Dietary Supplement Update
|
|
|
- Susan Amie Wilkerson
- 5 years ago
- Views:
Transcription
1 Dietary Supplement Update Daniel Fabricant, Ph.D. Director, Division of Dietary Supplement Programs, Office of Nutrition, Labeling and Dietary Supplements, Center for Food Safety and Applied Nutrition, Food and Drug Administration 1 The Menu New Dietary Ingredient (NDI) guidance 21 CFR Part 111 cgmps Adverse event reporting (AERs) Tainted Supplements Other matters The take away Questions 1
2 NDI fun facts There are about 55,600 dietary supplement products on the market, and the Institute of Medicine has estimated that 1,000 new dietary supplements are introduced to the market each year, yet FDA has only received approximately 700 NDI notifications since we began reviewing NDI notifications some 16 years ago. More NDI fun facts We have acknowledged 158 of those notifications. An acknowledgement means that we do not object to the marketing of the ingredient in a dietary supplement under the conditions of use proposed in the notification. To date we have objected to 442 notifications. A number of objections still find their way onto the market. 2
3 NDI Guidance Under the FDA Food Safety and Modernization Act (FSMA), FDA is required to publish NDI guidance not later than 180 days after the date of enactment. The guidance is intended to inform and assist manufacturers, distributors, and other industry entities in deciding when a premarket safety notification for a dietary supplement containing a new dietary ingredient (NDI) is necessary and in preparing premarket safety notifications. 3
4 NDI guidance Guidance represents the FDA's current thinking on this topic. It does NOT create or confer any rights for or on any person and does not operate to bind FDA or the public. 21 CFR Part 111 Official Action Indicated 25% for FY10 Letter to Industry- December 15, 2010 ( ResourcesForYou/Consumers/ BuyingUsingMedicineSafely/ MedicationHealthFraud/UCM pdf) New Food GMPs Preventive Control/HACCP (Sec. 103) & Intentional Adulteration (Sec. 106) 4
5 Number of CAERS cases (individuals)^ consuming dietary supplements and experiencing an adverse event January 1, 2007 through December 31, 2010 Number of CAERS Cases Year From Voluntary Reports From Mandatory Reports $ Both Voluntary and Mandatory Reports Total Cases Per Year * Total Cases Per Report Type ^For , counts were obtained using FDA Received Date and year of first report for a case if there were multiple reports for a case (e.g., both a voluntary report and a mandatory report; follow-up reports); CAERS was searched on May 26, 2011 for these counts; *There were two cases with a voluntary report in 2007 that also had information submitted under a mandatory report; one in 2009 and one in $-Submission will not be construed by FDA as an admission that the dietary supplement involved caused or contributed to the adverse event being reported. Soladek Received seven reports of serious health problems occurring in consumers using the product. The problems include decreased renal function, elevated levels of calcium in the blood, fatigue, heart arrhythmia, vomiting, and diarrhea. Symptoms of vitamin D toxicity include weakness, fatigue, headache, nausea, vomiting, diarrhea, changes in mental status, increased blood pressure, abnormal heart rate or rhythm, kidney damage, and coma. Symptoms of vitamin A toxicity include anemia, anorexia, alopecia, joint pain, bone weakness, bulging eyes, liver abnormalities, and birth defects. Other related products 5
6 Serious Health Risks In the past few months, there have been reports in all three high risk product categories of serious adverse events including two deaths. Health risks are compounded by false and misleading marketing: Undeclared ingredients -- at times, up to 10 times the recommended starting doses False claims to be natural, herbal, or safe Inadequate or absent safety warnings Hidden ingredients may interact with other medications a consumer may be taking Poor manufacturing quality controls Professional careers adversely affected by positive drug tests (e.g. amphetamines, anabolic steroids) police, firefighter, military, athletes 11 Hidden Ingredients Prescription drug ingredients, including controlled substances Analogues of prescription drug ingredients Unapproved scheduled controlled substances Ingredients with an IND and under clinical investigation Novel ingredients that have never been studied in humans Ingredients approved as drugs in other countries Ingredients that have been removed from the US market for safety reasons 12 6
7 Weight Loss Products More than 40 recalled products Consumer alerts concerning more than 70 products Hidden Ingredients: sibutramine controlled substance and obesity drug bumetanide a potent diuretic fluoxetine an antidepressant rimonabant not approved for marketing in US cetilistat an unapproved experimental obesity drug fenfluramine stimulant drug withdrawn from US market propranolol beta-blocker drug Examples: Slim 30 SoloSlim Que She 2 Day Diet StarCaps Venom Hyperdrive 13 Masquerades as Legitimate Supplement 14 7
8 Sexual Enhancement Products More than 70 recalled products 4 consumer warnings Hidden Ingredients: sildenafil erectile dysfunction drug (Viagra) tadalafil erectile dysfunction drug (Cialis) vardenafil erectile dysfunction drug (Levitra) piperidenafil analogue of vardenafil sulfoaildenafil analogue of sildenafil benzamidenafil - a newly discovered PDE5 inhibitor Examples: Erectzia Vigor 100 Aziffa Mojo Zotrex Zilex Prolatis 15 Masquerades as Legitimate Conventional Food 16 8
