place Louis-R.-Renaud Laval, Quebec H7V 0A3 Date of Revision: April 15, 2014 Submission
(Adams 8c Purves 1958), or LATS-protector (LATS-P) (Adams 8c Kennedy. 1967). The failure of the McKenzie (1958) mouse bioassay to detect LATS in
 Department of Endocrinology, Royal Prince Alfred Hospital, and Department of Medicine, University of Sydney, Sydney, Australia THE THYROTROPHIN RECEPTOR IN HUMAN THYROID PLASMA MEMBRANES: EFFECT OF SERUM
Department of Endocrinology, Royal Prince Alfred Hospital, and Department of Medicine, University of Sydney, Sydney, Australia THE THYROTROPHIN RECEPTOR IN HUMAN THYROID PLASMA MEMBRANES: EFFECT OF SERUM
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Separation of Plasma and Serum and Their Proteins from Whole Blood
 Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
Bone and Mineral. Comprehensive Menu for the Management of Bone and Mineral Related Diseases
 Bone and Mineral Comprehensive Menu for the Management of Bone and Mineral Related Diseases Innovation to Assist in Clinical Diagnosis and Treatment DiaSorin offers a specialty line of Bone and Mineral
Bone and Mineral Comprehensive Menu for the Management of Bone and Mineral Related Diseases Innovation to Assist in Clinical Diagnosis and Treatment DiaSorin offers a specialty line of Bone and Mineral
Effect of Orchiectomy on Pituitary Secretion of ACTH MARY D. COYNE AND JULIAN I. KITAY
 Excerpted from: Journal Title: Endocrinology. Volume: 89 Issue: 4 October 1971 Pages: 1024-8 Effect of Orchiectomy on Pituitary Secretion of ACTH MARY D. COYNE AND JULIAN I. KITAY Department of Physiology,
Excerpted from: Journal Title: Endocrinology. Volume: 89 Issue: 4 October 1971 Pages: 1024-8 Effect of Orchiectomy on Pituitary Secretion of ACTH MARY D. COYNE AND JULIAN I. KITAY Department of Physiology,
Calcium metabolism and the Parathyroid Glands. Calcium, osteoclasts and osteoblasts-essential to understand the function of parathyroid glands
 Calcium metabolism and the Parathyroid Glands Calcium, osteoclasts and osteoblasts-essential to understand the function of parathyroid glands Calcium is an essential element for contraction of voluntary/smooth
Calcium metabolism and the Parathyroid Glands Calcium, osteoclasts and osteoblasts-essential to understand the function of parathyroid glands Calcium is an essential element for contraction of voluntary/smooth
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
EFFECT OF THYROID HORMONES ON HISTAMINE FORMATION IN THE RAT
 Brit. J. Pharmacol. (1961), 17, 479-487. EFFECT OF THYROID HORMONES ON HISTAMINE FORMATION IN THE RAT BY T. BJURO, H. WESTLING AND H. WETTERQVIST From the Department of Clinical Physiology, University
Brit. J. Pharmacol. (1961), 17, 479-487. EFFECT OF THYROID HORMONES ON HISTAMINE FORMATION IN THE RAT BY T. BJURO, H. WESTLING AND H. WETTERQVIST From the Department of Clinical Physiology, University
Approach to a patient with hypercalcemia
 Approach to a patient with hypercalcemia Ana-Maria Chindris, MD Division of Endocrinology Mayo Clinic Florida 2013 MFMER slide-1 Background Hypercalcemia is a problem frequently encountered in clinical
Approach to a patient with hypercalcemia Ana-Maria Chindris, MD Division of Endocrinology Mayo Clinic Florida 2013 MFMER slide-1 Background Hypercalcemia is a problem frequently encountered in clinical
Metabolism and Kinetics of Adrenocortical Steroids. -A Consideration on Corticosteroid Therapy-
 Metabolism and Kinetics of Adrenocortical Steroids -A Consideration on Corticosteroid Therapy- Yoshitaka Araki First Department of Internal Medicine, Faculty of Medicine, University of Tokyo, Tokyo I.
Metabolism and Kinetics of Adrenocortical Steroids -A Consideration on Corticosteroid Therapy- Yoshitaka Araki First Department of Internal Medicine, Faculty of Medicine, University of Tokyo, Tokyo I.
