EDCTP Portfolio Evaluating clinical trials in Africa
|
|
|
- Victor Short
- 5 years ago
- Views:
Transcription
1 EDCTP Portfolio Evaluating clinical trials in Africa - 1 -
2 1.1 Munguambe Pare Toe Asante
3 JCMS 2010 call for Evaluating the Impact of Clinical Trials in Africa supported by EDCTP. Project Acronym (Coordinator) Health Systems Disease Area Trials under study Trial Sites Status Munguambe Malaria/TB A phase IIb proof-of-concept efficacy trial of the RTS,S/AS candidate malaria vaccine in children 1 to 4 years in Mozambique, and in infants in Tanzania A trial of the RTS, S/AS candidate malaria vaccine in newborns in Mozambique and Tanzania A phase III efficacy trial of the RTS,S malaria vaccine candidate in children aged 5 to 17 months, and infants aged 6 to 12 weeks in Gabon, Tanzania, and Mozambique Trial to evaluate alternative antimalarial drugs to sulfadoxine-pyrimethamine (SP) for intermittent preventive treatment in pregnancy (IPTp) in the context of insecticide treated nets in Gabon, Tanzania, and Mozambique (EDCTP funded) Intermittent preventive treatment of malaria in infants (IPTi) with SP in Gabon, Tanzania, and Mozambique A phase II trial of GMZ2.4 malaria vaccine candidate in children 1-5 years in Gabon In vivo trial of combination therapy for treatment of malaria in Tanzania Phase II assessment of TB therapy in Tanzania (EDCTP funded) Pare Toe Malaria Burkina Faso and Zambia: PREGACT (Donor: EDCTP. Status: ongoing) Multicentre trial Safe and efficacious artemisininbased combination treatments for African pregnant women with malaria (PREGACT, NCT ) Burkina Faso Malactres trial (Funder FP7. Status: completed at the end of 2010) In vivo and in vitro efficacy of the recommended first line antimalarial treatments (artemetherlumefantrine and 1. Mozambique - Manhiça study area, which includes the Manhiça District Hospital and 5 health centres (Maragra, Ilha Josina, Taninga, Malavele, and Palmeira). 2. Tanzania - Bagamoyo and Kisarawe Districts. Bagamoyo District - Bagamoyo District Hospital. 3. Gabon - Ogooué et Lacs District - the Albert Schweitzer District Hospital, the Lambarene Regional District Hospital, the Fougamou Regional Rural Hospital, the Makouke Health care centre and 10 dispensaries. 1. Burkina Faso Nanoro: Institut de Recherche en Science de la Santé (IRSS) and Centre Médical avec Antenne chirugicale (CMA). Bobo Dioulasso: IRSS/Centre Muraz and Dafra district hospital. 2. Zambia Nchelenge district 3. Ghana - Kassena-Nankana district: Navrongo Health Research Center. Oongoing Ongoing - 3 -
4 amodiaquine-artesunate) in children with uncomplicated malaria in Burkina Faso (Malactres, NCT ) is carried out in Bobo Dioulasso (Burkina Faso). Ghana, Study 13-Rectal Artesunate. (Funder WHO/TDR and EU. Status: ended). Multi-center double-blind randomisedcontrolled trial that evaluated the effect of rectal artesunate on child mortality in the Kassena-Nankana district of northern Ghana. Asante Malaria Ghana and Kenya : Kintampo and Kilifi Malaria Vaccine Trial phase II efficacy, safety and immunogenicity trial of GSK s RTS,S malaria vaccine with the aim of integrating into routine immunization for infants. Phase I Safety and immunogenicity of heterologous prime-boost with the candidate malaria vaccines AdCh63 ME-TRAP and MVA ME- TRAP in healthy adults in a malaria endemic area the phase IIb and malaria drug trials that preceded the RTS,S vaccine Burkina Faso: Since 2003, CNRFP has conducted three phase I trials among children months old and adults years respectively. Also as part of an EDCTP funded integrated Project IP_08_31100 (MVVC) CNRFP will implement a Phase IIb clinical trial entitled Integrating capacity building and networking in the design and conduct of Phase I and II clinical trials of viral vectored candidate malaria vaccines in East and West African children and infants. This multi-site Phase II trial also involves KEMRI-Kilifi, Kenya. This clinical trial will commence in 2011 and will end in Ghana Kintampo: Kintampo Health Research Centre 2. Kenya Kilifi: KEMRI Kilifi 3. Burkina Faso Ouagadougou: Centre Nacional du Recherche et de formation sur le Paludisme (CNFRP) Ongoing - 4 -
5 1.1 Munguambe EDCTP Project Coordinator: Khatia Munguambe (Manhiça Health Research Center, Mozambique) EDCTP Call Title: JCMS Evaluating the Impact of Clinical Trials in Africa EDCTP Project Title: Evaluating the impact of clinical trials on health services delivered to women and children in three countries (Mozambique, Gabon and Tanzania) of sub-saharan Africa EDCTP Project Code: JC EDCTP Project Start Date: 30 December 2011 EDCTP Project End Date: 29 December 2013 Collaborators: Salim Abdulla (Ifakara Health Research and Development Centre, Tanzania) Selidji Todagbe Agnandji (Albert Schweitzer Hospital, Gabon) John Aponte (Hospital Clinic of Barcelona, Spain) Caterina Guinovart (Hospital Clinic of Barcelona, Spain) Bertrand Lell (Albert Schweitzer Hospital, Gabon) Elisa Sicuri (Centre de Recerca en Salut Internacional de Barcelona (CRESIB), Spain) Site Principal Khatia Munguambe (Mozambique) Investigator(s): Salim Abdulla (Tanzania) Selidji Agnandji (Gabon) Trial/Study title: Evaluating the impact of clinical trials on health services delivered to women and children in three countries (Mozambique, Gabon and Tanzania) of sub-saharan Africa Goal: This study aims to understand the influence of clinical trials targeting women and children on healthcare delivered to these population groups in clinical trial sites in 3 sub-saharan African countries: Mozambique, Tanzania and Gabon Primary Objective(s): 1. The qualitative indicators will be evaluated through data generated by focus group discussions with community members, and case studies of the above listed clinical trials 2. The quantitative assessment will be done through a community based household survey and a health facility survey. Clinical Trial/Study site(s): Manhiça study area, which includes the Manhiça District Hospital and 5 health centres (Maragra, Ilha Josina, Taninga, Malavele, and Palmeira) where clinical trials are being or have been conducted. The control cluster will include the rest of the district, not covered by DSS, with Xinavane Rural Hospital and 4 other health centres (Munguine, Maluana, Calanga, and 3 de Fevereiro) Bagamoyo (74 Km North of Dar-es-salaam) and Kisarawe (40 Km South-west of Dar-es-salaam) Districts. The intervention cluster will be Bagamoyo District - Bagamoyo District Hospital. The control cluster will be Kisarawe district, with Kisarawe District hospital and its surrounding dispensaries, where there is no research infrastructure and no ongoing clinical trials Lambarené study population: Ogooué et Lacs (250 km from Libreville) and Mouila (580 Km from Libreville). The intervention cluster will be Ogooué et Lacs District - the Albert Schweitzer District Hospital, the Lambarene Regional District Hospital, the Fougamou Regional Rural Hospital, the Makouke Health care centre and 10 dispensaries, all previously and/or currently involved in - 5 -
6 clinical trials. The control cluster will be Mouila District with a population of 15,000. Collaborating site(s): Institute of Tropical Medicine, University of Tuebingen (Germany) Hospital Clinic of Barcelona CRESIB (Spain) Study design: Cross-sectional cluster survey comparing intervention and control groups Number of subjects: There will be a total of 6 clusters: one intervention and one control cluster per site. Cofunders: NACCAP (Netherlands) ISCIII (Spain) University of Teubingen (Germany) Status: Ongoing Results and Outcomes: Quantitative and qualitative studies have both started at all sites