9 Body Building Products More than 90 recalled products A public health advisory issued about certain body building products Steroid Ingredients: Many products contain undeclared listed anabolic steroids Aromatase inhibitors estrogen blockers Prohormones and SARMs Long chemical names that are confusing to consumers: Ø 19-Norandrosta-4,9-diene-3,17 dione Ø 17α-methyl-etioallocholan-2-ene-17b-ol Ø 4-hydroxyandrostenedione (4-OHA) Ø 2α,3α-epithio-17α-methyl-17β-hydroxy-5α- etioallocholane Ø Androsta-1,4-dien-3,17-dione Examples: TREN-Xtreme Straight Drol Epi-Tren ESTRO Xtreme Methadrol Reversitol Rage RV5 17 Masquerades as Legitimate Supplement 18 9
10 Tainted Supplement Pathway to Market FOREIGN DOMESTIC Finished Product Raw Material U.S. Border Importer Contract Manufacturer(s) (bottler, labeler, ingredient manufacturer, encapsulator) Niche/Community Distributors Own Label Distributors Large Distributors Consumer Notable Recent Actions 2011 Recalls and Alerts U-Prosta prostate health contained undeclared terazosin (FDA drug for BPH) Black Ant sexual enhancement sildenafil (Viagra) Svelte 30 weight loss sibutramine Extenze counterfeit tadalafil and sibutramine Celerite weight loss sibutramine Nite Rider sexual enhancement sildenafil Fruta Planta weight loss sibutramine Passion Coffee sexual enhancement analog of sildenafil Rock Hard Extreme sexual enhancement analog of sildenafil STUD capsules sexual enhancement analog of sildenafil Regenerect Sexual enhancement- analog of sildenafil 20 10
11 Notable Recent Actions Recent Criminal Prosecutions: In early 2010, VMG Global pleaded guilty to a corporate felony and was fined for selling anabolic steroids under the names Tren Xtreme, Mass Xtreme, and others. In December, Mimi Trieu pleaded guilty to an 18 count indictment charging her with the illegal importation and distribution of more than four million diet pills that contained a controlled substance, unapproved drugs, and a possible carcinogen. In January, a man from Kunming China pled guilty to charges relating to trafficking counterfeit versions of the weight loss drug Alli, as well as unapproved weight loss related drugs marketed in the US as dietary supplements. In early February, several individuals were charged with the misbranding and dispensing of steroids sold as dietary supplements 21 Notable Recent Actions Consumer and Industry Outreach Early March New tainted weight loss supplement videos Widget Weight loss fraud website
12 12
13 Other Issues Recalls Antibiotic dietary supplements NTP/NCTR Aloe Other ingredients 26 13
14 Other Issues Conventional Food/Dietary Supplement Regulatory Line Melatonin products Liquid DS Guidance Pesticides 28 14
15 Thanks OCRA Board CDER: Brad Pace; Elizabeth Miller CFSAN: Bob Moore ORA: Gary Coody Questions? 15
STATEMENT OF JOSHUA M. SHARFSTEIN, M.D. PRINCIPAL DEPUTY COMMISSIONER U.S. FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 STATEMENT OF JOSHUA M. SHARFSTEIN, M.D. PRINCIPAL DEPUTY COMMISSIONER U.S. FOOD AND DRUG
DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 STATEMENT OF JOSHUA M. SHARFSTEIN, M.D. PRINCIPAL DEPUTY COMMISSIONER U.S. FOOD AND DRUG
Adverse Event Reports and Dietary Supplements
 Adverse Event Reports and Dietary Supplements Supply Side East April 28, 2010 Michael McGuffin President, American Herbal Products Association mmcguffin@ahpa.org DS & OTC * Consumer Protection Act Sponsors:
Adverse Event Reports and Dietary Supplements Supply Side East April 28, 2010 Michael McGuffin President, American Herbal Products Association mmcguffin@ahpa.org DS & OTC * Consumer Protection Act Sponsors:
CDER Compliance Update
 CDER Compliance Update Douglas Stearn, JD Deputy Director for Policy and Analysis FDA/CDER/Office of Compliance FDLI Enforcement, Litigation and Compliance Conference Washington, DC December 12, 2012 1
CDER Compliance Update Douglas Stearn, JD Deputy Director for Policy and Analysis FDA/CDER/Office of Compliance FDLI Enforcement, Litigation and Compliance Conference Washington, DC December 12, 2012 1
1. Dream Body Slimming Capsule, Asset Extreme, SlimEasy Herbal, SlimExtra Herbal
 1 of 5 1/26/2014 9:52 AM Melanie Haiken, Contributor I'm insatiably curious about how to stay healthy & where to go next. HEALTH 1/26/2014 @ 9:00AM 996 views This week, the FDA issued two new warnings
1 of 5 1/26/2014 9:52 AM Melanie Haiken, Contributor I'm insatiably curious about how to stay healthy & where to go next. HEALTH 1/26/2014 @ 9:00AM 996 views This week, the FDA issued two new warnings
Southern California Section & Western Compendial Discussion Group
 Association for Official and Analytical Chemists Southern California Section & Western Compendial Discussion Group October 6, 2011 Compliance Officer Bill Vitale, Food and Drug Administration Los Angeles
Association for Official and Analytical Chemists Southern California Section & Western Compendial Discussion Group October 6, 2011 Compliance Officer Bill Vitale, Food and Drug Administration Los Angeles
Food Recalls: May 25, 2016 GREENBERG TRAURIG, LLP ATTORNEYS AT LAW Greenberg Traurig, LLP. All rights reserved.
 Food Recalls: 2016 Greenberg Traurig, LLP. Attorneys at Law. All rights reserved. Greenberg Traurig is a trademark and trade name of Greenberg Traurig, LLP and Greenberg Traurig, P.A. This presentation
Food Recalls: 2016 Greenberg Traurig, LLP. Attorneys at Law. All rights reserved. Greenberg Traurig is a trademark and trade name of Greenberg Traurig, LLP and Greenberg Traurig, P.A. This presentation
NDI: LOOKING BACK & AHEAD
 NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
USP Annual Scientific Meeting. Welcome to USP
 USP Annual Scientific Meeting Welcome to USP USP s Mission To improve global health through public standards and related programs that help ensure the quality, safety, and benefit of medicines and foods.
USP Annual Scientific Meeting Welcome to USP USP s Mission To improve global health through public standards and related programs that help ensure the quality, safety, and benefit of medicines and foods.