Pseudohypoparathyroidism Showing Positive Phosphaturic and Negative Cyclic AMP Excretion Response to Parathyroid Hormone
 Pseudohypoparathyroidism Showing Positive Phosphaturic and Negative Cyclic AMP Excretion Response to Parathyroid Hormone KIICHIRO HIGASHI, KENICHI HONDA*, MITSUO MORITA*, TERUHISA UMEDA*, TATSUYA SHIMADA,
Pseudohypoparathyroidism Showing Positive Phosphaturic and Negative Cyclic AMP Excretion Response to Parathyroid Hormone KIICHIRO HIGASHI, KENICHI HONDA*, MITSUO MORITA*, TERUHISA UMEDA*, TATSUYA SHIMADA,
Thyrocalcitonin and the Response to Parathyroid
 Journal ofclnical Investigation Vol. 46, ao. 1, 1967 Thyrocalcitonin and the Response to Parathyroid Hormone * CONSTANTINE ANAST, CLAUDE D. ARNAUD, HOWARD RASMUSSEN,t AND ALAN TENENHOUSE (From the Department
Journal ofclnical Investigation Vol. 46, ao. 1, 1967 Thyrocalcitonin and the Response to Parathyroid Hormone * CONSTANTINE ANAST, CLAUDE D. ARNAUD, HOWARD RASMUSSEN,t AND ALAN TENENHOUSE (From the Department
David Bruyette, DVM, DACVIM
 VCAwestlaspecialty.com David Bruyette, DVM, DACVIM Disorders of calcium metabolism are common endocrine disorders in both dogs and cats. In this article we present a logical diagnostic approach to patients
VCAwestlaspecialty.com David Bruyette, DVM, DACVIM Disorders of calcium metabolism are common endocrine disorders in both dogs and cats. In this article we present a logical diagnostic approach to patients
THE RENAL HANDLING OF PHOSPHATE IN THYROID DISEASE
 THE RENAL HANDLING OF PHOSPHATE IN THYROID DISEASE B. MALAMOS, P. SFIKAKIS and P. PANDOS Department of Clinical Therapeutics of the Athens Medical School, Alexandra Hospital, Athens, Greece (Received 18
THE RENAL HANDLING OF PHOSPHATE IN THYROID DISEASE B. MALAMOS, P. SFIKAKIS and P. PANDOS Department of Clinical Therapeutics of the Athens Medical School, Alexandra Hospital, Athens, Greece (Received 18
TSH ELISA Kit Medical Device Licence No.: 16419
 TSH ELISA Kit Medical Device Licence No.: 16419 Enzyme immunoassay kit for the quantitative determination of TSH concentration in serum. Catalog Number: SL100305 Catalog Number: SL100306 Catalog Number:
TSH ELISA Kit Medical Device Licence No.: 16419 Enzyme immunoassay kit for the quantitative determination of TSH concentration in serum. Catalog Number: SL100305 Catalog Number: SL100306 Catalog Number:
Metabolic Studies of Human Bone In Vitro.
 Journal of Clinical Investigation Vol. 44, No. 11, 1965 Metabolic Studies of Human Bone In Vitro. II. Changes in Hyperparathyroidism * BARRY FLANAGAN t AND GORG NICHOLS, JR. t (From the Department of Medicine,
Journal of Clinical Investigation Vol. 44, No. 11, 1965 Metabolic Studies of Human Bone In Vitro. II. Changes in Hyperparathyroidism * BARRY FLANAGAN t AND GORG NICHOLS, JR. t (From the Department of Medicine,
Endocrine Regulation of Calcium and Phosphate Metabolism
 Endocrine Regulation of Calcium and Phosphate Metabolism Huiping Wang ( 王会平 ), PhD Department of Physiology Rm C516, Block C, Research Building, School of Medicine Tel: 88208252 Email: wanghuiping@zju.edu.cn
Endocrine Regulation of Calcium and Phosphate Metabolism Huiping Wang ( 王会平 ), PhD Department of Physiology Rm C516, Block C, Research Building, School of Medicine Tel: 88208252 Email: wanghuiping@zju.edu.cn
See external label 2 C-8 C = C-REACTIVE PROTEIN (CRP) LATEX SLIDE TEST
 CORTEZ DIAGNOSTICS, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
CORTEZ DIAGNOSTICS, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
FEBS 1138 January Paul R. Buckland and Bernard Rees Smith
 Volume 166, number 1 FEBS 1138 January 1984 A structural comparison receptors by of guinea pig thyroid and fat TSH photoaffinity labelling Paul R. Buckland and Bernard Rees Smith Endocrine Immunology Unit,
Volume 166, number 1 FEBS 1138 January 1984 A structural comparison receptors by of guinea pig thyroid and fat TSH photoaffinity labelling Paul R. Buckland and Bernard Rees Smith Endocrine Immunology Unit,
(Received 9th January 1974)
 RELEASE OF LH AND FSH IN THE NORMAL INTACT RAM BY SYNTHETIC LH-RF AND THE EFFECT OF PRETREATMENT WITH TESTOSTERONE PROPIONATE C. R. N. HOPKINSON, H. C. PANT and R. J. FITZPATRICK Department of Veterinary
RELEASE OF LH AND FSH IN THE NORMAL INTACT RAM BY SYNTHETIC LH-RF AND THE EFFECT OF PRETREATMENT WITH TESTOSTERONE PROPIONATE C. R. N. HOPKINSON, H. C. PANT and R. J. FITZPATRICK Department of Veterinary
RELATIONS BETWEEN INSULIN AND PITUITARY HORMONES IN AMINO ACID METABOLISM
 RELATIONS BETWEEN INSULIN AND PITUITARY HORMONES IN AMINO ACID METABOLISM BY WILLIAM D. LOTSPEICH* WITH THE TECHNICAL ASSISTANCE OF JOAN B. SHELTON (From the Department of Physiology, Syracuse University
RELATIONS BETWEEN INSULIN AND PITUITARY HORMONES IN AMINO ACID METABOLISM BY WILLIAM D. LOTSPEICH* WITH THE TECHNICAL ASSISTANCE OF JOAN B. SHELTON (From the Department of Physiology, Syracuse University
these peptides in peripheral serum if their survival times were, as estimated by several other workers, 5-10 times that of hpth(1-84).