7 1.2 Pare Toe EDCTP Project Coordinator: Léa Pare Toé (Centre Muraz, Burkina Faso) EDCTP Call Title: JCMS Evaluating the Impact of Clinical Trials in Africa EDCTP Project Title: Impact of clinical trials on the health behaviours of the communities and the quality of the health services in West and South Africa (Burkina Faso, Ghana and Zambia) EDCTP Project Code: JC EDCTP Project Start Date: 4 October 2011 EDCTP Project End Date: 3 August 2013 Collaborators: James Akazili Ghana (Navrongo Health Research Centre, Ghana) Frank Baiden (Kintampo Health Research Center, Ghana) Victor Chalwe (Tropical Diseases Research Centre, Zambia) Umberto D'Alessandro (Prince Leopold Institute of Tropical Medicine (ITM), Belgium) K. Maxime Drabo (Centre Muraz, Burkina Faso) Derrick Elemu (University of Zambia (UNZA), Zambia) Abraham Hodgson (Navrongo Health Research Centre, Ghana) Koen (Peeters ITM, Belgium) Rafaella Ravinetto (ITM, Belgium) Nancy Soko (Tropical Diseases Research Centre, Zambia) Halidou Tinto (Centre Muraz, Burkina Faso) Site Principal Investigator(s): Trial/Study title: Goal: Primary Objective(s): Lea Pare Toe (Burkina Faso) Victor Chalwe (Zambia) Abraham Hodgson (Ghana) Impact of clinical trials on the health behaviours of the communities and the quality of the health services in West and South Africa (Burkina Faso, Ghana and Zambia) Carry out a comprehensive evaluation of the impact of public health oriented clinical trials on local communities and the corresponding health system. To achieve this, the project will address the following research questions: Does the implementation of clinical trials have an impact on access to care and health facility utilization, i.e. frequency of attendance, early attendance or delay, increased/decreased trust, etc? In case of positive changes, can these be maintained over time and beyond the presence of the research team? If negative or no changes, is there anything that could be done to improve the situation? What is the impact on health staff and health services in terms of: quality of care for all patients; skills and motivation of the health staff; other possible factors of change. Is there any indirect benefit (development of trade, employment or local infrastructure) related to the implementation of the trials, and if yes, can these be maintained? - To analyse the impact of clinical trials on the quality of care and the community s health behaviour - To describe the skills and motivation of the health staff in the context of clinical trial implementation versus the context of no clinical trial - To describe the quality of care for all patients in the context of clinical trial implementation versus the context of no clinical trial - To analyse patients and communities perception of the various benefits and constraints of participation in clinical trials (e.g., free care, loss of time, reimbursements, home visit by - 7 -
8 health workers, risks of participating) - To analyse changes in the patterns of individual/family care seeking (medical, traditional, consultation of various health providers, etc.) during and after a clinical trial. Clinical Trial/Study site(s): Institut de Recherche en Science de la Santé (IRSS) and Centre Médical avec Antenne, Nanoro (Burkina Fasp) chirugicale (CMA). Bobo Dioulasso: IRSS/Centre Muraz and Dafra district hospital Tropical Diseases Research Centre Ndola and University of Zambia, Lusaka, Nchelenge district (Zambia) Kintampo Health Research Center and Navrongo Health Research Center, Kassena-Nankana district (Ghana). Collaborating site(s): Institute of Tropical medicine Prince Leopold (Belgium) Study design: Quantative and qualitative data analysis Cofunders: NACCAP (Netherlands) SIDA (Sweden) Status: Ongoing Results and Outcomes: All qualitative surveys were completed with in total 141 interviews realised in Ghana, 221 in Zambia and 333 in Burkina Faso. The quantitative surveys are ongoing in all three countries
9 1.3 Asante EDCTP Project Coordinator: Kwaku Poku Asante (Kintampo Health Research Center, Ghana) EDCTP Call Title: JCMS Evaluating the Impact of Clinical Trials in Africa EDCTP Project Title: An evaluation of the impact of malaria clinical trials on the delivery of health care, particularly for women and children, in sub-saharan Africa EDCTP Project Code: JC EDCTP Project Start Date: 27 October 2011 EDCTP Project End Date: 26 April 2013 Collaborators: Traore Abdoulaye (Centre national de recherche de Formation sur le Paludisme (CNRFP), Burkina Faso) Bright Akpalu (Kintampo Health Research Center, Ghana) Konate Amadou Tidiana (CNRFP, Burkina Faso) Yaro Jean-Baptiste Bibié (CNRFP, Burkina Faso) Daniel Chandramohan (London School of Hygiene and Tropical Medicine (LSHTM), UK) Roma Chilengi (African Malaria Network Trust, Tanzania) Lawrence Gyabaa Febir (Kintampo Health Research Center, Ghana) Egeruan Babatunde Imoukhuede (European Vaccine Initiative (EVI), Germany) Caroline Jones (Kenya Medical Research Institute (KEMRI), Kenya) Malick Lankoandé (CNRFP, Burkina Faso) Deborah Mogaka (KEMRI, Kenya) Sassy Molyneux (KEMRI, Kenya) Issa Ouedraogo Nebie (CNRFP, Burkina Faso) Seth Owusu-Agyei (Kintampo Health Research Center, Ghana) Benjamin Sombie (CNRFP, Burkina Faso) Charlotte Tawiah-Agyemang (Kintampo Health Research Center, Ghana) Alfred Tiono (CNRFP, Burkina Faso) Jayne Webster (LSHTM, UK) Site Principal Investigator(s): Trial/Study title: Goal: Primary Objective(s): KP Asante (Ghana) Roma Chilengi (Kenya) Amadou Tidiana (Burkina Faso) Alfred Tiono (Burkina Faso) An evaluation of the impact of malaria clinical trials on the delivery of health care, particularly for women and children, in sub-saharan Africa. The study is designed to assess the impact of clinical trials on the communities and facilities where they are conducted, with emphasis on the impact on women and children. The proposed research seeks to address the following principal questions: 1. What are the range of inputs (infrastructural and human resource capacity) that malaria clinical trials bring to trial centres in SSA? 2. What are the expectations and perceptions among health providers, community members, public health service providers, and policy makers of the impacts of clinical trials especially on health services provided for women and children; and specifically with regard to: 1) delivery of routine health care 2) quality of care 3) policy change? 3. What are the key factors that limit or enhance positive and negatives impacts associated with trials (e.g. local and - 9 -
10 international regulations, type of trial, type of implementing institution, level of embeddedness in health systems, nature of the local health system, community engagement strategy, perceptions of the key stakeholders etc)? 4. What are the ways in which clinical trials in SSA are embedded within health facilities and wider health systems and the perceptions of key stakeholders such as health service providers, researchers and policy makers on the different approaches taken? 5. Do the range of inputs, perceptions of community and key health stakeholders, and impacts of clinical trials in SSA change over the life of the trial and in post-trial contexts, and why? Clinical Trial/Study site(s): Kintampo Health Research Centre (Ghana) KEMRI Kilifi (Kenya) Centre National de Recherche et de Formation sur le Paludisme, Ouagadougou (Burkina Faso) Collaborating site(s): London School of Hygiene and Tropical Medicine (UK) KEMRI-Wellcome Trust Research Programme (UK) European Vaccine Initiative (Germany) Study design: Quantative and qualitative data analysis Cofunders: NACCAP (Netherlands) SIDA (Sweden) MRC (UK) Status: Ongoing Results and Outcomes: Qualitative data collection has started and ongoing in Kintampo; data collection is pending in CNRFP and KEMRI Kilifi. Publications:
Status of RTS,S/AS01 malaria vaccine candidate
 Status of RTS,S/AS01 malaria vaccine candidate Multi-center RTS,S malaria vaccine efficacy trial Phase 3, randomized, controlled, double-blind trial conducted in 11 centers in 7 African countries 15,460
Status of RTS,S/AS01 malaria vaccine candidate Multi-center RTS,S malaria vaccine efficacy trial Phase 3, randomized, controlled, double-blind trial conducted in 11 centers in 7 African countries 15,460
Evaluation of 4 artemisinin-based combinations for treating uncomplicated malaria in African children Preliminary results
 Evaluation of 4 artemisinin-based combinations for treating uncomplicated malaria in African children Preliminary results Umberto D Alessandro and the 4ABC study group Objectives Main To compare the safety
Evaluation of 4 artemisinin-based combinations for treating uncomplicated malaria in African children Preliminary results Umberto D Alessandro and the 4ABC study group Objectives Main To compare the safety
NAVRONGO HEALTH INFORMING HEALTH POLICY THROUGH RESEARCH
 NAVRONGO HEALTH RESEARCH CENTRE INFORMING HEALTH POLICY THROUGH RESEARCH Strategic Location of Ghana DSS Sites Burkina Faso Cote d'ivoire Togo Dangbe West Kassena-Nankana Kintampo 50 0 50 100 150 200 250
NAVRONGO HEALTH RESEARCH CENTRE INFORMING HEALTH POLICY THROUGH RESEARCH Strategic Location of Ghana DSS Sites Burkina Faso Cote d'ivoire Togo Dangbe West Kassena-Nankana Kintampo 50 0 50 100 150 200 250
Development of a Malaria Vaccine for Sub-Saharan African Children December 3, 2009 Lode Schuerman
 Development of a Malaria Vaccine for Sub-Saharan African Children December 3, 2009 Lode Schuerman Copyright John-Michael Maas, Darby Communications Agenda Malaria Disease burden Prevention and vaccine
Development of a Malaria Vaccine for Sub-Saharan African Children December 3, 2009 Lode Schuerman Copyright John-Michael Maas, Darby Communications Agenda Malaria Disease burden Prevention and vaccine
Stakeholders meeting on Malaria
 Plans and progress towards EDCTP2 Stakeholders meeting on Malaria 19 September 2013 Austrian Ministry of Science and Research First district of Vienna, Freyung 3, Vienna, Austria. Michael Makanga, MD PhD.