CDER Compliance Update
 CDER Compliance Update Donald D. Ashley, JD 22 nd Annual GMP by the Sea August 30, 2017 www.fda.gov www.fda.gov 2 Office of Compliance Structure Office of Compliance Office of Drug Security, Integrity
CDER Compliance Update Donald D. Ashley, JD 22 nd Annual GMP by the Sea August 30, 2017 www.fda.gov www.fda.gov 2 Office of Compliance Structure Office of Compliance Office of Drug Security, Integrity
Hello from the other Side of the Dietary Supplement Fence
 Hello from the other Side of the Dietary Supplement Fence Vasilios H. Frankos, Ph.D. Senior Vice President, Product Science, Safety and Compliance Herbalife International FDA Concerns that Impact the Safety
Hello from the other Side of the Dietary Supplement Fence Vasilios H. Frankos, Ph.D. Senior Vice President, Product Science, Safety and Compliance Herbalife International FDA Concerns that Impact the Safety
Border Issues and Statistics: Regulatory Activities. Mr. Robert Deininger Southwest Import Director AFDO San Antonio, Texas
 Border Issues and Statistics: Regulatory Activities Mr. Robert Deininger Southwest Import Director AFDO San Antonio, Texas U.S. Food & Drug Administration Mission Statement The Food & Drug Administration
Border Issues and Statistics: Regulatory Activities Mr. Robert Deininger Southwest Import Director AFDO San Antonio, Texas U.S. Food & Drug Administration Mission Statement The Food & Drug Administration
Overview of FDA Oversight and Enforcement on Drug Compounding
 Overview of FDA Oversight and Enforcement on Drug Compounding Ruey Ju, Pharm.D., J.D. Senior Advisor for Compounding Compliance and Enforcement (Acting) Center for Drug Evaluation and Research Today s
Overview of FDA Oversight and Enforcement on Drug Compounding Ruey Ju, Pharm.D., J.D. Senior Advisor for Compounding Compliance and Enforcement (Acting) Center for Drug Evaluation and Research Today s
FDA Foods Program Update
 FDA Foods Program Update Comments by Stephen F. Sundlof, D.V.M., Ph.D. Director Center for Food Safety and Applied Nutrition JIFSAN Annual Meeting, College Park, MD March 25, 2010 The Food Safety Working
FDA Foods Program Update Comments by Stephen F. Sundlof, D.V.M., Ph.D. Director Center for Food Safety and Applied Nutrition JIFSAN Annual Meeting, College Park, MD March 25, 2010 The Food Safety Working
Regulatory Update: Food Safety and Nutrition
 Regulatory Update: Food Safety and Nutrition Ricardo Carvajal Hyman, Phelps & McNamara, P.C. www.hpm.com www.fdalawblog.net North American Millers Association March 2015 Today s Agenda FSMA Update Biological
Regulatory Update: Food Safety and Nutrition Ricardo Carvajal Hyman, Phelps & McNamara, P.C. www.hpm.com www.fdalawblog.net North American Millers Association March 2015 Today s Agenda FSMA Update Biological
Center for Food Safety & Applied Nutrition: Update
 Center for Food Safety & Applied Nutrition: Update Comments by Ted Elkin Deputy Director for Regulatory Affairs FDA/Center for Food Safety and Applied Nutrition AFDO 2017 Annual Meeting June 20, 2017 Foods
Center for Food Safety & Applied Nutrition: Update Comments by Ted Elkin Deputy Director for Regulatory Affairs FDA/Center for Food Safety and Applied Nutrition AFDO 2017 Annual Meeting June 20, 2017 Foods
Putting the Nutritional Supplement Industry to the Test: Looking for Transparency
 Putting the Nutritional Supplement Industry to the Test: Looking for Transparency Frederick H. Fern @RickFern_HB ffern@harrisbeach.com Marina Plotkin @MarinaPlotkin_HB mplotkin@harrisbeach.com Putting
Putting the Nutritional Supplement Industry to the Test: Looking for Transparency Frederick H. Fern @RickFern_HB ffern@harrisbeach.com Marina Plotkin @MarinaPlotkin_HB mplotkin@harrisbeach.com Putting
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
FDLI ANNUAL CONFERENCE May 4, 2018
 FDLI ANNUAL CONFERENCE May 4, 2018 DIETARY SUPPLEMENTS RETAILER ISSUES AND LIABILITY Jean Frydman, Partner, Chair FDA Practice Fox Rothschild, LLP FDA Concern is Safety Only DHSEA Act of 1994 Know what
FDLI ANNUAL CONFERENCE May 4, 2018 DIETARY SUPPLEMENTS RETAILER ISSUES AND LIABILITY Jean Frydman, Partner, Chair FDA Practice Fox Rothschild, LLP FDA Concern is Safety Only DHSEA Act of 1994 Know what
RESULTS AT A GLANCE. FDA Oversight of Tobacco Manufacturing Establishments. HHS OIG Data Brief August 2017 OEI
 HHS OIG Data Brief August 2017 OEI-01-15-00300 FDA Oversight of Tobacco Manufacturing Establishments RESULTS AT A GLANCE The Tobacco Control Act authorized FDA to regulate domestic tobacco manufacturers
HHS OIG Data Brief August 2017 OEI-01-15-00300 FDA Oversight of Tobacco Manufacturing Establishments RESULTS AT A GLANCE The Tobacco Control Act authorized FDA to regulate domestic tobacco manufacturers
LIVE INTERACTIVE YOUR DESKTOP. Food Recall Process. Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement
 LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Food Recall Process Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement Wednesday, December 9, 2009 LEGAL ISSUES Code of Federal Regulations
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Food Recall Process Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement Wednesday, December 9, 2009 LEGAL ISSUES Code of Federal Regulations
Learn to reduce your risk if you use dietary supplements. Presented by Amy Eichner, PhD for USA Swimming, Dec
 Learn to reduce your risk if you use dietary supplements Presented by Amy Eichner, PhD for USA Swimming, Dec 6 2017 Introduction PERFORMANCE VERSUS SAFETY 2 What we hear all the time Is my supplement safe?
Learn to reduce your risk if you use dietary supplements Presented by Amy Eichner, PhD for USA Swimming, Dec 6 2017 Introduction PERFORMANCE VERSUS SAFETY 2 What we hear all the time Is my supplement safe?