 Immunoheterogeneity of Parathyroid Hormone in Venous Effluent Serum from Hyperfunctioning Parathyroid Glands JAMES A. FLUECK, FRANcis P. Di BELLA, ANTHONY J. EDIS, JEAN M. KEHRWALD, and CLAUDE D. ARNAUD,
Immunoheterogeneity of Parathyroid Hormone in Venous Effluent Serum from Hyperfunctioning Parathyroid Glands JAMES A. FLUECK, FRANcis P. Di BELLA, ANTHONY J. EDIS, JEAN M. KEHRWALD, and CLAUDE D. ARNAUD,
Hypoparathyroid Patients *
 Journal of Clinical Investigation Vol. 44, No. 6, 1965 Effects of Serum Calcium Level and Parathyroid Extracts on Phosphate and Calcium Excretion in Hypoparathyroid Patients * EUGENE EISENBERG t (From
Journal of Clinical Investigation Vol. 44, No. 6, 1965 Effects of Serum Calcium Level and Parathyroid Extracts on Phosphate and Calcium Excretion in Hypoparathyroid Patients * EUGENE EISENBERG t (From
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
PARATHYROID, VITAMIN D AND BONE
 PARATHYROID, VITAMIN D AND BONE G M Kellerman Pathology North Hunter Service 30/01/2015 BIOLOGY OF BONE Bone consists of protein, polysaccharide components and mineral matrix. The mineral is hydroxylapatite,
PARATHYROID, VITAMIN D AND BONE G M Kellerman Pathology North Hunter Service 30/01/2015 BIOLOGY OF BONE Bone consists of protein, polysaccharide components and mineral matrix. The mineral is hydroxylapatite,
SYSTEM ACCELERATING DECISION-MAKING
 4 SYSTEM ACCELERATING DECISION-MAKING Accurate and fully automated TM System offers fast result. With simple 3 steps operation and wide range of disease markers, the system supports your quick decision-making.
4 SYSTEM ACCELERATING DECISION-MAKING Accurate and fully automated TM System offers fast result. With simple 3 steps operation and wide range of disease markers, the system supports your quick decision-making.
MIACALCIC (salcatonin)
 1 MIACALCIC (salcatonin) DESCRIPTION A synthesised polypeptide hormone structurally identical with salmon calcitonin. It contains 32 amino-acids in linear sequence with a disulphide bridge at position
1 MIACALCIC (salcatonin) DESCRIPTION A synthesised polypeptide hormone structurally identical with salmon calcitonin. It contains 32 amino-acids in linear sequence with a disulphide bridge at position
Rho, or to the "partial" or "blocking antibody"
 THE IMPORTANCE OF RH INHIBITOR SUBSTANCE IN ANTI-RH SERUMS' By LOUIS K. DIAMOND AND NEVA M. ABELSON (From the Infants' and the Children's Hospitals, the Department of Pediatrics, Harvard Medical School,
THE IMPORTANCE OF RH INHIBITOR SUBSTANCE IN ANTI-RH SERUMS' By LOUIS K. DIAMOND AND NEVA M. ABELSON (From the Infants' and the Children's Hospitals, the Department of Pediatrics, Harvard Medical School,
See external label 2 C-8 C Σ=96 tests Cat # 6101Z. Cortisol. Cat # 6101Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Tymlos (abaloparatide) NEW PRODUCT SLIDESHOW
 Tymlos (abaloparatide) NEW PRODUCT SLIDESHOW Introduction Brand name: Tymlos Generic name: Abaloparatide Pharmacological class: Human parathyroid hormone related peptide analog Strength and Formulation:
Tymlos (abaloparatide) NEW PRODUCT SLIDESHOW Introduction Brand name: Tymlos Generic name: Abaloparatide Pharmacological class: Human parathyroid hormone related peptide analog Strength and Formulation:
Rat C-Peptide EIA. Cat. No. YII-YK010-EX FOR LABORATORY USE ONLY
 Rat C-Peptide EIA Cat. No. YII-YK010-EX FOR LABORATORY USE ONLY TOYO 2CHOME, KOTO-KU, TOKYO, 135-0016, JAPAN http://www.cosmobio.co.jp e-mail : export@cosmobio.co.jp 1 Phone : +81-3-5632-9617 FAX : +81-3-5632-9618
Rat C-Peptide EIA Cat. No. YII-YK010-EX FOR LABORATORY USE ONLY TOYO 2CHOME, KOTO-KU, TOKYO, 135-0016, JAPAN http://www.cosmobio.co.jp e-mail : export@cosmobio.co.jp 1 Phone : +81-3-5632-9617 FAX : +81-3-5632-9618
however, and the present communication is concerned with some of
 THE AGGLUTINATION OF HUMAN ERYTHROCYTES MODIFIED BY TREATMENT WITH NEWCASTLE DISEASE AND INFLUENZA VIRUS' ALFRED L. FLORMAN' Pediatric Service and Division of Bacteriology, The Mount Sinai Hospital, New