Plans and progress towards EDCTP2 Stakeholders meeting on Malaria 19 September 2013 Austrian Ministry of Science and Research First district of Vienna, Freyung 3, Vienna, Austria. Michael Makanga, MD PhD.
RTS,S malaria candidate vaccine reduces malaria by approximately one-third in African infants
 Embargoed until Friday 9 th November, 0900 London (GMT); 1000 Brussels (CEST); 1100 Cape Town; 0400 (EST) New York RTS,S malaria candidate vaccine reduces malaria by approximately one-third in African
Embargoed until Friday 9 th November, 0900 London (GMT); 1000 Brussels (CEST); 1100 Cape Town; 0400 (EST) New York RTS,S malaria candidate vaccine reduces malaria by approximately one-third in African
Enhancing Immunogenicity of Recombinant Vaccines: Chemical Conjugation
 Enhancing Immunogenicity of Recombinant Vaccines: Chemical Conjugation Malaria vaccine development and a role for conjugates Ashley J Birkett, PhD Director, Research & Development New Cells, New Vaccines
Enhancing Immunogenicity of Recombinant Vaccines: Chemical Conjugation Malaria vaccine development and a role for conjugates Ashley J Birkett, PhD Director, Research & Development New Cells, New Vaccines
Malaria. Dr. Salim Abdulla, Director Ifakara Health Institute, Dar-es-salaam, Tanzania
 Malaria Dr. Salim Abdulla, Director Ifakara Health Institute, Dar-es-salaam, Tanzania Despite 42% reduction since 2000, a child dies every minute in Africa from malaria 207 million malaria cases in 2012,
Malaria Dr. Salim Abdulla, Director Ifakara Health Institute, Dar-es-salaam, Tanzania Despite 42% reduction since 2000, a child dies every minute in Africa from malaria 207 million malaria cases in 2012,
Malaria in pregnancy programmes: challenges and priorities in antimalarial drug development for African pregnant women
 7 th MIM Pan African Malaria Conference 15-20 April 2018 Dakar, Senegal The power of sharing science EDCTP session on Malaria in pregnancy programmes: challenges and priorities in antimalarial drug development
7 th MIM Pan African Malaria Conference 15-20 April 2018 Dakar, Senegal The power of sharing science EDCTP session on Malaria in pregnancy programmes: challenges and priorities in antimalarial drug development
JTEG s Summary of RTS,S/ AS01 Clinical Trial Data
 JTEG s Summary of RTS,S/ AS01 Clinical Trial Data Peter Smith On behalf of the Joint Technical Expert Group (JTEG) based on Cohen et al 2010 RTS,S/AS01 Malaria Vaccine l Based on large segment of P. falciparum
JTEG s Summary of RTS,S/ AS01 Clinical Trial Data Peter Smith On behalf of the Joint Technical Expert Group (JTEG) based on Cohen et al 2010 RTS,S/AS01 Malaria Vaccine l Based on large segment of P. falciparum
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018
 Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Kaya HDSS, Burkina Faso
 Kaya HDSS, Burkina Faso Site Map Brief Introduction Kaya HDSS is located in the North Central region of Burkina Faso (see maps), in the health District of Kaya. It is about 100 km from the capital Ouagadougou.
Kaya HDSS, Burkina Faso Site Map Brief Introduction Kaya HDSS is located in the North Central region of Burkina Faso (see maps), in the health District of Kaya. It is about 100 km from the capital Ouagadougou.
EDCTP Executive diseases of poverty in Africa. It is certainly a very vital platform for networking,
 Vol 3, No 1 EDCTP newsletter January 2008 Executive Director s Note News about Calls and Grants Calls open for application - HIV Treatment - HIV Microbicides - HIV Vaccines Funded projects TB treatment
Vol 3, No 1 EDCTP newsletter January 2008 Executive Director s Note News about Calls and Grants Calls open for application - HIV Treatment - HIV Microbicides - HIV Vaccines Funded projects TB treatment
WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations
 WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations 12-15 February 2018 Salle XI, ILO Building, Geneva, Switzerland Country
WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations 12-15 February 2018 Salle XI, ILO Building, Geneva, Switzerland Country
New vaccine technologies: Promising advances may save more lives
 New vaccine technologies: Promising advances may save more lives Vaccine Technology III June 10, 2010 PATH s vision A world where innovation ensures that health is within reach for everyone. 2 PATH s mission
New vaccine technologies: Promising advances may save more lives Vaccine Technology III June 10, 2010 PATH s vision A world where innovation ensures that health is within reach for everyone. 2 PATH s mission
Malaria Vaccine Pipeline
 Malaria Vaccine Pipeline Perspectives and Challenges Carla Botting World Vaccine Congress Asia 2008 3 June 2008 Discussion Points Scope of the problem: the burden and challenge of malaria Malaria vaccine
Malaria Vaccine Pipeline Perspectives and Challenges Carla Botting World Vaccine Congress Asia 2008 3 June 2008 Discussion Points Scope of the problem: the burden and challenge of malaria Malaria vaccine
Manuscripts for the WHO Evidence Review Group for malaria in pregnancy (MiP-ERG), July 2015
 Malaria Policy Advisory Committee Meeting 5 7 March 2015, Geneva, Switzerland Background document for Session 4 Manuscripts for the WHO Evidence Review Group for malaria in pregnancy (MiP-ERG), July 2015
Malaria Policy Advisory Committee Meeting 5 7 March 2015, Geneva, Switzerland Background document for Session 4 Manuscripts for the WHO Evidence Review Group for malaria in pregnancy (MiP-ERG), July 2015
Navrongo DSS area Kassena-Nankana District. Kilometre
 NAVRONGO HDSS, GHANA Site Map Upper East Region G H A N A Navrongo DSS area Kassena-Nankana District Ghana 0 25 50 Kilometre 0000 5050 100 Kilometre Location of Navrongo HDSS (Monitored Population 149,000)
NAVRONGO HDSS, GHANA Site Map Upper East Region G H A N A Navrongo DSS area Kassena-Nankana District Ghana 0 25 50 Kilometre 0000 5050 100 Kilometre Location of Navrongo HDSS (Monitored Population 149,000)
BRIEFING ON RTS,S/AS01 MALARIA VACCINE FOR THE SEPTEMBER 2012 MEETING OF MPAC
 BRIEFING ON RTS,S/AS01 MALARIA VACCINE FOR THE SEPTEMBER 2012 MEETING OF MPAC Introduction Date: 12 August 2012. Author: WHO Secretariat with input from JTEG Chair The most advanced vaccine candidate against
BRIEFING ON RTS,S/AS01 MALARIA VACCINE FOR THE SEPTEMBER 2012 MEETING OF MPAC Introduction Date: 12 August 2012. Author: WHO Secretariat with input from JTEG Chair The most advanced vaccine candidate against
APPENDICES BACKGROUND PAPER ON THE RTS,S/AS01 MALARIA VACCINE SEPTEMBER 2015
 APPENDICES BACKGROUND PAPER ON THE RTS,S/AS01 MALARIA VACCINE SEPTEMBER 2015 PREPARED BY THE JOINT TECHNICAL EXPERT GROUP ON MALARIA VACCINES (JTEG) AND WHO SECRETARIAT 1 Appendix 1. Clinical studies conduc
APPENDICES BACKGROUND PAPER ON THE RTS,S/AS01 MALARIA VACCINE SEPTEMBER 2015 PREPARED BY THE JOINT TECHNICAL EXPERT GROUP ON MALARIA VACCINES (JTEG) AND WHO SECRETARIAT 1 Appendix 1. Clinical studies conduc
Copyright White et al. Open Access article distributed under the terms of CC BY.