Overview of Dietary Supplement GMP Inspection Trends Quality Session 6
 Overview of Dietary Supplement GMP Inspection Trends Quality Session 6 Presented by: Dean R. Cirotta, MBA President & Chief Operating Officer EAS Consulting Group, LLC 1700 Diagonal Road, Suite 750 Alexandria,
Overview of Dietary Supplement GMP Inspection Trends Quality Session 6 Presented by: Dean R. Cirotta, MBA President & Chief Operating Officer EAS Consulting Group, LLC 1700 Diagonal Road, Suite 750 Alexandria,
PUBLIC HEALTH RISK WITH HERBAL MEDICINES: AN OVERVIEW
 PUBLIC HEALTH RISK WITH HERBAL MEDICINES: AN OVERVIEW Introduction 1. There may be an erroneous perception in some quarters that the practice of herbal medicines poses few safety issues. In fact, given
PUBLIC HEALTH RISK WITH HERBAL MEDICINES: AN OVERVIEW Introduction 1. There may be an erroneous perception in some quarters that the practice of herbal medicines poses few safety issues. In fact, given
FSMA, FSVP, and FCS. Deborah Attwood May 12, 2016 Global Food Contact 2016
 FSMA, FSVP, and FCS Deborah Attwood May 12, 2016 Global Food Contact 2016 Food Safety Modernization Act (FSMA) Became law in January 2011 Improves safety and security of food supply through prevention,
FSMA, FSVP, and FCS Deborah Attwood May 12, 2016 Global Food Contact 2016 Food Safety Modernization Act (FSMA) Became law in January 2011 Improves safety and security of food supply through prevention,
2012 Emerging Trends and Key Issues Report Product Recall & Contamination Risk Management CONTENTS
 2012 Emerging Trends and Key Issues Report Product Recall & Contamination Risk Management The 2012 Emerging Trends and Key Issues Product Recall & Contamination Risk Management Report is published annually
2012 Emerging Trends and Key Issues Report Product Recall & Contamination Risk Management The 2012 Emerging Trends and Key Issues Product Recall & Contamination Risk Management Report is published annually
Mark M. Yacura. Partner
 Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011
 Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
FDA Basics FDA s Import Operations: How FDA Regulates Imported Products
 FDA Basics FDA s Import Operations: How FDA Regulates Imported Products Carlos W. Hernandez Compliance Officer U.S. Food and Drug Administration Global Regulatory Operations and Policy Office of Regulatory
FDA Basics FDA s Import Operations: How FDA Regulates Imported Products Carlos W. Hernandez Compliance Officer U.S. Food and Drug Administration Global Regulatory Operations and Policy Office of Regulatory
FDA Laws & Pharmacy Practice
 Objectives FDA Laws & Pharmacy Practice Tom Hazlet Pharmacy 543 Autumn 2006 Be able to discuss the evolution of food and drug law in the United States Be able to discuss the ways in which pharmacists and
Objectives FDA Laws & Pharmacy Practice Tom Hazlet Pharmacy 543 Autumn 2006 Be able to discuss the evolution of food and drug law in the United States Be able to discuss the ways in which pharmacists and
FDLI s Enforcement, Litigation, and Compliance Conference. Center for Tobacco Products Office of Compliance and Enforcement 2017 Update
 FDLI s Enforcement, Litigation, and Compliance Conference Center for Tobacco Products Office of Compliance and Enforcement 2017 Update Ann Simoneau, Director Office of Compliance and Enforcement Center
FDLI s Enforcement, Litigation, and Compliance Conference Center for Tobacco Products Office of Compliance and Enforcement 2017 Update Ann Simoneau, Director Office of Compliance and Enforcement Center
FDA Food Contact Fundamentals
 FDA Food Contact Fundamentals Deborah Attwood 2017 SPE International Polyolefins Conference March 1, 2017 Legal Highlights for Food 1906: Pure Food and Drug Act 1938: Federal Food, Drug and Cosmetic Act
FDA Food Contact Fundamentals Deborah Attwood 2017 SPE International Polyolefins Conference March 1, 2017 Legal Highlights for Food 1906: Pure Food and Drug Act 1938: Federal Food, Drug and Cosmetic Act
Vitamins & minerals Botanicals Amino acids Probiotics Glandular extracts
 Safety of Dietary Supplements What You Need to Know About the Supplements Your Patients are Taking Pieter A. Cohen, MD Assistant Professor of Medicine, Harvard Medical School What are dietary supplements?
Safety of Dietary Supplements What You Need to Know About the Supplements Your Patients are Taking Pieter A. Cohen, MD Assistant Professor of Medicine, Harvard Medical School What are dietary supplements?
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Products
 Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Nutrition Labeling Laws for Food and Supplements Timeline
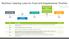 Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Refer to the manufacturers prescribing information or instructions for use before taking any medications.
 Medicines Medicines play an important role in treatment for patients who have had a heart attack or who have heart failure. In most cases, people who have had a heart attack or who have heart failure require
Medicines Medicines play an important role in treatment for patients who have had a heart attack or who have heart failure. In most cases, people who have had a heart attack or who have heart failure require
GROCERY MANUFACTURERS ASSOCIATION. Science Forum. Connecting Sound Science and Responsible Solutions
 GROCERY MANUFACTURERS ASSOCIATION Science Forum Connecting Sound Science and Responsible Solutions FSMA Implementation Michael M. Landa Director, Center for Food Safety and Applied Nutrition Food and Drug
GROCERY MANUFACTURERS ASSOCIATION Science Forum Connecting Sound Science and Responsible Solutions FSMA Implementation Michael M. Landa Director, Center for Food Safety and Applied Nutrition Food and Drug
FSMA & The Dietary Supplements Industry
 FSMA & The Dietary Supplements Industry Overview FDA Food Safety Modernization Act (FSMA) Preventive Controls for Human Food (PCHF) Foreign Supplier Verification Programs (FSVP) Produce Verification activities
FSMA & The Dietary Supplements Industry Overview FDA Food Safety Modernization Act (FSMA) Preventive Controls for Human Food (PCHF) Foreign Supplier Verification Programs (FSVP) Produce Verification activities
U.S. FDA Perspective on Food Supplements/TM
 U.S. FDA Perspective on Food Supplements/TM IKHLAS A. KHAN National Center for Natural Products Research, Department of Pharmacognosy and Research Institute of Pharmaceutical Sciences, School of Pharmacy,
U.S. FDA Perspective on Food Supplements/TM IKHLAS A. KHAN National Center for Natural Products Research, Department of Pharmacognosy and Research Institute of Pharmaceutical Sciences, School of Pharmacy,
Update FDA/Center for Food Safety and Applied Nutrition
 Update FDA/Center for Food Safety and Applied Nutrition Comments by Susan Mayne, Ph.D. Director Center for Food Safety and Applied Nutrition FDLI Annual Conference May 3, 2018 Food Safety, Nutrition and
Update FDA/Center for Food Safety and Applied Nutrition Comments by Susan Mayne, Ph.D. Director Center for Food Safety and Applied Nutrition FDLI Annual Conference May 3, 2018 Food Safety, Nutrition and
Inter-Agency Overlap and Jurisdictional Boundaries
 Inter-Agency Overlap and Jurisdictional Boundaries FDLI Food Advertising, Labeling, and Litigation Conference September 14, 2017 Jessica P. O Connell Covington & Burling LLP jpoconnell@cov.com 1 FDA Approach/Perspective
Inter-Agency Overlap and Jurisdictional Boundaries FDLI Food Advertising, Labeling, and Litigation Conference September 14, 2017 Jessica P. O Connell Covington & Burling LLP jpoconnell@cov.com 1 FDA Approach/Perspective
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years
 The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
Legal Framework: Counterfeit Medicines.