THE AGGLUTINATION OF HUMAN ERYTHROCYTES MODIFIED BY TREATMENT WITH NEWCASTLE DISEASE AND INFLUENZA VIRUS' ALFRED L. FLORMAN' Pediatric Service and Division of Bacteriology, The Mount Sinai Hospital, New
THE SITE OF STEROL AND SQUALENE SYNTHESIS IN THE HUMAN SKIN123
 THE SITE OF STEROL AND SQUALENE SYNTHESIS IN THE HUMAN SKIN123 N. NICOLAIDES, PH.D. AND STEPHEN ROTHMAN, M.D. In earlier work (1) it was demonstrated that human scalp skin is an efficient organ for synthesizing
THE SITE OF STEROL AND SQUALENE SYNTHESIS IN THE HUMAN SKIN123 N. NICOLAIDES, PH.D. AND STEPHEN ROTHMAN, M.D. In earlier work (1) it was demonstrated that human scalp skin is an efficient organ for synthesizing
(Received for publication July 5, 1944) course of the malarial fever and also during the period
 SIGNIFICANCE OF CEPHALIN-CHOLESTEROL FLOCCULATION TEST IN MALARIAL FEVER1 By SAMUEL A. GUTTMAN, HARRY R. POTTER, FRANKLIN M. HANGER, DAVID B. MOORE, PAUL S. PIERSON, AND DAN H. MOORE (From the Neurological
SIGNIFICANCE OF CEPHALIN-CHOLESTEROL FLOCCULATION TEST IN MALARIAL FEVER1 By SAMUEL A. GUTTMAN, HARRY R. POTTER, FRANKLIN M. HANGER, DAVID B. MOORE, PAUL S. PIERSON, AND DAN H. MOORE (From the Neurological
Human Aldosterone ELISA Kit
 Human Aldosterone ELISA Kit Cat. No.:DEIA2002 Pkg.Size:96T Intended use For determination of Aldosterone by enzyme immunoassay in human serum by enzyme immunoassay. Hydrolysis is necessary for the determination
Human Aldosterone ELISA Kit Cat. No.:DEIA2002 Pkg.Size:96T Intended use For determination of Aldosterone by enzyme immunoassay in human serum by enzyme immunoassay. Hydrolysis is necessary for the determination
Parathyroid hormone (serum, plasma)
 Parathyroid hormone (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Parathyroid hormone (PTH) 1.2 Alternative names Parathormone 1.3 NMLC code 1.4 Description of analyte PTH is an
Parathyroid hormone (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Parathyroid hormone (PTH) 1.2 Alternative names Parathormone 1.3 NMLC code 1.4 Description of analyte PTH is an
JOSEPH P. DAILY Armour Pharmaceutical Company Kankakee, Illinois
 PHARMACEUTICALS AND MEDICDIALS OF ANIMAL ORIGIN* JOSEPH P. DAILY Armour Pharmaceutical Company Kankakee, Illinois Thrcughout history, the inter-dependency between man arid animals has been profound. Man
PHARMACEUTICALS AND MEDICDIALS OF ANIMAL ORIGIN* JOSEPH P. DAILY Armour Pharmaceutical Company Kankakee, Illinois Thrcughout history, the inter-dependency between man arid animals has been profound. Man
Metabolism of echitamine and plumbagin in rats
 J. Biosci., Vol. 3, Number 4, December 1981, pp. 395-400. Printed in India. Metabolism of echitamine and plumbagin in rats B. CHANDRASEKARAN and B. NAGARAJAN Microbiology Division, Cancer Institute, Madras
J. Biosci., Vol. 3, Number 4, December 1981, pp. 395-400. Printed in India. Metabolism of echitamine and plumbagin in rats B. CHANDRASEKARAN and B. NAGARAJAN Microbiology Division, Cancer Institute, Madras
Cambridge CB2 3EG. ['25I]L-thyroxine. Experiments were performed after 24 hr had elapsed.
![Cambridge CB2 3EG. ['25I]L-thyroxine. Experiments were performed after 24 hr had elapsed. Cambridge CB2 3EG. ['25I]L-thyroxine. Experiments were performed after 24 hr had elapsed.](/thumbs/95/125304458.jpg) J. Physiol. (1971), 212, pp. 447-454 447 With 2 text-ftgurea Printed in Great Britain AN EXAMINATION OF THE EXTENT OF REVERSIBILITY OF THYROXINE BINDING WITHIN THE THYROXINE DISTRIBUTION SPACE IN THE RABBIT
J. Physiol. (1971), 212, pp. 447-454 447 With 2 text-ftgurea Printed in Great Britain AN EXAMINATION OF THE EXTENT OF REVERSIBILITY OF THYROXINE BINDING WITHIN THE THYROXINE DISTRIBUTION SPACE IN THE RABBIT
Human Thyroid Stimulating Hormone CLIA kit
 Human Thyroid Stimulating Hormone CLIA kit Cat. No.:DEEL0223 Pkg.Size:96 tests Intended use For the direct quantitative determination of Thyroid Stimulating Hormone in human serum by chemiluminescence
Human Thyroid Stimulating Hormone CLIA kit Cat. No.:DEEL0223 Pkg.Size:96 tests Intended use For the direct quantitative determination of Thyroid Stimulating Hormone in human serum by chemiluminescence