 Immunogenicity of the RTS,S/AS0 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial Michael T White, Robert Verity,
Immunogenicity of the RTS,S/AS0 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial Michael T White, Robert Verity,
Moderate efficacy malaria vaccines as part of comprehensive malaria control and elimination
 Moderate efficacy malaria vaccines as part of comprehensive malaria control and elimination Progress and Limitation of Existing Vector Control Tools Draper & Smith 1960 TRSTMH 54: 342 Cannot afford to
Moderate efficacy malaria vaccines as part of comprehensive malaria control and elimination Progress and Limitation of Existing Vector Control Tools Draper & Smith 1960 TRSTMH 54: 342 Cannot afford to
Malaria vaccine development
 Malaria vaccine development Bernhards Ragama Ogutu Malaria Clinical Trials Alliance INDEPTH-Network INDEPTH@ 10 AGM 24 Sept 2008 Dar es salaam, Tanzania INDEPTH Network Outline Focus on P. Falciparum Need
Malaria vaccine development Bernhards Ragama Ogutu Malaria Clinical Trials Alliance INDEPTH-Network INDEPTH@ 10 AGM 24 Sept 2008 Dar es salaam, Tanzania INDEPTH Network Outline Focus on P. Falciparum Need
Malaria Control in Togo
 Malaria Control in Togo Introduction * Dr Kodjo Morgah In Collaboration with Dr Koubagnine Takpa (Director, EPI) Dr Jérome Agbekou (DPC/WHO/Togo) Dr Stephan Tohon (ICP/MAL/WA) Dr Jean Pierre E. Batchassi
Malaria Control in Togo Introduction * Dr Kodjo Morgah In Collaboration with Dr Koubagnine Takpa (Director, EPI) Dr Jérome Agbekou (DPC/WHO/Togo) Dr Stephan Tohon (ICP/MAL/WA) Dr Jean Pierre E. Batchassi
TRANSLATING RESEARCH INTO POLICY AND PRACTICE - Examples from HDSS Centres
 TRANSLATING RESEARCH INTO POLICY AND PRACTICE - Examples from HDSS Centres Four HDSS centres were selected to provide examples of their work in translating research into policies or practice. As stated
TRANSLATING RESEARCH INTO POLICY AND PRACTICE - Examples from HDSS Centres Four HDSS centres were selected to provide examples of their work in translating research into policies or practice. As stated
Stakeholders opinions and questions regarding the anticipated malaria vaccine in Tanzania
 DOI 10.1186/s12936-016-1209-6 Malaria Journal RESEARCH Open Access Stakeholders opinions and questions regarding the anticipated malaria vaccine in Tanzania Sally Mtenga 1*, Angela Kimweri 1, Idda Romore
DOI 10.1186/s12936-016-1209-6 Malaria Journal RESEARCH Open Access Stakeholders opinions and questions regarding the anticipated malaria vaccine in Tanzania Sally Mtenga 1*, Angela Kimweri 1, Idda Romore
Studies of Epilepsy Epidemiology in Demographic Sites (SEEDS) Kenya Medical Research Institute/WellcomeTrust Kilifi, Kenya INDEPTH SEEDS Group
 Studies of Epilepsy Epidemiology in Demographic Sites (SEEDS) Charles Newton Kenya Medical Research Institute/WellcomeTrust Kilifi, Kenya INDEPTH SEEDS Group Specific Aims prevalence of active convulsive
Studies of Epilepsy Epidemiology in Demographic Sites (SEEDS) Charles Newton Kenya Medical Research Institute/WellcomeTrust Kilifi, Kenya INDEPTH SEEDS Group Specific Aims prevalence of active convulsive
Supplementary figures
 Weight, kg Lumefantrine concentration μg/ml Supplementary figures 8 7 6 5 4 3 2 model data 1 0 0 5 10 15 Days Supplementary Figure 1. Plasma lumefantrine concentrations in African children [1] and model-fitted
Weight, kg Lumefantrine concentration μg/ml Supplementary figures 8 7 6 5 4 3 2 model data 1 0 0 5 10 15 Days Supplementary Figure 1. Plasma lumefantrine concentrations in African children [1] and model-fitted
Maternal, Child and Reproductive Health Initiative
 Maternal, Child and Reproductive Health Initiative Maternal, Child and Reproductive Health Initiative The Maternal, Child and Reproductive Health (MCRH) Initiative works in developing countries to improve
Maternal, Child and Reproductive Health Initiative Maternal, Child and Reproductive Health Initiative The Maternal, Child and Reproductive Health (MCRH) Initiative works in developing countries to improve
Implementing the Abuja Declaration and Plan of Action: the journey so far
 Implementing the Abuja Declaration and Plan of Action: the journey so far The Abuja Declaration African leaders who met on 25 April 2000 in Abuja, Nigeria, laid out the foundation for a sustained battle
Implementing the Abuja Declaration and Plan of Action: the journey so far The Abuja Declaration African leaders who met on 25 April 2000 in Abuja, Nigeria, laid out the foundation for a sustained battle
Site ANRS/UFR-SDS Université de Ouagadougou, Ouagadougou, Burkina Faso; 3
 Establishment of a HIV Vaccine Cohort among Clandestine Female Sex Workers in Ouagadougou, Burkina Faso: Situation Analysis of Sexual and Reproductive Health and HIV Needs Abdramane BERTHE 1, Nicolas MEDA
Establishment of a HIV Vaccine Cohort among Clandestine Female Sex Workers in Ouagadougou, Burkina Faso: Situation Analysis of Sexual and Reproductive Health and HIV Needs Abdramane BERTHE 1, Nicolas MEDA
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Fernandes S, Sicuri E, Kayentao K, et al. Cost-effectiveness
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Fernandes S, Sicuri E, Kayentao K, et al. Cost-effectiveness
ACTIVITY REPORT
 ACTIVITY REPORT 2009-2010 ACTIVITY REPORT 2009-2010 Copyright 2011 by Fundação Manhiça Published by Fundação Manhiça Rua 12 Manhiça Mozambique www.manhica.org Editorial Committee Teresa Machai Tânia Machonisse
ACTIVITY REPORT 2009-2010 ACTIVITY REPORT 2009-2010 Copyright 2011 by Fundação Manhiça Published by Fundação Manhiça Rua 12 Manhiça Mozambique www.manhica.org Editorial Committee Teresa Machai Tânia Machonisse
PROGRESS REPORT ON CHILD SURVIVAL: A STRATEGY FOR THE AFRICAN REGION. Information Document CONTENTS
 29 June 2009 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, 31 August 4 September 2009 Provisional agenda item 9.2 PROGRESS REPORT ON CHILD SURVIVAL: A
29 June 2009 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, 31 August 4 September 2009 Provisional agenda item 9.2 PROGRESS REPORT ON CHILD SURVIVAL: A
66th Annual meeting of the American Society of Tropical Medicine and Hygiene Baltimore, USA
 66th Annual meeting of the American Society of Tropical Medicine and Hygiene Baltimore, USA 5 9 November 2017 EDCTP Wellcome Trust symposium on Challenges and opportunities of conducting clinical trials
66th Annual meeting of the American Society of Tropical Medicine and Hygiene Baltimore, USA 5 9 November 2017 EDCTP Wellcome Trust symposium on Challenges and opportunities of conducting clinical trials
Non-State Providers in Strengthening Health Systems Towards Universal Health Coverage PROJECT SUMMARIES
 Bosnia and Herzegovina Engaging private healthcare providers in implementation of mandatory safety standards in the Republic of Srpska Budimka NOVAKOVIC Medical Faculty, University of Novi Sad Healthcare
Bosnia and Herzegovina Engaging private healthcare providers in implementation of mandatory safety standards in the Republic of Srpska Budimka NOVAKOVIC Medical Faculty, University of Novi Sad Healthcare
European & Developing Countries Clinical Trials Partnership. project portfolio. Networking grants
 European & Developing Countries Clinical Trials Partnership project portfolio Networking grants 1 Networking grants... 2 1.1.1 Marleen Temmerman... 4 1.1.2 Bob Colebunders... 5 1.1.3 Daniel Kyabayinze...