 Legal Framework: Counterfeit Medicines. AIFA and the actions against drugs counterfeiting Marcello Chiavoni Zagreb, November 2014 Public Declaration of transparency/interests* The view and opinions expressed
Legal Framework: Counterfeit Medicines. AIFA and the actions against drugs counterfeiting Marcello Chiavoni Zagreb, November 2014 Public Declaration of transparency/interests* The view and opinions expressed
Regulation of Dietary Supplements
 (name redacted) Analyst in Health Policy December 16, 2014 Congressional Research Service 7-... www.crs.gov R43062 Summary Many Americans take dietary supplements (e.g., vitamins, herbs, sports nutrition
(name redacted) Analyst in Health Policy December 16, 2014 Congressional Research Service 7-... www.crs.gov R43062 Summary Many Americans take dietary supplements (e.g., vitamins, herbs, sports nutrition
Roseann B. Termini, Esq.
 Food Labeling and Food Safety Kind, Naked and Legal Issues... Do you Know who Really regulates Your Pizza and Where Your Beef is From? What is Smart About FOP Labelling? Roseann B. Termini, Esq. www.fortipublications.com
Food Labeling and Food Safety Kind, Naked and Legal Issues... Do you Know who Really regulates Your Pizza and Where Your Beef is From? What is Smart About FOP Labelling? Roseann B. Termini, Esq. www.fortipublications.com
Food and Product safety
 Food and Product safety Role of EU customs Laboratories 6 nov 2015 gcm.koomen@belastingdienst.nl EU Mission of customs authorities Protecting the financial interests of the Community and its Member States;
Food and Product safety Role of EU customs Laboratories 6 nov 2015 gcm.koomen@belastingdienst.nl EU Mission of customs authorities Protecting the financial interests of the Community and its Member States;
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS
 WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
AFDO Conference June 9, Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide
 AFDO Conference June 9, 2009 Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide Government Agencies 483 observations A violation of the FD&C Act involving
AFDO Conference June 9, 2009 Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide Government Agencies 483 observations A violation of the FD&C Act involving
Getting Your Ingredient to Market: Understanding Your Regulatory Options Venable LLP
 Getting Your Ingredient to Market: Understanding Your Regulatory Options 2013 Venable LLP 1 Overview of the Governing Laws 1. The Federal Food, Drug, and Cosmetic Act ("FD&C Act"): Requires foods, cosmetics,
Getting Your Ingredient to Market: Understanding Your Regulatory Options 2013 Venable LLP 1 Overview of the Governing Laws 1. The Federal Food, Drug, and Cosmetic Act ("FD&C Act"): Requires foods, cosmetics,
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations V-SAB Medical Labs,
Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations V-SAB Medical Labs,
Fundamentals of Pharmacology for Veterinary Technicians Chapter 1
 1906 1914 1933 1937 1938 1941 1951 1965 1968 1970 1970 1972 1972 1983 1984 1988 1994 1994 1996 1997 2001 2003 2008 The original Pure Food and Drug Act is passed by Congress on June 30 and signed by President
1906 1914 1933 1937 1938 1941 1951 1965 1968 1970 1970 1972 1972 1983 1984 1988 1994 1994 1996 1997 2001 2003 2008 The original Pure Food and Drug Act is passed by Congress on June 30 and signed by President
NOT FOR HUMAN CONSUMPTION: HOW TO SPOT HIGH-RISK MERCHANTS HIDING IN PLAIN SIGHT
 NOT FOR HUMAN CONSUMPTION: HOW TO SPOT HIGH-RISK MERCHANTS HIDING IN PLAIN SIGHT AGENDA 1 COSMETIC MERCHANTS 2 ADULT PRODUCT MERCHANTS 3 RESEARCH INGREDIENT MERCHANTS 4 BOTANICAL AND FLORAL MERCHANTS 5
NOT FOR HUMAN CONSUMPTION: HOW TO SPOT HIGH-RISK MERCHANTS HIDING IN PLAIN SIGHT AGENDA 1 COSMETIC MERCHANTS 2 ADULT PRODUCT MERCHANTS 3 RESEARCH INGREDIENT MERCHANTS 4 BOTANICAL AND FLORAL MERCHANTS 5
4/26/2013. Libia Lugo Drug Specialist San Juan District Office Investigations Branch
 Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
Science IN THE Public Interest ) ihr nonprofit puhliihc~?/ Nutrition Action FIea!thIcttcr
 CEXTER FOR Science IN THE Public Interest ) ihr nonprofit puhliihc~?/ Nutrition Action FIea!thIcttcr August 20,2007 Linda Silvers FDA Center for Drug Evaluation and Research Office of Compliance MM2 Room
CEXTER FOR Science IN THE Public Interest ) ihr nonprofit puhliihc~?/ Nutrition Action FIea!thIcttcr August 20,2007 Linda Silvers FDA Center for Drug Evaluation and Research Office of Compliance MM2 Room
Levitra According to its FDA-approved product labeling (PI), Levitra is indicated for the treatment of erectile dysfunction.