EGTA, and Urinary Electrolyte Excretion *
 Journal of Clnical Investigation Vol. 46, No. 5, 1967 Thyrocalcitonin, EGTA, and Urinary Electrolyte Excretion * HOWARD RASMUSSEN,t CONSTANTINE ANAST, AND CLAUDE ARNAUD (From the Department of Biochemistry,
Journal of Clnical Investigation Vol. 46, No. 5, 1967 Thyrocalcitonin, EGTA, and Urinary Electrolyte Excretion * HOWARD RASMUSSEN,t CONSTANTINE ANAST, AND CLAUDE ARNAUD (From the Department of Biochemistry,
THE INSULIN-GLUCOSE TOLERANCE TEST IN PITUITARY GROWTH RETARDATION
 Arch. Dis. Childh., 1965, 4, 58. THE INSULIN-GLUCOSE TOLERANCE TEST IN PITUITARY GROWTH RETARDATION BY OLAV TRYGSTAD From the Paediatric Research Institute, University Hospital, Rikhospitalet, Oslo, Norway
Arch. Dis. Childh., 1965, 4, 58. THE INSULIN-GLUCOSE TOLERANCE TEST IN PITUITARY GROWTH RETARDATION BY OLAV TRYGSTAD From the Paediatric Research Institute, University Hospital, Rikhospitalet, Oslo, Norway
Reduced sulphation factor in undernourished children
 Archives of Disease in Childhood, 1973, 48, 596. Reduced sulphation factor in undernourished children D. B. GRANT, JUDY HAMBLEY, DOROTHY BECKER,* and B. L. PIMSTONE* From the Clinical Research Centre,
Archives of Disease in Childhood, 1973, 48, 596. Reduced sulphation factor in undernourished children D. B. GRANT, JUDY HAMBLEY, DOROTHY BECKER,* and B. L. PIMSTONE* From the Clinical Research Centre,
Chapter 13 worksheet
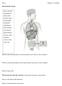 Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
WHO International Biological Reference Preparations Held and distributed by the WHO International Laboratories for Biological Standards
 WHO International Biological Preparations Activin A, human, recombinant. Lyophilized. 5 units / Reagent, 1998 No. 897 49th NIBSC 91/626 98.1882 Arginine vasopressin. Lyophilized. 8.2 IU / Standard, 1978
WHO International Biological Preparations Activin A, human, recombinant. Lyophilized. 5 units / Reagent, 1998 No. 897 49th NIBSC 91/626 98.1882 Arginine vasopressin. Lyophilized. 8.2 IU / Standard, 1978
The Role of Nonprecipitating Insulin Antibodies in Diabetes
 The Role of Nonprecipitating Insulin Antibodies in Diabetes Joseph H. Skom, David W. Talmage J Clin Invest. 1958;37(6):787-793. https://doi.org/1.1172/jci13665. Research Article Find the latest version:
The Role of Nonprecipitating Insulin Antibodies in Diabetes Joseph H. Skom, David W. Talmage J Clin Invest. 1958;37(6):787-793. https://doi.org/1.1172/jci13665. Research Article Find the latest version:
SONE ON THE DISTRIBUTION AND PERIPHERAL
 THE INFLUENCE OF ACTH, CORTISONE, AND HYDROCORTI- SONE ON THE DISTRIBUTION AND PERIPHERAL METABOLISM OF THYROXINE' By SIDNEY H. INGBAR2 AND NORBERT FREINKEL 8 (From the Thorndike Memorial Laboratory, Second
THE INFLUENCE OF ACTH, CORTISONE, AND HYDROCORTI- SONE ON THE DISTRIBUTION AND PERIPHERAL METABOLISM OF THYROXINE' By SIDNEY H. INGBAR2 AND NORBERT FREINKEL 8 (From the Thorndike Memorial Laboratory, Second
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 2 Available Product Indication Dosing and Administration Natpara (parathyroid hormone)
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 2 Available Product Indication Dosing and Administration Natpara (parathyroid hormone)
Distribution of alkaline phosphatase in serum protein fractions
 J. clin. Path. (1962), 15, 195 Distribution of alkaline phosphatase in serum protein fractions N. H. KORNER From the Department of Medicine, St. Vincent's Hospital, Sydney, Australia synopsis A technique
J. clin. Path. (1962), 15, 195 Distribution of alkaline phosphatase in serum protein fractions N. H. KORNER From the Department of Medicine, St. Vincent's Hospital, Sydney, Australia synopsis A technique
STUDIES OF PLASMA VOLUME USING HUMAN SERUM ALBUMIN TAGGED WITH RADIOACTIVE IODINE 131
 STUDIES OF PLASMA VOLUME USING HUMAN SERUM ALBUMIN TAGGED WITH RADIOACTIVE IODINE 131 Kenneth R. Crispell,, Blanche Porter, Robert T. Nieset J Clin Invest. 1950;29(5):513-516. https://doi.org/10.1172/jci102288.