European & Developing Countries Clinical Trials Partnership project portfolio Networking grants 1 Networking grants... 2 1.1.1 Marleen Temmerman... 4 1.1.2 Bob Colebunders... 5 1.1.3 Daniel Kyabayinze...
Site Map. Manhica HDSS, Mozambique
 Site Map Manhica HDSS, Mozambique Brief Introduction to Manhiça HDSS The Manhiça Health Research Center (Centro de Investigação em saúde de Manhiça, CISM in Portuguese) was established in 1996 as part
Site Map Manhica HDSS, Mozambique Brief Introduction to Manhiça HDSS The Manhiça Health Research Center (Centro de Investigação em saúde de Manhiça, CISM in Portuguese) was established in 1996 as part
The authoritative academic voice on malaria research. Improving health worldwide
 The authoritative academic voice on malaria research Improving health worldwide At the 6 th MIM Conference Durban, 6-11 October 2013 Where we work in Africa Economic evaluations of four prevention trials
The authoritative academic voice on malaria research Improving health worldwide At the 6 th MIM Conference Durban, 6-11 October 2013 Where we work in Africa Economic evaluations of four prevention trials
The human immunodeficiency virus/acquired immune
 Vol. 7(4), pp. 44-48, May 2015 DOI: 10.5897/JAHR2014.0320 Article Number: 70F676253212 ISSN 2141-2359 Copyright 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/jahr
Vol. 7(4), pp. 44-48, May 2015 DOI: 10.5897/JAHR2014.0320 Article Number: 70F676253212 ISSN 2141-2359 Copyright 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/jahr
Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data
 Efficacy of malaria vaccines: individual-participant pooled analysis of phase 2 data Philip Bejon, Michael T White, Ally Olotu, Kalifa Bojang, John P A Lusingu, Nahya Salim, Nekoye N Otsyula, Selidji T
Efficacy of malaria vaccines: individual-participant pooled analysis of phase 2 data Philip Bejon, Michael T White, Ally Olotu, Kalifa Bojang, John P A Lusingu, Nahya Salim, Nekoye N Otsyula, Selidji T
Malaria Vaccine Implementation Programme (MVIP) update and framework for policy decision. Mary J Hamel, WHO MPAC, 11 April 2018
 Malaria Vaccine Implementation Programme (MVIP) update and framework for policy decision Mary J Hamel, WHO MPAC, 11 April 2018 MVIP update Background to MVIP MVIP Updates Regulatory Vaccine introduction
Malaria Vaccine Implementation Programme (MVIP) update and framework for policy decision Mary J Hamel, WHO MPAC, 11 April 2018 MVIP update Background to MVIP MVIP Updates Regulatory Vaccine introduction
The Challenge of Malaria
 The Challenge of Malaria Prof. Awa-Marie Coll-Seck Executive Director of RBM Partnership Donors Forum (co-organized by MMV & CRESIB) Barcelona, 15 March 2010 A DECADE OF ROLLING BACK MALARIA: WHERE ARE
The Challenge of Malaria Prof. Awa-Marie Coll-Seck Executive Director of RBM Partnership Donors Forum (co-organized by MMV & CRESIB) Barcelona, 15 March 2010 A DECADE OF ROLLING BACK MALARIA: WHERE ARE
United Nations Millennium Declaration: the 8 Millennium development Goals
 CIH Strategy To develop strong research clusters within specific health research areas in fields of global importance, and to contribute to improvement of health policy (support, care and prevention/health
CIH Strategy To develop strong research clusters within specific health research areas in fields of global importance, and to contribute to improvement of health policy (support, care and prevention/health
Lessons learned from the IeDEA West Africa Collaboration
 Lessons learned from the IeDEA West Africa Collaboration François DABIS with the contribution of Didier Koumavi EKOUEVI INSERM U-897, Bordeaux, France, Programme PACCI, ANRS site, Abidjan, Côte d Ivoire
Lessons learned from the IeDEA West Africa Collaboration François DABIS with the contribution of Didier Koumavi EKOUEVI INSERM U-897, Bordeaux, France, Programme PACCI, ANRS site, Abidjan, Côte d Ivoire
Diagnostics and control interventions for taeniosis and cysticercosis in Zambia. Chummy Sikalizyo SIKASUNGE
 Diagnostics and control interventions for taeniosis and cysticercosis in Zambia Chummy Sikalizyo SIKASUNGE Stakeholder meeting on T. solium Taeniais/cysticercosis Diagnostic tools, WHO HQ, Geneva, Switzerland,
Diagnostics and control interventions for taeniosis and cysticercosis in Zambia Chummy Sikalizyo SIKASUNGE Stakeholder meeting on T. solium Taeniais/cysticercosis Diagnostic tools, WHO HQ, Geneva, Switzerland,
1 introduction strengthening and ac- celerating future vaccine innovation and development for diseases of poverty.
 European Vaccine Initiative 1 Introduction Infectious diseases are key contributors to the devastating poverty in much of the world today. Every year diseases of poverty kill almost 9 million people, many
European Vaccine Initiative 1 Introduction Infectious diseases are key contributors to the devastating poverty in much of the world today. Every year diseases of poverty kill almost 9 million people, many
ORIGINAL RESEARCH. Stanley Mwita 1, Angela Jesse 2, Deogratius Katabaro 1, Carol Marwa 3, Deodatus Ruganuza 4 INTRODUCTION.