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Fadwa Almanakly Associate Director, Global Regulatory Affairs Bayer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Fadwa Almanakly Associate Director, Global Regulatory Affairs Bayer
IMPORTANT INFORMATION
 IMPORTANT INFORMATION A patient s guide to treating Premature Ejaculation with PRILIGY (dapoxetine) This guide is intended only for patients who have been prescribed Priligy by their Physician. If you
IMPORTANT INFORMATION A patient s guide to treating Premature Ejaculation with PRILIGY (dapoxetine) This guide is intended only for patients who have been prescribed Priligy by their Physician. If you
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
14 th International Conference of Drug Regulatory Authorities 30 th November December 2010 Singapore
 14 th International Conference of Drug Regulatory Authorities 30 th November 2010 3 December 2010 Singapore CURRENT REGULATORY CHALLENGES RELATING TO HERBAL MEDICINES: ADULTERATION OF HERBAL MEDICINES
14 th International Conference of Drug Regulatory Authorities 30 th November 2010 3 December 2010 Singapore CURRENT REGULATORY CHALLENGES RELATING TO HERBAL MEDICINES: ADULTERATION OF HERBAL MEDICINES
Mini Summit XIV: Clinical Trial Disclosure and Results Reporting Liability under FDAAA, Section 801
 Mini Summit XIV: Clinical Trial Disclosure and Results Reporting Liability under FDAAA, Section 801 Jarilyn Dupont, JD Director of Regulatory Policy, Office of Policy Office of Commissioner, U.S. Food
Mini Summit XIV: Clinical Trial Disclosure and Results Reporting Liability under FDAAA, Section 801 Jarilyn Dupont, JD Director of Regulatory Policy, Office of Policy Office of Commissioner, U.S. Food
The Family Smoking Prevention and Tobacco Control Act s Grant of Authority to the FDA and the Impact on the Grower
 The Family Smoking Prevention and Tobacco Control Act s Grant of Authority to the FDA and the Impact on the Grower Victor L. Moldovan McGuireWoods, LLP The Act The Act governs all manufacturers and importers
The Family Smoking Prevention and Tobacco Control Act s Grant of Authority to the FDA and the Impact on the Grower Victor L. Moldovan McGuireWoods, LLP The Act The Act governs all manufacturers and importers
Report of the Task Force on Drug Diversion through Institutional Outlets
 Report of the Task Force on Drug Diversion through Institutional Outlets Members Present: Wiki Erickson (TX); David Flashover (NY); Donald Gibson (MN); Susan Ksiazek (NY); Wallace Nelson (NC). Not Present:
Report of the Task Force on Drug Diversion through Institutional Outlets Members Present: Wiki Erickson (TX); David Flashover (NY); Donald Gibson (MN); Susan Ksiazek (NY); Wallace Nelson (NC). Not Present:
Public Hearing Before U.S. Food and Drug Administration
 Substances in Food and Labeling; Regulatory Framework for Foods that Companies are Marketing as Functional Foods Public Hearing Before U.S. Food and Drug Administration Ilene Ringel Heller Senior Staff
Substances in Food and Labeling; Regulatory Framework for Foods that Companies are Marketing as Functional Foods Public Hearing Before U.S. Food and Drug Administration Ilene Ringel Heller Senior Staff
FDA Food Safety Modernization Act (FSMA) January 4, 2011
 FDA Food Safety Modernization Act (FSMA) January 4, 2011 FDA Food Safety Modernization Act (FSMA) The Most Sweeping Reform of the U.S. Food Safety Laws in More than 70 Years FDA Food Safety Modernization
FDA Food Safety Modernization Act (FSMA) January 4, 2011 FDA Food Safety Modernization Act (FSMA) The Most Sweeping Reform of the U.S. Food Safety Laws in More than 70 Years FDA Food Safety Modernization
Reishi D. International, Inc. 2/6/18
 Reishi D. International, Inc. 2/6/18 San Francisco District Office 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Via UPS Overnight February 7, 2018 Mr. Zheng Xiong Li, CEO Reishi D. International, Inc.
Reishi D. International, Inc. 2/6/18 San Francisco District Office 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Via UPS Overnight February 7, 2018 Mr. Zheng Xiong Li, CEO Reishi D. International, Inc.
Resolution 59/8 Promotion of measures to target new psychoactive substances and amphetamine-type stimulants
 Resolution 59/8 Promotion of measures to target new psychoactive substances and amphetamine-type stimulants The Commission on Narcotic Drugs, Deeply concerned about the combination of the diversity of
Resolution 59/8 Promotion of measures to target new psychoactive substances and amphetamine-type stimulants The Commission on Narcotic Drugs, Deeply concerned about the combination of the diversity of
U.S. FDA Regulations for Exporting Food to the United States. Presented by: David Lennarz President, Business Development & Operations
 U.S. FDA Regulations for Exporting Food to the United States Presented by: David Lennarz President, Business Development & Operations Overview FDA Overview Food Facility Registration Prior Notice Food
U.S. FDA Regulations for Exporting Food to the United States Presented by: David Lennarz President, Business Development & Operations Overview FDA Overview Food Facility Registration Prior Notice Food
FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS
 FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS Presented by W. Patrick Noonan And Chris Noonan For NUTRITION INDUSTRY ASSOCIATION W. Patrick Noonan, P.C. 21800 Oxnard Street, Suite 840 Woodland Hills,
FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS Presented by W. Patrick Noonan And Chris Noonan For NUTRITION INDUSTRY ASSOCIATION W. Patrick Noonan, P.C. 21800 Oxnard Street, Suite 840 Woodland Hills,
If you wake up to urinate 2 or more times a night, ask your doctor about NOCTIVA
 If you wake up to urinate 2 or more times a night, ask your doctor about NOCTIVA IMPORTANT SAFETY INFORMATION WARNING: HYPONATREMIA See full prescribing information for complete boxed warning. NOCTIVA
If you wake up to urinate 2 or more times a night, ask your doctor about NOCTIVA IMPORTANT SAFETY INFORMATION WARNING: HYPONATREMIA See full prescribing information for complete boxed warning. NOCTIVA