STUDIES OF PLASMA VOLUME USING HUMAN SERUM ALBUMIN TAGGED WITH RADIOACTIVE IODINE 131 Kenneth R. Crispell,, Blanche Porter, Robert T. Nieset J Clin Invest. 1950;29(5):513-516. https://doi.org/10.1172/jci102288.
suffering from hyperthyroidism before and after treatment have shown variable J. Physiol. (1958) I42,
 447 J. Physiol. (1958) I42, 447-452 BONE CALCIUM AND SODIUM CONTENT AND THE EXCHANGE OF RADIOSODIUM IN BONES FROM RATS TREATED WITH THYROXINE AND PARATHORMONE BY D. S. MUNRO, R. S. SATOSKAR AND G. M. WILSON
447 J. Physiol. (1958) I42, 447-452 BONE CALCIUM AND SODIUM CONTENT AND THE EXCHANGE OF RADIOSODIUM IN BONES FROM RATS TREATED WITH THYROXINE AND PARATHORMONE BY D. S. MUNRO, R. S. SATOSKAR AND G. M. WILSON
AROMASIN 25mg (Tablets)
 APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
Specificity and Sensitivity of Radioimmunoassay for Hepatitis
 APPLIED MICROBIOLOGY, Oct. 1974, P. 600-604 Copyright 0 1974 American Society for Microbiology Vol. 28, No. 4 Printed in U.S.A. Specificity and Sensitivity of Radioimmunoassay for Hepatitis B Antigen GILBERT
APPLIED MICROBIOLOGY, Oct. 1974, P. 600-604 Copyright 0 1974 American Society for Microbiology Vol. 28, No. 4 Printed in U.S.A. Specificity and Sensitivity of Radioimmunoassay for Hepatitis B Antigen GILBERT
CHISCG1: Short Synacthen Test for the Investigation of Adrenal Insufficiency
 Pathology at the Royal Derby Hospital Short Synacthen Test Standard Clinical Guidelines Chemical Pathology Department Valid Until 31 st August 2011 Document Code: CHISCG1 Short Synacthen Test for the Investigation
Pathology at the Royal Derby Hospital Short Synacthen Test Standard Clinical Guidelines Chemical Pathology Department Valid Until 31 st August 2011 Document Code: CHISCG1 Short Synacthen Test for the Investigation
Estimation of the Secretion Rate of Thyrotropin in Man *
 Journal of Clinical Investigation Vol. 46, No. 6, 1967 Estimation of the Secretion Rate of Thyrotropin in Man * W. D. ODELL,t R. D. UTIGER, J. F. WILBER, AND P. G. CONDLIFFE (From the Endocrinology and
Journal of Clinical Investigation Vol. 46, No. 6, 1967 Estimation of the Secretion Rate of Thyrotropin in Man * W. D. ODELL,t R. D. UTIGER, J. F. WILBER, AND P. G. CONDLIFFE (From the Endocrinology and
PREPARATION STANDARD MATERIAL HELD AT CODE WHO/BS DOCUMENT. Native hormone purified from urine
 WHO International Biological Reference Preparations Held and Distributed by the WHO International Laboratories for Biological Standards ENDOCRINOLOGICAL SUBSTANCES Activin A, human, recombinant, Lyophilized,
WHO International Biological Reference Preparations Held and Distributed by the WHO International Laboratories for Biological Standards ENDOCRINOLOGICAL SUBSTANCES Activin A, human, recombinant, Lyophilized,
Radioimmunoassay of Herpes Simplex Virus Antibody: Correlation with Ganglionic Infection
 J. gen. Virol. (I977), 3 6, ~ 371-375 Printed in Great Britain 371 Radioimmunoassay of Herpes Simplex Virus Antibody: Correlation with Ganglionic Infection By B. FORGHANI, TONI KLASSEN AND J. R. BARINGER
J. gen. Virol. (I977), 3 6, ~ 371-375 Printed in Great Britain 371 Radioimmunoassay of Herpes Simplex Virus Antibody: Correlation with Ganglionic Infection By B. FORGHANI, TONI KLASSEN AND J. R. BARINGER
Thyroid, antithyroid, parathyroid & Calcium metabolism. Suharti K Suherman Dept. of Pharmacology & Therapeutic Medical Faculty, Univ.
 Thyroid, antithyroid, parathyroid & Calcium metabolism Suharti K Suherman Dept. of Pharmacology & Therapeutic Medical Faculty, Univ. of Indonesia Thyroid secreted by thyroid gland source of 2 different
Thyroid, antithyroid, parathyroid & Calcium metabolism Suharti K Suherman Dept. of Pharmacology & Therapeutic Medical Faculty, Univ. of Indonesia Thyroid secreted by thyroid gland source of 2 different
PROTEIN SYNTHIESIS DURING WORK-INDUCED GROWTH OF SKELETAL MUSCLE
 Published Online: 1 March, 1968 Supp Info: http://doi.org/10.1083/jcb.36.3.653 Downloaded from jcb.rupress.org on October 15, 2018 PROTEIN SYNTHIESIS DURING WORK-INDUCED GROWTH OF SKELETAL MUSCLE ALFRED
Published Online: 1 March, 1968 Supp Info: http://doi.org/10.1083/jcb.36.3.653 Downloaded from jcb.rupress.org on October 15, 2018 PROTEIN SYNTHIESIS DURING WORK-INDUCED GROWTH OF SKELETAL MUSCLE ALFRED
Inpatient Pediatric Endocrinology. Tala Dajani MD MPH Pediatric Endocrinology of Phoenix
 Inpatient Pediatric Endocrinology Tala Dajani MD MPH Pediatric Endocrinology of Phoenix Objectives Identify calcium disorders in the hospital Distinguish between temporary versus permanent glucose problems