 ORIGINAL RESEARCH www.ijcmr.com in Treatment of Uncomplicated Malaria in Private Medicines Outlets: Practice of Medicines Dispenser s in Mwanza Region, Tanzania Stanley Mwita 1, Angela Jesse 2, Deogratius
ORIGINAL RESEARCH www.ijcmr.com in Treatment of Uncomplicated Malaria in Private Medicines Outlets: Practice of Medicines Dispenser s in Mwanza Region, Tanzania Stanley Mwita 1, Angela Jesse 2, Deogratius
Fighting Harder and Smarter Against Malaria. Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010
 Fighting Harder and Smarter Against Malaria Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010 Outline Burden of malaria Global support for rolling back malaria
Fighting Harder and Smarter Against Malaria Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010 Outline Burden of malaria Global support for rolling back malaria
INDEPTH effectiveness and Safety studies for antimalarials(iness)
 INDEPTH effectiveness and Safety studies for antimalarials() Fred Binka Principal Investigator on behalf of The Team Objectives Objective 1: to develop and maintain phase IV effectiveness studies of antimalarials
INDEPTH effectiveness and Safety studies for antimalarials() Fred Binka Principal Investigator on behalf of The Team Objectives Objective 1: to develop and maintain phase IV effectiveness studies of antimalarials
Addressing Malaria in Pregnancy: A Comprehensive Approach to Maternal and Newborn Health Outcomes
 Addressing Malaria in Pregnancy: A Comprehensive Approach to Maternal and Newborn Health Outcomes Malaria is a major public health crisis, especially in sub-saharan Africa, where 90% of all malaria-related
Addressing Malaria in Pregnancy: A Comprehensive Approach to Maternal and Newborn Health Outcomes Malaria is a major public health crisis, especially in sub-saharan Africa, where 90% of all malaria-related
public facility in the same area context of AMFm
 A CASE CONTROL STUDY OF FACTORS INFLUENCING CARE SEEKING FOR MALARIA IN CHEMICAL SHOPS INVOLVED IN THE DANGME WEST CLUSTER RANDOMIZED TRIAL OFVRAPID DIAGNOSTIC TESTS FOR MALARIA (Dangme CommRDT Study)
A CASE CONTROL STUDY OF FACTORS INFLUENCING CARE SEEKING FOR MALARIA IN CHEMICAL SHOPS INVOLVED IN THE DANGME WEST CLUSTER RANDOMIZED TRIAL OFVRAPID DIAGNOSTIC TESTS FOR MALARIA (Dangme CommRDT Study)
Malaria Vaccine Implementation Programme Framework for Policy Decision
 Malaria Vaccine Implementation Programme Framework for Policy Decision Mary J Hamel, IVR SAGE 17 April 2018 1 Questions for SAGE on the Framework for Policy Decision 1. Does SAGE agree with the approach?
Malaria Vaccine Implementation Programme Framework for Policy Decision Mary J Hamel, IVR SAGE 17 April 2018 1 Questions for SAGE on the Framework for Policy Decision 1. Does SAGE agree with the approach?
Proportion (%) of population with haemoglobin below:
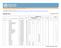 Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Anaemia The database on Anaemia includes data by country on prevalence of anaemia and mean haemoglobin concentration BURKINA
Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Anaemia The database on Anaemia includes data by country on prevalence of anaemia and mean haemoglobin concentration BURKINA
Malaria parasite vaccine development Strategies & Targets
 Malaria parasite vaccine development Strategies & Targets Tulane University Ahmed Aly Most malaria disease deaths are among children and pregnant women A child or a pregnant woman dies of malaria nearly
Malaria parasite vaccine development Strategies & Targets Tulane University Ahmed Aly Most malaria disease deaths are among children and pregnant women A child or a pregnant woman dies of malaria nearly
Progress Towards the Child Mortality MDG in Urban Sub-Saharan Africa. Nyovani Janet Madise University of Southampton
 Progress Towards the Child Mortality MDG in Urban Sub-Saharan Africa Nyovani Janet Madise University of Southampton United Nations Expert group Meeting on Population Distribution, Urbanization, Internal
Progress Towards the Child Mortality MDG in Urban Sub-Saharan Africa Nyovani Janet Madise University of Southampton United Nations Expert group Meeting on Population Distribution, Urbanization, Internal
Ending Malaria in Nigeria: The WHO Agenda
 Nigeria Institute of Medical Research 2016 World Malaria Day Lecture 27 April, 2016 Ending Malaria in Nigeria: The WHO Agenda Dr Tolu Arowolo Malaria Containment Programme, WHO, Nigeria arowolot@who.int
Nigeria Institute of Medical Research 2016 World Malaria Day Lecture 27 April, 2016 Ending Malaria in Nigeria: The WHO Agenda Dr Tolu Arowolo Malaria Containment Programme, WHO, Nigeria arowolot@who.int
Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana
 11 Hofmeyr GJ, Nikodem VC, de Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour a randomised placebocontrolled trial. S Afr Med J 2001;91:432-5. 12 Lumbiganon P, Villar
11 Hofmeyr GJ, Nikodem VC, de Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour a randomised placebocontrolled trial. S Afr Med J 2001;91:432-5. 12 Lumbiganon P, Villar
Malaria Funding. Richard W. Steketee MACEPA, PATH. April World Malaria Day 2010, Seattle WA
 Malaria Funding Richard W. Steketee MACEPA, PATH April World Malaria Day 2010, Seattle WA Malaria Funding Is there a plan? Is there money? Where does the money come from? Is the money moving efficiently?
Malaria Funding Richard W. Steketee MACEPA, PATH April World Malaria Day 2010, Seattle WA Malaria Funding Is there a plan? Is there money? Where does the money come from? Is the money moving efficiently?
How to scale up delivery of malaria control interventions:
 How to scale up delivery of malaria control interventions: A systematic review using insecticide-treated nets, intermittent preventive treatment in pregnancy, and artemisinin combination treatment as tracer
How to scale up delivery of malaria control interventions: A systematic review using insecticide-treated nets, intermittent preventive treatment in pregnancy, and artemisinin combination treatment as tracer
RTS,S/AS01 Candidate Malaria vaccine Summary for the SAGE meeting
 RTS,S/AS01 Candidate Malaria vaccine Summary for the SAGE meeting October 2009 This document is submitted to SAGE upon request from WHO for the sole purpose of publication in the Yellow book in preparation
RTS,S/AS01 Candidate Malaria vaccine Summary for the SAGE meeting October 2009 This document is submitted to SAGE upon request from WHO for the sole purpose of publication in the Yellow book in preparation
Estimating the potential public health impact of seasonal malaria chemoprevention in African children
 Received 16 Feb 212 Accepted 3 Apr 212 Published 6 Jun 212 DOI: 1.138/ncomms1879 Estimating the potential public health impact of seasonal malaria chemoprevention in African children Matthew Cairns 1,
Received 16 Feb 212 Accepted 3 Apr 212 Published 6 Jun 212 DOI: 1.138/ncomms1879 Estimating the potential public health impact of seasonal malaria chemoprevention in African children Matthew Cairns 1,
Antimalarials in the WHO Essential Drugs List for Children Reviewer No.1
 Antimalarials in the WHO Essential Drugs List for Children Reviewer No.1 Part I: Evaluation of the current list Proposed grouping from the March 2007 meeting 6.5.3 Antimalarial medicines 6.5.3.1 For curative
Antimalarials in the WHO Essential Drugs List for Children Reviewer No.1 Part I: Evaluation of the current list Proposed grouping from the March 2007 meeting 6.5.3 Antimalarial medicines 6.5.3.1 For curative
Seasonal Malaria Chemo-prevention (SMC) From Doctoral Research to Health Policy and Regional Scaling-Up. Dr. Badara Cissé Med.