Food and Drug Law. Fall Syllabus. Professor Jesson. Hamline University School of Law
 Food and Drug Law Fall 2009 Syllabus Professor Jesson Hamline University School of Law General course Information: Course: Food and Drug Law Credits: 2 Classroom: Law 103 Time: Thursdays, 4-5:50 p.m. Professor:
Food and Drug Law Fall 2009 Syllabus Professor Jesson Hamline University School of Law General course Information: Course: Food and Drug Law Credits: 2 Classroom: Law 103 Time: Thursdays, 4-5:50 p.m. Professor:
FDA Laws & Pharmacy Practice
 Objectives FDA Laws & Pharmacy Practice Tom Hazlet Pharmacy 543 October 6, 2004 Be able to discuss the evolution of food and drug law in the United States Be able to discuss the ways in which pharmacists
Objectives FDA Laws & Pharmacy Practice Tom Hazlet Pharmacy 543 October 6, 2004 Be able to discuss the evolution of food and drug law in the United States Be able to discuss the ways in which pharmacists
The Shifting Federal Regulation of Cannabis Products
 The Durham Bar 2019 CLE Program The Shifting Federal Regulation of Cannabis Products Erica M. Jackson, FDA Partner K&L Gates February 6, 2019 Copyright 2018 by K&L Gates LLP. All rights reserved. OVERVIEW
The Durham Bar 2019 CLE Program The Shifting Federal Regulation of Cannabis Products Erica M. Jackson, FDA Partner K&L Gates February 6, 2019 Copyright 2018 by K&L Gates LLP. All rights reserved. OVERVIEW
PLAN TO REGULATE THE COMPLEMENTARY MEDICINES INDUSTRY IN SOUTH AFRICA
 PLAN TO REGULATE THE COMPLEMENTARY MEDICINES INDUSTRY IN SOUTH AFRICA René Doms FPS Healthcare Regulatory Consultant Dip Pharm Adv Dip (B&A) BIuris LLB South African Registered Pharmacist Fellow of the
PLAN TO REGULATE THE COMPLEMENTARY MEDICINES INDUSTRY IN SOUTH AFRICA René Doms FPS Healthcare Regulatory Consultant Dip Pharm Adv Dip (B&A) BIuris LLB South African Registered Pharmacist Fellow of the
What Regulators and Other Attorneys Will Not Tell You About FDA, FTC, and Class Action Lawsuits Venable LLP
 What Regulators and Other Attorneys Will Not Tell You About FDA, FTC, and Class Action Lawsuits 2012 Venable LLP 1 Agenda Current Enforcement Trends What is a Claim? Substantiation Hot Topic Claims FDA
What Regulators and Other Attorneys Will Not Tell You About FDA, FTC, and Class Action Lawsuits 2012 Venable LLP 1 Agenda Current Enforcement Trends What is a Claim? Substantiation Hot Topic Claims FDA
Mixing male enhancement pills
 Mixing male enhancement pills Wegmans Recalls Traditional Medicinals Teas Due to Salmonella Risk (Posted: 4/26/2018). FDA Warns Dr. Carolyn Dean of Drug Claims Made for Magnesium, Calcium & Other Mineral
Mixing male enhancement pills Wegmans Recalls Traditional Medicinals Teas Due to Salmonella Risk (Posted: 4/26/2018). FDA Warns Dr. Carolyn Dean of Drug Claims Made for Magnesium, Calcium & Other Mineral
Featured Topic: New Research on Grape Seed and Cancer (4 slides)
 Featured Topic: New Research on Grape Seed and Cancer (4 slides) New Research on Grape Seed Extract and Cancer Researchers exposed colon cancer cells to a specific, tannin-free grape seed extract containing
Featured Topic: New Research on Grape Seed and Cancer (4 slides) New Research on Grape Seed Extract and Cancer Researchers exposed colon cancer cells to a specific, tannin-free grape seed extract containing
Premarket Notification: Analysis of FDA Recall Data
 Premarket Notification: Analysis of FDA Recall Data Director, Medical Device Safety Institute Beth Israel Deaconess Medical Center Boston, MA IOM 510(k) Workshop July 28, 2010 Disclosure FDA Consultant
Premarket Notification: Analysis of FDA Recall Data Director, Medical Device Safety Institute Beth Israel Deaconess Medical Center Boston, MA IOM 510(k) Workshop July 28, 2010 Disclosure FDA Consultant
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Distillers Grains Symposium
 Distillers Grains Symposium REGULATORY ISSUES/FSMA IMPACTS ON PRODUCTION & SALE OF DDGS Leah Wilkinson American Feed Industry Association lwilkinson@afia.org Dallas, TX May 15, 2014 Topics to be Presented
Distillers Grains Symposium REGULATORY ISSUES/FSMA IMPACTS ON PRODUCTION & SALE OF DDGS Leah Wilkinson American Feed Industry Association lwilkinson@afia.org Dallas, TX May 15, 2014 Topics to be Presented
We are not the Boogeyman! Detective A. McMillan Prince William County Police Narcotics Unit
 We are not the Boogeyman! Detective A. McMillan Prince William County Police Narcotics Unit Objectives Provide a background of the law enforcement initiative with the current heroin problem and how we
We are not the Boogeyman! Detective A. McMillan Prince William County Police Narcotics Unit Objectives Provide a background of the law enforcement initiative with the current heroin problem and how we
Physician Off-Label Marketing. FDA Regulations Governing Manufacturers
 February 2012 Physician Off-Label Marketing As physician reimbursement decreases, physicians are increasingly looking to other means to replace lost income and control more of the healthcare dollar. From
February 2012 Physician Off-Label Marketing As physician reimbursement decreases, physicians are increasingly looking to other means to replace lost income and control more of the healthcare dollar. From
Counterfeit Drugs Affect Even Legitimate Sources: Hospitals and Pharmacies
 Counterfeit Drugs Affect Even Legitimate Sources: Hospitals and Pharmacies Charlie Cichon, Executive Director National Association of Drug Diversion Investigators Homeland Security 2014 Each year, more
Counterfeit Drugs Affect Even Legitimate Sources: Hospitals and Pharmacies Charlie Cichon, Executive Director National Association of Drug Diversion Investigators Homeland Security 2014 Each year, more
Counterfeit Pharmaceuticals. in Canada. The counterfeit criminal market. in Canada. No country is immune from counterfeit drugs
 Criminal Intelligence Service Canada August 2006 Counterfeit Pharmaceuticals in Canada The counterfeit criminal market in Canada Counterfeit goods can be categorized into industry sectors: entertainment
Criminal Intelligence Service Canada August 2006 Counterfeit Pharmaceuticals in Canada The counterfeit criminal market in Canada Counterfeit goods can be categorized into industry sectors: entertainment
Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products
 Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Food Commissaries under FSMA and the US FDA model Food Code
 Food Commissaries under FSMA and the US FDA model Food Code Introduction A food commissary is a facility or operation that procures and/or produces foods intended for distribution. A retail or foodservice
Food Commissaries under FSMA and the US FDA model Food Code Introduction A food commissary is a facility or operation that procures and/or produces foods intended for distribution. A retail or foodservice
cgmp (21 CFR 111) Regulation and Compliance Overview
 cgmp (21 CFR 111) Regulation and Compliance Overview Neogen Effective Compliance Seminar September 23, 2014 Michael McGuffin President, American Herbal Products Association mmcguffin@ahpa.org Regulation
cgmp (21 CFR 111) Regulation and Compliance Overview Neogen Effective Compliance Seminar September 23, 2014 Michael McGuffin President, American Herbal Products Association mmcguffin@ahpa.org Regulation
Overview of Regulatory Science of Food Contact Substances
 Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
GDUFA II User Fees. Update on Implementation
 GDUFA II User Fees Update on Implementation Donal Parks, Director Division of User Fee Management and Budget Formulation OM CDER US FDA November 8, 2017 Outline Refresher on GDUFA II fee structure What
GDUFA II User Fees Update on Implementation Donal Parks, Director Division of User Fee Management and Budget Formulation OM CDER US FDA November 8, 2017 Outline Refresher on GDUFA II fee structure What
Problems with the 1906 Act
 Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
ED treatments: PDE5 inhibitors, injections and vacuum devices
 ED treatments: PDE5 inhibitors, injections and vacuum devices Martin Steggall Clinical Nurse Specialist (Erectile Dysfunction and Premature Ejaculation) Barts Health NHS Trust; Associate Dean, Director
ED treatments: PDE5 inhibitors, injections and vacuum devices Martin Steggall Clinical Nurse Specialist (Erectile Dysfunction and Premature Ejaculation) Barts Health NHS Trust; Associate Dean, Director
THE FDA DRUG APPROVAL PROCESS Under the Federal Food, Drug, and Cosmetic (FD&C) Act, FDA is responsible for ensuring that all new drugs are safe and
 THE FDA DRUG APPROVAL PROCESS Under the Federal Food, Drug, and Cosmetic (FD&C) Act, FDA is responsible for ensuring that all new drugs are safe and effective. Before any drug is approved for marketing
THE FDA DRUG APPROVAL PROCESS Under the Federal Food, Drug, and Cosmetic (FD&C) Act, FDA is responsible for ensuring that all new drugs are safe and effective. Before any drug is approved for marketing
Determining Whether A Dietary Supplement Study Requires an Investigational New Drug (IND)
 Determining Whether A Dietary Supplement Study Requires an Investigational New Drug (IND) 02/02/16 description Does a study that claims their dietary supplement promotes healthy joints and cartilage or
Determining Whether A Dietary Supplement Study Requires an Investigational New Drug (IND) 02/02/16 description Does a study that claims their dietary supplement promotes healthy joints and cartilage or
DSQC Overview. Scott Kuzner, Ph.D. USP Global External Affairs Director
 DSQC Overview Scott Kuzner, Ph.D. USP Global External Affairs Director A multi-stakeholder and cross-sector collaborative aimed at improving the quality and safety of products marketed as dietary supplements.
DSQC Overview Scott Kuzner, Ph.D. USP Global External Affairs Director A multi-stakeholder and cross-sector collaborative aimed at improving the quality and safety of products marketed as dietary supplements.
Michelle Sharp, PharmD Director, US Regulatory Affairs Lilly Corporate Center Indianapolis, IN 46285
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Michelle Sharp, PharmD Director, US Regulatory Affairs Lilly Corporate
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Michelle Sharp, PharmD Director, US Regulatory Affairs Lilly Corporate
Substantiation and Risk Management For Dietary Supplement Claims
 Substantiation and Risk Management For Dietary Supplement Claims Jeremy Appleton Director of Scientific Affairs Schwabe North America/Nature s Way Claims and Consequences What are label claims? What are
Substantiation and Risk Management For Dietary Supplement Claims Jeremy Appleton Director of Scientific Affairs Schwabe North America/Nature s Way Claims and Consequences What are label claims? What are
FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality
 FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality Lawrence Deyton, M.S.P.H., M.D. Director, FDA Center for Tobacco Products June 11, 2012 FDA s Vision To make tobaccorelated
FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality Lawrence Deyton, M.S.P.H., M.D. Director, FDA Center for Tobacco Products June 11, 2012 FDA s Vision To make tobaccorelated
Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States
 Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States SOUTH SAN FRANCISCO, Calif. February 14, 2012 Roche and Genentech have been informed that a counterfeit product,
Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States SOUTH SAN FRANCISCO, Calif. February 14, 2012 Roche and Genentech have been informed that a counterfeit product,
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE James Manuso, Ph.D. Chairman, President, and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE James Manuso, Ph.D. Chairman, President, and Chief Executive Officer
Talon Compounding Pharmacy 10/3/17
 Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
GSCI 2202 FOOD FOR HEALTH. Eat to compete: Dietary Supplements
 GSCI 2202 FOOD FOR HEALTH Eat to compete: Dietary Supplements Dietary supplements on the market are: NOT regulated Could contain illegal substances Could be costly Could be harmful Current laws on dietary
GSCI 2202 FOOD FOR HEALTH Eat to compete: Dietary Supplements Dietary supplements on the market are: NOT regulated Could contain illegal substances Could be costly Could be harmful Current laws on dietary