Inpatient Pediatric Endocrinology Tala Dajani MD MPH Pediatric Endocrinology of Phoenix Objectives Identify calcium disorders in the hospital Distinguish between temporary versus permanent glucose problems
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit
 Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
NEW ONE-STAGE PROCEDURES FOR THE QUANTITATIVE DETERMINATION OF PROTHROMBIN AND LABILE FACTOR*
 NEW ONE-STAGE PROCEDURES FOR THE QUANTITATIVE DETERMINATION OF PROTHROMBIN AND LABILE FACTOR* MARIO STEFANINI, M.D.f From the Department ofbiochemistry, Marquette University School of Medicine, Milwaukee,
NEW ONE-STAGE PROCEDURES FOR THE QUANTITATIVE DETERMINATION OF PROTHROMBIN AND LABILE FACTOR* MARIO STEFANINI, M.D.f From the Department ofbiochemistry, Marquette University School of Medicine, Milwaukee,
C-Peptide I and II (Rat) ELISA
 C-Peptide I and II (Rat) ELISA For the quantitative determination of rat C-peptide in plasma, serum, urine, and cell culture supernatant Please read carefully due to Critical Changes, e.g., blot plate
C-Peptide I and II (Rat) ELISA For the quantitative determination of rat C-peptide in plasma, serum, urine, and cell culture supernatant Please read carefully due to Critical Changes, e.g., blot plate
Boston City Hospital, and the Department of Medicine, Harvard Medical School, Boston)
 THE EFFECT OF FOREIGN SURFACES ON BLOOD COAGULATION ", 2 BY EUGENE L. LOZNER AND F. H. L. TAYLOR WITH THE TECHNICAL ASSISTANCE OF HARRIET MAcDONALD (From the Thorndike Memorial Laboratory, Second and Fourth
THE EFFECT OF FOREIGN SURFACES ON BLOOD COAGULATION ", 2 BY EUGENE L. LOZNER AND F. H. L. TAYLOR WITH THE TECHNICAL ASSISTANCE OF HARRIET MAcDONALD (From the Thorndike Memorial Laboratory, Second and Fourth
satisfactorily as a means of altering experimentally the ph of the upper
 THE REACTION QF HUMAN DUODENAL CONTENTS TO ACID AND ALKALINE MEAT MIXTURES By STACY R. METTIER (From I1e Thorndike Memorial Laboratory, Boston City Hospital, and the Department of Medicine, Harvard Medical
THE REACTION QF HUMAN DUODENAL CONTENTS TO ACID AND ALKALINE MEAT MIXTURES By STACY R. METTIER (From I1e Thorndike Memorial Laboratory, Boston City Hospital, and the Department of Medicine, Harvard Medical
III. TOXICOKINETICS. Studies relevant to the toxicokinetics of inorganic chloramines are severely
 III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
GROWTH HORMONE AND AMINO ACIDS
 GROWTH HORMONE AND AMINO ACIDS Certain amino acids when combined with one another and ingested will cause an increase in growth hormone levels in humans. The increase in the body's production of growth
GROWTH HORMONE AND AMINO ACIDS Certain amino acids when combined with one another and ingested will cause an increase in growth hormone levels in humans. The increase in the body's production of growth
9.2 Hormonal Regulation of Growth
 9.2 Hormonal Regulation of Growth Hormonal Regulation of Growth Pituitary gland regulates growth and development Thyroid gland regulates metabolic rate (exception: some hormones for growth and development)
9.2 Hormonal Regulation of Growth Hormonal Regulation of Growth Pituitary gland regulates growth and development Thyroid gland regulates metabolic rate (exception: some hormones for growth and development)
had no effect on the production of aldosterone, corticosterone, or cortisol after
 INHIBITION OF THE EFFECTS OF ANGIOTENSIN II ON ADRENAL STEROID PRODUCTION BY DIETARY SODIUM BY WARREN W. DAVIS,* LAWRENCE R. BURWELL,t AND FREDERIC C. BARTTERt ENDOCRINOLOGY BRANCH, NATIONAL HEART INSTITUTE,
INHIBITION OF THE EFFECTS OF ANGIOTENSIN II ON ADRENAL STEROID PRODUCTION BY DIETARY SODIUM BY WARREN W. DAVIS,* LAWRENCE R. BURWELL,t AND FREDERIC C. BARTTERt ENDOCRINOLOGY BRANCH, NATIONAL HEART INSTITUTE,
GSI Canine IL-5 ELISA Kit-2 Plates DataSheet
 Interleukin5 (IL5) is a secreted glycoprotein that belongs to the α-helical group of cytokines (1, 2, 3). Unlike other family members, it is present as a covalently linked antiparallel dimer (4, 5). IL-5
Interleukin5 (IL5) is a secreted glycoprotein that belongs to the α-helical group of cytokines (1, 2, 3). Unlike other family members, it is present as a covalently linked antiparallel dimer (4, 5). IL-5
ALPHAFETOPROTEIN IN LWER DISEASE
 ALPHAFETOPROTEIN IN LWER DISEASE Pages with reference to book, From 234 To 236 Waquar uddin Ahmed, Sarwar J. Zuberi ( PMRC Research Centre, Jinnah Postgraduate Medical Centre, Karachi-35. ) Abstract Alpha