 Seasonal Malaria Chemo-prevention (SMC) From Doctoral Research to Health Policy and Regional Scaling-Up Dr. Badara Cissé Med. epidemiologist Objectives Define the concept and recall natural history Summarise
Seasonal Malaria Chemo-prevention (SMC) From Doctoral Research to Health Policy and Regional Scaling-Up Dr. Badara Cissé Med. epidemiologist Objectives Define the concept and recall natural history Summarise
Executive Summary. Emergency meeting EBOLA Lessons learned from past Ebola outbreaks to inform current risk management. Dar es Salaam, Tanzania
 Executive Summary Emergency meeting EBOLA Lessons learned from past Ebola outbreaks to inform current risk management Dar es Salaam, Tanzania 1st 2nd September 2014 London, Lyon, Dar, 7th September 2014
Executive Summary Emergency meeting EBOLA Lessons learned from past Ebola outbreaks to inform current risk management Dar es Salaam, Tanzania 1st 2nd September 2014 London, Lyon, Dar, 7th September 2014
WHO/GMP TECHNICAL EXPERT GROUP ON PREVENTIVE CHEMOTHERAPY, Geneva 4-6 May 2011
 WHO/GMP TECHNICAL EXPERT GROUP ON PREVENTIVE CHEMOTHERAPY, Geneva 4-6 May 2011 Background Report of the Technical consultation on Seasonal Malaria Chemoprevention (SMC) / Chimio-prévention saisonnière
WHO/GMP TECHNICAL EXPERT GROUP ON PREVENTIVE CHEMOTHERAPY, Geneva 4-6 May 2011 Background Report of the Technical consultation on Seasonal Malaria Chemoprevention (SMC) / Chimio-prévention saisonnière
IHI Biomedical Sciences Group. Joseph Mugasa, PhD, PD. Stakeholders Meeting
 IHI Biomedical Sciences Group Joseph Mugasa, PhD, PD Stakeholders Meeting Jan 2013 Overview Biomedical group carries out a range of laboratory studies with the aim of understanding the pathogenesis, diagnosis
IHI Biomedical Sciences Group Joseph Mugasa, PhD, PD Stakeholders Meeting Jan 2013 Overview Biomedical group carries out a range of laboratory studies with the aim of understanding the pathogenesis, diagnosis
Francine Ntoumi PhD, HDR, FRCP Coordinator Fondation Congolaise pour la Recherche Medicale, Brazzaville, Congo
 Pan-African Network for Rapid Research, Response, Relief and Preparedness for Infectious Diseases Epidemics Francine Ntoumi PhD, HDR, FRCP Coordinator Fondation Congolaise pour la Recherche Medicale, Brazzaville,
Pan-African Network for Rapid Research, Response, Relief and Preparedness for Infectious Diseases Epidemics Francine Ntoumi PhD, HDR, FRCP Coordinator Fondation Congolaise pour la Recherche Medicale, Brazzaville,
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/41537 holds various files of this Leiden University dissertation Author: Mombo-Ngoma, Ghyslain Title: Parasitic infections during pregnancy : birth outcomes
Cover Page The handle http://hdl.handle.net/1887/41537 holds various files of this Leiden University dissertation Author: Mombo-Ngoma, Ghyslain Title: Parasitic infections during pregnancy : birth outcomes
Women by area: Urban F Women by area: Rural F
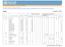 Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Vitamin A Deficiency The Vitamin A Deficiency database includes data by country based on xerophthalmia and/or serum or plasma
Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Vitamin A Deficiency The Vitamin A Deficiency database includes data by country based on xerophthalmia and/or serum or plasma
H. Tinto 1,2, E. B. Zoungrana 1, S. O. Coulibaly 1, J. B. Ouedraogo 1,2, M. Traoré 1,2, T. R. Guiguemde 1, E. Van Marck 3 and U.
 Tropical Medicine and International Health volume7no11pp925 930november2002 Chloroquine and sulphadoxine-pyrimethamine efficacy for uncomplicated malaria treatment and haematological recovery in children
Tropical Medicine and International Health volume7no11pp925 930november2002 Chloroquine and sulphadoxine-pyrimethamine efficacy for uncomplicated malaria treatment and haematological recovery in children
Executive summary... 1 Background... 3 EPI Serology Study Design... 5 Results Pooled Analysis... 12
 WHO Advisory Committee on serological responses to Expanded Programme on Immunization vaccines in infants receiving Intermittent Preventive Treatment for malaria (IPTi) FINAL REPORT October 8, 2009 Contents
WHO Advisory Committee on serological responses to Expanded Programme on Immunization vaccines in infants receiving Intermittent Preventive Treatment for malaria (IPTi) FINAL REPORT October 8, 2009 Contents
Targeting Poverty and Gender Inequality to Improve Maternal Health
 Targeting Poverty and Gender Inequality to Improve Maternal Health presented by Rekha Mehra, Ph.D. Based on paper by: Silvia Paruzzolo, Rekha Mehra, Aslihan Kes, Charles Ashbaugh Expert Panel on Fertility,
Targeting Poverty and Gender Inequality to Improve Maternal Health presented by Rekha Mehra, Ph.D. Based on paper by: Silvia Paruzzolo, Rekha Mehra, Aslihan Kes, Charles Ashbaugh Expert Panel on Fertility,
Driving access to medicine
 Driving access to medicine An example from the Novartis Malaria Initiative Hans Rietveld Director, Market Access & Capacity Building Novartis Malaria Initiative Social Forum - Geneva February 20, 2015
Driving access to medicine An example from the Novartis Malaria Initiative Hans Rietveld Director, Market Access & Capacity Building Novartis Malaria Initiative Social Forum - Geneva February 20, 2015
Learning from the past: How prepared are we for future HIV vaccine efficacy trials in Uganda
 Learning from the past: How prepared are we for future HIV vaccine efficacy trials in Uganda Pontiano Kaleebu MRC-UVRI Uganda Research Unit on AIDS & UVRI-IAVI HIV Vaccine Programme Past 1987 Cohort studies
Learning from the past: How prepared are we for future HIV vaccine efficacy trials in Uganda Pontiano Kaleebu MRC-UVRI Uganda Research Unit on AIDS & UVRI-IAVI HIV Vaccine Programme Past 1987 Cohort studies
Issue 9: January March, 2017 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 9: January March, 2017 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key activities undertaken in First
Issue 9: January March, 2017 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key activities undertaken in First
1978 WHO primary health care 2000
 2008 Vol. 20 No. 2183 1 20 3 1911 1 17 19 1 2 1978 WHO primary health care 2000 2 Key wordsmdg 222 0036 3211 184 2008 1 1 2015 1 1 1990 1 2 2015 1990 2 3 4 5 2015 5 1990 3 1 5 6 HIV 7 HIV 2015 8 2015 7
2008 Vol. 20 No. 2183 1 20 3 1911 1 17 19 1 2 1978 WHO primary health care 2000 2 Key wordsmdg 222 0036 3211 184 2008 1 1 2015 1 1 1990 1 2 2015 1990 2 3 4 5 2015 5 1990 3 1 5 6 HIV 7 HIV 2015 8 2015 7
Women s Paid Labor Force Participation and Child Immunization: A Multilevel Model Laurie F. DeRose Kali-Ahset Amen University of Maryland
 Women s Paid Labor Force Participation and Child Immunization: A Multilevel Model Laurie F. DeRose Kali-Ahset Amen University of Maryland September 23, 2005 We estimate the effect of women s cash work
Women s Paid Labor Force Participation and Child Immunization: A Multilevel Model Laurie F. DeRose Kali-Ahset Amen University of Maryland September 23, 2005 We estimate the effect of women s cash work
Seasonality in malaria transmission: implications for case management with long acting artemisinin combination therapy in sub Saharan Africa
 DOI 1.1186/s12936-15-839-4 RESEARCH Open Access Seasonality in malaria transmission: implications for case management with long acting artemisinin combination therapy in sub Saharan Africa Matthew E Cairns
DOI 1.1186/s12936-15-839-4 RESEARCH Open Access Seasonality in malaria transmission: implications for case management with long acting artemisinin combination therapy in sub Saharan Africa Matthew E Cairns
Malaria Morbidity in High and Seasonal Malaria Transmission Area of Burkina Faso
 Malaria Morbidity in High and Seasonal Malaria Transmission Area of Burkina Faso Alphonse Ouédraogo 1, Alfred B. Tiono 1, Amidou Diarra 1, Souleymane Sanon 1, Jean Baptiste Yaro 1, Esperance Ouedraogo
Malaria Morbidity in High and Seasonal Malaria Transmission Area of Burkina Faso Alphonse Ouédraogo 1, Alfred B. Tiono 1, Amidou Diarra 1, Souleymane Sanon 1, Jean Baptiste Yaro 1, Esperance Ouedraogo
Mass drug Administration When Is It Useful