ALPHAFETOPROTEIN IN LWER DISEASE Pages with reference to book, From 234 To 236 Waquar uddin Ahmed, Sarwar J. Zuberi ( PMRC Research Centre, Jinnah Postgraduate Medical Centre, Karachi-35. ) Abstract Alpha
Immunoglobulins in chronic liver disease
 Gut, 1968, 9, 193-198 Immunoglobulins in chronic liver disease TEN FEIZI From the Department of Medicine, Royal Free Hospital, London The association of high gamma globulin levels with hepatic cirrhosis
Gut, 1968, 9, 193-198 Immunoglobulins in chronic liver disease TEN FEIZI From the Department of Medicine, Royal Free Hospital, London The association of high gamma globulin levels with hepatic cirrhosis
Phosphatase Activity of Drosophila Salivary Glands
 Phosphatase Activity of Drosophila Salivary Glands BY W. L. DOYLE (From the Department of Anatomy, University of Chicago) THE presence of alkaline phosphatase in chromosomes has been demonstrated by means
Phosphatase Activity of Drosophila Salivary Glands BY W. L. DOYLE (From the Department of Anatomy, University of Chicago) THE presence of alkaline phosphatase in chromosomes has been demonstrated by means
usually contain somatic protein as the principal antigen, determination of the Heidelberger and Kendall (1932) developed a method for the quantitative
 A MOUSE PROTECTION METHOD FOR THE ESTIMATION OF ANTIGENIC PNEUMOCOCCAL POLYSACCHARIDE IN SOLUTION CURTIS SANDAGEl AND ORTON K. STARK Miami University, Oxford, Ohio Received for publication May 5, 1947
A MOUSE PROTECTION METHOD FOR THE ESTIMATION OF ANTIGENIC PNEUMOCOCCAL POLYSACCHARIDE IN SOLUTION CURTIS SANDAGEl AND ORTON K. STARK Miami University, Oxford, Ohio Received for publication May 5, 1947
venous blood samples were drawn. The plasma was separated rapidly by centrifugation and assayed within one
 THE ANTIDIURETIC ACTIVITY OF PLASMA OF PATIENTS WITH HEPATIC CIRRHOSIS, CONGESTIVE HEART FAIL- URE, HYPERTENSION AND OTHER CLINICAL DISORDERS' By MARVIN STEIN,2 ROBERT SCHWARTZ, AND I. ARTHUR MIRSKY (From
THE ANTIDIURETIC ACTIVITY OF PLASMA OF PATIENTS WITH HEPATIC CIRRHOSIS, CONGESTIVE HEART FAIL- URE, HYPERTENSION AND OTHER CLINICAL DISORDERS' By MARVIN STEIN,2 ROBERT SCHWARTZ, AND I. ARTHUR MIRSKY (From
: /18
 612.461.23: 616-001.17/18 SOME OBSERVATIONS ON THE COMPARATIVE EFFECTS OF COLD AND BURNS ON PROTEIN METABOLISM IN RATS. By G. H. LATHE 1 and R. A. PETERS. From the Department of Biochemistry, Oxford. (Received
612.461.23: 616-001.17/18 SOME OBSERVATIONS ON THE COMPARATIVE EFFECTS OF COLD AND BURNS ON PROTEIN METABOLISM IN RATS. By G. H. LATHE 1 and R. A. PETERS. From the Department of Biochemistry, Oxford. (Received
IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS
 22 IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS Michael P. Lerner*, J. H. Anglin, Peggy L. Munson, Peggy J. Riggs, Nancy E. Manning, and Robert E. Nordquist Departments
22 IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS Michael P. Lerner*, J. H. Anglin, Peggy L. Munson, Peggy J. Riggs, Nancy E. Manning, and Robert E. Nordquist Departments
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Natpara (parathyroid hormone) Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Natpara (parathyroid hormone) Prime Therapeutics will review Prior Authorization
Natpara (parathyroid hormone) Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Natpara (parathyroid hormone) Prime Therapeutics will review Prior Authorization
Awaisheh. Mousa Al-Abbadi. Abdullah Alaraj. 1 Page
 f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
absorption is confined to the dark period. cyclic variation related, among other factors, to the calcium intake.
 J. Physiol. (1972), 222, pp. 559-567 559 With 3 text-figures Printed in Great Britain DIURNAL VARIATION OF PLASMA CALCIUM AND CALCITONIN FUNCTION IN TH RAT BY GIRARD MILHAUD, ANN-MARI PRAULT-STAUB AND
J. Physiol. (1972), 222, pp. 559-567 559 With 3 text-figures Printed in Great Britain DIURNAL VARIATION OF PLASMA CALCIUM AND CALCITONIN FUNCTION IN TH RAT BY GIRARD MILHAUD, ANN-MARI PRAULT-STAUB AND
and fibrinogen (2). The present
 THE COAGULATION DEFECT IN HEMOPHILIA: THE CLOT PRO- MOTING ACTIVITY IN HEMOPHILIA OF BERKEFELDED NORMAL HUMAN PLASMA FREE FROM FIBRI- NOGEN AND PROTHROMBIN' By EUGENE L. LOZNER, ROBERT KARK, AND F. H.
THE COAGULATION DEFECT IN HEMOPHILIA: THE CLOT PRO- MOTING ACTIVITY IN HEMOPHILIA OF BERKEFELDED NORMAL HUMAN PLASMA FREE FROM FIBRI- NOGEN AND PROTHROMBIN' By EUGENE L. LOZNER, ROBERT KARK, AND F. H.