 Mass drug Administration When Is It Useful Kwaku Poku Asante, MD MPH PhD Kintampo Health Research Centre, Ghana Malaria Control and Elimination 8-9 December 2016, Congress Center Basel, Switzerland MDA
Mass drug Administration When Is It Useful Kwaku Poku Asante, MD MPH PhD Kintampo Health Research Centre, Ghana Malaria Control and Elimination 8-9 December 2016, Congress Center Basel, Switzerland MDA
AFRICA. The continent of All challenges
 AFRICA The continent of All challenges Part of Resources and burden of the disease in Africa Africa=25% Rest of the World=75% Africa=0.6% Rest of the World=99.4% 0% 20% 40% 60% 80% 100% 57 Critical shortage
AFRICA The continent of All challenges Part of Resources and burden of the disease in Africa Africa=25% Rest of the World=75% Africa=0.6% Rest of the World=99.4% 0% 20% 40% 60% 80% 100% 57 Critical shortage
Issue 8: January December 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 8: January December National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key Activities Undertaken in 2 Malaria
Issue 8: January December National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key Activities Undertaken in 2 Malaria
Summary World Malaria Report 2010
 Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
The Global Fund & UNICEF Partnership
 The Global Fund & UNICEF Partnership Prof Michel D. Kazatchkine Executive Director UNICEF Executive Board February 9 th, 2011 The Global Fund Millennium Development Goals 1. Eradicate extreme poverty and
The Global Fund & UNICEF Partnership Prof Michel D. Kazatchkine Executive Director UNICEF Executive Board February 9 th, 2011 The Global Fund Millennium Development Goals 1. Eradicate extreme poverty and
MMV s Access & Product Management Strategy
 MMV s Access & Product Management Strategy Siem Reap, Cambodia 24-26 February 2015 George Jagoe, EVP, Access & Product Mgmt Defeating Malaria Together OUR MISSION to reduce the burden of malaria in disease-endemic
MMV s Access & Product Management Strategy Siem Reap, Cambodia 24-26 February 2015 George Jagoe, EVP, Access & Product Mgmt Defeating Malaria Together OUR MISSION to reduce the burden of malaria in disease-endemic
abstract n engl j med 374;26 nejm.org June 30,
 The new england journal of medicine established in 1812 June 30, 2016 vol. 374 no. 26 Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children Ally Olotu, Ph.D., Gregory Fegan, Ph.D.,
The new england journal of medicine established in 1812 June 30, 2016 vol. 374 no. 26 Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children Ally Olotu, Ph.D., Gregory Fegan, Ph.D.,
*Corresponding author. 1 Mailman School of Public Health, Columbia University, New York, USA 2 Ifakara Health Institute, Dar es Salaam, Tanzania
 Do women bypass village services for better maternal health care in clinics? A case study of antenatal care seeking in three rural Districts of Tanzania Christine E. Chung 1*, Almamy M. Kante, 1,2, Amon
Do women bypass village services for better maternal health care in clinics? A case study of antenatal care seeking in three rural Districts of Tanzania Christine E. Chung 1*, Almamy M. Kante, 1,2, Amon
STATISTICS DATAS AND GEOSPATIAL INFORMATIONS IN THE SERVICE OF ACHIEVING THE OBJECTIVES OF SUSTAINABLE DEVELOPMENT IN BURKINA FASO
 UNITED NATIONS ECONOMIC COMMISSION FOR AFRICA (ECA) International Workshop on Global Fundamental Geospatial Data Themes for Africa STATISTICS DATAS AND GEOSPATIAL INFORMATIONS IN THE SERVICE OF ACHIEVING
UNITED NATIONS ECONOMIC COMMISSION FOR AFRICA (ECA) International Workshop on Global Fundamental Geospatial Data Themes for Africa STATISTICS DATAS AND GEOSPATIAL INFORMATIONS IN THE SERVICE OF ACHIEVING
Community knowledge, perceptions and practices on malaria in Mpwapwa District, central Tanzania
 Tanzania Health Research Bulletin (2004), Vol. 6, No. 2 37 Community knowledge, perceptions and practices on malaria in Mpwapwa District, central Tanzania L.E.G. MBOERA 1, M.L. KAMUGISHA 2, V. BARONGO
Tanzania Health Research Bulletin (2004), Vol. 6, No. 2 37 Community knowledge, perceptions and practices on malaria in Mpwapwa District, central Tanzania L.E.G. MBOERA 1, M.L. KAMUGISHA 2, V. BARONGO
Ebola - retour d expérience sur la mise en place d essais vaccinaux
 De fis Scientifiques et Economiques du De veloppement de Vaccins pour les Pays du Sud 2 & 3 Décembre 2015, Institut Pasteur, Paris Ebola - retour d expérience sur la mise en place d essais vaccinaux Pr
De fis Scientifiques et Economiques du De veloppement de Vaccins pour les Pays du Sud 2 & 3 Décembre 2015, Institut Pasteur, Paris Ebola - retour d expérience sur la mise en place d essais vaccinaux Pr
EDCTP Third Forum PARTNERSHIP AND AFRICAN LEADERSHIP IN VACCINE RESEARCH AND HIV/AIDS DRUG. MBOUP Souleymane
 EDCTP Third Forum PARTNERSHIP AND AFRICAN LEADERSHIP IN VACCINE RESEARCH AND HIV/AIDS DRUG MBOUP Souleymane HIV VACCINE IN AFRICA In 2005, 13 new trials of preventive AIDS vaccine candidates began in 9
EDCTP Third Forum PARTNERSHIP AND AFRICAN LEADERSHIP IN VACCINE RESEARCH AND HIV/AIDS DRUG MBOUP Souleymane HIV VACCINE IN AFRICA In 2005, 13 new trials of preventive AIDS vaccine candidates began in 9
Jaderson Lima, MD On behalf of François Bompart, MD
 Challenges and Successes of the FACT Project through Innovative Partnerships for the Development of Artesunate Combination Therapies for Malaria 5º. ENIFarMed São Paulo Brazil August 2011 Public-private
Challenges and Successes of the FACT Project through Innovative Partnerships for the Development of Artesunate Combination Therapies for Malaria 5º. ENIFarMed São Paulo Brazil August 2011 Public-private
GRADE tables to assist guideline development and recommendations. Plain Language Summary of Results
 Seasonal Malaria Chemoprevention (formally known as Intermittent Preventive Treatment in children) for preventing malaria morbidity in children aged less than 5 years living in areas of marked seasonal
Seasonal Malaria Chemoprevention (formally known as Intermittent Preventive Treatment in children) for preventing malaria morbidity in children aged less than 5 years living in areas of marked seasonal
Profile: Manhiça Health Research Centre (Manhiça HDSS)
 Published by Oxford University Press on behalf of the International Epidemiological Association ß The Author 2013; all rights reserved. International Journal of Epidemiology 2013;42:1309 1318 doi:10.1093/ije/dyt148
Published by Oxford University Press on behalf of the International Epidemiological Association ß The Author 2013; all rights reserved. International Journal of Epidemiology 2013;42:1309 1318 doi:10.1093/ije/dyt148
Comparison of the cost effectiveness of LLINs, SMC, the RTS,S vaccine and RTS,S plus IPTi in African settings.
 Comparison of the cost effectiveness of LLINs, SMC, the RTS,S vaccine and RTS,S plus IPTi in African settings. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease Epidemiology,
Comparison of the cost effectiveness of LLINs, SMC, the RTS,S vaccine and RTS,S plus IPTi in African settings. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease Epidemiology,
Tanzania s Progress in Combating Malaria: Achievement and Challenges
 Tanzania s Progress in Combating Malaria: Achievement and Challenges DR RENATA A MANDIKE DEPUTY PROGRAMME MANAGER NATIONAL MALARIA CONTROL PROGRAMME, MINISTRY OF HEALTH, COMMUNITY DEVELOPMENT, GENDER,
Tanzania s Progress in Combating Malaria: Achievement and Challenges DR RENATA A MANDIKE DEPUTY PROGRAMME MANAGER NATIONAL MALARIA CONTROL PROGRAMME, MINISTRY OF HEALTH, COMMUNITY DEVELOPMENT, GENDER,
THE PRESIDENT S MALARIA INITIATIVE
 Executive Summary THE PRESIDENT S MALARIA INITIATIVE Sixth Annual Report to Congress April 2012 Maggie Hallahan Photography Executive Summary Over the past five years, many African countries have reported
Executive Summary THE PRESIDENT S MALARIA INITIATIVE Sixth Annual Report to Congress April 2012 Maggie Hallahan Photography Executive Summary Over the past five years, many African countries have reported
Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights Page 1 Malaria Burden Page 2 Key Activities undertaken
Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights Page 1 Malaria Burden Page 2 Key Activities undertaken
